Note: Descriptions are shown in the official language in which they were submitted.
13~ ~322
FIELD OE THE INVENTION
This invention relates to a cyclohexanedione
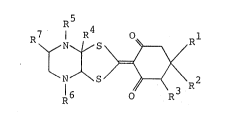
derivative represented by the general formula
~r~- J
wherein Rl, R4 and R7 represent independently hydrogen
atom or Cl - C8 alkyl group, R2 represents hydrogen atom;
Cl - C8 alkyl group; C2 - C16 alkenyl group; C3 - C8
cycloalkyl group; C2 - C7 alkoxycarbonyl group; Cl - C6
alkylthio Cl - C6 alkyl group; a phenyl group; a phenyl
group substituted with 1 to 3 groups selected from the
group consisting o~ halogen atom, Cl ~ C6 alkyl group, Cl -
C6 haloalkyl group, Cl - C6 alkoxy group, Cl - C6
alkylthio group, C3 - C8 cycloallcoxy group, a benzyloxy
group and a benzyloxy group subst~ituted w~ith C - C6
~ ry/ ~r ~ ,~n~ :
alkoxy group; naphthyl group or a-~e~e~e~e~ group, R3
represents hydrogen atom, Cl: - C8 alkyl group or C2 - C7
alkoxycarbonyl group, and R5~and R6j which may be the same
or different, represent hydrogen atom, Cl - C6 alkyl
group, C2 - C6 alkenyl group, C2 - C7 alkylcarbonyl group
: or C2 - C7 haloalkylcarbonyl group and its pharmaceutically
-- 1 -- .
3~
~ 3:~ ~322
1 acceptable salts, a process for producing the same and a
pharmacel~tical composition containing the same.
BACKGROUND OF THE INVENTION
It has been disclosed tnat cyclohexanedione
~- 5 derivatives shown below are effective for the treatment of
~v~ damage (See U.S. Patent Mo. 4,668,799)
1. 2-(1,3-dithiol-2-ylidene)-1,3-cyclohexanedione.
2. 2-(1,3-dithiol-2-ylidene)-4-methyl-1,3-cyclohexane-
dione.
3. 2-(1,3-dithiol-2-ylidene)-4-(2-methylethyl-1,3-cyclo-
hexanedione.
4. 2-(1,3-dithiol-2-ylidene)-5,5-dimet]lyl-1,3-cyclohexane-
dione.
There is still a desire, however, for a compound capable
of curing and/or preventing liver disorders at a conside-
rably lower dosage which will provide a more safety margin
for treating both men and animals.
SUMMARY OF THE INVENTION
~ The object of the present invention is to
provide a cyclohexanedione derivative represented by the
aforementioned general formula (Ij.
The other object of the present invention is to
provide a pharmaceutical composition containing as an
active ingredient a compound shown by said general formula
(I).
The furt'ner other object of the present
-- 2 --
3 ~ ~
1 invention is to provide a method for treating liverdisorders in men and animals by administrating said
composition to them parenterally or orally.
The further other object of the present inven-
tion is to provide a method for producing a compoundrepresented by said general formula (I).
The terms ~alkyl and allcenyl" as used herein
denote both straight-chain and branched alkyl and alkenyl
groups, respectively.
.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
The compounds represented by the afore-mentioned ~-
general formula (I) and their salts are novel compounds
not described in the literatures; they have, for example,
a liver function activating effect, and hence is useful as
active ingredient for a pharmaceutical composition for
treating hepatic disorders in men and animals.
The compound of general formula (I) can be
produced, for example, by methods A and B as shown in the
following scheme:
:: :
Method A
. ~.
R
R ~1) R HNCCH2NHR (III)
O\ ¦ ~S03Na H
C
HO 53Na MS ~ R2
3 --
.
~ 3 ~ 2
R7 1 O R (I)
l R2 R3 R4 R5 R6 and R7 have the same meanings
as defined above, M represents alkali metal atom.
That is, the compound of the general formula (I)
can be produced by reacting the compound of the general
formula (II) with the compound of the general formula
(III) in a suitable solvent at a temperature in the range
of from -20C to 50C and followed by the reaction with
the compound of the general formula (IV) at a temperature
in the range oE from -20C to 80C.
The solvents which can be used in the present
reaction are preferably water or solvents consist of water
and an organic solvent. For the organic solvent, there
can be exemplified, for example, dimethylforamide,
dimethy1sulfoxide, hexamethylphosphoroamide and N,N-
dimethylethyleneurea or in combination oE these solvents.
The reaction time depends upon the~reaction temperature
and reaction scale, but it may properly be selected from l
to 2~hours. As to the molar rati~o of the reagents in
pract1c1ng the present reaction, they are used in equi-
mola~r amounts because the present reaction are anequimolar reaction, but e1ther one of them may be used in
~ excess of the other.
: ~ : :
~ - 4 -
~L3~3~
1 The compound of general formula (II) shown below
can be obtained by reacting a compound of general formula
(V) with an equivalent or a slightly excess of sodium
bisulfite in water at a temperature in the range of from
0C to 80C.
4 R
~ C~ HO \¦ ~SO3Na
+ 2NaHS03 >
~,C~ Ho~ CH~So N
(V) (II)
wherein R4 has tne same meaning as defined above. The
compound of the general formula (IV) shown below can be
obtained by reacting a cornpound of general formula (VI)
with carbon disulfide in a suitable solvent in the
presence of a base at a temperature in the range of from
-20C to 60C:
R base MS ~ R
(VI) (IV)
whereln Rl, R2, R3 and M have the same meaning as defined
above.
In most case, the compound of the general
formula (IV) can be used without being separated from the
: - 5 -
~31~3~
1 reactiOn mixture.
For a base usable in preparing the compound of
the general formula (IV), there can be exemplified, for
example, a hydroxide such as sodium hydroxide, potassium
hydroxide and a carbonate such as sodium carbonate,
potassium carbonate.
~ or a solvent, there can be exemplified, for
example, dimethylformamide, dimethylsulfoxide, hexamethyl-
phosphoroamide and N,N-dimethylethyleneurea etc., and in
combination of these solvents or in combination of water
with above organic solvents.
Method B
RSX (VII) or
N S 3 R R6X (VIII)
R8 0 R
R R4
~ N ~ S Rl
: N S ~ R2
R6 (or ~8) o R
- (I)
1 h ein Rl R2 R3 R4, R5, R6 and R7 have the same
meanings as defined above, R8 represents hydrogen atom or
Cl - C5 alkyl group, and X represents halogen atom.
That is, the compound of the general ormula (I)
can be produced by reacting the compound of the general
formula (I'), which was prepared by the method A with the
compound of the general formula (VII) or (VIII) in an
inert solvent at a temperature in the range of from -20C
to the boiling point of the solvent used.
Solvents which can be used in this reaction may
be any of those not disturbing the reaction, and include
for example ethers (e.g. diethyl ether, tetrahydrofuran,
dioxane), aromatic hydrocarbons (e.g. benzene, toluene,
xylene), halogenated hydrocarbons (e.g. chloroform, carbon
tetrachloride). These solvents may be used alone or in
combination. Bases which can be used in this reaction are
inorganic bases such as sodium hydroxide, potassium
hydroxide, lithium hydride, sodium hydride, etc. and
organic bases such as, triethylamine, pyridine etc.
As to the amount of the base and the compound of
the general formula (VII) or (VIII) used in this reaction,
it suffices to use 2 mole per mole of the compound of the
general formula (I') when R8 represents hydrogen atom in
general formula (I') and an amount equimolar to the com-
pound of the general formula (I') when R8 represents
Cl - C5 alkyl group, but amounts in excess thereof will do.
T'ne reaction time depends upon the reaction
temperature and reaction scale, but lt may properly be
-- 7
q ~3~
1 selected from a range of 30 minutes to 8 hours.
Further, the salt of the compound of the general
formula tI) was obtained by reacting the compound of the
general formula (I) with the acid.
The salt of the compound of the general formula
(I) may be any of pharmaceutically acceptable salt. For
the acids usable in preparing the salt, there are exempli-
fied, for example, inorganic acids such as hydrogen
chloride, sulfuric acid, phosphoric acid etc., organic
carboxylic acids such as acetic acid, succinic acid,
fumaric acid, tartaric acid and organic sulfonic acids
such as methanesulfonic acid, heptanesulfonic acid,
benzenesulfonic acid, toluenesulfonic acid. For the
solvents, there are exemplified, alcohol, chloroform,
dichloromethane, ethyl acetate and the like.
The compound of the general formula (I) and its
salt can be separated by a conventional method.
Representative examples of the compound of the
general ~ormula (I) and their salts will be shown in Table
1, but the derivatives are not limited to these examples.
Among the compound of the present invention of
which the typical example are shown in Table 1 below, the
prefered compounds are those whose R2 represents hydrogen
atom; lower (Cl - C6) alkyl group; alkenyl group; cyclo-
alkyl group; a phenyl group; a phenyl group substitutedwith 1 to 3 groups selected from the group consisting of
halogen atom, lower (Cl - C6) alkyl group, lower (Cl - C6)
haloalkoxy group, lower (Cl - C6) alkoxy group,
-- 8
25711-502
cycloalkoxy group, a benzyloxy group and a benzyloxy group
substituted with lower (Cl-C6) alkoxy group; naphthyl group or a
heterocyclic group, and R5 and R6, which may be the same or
different, represent hydrogen atom, lower (Cl-C6) alkyl, lower
(C2-C6) alkenyl group, alkylcarbonyl group or haloalkylcarbonyl
group and R , R , R4 and R represent hydrogen or lower (Cl-C6)
alkyl. More preferred ones are those whose R2 represents
hydrogen atom; Cl-C4 alkyl group; a phenyl group; a phenyl group
substituted with 1 to 2 groups selected from the group consisting
of halogen atom, Cl-C4 alkyl group and Cl-C4 alkoxy group; or
furyl group, Rl, R3, R4, R5, R6 and R7 represents hydrogen atom
or Cl-C4 alkyl group.
~, _ g _
~3~QJ~2~
~ ~ ~ ~J ~
C) In U~ U~ ~ ~
~ ~ ~ ~ c~ ~ ~ ~ c
~ O'JJ l O l O l l l O
C~ ~ C ~ u~ ~ Ll~ ~r Lr) ~r ~
J- ~ X It
~ o ~ . . . . . .
S ~ O a) C CIJ R~ ~J Q~ Q~ Q~
_ _ E E __ E E E
m m m m m m
_._ _ ~1 _ , _ ~`
X
X :C
~ ~ ~ ~ ~ ~ C~ C
. __ _ _ __
In ~ U~
~ ~ ~ ~ m
u~ 3: ~ ~ m ~ v
~; c~ v ~ v m ~ c
... _ _
a~ ~; ~5 ~ ~ ~ ~ m tc
_ _
P~ m m m m m m m
m ~ ~ ~ 5: x~ m
:: :: ~ ~ : : :
:: .. - - -
~ ~ ~ ~ m m ~ m m u m m
~: : __ _
~: ~ ~Z ~ _ ~ r In ~D ~_
-- 10 --
3 ~ 2 2
. V V ~,v ov ov V V V
In ~g ~ ~ ~ ~ O ~
~r ~r ~ In U~ ~r u~ I_
~ ~ ~ ~ ~ ~ V l l C
U~ o U~ ~ ~ o
~r ~ ~ n ~D ~ Ln ~r
~1 ~ ~ ~ ~ ~ ~ ~ ~
~ Ql ~ R~ ~ o Q. ~
E E E E~ E E E~ E E~
_ ~ 'X~ ._
::C ~ ~ ~ ~r: V V
_ _ _
U~ ~ Ln
~C r~ ~ ~ ~:
V ~5 ~ ~ ~ ~r
V l ~ V V ~C V V
~ ~:: O=V O=c)
_ _ . _ .. _ . _ _ _
~ :C~ U~
r~ r~ ~ ~
V ~ :C ~ ~ ~r
V V V ~ V V
~5 ~ C O=V
C ' _ -r~ ~ !~7~ _ ..
::~ ~ X V V V X ~ ~C
_ _ _ _ _ __
~ ~ ~C :C ~:: ~n :~ ~ ~ ~
Q _ ___
El
a v ~ ~ v :
: :
:
~ ~ ~ ~ ~ ~ .
V V :I: :3: V V V V ~C
- -
: ~ --~ ~ _l ~ ~ ~1 ~ U>
~ ~ o - u ~ ~ ~
03 ~r I ~D CO l ~ O
~ ''1' ~ C~ ~ ~ I_ ~ l S~
co ~ L(~ L~ o Ln u~ ~r
. . ~ ~ ~ ~ ~ ~ u~ l
l- o~ Q~
. . . ~ a ~ a . ~ a ~ a .
C C ~ C C
__ _ . __
m 3:: m 5: m 5: x :~ 3::
_ _ __
~ ~ ~ ~7 ~ ~ ~
E4 ~ ~C m ~ ~ :~: ~::
0=~1 C~ V _ O _~ V V
_
~ r~7 ~~7 ~ ~ ~ ~ r~
~ ~C ~ X _ ~ :C :C ~C
~ C~ C~ C~ ~ C~ V V O V
C _ ... __ _
O X ~ X m ~ ~ ~ ~ ~
_ _ _
~ ~ ~C~
R ::C ~ :C ~ ::C m m m V
~ _ _
~ m
~ ~ ~ \/
I ~7
~ ~ ~ U ~ ~ ~
::~ ~T~ ~ l o ~ U
C~ V : C ; ~)~ ~ :
: ; c~-vv V-V~
. 1_ 1 , .
:~
v ~r m ::c m ~ t:C ~n ~
: : __
l_ CO a~ o ~ ~ ~ ~r u~
~ ~ ~ : ~ ~ ~ ~ ~
~ _ _ ._ . .
- 12 -
~ 3 ~
ov V V V V V V V
U,~ ~D ~ l C~ ~, l I
.D ~ ~ ~ ~ ) U~
P~P~ P~ P~ P~ PJ ~ P~
._ __ .__ . _ .. __ __
m ~ m ,~ m m ~ m
~", ~ ____ _
3: u ~ o m 5: o ~)
.._ ~ ~ ~ _ .
r~5 ~ ~ ~ ~ m m m
~ . _ _ _ . _ __ ._
C) ~ ~ ~ ~ :C
.__ _ .._ .._ . _ ._
Q m m ~ m m m ~ x
~ ~ ~ ~ v ~ ~v ~ ~v
~ ~--- --- ---- - --
P: m ~ ~ ~:: 3:: ~ m
_ -_ _ . _ .
~D I~ ) ~ O
._. _ _ _ .._ ._ ..__
-- 13 --
~31~J2
ou ov u u u
l l l l o ~ v o
~: ~ o ~ co ~ ~: ~
~ P~ ~ n~ P~ ~ ~ Q~
- - -- ~ - ---- --- --
:~ m m ~ ::a m ::c
_~ ... _ ~ .
m
c~ ~ m
~ ._ . , _ _
m ~ ~ ~ :r ~ .
~ m c~ ~ ~ m
._ ._
o m m m x m ~ m
' _ ._
::c ~c m
E~ . __ ._
~ ~ ~ ~ ~ ~ ~E4
o _~__ ~ - _ _
m ~ ~ ~: ~:: ::~ ~
_ _~ _ _ .. _
U~ U~ ~ CO C~ o
~ ~ ~ ~ ~ - _
-- 14 --
2 2
. V V V V ~5
~ ~ ~ In O
~ ~, V ~ ~ ~. ~ ~
~ ~ U~ ~ ~ ~ o
~ ~ ~ ~ ~ ~ ~ l
. ~ ~ Q~
~ E~ ~ ~ ~ e ~
~ ~_ . _
x m ~ ~ ~
... ._ W~
5: ~ ~ ~-
::C ~ ~ V ~, X
V V V V ~ ~ U
. .. . .__
m ~
V ~ ::C
. .. V V ,~_ V
s:~
X ~ ~ ~ :~ ~ V
.. .__ _ .. _
a) ~ ~ ~ ~ P~ :c ~
R .. _ _ .__ __
V V V V X~ V
~ ~ ~ ~ ~ ~ '~
..__ -._. _
5: X ~ ~ ~C :r:
.__ : .
~ ~ ~ ~ U~ ~t7
~, ~r ~ ~ ~ .~
-- 15 --
~ 3 ~ ~ ?~ J
~ ~ ~ ~ ~
~ F co ~ ~ ~ ~ l
_ .. .__
m ~ ~ m ~ m m
~ . ._ .
~ 3: ~ r~ r~ r~
3 u~ c~ ~ c~ 5
._ _ ._ . _ .
C~ C~ h~ C~ m ~:: c~
._ .._ :.._ _ ..
C~ X ~ X ~C ~ ~ ::C
._ . ._ r~
Q ~ !T! 3:~ X ~:C ~:: C)
E~ __ ........... ._
:C~ :C~ ~tc~ ~ ~
N N ~r~ )
' _ ~
e~ ~ :I: ~C m ~
_ ._
~ a~ o ~ ~ r~ ~
~r ~r In U~ ~ In n
-- 16 --
~3~ ~v~2
ov CJ ~. ~, ~ ~, o V
o
r~ ~o ~ ~ ~ l
Q~ Q~ L~ Q~ Q~ Q~ ~J Q~
. ~ ~ ~ ~ ~ ~ ~ ~
_ __ _ ._ . ._ ..
X X ~ ~ ~ ~: ~ ~C
._ .__ . . __ _ , _
~ ~ x ~ ~ ~ :~ m
~ ~>~' l ,i ~ ~: ~
._ ._ . ___ _
~ m x~ ~ ~ ~ ~u~ ,_
5: ~ ~ ~ X ~ ~ ~
_ l V ._ _
~C ~ 3~ ~ :C~) ::C X X
. _ _ _
:~ ~ :C ~' X :~ ~ ~
E~ _ _ ... _ _ .. _ ._
~C~ ~U~
~C C~ C~ ~>~
o o o o o o o o
~D ~ ~ ~ ~ ~ ~ ~
: ~. _
~C ~ ~: ~ P~ 5: ~q
...__
~ ~ ~ n c~ o ~ ~
-- 17 --
~ 3
a~
.~ Q. Q. ~ Q
~ ~ ~ ~ ~ ~.
:~ :C X V V m
._ ~ , _
~ X m V V ~
_ __ .
o V ~ ~ X ~C 5:
_ __ . __
~ ~ ~r ~C m ~:C m
Q ._ ~_
~ m :~ ~ ~ ~ ~ r~
O O U O ~ O U O
_, __ .__
: ~ m ~c~: :~ !r
: ._ . .__
~ ~ U~ U~ ~ CO
:; ~ ~ ~ ~ ~- __ _
~ 18 -
~ (-~ c~ u ~ ~J c~
~ ~ ~ ~ O ~D I~ o ~
~ ~ ~ o ~ ~ ~ ~ o
r~ c~ I t~ ~ ~1 l 'J
~1 ~ o a~ o ~ ~ ~
~ ~ ~ G~ ~ ~ ~ l_ l
R~ ~ Q~R~ R~ R~ R~ C~
. ~ ~ ~ ~ ~ ~ ~
__ __ . .__
~ :c ~:~:: m ~ x ~
._ .. _ 11:~ _
~ ~ ~ .~ ~ ~ ~ ~
V~ ~ ~ ~ ~C C~ C~
.. _ .
U~ ~ ~
~:
X ~) ~ ~C ::C ~C
t~ ~ ~ ~ ~ V
_ _ ._
o ~ ~ ~ ~ ~ 5~ 5:
: ~ _ _ ...
~ ~7 ~ ~ :C ~C 5: X
E~ __ .. _ _ __ _
.
l ~ ~ ~ x~ ~c~ ~ ~
~ ~ v ~ ~
~:: :~ :~ 5:
-- ~----- -
a~ o I_ ~ ~ ~r L~
~ - ~ -- --
-- 19 --
2 ~
V U V V N V
O ~ V C~ ~~ ~ ~ ~~ V
t~ Lt~ n ~r ,_ u~ ~ ~r c.
o Ln ~ u~ r~ ~ ~r ~
~'
P~ P~ P~ P~ P~ P~ P~ P~
.. _ ._ ___
3~ V ~ 5: ~ ~C ~ ~::
._ .___ _ _ .___
r~
~C ~ ~ ~ 3:
~ X V V ~ ~ O O
' _ ~ _ ~ .____
~ r~ X ~ :C
m ~ ~ x ~ rr
V ~ ~ C~ V ~ o C~
._ _ . ._ _ . ._
O ~C ~C X !rl 3 !T! X !r
r-l _......................... .. ____
Q ~:C h:~ ~:C 5: T! ~:C ~ ~1
.
~ N~ Vj ~ ~ ~ $ j ~
:
.._
5~ :r: :~ ~~ ~ X ~C
~ _ _ ..___
~ c~ ~ ~ o ~ ~ r~ ~
~ ~ I , 0 ,
.
~ v - ~ ~ :J ~ o
u~u:~ ~ r- ~ ~ u~ ~ ~ :
a) ~ ~ a) ~ a) ~ ~:
l l l o l l o l o l o
u~ ~r ~ ~ ~ ~o ~ l
P~ Q~ ~a) Q~ PJ~ ~ Q~
~ ~ ~ -~ ~ ~ ~ ~ ~-
-- - -- -- -- --
m m m m x m x
.. ... .
~) ~: ~: ~C ~ :C !r
_ . .
r~ ~ ~ ~
...... ...... .__
::c ~: m m ~ m :~
.. .__ ~ . ..__ .._ _
Q ~C m :c m m ~ x
E~ ... .._ .._ .
o~ o V ~ ~o: ~o ~o
: :
: _ .. ___ :.. __ . ~.. _ ~ _
5: ~ ~ x : m m :c
:...... _ : _
U~ U:) I C~ ~ O
. .~ ... __
:
-- 21 --
- ---- ~ l
o o o o ~
l ~ l l- - o - ~ ~ v
a) o ~ s::
. l . I ~n I u~ ~ l o
nu~ Oo~ O l L~ C~
r~ ~r ~ ~~ ~~ ~ u~
~ ~ ~ o~ 8~ ~ ~ ~ l
. o ~ ~ ~~ ~,. ~, .
P~ ~ ~ ~ ~~ a~~ a)R~
. ~ ~ ~ ~ ~ ~ ~
.. _ . .. ....
X~
:~ V V ~:
. . _ _ .. _ __
,.,
::C ~ ~C :C ~ ~
._ .__ . . ...
~:: ~ X ~ ~ ~ ~
._ _ .__ . _
~o, ~ ~ ~C ~ ~C 3~ ~C
~ . _ .. _ . ._
Q 5~ ~C :C ~:C ::C ~: ~C
~:: 5: ~ : ~ P~ ~' ~
. ,
~ r ~ ~D t- 0
a~ ~ ~ o~ o~
- - -
-- 22 --
.._ ~ o ~, V
~ _ ~ ~r co c~ ~
U~ U~ ~ o ~ ~
, U~ l l l l l o
o ~ ~ ~ o ~ o ~,
~C~ ~ ~ ~ ~ ~ ~ l
. ~ ~ ~IS 1:~J ~ P~- ~1 ~1
E~ -- Q.E~ E Ei ~ E~
. .. _ _ . .__ ... _
m ~ ~ ~ ~c
_ . ...__ .._
~~ ~ ~ ~ ~
h:: ~~ C~ ~ ~C
_ _ .._ .
~ ~ .~ ~ r~
:C X ~ X X :~
C> C~ C~ t~ ~ C~
._ ._
o 5: ~ X ~ ~C ~: t~
. ._ ._ . _ . . _
In U~ Lr~n
:C ~ tc~ ~
Q~ ~:C x O O ~O) O ::C
E~
:~ ~ :c ~ x ~
- ~ --- --- --- ---- ---
o ~ ~ ~7 ~r u~
~ o o o o o o
--- --- ~ - - -- -
-- 23 --
~ ~ ~ ~ o l ~J
~ ~ ~ ~ ~ ~: ~
~ ~ Q~ ~ ~ ~
E ___ E E E
m m m m m ~:
_ _ .. _ .
m m ~ m x 5:
y ~) ~ ~) c> m m ~)
. __
:~
m m o ~ 3 ~C ~:: m
o :-1 ~ ~a D:: 3
_ .. _ ~ . _ ~U~
R ~:C m m m m m o
E~ ... _ _
: ~ ~ ~ ~ ~ a~.
m ~m, o . ~/ x ~ ~c
~ l
. . .. _ ~ - . _
: ~ :: ~ :r m ~ x r~ .__:_ .. . __ ~____ ~__
~ ~ ~D O O~ ~ O ~_
`
-- 24 --
~ 3 ~ 2
U U U U U V
o a ~ o o ~D ~1 ~ ~1 V
~ ~ ~ ~ ~ ~, ~, U ~
~ In ~ l ~ ~ ~ CO L/ C~
O ~ ~ ~ I~ ~ > U~
r ~ ~ >1'
Q~ Q~ R~ ~~ ~ X ~ Q~ x
E E E E E E ~ O o? E ~ c) ~,q
_ _ _._ .. _ _ _
m r m ~ m m m
_ . . _ . .
m
~ r~ ~7
m U :C V ~ V :~:
c
ID~ .. _ .. ___ . .___
m c v m m m m
-
c . .
v m ~ m m m m ~r
. .. _ _ . _ . . .
u~ u~ u~ ~ u~
,~ ~ m ~ m
Q 8 o o 8 o m m
~ ~J v ~ v ~
~ ~ ~ 1 ~o ~
~-
m ~ m m ¦ m m
. _ ._ _
~7 ~ In ~ r- Q~ ~
: ~ ~ ~ ~ ~ ._
-- 25 -
o- ~ ~ ~
CO ~ ~1 In ~1 a~
a~ ll I c) ~ ~ I u
I u~ o ~ I o ~ I tn o r~
O 0 4 r-l ~ In 0 ~1
Q,~ ~ u~ ~ o ~D ~'1:1 C)
E~ ~ ~ rl ~ ~ ~ ~
O ,C S~ ~ ~ 0 5:: ~1
. C,) O ~ ~ O V . ~ O
12~ a~ x -1 ~ ~ X ~ Q~ X
. ~ ~ ~ . ~ ~
~ U D~ ~ ~ V ~ ~ ~ ~ V ~q
_ __. .__ _
~C ~
_ ._ ._ .. _ .__
~ ~C ~
._ - .. -- _
~C~
~5 ~ ~ ~)
- .. _ .
C) ~ :C ~::
._ _
~ ~C ~
Q .
E~
c~ ~ ~D~ a
: : . . .:_ ~. :
: ~ 3~ ~::
: ~0 _ _ ._
~ ~ .
-- 26 --
~ 3 ~ 2
1 The respective melting point of the compound numbers
118-122 in Table 1 shows the value of hydrochloric acid
salt of said compounds.
~ext, NMR spectra data of compound No. 100 is sho~n below.
NMR data (60 MHz, CDC13, ~ value)
1.12(3H, t, J=7.0Hz), 1.24(3H, d, J=7.0~z)
1.20~1.70(3H), 2.40(6H, S), 2.0-3.10(11H)
4.35(2H, S)
Cyclohexanedione derivatives represented by the
general formula (I~ and their salts caused no toxic
symptom nor death in mice or rats even after administrated
continually for two weeks at a does of 300 mg/kg/day to
the mice or rats, which reveals the marlcedly low toxicity
of the compound of this invention. For example, LD50
value (acute oral toxicity to male rat) of the compound
No. 55 is more than 1,000 mg/kg.
The compounds represented by the general formula
(I) and their salts are useful as a medicinal agent for
t~eating liver diseases. For example, while it is Icnown
that hepatic disorders can be experimentally produced in
healthy test animals by administering various agents such
as carbon tetrachloride to the animals, as d1sclosed for
example in U.S. Patent Mo. 4,118,506, it has been found
that the compounds represented by the general formula (I)
5 and th~eir salts give a marked e~fect of suppressing the
:
lowering o~ liver functions when administered orally or
parenterally (for example by injection) to test animals
which have hepatic disorders of var1ous pathologic models
- 27 -
~ 3 ~
1 experimentally produced therein. Accordingly, the
compounds represented by the general formula (I) and their
salts are useful as a medicinal agent for curing or
preventing hepatic disorders in men and animals. Thus, it
can be used as a curative for acute or chronic hepatic
disorders of men and animals produced by various causes,
for example, jecure adiposum, alcoholic hepatitis,
hepatitis, toxic liver disorders, cardic cirrhosls,
cholestatic liver disorder, or hepatocirrhosis which is
the final state of these diseases.
Accordingly, the term "a pharmaceutical
composition for treating hepatic disorders" as used in
this lnvention means a medicinal agent for curing and/or
preventing various disorders in liver by utilizing the
pharmacological actions manifested in liver as mentioned
above including the action of activating liver functions
and the action of preventing and curing hepatic disorders.
The compound represented by the general formula
(I) or its salt can be used as a medicinal agent for
treating hepatic disorders in the form as it is; it may
also be formulated, according to conventional pharma-
ceutical procedures, as a mixture thereof with a pharma-
ceutically acceptable diIuents and/or other pharmacologi-
cally active substances. Further, it may be formulated
into a dose unit form. Examples of the form which the
compound can take as a medicinal agent include: powders,
granules, tablets, dragée, capsules, pills, suspensions,
solutions, liquid, emulsions, ampulesr injections, and
- 28 -
1 3 ~
1 isotonic solutionsO
The modes of preparing the compound of tnis
invention into a pharmaceutical composition include one
wherein the compound represented by the general rormula
(I~ or its salt is contained as a mixture thereof with one
or more pharmaceutically acceptable diluents.
The "diluent" referred to herein means a
material other than the compound represented by the
general forJnula (I) and their salts. It may be in the
form of solid, semisolid, liquid, or ingestible capsules.
Examples of the diluents include excipients, fillers,
binders, moistening agents, disintegrators, surfactants,
lubricants, dispersants, buffering agents, flavoring
agents, odor correctives, coloring agents, flavors,
preservatives, solubilizing aids, solvents, coating
agents, and sugar-coating agents. However, they are not
limited to these. Further, they may be used as a mixture
of one or rnore kinds thereof. Sometimes, these pharma-
ceutically acceptable diluents are used as a mixture
thereof with other pharmacologicalLy active substances.
The pharmaceutical composition according to this
invention may be prepared by any method known in the art.
For instance, the active ingredie~nt is mixed wi~h a
diluent and made up, for example, into granules. The
resulting composition is then formed, for example, into
tablets. Preparations to be administered parenterally
should be made aseptic. Further, as occasion demands,
they should be made isotonic with blood.
- 29 -
1 In this invention, since the compound represent-
ed by the genera] formula (I) and their salts mentioned
above can be itself make a medicinal agent for treating
liver diseases, the active ingredient is generally
S contained in the composition in a proportion of 0.01 to
100% by weight.
~ hen the compound is made into a preparation in
the form of dose unit, the individual parts of the prepa-
ration which form said preparation may be either in the
same shape or in shapes different from each other. For
example, the following shapes are often adopted: tablets,
granules, pills, powders, dragée, capsules, ampules, and
the lil<e.
The medicinal agent for treating hepatic
disorders according to this invention can be applied to
men and animals for the purpose of preventing and treating
hepatic disorders therein, in a manner conventional in the
art. It is administered orally or parenterally. Oral
administration referred to herein includes sublingual
administration. Parenteral administration includes herein
administrations conducted by means of injections (includ-
ing, for example, subcutaneous, intramuscular or
intravenous injection and instillation).
The dose of the medicinal~agent of this
invention varies depending upon various factors including
whether it is applied to animals or men, difference in
susceptibility, age, sex, body weight, the method, time,
and interval of administration, the condition of diseases,
~ 30 -
~ 3 ~ 2
1 physical condition, the properties of the pharmaceutical
composition, the kind of the preparation, and the kind OL
the active ingredient.
Accordingly, sometimes those doses may be
sufficient which are lower than the minimum of the dose
range shown below, whereas sometimes it becomes necessary
to administer an amount exceeding the upper limit of the
dose shown below.
When the pharmaceutical composition is to be
administered in a large amount, it is preferably
administered divided in several doses per day.
In order to obtain effective results in
application to animals, the agent is advantageously
administered at a dose, in terms of the active ingredient,
15 in the range of 0.1 to 500 mg, preferably 0.1 to 30 mg,
per 1 kg of body weight per day in oral adminsitration,
and 0.01 to 250 mg, preferably 0.1 to 25 mg, per 1 kg of
body weight per day in the case of parenteral
administration.
The doses necessary for obtaining effective
results in application to men are, judged from the effec-
tive doses in animals and in consideration of difference
ln susceptibility and safety, advantageously selected, for
example, from the following dose range. In oral admini-
25 stration the dose is Ool to 200 mg, preferably 0.5 to 50
mg, per kg o~ body weight per day, and in parenteral
administration it is 0.01 to 100 mg, preferably 0.1 to 25
mg, per kg of body weight per day.
- 31 -
~3~^s
1 Example
This invention will be described in detail belo~
with reference to Example.s, but it is in no way limited
thereto.
First, synthesis examples of this invention are
shown below.
Example 1 2-~2,5-dimethyl-2,5~diaza-7,9~dithiabicyclo-
(4,3,0)-nonane-8-ylidene]-1,3-cyclohexanedione.
~compound No. 1)
To a suspension of 5.75 g (0.02 mole) of
glyoxal-sodium bisulfite in 20 ml of water was added drop-
wise 1.92 g`(0.02 mole) of N,N'-dimethylethylenediamine
with ice-cooling and the mixture was stirred until it
became a homogeneous solution. Then to this solution was
added with ice-cooling the dithiolate solution prepared in
the manner shown below: To a mixture of 2.24 g (0.02
mole) of cyclohexanedione and 1.6 g (0.021 mole) of carbon
disulfide in 15 ml of dimethylsulfoxide was added with
ice-cooling 2.8 g (0.05 mole) of powdered potassium
hydroxide and the mixture was stirred for 1 hour.
The reaction mixture was stirredl for addition-
al 30 minutes and the crystalline precipitated was
collected by filtration. This crystalline was washed with
water, isopropylalcohol and~hexane, and then recrystalized
from isopropylalcohol to give 3.0 g of the desired product.
Yield 50~; m.p. 158-159C.
- 32 -
1 Example 2 2-[2-methyl-2,5-diaza-7,9-dit'niabicyclo-
(4,3,0)-nonane-8-ylidene]-5,5-dimethyl-1,3-
cyclohexanedione. (compound No. 5)
To a suspension of 5.68 g (0.02 mole) of
glyoxal-sodium bisulfite in 30 ml of water was added drop-
wise with ice-cooling 1.5 g 10.02 mole) of N-methyl-
ethylenediamine. Then to this solution was added dropwise
with ice-cooling the dithiolate solution prepared in the
manner shown below; To mixture of 2.8 g (0.02 mole) of
dimedone and 1.6 g (0.021 mole) of carbon disulfide in 20
ml or dimethylsulfoxide was added at room temperature 2.7
g (0.044 mole) of powdered potassium hydroxide and the
mixture was stirred for 1 hour.
The reaction mixture was stirred for additional
one hour at room temperature. The crystalline precipitat-
ed was collected by filtration and washed with water and
hexane and recrystalized from chloroform-ether to give 1.5
g of the desired product.
Yield 24%; m.p. 156-157C.
0 Example 3 2-[2,5-dimethyl-2,5-diaza-7,9-dithiabicyclo-
(4,3,0)-nonane-8-ylidene]-5-phenyl-1,3-cyclo-
hexanedione. (compound No. 26~
: : To a suspension of 3.4 g (0.01 mole) of glyoxal-
sodium bisulfite in 20 ml of water was added drop~ise at
0C N,N'-dimethylethylenediamine and the mixture was
stirred for 1 hour.
Then to this solution was added at 0C the
- 33 -
J ~
1 dithiolate solution prepared in the manner sho~7n below; To
a solution of 1.9 g (0.01 mole) of 5-phenyl-1,3-cyclo-
hexanedione and 0.9 g (0.012 mole) of carbon disulfide in
10 ml of dimethylsulfoxide was added at 10C l.S g of
powdered potassium hydroxide and stirred for 1 hour.
After the completion of addition, the mixture
was stirred for 1 hour. The solid precipitated was
collected by filtration and washed with water and then
recrystalized ethyl acetate-hexane to give 2.0 g of
desired product.
Yield 53%; m.p. 149-153C.
Example 4 2-[2,5-di-n-butyl-2,5-diaza-7,9-dithiabicyclo-
(4,3,0)-nonane-8-ylidene]-5-phenyl-1,3-cyclo-
hexanedione. (Compound No. 29)
The mixture of 3.4 g (0.01 mole) of glyoxal-
sodium bisulfite and 3.0 g (0.018 mole) of N,N'-di-n-
butylethylenediamine in 30 ml of water was stirred for 1
hour at 0C. Then to this solution was added dropwise at
0C the dithiolate solution prepared by the reaction of
1.9 g (0.01 mole) of 5-phenylcyclohexane-1,3-dione with
0.9 g (0.012 mole) of carbon disulfide in 10 ml of
dimethylsulfoxide in the presence of 1.5 g (0.027 mole) of
powdered potassium hydroxide.
The reaction m1xture was st1rred for 2 hours.
Then 50 ml of water was added to the reaction solution.
The crystalline precipitated was collected by filtration,
washed with water and dried up and then recrystalized from
- 3~ -
2 2
1 ethyl acetate-n-hexane to give 1.7 g of the desired
product.
Yield 37~; m.p. 130-132C.
Example 5 2-[2,5-diaza-7,9-dithiabicyclo-(4,3,0)-nonane-
8-ylidene]-5-(4-methoxyphenyl)-1,3-cyclo-
hexanedione. (Compound No. 53)
To a suspension of 5.68 g (0.02 mole) of
glyoxal-sodium bisulfite in 30 ml of water was added drop-
wise at room temperature 1.44 g (0.024 mole) of ethylene-
diamine and the mixture was stirred until it became a
homogeneous solution.
Then to this solution was added with ice-cooling
the dithiolate solution prepared in the manner shown
below; To a solution of 4.09 g (0.02 mole) of 5-(4-
methoxyphenyl)-1,3-cyclohexanedione and 1.6 g (0.021 mole)
of carbon disulfide in 20 ml of dimethylsulfoxide was
added 2~8 g (0.05 mole) of powdered potassium hydroxide,
and the mixture was stirred for one hour.
The reaction mixture was stirred for additional
30 minutes at 0C. The crystalline precipitated was
collected by filtration, washed with water, and~dried and
recrystalized from chloroform-ether to give 1.45 g of the
desired product.
Yield 2~%, m.p 160-162r.
- 35 -
~ 3 ~ 2
1 Example 6 2-[2,5-dimethyl-2,5-diaza-7,9-dithiabicyclo-
(4,3,0)-nonane-8-ylidene]-5-(2-thienyl)-1,3-cyclo
hexanedione. (cornpound No. 82)
To a suspension of 2.84 g (0.01 mole) of
glyoxal-sodium bisulEite in 20 ml of water was added
dropwise at room temperature 1.05 g (0.01 mole) of N,N'-
dimethylethylenediamine and the mixture was stirred until
it became a homogeneous solution. Then to this solution
was added with ice-cooling the dithiolate solution prepar-
ed in the manner mentioned below; To a solution of 1.94 g(0.01 mole) of 5-thienyl-1,3-cyclohexanedione and 0.8 g
(0 01 mole) of carbon disulfide in 10 ml of dimethylform-
amide was added with ice-cooling 1.4 g (0.025 mole) of
powdered potassium hydroxide, and the mixture was stirred
for one hour.
The reaction mixture was stirred for additional
30 minutes. The crystalline precipitated was collected by
filtration, washed with water, dried and then
recrystalized from chloroform-ether to give 1.07 g of the
desired product.
Yield 28%; m.p. 165-166C.
Example 7 2-[1,2,5-trimethyl-2,5-diaza-7,9~dithiabicyclo-
( 4 r 3~o)-nonane-8-ylidenei-5l5-dimetllyl-l~3-
:,:
~ cyclohexanedione. (compound ~o. 12)
~ To a solution of 4.58 g (0.04 mole) of sodium
bisulrlte was added 3.96 g (0.02 mole) of 40% methyl-
glyoxal and the mixture was stlrred for 1 hour at room
- 36 -
~ 3 ~
1 temperature.
To this solution was added dropwise with ice-
cooling 1.94 g (0.022 mole) of N,N'-dimethylethylene-
; diamine and tne mixture was stirred for 1 hour. Then to
this solution was added with ice-cooling the dithiolate
solution prepared in the manner shown below; To a mixture
of 2.8 g (0.02 mole) of dimedone and 1.6 g (0.02 mole) of
carbon disulfide in 15 ml of dimethylsulfoxide was added
2.7 g (0.044 mole) of powdered potassium hydroxide, and
the mixture was stirred for one hour.
The reaction solution was stirred for additional
20 minutes and the solid precipitated was collected by
filtration. The solid was dissolved in chloroform, and
the solution was washed with water and dried over
anhydrous sodium sulfate. After chIoroform was evaporated
in vacuo, the residue was recrystalized from chloroform-
ether to give 0.55 g of the desired product.
Yield 8%; m.p. 161-162C.
Example 8 2-[3-methyl-2,5-diaza-7,9-dithiabicyclo-(4,3,0)-
nonane-8-ylidene]-5,5-dimethyl-1,3-cyclohexane-
dione. (compound No. 14)
To a suspension of 5.68 g (0.02 mole) o~
glyoxal-sodium bisul~ite in 20 ml of water was added
- dropwlse with ice-cooling 1.49 g (0.02 mole) of
; 25 1,2-propanediamine and the mixture was stirred until it
hecame a homogeneous solution. Then to this solution was
added with ice-cooling the dithiolate solution prepared in
~ 37 -
~ 3 ~ 2
1 the manner shown below; To a solution of 2.8 g (0.02 mole)
of dimedone and 1.6 g (0.02 mole) of carbon disulfide in
20 ml of dimethylsulfoxide was added with ice-cooling 2.7
g (0.044 mole) of powdered potassium hydroxide, and the
mixture was stirred for one hour.
The reaction mixture was extracted with ethyl
acetate, washed three times with ice-water and dried over
anhydrous magnesium sulfate. The solvent was evaporated
and the residue was recrystalized from ethyl
10~ acetate-hexane to give 0.8 g of the desired product.
Yield 13%; m.p. 150.0C.
:
Example 9 2-~2,5-acetyl-3-methyl-2,5 diaza-7,9-dithiabi-
cyclo-(4,3,0)-nonane-8-ylidene]-5,5-dimethyl-
1,3-cyclohexanedione. (compound No. 15)
To a solution of 0.4 g (0.0013 mole) of 2-[3-
methyl-2,5-diaza-7,9-dithiabicyclo-(4,3,0)-nonane-8-
ylidene]-5,5-dimethyl-1,3-cyclohexanedione in 5 ml of
pyridine was added dropwise with ice-cooling 0.79 g (0.01
mole) of acetyl chloride and the mixture was stirred for 1
hour.
The reaction solution was~extracted with ethyl
~acetate, washed with 2 N hydrochloric acid and water, and
then~dried over magnesium~sulfate. The solvent was
removed and the residue was washed with ether and
recrystalized from ethyl acetate-hexane to give 0 35 g of
the;desired product.
Yield 68%; m.p. 146-150C.
:
- 38 -
:
: :: :
~ 3 ~ d 2
1 Example 10 2-[2-metllyl-2,5-diaza-7,9-dithiabicyclo-
(4,3,0)-nonane-8-ylidene]-5,5-dimethyl-1,3-
cyclohexanedione dihydrochloride salt.
(compound 118)
Into a solution of l.n g (0.0032 mole) of 2-[2-
methyl-2,5-diaza-7,9-dithiabicyclo-(4,3,0)-nonane-~-
ylidene] 5,5-dimethyl-1,3-cyclohexanedione in 20 ml of
acetone was bubbled dry hydrogen chloride with ice-
cooling. After the crystalline was precipitated, to this
solution was added 20 ml of ether to crystalize further
the product dissolved in acetone.
The crystalline was collected by filtration and
was washed with ether to give 1.1 g of the desired product.
Yield 90~; m.p. 162-164C.
Now, Examples regarding pharmaceutical composi-
tions according to this invention will be described
below. In the Examples, "part" is all part by weight. It
is needless to say that the kinds and the proportions of
the compounding ingredients used in the composition
according to this invention can be changed variously
without being restricted by these Examples.
Example 11
Compound No. 4 ........................... 10 parts
~Heavy magnesium oxide ................... 10 parts
Lactone .................................. 80 parts
The above ingredients were mixed uniformly and
made into a medicinal preparation in the form of powders or
- 39 -
~ 3 ~ 2
1 fine granules.
Example 12
Compound No. 43 .......................... 10 parts
Synthetic aluminum silicate .............. 10 parts
Calcium hydrogen phosphate ................ 5 parts
Lactose .................................. 75 parts
The above ingredients were used to be rnade up
into powders in a similar manner to that in Example 11.
Example 13
Compound No. 53 .......................... 50 parts
Starch ................................... 10 parts
Lactose .................................. 15 parts
Crystalline cellulose .................... 20 parts
Polyvinyl alcohol ......................... 5 parts
~ater ................................... 30 parts
The above ingredients were uniformly mixed,
kneaded, then crushed, granulated, dried and sieved to
obtain granules.
Example 14
A mixture of 99 parts of the granules obtained
: in Example 13 and 1 parts of calcium stearate was
compression-formed into tablets of 10 mm diameter.
Example 15
Compound No. 79 .......................... 78 parts
- 40 -
.
~ 3 ~
1 Polyvinyl alcohol ....................... .2 parts
Lactose ..................... ........... 20 parts
Water ~ 30 parts
T'ne above ingredients were made up into granules
in the same manner as in Example 13. Ten parts o~
crystalline cellulose was added to 90 parts of the
granules obtained above, and the mixture was compression-
molded to obtain tablets of 8 mm diameter. The tablets
may be further made up into dra~ée by using, in appro-
priate amounts, a mixed suspension of syrup, gel~tin andprecipitated calcium carbonate, and a coloring agent.
Example 16
Compound No. 95 ......................... Ø5 part
Nonionic surfactant ..................... .2.5 parts
Physiological saline ........ ........... 97 parts
The above ingredients were mixed with warming,
then sterilized to obtain injections.
Example 17
The powders obtained in Example 11 were filled
into capsule containers available on the market to obtain
capsules.
The effect of the compound of the present inven-
tion will be illustrated by the following Test Example.
- 41 -
l ~est Example l
Effect of suppressing hepatic disorder caused by
carbon tetrachloride.
Test Method
The test compound was dissolved or suspended in
olive oil, and orally administered at a dose of 30 mg/kg
to mice t6 weeks of age, dd-strain, d), Six hours there-
after, carbon tetrachloride was orally administered in a
proportion of 0.05 ml/kg. The animals were sacriEiced 24
hours after the administration of carbon tetrachloride,
and the extent of liver injury was examined.
On the other hand, blood was collected from the
animal at the time of the sacrifice, and centriEuged to
obtain plasma. The plasma glutamic pyruvic transminase
(GPT) activity was determined according ot the method of
Reitman-Frankel. The activity was expressed in terms of
Karmen Units (R.U.). The conditions of the liver were
expressed in terms of liver injury index as follows.
Liver injury index Condition of liver
20 0 Healthy liver
2 Slightly affected
4 Evidently observed injury
,
6 ; Serious injury
Mice were used in groups of rive and the results
of test were represent~ed by the mean value. When the GPT
activity was 2,000 onits or higher, or further determina-
tion was made, the activity was calculated as 2,000
- 42 -
1 3 ~
1 ~nits for reasons of convenience.
Trle results obtained are shown in Table 2.
:
:
- . :
:
- 43 _
,
z~ ~ ~
Table 2 Effect of carbon tetrachloride
on liver injury
No. of compound Liver
of this invention ln~uryP-GPT (K.U.)
.. __
Administration of carbon 6.02000
tetrachloride alone
No treatment 0 12
. .___
1 0.2 14
.. _ _. _ .. _
2 0.4 15
: 4 0 11
. . ._ . _ . _ ._ ..
6 0 19
. _ . _ ._
7 0 16
.__ __ .
0 41
. _ _ . . . _
11 0.5 - 16
. ~ ..
12 0.4 33
14 0.4 18
_ _. .. __
18 1.2 22
21 0.1 23
0.8 15
_
26 0 12
... ~ _.
~ 28 0.2 39
~ ~ .. _
32 0.3 22
. ._ .. __
~ 36 1.5 268
- - . .. _ . .__
: ~: 41 0.4 213
. _ _ _
42 0 14
- cont'd -
- 44 -
1 3 ~ 2
Table 2 ( cont ' d )
, ._ _ . . __
43 0.2 24
... _ . _ .
47 0 24
.. _ _ .. _ .. ._
48 0.3 18
_ _~ .___
51 0 81
.. _ . ___ . ...... _
52 0.1 14
_ . _
53 0.1 13
. .. ~ _ _ ._
0.3 107
_ : ._ _..__ .. ._
56 2.5 50
. . . _ _ _ .. _ __ _ _ .. _ _
57 0.2 142
. . _. _ ._
59 0.1 - 16
0.1 26
. . . __ ._
61 1.5 494
. __ .. ___
63 0 15
.. _
1.6 347
. . ._ ~ . . _
78 0.2 18
. . . . __ . _
79 0 30
._ . . . . .
1.0 405
._ ._ .__ ._
82 0.3 16
_._ .. __ .. __ .. _
0 15
._
92 - 0.4 15
._ . __ .. _ _ ..
102 1.5 319
_ _ -- __ _ . _
103 _ 18
104 1.7 345
. . _ __ . _ ... . . .
: 105 0 _ 15
106 1.2 242
- cont ' d -
- 45 -
3 ?J 2
Table 2 ( cont ' d )
_ __ . .
108 1 . 0 24
. __ __ _ . _ . _
109 0 17
._ _ . _ , .
111 ,___ 1 0 . .. _
114 2 . 2 26
. ... __ ..... ._ .. __ .. ._
115 0 39
. _ . . __ .....
118 0 32
.. __ ._ _._ . . . ._
119 0 15
.... _ _ .. __ _ .. __ _
121 0 20
_ _ . __ ~ __
1 22 . . 231
-- 46 --