Note: Descriptions are shown in the official language in which they were submitted.
t317876
BI-DIRECTIONAI. L~TERAL CHRO~TOGRAPHIC TEST DEVICE
Back round of the Invention:
The invention relates to a diagnostic device for
performing solid phase immunoassays to detect the presence
of antigens or antibodies in biological or non-biological
5. fluids. The teachings of the invention are incorporated
in a bi-directional lateral chromatographic device for
use in solid phase immunoassays or for the non-immunological
detection or quantitation of proteins or substances in
biological or non-bioloqical fluids.
10. More particularly, the invention relates to
devices and methods which utilize filter means for testing
biological fluids to detect the presence of analytes such
as bacterial, viral, parasitic, or funqal antigens and
immunoglobulins, hormones, serum proteins, drugs and the
- 15. like.
$ypical of prior art devices presently in use
are the teachings of U.S. Patent 4,623,461, which discloses
a filter body located in a housing having an openin~ therein
for the reception of a suspension sample to permit the
20. upper face of the filter to trap colored or particulate
matter contained within the specimen and prevent such
1317876
matter from reaching the bottom face of the reaction zone,
which has been previously treated with a suitable reactant.
The perimeter of the filter is engaged with a suitahle
absorbent body and the absorbent body is intended to receive
5. the outward diffusion of liquids applied to the filter.
One of the disadvantages of the '461 construction
lies in the fact that the fluid flow from ~he point of
application of the suspension to the absorbent hody is uni-
directional and a subtantial accumulation of solids at the
10. point of application of the sample suspension is inevitable,
which will seriously i~pinge upon the resultant chroma-
tological or other type of test reading imparted by the
device.
U.S. Letters Patent 3,825,410 discloses a disposable
15. combined storage and reaction cell for use in the performance
o chemical and biological reactions which receives reactants
dispensed therein and maintains the same in stored condition
so that they remain stable. Reactants will not mutually
react until such time as it is required to initiate the
20. reaction.
The immobilization of the reactants is accomplished
by such procedures as freeze drying and the reaction is
initiated by the introduction of a sample to be analyzed,
~, ' \
1317876
whereafter separation of bound and free ligand can be
performed either within the unit itself or externally.
The reaction cell of the '410 patent may include
a filter so that the entire process of separation can be
5. completed within the reaction cell and the filter be removed
from the reaction cell and submitted for radioactivity or
other tracer counts.
~ J.S. Letters Patent 3,888,629 discloses a reaction
cell for performing various types of assays which incorpo-
LO. rates a matrix pad of absorbent material retaining the
necessary reagents for the reaction and serving as a site
in which the reaction totalIy occurs. A se~arable lower
chamber incorporates absorbent material abutting the matrix
pad to promote filtration through the pad after the reaction
lS. has taken place~ ~
Both patents are characterized by the mere uti-
lization of the filter as a pass-through device which is
time-consuming and which is hi~dered by the deposition of
solids out of the suspension sample.
20. Objects and Advanta~es of the Invention:
One of the objects of ~he invention is the pro-
vision of a diagnostic ~evice which incorporates a planar
--3--
1 3 1 7876
filter body havinq sample application, separation Ind
reaction zones, said filter body bein~ configured in such
a manner that the bulk o the solids in the suspension
sample are retained in the sample application zone and the
5. fluid ls caused to mi~rate bilaterally through the inter-
stices of the filter by the 1uid communication of absorbent
means with the application and reaction zones.
Conse~uently, the unilateral directional flow
which causes accumulation of solids in the reaction zone
10. in the previously discussed prior art devices is eliminated
in the test device of our construction because the rapid
bilateral flow achieved by the construction of the device
causes i~mediate deposition of solids out of the fluid
component of the suspension.
15. Another object of the invention is the provision
of a diagnostic test device of the aforementioned character
wherein the planar filter incorporates a sample application
zone which is relatively large and which is connected to
the reaction zone by a separation zone, the length of the
20. separation zone being proportioned to the character of the
suspension sample applied to the a~plication zone, and the
separation and application zones cooperate to retain the
bulk of the solids or particulate matter in the application
1 3 1 7876
and separating zones so that the immuno-reagent or chemical
test reagent deposited in the reaction zone, when subjected
to the test procedures and analytes, will have a minimum of
or no particulates embodied therein which would cause the
5. emission of high background signals, thu~ creating a
negative effect on the tes~ readout.
~ nother important object of the invention is the
provision of a composite housing filter conjugate which is
characterize~ by ease of assembly and application. The
10. test device of the invention is designed particularly for
use in the field or the testing of various human and
animal diseases or for various chemical tests involving
the utilization of blood samples from humans and animals,
and it is capable of giving test results equal to labora-
15. tory results within a matter of minutes depending upon thesample solution which is applied to the specific device.
Another object of the invention is the provision
of a method of performing a test by the utilization of the
device of the invention which incorporates a plurality of
20. simple steps which can be carried forth by non-laboratory
personnel in the field and which can provide such personnel
with an almost immediate readout of the presence or absence
of the sought-after infection or dru~, or the like.
1317876
According tG one aspect of the invention there is provided in a test device for
per~orming solid phase immunoassays, the combination of a planar fiber filter matrix, said
matrix including a test sample application zone, a separation zone and a reaction zone,
said separation zone separating said application zone from said reaction zone and being
sufficiently long to prevent migration of particulates in said sample into said reaction
zone, first absorption means contiguous to said sample application zone, and second
absorption means contiguous to said reaction zone whereby bilateral flow of the liquid
componerlt of said test sample occurs.
According to another aspect of the inveDtion there is provided in a test device for
performing solid phase immunoassays, the combination of a housing, a planar filter matrix
located in said housing, said filter matrix iDcorporating a test sample application ~one, a
separation zone integIal with said test sample application zone and a reaction zone
communicating with said separation zone, a first absorbent means located in said housing
in fluid communication with said application z~lne, and second absorbent means in said
housing in spaced relationship with said first absorbent means and in fluid communicatlon
with said Ieaction zone whereby bilateral flow of the fluid component of said test sarnple
occurs.
According to a further aspect of the invention there is provided in a
chromatographic test device for performing solid phase immunoassays, the combination a
planar filter matrix including a body portion having a plurality of laterally extending
integral arrns disposed in spaced relationship with one another, each of said anns having
1 3 1 7876
an expanded end portion, first absorbent means in fluid communication with said expanded
end portions and second absorbent means in fluid communication with said body portion
whereby bilateral flow of fluid deposited upon said expanded end portions may be
achieved.
According to yet a further aspect of the invention there is provided a method of
perfonning an immunoassay in coryunction with a chromatographic test device which
incorporates a planar filter matrix having sample application, separation and reaction
zones, and fiIst and second absorbent means in fluid comrnunication, respectively, with
said application and reaetion zones, the steps of applying a suitable volume of liquid
sample to said sample application zone, applying a suitable volume of wash reagent to
said sample application zone, permitting capillary forces within said filter matrix to
bilaterally draw the fluid portion of said sample primarily in the direction of said
separation zone, but secondarily in the direction of said first absorbent means, entrapping
particulates in said application and separation zones, permitting the fluid portion of said
sample to flow into said reaction zone which is treated with a reagent immobilized to the
fiber matrix, applying a suitable volume of wash reagent to said reaction zone to wash
away ur~eacted sample components in bilateral directions away ~rom the reaction zone and
toward the separation and sample application zones, applying a tracer reagent to said
reaction zone, washing said reaction zone, and applying chromatic-eliciting substrate to
said reaction zone to produce a c}~omatic reaction.
1317876
Brief Descri~tion of the Drawin~s:
~_.
Other objects and advantages of the invention will
be apparent from the following specificat:ion and the accom-
panying drawings, which are fox the purpose of illustration
5. only, and in which:
FIG. 1 is a top plan view of a ~ypical device of
the invention;
FIG. 2 is a device similar to FIG. 1 with the
exception that it incorporates a plurality of application
10. and reaction zones;
FIG. 3 is a top plan view showing the housing of
the device of FI~. 2 with the cover removed therefrom to ` .
illustrate the location of and configuration of the filter
and the relation thereof with tha absorbent means and the
15. particular design of the housing to encapsulate the filter
and absorbent means;
FIG. 4 is a view similar to FIG. 3 illustrating
removal of the filter to disclose the relationship of the
absorbent means with the housing and the particular con-
20. ~figuration thereof;
FIG. 5 i5 a vertical sectional view taken onthe broken line ~-5 of FIG. 2 and illustrates the eluid
relationship~of the filter with the absorbent means and
,
1317876
the filter and absorbent components with the specific
design of the housing and co~er therefor; anfl
FIG. 6 is an exploded view illustrating the
various components of the test device.
5. Description of the Preferred Embodiments of the Invention:
The chromatic assay device of the present ~n~en-
tion may be used to perform sol.id phase immunoassays for
the detection of antigens or antibodies, hereinafter
referred to as analytes, in biological and non-biological
10. fluids. The device may be non-immunologically used to
identify and/or quantitate proteins or substances in
biological and non-biologi~al fluids. The device may be
: utilized to perform assays such as competitive or non-
: competitive enzyme-linked immunoassays, enzyme-multiplied
lS. immunoassays, enzyme-inhibition assays, heterogeneous or
homogeneous fluorescent immunoassays, chemiluminescent
and bioluminescent assays, these assays utilizing various
labelled probes, and the like.
Obviously, the particular analyte test to be
20. used will depend upon the chosen sample and the desired
result to be achieved.
1 31 7816
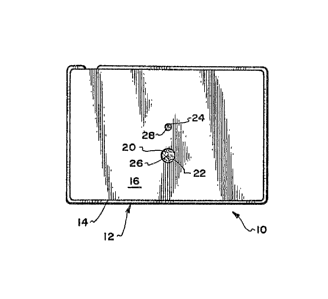
Referring to the drawings, and particularly to
FIG. 1 thereof, we show a test device 10 constructed in
accordance with the teachings of our invention which is
incorporated in a housing 12, said housing consisting of
5. a lower or bottom component 14 and a cover or closure 16.
The bottom component 14 of the housing may be fabricated
by injection molding from suitable synthetic plastic
materials such as polyethylene and, as will appear further
hereinbelow, is specifical.Ly designed to receive a flat
10. co-planar filter 20.
The closure 16 overlies the filter 20 and is
: secured to the housing by pressure-sensitive adhesive or
other adhering means and incorporates an application port
~ or openina 22 and a reaction port or opening 24, said ports
: 15. communicating, respectively, with the application zone 26
and the Eeaction zone 28~of the filter.
The test device 10 is designed for the performance
of a sinqle test, but, as best shown in FI~,. 2 of the draw-
ings,~a device 30 incorporating a multiplicity of application
20. ports 22 and reaotion ports 24 can be provided in a closure
or cover 32 which overlies a suitabl~ configured, as will
be explained in greater detail below, lower housing portion 34
and filter 36. The ports 22 and 24 respectively overlie
application and reaction zones~38fand 40 of the filter 36.
.
--8--
131~87~
The filters 20 and 36 are constituted by a planar
glass fiber matrix which is sandwiched be~tween the lower
component of the housing and the cover therefor.
As best shown in FIG. 3 of the drawings, the
5. filter 36 includes a plurality of application zones 38
. and reaction zones 40 which are maintained in fluid
.. ..
communication by separation zones 42. The separation
zones 4~ are of elongate configuration and establish
fluid communication between the application zones 38 and
lO. the reaction zones 40.
The application zon~s 36 are shown as being
roughly trapezoidal in configuration and provide a rela-
tively large area for the application of the test sample.
For a purpose which wlll be expl~ined hereinbelow, the
15. separation zones 42 are of relatively restricted width in
comparison with the width of the application zones 38.
Located in fluid communication with the appli-
cation zones 38 is first absorbent means 44 constituted
. by an elongated strip 46 of absorbent material. Similarly,
20. a second absorbent means 48 constituted by an elongated
strip 52 is disposed in fluid communication with the
multiplicity of reaction zones 40 provided by the filter 36
.
:
1 31 7876
. The lower or bottom portion 34 of the.housing
of the test device 30 is configured, as best shown in
FIGS. 4 and 5 of th~ drawings, to provide receptacles 55
for the application, separation and reac1:ion zones o.f
5. the filter 36 so that fluid flow is confined in the plane
of the filter 36 because of the sandwich created between
the closure 32 and the lower portion 34 of the ho~sing.
The receptacles are defined bv integrally molded lobes 58
in the body of the lower por~ion 34 of the housing and
lO. stringently confine the relevant portions of the filter
in the receptacles 56.
Juxtaposed to the receptacles 56 is a first
elongated rectangular well 60 for the reception of the
first absorbent means 44, and a corresponding well 62
15. is provided for the reception of the second absorbent
means 48. ~
The closure or cover 32 of the device 30 can
be fabricated from vinyl or other plastic sheet material
and may be adhesively or otherwise secured to the bottom
20. portion 34 of the housing of the test device 30.
The application, separation and reaction zones
are contiguous w;ith-in the co-planar surfaces of the glass
fiber matrix. A sample or samples applied to the sample
application zones 38 will mlgrate laterally by capillary
--10--
1317876
and chromatographic action. As will be described herein-
~elow in greater detail, the fundamental result achieved
by the test devices constructed in accordance with the
teachings of the invention is bilateral flow of the fluid
5. component of suspensions applied to the application zones 38.
During the bilateral migration, particulate matter
present within the sample volume, i.e., cellular components
of whole blood, salt crystals of urine or protein aggregates
of serum or plasma, etc., are filtered from the fluid portion
10. of the applied sample by particle size exclusion dictated
by the mean pore size of the glass fiber matrix. Since the
mean pore size of the glass fiber matrix is not an absolute
value, but, rather, represents a Poisson distribution of a
range of pore sizes, the length and width of the separation
15. zone will be influenced and dictated by the mean porosity
of the glass fiber matrix. Likewise, since the mean
diameter o particulates within the sample will vary, a
separation gradient will be realized within the body of the
separation zone, with larger particulates remaining closer
20. to the application zone, while smaller particulates will
mlgrate some distance from the application zone.
Therefore, the length and width of the separation
zone between the reaction and sample application zone~ must
1317876
be carefully established empirically in order to position
the reaction zone at a proper distance from the sample
application zone to prohibit an inhibitory quantum of
particulates from entering the reaction zone. If the
5. separation zone length is too short, some particulates may
enter the reaction one; if too long, the volume of filtered
sample fluid containing the desired analyte to be detected
may be insufficient for optimal detection.
Bilateral mi~ration of the fluid portion of the
lO. applied sample is also channeled in a direction l80 degrees
away from the separation zone and, subsequently, the
reaction zone, by the tapered constriction in the lateral
boundaries of the trapezoidally-shaped glass fiber matrix.
This constriction of the glass fiber matrix favors migra-
15. tion of the sample through the separation zone in thedirection of the reaction zone, yet still allows for some
migration Qf fluid awa~ from the separation and reaction
zones~ facilitating removal of unwanted or interfering
debris ~particulates, protein aggregates, unreacted test
20. reagents) from the reaction zone upon subsequent applica-
tion of wash solution and/or test reagents to the reaction
zone. In essence, this design functions as a safety valve
and reduces or eliminates back-washing of unreacted
1317~76
components into the reaction zone which may cause high
background gignals.
Sandwich relationship between the filter 36 and
the cover 32 with the bottom portion 34 of the housing of
5. the filter and with the associated ahsorbent means 44 and
48 is illustrated in the cross-sectional view of FIG. 5,
which, of course, is equally applicable to the test device
of FIG. 1, as well as the test device of FIG. 2. It will
be noted rom the showings of F~GS. 3 and 5 that a portion
10. of each application zone 38 is in fluid communication with
the correspondiny first absorbent means 44, as best shown
at 66 in FIGS. 3 and 5 of the drawings. Consequently, the
absorbent means 44 is in fluid communication with the
application zones 38 and causes a bilateral flow of fluid
15. from the sample being applied to the application zones
simultaneously with flow in the opposite direction from
the application zones 38 into the separation zones 42.
The bilateral flow through the separation zones 42
is facilitated by the location of the reaction zones 40
20. adjacent to a relatively large area of the filter 36,
shown, in the particular embodiment of the test device,
as generally rectangular in configuration and overlying
the second absorbent means 48.
1 3 1 7876
Consequently, bilateral flow established in this
manner reduces the hydraulic pressure in ~he application
zones 38 and causes rapid settling of par.ticulates or other
inclusions in the sample suspension, thus causing rapid
5. settling out of the particulates or othex detritus before
reaching the reaction zones 40.
It will also be noted that the secona absorbent
means 48 is of much larger dimensions than the absorbent
means 44, causing more rapid absorption of the excess ~luid
10. of the sample and causing the accentuation of the bilateral
flow phenomenon achieved b.y the ~ilter design and its
association with the first and second absorbent means.
It will be readily apparent to those skilled in
. the art that the configuration of the application zones 38
15. can be readily altered to accommodate the needs of the
particular samples being tested by the devices 10 and 30
and, furthermore, as specified hereinabove, the length and
width of the separation zones be empirically established
to con~orm to the bilateral flow patterns to be established
20. ~or the particular sample.
Moreover, the relativ~ dimensions and depth of the
absorbent means 44 and 48 can be altered to establish qreater
or lesser fluid communication between the application zones 38
and reaFtion zones 40, respectively.
-14-
1 3 1 7~76
A typical glass fiber matrix filter has its
source in Eaton-Dikeman Division of Filtration Sciences,
Mount Holly Springs, Pennsylvania. The weight is 71 gm/m2;
the depth is 0.43 mm; the mèan pore size is 0.6 micron (u~;
5. the mean fiber diameter is 0.7u ~0.25u to 1.5u); and the
composition is borosilicate glass.
The dimensions of the application zone are 8 mm
; in diameter, and the separation zone 4 mm X 9mm.
These dimensions are suitable to effect se~aration
10~ of cells, protein aggregates or other debris from a 30-40 ul
sample of human whole blood or serum a~plied to the sample
application zone followed by a wash volume of S0-60 ul
applied to the same. Modification of the preferred embodi-
ment is indicated i~ the whole blood sample is from an
15. animal other than a human, such as equine or bovine whole
blood, which generally has smaller red blood cell diameters
(5.5u or 5.9u, respectively) than human (6.9u-8.1u). In
this instance, a glass ~iber matrix of a smaller pore size
would be desirable, or, alternately, a longer or shorter
20. separation zone may be re~uired.
` Although the filters 20 and 36 are described as
fahricated in accordance with the previously set forth
specifications, it will be obvious to those skilled in the
.
--15--
`
1 3 ~ 7876
art that the filter means can be made of any porous material
capable of drawing liquid through its structure by capillary
action. The pores of the filter matrix should/ obviously,
be sufficiently small to accomplish filter separation of the
5. insolubilized components of the test sample from solubilized
components.
The filter may be composed of such materials as
glass fiber filter paper, nitrocellulose,-plastic, synthetic
polymer, cellulose, cellulose acetate, and various other
10. equivalent materials having the qualities and characteristics
described hereinabove.
Of course, it is desirable to utilize materials
which are inert and chemically non-reactive with the analytes
and washing solvents with which the test device lS to be
15. utilized.
The test devices 10 and 30, of course, have their
respective reaction zones 28 and 40 treated with specific
analyte reactants. ~ocalized regions o~ the respective
filters 20 and 36 are treated to provide the reaction zones
20. 28 and 40 to prepare the test devices 10 and 30 for use
with a predetermined test specimen without any preparatory
addi~ions to the test devices. For example, a binding
protein could be placed in the reaction zones to which an
antibody is bound, which antibody is im~unologically
25. reactivé with a specific antigen.
-16-
1 31 787~
Consequently, when a specimen is applied to the
application zones 26 and 3~ throu~h the application ports 22,
the fluid component of the suspension is immediately sub~ected
to the bilateral action achieved by ~he specific construction
5. of the test devices alluded to hereinabove.
The absor~ent material utilized in the first and
second absorbent means 44 and 48 may be of any suitable
material, such as hydrophilic polymers, particulate absor-
bents, glass fiber, carbon fiber, cellulose iber, wood pulp
10. or sponge material.
As previously mentioned, the size and shape of the
respective absorbent means 44 and 48 is dictated by the
volumetric considerations apDlicable to the specific test
for which the test devices 10 and 30 are designed, and
15. corresponding diminishment or enhancement of the absorptive
capacity of the first and second absorbent means 44 and 48
result from empirical calculations of the needs for the
establishment of greater or lesser bilateral flow of the
fluid components of the test specimen.
20. Method of the Invention
In practicing the method o the invention, a
suitable volume of sample is applied directly to the sample
application zone of the glass fiber matrix. A suitable
:
:
-17-
1317~76
volume of wash reasent is then applie~ to the same area of
the sample application zone.
Capillary and chromatographic forces within the
body of the glass fiber matrix draw the fluid portion of
5. the sample primarily in the direction of the separation
zone but, also, secondarily in the opposite direction.
The bilateral flow is defined by the lateral boundaries
of the glass fiber matrix and the fluid communication of
the application and reaction zones with their respective
10. absorbent means. As the fluid migrates through the separa-
tion zone, particles larger than the mean pore size of the
glass fiber matrix are restricted in their lateral migration
toward the reaction zone, so that only the fluid portion of
the sample reaches and flows into and through the reaction
15. zone. The analyte, contained within the fluid portion o
the sample reacts and binds with the specific complimentary
.
immuno-reagent (antigen or antibody) or chemical test
reagents, which have been immobilized to the glass fiber
matrix in the area of the reaction zone.
20. Subsequently, a suitable volume of wash reagent
is applied directly to th~ ~ This washes away
.... ,, ~. _
unreacted sample components which may interfere with subse-
quent steps, in bilateral directions, again defined by the
'
'
-18-
1317876
lateral boundaries of the glass fiber matrix, the directions
being 1) away from the separation and sample application
zones, and 2) toward the separation and sample application
zones, reversing the original direc~ion of flow.
5. Wash in the latter direction inhibits or prevents
previously filtered particulates from reaching the reaction
zone and actually acts as a "counter current" to back flush
potential interfering particulates present in the original
sample away from the reaction zone. The test analyte
10. present in the fluid portion of the sample is bound to the
complimentary ~m~no-reagent or chemical test reagent
immobilized to the glass fiber matrix at the reaction zone
site.
Subsequently, an immuno-reagent, or chemical test
15. reagent in the case of a biochemical test, complimentary
to the test analyte, conjugated with an enzyme or other
suitable tracer, such as a radionuclide or fluorescent dye,
is applied directly to the reaction zone. Unbound immuno-
reagent conjugate or chemical test reagent is washed from
20. the reaction zone in the lateral bi-directional mode
outlined above by the application of a suitable wash volume
applied directly to the reaction zone~
--19--
~;;
1 31 7876
Sequentially, a suitable substrate or chromogen
is added to the reaction zone. I the analyte was pre.sent
in the sample, it will be sandwiched between the immobilized
and enxyme conjugate immuno-reagents within the reaction
5. zone. The enzyme conjugated to the analyte bound immuno-
reagent acts upon the substrate or chromogen to produce a
colored product within the reaction zone which may be
viewed or measured with an instrument.
Exem lar Filter Construction and
P Y__
10. Methods of Utilizing Same
Example 1. Detection of Antibody to Rubella
Virus in Whole Blood
Inactivated Rubella virus antigen is immobilized
onto the reaction zone of the glass fiber matrix. This is
15. followed by the addition of a blocking agent such as 1.0
- bovine serum albumin or 0.5~ non-fat milk suspension to
the same area and allowed to dry.
The use of a blocking agent decreases the non-
specific binding of extraneous proteins present in the
20. fluid (serous) portion of whole blood to the reaction zone
of the glass fiber matrix.
-20-
1 31 787h
To perform an assay, approximately 30 microliters
of whole blood is applied to the sample application zone
of the device. This is followed by 60 microliters of a
wash solution consisting of 0.53 non-fat milk in a phosphate
5. buffered saline applied to the same area. Migration of the
whole blood sample through the separation zone will filter
and separate the cellulax components from the sample within
the area of the separation zone. Evidence of separation
of the fluid portion of the whole blood sample is observed
lQ. in the reaction zone by the appearance of serous fluids
wetting the reaction zone area.
60 microliters of the wash solution is then
applied to the wetted reaction zone. Upon absorption, the
.., ,. _ . . ...
wash step is repeated. It will be noticed that the serous
lS. components and pigments contained therein will be eliminated
via the lateral bi-directional mode described earlier from
the reaction zone by this wash procedure.
However, if the whole blood specimen contains
antibodies to the Rubella virus, the antibodies in the
20. serous portion of the blood sample will bind to the Rubella
virus antigens immobilized within the reaction zone of the
glass fiber matrix. Thenl 60 microliters of an affinity
purified rabbit anti-human IgG alkaline phosphatase con~ugate
-21-
1317876
is applied to the reaction zone. This will bind to the
antibody of the Rubella virus which may be present in the
blood sample and will be trapped by the immobilized an~igen
located in the reaction zone of the glass fiber matrix.
5. Unreacted en2yme conjugate is washed away as described above.
Finally, 60 microliters of a suitable`substrate
chromogen may be applied to the reaction zone. Appearance
of a colored product at the reaction zone is evidence of
enzyme activity and, therefore, indicative of antibody to
10. Rubella virus present in the whole blood sample.
Example 2. Determination of Human
Choriogonadotropin in Urine by
_ a "Sandwich" Technique
A polypeptide hormone, human choriogonadotropin
15. (HCG), is secreted into the maternal circulatory system by
the trophoblasts of the developing fetus. Ultimately, this
hormone is excreted in the maternal urine. Detection of
~CG in the urine is presumptive evidence of pregnancy. HCG
is collected, concentrated and purified by well known pub-
20. lished methods. The purified hormone may be used to generateantibodies (polyclonal or monoclonal) in the appropriate
species, i.e., rabbits or mice, respectively.
-22-
1 31 7~76
The antibody of HCG is immobilized to the reaction
zone of the glass fiber matrix. A blocking protein is then
applied to the reaction zone as described in the previous
example.
5. To determine if a urine specimen contains HC~7, a
few drops of the specimen are applied to the sampl~ appli-
cation zone of the glass fiber matrix of the device. This
is followed by a sufficient volume of a wash solution
applied to the same area to cause the sample to migrate
10. throu~h the separation zone towar~ and through the reaction
zone of the glass fiber matrix which contains the immobi-
lized antibody to HCG. If HC~, is present in the sample, it
will ~ind to the i~mobilized antibody located within the
reaction zone.
15. Alternately, sufficient volume of urine may be
applied to the sample application zone to cause the sample
to chromatograph through the reaction zone without the use
of a wash. In either case, migration of the urine sample
through the separation zone will filter out urine particu-
20. lates which may interfere in subsequent testing steps.
Then, a suitable volume of a washing solution is
applied to the reaction zone. Lateral, bi directional flow
of the wash solution will carry unreacted urine components
away from the reaction zone, i.e., away from the separation
-23-
1317876
and sample application zones as well as toward the separa
tion and sample application zones, reversing the original
dixection of flow. Movement toward the reaction zone of
wash fluid in the latter dixection prohibîts further mo~e-
5. ment of unwanted particulates by counter flow forces.
Indeed, subsequent addition of any wash or test reagent to
: the area of the reaction zone will force any trapped
particulates or debris located within the separation zone
away from the reaction zone.
lO. Applicatian to the reaction zone of an appropriate
enzyme labeled antibody to HCG (either polyclonal or mono-
clonal~ will bind to the HCG of the sample which has been
trapped by the immobilized antibody bound to the reaction
zone of the glass fiber matrix. Again, a wash solution is
15. applied, as indicated above, to wash away, in a lateral,
bi-directional mode, any unreacted enzyme conjugated anti-
body.
Subsequent addition of a suitable substrate
chromogen solution to the reaction zone will indicate the
20. presence of enzyme and, therefore, the presence of HCG, by
: the development of a colored product at the reaction zone.
The method described above in this example is typical of a
"sandwich technique", whereby the analyte, HC~. in this
- .
-24-
~317876
case, is sandwiched between two antibodies, one immobilized
to the glass fiber matrix of the reaction zone, the other
- conjugated to an enzyme or other suitable label. The
presence of the HCG analyte is indicated by the development
5. of color within the reaction zone.
Example 3. Determination of Human
Choriogonadotropin in Urine
by Competitive Inhibition
Immunoassa
Y
10. The test devices of the invention are not limited
to "sandwich" methodoLogy, but ma~ be applied to aompetitive
inhibition techniques as described by the following example.
Antibody immobilization, sample application and washing
methods and separation/chromatographic principles are as
15. described in the prevlous example. However, instead of
application of an antibody enzyme conjugate, one may apply
to the reaction zone an enzyme conjugate of the analyte,
i.e., HCG coupled to an appropriate enzyme.
If HCG is present in the sample~ it will bind to
20. a finite and limited number of available antibody binding
sites located and lmmobilized within the reaction zone of
the glass fiber matrix. If the sample contains substantial
amounts of HCG, then all available antibody binding sites
in the reaction zone will be saturated.
-25-
1317876
Upon subsequent application of an enzyme conjugated
to HCG (instead of enzyme conjugated to an anti-HCG antibody),
all available immobilized antibody binding sites are saturated
with the HCG from the sample and will not bind to the enzyme-
5. HCG conjugate. When a sui~able wash solution is applied,the enzyme-HCG conjugate will be washed away from the reaction
zone in a lateral, bi-directional fashion.
Application of a suitable substrate chromo~en
solution to the xeaction zone will not develop a color in
10. this ins~ance sinca no enzyme is available. If, howev~r,
the sample contains no or insufficient quantities of HCG, to
saturate all immobilized antibody bindin~ sites, then HC~
enzyme conju~ate will bind to the available immobilized
HCG binding sites and will not be washed away with subse-
15. quent washing steps.
Therefore, in this instance, upon subsequentapplication of a suitable substrate chromogen solution to
the reaction zone of the glass fiber matrix, some color
will develop, indicating the sample had little or no HC&
20. present. In a competitive inhibition assay as just des-
cribed, the absence of color development in the reaction
zone is indicative of the presence of the analyte (HCG) in
the sample, while~he presence of color development in the
-26
1317876
reaction zone indicates little or no analyte (HC~.) in the
sample. The device can also be used for competitive immuno-
assays of low molecular weight analytes, such as thyroid
hormones, therapeu~ic drugs, steroids and other low
5. molecular weight analytes.
Example 4. Detection of Glucose in
Whole Blood
__ _ _ _ _ _
The device may be used to perform assays to
indicate the presence or quantitation of analytes without
10. employing immunological methods and principles. For
example, one may detect the presence of glucose in whole
bLood by standard enzyme analytical techniques. In this
instance, a mixture of the enzymes glucose oxidase and
~ horseradish peroxidase is immobilized to the reaction
15. zone of the device. Whole blood is then applied to the
sample application zone.
A suitable wash solution is then applied to the
sample application zone to effect the bi-directional lateral
chromatographic separation of the fluid portion of the
20. sample ~rom the ce1lular components as described previously
to introduce the fluid portion containing glucose into the
reaction zoneO The immobilized oxidase acts upon the
giucose of the sample to produce D-glucono-3-lactone and
hydrogen peroxide.
-27-
~ 3 1 7876
The horseradish peroxidase, also i~mobilized
within the reaction zone, catalyses the hydrogen peroxide
in situ as it is generated. Subse~uent addition to the
reaction zone of a suitable chromogen test reagent will
5. react with the products of catalysis to produce a colored
product, the intensity of which is proportional to the
amount o glucose present in ~he original sample. The
intensity of color development may be observed visually
or detected by the use o~ suitable instrumentation. It
10. is evident from this example that the device of the
invention accomplishes other than immunoassays with equal
efféctiveness.
It will be readily apparent that the utilization
of the test devices manufactured in accordance with the
~; 15. teachings of this invention provides both more effective
and less time-consumin~ testing of various suspensions in
the field by relativel~ inexperienced personnel. ~he
bilateral mi~ration of the flui~ components of the various
samples applied to the application zones attributable to
20. the unique construction of the test devices prevents the
contamination of the reaction zones by the partlculates
in the suspension samples and also facilitates the migra-
tion of the fluid component of the sample to the reaction
zones.
~`
-2~-
1 3 1 7876
It is also contemplated by the invention that a
plurality of test devices manufactured in accordance with
the teachings of the invention and incorporating single
application, separation and reaction zones may be snapped
5. together or otherwise associated on a mounting board or
the like to permit a series o different tests to be
accomplished by juxtaposition of the single test devices.
It will be obvious to those skilled in the art
that various modifications of the test devices of the
10. invention may be made without departing from the sco~e of
the claims.
.
,
-29-