Note: Descriptions are shown in the official language in which they were submitted.
- 131i50~
PROCESS FOR THE PREPARATION OF VERY HI~H PURITY
SOR~ITOL SYRUPS
5The invention relates to a process for the prepa-
ration of very high purity sorbitol syrups.
At the present time, very high purity sorbitol
syrups are obtained by hydrogenation of dextrose syrups
prepared by dissolving crystalline dextrose in water.
10The richness of sorbitol syrups so obtained which
is sufficient, however rarely exceeds 98X after purifica-
tion.
The reason therefor is that in addition to traces
of hydrogenated polyholosides originating in the raw mate-
rial and traces of reducing substances which have escaped
the hydrogenation, there are formed during the hydrogena-
tion, isomers of sorbitol, namely particularly mannitol.
The presence of this group of products results in certain
drawbacks in the matter of crystallisation operations of
the sorbitol from these syrups and has consequences on the
characteristics of the crystalline sorbitol obtained, par-
ticularly from the point of view of its crystallinity.
In addition, the yield of the operation is not
entirely satisfactory since, to obtain sorbitol syrups ha-
ving this richness, which in any case rarely exceeds 98X,it is necessary to satisfy oneself with limited yields at
the level of the preceding step of preparation of the
crystalline dextrose, which is used for the preparation of
the subsequently hydrogenated dextrose syrup, and which is
obtained by crystallisation in two or three crops from
starch hydrolysates with a glucose content of the order of
94 to 96/. by weight ; in fact, said yields of dextrose are
generally limited to about 75 to 77/. in two crops and to
about 80 to 88/. after a third crop and the crystallisation
mother liquors or hydrols have a sometimes difficult sale
in commerce by reason of their color and their content of
. .
- i'
" 1311~03
impurities.
It has indeed already been proposed to suppress
the intermediate crystallisation step of the dextrose by
subjecting directly, within the same reaction vessel, a
cheaper raw material, namely a starch hydrolysate, not
only to reducing c:onditions, but also to hydrolysis by
means of a strong acid, which permits also to make use of
the conversion known in itself, of polyholosides such as
maltitol or isomaltitol into equimolecular amounts of
sorbltol and of glucose under the action of the strong
acid.
It is possible to mention in this respect French
Patent N 1 263 290 which proposes the use, as a catalyst,
of reduced nickel on a support constituted of infusorial
earth and according to which there is carried out in one
and the same reaction vessel, in substantially neutral
solution and at a moderately high temperature, a reduction
of the major part of the reducing sugars, after which a
strong inorganic acid such as phosphoric acid is added, in
20. a proportion of 0.05 to 1.0~ by weight on the basis of the
saccharides initially employed, the mixture constituted by
the thus acidified solution and the catalyst being sub-
jected to higher pressure and temperature, by means of
which the hydrolysis of the hydrogenated polyholosides
present in the hyd.rolysate and the hydrogenation of the
sugars then appearing are effected simultaneously.
Within the same order of ideas, but 15 years
later, Belgian Patent N ~37 201 described the simulta-
neous hydrogenation-hydrolysis-hydrogenation of starch
hydrolysates with use of a ruthenium catalyst supported on
zeolite of type Y; the hydrogenation reaction is effected
in two steps, the first being conducted at a temperature
comprised between 100 and 175 C and the second at a tempe-
rature of about 170 to 200-C. The pH conditions in which
the consecutive phases of the hydrogenation take place are
specified, the pH of the product of the reaction having to
13~1503
be comprised between 3.5 and 4.
The drawbacks of these processes reside not only
in the rapid deactivation of the catalysts and in the de-
naturation of the structure of the zeolites, but also in
S the fact that the syrups so obtained include numerous
impurities.
Thus,
- there occur partial anhydrysation reaction and
isomerisation of the polyols,
- the hydrolysis of the hydrogenated polyholoside
fraction is incomplete and manifested by a high value of
"total sugars",
- the proportions of "hexitans", of mannitol, of
"total non-sugar impurities" are large, the content of
true sorbitol being finally at the most equal to 94X,
which is, for example, very insufficient for the prepara-
tion of a crystalline sorbitol of suitable quality.
Another process suitable for the preparation of
sorbitol syrup without passage through the crystallisation
of the dextrose is that described in French Patent N-
2 052 202 filed by the Assignee. According to this pro-
cess, in which recourse is also had, as starting material,
to a starch hydrolysate which is hydrogenated, a fractio-
nation of the hydrogenated starch hydrolysate is carried
out by passage over a resin or a cationic molecular sieve,
preferably in calciurn form, which permits the isolation of
a fraction containing sorbitol of richness greater than
99Z .
This technique therefore leads to a sorbitol of
very high purity, but it proves however to be unsatisfac-
tory in practice. It results, in fact, in a very partial
yield of sorbitol, strictly dependant on the true dextrose
content of the starting starch hydrolysate, the latter
having for this reason to be as high as possible.
It follows that a priori none of the known proces-
ses permits at the same time the manufacture of sorbitol
131~503
syrup of high purity, suitable particularly for the
preparation of crystalline sorbitol, and an easy access
to high yield whose influence on the cost price of the
final product is readily seen.
It is to this problem that the Applicants have
had the merit of proposing a particularly effective
solution, according to which a raw material of which a
first part is constituted by a starch hydrolysate, is
subjected to a process comprising in combination
- a catalytic hydrogenation step of the raw
material,
- a step of chromatographic separation of the
raw material which has undergone hydrogenation into a
first fraction containing very pure sorbitol and which
is recovered and into a second fraction containing,
besides sorbitol, non-hydrogenated sugars as well as
hydrogenated di- and polysaccharides,
- an acid hydrolysis step of the abovesaid
second fraction, which provides a partially
hydrogenated starch hydrolysate which is recycled and
which constitutes a second part of the raw material
subjected to the hyrogenation step.
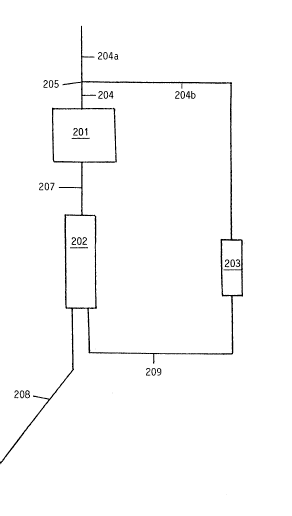
IN THE DRAWINGS
Figure 1 is a diagrammatic flow sheet
illustrating an installation for carrying out the
process in accordance with the invention;
~,
-4a- ~ 3 ~ 1~ a 3
Figure 2 is a diagrammatic view of an
installation suitable for carryi.ng out the
chromatographic fractionation step in accordance with
the invention;
Figures 3 and 4 are diagrammatic flow sheets
representing particular embodiments of the installation
proper to carry out the process in accordance with the
invention.
The process thus defined may be carried out by
means of the installation shown diagrammatically in
Figure 1 and which comprises:
- a vessel 201 within which the catalytic
hydrogenation step is carried out,
- a chromatographic separation installation
202 and
- a reaction vessel 203 within which the
hydrolysis step is carried out.
The vessel 201 is supplied with raw
material. through a pipe 204 formed by the
junction at 205 of a pipe 204a bringing in the
starch hydrolysate from a tank (not shown) and a
pipe 204b connected to the outlet of the vessel
203 and introducing the partially hydrogenated
1~115~
hydrolysate.
The outlet of the vessel 201 is connected to the
inlet of the vessel 202 through a pipe 207.
From the outlet of the ~essel 20Z, are conducted :
- the first fraction containing very pure sorbitol
through a pipe 208 to a storage tank Inot shown) and
- the second fraction containing sorbitol, non-
hydrogenated sugars as well as hydrogenated di- and poly-
saccharides through a pipe 209 to the inlet of the vessel
10 203.
The means proper to ensure the circulation of the
various syrups within the installation are not shown.
The starch hydrolysate which is part of the c~ons-
titution of the raw material has, preferably, a true
dextrose content comprised between 65 and 97X and, more
preferably still, between 70 and 95~/.. Particularly advan-
tageous starch hydrolysates are constituted by hydrols of
the first and of the second crop obtained in the crystal-
lisation of the dextrose.
In the foregoing and in the following, the percen-
tages indicated are understood, except when stated to the
contrary, with respect to the dry matter of the syrups.
The catalytic hydrogenation step is carried out in
manner known in itself, particularly on ruthenium or Raney
nickel catalysts. Preferably, it is carried out on a Raney
nickel catalyst, at a hydrogen pressure comprised between
40 and 70 kg/cm and at a temperature of about 100 to 150 C.
The chromatographic separation step can be effect-
ed, in manner known in itself, discontinously or conti-
nuously Isimulated mobile bed), on strongly acid absor-
bants of the cationic resin type, charged with alkaline or
alkaline earth ions or of the zeolite type, charged with
ammonium, sodium, potassium calcium, strontium, or barium
ions.
Examples of such chromotographic separation pro-
cesses are described in patents US 3 044 904, US 3 416 961,
6 1 3 ~ 3
US 3 692 582, FR 2 391 754, FR 2 099 336, US 2 985 589, US
02~ 331, US 4 226 977, US ~ 293 346, US 4 157 267, US
1~2 633, US ~ 332 633, US 4 405 4~5, US4 ~12 866, and US
4 4~2 ~381.
In a preferred embodiment, the chromatographic se-
paration step lS carried out by employing the process and
the lnstallation dlsclosed in US Patent N- 4 ~42 881 and
its corresponding French Application N 79 10563, published
October 14, 1983 under publication no. 2454830.
Whatever the chromatographic separation process
adopted, recourse is had, preferably, as adsorbant, to a
strong cationic resin placed in the calcium form and hav-
ing a proportion of divinyl-benzene from about 4 to about
1 0 1. .
Preferably, the acid hydrolysls step of the above-
said second fraction is carried out on a fixed acid cata-
lyst and it is conducted so that more than ~OX by weight
of the hydrogenated disaccharides and polysaccharides
present are hydrolysed.
It is possible to use, to do this, any type of
reaction vessel and any reagent or acid catalysts capable
of ensuring the hydrolysis of hydrogenated disaccharides
and polysaccharides.
Preferably, said hydrolysis is carried out conti-
nuously by passing the syrup containing the polyols to be
hydrolysed over a fixed bed comprising acid catalysts of
the alumino-silicate, silica, alumina or cationic resin
type, at a temperature and for a time sufficient to
achieve the above indicated desired result.
3n Advantageously, the hydrolysis is conducted on
strong cationic resins in the acid form, at a temperature
comprised between 50 and 120 C, the passage flow rate over
the acid catalyst bed being regulated so as to obtain a
hydrolysis ratio of the hydrogenated di- and polysacchari-
des at~least equal to 60l. and, preferably, at least equal
to 80/..
~'~
``"` 13i 1503
The content of dry matter of the above-said second
fraction which is subjected to the hydrolysis step and
which is recycled is generally comprised between 1.5~. and
30~ and, preferably, between 2.5 and 20'~.
The process thus described permits sorbitol syrups
to be obtained having a richness in sorbitol greater than
9~/., and even greater than 997., which are essentially cha-
racterlsed by a minimal content of reducing sugars. These
syrups, which have at the outlet from the vessel 202, a
content of dry matter comprised between 20~/. and 50'~ and,
preferably between 25~/. and 40~, can then be evaporated to
the dry matter content for marketing or be evaporated more
completely for the production of crystalline sorbitol.
The invention will be still better understood by
means of the examples which follow and of the illustrative
figures appended hereto, said e~amples relating to prefer-
red embodiments of the invention.
EXAMPLE 1
Preparation of pure sorbitol from a starch hydrolysate
prepared by a double enzymatic hydrolysis with -amylase
and amyloglucosidase and having a richness in true dex-
trose equal to 94.5~/., its composition being :
Dry Matter (DM) : 74.0 ~/.
De~trose : 94.5 ~/.
Z5 DP 2 : 4.0
DP 3 : 1.0
DP > 3 : o.s
it being understood that "DP" means the degree of polyme-
risation of a given constituent of the hydrolysate.
a~ Hvdroqenation ste~
The above-said hydrolysate, after purification on
ion exchange resins and on active carbon, is hydrogenated
on Raney nickel,~at a hydrogen pressure of 45 kg/cm and
at a temperature of 125'C.
It shows, after conventional purification, the
following composition :
"` 13~15~3
DP > 2 : 1.3
DP 2 : 5.6
Glycerln : 0.2
Mannltol : 1.O
Other hexitols : 0.4
Sorbltol : 93.5
Reducing sugars : 0.OB
Total sugars : 2.74
determined by the analytical method disclosed in the FOOD
CHEMICAL CODFX, 2nd Edition, page 791.
b) ChromatoqraDhic seParation steD
The above-said hydrogenated starch hydrolysate is
then sent to a continuous chromatographic separation ins-
tallation of which the details of constitution and of
operation are those disclosed 1n US Patent N 4 442 881
and in the corresponding French publication number 2454830 of
October 14, 1983, the said details only being taken up again here to
the extent that understanding of the present description requires it.
It includes, as shown in Figure 2 of the American
patent (taken up again here as Figur 2 for the ôetailed
explanation of which reference is made to the American
patent), eight columns or stages C1 to CB of 200 liters
each, lined with adsorbant of the strong cationic resin
type in the calcium form and of fine granulometry (0.2 to
0.4 microns).
By adjustment of the electrovalves, there are
formed a desorption zone I of two stages, an adsorptlon
zone II of four stages and an enrichment zone III for the
hydrogenated polyholosides of two stages.
A c.losing device maintains in the configuration
adopted the total fluid-tightness betweeh the zone III,
enrichment zone at the end of which are recovered the hy-
drogenated polyholosides and the zone I or desorption zone
of the sorbitol, at the head of which zone the desorption
water is lntroduced.
" 9 1311503
This closing device ensures the direction of
passage of the liquid phase over the selective adsorbant
and avoids especially contamination of the pure sorbitol
by traces of polyholosides, whose migration speed within
the resin is largely greater than that of the hydrogenated
di- or triholosides.
A kimer ~not shown) adjusted to 2i minutes 50
seconds ensures for the flow rates indicated below a water
supply sufficient to carry out de-sugaring of the first
stage of the desorption zone, and for the supply of a vo-
lume of hydrogenated starch hydrolysate or HSH compatible
with the volume of adsorbant and its capacity of absorp-
tion, so as to obtain an extraction ratio of the hydroge-
nated polyholosides greater than 99/ and an extraction
ratio of the sorbitol at least equal to 90'~ of the sorbi-
tol present in the supplied hydrogenated hydrolysate.
These proportions are kept constant by adjusting
the flow rate of the extraction pump ~not shown) of the
adsorbed sorbitol. The outflow of the polyholoside frac-
tion is effected at atmospheric pressure and its flowrate, constant, results from the difference between the
supply flow rates and the extraction flow rate.
The hydrogenated starch hydrolysate which is
introduced into the installation at the head of the
adsorption stage, has a dry matter content equal to 64.5/..
The temperature inside the separation columns is kept at
about 90-C.
In the schematic diagram of Figure 3 is shown the
installation of Figure 2 diagramatically at 202 (the same
references denoting the same elements for the common parts
as in Figure 1). The chromatography installation includes,
in addition to the constituent elements already shown in
Figure 1,a pipe 210 for recirculating excess water and a
pipe 211, through which are extracted from the circuit the
polyholoside fractions of DP > 4 extracted at a very low
DM.
10 i 1311503
Supply of the installation with water is effected
by a pipe 212.
The arrows borne on the pipes indicate the direc-
tion of flow~
The unit operates as follows:
- the hydrogenated starch hydrolysate or HSH intended
for chromatographlc fractlonation is led through the pipe
207 at a flow rate of 120 l/hour and has a dry matter
content of 64.5'~,
10 - the water is introduced through pipe 212 with a flow
rate of 330 l/hour,
- the pure sorbitol is recovered through the pipe 208
with a flow rate of 240 l/hour, its content of dry matter
being 34,15~,
- the total amount of liquids extracted from the ins-
tallation 202 is e~tracted with a total flow rate of 386
l/hour, comprising successively,
. a fraction of e~cess water of 176 l/hour which
is recirculated through the pipe Z10 to the head
of the installation,
. a polyholoside fraction removed through the pipe
211, whose flow rate is 114 l/hour and the DM
about 1~.,
. a polyholoside fraction led through the pipe 209
to the hydrolysis installation with a flow rate
of 96 l/hour, the content of DM being 3.5/
Analyses of the outflows of sorbitol and of hydro-
genated polyholosides are presented in Tables I and II.
ll 131~03
TA8LE I
ANALYSIS OF THE SOR8ITOL
Tlmes Brix DP 2 Glycerin Mannitol Sorbitol
~minutes) / . ...
0 56.50.4 0.3 1.0 98.3
3 47.50.3 0.3 0.9 98.5
6 41.50.3 0.3 0.8 ga-.6
12 27.00.2 0.2 0.5 99.1
21.50.2 0.1 0.5 99.2
18 16.0 _ 0.1 0.4 99.5
21 12.5 _ 0.1 0.3 99.6
23'50" 10.0 _ 0.3 99.7
From examination of Table I, it is seen that sor-
bitol has been extracted for 23 minutes 50 seconds with an5 average purity higher than 9BZ and an average content of
dry matter of 34.15Z.
The value of the "brix" gives the approximate per-
centage of dry matter ; it is determined by the refractive
index.
TABLE II
ANALYSIS OF THE POLYHOLOSIDES OUTFLOW
Times Brlx Rotatory DP > 3 DP 3 DP 2 Sorbitol
(minutes) (a) D Z Z Z Z
!~ ~n
12 _ ~0.166 +++ ++ +
14 0.7 +0.629 +++ ++ +
16 1.5 +1.038 35.9 30.9 22.610.6
18 2 +1.079 24.0 29.5 33.712.8
2.5 +2.209 21.0 26.0 43.79.3
22 3.4 +2.807 16.1 21.2 53.49.3
~5 23 50" ~.0 +2.604 13.5 19 D57.0 10.5
12 ' 1 3 il ~ 03
The specific rotatary power (a)D is deduced from theangle of ro-tation read on a polarimeter.
Detailed analysis of the results collected in Table II
and of the indications provided above with respect to
Figure 3, as regards the hydrogenated polyholosides, shows
in addition that:
- during the first 11 minutes, that is to say an
equivalent of 176 l/h, the polyholosides outflow has been
recyclable for the de-sugaring of the following stage,
- from minute 11 to minute 18, the extracted fraction,
containing the major portion of the hydrogenated
polyholosides of DP greater than or equal to 4 was elimi-
nated from the system, that is to say 114 l/h with 1X of
dry matter,
- then, from minute 18 to the moment corresponding to
Z3 minutes 50 seconds, the polyol fraction was recovered
for the hydrolysis step, that is to say 96 liters with a
dry matter of 3.6/
The overall balance, which appears on examining the
diagram of Figure 3 and Tables I and II, is summarised as
indicated below.
The chromatographic separation system was supplied as
follows: -
Hydrogenated starch Water
hydrolysate
Flow rate 120 l/h 330 l/h
Density 1.269
~/ dry matter . 64.5
Flow rate by weight 98.22 kg/h
There was drawn from this system :
Higher polyols Polyols
Sorbitol discarded recovered
Flow rate 240 l/h 114 l/h 96 l/h
Density 1.138 1.0 1.02
/ dry matter 34.15 1.0 3.6
Flow rate by weight 93.27 kg/h 1.14 kg/h 3.53 kg/h
`" 1311~0~
These results correspond to an extraction ratio of
sorbitol present in the hydrogenated starch hydrolysate
- higher than 99~..
c) Continuous hvdrolysis steD of
the recovered polvols
The fraction of polyols recovered is then led
through the pipe 209 into a hydrolysis vessel 203 consti-
tuted by a column identical with that equiping the chroma-
tographic separation installation illustrated by Figure 2.
This column is filled with a cationic resin of
very fine granulometry (U.1 - 0.2 micr~ns) placed in the
H form.
- The feed flow rate was adjusted to about 90 liters/
hour.
The hydrolysis vessel was kept at a temperature of
about 110-C.
Under these conditions, the hydrolysed syrup flows
out from the vessel 203 with a proportion of reducing
sugars equal to 17.0 grams/liter glucose equivalent, which
is equivalent to a hydrolysis ratio of 91.4-~, since an as-
say test of the reducing sugars carried out after complete
hydrolysis ~effected after two hours under reflux in the
presence of normal H2S0~) showed a glucose equivalent of
lB.6 grams per liter.
The chromatographic analyses of the syrup at the
inlet and. at the outlet from the hydrolysis vessel 203
gave the results indicated below.
The compcsition of the syrup led through the pipe
209 to the vessel 203 was as follows:
Sorbitol 9
Mannitol 0.8
DP 2 55
DP 3 15.2
DP > 3 20
14 ' ~ 3
and that of the syrup emergi.ng from the vessel 203 through
the pipe 204b, determined on three samplings, as follows:
Sorbitol 41 42 39.6
Mannitol Z.3 2.5 2.a
DP 2 6 7 5
DP 3 5 6.5 7.0
Glucose 46.4 52 45.6.
dl Recirculation throu~h ~ioe 204b of the svruo
thus hvdrolysed to the hvdro~enation vessel
201. which corresPonds to a dilution of the
startinq starch hvdrolvsate.
In this case, through the pipe 204 is conducted a
starting material constituted by starch hydrolysate not
yet treated and hydrolysed syrup emerging from the vessel
15 203.
In Figure 4, are shown diagramatically the princi-
pal elements of the corresponding installation, the same
elements being denoted by the same reference figures as in
Figures 1 and 3.
Two stages of purification 213 and of evaporation
purification 214 are shown in respectively the pipes 205
and 207.
To bring the raw material introduced into the
vessel 201 to a dry matter content of about 43/., which
z5 corresponds to the optimal conditions of the hydrogenation
reaction, through the pipe 204a is led, at a flow rate of
140.4 kglh, -the starch hydrolysate not yet treated and
having a content of dry matter of 70Z., which, after combi-
ning with the flow rate of 97.9 kg/h of syrup flowing from
the vessel 203 with a dry matter content of 3.6Z provides
a raw material of dry matter content 42.7/. with a flow
rate of 23a.3 kg/h.
After purification and evaporation at step 214.
there is thus led to the installation 202, through the
pipe 207, a hydrogenated syrup with a dry matter content
of 64.5/. at a flow rate of 120 l/h.
' 1311~0~
As already indicated with respect to Figure 3:
- the installation 202 is fed with 330 l/hour of water
coming through the pipe 212 to which is added the 176 l/
hour of excess water recycled through the pipe 210, i.e. a
total of 506 lthour ;
- from installation 202 is extracted:
. 11`4 l/hour of a fraction of higher polyols removed
from the system through the pipe 211 and having a
dry matter content of about 1~.,
10. 96 l/hour with a dry matter content of 3.6'~ of po-
lyols led to the hydrolysis vessel 203 through the
pipe 209.
The whole of the device thus described was placed
in continuous operation.
15In Tables III, IV and V below, are collected the
values recorded respectively with respect to the analyses
of the hydrogenated starch hydrolysate before chromatogra-
phic separation with the analyses of the sorbitol fraction
recovered and with the analyses of the fraction which have
to be subjected to hydrolysis, in the course of five
different successive samplings.
TA6LE III
HYDROGENATED HYDROLYSATE BEFORE CHROMATOGRAPHY
DP > 2 1.6 1.: 1.55 1.5 1.7
DP 2 ~.5 3.5 2.85 3.4 3.6
Glycerin 0.1 0.1 0.2 0.2 0.2
Mannitol 0.7 0.9 1.0 0.8 1.0
Other hexitols 0.3 0.3 0.4 0.2 0.4
SORBITOL 93.8 93.6 93.9 93.9 93.1
Reducing sugars 0.09 0.07 0.065 0.06 0.092
Total sugars 1.5 1.45 1.50 1.33 1.33
1311503
16
T LEAU IV
ANALYSIS OF THE SOR8ITOL FRACTION
P > 2 ~ O,z r :~1=0~ 25 '~
SORBITOL 98.6 98.2 98.4 98.~5 98.1
Manni-tol O . r O . 9 O . 9 O . 8 1 . O
Glycerin 0.2 0.2 0.2 O.Z 0.2
Other hexitols 0.3 0.4 0.3 0.3 0.4
Reducing sugars 0.017 0.028 0.010 0.020 0.012
Total sugars 0.11 0.09 0.14 0.12 0.17
TA8LE V
ANALYSIS OF THE FRACTION SUBJECTEO TO HYDROLYSIS
DP > 3 20.2 20.2 16.9 16.4
DP 3 ___, 18.4 16.1 14.1 13.8
DP 2 43.2 49.2 55.8 55.5
SORBITOL 17.6 13.5 12.1 13.3
Mannitol 0.6 1.0 1.1 1.0
Other hexitols _ _ _
Reducing sugars 0.83 0.30 0.25 0.35
Total sugars I 26.4 28.0 33.5 30.5
The sorbitol fraction so obtained was compared
with five liquid sorbitol grades obtained by hydrogenation
of dextrose according to the prior art and of which the
analyses are presented in Table VI below:
17 ' 1311~0~
TABLE VI
ANALYSES OF SOR81TOL SYRUPS OBTAINED 8Y
HYDROGENATION OF DEXTROSE ACCORDING TO THE PRIOR ART
5 DP > 2 ¦ 0.06 0 15 0.40 O Z5 0.30
DP 2 ¦ 0.40 D.40 0.40 0.6 0.50
SORBITOL 97.84 9a.a 97.55 97.35 97.40
Other hexitols 0.40 0.50 0.15 0.2 0.20
10 Mannitol 1-.0 0.80 1.1 1.1 1.1
Glycerin 0.30 0.03 0.4 0.5 0.5
Reducing sugars 0.05a 0.040 0.052 0.047 0.050
15 Total sugars 0.26 0.26 0.22 0.26 0.30
It can be observed, by comparing Tables IV and VI
that the sorbitol quality obtained by the process accor-
ding to the invention:
- has a higher average purity in sorbitol,
- has a content of products of DP equal or greater
than 2 which is distinctly lower,
- and has a content of reducing sugars distinctly
less (0.017 on the average against 0.050).
A ratio of reducing sugars, so low in the case of
the process according to the invention, could only be
obtained with great difficulty by direct hydrog0nation.
This ratio would need in fact a degree of hydrogenation of
99.98/., which can only be achieved with greatly prolonged
and, consequently, uneconomic hydrogenation periods.
EXAMPLE 2
This example discloses the production of pure sor-
bitol from a hydrol from a frist crop of crystallation of
dextrose, having a true dextrose content equal to 86.7'~.
a) Steo of catalvtlc hydroaenation of the hvdrol
The hydrol, obtained after crystallisation from a
first crop of dextrose monohydrate, has a dry matter con-
~` - 1311503
1R
tent of 74Z and the following composition:
DP > 3 2.7
DP 3. 1.8
DP 2 8.5
S Dextrose a6.70
Fructose 0.3.
After purification and hydrogenation, the composi-
tion of the syrup became:
DP > 2 4.6
DP 2 7.5
Glycerin
Mannitol 1.0
Other hexitols 0.Z
Sorbitol 86.7.
b) Ste~ of continuous liouid chromatoqraDhic
seDaration
This separation was carried out on the device and
according to the process described in Example 1 with res-
pect to Figure 3.
The c.hromatography installation was fed:
- with hydrogenated hydrol through pipe 207 at a
flow rate of 120 l/hour, the hydrol having a dry matter of
627. and a density d-1.267, i.e. 94.26 kg/hour,
- with water through the pipe 212 at a flow rate
of 330 l/hour,
- with a fraction of excess water recirculated
through the pipe 210 at a flow rate of 176 l/hour.
There was withdrawn from this installation:
- pure sorbitol through the pipe 20a at a flow
rate of 220 l/hour, the content of dry matter being 32Z
and the density 1.137, that is to say 80 kg/hour,
- a polyol fraction, discarded through the pipe
211, at a flow rate of 100 l/hour, the dry matter content
being 1.5Z and the density 1.00, that is to say 1.5 kg/
hour,
- a fraction of polyols subjected to hydrolysis
i~ll503
1 9
through the pipe 209 at a -flow rate of 130 l/hour, with a
dry matter content of 8.8Z and a density of 1.02, that is
to say 11.7 kg/hour.
The whole gave an extraction calculated on the
sorbitol of 96.2Z..
c) Contlnuous hvdrolvsis steP
The streams of recovered polyols were conducted
through the pipe 209 to the hydrolysis vessel 203 as
descrlbed in Example 1. The temperature is kept at 115 C
in side the said vessel 203. The degree of hydrolysis of
the polyholosides measured by the reducing sugars in
comparison with the product hydrolysed under reflux for
two hours in the presence of normal sulfuric acid, was
always higher than 90Z.
d) Dilution steo, bv means of the sVruo flowina
from vessel 203 and led throuqh the ~iDe 204b.
of the hvdrol introduced throuqh the ~iPe 204a
before Durification
In the integrated circuit as presented in Example
1, the hydrolysed polyol fraction was used for the dilu-
tion of the hydrol before puriflcation, the whole consti-
tuting the raw material subjected to hydrogenation.
From the practical point of view, 112 kg/h of hy-
drol with 74/ of dry matter are thus diluted with 130 l/h
of the hydrolysed polyols. The solution thus diluted
titrates 3f~.7Z of dry matter.
, After hydrogenation, purification and evaporation
to 62Z of dry matter, the syrup was sent to the continuous
chromatography installation.
-o-0-o-
After several days of operation, the results col-
lected in Tables VII (for three samplings), VIII lfor five
samplings), and IX (for five samplings) were obtained.
` 20 131t5~3
ARLE VII
ANALYSIS OF THE RAW MATERIAL CONSTITUTED 6Y THE
HYDROL DILUTED BY THE HYDROGENATED POLYHOLOSIDES
HYDROLYSED AND THEN HYDROGENATED
Nature of the Content in l.
constituent ir the syru~
DP > 2 4.6 2.9 5.6
DP 2 7.1 7.5 7.7
1 0 ~
Glycerin 0.1 0.2 0.1
Mannitol 1.1 0.9 1.0
Other hexitols 0.3 0.2 0.3
15 SOROITOL 86.8 88.3 85.3
Reducing sugars 0.08 0.06 0.07
Total sugars 4.36 4.7 5.5
TABLE VIII
ANALYSIS OF THE SOR6ITO- OBTAINED
Nature of the Content in /.
constituent in the sorbi tol
DP 3 _ _ _ _ _
_
25 DP 2 0.4 0.3 0.6 0.2 0.
Mannitol 1.0 1.1 0.9 0.7 1.1
Other hexitols 0.3 0.2 0.2 0.3 0.1
Glycerin 0.15 0.1 0.1 0.2 0.1
30 SORBITOL ¦ 98.15 98.3 98.2 98.6 98.6
Reducing sugars 0.025 0.010 0.022 0.010 0.040
Total sugars 0.11 0.15 0.20 0.09 0.13
- ~311~3
21
_BLE IX
ANALYSIS OF THE POLYOLS INTRODUCED BEFORE HYDROLYSIS
Nature of the Content in /.
constituent
S
DP > 3 18.7 20.2 21.3 16.9 16.4
DP 3 15.9 16.1 17.0 14.2 13.7
DP 2 48.0 49.2 51.9 55.8 57.4
SORBITOL 17.4 14.5 9.8 13.1 13.5
Reducing sugars 0.17 0.14 0.11 0.14 0.14
Total sugars 27.1 33. a 34.5 29.6 28.7
The more detailed composition of a sample of this
polyol fraction before hydrolysis is given in Table X :
TABLE X
Nature of the Content ln /
constituent
_
Sorbitol ` 12.8
Mannitol 1.9
DP 2 50.4
DP 3 14.9
DP 4 3.0
DP 5 2.1
DP 6 2.9
DP 7 2 8
DP 10 to DP 20 5.6
DP > 20 0.9
From all these analyses, it again emerges that the
quality of sorbitol produced under these conditions with a
total yield from a hydrol from a first crystallisation of
dextrose is at least equal to that obtained by hydrogena-
tion of a pure dextrose.
Reference will be made, by way of comparison, with
the compositions, given in Example 1, of sorbitol obtained
by dextrose hydrogenation.
i 1311503
22
T~e polyols extracted throughout the balanced ope-
ration of the chromatography had a true sorbitol content
less than 15-/., which indicates an effective extraction of
the sorbitol, higher than 90Z of the content of sorbitol
of the material processed.
EXAMPLE 3
This example illustrates the production of pure
sorbitol from a H2 hydrol of a second crystallisation crop
of dextrose, titrating about 78'Z of true dextrose.
Reference is made again to Figures 1`and 3.
a) Hvdroqenation steo
The syrup obtained after purification and hydroge-
nation had the composition indicated in Table XI:
TA8LE XI
Nature of the Content in Z
constituent
DP > 3 3.5
DP 3 3.1
DP 2 11.1
- 20 Mannitol 1.0
Other hexitols0.3
Sorbitol 78.0
Reducing sugars 0.48
. Total sugars7.1
z5 b) ChromatoqraPhv steo
The above-said syrup was supplied at a flow rate
of 103.2 l/h for a content of dry matter of 63.5~/. through
pipe 207 to the continuous liquid chromatography installa-
tion 202 described previously ; the density of the syrup
was 1.264.
The -Feed flow rate of water through the pipe 212
of this installation was 341 l/h, to which must be added a
flow rate of 166 l/h of recirculated water (excess water
cominq from the total de-sugaring during the preceeding
sequence of the first pure sorbitol desorption stage)
introduced through the pipe 210.
1311~03
The sorbitol extraction pump was regulated to 200
l/h to obtain at the same time excellent sorbitol quality
as well as maximum extraction ratio.
Hence 200 l/h of sorbitol was extracted with a dry
matter content of 28A2~.~ namely 62.04 kg/hour through the
pipe 208.
Through the pipe 209 was extracted a syrup which
had to be subjected to hydrolysis, the flow rate being 134
l/hour, the density 1.04 and the dry matter content 13.7~,
namely 18.9 kg/hour.
Through the pipe 211 was extracted a discarded
syrup with a flow rate of 160 l/hour, the dry matter con-
tent being 1.7'~, namely 1.87 kg/hour.
Analysis was made as a function of time of both te
sorbitol extracted from the installation and the output of
the polyol fraction.
The results are collected in Tablex XII and XIII.
TA8LE XII
ANALYSIS OF THE SORBITOL
Tlme 31 iK DP > 3 0P Z Mannitol ~ Sorbitol
0 56 0.2 D.5 1.1 0.3 97.9
2 54 0.2 0.4 1.0 0.3 98.0
4 48 0.2 0.4 1.0 0.4 97.9
6 4.0 0.1 0.3 1.0 0.3 98.1
835.5 0.1 0.2 1.0 0.3 98.4
1 n 31 0.2 1.0 0.3 98.5
12 26 0.2 0.9 0.4 98.5
14 21 0.3 0.9 0.3 98.4
16 16 0.3 0.8 0.3 ~38.6
0.2 0.7 0.3 98.9
22 8 0.2 0.6 0.3 98.9
30 2~ 6 0.2 0.6 0.3 98.9
1311~
24
TA3lE XIII
ANALYSIS OF THL POLYOLS FRACTION
24 Z3.0 21 16.7 54.6 1.4 0.4 6
c) Continuous hvdrolvsis ste~
The syrup recovered for the purposes of hydrolysis
at the outflow of the installation 202 is led to the hy-
drolysls vessel as described in Fxample 1.
The temperature therein was kept at 110 C. The
degree of hydrolysis of the polyholosides for a flow rate
of 130 l/h on a bed of 20 liters of adsorbant was always
higher than 907..
The degree of hydrolysis was, as in the preceeding
examples, obtained by comparing the ratio of reducing su-
gars expressed in glucose equivalents, found in the syrupflowinq out from the hydrolysis vessel, with that found in
a specimen subjected to the action of normal sulfuric acid
under reflux.
d) steP of dilution of the hydrol H2 introduced
throuqh the oioe 204a. before hydroqenation
with the svru~ flowinq from the hvdrolvsis
vessel and conducted throuah the Pipe 204b.
93.4 kglhour of hydrol H2 with 74/. of dry matter
was diluted with 134 l/hour of hydrolysed polyols with
35 13.7/ of dry matter, introduced through the pipe 204b, the
solution obtained representing 230 kg/hour of a syrup with
about 36/. of dry matter.
1311~03
Tllis syrup was hydrogenated, purified and evapo-
rated to 63.5/. of dry matter, then led to the continuous
chromatography installation 202.
After several days of operation, analysis was car-
ried out of five samplings of the raw material constituted
by the hydrol diluted with the syrup introduced through
204b (Table XIV) and hydrogenated, five samplings of the
sorbitol obtained ITable XV) and four samplings of the
syrup introduced through the pipe 209 (Table XVI).
TABLE XIV
HYDROL DILUTED WITH HYDROGENATED POLYHOLOSIDES
HYDROLYSED AND THEN HYDROGENATED
. _
Nature of the Contents in X
constituent
DP > 3 5.2 6.6 4.5 5.4 4.3
_
DF 3 3.3 4.3 3.1 2.8 3.2
DP 2 10.2 14.0 11.7 12.4 11.7
20 Mannitol 1.2 1.3 1.2 1.3 1.5
SORBITOL 79.8 75.4 79.2 79.0 79.0
Other heuitols 0.3 0.4 0.3 0.2 0.3
25 Reducing sugars 0.05 0.045 0.048 0.035 0.07
Total sugars 6.9 7.2 7.5 7.0 8.0
26 ~ 1311~03
TABLE XV
SOR0ITOL
Nature of the Contents in ~.
constituent
S _ _
DP 3 _ 0.1 0.05 0.05 0.1
.
DP 2 0.2 0.2 0.2 0.3 0.3
Mannitol 0.9 0.9 1.1 1.1 1.2
Other hexitols 0.5 O.Z 0.2 0.3 0.1
SORBITOL 98.4 98~6 98.45 98.25 98.3
_ . ..
Reducing sugars 0.011 0.027 0.028 0.01 0.03
. _
Total sugars 0.04 0.2 0.12 0.15 0.13
_ . .
Results of analyses collected in Tables XIV and
XV, show that the sorbitol quality produced under these
conditions from hydrol H2 had a purity at least equal to
that obtained by hydrogenation of a pure dextrose and .is
characterised by a very low content of reducing sugars.
TABLE XVI
POLYOL SYRUP INTROOUCED THROUGH THE PIPE 209
DP > 3 19.2 21.7 21.0 20.7
.
DP 3 15.9 16.9 16.~ 16.4
. .. __ _
DP 2 51.6 56.1 54.6 54.8
_
Mannitol 3 2 1.8 2
_ __
SORBITOL 10 2.9 5.8 5.7
. .
Reducing sugars 0.3 0.4 0.40 0.4
- _ .
Total sugars 35.0 38.2 36.4 32.2
:
From the results collected in Table XVI, it is
deduced that the polyols extracted throughout the balanced
27 ' 13~1503
operation of the chromatography installation had a sorbi-
tol content below 10~, which indlcated a very efficient
extraction of the sorbi-tol, higher than about 9~7. of the
content in sorbitol of the raw material.
As is self-evident and as emerges already besides
from the foregoing, the invention is in no way limited to
the embodiments and applications which have been more par-
ticularly envisaged ; it encompasses, on the contrary, all
modifications.