Note: Descriptions are shown in the official language in which they were submitted.
1 3n~540
Technical Field
This invention pertains to producing superconductive bodies, and apparatus
and systems comprising such bodies.
Back round of the Invention
S ~rom the discovery of superconductivity in 1911 to the recent past,essentially all known superconducting materials were elemental metals (e.g., Hg, the first
known superconductor) or metal alloys (e.g., Nb3Ge, probably the material with the
highest transition temperature Tc known prior to 1986).
Recently, superconductivity was discovered in a new class of materials. See
J.G. Bednorz and K.A. Muller Zeitschr. f. Phvsik B - Condensed Matter Vol. 64, 189
(1986), which reports superconductivity in lanthanum barium copper oxide. The disclo~ed
material had an onset temperature in the 30K range.
This report stimulated worldwide research activity, wl1ich very quickly
resulted in further significant progress. The progress has resulted, inter alia, to date in
the discovery that compositions in the Y-Ba-Cu-O system can have superconductivetransition temperatures above 77K, the boiling temperature of liquid N2 (Phvs. Rev.
Letters, Vol. 58, ~arch 2, 1987, p. 908; and ibid, p. 911). Furthermore, it has resulted in
the identification oE the material phase that is responsible for the observed high
temperature superconductivity, and in the discovery oE compositions and processing
techniques that result in the formation of bulk samples oE material that can be
substantially single phase material and can have Tc above 90K. See Canadian Patent
Application Serial No. 556,031, filed January 7, 1988, entitled "Devices and Systems Based
on Novel Superconducting Material".
Prior to the discovery of the nature oE the superconducting phase
and oE compositions and processing conditions that can result in substantially
single phase oxide superconducting material, the expressed belief in the field
was that the Y-Ba-Cu-O superconducting material is multiphase material, with
only a relatively small part (variously considered to be about 24~o or about 2~o)
being superconducting. Significantly, it was also speculated that the high
temperature superconductivity of the material may be associated with interfacialmanifestations, and may not be identified with perovskite or tetragonal layered
~,
~,
1 ;~'J8540
structures.
The excitement in the scientific and technical community that was
created by the recent advances in superconductivity is at least in part due to the
potentially immense technological impact of the availabili~y of materials that are
S superconducting at temperatures that do not require refrigeration with expensive
liquid He. For obvious reasons liquid hydrogen and neon would not be very
desirable refrigerants, and therefore it is liquid nitrogen (B.P. 77K) that is
considered to be the lowest boiling point convenient and inexpensive cryogenic
refrigerant. Attainment of superconductivity at liquid nitrogen temperature was
10 thus a long-sought goal which for a long time appeared almost unreachable.
Although Tc > 77K has now been attained, there still exists at least
one ba~ier that has to be overcome before the new oxidic high Tc
superconductive materials can be utilized in many technological applications. Inparticular, techniques for forming superconductive bodies of technologically
15 significant shape have to be developed.
To date known oxidic superconductive bodies are substantially three-
dimensional bodies (e.g., pellets, disks, tori), i.e., all three dimensions are of
substandally the sarne order of magnitude. Although such three-dimensional
bodies may have specialized udlity, widespread use of the new high Tc materials
20 will occur only if superconducdve thin films, and sheet-likc and filamentary
superconducdve bodies can be produced &om the material.
According to a so-far unconflrmed report in the press, one laboratory
has succeeded in producing a superconductive thin film from one of the oxidic
high Tc materials by an evaporation technique. Evaporation as well as other thin25 film deposition techniques generally can be used to deposit layers of limited thickness (typically substantdally less than 5 llm) onto a substrate.
No technique for producing superconductive bodies having a small
dimension g~eater than is obtainable by thin film techniques but less than is found
in the prior art three-dimensional bodies has yet been reported. Such bodies,
30 which can be free-standing or can be in contact with a substrate, typically have a
minimum dimension in the range from 5 or 10 ~m to about 1 mm, and have at
least one dimension which is much greater than the rninimum dimension. If a
body has two dimensions that are approximately equal and in the above range thenwe will refer to such a body as "filamentary". An example of a filamentary body
35 is a thin rod. If a body has, in addidon to the one small dimension, two large
~ ~0~540
- 3 -
dimensions of approximately equal magnitude, or one large dimension and one
intermediate dimension then we will refer to the body as "sheet-like". Exarnplesof sheet-l~e bodies are sheets, wide strips, shaped pordons of a sheet, and strips
or tapes in which at least the thickness is within the above range.
If filamentary and/or sheet-like superconducdng bodies could be
produced from the new high Tc oxidic materials it is certain that they would find
significant technological application. It will be recalled that prior art
superconductors are used, in addidon to thin film form, essentially only in
filamentary form (as wire~,and in strip-form. Furthermore, because of the novel
10 properties of the oxidic superconductors, it is likely that filamentary and sheet-like
superconducdve bodies would find uses in ways that were not possible or pracdcalwith the prior art metallic superconductors. Thought is given here, for instance, to
assemblies of sheet-like superconducdve elemen~s.
For an overview of somc potendal applicadons of superconductors
15 see, for instancc, B. B. Schwartz and S. Foner, editors, Superconductor
Applicadons: SQUlDS and Machines, Plenum Press 1977; S. Foner and B. B.
Schwartz, editors, Superconducdng Machines and Devices, Plenum Press 1974;
and S. Foner and B. B. Schwartz, Superconductor Material Science, Metallur~y,
Fabricatdon, and Applicadons, Plenum Press 1981. Among the applications are
20 detecdon and measurement apparatus based on, e.g., the Josephson effect or
electron tunneling, power transmission lines, rotadng machinery, and
superconducdve magnets for, e.g., fusion generators, MHD generators, particle
accelerators, levitated vehicles, magnedc soparation, and energy storage. The prior
art has considered these actual and potendal applicadons in terms of the prior art
25 (non-oxidic) supe~conductors. It is expected that the above and other applicadons
of superconducdvity would materially benefit if high Tc superconductors could beused instead of the previously considered reladvely low Tc superconductors.
Prio~ art cerarnic techniques can successfully produce three-
dimensional superconducdng bodies. However, workers in this field have so far
30 failed to produce superconducdng filamentary or shcet-like bodies by any
technique, including ceramic techniques.
A known technique, screen printing, has been used in the past to form
sheet-like bodies on ceramic substrates. See, for instance, B. Schwartz, CeramicBulletin Vol. 63(4), p. 577(1984). The bodies included conductors (including
35 conductors that comprise the conducting oxide RuO2), insulators and dielec~ics.
1 3~540
- 4 -
The technique was not used to form any superconducdve bodies.
In view of the immense potendal importance of high Tc
superconductive bodies of technologically useful (i.e.g, having at least one small
dimension and at least one relatively large dimension) shape, a technique for
5 producing such bodies would be of great significance. This applicadon discloses
such a technique.
Summarv of the Invention
In a b~oad sense the invendon is apparatus that compAses a
filamentary and/or sheet-like superconducdve body formed by a technique that
10 comprAses forming an oxide powder-containing "green" body and headng the
green body under condidons such that the resuldng ceramic body is a
superconductor. Significant consideration is to be given to the chemical
compadbility of the fiAng apparatus (especially the substrate that supports the
body) with the material of the body, and to those other firing condidons that can
15 have an effect on the composidon (especially the degrec of oxygen deficiency) of
the resuldng body.
Superconducdve bodies according to the invendon will frequently
comprise copper-containing mixed oxides, including oxides of the type
La2 xMxCu04 o (M being one or more of Ba, Sr, Ca, Pr, Y, and Bi, with
20 0.05 S x S 1.2, and 0 S o ~ 0.5), or of the type M2M'Cu3Og O (M is one or more
essentdally divalent metal ion, M' is one or more essendally trivalent metal ion,
> 1, and divergence from the nominal amounts of M and M' is a Qimum of
10%; frequently M comprises one or more of Ba, Ca, Sr, M' frequently comprises
one or more of Y, La, Eu, Lu, and Sc, and 1.5 S ~ S 2.5). The bodies typically
25 have a reladvely high superconducdng temperature Tc, generally > 30K.
Preferred embodiments are substandally single phase mateAal (typically > 75 or
even 95% by volume superconducdng mateAal).
Preferred cmbodiments become superconducdve at a temperature
Tc > 77K. An example of a mateAal with Tc > 77K is Ba2YCu306 9. There
30 have recently been reported clairns that indicadons of superconductivity have been
observed above 200K, at temperatures, as high as 240K, in some oxides (cuprates)of the type that is of concern herein. See, for instance, New York Times,
Saturday, March 28, 1987, page 6, which reports on observadons made at Wayne
State University. Similar claims have also been made by workers at Berkeley
35 University. The invendve method for making filamentary and sheet-like oxide
1 3~540
superconductive bodies is broadly applicable to forming such bodies from oxide powder
and is, in particular applicable to forming such bodies from cupra~e powders such as the
cuprate on which the Wayne State and Berkeley experiments were done.
Among the techniques for producing the green bodies are extrusion, screen
printing, tape casting, and slip casting. The inventive filamentary and sheet-like ceramic
superconductive bodies can be advantageously used in a variety of apparatus including
power transmission lines, rotating machinery such as electrical generators, magnets such as
may be used in fusion generators, MHD generators, particle accelerators, levitating
vehicles, and ion separation, or in ultra high-field magnets such as Bitter magnets.
Inventive bodies can also be used as strip lines or other interconnects, or can be directly
used as circuit components (e.g., a high-Q inductor). They may also find use as detectors
and measurement devices.
In accordance with one aspect of the invention there is provided a
filamentary or sheet-like superconductive body having at least one dimension in the range
from about 5 ,~Lm to about 1 mm, CHARACTERIZED IN THAT the superconductive
body is a ceramic body that comprises superconductive oxide material and that is produced
by a process that comprises a) producing an oxide powder and mixing the oxide powder
with a binder material; b) forming, from the mixture produced in a), a "green" body having
at least one relatively small dimension; and c) firing the green ceramic body in an oxygen-
containing atmosphere such that the superconductive ceramic body results.
Brief Description of the prawin~
FIG. I schematically illustrates one technique for forming a green body
according to the invention, the doctor blade technique;
FIG. 2 schematically depicts an inventive body consisting of a
superconductive tape and an insulating tape;
FIG. 3 schematically shows a "jelly-roll" magnet coil produced from
inventive material;
FIG. 4 depicts an inventive body that can be used to assemble a Bitter-type
magnet;
FIGS. 5 and 6 show the resistance and magnetization, respectively, of a
sample of inventive ceramic tape as a function of temperature;
A
1 3n~540
5 a
FIGS. 7 and 8 show X-ray diffraction patterns for the ceramic tape, and for
powder produced from the tape, respectively;
F~G. 9 gives Rutherford Backscattering Spectroscopy (RBS) data for a
sample of the inventive tape, and for a polished pellet of essentially the same composition;
S and
FIG. 10 illustrates a further embodiment of the invention, a high-Q
inductor coil.
1 303540
- 6 -
Detailed Description
Although in many cu~rently preferred embodiments the inventive
superconductive bodies consist substantially of perovskite, or a mixture of
perovskites, of the previously referred to M2M'Cu309 ~j-type, the invention is not
5 so limited. We believe that the techniques disclosed herein can, either direcdy or
with obvious changes, be used in general in the manufacture of filamentary and/or
sheet-like ceramic oxidic superconducdve bodies having at least one relatdvely
small dimension, generally in the approximate range 5 or 10 ~m to 1 mm.
Such bodies not only are of substandal technological significance but
10 their manufacture encounters a problem that is not present, or not of comparable
severity, in the manufacture of bodies that do not have such a "small" dimension.
The problem is the unpredictable outcome of the firing process with regard to
superconducdvity, including the amount of superconductdve material present in the
first body.
For instance, the processing condidons can result in a substandal
change in the oxygen content of all or a significant portion of the total volume of
the body, such that the affected portion of the body is semiconductdve. As a
further example, duAng processing the body might be in contact with an
inappropriatc substrate material, such that one or more atomic species diffuse into
20 the body from the substrate, resuldng in the absence of high Tc superconducdvity
in all or a substantial pordon of the body.
Preferred Materials
Among the currently preferred inventive bodies are substantially
single phasc perovskites of nominal composidon M3 mM'mCu309 0, with m
25 being preferably about 1. By "single phase" we mean that preferably at least
about 95 mol percent of the rnaterial of the body is a single phase, as deterrnined
by powder X-ray diffractdon. While such "truly" single phase material is to be
prefe~Tcd and will most likely be the ambidon for practical usage, under some
circumstances it may be acceptable (or even desirable) for greater amounts of
30 second phase material to be present, provided the body is a superconductor.
By "perovskite" is meant not only the prototype AB03 cubic
structure, but very significantly also material whose lattice shows distortion (e.g.,
orthorhombic) from the cubic symmetry. As indicated in the above generic
chemical formula, the preferred materials also depart from the nominal perovskite
35 also in terms of stoichiometry. Analyzed materials had o values of 1.9 to 2.~, but
`` 1 3n~540
a somewhat greater o range is expected to be compatible with superconductivity.
There are typically two significant compositional contributions to the
structure of these preferr d materials. These relate (a) to observed oxygen
deficiency which reduces the coordination number of a portion of the copper from5 six to five or possibly four, and (b) r~uxed occupancy of the "A site" (in theprototypical representation ABO3), i.e., occupancy by ions represented as M and
M' in the general formula above--gives rise to a further variadon. X-ray
diffraction studies, in indicating single phase material, transla~e into substantial
ordering of M and M' ions in selected compositions which, in turn, gives rise to a
10 unit cell size larger than that of the primitive cell corresponding with the single
formula unit ABO3. X-ray diffraction measurements of a prefe~red composition--
nominal Ba2YCu306 9, indicate a crystallographic unit cell of orthorhombic
symmetry of size a=3.87, b=3.86, c=11.67 Angstroms. This crystallographic cell
is a "supercell" of the cubic ABO3 and is of three times the volume, due to subtle
15 ordering effects. Other compositions may show different "supercells" or exhibit
"supercells" whose diffraction signatures are too weak to be observed by
convendonal x-ray powder diffracdon techniques. Such supercells are well known
in perovskite structure type materials. In compositions herein in which M and M'ions differ in size sufficiently (e.g., in terms of an ionic radius ratio of at least
20 1.2), these materials are truly ordered for compositions in which inclusion of
M/M' ions nominally follows the ratio 2/1, the repeadng unit includes three
primidve cells. While preferred composidons generally meet the ion size
requirements for ordering, other superconducting composidons do not.
Consideradons such as material cost and ease of processing may lead to selection25 of such composidons which, in these terrns, may be "disordered".
In terms of cridcal temperature, Tc, preferred composidons are those
in which M is primarily barium. Partial subsdtudon of barium by other ions, e.g.,
calcium and/or stron~ium, may be dictated by economic or processing
consideradons. In preferred materials M' may be primarily yt~rium although total30 or partial substitution of other elements has been useful. Europium, in particular,
yields increased values of Tc. Total subsdtudon by lutedum as well as lanthanum
has also been employed, as has (substandal) pardal subsdtution by scandium.
Pardal subsdtutions, e.g., at the 25 mole percent level, do not substantially affect
Tc in many instances. The 2:1 A site occupancy is considered essendal to fo~m
35 superconducting perovskite structures with small rare earths (Y, Eu, Lu).
1 3~54o
Experiments thus far with compositions deviating by as little as 10 percent, result in
multiphase material. For larger rare earths, e.g., La, considerable variation in the M/M'
ratio is tolerated structurally, although larger variation generally does not lead to
optimized superconductive characteristics. It is observed that partial substitution, like total
S substitution, generally gives rise to some change in the degree and type of distortion from
cubic perovskite. Again, it has generally been observed that reduction in distortion
corresponds with some lessening in Tc.
Barium and yttriurn are currently preferred M, M' occupants. Substitution,
whether partial or total, is preferably by ions that approach the size of the ions that are
replaced. Substitution of the divalent ion in the M site also meets such size criteria.
It is well known that copper-based perovskites can be -- generally are --
oxygen deficient. Materials used in the practice of the invention which have been
examined are no exception. Measurements made in the usual manner (thermogravimetric
analysis using hydrogen reduction at 950C) yield values oE ~ within the range of from 1.5
to 2.5. Conductivity is largely dependent upon electrons yielded by the coexistence of
both divalent and trivalent copper. The observed oxygen stoichiometry yields an average
copper valence centering on about 2.3. The average valence state is dependent upon
processing conditions. Specifically varying the temperature and time of oxygen anneal
varies this quantity.
Other preferred materials belong to the class having formula La2.,~M~CuO4.~,
where M is one or more Oe Ba, Sr, Ca, Pr, Y, and Bi with 0 ~ ~ < 0.5, and 0.05 < x < 1.2.
These materials generally deviate from cubic symmetry. Substitution of a minor amount
oE Cu by another element, e.g., Sn, is also contemplated. In general, materials oE this
class have been described in Canadian Patent Application Serial No. 556,030, Eiled
January 7, 1988, and can be prepared in substantially the same manner as materials in the
above described class of formula M2M'Cu309.".
Cornposition and Preparation of Some Preferred Materials
Material specification in accordance with the invention depends upon
the nature of the intended use. For power transmission, or other current-carrying
application, it is required that there be a continuous superconducting path. Fordetector and other device use (e.g., Josephson junction devices) in which tunneling
might be perrnitted or even required, it may be necessary only that there be
.,
1 30~540
g
sufficient superconducting phase ~o satisfy such use.
Appropriate starting rnaterials are mixtures of metallic oxides,
hydroxides, carbonates, hydrates, oxalates or other reactive precursors in the
appropriate ratio to obtain the desired final composition. Starting material may be
5 produced by wet or dry mixing, by co-precipitation of ma~erials from solution, or
by any other method which results in intimate mixture of reactive particles.
Mixtures ~f starting materials can be fired in air, oxygen or other
non-reducing ambient at ~mperatures sufficient to facilitate chemical reaction
between constituents and to begin formation of the desired phase. Firing
10 temperatures as noted are composition-dependent so that choice of temperaturemay radically affect Tc for certain compositions. Typically, temperatures are
between approximately 700 and 950 C for times of between a few hours and
several days until the desired phase is either fully or partially produced. The
"calcined" material is then formed into the ceramic body of desired shape by an
15 appropriate ceramic processing technique, as will be described in detail below.
The green body is fired at a temperature sufficiently high to complete
chemical reaction of components ("reactive sintering") if noe accomplished during
calcining, and for densification. This sintering is conducted so as to reduce voids
to the point where the density of the ceramic body is sufficient to allow obtaining
20 favorable electrical and rnechanical properties. For most favorable results, the
material is fired in an ambient environment with greater partial pressure of 2
than that of air (.2 atm.).
A significant aspect of the invention is the formation of a filamentary
or sheet-like ceramic superconductive body. In general, known techniques can be
25 used to form the given body. These include extrusion, screen printing, tape
casting, and slip casting.
The starting materials for each of these processes comprise finely
divided oxidic powder, solvent, and opdonally one or more of binder, plasdcizer,deflocculant, and wetting agent. Typically the stardng materials for the various30 processes may di~fer substantially only in consistency, due primarily to differences
in the amount of solvent used. I he starting material for extrusion ~pically is a
relatively stiff paste, and that for slip casting being a liquid slurry. Screening and
tape casting materials are of interrnediate viscosity, with the former being more
like a paste and the latter being more like a slurry. Typically the powder is about
35 25-75% by weight of the preparadon.
1 30~3540
- 10-
Some known nonaqueous solvents are acetone, ethyl alcohol, benzene,
bromochloromethane, butanol, diacetone, ethanol, isopropanol, methyl isobutyl
ketone, toluene, trichloroethylene, and xylene.
Known binders for use in nonaqueous systems comprise cellulose
5 acetate butyrate resin, nitrocellulose, petroleum resins, polyethylene, polyacrylate
esters, polymethylmetacFylate, polyvinyl alcohol, polyvinyl butryal resins, and
polyvinyl chloride.
Known plasticizers for use in nonaqueous systems comprise butyl
benzyl phthalate, butyl stearate, dibutyl phthalate, dimethyl phthalate, methyl
10 abietate, mixed phthalate esters, polyethylene glycol, polyalkylene glycol
derivatives, and tricresyl phosphate. Among known deflocculants for use in
nonaqueous systems are fatty acids (e.g., glyceryl trioleate), natural fish oils (e.g.,
menhaden), and synthetic surfactants (e.g., benzene sulfonic acids).
Known wetting agents for use in nonaqueous systems include
15 alkylaryl polyether alcohols, ethylether of polyethylene glycol, ethyl phenyl glycol, polyoxyethylene acetate, and polyoxyethylene ester.
More detail, including defoamers, binders, plasdcizers, deflocculants
and wetting agent for use in aqueous systems, can be found in F. F. Y. Wang,
editor, Treatise _ Materials Science and Technolo~y, Vol. 9, Ceramic Fabrication20 Processes, Academic Press (1979), especially p. 179.
After preparation of the paste or slurry, as thc case may be, the green
body is formed. Among the currently preferred techniques for forming a sheet-
like body are screen printing and tape casdng.
In the former a (typically patterned) sheet-like green body is formed
25 by the known screen prindng technique (see, for instance, J. Medernach, Hybrid
Circuit Technolo~, February 1987, p. 21) on a chemically compatible substrate.
A substrate is chcrnically compadble with the superconductive material thereon if,
during processing, substandally no chemical superconductivity-destroying
interactdon occurs between the substrate and the body thereon. For instance, we
30 have found tha~, at least for (La, Sr) copper oxide-type material, alumina isfrequently not a chemically compatible substrate material, whereas zirconium
oxide is generally chemically compatible.
Sheet-like bodies formed by screen printing can be, ~or instance, fine
lines used to interconnect electronic components, or conductor patterns that define
35 electrical components such as an inductor. Altematively such bodies can have
t 30~540
11 -
comparable extent in two dimension, e.g., to form a superconducting backplane orflux shield. Other possible uses of screen printed bodies include detectors and
Josephson junction devices. Ceramic superconductive bodies produced by screen
printing typically ha~e thickness between about S llm and about 100 ~lm.
S The tape casting technique ~also known as the doctor blade process) is
also well known to those skilled in the art. See, for instance, F. F. Y. Wang,
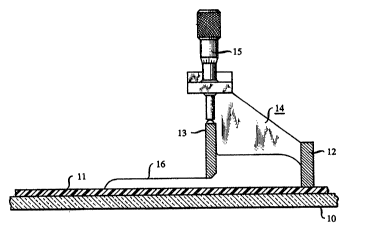
op. cit., pp. 173-197. The ~echnique is schematically depicted in F~G. 1, wherein
10 is a tempered glass bed, 11 is a carrier film (e.g., polytetrafluoroethylene,(PIFE), barrier 12 and doctor blade 13 define a reservoir for the slurry, and 1510 refers tO means for adjusting the gap between the doctor blade and the carrier
film. A strip of sluIry 16 is deposited on the carrier film by translation of the
casting head relative to the calTier film, the strip is dried, and the resulting flexible
tape (or portion thereof) is fired. Since in general the green tape has to be
supported during firing, the support has to be chetnically compatible with the
15 superconductive oxide material.
Chemical compadbility between the support and the body supported is
also essential for the filamentary bodies typically produced by extrusion, as well
as for the bodies produced by slip casting. (For a description of the extrusion
process, see, for instance, W. D. Kingery, editor, Ceramic Fabricadon Processes,20 MIT and John Wiley & Sons, 1958, pp. 107-111, and for a descripdon of the slip
casting process, see, for instance, ibid, pp. 45-51. Zirconium oxide is expected to
be chemically compatible with many, if not all, of the superconductive oxides ofinterest herein, and is a convenient substrate material. Any given combination of
superconductive oxide and substrate material can readily be checked for chemical25 compadbility, by firing the combinadon and by checking the resulting ceramic
body for superconducdvity.
As will be appreciated by those skilled in the art, bodies that comprise
superconducdve ceramics in combinadon with non-superconducdve (typically
insuladng) ceramics can also be produced. For instance, a larninate 20 consisting
30 of an insuladng tape 21 and a superconducdve tape 22 is shown in FIG. 2. Sucha laminate can be formed by tape casdng. If the combination of materials is not
chernically compatible, a barrier layer of chemically compatible ma~erial may berequired between 21 and 22. The laminate can be processed in conventional
manner, provided the two tapes have matched sintering parameters and therrnal
35 expansion. It may be desirable in some cases to impart a desired shape to the
~ 30,~5~0
- 12 -
laminate prior to firing. For instance, FIG. 3 schematically shows a "jelly roll"
laminated tape magnet coil that may be advantageously fired in this form.
A super onducting magnet of the "Bitter" type can be assembled from
alternating slotted tori (shown schematically in FIG. 4) formed from
5 superconductive tape and appropriate insulating material, respectively. Eventual
electrical continuity between the superconductive tori typically is established prior
to firing of the assembly by fusing of the overlapping ends of adjacent tori.
For some purposes (e.g., for power transmission) a thin continuous
strip of superconductive material can be advantageously used. Such a strip can be
10 formed by tape casting and can be fired either free standing or wound helically on
an appropriate mandrel. Firing can be continuous or in batch fashion. Exemplary
prior art transmission lines are described in detail in Superconductor A~icadons,
SQUIDS and Machines, op. cit., pp. 672-699. Cables that utilize high Tc
superconductive bodies according to the invention are expected to be similar in
15 principle but of significantly simpler construction, since LHe-cooling is notrequired. Advantageously one or more superconductive strips according to the
invendon are wound helically on a core element that provides mechanical support
for the strips, and may serve as a conduit for LN2. The superconducting strips are
surrounded by electrical insulation, thermal insulation, and rnechanical protection
20 layers, and optionally with a further refrigerant conduit, in a manner similar to the
prior art cables of FIG. 2 of the cited reference.
FIG. 10 schematically depicts an embodiment produced
advantageously by screen printing, namely, a high Q inductor 100 on an insulating
substrate 104. The inductor consists of a superconducting spiral winding 101,
25 with via holcs 102 and 103 filled with superconductive material that makes
contact with the indicated return path.
Example I: A thin sheet of superconductive cerarnic of the
Ba2YCu3O9 ~-type was prepared as follows. 114.385g of Y2O3 (98,706%
Y2O3), 394.680g BaCO3 (essendally 100% BaCO3), and 241.738g CuO (98.71%
30 CuO) was thoroughly mixed with about 3 1 of deionized water and milled for 4
hours in a 4 I polyethylene jar that was approximately half filled witb lcm
diarneter, lcm length ZrO2 milling media. The resulting slurry was filtered and
the filter cake was dried at 130C in air. The dried cake was passed through a
20-mesh sieve and the fragments calcined in fused silica boats in air, by raising
35 the temperature linearly with time in 4 hours from room temperature to 900C in
-``` 1 ~OP,540
- 13 -
2~ maintaining the temperature at 900C for two hours, and then furnace coolingto room temperature. The calcined powder was mixed with deionized water,
milled for 4 hours, lSltered, dried, and passed through a 20-mesh sieve, all
substantially as described above. 200g of the resulting powder was mixed with
5 200g of an acrylic binder dissolved in 1, 1, 1 trichloroethane solvent (Cladan No.
73140 obtained f om Cladan, Inc.,San Marcos, California) in a 1 1 polyethylene jar
half filled with the above ZrO2 milling media, and milled on a roller mill for 16
hours, followed by very slow rolling for 1 hour to remove entrained air.
The thus produced slur y was poured into the reservoir of a doctor
10 blade tape caster, with a gap 7.6 cm wide and 0.71 mm high. The slurry was cast
in conventional fashion onto a E~FE-coated plate by moving the casting head
across 1.8 m of the plate at 1.8 cm/sec. The resulting strip of slulTy was dried for
16 hours in air, yielding a flexible tape 112 ~lm thick and about 7.6 cm wide. Aportion of the flexible tape was placed on a ZrO2 substrate, covered with low
15 density A12O3 sheet that was coated with ZrO2, and placed in a conventional
muffle furnace. The tape was heated to 400C in 12 hours and held at that
temperature for 12 hours in 2 This resulted in substantial complete removal of
the organic binder from the tape. The tape was then sintered by raising the
temperature to 900C in 4 hours, raising to 950C in S hours, holding at 950C
20 for 5 hours, cooling to 600C in 2 hours, and cooling with the furnace to room
temperature. The sintering treatment was carried out in 1 atrnosphere of flowingoxygen. The resuldng tape was approximately 0.1 mm thick, and had substandal
flexibility and mechanical strength.
A variety of measurements were carried out on the tape. FIG. S
25 shows the normalized resistivity of the tape as a funcdon of temperature,
indicating that the midpoint of the transidon is 92K, with the resistance vanishing
at 91K. FIG. 6 shows the magnetdzation of the tape (in arbitrary units) as a
function of temperature, for an applied field normal to the plane of the tape. The
sample was cooled in zero Seld and heated in an applied field of 380 arnpere
30 turn/m (4.7 Oe). The Figore clearly shows the existence of superconducdvi~y
below 91K. FIG. 7 shows X-ray diffracdon data obtained from the tape, and
FIG. 8 shows similar da~ obtained from a powder sample of the tape material.
The two sets of data are essendally identical, indicating the composidonal
uniformity of the tape material. Analysis of the data shows that the material is35 the known superconductive phase of the Ba2YCu309 ~;-system, with ~ being
-` 1 30~540
- 14 -
about 2. FIG. 9 shows RBS data obtained from the sintered tape (curve 91) and
from a polished pellet of essentially the same composition (curve 92). As is well
known, RBS probes only the top layer (about 0.1 ~Lm) of a sample. ~IG. 9 shows
that the surface layer of the tape is of essentially the same composition as theS polished surface of the pellet. In particular, the closely similar heights of knees
92, 93, and 94 indicate that both samples have essendally the same concentradonsof Ba, Y, and Cu, respectively. Since the polished surface material was interiormaterial dunng fabrication of the pellet, the identity of the two RBS spectra
proves that even the top 0.1 Ilm of the tape consist of the known superconducting
10 phase. The data also shows that, to within the resolution of the method, no
contaminants have been introduced during the manufacture of the tape.
Exarnple lI: A cerarnic tape is produced substantially as described in
Exarnple I, except that 100 g of calcined powder is mixed with 15 g of 90 10
vinyl chloride-vinyl acetate copolymer binder, 8S g of methyl ethyl ketone
15 solvent, and 1 g of butyl benzyl phthalate plasticizer. The results obtained is
essentially as described in Example I.
Example m: A further ceramic tape (about 50 ~lm thickness,
composition Lal 8SrO 2CuO4 O, with ~ about 0.1) was produced substantially as
described in Example I, except as noted: 436.106 g hydrated lanthanum carbonate
20 (67.24% by weight La203 equivalent), 29.736 g strondum carbonate (99.3% b~w.
SrCO3), and 80.579 g of copper oxide (98.71% b.w. CuO) were mixed with
isopropanol. 80 g of the calcined powder was mixed with 80 g of the binder.
The tapc was heated, after binder burn-out, from 400C to 1100C in 1.75 hours,
and held at 1100C for 2 hours, cooled over 6 hours to 900C, followed by
25 cooling to room temperature in 2.15 hours. A pordon of the tape was fired
between two Pt sheets. This pordon was non-superconducdng. A further pordon
was fired while on a Pt substrate without being covered. A segment of this tape
which lifted off the substratc during firing was superconducdve (Tc about 33K),
whercas the segment that remained in contact with the Pt substrate was not
30 superconducting.
Exarnple IV: Four pieces of green tape, produced as described in
Example m, were laminated together by pressing at about 67 MPa (1000 psi) and
80C, then sintered, as described in Exarnple III, on a sheet of Pt. This laminate
was superconducting, with Tc of about 28.5K.
1 30~5~0
- 15 -
Example V: A filarnentary body is produced by extrusion as follows.
The paste is prepared substantially as in Exarnple I, except ~hat 100 g of the
calcined powder is mixed with 50 g of binder made by dissolving 20 g of
polyvinyl butyral in 80 g of anhydr~us ethyl alcohol. This paste is extruded in a
5 c~nventionàl manner at about 1675 MPa (25 kpsi) through a O.S rnm die. The
resulting filamentary green body is dried, wound helically onto a loose cylindrical
mass of fibrous zirconia, and fired subs~antially as described in Example I. Theresulting helical filament is superconducting. The filament is then slipped over a
tubular core, thereby producing a superconducting solenoid.
Example VI: A screen printing ink ~paste) is produced as follows.
The paste is prepared substantially as in Example I, except that 65 g of the
calcined powder is mixed with 15 g of ethylene cellulose binder and 10 g of
terpinol solvent on a 3-roll shear rnixer while further terpineol is added to yield a
paste of approximately room temperature viscosity of 3xlOS poise. This pastc is
15 screen printed in the conventional manner onto an unfired ceramic substrate to
yield conductor lines and filled vias such as are shown in FIG. 10. After firing as
dcscribed in Example I the sheet-like body is superconductive, forming a high Q
planar inductor.