Note: Descriptions are shown in the official language in which they were submitted.
3~L2~ 72
- 1
METHOD FOR REDUCING SHEETING
~URING POLyMERIZATION OF ALPHA-OLEFINS
8ACKGROUND OF THE INVENTION
Field of the Invention
Th~s invention relates to a method for
reducing shseting during polymerization of
alph~-olefins ~nd more particularly to a method for
reducing sheeting during polymerization of
polyethylene utilizing titanium b~sed catalysts or
vanadium based catalysts with alkyl aluminum
cocatalysts.
Summar~ o~ the Prior Art
Conventional low density polyethylene has
I been historically polymerized ~n heavy walled
; 15 autoclaves or tubular re~ctors at pressures as high
as 50,000 psi and temperatures up to 300C or
higher. The molecular structure of high pressure,
low denslty palyethylene (HP-LDPE~ is high complex.
The permutations in the arra~gement of their simple
building blocks are essentially ~nfinite. HP-LDPE's
~re characterized by an intricate long chain
branched molecular architecture. These long chain
branches have 8 drsmatic ef~ect on the melt rheology
of these resins. ~P-LDPE's also possess a spectrum
of shart chain branchesJ generally l to 6 carbo~
atoms in length. These short chain br~nches disrupt
cryst~l ~ormation and depress resin ~ensity.
; ~ More recently, technology has been provided
whereby low density polyethylene can be produced by
~luidized bed techniques at low pressures and
;' .
.
D-15407
.: :
'"' '
~312~ ~2
temperatures by copolymerizing ethylene with vsrious
slpha-olefins. These low pressure LDFE (LP-LDPE)
resins generally possess little, iE any, long chain
branching and sre sometimes referred to as linear
LDPE resins. They sre short chain branched with
branch length and frequency controlled by the type
and amount of comonomer used during polymerization.
As is well known to those sX~lled in the
art, low pressure t high or low density polyethylenes
can now be conventionally pro~ided by a fluidized
bed process utilizing several families of catalysts
to produce a full range of low density and high
density products. The appropriate selection of
catalysts to be utilized depends in part upon the
type of end product desired, i.e., high density, low
density, extrusion grflde, film grate resins and
other criteria.
The various types of catalysts which may be
used to produce polyethylenes in fluid bed reactors
can generally be typed as follows:
TYPe I. The silyl chromate catalysts disclosed in
U.S. Patent No. 3,324,101 to Baker and Carrick and
U.S. Patent No. 3,324,095 to Carrick, Karapinks and
Turbet. The silyl chromate catalysts are
characterized by the presence therein of a group of
the formula:
R O
Si 0-- Cr O
R 0
D-15407
.
13~ 2172
- 3
wherein R is a hydrocsrbyl group having from i to 14
c~rbon ~toms. The preferred silyl chromste
cat~lysts are th~ b~s(tri~rylsilyl) chromates and
: more preferably bis~triphenylsilyl) chromate.
This catalyst i5 used on a support such as
. silica, ~lumin~, thor~, zircon~ and the like,
other supports such as carbon blacX,
~ : micro-crystalline cellulose, the non-sulfon~ted ion
-~ exchange resins ~nd the like may be used.
TyPe II. The bis(cyclopentadienyl) chromium (II)
compounds disclosed ~n U.S. Patent No. 3,879,368.
These bis(cyc~spent~dienyl) chromium ~II) compounds
have the following formuls:
~R )~ )n"
Cr
~H)5~n' (~)S-n"
`~ wherein R' ~nd ~" may be the same or different C
to C20, inclusive, hydroc~rbon radicals, and n'
and n" may be the same or different integers of 0 to
5, incluslve. The R' and R" hydrocarbon r~dicals
may be s~tursted or unsatur~ted, ~nd can ~nclude
: aliph~tic, al~cyclic snd aromatic rad~cals such as
methyl, ethyl, propyI, butyl, pentyl, cyclopentyl,
cyclohexyl, allyl, ~henyl ~nd naphthyl r~dicals.
These catalysts are used on a support as
: heretofo~e described.
Type III. The catalysts 8S described in U.S. Patent
No. 4,011,382. These cat~lysts contain chromium snd
D-15407-
`" _ 4 1~ 72
based on the combined weight of the support and the
chromium, titanium and fluorine, about 0.05 to 3.0,
and preferably about 0.2 to 1.0 weight percent of
chromium (calculated as ~r), about 1.5 to 9.0 and
preferably about 4.0 to 7.0, weight percent of
titanium (calculated as Ti), and 0.0 to about 2.5,
and preferably about 0.1 to 1.0 weight percent of
fluorine (calculated as F)
The chromium compounds which may be used
for the Type III catalysts include CrO3, or any
compound of chromium which is oxidizable to CrO3
under the activation conditions employed. At least
a portion of the chromium in the supported,
activated catalyst must be in the hexavalent state.
Chromium compounds other than CrO3 which may be
used are ~isclosed in U.S. Patent No. 2,825,721 and
U.S. Patent No. 3,622,521 and include chromic acetyl
acetonate, chromic nitrate, chromic acetate, chromic
chloride, chromic sulfate, and ammonium chromate.
` The titanium compounds which may be used
include all those which are oxidizable to TiO2
under the activation conditions employed, and
include those disclosed in U.S. Patent No. 3,6Z2,521.
The fluorine compounds which may be used
include HF, or any compound of 1uorine which will
yield HF under the activation conditions employed.
The inorganic oxide materials which may be
used as a support in the catalyst compositions are
D-15407
1'~12~ 7~
The inorganic oxide materials which may be
used as a support in the catalyst compositions are
porous materisls having a high surface area, that
i~, a surface area in the ran8e of about 50 to lOOO
S square meter~ per gram, and an average particle size
of about 20 ~o 200 microns. The ~norganic oxides
which may be used include silica, alumina, thoria,
zirconia and vther romparable inorganic oxides, as
well as mixtures of such oxides.
Type IV. The catalysts as described in U.S. Patent
No. 4,302,566 in the names of F.~. Karol et al, and
entitled, "Preparation o~ Ethylene Copolymers in
Fluid Bcd Reactor" and ~ssigned to the same assignee
~ as the present application. These catalysts
: 15 comprise at least one titanium compound, at least
one magnesium compound, ~t least one electron donor
compound, at least one activator compound and at
least one inert carrier ma~erial.
The titanium compound has the structure
20 , Ti (OR)aXb
wherein R is a Cl to C14 aliphatic or aromatic
hydrocarbon radical, or COR' where R' is a Cl to
C14 sliphatic or aromatic hydrocarbon radicali X
is Cl, Br, or I; a is O or l; b is 2 to 4 inclusive;
and a+b = 3 or 40
The titanium compounds can be used
individually,or in combinstion thereof, ~nd would
~nclude TiC13, TiC14, Ti(OCH3~C13,
Ti~oc6H5)cl3~ Ti(OCOCH3~)C13 and
Ti(OC~c6Hs)cl3
The magnesium compound has the structure:
MgX2
-
~-15407
. ,
wherein X is Cl, Br, or I. Such magnesium compounds
can be used individually or -in combinations thereof
and would include MgC12, M~Br2 and MgI2.
Anhydrous MgC12 is the preferred magnesium
compound.
-The titanium compound and the magnesium
compound sre generally used in a form which will
facilitate thei~ dissolution in the electron donor
compound.
The electron donor compound is an organic
compound which is li~uid at 25C and in which the
titanium compound and the magnesium compound are
partially or completely soluble. The electron donor
compounds are known as such or as Lew~s bases.
The electron donor compounds would include
such compounds as alkyl esters of aliphatic and
aromatic carboxylic acids, aliphatic ethers, cyclic
ethers and aliphatic ketones.
The cstalyst may be modified with a boron
halide compound hflving the structure:
BR X ' 3
wherein R is an aliphatic or aromatic hydrocarbon
radical containing from 1 to 14 carbon atoms or OR',
wherein R' is also an aliphatic or aromatic
hydrocarbon radical containing from l to 14 carbon
atoms; X' is selected from the group consisting of
Cl and Br, or mixtures thereof, and; c is O vr 1
when R is an ~liphatic or aromatic hydrocarbon and
O, 1 or 2 when R is OR'.
The boron halide compounds can be used
individually or in combination thereof, and would
include BC13, ~Br3, B~C2H5)C12.
D-15407
. .
~ ~-
7 2
B(oc2Hs)cl2~ B(C2H5)
B(C6H5)C12~ B(OC6H5)Cl2'
(C6H13)C12' B()C6Hl3)C12, and
B(OC~H5)2Cl. Boron trichloride is the
particularly prererred boron compound.
The ~ctivator compound has the structure:
Al(~") X'dH
where~n X' is Cl or ORl; Rl and R" are the same
or di~ferent and are Cl to C14 saturated
hydrocarbon radicals, d is 0 to 1.5, e is 1 or 0,
and c~d+e = 3.
Such activator compounds can be used
individually or in combinations thereof.
The carrier materials are solid~
particulate materials and would include inorganic
materials such as oxides of silicon and aluminum and
molecular sieves, ~nd organic materials such as
ole~in polymers, e.g., polyethylene.
TYPe V. Vanadium based catalysts. These type
catalysts generally include vanadium as the sctive
ingredient, one such type catalyst generally
comprises a supported precursor, a cocatalyst and a
promoter. The supported precursor consists
essentially of a vanadium compound and modifier
impregnated on a solid, inert carrier. The vanadium
compound in the precursor is the reaction product of
a vanadium trihalide and an electron donor. The
halogen in the vanadium trihalide is chlorine,
bromine or iodine, or mixtures thereo~. A
particularly preferred vanadium trihalide is
vanadium trichloride, VC13.
The electron donor is a liquid, organic
Lewis base in which the vanadium trihalide is
,
D-15407
,.. .
~ ~, "~
~ ~3~ ~7~
- 8 -
soluble. The ~lectron donor is selected from the
group consisting of alkyl esters of aliphatic and
aromatic carboxylic scids, aliphatic esters,
aliphatic ketones, aliphatic amines, aliphatic
slcohols, alkyl and cyclo21kyl ethers, and mixtures
thereof. Preferred eleotron donors are alkyl and
eyeloalkyl ethers, including particularly
tetrahydrofuran. Between about 1 to about 20,
preferably between sbout 1 to about 10, and most
preferably about 3 moles of the electron donor are
complexed wi~h each mole o vanadium used.
The modifier used in the precursor has the
formula:
MXa
wherein:
M is either boron or AlR(3 a) and wherein
each R is independently alkyl, provided
that the total number of aLiphatic carbon
stoms in any one R group may not exceed 14;
X is chlorine, bromine or iodine; and
a is 0, l or 2, with the provision that when
M ig boron a is 3.
Preferred modifiers include Cl to C6
alkyl aluminum mono- and ti- chlorides and boron
trichloride. A particularly preferred modifier is
diethyl aluminum chloride. About 0.1 to about 10,
and pref erably abo~t 0.2 to about 2.5, moles of
modifier are used per mole of electron donor.
The carrier is a solid, pa~tic~late porous
material inert to the ~olymerizAtion. The carrier
consists essentially of silica or alumina, i.e.,
oxides of silicon or aluminum or mixtures thereof.
: .
D-15407
' . . ~,.
.
7 2
_ 9
Optionally, the carrier may contsin additional
materials such as zirconia, ~thoria or other
compounds chemically inert to the polymerization or
mixtures thereof.
The carrier is u~ed as ~ dry powder having
sn average p~rticle size of between about 10 to
about 250, pre~erably about 20 to about 2~0, and
most prefersbly about 30 to about 100, microns. The
po~ous carrier has a ~urfsce sre~ of 8reater than or
equal to about 3, and preferably gre~ter than or
equ~1 to about SO, m /g. A preferred carrier is
silica having pore sizes of greater than or equal to
about 80, and preferably greater than or equal to
about 100, angstroms. The carrier is predried by
heating to remove water, preferably at a temperature
of greater than or equal to about 600C.
The mount o~ carrier used is that which
will provite a vanadium content of between about
0.05 to about 0.5 mmoles o~ vanadium per gram (mmole
V/g), and preferably between about 0.2 to about 0.35
mmole V/8, ~nd most preferably about 0.29 mmole V/g.
l'he carrier is ordinarily free of
prep~rative chemical treatment by reaction with an
alkylaluminum compound prior to the formation of the
supported precursor. Such treatment results in the
formation o aluminum alkoxides chemically bonded to
the carrier molecules. It~has been discovered that
the use of such a treated carrier in the catalyst
composition and process is not only nonessential,
but instead results in undesirable agglomeration
when used in the preparation o~ high density
polyethylene
.
~ D-15407
:~ `
'
- i
,
, ' ' - ~ :
',. . .
7 2
- 10 -
(>0.94 ~Icc), resulting in a chunk-like,
non-fr~ely flowing product,`~
The cocatalyst wh~ch can be employed for
: the Type IY and Type V catalysts has the formula::~: 5 AlR3
;~ wherein R is as previously de~ined in the definition
o~ M. Preferred cocatalysts include C2 to C8
trialkylaluminum compounds. A particularly
pre~erred cocatslyst ~s triiso~utyl alum~num,
Between ~bout 5 to ~bout 500, and preferably between
about ~0 to about 50, moles of cocatalyst are used
per mole of vanad~um.
The promoter has the formula:
R'bCx (4_~)
wherein:
R' is hydrogen or unsubstituted or
h~losub~tituted lower, i,e,, up to sbout
C6 containing, alkyl;
X' is halogen; snd
b is 0, 1 or 2,
Between about 0,1 to about 10, and prefersbly
~ between about 0,2 to about 2, moles of promoter are
: uset per mole o~ cocatalyst,
The catalyst is produced by first preparing
the supported precursor, In one embodiment, the
vanadium compound is prepared by dissolving the
vanadium trihalide in the electron donor at
: temperature between about 20CC up to the bo~ling
point of the elect.on donor for:a few hours,
~, ~ 30 Preferably, mixing occurs:at about 65C for about 3
hours or more, The vanadium compound so produced is
: then impregnated onto the carrier, Impregnation may
.
~ D-15407
:~ ' .
, ~ .
`'.. : : '
. .
~2~7~
b~ effected by adding the carrier as a dry powder or
8S a slurry in the electron donor or other inert
solvent. The liquid is removed by drying at less
than about 100C for a few hours, preferably between
about 45 to about-90C for about 3 to 6 hours. The
modifier, dissolved in an inert solvent, such as a
hydroearbon, is then mixed with the vanadium
impregnated carrier. The liqu~d is removed by
drying ~ temperatures of less than about 70C for a
few hours, preferably between about 45 ~o about
65C f or ~hout 3 hours.
The cocatslyst and promoter are added to
the supported precursor either before and/or during
the polymerization reaction. The cocatalyst and
promoter are add~d either together or sepsrately,
and either simultaneously or sequentially during
pol~merization. The cocatalyst and promoter are
preferably added separately as solutions in inert
solvent, such as isopentane, during polymerization.
In general, the above catalysts are
introduced together with the polymerizable
materials, into a reactor having an expanded section
sbove a straight-sided section. Cycle gas enters
the bottom of the reactor and passes upwsrd through
a gas distributor plate into a fluidized bed located
in the straight-sided section of the vessel. The
gas distributor plate ser~es to ensure proper gas
d~stribution snd to support the resin bed when gas
flow is stopped.
G~s leaving the fluidized bed entrains
resin particles. Most of these particles are
d~sengaged as the gas passes through the expanded
section where ~ts veloc~ty is reduced.
D-15407
~3~ 7~
In order to satis~y certain end use
appllcations for ethylene resins, such as ~or film,
in~ection molding and roto-molding applications,
c~t~lyst Types IV and V with fllkyl aluminum
cocatalysts have been used. However, attempts to
produce certa~n ethylene resins utilizing alkyl
aluminum cocatalysts with the Type IV and V
catalysts supported on a porous silica substrate in
certain fluid bed re ctors, hsve not been entirely
satlsfactory from a practical commercial
standpoint. This is prim~rily due to the formation
of "sheets" in the reactor after a period of
operat~on. The "sheets" can be characterized 8S
constituting a fused polymeric material.
It has been ~ound that a static mechanism
is a contributor to the sheeting phenomena whereby
catalyst and resin particles adhere to the reactor
walls due to static ~orces. If allowed to reside
long enough under a reactive environment, excess
temperatures can re~ult in particle fusion.
N~merous causes for static ch~rge exist. Among them
are generation due to frictionsl electrification of
dissimilar materials, llmited static dissipation,
introduction to the process of minute quantities of
prostatic agents, excessive catalyst Pctivities,
etc. Strong correlation exists between sheeting and
the presence of excess static charges e~ther
negative or positive. This is evidenced by sudden
_hanges in static leYels followed closely by
deviation in temperatures at the reactor wall.
These temperature deviations are either high or
low. Low temperatures indicate particle adhesion
~ ~
D-15407
- ~ ,
: . ..
' :.:
,
2~72
- 13 -
causing an insulating effect from the bed
temperature. High deviations indicate reaction
taking place in zones of limited heat transfer.
Following this, disruption in fluidization patterns
ls generally evident, ~atalyst feed interruption can
occur, product discharge system pluggage results,
and thin fused agglomerates ~sheets) are noticed in
the granular produc~.
The sheets vary widely in size, but are
similar in most respects. They are usually about
1/4 to 1/2 inch thick and are from about one to five
feet long, with a few specimens even longer. They
have a width of about 3 inches to more than 18
inches. The sheets have e core composed of fused
polymer which is oriented in the long direction of
the sheets and their surfaces are covered with
granular resin which has fused to the core. The
edges of the sheets can have a hairy appearance from
strands of fused polymer.
It is therefore an object o the present
invention to provide a method for substantially
reducing or eliminat~ng the amount of sheeting which
`~ occurs during the low pressure fluidized bed
polymerization of alpha-olefins utilizing titanium
based compounts or vanadium based compounds as
catalyst with alkyl aluminum as cocatalysts.
; Another obiect is to pro~ide a process for
reducing sheeting in fluidized bed reactors utilized
for the production of polyolefin resins wherein
- 30 titanium or van~dium based catalysts with alkyl
aluminum cocatalysts are employed.
D-15407
;,", ',,
. : ,
,
:' "`" ` -...
.
17~
- 14 -
These and other ob~ects will become readily
apparent from the following-descri~tion taken in
con~unction with the accompanying drawing which
generally indicates a typical gas phase fluidized
bed polymerizAtion process for producing high
den~ity ~nd low density polyolefins slightly
modified to reflect the present invention.
~roadly contemplated, the present inYention
provides a method or reducing sheet~ng during
polymerization of alpha-olefins in a low pressure
fluid~zed bed reactor utilizing titanium or vanadium
based compounds as catalysts together with alkyl
aluminum cocatalysts which comprises introducing
water into said reactor in an amount sufficient to
maintain the electrostat~c levels at the site of
possible sheet formation at levels ~hich a~oid
sheeting without substantially altering the
effectiveness of said catalysts.
The amount of water which is fed to the
reactor depends on the stat~c voltage within the
reactor and can generally range in an amount of 0.1
to about 2 ppm based on ethylene feed. In general,
the nitrogen flow control is such as to permit a
nitrogen flow of about 0 to about ll lbs/hr for an
ethylene feed range of about 0 to about 50,000
lb/hr. The water cylinder temperature in the
O'Brien box can generally be in a range of about
10C to about 40C. Nitrogen pressures can
generally range from about 200 to 400 psig
prefer~bly about 320 to about 370 psig.
The critical static voltage level for sheet
; formation is a complex ~unction of resin sintering
.~
:,
D-15407
. .
.
. , .
~L31~7~
temperature, operating temperature, drag forces in
the fluid bed, resin particle size distribution and
recycle ~as compositlon. The static vol~age can be
. reduced by a variety o~ techniques such as by
; 5 tresting the reactor surface to reduce static
electric generation by inlection of an antistatic
agent to inc~ease particl~ surface electrical
. ~. conductivity thus p~omoting particle dischargin~; by
installation of appropriate devices connected to the
reactor walls which are designed to promote
~ electrical dischargin~ by creating areas of high
: localized field stren~th, and by neutralization of
charges by the in~ection or creation of ion pairs,
ions or charged particles of the opposite polarity
from the resin bed.
According to the present invention, the use
of water add back to the gas phased low pressure
polyethylene process will assist in the reduc~ion of
agglomerate formation in the fluidized bed. This is
accomplished by a reduction in the levels of
positive. static voltage which lowers particle
adhesive forces in the re~ction system.
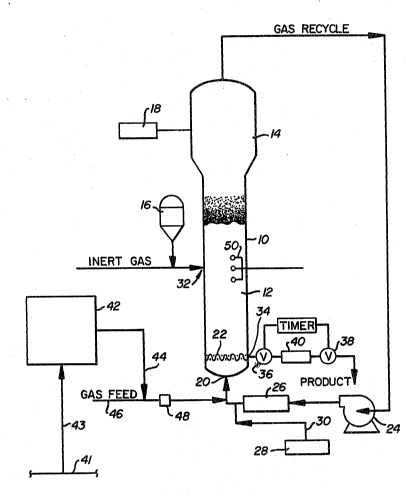
Re~errin~ particulsrly to the sole figure
of the drawing, a conventional fluidized bed
reaction system for polymerizing alpha-olefins
slightly modified to provide for water add back,
includes a reactor 10 which consists o~ a reaction
zone 12 and a velocity reduction zone 14.
The react$on zone 12 includes a bed of
: 30 growing polymer particles, formed polymer particles
:` and a minor amount of catalyst particles fluidized
~`i by the continuous flow of polymerizable and
D-15407
~:
.
, ; - ~ ,. ; .. - .
' " :-,
72
- 16 -
modifying gaseous components in the form of make-up
feed and recycle gas through the reaction zone. To
maintain a viable fluidized bed, the mass ~as flow
rate through the bed is normally maintained above
the minimum flow required for fluidization, and
preferably from about 1.5 to about 10 times Gmf
and more preferably from about 3 to ~bout 6 times
Gmf. G~f ~s used in the accepted form as the
abbrevi~tion for the minimum gas flow required to
achieve fluidlzation, C.Y. W~n and Y.H. Yu,
"Mechanics of Fluidization", Chemical Engineering
Progress Symposium Series, Vol. 62, pg. 100-111
(1966).
It is highly desirable that the bed always
contalns particles to prevent the formation of
localized "hot spots" and to entrap and distribute
the psrticulate catalyst throughout the reaction
zone. On start up, the reactor is usually charged
with a base of particulate polymer particles before
g~s flow is initiated. Such particles may be
identical in nature to the polymer to be formed or
different therefrom. When different, they are
withdrawn with the desired formed polymer particles
as the first product. Eventually, a fluid~zed bed
of the desired polymer particles supplants the
start-up bed.
The appropriate catalyst used in the
fluidized bed is preferably stored for service in a
reservoir 16 under a blanket of a gas which is inert
to the stored m terial, such as nitrogen or argon.
Fluidization is achieved by a high rate of
gas r~cycle to and through the bed, typically in the
D-15407
- 17 -
order of about 50 times the rate of feed of make-up
gas. The fluidized bed has~-the general appearance
o~ ~ dense mass of viable particles in possible
free-vortex flow es created by the percolation sf
g8s through the bed. The pressure drop through the
bed is equal to or slightly greater th~n the mass of
the bed divided by the cross-s2ctional area. It is
thus dependent on the geometry of the reactor.
Make-up gas ~s fed to the bed at a rate
equ~l to the rate at which particulste polymer
product is ~ithdrawn. The composition of the
make-up g~s is determined by a gas analyzer 18
pos~tioned above the bed. The gas analyzer
determines the composition of the ~as being recycled
and the composition of the,make-up gas is adjusted
accordingly to maintain An essentially steady stste
gaseous composition within the rear.tion zone.
To insure complete fluidization, the
recycle gas and, where desired, part or all of the
; 20 make-up gas ~re returned to the reactor at bsse 20
~ b,elow the bed. Gas distribution plate 22 positioned
,, above the point of return ensures proper gas
distribution and also supports the resin bed when
~ ~as flow is stopped.
;;~ 25 The port~on of the gas stream which does
not resct in the bed constitutes the recycle gas
which is removed from the polymeri~ation zone,
preferably by psss~ng it into velocity reduction
~one 14 above the bed where entrained particles are
, 30 given an opportunity to drop back into the bed.
The recycle gas is then compressed in a
compressor 24 and thereafter passed through a heat
~'
~ ~ ,
D-15407
~, :
.
'~
., ''
7 2
- 18 -
exchanger 26 wherein it is stripped of heat of
reaction before it is returned to the bed. By
constantly removing heat o~ reaction, no noticeable
tempetatu2e gradient appears to exist within the
upper portion 9~ the bed. A temperature gradient
will exist in the bottom of the bed in 8 layer of
about 6 to 12 tnches, between the temperature of the
inlet gas and the temperature of the remainder of
the bedO Thus, it has been observed that the bed
acts to almost immediately ad~ust the temperature of
the recycle gas above this bottom layer of the bed
zone to make it conform to the temperature of the
rem~inder of the bed thereby maintaining itself st
~n essentially constant temperature under steady
conditlons. The recycle is then returned to the
reactor at its base 20 and to the ~luidi~ed bed
through distribution plate 22. The compressor 24
can also be placed downstream of heat exchanger 26.
Hydrogen msy be used as a chain transfer
agent for conventional polymerization reactions of
the types contemplated herein. In the case where
ethylene is used as a monomer the ratio of
hydrogen/ethylene employed will vary between 0 to
~ about 2.0 mo}es of hydrogen per mole of the monomer
- 25 in the gas stream.
Any gas inert to the catalyst and reactants
can also be present in the gas stream. The
cocstalyst is ~dded to the gas recycle stream
upstream of its connection ~ith the reactor ~s ~rom
d$spenser 28 through line 30.
As is well known, it is essential to
operate the fluid bed reactor at a temperature below
D-15407
~2~2
_ ~9
the sintering temperature of the polymer particles.
Thus to-insure that sinteri~g will no~ occur,
operating tem~eratures below sintering temperature
~re desired. For the production of ethylene
polymers an operating temperature of rom about 90
to 100C ~s preferably used to prepare products
hsving a density of about 0.94 to 0.97 while a
temperature of about 75 to 95C is preferred for
products having a density of about 0.91 to 0.94.
; 10 Norm~lly the fluid bed reactor is operated
at pressures of up to about 80-110 psi for high
density &nd 65-95 for low and medium density.
The catalyst is inJected ~nto the bed ~t a
rate equal to its consumption at a point 32 which is
abo~e ~he distribution plate 22. A gas which is
inert to the catalyst such as nitrogen or argon is
used to carry the catalyst into the bed. Injecting
the catalyst at a po~nt above distribution plate 22
is an important feature. Since the catalysts
normally used are highly active, in~ection into the
area below the distribution plate may cause
polymerizstion to begin there and eventually cause
plugging of the distribution plate. In~ection into
the viable bed, instead, aids in distributing the
catalyst throughout the bed and tends to preclude
the formation of localized spots of high catalyst
concentration which may result in the formation of
"hot spots".
Under a given set of operatin~ conditions,
the fluidized bed is maintained at essentially a
; constant height by withdrawing a portion of the bed
as product at 8 rate equal to the rate of ~ormation
D-15407
:, .... :
- .:
. . : -
1~2~72
- 20 -
of the psrticulate polymer product. Since the rate
of hest generation is directly related to product
formation, a measurement of the temperature rise o~
the gas across the reactor (the difference between
inlet g~s temperature and exit ~as temperature) is
determ~native of the rate of particulate polymer
~ormation at a oonstant gas velocity.
The partic~late polymer product is
prefer~bly withdr~wn at a point 34 at or close to
distribution plate 22. The particulate polymer
product is conven~ently snd preferably withdrawn
through the sequential oper~tion of a pair of timed
valves 36 ~nd 3S defining a segregation zone 40.
While Yalve 38 is closed, valve 36 is opened to emit
a plug of gas ~nd product to the zone 40 between it
and valve 36 which is then closed. Valve 38 is then
opened to deliver the product to an external
recovery zone and after delivery, valve 38 is then
closed to await the next product recovery opera~ion.
Finally, the fluidized bed reactor is
equipped with an adequate venting system to allow
venting the bed during the start up and shut down.
The reactor does not require the use of stirring
means andlor wall scraping means.
The re~ctor vessel is normally constr~cted
of carbon steel and is designed for the operating
conditions stated above.
In order to better illustrate the problems
incident to the util$zation of the Type IV
catalysts, reference is again made to the drawing.
The titsnium based cata}yst (Type IV) is introduced
into the reactor 10 at point 32. Under conventional
D-15407
.,
,-
:~ ,
" ~3~ ~72
- 21 -
operations on certain resins, after a period of
~ime, sheets begin to form in reactor-10, at a site
in the reactor proximate the wall of the resctor and
located about a distance of one-h~lf the reactor
diameter up ~rom the base of the fluid bed. The
sheets of fused resin begin to appear in segregation
zone 409 rapidly plugging the system, causing the
reactor to be shut down. More characteristicslly
the sheeting begins after production equivalent to 6
to 10 times the weight of the bed of resin in
reactor 10.
Many possible causes were investigated in
attempting to discover and eliminate the sheeting.
In the course of the investigation, thermocouples
were ins~alled ~ust inside the reactor walls at
elevations of 114 to }l2 reactor diameter above the
gas dlstribution plate. Under conventionsl
operationsl "skin" thermocouples indlcate
temperatures equal to or slightly lower than the
temperature of the fluidized bed. When sheeting
occurs, these thermocouples m~y indicate tempersture
excurslons of up to 20C ~bove the temperature of
the fluidized bed thus providing reliable indication
of the occurrence of sheeting. In addition, an
electrostatic voltmeter was used to measure voltage
on a 112 inch spherical electrode located ~n the
fluid bed 1 inch radially from the reactor wall and
usually 5 to 6 feet above the g~s distributor
plste. The location was selected because sheet
formation was observed to initiste in a band ranging
~rom 1/4 to 3l4 reactor dismeter in elevation sbove
the ~ase of the fluid bed. As is well known for
D-15407
.
; ~
. , " ' ~ . .. .. . .
,
13 ~ rl ~
deep fluidized beds, this corresponds to the re~ion
of least mixing intensity ne-ar the wall, i.e., a
null zone where particle motion near the wall
changes from generally upwsrd to generally
downward. The possible c~uses investigated included
factor~ affecting mixing in the fluidized bed,
reactor operating conditions, c3talyst and resin
pa~ticle size, part~cle size distribution, and
others. A correlati~n was found between sheeting
and buildup of static electric charge on the resin
particles proximate the re~ctor walls. When the
static voltage level of resin particles at
particular sites proximate the reactor wall in a
fluidized bed reactor is low, the reactor runs
normally and no sheets are formed. When the static
voltage level exceeds 8 critical level at those
sites, uncontrolled sheeting occurs and the resctor
must be shut down.
It was further discovered that sheeting
could be substantially reduced and in some cases
entirely eliminated by controlling static voltage in
the ~luidized bed at a site proximate the reactor
walls below the critical level for sheet formation.
This critical level for sheet formation is not a
fixed v~lue, but is a complex function de~endent on
variables including resin sintering temperature,
operstin~ temperature, drag forces in the fluid bed,
resin particle size distribution and recycle g8S
composition.
The critic~l voltage le~el Vc for sheeting
~; of ethylene homopolymers, ethylene-butene and
ethylene-hexene copolyme~s is primarily a function
D-15407
: :`
7 ~
- 23 -
of the ~esin sintering *emperature, the reactor bed
temperature and the concentration of hydrogen in the -
recycle ~as.
The sintering temperature of the resin
under reactor operating conditions ~s the
te~perature at which ~ settled bed o~ resin in
contact with a ~8S having the same composition as
the reactor recycle gas used in producing the resin
will sinter and form ~gglomerates when
refluidization is attempted after allowing the ~ed
to remsin settled for fifteen minutes. The
sintering temperature is decreased by decreasing the
resin denslty, by increasing the melt index and by
increasing the amount of dissolved monomers and
monomer type.
The constants in the equation were
determined from data collected during reactor
operation when the re~ctor ~ust began to exhibit
sheeting symptoms through skin thermocouple
temperature excursions above the bed temperature.
The voltage indicated on the voltage probe described
earlier varies with time due to the random nature of
a fluidized bed. Thus the crit~cal voltage, Vc, is
expressed as A time averaged voltage. Voltage
measurements are difficult to interpret because
additional static electric charge is generated when
sheet, formed because of a static chsrge,
separates from the reactor wall~ In atdition, the
sheeting phenomena can start ac a very local
phenomenon and spread further clouding
interpretation of voltage readings.
Although the sheeting mechanism is not
fully understood, it is belieYed th t static
D-15407
'
., ~ , ,
, ,. .. ~ :
.
.
~2~72
- 24 -
electricity generated in the fluid bed charges resin
pArticles. When the charge ~n the par*icles reaches
the level where the electrostatic forces trying to
hold the chsrged particle near the reactor wall
exceed the dr g forces in the bed trying to move the
psrticle away from the w811, R layer o~ cstalyst
contsining, polymerizing resln particles forms a
non-fluidized layer nes~ the reactor wall. Heat
removal from this layer is not sufficient to remove
the heat of polymerization because the non-fluidized
layer near the wall has less contact w~th ~he
fluidizing gas than do particles in the fluidized
portion of the bed. The heat of polymeriz~tion
increased the temperature of the non-fluidized layer
near the reactor wall unt~l the particles melt and
fuse. At this point other particles from the
fluidi~ed bed will stick to the fused layer and it
will grow in size until it comes loose from the
reactor wall. The separation of ~ dielectric from a
conductor (the sheet from the reactor wall) is known
to 8enerate additional static electricity thus
accelerAting subsequent sheet ~ormstion.
The srt teaches various processes whereby
~ static voltage can be reduced or eliminated. These
; 25 comprise (1) reducing the rate of charge generation,
(2~ increasing the rate of disch3rge of electrical
chsrge, and (3) neutralization of electrical
chsrge. Some processes suited for use in a
fluidized bed comprise (1) use of an additive to
inc~ease the conductivity of the particles thus
providing a path for discharging, ~2) installation
of grounding devices in a fluidized bed to provide
D-154~7
. .,
~3~2~7~
- 25 -
additionQl area for disch~rging electrostatic
chsrges to ~round, (3) ioniz- tion of g~s or
p~rticles by electrical dischsrge to generate ions
: to neutrslize electrostatic charges on the
particles 9 ~nd (4) the use o~ r~tioactive sources to
: produce radiation thAt ~ill create ions to
neutralize elec~rostatic rhsrges on the particles.
The application of the~e techniques ~o a commercial
scale, ~luidized bed, polymeriz~tion reactor m~y not
be feasible or practical. Any ~dditive used must
not act ~s 8 poison to the polymerizætion cat~lyst
and must not ~dversely affect.the quality of the
product. It had been previously thought that wster,
the most widely used additi~e to reduce static on
particles, c~nnot be used since it is 8 severe
catalyst poison.
We h~ve found however that in certain
specific reactions, i.e., when Type IV and ~
cat~lysts with alkyl aluminum cocatalysts are used
in the fluidized polymerizstion process that the
additlon of controlled minute quantities of water to
the reactor drsmatically reduces the incidence of
sheeting without producing severe detrimental
effects to the catRlysts. The amount of water fed
to the reactor depends on the static chsr~es present
in the reactor.
Water addition csn be accomplished by a
simple modification to the conventionsl procedures.
Thus referrin" ~gain to Fig. 1, ~n inert gas such as
dry nitrogen from ~ nitrogen supply source, 41, is
introduced into what is commonly referred to in the
art an "O'Brien box" represented by Figure 42. The
.~
D-15407
.
.
. .
`` ~L312172
- 26 -
O'Brien box ~enerally contains one or more water
tanks containing distilled w~ter and is equipped
with temperat~re and flow control means all of which
~re not shown. The nitrogen is bubbled through one
of the w~ter tanks which is a one liter st~inless
steel cyllnder of distilled water in the temperature
controlled housing. Water saturated nitrogen
leaving the Q'Brien box 42, through line 44, is then
flow controlled thro~gh heat traced tubing to enter
the ole~in feed line such as ethylene feed line, 46,
to the reaction cycle. Resultant water
concentration in the ethylene is generally less than
one part per million by volume. A moisture
analy~er, 48, on the ethylene feed line 46 can be
used for confirmation of water addition. R typical
range for nitrogen flow control is about 0 to ll
lbs/hr for sn ethylene ~eed range of 0 to 50,000
lbs/hr. With water cylinder temperature of 20C and
: 350 psig nitrogen a ran8e of 0 to .3 ppm water isreali~ed. Ad~ustments in water temperature or
nitrogen pressure can permit varying this range to
desired levels.
Merely as illustrative the following
information shows the water add back flow to the
; 25 reactor concentration calculations:
1. Determine vapor pressure of water at
temperature in O!Brien box (PH20).
2. Determine flow of n~trogen with
integrsl orifice (WN~).
3. Determine nitrogen pressure from
reactor bottom head pressure (DN2) plus the
pressure drop in the line.
D-15407
:~
i: :
1 312 3 12
4. Determine ethylene flow to reactor
,. (WC2H4~'
5. Assume nitrogen to be saturated with
water:
P x WN2 ~ 18 = Water flow ~WH20)
PN2 28
I0 WH20 x 28 x I~ = PPM H2O In ethyIene feed
At 20C PH20 = 0.339 ps~a
PN2 = 325 psi~ when reactor at 300 psig.
WNz - 0 to 11.36 ppm typically flow at 3 ppm
Wc2~4 = 18,000 pph
Water concentration = (.339) (3)_(10) =0.174 ppm
325 18,000
Static voltage in the reactor can be
monitored near the reactor wall by one or more
ststic voltage indicator~ 50 inserted into the
reactor bed approxim~tely five feet above the
distributor plate in the range of -15,000 to ~15,000
volts. With reaction in progress, changes in static
voltage levels erom neutral to positive can be
counteracted by feed oP the moisture laden nitrogen
to the ethylene stream. I~ this is not performed,
impending agglomerate formation will likely create a
process upset. Care must be exercised to avoid
excessive water levels which can result in unwanted
negative st~tic vol*age levels.
The system is operated with various flow
and check valves wh~ch are common ~n the srt and
- hence not illustrated. In ~ddtion, line 44 is
D-15407
:
,
.; ~
.
` ~ 31~72
- 28 -
preferably insulated ~nd steam traced prior to
entering the gas feed line 4~. ~ -
The polymers to which the present invention
is primarily directet and which cause the sheeting
problems aboYe reerred to ln the presence of
titanium catalysts are linear homopolymers of
ethylene or linear copolymers of a major mol percent
(>90%) of ethylene, ~nd a~minor mol percent (<10~)
of one or more C3 to C8 alpha olefins. The C3
to C8 alph~ olefins should not contain any
brsnching on any of their carbon atoms which is
closer than the fourth carbon atom. The preferred
C3 to C8 alpha olefins are propylene, butene-l,
pentene-l, hexene-l, 4-methylpentene-1, heptene-l,
and octene-l~ This descript~on is not intended to
exclude the use of this invention with alpha olefin
homopolymer and copolymer resins in which ethylene
is not a monomer.
; The homopolymers and copolymers have a
density ran~ing from about 0.97 to 0.91~ The
density of the copolymer, at a given melt index
level is primarily regulated by the smount of the
C3 to C8 comonomer which is copolymerized with
the ethylene. Thus, the addition of progressively
large~ amounts of the comonomers to the copolymers
results in a progressive lowering of the density of
the copolymer. The amount of each of the various
C3 to C8 comonomers needed to ach~eve the same
result will ~ary from monomer to monomeri under t~he
same reaction conditions. In the absence of the
comonomer, the ethylene would homopolymerize.
The melt index of a homopolymer or
copolymer is a re~lection o~ its molecular weight.
,
D-15407
, :
:
, .
~3~2~2
- 29 ~
Polymers having 8 relPtively high molecular weight,
have reiatively high viscosities flnd low melt index.
In a typical mode of ut~lizing the subject =-
invention to reduce she~ting, a reactor vessel such
as ~hown in Figure 1 and which is susceptible to
sheeting problems by the polymerization of the above
described materials utilizing Type IV and Type V
catalysts with an alkyl aluminum cocataly~t is
part~lly filled with gr~nular polyethylene resin
which is purged with a non-resctive gas such as
nitrogen and is fluidi2ed by circulating said
non-reacting gas through t~e reactor at a velocity
aboYe the minimum fluidizing velocity (Gmf) of the
granular polyethylene and preferably at 3 to 5 Gmf.
The reactor is brought up to operational
temperatures by the gas and the resction is started
by introducing the catalyst and cocatalyst to the
resctor~ During reaction, static voltage levels
approach those levels which cause sheeting, then the
pressurel temperature and flow control in the
O'Brien box are increased to permit nitrogen to
become water saturated. The water s~turated
nitrogen is then directed to the gas feed line and
introduced into the reactor. The voltage levels in
the reactor are monitored responsive to the water
laden gas feed st~eam and the procedure is continued
until the static ~oltage levels are brsught to
levels of non-sheeting.
Having set ~orth the general nature of the
invention, the fvllowing examples illustrate some
specific embodlments of the 1nyention~ It is to be
D-15407
:
..
~ ~2~72
- 3~ -
understood, howe~er, that this invent~on is not
limited to the ex~m~les, si~ce the invention may be
practiced by the use of various modifications.
Ex~mples 1 and 2 were conducted in a
conventionsl bed reactor. The catalyst used was a
Zi~gler type, ~it~n~um based eatalyst supported on
porous silica produced as described earli~r as Type
IV. Thè coc~talyst u~ed was triethyl aluminum. The
products made in the examples were copolymers of
ethylene and l-butene. HydrGgen was used as a chain
transfer sgent to control the melt index of the
polymer.
ExamPle 1
A fluidized bed reactor was started up at
operat~ng condltions designed to produce a ilm
gr~de low density ethylene copolymer product having
a density of 0.918, a mel~ index of 1.0, and a
sticking temperature of 104C. The reaction was
` 20 started by feeding catalyst to the reactor
prech~rged ~ith a bed of granular resin similar to
the p~otuct to be made. The cfltalyst was a mixture
of S.S parts titanium tetrachloride, 8.5 parts
magnesium c~loride and 14 parts tetrshydrofuran
deposited on 100 parts Davison 8rade 955 silica
; which had been dehydrated at 600C ~nd treated with
four parts triethylaluminum prior to deposit~on and
was activated~with thirty-five psrts tri-n-hexyl
~aluminum subsequent to deposition. Prior to
starting catalyst feed 9 the resctor and resin bed
~ere brought up to the operating tempersture of
85C, were purged o~ impurities by circulating
nitrogen through the resin bed. Ethylene, butene
D-15407
. '
.,
` ~31~72
- 31 -
and hydro~en concentrat1ons were established at 53,
24, and~ , respectively. -Cocatalyst was fed at a
r~te of 0.3 parts triethylaluminum per part of
catalyst.
Reac~or star~-up was normal. After
producing product for twenty-nine hours and
equiYalent to 6-112 times the weight of the
f}uidized bed, temp~rature excurs~ons of 1 to 2~C
~bove bed temperature were observed using
thermocouples l~cated ~ust inside the reactor wall
at an elevation of 1/2 reactor diameter ~bove the
~as distributor plate. Prior experience had shown
that such temperature excursions ~re a positive
indication that sheets of resin are being formed in
the fluidized bed. Concurrently, bed voltsge
(measured using an electrostatic voltmeter connected
to a 112 inch diameter spherical electrode located
one inch ~rom the reactor wall at an elevation of
1/2 reactor diameter Rbove the gas distributor
plate) increased from a readin8 of approximately
~1500 to ~2000 volts to a'rea~ing of over +S000
volts and then dropped back to ~2000 volts over ~ 3
minute period. Temperature and voltage excursions
continued for approxim8tely 12 hours and increased
in frequency and magnitude. During this period,
sheets of ~used polyethylene resin began to show up
in the resin product. Evidence of sheeting became
more severe, i.e., temperature excursions increased
to ~s high as 20C above bed temperature and stayed
high for extended periods of time and voltage
' excursions slso bec~me more ,frequent. The reactor
was shut down because of the extent of sheeting.
.
D-15407
'
, ~ ' "" ; '
~312172
- 32 -
ExamE~e 2
The fluldized bed reactor used in Exsmple 1
w~s stsrted up ~nd operated to produce a linear low
density ethylene copolymer suitable for extrusion or
rvtational molding and having a density of 0.934, a
melt lndex of 5 and a sticking temperatu~e of
; 118C. The reaction W8S started by feeding catalyst
similar to the catalyst in Example 1 except
sctivated with 28 parts tri-n-hexylaluminum, to the
resctor precharged with ~ bed of granular resin
similar to the protuct to be made. Prior to
starting catalyst feed the reactor and the resin bed
were brought up to the operat~ng temperature o~
85C, and were purged of impurities with nitrogen.
The concentration of ethylene ~52~), butene (14~),
hydrogen ~21%) were introduced into the reactor.
Cocatalyst triethylaluminum was fed at 0.3 parts per
part of catalyst. The reactor was operated
continuously for 48 hours and during that period
produced resin equivalent to 9 times the amount of
resin contained in the bed. After this 48 hour
period of smooth operation9 sheets of fused resin
be8an to come out of the reactor with the normal,
granular ptoduct. At this time voltages ~easured
l/Z reactor diameter ~bove the distributor plate
averaged +2000 volts and ranged from 0 to ~10,000
volts, while the skin thermocouples at the same
elevat~on indicated excursions of ~15C above the
bed temperatu~. Two hours after the ~irst sheets
were noted in the product ~rom the reactor, it was
necessary to stop ~eeding catalyst and cocatalyst to
the reactor to reduce the resin production rate
,
D-15407
.
,,, : :
,; ..
`:` : '
~31~172
because sheets were plugging the resin discharge
system. One hour later, çatalyst ~nd cocatalyst
feeds were restarted. The production of sheets
continued and after two hours catalyst and
cocatalyst feed were again stopped and the reaction
WdS termin~ted by in~ecting carbon monoxide. The
voltage at this ~ime was > ~12,000 ~olts and the
thermocouple excursions continued until the poison
was ~n~ected. In totsl, the re~ctor was op~rated
for 53 hours and produced 10-1/2 bed volum~s of
resin before the reaction was stopped due to
sheeting.
The following Example illustrstes the
prevention of sheeting by adding water to the gas
feed during periods of high volta8e in the reactor.
ExamPle 3
The reac~or of Examples 1 and 2 were
modified AS shown ~n Figure 1 ~nd a high density
film grade polyethylene resin of 0.946 density, 7.5
flow ind~x, and sticking temperature of 124C was
continuously produced. The product was an
ethylene-hexene copolymer utilizing a vanadium based
c~talyst with an aluminum alkyL cocatalyst and
halogen promoter for polymerization.
The catalyst contained 0.29 millimoles vanadium per
gram of precursor and 1.2~ aluminum added in the
form of diethylaluminum chloride on a Davison silica
support o~ 30-130 micron s~ze. Reaction proceeded
with a bed temperature of 98C, 7~% ethylene t 1.670
hydrogen, lq2~ hexene, and the remaining
concentration inert gases of nitrogen, methane,
.
'
~-~5407
' ', ~ '' ~ '
" , '
,
~ 3~2~7~
- 34 -
isopentane, etc. under a reactor pressure of 315
psia. Cocatalyst was controlled by feeding - -
tr~ethylaluminum to m~intain 200 ppmv in the resin
produced. Freon was fed AS a promoter to maintain a
r&tio o 0.7 moles freon to each mole of teal.
Production r~te was su~tained at approximately
20,000 pph or a space time yield of 5 mlbs/hr/~t3
o~ bed volume.
~uring production gradual incrPasP in
sta~ic voltage levels measured 5 ft above the
distributor plate at the reactor wall began about 18
hours a~ter st~ble production was ~chieved~ Voltage
build up appeared with small static spikes from 0 to
~00-300 volts every 1 to 5 minutes. The trend
cont~nuet upwsrd with a ~ase line shift ~rom 0 to
1000-5000 volts ant static spikes to 10,000-15,000
volts with an increasing frequency. Associated with
the stQtic were deviations in skin temperatures
measuret 3 to 6 ft above the distributor plate at
the reactor wall. These deviations were generally
negatiYe indicating an insulating effect due to
resin accumulation adhered to the wall. If sllowed
to continue sheet formation will occur eventually
leading to a reactor shutdown from plugged discharge
systems or blockage at the distributor plate
resulting in 8 large agglomerate formation due to
loss o 1uidization.
At this point water was added by
establishing approximately 5 pounds per hour
nitrogen feed through the cylinder containing
;~ distilled water at 20~C and 350 psig to the ethylene
feed line t~ the reactor. Resultant water
D-15407
-,. :
131 2~72
- 35 -
concentration in the ethylene feed was 0.2 ppmv.
Adiustments were msde to control the static level
ne~r zero. Care was exercised to avoid excessive
water ~eed which can result in unw~nted negative
~tatic excursions which can also lead to a sheeting
ineldent.
The static le~el was brought to control
near zero ~nd sheet formation was avoided and stable
reactor operation was maintained w~thout unwanted
shutdowns from sheeting incidents.
Example 4
The-mod~fied reactor of Exampl~ 3 was
utilized to produce a linear low density film
resin. ~he resin produced was a 0.917 density, 2.7
melt index ethylene-hexene copolymer with a sticking
temperature of 102~C. The catalyst used was a
titanium based on a silica support. Loading o~
titanium was 0.25 millimoles per gram of precursor.
Magnesium chloride, diethylaluminum chloride, and
tri-normal hexylaluminum were added in molar ratios
of 3, .02, .02 respectively to the titanium
content. Silica support was Davison 955 with a
micron si2e range o~ 10-80. Reaction is proceeded
with a bed temperatur~ of 76C, 29~ ethylene, 11%
hydrogen, 5% l-hexene, and the remsining
concentrations inert nitrogen, ethane, methane, snd
isopentane. Cocatalyst was fed to control 300 ppmw
triethylaluminum in the resin. Catalyst
productivity under these conditions was 2203 pounds
o~ polyethylene produced per pound of catslyst.
Production r~tes were 18,000 pounds per hour or 4.5
space time yield.
D-15407
:,
~3~2~
- 3~ - -
A sudden incre~se in ethylene concentration
resulted in a pronounced increase in catalyst
~; activity. Static voltage near the reactor w811
; ~ncreased from near zero to 6000 volts over a ten
: 5 minute period. Sk~n temperature-e at the wall showan ~ncrease indic~ting the sudden ~ormat~on of
polymer sheets along the wall of the reactor at the
6 ft leve} above the dist~ibution plate. If allowed
to oon~inue, 8 reactor shutdown was ~mminent due to
plugged product discharge systems.
. Water add b~ck was beg~n with nitrogen flow
of 4 pph through the water cylinder in the
temperature controlled housing at 20C. Resultant
water concentration in the ethylene was less thsn
0.2 ppm. Static voltage quickly returned to near
zero. Reactor skin temperature deviations subsided
within ten minutes and normal reactor production
resumed.
: `
~ :,
~,
D-15407
i