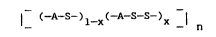Note: Descriptions are shown in the official language in which they were submitted.
1 3 ~
PROCESS FOR THE PREPARATION
OF COPOLY(ARYLENE SUL~IDE)
The invention relates to a process for the
preparation of a copoly(arylene sulfide) by heating a
diiodoaromatic compound in the presence of elemental
sulphur.
Poly(arylene sulfide) (PAS) resins are thermo-
setting-thermoplastic polymeric materials with good
thermal stability, unusual insolubility, resistance
to chemical environments and inherent flame
resistance. PAS resins additionally have good
electrical insulative properties which make them
ideal for electrical and electronic applications.
Their excellent resistance to chemical degradation
makes them ideal for use in chemical environments
which involve organic solvents and strong mineral
acids, such as coatings for pipes, tanks, pumps and
other equipment.
Poly(phenylene sulfide) (PPS) is a commercial
product which is generally produced by reacting
p-dichloro-benzene with sodium sulfide in a polar
organic solvent to produce PPS and the by-product
sodium chloride. This process is known as the
Macallum polymerization procedure and the basic
process is disclosed in U.S. 2,513,188 and U.S.
2,583,941. An improvement on the Macallum polymeri-
zation procedure involves adding N-haloamides as
catalysts in the procedure (U.S. 3,285,882). The
Macallum polymerization utilizes only chloroaromatic
compounds.
The PPS which is formed in the Macallum process
has only a modest molecular weight on the order of
10,000-40,000 and has relatively low melt viscosity.
Higher molecular weights can be obtained by heating
the PPS in the presence of oxygen. During heating,
~k
7 I r
-- 2 --
the molecular weight of the PPS increases due to a
variety of chemical reactions including oxidation,
crosslinking and chain extension. These curing
reactions result in polymers which have inherent
brittleness and reduced drawing capability while only
achieving modest increases in molecular weight.
Additionally, PPS which is produced by polymerization
in the presence of sulfide and/or hydrosulfide salts,
such as sodium sulfide and sodium hydrosulfide, has a
residual content of inorganic salt present in the
polymer. These residual salts are, for example,
sodium chloride and sodium sulfide resulting from the
combination of the sodium cation with chlorine or
sulfide from the starting materials. The presence of
these residual salts in the polymer increases the
corrosive nature of the polymer and can cause a
deterioration in the drawing or spinning character-
istics of the polymer. Residual salts can also
result in breakages in the spun fibers and addi-
tionally contribute to plugging and clogging of thespinnert holes.
An additional problem with poly(arylene sulfide)
produced by the Macallum process is the effect of
residual salts on the electrical properties. The
presence of residual salts results in polymers with
increased moisture adsorption and electrical
activity, which are detrimental to applications
requiring highly insulating characteristics.
Although extensive extraction reduces the salt
content of PPS produced by the Macallum process,
complete removal of these salts is commercially
infeasible.
An additional problem with PPS produced by the
Macallum process is the high rate of crystallization
of these polymers. Although some applications do
~ 3 1~
- 3 -
require high rates of crystallization, many appli-
cations require much slower rates of crystalliza-
tion. These polymers contain no substantial
quantities of disulfide units.
U.S. 4,645,826 discloses a process of preparing
"ultra-high molecular weight" linear PAS by first
preparing a prepolymer with a melt viscosity between
5-3,000 poise and then performing a liquid-liquid
two-phase polymerization. Only dichloroaromatic
compounds are disclosed and the prepolymer is formed
using a conventional alkaline metal sulfide. The
"ultra-high molecular weight" polymers have melt
viscosities of only tens of thousands of poise. The
prepolymer is formed by a standard Macallum polymeri-
zation in the presence of an alkali metal sulfide.
Accordingly, the polymers produced will suffer from
the problems associated with residual salt content
noted above. These polymers are also thought to
contain no substantial quantities of disulfide units.
U.S. 4,645,825 also discloses poly(arylene
sulfide) produced using dichloroaromatic or dibromo-
aromatic compounds and polymerizing in the presence
of conventional alkaline metal sulfides or hydro-
sulfides. Although polymers with relatively high
molecular weights and melt viscosities can be
produced by this process, the presence of residual
inorganic salts in the polymer results in inferior
corrosion characteristlcs as well as poor spinning
and drawing capability. These polymers are also
thought to have no substantial quantities of
disulfide units.
We have now discovered a process which can be
used to prepare a poly(arylene sulfide) which does
not contain substantial quantities of alkali metals
and has an adjustable rate of crystallization. The
polymers prepared using the process of the invention
1~J1 $7
~ 4 --
do not contain substantial quantity of alkali metals
simply because no alkali metal is used in the process
used to prepare the polymer. Although Applicants do
not wish to be limited to any particular theory, it
is believed that the variable rate of crystallization
of the copolymer prepared using the process is due to
the presence of small amounts of (-A-S-S-) or
disulfide units in the polymer chain. Thus, the
polymers prepared by this process can be considered
to be copolymers. The presence of these disulfide
units in the copolymer do not materially affect other
important properties of the polymer, such as glass
transition temperature, solvent resistance, thermal
stability, and oxidative stability.
The vast majority of units in the copolymer
prepared by the process of this invention are the
(-A-S-) unit and the number of (-A-S-S-) or disulfied
units are small compared to the number of (-A-S-)
units. Generally, the number of (-A-S-S-) units is
20 in the range of 0.5 to 0.001, based on the combined
number of both (-A-S-) and (-A-S-S-) units. Thus,
the copolymer prepared by the process of the
invention can be represented as
(-A-S~ X(-A S S )x
where x is in the range of 0.5 to 0.001. The
sequence of (-A-S-) and (-A-S-S-) units is thought to
be random throughout the molecular chain. When x is
in the range of 0.5 to 0.2 the polymers obtained when
A is p-phenylene are amorphorus and can be
crystallized only with difficulty. When x is in the
range of 0.2 to 0.1 the polymers obtained can be
thermally crystallized and have crystalline melting
points of 230-260 degree C. When x is in the range
of 0.1 to 0.05 the polymers obtained have moderate
- 5 -
crystallization rates and the crystallized polymers
can be annealed to high crystalline melting points
(280-290C) and show Tch (temperature of crystalli-
zation on heating) and Tcc (temperature of crystalli-
zation on cooling) at increasingly lower and highertemperatures, respectively, indicative of increasing
rates of crystallization. When x is in the range of
0.05 to 0.001 the crystallization rate increases
rapidly with decreasing x.
The following table more clearly shows the
effect of disulfide units on the crystallization rate
of poly(phenylene sulfide):
X T~ Tcc Tch Tm T 1/2 (130C)
-
0.25 88 -- - 238
150.14 90 - - 251
0.12 94 - - 255 132 Seconds
0.10 92 168 - 243
0.064 94 142 231 280
0.055 95 140 226 278
200.049 95 126 240 280
0.000 91 126 242 278 12 Seconds
The size of the polymer chain can conveniently
be expressed as the total number of each kind of unit
in the'chain. Therefore, the copoly(arylene sulfide)
prepared by the process of this invention can be more
specifically expressed'as corresponding to the
structure
I (-A-S-)l_X(-A-s S )x _I n
wherein n, the degree of polymerization, is at least
200 and is preferably in the range of 500 to 5,000 as
determined by melt viscosity measurement at 300C.
The degree of polymerization when A is p-phenylene
7 `
can be calculated using the relationship log(n) =
1.473 + 0.2873 x log(melt viscosity) where melt
viscosity is measured in poise.
In the process of the present invention a
diiodoarylene compound corresponding to the
structure
I-A-I
where A is a divalent arylene radical is reacted with
elemental sulfur to produce a substantially linear
copoly(arylene sulfide) having both (-A-S-) units and
(-A-S-S-) units.
Diiodoaromatic compounds which can be utilized
in the present process include unsubstituted or
substituted aromatics which have two iodine substitu-
ents. Suitable diiodoaromatic compounds includehydrocarbon aromatics, nitrogen-containing aromatics,
sulfur-containing aromatics and oxygen-containing
aromatics. Typical hydrocarbon aromatics include
benzene and biphenyl, and condensed rin8 aromatics
such as naphthalene and anthracene. Typical sulfur-
containing aromatics include, for example, thiophene
and benzothiophene. Typical nitrogen-containing
aromatics include pyridine and quinoline. Suitable
oxygen-containing aromatics are, for example, furan,
dibenzofuran, etc. Substituted diiodoaromatic
compounds suitable for use with the present invention
include aromatic sulfones, diarylethers, diaryl-
carbonyls, diarylsulfides and the like.
The aromatic starting materials may be substi-
tuted by one or more alkyl groups, preferably alkylgroups having from 1-6 carbon atoms. Specially
preferred alkyl groups are methyl, ethyl, propyl and
butyl groups. There is no limitation on the spatial
arrangement of the substituents, for example, the
substituents may be on a carbon adjacent to an iodine
131 q ~ r~7 ~
bearing carbon or may be on a carbon atom further
removed from the iodine bearing carbon.
Additional substituents on the aromatic
compounds may include phenyl, halogen, hydroxy,
nitro, amino, Cl_6 alkoxy, and carboxylate and
carboxylic acid substituents, as well as aryl
sulfones and aryl ketones.
Preferred diiodoaromatic compounds are the
diiodobenzenes, diiodonaphthalenes, diiodobiphenyls,
diiododiphenyl ethers and diiodotoluenes which may be
unsubstituted or substituted with any of the
substituents noted above.
Specific diiodoaromatic compounds suitable for
the present invention include p-diiodobenzene,
m-diiodobenzene, p,p'-diiodobiphenyl, m,p'-diiodo-
biphenyl, p,p'-diiododiphenyl sulfone, p,p'-diiodo-
diphenyl ether, 2,6-diiodonaphthalene, and
p,p'-diiodobenzophenone. p-Diiodobenzene,
p,p'-diiodobiphenyl, and p,p'-diiododiphenyl ether
are most preferred.
The diiodoaromatic starting materials of the
present invention may be prepared by any suitable
process. For example, the diiodoaromatic compounds
may be prepared by standard liquid or gas phase
iodination reactions.
Sulfur is reacted as elemental sulfur and may
consist of any of the standard forms which are
possible for elemental sulfur. That is, the sulfur
may be present in any of its allotropic modifications
such as orthorhombic cyclooctasulfur (S8) or any
other cyclic elemental sulfur such as any of the
cyclosulfur species having 6-12 sulfur atoms.
Additionally, any crystalline form of sulfur may be
used in the present reaction. Surprisingly,
impurities in the elemental sulfur do not appear to
affect the efficiency or selectivity of the present
r~
- 8 -
polymerization reaction. The sulfur preferably has a
purity of 98%-100%, although sulfur having a lower
degree of purity may be used. This lack of
sensitivity to the presence of impurities in the
sulfur is advantageous to the present process when
used as a commercial process since highly purified
sulfur is not required and the associated expense is
not incurred.
In the process of the present invention sulfur
reacts wlth a diiodoaromatic compound, eliminating
elemental iodine and forming the PAS as shown below.
2 > (-Ar-S-)n + nI2
The formation of polymer is not sensitive to the
relative stoichiometry of the diiodoaromatic compound
and sulfur. Accordingly, an excess of sulfur or an
excess of diiodoaromatic compound may be used in the
polymerization process. When excess sulfur is used,
some disulfide linkages are observed in the polymer.
Decreasing amounts of sulfur result in decreasing
levels of disulfide linkages in the final polymer.
When the diiodoaromatic compound is present in
excess, polymerization to high polymer can still
occur, if the excess diiodoaromatic compound is
removed during final polymerization.
The polymerization reaction is preferably
carried out in the absence of solvent by merely
heating and reacting the sulfur and diiodoaromatic
compound. Under these conditions, the diiodoaromatic
compound itself acts as a solvent for the sulfur
which is melted thereby forming a substantially
homogeneous solution enabling a facile and complete
reaction.
In another embodiment, the diiodoaromatic
compound can be dissolved in an organic solvent which
is inert to the reaction conditions, i.e., which is
13~
inert to reaction with iodine and sulfur. High
boiling inert aromatic solvents are preferred such
as, for example, aromatic hydrocarbons, diaryl-
sulfides, diarylethers and diarylsulfones. It is
preferable to use a solvent which corresponds to the
diiodoaromatic compound which is being polymerized.
Thus, for example, in the polymerization of diiodo-
benzene with sulfur, one might use benzene, toluene
or naphthalene as a solvent.
It is also possible to perform the polymeriza-
tion reaction of the present invention by solid state
polymerization. Solid state polymerization enables
very high molecular weights and melt viscosities to
be achieved. After an initial melt polymerization
(or alternatively solution polymerization) has been
performed, the product is cooled to a solid state.
Further heating and polymerization in the solid state
under vacuum or inert gas flow dramatically increases
the molecular weight allowing weight average
molecular weights in excess of 100,000 to be
achieved. It is significant to note that substan-
tially no cross-linking occurs during the solid state
or melt polymerization processes. The very high
molecular weight copolymers which are produced after
the solid state polymerization are still substan-
tially linear and have excellent film and fiber
forming properties.
During the polymerization reaction between the
diiodoaromatic compound and sulfur elemental iodine
is produced and evolves from the reaction melt or
solution, or solid. Removal of the elemental iodine
provides a driving force for completion of the
polymerization reaction. The iodine may be removed
by passing a stream of air or ~n inert gas such as
nitrogen or argon over or through the reaction mass
at atmospheric or superatmospheric pressure or
1 3 ~
- 10 -
alternatively by applying a vacuum to the reaction
apparatus. The elemental iodine may be collected and
used as a commercial product or as a reactant for
further chemical processes. The present reaction,
therefore, does not result in wasted reaction
products since both the PAS and elemental iodine are
useful commercial chemical products.
The polymerization reaction is generally
conducted at a temperature above 175C. Although the
reaction may be conducted at temperatures below
175C, the polymerization reaction is much slower.
There is no particular upper temperature limit on the
polymerization reaction, which may be conducted at
any temperature below the decomposition temperature
of the diiodoaromatic compound. For most polymeri-
zation reactions, temperatures in the range of
175-400C will be suitable, although for particular
diiodoaromatic compounds temperatures in excess of
400C may be used. Particularly preferred
temperature ranges are from 180-350C.
The reaction is generally conducted for a period
of at least one-half hour and is continued for up to
10 hours or longer, and reaction times approaching
infinity are theoretically possible~ The exact
reaction time will depend on the diiodoaromatic
compound, the engineering requirements of the
process, and the specific molecular weight, viscosity
and physical properties of the desired product.
The polymerization reaction may be carried out
in a batch reaction vessel or may be carried out as a
semi-continuous or continuous process. Agitation of
the reaction mixture is optional, however agitation
or stirring assists in the production and yield of
the polymeric product. Agitation of the reaction
mixture may be accomplished by any known method, such
as mechanical stirring or by passing a stream of
inert gas through the reaction mixture.
In a preferred embodiment, the polymerization
reaction is conducted on a continuous basis with the
diiodoaromatic compound and sulfur being combined in
a continuous staged reactor to form a reaction melt.
An inert gas such as nitrogen or argon is passed
through the melt, preferably in a countercurrent
direction, thereby accomplishing agitation and mixing
of the reaction melt and at the same time removing
the elemental iodine which is evolved and sweeping it
out of the reactor. Alternatively, a vacuum may be
applied to the reactor to remove the elemental iodine
as it is generated. It should be noted that the
reaction proceeds equally well under batch conditions
and combinations of batch and continuous processes
are considered to be well within the scope of the
present invention.
The copolymer produced by the process of this
invention is useful for preparation of various shaped
articles such as pellets, fibers and molded
articles. The polymer can be prepared into these
shaped articles by conventional processes, such as
injection molding and melt spinning.
Since there are no alkali metal containing
materials in the reaction, there are no substantial
quantities of alkali metal in the polymer matrix.
Typically, there is less than 100 weight parts per
million alkali metal, preferably less than 10 weight
parts per million, based on the weight of the
copoly(arylene sulfide). The absence of substantial
quantities of alkali metal greatly enhance the
capability of the polymer to be melt processed,
particularly melt spun into fibers.
The copoly(arylene sulfide) and particularly the
copoly(phenylene sulfide) produced by the process of
~31~ ~
- 12 -
this invention have an adjustable rate of crystalli-
zation, due to the presence of the disulfide
linkages. Since the concentration of disulfide
linkages can be varied over a wide range, the rate of
crystallization can be readily adjusted to suit the
technological application without unduely sacrificing
other desirable characteristics of the polymer. In
addition, the rate of crystallization can be further
enhanced by the addition of conventional nucleating
aids such as talc, terephthalic acid, silica or the
like for those applications where extremely fast
rates are desired.
Other features of the invention will become
apparent in the course of the following descriptions
of exemplary embodiments which are given for illus-
tration of the invention and are not intended to be
limiting thereof.
EXAMPLES
The reactions described in Examples 1-17 were
carried out in a stirred flask fitted with a vacuum-
jacketed Vigreux column and a receiver cooled in dry
ice. The temperature/pressure profile used was
230C/120 Torr for three hours, 230C/4 Torr for
another hour, then raising the temperature to 250C
for one hour, and finally raising the temperature to
280-300C for the last 1-3 hours. Fiber-forming
capabilities of these polymer were established by
drawing strands from the polymer melt. Some polymers
were tested to determine the value of x or the number
of (-A-S-) units and (-A-S-S-) units in the polymer
chain. In some cases the weight parts per million
alkali metal and crystallization rate were determined.
The weight parts per million alkali metal, based
on the weight of the poly(arylene sulfide) were
determined by atomic adsorption analysis.
~ 3 ~ ~ ~ 7 ~
- 13 -
The crystallization rate was determined by
differential scanning colorimetry half-times or by
comparing the Tcc and Tch for the polymer in question
to that of a polyphenylene sulfide homopolymer. All
DSC analyses were run at 20C/min scan rate under
N2 ~
The degree of polymerization (n) was determined
by measuring melt viscosity and applying the
relationship log(n) = 1.473 + 0.2873 x log(melt
viscosity).
Melt viscosity was determined on a Rheometrics
Mechanical Spectrometer (Model RMS-7220) at 300C and
25 radians/second. All samples were predried in a
vacuum oven and run under air.
The value of x for moderate valves of x were
determined by elemental analysis and calculation
based on the excess sulfur present. For low values
of x the values can be determined by digestion of the
polymer by concentrated nitric acid, which oxidizes
all disulfide linXages to sulfonic acid. Titration
for sulfonic acid determines the amount of disulfide
present.
ExamPle 1
200.0 Grams of p-diiodobenzene and 20 0 grams of
elemental sulfur (3.0% excess) were reacted as noted
above. A hard, glassy polymer was obtained which
could be pressed into rigid, creasable films. The
fibers obtained could be knotted without breaking.
The material was insoluble in 1,2,4-trichlorobenzene
at 100C, and dissolved slowly over two hours in
chloronaphthalene at 210C. The infrared spectrum of
the pressed film was identical to that for authentic
poly(phenylene sulfide). Elemental analysis of the
polymer was consistent with the empirical formula
(C6H4Sl 10) The yield of the polymer was
~ 3~7'~
- 14 -
60 grams. The alkali metal content was <5 ppm.
The polymer was thermally crystallizable with a Tm of
243C, a Tg of 91.4C and Tch of 168C (first
cycle). The value of x was 0.10. The melt viscosity
was 20,000 P, giving a value of n of 511. Amorphorus
pressed films had a density of 1.331 g/cc and
contained 890 ppm total iodide. Thermal gravimetric
analyses showed 5% weight loss of 475C (scanning at
10C/min, in air and N2).
ExamPle 2
50.0 Grams of 2,6-diidonaphthalene and
4.35 grams of elemental sulfur (3.0% excess) were
reacted as noted above. A hard, glassy brittle
polymer was obtained. DSC found a Tg of 182C. The
alkali metal content was <5 ppm.
ExamPle 3
50.0 Grams of 4,4'-diiodobiphenyl and 4.06 grams
of elemental sulfur (3.0~ excess) were reacted as
noted above. After two hours, the reaction mixture
became solid. Reaction was continued at 250C for
two more hours, then the product was removed and
powdered. The powder was then solid-state
polymerized at 250C for 24 hours. The powder
yielded a creasable film when melt-pressed at 450C.
ExamPle 4
205.0 Grams of p-diiodobenzene and 20.0 grams of
sulfur (0.25% excess) were reacted as noted above.
The resulting polymer could be drawn into long fibers
and pressed into creasable films. The material had a
measured Tg of 88-91C.
1 3 ~
- 15 -
ExamPle 5
211.0 Grams of 4,4'-diiododiphenyl ether and
16.0 grams of sulfur (stoichiometric amount of
sulfur) were reacted as noted in Example 1. 98 Grams
of a very tough, glassy polymer were obtained. The
material had a measured Tg of 100C.
ExamPle 6
205.0 Grams of p-diiodobenzene, 0.10 grams of
iodonitrobenzene, and 10.0 grams of sulfur (50~ of
theoretical) were reacted as noted above, except the
final vacuum was < 1 torr. A large amount of
p-diiodobenzene distilled out under polymerization
conditions. The final polymer was highly viscous,
and yielded creasable pressed films. The polymer
yield was 31.9 grams. DSC found 8 Tg of g4C; the
density of the amorphous film was 1.34 grams/cc.
ExamPle 7
153.7 Grams p-diiodobenzene (0.466 moles),
63.1 grams 4,4'-diiodobiphenyl (0.155 moles)
20.0 grams sulfur, and 0.10 grams p-iodonitrobenzene
were polymerized as in Example 6. The material
yielded a tough, creasable film with a measured Tg of
125C. The final polymer contained 400 ppm total
iodine.
ExamPle 8
102.5 Grams p-diiodobenzene (0.31 moles),
126.14 grams 4,4'-diiodobiphenyl (0.31 moles)
20.0 grams sulfur (0.62 moles), and 0.1 grams p-iodo-
nitrobenzene were polymerized as in Example 6. The
material yielded creasable films with a measured Tg
of 152C. Elemental analysis expected for this
polymer is C = 73.95%, H = 4.11%. Found C = 72.2~%,
H = 4.05%).
~3~7 ~
- 16 -
ExamPle 9
51.25 Grams p-diiodobenzene (0.155 moles),
51.25 grams m-diiodobenzene (0.155 moles) 10.0 grams
sulfur (0.311 moles), and 0.1 grams p-iodonitro-
benzene were polymerized as in Example 6. Thematerial yielded brittle films with a measured Tg of
68C. Infrared analysis of these films showed peaks
at 777 and 876 cm 1 unique to meta-substituted
benzene, and a peak at 812 cm 1 unique to
para-substituted benzene.
ExamPle 10
205.0 Grams of p-diiodobenzene, 19.0 grams of
sulfur (95% of theory) and 0.10 grams p-iodonitro-
benzene were polymerized as in Example 6. The finalpolymer yielded tough, creasable films. Infrared
analysis of the pressed film showed the character-
istic strong peak at 812 cm 1 characteristic of
para-substituted benzene, and no detectable peaks at
777 and 876 cm 1, characteristic of meta
substituted benzene. The infrared spectrum was
indistinguishable from that of a pressed film of
Ryton (trademark) P-6. This demonstrates that
substantially no positional isomerization of the
aromatic compound occurs during polymerization.
ExamPle 11
200.0 Grams of p-diiodobenzene, 19.5 grams
sulfur (0.3% excess) and 0.4 grams 1,3-diiodo-
5-nitrobenzene were polymerized in Example 6. Long
fibers could be pulled from the melt, and pressed
films were tough, creasable, and thermally crystalli-
zable. DSC analysis revealed a Tg of 94C and a Tm
of 255C (first cycle). The melt viscosity at 300C
was 70,000 P, giving a degree of polymerization (n)
of 733. The half-times for crystallization from the
13 1 ~
glass were 135 seconds at 120C, 132 seconds at
130C, 130 seconds at 140C, and 120 seconds at
160C. DSC found no Tcc or Tch. Elemental analysis
found C = 64.30%, H = 3.68%, I = 646 ppm, Cr = 9 ppm,
Ni = 14 ppm, Fe = 110 ppm. The empirical formula for
P Y 8 4 1.12'
ExamPle 12
A sample of commercial Ryton P-6 PPS was
analyzed. The melt viscosity at 300C was 770 P
DSC analysis revealed a Tg of 91.0C and a Tm of
278C (second cycle), Tm = 282C (first cycle), Tch =
126C, Tcc = 242C. The half-times for crystalli-
zation from the glass were 24 seconds at 120C and 12
seconds at 130C. A sample of Ryton P-4 had a melt
viscosity of 3500 P at 300C and DSC behavior
identical to the Ryton P-6. Thermal gravimetric
analysis showed 5~ weight loss at 500C (scanning at
10C/min in air and nitrogen). Elemental analysis
found C = 66.37%, H = 3.70%, Cl = 0.20%, Na = 0.13%,
K = 48 ppm, Ca = 326 ppm, Mg = 149 ppm, Fe = 40 ppm,
Ni = 11 ppm, Cr = 6 ppm, corresponding to an
empiric 6 4 1.00
basis. The mole fraction of disulfide units (x) is
0.000.
ExamPle 13
410.0 Grams of p-diiodobenzene (1.24 moles),
38.0 grams sulfur (1.19 moles) and 0.2 grams p-iodo-
3Q nitrobenzene were polymerized as in Example 6, except
the final reaction temperature was 250C. The
polymer obtained could be pressed into brittle
films. DSC showed a Tg of 85C.
1 3 ~
- 18 -
ExamPle 14
80 Grams of the polymer obtained in Example 13
was granulated and crystallized by contacting with
toluene. After drying the solid polymer was divided
into 4 portions and was solid-state polymerized under
vacuum at 210C for 3, 6, 12, and 24 hours. Melt
viscosity was measured on these samples at 300C.
The results are tabulated below:
10 Solid--
Stating Melt
Time, Vis-
Hour cositY n ~ Tm C,H x
3 5566 354 88C 230C62.25 3.56 .233
6 7003 378 88C 234C63.31 3.58 .175
15 12 13151 453 88C 238C61.94 3.52 .252
24 18440 500 88C 242C61.85 3.55 .256
These polymers exhibited no Tcc or Tch under the DSC
conditions, consistent with a difficultly crystalli-
zable polymer.
ExamPle 15
410.0 grams p-diiodobenzene (1.24 moles),
38.0 grams sulfur (1.19 moles) and 0.2 grams p-iodo-
nitrobenzene were polymerized as in Example 6 except
that the final temperature was 275C. The resultant
polymer had a melt viscosity of 11,450 poise at
300C. The polymer was thermally crystallized at
175C, then solid-state polymerized at 210C for
21 hours. The resultant polymer had a melt viscosity
at 300C of 40,180 poise and a value of n of 625.
The polymer had a Tg of 90.2C, Tm of 251C, no Tcc
or Tch on second DSC cycle. Elemental analy~ls found
C = 64.00%, H = 3.65%, consistent with the formula
C6H4S1 14; therefore x = 0.14 in this polymer.
13~ 7:
- 19 -
ExamPle 16
410.0 Grams of p-diiodobenzene (1.24 moles),
38.0 grams of sulfur (1.19 moles) and 0.2 grams
p-iodonitrobenzene were polymerized as in Example 6
(300C final temperature). The polymer had a
measured melt viscosity of 48,830 poise at 300C.
Solid-state polymerization as in Example 15 yielded a
polymer with a melt viscosity at 300C of
130,900 poise and a value of n of 877. The final
polymer had a Tg of 89.1C, Tm of 250C, no Tch or
Tcc on second DSC cycle. Elemental analysis found
C = 63.97, H = 3.69; therefore x = 0.135 for the
polymer.
Example 17
410.0 grams p-diiodobenzene, 38.0 grams sulfur,
and 0.8 grams diiodonitrobenzene were polymerized as
in Example 6, except that the final reaction
temperature was 250C. The low molecular weight
polymer crystaIlized on cooling. DSC analysis of
this prepolymer showed a Tg of 73C, Tm of 248C, and
Tch (temperature of crystallization on heating) of
175C. The prepolymer was ground and solid-state
polymerized for 16 hours at 260C. The solid-state
polymerized PPS obtained had a measured Tg of 94C
and Tm of 288C, and a melt viscosity of 69,080 poise
at 300C with a value Df n of 730.
ExamPle 18
410.0 grams of p-diiodobenzene (1.24 moles)
34.00 grams of sulfur (1.06 moles) and 0.80 grams
diiodonitrobenzene were polymerized as in examples
except that the final polymerization temperatures
were 250C. The low molecular weight polymer
crystallized rapidly on cooling and exhibited a
7~
- 20 -
second cycle Tg of 84.5C, Tch of 162.6C, Tm of
272C, and a Tcc of 197C. 10 grams of this polymer
was ground to pass a 3 mm screen and was then solid-
state polymerized at 240C for 23 hours under
vacuum. The resultant polymer had a first cycle Tm
of 285.3C, and a second cycle Tg of 94.7C, Tch of
170.5C, Tm of 267.3C, and Tcc of 177.8C.
Elemental analysis found C = 65.58%, H = 3.56%,
consistent with the formula C6H4S1 057. For
this polymer x = 0.057.
ExamPle 19
10 grams of the prepolymer of Example 18 was
solid state polymerized at 260C for 25 hours under
vacuum. The resultant polymer a first cycle Tg of
101C, Tm of 288C, second cycle Tg of 98.5C, Tch of
145.4C, Tm of 265.4C, and Tcc of 198.8C.
ExamPle 20
410.0 grams p-diiodobenzene, 36.0 grams sulfur,
and 0.80 grams diiodonitrobenzene were reacted as in
Example 6, except that the final temperature was
250C. This polymer which crystallized rapidly on
cooling, showed a second cycle Tg of 78C, Tch of
135C, Tm of 271C, and Tcc of 212C. Solid-state
polymer-ization of the ground polymer at 240C for
20 hours yielded a polymer with a first cycle Tm of
284C, and second cycle Tg of 95.4C, Tch 171.6C, Tm
of 271C, and Tcc of 189C. Melt viscosity at 300C
was 107,900 poise, corresponding to a molecular
weight of 179,270 and a degree of polymerization (n)
of 830.
ExamPle 21
410.0 grams p-diiodobenzene, 36.00 grams sulfur,
and 0.80 grams diiodonitrobenzene were reacted as in
~ 3 ~
- 21 -
Example 6 with the final polymerization temperature
300C. The resultant high molecular weight polymer
crystallized on cooling. Solid state polymerization
at 240C under vacuum for 20 hours yielded a polymer
with a Tg of 98.1C and a Tm of 280C. Melt
viscosity at 300C was 410,069 poise, corresponding
to a molecular weight of ~63,000 and a degree of
polymerization (n) of 1,218.
Example 22
410.0 grams p-diiodobenzene, 32.00 grams sulfur,
and 0.80 grams diiodonitrobenzene were reacted as in
Example 6 except that the final reaction temperature
was 250C. After grinding, the prepolymer was
solid-state polymerized at 260C for 24 hours under
vacuum. The resulting polymer had a first cycle Tm
of 291.8C, second cycle Tg of 94C, Tch of 126C, Tm
of 279.5C, and Tcc of 240.0C. Elemental analysis
found C = 65.73%, H = 3.57%, corresponding to an
empirical formula of C6H4S and an x of
0.049.
ExamPle 23
410.0 grams p-diiodobenzene, 32.00 grams sulfur,
and 0.80 grams diiodonitrobenzene were reacted as in
Exampl~ 6, with the final polymerization temperature
300C. The high viscosity polymer crystallized rapidly
on cooling and yielded tough pressed films. DSC of
this polymer found first cycle Tm of 278C, and second
cycle Tg of 94.1C, Tch of 142.1C, Tm of 279.8C, and
Tcc of 230.9C. Elemental analysis found C = 65.46%,
H = 3.53% corresponding to an empirical formula of
C6H4S1 064 and x equals 0.064.
~L3~ ~ ~7 ~
- 22 -
ExamPle 24
10 grams of the ground polymer from Example 22
was solid-state polymerized at 260C for 24 hours
under vacuum. The polymer obtained had a first cycle
Tm of 284.2C, and a second cycle Tg of 95.0C, Tch
of 140.3C, Tm of 278.3C, and a Tcc of 226.2C.
Elemental analysis found 65.66%C, 3.49%H, correspond-
ing to an empirical formula of C6H4S1 055, and
x equals 0.055.
Obviously, numerous modifications and variations
of the present invention are possible in light of the
above teachings. It is therefore to be understood
that within the scope of the appended claims, the
invention may be practiced otherwise than as
specifically described herein.