Note: Descriptions are shown in the official language in which they were submitted.
131414q
FIELD OF INVENTION
This invention relates to methods of recovering arsenic values
from arsenic bearing materials.
BA,C~OUND OF THE INVENTION
Ores containing arsenic have to be subjected to an arsenic removal
step prior to the extraction of other values. A typical example is gold ore -
arsinopyrite.
The removal of arsenic is most often accomplished by roasting.
When roasted, Arsenic is oxidized to As203, and at the roasting
temperature leaves the Roaster as a vapour together with the oxidation
products of Sulphur, - Sulphur Dioxide and Sulphur Trioxide. The presence
of Sulphur oxides with Arsenic complicates the subsequent Gas Cleaning step,
resulting in an impure (crude) Arsenic Trioxide dust being trapped in a bag-
house, following precipitation from the gas stream by the addition of cold air.
The off-gas is usually discharged to the atmosphere with consequent
environmental problems.
A major consumer of Arsenic Trioxide (As203) is the wood
preservative industry. Since As203 from the roasting operation is not of a
sufficient purity, a further refining step has to be employed.
Refining is usually accomplished by one of two approaches:
(a) Hot Water Leach, by one of two approaches.
(b) Ammonia Leach
(a~ Hot Water Leach:
Approach 1
Hot water is used to dissolve the As203 from the crude feed
material. Undissolved solids are separated from the solution by filtration, and
the filtered solution is either cooled or evaporated in a crystallizer to form
As203 crystals.
~k
-- 2 --
1314149
Approach 2
Due to the positive effect of temperature on solubility of As203,
pressure leaching may be employed with a higher water temperature hence
obtaining a higher solubility of As203. The cost of energy with this approach
S is improved over Approach 1 but still remains very high.
(b) Ammonia Leach:
The Ammonia Leach process has an advantage over the hot water
leach approach in that the solubility of As203 is higher in amrnonia solution.
However the only user of this process of which the inventor is presently aware
has presently ceased operations due to technical difficulties.
Both approaches additionally have a common problem. Re-
contamination of the residue is caused by a reaction between dissolved Arsenic
and the Iron content of the residue, resulting in a slimy material which is
difficult to filter. Since this residue contains in some cases significant amounts
of precious metals, this is a serious problem. Further these approaches are
generally complicated and have a high operating and capital cost.
U.S. patent 3923478 teaches pyrites-roast gas at 350-400 degrees
C composed mainly of S02 with As203, iron oxide dust, S03, and other
impurities being scrubbed in a closed system with a solvent (preferably
aqueous) for As203. The product is obtained by concentration and
crystallization and unwanted deposits formed are removed by dissolution in
unsaturated As203 solution. Using systems in parallel and switching liquid
flows makes possible continuous operation, it is alleged, with minimal solid or
liquid effluent.
Thus a process and an apparatus is asserted as having been
provided for recovering arsenic trioxide from a gas by washing out the oxide
from the gas with a liquid having the ability of dissolving arsenic trioxide.
The purported invention is characterized that non-desired deposits in the
apparatus formed in connection with a saturated solution are removed by
... ..... .
3 l 5 1 4 1 4 9
dissolution with an unsaturated solution of arsenic trioxide. See also
corresponding Canadian Letters patent 1033538.
U.S. patent 4588564 relates to a process for recovering arsenic
trioxide from scrubbing water used to scrub the flue gas of sulfide ore
5 smelting. The crude arsenic trioxide crystals collected from the scrubbing
solution which contains plaster as an impurity are purified by treating with
hydrochloric acid of a concentration of 50-150 g/l at a temperature not higher
than 30C.
U.S. patent 4605812 purports to teach a process for the removal
10 of arsenic from gases whereby arsines are removed from streams of
hydrocarbons or inert gases by contacting the streams with copper (II)
chromite catalyst.
U.S. patent 4615731 purports to teach a hydro metallurgical
process for treating a feed comprising an aqueous acidic solution containing
15 dissolved therein one or more precious metals selected from the group,
platinum group metals and gold and one or more of the nuisance elements
bismuth lead, tin, arsenic and antimony, to separate the precious metals from
the nuisance elements comprising:
(i) treating the aqueous acidic solution with sulfur dioxide in
20 the presence of selenium and a halide to reduce and precipitate selectively
selenium and precious metals, and
(ii) separating the precipitated components from the remaining
solution; thereby separating selenium and precious metals from the nuisance
elements.
U.S. patent 4489046 purports to teach a method for converting an
arsenic-containing waste product to a depositable, substantially arsenic-free
form by fuming-off the arsenic content thereof. The method comprises
melting the waste product under oxidizing conditions in a furnace to form an
oxidic slag melt; causing turbulence of the melt, while maintaining a reducing
` 30 atmosphere supporting the formation of arsenic (III) oxide at the furnace
-4- 1314149
temperature driving-off arsenic content of the waste product substantially in
the form of gaseous arsenic (III) oxide; separating the formed oxide by
condensation and recovering the same and removing from said furnace a
substantially arsenic-free depositable slag.
U.S. patent 4244735 purports to teach a process for the
hydrometallurgical recovery of metals, such as, lead, silver, gold, antimony,
and bismuth from materials such as flue dust in the presence of arsenic,
comprising precipitating arsenic as an insoluble ferric-arsenic compound in
the first processing step, carrying the insoluble arsenic compound through a
chloride leach step, in which it is insoluble, to recover the metals, and
disposing of the residue in which the arsenic has been fixed with ferric ions torender it non-polluting, or alternatively, recovering the arsenic by caustic
leach and crystallization.
Swiss patent 273779 purports to teach a continuous refining
process which it is purported, can be done more intensively and is carried out
in equipment which consists of a lined vessel, screw feeder with a rotating discmounted on it and bearing fixed knives, screening plates. Silite heaters and a
discharging screw, moving inside the vessel. The product to be refined is fed
on to the rotating disc of the screw feeder having a sealed hopper. The
distance between the cover and the disc is relatively small (i.e. 1/5 to 1/8 of
the diameter of the disc), as a result of which uniform heating of the reaction
zone is obtained and dust-formation of the incoming material is eliminated.
The product to be refined is fed on to the disc, which is red-hot (500-600
degrees C), and the Arsenic trioxide is purported to immediately begin to
evaporate, which causes the original thickness of the layer of material to
diminish. The solid residue, which comprises about 10% of the original
material put on the disc, is removed by the fixed knife and the screw being
conveyed to the hopper. In this way the disc is cleaned ready for the fresh
deposit of material. The vapours of the trioxide go along into a crystallizer of
~ .
-s 1 31 4 1 4q
the usual type. The device operates under minimum vacuum (0.5-1.0 mm of
water column).
German reference 131850 purports to teach that Sulphur dioxide
gases containing As203, halides and dust, are cleaned by washing with
5 circulating H2S04 solution of which the concentration is adjusted to such a low
value that the halides are being dissolved, separating the H2S04 solution and
subjecting this solution to a vacuum evaporation to evaporate the halides and
crystalize As203. The purified H2S04 is recycled.
Once again these approaches are generally complicated and have
10 high operating costs and capital costs.
It is therefore an object of this invention to provide an improved
process for the recovery of Arsenic values.
It is a further object of the invention to provide such process at
reduced operating and reduced capital costs.
Further and other objects of the invention will be realized by
those skilled in the art from the following Summary of the Invention and
Detailed Description of Embodiments thereof.
SUMMARY OF THE INVENTION
According to one aspect of the invention, a process is provided
20 for the recovery of Arsenic values from Arsenic containing material which
may also contain Sulphur in one embodiment the Arsenic being in the form of
As203, the process comprising the step of evaporating (in a wet or dry state)
the Arsenic values from the Arsenic containing material into a gas stream
which gas stream does not react with the Arsenic values. The gas stream is
25 then cooled to precipitate the Arsenic. The residue from which the arsenic
was evaporated may then be treated for removal of the metal values. The
simplicity of the process is reflected in relatively low operating costs and
relatively low capital expenditures. Without the presence of Sulphur, the
As203 vapour can be well cleaned of any carry-over solids (prior to
30 precipitation) giving a high purity As203 product, acceptable to for example
-6- 1314149
the wood preserving industry. Preferably the evaporation of the Arsenic
values is as arsenic trioxide (As203) near its boiling point.
According to another aspect of the invention the evaporation of
the arsenic trioxide is performed in:
S (a) A chamber heated by a combustion of oil or hydrocarbon
gas.
(b) Fluid bed heated by combustion of oil or hydrocarbon gas
or by electrical energy.
(c) Electrical plasma reactor.
According to another aspect of the invention the arsenic values
(for example the arsenic trioxide) are precipitated from the clean arsenic
trioxide vapour containing gas in a fluidized bed of arsenic trioxide particles
cooled by water evaporation. Thus the As203 is deposited on the surface of a
cold particle causing it to grow to a form of little balls. The size of the balls
of As203 may be controlled by the amount of precipitation onto the coarse
particles. Thus "dusting" during handling of the "balled" material is reduced.
According to another aspect of the invention the process may be
carried out u~ilizing electrical energy for evaporation of arsenic trioxide in an
evaporator into a gas stream and after separation of the residue and
precipitation of arsenic trioxide, the clean gas stream may be recycled back to
the evaporator thus minimizing possible atmospheric emissions.
Thus, according to another aspect of the invention the process
may be carried out in a closed circuit thus substantially eliminating emissions
giVillg rise to substantial and significant environmental benefits.
According to another aspect of the invention the process may be
carried out utilizing combustion of oil or hydrocarbon gas for evaporation of
arsenic trioxide in the evaporator. Due to production of combustion products
with this process the gas after cleaning can be vented.
-7- l 31 41 49
The invention will now be illustrated with reference to the
following drawings of embodiments of the invention and the detailed
description of the embodiments thereof.
BRIEF DESCRIPTION OF THE DRAWINGS
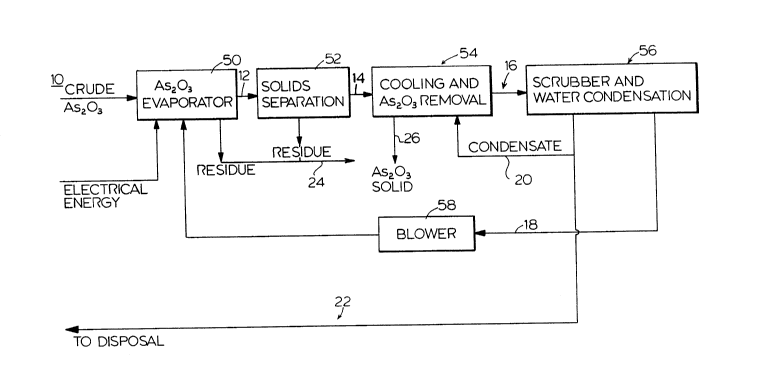
S Figure 1 is a flow sheet illustrating a process carried out
according to an embodiment of the invention.
Figure 2 is a flow sheet illustrating another process according to
another embodiment of the invention.
Figure 3 is a flow sheet illustrating another process according to
another embodiment of the invention.
DETAILED DESCRIPTION OF THE EMBODIMENTS
With reference to Figures 1 and 2, two processes are disclosed
schematically according to the flow sheets. The difference between the
processes relate to the energy source used to generate the heat in the
evaporator to evaporate the Arsenic trioxide (As203).
With reference to Figures 1 and 2, the crude material containing
arsenic trioxide 10 is exposed to heat in the evaporator 50. The arsenic
trioxide vapour is transported from the evaporator 50 by a stream of other
gas e.g. Nitrogen (See Figure 3) and the mixture leaves at a temperature of
250 - 500 degrees C (preferably 350 - 450 degrees C.) 12.
The evaporator 50 could be a:
(a) heated chamber - (for example for fine material which
wouldn't stay in a fluid bed)
(b) fluid bed
heated by oil or hydrocarbon gas combustion, (Figure 1), or by electrical
energy (Figure 2). The carry-over solids (residue) 24 are removed from the
hot gas stream in a solids separator 52, preferably in a double filtration
baghouse.
The separated residue 24, usually containing other metallic values
is then available for further treatment as the major portion of arsenic was
-8- l 31 41 49
removed. The clean gas 14 containing As203 vapour, or As203 vapour and
water vapour and other gases, depending on the source of energy used in the
evaporator, is then cooled in cooling and As203 removal section S4 by
injection of water, preferably condensate 20 from the scrubbing circuit to a
temperature:
(a) above boiling point of water (110-200 degrees C.) and the
precipitated Arsenic trioxide is then removed in the cold baghouse.
b) just above point of Arsenic trioxide precipitation
(depending upon concentration of arsenic trioxide in the vapour), generally
between 300 degrees C. to 460 degrees C., and the precipitation is
accomplished by further cooling in a fluid bed of Arsenic trioxide by spray of
water (preferably condensate from scrubbing circuit) 20, to produce a
desirable coarse product 26. (See Figure 3). The remaining gas is then
scrubbed of Arsenic trioxide in a scrubber - cooler 56 and excess water
vapour is condensed. The condensate 20 is recycled for cooling of the hot
clean gas 14. The surplus 22 will be disposed of. The scrubbed cooled gas is
then:
(a) recycled to the evaporator as Arsenic trioxide vapour
carrier via 18 - if electric energy is utilized for heating of the evaporator.
(b) sent to stack. (See 18 of Figure 2).
With reference to Figures 1 and 2, equipment suitable for use
(1) as the evaporator 50 may comprise:
(a) Fluid bed heated by an electrically preheated circulating
gas.
(b) Fluid bed heated by inserted electrical heaters.
(c) Fluid bed heated by combustion of oil or hydrocarbon gas.
(d) Evaporation chamber heated by either electricity or by
combustion of oil or hydrocarbon gas fine grained crude.
(2) for Solids separation 52 may comprise:
(a) High temperature bag-house, preferably two in series.
9 1 ~ 1 4 1 4 9
(3) For cooling and As~03 removal referred to at 54, the equipment
may comprise:
(a) A fluid bed of coarse As203 particles cooled by spraying
with a scrubber solution or water 20. As203 deposits on the cold
particles thus allowing the production of a coarse particle As203
product. The remaining As203 dust is removed in a cold bag-
house and can be sent to either product storage or back for
evaporation.
(b) Injection of scrubber condensate directly to hot gas-
adiabatic cooling. The precipitated As203 is removed from the
gas stream in a cold bag-house and sent to product storage.
(c) combination of both - cooling to temperature of As203
saturation by injection of condensate or water, then precipitation
of As203 as per (a).
(4) As Scrubber and Water Condenser shown at 56:
(a) Scrubbing traces of As203 from the gas stream is
accomplished in a tower or a venturi using the condensate from
the following condenser. The amount of condensate to be used
for scrubbing is determined by the consumption of Arsenic
bearing scrubber solution used for gas cooling.
(b) As a Water condenser:
The water condenser can be a tray or preferably a packed tower
which is cooled, depending on scrubber efficiency, by:
- directly injected cooling water - recycled, indirectly cooled
condensate
Depending on the temperature of the cooling water, the Gas
Cooling-Water Condenser may employ an additional cooling (for example,
heat pump) so that a minimum of water vapour is recycled to evaporate.
The operating conditions may be as follows:
(1) Evaporator 50
!; ~
-lo- 1314149
Temperature 300-600 degrees C., preferably 350-450 degrees C.
Pressure in freeboard - approximately minus 1 inch of H20.
(2) Hot bag-house
Temperature 300-400 degrees C. Pressure - approximately minus
.5 psig.
(3) Precipitator
Temperature 80-150 degrees C. Pressure - approximately minus
1 psig.
(4) Cold bag-house
Temperature 80-150 degrees C., preferably 110-120 degrees C.
(5) As203 Scrubber
Temperature 65-80 degrees C., preferably 71 degrees C.
Pressure - approximately minus 2.1 psig.
(6) Water Condenser
Temperature 5-10 degrees C., preferably lowest possible.
As many changes can be made to the embodiments without
departing from the scope of the invention, it is intended that all material
contained herein be interpreted as illustrative of the invention and not in a
limiting sense.
. .