Note: Descriptions are shown in the official language in which they were submitted.
1 3 1 40 1 5
~ QM 33709
"Wall of Plastics Material With Embedded Conn_ctors
Joined To Electrodes"
This invention relates to an electrode for u6e
in an electrolytic cell, and in particular to a bipolar
electrode for use in an electrolytic cell, although the
invention i6 not limited to such bipolar electrodes.
Monopolar electrodes for use in electrolytic
cells may take a variety of forms. ~hus, the electrode
may consist of a single metal plate, which may be
perforated, for example a punched plate, or it may
consict of a metallic mesh, which may ~e woven or
unwoven, or it may be a Qheet of expanded metal. The
electrode may consist of a pair of ~uch plates, meshes
or sheets which are spaced apart and which provide a
pair of spaced apart outwardly-facing active electrcde
surfaces, and the active electrode ~urfaces may have a
coating of an electroconducting electro-catalytically-
active material. An electrode o~ thi~ latter type
provides a space for liquors in the electrode
compartment~ of the cell, particularly when the active
electrode surfaces are close to or in contact with a
separator, that is with a hydraulically permeable
diaphragm or a hydraulically Lmpermeable ion-exchange
membrane positioned between an anode and an adjacent
cathode. Ihe monopolar electrode must be provided with
means for feeding electrical current to the electrode~
A bipolar electrode for u~e in an electrolytic
cell mu~t fulfil a ~umber of ~eparate requirements.
Thu~, it must provide a barriex wall which in the
electrolytic cell separates an anode compartment from
an ad~acent cathode comparbment and which thus
~eparates the liquor in ~he anode compartment from ~he
liquor in the cathode compartment. The bipolar
electrode mu~t have an active anode ~urface on one ~ide
of the barrier waIl and an active cathode ~urface on
` '::
"
` :
1314015
the opposite side of the barrier wall. These active
anode surfaces and active cathode ~urfaces may have a
coating of an electroconducting electrocatalytically-
active material. It is preferred that the activè
anode surface, and the active cathode ~urface, each be
displaced from the barrier wall in order to form a
~pace for the anolyte and catholyte liquors between the
active anode surface and the barrier wall and between
the active cathode surface and the barrier wall
respectively. This is particularly desirable w~en the
active anode surface and active cathode surface are
close to or in contact with a separator positioned
between an anode surface of one bipolar electrode and a
cathode surface of an adjacent bipolar electrode. The
bipolar electrode must also be provided with means for
feeding electrical current from one electrode surface
to the other electrode surface across the barrier
wall.
There are many forms of such bipolar
electrodesO
In GB Patent 1503799 there is described a
bipolar electrode which comprises a barrier wall made
of a titanium plate and an iron plate which plates
have been explosion bonded together, a titanium anode
displaced from and electrically connectad to the
titanium plate of the barrier wall, and an iron cathode
displaced from and electrically connected to the iron
plate of the barrier wall, the iron cathode bein~
displaced from the iron plate of the barrier wall by a
30 ~ distance of at least 10 mm. The electrical connection
between the titanium anode and the titanium plate of
the barrier wall is provided by a plurality of titanium
Rheets welded to the anode and to the plate of the
1314015
--3--
barrier wall and positioned vertically therebetween.
Similarly, the electrical connection between the iron
cathode and the iron plate of the barrier wall is
provided by a plurality of iron 6heets welded to the
cathode and to the plate of the barrier wall and
positioned vertically therebetween.
US Patent 3755108 describes a bipolar
electrolytic cell which comprises a plurality of
bipolar units each of which comprises a metallic
barrier wall, anodes mounted vertically on one side of
the barrier wall, and cathodes mounted vertically on
the opposite side of the barrier wall. In the
electrolytic cell the bipolar units are so arranged
that the anodes of one bipolar unit are interleaved
with the cathodes of an adjacent bipolar unit, with a
separator, which may be a hydraulically permeable
diaphragm or a hydraulically impermeable ion-exchange
membrane, positioned between adjacent anodes and
cathodes.
Electrolytic cells have widespread applications,
and they are used in particular on a large ~cale
throughout the world in the production of chlorine and
alkali metal hydroxide, or in the production of alkali
metal chlorate or hypochlorite, by the electrolysis of
aqueous alkali metal chloride solution.
Th electrolytic cell may be of the so-called
tank type comprising, for example, a cathode box having
a plurality of foraminate cathode fingers with an anode
positioned in the gap between adjacent cathode ~ingers,
or it may be of the filter press type and comprise a
large number of alternating anode~ and cathodes, for
; example, fifty anodes alternating with fifty cathodes,
although the cell may comprise even more anodes and
1 3 1 ~0 1 5
cathodes, for example up to one hundred and fifty
alternating anodes and cathodes. The electrode of the
present application is particularly suited for use in
an electrolytic cell of the filter press type.
Where the electrolytic cell i5 used in the
production of chlorine and alkali metal hydroxide the
cell comprises a separator, which may be a
hydraulically-permeable microporous diaphragm. Where
aqueous alkali metal chloride solution is electrolysed
in a cell containing a diaphragm the solution is
charged to the anode compartments of the cell and
chlorine produced in the electrolysis is removed
therefrom, the solution passes through the diaphragm to
the cathode compartments of the cell and hydrogen and
aqueous alkali metal hydroxide 601ution produced by
electrolysis are removed therefrom.
~ere the electrolytic cell contains an
essentially hydraulically impermeable cation-exchange
membrane aqueous alXali metal chloride ~olution is
charged to the anode compartments of the c811 and
chlorine produced in the electrolysis and depleted
alkali metal chloride solution are removed from the
anode compartments, alkali metal ions are transported
across the membranes to the cathode compartments of the
2S cell to which water or dilute aqueous alkali metal
hydroxide solution may be charged, and hydrogen and
alkali metal hydroxide solution produced by the
reaction of alkali metal ions with hydroxyl ions are
removed from the cathode compartments of the cell.
Electrodes for use in electrolytic cell~,
~- including bipolar electrodes, are known w~ich comprise
organic plastics material.
_5_ 1 31 4 01 5
U5 Patent 4141801 describes a fuel cell anode
electrode made by pressing a paste of noble metal
powder, graphite, and polytetrafluoroethylene onto a
screen current collector and drying the electrode 80
formed.
US Patent 3600230 describes a gas electrode for
u~e in a gas-depolarising current generating cell which
comprises a metallic grid or screen, a porous
conductive layer of a hydrophobic resinous material and
conductive fibrous material in contact with one surface
of the grid or screen, and a catalytically-active layer
in contact with the outer ~urface of the porous
conductive layer.
US Patent 4350608 describes a cathode formed by
compressing a mixture of carbon black and
polytetrafluoroethylene optionally onto a core of a
metal mesh.
UK Patent application 2039954A describes a
bipolar current collector which consists of a moulded
aggregate of graphi$e and t~ermoplastic fluoropolymer.
The present invention relates to an electrode
for use in an electrolytic cell which comprises a wall
of an organic plastics material. The electrode of the
invention is readily produced and, because it comprisPs
a wall of a plastics material, it can readily be sealed
by plastics processing techniques to a wall of an
adjacent electrode, or to a frame-like gasket of a
plastics material positioned between adjacent
electrodes. Such techniques cannot, of course, be used
to seal together adjacent electrodes of the types
hereinbefore described which consist of a wall of
metal or metals, for example, as in the electrodes
1 3 1 40 1 5
described in GB Patent 1503799 and in US Patent
375S108. Furthermore, and unlike the electrodes
consisting of a metal or metals hereinbeore described,
the wall of plastics material is of light weight and
may have some flexibility which also aids in sealing to
a wall of an adjacent electrode, or to a gasket of a
plastics material positioned between adjacent
electrodes.
The present invention provides an electrode
which comprises
a wall of plastics material,
an electrically-conductive electrode surace on one
side of the wall and displaced therefrom,
an electrically-conductive electrode surface on the
opposite side of the wall and displaced therefrom,
at least one electrically-conductive connecting
member in electrical contact with one of the
electrode surfaces,
at least one electrically-conductive connecting
member in electrical contact with the other of the
electrode surfaces,
and in which the electrically-conductive connecting
members are embedded in the wall of plastics
material and are in electrical contact with each
~5 other.
: Although use of the electrode in an electrolytic
cell for the~production of chlorine and aqueous alkali
metal hydroxide solution by the electrolysis of aqueous
alkali metal chloride solution has been described it is
to be understood that the electrode is not limited to
use in an electrolytic cell for such electrolysis. By
suitable choice of material~, and in particular of the
~ 3 1 4 0 1 5
wall of plastics material and of the electrode
surfa`ces, it may be used in an electrolytic cell in
which many different types of electrolyses may be
effected.
The electrode of the invention may be a
monopolar electrode or a bipolar electrode.
Where the electrode is a monopolar electrode it
may be an anode or a cathode and should be provided
with means of feeding electrical current to the
electrode. The ~onopolar electrode, when installed in
an electrolytic cell, should permit passage of liquid
from one side of the wall of plastics material to the
other, and in order to permit ~uch passage of liquor
the wall may be perforated.
Where the electrode is a bipolar electrode the
wall of plastics material should serve as a barrier
wall which prevents pas~age of liquor from one side of
the wall to the other, that is from an anode
compartment on one side of the wall to a cathode
compartment on the other side of the walI. In a
bipolar electrode the electrode surface on one side of
the wall serves as an anode and the electrode æur~ace
on the oppo~ite ide of the wall serves as a cathode.
The invention also provides an electrolytic cell
which comprises a plurality of electrodes as
hereinbefore described. Where the electrode is a
monopolar electrode the electrodes serve as anodes and
cathodes, and optionally a æeparator may be positioned
between each anode and adjacent cathode. Where the
electrode is a bipolar electrode a separator may
optionally be positioned between adjacent electrodes,
- that iæ between an anode of one bipolar electrode and a
1 3 1 40 1 5
cathode of an adjacent bipolar electrode. The
electrolytic cell will be provided with means for
charging electrolyte to the electrolytic cell and with
means for removing products of electrolysis from the
electrolytic cell.
The wall of the electrode is of a plastic
material which will generally be electrically non-
conductive. The wall is suitably in the form of a
sheet of plastics material.
There is no particularly preferred thickness for
the wall. It should of course be sufficiently thick as
to provide a degree of structural integrity and~ in the
case of a bipolar electrode, to act as a barrier
between the liquors on opposite sides of the wall.
However, there is no particular advantage to be gained
by having a thick wall, and in general a thicXne~s in
the range 0.2 cm to 2 cm will suffice, although these
thicknesses are not to be taken as being in any way
limiting. The wall is suitably flexible and preferably
resilient as this aids in fvrming leak tight 6eals when
the electrode is inctalled in an electrolytic cell.
Unless the context dictates otherwise the anode
surface and the cathode surface will hereafter be
referred to as the electrode surfaces.
The electrode surfaces, which are electrically
conductive and will generally be of metal, may take
various forms. They may be non porous, e.g. in the
form of a non-porous sheet, but more usually they will
be foraminate, e.g. in the fo~m of a foraminate eheet.
The foraminate ~heet may, for example, be in the form
of a perforated pl te, e.g. a punched plate, or a mesh,
which may be a woven or unw~ven mesh, or an expanded
substrate, e.g. an expanded metal.
' ~
9 1314015
The electrode surfaces of the electrode are each
in electrical contact with at least one electrically-
conductive connecting member. The purpo6e of these
electrically-conductive connecting members is to
conduct current from one electrode surface to the
other, for example in a bipolar electrode from the
anode surface of the electrode to the cathode surface
of the electrode. In order to aid current distribution
over the electrode surfaces it is preferred that the
electrode surfaces are each in electrical contact with
a plurality of electrically-conductive connecting
members, which are ~paced apart and which are
preferably substantially evenly spaced apart.
The electrically-conductive connecting members
which are in electrical contact with the electrode
surfaces may be in direct or indirect contact with each
other. Thus, they may make indirect contact with each
other by each being in electrical contact with a
separate electrically conducting member, for example a
sheet, e.g. a foraminate sheet, which may be of metal,
embedded in the wall of plastics material. The use of
such an embedded sheet aids in current distribution.
In the case of a monopolar electrode the embedded sheet
may project beyond the edge of the wall of plastics
material and thus provide a means by which electrical
current may be fed to the electrode.
- The electrode of the invention may take a
variety of different forms, and the electrode surfacP
and the electrically-conductive connecting member of
the electrode may be of unitary construction or they
may be of separate construction and electrically
connected to each other.
-10- 13l~ol5
For example, the electrode surface and the
associated electrically-conductive connecting members
may be formed of a corrugated sheet, which i~ suitably
foraminate, with the part of the sheet at or near to
the peaks of the corrugations projecting from the wall
of plastics material and serving as the electrode
surface and the part of the sheet at or to near to the
troughs of the corrugations serving a~ the connecting
members and bein~ embedded in the wall of plastics
material. The corrugations embedded in the wall of
plastics material which are electrically connected to
an electrode surface on one side of the wall are in
electrical contact with the corrugations embedded in
the wall which are electrically connected to the
electrode surface on the opposite side of the wall. In
order to provide a plurality of electrical contacts and
to aid current distribution, and particularly where
direct electrical contact is established between the
corrugations of the sheets, the corrugated sheet
providing one electrode surface may be positioned such
that the corrugations are transverse to, for example
substantially at right angles to, the corrugations of
the corrugated sheet providing the opposite electrode
surface. The electrode may be con~tructed ~y pressing
corrugated sheets into the surface of a heat softened
; sheet of plastics material from opposite ides of the
sheet until electrical contact, which may be direct or
indirect, is established betwePn the corrugated sheets.
The ~heet of plastics material may then be allowed to
harden.
The corrugated sheet is not necessarily of
symmetrical fo~m, or even of substantially Rymmetrical
form. For example, it may be unsymmetrical in that
,.
31~015
those parts of the corrugated sheet at or near the
peaks thereof which project from the wall of plastics
material and which ~erve as the electrode surface may
co~er a relatively large area, and may be flat, and
those parts of the corrugated sheet at or near the
troughs thereof which are embedded in the wall of
plastics material and which serve as the electrically-
conductive connecting mem~ers may cover a relatively
small area.
In another embodiment of the electrode of the
invention the electrode surfaces comprise sheets, which
are preferably foraminate and the electrically-
conductive connecting members comprise a projection or
projections upstanding from the sur~ace of each sheet.
The æheet preferably comprises a plurality of such
projections on each sheetO The electrode may be
constructed by pressing the projections attached to the
sheets into the surface of a heat softened sheet of
plastics material from opposite sides thereof until
electrical contact, which may be direct or indirect, is
establi~hed between the projections. In a preferred
embodiment, which assists in obtaining good electrical
contact between the projections embedded in the wall of
plastics material, the wall of plastics material
comprises an aperture or a plurality of apertures
therein, and the electrode is constructed by
positioning the pro~ections attached to the electrode
surfaces through the apertures and in contact with each
other and ~ealing the projections to each other, e.g.
by welding. The apertures in the wall are then sealed,
e.g. by application of a plug of heat-softened plastics
material, in order to maintain the electrode surfaces
in the desired position r~lative to the wall of
-12- ~31~015
plastics material, and in the case of a bipolar
electr~de, in order that the wall may function as a
barrier wall.
In the foregoi~g description of embodiments of
the electr~de of the invention the electxically-
conductive connecting members have been described as
being of separate construction. However, the
electrically-conductive connecting members attached to
the electrode surfaces on opposite sides of the wall of
plastics material may be of unitary construction. For
example, the preferred embodiment of electrode
previously described in which the wall comprises an
aperture, or a plurality of apertures therein, and the
electrode is constructed by positioning the projections
attached to the electrode surfaces through the
apertures and in contact with each other and ~ealing
the projections to each other, e.g. by welding, may be
constructed by positioning the projections a~tached to
one of the electrode surfaces through the apertures in
the barrier wall and sealing the projections into
electrical contact with the opposite electrode surface,
e.g. by welding. In this case ~he electrically-
conductive connecting members attached originally to
on~ electrode surface serve as the connecting members
between the electrode surfaces.
~he electrically-conductive electrode surfaces
are displaced fr~m the wall of plastics material. The
amount o this displacement may be ~mall, or example,
such that the electrode surfaces are merely slightly
upstanding from the surface of the wall. However, it
is preferred that the electrode surfaces be di~placed
so as to leave a gap between the electrode ~urfaces and
the wall which gap provides a space which serves as an
-13- 1314015
electrode compartment. This i6 particularly necessary
where the electrolytic cell comprises a separator which
is near to or in contact with the anode and cathode
surfaces of adjacent electrodes. The electrode
surfaces may be displaced from the wall of plastics
material by a distance of, for example 2 mm to 20 mm,
although these specific displacements are not intended
to be limiting.
In a preferred embodiment the projected area of
the electrode surface is less than the projected area
of the wall of plastics material such that, for
example, in plan view the wall forms a frame-like
section around the electrode surface. In the
electrolytic cell frame-like gaskets, e.g. of plastics
material, may be positioned on this frame-likç part of
the wall and surround the electrode surfaces.
Alternatively, the wall of plastics material of the
electrode and the gaskets may be of unitary
construction in that the wall may have a greater
~hickness in the region of the frame-like part than in
the part adjacent to the electrode surfaces. The
frame-like part of the wall may extend to the plane of
the electrode surfaces or extend beyond the p~ane of
the electrode surfaces.
The wall of plastic~ material may be of a
thermoplastic material, or of a thermosetting material,
the nature of the material depending at least in part
on the type of electrolysis which is to be effected in
the electrolytic cell. The plastics material may be,
for example, a polyolefin, e.g. polyethylene or
polypropylene. It may be an aromatic polymer, e.g.
polystyrene, or a polymer containing ~uch aromatic
;
.
-14- 1 31 401 5
groups, e.g. an acrylonitrile-butadiene-styre~e
polymer. It may be a halogenated polymer, for example
a chlorine-containing polymer, e.g. polyvinyl chloride
or chlorinated polyvinyl chloride, or a fluorine-
containing polymer, e.g. polyvinyl fluoride,
polyvinylidene fluoride, or polytetrafluoroethylene. ,
The plastics material may be an elastomer, for example,
polybutadiene, polyisoprene, polychloroprene, an
ethylene-propylene copolymer, an ethylene-propylene-
diene copolymer, or an acrylonitrile-butadiene-styrene
polymer aæ hereinbefore described.
Where the liquors in the electrolytic cell are
particularly corrosive, for example in a cell for the
electrolysis of aqueous alkali metal chloride solution,
corrosion resistant plastics materials are preferred,
~or example, fluorine-containing plastics materials or
plastics materials faced with or filled with such
1uorine-containing materials.
Examples of thermosetting plastics materials
include polyester resins and epoxy resins.
The electrically-conducting electrode surfaces
will generally be metallic, the nature of the metal
depending on the type of electrolysis which i8 to be
effected in the electrolytic cell. Wh~re aqueous
alkali metal chloride solution is to be electolysed and
the electrode surface is to function as an anode
surface it is suitably made of, or at least has an
active area of, a film forming metal or alloy, for
example of zirconium, niobium, tungsten or tantalum.
T~le anode ~urface preferably has at least an active
area of titanium, and the anode surface suitably
carries a coatin~ of an electroconducting
electrocatalytically-active material. The coating may
.:
-15- 1314015
comprise one or more platinum group metals, that is
piatinum, rhodium, iridium, xuthenium, osmium or
palladium, and/or an oxide of one or more of these
metals. The coating of platinum group metal and/or
oxide may be present in admixture with one or more
film-forming metal oxides, e.~. titanium dioxide,
preferably in the form of a solid solution.
Electroconducting electrocatalytically-active materials
for use as anode coatings in an electrolytic cell for
the electrolysis of aqueous alkali metal chloride
solution, and methods of application of such coatings,
are well known in the art.
Where aqueous alkali metal chemical solution is
to be electrolysed and the electrode surface is to
function as a cathode the cathode surface is suitably
made of, or at least has an acti~e area of iron or
steel or other suitable metal, e.g. nicXel. The
cathode surface may carry a coating of an
electroconducting electrocatalytically-active material,
~0 e.g. a platinum group metal and/or oxide there~f, which
lowers the hydrogen overvoltage of the cathode urface.
The electrolytic cell may be of the diaphragm or
membrane type. In the aiaphragm type cell the
separator positioned between an anode and an ad~acent
cathode, or between an anode ~urface of a bipolar
electrode and a cathode urface of adjacent bipolar
electrode, to form separate anode compartments and
cathode compartments in the cell are microporous and in
use the electrolyte passes through the diaphragm from
the anode compartments ~o the ca~hode compartments.
Thus, in the case where aqueous alkali metal chloride
solu~ion i6 electrolysed ~he cell liquor which i8
produced comprises an aqueous solution of alkali metal
:
-16- 1314015
chloride and alkali metal hydroxide. In the membrane
type electrolytic cell the separators are es6entially
hydraulically impermeable and in use ionic 6pecies are
transported across the membranes between the
S compartments of the cell. Thus, where the membrane is
a cation-exchange membrane cations are tsan~ported
across the membrane, and in the case where aqueou
alXali metal ~hloride solutio~ i6 electrolysed the cell
liquor comprises an aqueous solution of alkali metal
hydroxide.
h~ere the separator to be used in the
electrolytic cell i~ a microporous diaphragm the nature
of the diaphragm will depend on the nature of the
electrolyte which i~ to be ele~trolysed in the cell.
qhe diaphragm should be resistant ~o degradation by the
electrolyte and by the products of electr~lysi~ and,
where an aqueous solution of alkali metal chloride i~
to be electroly6ed, the diaphragm is suitably made of a
fluorine-containing polymeric material a such
ma~erial~ are generally resistant to degradation by the
chlorine a~d alkali metal hydroxide s~lution pr~duced
in the electroly~is. Preferably, the microporous
diaphragm i8 made ~f polytetrafluoroethylene, although
other materials which may be u~ed include, fsr exEmple,
tetra~luoroethylene-hexafluoropropylene copolymers,
vinylidene fluoride polymer and copolymers, a~d
fluorinated ethylen2-propyl~ne c~polymers.
Suitable microporous diaphragms are ~hose
descrlbed, for example, in UX Patent ~o 1503915 in
which there i~ de~cribea a microporou~ ~iaphragm of
polytetra-fluoroethylene having a micro~ruc~ure of
.,
-17- 1314015
nodes interconnected by fibrils, and in UK Patent
~o 1081046 in which there is deæcribed a microporous
diaphragm produced by extracting a particulate filler
from a sheet of polytetra1uoroethylene. Other
suitable microporous diaphragms are described in the
art.
Where the separator to be used in the cell is an
ion-exchange membrane the nature of the membrane
will also depend on the nature of the electrolyte which
is to be electrolysed in the cell. The membrane æhould
be resistant to degradation by the electrolyte and by
the products of elctrolysis and, where an aqueous
solution of alkali metal chloride i8 to be
electrolysed, the membrane is suitably a cation-
exchange membrane made of a fluorine--containing
polymeric material containing cation-exchange groups,
for example, sulphonic acid, carboxylic acid or
phosphonic acid groups, or derivatives thereof, or a
mixture of two or more such groups.
Suitable cation-exchange membranes are those
described, for example, in UK Patents Nos 1184321,
1402920, 1406673, 1455070, 1497748, 1497749, 151~3g7
and 1531068.
The separators may be mounted on suitably shaped
plates, which may act as sealing gaskets, positioned
be~ween adjacent electrode~, or alternatively the
separators may merely be held in position by clamping
between adjacent electrodes.
The electrolytic cell may contain gaskets, which
may be of the same plastics material as the wall of the
electrode, or which may be of a different plastics
material. The gaskets are preferably pliable and more
preerably resilient.
-18- 1314015
In assembling the electrolytic cell the
component parts may be positioned on tie rods and
clamped together, or they may be sealed together, e.g.
by use of adhesives or by use of thermal welding, in
the case where the plastics material is capable of
being thermally welded.
The anode compartments of the electrolytic cell
are provided with means for feeding electrolyte to the
anode compartments, and with means for removing
products of electrolysis from the anode compartments.
Similarly, the cathode compartments of the electrolytic
cell are provided with means for removing products of
electrolysis from the cathode compartments, and
optionally with means for feeding water or other fluid
to the cathode compartments.
For example, where the electrolytic cell is to
be used in the electrolysis of aqueous alkali metal
chloride solution the anode compartments are provided
with means for feeding the aqueous alkali metal
chloride solution thereto and with means for removing
chlorine and optionally with means for removin~
depleted aqueous alkali metal chloride solution
therefrom, and the cathode compartments are provided
with means for removing hydrogen and cell liquor
containing alkali metal hydroxide therefrom, and
optionally, and if necessary, with means for feeding
water or other fluids thereto. Although such means may
be provided by æeparate pipes leading to or from each
of the respective compartments such an arrangement
would be unnecessarily complicated and cumbersome, and
in a preferred embodiment of the electrolytic cell the
wall of plastics material of the electrode, and of the
separate gaskets, if present, comprises a plurality of
1314015
openings, e.g. in a frame~like part thereof, which in
the electrolytic cell define a plurality of
compartments lengthwi~e of the cell which ~erve as
headers from which, and to which, liquors may be
passed. The liquors may be distributed from the
headers to the electrode compartments, and to the
headers from the electrode c~mpartments, by means of
channels, e.g. slots, appropriately positio~ed in the
wall of the plastics material of the electrode and/or
in the gaskets, if present.
The invention will now be described with the aid
of the following figures in which
Figure 1 is an isometric exploded view of a bipolar
electrode of the invention~
Figure 2 is a cross-sectional view in the direction A of the
bipolar electrode of Figure 1,
Figure 3 i~ an isometric exploded view of a bipolar
electrode of the invention,
Figure 4 is a cross-sectional view in the direction B of the
bipolar electrode of Figure 3,
Figure 5 is an i~ometric exploded view of a bipolar
electrode of the invention,
Figure 6 is a cross-sectional view in the direction C of the
bipolar electrode of ~igure S, and
Figure 7 is an i~ometric.partially exploded view of an
electrolytic cell incorporating the bipolar electrode
of Figure 4.
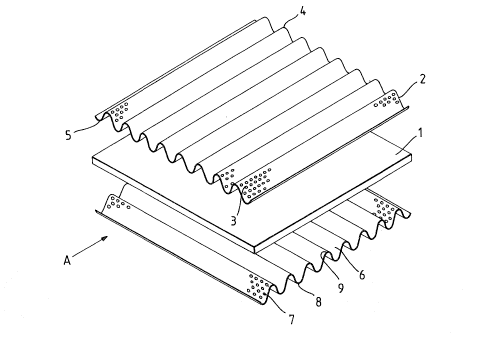
Referring to Figure~ 1 and 2 the bipolar
electrode comprises a sheet 1 of thermoplastic polymer
material w~ich ~erves a a barrier wall ~n the-
electrode, a first corru~ated metallic 6heet 2 having
perforation6 3, the peak~ 4 of which serve a~ an
electrode ~urface and the trough~; 5 of which serve as
~'
.
-20- 1 31 401 5
electrically conductive connecting mernbers, and a
~econd corrugated metallic fiheet 6 having perforations
7, the corrugations of ~heet 6 being positioned at
right angles to those of the 6heet 2, and the peaks 8
of which ~erve as an electrode ~urface and the trough~
9 of which ~erve as electrically-conductive connecting
members.
The bipolar electrode was as6embled by heat
softening the sheet of thermoplastics material 1 and
pressing the corrugated metallic sheets 2 and 6 into
the heat-softened sheet 1 until the troughs 5 of sheet
2 and the troughs 9 of sheet 6 contact each other
thereby forming the required electrical connection~.
As the corrugations of corrugated shee.t 2 are
positioned at right angles to those of the corrugated
sheet 6 a plurality of electrical connectionfi are
formed. Finally, the ~heet of thermoplastic6 material 1
w~s ~ealed, by heat ~ealing, to a frame-Iike member 10
of the same thermoplastics material, the frame-like
member 10, which i6 not shown in Figure 3, projecting
from the plane of the ~heet 1 up to the planes of the
peaks 4 and 8 of the corrugations of the corrugated
metaIlic sheets 2 and 6 respectively.
Referring to Figures 3 and 4 the bipolar
electrode comprises a metallic sheet 20 ha~in~
perforations 21 sandwiched between sheet~ ~2 and 23 of
thermoplastic~ material. The sheet~ 20, 22 and 23
6erve as a barrier wall in ~he bipolar electrode. ~he
electrode a1BO comprise~ a fir~t corrugated metallic
sheet 24 havinq perforatio~s 25, ~e pea~ 26 of which
serve as an electrode ~urface and the troughs 27 of
which ~erve a~ electrically conductive connecting
. ~ .
:,
-21- 1314015
members, and a second corrugated metallic sheet 28
having perforations 29, the peaks 30 of w~ich serve as
an electrode surface and the troughs 31 of which serve
as electrically-conductive connecting members.
The bipolar electrode was assembled by heat
softening the ~heets of thermoplastics ~aterial 22 and
23 and sandwiching the metallic sheet 20 between the
sheets 22 and 23, and pressing the corrugated sheets 24
and 28 into the heat so~tened sheets 22 and 23
xespectively until the troughs 27 of sheet 24 and the
troughs 31 of sheet 28 contact the sheet 20 thereby
forming the required electrical connections. Finally,
the sheets of thermoplastics material 22 and 23 were
sealed, by heat sealing, to a frame-like member 32 of
the same thermoplastics material, the frame-like member
32, which is not shown in Figure 3, projecting from
the plane of the sheets 22 and 23 up to the planes of
the peaks 26 and 30 of the corrugation6 of the
corrugated metallic sheets 24 and 28 respectively.
Referring to Figures 5 and 6 the bipolar
electrode comprises a sheet 40 of thermoplastics
material, a metallic sheet 41 having perforations 42,
and projections 4~ on one face of the sheet 41, and a
metallic sheet 44 having perforations 45, and
projections 46 on one face of the sheet 44. Prior to
assembly of the electrode the ~heet 40 comprises
openings 47.
The bipolar electrode was assembled by placing
the projections 43 of metallic sheet 41 through the
openings 47 in sheet 40 and sealing the projections 43
to the projections 46 on metallic sheet 44, e.g. by
welding or by bra7ing. The openings 47 were then
-22- 1314015
sealed by placing a plug 48 of thermopla~tics material
in each of the openings 47 in order that the sheet 40
may form a barrier wall in the bipolar electrode.
Finally, the sheet of thermoplas~ics material 40 was
sealed, by heat sealing, to a frame-like member 49 of
the same thermoplastics material, the frame-like member
49, which is not shown in Figure 5, projecting from
the plane of the sheet 40 up to the planes of the
sheets 41 and 44 respectively.
In the electrolytic cell one of the metallic
sheets of the bipolar electrode will serve as an anode
and the other as a cathode and the surface of each
sheet may have a coating of a suitable
electrocatalytically-active electroconducting material.
Titanium is a suitable metal for an anode sheet and
nickel is a suitable metal for a cathode ~heet.
In the embodiment illustrated in Figure 7 the
bipolar electrode in the electrolytic cell is of the
type described with reference to Figures 3 and 4.
The electrolytic cell~comprises a frame-like
member 60 of an acrylonitrile-butadiene-styrene
polymeric material (ABS) having a central opening in
which a bipolar electrode 61 is positioned.
The frame-like member 60 has four openings 62,
63, 64, 65 which serve as locations for tie rods used
in assembly of the electrolytic cell, as hereinafter
described.
~he frame-like member 60 comprises a
horizontally disposed opening 66 through the thickness
of the frame-like member 60 and a vertically disposed
channel 67 which leads from the opening 66 to one face
of the bipolar electrode 61, and a hori~ontally
disposed opening 68 through the thickness of the
-23- 131~015
frame-liXe member 60 and a vertically di~posed channel
(not shown) w~lich leads from the opening 68 to the
oppo~ite face of the bipolar electrode 61.
Similarly, the frame-like member 60 compri6es
four horizontally disposed openings 69, 70, 71, 72
throu~h the thickness of the frame-like member 60 and
four channels 73, 74, 75, 76 respectively as~ociated
with said openings, the channels 74, 75 leading from
one face of the bipolar electrode 61 to the opening6
70, 71 respectively, and the channels 73, 76 leading
from the opposite face of the bipolar electrode 61 to
the openings 69, 72 respectively.
The electrolytic cell also comprises a
frame-like member 77 of ABS polymeric material having a
central opening in which a cation-exchange membrane 78
is positioned. The membrane 78 has an area slightly larger
than the central openinq in the frame-like ~ember 77 and may
be affixed thereto by mean~ of an ad~esive.
~ ternatively, the membrane 78 may be ~andwiched
between a pair of frame-like section6 which are bonded
together to form the frame-like member 77. The frame-
like member 77 comprises four openings 79, 80, 81 (one
not shown), corresponding in position to the openings
62, 63, 64, 65 in the frame-liXe member 60 and which
serve a6 locations for tie rods used in a6sembly of the
electrolytic celi, and six horizontally di~po~ed
openin~s 82, 83, 84, 85 (two not ~hown) corre~ponding
in position to the opening~ 69, 70, 71, 72, 66 and 68
in the frame-like member 60.
In assembling the electrolytic cell a frame-like
member 60 i~ positioned on four tie tod6 through the
opening~ 62, 63, 64, 65 and a ~ace of ~h~ member 60 i~
.
.
~, . . ~ , .
-24- 1 31 ~01 5
coated with an adhesive comprising ABS polymeric
material in an organic solvent, e.g. perchlorethylene.
A frame-like member 77 is then positioned on the tie
rods and contacted with the adhesive-coated face of the
frame-like member 60. The opposite face of the
frame-like member 77 is similarly coated with adhesive
and another frame-like member 60 is positioned on the
tie rods and contacted with the adhesive coated face of
the frame-like member 77. In this way a ~tack of
frame-like members 60 comprising bipolar electrodes 61
and frame-like members 77 comprising cation-exchange
membranes is built up, the stack is held in compression
until the frame-like members are firmly bonded
together, and the tie rods are removed.
In the electrolytic cell the horizontally
disposed openings 66, 68, 69, 70, 71, 72 in the
frame-like members 60 and the corresponding openings
(two not shown) 82, 83, 84 and 85 in the frame-like
members 77 together form channels lengthwise of the
cell through which, respectively aqueous alkali metal
chloride solution may be charged to the anode
compartments of the cell, water or dilute aqueous
alkaii metal hyd.roxide ~olution may be charged to the
cathode compartment of the cell, hydrogen produced by
electrolysis may be removed from the cathode
compartments, chlorine produced by electrolysis may be
removed from the anode compartments, depleted aqueous
alkali metal chloride solution may be removed from the
anode compartments, and aqueous alkali metal hydroxide
solution produced by electrolysis may be removed from
the cathode compartments.
Assembly of the electrolytic cell i6 completed
by sealin~ end plates (not shown) to each end of the
-25 1314015
cell, completing electrical connections, and co~necting
to appropriate headers he channels of which the
openings 66, 68, 69, 70, 71, 72 form a part.
In operation in the electrolysis of aqueous
alkali metal chloride solution the solution is charged
to the anode c~mpartments of the electrolytic cell
through the lengthwise channel of which opening 66
forms a part and through vertically disposed channel
67, and depleted alkali metal chloride solution and
chlorine produced in the electrolysis are removed from
the anode compartments, respectively, through the
channel 75 and the lengthwise channel of which opening
71 forms a part, and through channel 74 and the
lengthwise channel of which opening 70 forms a part.
Water or dilute alkali metal hydroxide ~olution
is charged to the cathode compartments of the
electrolytic cell through the lengthwise channel of
which opening 6~ forms a part and through a vertically
disposed channel (not shown), and alkali metal
hydroxide solution and hydrogen produced in the
electrolysis are removed from the cathode compartments,
respectively, through the channel 76 and the lengthwise
channel o which opening 72 forms a part, and through
channel 73 and the lengthwise channel of which opening
69 forms a part.