Note: Descriptions are shown in the official language in which they were submitted.
3~5~
SPEC IFICATION
POROUS POLYPROPYLENE MEMB~ANE ~ND METHOD
FOR PRODUCTION THEREOF
BACKGROUND OF ~HE INVENTION
5 tFiled of the Invnetion]
This invention relates to a porous
polypropylene membrane and a method for the production
thereof. This invention also relates to a flat film
type porous polypropylene membrane to be used for blood
10 plasma separation, i.e. separation of blood into blood
cells and blood plasma, and for removal of bacteria from
blood and a method for the production thereof. More
particularly, this invention relates to a flat-film type
porous polypropylene membrane which, when used for the
15 blood plasma separation, exhibits a high blood plasma
separa-tion speed and has only a sparing possibility of
incurring such adverse phenomena as leakage of part of
the blood cells in the blood plasma after the
blood-plasma separation and hemolysis and a method for
20 the production thereof.
[Description of the Prior Art]
Heretofore, vrious permeable membranes have
been adopted for the purpose of separating blood into
blood cells and blood plasma. These permeable membranes
25 are used for blooa plasma purification aimed at removal
of abnormal protei~s, antigens, antibodies, and immune
complexes in such diseases due to abnormal immunity as
systemic lupus erythematosus, rheumatoid arthritis,
glomerular nephritis, and myasthenia gravis, for
30 manufacture of blood plasma preparations for component
transfusion, and for pretreatment of artificial kidneys,
for example. As examples of the permeable membranes
heretofore used for the blood plasma separation
mentioned above, there can be cited a cellulose acetate
35 membrane (applicant's Japanese patent Unexamined
Publication SHO 54(1979)-15,476, publishe~ February 5,
1979) and a polyvinyl alcohol membrane, a
--1--
'.
~. 3 11 ~
polyester membrane, a polycarbonate membrane, a
polymethyl methacrylate membrane, and a polyethylene
membrane (applicant's Japanese Patent Unexamined
Publication SH0 57(1982)-84,702, published May 27, 1982).
5 These parmeable membranes are
deficient in mechanical strength, porosity, and plasma
separating ability. When these permeable membranes are
used in the blood plasma separation, owlng to the
clogging of the micropores therein, the erythrocytes
lO are injured and the components of complement in the
blood plasma are activated and the separated blood
plasma is seriously injured as the result.
A permeable membrane has been proposed which
is produced by mixing a polymer such as a crystalline
15 polyolefin or polyamide which is sparingly soluble in a
solvent and is stretchable and a compound partially
compatible wi-th the polymer and readily soluble in the
so]vent, molding the resultant mixture in the form of
film, sheet, or a hollow article, treating the shaped
20 article with the solvent, drying the treated shaped
article, and then uniaxially or biaxially stretching the
dried shaped article to an extent fallng in the range of
50 to 15,000% (applicant's Japanese Pa~ent Publication SHO
57~1982)-20,970, published May 4, 1982). Since this
25 membrane has been
stretched for the purpose o~ increasing pore diameter,
it is susceptible of thermal shrinkage so much that,
when the permeable membrane is used in a medical device,
it will not be ab]e to be safely sterilized in an
30 autoclave. Moreover since the micropores are formed by
stretching in the permeable membrane, they are linear
micropores substantially parallel to the direction of
thickness of the membrane. Since the micropores have a
substantially uniform shape in the opposite surfaces and
35 in the interior of the wall of the membrane, they are
inevitably clogged with proteins and blood cells when
the permeable membrane is used in the blood plasma
separation.
_~ -2-
., .
11 3~6~
As concerns permeable membranes for use in the
blood plasma separation, polyolefin type macromolecules
hav~ been attracting attention as materials experiencing
activation of complements only to a nominal extent and
5 e~celling in bio-adaptability. At present, studies are
underway on the feasibility of permeable membranes using
such polyolefin type macromolecules. For example, there
has been discloses a method for the produc-tion of a
porous membrane, which comprises preparing a molten
lO mixture consisting of lO to 80% by weight of a paraffin
and 90 to 20% by weight of a polypropylene resin,
extruding the molten mixture through a die in the form
of a film, a sheet, or a hollow fi.ber, suddenly
solidifying the molten extruded mixture in water kept at
15 a temperature of not more than 50C, and then separating
the paraffin from the shaped article by extraction
(applicant's Japanese Patent Unxamined Publication SHO
55(1980)-60,537, published May 7, 1980). The porous
membrane which is obtained
20 by this method, however, cloes not fit speedy blood
plasma separation because the membrane has been suddenly
cooled with water, a substance of a large specific heat,
and, as the natural consequence, the pores formed in
the surfaces and those formed in the interior of the
25 membrane have small diameters and the porosity is low
and the speed of permeation is proportionately low.
As means of cooling and solidlfying the
aforementioned molten mixture, there have been proposed
a method which uses a metallic roller and a method which
30 uses a cooling and solidifying liquid such as a paraffin
possessing highly desirable compatibility with the
aforementioned organic filler (ap~licant's Japanese Patent
Application SH0 60(1985)-237,069 corresponding to Japanese
Patent Unexamined' Publication SHO 62(1987)-97,603,
35 published May 7, 1987). The former method
produces a porous membrane which possesses surface pores
of an extremely small diameter and, therefore, passes
blood plasma only at a low speed. In the latter method,
since the cooling and so].idifying liquid has a small
3--
,
1 3 ~
specific heat as compared with water and, therefore,
promotes the crystallization of polypropylene at a
proper cooling rate, the membrane is enabled in the
interior thereof to form micropores of a large diameter
5 enough for the purpose of blood plasma separation and is
suffered in the surface regions thereof to form a very
large reticular structure which is believed to arise
becaue the polypropylene in the surface regions is
dissolved out into the cooling and solidifying liquid
10 before it is allowed to solidify. In the porous
membrane possessing such surface layers as described
above, the surface layers each function as a prefilter.
Thus, the porous membrane is capable of carrying out the
blood plasma separation at a highly desirable speed
15 without surffering proteins and blood cells to clog the
micropores. When this porous membrane is brought into
contact with blood, however, it is liable to occlude
blood cells, which may possibly be forced to liberate
homoglobin under application of pressure.
An object of this invention, therefore, is to
provide an improved porous polypropylene membrane and a
method for the production thereof. ~nother object of
this invention is to provide an improved flat-film type
porous polypropylene membrane and a method for the
25 production thereof. A further object of this invention
is to provide a flat-film type porous polypropylene
membrane to be used for blood plasma separation aimed at
separting blood into blood cells and blood plasma and
for removal of bacteria from blood and a method for the
30 production thereof. Yet another object of this
invention is to provide a flat-film type porous
polypropylene membrane which, while being used for blood
plasma separation, permits the blood plasma separation
to proceed at a high speed, suffers the separated blood
.
.: :
: : :
plasma to be injured only sparinglyr and has little
possihility of entailing occlusion of blood cells or
hemolysis and a methocl for the production thereof.
[Deisclosure of the Invention]
The objects described above are accomplished
by a flat-film type porous polypropylene membrane
possessing a microreticular structure, characterized by
the fact that either or both of the opposite surface
region of the porous membrane forms a surace layer
10 possessing an average pore diameter in the range of 0.1
to 5.0~m, a bubble point of not more than 2.0 kgf/cm2, a
porosity in the range of 60 to 85~, and a water
permeability of not less than 100 ml/min.mmHg.m2 and the
membrane posesses a wall thickiness in the range of 30
15 to 300~m.
This invention also discloses a flat-film type
porous polypropylene membrane, wherein the bubble point
of the membrane is not more than 1.8 kgf.cm~. This
invention further discloses a flat-film type porous
20 polypropylene membrane, whrein the water permeability is
not less than 140 ml/min.mmHg.m2. Further this
invention discloses a flat-film type porous
polypropylene membrane, wherein the shrinkage ratio
after 120 minutes' heat treatment at 121C is not more
25 than 6.0~.
The object descrbied above are further
accomplished by a method for the production of a
flat-film type porous polypropylene membrane,
chaxacterized by mixing 100 parts by weight of
30 polypropylene, 200 to 600 parts by weight of an organic
filler uniformly dispersible in the polyperopylene in
; the molten state, and 0.1 to S.0 parts by weight of a
; crystalline seed forming agent, discharging the
resultant mixture in a molten state through a die
35 thereby producing a molten membrane in the form of a
flat film, cooling and solidifying the molten membrane
by contact with a cooling and solidifying liquid
exhibiting no compatibility to the organic filler and
possessing a specific heat capacity in the range of 0.2
to 0.7 cal/g, and then bringing the cooled and
S solidified membrane into contact with an extractant
incapable of dissolving the polypropylene and capable of
dissolving the organic filler thereby removing the
organic filler from the membrane by extraction.
This invention also discloses a method for the
10 production of a flat-fim porous polypropylene membrane,
wherein the porous polypropylene membrane obtained after
the aforementioned removal of the organic filler by
extraction is fixed in a presciribed length is subjected
to a heat treatment at a temperature in the ranye of
` 15 110 to 140C. This invention further discloses a
method for the production of a flat-film type porous
polypropylene membrane, wherein the contact of the
molten membrane with the cooling and solidifying is
effected by disposing a guide roller in the cooling and
20 soldifying liquid, allowing part of the guide roller to
emerge from the surface of the cooling and solidlfying
liquid, discharging the aforementioned mixture onto the
guide roller,~ and allowing the mixture to be led into
the cooling and solidifying liquid by the rotation of
25 the guide roller. Further this invention discloses a
method for the production of a flat-film type porous
polypropylene membrane, wherein the cooling and
solidifying liquid is a polyether. This invention also
discloses a method for the production of a flat-film
30 type porous polypropylene membrane, whrein the
polypropylene is a polypropylene possessing a melt index
in the range of 5 to 40 and having mixed therewith 0 to
50% by weight of a polypropylene possessing a melt index
in the range of 0.0~ to 5. This invention also
35 discloses a method ~or the production of a flat-film
type porous polypropylene membrane, wherein the
--6--
~ 3 ~
crystalline seed forming agent is incorporated therein
in an amount in the range of 0.1 to l.0 part by weight.
This invention further discloses a method for the
production of a flat-film type porous polypropylene
5 membrane, wherein the crystalling seed forming agent is
an organic heat-resistant substance possessing a melting
point of not less than 150C and a gelling point of not
less than the crystallization startin~ point o~ the
polypropylene. Further, this invention discloses a
lO method for the production of a flat-film type porous
polypropylene membrane, wherein the extractant is a
halogenated hydrocarbon or a mixture of the halogenated
hydrocarbon with a'ketone.
[Brief Description of the Drawinys]
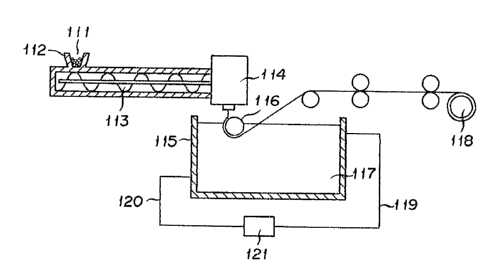
Fig. 1 is a schematic diagram illustrating a
typical apparatus to be used in working the method of
production of flat-film type porous polypropylene
membrane of this invention. Fi~. 2 is a circuit diagram
for the measurement of the highest blood plasma
20 separtion speed~ Figs. 3 and ~ are electron microscope
photographs illustrating textures of typical flat-film
type porous polypropylene membranes of the present
invention. Figs. 5 and 6 are electron microscope
photographs illustrating textures of flat-film-type
25 porous membranes used for comparative experiments. Fig.
7 is a graph showi,ng the relation between the blood
plasma separatin speed (Qf) and the total membrane
pressure (T.M.P.). Fig. 8 is a graph showing the
relating between the total membrane pressure and the
30 amount of free hemoglobin (~Hb). Fig. 9 a through c
illustrate realtions of permeation of various components
of blood plasma vs the blood plasma separatin speed
(Qf). Figs. 7 through 9 illustrate the data of Example
l with blank cycles (O ) and the data of Control l with
35 solid circles (~).
~Description of Preferred Embodiments]
~ 3 ~
~ ow, the present invention will be described
below with reference to working examples. To ~acilitate
comprehension of this invention, paragraphs titled
"Flat-Film Type Porous Polypropylene Membxane," "Method
5 for the Production of Flat-Film Type Porous
Polypropylene Membrane," and "Example" will he included
in the following part of the text hereof.
Flat-Film Type Porous Polypropylene ~embrane
The flat-film type porous polypropylene
10 membrane of the present invention i5 a flat-film type
porous membrane formed substantialy with polypropylene
in a wall thickness in the range of 30 to 300 ~m,
preferably 60 to 200 ~m. The microstructure of this
flat-film tyep porous polypropylene membrane is variable
!,~ 15 with the conditions for production of the porous
membrane. Generally, however, it is enabled to acquire
such a st~ucture as shown by the photographs of Figs. 3
and 4 taken under a scanning electron micrograph by
using, as a cooling and solidifying liquid to be fully
20 described later, a solution exhibiting no compatibility
with the~organic filler and possessing specific heat
capacity in the range of 0.2 to 0.7 cal/g.
Specifically, the flat-film type porous polypropylene
`membrane of this insvention possesses a microreticular
25 structure which is formed with interlaces threads each
consisting of interconnected particles of polypropylene.
In either or both of the opposite surface parts of the
membrane, a surface layer of practically the same
microreticular structure as in the interior of the
30 membrane is formed. The flat-film type porous
polypropylene membrane of this invention possesses pores
such that the pores in the interior thereof have the
same diameter as those in the porous membrane obtained
by using paraffin as the cooling and solidifying liquid
35 (Figs. 5 and 6) and the pores in the surface parts
thereof, unlike those in the porous membrane using
--8--
~ !
paraffin as the cooling and solidifying liquid, have
roughly the same diameter as those in the interior of
the membrane. Surprisingly, it has been found that the
flat-film type porous polypropylene membrane of this
5 invention which possesses such a microstructure as
described above exhibits as high permeation speed and
separation ability as the porous membrane obtained by
using paraffin, for example, as the cooling and
solidifying liquid and has a very remote possibility of
10 inducing occlusion of blood cells or hemolysis in
consequence of contact with blood.
In the flat-film type porous polypropylene
membrane of the present invention, the pores formed
therein are desired to have an average diameter in the
15 range of 0.1 to 5.0 ~m, preferably 0.2 to 3.0 ~m. If
the average pore diameter is less than O.l~m, the
membrane is liable to exhibit an insufficient permeation
speed to the blood plasma and the pores are liable to be
clogged. Conversely, if the average pore diameter
20 exceeds 5.0 ~m, the porous membrane has the possibility
of permitting not only the blood plasma component but
also the blood cell component (erythrocytes leukocytes,
and platelets) to permeate therethrough. So long as the
average pore diameter f~lls in the aforementioned range,
25 the porous membrane is capable of passing not less than
95% of the total protains, namely the blood plasma
component, without passing the blood cell component.
The texm l'average pore diameter" as used herein means
the average diameter of all the pores contained
30 throughout the entire volume of the membrane as actually
mesured with a mercury porosimeter and not the average
diameter of the pores contained only in the surface
layers. In the flat-film type porous polypropylene
membrane of the present invention, the bubble point is
35 required not to exceed 2.0 kgf/cm2, preferably 1~8
kgf/cm2. ~he term "bubble ~oint" as used herein means
~L 3 ~
to define the largest allowable pore diameter of the
membrane. If the bubble point exceeds 2.0 kgf/cm2, the
pores in the membrane have too small diameters for the
porous membrane to fit speedy filtration of blood plasma
5 and exhibit sufficient permeability to the blood plasma
component.
Further, in the flat-film type porous
polypropylene membrane of the present invention, the
porosity is in the range of 60 to 85%. If the porosity
10 is less than 60%, the poxous membrane is liable to
exhibit insufficient permeability and offer no
sufficient blood plasma separation speed. Conversely,
if the porosity exceeds 85%, the porous membrane to be
produced is liable to acquire no sufficient working
: 15 strength. Further in the flat-film type porous
polypropylene membrane of the present invention, the
amount of water to be passed therethrough is required to
exceed 100 ml/min.mmHg.cm , preferably 140
ml/min.mmHg.cm . If the amount of water passed
20 therethrough is less than lOOml/min~mmHg.cm2, the porous
membrane is liable to offer no sufficient blood plasma
seapration speed. The flat-film type porous
polypropylene membrane of this invention is required to
`have a waLl thickness in the range of 30 to 300 ~m. If
25 the wall thickness is less than 30~m, the porous
membrane is liable to be deficient in strength.
conversely, if the wall thickness exceeds 300~m, the
module to be obtained by incorporating a multiplicity of
such porous membranes is liable to occupy too large a
30 volume ot suit practical use.
The shrinkage which the flat-film type porous
polypropylene membrane of this in~ention exhibits after
120 minutes' heat treatment at 121C is required not to
exceed 6.0~, preferably 3,0%. The expression "120
35 minutes' heat treatment at 121C" represents the
high-pressure steam sterilization specified by the
--10--
.
~ 3 ~
Japanese Pharmacopoeia. The term "shrinkage" as used
herein means the degree of change in amount of the
porous membrane before and after the aforementioned heat
treatment. Since the flat-film type porous
5 polypropylene membrane of the present invention is a
flat film in shape, the foregoing requirement dictates
that the change to be brought abut by the heat treatment
in the length of the porous membrane in the dirction
perpendicular to the axis of molding should be not more
10 than 6.0%. If the shrinkage exceeds 6.0%, the porous
memebrane is liable to offer no sufficient separation of
the blood component because of decrease in the amount of
water to be passed and decrease in the blood plasma
separation speed.
15Method for Production of Flat-Film type
Porous Polypropylene Membrane
The flat-film type porous polyrpopylene
membrane of the present invention which possesses the
characteristic properties described above is produced,
20 for example, as follows.
As illustrated in Fig. 1, a composition 111
consisting of polypropylene, an organic filler, and a
crystalline seed forming agent is fed through a hopper
` 112 to a mi~er such as, for example, a twin-screw
25 extruder 113 to be melted and blended therein and
extruded therefrom, forwarded to a T die 114, discharged
in the form of a flat film therefrom, brought down
toward a guide roller 116 disposed inside a cooling tank
115 holding therein a cooling and solidifyin liquid 117
30 and allowed to contact the guide roller 116 at a Ievel
higher than the surface of the cooling and solidifying
liquid 117, and then led into the cooling and
solidifying liquid 117 by the rotation of the guide
roller 116. In this embodiment, the contact of the
35 molten membrane with the cooling and solidiying liquid
is effecte by use of the guide roller. Optionally, the
molten membrane may be dischargea directly into the
cooing and solidifying liquid instead. The molten
membrane is completely cooled and solidified while it is
travelling through the interior o~ the cooing tank 115
5 and then taken up on a ta~eup roller 118. In this
while, the cooling and solidifying liquid 117 which is
supplied via a line 119 is discharged via a line 120,
then cooled to a prescribed temperature by a cooling
device (such as, for example, a heat exchanger) 121, and
10 recycled. The membrane taken up as described above was
then led into an extraction tank (not shown) filled with
an extractant, there to be deprived of the organic
filler by extraction. The membrane emanating from the
extraction tank, when necessary, is subjected to
15 re-extraction, drying, and heat treatment, for example,
before it is rewound. For the purpose of stabilizing
the construction and the permeation property of the
porous membrane to be produced the membrane is desired
to be subjected to the heat treatment as fixed in a
20 prescribed length. The extraction of the organic filler
from the membrane may be effected by the use of an
extractin tank disposed before the step of the
rewinding.
The polypropylne to be used as one of the raw
25 materials in the method of this invention need not be
limited to homopol~ymer of propylene. It may be a block
copolymer using propylene as the main component and
additionally incorporating therein another monomer (such
as, for example, polyethylene). Desirably, the
30 polypropylene is required to possess a melt index (M.I~)
in the range of 5 to 70, preferably 10 to 40. Fuxther
for the purpose of enhancing the strength of the
membrane, the polypropylene to be used in the
composition is desired to have a large molecular weight,
35 namely, a low M.I. Fox example, a mixture consisting of
a species of polypropylene having a M.I. in the range of
;
: : .
. ~:
0.05 to 5 is used to advanrage. In all the species of
polypropylene enumerated above, the homopolymer of
propylene proves to be particulaxly advantageous,
especially so where the homopolymer possesses high
5 crystallinity.
The organic filler is required to be uniformly
dispersible in the polypropylene which is in a molten
state and to be easily soluble in an extractant to be
used later. Examples of the filler which fulfils this
10 requirement include liquid paraffin (number average
molecular weight 100 to 2,000), ~ -olefin oligomers
[such as, for example, ethylene oligomer (number average
molecular weight 100 to ~,000), propylene oligomer
(number average weight 100 to 2,000), and ethylene
15 oligomer (number average molecular weight 100 to
2,000)], paraffin wax (number average molecular weight
200 to 2,500), and various hydrocarbons. On the organic
fillers enumerated above, the liquid paraffin proves to
be particularly desirable.
The mixing ratio of the polyrpopylene and the
aforementioned organic filler is such that the proportin
of the organic filler to 100 part~ by weight of the
propylene is in the range of 200 to 600 parts by weight,
peferably 300 to 500 parts by weight. I fhte proportion
25 of the organic filler is less than 200 parts by weight,
the flat~ilm typè porous polypropylene membrane to be
produced possesses unduly low porosity and water
permeability and fails to acquire sufficient permeation
property. If this proportion exceeds 600 parts by
30 weight, the produced membrane exhibits unduly low
viscosity and deficiency in workability. For the
formulation of raw materials mentioned aboe, the mixture
consisting of raw materials in a prescribed percentage
composition is prepared (designed) by the premix method
35 which comprises melting and blending the mixture,
-13-
-: ~
L 6 ~
extruding the resultant blend, and pelletizing the
extruded blend by the use of biaxial type extruding
machine, for example.
The crystalline seed forming agent to be
5 included in the raw materials for this invention is an
organic heat-resistant substance possessing melting
point of not less than 150C, preferably in the range of
200 to 250C, and a gelling point of not less than the
crystallization starting piont of polyolefin~ The
10 crstalline seed forming agent of this description is
used as one of the raw materials for the purpose causing
contraction of the particles of polypropylene thereby
controlling the gap b~tween the solid phases, namely the
diameter of micropores to be formed. As examples of the
lS crystalline seed forming agent, there can be cited
1,3,2,4-dibenzilyaene sorbitol,
1,3,2,4-bis(p-methylbenzilydene~ sorbitol,
1,3,2,4-bis(p-ethylbenzilydene)sorbitol,
bis(4-t-butylphenyl)sodium phosphate, sodium benzoate,
20 adipic acid, talc and kaoline. Among other crystalline
seed forming agents enuemxated above,
1,3,2,4-dibenzylidene sorbitol,
1,3,2,4-bis(p-ethylbenzilydene) sorbitol, and
1,3,2,4-bis(p-methylbenzylidene) sorbitol prove to be
25 advantageous because they do not appreciably dissolve
out into the blood.
The mixing ratio of the propylene and the
aforementioned crystalline seed forming agent is such
that the proportion of the crstalline seed forming agent
30 to 100 parts by weight of the polypropylene i5 in the
range of 0.1 to 5 parts by weight, preferably 0.2 to 1.0
part by weight.
As the cooling and solidifying liquid for this
invention a solution exhibiting no compatibility with
35 the organic filler being used and possessing a specific
heat capacity in the range of 0.2 to 0.7 cal/g,
-14-
, ~
.
'J~ ~3
preferably 0.3 to 0.6 cal/g. As concrete e~amples of
the cooling and solidifying liquid, there can be cited
polyethers such as polyethylene glycol and water-soluble
paraffins which are insoluble in the oragnic filler.
5 Among other cooling and solidifying liquids enumerated
above, various species of polyethylene glycol prove to
be particularly advanrageous, especially so when their
average molecular weights fall in the range of 100 to
400. The species of polyethylene glycol possessing an
10 average molecular weight in the range o~ 18~ to 330
proves to be the most desirable selection. Such a
liquid as exhibiting no compatibility with the organic
filler being used and possessing a specific heat
capacity in the range of 0.2 to 0.7 cal/g is used as the
- 15 cooling and solidifying liquid for the following reason.
When a compound identical or similar to the
aforementioned organic filler is used as the cooling and
solidifying liquid, namely when a species of liquid
paraffin is used as the organic filler and another
20 species of liquid paraffin possessing number average
molecular weight approximating that of the first species
of li~uid paraffin is used as the cooling and
solidifying liquid, the produced membrane is enabled to
contain pores in a prescribed density without entailing
25 any appreciable migration of the organic filler in the
molten membrane. Further, since the specific heat is
not unduly large, the polypropylene is crystallized
smoothly at a proper cooling speed to form particles
stably. During the course of this cooling, the
30 polypropylene dissolves out into the cooling and
solidifying liquid before the polypropylene in the
surface parts is solidified. As the result, a very
large reticular structure is formed in the surface
parts. When an inactive liquid exhibiting no
35 compatibility with the organic filler and possessing a
large specific heat capacity of about 1.0 cal/g, is used
-15-
,~
as the cooling and solidifying liquid, the polypropylene
is quickly cooled, the phase separation between the
polypropylene is qulckly cooled the phase separation
between the polypropylene does not suffciently proceed,
5 and the pores in the surface parts and those in the
interior of the membrane are both small and the porosity
of the membrane is 1ow becaue the cooling effect of the
liquid is high.
In contast, when a liquid exhibiting no
10 compatibility with the aforementioned organic filler and
possesisng a specific heat capacity in the range of 0.2
to 0.7 cal/g is used as the cooling and solidifyng
liquid, the propylene is not suffered to dissolve out in
the surface parts, the cooling speed of the
t 15 polypropylene is proper, and the polypropylene is
smoothly crstallized even in the surface parts with the
composition ratio thereof retained intact. As the
result, there can be formed a reticular structure which
is not enlarged unduly even in the surface parts and
20 which is enabled to contain amply large pores fit for
blood plasma separation even within the interior
thereof.
The temperature of the cooling and solidifying
liquid is desired to be in the range of 10 to 80C,
25 preferably 30 to 60C, for the following reason~ If
this temperature is less than 10C, since ~the cooling
and solidifying speed is so fast that the micropores to
be formed consequently have a very small diameter.
Conversely, if the temperature exceeds 80C, the cooling
30 and solidifying treatment does not proceed amply and the
molten emmbrane is liable to break in the cooling and
solidifying liquid.
The membrane which has been throughly cooled
and solidified in the cooling and solidifying tank is
35 brought into contact with the extractant to permit
removal of the organic filler therefrom by extraction.
-16-
.. ~ .
..
~3~ ~~
This solution and extraction of the organic filler may
be effected by the extraction tank method or the shower
method which comprises advanting the membrane on a belt
conveyor and causing the extractant to fall in the form
5 of shower OlltO the membrane in motion.
As the extractant, any liquid can be used on
the sole condition that it should be incapable of
dissolving the polypropylene forming the backbone of the
porous membrane and acapable of dissolving and
10 extracting the organic filler. Examples of the liquid
answering the description include such halogenated
hydrocarbons as tetrachloromethane,
1,1,2-trichloro-1,2,2-trifluoroethane,
trichlorofluoromethane, dichlorofuluoromethane,
15 1,1,2,2-tetrachloro-1,2-difluoroethane,
trichloroethylene, and perchloroethylene. In the
liquids enumerated above, chlorofluorinated hydrocarbons
prove to be desirable in terms of ability to extract the
organic filler and safety in the human system. Where a
20 sorbitol is used as the crystalline seed forming agent,
a ketone may be incorporated in the extractant so as to
remove the sorbitol from the porous membrane during the
course of extraction and thereby to preclude the
otherwise possible exudation of the sorbitol from the
25 surface of the porous membrane after the molding.
The falt-film type porous polypropylene
membrane which is obtained as described above, when
; necessary, may be further subjected to a heat treatment.
; This heat treatment is carried out at a temperature 10
30 to 15C lower than the melting point of the
polypropylene, specifically a temperature in the range
of 110 to 150C, preferably 130 to 140C, for a period
in the ragnge of 30 to 180 secondst prefarably 60 to ~0
seconds. Preparatory to the heat treatment, the porous
35 membrane must be fixed in a prescribed length. ~he
flat-film type porou polypropylene membrane which is
-17-
~ 3 ~
produced as described above is useful as a membrane for
the separation of blood into blood cells and blood
plasma and as a microfiler for the removal of bacteria
from blood. It is used particularly advantageously as a
5 membrane for the separation of blood plasma where the
separted blood plasma is put to use as in the treatment
of donorpheresis.
Example
As an aid for further facilitating the
10 comprehension of this invention, a few working exampls
will be cited below. There exampls are offered purely
for the purpose of illustrating this invention and are
not meant to restrict the scope of this in~netion in any
respect.
15 Examples 1 through 3 and Controls 1 through 3:
By the use of a twin-screw extruder (produce
dby Ikegai Iron Works, Ltd. and marketed under trademark
designation of PCM-30-25), 100 parts by weight of a
mixture of two polypropylne species possessing melt flow
20 indexes of 30 and 0.3 (mixing ratio 100 : 40 by weight),
varying proportions of liquid paraffin (nul~er average
molecular weight 324), and
1,3,2,~-bis(p-ethylbenzylidene)sorbitol as a crystalline
seed forming agent indicated in Table 10 were melted and
25 kneaded and pelletized. By the aforementioned extruder,
the pellets were melted at a varying temperature in the
range of 150 to 200C, extruded through a T die 0.6 mm
in slit width into the air, allowed to fall onto a guide
roller in a colling liquid tank disposed directly below
30 the T die, led into the cooling and solidifying liquid
by the rotation of the roller to be cooled and
solidified therein, and thereafter taken up. The kind
and temperature of the cooling and solidifying liquid
used in this case were as shown in Table 1. From the
35 film thus taken up, a square (about 200 x 200 mm) was
cut off, fixed in both the longitudinal and lateral
-18-
,:
.. . . .
,,: , ' .
directions, immersed four times in
1,1,2-trichlor-1,2,2-trifluoroethane (liquid
temeprature 25C) for 10 rninutes each to effect
expulsion of the liquid para~fin, and then heat trea-ted
5 in the air at 135C ~or 2 minutes.
The flat-film type porous polypropylne
membrane consequently obtained was tested for wall
thickness, bubble point, porosity, water permeation, and
highest blood plasma separation speed~ The results are
10 shown in Table 1.
Then, to determined the microstructure of the
flat-film type porous polypropylene membrane, various
portions of the membrane were observed under a scanning
electron microscope (produced by JEOL and marketed under
15 product code of "JSM-840"~. Fig. 3 is a photomicrograph
of the surface (x 1,000) of the flat-film type porous
polyrpopylene membrane of Example 1, Fiy. 4 of the
partial cross section (x 2,500) of the flat-film type
prous polypropylene membrane of Example 1, Fig. 5 of the
20 surface (x 1,000) the flat-film type porous
polypropylene membrane of Control 1, and Fig. 6 of the
partial cross section (x 3,000) of the flat-film type
porous polypropylene membrane of Control 1, respectively
taken under the electron microscope. It is clearly
25 noted from Fig. 3, and Fig. 4 that the flat-film type
porous polypropylene membrane of Exmaple I according
with the present invention possessed practically equal
reticular structure in the surface parts and in the
interior of the membrane, the surface layers had a
30 virtually negligible thickness (about 0.5~ of the total
membrane thickness), and the reticular structure had
attained full development even in the interior of the
membrane. In contrast t the flat-film type porous
polypropylene membrane obtained by used liquid paraffin
35 as the cooling and solidifying liquid (control 1), as
clearly noted from Fig. 5 and Fig. 6, possessed as fully
trade-marlc
--19--
~. 3 ~
developed A reticular structure in the interior of the
membrane as in the membrane of E~maple 1, possessed
fairly rough reticular s-tructure in the surface paxts,
and had surface layers of a fairly large -thickness
5 (about 24.0% of the total thickness of the membrane).
The comparison offers a definite evidence that the
falt-film type porous polypropylene membrne of Example l
according with the present invention ~uffered sparingly
from occulsion of blood cells~
Separately, life-size laminate modules
severally incorporateing therein the falt-film type
porous polypropylene memebrane of E~ample l and Control
l were operated t~ effect blood plasma separation of
bovine blood, to compare the flat-fim type porous
15 polypropylene membrane in ability o~ blood plasma
separation. Results are shown in Fig. 7 - 9. Fig. 7
shows the relation between the speed of blood plasma
separation (Qf) and the total intermembranous pressure
(T.M.P.) and Fig. 8 the relation between the T.M.P~ and
20 the amount of free hemoglobin ( ~ Hb).
Control 4:
For the purpoe of comparison, a commercially
availabel cellulose acetate membrane (C~; produced by
Toyo Filter Paper Co., ltd.) was similarly tested for
25 thickness of membrane, bubble point, porosity, water
permeation, and highest blood plasma separation speed.
The results are shown in Table l.
* trade-mark
-20-
,
o 1 3 ~
S U~ ~ ~ ~ o I I o o o
~ E
~, ~
~ e
41 0 ~ N ) 0~ O Ul O O
E E E
g ~ E
~,. ~ 1 u'l N
O^ ~D ~O ~O (` i` U~ ~
~ dP
~ ~ ~ ~1 0 0 0
m
~ ~ ~ o ~ o O o
E t~ E~ ,~ ~ _~ ,1 ,~ ~1 ,
_l~ r.
o ~ 0 ~,
ul ~ R o o o o o o
E u ,1 ~
~1 0 ,1 0 Q.
r .
~ O C~
U-- ul o In a:\ o o
~ ~J ~ ~) N
aJ S --1
CJ ~.,1
E~ O
~ O O ~ ra
q~ho ho ''I h
tJ' ~ q.l RE~
~1 C) ~ O q I h :~
~' S R, h h P. U
a) O : : .1N `1 C: ~1 ~1
O O ,,1 ~r-l N ,.
u ~ ~ ~~ ~ a~
~n
o
~ S O O ~D O O O ~
h h ~1 o o ~ o o Ul o
3 ~ 1 U
~ _
~I N ~ ~I N ~r) ~
O O O O
h h h h
X X X O O O O
E 1 1~ W [.) U U
-21 -
.
.
,
131~
It is clearly noted from Table 1 that the
flat-film type porous polypropylene membranes of the
present invention (Examples 1 through 3) exhibited high
porosity and water permeability and also possessed high
5 blood plasma separation speeed.
Further the flat-film type porous
polypropylene membranes of this invention, owing to
their membranous structure, suffered sparingly from
occlusion of blood cells and they had an advatnage of
10 hardly admitting of hemolysis as shown in Figs. 7 and 8.
In contrast, the flat-film type porous polypropylene
membranes of Conotrols 1 through 3 which used liquid
paraffin or halo~enated hydrocarbon as the cooling
liquid were liable to induce hemolysis and ocnsequently
: 15 were not allowed to use any large intermembranous
pressure, thouyh they exhibited as high blood plasma
eparation speed as the flat-film type porous
polypropylene membranes of the present in~ention. In
terms of the permeability to the componenets of blood
20 plasma, the flat-film type porous polypropylene membrane
of this invention (Example 3~ was favorably comparable
with that of Control 1 which had a large surface pore
- diameter.
The various terms used in the present
25 specification concerning the Flat-film tyep porous
polypropylene membrane and the methods used for the
determination of the properties mentioned herein are
- de~iend below.
Bubble point
This property was determined in accordance
with the method of correction specified in ASTM F316,
using a stainless steel holder 47 mm in diameter and
-~ isopropyl alcohol as a liquid alcohol. The pressure
applied was continuously increased until a continuous
35 line of nitrogen bubbles began to rise uniformly and
-22-
'
. .
. ~
.
.~
~ ~ 3~
incessantly from the center of the filter through the
isopropyl alcohol. The pressure at this point was
reported as bubble point.
Thickness of membrane
This property was determined by actually
measuring the thickness of a given membrane with the aid
of micrometer.
Porosity
~ ~ . . . = _
A given flat-film type porous polypropylene
10 membrane was immersed in ethanol and then hydrated by
means of displacement of ethanol with wa-ter and the
weight of the water (Wp) conse~uently contained therein
was determined. The porosity was calculated in
accordance with the fol.lowlng formula:
(Wp-Ww) x 100
(Ww/P) + (Wp-Ww)
wherein Ww stands for the weight of the membrane in the
dry state and P g/ml for the density of the polymer.
Water permeation
.
Water at 25C was caused to permeate a give
membrane measuring 1.45 x 10 3 m2 in area under
application of a pressure of 0.7 kgf/cm2. The -time
required for 100 ml of water to pass through the
membrane was clocked and reported as water pemation.
25 Highest blood pLasma separation speed (Qf max)
This property was determined by use of a
circuit illustrated in Fig. 2. In a module 30
possessing a membrane surface area of 0.4 m2~ fresh
bovine blood incorporating therein heparin of a
30 hematocrit value of 40~ (5,000 U/liter) was circulated
in a flow volume of lOOml/min at a pressure loss of 30
mmHg, with the flow volume of filtration pump
successively increased from 10 ml/min to 10, 15, 20, 25,
30, 40, and 42 at intervals of 30 minutes. The amount
.
-23-
;'
6 ~
of filtrate immediately before the increase o~ T~M.P.
within an interval of 30 minutes surpassed 20 mmHg was
found and reported as Qf max.
in + POut/2 - Pfil is presumed.
5 In ~ig. 2, Gl, G2, and G3 each denote a pressure meter,
the pressure of Gl is expressed as Pin, that of Ga as
Pfil, and that of G3 as PoUt respectively, Every P
indicated in the diagram stands for a pump.
Average pore diameter
This property was determined by actually
measuring the size of pores, in a given membrane with a
mercury porosity.
~Industrial Utility of the Invention~
As described above, this invention concerns a
15 flat-film type porous polypropylene membrane possessing
a microreticular structure, characterized by the fact
that either or both of the opposite surface region of
the porous membrane forms a surface layer possessing an
average pore diameter in the range of 0.1 to 5.0~m, a
20 bubble point of not more than 2.0 kgf/cm2, a porosity in
the range of 60 to 85%, and a water permeability of not
less than 100 ml/min.mmHg.m2, and the mem~rane possesses
a wall thickness in the range of 30 to 300~m. Thus, the
flat-film type porous polypropylene membrane exhibits
25 high porosity and water permeability. When it is used
for blood plasma separation, it suffers sparinyly from
clogging of pores with proteins or blood cells, effects
separation of blood plasma at a high speed, and entails
only slight occlusion of blood cells and hardly includes
30 hemolysis. Owing to these features, the falt-film type
porous polypropylene membrane is used advantageously for
blood plasma separation, i.e. the separtion of blood
into blooa cells and blood plasma~. It is particularly
useful as a membrane for bloood plasma separation where
35 the separated blood plasma is put to use as in the
donorpheresis. The flat-film type porous polypropylene
-~4-
`:"
'
' ' ' ' ~
' . . ' '
~ 3 ~
membrane of this invention is enabled to manifest these
highly desirable properties still more to advantage when
the bubble point is not more than 1.8 kgf/cm2, the water
permeation not less than 140ml/min.mmHg.m2, and the
5 ratio of shrinkage due to 120 minutes' heat treatment at
121C is not more than 6.0%.
This invention also concerns a method for the
production of a flat-film type porous polypropylene
membrane, characterized by mixing 100 parts by weight of
10 polypropylene, 200 to 600 parts by weight of an organic
~iller uniformly dispersible in the polypropylene in the
molten state, and 0.1 to 5.0 parts by weight of a
crystalline seed ~orming agent, discharging the
resultant mixture in a molten state through a die
15 thereby producing a molten membrane in the form of a
flat film, cooling and solidifying liquid exhibiting no
compatibility to the organic filler and possessing a
specific heat capacity in the range of 0.2 to 0.7 cal/g,
and then bringing the cooled and solidified membrane
20 into contact with an extractant incapable of dissolving
the polypropylene and capable of dissolving the organic
filler thereby removing the organic filler form the
membrane by extraction. This method is capalbe of
easily producing the flat-film type porous polypropylene
25 membrane possessing the aforementioned outstanding
properties. The properties of the flat-film type porous
polypropylene membrane are stabilized to a greated
extent when the method described above further comprises
; causing the flat-film type porous polypropylene membrane
30 which results from the removal of the organic filler by
extraction to be fixed in a prescribed length and
subjected to a heat treatment at a temperature in the
range of 110 to 140C. The flat-film type poxous
polypropylene membrane o~ high grade can be obtained
35 easily when the contact of the molten membrane with the
cooling and solidifying liquid is effected by having a
-25-
.
11 3 ~ 3 1~
guide roller disposed in the cooling and solidfying
liquid, discharging the molten mix~ure onto the guide
roller, and causing the molten mixture to be led into
the cooilng and solidifying liquid by the rotation of
5 the guide roller. The properties of te flat-film type
porous polypropylene membrane are further enhanced when
the cooling and solidifying liquid is a polyether, the
polypropylene consists of a species of polypropylene
possessing a melt index in the range of 5 to 40 and 0 to
10 50% by weight of another species of polypropylene
possessing a melt index in the range of 0.05 to 5, the
crystalline seed forming agent is incorporated in the
mixtrue in a proportion falling in the range of 0.2 to
1.0 part by eight, the crystalline seed forminng agent
-~. 15 is an organic heat-resistant substance possessing
melting point of not less than 150C and a gelling point
not less than the crystallization starting point of
polypropylene, and the extractant is either a
halogenated hydrocarbon or a mixtue of a halogenated
20 hydrocarbon with a ketone.
-26-