Note: Descriptions are shown in the official language in which they were submitted.
- 3
ELEC T~< ICAL G ENERATI NG PLANT
This invention relates to an electrical generating
plant which comprises a fuel cell which requires a supply
of gaseous hydrogen and a supply of gaseous oxygen to
produce an electrical output.
The present invention has been developed
primarily r though not exclusively, with a view to provide
an electrical generating plant which is particularly
suitable for use in a submarine. For the purposes of the
present specification, there will be described the
application of an electrical generating plant according
to the invention for use in a submarine, though it should
be understood that the invention has general application
for use in other environments in which supplies oE
air/oxygen may be limited or not available, including use
in space vehicles, such as orbital craft and artificial
satellites, or for emergency use in hazardous
environments, such as rescues in mines.
It is common practice for ocean-going submarines,
which are not powered by a nuclear reactor, to use a
diesel engine for propulsion when on the surface or
snorting, and batteries when fully submerged. The main
problem with diesel/electric submarines is that battery
capacity severely limits the fully submerged endurance of
the vessel, and there is therefore a requirement for a
power supply which is capable of extending the submerged
operational capability for a submarine within the
physical and operational restraints imposed by a
submarine. Such restraints include limits on the space
and weight of the power conversion plant, volumetric
requirements of fuel(s) and buoyancy problems created by
the increasing ullage as the fuel(s) is consumed.
One possible power system, known as a Re-Cycle
Diesel System uses a conventional diesel engine
aspirating oxygen diluted with steam and/or e~haust gas
-` ~31126~
in a self-contained closed cycle. While such a system
extends the endurance of a submarine based upon batteries
alone, it suffers from the disadvantages of total weight,
volume of fuel and oxidant required, disposal of
5 combustion products and noise.
Though other variations of diesel-engined energy
conversion systems are known, none offer significant
advantages for this application. The efficiency of
energy conversion of a diesel/generator is 20-30%.
However, fuel cells, which directly convert chemical to
electrical energy, have a proven energy conversion
efficiency of 50-60%. Thus, if a reliable fuel cell
capable of producing the required power output could be
combined with a satisfactory means of storage for the
reactants, a suitable power producing system for a
submerged submarine could be provided.
Various types of fuel cell have been the subject
of considerable development effort and now offer high
specific performance with demonstrated reliability. A
first type of cell combines high purity gaseous oxygen
and hyarogen to form water with the production of
substantial quantities of electrical power. Such a fuel
cell can be started up easily, power output may be varied
automatically and the operation of the cell is virtually
silent. The problem is the provision of high purity
gaseous oxygen and hydrogen. Oxygen and hydroyen are
both permanent gases and thus cannot be liquified at
normal temperatures by the application of pressure. The
gases may thus be stored either under high pressure in
cylinders, which invokes a penalty for the weigh~ of the
containers an~ the volume of gases that could be stored,
- ox as cryogenic liquids, which may generate space
problems, buoyancy problems due to changing ullage and
problems associated with shock loading. Hydrogen may
also be stored in solid form in chemical combination as a
131 1264
metal hydride, but this also poses weight problems.
A second type of fuel cell can utilise oxygen
~rom air and the use of such a cell is equally
advantageous. This type of cell could be used when a
submarine was on the surface or snortlng using normal
air. When the vessel is submerged, this type of cell
could be operated using the air inside the submarine,
provided that the oxygen content in this air is
maintained.
SUMMARY OF TH~ INVENTION
According to an aspect of the invention there
is provided an electrical generating plant having dual
modes of operation and comprising:
a ~uel cell which requires a supply of gaseous
hydrogen and a supply of gaseous oxygen in order to
generate an electrical output;
first supply means for supplying to the plant
a hydrogen-containing compound which is liquid at NTP
and which can undergo an endothermic reaction to
liberate gaseous hydrogen;
second supply means selectively operable for
supplying, to the plant, liquid hydrogen peroxide in a
first mode of operation and air in a second mode of
operati.on;
~5 a reformer, connected to the first supply
means, in which said compound can undergo said
endothermic reaction and liberate gaseous hydrogen;
a decomposer which is selectively connectible
to said second supply means, in the first mode of
operation, and which is arranged to decompose the
hydrogen peroxide exothermically so as to liberate
gaseous oxygen;
means ~or transmitting to the hydrogen-
containing compound all or part of the heat which is
given-off in the decomposer in the first mode of
,',. . .
1 3 1 1 264
operation, so as to maintain the endothermic reaction in
the reformer;
means for supplying heat to the hydrogen-
containing compound, in the second mode of operation, so
as to maintain the endothermic reaction in the reformer:
means for supplying the gaseous hydrogen
given-off in the reformer to the fuel cell; and
means for supplying to the fuel cell the
gaseous oxygen which i~ given-off in the decomposer in
the first mode of operation, and means for supplying the
air to the fuel cell in the second mode of operation,
whereby an electrical output is obtainable from the fuel
cell in the first or the second modes of operation.
The hydrogen peroxide is decomposed
exothermically in the decomposer, and preferably means
is provided for supplying heat from the decomposer to
the reformer, so as to maintain the endothermic reaction
in the reformer.
It should be particularly noted that the heat
available from the decomposition of hydrogen peroxide in
; the decompos r is o~ a high quality i.e. concentrated
high temperature heat, which is especially suitable for
use in promoting and maintaining the reforming reaction
of tha hydrogen-containing compound in the reformer.
This provides for efficient operation of the
electrical generating plant in the first mode of
operation, in that what would otherwise be waste heat
given-off in the decomposer, is used as a heat source
for maintaining the endothermic reaction in the
reformer.
Another aspect of this invention is as
follows:
A submarine electrical generating plant for
the electrical driving of the submarine drive train and
having dual modes of operation; said plant comprising:
~.
. ,
`` 131 12~
6a
a plurality of fuel cells which requires a
supply of gaseous hydrogen and a supply of gaseous
oxygen in order to generate an electrical output;
first supply means for supplying methanol to
the plant;
second supply means selectively operable for
supplying, to the plant, liquid hydrolg~n peroxide in a
first mode of operation and air in a second mode of
operation;
a reformer, connected to the first supply
mean~, in which the methanol can undergo an endoth~rmic
reforming reaction so as to liberate gaseous hydrogen;
a decomposer which is selectively connectible
to said second supply means, in the first mode of
operation, and which is arranged to decompose the
hydrogen peroxide exothermically so as to liberate
gaseous oxygen;
means for transmitting to the methanol prior
to and/or during its reception by the reformer, the heat
which is given-off in the decomposer in the first mode
of operation, so as to maintain the endothermic reaction
in the reformer;
means for supplying heat to the methanol in
the second mode of operation, prior to and/or during its
reception by the reformer, 50 as to maintain the
endothermic reaction in the reformer;
means for supplying the gaseous hydrogen
given~off in the reformer to the fuel cells; and
means for supplying to the fuel cell the
gaseous oxygen which is given-o~f in the decomposer in
the first mode of operation, and means for supplying the
air to the fuel c811s in the second mode of operation,
; whereby an eLectrical output is obtainable from the fuel
cells in the first or the second modes of operation.
When an electrical generating plant according
to the invention is installed in a submarine, it may be
. ~j
6 ~
6b
used to supply the entire motive power for the submarine,
both underwater, and also in surface/snorting operation.
In underwater operation, hydrogen peroxide may be supplied
to the decomposer (in the first mode of operation) and the
heat given-off in the decomposer is supplied to the
hydrogen-containing compound in order to maintain the
endothermic rPaction. Alternatively, the heating
~ ~ ~ 1 26i~
requirements Eor the reforming reaction may be met wholly
or in part by other sources of waste heat, or by part of
the electrical output from the fuel cell.
However, in the surface/snorting operation of the
5 submarine, it is no longer necessary to use hydrogen
peroxide as a source of oxygen for the fuel cell, which
can be derived from the atmosphere. This then enables
the supply of hydroaen peroxide to be conserved.
However, the lack of availability of heat from the
10 exothermic reaction of hydrogen peroxide in the
decomposer (to maintain the endothermic reaction in the
re~ormer) has to be met from other sources. This may be
derived, for example, from burning a portion of the
hydrogen-containing compound, which is preferably
15 methanol, or any suitable chemical ~ith an adequate heat
of combustion e.g. fuel oil.
Alternative]y, a portion of the electrical power
output produced by the fuel cell may be used to provide
the heating requirements of the reformer. In addition,
20 or alternatively, other sources of waste heat, occurring
elsewhere-~in the plant, may, where practicable, be used
in preference to burning the source of hydrogen and/or
the use of electri~al power. It is further possible that
all three sources of heat may be used, i.e. waste process
25 heat, chemical and electrical energy could be used either
singly, in any combination of two, or all three together
- to provide the process heat requirements in the most
practical and/or economic way.
The preferred hydrogen-containing compound to be
30 used in the electrical generating plant is methanol,
though other alcohols, or hydrocarbons may be used,
provided that they are liquid at ~TP and can undergo an
endothermic reforming reaction to liberate gaseous
hydrogen.
When, as is preferred, methanol is used as the
` : :
~ 3 1 1 264
--8--
hydrogen-containing compound~ this is particularly
suitable havi~g a specific gravity of about 0.8 at NTP
and a boiling point of 64.7C. The reforming reaction
requires the addition of pure water to the methanol, and
5 advantageously this is derived from the pure water
produced during the operation of the fuel cell.
The hydrogen peroxide, which is used as a source
of oxygen, may be catalytically decomposed into oxygen
and water. Hydrogen peroxide is a highly reactive
10 compound and is usually supplied in aqueous solution.
Certain concentrations of hydrogen peroxide may be used,
but 85% is preferred~ Such a concentration would have a
specific gravity of about 1.36 and be a liquid at NTP.
The exothermic decomposition of the hydrogen
15 peroxide reaction may be used to provide some or all of
the necessary heat input for the endothermic methanol
reforming reaction in the first mode of operation,
thereby, as indicated above, maximising the thermal
economy of the operation of the generating plant and
reducing/eliminating the requirement to use an
alternati~e source of heat, such as electricity derived
from the fuel cell.
Advantageously, the exothermic decomposition and
the endothermic reforming reactions are carried out in
close proximity in order to maximise heat transfer. In a
possible arrangement, both reactions could take place in
a single vessel, separated only by a highly thermally
conductive member, though other high efficiency means of
heat transfer may be employed as desired.
It is also preferable to arrange that the hot
product streams leaving the reformer/decomposition
vessel(s), plus other sources of heat e.g. the fuel cell,
catalytic oxidisers and the like should be used to
preheat the reactants i.e. methanol and water, prior to
reaching the reforming reaction stage.
131 126~
g
The invention therefore provides an electrical
generating plant which utilises a fuel cell, which is
capable of high power output and high efficiency of
energy conversion, and yet without requiring cryogenic or
5 high pressure storage of hydrogen and oxygen sources. By
providing, as is preferred, for the thermal requirements
of the reforming reaction to be met in the first mode of
operation, at least substantially , by the heat produced
by the decomposition reaction, together with other
10 thermal economies, virtually the entire output of the
fuel cell is available for use, in tllat the plant
operates in substantially thermally self-sufficient
manner in the first mode of operation.
BRIEF DESCRIPTION OF THE DRAWINGS
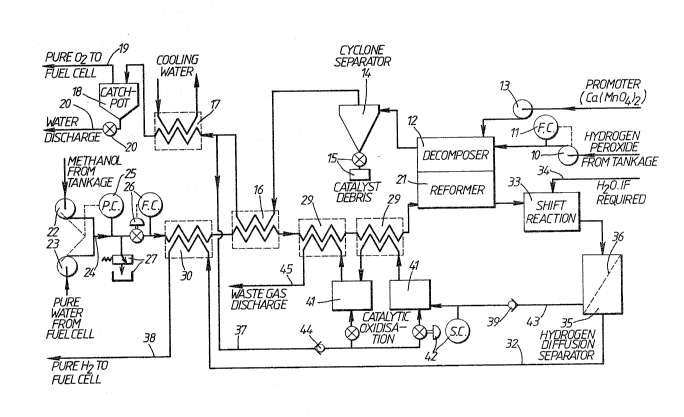
Figure 1 is a diagrammatic illustration of an
electrical generating plant for use in a submarine,
including apparatus for producing oxygen and hydrogen
gases for supply to a fuel cell;
Figure 2 is a diagrammatic illustration of the
20 combination of oxygen and hydrogen in the fuel cell to
produce electrical power;
Figure 3 is a diagrammatic illustration of
catalytic oxidation of purges of oxygen and hydrogen
gases;
Figure 4 is an illustration, similar to Figure 1,
of an embodiment of electrical generating plant according
to the invention;
Figure 5 is an overall heat and mass balance
diagram for the simultaneous production of oxygen and
30 hydrogen in the plant; and
Figure 6 is an overall heat and mass balance
diagram, relevant to the plant o~ Figure 4, for the
production of hydrogen using an alternative heat source
to maintain the reaction.
1 31 1 264
--10--
DESCRIPTION OF PREFERRED EMBODIMEr;lT
The fuel cells which are employed in an electrical
generating plant according to the invention are of the
type which require the provision of pure supplies of
gaseous hydrogen and oxygen, or gaseous hydrogen and air
(as a source of gaseous oxygen), in order to generate an
electrical output.
Fuel cells of the above type which may be used
include the following:
Solid Polymer Electrolyte
ir/Hydrogen or Oxygen/Hydrogen
Low temperature 6 - 105 C
Phosphoric Acid Electrolyte
Air/Hydrogen or Oxygen/Hydrogen
15 Operating temperature 150 - 190C
Alkaline (Potassium Hydroxide) Electrolyte
Oxygen/Hydrogen ~can use air if Carbon Dioxide is first
scrubbed out)
Low temperature 60 - 90 C
The chemical reactions will now be described which
take place, in order to generate supplies of gaseous
hydrogen and oxygen to the fuel cell, and to convert the
chemical energy in the fuel cell into electrical energy.
A gaseous supply of pure oxygen is derived from
hydrogen peroxide which decomposes according to the
equation:
2 ~~> 2H2O ~ 2 + heat (l)
Hydrogen peroxide is an unstable chemical and
requires only a small means of activation to initiate
decomposition. Thus, for safety reasons, it is usually
available as an aqueous solution. It is found that an
85% solution vf hydrogen peroxide provides the required
levels of decomposition heat while reducing the handling
and storage risks to acceptable levels. A catalyst is
used to ensure that the decomposition reaction (l) occurs
1 31 1 264
in a reaction vessel 12 ~described below with reference
to Figure 13 and is as nearly complete as possible.
A gaseous suppLy of pure hydrogen is derived from
a reforming reaction of any suitable alcohol or
5 hydrocarbon, provided that such supply is liquid at NTP
and can undergo an endothermic reforming reaction to
liberate gaseous hydrogen. The preferred
hydrogen-containing compound is a reformable alcohol, and
especially methanol in vapour form which is reformed with
10 steam in the presence of a catalyst according to the
overall reaction equation:
CH30H ~ H20 + heat --> C02 + 3H2 ~2)
The methanol reaction is, in factr a two part
reaction, which comprises decomposition according to the
15 equation:
CH30H --> C0 ~ 2H2 (2A)
followed by a shift reaction according to the equation:
C0 + H20 --> C02 + H2 (3)
The overall reaction is strongly endothermic and
20 thus requires considerable heat to achieve an acceptable
rate of hydrogen production. To facilitate the heat
exchange between the decomposition and reforming
reactions, it is advantageous for the two reactions to
occur in very close proximity, preferably in the same
vessel, and separated only by a heat transfer member, as
described in more detail below. A concentric vessel with
one reaction occurring in the bore and the other in the
annulus and a highly conductive annular member separating
the two is ideal. Another suitable form of vessel for
this purpose could be similar to a tube and shell heat
exchanger with, for example, the decomposition reaction
occurring outside the tubes and the reforming reaction
inside the tubes. The particular design of the vessel
would also depend on the types of catalysts to be used
and whether the passage of the two flow currents is to be
,
131 1264
-12-
co-current or counter--current. Alternatively, the
reactions could be performed in separate vessels with a
highly efficient means of heat transfer between the two
vessels, for example heat pipes and the like, ~r by means
5 of electrical heating.
Once hydrogen gas has been pro~luced, it must be
purified before it can be passed to the fuel cell. The
means of purification will be described hereinafter, but
the reason for this requirement is that the active
10 elements in a fuel cell can easily be "poisoned" by the
presence of impurities, thus reducing the operational
- efficiency of the cell. Hydrogen and oxygen react in a
fuel cell to produce pure water, heat and electrical
energy according to the equation:
2H2 + 2 ~~> 2H2o + heat + electrical energy (4)
The pure water produced may be used as diluent for
the methanol prior to reforming, and any excess water may
be used for domestic purposes by the crew of a submarine,
when the electrical generating plant is provided therein~
One embodiment of electrical generating plant
according to the invention, for use in a submarine, is
shown in Figure 4. However, description will first be
made of the plant shown in Figures 1 and 2, in which
methanol and liquid hydrogen peroxide are used as the
25 starting materials for generating gaseous hydrogen and
oxygen respectively for supply to a fuel cell which forms
part of the plant.
Referring now to Figure 1, hydrogen peroxide is
drawn from tankage by a pump 10 and passed into a
30 decomposer 12. A promoter, such as calcium permanganate
may also be added by pump 13 to start the hydrogen
peroxide decomposition when the decomposer 12 is cold.
The tankage (not shown) for the hydrogen peroxide as well
as for the methanol preferably comprises flexible bags
35 located outside the pressure hull, but within the
~31 1264
-13-
hydrodynamic casing of the submarine. There are several
advantages for such a method of storage, e.g. space
saving within the pressure hull, depth pressure
effectively 'pumps' the liquid into the suhmarine and
5 there is no ullage because the bag just collapses as
liquid is withdrawn thus substantially reducing buoyancy
problems.
In the decomposer 12, the hydrogen peroxide
contacts a first catalyst which ensures that the majority
10 of the hydrogen peroxide decomposes. As this is a very
violent reaction producing considerable heat r the
conditions placed on the catalyst are very arduous so
that the product gases are then passed via a cyclone 14,
to separate any catalyst debris from the gas stream and
15 collected via the valve in a container 15. From cyclone
14, the gases pass through a heat exchanger 16 where they
are cooled by transferring heat to a methanol/water
inflow to the apparatus. After further cooling in a heat
exchanger 17, the decomposed hydrogen peroxide, which now
20 consists of mostly liquid water, oxygen and possibly some
residual h~drogen peroxide, passes to a catchpot 18,
(which may be catalysed if necessary), where liquid water
is connected. Pure saturated oxygen passes out via pipe
19 while waste water leaves via valve and pipe 20. The
25 operation of valve 20 is by level controller. In
practice the decomposition of hydrogen peroxide in
decomposer 12 may be so complete that further catalyst in
catchpot 18 is not necessary; however if the conversion
efficiency is not as high as expected, e.g. when starting
3a upl additional catalyst is required.
Methanol from tankage (not shown) and pure water
are passed by pumps 22 and 23 respectively to a mixing
pipe 24 where mixing occursO A proportional controller
25 measures the proportions of the mixture and controls
the operation of one/both pumps 22 and 23 to achieve the
131 1~64
-14-
desired methanol/water ratio, A flow controller (F.C.)
and valve 26 regulates the flow of methanol/water mixture
via heat exchangers 3~,16 and 29, to a reformex 21 where
reaction 2 (i.e.2A) occurs. The decomposer 12 and the
5 reformer 21 are shown side-by-side to emphasise the
thermal interdependence of the two reactions. the
reactions may be conducted in the same vessel separated
by a highly conductive membrane or in adjacent vessels
with enhanced thermal transfer, e.g. via heat pipes, and
10 the like to provide means for transmitting heat given-off
in the decomposer 12 to the reformer 21 in order to
maintain the endothermic reaction therein. The flow
controller/valve 26 in the methanol/water line is linked
via a central controller (not shown) to flow
15 monitor/controller 11 in the hydrogen peroxide line so
that, under normal operational conditions, the flows can
be balanced to equate the thermal requirements of the
reforming and decomposition reactions and/or the rates of
oxygen and hydrogen prGduction. The pressure relief and
20 tankage 27 i5 provided from pipe 24 as a safety
precaution.
The pure water, passed by pump 23, is produced by
the fuel cell (Figure 2) and is held in an intermediate
storage tank tnot shown) until required.
For safet~ reasons, it may be desirable to
separate the decomposer 12 and the reformer 21 from close
proximity to each other. However, heat given-off in the
decomposer 12 is still used usefully, in that the
incoming flow of methanol/water to the reformer 21 is
30 pre-heated in the heat exchanger 16. When the decomposer
12 and the reformer 21 are separated, additional heatiny
may be required in the reformer 21 so as to cause the
reaction to go to a reasonable degree of completion.
This may be provided by any suitable means, such as
electrical heating. It is envisaged that substantial
1 3 1 1 264
-15-
pre-heating of the methanol/water inflow may take place
via the heat exchangers 30, 16 and 29, say up to 80% of
requirements, with the remainder of the required heating
(to maintain the endothermic reaction~ taking place in
5 the reformer 21.
It will be noted that the methanol/water flow is
heated by passing through three heat exchangers 30, 16
and 29. The order in which these heat exchangers come
along the methanol/water pipe run will depend on the
10 particular temperature of hot and cold fluids at each
point; the number and order of heat exchangers 30, 16 and
29 will be determined to obtain maximum thermal economy.
Electric heating (not shown) may also be provided, if
required~ e.g. for start up or in the reformer 21. Heat
15 exchangers 30, 16 and 29 may operate under co-current or
counter-current flow, as determined by thermal efficiency
requirements. Similarly the flow of the hydrogen
peroxide and methanol/water streams through the
decomposer 12 and the reformer 21 may be either
20 co-current or counter-current.
Chëmical reactions seldom go to completion, i.e.
100% conversion of the reactants, and often side
reactions occur. This is the case with the
methanol/water reforming. If the shift reaction
25 (Equation 3) has not occurred sufficiently in
reformer/decomposer 12, an additional opportunity for the
reaction to take place must be provided. In Figure 1,
the products of the reforming reaction, consisting of
hydrogen, carbon dioxide, carbon monoxide, unreformed
30 methanol and steam leave the re~ormer 21, and pass to a
shift reaction vessel 33. Additional pure steam along
supply line 34 may be added, if re~uired, and the shift
reaction proceeds, in the presence of a catalyst if
needed, according to Equation 3, changin~ most of the
; 35 unconverted carbon monoxide to carbon dioxide with the
,
131 1264
-16-
production of further hydrogen.
The yases now pass into a separation vessel 35
where the hydrogen gas is separated from the other gases.
Because it has the smallest atomic volume of all
elements, hydrogen gas will diffuse through the crystal
structures of some substances, whereas other gases
cannot. The metal palladium is unique in having a
crystal lattice just large enough to permit the passage
of hydrogen, yet not large enough for any other gas to
10 pass. Thus a diffusion membrane 36 of palladium, or its
alloys, separates the two parts of diffusion separation
vessel 35, allowing only hydrogen to pass through
membrane 36 under a pressure differential. From
diffusion separation vessel 35, via pipe 32, the pure
hydrogen gas is cooled in cooler 30 and passed via pipe
38 to the fuel cell (not shown).
The other gases which leave the diffusion
separation vessel 35 pass along pipe 43 and consist
mainly of carbon dioxide with smaller amounts of carbon
20 monoxide, hydrogen, steam and methanol vapour. These are
all waste gases. It will be noted that not all the
hydrogen gas is removed in the diffusion separation
vessel 35; this is because diffusion is a physical
process and the timescale required to achieve dynamic
25 equilibri~m would be unacceptable for the rate of
hydrogen production required. Of the gases in pipe 43,
carbon monoxide and hydrogen are sparingly soluble in
water and therefore cannot be discharged from a submarine
without fear of bubbles rising to the surface and noise
30 yeneration due to bubble collapse thus betraying the
position of the vessel. As onboard storage of gas under
pressure has been rejected previously, it is necessary to
chemically convert these gases to soluble forms. This
can be done by catalytic oxidaton to carbon dioxide and
35 water, with the oxygen for this requirement coming from
:
:~
-
131 126~
-17-
decomposed hydrogen peroxide via pipe 37, and according
to the following equations:
2H2 ~ 2 ~~> 2H20 + heat (5)
2C0 + 2 ~~> 2C02 + heat (6)
2CH30H ~ 32 ~-> 2C02 ~ 4H20 + heat (7)
The gases from the separator 35 pass via a
non-return valve 39 and along pipe 43 to a first
catalytic oxidiser 41. A stoichiometric control system
42 regulates the process. It consists of a meter (SC)
10 upstream of the catalytic oxidiser 41 to measure the
concentrations of hydrogen and carbon monoxide in the
gas, and a control valve 42 to admit oxygen from a pipe
37 via a non-return valve 44. Depending on the
efficiency of the reforming and shift reactions, more
15 than one catalytic oxidiser 41 may be required; two are
shown in Figure 1.
The heat generated in the catalytic oxidisation is
transferred via heat exchanger(s) 29 to the
methanol/water stream entering the reformer 21. After
20 passing through heat exchanger(s) 29, the gases are
dissolved and discharged overbaard via pipe 45.
It will be noted that as the methanol is stored
externally to the pressure hull, it will thus be at
diving depth pressure. Pump 22 will raise this pressure
25 by a small amount to give the operating pressure in the
system shown in Figure 1. Throughout the system, the
pressure will be virtually maintained so that the gas may
be dissolved and discharged from pipe 45 directly
overboard (probably through a non-return valve (not
shown)), without the need for pumping. The same
situation applies to pump 10 in the hydrogen peroxide
.
line. Pumps 23 and 13 will have to raise water and
calcium pexmanganate respectively from ambient pressure
up to system pressure, but in both cases only small
volumes need be handled. Thusr from a pumping aspect,
1 3 1 ~ 264
only a minimum energy need be expended to operate the
system. Like the thermal economy of the system, the
pumping requirements are also designed to maximise the
output of useful energy from the plant.
Referring to Figure 2, pure oxygen and hydrogen
gases enter a fuel cell 50 via pipes :L9 and 38
respectively. Although "fuel cell 50" is referred to in
the singular, it will be understood that a plurality of
fuel cells will be used in real installations; the cells
10 may be arranged in series or parallel or any required
combination. Reaction 4 occurs producing electrical
energy (shown at the top of the figure), water and heat.
The rate of reaction is controlled by the valve in pipe
38 admitting hydrogen to the fuel cell 50. Though
15 difEusion membrane 36 of separator 35 (Figure l) shoula
pass only hydrogen gas, flaws in the metal may lead to
small amounts of other gases also passing. As these
impurities would tend to build up in fuel cell 50 due to
not reacting , a purge 51 of hydrogen is provided for use
20 on a continuous or intermittent basis, as required.
Steam and unused oxygen leave the fuel cell via
outlet pipe 52 and the steam condenses in a cooler 53.
Pure water is separated in a catchpot 54 and passed via a
level controller/valve 5g and a pipe 60 to storage (not
shown) where it is used for methanol dilution, (via pump
23, Figure l) in supply line 34 to vessel 33 (Figure l)
or as potable water. The unused oxygen leaves catchpot
54, via pipe 55, and is circulated by pump 56 and pipe 57
back to the fuel cell. As members 52,53,54,55,56 and 57
form a closed loop, a purge 58 is provided to prohibit
the build-up of impurites. The oxygen circulation around
loop 52,53,54,55,56 and 57 also provides some cooling for
fuel cell 50 via cooler 53. The main source of cooling
for the fuel cell is by separate system 50A and the heat
from this source may be used elsewhere in the process.
131 1264
--19--
As neither hydrogen or oxygen are appreciably
soluble in sea water, the purge gases cannot be
discharged overboard and must thus be disposed of in
another way. In Figure 3, two metering devices 70
5 monitor the flows in pipe lines 51 and 58 (Figures 2 and
3), and send signals to a stoichiometric controller
(S.C.) 71 which activates pump 72 to pump in air from the
submarine atmosphere whenever the hydrogen-oxygen ratio
exceeds a preset value. The air from pump 72 also acts
as a diluent and thus limits the temperatures reached in
a catalytic oxidiser 74. The two gas ~lows then pass via
non-return valves 73 to catalytic o~idiser 74, where
reaction 5 occurs. After condensing the steam in a
cooler 75, the resulting water is collected in a catchpot
76 and discharged via level controller/valve 78 to
tankage 77; it would be potable, but would not be used
for methanol dilution. The mixture of air/oxygen from
catchpot 76 is discharged back to the atmosphere of the
submarine via pipe 79.
The electrical generating plant just described
offers a ~ompletely self-contained power generation
system operable without requiring any external
air~oxygen~ The oxygen from the submarine atmosphere
passed by pump 72 ~Figure 3) could equally well come from
oxygen purge line 58 ~ opening the valve in this pipe a
little more. The process either recycles or discharges
its own waste products and thus does not contaminate the
environment. Additionally, if the hydrogen peroxide
decomposition reaction alone is run, or is run at a rate
higher than stoichiometric requirements, oxygen may be
added to the submarine atmosphere via pipe 79.
To start up the process, hydrogen peroxide is
admitted to decomposer 12 together with some of the
calcium permanganate promoter. This will heat up the
decomposer 12 and the heat exchanger 16. Then
131 12~4
-20-
methanol/water is passed through heat e~changer 16 and
into reformer 21 where, because of the low temperature,
the rate of methanol conversion is low. This results in
a high proportion of methanol being oxidised in the
5 catalytic oxidiser 41 and hence high heat transfer in the
heat exchanger 29, leading to higher methanol/water input
temperatures in the reformer 21. Thus the rate of
conversion of methanol and the heat input to the
methanol/water in heat exchangers 16 and 29 will result
10 in a stable equilibrium being achieved after a period of
operation and maintained thereafter.
Where changes in electrical power output are
required, this could be achieved by changes in input
flows of hydrogen peroxide and methanol/water via an
15 automatic control system. If required, small oxygen and
hydrogen reservoirs (not shown) may be provided in pipes
19 and 38 respectively. Any time lag between power
demand and power output may be made up/absorbed by the
submarine's batteries. The system is well suited to
-~ 20 automatic control in an unmanned environment.
~ further point, which is very important for
operation in a confined environment, is that the system
is almost completely silent. This is particularly useful
in a submarine trying to avoid detection, ~ut also
; 25 important for the health and safety of personnel working
in or near the same location as the system.
Though this specification has been written with
reference to use of the electrical generating plant in
submarines, its application extends to many other fields,
30 e.g. submarine habitats for oil exploration, mining, fish
farming, rescue equipment for use in mines, caves,
emergency equipment for use where naked flames may be
prohibited, e.g. oil rigs, petroleum refineries, space
exploration and colonisation.
It should be understood that Figures 1 to 3 are
131 126~
-21-
somewhat schematic flow diagrams, and that detaile~
aspects of the apparatus may be modified as further test
work is performed.
Figure 5 is an overall heat and mass balance
5 diagram and assumes process heat recovery via heat
exchangers etc, (as shown generally in the other
figures3, for the simultaneous productlon of oxygen and
hydrogen from the decomposition of hydrogen peroxide and
the reforming of methanol respectively, in the plant as
10 shown in Figures 1 to 3.
Currently, two basic types of fuel cell are
possible:-
(i) A first type which requires high purity gaseous
hydrogen and high purity gaseous oxygen, and
(ii) A second type which requires a high purity gaseous
hydrogen and a source of oxygen which may be
either the pure gas or an impure gas such as air
If fuel cells of the first type are used, the flow
diagrams may be very similar to those shown in Figures
20 1,2 and 3. If, however, fuel cells of the second type
are used, a modification of the flow diagram of Figure 1
will be required.
Referring now to Figure 4, there is shown an
embodiment of electrical generating plant according to
25 the invention which is intended for use as an electrical
source of energy for driving the drive train of a
submarine. The plant of Figure 4 has dual modes of
operation, as will be evident from the subsequent
detailed description, in which parts corresponding with
30 those already described with reference to Figures 1 to 3
are designated by the same reference numerals, and will
not therefore be described in detail again.
When the submarine is operating underwater, the
electrical generating plant shown in Figure 4 will
:~ 35 operate generally similarly to the plant of Figures 1 to
,
131 1264
-22-
3, in that liquid hydrogen peroxide is supplied to the
decomposer 12 to provide (1) a supply oE gaseous oxygen
to the fuel cell and (2) a source of heat which may be
used to promote the reforming reaction in the reformer
5 21, and methanol is supplied to the reformer so as to
undergo the endothermic reaction which liberates gaseous
hydrogen for supply to the fuel cell.
However, when the submarine is operating on the
surface or snorting, the supply of hydrogen peroxide can
10 be conserved by switching the operation of the plant so
that aspirated air can be used as the source of gaseous
oxygen for the fuel cell.
Therefore, an input of air (not shown) is provided
to the pipe 19. ~owever, when air is used as the sole
source of oxygen, heat must be provided for the reforming
reaction in the reformer from alternative heat sources.
This can be achieved by providing a fuel burner to burn
fuel, such as the methanol, or other fuels.
The combustion could take place in one of three
places:
(i) Tn or close to decomposer 1?.
(ii) In a separate combustor or in a catalytic oxidiser
46 with high heat transfer connections to reformer
21.
(iii) In catalytic oxidiser(s) 41.
In all cases, the methanol (or other suitable
fuel, e.g. diesel oil) and air would be admitted to the
appropriate vessel and catalytic combustion would occur.
Only if a co~bustor was used in (ii) above would there
actually be any flame. Methanol would be a preferred
fuel for combustion in decomposer 12 as there would be no
impurities (e.g. SO2 if diesel fuel were used) to affect
the fuel cell where poisoning of the electrodes can
easily occur.
Three separate points are given above and shown on
131 1264
-23-
Figure 4 where the methanol (or other suitable fuel)
could be burnt. Each option will be considered in turn.
(i) Oxidation of fuel in hydrogen peroxide decomposer
12.
The advantage of this option is that no or minimal
additional plant is required and the hydrogen
peroxide and methanol decomposition reactions
could be alternated rapidly whenever the submarine
dived or surfaced. Additional air would be added
to ensure complete combustion and provide for the
oxygen requirements of the fuel cell. Under
these circumstances all the combustion products as
-
well as the additional air would pass through the
whole system, i.e. via pipe 19 and fuel cell 50 to
be rejected via purge 58 and pipe 79. This would
result in the risk of poisoning the fuel cell
catalyst; there would also be a reduced partial
pressure of oxygen in the fuel cell. It may thus
be necessary to exhaust the products of the
methanol combustion after heat exchanger 16 and
introduce a separate air supply into oxygen pipe
19 .
tii) Combustion or catalytic oxidation in separate
vessel 46
In this case a separate vessel preferably in
intimate thermal contact with reformer 21 would be
used. The extra volume of such a vessel would be
a disadvantage in a submarine but it would keep
combustion products away from the fuel cell. The
3~ hot combustion gases would be exhausted via
catalytic oxidiser(s) 41, heat exchangers 29 and
pipe 45. A separate supply of air would be
admitted to oxygen pipe 19 for fuel cell 50.
(iii) Combustion in catalytic oxidisers 41
Here again the combustion products would be kept
~: :
131 1264
-24-
free from the fuel cell and additional air admitted to
pipe 19. Though no additional plant is required, the
heat of combustion is applied via heat exchangers 29 and
not directly to reformer 21. Electrical heating in
5 reformer 21 could be used as a supplement.
In practice, a combination of more than one of the
three alternatives could be used, supplemented by
electric heating if necessary. On a submarine,
one of the most important factors would be the
ability to change from the air system (mode II) to
the hydrogen peroxide one ~mode I) in a minimum of
time.
When the second type of cells are used, fuel cells
could become the main form of propulsion for all
15 conditions, i.e. there would be no need for a main diesel
engine. Fuel supplies would thus consist mostly of
methanol, or another reformable hydrocarbon, plus a
smaller quantity of hydrogen peroxide for use only when
fully submerged.
Figure 6 is an overall heat and mass balance
diagram f~r the production of hydrogen by reforming
methanol using an alt`ernative heat source and assumes
process heat recovery via heat exchangers etc, as shown
in the Figure, (to that as described above with reference
25 to Figures 1 to 3), for maintaining the endothermic
reaction in the reformer arrangement of Figure 4. This
alternative heat source is assumed to be derived from
heat generated by a combustion process, as shown in
Figure 6.
: , . `'
'
~ - 35
~::
!
s,
... , ~. .