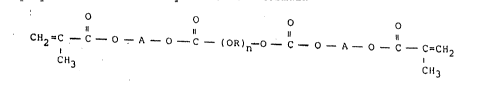Note: Descriptions are shown in the official language in which they were submitted.
~ - t 309 1 05
pEI`II 17 .1
CAN~DA
DENTAL llESIN ~!AI~ ;b
¦This invention relates to polymeric condensation
products which have been found to be useful in restorative
dentistry. More particularly, it relates to polymeric
condensation products wtlich can be used as a principal
component o~ resinous adhesives, and can be used in dental
reRtorative agents. The resLnou~s adhesives of this
invention are suitable ~or bonding to virtually all types of
dental surfaces, including enamel, dentin, portcelain and
metallic sur~aces. They are especially useful in a system
for bonding dental restorative materials to exposed dentin.
Filled compositions contalning the resinous adhesives as
components are useful, depending on filler content, as crown
¦ and bridge materials, as luting agents or cements, as
orthodont c ealants, etc.
- 1 309 1 05
In recent years, materials used for dental restoration
have comprised principally methacrylate polymers. Typical
of these polymeric substances are the acrylic resinous
materials disclosed in U.S. Patents No. 3,066,112, No.
3,179,623, No~ 3,194,784, No. 3,751,399 and No. 3,926,906.
Especially noteworthy is the compound which is the
condensation product of bisphenol A and glycidyl
methacrylate, 2,2'-bis [4-(3-methacryloxy-2-hydroxy
propoxy)~phenyl]-propane (hereinafter abbreviated to "BIS-
GMA"). Other methacrylate polymers, such as ethylene glyco~
dimethacrylate, diethylene glycol dimethacrylate,
triethylene glycol dimethacrylate and tetraethylene glycol
dimethacrylate, are also in general use as diluents,
polyurethanedimethacrylate is also used as a principle
lS polymer in dental restorative materials o~ this type. Since
BIS-GMA is highly viscous at room temperature, however, it
is generally diluted with a monomer having a lower viscosity
such as the aforementioned alkylene glycols or other
compatible materialsl including trimethylol propyl
trimethacrylate, 1,6-hexanediol dimethacrylate, 1,3-
butanediol dimethacrylate, and the like.
~ U ~1 UJ
When these acrylic re inous materials were first
developed, they were used unfilled for dental restorative
purposes. However, because thcsc acrylic materials exhibit
high coefficients of thermal expansion relative to the
coefficient of thermal expansion for tooth structure, these
unfilled substances soon proved to be less than
satis~actory. The disparity in thermal expansion, coupled
with high shrinkage upon polymerization, resulted in poor
marginal adaptability and ultimately led to secondary decay.
Furthermore, the wear and abrasion characteristics and the
overall physical, mechanical and optical properties of these
unfilled acrylic resLnous materials were quite poor. Thus,
from the o~ltset, composite dental restorative materials
containing these methacrylate resins and fillers were
developed. The fillers are generally inorganic filler
materials based on silica~ silicate glass or ~uartz.
Over the years there have been a number of refinements
in the resin matrix component, in the ~iller component, and
in the other additives -- notably antioxidants, ultraviolet
absorbers, polymerization initiators, polymerization
accelerators, etc. -- in dental restorative materials.
There are now available materials which exhibit high
diametral tensile strength, excellent optical properties and
polishability, and low water absorption wh~le, at the same
1 309 1 05
time, complying with all of the requirements specified in
ADA Specification No. 27 for direct filling resins.
Particularly suitable restorative materials are the new
compositions having improved inorganic filler materials such
5 as those disclosed in my co-pending application Serial No.
636,970 filed on August 2, 1984, now U.S. Patent No.
4,547,531, disclosing self-curing 2-component compositions,
and my application Serial No. 677,595 filed on December 3,
1984, now U.S. Patent No. 4,544,359, disclosing visible
light curable compositions.
All of these dental restorative materials are required
to adhere permanently to the tooth structure. GeneralIy,
the tooth surface is treated with an acid such as 30 - 50
wt. 3 orthophosphoric acid, which etches the enamel tooth
sur~ace and exposes enamel rods in honeycomb prismatic
structure thereon, ~here adhesion of the cured polymeric
material is improved via micromechan`ical interlocking. In
addition to this process o etching, the prior art teaches
the use of various additives and improved monomeric mixtures
which are designed to provide improved adhesion.
Although the acid etching technique by itself or
coupled with modification of the resinous material to
increase adhesive capability has been beneficial in
effectin~ the bonding of dental restorative materials to
tooth enamel, there has heretofore been no completely
.
.; ' . .
- 1 30q 1 05
satisfactory process for adhering such restorative materials
to dentin. Acid etching is not suitable for bonding dental
restorative materials to dentin because the tubular
structure of dentin provides passageways to the tooth pulp.
The acid would cause great inflammati'on and pain to the
patient anfl ulti~atelv lead to ~ul~al necrosis. Furthermore,
the hi~h percentage or orqanic (protein) màterial in the dentin
results in a lower degree of adhesion as compared with
enamel.
There has recently been proposed a method for improving
adhesion o~ dental composite materials to dentin surfaces
which involves the successive application of (a) an acidic
salt such as erric oxalate or ferric citrate, (b) the
adcluck of N-(p-tolyl)~glycine and glycidyl methacrylate, the
addition reaction product oE N-phenylglycine and glycidyl
methacrylate or N-phenylglycine itself, and ta) the addition
. reaction product of pyromellitic acid dianhydride and 2-
hydroxyethylmethacrylate, the addition reaction product of
3,3',4,4'-benzophenonetetracarboxylic dianhydride and 2-
hydroxyethylmethacrylate, or 4-methacry10xyethyltrlmellitic
anhydrlde. This multi-step process has, however, not proved
satisfactory.
In addition~to much desired improvements in dentin
bonding systems, it is also desirable to attain improved
bonding to other dental surfaces, sUch as enamel, metallic
a1loy~ and particularly pjrrelaln.
S
,~ .,
- 1 309 1 05
Despite recent advances in the development of filled
dental restorative materials which have led to composites
having higher resistance to abrasion, better handling
characteristics, more satisfactory visual appearance, there
also remains considerable room for improvement in these
areas, specifically desired are improved filled
compositions for use as cro~n and bridge materials, denture-
base materials, luting agents or cements and orthodontic
a~p~iance sealants.
Accordingly, it is one primary object of this invention
to provide a dentin bonding system which employs a non-
irritatinc~ pretreatment to yield a proper surface for
application of a dental adhesive, It is another primary
object o this invention to provide a dental adhesive which
is suitablo or bonding to a properly prepared dentin
surace. It is a further object of this invention to
provide new adhesive materials which are particularly suited
for application, with appropriate pretreatmentr to exposed
dentin surfaces. Other obiects include the provision of
improved adhesive materials for bonding to enamel, metallic
alloys and por~elain. Still another object is to provide
improved filled compositions for use as luting agents,
denture-base material, orthodonticsealants and other dental
restorative materials. Other ob]ects of this invention will
become apparent from the following specification.
- 1 3091 05
According to the invention, there is provided a
dental resin adllesive whicll comprises BIS-G~l~ in an arnount
rangir-g Erom about 20 to about 80 weight percent and a
polycarbonate dimethacrylate of the formula
O O O O
11 ll ll ll
C112-C - C - O - A - o - C - (OR)~l-O - C - O - ~ - O - C - C=C112
c,l3 C113
wherein A is Cl- C6 alkylen, R is C2- C5 alkylene having at
least 2 carbon atoms in its principal chain and n is an integer
from l to 4, said polycarbonate dimetllyacrylate l)eing present
in an amount ranging Erom about 20 to about 80 weight percent.
The resinous adhesive compositions disclose~ herein
comprise ~S~-G~ and polycarbonate dimethclcrylate
condensation products which result from the con(3ellsat1OIl,
under eareEully controlled eonditlons, of two parts by
welght of c!n hyc3roxyalkylmethaerylate of the formula I
()
t'~2 = C - C - O - ~ - Oll
C~13
in whieh A 1s Cl - C6 alky1ene, anc3 one part by weight oE a
bis(ellloroEormate) oE tlle Eormula II
C1 - C - (~)n - O - C - Cl
O O
in whlch R is C2-C5 alkylene having at least two carbon
atoms in its principal chain and n ls an lnteger Erom 1 to
4. of particular interest is the novel condensation product
of 2-hydroxyethylmethacrylate and trlethylene glycol
bis(chloroEormate). rl-ese new adheslve composltions whlch
are unfilled, eonstitute the dental adhes1ve materials of
this invention. These dental adhesives may be applied to
all types of dental surfaces, lncluding enamel, dentin,
porcelain and metallic surfaces but are particularly sulted
Eor 9pplic9tion 0 pretre t9~ d-~tln 9urf9c99
- . .
, ~ ' ` ~ ' '.. ,
- '.
,
' .
-- 130'~05
The dental adhesives of this invention include both
visible light curable and self-curing compositions. The
visible light curable compositions include, in addition to
BIS-GMA and the polycarbonate dimethacrylate condensation
product, the usual polymerization initiators, polymerization
accelerators, ultraviolet absorbers, fluorescent whitening
agents, etc. In the self-curing adhesive compositions, the
polymerization accelerator can be included in the adhesive
composition itself or can be present in a liquid composition
which is used for pretreatin~ the exposed dentin.
Methods for bonding dental restorative materials to an
exposed dentin surface are also provided. The surface is
pretreated by application of 3% ~12O2, 17~ EDTA or 5% NaOCl in
non-vital teeth followed by an alcohol solution of an alkali
metal salt of benzenesulfinic acid with subsequent evaporation
o~ the alcohol from the solution. The treated dentin surface
is then coated with a resinous adhesive according to this
invention. The adhesive is then cured and an appropriate
dental restorative material is applied. Where the adhesive is
a sel~-curing composition, the polymerization accelerator can
either be included in the adhesive itself or be incorporated
into the alcohol pretreatment solution, in which case the
accelerator remains on the dentin surface after evaporation of
the alcohol.
In addition to the unfilled resinous adhesive
compositions, there are also provided filled compositions
comprising the new BIS-GMA and pol~carbonate dimethacrylate
condensation products as a principal component and varous
additives. Such filled compositions are useful for a variety
,~. _ p~ _
- 1 309 1 05
of dental treatment and restorative functions including the
crown and bridge materials luring agents, or cements, denture-
base materials, orthodontic sealants, and dental restorative
materials.
According to one aspect of the invention, a dental
restorative composition comprises about 20 to about 98 percent
by weight resinouscomponent comprising from about 25 to about
35 weight percent o~ BIS-GMA, and from about 65 to about 75
weight percent of a polycarbonate di.methyacrylate o~ the
formula
O O O O
Il 11 11 ll
CH2=C - C - O - A - O C - (~)n - C - O - A - O - C - C=C~12
CH3 CH3
where A is Cl-C6 alkylene, R is C2-C5 alkylene having at least
2 carbon atoms in its principal chain and n is an integer from
1 to ~, and about 80 to about 2 percent by weight in organlc
~iller.
g
.
,
,
,
- 1 309 1 05
Tb~ pu.y-~rb~---e ~ILm~ -y a~e n~....ti--- ~ d.ct-
usable in this invention are obtained by the condensation
reaction o~ an hydroxyalkylmethacrylate of the general
formula I
O
CH2 = C - C - O - A - OH
C~13
in which A is Cl - C6 alkylene, and a bi~tchloroformate) of
the general formula II
Cl - C - (OR)n - O - C - Cl II
in which R is C2-C5 alkylene having at least two carbon
atoms in its principal chain and n is an integer from 1 to
4. By "principal chain" is meant the chain of carbon atoms
serving as a bridge hetween the oxygen atoms.
In the hydroxyalkylmethacrylate, the group A can be,
for example, methylene, ethylene, propylene, 2,2-
dimethylpropylene, butylene, etc. Preferred compounds are
those in which A has 2 or 3 carbon atoms, such as 2-
hydroxyethylmethacrylate and 2-hydroxypropylmethacrylate,
- 1 30~ 1 05
with 2-hydroxyethylmethacrylate particularly preferred. In
the bis(chloroformate), examples of the group R include
ethyiene, l-methylethylene, 1,2-dimethylethylene, propylene,
2-methylpropylene, 2,2-dimethylpropylene, butylene, etc.
The preEerred groups for R are ethylene, propylene, and with
them the preferred values for the integer n are 2 and 3.
Particularly preferred as the bis(chloroformate) reactant i5
triethyleneglycol bis(chloroormate)
(~ C(OCH2CH2)30-C-Cl III
but others, ~uch as diethylene glycol bis(chloroformate),
tetraethylene glycol bis(chloroformate), dipropylene glycol
bis(chloroformate) and tripropylene glycol
bis(chloroformate) are also quite suitable. In general, the
bis(chloroformates) for use in the practice of this
invention include those of the aforementioned generic
formula which are liquid at temperatures employed in the
condensation reaction.
The hydroxyalkylmethacrylates and the various
bis(chloroformates) required as starting materials or the
polycarbonate dimethacrylate condensation products are
either available commercially or can be easily prepared by
known methods. The bis(chloroformate) starting
- 1 ~091 05
materials can be prepared, for example, by reaction of
phosgene with the appropriate glycol according to methods
well known in the art. Triethylene glycol
bis(chloroformate) and certain other bis~chloroformates) can
be obtained from PPG Industries (Chicago, Illinois).
The polycarbonate dimethacrylate condensation products
are prepared by reacting two moles of the
hydroxyalkylmethacrylate and one mole of the
bi~(chloroformate). Said condensation products have the
general formula IV
O O o o
11 , 11 11 ll
CH23C`- C - O - A ~ O - C - (OR)n O - C - O - A - o - C - C=CH2
CH3 CH3
in which A, R and n are defined above. ;.
The preferred polycarbonate dimethacrylate condensation
products are those which, based on Formula IV, have a
molecular weight between about 418 and 506. Particularly
preerred is the novel polycarbonate dimethacrylate obtained
with triethylene glycol bis(chloroformatej and 2-
hydroxyethylmethacrylate, which has the formula V
O O o o
11 11 : 11 11
2 ~ C o (C~2)~2o-c(oc~2cH2)3o-c-o(cH2)2-o-c-c=c~l2
¦ CH3 CH3
- 1 309 1 05
with a molecular weight oE 462.
The polycarbonate dimethacrylate condensation product
is obtained by adding the bis(chloroformate) slowly by drop-
wise addition to the hydroxyalkylmethacrylate in a suitable
solvent such as pyridine. The solution should be well
stirred during the condensation reaction and the temperature
should be maintained between about -5C and 10C, preferably
between about -2C and 4C. Conveniently, the reaction can
be done in an ice bath, thus maintaining a tcmperature of
about 3 to ~C. These lo~ temperatures are necessary in
order to prevent polymerization. After the condensation
reactlon is complete, the product is separated from solvent,
unreacted starting materials, by-products, etc., by methods
well known in the art,
Many of the condensation products included within the
scope o~ formula (IV) are known from United States Patent
Wo. 3,716,571 issued February 13, 1973, and from Soviet
Author's Certiicate No. 732,291 publiahed on May 8, 1980.
They have heretofore found utility as binder compositions
in gloss-reinforced plastics, electric insulating
compositions, heat resistant compositions, etc., but not as
components in dental adhesives or dental restorative
compositions. The specific condensation product of
triethyleneglycol bis(chloroformate) and 2-
hydroxypropylmethacrylate lS a novel substance.
Aromatic polycarbonates derived frcm bisphenol-A by
reaction with diphenyl carbonate or phosgene are known
`~ 1 30q 1 0~
71529-51
up to almost any pressure attainable with production apparatus.
Since extremely high pressure apparatus is quite expensive,
pressures to about 500 at~ospheres are suggested. Most
desirably, the pressure should be in the range of from about
100 to about 400 atmospheres, particularly when employing the
aforesaid preferred temperature range. It has been found,
however, that the glycol aldehyde selectivity decreases
slightly as a result of decreasing the total pressure from
about 225 atmospheres to 100 atmospheres. Lowering the total
pressure to 500 psig (31 atmospheres) results in a substantial
decrease in glycol aldehyde selectivity. ~ reaction pressure
of 1,400 to 4,000 psig is preferred.
The reaction pressures represent the total pressure
gases aontained in the reactor, i.e., aarbon monoxide and
hydrogen, and, if present, any inert diluent gas such as
nitrogen. As in any gaseous system, the total pressure is the
sum of partlal pressures of component gases~ In the present
reactlon, the molar ratio o~ hydrogen to carbon monoxlde can
range from about 1~10 to about 10.1, with the preferred ratio,
from about l~S to about 5:1, and the reaction pressure can be
achieved by adjusting the pressure of these gases in khe
reactor. For best results, the molar ratio of carbon monoxide
to hydrogen is main~ained at. high values where partial
pressures o~ carbon monoxide favour production of glycol
aldehyde. Thus, to produce glycol aldehyde, the partial
pressure of carbon monoxide is usually adjus~ed to be about 3
to about 10 times that of hydrogen.
- 1 30q 1 05
¦ products and have been used as denture-base materials; see
¦ Stafford et al, "Polycarbonates: A Preliminary Report on
¦ the Use of Polycarbonates as a Denture Base Material",
I Dental Practitioner 17, 217-23 (1967) In contrast to the
I .
relatively low molecular weight condensation products usable
in this invention, these previously-described
polycarbonates have phenylene groups rather than the
alkylene groups and have preferred molecular weights in the
range o 30,000 to 200,000.
The subject polycarbonate dimethacrylates are suitable
for incorporation into dental adhesives, including those
which are visible li~ht curable and those which are self-
curing. Typically, these polycarbonate dimethacrylates are
incorporated into a reslnous composition having from about
20 to about 60 weight percent of BIS-GMA and from about 40
to about 80 weight percent of the polycarbonate
dimethacrylate. The preferred ranges for BIS-GMA are 25 to
35 weight percent, particularly about 30 weight percent, and
the preferred ranges for the polycarbonate dimethacrylates
are from 65 to 75 weight percent, particularly about 70
weight percant. In addition to these monomeric materials,
the resinous adhesive compositions of this invention will
also typically include polymerization initiators,
polymerization accelerators, ultraviolet light absorbers,
anti-oxidants, and other additlves well known in the art.
Although a polymerization initiator and a polymerization
accelerator are generally used in these resinous adhesive
compositions, the presence o a polymerization accelerator
- 130~105
¦ in the self-curing adhesive compositions of this invention
¦ is optional. One of the features of the self-curing dentin
¦ bonding system of this invention is that the polymerization
accelerator can be incorporated into the dentin pretreatment
composition rather than into the resinous adhesive
composition.
.
- 1 30~ 1 05
The polymerization initiators usable in the resinous
adhesives o~ this invention are the usual initiators known
in the art. For example, visible light curable compositions
employ light-sensitive compounds such as benzil, diketones
and in particular, dl~camphoroquinone in amounts ranging
from about O.OS to 0.5 weight percent. Self-curing
compositions will generally contain free radical
polymerization initiators such as, for-example, a peroxide
in amounts ranging from about 2 to about 6 weight percent.
Particularly suitable free radical initiators are lauroyl
peroxide, tributyl hydroperoxide and, more particularly
benzoyl peroxide.
The polymerization accelerators usable in the
compositions of this invention are the various organic
tertiary amines well known in the art. In visible light
cured compositions, the tertiary amines are generally
acrylate derivatives such as diethylamino ethylacrylate,
dimethylamino ethylmethacrylate and, particularly,
diethylamino ethylmethacrylate in amounts ranging from about
0.05 to about 0.5 weight percent. In the self~curing
compositions, the tertiary amines are generally aromatic
tertiary amines, such a~ dimethyl-p-toluidine,
dihydroxyethyl-p-toluidine, and the like, in amounts ranging
from aboutO.05to about 4.0 weight percent. These can
.
16
- 1 309 1 05
optionall be incorporated n a pretreatment ~o1ution rather
than in the resinous adhesive,
¦ It is preferred also to employ an ultraviolet absorber
in these resinous adhesives in amounts ranging from about
0.05 to about 5.0 weight percent. Such UV absorbers are
particularly desirable in the visible light curable
compositions in order to avoid discoloration of the resin
from any incident ultraviolet light. Suitable UV absorbers
are the various benzophenone~, particularly UV-9 and UV-S411
available rom American Cyanamid Company, and benzotriazoles
known in the art, particularly 2-(2'-hydroxy-5'-
methylphenyl)-benzotriazole, sold under the trademark
TINUVIN P by Ciba-Geigy Corporation, Ardsley, New York.
Typical visible light curable dental adhesives
according to this invention comprise:
65 - 75 weight percent of the polycarbonate
. dimethacrylate condensation product of triethylene
glycol bis(chloroformate) and 2-
hydroxyethylmethacrylate,
25 - 35 weight percent of BIS-GNA,
0.05 - 0.35 weight percent of dl-camphoroquinone,
0.05 - 0.5 weight percent of diethylamino
ethylmethacrylate, and
0.05 - 5 weight percent of TINUVIN P ultraviolet
absorber
- 1 309 1 05
in sp~cieic amoants within these ranges to yield a 100~ by
weight polymerization system.
Typical adhesives for use in self-curing systems
comprise:
65 - 75 weight percent of the polycarbonate
dimethacrylate condensation product of triethylene
glycol bis(chloroformate) and 2-
hydroxyethylmethacrylate,
25 - 35 wei~ht percent of BIS-GMA,
3.2 - 5.0 weight percent of benzoyl peroxide, and
0.05 -4,5wQight percent of TINUVIN P ultraviolet
absorber
in specieic amounts within these ranges to yield a 100% by
weight polymerization system. In addition, where the
polymerization accelerator is incorporated in the resinous
adhesive rather than in the dentin pretreatment composition,
the adhesive additionally conta~ins from 0.05to 4 weight
percent of dihydroxyethyl~p-toluidine.
It is also possib~le to use the polycarbonate
dimethacrylate condensation products in adhesives which are
both self-curing and visible light curable. In this
"combination" system the resinous adhesive composition is
;identical to the visihle lig~ht cura~ble composition described
above with the addltion of about 3.2 to about 5.0 weight
percent of benzoyl peroxide.~
.
18
1 30q 1 05
This invention also includes methods for bonding dental
restorative material to various surfaces, such as enamel,
rr~e,~lî~
porcelain,-~e~ e alloy and particularly to exposed dentin
surfaces. For such dentin surafces, the methods include
S pretreatment of the dentin surfaces and coatin~ of the
treated surfaces with the resinous adhesive compositions of
this invention.
When dentin surfaces are exposed as a result of cutting
and abrasion which occurs during treatment, there is formed
on the dentin surface a "smear layer" composed principally
of organic material. This smear layer is believed to cause
partial filling of the dentin tubules as well as to obstruct
the orifice~ o said tubules. Any pretreatment of the
dentin surface must be preceded by removal of the smear
lS layer, It has been ound that this gmear layer is readily
removed by application, for example, of a 5 percent aqueous
~olution of ~odium hypochlorite, a 17 percent aqueous
solution of ethylenediaminetetraacetic acld or a 6 percent
citric acid gel. The methods of this invention assume prior
removal of the smear layer.
In applying the resinous adhesive compositions of this
invention to dentin surfaces, the first step after the usual
prophylaxis with, for example, hydrogen peroxide, is a
pretreatment of the dentin surface with an alcohol solution
containing an alkali metal salt of benzenesulfinic acid,
preferably sodium benzenesulfinate salt. If the dental
19
.,
.
~ ` ~
-- - 1 309 1 05
restorative composition to be used is a self-curin~
composition, the alcohol pretreatment solution for the
dentin can also include an or~anic tertiary amine,
~referably an aromatic amine such as dihydroxyethyl-p-
toluidine. Typical alcohol solutions for use in thispretreatment contain from about 3 to about 5 weicJht percent
of sodium benzenesulfinate, and from about 1 to about 2-1/2
wei~ht percent of dihydroxyethyl-p-toluidine, the remainder
bein~ ethanol.
The alcohol solution is applied to the dentin surface
and is then evaporated. Evaporation may be effected by a
dry air stream or other methods well known in the art. This
pretreatment step fulfills the function of (1) prophylaxis
and (2) improvin~ the dentin surface for acceptance of
resinous adhesive. The benæenesulfinic acid salt causes a
chelatin~ polymerization promotion of the dentin surface
with the adhesive and results in partial fillin~ of the
tubules o:E the dentin. Thus, the dentin surfaae is made
considerably rou~her and this provides for enhanced
adhesion. This enhanced susceptibility is obtained without
the use of irritatin~ substances such as phosphoric acid.
If the alcohol solution also contains an aromatic
tertiary amine, this is deposited on the surface of the
dentin and functions as a polymerization acceleratinq a~ent
- 130qlO5
when a resinous adhesive, without accelerator, is applied in
the third step.
The resinous adhesive compo~sitions of this invention
are then coated onto the pretreated dentin surface,
according to methods well known in the art. If the resinous
adhesive composition is a light curable composition,
polymerization is effected by exposure to a visible light
source, for example a 150 watt halogen light source or any
visible light source which is capable oE generating light
within wavelengths ranging Erom about 250 to about 750
nanometers, preferahly from 4S0 to 500 nanometers and more
preferably~i~rom 4fi8 to ~92 nanometers, for about 20 to 50
seconds. If the resinou~s adhesive composition is a self-
curing composition, a period of about 3 to 6 minutes should
be allowed for polymerization.
The adhesive composition of this invention is also
suitable for use on a properly prepared enamel surface and
can be simultaneously applied to a pretreated dentin surface
and adjacent areas of an enamel surface. Such enamel
surfaces can be subjected to etching treatment with, for
3~ 50 I/~to ~0
example,~orthophosphoric acid. Obviously, steps should be
taken to shield the exposed dentin from the enamel etching
treatment
21
I -- 1 309 1 05
The compositions of this invention are employed in the
following manner as a dentin adhesive bonding system where
the smear layer has been removed from the dentin surface and
adjacent exposed enamel has been acid etched by the use of
well-known etching materials such as, for example,
orthophosphoric acid gel. Pulp protection is accomplished
by use of, for example, a calcium hydroxide composition.
After ~craping any debris from the dentin surface, it
is cleaned with an oil-free dental pumice and water. A
3~ hydrogen peroxide solution is then applied as prophylaxis
and the dentin sur~ace is washed and dried.
The restoration site is then isolated with a Mylar
polyester strip for proper gingival margins, gingival
pappilae and adjacent tooth preparation via sliding
preshaped/formed contour strip into gingival sulcus. The
polyester strip is tightened to seal the gingival margin,
thus isolating saliva and gingival cervl~ul~r fluid flow to
the restored area. In addition, dental wedges are
recommended in order to separate teeth, facilitate
interproximal contact and hold the polyester strip in
position. Two or three drops of a liquid solution of sodium
benzene sulfinate in ethanol are brushed over the dentin
surface. If a self-curing adhesive system is used, this
alcohol solution can additionally contain dihydroxy-p-
toluidine~ The ethanol should be permitted to evaporate,
with air drying recommended for this purpose.
~ - 1 3091 05
The resinous adhesive composition is then applied over
the etched peripheral enamel beyond cavo-surface margin and
over the dry, prepared and conditioned dentin surfaces. An
extremely thin layer of resinous adhesive is obtained by
removing excess material from enamel and dentin surfaces
with a dry brush and jet of oil-fres air. If the adhesive
composition is visible light curable, it should be exposed
to a visible light source such as, for example, spectra-
Lite, Eor about 40 seconds. If a self-curing adhesive
composition is used -- with the polymerization accelerator
either on the pretreated dent:in surface or in the resinous
adhe~sive itself -- a yeriod of about three to five minutes
should be allowed Eor yolymerization. Immed;ately-t~lereafter, th
dental restorative paste should he placed onl:o the aclhesive.
Alternatively, the restorative paste and the adhesive can be
cured simultarleously.
In aclclit~oll to use Eor bondiny restorative materials in
. situations where exposed dentin is encountered, the resinous
adhesive comyositions oE tlli~s invention are al.so applicable
for the bonding oE enarnel, porcelain and metallic alloys,
each to each other, or for the bonding of other dental
restorative material~s, including all types of acrylic base
materials and various crown ancl hridae alloy compositions, -to
enamel, porcelain and me-tallic surfaces. They are applied to the
surfaces to be adhered bv methods well known in the art.
Typically, the surfaces are prepared by, for example,
etching or sand blasting.
* trade mark -or apparatus v,ilable from Jenerlc/Pentron
. .. , ~
,
-- 1 309 1 05
The subject adhesive materials are particularly
suitable also for promoting the adhesion of various dental
restorative materials to porcelain surfaces. Generally, the
porcelain surface is etched by, for example, hydrofluoric
acid and the resinous adhesive compositions of this
invention applied to the etched surfaces. The adhesive is
then cured by methods well known in the art such as, for
example, heat curing or visible light curing. If visible
light curing is desired, the adhesive compositian should
also contain the various visible light curing additives
dlscussed above. The dental restorative material is then
applied to tho cured adhesive. Such restorative materials
include virtually all oE the materials currently used in
dentistry and can also include the novel filled compositions
of this invention.
When using the resinous compositions of this in~ention
as a porcelain adhesive, it is preferred that there be a
silane coupling agent used between the etched surface of the
porcelain and the resinous adhesive composition. The
silane coupling agent is brushed onto the etched porcelain
surface prior to application of the resinous adhesive.
Useful silane coupling agents may be selected from members
of organosilicon monomers such asaminoalkyl(trisalkoxy)silanes
which are characterized by the formula R-SiX3, wherein R is
an organofunctional group attached to silicon in a
hydrolytically stable manner and X designates hydrolyzable
groups which are converted to silanol groups upon
hydrolysis. Most commonly, R comprises 3-aminopropyl or 3-
24
-- 1 30q 1 05
ureidopropyl moiety WtliCtl may be further separated from the
silicon group by one or two -Nil(~ll2)-~ moieties wllereill n=l-
2. Preferably X is an alkoxy group selected ~rolll tlle group
conslsting of methoxy, ethoxy, 2-methoxyetlloxy or is acetoxy
speciflcally y - metllacryloxypropyltrillletlloxysilane ~-174*
Union Carbide. Preferred silane coupllng agellts are
commerclally available from Unioll Carbide as tile ~110~-~1160*
series which includes 3-aminopropyltri-ethoxysilalle, 3-
aminopropyltrimethoxysilane (also available from Dow Corning
as 2-6020), N-2 aminoethyl-3-amillopropyl-trillletlloxysilalle,
or 3-ureidopropyltriethoxysilane. With such silane-couplillg
agentsr the cure system for the resinous adhesive should be
a visible llght cure system, rattler than a heat cure system
since t~le heat necessary to efect the cure will cause
degredatlon of the silane. ~owever, tlle 1asll point of
pre~erred sllane ~-17~*i9 275 C well above l~eat curing
temperature range.
The filled COlllpO9itiOns oE tl~is inventioll can lnclude
all of the inorganic fillers currently used in delltal
re9torative materials, tlle amount o sucl~ flllcr belng
determined by the specific unctioll of the illcd materials.
Thus, for example, wllere crown and bridge materials are
belng prepared, th2 resinous compositolls of this inventio
are present lllamounts ranging from about 20 to about ~0
welgllt parcent, and the filler materials are prc~serlt in
amounts ranglng from about 60 to about 80 weigllt percent.
Typical compositions ~or crown and bridge materials are
about 3~ of the resinous material and about 70~ of the
filler. For luting cements, ttle resinous compositions of
this invention are present in amouots ranging froln about 43
* tracle mar~s of Union ~arbide
-- 1 309 1 05
to about 55 weiyht percent, the fillers comprising from
about 45 to about 57 weight pereent. For orthodontic
sealant and orthodontic cement compositons, there will
typically be from about 90 to about 98~ of resinous
eomponent and about 2 to about 10~ of filler.
The filled compositions of this invention can, in
general, inelude any suitable filler material such as a
siliea, silieate glass filler or ~uartz ~hich is ea~able of~ein( ?
eovalently bonded to the resin matrix itself or to a
eoupling agent whieh is covalently bonded to both.
Particularly suitable as fillers for dental
restorative m~terials pre~ared in accordance with tllis
invention are those having a particle size ranging from
about 0.01 to aboutO.07 microns and prepared by a series of
milling steps eomprisin~ wet milling in an aqueous medium,
surfaee eteh milllng and silanizing milling in a silane
solution. Sueh inor~anie filling materials are disclosed in
U.S. Patents No. 4,544,359 and No. 4,547,531. ~s
with the unfilled eompositions, the filled and partially
filled eompositions can be prepared in both visibLe light
eurable formulations and self-curing (paste-paste)
formulations. The filled eomposite restorative materials
ean be prepared by admixing from about 20 to 30~ by weight,
preferably 20-26% by weight, of either the unfilled visible
light eurable dental adilesive or tile unfilled self-euring
adhesive eompositions with from about 65 to about ~5~ by
26
.,
- 1 309 1 05
weight, preferably about 75 to 83% by weight of inorganic
filler material.
The composite dental restorative material of the
present invention preferably comprises an inorganic filler
having an average particle size diameter of from about 0.5
to 5 microns homogeneously dispersed in an oryanic
polymerizable monomeric matrix comprising a polycarbonate
dimethacrylate. In addition, a relatively small amount of
fumed silica is also preclispersed within the monomeric matrix.
The inorganic filler primarily comprises an X-ray opaque
alkali metal or alkaline ear~h metal silicàte such as
lithium silicate, barium silicate and the like. For
purposes of illustration, and as the preerred silicate
species, barium silicate will hereinafter be employed as
being typical of the alkali metal or alkaline earth metal
silicates which can be suitably employed in the present
~ r
invention. The barium~silicate exhibits substantially the
same index of refraction as that of the organic monomeric
matrix in which it is dispersed. The filler may
additionally contain a relatively small amount of
borosilicate glass which imparts greater compressive
strength to the resulting composite and enhances the
translucency thereof thereby enabling better blending of the
restorative material with the adjacent teeth. In addition,
the presence of the borosilicate glass helps narrow the gap
between the refractive indices of the barium silicate and
the organic monomeric matrix.
- 130~105
The ability to provide a composite dental material
having improved properties is achieved by the method by
which the inorganic filler is prepared. This method
involves a sequence of milling operations which includes wet
milling to reduce the barium silicate and borosilicate to
the requisite particle size and assure a very narrow
particle size distribution and to uniformly disperse the
borosilicate glass particles throughout the bulk of the
barium silicate, Thereafter, the wet milled filler is
subject to a further milling operation to etch the surface
thereof which has been found to impart a
dramatic increase to the diametral tensile strength of the
resulting composite. Subsequently, the so treated filler is
subjected to a final milling operation during which it is
silanized in order to render it compatible with the resin in
which it will ultimately be dispersed.
Details of the preparation of the inorganic filler,
. which comprises a mixture of from about 5 to about 20% by
weight of borosilicate glass and from about 80 to abut 95%
by weight barium silicate, and has an average particle size
~diameter of from about 0.5 to about 5 microns, may be found
in the aforementioned ~.SO Patents No. 4,544,539 and No.
4,547,531-
This invention will be better understood by referenceto the following examples which are included here for
illustrative purposes only and are not to be construed as
limitations,
- 1 3091 05
EXAMPLE 1 - Preparation of Condensation Product of 2-
Hydroxyethylmethacrylate and Triethylene Glycol
bis(Chloroformate).
~ A) The apparatus used was a round-bottom flask
containing a magnetic stirring bar and fitted with a unnel
and drying tube; said apparatus was fitted on an ice bath
and stirring plate. 110 ml of pyridine was poured into the
flask and 78.86 grams of 2-hydroxyethylmethacrylate (0.606
moles) was slowly added. To this was added very slowly --
over a period of about two hours -- 82.S grams ~0.300 moles)
o~ triethylene glycoi bis(chloroformate), A white
precipitate began formin~ almost immediately, After the
addition was completed, the ice bath was removed and the
mixture allowed to stir overnic~ht at room temperature.
(B) The mixture was then slowly added to 77 ml of
concentrated hydrochloric acid containing about 120 g of
ice.
(C) The mixture from Step (B) was placed in a
separatory funnel and oil and aqueous/acid layers were
separated, The aqueous/acid layer waæ extracted twice with
150 ml of ethyl acetate (total 450 ml). The oil layer and
the ethyl acetate extractants were combined into an organic
layer~
(D) The organic layer was washed f iV9 times with 100
ml of 1 molar HCl, once with 100 ml of water, twice with
100 ml of 5% NaOH, once with 100 ml of water, and once with
100 ml of saturated NaCl solution, The product was then
dried overnight with MgSO4.
- 1 309 1 05
(E) The dried product was decolorized with Carbon
Norlte and filtered by gravity filtration. The solvent was
removed on a roto-evaporator at a temperature of 40C and
the product, a clear water white solution was obtained.
0.03~ of 2,6-di-tert.butyl-4-methylphenol was added as a
polymerizatlon inhibitor.
(F) An IR spectrum and size exclusion chromatographic
plot shows the molecular weight to be 462.
In like manner, the following additional
polycarbonate dimethacrylates are prepared.
EXAMPLE -A- -(OR)n~ Mol. Wt.
NO.
2 CH2 ~OCH2CH2)3 434
3 CH2CH2CH2 (OCH2C~2)3 490
4 CH2CH2 (OCH2C~2)2 418
CH2CH2 (ocH2cH2)4 506
6 CH2 (C~l2cH2cH2)3 504
7 Ci~2 1OCH(CH3)CH2]2 418
8 CH2 (OCH2CH2)4 478
9 CH2 (ocH2cH2cH2c~2)4 446
CH2CH2 ~OCH2CH(CH3)cH2]2
11 CH2 (0CH2CH2CH2cH2c1l2)2
12 CH2-CH2 [OCH2C(CH3)2CH2]2 502
13 CH2-CH2-CH2-cH2 (OCH2CH2)2 474
14 CH2-CH(CH3) 2 2 2 446
CH2-CHtCH3) (OCH2CH2CH2)2 474
`S ~ 30
,
:
.
~ 1 309 1 05
EXAM _E 16 - Self-Curing Dentin Adhesive Bondiny System.
A two-component self-curing dentin adhesive bonding
system having the followiny constituents was prepared. The
first component is a liquid composition having:
~5.60grams of ethanol,
4.00 grams of sodium benzene sulfinate, and
o ~o grams of dihydroxy;ethyl-p-toluidin~
The second component is a resin paste composition having:
70.96 grams of a polycarbonate dimethacrylate which
is the condensation reaction product of 2-
hydroxyethylmethacrylate and triethylene glycol
bis(chloroeorlnate),
29.03 grams of BIS-GMA,
0.0373 grams o~ 2,6-ditert.but:yl-4-1nethylphenol
(inhibitor),
0.75 grams of TINUVIN P (ultraviolet absorber from
. Ciba-Geigy Corporation, Ardsley, New York),
4.00 grams oE benzoyl peroxide (initiator), and
0.0097 grams of UVITEX OB*(fluorescent whitening agent
from Ciba-Geigy Corporation, Ardsley, New York).
The liquid composition is used as a pretreatment for
exposed dentin surfaces, while the paste composition is an
example of a resinous adhesive accordiny to thls invention.
* -trade mark
31
- 1 309 1 05
EXAMPLE 17 - Visible Light Cur~ble Dentin Adhesive Bonding
System.
A two-component visible light curable dentin adhesive
bonding system is prepared, whose first component is
identical to the liquid component of Example 16. The second
component is identical to the second component of Example 16
except for the absence of the dihydroxyethyl para toluidine
and 0.1650g d,l-camphoroquinone and 0.2300g
diethylaminoethylmethacrylate to substitute for the
benzenyl peroxide.
EXAMPLE 18 - Preparation of Filler Material.
Filler material suitable for use in the dental
re~torative compositions of this invention was prepared as
follow~.
~oro~ilicate glass rods, available from Corning Glass
Work9, Corning, New York, are cut into cylindrical form.
The resulting cylinders are loaded into a 5 gallon glass
carboy until the carboy is half filled. The carboy is then
filled with water, sealed and tumbled at 175 rpm for 168
hours to condition the glass rods.
The conditioned borosilicate glass rods are recovered
and loaded into a 5 gallon polyvinylidene fluoride lined
grinding vessel adapted for combined oscillatory and
vibratory motion. The grinding vessel is loaded with the
glass rods until three-quarters filled. Three kilograms of
32
. ~,
.
- 1 3091 05
X-ray opaque barium silicate glass frit ~ESSCHEM T-3000
available from Esschem Corporation, Essington, Pennsylvania)
having an average particle size diameter of 10 microns are
added to the grinding vessel and then water is added to fill
the grinding vessel. The vessel is then sealed and vibrated
for 24 hours whereupon the barium silicate frit is ground to
an average particle size diameter ranging between about 5and
6 microns and sufficient borosilicate glass is abraded off
the rods to provide a barium silicate borosilicate glass
mixture comprising about 89% barium silicate and about 11
borosilicate glass.
The resulting aqueous slurry is recovered and strained
through a series of 200, 400 and 600 mesh screens. The
resulting filtrate is subjected to vacuum filtration and
then dried in a convection oven at 120C for 24 hours. The
dried, milled filler is recovered and crushed and ground
with a mortar and pestle to a fine powder.
Three kilograms of the dried, milled filler are charged
to a glass carboy which is filled to one-half it volume with
conditioned borosilicate glass rods prepared as described
above. The carboy is then filled with six liters of a clear,
colorless, aqueous solution of sodium hydroxide exhibiting a
pH of 12 which is buffered with NaaHPOy. The loaded carboy
is ~ealed and tumbled at 175 rpm for ~ hours. Thereafter,
* trade mark
. ~ 33
.,, ~ ~
-- 1 309 1 05
the resulting milled filler is recovered and subjected to
vacuum filtration. The filter cake is washed with water
until pH indicators in the filtrate indicate neutrality
(pH=5.5-7.0) has been essentially obtained. The recovered
filter cake is then dried in a convection oven at 120C for
24 hours. The neutralized filter cake is ground to a fine
powder with a mortar and pestle.
Silanization of the filler thus obtained is effected by
filling a 5 gallon glass carboy to one-half its volume with
borosilicate glass rods conditioned in the manner described
hereinabove. Six kilograms of a solution of 8~ silane in
methanol is charged to the carboy along with 3 kilograms of
the milled filler recovered from the aqueous etchant milling
step. The carboy is sealed and tumbled for 6 hours at 175
rpm. The silanized slurry is recovered and subjected to
vacuum filtration. The resulting filter cake is dried in a
vacuum oven for one hour at 120C and then pulverized with
mortar and pes~tle giving rise to s;ilanized filler particles
having an average particle size of 2.3 microns.
Silanization results in 4.s~ silane being coupled to the
filler particles.
` This filler material can be used with resin matrices
containing polycarbonate dimethacrylates in either self-
curing or visible light curing ccmpositions.
- 1 309 1 05
EXAMPLE 19 - Visible Light Curable Filler Dental Restorative
Compo~ition.
A monomeric matrix composition is prepared by admixing
the following ingredients:
100 grams of the monomeric matrix composition is
prepared by admixing the following:
29.00 grams BIS-GMA
70.00 grams PCDMA (condensation product of 2-
hydroxymethylmethacrylate and triethyl~ne glycol
bis(chloroformate)
0.99 gram TINUVIN P
0.16 grams dl-camphoroquinone
0.23 grams DEA-EMA
0.0097 grams 2,2'-(2,5-thiophenediyl) bis(5-tert-
butylbenzoxazole)
A filled composite re~toration material of the present
invention especially suitable for anterior dental
application~ i~ prepared by admixing 30~ by weight of the
foregoing monomeric matrix composition with 68% by weight of
the inorganic ~iller of Example 18 and 2~ by weight of
colloidal fumed silica having an average particle size of
about 0.04 micron. The resulting composite is a homogeneous
paste comprising the monomeric composition as the matrix
with the inorganic filler and the fumed silica uniformly
dispersed therein.
~. , ., . , . , ~ .
1 30q 1 05
EXAMPLE 20 - Self-Curing Paste-Paste Dental Restorative
Composition.
An initiator resin system is prepared by admixing the
following:
29.00 grams BIS-GMA
70.00 grams PCDMA
0.15 grams BHT
4.00 grams LUCIDOL benzoyl peroxide.
0.00097 grams 2,2'-(2,5-thiophenediyl)bis(5-tert-
butylbenzoxazole)
The initiator paste system is obtained by admixing 21~
by weight o~ the above liquid monomeric compos;tion with 7g%
by weight of the inorganic filler of Example 18 in a
planetary mixer under vacuum forming a homogeneous paste.
The paste 1~ passed through a two roll stainless steel mill
to ensure homogeneity.
An accelerator resin system is prepared by admixing the
ollowing:
29,~0 grams BIS-GMA
70.00 grams PCDMA
0.15 gramq ~HT
loSO grams dihydroxyethyl p toluidene (m.p.:53.5-
54~5C)
4,00 grams UV-9 benzophenone
The heat cure single paste dental restorative excludes
~. i . `"'.1
i 36
~1
~,, ,
~ : .
:
1 309 1 05
any accelerator such as t-amines; dihydroxyethylpara-
~oluidine or diethylaminoethylmethacrylate as well as the
visible light cure initiator d,l-camphoroquinone with the
presence of the heat-cure initiator only specifically
benzoyl peroxide.
The accelerator paste system is obtained ~y admixing
30% by weight of the above liquid monomeric composition with
70~ by we~ght of the inorganic filler of Example 18 in a
planetary mixer under vacuum forming a homogeneous paste.
The paste is passed through a two roll stainless steel mill
to ensure homogeneity.
Essentially equal amounts of the foregoing initiator
paste system and accelerator paste system are uniformly
admixed for about one minute to form the filled composite
restorative material of the present invention.
. ~
~.~1
~ - 37