Note: Descriptions are shown in the official language in which they were submitted.
1 31 5055
PC-1274
ATOMIZATION PROCESS
The present invention is concerned with metal atomization and
more particularly with atomization of metal to produce metal powder
having irregularly shaped particles of relatively low oxygen content.
BACKGROIJND OF THE INVENTION
Atomlzation of metal is a process whereby a stream of molten
metal (including alloys) is broken up into particulates by means of
an intercepting, fast moving stream or mass of an atomizing agent
usually a gas or a liquid.
Gas atomization generally employs as the atomizlng agent a
gas which i8 inert to the metal being atomized e.g., argon when
atomizing a nickel-chromium alloy. On occasion, a gas reactive with
the molten metal can be used provided that the product of gas-metal
interaction forms a protective film aroun~ the small particulates
which are produced when the gas and molten metal streams intersect.
An example of use of a reactive gas i8 the use of air in atomizing
alumlnum. By and large however, an inert gas, e.g., argon, ls
: i
-'
, :
., : .
-`` 1 3 1 ~U55
2 PC-1274
emploved in atomizin~ reactive metals. A problem with gas
atomization is that the heat capacity of a gas is very ]ow and
relativelv little heat is extracted from the mo]ten metal stream when
that stream is intersected by an argon gas stream. The result of
this is that the part~culates produced by the atomization process are
molten particulates which solidify whlle falling under the influence
of gravitv in the atomization chamber. The ultimate product is a
powder having mainly spherical particles or streamlined
quasi-spherical particles which, if fall time is not great enough,
sometime te~d to bond together in the mass of powder product at the
bottom of the atomizing chamber. On occasion, persons in the prior
art have suggested the use of a water bath at the bottom of a gas
atomizing chamber to serve as a quench means so as to prevent bonding
in the powdered mass.
For powder metallurgical purposes, spherically or
quasi-spherically shaped powder particles are not particularlv
advantageous. Compaction of powder made up of such particles under
pressure does not generally produce a coherent mass having any
'~' measurable green strength unless the compaction pressure
substantially exceeds the yield strength of the particular metal
throughout the compacted mafis. It is well known that metal powder
:-: being compacted in the first step of producing an article by powder
metallurgical techniques does not act as a Newtonian liquid would
under pressure. Inter-particle effects such as bridging, complex-
ities of compacted shapes and the like tend to dissipate applied
compacting pressure. If one must rely upon deformation of particles
from a spherical shape to produce a compact having reasonable green
strength, enormous compacting pressures must be used to effect solid
state welding especially with strong alloys such as nickel-chromium
alloys. Consequently, when employing metal powders made up of
spherical or quasi-spherical particles, the art has resorted to
canning the powder~ before compaction. While this procedure is
operable and practical, it normally is expensive since the can must
be provided, a protective atmosphere (including vacuum) generally is
provided in the can and, ultimately, the can must be removed.
Another common atomization process is liquid atomization. In
this process a liquid, usually water, is employed as a fast moving
" .
.: ., : .
:~' ' ' :' ' -
.
.
-,: .
1 3 1 5055
3 61790-1613
stream to intersect the molten metal stream. ~1ater, in contrast
to gas, has a high heat capacity. Thus particulates formed by
interaction of the molten metal stream and the water stream are
instantaneously solidified into irregularly shaped particles. The
difficulty with such a process for producing a powder for
metallurgical use is that the product powder often contains a high
level of impurity resulting from chemical reaction with the
atomizing liquid. For example, powder produced by ~ater
atomization generally contains high oxygen levels even when the
water contains oxldation inhibitors such as alcohol. ~or many
purposes the high oxygen content of the powder makes it useless
for the intended purpose unless an expensive, sub~equent reduction
operation is carried out. If, as has been previously suggested,
hydrocarbon liquid is used as the atomizing medium, the resultant
powder can have high carbon or carbide content generated by
hydrocarbon cracklng when hydrocarbon liquid intersects a molten
metal stream having a temperature above about 700C.
Metal fragmentation processes other than gas or liquid
atomization which act on or produce molten metal fragments in a
gas phase are known. Among these processes are included
fragmentation of metal thrown off a rotating electrode of an
electrical arc and fragmentation of metal occurring by pouring
molten metal onto a rapidly moving (e.g., rotating) surface from
which fragmentable films or droplets are projected. The present
invention, may, under specific circumstances be applicable to
these other fragmentation processes.
,
1 31 5055
3a 61790-1613
PURPOSE OF THE INVENTION
The present invention has for its purpose the provision
of a process of gas atomization whereby particles similar to
irregularly shaped powder particles of the liquid atomization
process are produced with relatively low levels of contaminants
approaching or equallin~ the contaminant level of normally gas
atomized metal powder.
The invention provides a process for atomization of
metal comprising impacting particulates of gas atomized molten
metal traversing an inert gaseous phase into an aqueous liquid
quench medium containing an oxidation inhibitor, the position of
the gas-liquid interface of said quench medium having been
adjusted such that, a significant proportion of said particulates,
deformed from spherical shape, are recoverable from said liquid
quench medium.
In preferred embodiments the liquid quench medium is
positioned at rest less than about 25 cm below the zone of
atomization. Preferably the aqueous liquid quench medium contains
an effective amount of an organic oxidation inhibitor such as a
lower alcohol or a water-soluble carbohydrate.
`
.
" 1 31 ~055
4 PC-1274
~ _ IN~
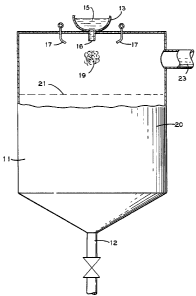
Figure 1 of the drawin~ is ~ schematic crosfi-section~l view
of a ~as-liquid atomizer such as can be used for carrvinR 0l1t the
process of the present invention.
Figure 2 of the drawin~ is a 5 X plloto~,raph of ~etal powder
produced by the process of the present invention.
GENERAL DESCRIPTION OF THE INVENTION
The present invention contemplates a process of atomization
of metal wherein molten metal, after disintegration into molten
particles in an inert or reducing gas phase, is quenched in a liquid
quench medium containing an oxidation inhibitor to provide
irregularly shaped particles. The position of the gas-liquid inter-
face of the quench medium onto which particulates impact is adjusted
with respect to the locus of metal disintegration such that a
sigDificant portion of particulates traversing the gas phase and
recovered from the quench medium have a shape other than spherical.
The principles of the present invention have been elucidated
by experimentation with gas-atomized nickel-iron alloy using argon as
the atomizing fluid and a bath of alcohol-water. It has been found
that if the at-rest level of the quench medium bath surface
containlng about 2% to about 10% by volume isopropanol as the liquid
quench medium is less than about 25 centimeters (cm.) below the
atomization zone, i.e., the spot where a metal stream and high
velocity streams of argon intersect, the powder produced has a
significant proportion of irregularly shaped particles and, as a
whole, has a, low oxygen content. If the at-rest quench medium bath
surface is substantially lower than 30 cm. below the atomization zone
the resultant powder particles are spherical. The quench medium can
be not only a bath below the atomization zone but also it can be a
curtain of liquid sprayed so as to approximate the positlon of the
surface of a quench bath under gas atomization conditions.
The present invention is particularly adapted for the
atomization of metals and alloys which in the main have oxides which
are reducible by hydrogen at temperatures below about 1000C. Alloys
, .,
--` 1 31 5055
PC-1274
especially operable in the process of the presellt invention are those
which have a~ a major or principal constituent a metal from the group
consisting of copper, iron, nickel and cobalt and which may incl~de
minor amounts of metals such as chromium, aluminum, titanium,
molybdenum, tungsten, etc.
While applicant is not fully aware of ~ll the factors which
are involved in the process of the present invention, it is believed
that by limiting the time of flight of a molten particle between the
atomization zone 19 of a conventional gas atomizer as schematicallv
depicted in Figure l of the drawing and the locus of quenching 21 a
particle as it hits the exposed surface of the quench medium can be
in a molten, mushy or highly plastic state. In such a condition, the
forces involved in impact can cause deformation of the particulate
from the spherical shape. With respect to Figure l, those skilled in
the art will recognize in schematic the various parts of a
conventional gas atomizer 11 including tundish 13, body of molten
metal 15, pouring nozzle 16, gas nozzles 17 and gas vent port 23. In
the practice of the present invention the level of quench medium 20
is ad~usted so that the gas-liquid interface 21 (or locus of
quenching 21) at rest is usually less than 25 cm below atomization
zone 19. At the end of or periodically or continuously during
atomization, metal powder is recovered from the liquid quench medium
20 using recovery means 12 and conventional liquid-solld separation
process.
The dynamic conditions existing during atomization are quite
different from those schematically depicted in Figure l of the
drawing. For one thing the interface 21 between quench medium 20 and
the gas phase is far from flat. It is highly deformed by rapid flow
of gas emerging at supersonic speed from ~ets 17 under pressures of 8
or more atmospheres gauge.
Further, in atomization zone l9 a range of size of
particulates 18 produced. Small particulates can be levitated by gas
and held in gas suspenslon for longer periods of time than large
particulates. It is known that convection and radiation cooling of
small particulateæ 18 much faster than cooling large particulates.
This results in the phenomenon that often the process of the present
inventlon produces powder in which the smaller sized powder fractions
:` :
;` .
: . .
.
.
" -,
.. ..
--`` 1 3 1 5 055
fi PC-1274
tend to be spherical and the ]flrger sized powder fractiorls tend to be
irregular in shape. This phenomenon is shown in Figure 2 of the
drawing which is a 5 power photographic view of a powder of nickel-
iron alloy produced by the process of the present invention. For use
in powder metallurgy such a product is perfectly satisfactory without
using canning because upon compaction the larger irregular particles
interlock holding the smaller spherical partic]es within an inter-
locked skeleton.
Another phenomenon that can occur during actual operation of
a gas atomizer under conditions specified in the present application
is the collision of molten metal particulates with spray droplets of
the quench medium. Under the applied pressure of 8 or more
atmospheres, gas issues from jets 17 at high velocity and can pick up
spray at interface 21. Occasionally a droplet of quench medium can
collide with a mo]ten metal particulate resulting in either freezing
the shape of the particulate prior to spheroidizing or more likely,
distorting a spherically shaped particulate by exceedingly rapid
almost explosive local generation of gas from the droplet of quench
medium. In view of the foregoing, the product powder resulting from
the process of the present invention can have a complex combination
of particles of clearly non-spherical irregular shape.
Carrylng out the process of the present invention is not
limited to the apparatus depicted in Figure 1 of the drawing. Gas
atomization chamber 11 can include deflection plates either above or
below interface 21; quench liquid 20 can be circulated to enhance or
oppose vortexlng induced by gas from ~ets 17; sonic or ultra-sonic
vibration can be used along with or in place of the disintegrating
gas and other means of disintegration such as centrifugal shotting in
assoclation with a peripheral curtain of quench liquid can be used in
place of gas atomization.
; When carrying out gas atomization in accordance with the
inventlon it is advantageous to use as the atomization medium
substantially pure argon gas introduced as a plurality of high
velocity gas streams which intersect a downwardly moving stream of
molten metal at one or more points in space. Argon gas is employed
; at a rate of about 0.033 to about 1.3 standard cubic meter (sm3) per
kilogram of metal atomized. Prior to the start of atomization the
. `
~", ......
t 3~ $055
7 PC-1274
chamber in which atomization is to take place shou~d be filled with
the atomization gas, e.g., argon and, during atomization, steps
should be taken to assure that a slight positive internal pressure
exists in the atomization chamber to prevent influx of ambient air.
If the metal or alloy being atomized is carburization resistant or
can tolerate small amounts of carbon, the argon can be diluted with a
hydrocarbon gas such as butane to thereby reduce the cost of atom-
ization gas and provide a reducing atmosphere at the instant of
atomization. In the case of atomization of an alloy such as aluminum
bronze, the hydrocarbon ~as, e.g., butane, can comprise a major part
or all of the atomizing gas. Other gases which may be used for
atomization, depending upon the metal being atomized include
nltrogen, helium, methane, propane and carbon monoxide.
Except in the case of aluminum it is generally important that
the atmosphere in the atomization chamber be substantially devoid of
free oxygen, e.g., from the air. This is to prevent rapid oxidation
of metal at the instant of atomization and during passage of atomized
particles through the gaseous medium in the atomizing chamber. If
oxide forms, it is usually difficult to reduce the oxide to metal
under conditions prevailing in the atomizer chamber because (l) the
metal temperature is always lower after atomization than at the time
of atomizatlon and (2) the time interval between atomization and
quench i9 very short.
The liquid quench medium used in the gas atomization process
of the present invention is advantageously a water solution of about
10% or less by volume of isopropanol e.g., 2 or 3 to 10% by volume of
isopropanol held at a temperature below about 66C. This quench
medium is advantageous in that its total vapor pressure (water plus
isopropanol) is less than about 0.5 atmosphere, water is cheap and
isopropanol is relatively inexpensive, readily available and
effective to inhibit oxidation of many common metals e.g., nickel,
iron, copper and the like. Other readily oxidizable, water-soluble
organics can be substituted in part or in whole for isopropanol but
are generally not preferred because of cost, toxlcity, volatility or
odor considerations. Such other water-soluble organic compounds
include but are not limited to methanol, ethanol, propanol, acetone,
formaldehyde, acetaldehyde, glucose, invert sugar, hexatols,
. . . . .
. . .
`" 1315055
~ ~C-1274
sorbitols, mannitol, dulcitol, other reducin~ carbohydrate~s,
benzaldehyde, hydroq-linone, ascorbic acid and it9 SaItR, phenol,
gallic acid and its alkali metal salts, resorcinol and salicylic acid
and its alkali metal salts. If desired an aqueous quench medium can
contain a water-insoluble oxidation inhibitor as a dispersed phase.
For metal which must be rigorously free of oxide but which can
tolerate carbon, it is possible to use a liquid hydrocarbon such as
purified mineral oil as a quench medium. Care should be taken
however that undesirable impurities, notably sulfur, e.g., in the
form of sulfur-containing compounds, should be at a very low ]evel in
mineral oil used for this purpose.
The liquid quench medium employed in the atomizing process of
the present invention should have a reasonably high heat capacity
e.g., a specific heat above about 0.5 cal. per mol. deg. and be
substantially unreactive with respect to the metal being atomized.
The temperature of the liquid quench medium should be maintained such
that the total vapor pressure of the medium is below about 0.5
atmosphere.
EXAMPLE I
A 13.6 kg heat of 42% nickel, 58% iron, 0.05% carbon alloy
was melted in air and fed to a tundlsh above an atomizing chamber at
a temperature of about 1650C. Prior to thls the atomizing chamber
was filled to a point about 20 to 25 cm below the atomization zone
with water containing 7.5 volume percent isopropanol. The metal was
atomized by allowing a molten metal stream about 0.76 cm in diameter
to run from the bottom of the tundish into the atomizing chamber
where it was intersected at the atomization zone by 8 jets of argon
gas emanating from a gas stream under a pressure of about 11
atmospheres absolute. The powder produced from this heat contained
0.08% oxygen and was irregular in shape.
In a parallel experiment using a quench bath of water
containing 8.6 volume percent isopropanol established 27.5 to 32.5 cm
below the atomization zone and using a gas pressure of 9 atmo8pheres
ab801ute, resultant powder contained 0.18% oxygen and was very
rounded and spherical in shape.
"~
,
,
~ : `
., :
-
1 3 1 50~5
9 PC-]274
~XAMI'I,~ II
A 14 lcilogram air me]ted heat of an alloy nomina]lY in weight
percent containing 30-35% nicke].. 19-23% chromium, 0.1% maximum
carbon, 1.5% maximum manganese, 1% maximum silicon, 0.75% maximum
copper, 0.15%-0.6% aluminum, 0.15-0.6% titanium, balance essentially
iron was atomized using argon at about 15 atmosphere gage using a
water-isopropanol (9%) quench liquid about 12-13 cm below the
atomization zone when at rest. The resultant powder was very
irregular and, after annea].ing in hydrogen at 980C was compactable
under a pressure of about 3945 atmospheres to form a disc having good
green strength.
EXAMPLE III
A 14 kilogram air-melted heat of alloy containing in weight
percent about 35% nickel, 20% chromium, 4% aluminum, 5% cobalt, 0.4%
titanium, 0.1% yttrium, 0.33% silicon, balance essentially iron was
: atomized in the same manner as was the alloy of Example II. The
resultant powder contained pancake-like particles, was compactable as
atomized and contained about 0.07% oxygen.
While in accordance with the provisions of the statute, there
ls illustrated and described herein specific embodiments of the
invention, those skilled in the art will understand that changes may
be made in the form of the invention covered by the clalms and that
certaln features of the invention may sometimes be used to advantage
wlthout a corresptDd1ng use of the other feature6.
..
,
~' .
,.,- ''''''`~ ' '' .