Note: Descriptions are shown in the official language in which they were submitted.
~ 3 ~ 9
SPECIFICATION
POROUS POLYPROPYLENE HOLLOW FIBER MEMBRANE,
METHOD FOR PRODUCTION T~IEREOF AND ARTIFICIAL LUNG
Background of the Invention
5 [Field of the Invention]
This invention relates to a porous
polypropylene hollow fiber m~mbrane, a method ~or the
production thereof, and an artificial lung using the
hollow fiber membrane. More particularly, this
10 invention relates to a porous polypropylene hollow fiber
membrane possessing a high gas-exchange capacity, a
method for the production thereof, and an artificial
lung using the hollow fiber membrane. Still more
paxticularly, this invention relates to a porous
15 polypropylene hollow fiber membrane which, while being
used in an artificial lung of the type passing blood
inside or the type passing blood outside the hollow
fiber membrane, exhibits a high gas-exchange capacity
without inflicting damage upon blood components,
20 inducing an increase in the pressure loss, or suffering
from leakage of blood plasma during a protracted
service, a method for the production thereof, and an
artificial lung using the hollow fiber membrane.
[Description of the Prior Art~
Generally in the cardiac operation~ an
artificial lung of hollow fiber membrane is used as
inserted in the extra-corporeal circulatory path for the
purpose of leading a patient's blood out of his body,
adding oxygen to the blood, and removing carbon dioxide
30 gas from the blood. The hollow fiber membranes
available in the artificial lungs of this nature come in
the two types, namely the homogeneous membrane and the
porous membrane. The homogeneous membrane effects
passage of a gas by allowing the molecules of the gas to
35 be dissolved and dispersed in the membrane. A typical
example of the homogeneous membrane is silicone rubber,
which has been commercialized as MERA SILOX (Senko Ika
* trade-mark
; ,.... .
~31~
Kogyo K.K.),for instance. Because of -the restriction
imposed by the gas permeahility, silicone rubber is the
only practicable homogeneous membrane known to the art.
The silicone rubber membrane, by reason of strength, is
5 not allowed to have a wall thickness less than lO0 ~m.
I-t, therefore, has limited gas permeability and exhibits
particularly poor permeability to carbon dioxide gas.
Worse still, the silicone rubber has a disadvantage that
it is expensive and deficient in workability.
iO In contrast, the porous membrane is such that
the micropores contained in the membrane are notably
large as compared with the molecules of a gas given to
be passed and, therefore, the gas passes the micropores
in the form of volume flow. Various artificial lungs
15 using microporous polypropylene membranes and other
similar porous membranes have been proposed. It has
been proposed, for example, to manufacture porous
polypropylene hollow fibers by melt spinning
polypropylene with a nozzle for the production of hollow
20 fibers at a spinning temperature in the range of 210 to
270C at a drart ratio in the range of 180 to 600, then
subjecting the spun fibers to a first heat treatment at
a temperature of not more than 155C, stretchiny the hot
spun fibers to an extent in the range of 30 to 200~ at a
25 temperature below 110C, and subjecting the stretched
fibers to a second heat treatment at a temperature
exceeding the temperature of the first heat treatment
and not exceeding 155C (Japanese Patent Publication SHO
; 56(1981)-52,123 published December 10, 1981 in the name of 30 the applicant). In the porous hollow ~ibers obtained
as described above, since the micropores are physically
formed by stretching polyprop~lene hollow fibers, they
are linear micropores substantially horizontal to the
direction of the membrane thickness~ Further, these
35 micropores are formed by producing cracks in the axial
direction of hollow fibers in conformity with the degree
of stretching, they have a cross section of the shape of
a slit. Moreover, the micropores continuously run
~ 2 --
1 3 ~
substantially linearly through the membrane and account
for a high porosity. The porous hollow fibers described
above, therefore, have a disadvantage that they exhlbit
high permeability to steam and, when used for
5 extracorporeal circulation of blood for a long time,
suffers leakage of blood plasma.
As a porous membrane incapable of incurring
leakage of blood plasma, a porous polyole~in hollow
fiber has been proposed which is produced by mixing a
10 polyolefin, an organic filler uniformly dispersible in
the polyolefin while the polyolefin is in a molten state
and readily soluble in an extractant -to be used later,
and a crystalline core forming agent, discharging the
resultant mixture in a molten state through annular
15 spinning orifices and, at the same time, introducing
inactive gas into the interiors of the hollow threads of
the mixture, cooling and solidifying the hollow threads
by contact with a cooling and solidifying liquid
incapable of dissolving the aforementioned polyolefin,
20 and then bringing the cooled and solidified hollow
threads into contact with the aforementioned extractant
thereby removing the aforementioned organic filler by
extraction from the hollow threads (see applicant's U.S
Patent No. 4,708,800, issued November 24, 1987). The
25 polypropylene
hollow fiber membrane, one version of the aforementioned
hollow fiber membrane which is obtained by using, as a
cooling and solidifying liquid, a cooling and
solidifying liquid capable of dissolving the organic
30 filler to be used as desirable for the process, however,
has a small pore density per unit area and possibly
offers an insufficient gas-e~change capacity for use in
an artificial lung, though it has no possibility of
incurring leakage of blood plasma because the pores are
35 small and complicate in shape. There is another
possibility that the low molecular component of the
po]yolefin will mingle into the cooling and solidifying
liquid capable of dissolving the organic filler,
- 3 -
: .
~3~. 3~
eventually adhere to the inner wall o~ the cooling bath,
and cause the shape of the hollow fibers to vary with
elapse of time.
As an amendment of such drawbacks as mentioned
5 above, there has been proposed a porous polyole~in
hollow fiber membrane produced by a process which
comprises mixing polypropylene, an organic filler
uniformly dispersible in the polypropylene while the
polypropylene is in a molten state and readily soluble
10 in an extractant to be used later,and a crystalline core
forming agent, discharging the resultant mixture in a
molten state through annular spinning orifices thereby
forming hollow threads, cooling and solidifying the
hollow threads by contact with a liquid of the
15 aforementioned orgniac filler or a compound similar
threreto, and then bringing the cooled and solidified
hollow threads into contact with the extractant
incapable of dissolving the polypropylene thereby
removing the aforementioned organic filler from the
20 hollow threads by extraction (see applicant's U.S. Patent
No. 4,770,852, issued September 13, 1988). The hollow
fiber
membrane which is obtained by this method is free from
the drawbacks enumerated above. During the course of
25 cooling, however, the organic filler or the cooling and
solidifying liquid is locally deposited on the outermost
surfaces of the hollow fibers which have not yet been
thoroughly cooled and solidified, to lower the ratio of
distribution of the polypropylene composition on the
30 outermost surfaces and consequently enlarge the pores in
the outer surfaces of the hollow fibers and cause the
polypropylene to continue in the form of a heavily
rugged network. When the hollow fibers of this nature
are used in an artificial lung of the type adapted to
35 pass blood inside the hollow fibers and blow an
oxygen-containing gas outside the hollow fibers to
effect addition of oxygen to the blood and removal of
carbon dioxide gas from the blood, no problem is raised.
~r~ ~ 4
.
.
. .
.
. . .
~ 3 ~
Conversely when the hollow fibers are used in an
artificial lung of the type adapted to flow blood
outside the hollow fibers and blows an oxygen-containing
gas inside the hollow fibers, they entail a disadvantage
5 that the outer surface of the hollow fibers, owing to
their quality described above, inflict an injury on the
blood cells and aggravate the pressure loss. Further,
the artificial lung using such a hollow fiber membrane
as described above, without reference to the choice
10 between the two types of artificial lung, has a
disadvantage that during the course of assembly of the
artificial lung, the individual hollow fibers
conglomerate to impair the workability thereo~ and
jeopardize the effect of potting.
An object of this invention, therefore, is to
provide an improved porous polypropy~ene hollow fiber
membrane, a method for the production thereof, and an
artificial lung using the hollow fiber membrane. A
further object of this invention is to provide a porous
20 polypropylene hollow fiber membrane possessing a high
gas-exchange capacity, a method for the production
thereof, and an artifiial lung using the hollow fiber
membrane. Still another object of this invention is to
provide a porous polypropylene hollow fiber membrane
25 which, while being used in an artificial lung of either
of the type passing blood inside or the type passing
blood outside, induces no leakage of blood plasma and
retains a high gas-exchange capacity intact through a
protracted service without impairing blood cells or
30 aggravating pressure loss and which, therefore, is
useful for an artificial lung, a method for the
production thereof, and an artificial lung using the
hollow fiber membrane. ~et another object of this
invention is to provide a porous polypropylene hollow
35 fiber membrane which possesses a smooth outer surface
and defies conglomeration of individual hollow fibers
-- 5 --
~ 3 ~ 9
thereof during the course of assembly of an artificial
lung, a method for the production thereof, and an
artificial lung using the hollow fiber membrane.
CDisclosure of the Invention]
The various objects described above are
accomplished by a porous polypropylene hollow fiber
membrane wherein the solid phase in the inner surface
region thereof is formed with particles of polypropylene
closely fused and joined to give rise to a continuous
10 phase while partially exposed through the surface
thereof, the solid phase in the interior and the outer
surface region thereof is formed with particles of
polypropylene interconnected in the direction of axis of
fiber to give rise to a multiplicity of lumps of
15 polypropylene, and the interstice between these solid
phases has continuous pores interconnect~d in the form
of a three-dimensional network.
This invention also discloses a porous
polypropylene hollow fiber membrane, wherein the index
20 of birefringent thereof in the direction of axis is in
the range of 0.001 to 0.01. This invention further
discloses a porous polypropylene hollow fiber membrane,
wherein the porosity thereof is in the range of 10 to
60~ and the aperature ratio of the inner surface region
25 thereof is in the range of 10 to 30~ and the oxygen gas
flux is in the range of 100 to 1,500 liters/min.m2.atm.
This invention further discloses a porous polypropylene
hollow fiber membrane, wherein the inside diameter is in
the range of 150 to 300~m and the wall thickness in the
30 range of 10 to 150~m. This invention also discloses a
porous polypropylene hollow fiber membrane, wherein the
average diameter of the particles of polypropylene is in
the range of 0.1 to 2.0~m and the average diameter of
the pores in the inner surface region is in the range of
35 0.1 to 1.0 ~m. ~ This invention further discloses a
porous polypropylene hollow fiber membrane, wherein the
membrane used in an artificial lung is su~stantially
-- 6 --
-
'
,
.
13~59
free from leakage of blood plasma or degradation of
gas-exchange capacity within 30 hours o~ service.
Further this invention discloses a porous polypropylene
hollow fiber membrane, wherein the membrane used in an
5 artificial lung sparingly inflicts injury on blood
cells.
The objects described above are accomplished
by a method ~or the production of a porous polypropylene
hollow fiber membrane, which is characterized by mixing
10 polypropylene, an organic filler uniformly dispersible
in the polypropylene in a molten state and easily
soluble in an extractant to be used latert and a
crystalline seed forming agent, discharging the
resultant mixture in a molten state through annular
15 spinning orifices, cooling and solidifying the resultant
hollow threads by contact with a cooling and solidifying
liquid having no compatibility with the aforementined
organic filler and possessing a specific heat capacity
in the range of 0.2 to 0.7 cal/g, and then bringing the
20 cooled and solidified hollow threads into contact with
an extractant capable of dissolving polypropylene
thereby removing the organic filler therefrom by
extraction.
This invention also discloses a method for the
25 production of a porous polypropylene hollow fiber
mem~rane, wherein silicone oil or polyethylene glycol is
used as the cooling and solidifying liquid. This
invention also discloses a method for the production of
a porous polypropylene hollow fiber membrane, wherein
30 silicone oil possesses viscosity in the range of 2 to 50
cSt at ~0C. This invention also discloses a method for
: the production of a porous polypropylene hollow fiber
membrane, wherein polye~hylene glycol possesses an
average molecular weight in the range of 100 to 400~
35 This invention further discloses a method for the
production of a porous polypropylene hollow fi~er
membrane, wherein liquid paraffin is used as the organic
-- 7 --
~31~
filler. This invention further discloses a method for
the productiorl of a porous polypropylene hollow fiber
membrane, wherein the amount of the organic filler to be
incorporated is in the range of 35 of to 150 parts by
5 weight, based on lO0 parts by weicJht of polypropylene.
This invention also discloses a method for the
production of a porous polypropylene holl~w fiber
membrane, wherein the cryst:alline seed forming agent is
an organic heat-resistant substance possessing a melting
lO point of not less than 150C and a gelling point not
less than the crystallization starting point of
polypropy]ene. Further, this invention discloses a
method for the production of a porous polypropylene
hollow fiber membrane, wherein the amount of the
15 crystalline seed :forming agent to be incorporated is in
the range of 0.1 to 5 parts by weight, based on lO0
parts by weight of polypropylene.
The objects described above are further
accomplished by an artificial lung provided with a
20 hollow fiber membrane as a gas-exchange membrane,
characterized by the fact that the hollow fiber membrane
is a porous polypropylene hollow fiber membrane wherein
the solid phase in the inner surface region thereof is
formed with particles of polypropylene closely fused and
25 joined to give rise to continuous phase while partially
exposed through the surface thereof, the solid phase in
the interior and the outer surface region thereof is
formed with particles of polypropylene interconnected in
the direction of axis of fiber to give rise to a
30 multiplicity of lumps of polypropylene, and the
interstice between these solid phases has continuous
pores interconnected in the form of a three-dimensional
network.
This invention further discloses an artificial
35 lung, wherein ~he index of birefringent of the porous
polypropylene hollow fiber used therein in the direction
of axis is in the range oi O.OOl to 0.01. This
.
~ 3 1 ~
invention also dlscloses an artificial lung, wherein the
porosity of the porous polypropylene hollow fiber used
therein is in the range of 10 to 60% and the aperature
ratio of the inner surface region thereof is in the
5 range of 10 to 30% and the oxygen gas flux is in the
range of 100 to 1,500 liters/min.m2.atm. This invention
further discloses an artific:ial lung, wherein the inside
diameter of the porous polypropylene hollow fiber used
therein is in the range of 150 to 300 ~m and the wall
10 thickness in the range of 10 to 100 ~m. This invention
further discloses an artificial lung provided with a
hollow fiber membrane and adapted to circulate blood
inside the hollow fiber membrane and blow an
oxygen-containing gas outside the hollow fiber membrane.
15 Further this invention discloses an artificial lung
provided with a hollow fiber membrane and adapted to
circulate blood outside the hollow fiber membrane and
blow an oxygen-containing gas inside the hollow fiber
membrane. This invention further discloses an
20 artificial lung which is substantially free from leakage
of blood plasma or degradation of gas-exchange capacity
within 30 hours of extracorporeal circulation of blood.
This invention also discloses an artificial lung which
sparingly inflict injury on blood cells during the
25 extra-corporeal circulation of blood. This invention
further discloses an artificial lung using a hollow
fiber membranej wherein the particles of polypropylene
of the hollow fiber membrane possess an average particle
diameter in the range of 0.1 to 2.0 ~m and the pores in
30 the inner surface region of the hollow fiber membrane
possess an average diameter in the range of 0.1 to 1.0
~m.
~Brief Description of the Drawings]
Figs. 1 through 6 are electron microscope
35 photographs illustrating te~tures of porous
polypropylene hollow fiber membranes of the present
invention. Figs. 7 through 19 are electron microscope
_ g _
photographs illustrating textures o~ conventional porous
hollow fiber membranes. Fig. 20 is a schematic cross
section of an apparatus to be used for the method of
production of a porous polypropylene hollow fiber
S membrane the present invention. Fig. 21 is a semi-cross
secction illustrating a typical hollow fiber membrane
type artificial lung as one emobidment of this
invention. Fig. 22 is a cross section illustrating
portions of the artificial lung relative to the hollow
10 fiber membrane filling ratios.
~Description of Preferred Embodiments]
Now, the present invention will be described
below with reference to working examples. To facilitate
comprehension of this invention, paragraphs titled
15 "Porous polypropylene Hollw Fiber Membrane~" "Method for
the Production of Porous Polypropylene Hollow Fiber
Membrane," and "Example" will be included in the
following part of the test hereof.
Porous Polypropylene Hollow Fiber Membrane
The porous polypropylene hollow fiber membrane
of the present invention is a hollow fiber membrane of
polypropylene substantially circular in cross section,
possessing an inside diameter in the range of 150 to 300
~m, - preferably 180 to 250 ~m, a wall thicXness in the
25 range of 10 to 150 ~m, desirably 20 to 100 ~m, and more
desirably 40 to 50 ~m. The microstructure of this
hollow fiber membrane of polypropylene is variable with
the production conditions of the hollow fiber membrane.
Generally, it acquires a microstructure as shown in the
30 scanning electron microscope photographs of Figs.
through 6 by using as the cooling and solidifying liquid
of the nature to be described later a solution showing
no compatibility to the organic ~iller and possessing a
specific heat capacity in the range of ~.2 to ~.7 cal/g~
35 To be specific, on the inner surface side, the solid
phase is formed with particles of polypropylene closely
fused and joined while partly exposed through the
-- 10 --
surface, namely, they are fused and then cooled and
solidified to give rise to a continuous phase (Figs. 2
through 6). In the interior of the membrane, the solid
phase is formed with a multiplicity of particles of
5 polypropylene randomly gathered without any
directionality in the circumferential direction (Fig. 3)
and interconnected in the dir~ction of axis of fiber to
give rise to lumps of polypropylene, which are
interlaced (Fig. 4). The solid phase in the interior of
10 the membrane, therefore, is believed to be ~ormed with
an aggregate of a multiplicity of lumps of
polypropylene, which each consist of particles o~
polypropylene interconnected in the direction of axis of
fiber. Further on the outer surface region similarly to
15 the interior of the membrane, the solid phase is formed
with an aggregate of multiplicity of lumps of
polypropylene, which each consist of particles of
polypropylene interconnected in the direction of axis of
fiber (~ig. 1). Then, in the interstice between these
20 solid phases mentioned above, pores extending from the
inner surface to the outer surface of the thick wall
portion inclusive of the inner surface and the outer
surface of the hollow fiber are interconnected not
linearly but in a complicate reticular pattern to give
25 rise to continuous pores of the form of a
three-dimensional shape. The complexicity of the
arrangement of these continuous pores is evinced by the
fact that the ratio of bireringent in the direction o~
axis o~ the porous polypropylene hollow fiber membrane
30 of this invention is extremely low so as to fall in the
range of 0.001 to 0.01 and the property of orientation
of the polypropylene crystals is small.
When the porous polypropylene hollow fiber
membrane of the present invention co~structed as
35 described above is used in an artificial lung adapted to
pass blood inside the hollow fiber, it neither inflicts
any injury upon the blood cells nor induces any
13~159
aggravation of pressure loss because the inner surface
of the membrane consists of the continuous phase formed
with particles of polypropylene closely fuse~ and joined
while partially exposed through the surface and the
5 remaining part of pores and possesses the quality of
smoothness. In contrast, when the porous polypropylene
hollow fiber membrane is used in an artificial lung
adapted to pass blood outside the hollow fiber, it
neither inflicts any injury on the blood cells nor
10 induces any aggravation of pressure loss because the
outer surface of the membrane consists of the solid
phase formed with an aggregate of a multiplicity of
lumps of polypropylene each ha~ing paxticles of
polypropylene arranged orderly in the direction of fiber
15 and the remaining part of pores and possesses the
quality of smoothness. Further the pores of the porous
polypropylene hollow fiber membrane which serve as the
routes for a gas when the porous polypropylene hollow
fiher membrane is used in an artificial lung are
20 continuous pores interconnected complexly and
reticularly in a three-dimensional network. When the
extra-corporeal circulation of blood is effected either
inside or outside the hollow fiber membrane, the blood
plasma component of the blood cannot pass through such
25 long complexly interlaced routes. Thus, the artificial
lung shows substantially no sign of leakage of blood
plasma or degradation of gas-exchange capacity after 30
hours' extra-corporeal circulation of blood, for
example.
For the porous polypropylene hollow fiber
membrane of this invention to be advantageously used in
an artificial lùng, the porosity is required to fall in
the range of 10 to 60%, preferably 30 to 55~, the
aperature ratio of the inner surface in the range of 10
35 to 30%, preferably 12 to 20%t and the oxygen gas flux in
the range of 100 to l,S00 liters/min.m2.atm, preferably
300 to 800 liters/min~m .atm. If the porosity is less
- 12 -
1 3 ~
than 10~, the membrane has the possibility of exhibiting
an insufficient gas-exchange capacity. Conversely if
the porosity exceeds 60~, the membrane has the
possibility of leaking blood plasma. If ~he aperature
5 ratio is less than 10%, the membrane has the possibility
of exhibiting an lnsufficien~ gas-exchange capacity
because of insufficient formation of continuous pores in
the part of pores of the membrane. Conversely, if the
apperature ratio exceeds 30~, the membrane has the
10 possibility of suffering from leakage of blood plasma
because of the lack of the complexity of continuous
pores. If the oxygen gas flux deviates from the range
of 100 to 1,500 liters/min.m2.atm, the membrane has the
possibility of failing to fulfil the function as a
15 gas-exchange membrane. The sizes and distribution
degrees of the particles of polypropylene and the
continuous pores, i.e. the interstices between the
adjacent particles of polypropylene, which make up the
porous polypropylene hollow fiber membrane of the
20 present invention can be controlled to their
respectively desirable conditions by the production
conditions of the membrane and the composition of raw
materials used therefor. Generally, the particles o~
polypropylene are required to possess an average
25 diameter in the range of 0.1 to 2.0 ~m, preferably 0.2
to 1.5 ~m and the pores opening in the inner surface to
possess an average diameter in the range of 0.1 to 1.0
~m, preferably 0.3 to 0.6 ~m.
Method for Production of porous_polypropylene hollow
30 fiber membrane
The porous polypropylene hollow fiber
described above is produced, or example, as follows.
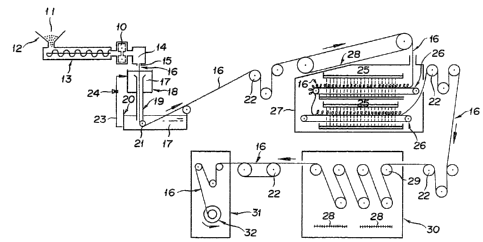
As illustrated in Fig. 20, a mixture 11 of
polypropylene, an organic filler, and a crystalline seed
35 forming agent is fed through a hopper 12 into a kneader
such as, for example, a uniaxial extruding machine 13,
there to be fused, blended, and extruded. Then, the
~ - 13 -
~ 3 ~ '3
extruded mixture is forwarded to a spinning device 14,
discharged through an annular spinning orifice (not
shown) of`a spinneret 15 into a gaseous atmosphere such
as, for example, air. A hollow thread 16 emanating from
5 the spinneret 15 is introduced into a cooling tank 18
filled with a cooling and solidifying liquid 17, and
cooled and solidified by contact with the cooling and
solidifying li~uid 17. In this case, the contact of the
hollow thread 16 and the cooling and solidifying liquid
10 17 is desired to be effected by causing the
aforementioned cooling and solidifying liquid 17 to flow
down the interior of a cooling and solidifying liquid
flow tube 19 disposed as directed downwardly through the
bottom of the aforementioned cooling tank 18 as
15 illustrated in Fig. 20 and allowing the aforementioned
hollow thread 16 to fall down along the flow of the
cooling and solidifying liquid and come into parallel
contact therewith. The cooling and solidifying liquid
17 which has flowed down is received and stored in a
20 solidifying tank 20. The hollow thread 16 is vertically
introduced into the solidifying tan~ 20 and caused to
chagne to course of travel by a deflection bar 21 so as
to be solidified through ample contact therewith. The
cooling and solidifying liquid lÇ accumlating in the
25 solidifying tank 20 is discharged via a circulation line
23 and circulated by a circulation pump 24 to the
aforementioned cooling tank 18. Subsequently, the
solidified hollow thread 16 is led to a shower conveyor
type extruding machine 27 onto which an extractant 25
30 capable of dissolving the aforementioned organic filler
and incapable of dissolving the polypropylene is dropped
in the form of shower. In this extruding machine 27,
the hollow thread 16 is brought into ample contact with
tha extractant and ~onsequently deprived of the
35 remaining organic filler while it is being advanced on a
belt conveyor 26. The hollow thread which is led out o~
the extruding ma~hine 27 by a drive roll 22, when
- 14 -
~31~
necessary, is passed through the steps of re-extraction
and heat treatment for drying and is finally taken up in
a roll.
The polypropylene to be used as one of the raw
5 materials for this invention need not be limited to
homopolymer of propylene. It may be a block polymer
using propylene as the main component and additionally
incorporating therein another monomer. The
polypropylene is required to possess a melt index (M.I.)
10 in the range of 5 to 70, preferably 10 to 40. In the
various forms of polypropylene mentioned above, the
homopolymer of propylene proves to be particularly
desirable. In the various species of homopolymer of
propylene, that which has a high degree of crystallinity
15 proves to be most desirable.
The organic filler is required to be uniformly
dispersible in the polypropylene which is in a molten
state and to be easily soluble in an extractant to be
used later. Examples of the filler which fulfils this
20 requirement include liquid paraffin (number average
molecular weight 100 to 2,000), ~ -olefin oligomers
[such as, for example, ethylene oligomer (nu~er average
molecular weight 100 to 2,000), propylene oligomer
(number average molecular weight 100 to 2,000), and
25 ethylene oligomer (number average molecular weight 100
to 2,000)], paraffin wax (number average molecular
weight 200 to 2,500), and various hydrocarbons. In the
organic fillers enuemrated above, the liquid paraffin
proves to be particularly desirable.
The mixing ratio of the polypropylene and the
aforementioned organic filler is such that the
proportion of the organic filler to 100 parts by weight
of the propylene is in the range of 35 to 170 parts by
weight, pxeferably 80 to 150 parts by weight. If the
35 proprotion of the organic filler is less than 35 parts
by weight, the produced hollow fiber membrane cannot
manifest sufficient permeability to gas because part of
~;4~
the membrane is ~ormed with a continuous phase of
polypropylene. Conversely, if the proportion exceeds
170 parts by weight, the moldability of the mixture in
the form of a hollow fiber is degraded because the
5 viscosity of the mixture is unduly lowered. For the
formulation of raw materials mentioned above, the
mixture consisting of raw materials in a prescribed
percentage composition is prepared (designed) by the
premix method which comprises melting and blending the
10 mixture, extruding the resuLtant blend, and pelletizing
the extruded blend by the use of a biaxial type
extruding machine, for example~
The crystalline seed forming agent to be
included in the raw materials for this invention is an
15 organic heat-resistant substance possessing a melting
point of not less than 150C (preferably in the range of
200 to 250C) and a gelling point of not less than the
crystallization starting point of polyolefin. The
crystalline seed forming agent of this description is
20 used as one of the raw materials for the purpose of
causing contraction of the particles of polypropylene
thereby narrowing the interstices namely the continuous
pores between the particles and heightening the pores
density. As examples of the crystalline seed forming
25 agent, there can be cited 1,3,2,4-dibenzilydene
sorbitol, 1,3,2,4-bis(p-methylbenzilydene) sorbitol~
1,3,2,4-bis~p-ethylbenzilydene) sorbitol,
bis(4-t-butylphenyl) sodium phosphate, sodium benzoate,
adipic acid, talc and kaoline.
Among other crystalline seed forming agents
cited above, benzilyidene sorbitols, especially
1,3,2,4-bis(p-ethylbenzilydene) sorbitol and
1,3,2,4-bis(p-methylbenzilydene) sorbitol prove to be
particularly desirable because they are not
35 significantly dissolved in the blood.
- 16 -
The mixing ratio o~ the propylene and the
aforementioned crystalline seed forming agent is such
that the proportion of the crystalline seed forming
a~ent to 100 parts by weight of the polypropylene is in
5 the ran~e of 0.1 to 5 parts by weight, preferably 0.2 to
1.0 part by weight.
The mixture of raw materials prepared as
described above is fused and blended in a uniaxial
extruding machine, for example, at a temperature in the
10 range of 160C to 250C, preferably 180 to ~20C,
extruded through an annular orifice of a spinning device
into gaseous atmosphere, when necessary, by the use of a
gear pump enjoying high accuracy of measurement, to give
rise to a hollow thread. To the center of the interior
15 of the annular orifice mentioned above, an inactive gas
such as, for example, nitrogen, carbon dioxide, helium,
argon, or air may be delivered through spontaneous
suction or, when necessary, forced introduction.
Subsequently, the hollow thread discharged through the
20 annular orifice is allowed to fall down into contact
with the cooling and solidifying liquid held inside the
cooling tank. The distance of this fall of the hollow
thread is in the range of 5 to 1,000 mm, preferably 10
to 500 mm. If this distance is less than 5 mm, the
25 hollow thread is caused to pulsate and is possibly
crushed at the time the hollow thread enters the cooling
and solidifying liquid. Inside this cooling tank, the
hollow thread has not yet been thoroughly solidified and
it is liable to be deformed by an external force because
30 the central part of the membrane is formed with a gas.
The aforementioned hollow thread can be forced to move
and the deformation of the hollow thread by the external
force (such as fluid pressure) can be precluded by
allowing the aforementioned solidifying liquid 17 to
35 flow down the interior of the cooling and solidifying
tube 19 disposed as directed downwardly through the
bottom of the cooling tank 18 as illustrated in Fig. 20
- 17 -
.
and allowing the hollow thread to fall parallelly to the
flo~ of the llquid. For the flow of the cooling and
solid.ifying liquid to fulfil the purpose thereof, the
flow rate obtained by gravitational attraction suffices.
5 The cooling temperature used in this case is in the
range of 10 to 90C, preEerably 20 to 75C. If the
cooling temperature is less than 10C, the cooling and
solidifying speed is unduly high and the greater part of
the thick wall part of the membrane assumes the form of
10 a closely packed layer and the gas-exchange capacity of
the membrane is proportionately lowered. If this
temperature exceeds 90C, the hollow thread is not
suf~iciently cooled and solidified and is possibly
broken within the cooling and solidifying tank.
As the cooling and solidifying liquid for this
invention, a solution exhibiting no compatibility with
the organic filler being used and possessing a specific
heat capacity in the range of ~.2 to 0~7 cal/g,
preferably 0.3 to 0.6 cal/g~ As concrete examples of
20 the cooling and solidifying li~uid, there can be cited
silicone oils such as dimethyl silicone oil and
methylphenyl silicone oil possessing a kinetic viscosity
in the range of 2 to 50 cSt, preferably 8 to 40 cSt, and
polyethylene glycols possessing an average molecular
25 weight in the range of 100 to 400, preferably 180 to
330. Such a liquid as exhibiting no compatibility with
the organic filler being used and possessing a specific
heat capacity in the range of 0.2 to 0.7 cal/g is used
as the cooling and solidifying liquid for the ~ollowing
30 reason.
When liquid paraffin is used as the organic
filler and a halogenated hydrocarbon is used as the
cooliny and solidifying liquid capable of dissolving the
organic ~ilIer mentioned ablove, it is inferred that the
35 organic filler will be dissolved and extracted out and
will pass from the inner to the outer side of the hollow
thread while the phase separation of the polypropylene
- 18 -
~ 3 ~
and the organic filler is proceeding in the cooling and
solidifying liquid, the proportion of the organic filler
near the inner surface of the hollow thread is lowered
after the hollow thread has been thoroughly cooled and
5 solidified, and the ratio of openings in the inner
surface is unduly lowered and the gas-exchange capacity
of the membrane is suffered to fall after the organic
filler has been thoroughly clissolved and extract0d out.
Further, in the present case, there is the possibility
10 that even the low molecular weight component of the
polypropylene in the hollow thread is extracted out and
suffered to accumulate and deposit on the inner wall of
the cooling and solidifying liquid flow tube 19 shown in
Fig. 20 and induce reduction of the inside diameter of
15 the cooling and sol.idifying liquid flow tube 19 and
consequent deformation of the hollow thread. When a
compound identical or similar to the aforementioned
organic filler is used as the cooling and solidifying
agent, namely when a species of liquid paraffin is used
20 as the organic filler and another species of liquid
paraffin having a number average molecular weight
approximating that of the first liquid paraffin is used
as the cooling and solidifying agent, the organic filler
(liquid paraffin) in the hollow thread is allowed to
25 give rise to pores in a prescribed density without being
significantly migrated within the hollow thread and the
specific heat i5 not unduly large and, as the result,
the polypropylene is crystallize~d at a proper cooling
speed and enabled to acquire a~ stable form finally.
30 During the course of the cooling,~ however, the organic
filler.or the cooling and solidifying liquid is sufered
to occur locally on the outermost~ surface of the hollow
thread which has not yet ~een~thoroughly cooled and
solidified and the ratio ::of the polypropylene
35 composition is lowered on the outérmost surface, and, as
the result, the pores in the outèr surface of the hollow
thread are large and the solid~phase finally assumes a
-- 19 --::
~l 3 ~ 9
heavily rugged surface condition because it is formed
with particles of polypropylene spread out in the form
of a network. When an inactive liquid which is
incompatible with the organic filler and possesses a
5 large specific heat capacity is used as the cooling and
solidifying liquid, namely when liquid paraffin is used
as the organic filler and water having a large specific
heat capacity of about 1.0 cal/g is used as the cooling
and solidifying agent, there is the possibility that the
10 polypropylene will be quickly cooled and the outer
surface will assume a state of low crystallinity because
the cooling effect of water is high. The possible
consequence is that the polypropylene will fail to form
minute particles and the produced hollow fiber membrane
15 will contain unduly small pores in the outer surface
thereof and exhibit a small gas-exchange capacity. If
the cooling and solidifying liquid to be used possesses
a small specific heat capacity, there is the possibility
that no sufficient cooling effect will be obtained and
20 the extruded mixture will not be converted into a hollow
threaa as desired.
In contrast, when a solution exhibiting no
compatibility with the aforementioned organic filler and
possessing a specific heat capacity in the range of 0.2
25 to 0.7 cal/g is used as the cooling and solidifying
liquid, the outer surface of the hollow fiber membrane,
similarly to the interior thereofj is formed with an
aggregate of a multiplicity of lumps of polypropylene
each having minute particles of polypropylene
30 interconnected in the direction of axis of fiber and is
allowed to assume a smooth surface condition because the
polypropylene is cooled at a proper speed and the
polypropylene is crystallized smoothly while keeping a
proper polypropylene composition ratio even in the outer
35 surface, while the organic filler is not locally
distributed on the outer surface of the hollow fiber.
- 20 -
'
The hollow thread which has been cooled and
solidified in the cooling and solidifying tank is
~orwarded`to an extracting machine, for e~ample, as
passed around a deflection bar to dissolve and extract
5 the organic filler. The means ~or dissolving and
extracting the organic filler need not be limited to the
shower method which resides in causing the extractant to
fall in the form of shower onto the hollow thread being
forwarded on the belt conveyor as shown in Fig. 20. The
10 e~traction tank method or the rewinding method which, at
the time that the hollow thread once wound up in a roll
is rewound on a skein frame, immerses the skein in the
extractant on the sole condition that the treatment
should bring the hollow thread into contact with the
15 extractant. Optionally,two or more such methods may be
used in combination.
As the extractant, there can be used any of
the liquids which are incapable of dissolving the
propylene which forms the backbone of the hollow fiber
20 membrane and capable of dissolving and extracting the
organic filler. Examples o~ the liquid so usable
include alcohols such as methanol, ethanol, propanols,
butanols, pentanols, hexanols, octanols, and lauryl
alcohol and halogenated hydrocarbons such as
25 1,1,2-trichloro-1,2,2- trifluoroethane,
trichloro~luoromethane, dichlorofluoromethane, and
1,1,2,2-tetrachloro-1,2- difluoroethane. In all these
extractants, halogenated hydrocarbons prove to be
desirable from the standpoint of extraction capacity and
30 chloro-fluorinated hydrocarbons prove to be especially
desirable from the ~tandpoint of safety on the human
system.
The hollow fiber membrane which is obtained as
described above, when necessary~ is further subjected to
35 a heat treatment. The heat treatment is carried out in
a gaseous atmosphere such as air, nitrogen or carbon
dioxide at a temperature in the range of 50 to 160C,
- 21 -
1 3 ~ O ~ 9
preferably 70 to 120C, for a period in the range of 5
seconds to 120 minutes,preferably 10 seconds -to 60
minutes. By this heat treatment, the hollow fiber
membrane is structurally and dimentionally stabilized.
5 Further, in this case, the hoJlow fiber membrane may be
stretched prior to or during the heat treatment~
Artificial Lung
The porous polypropylene hollow fiber membrane
which is obtained as described above is used most
10 suitably in a hollow fiber membrane type artificial
lung.
The hollow fiber membrane which is obtained by
the conventional stretching method possesses gas
permeability more than is necessary for an artificial
15 lung. When it is used in an artificial lung adapted to
circulate blood inside the hollow fiber, the oxygen
addition ability encounters a large boundary membrane
resistance on the blood side and the resistance of the
hollow fiber membrane has no rate-determining effect
20 and, in the meantime, the ability to remove carbon
dioxide gas depends on the resistance of the hollow
fiber membrane and the premeability to gas is excessive.
When the hollow fiber membrane is used in an artificial
lung adapted to circulate blood outside the hollow
25 fiber, the gas-exchange capacity also depends on the
resistance of the hollow membrane but the permeability
is excessive.
The hollow fiber membrane of the present
invention in its simple form possesses gas permeability
30 lower than that of the conventional stretching method.
It, however, acquires quality enough for the membrane to
be used efficiently in an artificial lung. Moreover,
since it is produced by the extraction method, it
produces no pinhole and induces no leakage of blood and,
35 therefore, is capable of preventing the gas-exchange
capacity from f 211 ing.
- 22 -
~ 3 ~
In the hollow fiber membrane which is obtained
by using, as the cooling and solidifying liquid, a
liquid identical or similar to the organic filler,
particles of polypropylene are interconnected in the
5 form of a network and consequently caused to assume a
heavily rugged surface. Thus, there ensues the
possibility that while the lndividual hollow fibers are
assembled to form an artificial lung, they will cohere
fast possibly to the extent of rendering the work of
10 assembly complicate and preventing the adhesive agent
from being dispersed evenly around the individual hollow
fibers and inducing defective potting.
In the hollow fiber membrane which is obtained
by the method of the present invention, the outer
15 surface of the membrane, similarly to the interior
thereof, is formed with an aggregate of a multiplicity
of lumps of polypropylene each having particles of
polypropylene interconnected in the direction of axis of
fiber and, therefore, is allowed to assume a smooth
20 surface condition. Thus, while a multiplicity of such
hollow fibers are assembled ~o form an artificial lun~,
the aforementioned drawbacks are not encountered. When
the artificial lung is adapted to circulate the blood
outside or inside the hollow fiber membranes, the
25 membranes neither inflict any injury on the blood cells
nor aggravate pressure loss.
Fig. 21 illustrates the condition of assembly
of a hollow fiber m mbrane type artificial lung as one
version of the hollow fiber membrane type artificial
30 lung of the present invention. A hollow fiber membrane
type artificial lung 51 is provided with a housing 56.
This housing 56 comprises a cylinderical main body 57
and annular fitting covers 58, 59 fastened with a male
screw to the opposite ends o~ the tubular main body.
35 Inside the housing 56, a large number falling in the
range of 10,000 to 65,000 of hollow fiber membranes 1
obtained as described above are disposed parallelly
- - 23 -
~ " ' ' '
'
along the longitudinal direction of the housing 56 as
individually separated throughout the entire interior.
The oppoisite ends of these hollow fiber membranes 1 are
water-tiyhtly supported with diaphragms 60, 61 inside
5 the fitting covers 58, 59 in such a manner that the
openings thereof will not be blocked up. The diaphragms
60, 61 mentioned above define an oxygen-containing gas
chamber 62 as a first substance transfer fluid flow
space in conjunction with the outer surfaces of the
10 hollow fiber membranes 1 and the inner surface of the
housing 56 and will block Up the oxygen-containing gas
chamber 62. They also function to isolate the
oxygen~containing gas chamber 62 from blood flow spaces
(not shown) which are formed inside the aforementioned
15 hollow fiber membranes 1 for a second substance transfer
fluid.
The fitting cover 58 is provided with an inlet
63 for supply of an oxygen-containing gas as the first
substance transfer fluid. The other fitting cover 59 is
20 provided with an outlet 64 for discharge of the
oxygen-containing gas.
The tubular main body 57 of the aforementioned
housing 56 is desired to be provided on the inner
surface thereof halfway along the direction of axis with
25 a projected constricting part 65. This constricting
part 65 is integrally formed with the tubular main body
57 on the inner surface and adapted to squeeze- the
overall outer periphery of a hollow fiber bundle 66
consisting of the multiplicity of hollow fiber membranes
30 1 inserted inside the tubular main body 57~ As the
result, the aforementioned tubular fiber bundle 66 is
constricted at the center in the direction of axis to
form a contricted part 67 as illustrated in Fig. 21.
The packing ratio of the hollow fiber membranes 1,
35 there~ore, varies along the direction of axis and
reaches the maximum at the centerO For the reason to
described later on, the packing ratios of varying parts
24
.
- ': .
-
, - :
.
~31~
of the hollow fiber bundle 66 are desired to be as
follows. First, the packing ratio A ln the constricted
part 67 at the center is approximately in the range of
60 to 80~, the packing ratio B within the tubular main
5 body 57 except for the constricted part 67 in the range
of 30 to 60~, and the packing ratio C at the opposite
ends of the hollow fiber bundle 66, namely on the outer
surfaces of the diaphragms 60, 61 in the range of 20 to
40% as illustrated in Fig. 22.
Now, the formation of the aforementioned
diaphragms 60, 61 will be described below. As described
above, the diaphragms 60, 61 fulfil an important
function of isolating the interiors from the exteriors
of the hollow fiber membranes 1. Generally, these
15 diaphragms 60, 61 are formed by centrifugallycasting a
macromolecular potting material of high polarity such
as, for example, polyurethaner ~ilicone, or epoxy resin
in the inner wall surfaces at the opposite ends of the
housing 56 and allowing the cast macromolecular material
20 to harden. To be more specific, a multiplicity of
hollow-fiber membranes 1 of a length greater than that
of the housing 56 are prepared and, with the openings
thereof at the opposite ends blocked up with highly
viscous resin~ parallelly disposed inside the tubular
25 main body 57 of the housing 56. Subsequently, the
opposite ends of the hollow fiber membranes 1 are
completely concealed with pattern covers of a diameter
larger than that of the fitting covers 58, 59 and the
housing 56 is set rotating about the axis thereof and,
30 at the same time, the macromolecular potting material is
cast into the housing 56 through the opposite ends
thereofO After the cast resin has been hardened, the
aforementioned pattern covers are removed and the outer
surface parts of the resin are cut off with a sharp
35 blade to expose the open ends of the hollow fiber
membranes 1 from the surfaces. In this manner, the
diaphragms 60, 61 are formed.
- 25 -
The outer surfaces of the aforementioned
diaphragms 60, 61 are respectively covered with flow
path forming members 68, 69 each possessed of an
annularly raised part. These flow path forming members
5 68, 69 severally consist of liquid distribution members
70, 71 and thread rings 72, 73. An inlet chamber 76 and
an outlet chamber 77 for the blood as the second
substance transfer fluid are formed by causing annular
ridges 7~, 75 disposed near the peripheral parts of the
10 liquid distribution members 70, 71 to be pressed by the
edge surfaces on the diaphragms 60, 61 mentioned above
and helically fastening the thread rings 72, 73 to the
fitting covers 58,59. In the flow path forming members
6~, 69, an inlet 78 and an outlet 79 for the blood as
15 the second substance transfer fluid are formed.
Gaps are formed around the diaphragms 60, 61
as defined by the diaphragms 60, 61 and the flow path
forming members 68, 69. These gaps are sealed as held
in contact with the diaphragms 60, 61 by injecting a
20 filler 84 or 85 through at least either of the two holes
80, 8-2~ and 81, 83 communicating with the gaps. The
sealing may be otherwise effected through the medium of
an O-ring (not shown).
The hollow fiber membrane type artificial lung
25 of the present embodiment is of a type adapted to use an
oxygen-containing gas like air as the first substance
transfer fluid and blood as the second substance
transfer fluid, namely to feed the oxygen-containing gas
outside the hollow fiber membranes and circulate blood
30 inside the hollow fiber membranes to effect desired
exchange of gases. Alternatively, the follow fiber
membrane type artificial lung of this invention may be
of another type adapted to circulate blood outside the
hollow fiber membranes and feed the oxygen-containing
35 gas inside the hollow fiber membranes to effect desired
exchange of gases. In the latter type, the hollow fiber
membrane type artificial lung has entirely the same
- 26 -
.~ - ' ~, ~' .', .'
,
,
~ 3 ~
construction as -that of the presen-t embodiment and is
operated by using the blood as the ~irst substance
transfer fluid and the oxygen-containing gas as the
second substance transfer fluid.
Example
As an aid for further facili-tatin~ the
comprehension of this invention, a ~ew working examples
will be cited below. These examples are of~ered purely
for the purpose of illustrating this invention and are
10 not meant to restrict the scope of this invention in any
respect.
Examples 1 and 2 and Controls 1 through 3
In a twin-screw extruder (produced by Ikegai
Iron Works, Ltd. and marketed under trademark
15 designation of PCM-30-25), 100 parts by weight of
propylene homopolymer possessing a melt index (M.I.) of
23, a varying proportion of liquid paraffin (number
average molecular weight 324) indicated in Table 1, and
0.5 part by weight o~ dibenzylidene sorbitol were melted
20 and blended and extruded. The extruded mixture was
pe]letized. By the use of a device illustrated in Fig.
20, namely a single screw extruder (produced by
kasamatsu Seisakusho and marketed under produce code of
"W0-30"), the pellets were melted at a varying
25 temperature indlcated in Table 1 and discharged through
an annular spinning hole possessing a core diameter of 4
mm, an inside diameter of 6 mm, an outside diame~er of 7
mm, and a land length of 15 mm at a varying discharge
volume indicated in Table 1 into the air to cause fall
30 of a continuous hollow thread 16. The distance of -this
fall was varied as shown in Table 1. Then, the hollow
thread 16 was brought into contact with a varying
cooling and solidifying liquid indicated in Table 1 held
in a cooling tank 18 and then cooled by parallel-flow
35 contact with the cooling and solidifying liquid 17
spontaneously flowing down the interior of the cooling
and solidifying liquid flow tube 19. In this case, the
* trade-marks
- 27 -
~ 3 ~
temperature of the coolin~ and solidifying liquid was
varled as shown in Table 1. Then, the aforementioned
hollow thread 16 was led into the cooling and
solidifying liquid inside a solidification tank 20,
5 caused to change the course of its travel by a
deflection bar 21, then led to a drive roll 22 operated
at a varying winding speed indicated in Table 1,
continuously treated on a shower conveyor type
extracting machine 27 with Freon* 113
10 (1,1,2-trichloro-1,2,2-trifluoroethane) to effect
through re~oval of the aforementioned liquid paraffin by
extraction, passed around a drive roll 22, passed
through a heat-treating device 30 under varying
tempera-ture and time conditions indicated in Table 1,
15 and taken up on a bobbin 32 by a winder 31. Then hollow
fiber thus taken up on the bobbin 32 was rewound on a
skein by a rewinding device to obtain a hollow fiber
bundle about 30 cm in length. The hollow fiber membrane
thus obtained was examined with respect to shape (wall
20 thickness), porosity, opening ratio in the inner
surface, gas flux, ability to add oxygen gas, ability to
remove carbon dioxide gas, leakage of blood plasma, and
speed o b].ood plasma permeation~ The results are shown
in Tables 2 and 3.
~5 To determine the microstructure of the hollow
fiber membrane obtained, various portions of the hollow
fiber membrane were observed under a scanning electron
microscope (produced by JEOL and marketed under product
code of JSM-840 ). Specifically, Fig. 1 a
30 photomicrograph of the outer surface (x 10,000) of the
hollow fiber membrane of Example 1, Fig. 2 of the inner
surface (x 10,000) of the hollow fiber membrane of
Example 1, Fig. 3 of the cross section (x 10,000) of the
hol].ow fiber membrane of Example 1, Fig. 4 of the
35 longitudinal cross section ~x 10,000) of the hollow
fiber membrane of Example 1, Fig. 5 of the outer surface
(x 10,000) of the hollow fiber membrane of Example 2,
* trade-marks
- 28 -
~ 3 ~
Fig. 6 of the inner surace (x 10,000) of the hollow
fiber membrane of Example 2, Fig. 7 o~ the outer surface
(x 10,000) of the hollow fiber membrane of Control 1,
Fig. 8 of the inner surface (x 10,000) of the hollow
5 fiber emmbrane of Control 1, Fig. 9 of the cross section
(x 10,000) of the hollow ~iber membrane of Control 1,
Fig. 10 of the longi-tudinal cross section (x 10,000) of
the hollow fiber membrane of Control 1, Fig. 11 of the
outer surface (x 10~0000 of the hollow fiber membrane of
10 Control 2, Fig. 12 of the inner surface (x 10,000) of
the hollow fiber membrane of Control 2, Fig. 13 of the
cross section (x 3,000) of ~he hollow fiber membrane of
Control 1, Fig. 14 of the outer surface (x 3,000) o~ the
hollow fiber membrane of Con-trol 3, and Fig. 15 of the
15 cross section (x 3,000) of the hollow fiber membrane of
Control 3, respectively taken under an electron
microscope. In each of the microphotographs, the
direction of axis of fibers in the relevant hollow fiber
membrane is shown on the right.
The hollow fiber membranes of Example 1 and
Control 1 were tested for ratio of birefringent as an
index of crystal orientation. The results are shown in
Table 4.
Modules of the type adapted to pass blood
25 outside hollow fiber membranes were assembled with the
hollow fiber membranes of Example 1 and Control 1 and
tested for hemolysis of blood and pressure loss of
blood. The results are shown in Table 5.
Control 4
For the purpose of comparison, a commercially
available artificial lung-grade polypropylene hollow
fiber membrane produced by the stretching method was
tested for shape (inside diameter/wall thickness),
porosity, oepning ratio in inner surface, gas flux,
35 ability to add oxygen gas, ability to remove carbon
dioxide gas, leakage of blood plasma, and blood plasma
permeation speed in the same manner as in Examples 1 and
- 29
2 and Controls l through 3. The results are shown in
Tables 2 and 3. The microstructrue of the hollow fiber
membrane was observed under a scanning electron
microscope (made by JEOL and marketed under product code
5 of "JSM 840"). Fig. 16 is a photomicrograph of the
outer surface (x 10,000) of this hollow fiber membrane,
Fig. 17 of the inner surface (x 10,000) thereof, Fig. 18
of the cross section (x 10,000) thereof, and Fig. 19 of
the longitudinal cross section (x 10,000) thereof, taken
10 under the electron micrograph. In each of these
figures, the direction of axis fibers in the hollow
fiber membrane is shown Oll the right.
The hollow fiber membrane was tested for ratio
of birefringent as an index of crystal orientation. The
15 results are shown in Table 4. A module of the type
adapted to pass blood outside the hollow fiber membrane
was assembled using the hollow fiber membrane and tested
for hemolysis of blood and pressure loss of blood in the
same manner as in Example l and Control l.
- 30 -
~ 3 ~
Ll ~ ~ ~ u~ u7
- ~ r ~ E~ o~ ~ ~ _~
~1 o o o o
E~ ~ JJ ~ 0 0
n~ ~--U o o o o o
o Ul O O O r~ I~
~ _
r,
.,~ ~ .~J
~e O O O O O
r, ~ ~ co 0 0 o ~
~ ~ e ,,
3 ~ ~
J, ~a r rr~ ~O O ~D
r~-.~ ~ o
13
O 111 Id ~t~l 0 0 N ~
~e ~ ~ ~
U~
r.~ u~ O O ~ o
0 0 ~7 ("7 ~ N
V~ ~
e
a o--
L~ ~ r,u
r, .,~0
,_ O O u~
)~ O'~I ~1 U) 1/~ ~ N r~
o o
_,e ~
r. o -~
E~ o nl u
a o
a~ o QJ
rN r,
. O~
G o o O ~ ~ JJ
_I N rl IL1 ~ N O O
11 0 ~ 1~ 0 0
r, r, ~ ~ U ~ -I ~
rl ~ ~ ~ H la 3 .C --I
~4~ .-1-- J H
r,,~
rl ~ ~ 8
O ~ ~ U ~ N N h
O O ~ ; N
u ul --I ~ O ~r ~I N V
O O
r. ~ ~
.1 v ,a .4--
3.~ ~
h r ~ ~ NO ON
O ~ 1~5 ~ O ,~
e - 3
r-- o o o o o
~1 ~1 U 0 co 0 ~1 o
~J O ~ ~ ,i r-l N N ,~
~ 0~-- ~1 .
_I N _I N ~1
~1 0 0 0
r, r, r
X X o o o
~3 U U U *
r~ 3 1 -
,
~ 3 ~
Table 2
Shape
Inside Wall Gas flux Prosity
diameter thlciness (~m) (lit/min.m .atm) (%)
5 Example 1 200/45 432 41.1
Example 2 200/45 361 42.8
Control 1 200/45 416 38
Control 2 209/26 16~9 17.8
Control 3 177/44 0 -
10 Control 4 200/25 1200 45
- 32 -
~ 3 ~
^
Q) E
E . c~ u~
Ul ~
(~ O ~3 r~ I N
~D 1` ~ ~
O h
o
O~C X ~:
5:1U h a) h ~ o U h U
q~O O ~ q~ O rd
Oq~ S ~ S r~ S X
O O u~
~d E ~ rl r` v
X ~'u~ h rl h aJ ~ ~ 5~ 0
Q~ ~~ ~ h ~ Id
o q~o ~ ~ a~ o ,~ O
z ~z ~ li, h S 1:'. nl S N
') Ul
G~,~d
O
E~h ,1
X^
rl Eo
.u) I` r~ a~
~ ~r ~ ~ ~r
,1 o E
~1 .
~ h ~E
~, _,
o~
o ~
J~ ~ E
-.~,~ C~ W o~
-( O EN O
E ~r ~r
,~_
O
h --
h o ~ ~
Q U~ r~ u~ o
H
0-,1 Ul
r-~~I N ~)
~1 0 0 0
Q. h h
X O O o
1~ U O ~.
-- 33 --
. ` ~
~ 3 ~
Table 4
Ratlo of birefringent(l~n)
Example l 0.004
Control l 0.003
5 Control 4 0.014
Completely oriented polypropylene 0.035 (As reported
in literature)
Table 5
Hemolysis (amount of free Pressure loss
hemoglobin in blood)
~Hb(mg/dl) ` ~p (mmHg)
_~ __ _
Example l 53 33
Control 1 122 48
Control 4 51 32
It is clearly noted from the results shown in
Tables. 2 through 4 that the hollow fiber membranes of
Examples 1 and 2 according with the present invention
exhibited as proper properties for artificial lung-grade
hollow fiber membranes as the hollow fiber membrane of
20 Control 1 and possessed smooth outer surface conditions.
Thus, even in the modules adapted to circulate blood
outside hollow fiber membranes, they induced neither
hemolysis nor pressure loss so heavily as the
countertype module of Control l as noted from Table 5.
25 When the hollow fibers wound on bobbins in Examples l
and 2 and Controls 1 and 2 were observed, the fibers
spun simultaneously in Controls 1 and 2 were liable to
cohere fast, whereas the fibers spun in Examples 1 and 2
were found to induce absolutely no such phenomenon.
30 Further in Control 2, the low molecular component of
polypropylene adhered to the interior of the cooling
bath and continued to accumulate thereon to cause a
- 34 -
~ 3 ~ 9
gradual decrease in the diameter of the tube. In
Examples 1 and 2, absolutely no such phenomenon was
observed.
Then the cooling and solidifying liquids used
5 in Examples 1 and 2 and Controls 1 through 3 were tested
for compatibility with organ:ic filler (liquid paraffin)
and specific heat capacity and the hollow fiber
membranes produced respectively were observed with
respect to the outer surface condition. The results are
10 shown collectively in Table 6.
- 35 -
~31~
S ~ ~r
la ,, u~
h ~ ~11
O 6~
O i) a~
O
~ o
O ~ ~~ E
Q. 3 0Q. h ~J .q
h h C~ h ~ ~ ~ ~ 0
~: ~ ~ U1~ h
U ~ L O h :~
tr~ O ~tr o u~ O ~ ~ Q,'O Q)
~1 ~ 1 C ~ q~
U h h O ~ h O ~ U ~ O U
h ~ h ~~ o ~ C) .Y h ~1
~1 ~ ~ O ~ C ~
::1 ~ ~ o ~ X o o 3 111 oD-
h o h,U ~ h ~ O O ~ ~I h
:1 0 ~ h O h ~3 'a 0 0 0 C~ ~ C q-l .Y O
OS O ~~ 0 ~0 ~ ~ U ~ ~ rl O
~/ ~
~o q~ ~1 ~ ~ ~ ~ _~
~1 'U 0~ v~ ~ ~ o
~1 ~ 0 Q, 0 O o o o ,~
Q ~ U0 U
r
V
~ ~ X O O X
UO R ~ 0
~1
C h O X
--OJ ~ h ~ o o
O ~J V o u~ u~
UV 0 -
.,.1 o 0 0 C
~1 O h O r~
Ql ~`I O
ti~ r 1l ~
C C r~ r '~ ~lZ 0
:-- ~1-- Y ~ 0 ~ 1
~ q~ s Q~ . o ~1
C '~ V ~o'~ ~ V 'U N
. ~ ,0 U~ ~ ~ OC h
O O O ~
O ~ rl o ~1 ~ h 0
O O O
~1~ D. h h h
~3 0 ~ V ,~
X X O O O
1~ ~1 U U U
-- 36 --
, ....
.3 g
It is noted from Table 6 that when a cooling
and solidifying liquid exhibiting compati~ility with
liquid paraffin serving as an organic filler was used, a
solid phase of polypropylene in the outer surface formed
5 a continuous network and imparted heav~ ruggedness to
the surface. ~hen water was used, the polypropylene was
quickly cooled and suf~ered to form a skin layer o~
polypropylene because the water possessed an excessively
large specific heat capacity in spite of incompatibility
10 with liquid paraffin. when the specific heat capacity
was so small as that of
1,1,2-trichloro-1,2,2-trifluoroethane (Freon 113), the
polypropylene was cooled so slowly that the
crystallization of polypropylene excessively proceeded
15 to give rise to giant lumps o~ polypropylene. In
contrast, when liquids exhibiting no compatibility with
liquid paraffin and possessing specific heat in the
range of 0.3 to 0.7 cal/g as in Examples 1 and 2, the
outer surface of the hollow ~iber was smooth and
20 contained pores sufficiently.
The ~arious terms used in the present
specification concerning the porous polypropylene hollow
fiber membrane and the methods used for the
determination of the properties mentioned herein are
25 defined below.
Shape (inside diameter/wall thickness)
The hollow fibers randomly selected from a
given lot were cut laterally to obtain rings 'about 0.5
mm in length with a sharp ra20r blade. The sectionis of
30 ~he rings were projected with a universal projector
(produced by Nippon Rogaku K.K. and marketed under
trademark designation of "Nikon Profile Projector V-12")
and the outside diameters, dl, and inside diameters,d2,
of the projected sections were measured with an
35 instrument (produced by Nippon Kogaku k.R. and markete~
under trademark designation of "Nikon digital Counter
CM-6S") and the wall ~hicknesses, t, were calculated by
- 37 -
~. .
,
- -
the formula, t = (dl - d2)/2. The numerical values thus
ob-tained with respect to the ten hollow fibers were
averaged -to be reported.
Porosity (~)
About 2 g of hollow fibers taken from a given
lot were cut laterally into rings not more than 5 mm in
length with a sharp razor blade. In a mercury
porosimeter (produced by and marketed under product
code of "65A"), the rings were subjected to a final
10 pressure of lrOOO kg/cm2 to find the total amount of
pores (volume of pores in a unit weight of hollow
fibers) and determine the porosity.
Aperature ratio of inner surface (~)
-
The inner surface of a given hollow fiber was
15 photographed under a scanning electron microscope
(produced by JEOL and marketed under product code of
*
"JSM-840") at 3,000 magnifications. The photograph was
enlarged on a quarter printing paper (about 7,500
magnifications on the printing paper). On the product
20 print, four linear lines were randomly drawn each in the
direction of axis of fibers and the direction
perpendicular thereto. The ratio of the sum of lengths
of pores intersected by the linear lines to the total
length of the linear lines was reported as the Aperature
~5 ratio of the inner surface.
Oxygen gas flux
.... _ _
A miniature module 1~ cm in available length
and 0.025 m in membrane surface was produced using
given hollow fibers. With one end of the module
30 tightly closed, 1 atmosphere of oxygen pressure was
applied on the interior of hollow fibers. After the
system assumed the steady state, the flow volume of
oxygen gas was read from the flow meter (produced by
Kusano Rikagakukiki Seisakusho and marketed under
35 trademark designation of "Floatmeter") and reported as
oxygen gas flux.
* trade-marks
- 38 -
', ~ ,,
.
131~9
r~bility to add oxygen gas and ability to remove carbon
dio~ide gas
An artificial lung module 130 mm in available
length and 1.6 m of membrane surface was produced using
5 given hollow fibers. Via a single path, bovine blood
(standard venous blood) was passed at a flow volume of
1.6 liters/min. inside hollow fibers and pure oxygen was
passed at a flow volume of 1.6 liters/min. outside the
hollow fibers. In this while, the bovine blood was
10 tested for pH, carbon dioxide partial pressure (Pco )~
and oxygen gas partial pressure (PO )at the inlet and
outlet of the artificial lung w2ith an instrument
(produced b~ Radiometer Corp. and marketed under product
cope of "~GA3") and the differences of partial pressured
15 at the inlet and outlet of the artificial lung.
Further details on the use of the artificial
lung module are shown in Table 7. The attributes of the
standard arterial blood are shown in Table 8.
* trade-mark
- 39 _
. ~
~" ' ' ~ .
,
0
0 ~ 3 ~ ~ ~ 5 9
h ~ ~7 0
~ d' r~7
~ 0 ~
dP ~ W
h I
l 0 WW N
O ~ O
,1 ~ o ~ ~ O
0 1~ U~ ~ O .r~
h ~ .,1 0
O ~
C~ rl
o ~ WW ~0 ~
0 W ~
h h 0 0
O h
k
h h
O ~ 0
,, O n
~ _~ ,,
aJ O O 0 3 ~
~1 ~ _ h O :>.
R ~ ~D h
.q ~o
0 ~ ~:
a) ~ ~I w
~: _( ,1 ~ O ~
0 rl
1` E; w h
q~ : O R
~ O 0 0 0
.R h O w ~ Q~ h
E-/ ~ o O 0
h ~ h
:~ O ~ a~
Z S q-l ~1 ~ E
X ,-1
O
0 0 0
'1:5 h W
J ~
O ~ U rl
. ~ o U~ W
o~ ~r S~
k --~ N N
w 0 E~ o o
a o o
H '~I _ N N
~ q~
0 U
h 0
hr~l ~0 ~3
~ ,~
~W-
0c ~ol
h
W O
a) u ,i
~1 0
N U
-- 40 --
. .
Table ~
Blood Fresh heparin-added bovine blood
Hematocrit value 35% (as prepared with physiological
Saline solution)
5 Hemoglobin concen- 12 + 1 g/dl
tration
Base Excess 0 -~ 2 mEq/liter (as prepared with
sodium hydrogen carbonate)
Degree of oxygen 65 + 5%
10 saturation
Carbon dioxide 45 + 5 mmHg
partial pressure
Temperature 37 + 2C
Leakage of blood plasma
An artificial lung module similar to that used
for the determination of the abili~y to add oxygen gas
and the ability to remove carbon dioxide gas was
prepared, inserted in a partial V-A bypass circuit
established by the jugular vein-}ugular artery
20 cannulation in a mongrel dog (weighing about 20 kg), and
used for 30 minutes extracorporeal circulation of blood
to measure the amount of blood plasma leaking from
within the hollow fibers. Where there was no
discernible leakage of blood plasma, drops of steam
25 condensate formed outside the hollow fibers were tested
for protein reaction to ensure detection of even slight
leakage of blood plasma.
Blood plasma permeation speed
A miniature module similar to that used for
30 the determination of gas flux was prepared, succesively
immersed in aqueous 100~, ~0%, 50%, and 0% ehtanol
solution, for 2 hours each. The blood plasma obtained
b~ centrifuging bovine blood was circulated through this
miniature module~with the intermembranous pressure ~TMP)
- 41 -
~3~1L~9
ad~usted to 0.225 kg/cm2. The amount of blood plasma
which had permeated the membrane was measured with a
measuring cylinder.
Ratio of birefringent (~ n) (retardation method)
From 10 hollow fiber membranes randomly
extracted from a given lot, central portions 3 cm in
length were cut out. The segmen-ts thus obtained had
their ends on one side cut aslant and used as a sample.
The hollow fiber membranes thus prepared were placed on
10 a slide glass, wetted with an immersing liquid (li~uid
paraffin), and the slide glass thus prepared was set on
a rotary stage in a polarlzing microscope. With a
monochromatic light source or a light source equipped a
filter as substitute, under a cross nicol exclusive of a
15 compensator, the specimen on the rotary stage was
rotated until the brightest position (reached by a 45
rotation from the darkest position) and immobilized at
this brightest position. Then, the compensator was
inserted, and -the annalyzer was rotated to find the
20 angles producing black the darkest ( ~ ) black, and the
retardation (R) was calculated in accordance with the
formula represented below, further the ratio of
birefringent of the hollow ~iber membrane was calculated
in accordance with the following formula. The average
25 of the numerical values obtained for 10 samples was
reported.
Retardation R = 1~0 ~
wherein ~ stands for the wavelength of which the
light is used.
Ratio of birefringent = ~n = 1 d
wherein d stands for the thickness of sample
(compensated with the porosity)~
Conditions of determination:
Polarizing microscope Nikon OPTIPHTO-POL
~avelength of Light source 546 nm
Compensator Senarmont type compensator
- 42 -
~31~
Incidentally, completely oriented
polypropylene has been reported in literature to possess
a ratio of birefringent n, of 0.35.
~emolysis and pressure loss
~_ . .
An artificial ].un~ module of the mode adapted
to circulated blood outside hollow fiber membranes and
satisfying the specification shown in Table 9 was
prepared using given hollow fiber membranes and used for
6 hours' circulation of fresh heparin-added bovine blood
10 to determine the amount of free hemoglobin in the blood
by the TMB method. The condition of hemolysis in the
blood was rated by comparing the amount of free
hemoglobin mentioned with that obtained by circulating
the same bovine blood through a circuit incorporating no
15 artificial lung. During the operatioin of the module,
the pressure loss was measured before and after the
ar-ti.ficial lung.
Table 9
Number of hollow fiber membranes 48,160
Available length 80 mm
Overall length 135 mm
Packing ratio in the central part 48
(Part A)
~Industrial Utility of the Invention3
As described above, this invention concerns a
porous polypropylene hollow fiber memb~ane wherein the
solid phase in the inner surface region thereof is
formed w.ith particles of polypropylene closely fused and
joined to give rise to a continuous phase while
30 partially exposed through the surface th~reof, the solid
phase in the interior and the outer surface region
thereof is formed with particles of polypropylene
interconnected in the direction of axis of fiber to give
rise to a multiplicity of lumps of polypropylene, and
- ~3 -
.
131~
the interstice between these solid phases has continuous
pores interconnected in the form of a three-dimensional
network. When this porous polypropylene hollow fiber
membrane is used in an artificial lung, therefore, it
5 induces no leakage o~ blood plasma and yet retains a
high gas-exchange capacity even during a protracted
servi~e and, without reference to the choice of the type
of the artificial lung on account of the mode of
circulation of blood either inside or outside the hollow
10 fiber membrane, neither imparts any inuury to blood
cells nor aggravates pressure loss of the blood. Since
the porous polypropylene hollow fiber membrane possesses
a smooth outer surface, it proves to be highly
advantageous in respect that it is free from various
15 drawbacks otherwise incurred during the assembly of an
artificial lung such as cohesion of adjacent hollow
fiber membranes or impairment of the work of potting due
to adhesive agent. These characteristic features are
manifested all the more to advantage when the ratio of
20 birefringent in the direction of axis thereof is in the
range of 0.001 to O~Olr the porosity in the range of 10
to 60~, the opening ratio in the inner surface in the
range of 10 to 30~, the oxygen gas flux in the range of
100 to 1,500 liters/min.m2.atm, the inside diameter in
25 the range of 150 to 300 ~m, the wall thickness in the
range of 10 to 150 um, the average diameter of
polypropylene particles in the range of 0.1 to 2.0 ~m,
and the average pore diameter in the inner surface in
the range of 0.1 to loO ~m.
This invention further concerns a method for
the production of a porous polypropylene hollow fiber
membrane, which is characterized by mixing
polypropylene, an organic filler uniformly dispersible
in the polypropylene in a molten state and easily
35 soluble in an extractant to be used later, and a
crystalline seed forming agent, discharging the
resultant mixture in a molten state through annular
- ~4 -
1 3 1 ~
spinning orifices, cooling and solidi.fying the resultant
hollow threads by contact with a cooling and solidifying
liquid having no compatibility with the aforementioned
organic filler and possessing a specific heat capacity
5 in the range of 0.2 to 0.7 cal/g, and then bringing the
cooled and solidified hollow threads into contact with
an extractant capable of dissolving polypropylene
thereby removing the organic filler therefrom by
extraction. While the spinni.ng dope obtained by melting
10 and uniformly dispersing the raw materials is cooled and
solidified, therefore, the phase separation of the
polypropylene and the organic filler in the spinning
dope can be effected at a proper cooling speed wi-thout
inducing any local presence of the organic filler in the
15 outer surface part and, as the result, numerous
micropores can be produced in the interstices of
properly crystallized and grown particles of
polypropylene and, moreover, the outer surface part as
well as the thick wall part of the hollow fiber can form
20 a solid phase having particles of polypropylene orderly
arranged in the direction of axis of fiber and assume a
smooth surface. ~s the result, there can be produced a
hollow fiber membrane which exhibits the aforementioned
outstanding properties stably and uniformly. By the
25 method of this invention, the porous polypropylene
hollow fiber membrane possessing still better properties
can be obtained when a silicone oil or polyethylene
glycol, preferably a silicone oil possessing a viscosity
in the range of 2 to 50 cSt or a polyethylene glycol
30 possessing an average molecular weight in the range of
100 to 400, is used as the cooling and solidifying
liquid, liquid paraffin is used as the organic filler,
the proportion of the organic filler to 100 parts by
weight of polypropylene is in the range of 35 to 150
35 parts by weight, an organic heat-resistance substance
possessing a melting point of not less than 150C and a
gelling point of not less than the crystallization
~ 3 ~
starting point of polypropylene is used as the
crystalline seed forming agent, and the proportion of
the crystalline seed forming agent -to 100 parts by
weight of polypropylene is in the range of 0.1 to 5
5 parts by weight.
This invention a:Lso concerns an artificial
lung provided with a hollow fiber membrane as a
gas-exchange membrane, characterized by the fact that
the hollow fiber membrane is a porous polypropylene
10 hollow fiber membrane wherein the solid phase in the
inner surface region thereof is formed with particles of
polypropylene closely fused and joined to give rise to a
continuous phase while partially exposed through the
surface thereof, tKe solid phase in the interior and the
15 outer surface region thereof is formed with particles of
polypropylene interconnected in the direction of axis of
fiber to give rise to a multiplicity of lumps of
polypropylene, and the interstice between these solid
phasses has continuous pores interconnected in the form
20 of a three-dimensional network. In the artificial lung
of either the type adapted to circulate the blood inside
the hollow fiber membrane and blow the oxygen-containing
gas outisde the hollow fiber membrane or the type
adapted to circulate the b]ood outside the hollow fiber
25 membrane and blow the oxygen-containing gas inside the
hollow fiber membrane, therefore, the ability of the
membrane to add oxygen and the ability to remove carbon
dioxide gas are not degraded even during a protracted
service in the e~tra-corporeal circulation of blood, no
30 leakage of blood or blood plasma is induced, and neither
inflictio~ of injury upon blood cells nor aggravation of
pressure loss is entailed. Thus, the artificial lung
deserves to be esteemed highly. Typically in 30 hours'
extra-corporeal circulation of blood, the artificial
35 lung of the present invention incurs neither leakage of
blood plasma nor degradation of gas-exchange capacity.
The properties of the artificial lung are manifested
~ ~6 -
1 3 ~ 9
more to advantage when the ratio of birefringent in the
direction of axis of fiber is in the range of 0.001 to
0.01, the porosity in the range of 10 to 60~, the
opening ratio in the inner surface in the rang~ of 10 to
5 30~ , the oxygen gas flux in the range of 10 to 1,500
liters/min.m~.atm., and inside diameter in the range of
150 to 300 um, the wall thickness in the range of 10 to
100 ~m, the average diameter of polypropylene particles
in the range of 0.1 to 2.0 um, and the average pore
10 diameter in the inner surface in the range of 0.1 to 1.0
um.
- 47 -