Note: Descriptions are shown in the official language in which they were submitted.
~ 13~480
27400-76
The present invention relates to pellet-containing
pharmaceutical compositions.
Slow-release pellets containing an active substance
are important in the area of orally administered
medicaments. Usually, these pellets are introduced
into gelatine push--fit capsules which the patient
takes without opening. A major disadvantage of
this way of administering an active substance is
that the dosage of the slow-release drug can only
be varied by an integral multiple Gf the dose contained
in a single push-fit capsule. Thus, it is not
possible for the patient to take a fraction of
a dose unit of the drug under controlled conditions,
i.e. to take a predeterminable and reproducible
dose. There is also no guarantee that the remaining
raction of the dose unit will be kept together
saely.
Furthermore, it may be noted that for reasons of
drug safety, many push-fit capsules are provided
with a safety seal which results in the capsule
being destroyed if it is opened.
Moreover, many patients find it distinctly unpleasant
to swallow a drug in the form of a commercial gelatine
capsule, since the capsule produces the sensation
of a foreign body on being swallowed. ~his sensation
can be so pronounced that it completely stops the
swallowing mechanism. Clearly, in such a case
it would be advantageous to he able to dissolve
the capsule in a small amount of water and to swallow
the pellets thus released as an "aqueous suspension".
:
1 3~4480
-- 2
~lowever, though this is hitherto not extensively
known, commercial gelatine capsules will not dissolve
in water at ambient temperature.
It is known to those skilled in the art that such
pellets cannot be pressed to form tablets since
the high pressure which has to be exerted in the
press in order to achieve compaction leads to at
least a partial destruction of the body of the
]0 pellets which may adversely affect active substance
release behaviour of the pellet. The few attempts
that have been made to develop a pellet which can
withstand the high pressures required in tablet-
pressing have entailed drastic limitations in the
choice of the coating materials and the si~e of
the pellets (see DE-A-23 36 21~).
It is therefore an aspect of the present invention
to provide a pharmaceutical or veterinary composition
made from pellets, which is manufactured by a process
during which the high press pressures usually required
for tablet-pressing do not have to be exerted.
Thus, according to one aspect of the present invention,
we provide a delayed release pharmaceutical or
veterinary composition comprising pellets which
comprise an active substance and which are held
in a fusible matrix comprising a physiologically
acceptable binder.
In the composition the pellets are embedded in
a fusible (sinterable) matrix, and the composition
optionally comprises means such as, for example,
notches for easier subdivision.
The composition is preferably in the form of a
moulding, more preferably in the form of a tablet
0
27400-76
or block of tablets, and preferably has a porous inner structure.
~ he t~rms fus:ible or sinterable matrix relate to a
matrix which in the course of its manufacture is consolidated
either by solidification of the melt or by sintering Ibrief
heating) of a pulverulent material.
The term mou].ding, in the context of the present
invention, encompasses not only tahlet-like or capsule-like shap~s
but also all shapes which are suitable for oral administration or
capable o~ division into forms suitable for oral administration.
For the composition of the lnvention pellets produced by
conventional processes, such as dragee-making procasses or coating
processes, are suitable. These include unretarded (that is to say
directly soluble) pellets, pellets which have a coating resistant
to gastric juices, for instance a la~quer coating, and slow-
release pellets. The matrix comprises binders and, if
appropriate, addi.tives such as are conventlonally used in
galeni~al preparations. To allow ready disintegration of the
composition to release the active substance containiny pellets
from the matrix, the hinder should preferably be water-soluble or
at least water-emulsi~iable, possibly depending on the pH, and
should preferably have a melting point below 100C.
Preferred as binders are sugar alcohols Isuch as, for
example, mannitol, xylitol and sorbitol~ as well as their esters,
acids encountered in foodskuffs (such as, for example, tartaric
acid, citric acid, malic acid, lactide and lact.ic acid), fatty
ac~ds as well as their monoglycerides or diglycerides,
:, ~
A
1 3 ~
~,
sugar mixtures (such as, for e~ample, monosaccharides
or disaccharides or glucose syrup), monoglycerides
or diglycerides and their derivatives, and mixtures
of the said substances.
For excipients having high melting points, it is
preferable to use eutectic mixtures having a melting
point below 100C.
One suitable binder for a fusible matrix is a mixture
of sugar, lactose, sorbitol, starch syrup and water;
this mixture is referred to as "Basic Mix" in the
literature. "Basic Mix" is a glassy mass having
a softening point of 55 to 90C and a residual
water content of about 0.5 to 2%.
Particularly preferred as binders are polyethylene
glycols, such as, for example, Polywa~ 1500, Polywax
* *
6000 and Polywax 20,000, which have a solidification
point of between 25 and 60C.
Compositions according to the invention which readily
disintegrate in an aqueous medium, releasing t'ne
pellets, are especially preferred since these may
be broken down before administration to produce
a suspension of the pellets which may be swallowed
more comfortably than a tablet. In this regard,
mouldings which contain poLyethylene glycols as
binders are preferred since the moulding rapidly
disintegrates in an aqueous medium within a short
time and the pellets can be swallowed comfortably
as a kind of "aqueous suspension".
The invention thus also provides a pharmaceutical
or veterinary form for pellets which form disintegrates
in an aqueous medium within an acceptable period of
time thereby releasing the pellets for easier ingestion.
*Trade Mark
~3~4~8~
Where the pellets are provided with a polymer shell
to give delayed or controlled release of the active
substance, the binder chosen should be one compatible
with the polymer shell.
For use with certain active substances, the binder
is preferably resistant to gastric juice.
According to another aspect of the present invention
we provide a process for the preparation of a delayed
release pharmaceutical or veterinary composition
comprising pellets which comprise an active substance
and which are held in a matrix of a fusible, physiologically
acceptable binder, comprising moulding or extruding
a forming composition comprising said pellets and
an at least partially fused physiologically acceptable
binder.
In the case of extrusion the composition may be
made by the extrusion of pellets in the binder,
optionally with the extruded column being nipped
at intervals to provide dividing lines defining
individual tablets. The binder or mixture of binders
should preferably have a broad softening point.
The mixing of the pellets with the binder and the
subse~uent mechanical extrusion should not be so
violent as to cause brealcing up of the extrusion
or rupture of the slow-release coating.
According to a further aspect of the present invention
we provide a process for the preparation of a moulding
containing pellets which comprise an active substance
and which are held in a matrix of a fusible, physiologically
acceptable binder, said process comprising one
of the following:
(a) introducing the pellets into moulds and subsequently
~31~80
-- 6
f illing said moulds by pouring in a fused
binder;
(b) coating the pellets with a binder, introducing
the coated pellets into moulds and then heating
briefly;
(c) mixing the pellets with a binder, introducing
the mixture into moulds and then heating
briefly; or
(d) introducing the pellets together with a binder
and a blowing agent into moulds and heating
briefly.
In process (a) pellets which may, for example,
have been manufactured by processes known from
the literature and are provided with a slow-release
coating are introduced into suitable moulds and
an adequate amount Oe the molten binder is then
poured into the mould. The amount of binder, which
may contain galenical additives, that is to be
used is conveniently between 5 and 50% by weight,
preferably 10 - 25~ by weight, relative to the
total weight of the pellets. When the material
has cooled, the finished mouldings are taken from
the mould and are packaged. Where appropriate,
a release agent may be applied to the mould beEorehand
to permit easier release. Mculds which permit
simple removal of the moulding when it has cooled
are particularly suitable for use in this process.
In process (b) the binder is applied as a coating~
in a suitable thickness, to pellets which may conveniently
already have been retarded, e.g~ by the provision
of a release delaying shell. Coating with the
binder may be done, for example, by a dragee-coating
process. The optimum thickness of the binder layer
1~14480
-- 7
is essentially dependent on the size of the pellet
and can be determined by simple experiments. The
amount of binder used is conveniently between 5
and 50% by weight, preferably between 10 and 25%
by weight, based on the total weight of the pellets.
The pellets coated with the binder are introduced
into moulds and are bonded together by brief heating,
during which the binder briefly melts, at least
at its surface. By brief heating it is meant that
the heating should be sufficient for the binder
on the surface of the coated pellets to melt sufficiently
for adjacent pellets to be bound together on subsequent
cooling. The heat re~uired for melting the binder
may, in the case of suitably designed moulds, be
applied via the mould, which, where appropriate,
may additionally be provided with cooling devices.
If it is not possible to apply heat via the mould,
the energy required for fusion may be applied from
outside, for example by using microwave radiation
or by using a heating device such as, for example,
a simple oven. It is merely necessary to ensure
that the retard layer of the pellets is not damaged
by excessive heating.
In process (c) the pellets, conveniently already
in retard form, are mixed with the binder and the
- mixture is introduced into a mould. Further processing
may be carried out as described for process (b)
above.
In process (d) the pellets, conveniently already
in retard form, are introduced, together with a
binder, into a mould. Upon heating, the binder
is uniformly distributed between the pellets by
decomposition of the blowing agent, so that after
cooling the desired moulding is obtained. Suitable
131~48~
-- 8
as blowing agents are compounds which are pharmacologically
safe and have a decomposition point in the range
oE the melting point of the binder. Further, low-
boiling solvents (such as, for example, hydrocarbons,
halogenated hydrocarbons, perhalogenated hydrocarbons
and alcohols) are suitable as blowing agents, provided
they are compatible with the slow-release coating
on the retarded pellets. If specially designed
moulds are used, an inert gas, for example compressed
air or nitrogen, may alternatively be used as the
blowing agent.
On heating (sintering) the moulding, especially
in the case of processes (b) and (c) as hereinbefore
described, the softening of the loose mass of pellets
causes a slight contraction in volume. To avoid
crack formation and in order to achieve a uniform
appearance it is advantageous to exert a slight
pressure on the moulding while it is still warm,
so as to assist the desired moulding action. However,
it is, of course, obvious that the pressures exerted
should be so slight that the pellets, and especially
the slow-release coating of the pellets in retard
formt are not damaged. Thus slight pressure is to
be taken as meaning a pressure typically sufficient to
cause smoothing down, polishing or densification. In
a suitable embodiment it may suffice to vibrate the
mould briefly in order to achieve densification.
In a particularly preferred embodiment of the process,
blister foils (e.g. of thermally formed plastics
film) with appropriate recesses for the mouldings
(and, where appropriate, additional shapings to
form division notches) are used.
The filling of these blister foils and the manufacture
of the mouldings may be carried out in accordance
with any one of processes (a~ to (d), the recesses
in the foil simultaneously serving as the moulds.
~3~8Q
g
When the mouldings have cooled, the blister foil
is sealed with aluminium foil. When required for
use, the mouldings are pressed out through the
aluminium foil.
A moulding produced according to any one oE processes
(b), (c) or (d) can, depending on the amount of
binder used, have an uneven surface. However,
this has no effect on the active substance release
characteristics. If desired, an approximate]y
uniform surface can be obtained on the moulding
by a further application of binder or of a lacquer.
Mouldings produced by process (b) have a Porous
inner structure. The bonding between the pellets
occurs only at the points of contact of the binder
coatings. Such mouldings therefore have particularly
advantageous disintegration characteristics, since
water or the relevant digestive fluids can more
rapidly penetrate and dissolve the binder.
To delay disintegration of the mouldings, they
may additionally be coated with a lacquer which
is resistant to gastric juice or which has a retarding
action. In this regard, it is also possible to
manufacture the compositions of the invention using
binders which are resistant to gastric juice, so
that the pellet release only takes place on passage
through the intestines.
The pharmaceutical composition is preferably divisible.
Such divisibility may be achieved, for example,
by providing dividing notches by using moulds of
appropriate shape, or subsequently by means of
a heated tool, or by working the composition while
it is still soft, i.e. not yet cooled, by means
o~ a tool of appropriate design.
- 131~0
-- 10 --
The present invent;on thus provides a pharmaceutical
or veterinary form for pellets which can be subdivided
one or more times and which permits such division
with retention of the properties characteristic
o~ the pellets, such as, for example, slow release.
An important advantage of the invention is that
in the course of the manufacturing process according
to the invention the pellets do not suffer any
mechanical damage; accordingly, even after the
composition has been subdivided, the pellets remain
fully preserved~ Indeed, mouldings may be produced
according to the invention from pellets which are
not provided with a slow-release or gastric juice-
resistant coating~
The invention will now be illustrated by means
of the following non-li.miting Examples and with
reference to the accompanying Drawings in which:
Figure 1 shows moulded tablets prepared according
to Example l;
Figure 2 shows moulded tablets prepared according
to Example 2;
Figure 3 shows a capsule-shaped tablet provided
with a dividing notch;
Figure 4 shows moulded tablets prepared according
to Example 4;
Figure 5 shows moulded tablets prepared according
to Example 5;
Figure 6 shows moulded tablets prepared according
to Example 6;
Figure 7 shows moulded strings of tablets;
Figure 8 shows moulded strings of tablets
provided with an additional outer coating o~ polyethylene
glycol powder;
Figure 9 shows moulded strings of tablets
having undercuts, the right hand side showing spherical
~31~0
-- 11. --
mouldings, the left hand side show;ng divisible
mouldings; and
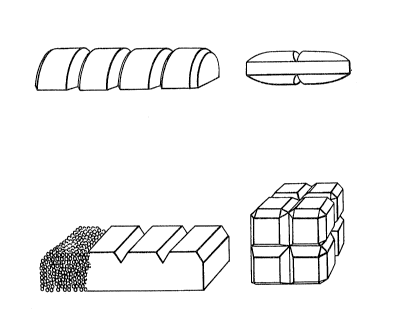
Figure 10 shows further possible shapes for
the compositions of the invention.
It should be noted that the compositions shown
in the Figures are shown on an enlarged scale.
All percentages and ratios referred to herein are
by weight unless otherwise stated.
131~80
- 12 -
Example 1
Pellets, manufactured by a conventional process
by introducing an active substance into so-called
"starter pellets" (which may be purchased) comprising
sugar and/or corn starch, are introduced into the
capsule receiving wells of a blister foil and Polywax
6000 is poured in. A typical tablet with a volume
of about 0.68ml preferably contains 450-600mg of
pellets. The pellets are pharmaceutical pellets
containing an active substance such as theophylline
and have a size o 0.8-2mm. lOO such pellets weigh
140mg and 100 such pellets with a retard coat weigh
160mg. Figure 1 shows the resultant tablets.
Example 2
Pellets from the same batch as for Example 1, mixed
in the ratio of 1:1 with Polywax 6000 powder, are
introduced into a capsule-like mould. The mould
contents are fused at about 65C and pressed together.
The mould is removed ater cooling, releasing tablets
as shown in F'igure 2.
Example 3
A dividing notch or notches may be introduced,
producing tablets as shown in Figure 3.
Example 4
Pellets from the same batch as for Examples 1 and
2, together with 20% of Polywax 20,000, are warmed
in a beaker and then stirred with a glass rod until
solidification is complete~ The pellets thus coated
with Polywax are introduced into a capsule-like
mould having an upper and lower part and are slowly
heated to about 65C, in the course of which the
4 8 ~
- l3 -
mould is slowly pushed together. A~ter cooling,
the mould is removed, releasing tablets as shown
in Figure 4.
Example 5
15 - 20~ of Polywax 20,000 and a small amount of
ammonium bicarbonate are introduced into the lower
part of a two-part mould, and pe]lets, produced
by a conventional process, are then introduced
into the lower part of the mould. The quantity
of ammonium bicarbonate used in strongly dependent
on the viscosity of particular binder used. The
quantity required can be determined by simple experiments.
In this particular case about 5m~ of ammonion bicarbonate
per moulding is used. The upper part of the mould,
which is provided with a plurality of aperatures,
is then pushed on to the lower part and sintering
is carried out at about 65C. The e~cess gas and
Polywax is able to escape. Figure 5 shows Examples
of tablets produced by this process.
_x~ e_
The mouldings are produced in the same manner as
described in Example 4 except that the mixture
of Polywax coated pellets introduced into the upper
and lower parts of the mould contained 20 and 30%
respectively of Polywax 20,000. Figure 6 shows
examples of such products.
Example 7
In addition to the Examples described above, rod
shaped mouldings have been produced in order to
emphasise the broad range of overall shapes possible
for divisible compositions according to the invention.
.
Placebo pellets, having a weight of 25mg per 100
pellets, corresponding to a sieve fraction of 0~6 - 0.71 mm
are provided, in a dragee-making kettle, with a
coating of 20% by weight of polyethylene glycol
1500. These pellets have no active substance.
... . . . .
~314480
- 14 -
Moulds are produced by casting from silicone rubber,
with the aid of precision-engineered rod patterns
made from aluminium.
On directly sintering the above pellets in a microwave
oven and lightly pressing the mould in by hand,
the rods shown in Figure 7 are produced.
In order to seal the interstices between the pellets,
the compositions of the invention may also be produced
which consist of pellets provided with an additional
"outer phase" or coating of polyethylene glycol
powder. Such compositions are illustrated in Figure
8.
The use of plastically resilient moulds as described
above also permits the manufacture of mouldings
having under-cuts such as are shown in Figure 9.
The term "under-cut" refers to the situation when
the mould opening is smaller than the greatest
width of the moulding. Thus in order to remove
the moulding the mould must either be broken or
it must be elastic (e.g. made of silicone rubber).