Note: Descriptions are shown in the official language in which they were submitted.
~ ~.
1 3 ~ 6
METHOD AND DEVICE FOR T~E SEPARATION AND
ISOLATION OF BLOOD OR BONE MARROW COMPONENTS
1 Background of the_Invention
The invention concerns a method and device
for the separation of blood or cells of blood-forming
organs, such as bone marrow, into th~ir components
and for the isolation of those components by means of
centrifuging in which a source reservoir, which is
connected with one or more recipient reservoirs via
an outflow opening, is applied as a container for the
blood or the bone marrow.
Blood consists of four components which, in
order of increasing specific gravity, are: blood
plasma, blood platelets, white blood cells and red
blood cells. For the red blood cells further
distinction is made between the old cells - the
gerocytes and the newly-formed cells - the neocytes.
The average lifetime of a red blood cell is
approximately 90 days. "New" cells will therefore
still be able to live for a relatively long time,
which can be of great importance in the case of blood
transfusion. The specific yravity of the red blood
cells increases as they get older, so that with the
aid of centrifuging it is possible to achieve a
certain distribution of the red blood cells according
to age. White blood cells and blood platelets -
3k
~ ~ . -
~ 3 0 ~
--2--
together called 'buffycoat' - constitute in total
approximately 1~ of the volume of normal blood.
Approximately 45% of the total volume is taken up by
red blood cells and after centrifuging approximately
50% of the neocytes are situated in a layer thereof,
which layer comprises approximately 10% of the total
outgoing volume.
In the prior art, both the components which
constitute the buffy-coat and the neocytes are
separated from one another according to a known
procedure, and thereafter isolated from one another.
For this purpose see, for example, European Patent
No. 0026417. A difficulty in this connection is the
isolation of the relatively small fractions - the
white cells, the platelets and the neocytes - without
much loss occurring through, for example,
contamination in the adjoining surfaces.
In European Patent No. 0026417 a method is
described for the separation and isolation of blood
components. After separation by centrifuging the
layers are successively pumped out of the source
reservoir and then collected. The pumping out is
done by exerting a sideways pressure on the flexible
source reservoir with the aid of a pressure cushion.
Liquid is then pressed out of the reservoir. A
description is given of how in that way blood plasma
is transferred to an adjacent recipient reservoir.
A device for separating and isolating blood
components described in the above-discussed European
30 Patent No. 0026417 consists of a centrifuge with one
or more containers mounted at a certain radial
distance from the centrifugal axle which rotate
- together with the centrifuge during use. Each
; contains a source reservoir with an outflow opening
1 30~Q~
-3-
1 which is in the main directed at the centrifugal
axle, with which the reservoir is joined via an
outflow pipe with one (or more) recipient
reservoir(s) as a closed system. This centrifuge is
equipped with a pump mechanism with which, after they
have been separated, the components are pumped out of
the source reservoir to the recipient reservoir(s).
In "Nature", vol. 217, page 816 et seq., a
method is described with which the said disadvantage
is removed, with the aid of a "continuous-flow"
process. Thereby the components are both separated
and isolated during centrifuginq. Contamination
after centrifuging does therefore not occur there.
This described process, however, also has
disadvantages: the necessary supply and drain pipes
with their accompanying channels and rotating seals
lead to a costly and complex construction. Another
disadvantage is that in one centrifugal process there
can only be worked with one reservoir, which leads to
a low processing capacity.
Summary o~ the Invention
According to one aspect of the invention,
; methods and apparatus are disclosed in which a source
reservoir is centrifuged and fractional components
thereof are pumped out into a co-rotating receiving
reservoir during centrifuging.
This method according to the invention makes
the pumping out of components durinq centrifuging
possible in a simple way without the said
disadvantages occurring, and has for that purpose the
characteristic that after a certain centrifuging
time, pumping takes place during centrifuging with
the aid of a pump mechanism which rotates with the
,~ . ' ;
.., :
:
~ ^~ 1 3080~6
1 centrifuge, and that the entire liquid system: the-
recipient reservoir(s), the source reservoir and the
joining pipe(s) rotate together, whilst the
centrifuge is kept in balance.
Because of the fact that the pump rotates
together with the centrifuge, the costly pipes and
rotating seals have become unnecessary.
Imbalance of the centrifuge can be avoided
by either situating the recipient reservior(s) at
approximately the same place with relation to the
centrifuge axis as the source reservoir, or by
ensuring in another way, for example via a separate -
also co-rotating - liquid system, such that in the
place of the source reservoir the weight of liquid
which flows out of it is compensated.
When a high rotational speed of the
centrifuge is necessary for obtaining and maintaining
a well defined separation surface between the layers
during pumping, the difficulty arises with this
method that a vacuum can be formed in the joining
pipe between the source and the recipient reservoir,
due to which further pumping out of the components
may be impedèd.
This difficulty can, according to a further
characteristic of the method according to the
invention, be overcome by ensuring that during
centrifuging the liquid in the system is subjected to
an extra pressure.
Such a pressure is obtained according to one
way of implementing the method by introducing extra
liquid into a system of flexible reservolrs which can
only expand to a limited degree. The limitation of
the expansion can be effected for example if both
reservoirs are situated in a closed container. When
'~''
~ ~ '
`~ :
- .. ,:
.
:........... . ~
., ~ .
1 30~0~6
--5--
1 extra liquid is then brought into the system the
reservoirs will expand with their flexible walls and
fill the container completely, after which the
desired extra pressure in the system occurs with only
a small excess of liquid. Plastic disposable
reservoirs, for example, could be used as
reservoirs. The extra fluid can, for example, be
introduced into the system from an extra reservoir
which is in fluid pressure communication via a
delivery pipe with the recipient reservoir, and
which, in relation to the expansion possibilities of
the system, is filled with sufficient liquid, and
from whereout, if a vacuum occurs in the system,
liquid can be drawn into the system.
Such an extra pressure can also be created
by externally increasing the pressure on the system,
or on part of it. Of course, in order to obtain the
desired effect, it is necessary that at least a part
of the system, for example a reservoir, has a
flexible wall, from where the extra pressure to the
system can be given.
The said methods are especially applied when
separating blood into its components and when
separating bone marrow cells.
The said components of blood are: blood
plasma, red blood cells, blood platelets and white
blood cells, with specific gravities Gf 1,03; 1,10;
1,05 and 1,07 g/ml. The white blood cells can be
divided again into mononuclears and granulocytes.
30 The demand for the different components with a high
; purity is high. In order to avoid undesired
immunological reactions with patients as a result of
transfusion and transplantations, one desires namely
to administer a patient with only those components
1 30~3086
l which are necessary. As only approximately one
percent volume of blood exists of blood platelets and
white blood cells together, and the blood platelets,
the mononuclear white cells and the granulocytes must
each be isolated out of this mixture, a method
according to which contamination of a component with
cells of another component is avoided to a great
degree is difficult to achieve, whilst the need
therefore is nevertheless great.
Besides the greater purity of the isolated
components the advantages of the methods according to
the invention are that the yield, or quantity of the
component which can be extracted out of a certain
quantity of the source mixture is considerably higher
than according to the known methods, and that more
units can be processed at the same time in a
centrifuge with more source reservoirs, as a result
of which more blood can be separated per unit of time.
When separating and isolating blood
components it appears to be important, in order not
to disturb the dividing surfaces during pumping
between the layer containing the blood platelets and
~ the white blood cells on the one hand, and the layers
; containing the blood plasma and the red blood cells
on the other hand, to centrifuge at a high rotational
speed; for example with a centrifuge with an arm
length of 26 cm, at more than 500 revolutions per
minute ~rpm). At approximately 800 rpm the problem
of the vacuum in the joining pipe began to occur and
it was necessary to increase the pressure in the
systemO At 2000 rpm the necessary extra pressure
appeared to be 6 atm., which was achieved by
~; introducing more liquid - a physiological salt
solution - into the system.
.,
. .
. . ~.
1 30~086
1 The invention also includes a device for the
execution of the method according to the invention.
This device consists of a centrifuge with one or more
containers at a certain radial distance to the
centrifuge axis which rotate in use together with the
centrifuge, and which each either serve as a source
reservoir, or contain a source reservoir, whereby the
source reservoir has an outflow opening which at
least in the main is directed radially and which is
joined as a closed fluid system with a recipient
; reservoir by a tube which is in the main radially
directed, and with a co-rotating pumping mechanism
for pumping liquid out of the source reservoir to the
recipient reservoir(s).
The containers are each situated for example
at the end of an arm which extends radially from the
rotation axis. From the source reservoir runs a
tube, in which a flow of the liquid to the recipient
reservoir(s) is brought about by the co-rotating pump
mechanism.
In order to ensure that the centrifuge
; remains in balance during pumping one must ensure
that the mass at the end of the arm of the centrifuge
always remains the same. For this purpose liq~id is
continually introduced into a container durinq
pumping.
A suitable solution is obtained with a
device of which the pump mechanism consists of a
second co-rotating liquid system (II) with a flexible
recipient reservoir which fills a container together
with the, also flexible, source reservoir of the
first liquid system (I), and which contains a liquid
in its source reservoir which is situated outside the
container with a density which is just a little
.
'~
.. :,;; :. . ,.. . , . , ,, . "
,.
~ ~ .
1 30~8~
1 larger than that of the liquid which must be
centrifuged. When this heavier liquid is pressed
into the container by the centrifugal force in the
recipient reservoir III), because of the fact that
the reservoirs are closed in, an equal amount of
fluid is pressed out of the source reservoir (I) of
the first system. The total mass at the end of an
arm thus remains approximately constant.
The two systems indicated here with I and II
are joined together in such a way that source
reservoir (I) and recipient reservoir (II) and
situated in one container and source reservoir (II)
and recipient reservoir (I) are situated in another
container.
A co-rotating pump can in principle be
situated anywhere in the centrifuge, for example also
in the container of the reservoirs. Thus a conical
shaped "cap", which is movable in a radial direction
and which rests on the the source reservoir, can
serve as a "pump", if the specific gravity of that
cap lies between that of the two components which are
to be separated. The recipient reservoir then lies
against the radially inward side of the cap. As a
component flows out the source reservoir into the
recipient reservoir, the cap is pushed outwards in a
radial direction and it will function as a pump.
A simple solution for the balancing problem
; is achieved if, in the device according to the
invention, a container contains both the source
reservoir and the corresponding recipient
reservoir(s). The total quantity of liquid in the
container does not then change.
For centrifuging at a high rotational speed,
in order to avoid the forming of a vacuum in the
~ ` -: ' '
1 308086
1 system, a device according to the invention is
equipped with a source reservoir and recipient
reservoir(s), both with flexible walls, which are
situated in a container which contains them
completely and which they approximately fill when in
use, whereby an extra reservoir, which is filled with
a liquid when in use, is coupled in fluid pressure
communication with the system with an open join in
the section between the container and the centrifuge
axle.
The make-up fluid in the reservoir which is
preferably a saline solution having a density greater
than the various components of the fluid mixture, may
be adapted to flow into the source reservoir for
displacing the lighter separated components
therefrom, or may be adapted to flow into a separate
pressure vessel or balloon which contacts the source
reservoir and applies pressure thereto to express the
separated components thereof.
Various designs can be chosen as pump
mechanisms. According to one preferred design the
device contains a usual type of peristaltic pump,
which rotates together with the centrifuge, and whose
drive shaft is situated in the extension of the
rotating shaft of the centrifuge, mechanically
coupled to it, e.g., by a clutch, which coupling can
be disconnected during centrifuging. The coupling
with the centrifuge shaft is for example via the pump
housing. The clutch can be disconnected by
disengaging the drive shaft during centrifuging with
the aid of a pressure plate which is fixed at a
stationary point, for example the lid of the
centrifuge. The pump housing will then rotate around
its now stationary drive sh~t and the pump will
`,
~'.
,
1 30~0~6
-10--
1 therefore pump.
Other designs of the pump mechanism are also
possible. For example the pump mechanism can be a
barrel (II) on or near the centrifuge shaft which
rotates together with the centrifuge, and which is
filled with a liquid with a larger density than that
of the heaviest component of the mixture which is to
be separated. This barrel is joined via a pipe with
a flexible recipient reseevoir (II), discussed above,
which is situated together with the flexible source
reservoir (I) of the mixture in a closed container at
the end of the centrifuge arm. During centrifuging
this liquid will then flow to the recipient reservoir
(I) in this container. As this reservoir is filled,
the source reservoir (I) is compressed and liquid
will be pushed out of it to a recipient reservoir (I)
on or near the centrifuge shaft. In order to avoid
the centrifuge getting out of balance a liquid must
be chosen as pumping liquid with a density which is
just a little larger than that of the heaviest
component of the mixture.
Different mechanical elements and
configurations are employed in several aspects of the
invention to control the rate of pumping during
centrifuging, so as to isolate precise components of
the fluid being treated.
A method according to the invention aims
firstly at being able to "treat" as much blood as
possible in one centrifugal processing run, with as
large a quantity as possible per quantity of blood of
each component of a certain high purity, and secondly
at keeping the duration of one centrifugal processing
run as short as possible. In order to achieve the
first thing it is important, among other things, that
1 3080~
--11--
1 an optimum use is made of the space which a
centrifuge offers for the placement of source
reservoirs.
One method according to the invention is
based on the insight, that is is possible to achieve
a pump mechanism which does not take up any space in
the sense mentioned. This method has for that
purpose the characteristic that the pumping-out is
effected by reducing the volume of the source
reservoir by pressing in the radially outer wall of
this reservoir. This can be achieved in two ways.
The first means of achieving this according to the
invention is that the pressing-in is effected by
allowing the source reservoir to move radially
outward under influence of the centrifugal force,
with its outer wall against an elevation in the floor
of the container, and the second means of achieving
it is that the pressing-in is effected by moving an
elevation in the floor of the container radially
inward, and pressing-into the outside wall of the
source reservoir.
In order to further achieve a shortest
possible duration of one centrifugal processing run
it is important that pumping out is always done at as
high a speed as possible. As the buffy-coat layer is
a relatively thin layer, there is an upper limit to
the speed at which it can be pumped out. Once the
upper limit is exceeded the buffy coat layer will, on
reaching the outflow opening, be "broken" and red
blood cells will also be pumped out together with the
buffy coat.
In order to achieve an optimum pumping-out
process as far as duration is concerned the method
according to the invention has a further
1 30~n,~6
-12-
1 characteristic that the speed with which the
reservoir is pressed-in is relatively high when
pumping out a relatively voluminous component, and
relatively low when pumping out a component which is
of relatively little voluminousness.
For the execution of the method accoxding to
the invention such A device has the characteristic
that the side walls of the source reservoir converge
~ in an approximately funnel-like shape to the outflow
; 10 opening and that a mechanism is present to reduce the
volume of the reservoir from the radially outer side
of the reservoir with an adjustable speed. The
funnel shape at the radially inner end serves to be
able to efficiently isolate the components. If only
a small amount of a layer has remained behind in the
reservoir, the funnel shape ensures that as the layer
approaches the outlet it becomes so thick, that it
can be pumped out without being mixed with a
following layer. This is especially true for the
buffy-coat layer, which has but a very small
thickness in total.
The mechanism to adjust the speed with which
the reservoir is compressed serves to obtain an
optimum speed, that is to say to adjust the rate of
outflow from the reservoir for each layer according
to the layer thickness.
The funnel shape can be effected by
constructing the radially inner end of the otherwise
flexible reservoir of a stiff material. In a
preferred design of the device according to the
invention there is a funnel shaped stiff cap fitted
over the inner end of the reservoir. The cap has an
opening, and its jacket is open from its outer to its
inner end over a width which is at least equal to the
.:
. ' ` .
.
:
1 ~0~0,36
l diameter of the inflow pipe. The openiny in the
jacket serves to enable the cap to be placed on the
reservoir together with its permanently joined
outflow pipe. Such a cap is preferably conical.
A design of a device according to the
invention, suitable for the method in which the
source reservoir moves radially outwards, has the
characteristic that the outside wall of the container
has an elevation on its inside side which when at
rest lies against the radially outside wall of the
source reservoir, which is situated in a mainly
cartridge-shaped housing containing the side walls of
the reservoir, which is movable by sliding in a
radial direction along the inside of the side walls
of the container. The cartridge around the reservoir
serves to make it possible that the reservoir can
move in the container. Housing and cap are
preferably joined to one another, for example by
means of a screw closure.
In order to make better use of the space
which a centrifuge has to offer to reservoirs for the
blood which is to be separated, the walls of the
housing converge, according to the a further
preferred design of the device, radially from the
outside to the inside, while the side walls of the
container also converge towards the inside
approximately parallel to those of the housing, and a
wall or part of a wall of the container is removable
over a width corresponding to the width or the
diameter of the source reservoir. Such containers
can be arranged as sectors of a flat disk, with the
centrifuge shaft at its center.
In order to create room for the recipient
reservoirs, the containers of the source reservoirs
~`-` 1 308(~v
-14-
1 are preferably executed in a conical shape. The
recipient reservoir(s) can then be placed per source
reservoir on the outer wall of the cone.
In a construction with a converging housing
in a converging container the housing must be guided
when it moves in a radial direction. In a desiqn of
a device this happens by supporting devices, which
consist of a radially directed rod which is fixed at
one end to the inward portion of the container, and
at the other end is joined in a sliding manner to the
inside end of the housing and to guiding surfaces on
the back inside face of the outer circumference of
the container which extend approximately radially
inwards, with a distance between them corresponding
to the distance between the housing walls at the
outer side of the housing and running inward to at
least above the elevation.
In order to regulate the rate at which fluid
is pumped, and thus the rate at which the outside
wall of the source reservoir is compressed, a design
of the mechanism which serves for that purpose is
characterized by the fact that the outside face of
the reservoir extends over the adjoining surface of
the elevation in a ring shaped part, and that under
this part and around the elevation two or more
inflatable rings are situated, lying over each other
as viewed in a radial direction, and equipped with
valves with independently regulatable flow openings.
This design makes two pump speeds possible. When the
first layer - the plasma - is pumped out the speed
can be high. The valve in the first air-filled ring
can be adjusted in such a way that the outflow is
high, whilst the other valve is closed. When the
buffy-coat has advanced to the outflow opening the
1 30~0~6
-15-
1 speed must be reduced. At that moment, the valve
which until then was open must be closed and the
other, narrower, valve is opened. The result is a
lower pumping rate.
Opening and closing of the valves can for
example be controlled electronically with the aid of
sensors.
If, instead of using the centrifugal force
which moves the source reservoir radially outward,
use is made of a body which presses into the outer
face of the reservoir from outside, the outside wall
for example is equipped with an opening, through
which an elevation, of which the inner face coincides
with the outer side of the container when at rest,
can be moved inwards in a radial direction.
Especially when separating, isolating and
keeping isolated components which together form the
relatively small buffy-coat fraction , problems
arise. These components of the buffy coat are the
white blood cells and the blood platelets. This is
also valid for the thin topmost or lightest layer of
the red blood cells: the neocytes. In order to
achieve a good separation in the recipient reservoir
and to ensure that isolation is possible after
separation without contamination occurring, special
demands must be made of the recipient reservoir.
These demands are fulfilled in a design of the device
according to the invention which has the
characteristic for that purpose that a recipient
reservoir consists of a tube which is connected to
the outflow opening-and which opens up with its other
end into a chamber, whereby the contents of the tube
are at least equal to the volume of the platelets,
white cells and possibly neocytes which are present
:`
1 3080~
-16-
1 in the blood or bone marrow, whilst the diameter is
at largest so large, that after separation and when
at rest, no mixing of the components occurs and that
provisions are available at the radially inward
portion o~ the source reservoir for placing the tube
in such a manner that during centrifuging the g-value
in the tube decreases along the length of the tube.
In particular it is desired to support the
tube in such an orientation that a sufficient
centrifugal gradient is developed along the tube to
maintain a separation between the different
fractional blood components therein.
In the chamber the plasma is collected
first. After that the buffy coat, arrived at the
radially inward end of the source reservoir and
divided into both its components, is pumped into the
tube, which can contain both components and if
desired also the neocytes. These two or three
components are then situated one outside the other in
the tube. In order to ensure that the separation
remains intact it is necessary that the centrifugal
force value in the tube continually decreases in the
direction of the inflow into that tube. This means
in practice that if the tube is stored in one way or
another, for example rolled onto a reel, this must be
done in a manner adapted for that purpose. Such a
reel is then situated radially inward from the
outflow opening and is preferably mounted on the
stiff cap over the inner end of the source reservoir.
In order to easily collect the components
which successively fill the tube, it is advantageous
to have connected the tube to a closable accessory
chamber in a zone of the tube where a particular
component is situated. By opening ~he entrance to
j.
: ~
1 ~0~086
1 that chamber the relevant component can flow into the
chamber, after which the chamber is closed again.
A purpose of the invention is to be able to
isolate in an efficient manner especially also the
said components which are present in relatively small
quantities after they have been separated in the
source reservoir. The device according to the
invention is for that purpose characterized in that
the outflow pipe is a flexible tube with a capacity
which is at least equal to the total volume of the
white blood cells, blood platelets and possibly
neocytes which are present in the source reservoir,
and in that the device is equipped with such
facilities for storing the tube that during
centrifuging the centrifugal force in the tube
continually decreases from the outside to the inside
seen in the direction of the centrifuge arm.
The tube forms the connection between the
source reservoir and the recipient reservoir(s).
When, after separation of the components in the
source reservoir, the liquid is pumped out in one way
or another via an opening in that reservoir, directed
towards the centrifuge shaft, the relatively light
blood plasma will come into the tube first and from
there into the recipient reservoir. After that, the
buffy-coat layer will flow into the tube with the
platelets at the inside, followed by the white cells
- and outside of them the neocytes or at least the
neocyte-rich red blood cells will follow.
To achieve that the centrifugal force in the
tube continually decreases, during centrifuging, in
the direction of flow, the tube can, in one design of
the device according to the invention, be wound on a
reel, whose axis is perpendicular to the centrifuge
':
~ `
1 30~0~6
-18-
l shaft and whose cross section perpendicular to the
axis is circular, whilst the adjacent windings of the
; tube continually form a positive angle with the
iso-g-lines, whose the size of the angle varies with
the thickness of the tube, the diameter of the reel
and the distance to the centrifugal axis.
By iso-g-lines is understood: closed lines
with equal centrifugal force value around the surface
of the reel. These lines are not the same as the
circle-shaped cross sections of the reel.
An iso-g-surface is the surface of a
cylinder having the centrifuge shaft as its central
axis. An iso-g-line is the intersection of such a
surface with the reel surface, and is thus a curved
line about the reel of equal radial distance from the
axis of the centrifuge. As such, an iso-g-line on
the reel follows a roughly saddle-shaped contour
about the reel. When the tube is wound about the
reel spool-like, so as to always make a positive
angle with the iso-g lines, a centrifugal gradient
will operate on the contents of the tube to inhibit
the mixing of components, which might otherwise occur.
In one embodiment, the windings of the tube
lie, seen in the direction of the axis of the reel,
next to one another, due to the fact that the
increase in thickness of an edge flange, over one
turn in the direction of winding, is equal to the
thickness of the tube.
For a good functioning of the device
according to the invention the dimensions given to
the tube, in which the components which are to be
isolated after being pumped out of the source
reservoir are situated, are of great importance.
If the device according to the invention is
-
- '
: .
~ 3~80~,~
-19-
1 used for the separation and isolation of white blood
cells and platelets out of blood, a preferred design
of such a device is characterized in that the volume
of the tube is equal to at least 1% of the volume of
the blood in the source reservoir, and in that the
inner diameter of the tube is equal to 5 mm at the
most.
If the diameter of the tube is larger than 5
mm it appears that after the centrifuging process has
ceased a relatively substantial contamination by
mixing occurs in the adjoining surfaces of both
separated components.
If the device according to the invention is
used not only for the separation and isolation of
white cells and platelets out of blood but also of
neocytes, the volume of the tube must be at least 11%
of the volume of the blood in the source reservoir.
The requirement, that in order to avoid contamination
in an adjoining surface the diameter of the tube is
at most 5 mm, does not apply for that length of the
tube in which the neocytes are situated. In the
event that the quantity of blood in the source
reservoir is for example 500 ml, the tube can consist
of a first piece with a length of 70 cm and a
diameter of 1 cm. An advantage of such a division of
the tube into two pieces is that it is not necessary
to work with a long, and therefore difficult to
handle, tube of 250 cm long.
It is observed that if the source material
is bone marrow instead of blood, the volume of the
tube must be at least 4% of the outgoing volume of
the bone marrow for collecting and thereafter
isolating the components of the buffy coat. The
reason is that bone marrow contains relatively many
' , .
: ~
1 308086
-20-
1 more white blood cells. Less stringent requirements
are made of the diameter of the tube. In the case of
bone marrow this can therefore be for example 1 cm.
An alternative to a thin tube with a reel is
a tube with bulges in the place where the respective
platelets, white cells and neocytes collect. These
bulges are divided by narrower parts of the tube and
can be clamped off there.
In order to reduce the chance of
contamination of the components with a relatively
small volume, both by each other and of the platelets
by blood plasma and of neocyte-rich by neocyte-poor
blood, it is important to maximize the speed with
which these components flow into the tube. In order
to be able to complete the process of separation and
isolation in as short a time as possibLe anyway it is
advantageous, according to a further characteristic
of the closed system according to the invention, to
equip this with, or to have it work in combination
with, means with which depending on the component
which is leaving the source reservoir, the rate at
which that component flows out of the reservoir can
be regulated. Such a means can for example include a
sensor, which depending on the component which it
"sees passing" regulates the outflow rate.
A preferred design of the system according
to the invention, if both neocytes and white blood
cells and platelets must be isolated, is
characterized in that the source reservoir is
equipped with a preferably spherical body with a
specific gravity which is smaller than that of the
neocytes and larger than that of the white blood
cells, and in that the outflow opening of the source
reservoir to the tube has such a shape that the
. .. . .
1 30~0~6
-21-
1 opening is partially closed off by the spherical
shaped body when the body lies against it.
When the buffy-coat components and
thereafter the neocytes have flowed into the t~be,
the small spherical body will partially close off the
outflow opening so that the further inflow of the red
cells into the tube will occur more slowly and the
chance of mixing in the tube will be reduced.
In another preferred design the means is a
sensor which is present in the device and which
registers the flowing out of the first cells of a
component and in combination therewith regulates the
outflow rate.
The outflow rate can also be reduced during
centrifuging if a source reservoir with a certain
shape is applied. Such a source reservoir has at the
end where it is connected with the outflow pipe a
narrowing of such a shape that, when the blood
platelets begin to flow into the outflow pipe during
centrifuging, the outflow rate is reduced. The mass
; of red cells, white cells and blood platelets
remaining in the source reservoir after the flowing
out of the blood plasma has such a viscosity that,
when this mass is forced to change shape, the rate of
flow decreases at a certain quantity of supplied
energy. By adjusting the shape of the narrowing to
; the said viscosity and to the quantity of the said
cells the result can be achieved that at the moment
upon which the blood platelets have reached the
outflow opening the outflow rate begins to decrease.
By a narrowing is meant in this connection: any
shape of the source reservoir which leads to an
increasing flow resistance when the relevant mass
flows into the relevant part of the reservoir. For
~ 30~086
-22-
1 example, the outflow side of an otherwise cylindrical
reservoir can have a conical shape. The angle of the
walls is then chosen in such a way that the result is
a desired reduction of the outflow rate.
For isolating the components which are
present at any given time in the tube various methods
can be applied. The tube can, for example, be
divided into compartments with the aid of clamps
which grip at the location of an adjoining surface
between components, after which the contents of each
compartment are pressed out into a chamber which is
intended for that purpose.
In a preferred design according to the
invention the compartment of the tube in which the
neocytes are collected is connected in a closable
manner with an auxiliary chamber, in which after the
centrifuging process has been stopped the neocytes
are collected. Such a chamber can also be used at
the platelets compartment and at the compartment for
the white blood cells.
For isolating the different components which
are situated in a tube it is advantageous to use a
plateau, or table, on which the filled tube, with the
auxiliary chamber(s) which may be coupled thereto,
~ 25 can be fixed after this has been unrolled. Such a
; plateau is equipped with clamps which can be slid
over a rail running alongside the tube which is fixed
onto the plateau. These clamps can be clamped onto
the tube at the location where a dividing surface
between two components is situated in the tube, so
that the already mentioned compartments are formed.
The invention will be further explained with
the aid of the drawings, which schematically show
embodiments of a device according to the invention,
: :
.
:
.
. ..
. . : ,
:,
::
1 30808~
-23-
1 and in which situations are shown which occur during
execution of the method.
Brief Description of Drawings
Fig. 1 is a view from above of a hori~ontal
cross section through a device illustrative of one
aspect of the invention;
Fig. 2 is a side view of the device of fig.
l;
Fig. 3 shows in detail the situation in the
container after centrifuging for some time, and
before pu~ping begins;
Fig. 4 shows the situation during pumping;
Fig. 5, viewed from above and perpendicular
to the centrifugal axis, is a cross section through a
centrifuge, in which two different design examples of
centrifugal units are schematically drawn;
Fig. 6 shows in more detail the same cross
section of one of the centrifugal units shown in fig.
5;
Fig. 7 shows a similar detailed cross
section through the other centrifugal unit drawn in
fig. 5, in which however the pump mechanism is
another than that shown in fig. 5;
Fig. 8 shows a cros~ section through a
conical centrifugal unit according to fig. 7, through
the middle of the unit, but now parallel to the
centrifugal axis;
Fig. 9 schematically shows the closed system
of source and recipient reservoirs;
Fig. 10 shows a reel in perspective for
winding up the tube section of the recipient
reservoir, whereby the reel forms a whole with a cap
which is placed over the radially inward end of the
1 3~8~
-24-
l source reservoir;
Fig. ll shows schematically a closed system
according to the invention, in which the tube
consists of two parts of differing thickness and in
which the source reservoir contains a spherical body;
and
Fig. 12 shows also schematically a closed
system according to the invention, whereby parts of
the tube are equipped with closable auxiliary
chambers and the whole is fixed onto a plateau
equipped with a rail with clamps.
Detailed Description
In fig. l the container l contains a source
reservoir 2 and a recipient reservoir 3, both made of
flexible material, for example as plastic
disposables, joined to each other by a pipe 4. The
pipe 4 runs via a pump 6 which is mounted above the
centrifuge shaft 5. In fig. l a centrifuge with four
"arms" is shown. The drawn pipes 4', 4" and 4"'
correspond to arms other than the drawn arm.
The pipe 4 is equipped with a valve or
closure 7, with which the pipe can be closed off when
a certain component has passed out of the mixture.
The closure 7 reacts on a signal from an "eye" which
may, for example, be a light source and photodetector
arranged about the pipe and which detects the passage
of a dividing layer between the components.
An extra reservoir 8 is coupled with the
pipe 4, filled with a physiological salt solution.
When a vacuum begins to form in the system at a
certain rotational speed, this solution will be
sucked into the system. When the source reservoir 2
and recipient reservoir 3, partially as a result of
1 3~086
-25-
1 the extra liquid which is brought to the system out
of reservoir 8, "fill" the container, a pressure will
be built up in the system such that the undesired
vacuum will be compensated for.
Figure 2 shows the same device in a side
view. With 9 i5 given the arm of the centrifuge on
the end of which the container, hinging around an
axle 10, is mounted. With 11 is meant the drive
shaft of the pump~ which is mechanically coupled with
the pump housing 6 and thereby with the centrifuge
shaft 5. This drive shaft 11 can be be disengaged
during centrifuging with the aid of a pressure piece
12.
Figure 3 shows the situation in the
container 1 after blood has been centrifuged for some
time. The blood is separated into red blood cells
13, blood plasma 15 and therebetween, in a layer 14,
the so called "buffy coat", consisting of blood
platelets and white blood cells.
Figure 4 shows the situation after pumping
has taken place for some time after centrifuging.
The plasma 15 leaves the source reservoir 1 as the
first component and comes into the recipient
reservoir 3. Next comes the buffy-coat. By stopping
the pumping when the buffy coat is situated in the
narrow pipe 4, and thus forms a relatively thick
layer, it becomes possible to effect a separation
between blood platelets 14" and the rest of the white
blood cells 14'.
In Fig. 5, 16 is the centrifuge shaft, to
which centrifuge units 17 and 18 are attached. One
centrifuge contains in general one type of centrifuge
unit, therefore, for example, either all units of
type 1 or of type 2. For purposes of this
:`
.
.
1 30~080
-26-
1 discussion, "type 1" refers to the design of unit 18,
and "type 2" refers to the design of unit 17. The
centrifuge units 17 and 18 each consist of containers
19 respectively 20, with radially outer walls 21,
respectively 22. In the containers 19 respectively
20 are flexible, for example plastic, source
reservoirs 23 respectively 24 for the blood which is
to be centrifuged. The walls of the containers 23
and 24 converge at the radial inner end in a funnel
shape to the outflow openings 25 respectively 26.
The outflow openings 25 respectively 26 open out into
recipient reservoirs 27 respectively 28, of which a
part is drawn. In order to guarantee the sterility
of the contents, the source reservoir and the
recipient reservoir in each unit are connected to
each other via an outflow tube. With 29 and 30 the
elevations are given, which move inwards, that is to
say in the direction of the centrifugal axis, in
relation to the respective outside walls 31 and 32
during the pumping out. The elevation 29
illustrated in the type I is permanently connected
with the outside wall 21 of the container. During
the pumping out, the reservoir 23, which is situated
in a housing which moves together with reservoir tnot
drawn), moves outwardly and will extend over each
side of the elevation 29. In the type 2 the
elevation 30 is situated outside the outer wall 22 of
the container 20. This outer wall 22 is e~uipped
with a hole, through which the elevation 30 can be
moved in the direction of the centrifugal axis 16,
thereby pushing in the outer end of the reservoir
24. The means by which the elevation 30 can be moved
inward are not drawn~ This can be done, for example,
hydraulically. The different ways of pumping out:
:, .
', :, .
.. : .
" ' ' - l " '
~ \
1 3~808~
1 either by pressing the elevation into the outer end
of the reservoir, or by pressing the reservoir
against the elevation can both be applied to either
of the container types.
With the use of centrifugal unit of type 1
the space available in the centrifuge can be used
better than with use of units of the type 2. With
type 1, 12 standard units for example can be placed
in one circular disk.
In Fig. 6 a same cross section as in ~ig. 5
is shown in detail of a design of a centrifugal unit
of type 2. The source reservoir 23 shows a wall
section 33 converging at the inner end in a funnel
shape. This wall section is held in shape by the cap
34 which lies over it. This cap 34 is equipped at
its inner end with a hole 35 which when in use lies
over the outflow opening 25, so that the outflow pipe
36 can pass through it. In order to be able to place
the cap 34 onto a source reservoir 23 the jacket
thereof must contain an opening (not drawn) extending
from the hole 35 outwardly.
The source reservoir 23 is supported on its
side walls 37 over preferably the whole height
thereof by a housing 38, which can slide from the
inside outwardly along the inner wall 39 of the
container 19. In the drawn example, cap 34 and
housing 38 are connected to each other by a screw
closure 40.
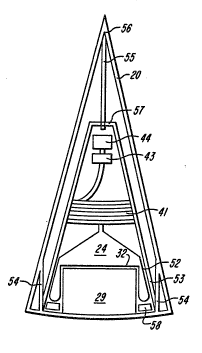
The recipient reservoir consists of a tube
41, which is connected at one end to the outflow
opening 25 of the reservoir 23, is wound around a
spool or reel 42 which is mounted on the cap 34, and
at its other end to one or more collecting chambers
(not drawn) for the components. At the recipient
~- .
.
1 3 0 ~ o
-28-
1 reservoir are included a sensor 43, which detects
when a following component "passes", and a valve or
closure, 44.
In order to be able to divide the blood in
the source reservoir 23 into its components, it is
first centrifuged~ without the liquid being pumped
out of the reservoir, while the closure 44 is
closed. When the separation is completed, the
closure 44 is opened. Due to the centrifugal force
the reservoir 23 will then move outwardly, sliding
with its housing 38 along the inside wall 39 of the
container 19. The flexible outer wall of the
reservoir is then dented in by the elevation 29 and
at places 45 and 46 extends over the sides of that
elevation in a ring shape and the fluid is pressed
out of the reservoir 23. After the first component -
the blood plasma - is thus pumped out, it is the turn
for the buffy co~t. As this has been pressed
inwardly during the pumping out of the plasma, this
will eventually be situated in the inmost tip of the
funnel. Due to the funnel shape a reduction of the
surface, and therefore an increase of the thickness
of that layer has come about. Nevertheless, in order
to avoid that fluid of the following component also
comes out when pumping, the pumping rate must be
relatively low; lower than is required when pumping
out the plasma.
In order to be able to regulate that rate,
two inflatable rings 47 and 48 with valves 49 and 50
are situated behind each other around the elevation
29. The rings 47 and 48 and the width of the valves
~; 49 and 50 can be given such dimensions, that as long
as plasma is being pumped out, ring 47 deflates at a
relatively high speed, and that when it is the turn
.
' ''`
,
.
1 3080~6
-29-
1 for the buffy-coat ring 48 deflates with a lower
speed.
Fig. 7 shows the cross section of
centrifugal unit 17 according to figure 5 in more
detail, with the difference that the relative
movement of the outside wall 32 in relation to the
elevation 29 is now achieved by pressing the source
reservoir 24 outwards against the elevation 29
instead of the other way around. An advantage of the
reservoir shape according to fig. 7 is the already
mentioned better degree of filling of the
centrifuge. In order to still be able to pump out
with the aid of the centrifugal force alone -
therefore by being able to allow the source reservoir
to move outwards - some special facilities are
necessary. In the container with converging walls 20
in order to make maximum use of the space available,
there is also a reservoir 24 with converging walls 52
which are laterally supported by a converging housing
53. This housing lies, in the starting position,
with its walls against the walls of the container
20. When this housing 53 begins to move under
influence of the centrifugal force it must be
guided. For that purpose the guides 54 are mounted
in the container 20, preferably also in the shape of
a cartridge shaped body, along which the wall of the
housing 53 slides. The housing is further guided in
radial direction by a rod 55 fixed at the front at 56
~` to the container 20 and at the other end fixed in a
sliding manner to the inward wall 57 of the housing
53, through which it protrudes.
With 58 the inflatable rings are
schematically given, corresponding with the rings 47
and 4~ drawn in fig. 6.
~ , .
'
~ . :
.
.,
1 30~30~36
-30-
1 Elements 43 and 44 are the already mentioned
sensor and the closure valve, respectively, in the
recipient reservoirl of which the tube 41 is drawn,
wound on a reel which is not drawn.
In fig. 7 a situation is drawn in which the
housing is situated in the most outward position.
Fig. 8 is a vertical cross section, parallel
to the centrifugal axis through a centrifugal unit as
drawn in fig. 7, but then conically shaped. An
advantage of the conical shape is that the collecting
chambers of the recipient reservoir, seen in the
direction of the centrifugal axis 5, can lie over the
container, whereby a maximum amount of space is
available for the reservoirs with the blood which is
to be centrifuged. The collecting chambers 59 for
the plasma and a second collecting chamber 60 for the
blood platelets are drawn.
The isolation of the plasma and the
platelets in the receiving chambers is explained
further with the aid of fig. 9 giving schematically
the closed system of the source reservoir 24 and the
recipient reservoir, consisting of the tube 41, the
collecting chamber 59 for the plasma and the
auxiliary chamber 60 for isolating the blood
platelets. When blood is present in reservoir 24,
this reservoir is closed off with a snap connection
68. The system is then placed in the centrifuge.
Then the snap connection 68 is broken and closure 44
(see fig. 8) takes over the closing function. The
centrifuging now takes place, and after the
buffy-coat is formed the closure 44 opens. Now
plasma and buffy-coat can be isolated as described.
When the platelets pass the sensor 43 closure 44
closes again. When this has been done for the la~t
1 3080~
-31-
1 centrifugal unit the centrifuge is stopped. The
system is then removed from the centrifuge and the
tube 41 is unrolled. The tube 41 is divided into
compartments corresponding to the presence of the
different components by placing clamps 65, 66, 67 and
68 at the boundaries of the components. By opening
snap connection 61, which closes the auxiliary
chamber 60 off from tube 41, the component 62 for
example (the platelets) can be pressed into auxiliary
chamber 60.
In order to ensure that the platelets, the
white cells and the neocytes are and remain separated
from each other in the tube 41 it is necessary that
during centrifuging the g-value which works on the
fluids decreases continually and evenly from the
entrance to the exit of the tube. For this purpose
the tube should be stored in a special way radially
inward from the outflow opening. A reel around which
the tube is wound in adjacent windings must have a
special shape for this purpose.
Fig. 10 shows in perspective how one reel
42, in the drawn position permanently joined to a cap
34, should look in order to comply with the
aforegoing requirements with regard to the g-value.
The tube 41 is regularly wound around the reel part
69 from the cap 34, lying between the standing edges
55a and 55b.
In fig. 10 is also shown how in the jacket
71 of a cap 34 the opening 72 is placed which makes
it possible to place the cap on a source reservoir
24. In order to obtain a correct winding, the reel
is constructed in such a way for example that the
thickness 74 of the edge 55a continually increases in
the direction of winding from the place 73, where the
1 7CI~0~6
--32--
tube 41 enters the reel, so that this has increased
after one winding by the diameter of the tube to
thickness 75.
In fig. 10 reel 42 is drawn with a reel-axis
5 80, and is connected to a conical cap 34, which when
in use falls over the inward face of the source
reservoir. The - not drawn - flexible tube which is
connected to the outflow opening of the source
reservoir is wound in adjacent windings around the
10 reel surface 69, which is limited by the standing
edges 55a and 55b. The tube enters the reel via the
opening 73. The thickness 74 of the edge 55a
increases continually from the opening 73 in the
direction of winding 81 to a thickness 75 whereby the
15 difference between the thickness 74 and 75
corresponds with the diameter of the tube used. If,
when winding on the tube, it is laid against the edge
55a and following windings are always laid against
the previous windings, a spiral shaped winding of the
20 tube on the reel surface 69 is achieved in a simple
manner, whereby the g-value in the tube continually
decreases. The shape of the reel surface 69, which
has a circular cross section perpendicular to the
reel axis 80, is determined by the condition, that
25 ~he distance of the tube to the centrifugal axis,
which when in use stands perpendicular to the reel
axis 80, continually decreases from the beginning up
to the end of the tube.
In fig. 11 the closed system according to
30 the invention is shown schematically, as that is used
for blood as a source material. Before centrifuging,
the blood which is to be separated is situated in
source reservoir 86. After the desired separation
has been brought about in this reservoir by
~ ,. , . - ~
~. ; .
1 30~n~0
--33--
centrifuging, the contents of the reservoir are
pumped out of the reservoir via the outflow opening
87. First the blood plasma comes out and flows
through the flexible tube 88 to the recipient
5 reservoir 890 The tube 88 consists of a first part
88' with a maximum inner diameter of 5 mm and of
which the contents preferably amount to at least 1
of the total volume of the source material, and a
second part 88", of which the inner diameter is for
10 example 1 cm and whose contents are preferably at
least approximately 10% of the total volume of the
source material. The buffy-coat is collected in the
tube part 88' and the neocytes in the tube part 88".
The precise volumes of tube segments 88',
15 88" are such that for the standard source blood bag
86, the segments 88', 88" will hold all of the
platelet (respectively neocyte) fraction likely to
occur in the blood of a normal individual.
Referring to the spatial location radially
20 outward from the centrifuge axis as "upstream," it
will be seen that the larger diameter tube segment
88" is supported upstream of the smaller diameter
portion 88' during the centrifuging operation.
A small spherical body is indicated with 104
25 with a specific gravity which is smaller than that of
the neocytes and larger than that of the white blood
cells. This small spherical body 104 partially
closes off the outflow opening when the last white
blood cells have passed the outflow opening 87 during
30 the pumping out. The outflow speed is thereby
decreased, so that contamination to the surfaces of
the adjoining buffy-coat components is prevented.
When one or more tube segments supported to
provide a centrifugal gradient for effecting or
-
1 3n80~'
-34-
1 maintaining separation of fractional components are
removed from the centrifuge apparatus, the final
isolation of the separated components is easily
effected by unreeling the tube on a flat table or
plateau. The boundaries between adjacent fractions
in the tube are then visually ascertained, and by
providing pinch-off clamps at appropriate sites, each
faction is isolated. The isolated factions are
preferably then each expressed into a separate
chamber. Such a preferred arrangement is shown
schematically in figure 12.
In fig. 12 the closed system according to
fig. 11 is shown again with the source reservoir 86,
the flexible tube 88 and the recipient reservoir 89.
In this design the flexible tube 88 has one uniform
diameter. When after pumping for a certain time the
components - the blood platelets, the white blood
cells and the neocytes - are situated in the tube
separated from each other, the isolation must still
take place. This can be achieved by dividing the
tube into compartments 95, 96 and 97, corresponding
with the different components, with the aid of clamps
91, 92, 93 and 94. The contents of each compartment
are then collected in for example auxilliary chambers
98, 99, of which two are drawn, which are connected
with the appurtenant compartments via snap connection
100, 101.
In order to be able to easily isolate the
components which are present in the tube and divided
from each other, this can be fixed in an unrolled
position onto a plateau 102, which is equipped with a
rail 103, along which clamps 91', 92', 93' and 9~'
can be moved. These clamps can then be clamped on to
the tube at the place where a dividing surface
- '' ' -
;
1 3080~)
1 between components is situated in the tube 88,
thereby forming the said compartments 95, 96 and 97.
The contents thereof can thereafter be pressed into
the auxiliary chambers 98 and 99.
If use is made of a tube with bulges instead
of a thin tube with a reel, the compartments 95, 96
and 97 correspond with these bulges. The dimensions
for the bulges could be: for the platelets 2 cm
(inner diameter 1.5 cm), for the white cells 3 cm
(inner diameter 1.5 cm), and for the neocytes 4 cm
(inner diameter 4 cm). A plateau along which the
clamps are moved is not necessary in this case
because clamping off always takes place at the pieces
of the tube (1 cm long) which are situated between
the bulges.
In either case, it will be appreciated that
the source reservoir, the tube, and any bulges or
auxiliary chambers are formed as a closed sterile
system, in which, initially the tube and chambers not
actively utilized in a processing step are closed off
from the active components. Thus, for example, a
snap-connection will isolate the tube 88 from the
source reservoir 86, into which blood is initially
drawn from a blood donor. When placed in the
centrifuge for separation, the snap-connection is
broken, allowing flow of the separated plasma through
tube 88 into plasma-receiving reservoir 89. When the
separated white cells, platelets and possibly
neocytes have been stopped along the length of tube
88, additional snap-connections isolating the
auxiliary chambers 98, 99 may be broken and the
separated components expressed into those chambers,
which are then closed, e.g., by heat-sealing, in a
manner known in the art. Thus the entire process of
1 30~0~6
-36-
1 drawing blood, separating, and isolating the fine
components thereof is effected in a closed sterile
environment. This prolongs the life of the separated
buffy coat components over that obtained by previous
multi-process methods of isolation.
It will be appreciated that the invention
has been described with respect to particular
embodiments thereof, and that such description is by
way of illustration, and the invention is not limited
1~ thereto. The invention being thus disclosed, various
modifications will occur to those skilled in the art, `~
and such modifications are included within the spirit
and scope of the invention, as defined by the
following claims.
What is claimed is:
..:
.