Note: Descriptions are shown in the official language in which they were submitted.
~3~2~
PERVAPORATION OF PHENOLS
Backqround of the Invention
Phenol-contaminated water as a by-product of
various chemical proce~seq i~ a recognized industrial
problem, both in term~ of water toxicity and recovery
phenol3. ~ to toxlcity~ it i~ known that ph~nol~ are
~oxic to ~l~h ~t: conc~ntra~lon~ a~ low ~ 0.1 ppmt whil~
at 0.01 ppm, an extremcly disagreeable t~ste ls imparted
to water treated by hypochlorite to render it potable,
owing to the formation of chlorophenols. Residual
phenol concentrations from such source~ a~ ga~works,
coking plant~, refineries, coal processing plant~, tar
processing plants, pesticide plant~, phenol conver~ion
plant~, and phenoplast plastics material~ plant~ vary
from a few ppm to a~ high a~ 10~.
Known processes for the purification of pheno-
lated water are few, and may be broadly characterized a~
falling into one of two categorie~: (1) recovery pro-
ces~e3, or (2) chemical/biological destruction proce~se~.
In the first category, there are included processes such
a~ liquid-liquid solvent extraction (see, for example,
U.SO Patent No. 3,673,070), steam di~t~llation, absorp-
tion on activated charcoal or ion-exchange resins, and
foaming with surfactants. In the second category are
included processes such a~ treatment by activitated
~3~
sludges and bacterial bed~, oxidation by o~one, per-
manganate, chlorine, catalyzed hydrogen peroxide, and
electrolysis (see U.S. Patent No. 3,730,864). Another
process not falling into either category is that dis-
closed in U.S. Patent No. 3,931,000, comprising passing
an aqueous polysubstituted phenolic feed stream around
the outside of a bundle of hollow fibers while passing
sodium hydroxide solution into the hollow fibers, the
phenols passing through the fibers to form insoluble
sodium phenate salts which concentrate inside the hollow
fiber membrane, and are ~wept out o the ~yatem wlth tlle
qoclium hydroxldo ~lolution ~tream.
~ low~v~r~ none Oe tho above proce~se~ have been
totally effective, leaving a significant residual phenol
content, and all suffer from various serious drawbacks,
such as strict monitoring of the content and pH of the
feed stream in the case of bacterial bed treatment,
regeneration of absorbents, high cost of reactant~ in
the case of oxidation treatment, and production of unde-
~irable by-products (chlorophenols) in the case of
chlorination treatment.
Use of membranes for pervaporation ha~ been
limited. The only known commercially useful pervapora-
tion membrane is one for dehydrating ethanol and
propanol which comprises a composite of polyvinyl alco-
hol on a porou~ support of polyacrylonitrile. See 53
Desalination 327 (1985). Ion-exchange membrane3 have
_
been investigated as to pervaporation e~fects on aqueous
ethanol and lower carboxylic acid mixtures, with the
water having pervaporated preferentially. Boddeker,
!
~ 3 ~
Proc. 1st Int. Symp. Pervapo_ation ~Feb. 1986). And
~ilicone rubber membranes have been used for the selec-
tive pervaporation of halogenated hydrocarbons and
butanol from aqueous ~olutions thereof. See 8 J. Membr.
Sci 177 (1983). However, none of these membrane~ have
been incorporated into a pervaporation process that is
technically feasible.
It i8 therefore a principal objective of the
present invention to provide a ~imple, hi~hly efficient,
and inexpenlive method o~ purl~yin~ phenol-contamlnated
water.
It .L~ qually .tmportant ob~ect.tva o~ tha
present invention to provide a simple, highly efficient
and inexpensive method of recovering phenols from
aqueous phenolic solutions.
These and other objects that will become
apparent are achieved by the method of the present
invention, which is summarized and described in detail
below.
~0
Summary of the Invention
The present invention is based on the
discovery that certain cla~ses of nonporous polymeric
membranes, when used in a pervaporation mode, will
selectively transport and thereby enrich the phenols
content in the permeate of the process. This selective
permeation of phenols is unexpected in view of the much
lower volatility of phenols relative to that of water,
baqed upon which one would predict precieely the oppo3ite
~3~2~
order of transport. An integral part of the invention
lies in the related discovery that the phenolic enrich-
ment factor strongly increases with decreasing phenolic
- concentration of the feed stream, thus permitting a pro-
cess by which, through a limited number of consecutive
pervaporation steps, the phenolic content of the per-
meate can be raised to a concentration at which natural
phase separation spontaneously occurs. Still another
unique aspect of the present invention iq the di~covery
that, with increa~ing feed concentration~ the phenolic
enrichment ~ctor remain~ relatively con~tant an(l~ In
Aome ca~3a~ actual.ly incr~a~eA~
The present invention accordingly comprisQs a
single-or multiple-step method for both ridding water of
phenolic contaminants and recovering phenols of rela-
tively high concentration. The method includes two
distinct steps: (1) a pervaporation step, followed by
(2) a phase separation step.
The pervaporation step essentially comprises
contacting the feed side of a nonporous polymeric
membrane having certain characteristics detailed below
with an a~ueous phenol-containing feed stream while
maintaining on the permeate side of the membrane either
a sweep stream or a coarse vacuum, whereupon phenols in
the feed stream preferentially diffuse through the mem-
brane to form a phenol-rich permeate comprising phenols
and water in a vapor state and leaving a phenol-depleted
water retentate on the feed side of the membrane.
~3~5~
The phase separation step essentially
comprises condensing the pervaporated phenol-rich per-
meate of a certain concentration, that concentration
exceeding the concentration at which spontaneous phase
separation occurs into an upper phenol-poor fraction and
a lower phenol-rich fraction.
A }arge number of variations of the pervapora-
tion step and the phase separation step and combinations
of the two steps are feasible by combining and recycling
permeate~, retentates and separated fraction~ a9 feed~
and by con~ecutlv~ pervaporatlon ~eps~ thu~ permitting
custom-mado ~ppllc~tlon~ Oe th~ method to achl~vo a
deslred degree oE puriflcation of water or concentration
of phenols. And, as one skilled in the art will readily
appreciate, the present invention may be utilized in
connection with other known phenol separation methods.
Brief Descri~tion of the Drawings
FIGS. 1-5 comprise schematic flow diagrams of
exemplary applications of the present invention.
FIGS. 6-8 comprise graphs showing the per-
vaporation performance of three exemplary membranes of
the present invention.
Detailed Description of the Invention
According to the present invention, there is
provided a simple, efficient and inexpensive method for
the recovery of both phenols and phenol-purified water
from phenol-contaminated water, the method comprising a
--5--
~.3~2~
single- or multiple-step pervaporation separation or a
combination of pervaporation separation and phase
separation steps.
The pervaporation step comprises contacting
the feed side of a nonporou~ polymeric membrane having a
feed side and a permeate side with an aqueous phenol-
containing feed ~tream, said membrane not being degraded
by phenols and selected from the group consisting essen-
tially of elastomeric polymers and anion exchange poly-
mers, and maintaining on the permeate side o~ saidmombrane either an inert ga~ sweep strRam or a prea~ur~
oE 10 mm~lg or l~a~ wh~r~y ph~nol~ in ~id ~ r~am
sel~ctively diEfusc through ~id membrane to Eorm a
phenol-rich permeate comprising phenol and water in a
vapor state on the permeate side of said membrane, and
leaving a phenol-depleted water retentate on the feed
side of said membrane, followed by recovery of the
phenol-depleted water retentate.
The phase separation step may be utilized when
the phenolic concentration in the permeate has exceeded
a threshold concentration (about 10% by weight, depend-
ing upon the temperature of the condensate) at which
spontaneous phase separation of the condensed permeate
occurs into a phenol-poor fraction (also about 10% by
weight phenol) and a phenol rich fraction (about 70% by
weight phenol). The "phenol-poor" fraction is often
also referred to as "phenol in water, n while the
"phenol-rich" fraction is also referred to as "water
in phenol."
-6-
~3~2~
The terms "phenol," "phenols," and "phenolic"
are intended to include phenol, pyrocatechol, resorci-
nol, hydroquinone, naphthols, as well as substituted
phenols such as phlorol, cresols, and xyIenol~
sy "nonporous" membranes is meant membranes
capable of separations that are best described by the
solution-diffu~ion model, the class of membranes
generally comprehending tho~e with no discernable pore~
having a dlameter greater than 5 Angstroms~ Membl-anes
usable ln the prqclenk invenkion m~y no~ bo ~u3copl:ibl~
~o deg~adakiol~ by phnnol~ in ~ny concentration ~or
obviou~ rea~on~ Clas3~s o~ Qlastomeric polymer~
include silicone rubbers, polyesters, polyurethanes, and
soft segment copolymers containing flexible groupings
such as chains of rigid polyamide with flexible poly-
ether segments. Preferred examples of such elastomeric
polymers are a silicone-polycarbonate copolymer made by
General Electric Co. of Schenectedy, New York and sold
under the trade name "MEM-213,*" a polyether-polyamide
block copolymer made by Atochem S.A. of Paris, France,
and sold under the trade name "Pebax 5533,*" a polyester
base polyurethane made by Lord Corporation of Erie,
Pennsylvania, and sold under the trade name "Tuftane
TF-312," a polyether base polyurethane also made by Lord
Corporation and ~old under the trade name "Tuftane
TF-410," and a diol terephthalate-polyether diol
terephthalate block copolymer made by DuPont Company of
Wilmington, Delaware and 301d under the trade name
*
"Hytrel 5556."
* Trade-marks
3L3~255~
Anion exchange polymers include virtually any
polymer containing in 30me fashion the well-known anion
exchange functionality of a quaternized ammonium group,
as well as weak base anion exchange of the tertiary
amine types. Preferred example~ are a ~erie~ of polymer
films containing quaternized vinylbenzylamine groups
grafted onto polyethylene or polytetrafluoroethylene
made by RAI Research Corporation and sold under the
trade name "Raipore,*" includin~ "Raipore R-1035, n
"Ralpore R-4035~l "Raipore R-5035~" and "Ralpore
R-S035~1 ~k~
Membrarlo~ useful in the presQnt inventlon may
be either flat or tubular, such as tubular membranes and
hollow fibers, including asymmetric membrane~. In the
lS case of flat sheets, the dry thickness of the membranes
may vary from 2 to 200 micrometers~ 5-S0 micrometers
being preferred, the e~sentlal criterion being that the
membrane withstand the low pre~sure applied to it on the
perm~ate side of the membrane. Incorporation into
pervaporation modules or series of modules comprises a
convenient way of using membranes in the method of the
present invention. In the case of hollow ~ibers, incor-
poration into modules by potted bundles i9 the preferred
form of use, in the same fashion as such fibers are used
in the reverse osmosis art. Hollow fibers are best used
with a lumen-side feed.
The proces~ of the present invention may be
used on aqueous solutions of phenols having virtually
any phenolic concentration from a few ppm up to about
* Trade-marks
~L3~2~
10~ by weight* (all concentrations hereafter, when
specified a~ a percentage, refer to percent by weight).
Under pervaporation conditions, the membrane is in a
state of extreme anisotropic swellinl~, ranging from
fully swollen near the feed ~ide to near dryness at the
permeate side, resulting in an extremely steep concen-
tration profile within the membrane from very high at
the feed side to very low at the permeate side.
Subject to the stability of the membranes, the
aqueou~ phenollc feed solution may be at temperatures
rangin~ anywhore ~rom ~lbout 20C up to the boiltn~
pOillt oE w~ter~ htly ~lovnt~d t~mpera~ureY Oe 4so to
90C being preferred. The process of the present inven-
tion is therefore highly efficient, allowing the use of
low-grade, waste-type heat (temperatures of less than
100C) to be utilized to produce a relatively high grade
product. The linear crossflow velocity of the feed may
range from 10 to 100 cm/sec. When a sweep stream is
used on the permeate side of the membrane, the gas
should be both inert to phenols and water and noncon-
densableO Examples include air, nitrogen, argon and
helium. When a vacuum is maintained on the permeate
side, it should be les~ than 10 mmHg. It should be
noted that, in the process of the present invention,
the downstream or permeate side pressure is entirely
independent of the feed side pressure.
_
*The concentration above which, at 40C or below,
spontaneous phase separation occurs~
~3~1 2~
Conden~ation of the vaporized permeate
emerging from the permeate ~ide of the membrane may be
accomplished by any number of known method~, including
collection on a cold surface such as in a cold trap, or
~ubjecting the same to elevated pressurs.
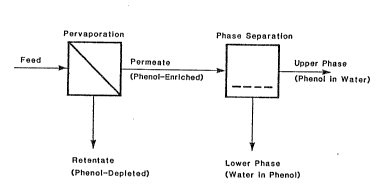
Referring now to the drawings, FIG. 1
comprise~ a schematic diagram illustrating both the
pervaporation step and the pha~e separation qtep of the
present invention, having the objectives of (a) removal
o~ phenol~ from wa~te water or proce3s water to produce
wat~r me~ting ~A~e dl~po~al or reu~e purlt~ reqllir~mon~7
and tb) cnrichm~nk an~ rocovery oE phenol to a "w~lter in
phenol" solution comprising roughly 70~ phenol and 30%
water. As shown therein, the aqueous phenolic feed
solution is directed to the feed side of a nonporous
polymeric membrane of the type described herein, repre-
sented by the diagonal line in the "Pervaporation" box.
An inert gas sweep stream or coar~e vacuum of 10 mmHg or
les3 is maintained on the downstream or permeate side of
the membrane, causing permeation or diffusion of the
liquid phase feed stream from the feed side of the mem-
brane to the permeate side of the membrane, the phenols
in the feed being transported in preference to water, 30
as to form a phenol-enriched vaporized permeate on the
permeate side of the membrane, and leaving on the feed
3ide of the membrane a phenol-depleted liquid retentate,
or phenol-purified water, the degree of purification
depending upon the particular separation characteristic~
of the membrane used, the membrane surface area, and the
--10-- ,
~L 3 ~
duration of contact of the feed with the membrane~ The
vaporized permeate is continually condensed in, for
example, a cold trap (not shown) into a phenol-enrichsd
aqueous liquid. When the concentration of phenols in
the permeate is about 10%, upon condensation of the
permeate, separation into two immiscible phases spon-
taneously occurs, shown schematically by the dashed
horizontal line in the 'IPhase Separation~ box of FIG. 1,
into an upper phenol-poor or "phenol in water" pha~e
comprising about 10~ phenol and ~0% water, and a lower
phenol-rich or "wa~er in phenol" pha~e compri~ing about
70~ ptlenol and 3Q~ w~to~ Tlle lower ph~3e may bQ
withdrawn from the procQs~, representing recovery of a
highly concentrated aqueous phenolic solution.
FIG. 2 schematically illu~trates a multi~stage
arrangement of pervaporation modules, allowing repeated
processing of the permeate resulting from each pervapo-
ration step wherein the condensed permeate of a given
stage con3titutes the feed of the next ~tage. As ~hown
in FIG. 2, the resulting retentate of each stage may be
combined to produce a single retentate exiting the pro-
cess stream and recoverable as phenol-depleted water,
while a single phenol-enriched permeate, that of the
last stage, is produced.
FIG. 3 shows a serial arrangement of pervapo-
ration modules, which, for the sake of simplicity in
illustration, represents two pervaporation steps,
wherein the retentate of a first pervaporation step
--11--
~C~2~5~
serve~ as the feed of a ~econd pervaporation step and
the downstream second permeate is recycled as part of
the feed to the first pervaporation ~tepO A~ may be
readily seen, such a serial arrangement need not be
limited to two pervaporation modules.
FIG. 4 illustrate~ another important advantage
of the method of the present invention, combining per-
vaporation and phase ~eparation, wherein the phenol-poor
(or "phenol in water") fraction from a first phase ~epa-
ration step comprises the f~ed to a second se~uel ofpervaporation and pha~ ~eparation ~tep~. In thi~
~cheme~ the "phonol in wa~r" eraction rQsul~ing ~rom a
combination of pervaporation and phase separation steps
such as shown in FIG. 1 comprises the "phenol in water"
feed having a phenolic concentration of about 10%, this
"phenol in water'l feed being pervaporated to yield a
phenol-enriched permeate which, upon condensation and
upon reaching a phenol concentration of about 10~,
undergoes spontaneous phase separation into an upper
phenol-poor (or "phenol In water") fraction comprising
about a 10~ aqueous phenolic solutiQn, and a lower
phenol-rich (or "water in phenol") fraction. The
"phenol in water" fraction, being identical in com-
position to the feed of the pervaporation step shown at
the left hand ~ide of FIGr 4, may be recycled to that
~ame feed.
Simultaneously with the production of the
phenol-enriched permeate, the pervaporation ~tep shown
in FIG~ 4 leaves a phenol-depleted retentate which, due
12-
~2~
to its relatively low phenol concentration, i~ suited to
be recycled as feed to the initial pervaporation ~tage,
as shown in FIG. 5, FIG. 5 essentially comprising the
combination of the ~cheme shown in FIG. 1 with that
shown in FIG. 4.
As mentioned above, the process of the present
invention, by virtue of the large number of permutation~
of steps available, may be used to tailor a predeter-
mined degree oP either phenol-purified water or phenolic
values. 0~ course, the ~electivity and Elux clensity o~
th~ mombrall~ chosen al~o con~ikut~ ~actor~ in:eluoncin~
the degre~ oE ~eparatlon achieved~
As is the case wlth membrane separations in
general, selectivity and flux in the membrane per-
vaporation separation of the present invention haveopposite tendencies, the greatest phenolic enrichment
being generally ob~erved at the lowest flux density.
For convenience herein, the selectivity of a giverl
membrane iq expressed as an "enrichment Eactor," that
factor comprising the ratio oE phenolic concentration in
the permeate to phenolic concentration in the feedr
The elastomeric polymeric membranes useful in
the present invention generally exhibit a moderately
increasing enrichment at high phenol concentration,
followed by a ~ignificant increase in enrichment with a
low residual phenol concentration, while flux density
remains nearly independent of phenol concentration,
declining ~lightly a~ phenolic depletion progre~ses.
-13-
The anion exchange membranes u~eful in the
present invention behave differently than the elasto-
meric membranes in that enrichment i~3 generally lower
and flux density generally higher than with the elasto-
meric membranes. Both phenol enrichment and fluxdensity increase at low phenolic concentrations in the
feed. But, when viewed as a function of the total con-
centration range entailed, the enrichment factor passes
through a minimum, whereas the flux denqity ~teadily
increases with phenol depletion of the feed~
In general, an increase of the temperature at
which porvaporation 1~ ~on~lc~ed ha~ the a~ect o~
lowerirlg the enrichlnont Eacto~ and increa~ing ~lux
density. Flux density is inver~ely proportional to
membrane thickne~s, while thickness appears to have no
impact on enrichment capability. Suitable phenol
depletion in a single stage pervaporation step may be
accomplished by highly selective membranes, wherea~ a
multi-stage pervaporation process i9 required to achieve
the same degree of phenol depletion with a le8s selec-
tive, more permeable membrane. In a multi-stage per-
vaporation process, each pervaporation ~tage is designed
to produce a retentate of a targeted residual phenol
concentration high yield of phenol-depleted retentate.
Example 1
Aqueous phenolic feed solutlons comprising
200 ppm of each of phenol (b.p. 181C), phlorol ~b.p.
196C) and xylenol (b.p. 212C), for a combined total
phenolic concentration of 600 ppm was fed at lo 2 L/min
13~2~
and 50C for about 2 hours via a rotary feed pump with a
flowmeter through two pervaporation cells in parallel,
the functional part of each cell comprising the non-
porou~ polymeric membranes noted in Table 1~ each
S membrane having a surface area of 45.5 cm2, and a dry
thicknes~ varying from 1 to 2 mils. The downstream or
permeate ~ide of each cell was connected via cold traps
to a vacuum pump which maintained a pressure of
5-10 mmHg on that 3ide of the cell, the cold trap~ being
immersed in liquid nitrogen to effect condensation of
the permeate. Down~tream pr~ure wa9 monitored by a
mercury manomotor ln clo~ proximity to the down~t:re~m
~ide oE the pervaporaton cell. Analysis for phenolic
enrichment was by both high pressure liquid chroma-
tography and by ultraviolet spectroscopy. The resultsare shown in Table 1, the enrichment factors being
expres~ed as noted above, and flux density being
expressed in kg/m2-day.
Table 1
Enrichment Flux
Membrane Factor Density
Hytrel 5556 24 4.9
MEM-213 60 5.5
Pebax 5533 150 5.2
Tuftane TF 312 14 2.8
Tuftane TF 410 30 3.8
Raipore R-1035 3 68
Raipore R-4035 6 18
Raipore R-5035L 3 32
Raipore R-5035H 5 13
-15-
13~2~
Example 2
Three of the membranes of Example l were
evaluated assuming two pervaporation cell3 in ~erie3, as
schematically shown in FIG. 3, and further as~uming the
continuation of pervaporation in discrete step~ of
increasing phenolic concentration of the feed just until
spontaneous phase separation of the condensed permeate
took place, the qeparation being one of an upper phenol-
poor pha~e ("phenol in water") comprising about 10%
phenol in waker and a lower phenol-rich pha~a ("water in
phonol") comprl~ing about 70~ phenol ln WatQr~ Th~
re~ult~ ar~ shown in Table 2~ with fe~d conc~ntration
being given in ppm phenols.
Table 2
Feed Concentration Enrichment Factor
Membrane Yielding Pha~e Sep'n at Phase Se~'n
Pebax 5533 1,700 57
MEM-213 3,200 31
Raipore ~-~035 11,500 8.5
Example 3
The ~ame three membranes of Example 2 were
used in a single pervaporation ~tage to determine the
relation~hip between the concentration of phenolics in
the feed and enrichment and flux den3ity. The values
obtained were plotted in the graph~ comprising
FIGS. 6-8.
As 3een in FIGS. 6 and 7, the two ela~tomeric
membranes show a fairly ~imilar pattern of enrichment
and flux with progres3ing phenol depletion of the feed,
-16-
~2~
i.e., there was a moderately increasing enrichment at
high phenol concentration in the feecl, followed by a
marked increa~e in enrichment toward low residual phenol
concentration, while flux density remained nearly inde-
pendent of phenol concentration in the feed, slightlydeclining as phenol depletion progre~lsed.
As seen in FIG. 8, the anion exchange membrane
exhibited a generally lower enrichment and higher flux
than the elastomeric membrane~. Both enrichment and
~lux incr~ed with decrea~ing eced concantr~tion~
~nrichm~nt paa~ g khrou~h a minimum, whlle ~lux
stQadily increa~ed.
Example 4
U~ing the data of Example 3, single-stage
pervaporation of aqueous phenolic feea ~olutions with
initial concentrationq of 5000 ppm, 1000 ppm and 200 ppm
were evaluated assuming an elastomeric nonporous poly-
meric membrane ~Pebax 5533) and the ~ame apparatus a~
that of Example 1 for examination of the pattern of
phenol depletion. The result~ are ~hown in Table 4,
with all concentration3 in ppm (mg/kg), the membrane
area in m2/1000 kg-day, and ~howing the fraction of feed
recovered as phenol-depleted retentate ~% Feed in
Retentate). As is apparent from Table 4, phenol deple-
tion i~ readily accompli~hed in a ~ingle ~tage.
-17-
~.3~2~
Table 4
Feed RetentateMembrane ~ Feed in
Conc. Conc~ Area Retentate
5000 10 35 92
5000 1 42 90
5000 0.1 49 89
1000 10 24 95
1000 1 31 93
1000 0.1 36 92
200 10 1~ 97
200 1 21 96
200 0.1 26 9S
Example 5
Multi-stage pervaporation of feed solution~
having the same concentrations as those of Example 4 was
: evaluated as~uming an anion exchange membrane of the
pre~ent invention (Raipore R-4035) in a series arrange-
ment of the type depicted in FIG. 2. Each stage wa~
de~gned to produce a retentate having the targeted
re~idual phenol concentration~ of 10 ppm and 1 ppm. The
results are shown in Table 5, the units of which are the
same as for Table 4 except that the membrane area given
comprises the combined areas of the membrane~ for each
. . . . .
~tage of pervaporation nece~ary to achieve the phenol
depletion shown.
-18-
~ 3 ~
Table_5
Feed Retentate Membrane ~ Feed in Number of
Co Conc. Area Retentate Stages Required
5000 10 178 92 13
5000 1 197 85 12
1000 10 69 89 7
1000 1 94 89 9
200 10 23 93 4
200 1 36 92 5
~ Slmult,lnQou~ anrlchmQnt oE ph~nol to ~ per-
meate concentration of 10~ (lOO,Q00 ppm), so as to cause
phase separation as discussed above, and depletion of
phenol from a water fraction was evaluated on feed
solution~ having the same concentrations a~ those of
Example 4 by an arrangement of the type shown in FIG. 3
assuming an elastomeric nonporous polymeric membrane
(Pebax 5533)~ Membrane areas ~or the two modules are
given separately in Table 6 in the same unit~ as in
Table 4. By recycling the phenol-enriched down~tream
permeate (permeate 2) to the feed stream, the phenol
concentration o~ the feed is increased such that single-
stage pervaporation yielded the targeted 10% phenolic
concentration. Thus, as seen in Table 6, the fraction
of the total membrane area required to deliver permeate
for recycling increa~ed as the initial feed con-
centration decreased.
--19--
~311 2~
Table 6
Feed Retentate Permeate Membrane Area % Feed in
Conc. Conc. Conc. _odule 1 Module 2 Retentate
5000 10 100,000 18 20 95
55000 1 100,000 18 26 95
5000 0.1 100,000 18 27 95
1000 10 100,000 4 2~ 99
1000 1 100,000 4 37 99
1000 0.1 100,000 ~ 4~ 99
10200 10 100,000 0.7 31 99 '
200 1 100~000 0.7 3~ 9
200 0.1 100,000 0.7 50 99
Example 7
Using actual data obtained in the previous
example3, an idealized process scheme of the type
illustrated in FIG. 5 utilizing both elastomeric and
anion exchange-type membranes of the present invention
was evaluated. An elastomeric-type membrane with high
qelectivity is used in a flrst pervaporation stage on a
dilute feed solution, while an anion exchange-type
membrane with moderate selectivity is used in a second
pervaporation stage to treat the "phenol in water" solu-
tion comprising the supernatant of the spontaneous phase
separation occurring in the first phase separation step.
Since thi~ "phenol in water" solution is to be subjected
to a second pervaporation stage as shown in FIG. 5,
phenol enrichment of the first pervaporation stage may
be limited to the concentration level of "phenol in
-20-
~3~2~
water" (about 10~ phenol), implying that very litle
"water in phenol" is being produced at this first per-
vaporation stage. Given appropriate procesq control,
the first phase separation step may be eliminated alto-
S gether, feeding the condensed first qtage permeate of
"phenol in waterN concentration directly into the ~econd
pervaporation stageO As would be expected given the low
initial phenol concentrations considered, the fraction
of the feed appearing as phenol-enriched permeate, to be
processed in the ~econd pervaporation ~tage~ mall.
~ed on the ~eparation charactoris~ic o tha anion
exchange m~mbrane Ralpore ~-~035~ the ~ollowln~ ma~
balance for the ~econd stage pervaporation is obtained:
Pervaporation of 100 kg of "phenol in water"
lS (10% phenol) at 50C yields
- 16 kg of permeate (60% phenol)
- 84 kg of retentate (0.5% phenol)
The retentate is recycled into the original feed stream
and thus remains in the proceq9. The permeate undergoes
phase separation as follows:
Pha~e separation of 16 kg of the above
permeate (60% phenol) yields
- 13.3 kg of "water in phenol" (70% phenol~
- 2.7 kg of "phenol in water" (10~ phenol)
The "phenol in water" fraction is recycled to the second
pervaporation ~tep, as shown in FIG. 5. The "water in
æhenol" fraction, combined with the corresponding frac-
tion of the first phase separation step, is con~idered
to be the phenol-enriched product of the overall
separation process.
The overall enrichment of phenol in the pro-
ces~ envisioned depends solely on the initial phenol
concentration of the feed stream, the exit concentration
of the phenol-enriched process stream being fixed by the
nature of the immi~cible water-phenol phases. The over-
all enrichment realized by such a process i3 illustrated
by the figure~ in Table 7, concentration again being
given in ppm phenol.
Table 7
Initial Overal.l
Feed Concenkration~nrichmenk Factor
200 3~S00
1,000 700
2,000 350
155,000 140
lOO,000 (10%) 7
The term.s and expressions which have been
employed in the foregoing specification are used therein
a~ terms of description and not of limitation, and there
is no intention, in the use of such terms and expres-
sions, of excluding equivalents of the features shownand described or portions thereof, it being recognized
that the scope of the invention is defined and limited
only by the claims which follow.