Note: Descriptions are shown in the official language in which they were submitted.
3 ~ 8 ~
This invention relates to a pacl~age containing a
blood facsimile reference solution for use in blood gas
electrolyte analysis and a method or producing a
packaged blood facsimile reference solution. This
~ 5 application is a di~ision of Canadian patent
: application serial No. 504,268 filed March 17, 1986.
In a variety of clinical situations it is
; important to measure certain chemical characteristics
of the patient's blood quch as pH, concentrations of
calci.um, po-tassium ions and hematocrit, -the partial
pres ure of 02 and C02 and the lilce. (See, for
example, Fundamentals of Clinical Chemistry, Tietz,
Edi-tor, page 135 et seq., Electrochemistry; page 849 et
seq., Blood Gases and Electrolytes; 1976, Saunders
~` 15 Company, Phila.). These situations range from a
routine vi~it of a patient in the physician's office to
monitoring during open-heart sur~ery for which
situa-tions of the required speed, accuracy and similar
performance charac-teristics vary with each situation.
Measurement of chemical characteristics of blood
during open-heart surgery provides the most demanding
set of criteria. Presently, blood gas analysis during
major surgery is provided by repeated tra.llsfer of
~ ~k
...
,,
~ 3 ~ 3 ~
-- 2
discrete blood samples -to a permanent lab-based blood
gas analyzer or by use of sensors placed in-line with
the extra-corporeal blood circuit of a hear-t-lung
machine employed -to bypass the patient's heart.
The transfer of discrete blood samples, required
by blood-gas analyzers inheren-tly increases the risk oE
contaminating the blood sample with the ambient airl
whicll may alter certain of the monitorecl
oharaoteristics. ~dditionally, since such analyzers
are complex and aostly clevices, they are typic:ally
located only in the hospital ].ab where -they need to be
operated by a skilled technician, resulting in
undesirable delay during surgery, critical care or
intensi~e care. Furtherg such analyzers employ bubble
tonometers to generate a suitable electrolyte referent
mixture by dissolving quantities of gases, stored in
pres~urized free~standing tanks, into -the electrolyte
solution. ~hile replacemen-t o-f theses gas tanks is
infrequerltly required, it is a cumbersome procedure.
Finally, these existing analyzers require cleaning to
decontaminate all exposed portions from the prior
patient's blood prior to subsequent use.
hlthough use of in-line sensors minimized the risk
_ 3 _ ~ 3 ~
of contamination during transfer and of delay, they
have a response wh:ich normally varies or "drifts"
during use; moreover this drift is not at a constant
rate. Present in-line sensors can only be calibrated
before they are placed in -the extra-corporeal circuit.
Thus, the inherent drift of these in-line sensors
cannot be monitored, resulting in reading of ever
decreasing reliability as time passes.
The system disclosed herein provides quicll, on-
si-le oontemporaneoll~ blood chernis-try analysis, with
minilnal risl~ o~` contamina-tion, and maintains its
accuracy over its useful life.
According to the present invention, -there is
provided a blood facsimile reference solution for use
in blood ~as electrolyte analysis, comprising ionic
po-tassium and calcium and tonometered at e]evated
-temperature l~ith oxygen and carbon dioxide, the package
content being free of voids.
According to another aspect of the invention,
there is provided a method of producing a packaged
blood facsimile reference solution containing oxygen
gas, carbon dio~ide gas and ionic potassium and
calcium, comprising constituting an aqueous buffered
3 ~ 3 ~
solution containing ionic potassium at a predetermined
concentration, subjecting the resulting solution to
tonometry with said gases, and following initiation of
tonometry admixing ionic calcium in predetermined
amount with the tonometered solution.
According -to a further aspect of the invention,
there is provided a method of producing a package of an
electrochemioally stable tonometered blood ~acsimi:Le
solution for storage and for use in b]ood/gas
monitoring at atmospheric pressure, comprising
~ packaging the solution in a sealed flexible gas-
; impermeable envelope free of voids while maintainirlg
the solution at sub-atmospheric pressure ranging from
about 625 to about 700 mm Hg and at temperature higher
16 than said use temperature.
A preferred blood chemistry analysis machine is
adapted to be connected to a blood collection device,
an extracorporeal shun-t, or arl ex vivo blood ~ource
such as a heart/lung machine used to sustain a patient
during surgery, intensive care, critical care and the
like. The machine is designed to allow small test
samples of flowing live ex vivo blood to be diverted
off-line from either the venous or arterial flow lines
:L3tl. 3~-$~
of a flowing blood source such as a heart/lung machine
directly in real time -to a chamber exposed to a bank of
solid state micro-electrodes which generate electr,~ical
signals proportional to chemical characteristics of the
real time flowing blood sample.
; The bank of eleotrodes is housed in a disposable
cartridge, adjacent to a thermal plate whicll maillt,airls
the tes-t sample at a constant temperature. Upon
insertion of the cartridge ;.nto a challlber of suitably
adapted blood chemistry analysis mac}lh~e, the
electrodes conneot l;o an electrode inter~ace wll,ic
selects on of` the plurality of electricul si~nals
generated by the sensors and passes the selected si~nal
to a microprocessor in the machine ~here it is
converted from analog to digital form suitable for
analysis, storage and display.
A metal plate in the cartridge connects -to a
thermal unit in the machine l~hich moni-tors the
-temperature of and genera-tes and -transmits heat to the
plate and through it to the sample in -the adjacent
electrode chamber in order to maintain the sample at a
constant temperature.
The car-tr,idge also contains at least one, and
.
- 6 - ~3~$~
preferable two containers of reference or calibrating
electrolyte solution (i.e., solution serving for
purposes of quality control including calibration,
sometimes referred to hereinafter as control or
calibration solution), as well as a reservoir suitable
to collect waste Eluids following assay. ~pon
insertion o-f the cartridge, a selec-tion valve in the
cartrid~e connec-ts to a shaft in the machine,
con-trolled by the microprocessor, to selectively all.ow
either oE the calibrati.rlg solutions or the test sampLe
to flow across the eleotrodes.
The force driving the fluid flow through the
cartridge (eg, by positive pressure or suc-tion) is
provided by a peristaltic pump formed when a set of
rotatable drive rollers in the machine pinch exposed
por-tions of tubing against the curved wall of the pump
slot on the cartridge. The rotation of the rollers
forces either the calibrating solutions or a test
sample from their respective sources through the
cartridge tubing across the electrode chamber and into
the was-te collec-tion reservoir. The rotation of the
drive rollers is controlled by the microprocessor.
In addition to the features already mentioned, the
~ 7 ~ ~3~
analysis machine houses an internal digital clock which
provides a time base for the operation of the system, a
bacl~-up battery power source, an operator keyboard, a
display and a printer.
In operation, after all connections are suitably
made, the selection valve and drive rollers cooperate
to cause the calibrating solution to flow into the
electrode chamber where a reading is taken and stored
in the microprocc?ssor. Subsequenl.Ly all(J in a s.illl;lar
manrler, a read.ing o:l` the test sample is taken, anal~zecl
by -the microprocessor and displayed. The assa~ed
fluids are directed into the waste collection
reservoir. The microprocessor controls and repeats
this cycle oE calibration and test sample assay at a
rate preselected and entered by the operator through
the control ]~eyboard. The keyboard also allows the
operator to take an immediate asqay at any time, e~en
while the machine is in its automatic cycle mode,
li.mited only by the recycling time of be-tween two and
three minutes. Following surgery, the cartridge and
the tubing connecting the venous and arterial flows of
the heart-lung machine to the cartridge are discarded
and the machine is ready for use with a new cartridge.
- 8 - ~3~3~$~
Further features and advantages will be more
apparent upon reading the following de-tailed
description and by reference to the drawings in which:
FIGURE 1 is a schematic diagram showing the major
components of a preferred embodiment of a blood gas
analysis system;
FIGURE 2 is an elevated side view of one
embodiment of the cartridge useful wi-th the system of
FIGURE 1; showirlg the insertiorl end Or tlle curtriclge in
tlle foreground;
FIGURE 3 ls a fragmentary perspeotive view of this
embodimen-t of the car-tridge;
FIGURE 4, which appears on the same shee-t as
FIGURE 2, is a side view of the trailing end of this
embodiment of the cartridge;
FIGURE 5 is an exploded view of the selection
valve contained in this embodiment of the car-tridge;
FIGURE 6a is a frontal view of -the elec-trode card
contained in this embodiment of' the cartridge;
20FIGURE 6b is a cross sectional view of this
electrode card; and
FIGURE 7, which appears on the same sheet as
FIGURES 2 and 4, is a fragmentar~ side view of -the end
:
- 9 - ~ 3 ~ 3
wall a-t the inser-tion end of this embodiment of -the
cartridge, showing the peristaltic pump slot;
FIGURE 8, which appears on the same sheet as
FIGURE 3, is an elevated side view of that portion of
the blood gas analysis machine useful with the system
of FIGURE 1, adapted to receive and connect suitably -to
certain features of -this embodiment of the cartridge;
and
FIGURE 9 is a rrontal view o~ one embo(lilllerlt of
the oontrol panel of the blood gas analysis machine
showing -the display and keyboard.
While the apparatus for chemical measurement of
blood charac-teristic of the present invention may be
used in a variety of clinical and experimental
environments, the preferred embodiment of the inven-tior
is described as being used in major surgery. This
description should not be taken to limit the
applicability of` the presen-t invention.
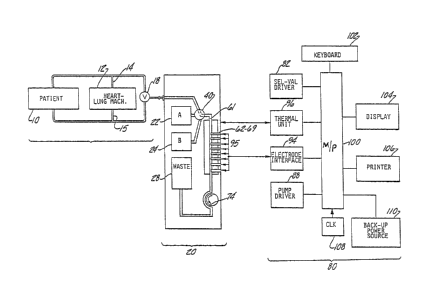
FIGURE 1 shows in schematic form a blood gas
analysis system suitable for use during surgery in
which a patient lO is sustained by a heart/lung machine
12.
lo ~ c $ ~)
This system allows a test sample of blood to be diverted
from either the venous flow 14 or the arterial flow 15
of the heart/lung machine 12, as selected by the system
using a two-way valve 18, directly to a cartridge 20
containing a bank of sensing electrodes fi2-69~ These
electrodes 62-69 generate electrical signals
proportional to distinct characteristics of the blood
sample. These signals are transmitted to a
microprocessor 100, contained within a blood chemistry
analysis machine 80 into which the cartridge 10 has been
inserted. After analyzing these signals, the
microprocessor 100 controls a display 104 to display the
values of these parameters of the blood sample to
provide the surgeon with information on the status of
the patient 10~
: The system operator uses a keyboard 102 to
program the microprocessor 100 with the desired
frequency of assays to be made by the system during
surgery~ The microprocessor 100 then controls the
selection valve driver means 82 in the machine 80 to
cooperate with a selection valve 40 in the cartridge 20
to allow the sequential fluid flow from a calibrating
solution bag 22 and a calibrating solution bag 24, both
contained in the cartridge 20, and then from the venous
:
3 ~ $ ~
flow 14 or arterial flow 15, into an electrode channel
61 exposed to a bank of electrodes 62-69. The distinct
reference solutions from the bags 22 and 24 provide a
two-point calibration of the electrodes 62-69. In a
similar manner, at the selected intervals, subsequent
assays of blood samples are made, most preceded by one-
point calibration, with occasional two-point caLibration
made to ensure continued accuracy. Upon completion of
the surgery or depletion of the calibrating solutions,
the cartridge 20 is discarded and replaced by a new
cartridge 20 Eor subsequent use of the system.
All of these features and additional features
are explained more fully herein.
Referring now to the FIGURES 2 and 3, there is
shown a box-like cartridge 20 which is preferably made
of rigid plastic. The dimensions of the cartridge
allow insertion into a blood gas electrolyte analysis
machine 80, shown in FIGURE 1, appropriately engaging
features to be described herein.
The main body of cartridge 20 is partially
enclosed to provide protection of its contents,
flexible bags which are two calibrations solution bags
- 12 - ~ $
22 and 24, and a waste collection bag 28. The
calibrating solution bags 22 and 24 have zero head space
and contain solutions and dissolved gases therein that
preferably are specially formulated as described
hereinafter, having known, distinct electrochemica1
characteristics. For a description of the technology of
packaged reference or calibration solution, see U.S.
Patent No~ 4,115,336. The third bag 28 or waste
collection bag begins in an empty state and is intended
to collect waste calibrating solution and blood products
following assays. Preferably, the calibrating solut~on
bags 22 and 24 are encircled by the two sides of waste
collection bag 28, as shown in FIG~RE 4.
The bags 22 and 24 each are gas impermeable
and contain an aqueous reference (i.e., calibration or
control) solution (a solution electrochemically
resembling blood with respect to dissolved gas and
electrolyte) having known values of the chemical
characteristics over a range of values that the system
is intended to monitor. The values of those
characteristics are different in the two bags so that by
sequential passage of the two calibrating solutions over
the electrodes 62-63, a two point calibration or bracket
- 13 - ~3~3~3
(e.g~ high and low) calibration of the measurement
characteristics of the electrodes may be made~
In order to maintain the concentratlon of
gases, such as oxygen and carbon dioxideg at a known
constant level in the bags 22 ancl 24, independent of
variations in ambient pressure and temperature, the
~ gases are added to the solutions~ during their
: packaging, in a special manner~ As a feature of the
invention, the packaging in one embodiment to be
described is performed under conditions of pressure and
temperature which are different from those that will be
encountered during normal use of the solutions, so that
advantageously the solutions may be fully saturated with
the gases at the time of packaging with the important
but hitherto unrealized result that these same solutions
will be suitably unsaturated during use~ Typically,
both the temperature will be higher and the ambient
pressure lower during the packaging procedure than will
ever be encountered in use~ For example, for a blood
facsimile formulation tonometered with oxygen, CO2 and
nitrogen, packaging may occur at a pressure above about
625 mgs~ to about 700 mm. Hg and at elevated
temperatures in the approximate range from 45 to 50
C~, higher temperatures being unnecessary~ During
- 14 ~ 3l~3
packaging, the 1iquids are fully saturated with the
gases and the packages are sealed in an effort to
minimize entrapped air. It is found that later when the
temperature and ambient pressure are at normal use
values, the packaged solutions will not be saturated but
their analyte concentrations will sti1l be at the known
values achieved during initial filling process. Since
the solutions are unsaturated, there is no tendency for
the gases in the solution to outgas into any gas bubbles
formed during use~
By way of illustration but without limitation,
a preferred embodiment of reference solutions for dual
monitoring as described above, comprises the following
solutions designated A and B and their respective
methods of tonometry~
Formulations And Tonometry Procedure
Calibration Reference Solution A: Na+, Ca+~, pCO~, P~
Prepared at 37C and at atmospheric pressure
tonometered with ~% CO2 -N2 gas~
All cornpounds are weighed, combined, and
diluted to volume except the calcium salt which is added
after tonometering has started~
. 15 ~ 3~ 3 ~L ~ ~
COMPOUND CONCENTRA~ION ~SS. 1.0 L
Buffer, 3-Morpholinopropane- 14.0 mmol/l 2.926 g
sulfonic Acid (MOPS)
Buffer, NaMOPS 36O0 mmol/l 8.316 g
~ suffer~ NaHC03 14.5 mmol/l 1.218 g
NaCL 110 mmol/l 6.430 g
NaN3 .01~ w/w 007 g
KCl 6.0 mmol/l .447 g
CaC~2-2~2 1.25 mmol/l .184 ~
1J 1. ON HCl ca 8 mmol/l ca 8 ml
25 wt. % Surfactant (BRIJ
35) aq. soln.
This gives parameter levels of:
mmol/l
. .
L5 pH PCO~ mm Hg PO~ mm Hg K~~-Radiometer K~-Beckman Ca++
7.330-7.3~5 15.5-19.0 116-120 5.6-5.8 5.60-5.75 .85-.95
Calibration Reference Solution 8: Na~ ~ ~ ; pH
Prepared at 50~C and at 700 mm Hg absolute
pressure tonometeted with 21~ 2 ~ 4~ C02-N2 gas.
All compounds except the calcium salt are
weighed, transferred and combined, and diluted to volume
with H20. The calcium salt is added after tonometering
has started.
- 16 - ~3~3~$~
COMPOUND CONCENT.RATION MASS. 1.0 L
Buffer: Imidazole 50 mmol/1 3.405 ~
Na~SO3 10 mmol/1 1~250 g
NaHC03 11.5 mmol/1 0~966 g
NaC1 93 mmol/1 5~44 ~
NaN .01~ w/w .007 g
KC13 2.0 mmol/1 .149 g
CaC12!2H20 0.25 mmol/1 ~037
1.0N HC1 23 mmol/1 23 ml
25 wt. ~ Surfactant (BRIJ ~.25 ml/1
35) aq. soln.
This gives parameter levels of:
mmol/1
-
pE~ PCO~ mm Elg ~2~ Ig K~-Radiometer K~~-Beckman _A~-~
6.890-6.910 4~-48 n.o 1.9~-1.9 1.83-1.98 .18-.22
Thus, the reference solution in packaged form
for use in blood/gas measuring or monitoring equipment
according to a preferred embodiment of the invention
comprises a flexible gas-impermeable void-free package
~20 of an aqueous solution electrochemically resembling
arterial blood or venous blood. The solution contains
electrolyte (ionic potassium and calcium) and dissolved
gas at known partial pressure~ The mentioned packaged
solution may thus be regarded as an electrochemical
facsimile of blood in a stable form such as that
exempliEied by reference solution B above~ The total
gas pressure in the packaged solution is in the range
from about 0.82 to about 0.92 atmospheres (625 to 700 mm
~3 ~ 3~$~
- 17 -
Hg) at use temperature, i.e., temperature encountered
during storage and monitorir,g~ The package may be a
ilexible bag, as indicated, or other suitable container
package~
It is found that the preparation of the above-
mentioned packaged blood facsimile involves previously
unrealized compatibility problems. In this regard, when
constituting the solution by conventional tonometry
procedures, one finds that the compounds are incompatible
in that ionic calcium separates unmanageably Erom the
solution as a non-ionic precipitate~
Therefore, another preferred aspect of the
invention eesides in a method of producing a packaged
blood facsimile reference solution containing oxygen gas,
carbon dioxide gas and ionic potassium and calcium,
without the unwanted precipitation of calcium~ The
method comprises constituting an aqueous buffered
solution containing ionic potassium at a predetermined
concentration, subjecting the resulting solution to
tonometry with the gases, and following initiation of
tonometry admitting ionic calcium in predetermined
amount with the tonometered solution, whereby the
resulting solution is stable with respect to the desired
- 18 - ~3~
ionic parameters and the solution can be suitably
packag~d~
Still another preferred method aspect of the
invention concerns a method of producing a package of an
electrochemically stable tonometered blood facsimile
solution, as indicated, for storage and for use in
blood/gas monitoring a~ normal atmospheric pressure. The
method comprises packaging the solution in a sealed
flexible gas-impermeable envelope free of voids (i.e.,
zero head space) while maintaining the solution at sub-
atmospheric pressure and at temperature higher than said
use temperature, as described hereinbefore. The packaging
can be done in any suitable way by packaging art means
which per se may be conventional.
A preferred embodiment of the package aspect of
the invention concerns a flexible gas-impermeable
package~ The package contains a blood facsimile
reference solution for use in blood gas electrolyte
analysis, comprising ionic potassium and calcium and
tonometered with oxygen and carbon dioxide, the package
contents being entirely liquid and free of voids or
bubbles under conditions of use.
Both calibrating solution bags 22 and 24
contain tube fittings 26, as shown in FIGURE 3 and 4,
which connect in turn to their calibrating solution
19- ~3~
tubes 23 and 25 respectively. The ca1ibrating solution
; tubes 23 and 25 subsequerltiy connect to a selection
valve 40 as described later.
The waste collection bag 28, suitable for
collection of waste blood products and calibrating
solutions following assay, is formed of a materia1 which
is semi-permeable to gases but impermeable to the liquid
component of blood and to the calibratillg solutions. It
is thus intended that only the liquid component of blood
and of the caLlbrating solution will occupy space in
the waste collection bag 28. In the preferred
embodiment, the bags 22, 24 and 28 are contained in the
main body of the cartridge 20 such that as the waste
collection bag 28 fills, it will occupy the space
created by the emptying of the calibrating solution bags
22 and 24.
The waste collection bag 28 also has a tube
fitting 26, shown in FIGURE 4J connected to a waste tube
76, which originates at the discharging end of the
electrode card 60. A check valve 77 (FIGURE 3) is
disposed in the flow line to the collection bag 28 to
prevent back-flow~
The trailing end of the cartridge 20, bei~g
the end opposite to the cartridge which is inserted into
- 20 - ~ 3~
the blood gas analysis machine 80, contains a blood
intake port 30, shown in FIGURES 2 and 3, connected by
tubing 16 to either the venous blood flow 14 or the
arterial blood flow 15 of a heart/lung machine 12 used
to sustain the patient 10 during surgery~ The system
operator controls the selection of a blood sample from
either the venous flow 14 or the arterial flow 15 by
use of a valve 18 in the tubing 16~ The blood intake
port 30 is connected by a blood intake tube 32, passing
through the interior of the cartridge 20 between the
bags 22 and 24, to the selection valve 40 at the
insertion end of the cartridge 20.
; As shown in FIG~RES 2 and 3, the insertion end
of the cartridge 20 includes a selection valve 40, an
: 15 electeode card 60, a peristaltic pump slot 74, and a
metal plate 70 In the preferred embodiment, this
insertion end of the cartridge 20 is protected by the
overhanging sides and top of the plastic encasing
material of the cartridge 20 but is exposed to the
connecting portions of the blood gas analysis machine
80.
Referring to FIGURES 2 and 3, the selection
valve 4~, the electrode card 60, and the peristaltic
pump slot 74 are all intended to connect with
appropr;ate contacts in the blood gas analysis machine
- 21 - ~3~3~
80b The insertion end wall 50, formed of plastic,
serves to provide partial protection to the bags inside
the main body of the cartridge 20, and to provide well-
positioned contact between the appropriate portions of
the cartridge 20 and the blood gas analysis machine 80
upon insertion of the former~
As shown in FIGURE S, the selection valve 40
has a rotating plug 42, formed of a thick ring of
plastic, which houses the electrode input tube 58,
cunning ultimately to the input end of the electrode
channel 6l. The rotating plug 42 is held flush
against the insertion end wall 50 by a bolt 46 passing
through the center of the plug 42 and through the end
wall 50 into the interior of the cartridge 20. As the
bolt 46 extends into the interior of cartridge 20, it
passes through a spring 48 ~hich seats against a nut 47
which in turn serves to seat the plug 42 flush against
the head 44 of the bolt 46. The spring 48 thus serves
to urge the rotating plug 42 against the insertion end
wall 50~ The exterior end of the bolt head is recess
le~g., Allen-recess) adapted to receive a drive shaft 84
in matching relation when the cartridge 20 is inserted
into the machine 80~
. .
- 22 - ~3~
The rotating plug 42 seats against that
portion of the insertion end wall 50 having three ports
52l 54 and 55~ A blood sample por~ 52 connects in the
interioe of the cartridge 20 to the blood intake tube
32; the calibration solution ports 54 and 55 connect in
the interior of the cartridge 20 to the ca1ibration
solution tubes 23 and 25 respectively~ Each end of the
; ports 52, 54 and 55 which contacts the rotating plug 42
is sealed by a rubber ring 56 to provide a leakproof
connecti.on to an electrode input tube 5B~
As seen in FIGURE 5, the selection valve 40
allows the microprocessor to direct the rotating plug 42
into a position aligning the electrode input tube 58
with either the blood intake tube 32, the calibrating
solution tube 23, or the calibrating tube 25; when
aligned with one of these tubes, the rotating plug 42
blocks the flow from the other two tubes~
Another feature of the insertion end of the
cartridge 10 is the electeode card 60, best shown in
FIGURES 6a and 6b. The electrode card 60 .is formed of
polyvinylchloride in a generally rectangular shape and
contains a bank of electrodes 62-69~ The electrode card
60 is fastened to the insertion end wall 50 such that
the electrode bank protrudes and connects with an
, .
., , , , , ~
:~3~ 3~
- 23 -
electrode interface 94, within the blood gas analysis
machine B0.
Preferably, each of the electrodes 62-69 are
distinctly formed planar solid state electrodes which
allow assay of different characteristics of human blood~
The distinct construction of each electrode 62-69
produces a plurality of voltages or currents proportional
to differerlt chemical characteristics of a test sample~
The electrodes 62-69 are formed in preformed circular
slots in the electrode card 60. These solid state
electrodes may be either of the ion-selective membrane
type, as is preferable, of the metal/metal-oxide type,
or of polarographic type, as is also preferable, all
well known to the prior art. Once the electrodes 62-69
are formed, their interior analyte sensing ends remain
exposed to an electrode channel 61 and to any sample
contained therein. The electrode channel 61 is
connected at one end to the electrode input tube 58 and
at the other end to the waste tube 76 and is adapted to
contain a sample being exposed to the electrodes 62-69.
In one preferred embodiment the flow path of the
electrode channels is rectilinear in cross-section
(e.g., lmm x 2mm~ having a total volume of ca. B0 ul.
The electrode card 60 is backed by a metal
; 25 plate 70 disposed adjacent to the electrode channel 61
:~3~ 3~$~
- 2~ -
which makes contact with a thermal unit 96 in the
machine 80, allowing the microprocessor lO0 to monitor
and control the temperature of the sample while in the
channel 61.
: 5 The exterior end of each of electrodes 62-69
is topped with an electrically conductive material.
This conductive material is then drawn out to the distal
end of the electrode card 60 to complete, upon insertion
: of the cartcidge 20, the contact between the electrodes; 10 assaying the sample and the electrode interface 94 which
connects to the microprocessor lO0 contained in the
machine 80. The microprocessor lO0 is programmed to
monitor, store, and display the assay results, among its
other functions.
FIGURE 7 illustrates the peristaltic pump slot
74 which is disposed in the insertion end of the
cartridge 20~ The peristaltic pump slot 74 is a concave
slot in the insertion end wall 50. The waste tube 76
running from the output end of the channel 61 to the bag
fitting 26 of the waste collection bag 28 is brought out
of the main body of the cartridge 20 through the
; insertion end wall 50 and suspended across the
peristaltic p~mp slot 74. ~pon insertion of the
cartridge 20, the drive rollers 90 in the machine 80
-- 25 - ~3~ 3~
pinch the exposed portion of the waste tube 76 against
the concave wall of the slot 74. The rotation of the
rollers 90 thus forces the test sample across the
channel 61 of the electrode card 60, through the waste
tube 7G, and into the waste collection bag 28.
In the preferred embodiment, the blood gas
analysis machine 80 houses a selection valve driver
means 82, a peristaltic pump driver means 88, a thermal
unit 96, an electrode interface 94, a microprocessor
lO0, an operator keyboard 102, a printer 106, a display
104l an internal digital clock 108, and a back-up
powèr soùrce 110, as seen in the schematic diagram of
FIGURE 1~
Power is provided to the blood gas analysis
machine 80 by connection to a standard electrical
outlet. A back-up power source 110, comprising a
standard battery device which can power the system to
maintain calibration for up to 30 minutes, is contained
within the machine 80.
An internal digital clock 108 contained in the
machine 80 is of standard design and provides a time
base for the operation of the system.
- 26 - ~3~ J~
The valve driver means 82, shown isl FIGURES l
and 8, which selectively directs either of the
calibrating solutions or the test samp1e to the
electrodes 62-69, preferably includes a rotatable shaft
. 5 84 which fits into the end of the bolt 46 of the
selection valve 40. The position of the shaft 84 is
controlled by the microprocessor lO0 through a solenoid
86.
The peristaltic pump driver means B8, shown in
FIGURES 1 and 8, which drives the fluid flow through
the cartric3ge 20 comprises the rotatable peristaltic
pump driver rollers 90 which contact a portion oE the
waste tube 76 suspended across the pecistaltic pump slot
74 when the cartridge 20 is inserted into the blood gas
analysis machine 80. The rotation of the driver rollers
90, powered by a motor 92, is controlled by the
microprocessor lO0.
The thermal unit 96 includes a resistance
heater and a thermistor which are controlled by the
n,icroprocessor lO0 to obtain a constant, predetermined
temperature of samples in the electrode channel 61.
Heat generated by the thermal unit 96 is conducted to
the metal plate 70 adjacent to the channel 61.
The electrode interface 94, within the machine
80, connects to the electrodes 62-69 when the cartridge
'"' . '
- 27 ~ 3~
20 has been inserted and selects one of the plucality of
signals generated by the electrodes 62-69~ This
selected signal passes through to the microprocessor 100
which converts the signal from analog to digital form
and then further processes the signal~
The microprocessor 100 is programmed to
control those means described above and to control the
printer 106 and the display 104; additionally, the
microprocessor 100 receives, analy~es, and stores the
calibrating and test sample signals from the electrodes
62-69.
The keyboard 102 is a standard keyboard device
having a touch sensitive membrane which is mounted on
the front panel and has a format as shown in FIGURE 9
The keyboard 102 allows the opecator to initiate the
input of the calibrating solution or the test sample ,
to enter patient and operator identification
information, to initiate print or display functions, to
set the clock, to set the temperature, and to enter such
data.
In the preferred embodiment, the display 104
is a standard, commercially available LED device, having
a format shown in FIGURE 9~ The display 104, controlled
by the microprocessor 100, provides a constant reading
.,.. , .;. ,,~
28 - ~3~
of pH and of CO2 and 2 pressures in mmHg for the last
sample from both the venous flow 14 and the arterial
flow 15, as well as the operator's choice of hematocrit,
K~ or Ca++ readings of the last sample. The display 104
can also provide readings of the current temperature9
oxygen saturation, base e~cess, total CO2, bicarbonate,
oxygen consumption rate, or total blood volume consumed
to date, as well as the status of the back-up power
system, all available at the operator's discretion~
The printer 106 is a standard printer, such as
a dot matrix or thermal printer, adapted to provide a
hard copy of the time, date, patient and operator ID
numbers, and temperature, as well as the values of all
parameters of blood characteristics which can be the
displayed by display 104, as described above.
In operation, power is provided to the blood
chemistry analysis machine 80, and a cartridge 20 is
inserted therein. The blood intake valve 30 is
connected by the tubing 16 to the venous blood flow 14
and the arterial blood flow 15 of a conventional
heart/lung machine 12 sustaining a surgical patient 10.
An automatically operated valve 18 allows the operator
, ~
~3~
to select between the venous flow 14 or the arterial
flow 15~ Inside ~he cartridge 20 and passir3g between the
calibrating solution bags 22 and 24, a blood intake tube
. 32 connects a blood intake vaLve 30 to a selection
valve 40.
Upon insertion of the cartridge 20 into the
blood gas analysis machine 80, the bolt head 44 of the
selection valve 40 connects to a shaft 84 in the machine
80, the electrode card 60 connects to the electrical
contacts 9S in the machine 80 which lead to a
microprocessor lO0 contained therein, and the
peristaltic pump slot 74 connects to the rotating drive
rollers 90 in the machine 80. A metal plate 70 in the
cartridge 20 connects to a thermal unit 76 of the
machîne 80, to monitor and control the temperature of
samples in the electrode channel 61.
To initiate the automatic cycle of periodic
analyses of the blood samples, the operator uses a
keyboard 102 to enter the desired frequency of assays
into the microprocessor lO0~ The microprocessor lO0 ~hen
directs the shaft 84, which is in contact with the nut
44, to rotate a pluy 4? of a selection valve 40,
aliyning an electrode input tube 58 with one of the
calibrating solution ports 54 or 55. The port 54 is
connected by tubing to one calibrating solution bag 22;
_ 30 ~ ~ 3~3~$~3
the por~ 55 is connected to another calibra~ing solution
bag 24. The microprocessor 100 selects first the
: calibrating solution in the first bag 22 and then the
calibrating solution in the second bag 24 to establish a
two-point calibration of the electrodes 62-69~ Once the
rotating plug 42 is appropriately positioned, the
rotation of the rollers 90 along a portion of a waste
tube 76, suspended across the peristaltic pump slot 74,
draws the appropriate calibration solution into the
channel 61 of the electrode card 60.
When either calibrating solution is in contact
with the electrodes 62-69, a plurali~y of voltages or
currents proportional to distinct ionic characteristics
or gas concentrations of the solution pass from the
electrodes 62-69 to an electrode interface 94 which
selects one of the plurality of the signals. This
selected signal passes to the microprocessor 100 which
converts it from analog to digital fo[m. In subsequent
. turns, the electrode interface 94 selects each of the
2~ other voltage signals~ After the two-point calibration,
the microprocessor 1~0 causes the rotating plug 42 to
align the electrode input tube 58 with the blood sample
port 52~ The drive rollers 90 then draw a blood sample
into the channel 61, at the same time Eoccing the
- 31 -
~ 3~3~ ~3j
calibration solution through the waste tube 76 and into
the waste collection bag 28~ The several vol~age and
current signals of the blood sample are measured, the
distinct parameters are valued according to the two-
point calibration and are displayed through appropriate
means on the blood gas analysis machine 80~
Additionally, the values of the distinct parameters of
the blood sample may be stored in the microprocessor 100
for subsequent recall and display.
Constant temperature of samples in the channel
61 is insured by preprogramming the microprocessor 100
to monitor and control the temperature of a metal plate
70 through a thermal contact 97 and a thermal unit 96.
The calibration solution and blood sampLe
assay sequence is repeated at intervals previously
selected by the operator~ For most subsequent interval
assays, a one-point recalibration of the electrodes 62-
69 is made; occasionally, a two-point recalibration is
initiated by the microprocessor 1~0 to ensure continued
accuracy
Alternatively, a discrete blood sample may be
connected to the blood intake port 30 and subjected to
the above-outlined sequence, enabling the system to
operate as a standard lab-based blood gas analyzer~
_ 32 ~
Following the exhaustion oE calibrating
solutions or following the termination of the surgical
procedure of a particular pa~ient, the spent cartridge
20 may be discarded and replaced with a new cartridge
for subsequent use of the blood gas analysis system.
Therefore it is seen that this blood gas
analysis system provides an economical, highly
automated, contamination-free means to provide a surgeon
with almost immediate inEormation on the surgical
patient's blood characteristics, which in turn reflects
the patlent's status.
.