Note: Descriptions are shown in the official language in which they were submitted.
CYANIDE RECOVERY PROCESS
Field of the Invention
The present invention relates cyanide removal and
recovery from cyanide-containing mixtures.
5 Background of the Invention
Cyanides are useful materials industrially and have
been employed in fi~lds such as electro-plating and
electro-winning of metals, gold and silver recovery from
ores, treatment of sulfide ore slurries in flokation,
10 tannery processes, etc. Due to environmental concerns,
it is desirable to remove or destroy the cyanide present
in the waste solutions resulting from such processes.
Additionally, in view of the cost of cyanide, it is
desirable to regenerate the cyanide for reuse.
Techniques for cyanide disposal or regeneration
(recovery) in waste solutions include: ion exchange,
oxidation by chemical or electrochemical means, and
acidification-volatilization-reneutralization (AVR).
The term cyanide recovery and regeneration are used
2 0 interchangeably herein .
U. S . Patent No. 4, 267 ,159 by Crits issued May 12,
1981, discloses a process or regenerating cyanide in
spent aqueous liquor by passing the liquor through a bed
of suitable ion exchange resin to segregate the cyanide.
U. S . Patent No . 4,708,804 by Coltrinari issued
November 24, 1987, discloses a process for recovering
cyanide from waste streams which includes passing the
waste stream through a weak base anion exchange resin in
order to concentrate the cyaxlide. The concentrated
cyanide stream is then subj ected to an
acidification/volatilization process in order to recover
the cyanide from the concentrated waste stream.
U . S . Patent No . 4,312,760 by Neville issued
January 26, 1982, discloses a method for removing
cyanides from waste water by the addition of ferrous
bisulfite which forms insoluble Prussian blue and other
reaction products.
U.S. Patent No. 4,537,686 by Borbely et al. issued
August 27, 1985, discloses a process for removing
cyanide from aqueous streams which includes the step of
oxidizing the cyanide. The aqueous stream is treated
with sulfur dioxide or an alkali or alkaline earth metal
sulfite or bisulfite in the presence of excess oxygen
and a metal catalyst, preferably copper. This process
is preferably carried out at a pH in the range of 5 to
12.
U.S. Patent No. 3,617,5~7 by Mathre issued
November 2, 1971, discloses a method for destroying
cyanide anions in aqueous solutions using hydrogen
peroxide (H202) and a soluble metal compound catalyst,
such as soluble copper, to increase the reaction rate.
The pH of the cyanide solution to be treated is adjusted
with acid or base to between 8.3 and 11.
Treatments based on oxidation techniques have a
number of disadvantages. A primary disadvantage is that
no cyanide is regenerated for reuse. Additionally,
reagent costs are high, and some reagents (e.g. H202)
react with tailing solids. Also, in both the Borbely et
al and Mathre processes discussed above, a catalyst,
such as copper, must be added.
U.S. Patent No. 3,592,586 by 5cott issued July 13,
1971, describes an AVR process for converting cyanide
wastes into sodium cyanide in which the wastes are
heated and the pH is adjusted to between about 2 and
about 4 in order to produce hydrogen cyanide (HCN). The
HCN is then reacted with sodium hydroxide in order to
form sodium cyanide. Although the process disclosed in
the Scott patent is described with reference to waste
produced in the electro-plating industry, AV~ processes
have also been applied to spent cyanide leachate
resulting from the processing of ores. Such spent
--2--
cyanide leachate typically has a pH greater than about
10.5 prior to its acidification to form HCN.
AVR processes employed in the mineral processing
field are described in the two volume set nCyanide and
the Environment" (a collection of papers from the
proceedings of a conference held in Tucson, Arizona,
December 11-14, lg84) edited by Dirk Van Zyl,
nCyanidation and Concentration of Gold and Silver Ores,"
by Dorr and Bosqui, Second Edition, published by McGraw-
Hill Book Company 1950, and "Cyanide in the Gold MiningIndustry: A Technical Seminar, n sponsored by
Environment Canada and Canadian Mineral Processor,
January 20-22, 1981. Another description of an AVR
process can be found in "Canmet AVR Process for Cyanide
Recovery and Environmental Pollution Control Applied to
Gold Cyanidation Barren Bleed from Campbell Red Lakes
Mines Limited, Balmerton, Ontario," by Vern M. McNamara,
March 1985. In the Canmet process, the barren bleed was
acidified with H2SO4 to a pH level typically between 2.4
and 2.5. SO2 and H2SO3 were also suitable for use in
the acidification.
AVR processes take advantage of the very volatile
nature of hydrogen cyanide at low pH. In an AVR
process, the waste stream is first acidified to a low pH
(e.g. 2 to 4) to dissociate cyanide from metal complexes
and to convert it to HCN. The HCN is volatilized,
usually by air sparging. The HCN evolved is then
recovered, for example, in a lime solution, and the
treated waste stream is then reneutralized. A
commercialized AVR method known as the Mills-Crowe
method is described in Scott and Ingles, nRemoval of
Cyanide from Gold Mill ~ffluents," Paper No. 21 of the
Canadian Mineral Processors 13 Annual Meeting, in
Ottawa, Ontario, Canada, January 20-22, 1981.
A process using AVR to recover cyanide values from
a liquid is described in Patent Cooperation Treaty
--3--
~ 3 ~
application PCT/AU88/00119, International Publication
No. W088/08408, of Golconda Engineering and Mining
Services PTY. LTD. The disclosed process involves
treating a tailings liquor from a minerals extraction
plant by adjusting the pH into the acid range to cause
the formation of free hydrogen cyanide gas. The liquid
is then passed through an array of aeration columns
arranged in stages so that the liquid flowing from one
aeration column in a first stage is divided into two or
more streams which are introduced into separate aeration
columns in successive stage~. In a recent paper
describing the process, it was stated that plant
shutdown would occur if pH went above 3.5. In a
commonly assigned application, PCT/AU88/00303,
International Publication No. W089/081357, a process for
clarifying liquors containing suspended solids is
disclosed. The feed slurry is acidified to a pH of 3 or
lower. Flocculants are added to cause the formation
flocs to enable the separation of the suspended solids
from the liquor. The clarified liquor can then be used
as a feedstock for the AVR process disclosed in the
other commonly assigned application.
The AVR processes described in the Scott patent and
the above-mentioned texts typically include the step of
adjusting the pH of the spent cyanide stream to within
the range from about 2 to about 4. There are several
problems with such processes. These AVR processes are
expensive due to the amount of acidifying agent required
to lower the pH to within this rangP. Also, such
processes require a substantial amount of base to
reneutralize the waste stream after the ~olatilization
of HCN and prior to disposal. Further, insoluble metal
complexes form at the acid conditions employed in these
processes. The above-mentioned references only disclose
a treatment of barren bleed which typically results from
Merrill-Crowe type cyanidation treatment of ore. This
--4--
~ 3 ~ 3
bleed does not contain solid tailings. Today many ores
are treated by a carbon-in-leach or carbon-in-pulp
cyanidation process. The tailings Prom such processes
include the solid barren ore in the spent leachate.
Typically the tailing slurries contain about 30% to 40~
by weight solids and about 100 to 350 parts per million
~ppm) cyanide. In the past, such tailings were
typically impounded and the cyanide contained therein
was allowed to degrade naturally. Due to environmental
concerns about cyanide, such impoundment is not a
desirable alternative in many situations. Therefore,
it is often necessary to treat the material in some
manner to decompose the cyanide. This is expensive due
to the costs associated with the treatment, as well as
the loss of cyanide values which results.
Therefore, it would be advantageous to remove
cyanide from a cyanide-containing waste stream in an
economical manner. Further, it would be advantageous to
provide a process for treating cyanide-containing
slurries which also contain ore tailings. It would be
advantageous if the amount of cyanide present in the
waste stream could be reduced. It would also be
advantageous to regenerate the cyanide for reuse.
It has now been found that when the HCN is
volatilized at pH ranges higher than those previously
employed, significant advantages are achieved. For
example, cost savings can be realiæed due to the reduced
amounts of reagents required to acidify and subsequently
raise the pH of the waste stream. Additionally, many
insoluble complexes which form under strong acid
conditions will not form in the pH range employed in the
present process. Further, the higher pH avoids or
minimizes scaling, for example, by calcium sulfate
and/or metal thiocyanates such as copper thiocyanate.
The pH ranges successfully employed in the present
invention are possible because the present invention is
-5-
~ 3 ~
preferably conducted on fresh carbon-in-pulp (CIP) or
carbon-in-leach (CIL) tails. In contrast, previous
acidification-volatilization-reneutralization (AVR)
processes were employed on decant water or on barren
bleed from Merrill-Crowe gold cyanidation processes. In
the present process, much of the cyanide in the waste
stream is in ionic form or only weakly complexed,
whereas in barren bleed there is significant complexing
including insoluble and strongly complexed forms.
Therefore, previous AVR processes optimized the acidic
precipitation of some of the metallo-complexes in order
to deal with such precipitates separately. Use of the
instant method for treating cyanide-containing slurries
has additional advantages when used in combination with
a CIL or CIP process. Recycling recovered cyanide and
the low levels of effluent cyanide permits higher
cyanide levels to be used in the leaching process which
provides higher recoveries of precious metal values.
Summary of the Invention
In accordance with the present invention, a process
is provided for regenerating cyanide from a cyanide-
containing mixture. The process includes the steps of:
(1) adjusting the pH o~ the cyanide-containing mixture
to between about 6 and about 9.5, ~2) volatilizing the
hydrogen cyanide (HCN) contained in the pH adjusted
mixture, and (3) contacting the volatilized HCN with
basic material.
In another embodiment, the instant invention
involves a process for regenerating cyanide from
alkaline, cyanide-containing solution while minimizing
equipment fouling due to solids precipitation. The
method comprises (a) adjusting the pH of the cyanide-
containing solution to between about 7 and about 9.5 to
provide a pH adjusted solution; (b) passing a gas
through the pH adjusted solution to remove HCN from the
--6--
r~ ~ ~
pH adjusted solution and form a HCN-gas mixture; and (c)
contacting the HCN-gas mixture with an aqueous alkaline
solution to form a cyanide-containing solution.
In another embodiment, the instant invention
comprises an apparatus for regenerating cyanide values
from an alkaline, cyanide-containing slurry. The
apparatus comprises a zone for adjusting the pH of the
slurry to a pH of between about 6 and about 9.5 to for~n
a pH adjusted slurry. An HCN volatilization zone is
adapted to receive the pH adjusted slurry and contact
the slurry with a volatilization gas to Porm a HCN-gas
mixture. A cyanide recovery zone is adapted to receive
the HCN-gas mixture and contact the mixture with a basic
material to form a cyanide salt.
In an another embodiment the instant invention
involves an improved method for recovering metal values
from an ore. The method involves leaching the ore with
a cyanide-containing solution at a pH of at least about
to provide a cyanide-containing slurry having
dissolved metal values. The cyanide-containing slurry
is contacted with activated carbon to load the carbon
with the dissolved metal values. The loaded carbon is
separated from the slurry to form a barren slurry having
reduced dissolved metal values. The pH of the barren
slurry is adjusted from above about 10 to between about
6 and about 9.5 to provide a pH adjusted slurry. A
volatilization gas is passed through the p~ adjusted
slurry to form a HCN-gas mixture. The HCN-gas mixture
is removed from the pH adjusted slurry and contacted
with a basic solution to form a cyanide-containing
solution. The cyanide-containing solution is then
returned to the leaching step.
Brief Description of the Drawings
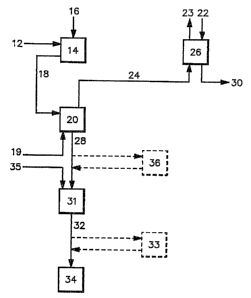
Figure 1 is a block diagram of one embodiment of
the present invention.
-7-
~ 3 ~
Figure 2 illustrates a preferred embodiment of the
cyanide recovery process of the present invention.
Figure 3 illustrates a carbon-in-leach process in
combination with the cyanide recovery process.
Figure 4 illustrates a carbon-in-pulp process in
combination with the cyanide recovery proc~ss.
Detailed Description of the Invention
The present invention concerns a process for
regenerating cyanide from cyanide-containing waste
streams. Tha process is preferably performed on
tailings slurries resulting from mineral recovery
processes, e.g. gold recovery processes employing
cyanide leach solutions, such as vat leach, carbon-in-
leach, and carbon-in-pulp processes. Such tailings
slurries typically have a pH of greater than about 10,
contain about 25% to 40% by weight solids and about 10
to 1000, more typically 100 to 600 ppm cyanide.
The recovery of cyanide from slurries is
advantageous for a number of reasons. Elimination of
sedimentation or clarification steps reduces both
capital and operating costs for the process. The
recovery of cyanide can significantly reduce operating
costs and the hazards associated with the manufacture,
transport and storage of the reagent. Reduction of the
total and weak acid dissociable (WAD) cyanide content
entering the tailings impoundment minimizes the toxic
effects of cyanide on wildlife and significantly reduces
the potential for generation of leachate containing
unacceptable levels of metals and cyanide. The
requirement for installing a lining in the tailings
impoundment can be eliminated for many applications.
The reduction of total cyanide to asceptable levels in
mine backfill can eliminate the need for wash plants in
some circumstances. The reduction of total cyanide and
metals concentration in the decant water and associated
-8-
cyanide waste waters significantly decreases the costs
while increasing the reliability and performance of
downstream treatment processes. The generation of
undesirable treatment byproducts such as ammonia and
cyanate can be minimized thereby reducing significant
capital outlays required for treatment of such
materials. Additionally, the recovery and recycle of a
substantial amount of cyanide from mineral recovery
streams particularly from vat leaching, CIL and CIP
tailings permits higher levels of cyanide to be used in
the leach resulting in higher and more rapid recovery of
precious metal values.
The cyanide f~ed streams from minerals recovery
processes are typically at a pH above 9 and normally
above 10. A first step in the cyanide recovery process
involves adjusting the pH of the stream of the cyanide-
containing mixture being treated to between about 6 and
about 9.5, more preferably between about 7 and 9, and
most preferably to about 8. This can be accomplished
through the use of an acidifying agent. Using a near
neutral or basic pH minimizes problems associated with
an increase in sulfate and total dissolved solids
concentrations which can result in precipitation of
materials such as calcium sulfate. Proper adjustment of
the pH results in the formation of HCN in solution. The
HCN is volatilized, preferably by contacting with air.
The volatilized HCN is then contacted with a basic
material, preferably in a solution having a pH between
about 11 and 12, to convert the HCN to a cyanide salt.
The tailings remaining after the HCN volatilization
step can be further treated to remove remaining cyanide
and/or metals and metal complexes. Such optional
treatment can include metal coagulation, pH adjustment
of the tailings in order to precipitate metal complexes,
and/or further cyanide removal by known treatments such
~3~7~$
as oxidation (e.g. with H22 or S02~ and/or biological
treatments.
As a result of the process of the present
invention, treated ore tailings have a greater long-~erm
stability. Potentially toxic species (e.g. silver) will
be less likely to be mobilized because of the lower
cyanide concentration in the tailings pond. Discharge
concentrations can be lowered and management
requirements after mine closure reduced.
Previous cyanide recovery processes have used a low
pH precipitation step. This is to be contrasted with
the present process which instead uses a pH in the range
of about 6 to about 9.5. An advantage of using a near
neutral or basic pH is that the formation of solids,
such as calcium sulfate, is minimized which avoids
scaling and fouling of equipment. This can be
particularly important when packed towers are used to
volatilize the HCN. Another advantage is that the
higher pH reduces the amount of acid required to be
added to initially acidify the waste stream. The amount
of base required to subsequently raise the pH of the
treated stream is also reduced.
With reference to Fig. 1, a cyanide-containing
waste stream 12 is treated in a pH adjustment zone 14 in
2~ order to obtain a stream having a pH between about 6 and
about 9.5 and more preferably between about 7 and about
9 and most preferably about 8. A cyanide-containing
slurry stream from any minerals recovery process can be
used as a feed for the instant cyanide recovery process.
In a preferred embodiment, the cyanide-containing waste
stream is a tailings slurry from a vat leach which can
use a precipitation method such as with zinc to recover
metal values, or, a carbon-in-pulp or a carbon-in-leach
metal recovery process which tailings normally have a pH
above about 10 and normally in the range of about 10.5
to 11.5, a solids content of between about 20% and 50%
--10--
by weight, more typically 25~ to 40% by weight and about
100 to 6~0 ppm cyanide. Normally, it is not
advantageous to lower the pH of the feed to below about
6. Based upon dissociation constants, more rapid
recovery of free cyanide and weakly bound cyanide, e.g.,
NaCN and Zn(CN)2, can be accomplished at a pH in the
range of 4.5 to 8.5, whereas ~or a weak acid dissociable
(WAD) cyanide, a pH of about 4.0 is optimal. However,
it has been found that the instant process provides a
high recovery of the ionic cyanide and unexpectedly, a
substantial recovery of the WAD cyanide even at a pH of
6 or above. For the reasons set forth hereinabove, a
near neutral or basic pH of between about 6 and about
9.5, more preferably about 7 and about 9, is preferred
to minimize precipitation problems. Additionally, at pH
ranges below about 3 or 4, some metal complexes, e.g.
Cu(CN)2, will precipitate and subsequently resolubilize
when the pH is increased. The dissolution of metals
such as iron, copper, nickel, etc. is also minimized
when a pH of at least about 6 is used.
The cyanide-containing stream 12 is acidified in
zone 14 by adding an acidifying agent 16. The
acidifying agent 16 is preferably H2SO4, but other
mineral acids can be used such as hydrochloric acid,
nitric acid, phosphoric acid, H2SO3, mixtures of H2SO3
and SO2, etc. or organic acids such as acetic acid, as
well as mixtures of acids. The particular acidifying
agent of choice depends on such factors as economics,
particularly the availability of acidic streams from
other processes, and the composition of the stream being
treated. ~or example, if the stream contains materials
which are detrimentally affected by an oxidizing agent,
nitric acid would probably not be useful. A potential
problem which was anticipated prior to the reduction to
practice of the present invention was the formation of
CaSO4 precipitates upon addition of H2SO4 to slurries
7 ~ ~
containing ore tailings. Surprisingly, this problem ~as
not found to be as severe as originally anticipated and
sulfuric acid can be readily used in connectlon with the
packed tower embodiment set forth hereinbelow. The
function of the acidifying agent 16 is to reduce the p~
in order to shift the equilibrium from cyanide/metal
complexes to CN- and ultimately to HCN. By employing
higher pH ranges than those used in prior art AVR
processes, the amount of acidifying agent 16 re~uired is
substantially reduced and the other advantages set forth
hereinabove can be obtained.
A pH adjusted stream is then transferred 18 from
zone 14 to a volatilization zone 20 as shown in Fig. 1.
In the volatilization zone 20, HCN is transferred from
the liquld phase to the gas phase using a volatilization
gas 19. Air i5 a preferred volatilization gas although
other gases such as purified nitrogen can be used. The
gas can also provide the turbulence required. Air can
be introduced into the pH adjusted mixture in the
volatilization zone 20 by any method well known in the
art. For example, a diffuser basin or channel can be
used without mechanical dispersion of the air.
Alternatively, an air sparged vessel and impeller for
dispersion can be employed. Baffles can be arranged in
the vessel, e.g., radially, to assist in agitation of
the slurry. In other alternative embodiments, a
modified flotation device or a countercurrent flow tower
with a grid, a plurality of grids, packing, a plurality
of trays, etc., can be used.
Volatilization of HCN by gas stripping involves the
passage of a large volume of low pressure compressed gas
through the acidified mixture to release cyanide from
solution in the form of HCN gas. Alternatively, the
mixture can be contacted with the volatilization gas,
e.g. in a countercurrent flow tower.
-12-
When a stripping reactor is used, the pH adjusted
mixture is transferred 18 from the initial pH adjustment
zone 14 to the stripping reactor (volatilization zone)
20. Incoming volatilization gas 19 is distributed
across the base of the stripping reactor 20 using gas
sparger units designed to prevent any solids from
entering the gas pipework on cessation of gas flow.
Preferably, coarse to medium sized bubbles are used to
provide sufficient gas volume and to minimize clogging
of gas ports with materials such as clay. The resulting
stripping gas stream is continuously removed 24 from the
enclosed atmosphere above the slurry in association with
removal of the extracted gas stream 23 which is
positively withdrawn from the scrubber zone 26 by a
device such as a fan. When the volatilization gas is
air, the preferred flow is from about 250 to about 1,000
cubic meters of air per cubic meter of pH adjusted
mixture per hour, more preferably, about 300 to 800 and
most preferably, about 350 to about 700 m3/m3~ This
flow is maintained for a time sufficient to remove the
desired level of HCN. The time required to accomplish
this removal depends on the air flow rate, the slurry
feed rate, the slurry depth in the stripping reactor,
the pH and the temperature of the mixture. Normally,
the stripping can be accomplished in a period of about 2
to 6 hours. Preferably, a flow rate of about 300 to 800
m3/m3 is used which corresponds to a flux of from 2.8 to
7.4 cubic meters air per square meter of pH adjusted
mixture per minute, based on a period of 3 to 4 hours.
While the key function of air in the system is to
provide an inert carrier gas and transport, the air also
has secondary effects. The first is to provide energy
to overcome barriers to HCN transfer to the gas phase.
Although HCN is very volatile, having a boiling point of
about 26~C, it is also infinitely soluble in water, and
HCN solutions have a high degree of hydrogen bonding.
~13-
Thus, there are significant resistances to the mass
transfer of HCN that can be overcome by using the
sparged air to provide the necessary energy in the form
of turbulence. Furthermore, the dissociation
equilibrium constants for most of the metal-cyanide
complexes are low at the desired pH ranges; therefore,
it is necessary for the CN~ concentration to be close to
zero in order to push the equilibrium far enough toward
CN- formation in order to substantially dissociate the
complexes. This can be achieved by efficient formation
of HCN from CN-, which is pH dependent, and then by
removal of HCN from the solution, which is energy
dependent.
As indicated above, preferred retention time in the
volatilization zone 20 is from about 2 to about 6 hours
with a stripping reactor. In a stripping reactor, the
liquid height in the reactor is preferably less than
about 3 meters. This preferred depth is due to the
function of air in the system and the possibility of
bubble coalescence if the depth is greater than about 3
meters. The necessary retention time can be achieved by
using a single reactor or a plurality of reactors
arranged in parallel, in series or a combination, as is
appropriate for the particular feed stream and
throughput. For example, multiple trains of reactors
can be arranged in parallel with a plurality of
stripping reactors arranged in series in each train.
The stream of volatilized HCN and volatilization
gas is removed from zone 20 and transferred into a
cyanide recovery zone 26. The apparatus useful in the
cyanide recovery zone should pxovide effective mixing of
the basic material being added and the stream of
volatilized HCN. Suitable apparatus includes a gas
sparger, preferably in an agitated vessel, which can
provide effective contact of the HCN containing gas with
the basic solution. More preferably, a conventional
-14-
1 3 ~
packed countercurrent scrubber is used (126 shown in
Fig. 2). Basic material, preferably in solution, is fed
22 to the recovery zone 26. The recovery solution is
preferably at a pH of at least about 11 and preferably
between about 11 and about 12, in order to absorb HCN
gas. Any basic material capable of providing a solution
having the desired pH can be used. Examples of such
materials include sodium hydroxide, potassium hydroxide,
calcium hydroxide, lime, calcium carbonate, sodium
carbonate, etc. Calcium-containing materials are
generally not preferred because of the potential for the
formation of CaSO4 scale. Sodium hydroxide is generally
preferred. The basic cyanide solution 30 can be
recycled, e.g. to a mineral recovery process such as a
gold cyanidation process.
~ he treated tailings which remain in reactor 20
after the HCN volatilization step can be removed 28 and
contacted in zone 31 with alkaline material 35 to
readjust the pH upward to a range of about 9.5 to about
10.5 in order to precipitate metals. Generally lime,
limestone or lime water are preferred basic materials
due to cost. The resulting pH adjusted tailings 32 can
then be impounded 34. Optionally, prior to the pH
adjustment step 31, complexed metals can be coagulated
36 tshown in phantom) by methods known in the art, for
example using FeCl3 or ~MT, an organic sulfide,
available from DeGussa Corporation, or mixtures thereof.
Additional cyanide can also be removed 33 (shown in
phantom) from the pH adjusted tailings 32, for example
by known oxidation techniques, e.g. using H2O2 or SO2, or
by know b ological processes.
A preferred embodiment of the process for removing
and recovering cyanide values from a slurry is shown in
Fig. 2. The pH of an incoming mill tailings slurry 112
is adjusted downward from a pH of above about 10 to
between about 6 and about 9.5. This is accomplished in
-15-
~Trademark
,~
~ 3 ~
a sealed, agitated reactor vessel 114 normally in
approximately a 5 to 20 minute time period. The ves~el
114 should be constructed of mat2rials compatible with
the abrasive nature of this process. The acidifying
agent 116, prefera~ly the H2S04 shown, is normally added
in the form of an aqueous solution normally containing
about 10 wsight percent acid. Once the pH of the slurry
has been adjusted to the range of about 6 to 9.5, the pH
adjusted slurry is transferred 118 to the volatilization
section 120. Preferably, at least one packed tower is
used in which the slurry is passed in countercurrent
flow to the volatilization gas.
A packed tower useful in the instant process
normally has a means Eor distributing the slurry
substantially uniformly across the top of the packing
material. The means is located near the top of the
tower and above the packing medium. It is preferred
that the distributing means minimize interference
between the slurry and rising volatilization gas to
minimize the flow disturbance and provide an effective
distribution of the slurry over a substantial cross-
sectional area of the packing material. For example, a
multiple weir, V-notch assembly can be used. The
distributing means can be made of any suitable material
such as steel or ceramic. The tower can also be
equipped with a demister. The demister functions to
suppress or disperse aerosols and can be formed from a
fine screen or grid, glass wool or other porous media,
etc.
The packing material useful in the tower can be any
mass-transfer media which provides a high void ratio,
i.e., a high surface area to volume ratio (e.g. square
meter per cubic meter). Preferably, the void ratio is
above 50%, more preferably above 80% and most preferably
above 85%. The openings in the packing material must be
sufficiently large to allow free passage of the
-16-
~ 3 ~
particles contained in the slurry. The height of the
packing i5 typically 3 to 10 meters, more preferably 4
to 8 meters, most preferably about 6 to 7 meters
depending on the desired pressure drop.
To maximize efficiency of the process, it is
important to control the viscosity of the slurry
entering the packed tower. It has been found that
increasing the viscosity of the slurry within an
operative range improves the mass transfer and removal
of hydrogen cyanide from the solution. However, if the
viscosity is too high, flow of the slurry through the
packing can be affected with subsequent operating
problems and a decrease in removal of the hydrogen
cyanide. The viscosity of the slurry is affected by the
percent solids contained in the slurry, the type of ore
being treated, and the temperature of the slurry.
Normally, the weight percent solids in the slurry should
not exceed about 60 weight percent. Preferably, no more
than about 50 weight percent solids should be contained
in the slurry. More preferably, the slurry should
contain between about 25 and 45 weight percent solids.
As set forth hereinabove, the packing material
should have a high void ratio. The packing can be any
material which can withstand the abrasion and ~perating
conditions in the packed tower. Preferred materials
include stainless steel, ceramic materials and plastic
materials, for example, polyethylene and polypropylene.
Examples of packing materials which have been found to
be e~fective include 50 mm and 75 mm Pall rings, Rashig-
rings, Tellerette', saddles, and grid, although it isanticipated that other packing materials can be used.
The tower can be constructed from any material capable
of withstanding the reaction conditions and the
chemicals which contact the internal surface of the
tower. The preferred materials include fiberglass,
steel (both mild and stainless) and concrete.
-17-
Trademark
~'
J
` ~3~ 7 j~J~
In an alternative configuration, a stripping
reactor 122 can be used as discussed for Fig. 1 and as
depicted in phantom in Fig. 2. Such a reactor would
normally be used in place of the stripping tower 120.
In operation of the stripping tower, the
volatilization gas, preferably air, is conveyed 119 to
the stripping tower 120. Although two towers are
depicted in Fig. 2, it is contemplated that, depsnding
on the amount of slurry to be treated and the size of
the tower, a single tow~r could be used. Alternatively,
a plurality of stripping towers can be used either in
parallel as depicted in Fig. 2 or in series or a
combination of parallel trains with each train
containing a plurality of towers arranged in series.
The towers can be arranged to provide a single pass of
the slurry as depicted in Fig. 2 or multiple passes with
the slurry being recycled.
In the operation depicted in Fig. 2, air is
introduced into the stripping tower in countercurrent
flow to the slurry. The air can be introduced by blower
123 shown in phantom or air can be forced through by
negation pressure induced by fan 150. ~he tower is
operated under a negative pressure with the air-HCN
mixture being positively removed through line 121 and
transported to a cyanide recovery section. In the
configuration of Fig. 2, the fan 150 is o~erated to
exceed the flow of stripping gas so that all of the
system above the packing in tower 120 through vessel 126
operates under negative pressure to minimize any leaking
of HCN. Preferably, the air is recycled as discus~ed
hereinbelow. Sufficient air is introduced into the
volatilization tower to provide a mean volume to volume
ratio of air to slurry of about 250 to 1,000, more
preferably in the range of 300 to 800, and most
35 preferably, in the range of 350 to 700. Preferably, a
pressure drop of about 15 millimeters (mm) to about 30
-18-
J~
mm water gauge per meter of packing height is
maintained. The pressure drop is the difference in
pressure between the top and bottom of the tower, the
air flow or air flux and the cross-sectional area of the
tower. The degree of flooding is based upon filling all
of the void space in the tower being considered 100%
flooding.
The slurry is fed to the packed tower at a rate
which maintains a desired pressure drop over the length
of the tower. Normally, the tower is operated in the
range of about 10% to about 70% of the flooding volume
and preferably, in a range of about 20% to about 50% of
the flooding volume.
The air-HCN mixture is conveyed 121 to the cyanide
recovery section 126. Preferably, the cyanide recovery
takes place in a packed tower by contacting the HCN with
a basic solution which is conveyed in countercurrent
flow to the HCN-containing gas. As discussed
hereinabove for Fig. 1, any appropriate basic material
capable of providing an aqueous solution with a pH of at
least about 11 can be u~ed. Sodium hydroxide is
preferred in ord~r to reduce calcium in the circuit and
reduce possible calcium sulfate precipitation and scale
formation. Ninimizing such scale formation can be
particularly important with the packed tower in order to
minimize packing media fouling. As depicted in Fig. 2,
in a preferred embodiment, sodium hydroxide solution 128
is added to vessel 125 where it is combined with cyanide
containing stream 127 from scrubber 126. caustic stream
30 129 is removed from vessel 125 by pump 140 and conveyed
141 to be used to scrub hydrogen-cyanide containing gas
in the cyanide recovery section 126. The air-HCN
mixture is drawn through the scrubber column. As
depicted in Fig. 2, the scrubber column is vertical but
the column can be horizontal or any other suitable
configuration. Additionally, although a single column
--19--
1 3 ~
is depicted, it is recognized that a plurality of
columns could be used as necessary to effectively scrub
the volume of gas. The columns can be arranged in
series or in parallel as desired. The column is
preferably packed with a media bed to provide efficient
contact between the HCN and the basic solution. The
media can be any packing capable of providing ~fective
contact between a gas and liquid, with such media being
well-known to those skilled in the art. A proportion of
the caustic-cyanide solution in vessel 125 bled of 130
to prevent the continuous build-up of cyanide removed
from the HCN-air mixture introduced 121. Sodium
hydroxide 128 is automatically dosed into the scrubber
liquid to maintain a constant pH thereby allowing for
the portion lost to bleed. Cyanide, now in the form of
a caustic solution of sodium cyanide bleed 130, is
returned to the mill circuit for reuse.
Scrubbed air is removed 160 from the scrubber 126
and is conveyed through fan 150 to line 162 for recycle
or venting to the atmosphere provided the air contains a
low enough level of hydrogen cyanide. Scrubbed air can
be discharged to the atmosphere by a line 164 Gas
monitoring equipment can be installed in connection with
line 162 to provide a continuous readout of performance
and can include detection of levels of cyanide.
Preferably, the scrubbing unit 126 allows for a minimum
of 98% HCN removal from the hydrogen cyanide-gas
mixture. On this basis, the concentration of HCN
exiting the scrubber bed is maintained at less than 10
milligrams per cubic meter. Preferably, the scrubbed
air is recycled to the volatilization section gas feed
119 through line 166.
The stripped tailings slurry i5 removed 138 from
the volatilization tower and transported to a
reneutralization section 131 which is preferably a
sealed, agitated vessel. The vessel 131 is constructed
20-
v~ . ~
f?~
of materials compatible ~ith the abrasive nature of this
process. A basic material 135 is added to provide the
desired pH level for the final slurry. Although any
suitable base such as sodium hydroxide or potassium
hydroxide can be used, it is preferred that sodium
carbonate, calcium oxide or calcium hydroxide be used to
minimize the cost. The normal residence time to
accomplish the reneutralization and retain the desired
pH level for the slurry is normally about 15 minutes to
1 hour. The necessary time depends upon the buffering
curve of the components contained in the slurry.
The adjusted slurry is removed 137 from the
reneutralization section and transported to a tailings
impoundment. Alternatively, the adjusted tailings can
be treated to remove the remaining cyanide or can be
transferred to a thickener (not shown) where the coarse
material is removed and deposited in an impoundment with
the decant being additionally treated to remove the
remaining cyanide. The treatment can be accomplished by
recycling the whole stream or decant into the feedstream
112 for the pH adjustment section.
Referring to Fig. 3, the use of the instant cyanide
recovery process in combination with a carbon-in-leach
process is depicted. Although the CIL process as
depicted has no cyanide leach without carbon, it is
contemplated that some CIL processes can use at least a
partial cyanide leach prior to introduction of the
carbon. The ore slurry 301 suitable for treatment by a
CIL process is prepared by well-known processes 303. An
oxidaticn process can he used to treat refractory ores.
The pH of the slurry is adjusted in zone 305 preferably
to above about 10, more preferably in the range of about
10.5 to 11 by adding a basic material 307, preferably
lime. The resulting alkaline slurry is transferred 309
to the carbon-in-leach process. A typical CIL process
is described in United States Patent No. 4,289,532 of
-21-
"` 131~7g~
Matson et al. (issued 1981).
In the carbon-in-leach circuit, the slurry is
simultaneously contacted with cyanide and granular
activated carbon in YeSse~ 311. ~he carbon moves
countercurrent with the flow of the slurry. Thus, in
Fig. 3, ~tream 309 enters the first mixing vessel 311
where it contacts a cyanide stream 313 which can contain
cyanide in the amount of between about 0.25 and 2.5
pounds o~ cyanide expressed as sodium cyanide per ton of
dry ore as disclosed in the Matson et al. '532 patent.
The cyanide can be added in solid form, but it may also
be added as a solution, for example, as a sodium cyanide
~olution having between about 10 and about 25 weight
percent sodium cyanide by weight. Other sources of
cyanide such as potassium cyanide and calcium cyanide
can be used, as is well known in the art. Additional
lime 307 can be added to maintain the pH above about 10
in order to decrease cyanide decomposition. A stream of
the slurry is removed 315 and transferred to a second
agitated vessel 317. Activated carbon is screened from
the slurry being transferred to vessel 317. Fresh
activated carbon is introduced 319 to vessel 317. A
slurry containing cyanide ore and activated carbon is
transferred 321 back to vessel 311. A slurry containing
loaded carbon is removed 323 from vessel 311 for
subsequent recovery of precious metals by methods such
as stripping and electro-winning which are well known in
the art. A slurry which has been screened to remove the
activated carbon is removed 325 from vessel 317 and
preferably conveyed to a separation device 327, such as
a screen, which remov~s any contained carbon as stream
329. The remaining ore tailings are transferred 331 as
a feed to the instant cyanide recovery process 333 which
is depicted in detail in Fig. 2. Sodium cyanide
containing solution (depicted as stream 130 in Fig. 2)
-22-
e
~L 3 ~ s ~
is removed 335 from the process and recycled to the CIL
process. Tailings 337 from the process are disposed of
as discussed hereinabove.
Use of the instant cyanide recovery process permits
the use of higher levels of cyanide in the CIL process.
The levels of cyanide used based on sodium cyanide can
be increased by up to 250%, more typically up to 100%,
most typically up to 50%.
Referring to Fig. 4, a carbon-in-pulp process is
depicted using the cyanide recovery process of the
present invention. A typical CIP process is described
in United States Patent No. 4,578,163 of Kunter et al.
(issued 1986). Ore is prepared in mill 401 and
transferred 403 optionally to a classification device
405, such as a cyclone, which classifies the ore into
sands and slimes. This classification is used where
necessary depending on the ore and whether the sand is
to be used as backfill. The sands are conveyed 407 to a
vat 409 where the pH of the sand is adjusted to the
desired pH range by the use of a basic material 411 such
as lime. The vat can be agitated or can be a stationary
bed. If a stationary bed of the sand is used, it can be
leached using a sodium cyanide solution 413 containing
about 0.045 to about 0.055 weight percent sodium cyanide
by percolating the solution by gravity throuyh the sand.
If the vat is agitated, then a solution containing about
l pound of cyanide per ton of ore is used. The sand
residue from the process is transferred 415 as a feed to
the cyanide recovery 416 process depicted in Fig. 2.
The recovered sodium cyanide solution (corresponding to
stream 130 of Fig. 2) i5 recycled 417 to be used as feed
for leaching the ore in the vat. The tailings are
removed 419 for subsequent treatment as discussed
hereinabove.
The slime which is separated from the sand by
apparatus 405 is transferred 421 to a carbon-in-pulp
--23--
~ 3 ~
process. Optionally, the ore slurry 403 can be
transfarred directly from mill 401 to vessel 423 as
depicted in phantom. The slime is introduced into the
pH adjustment vessel 423 to which a basic material such
as lime is added 425 to increase the pH typically to at
least about 10 and preferably at least about 10.5. The
resulting alkaline slurxy is transferred 427 to an
agitated vessel 429 to which cyanide 431 is added to
provide a final concentration of about 1 pound based on
sodium cyanide per ton of slurry. The pulp slurry fed
to vessel 429 preferably has a solids content of about
40 weight percent. Pulp from the cyanidation tank 429
is transferred 433 to at least one and normally, a
plurality of carbon-in-pulp vessels 435 and 439. As
depicted in United States Patent No. 4,578,163 of Kunter
et al., normally four or more carbon-in-pulp vessels are
operated in series to effect a countercurrent extraction
with the activated carbon. The activated carbon 437 is
fed to the final vessel 439 of the series. A slurry
containing activated carbon is transferred 441 from
vessel 439 to vessel 435. Simultaneously, a slurry,
from which the activated carbon has been separated, is
transferred 443 from vessel 435 to vessel 439. Loaded
activated carbon is removed 445 from vessel 435 and
precious metal ~alues are subsequently removed from the
carbon. A slurry stream, from which the activated
carbon is substantially removed, is transferred 447 from
vessel 439 to a separation means 449 which removes any
remaining activated carbon as a stream 451. The
remaining ta lings are transferred 453 to the cyanide
recovery process 455 which is depicted in detail in Fig.
2. A sodium cyanide solution (corresponding to stream
130 of Fig. 2) is transferred 457 to be recycled and
used in the carbon-in-pulp process. The tailings from
process 455 are removed 459 for disposal as discussed
hereinabovs.
-24-
p~
Although two separate cyanide recovery processes
are depicted in Fig. 4, a single cyanide recovery
process can be used if the different sizes of the
particles in the sand slurry and slime slurry permit.
Even if two separate processes are used, sodium cyanid~
solution can, of course, be recycled to either portion
of the process.
Use of the cyanide recovery process of the instant
invention similarly permits higher levels of cyanide to
be used particularly in the carbon-in-pulp. The level
of cyanide can be readily increased by at least about
50%, preferably up to 100% and preferably by at least
about 250%.
While not wishing to be bound by any mechanis~, it
is believed that the cyanide recovery process of the
present invention operates as follows.
When the pH of the kailings is adjusted to between
6 and 9.5, the CN- complexes (with the ~xception of Fe
and Co complexes) dissociate to form CN- and ultimately
HCN:
CN complexes ===== CN ===== HCN
These equations represent equilibrium reactions in which
the process of the present invention shifts the
equilibrium to the right-hand side. In the
volatilization section 20 of Fig. 1, the HCN in solution
is volatilized to HCN gas:
HCNsOlution ~~~~~ HCNgas
This preferably occurs under an overall pH of about 8
and a high energy environment of the volatilization
section 20. In the basic reaction chamber 26, the high
pH causes the equilibrium to shift back towards HCN in
solution:
HCNgas ~~-~----- HCNsOlution
Although the process has been described with
reference to tailings slurry from a carbon-in-leach or
carbon-in-pulp mineral recovery process, it is to be
-25-
'f '~7 ~
expressly understood that the process can also be
employed on other cyanide-containing streams, e.g. from
other mineral recovery processes, electro-plating
processes, etc.
The following experimental results are provided for
the purpose of illustration of the present invention and
are not intended to limit the scope of the invention.
EXAMPLES
A. Equipment
The apparatus employed in Examples 1 and 2 consists
of two 3' plexiglass columns six inches in diameter,
connected in series, and sealed on both ends with
plexiglass plates. The two columns are connected by
tubing to permit the flow of air into the bottom of the
first column, up through the column where it exits at
the top, and then enters the bottom of the second
column, flows through the column and exits at the top of
the second column. A flow meter was employed to measure
the flow of air entering the bottom of the first column.
The column nearest the flow meter operated as the
acidification-volatilization column, while the second
column operated as the absorption column. Tubing was
attached to the absorption column and ran into a fume
hood to vent the air and any cyanide not absorbed.
The aeration system was capable of producing a
continuous flow of air in the range of 0-lO scfm at
pressures of 10-20 psi. A compressor was employed for
this purpose. The compressor was attached to the flow
meter via tubing which was then attached to the first
column. A regulator between the compressor and the flow
meter was employed to regulate and record the pressure
being applied to the system.
A pipe was attached in each bottom plate of the two
columns to facilitate sampling and draining of the
columns during and following an experiment.
-26-
:~ 3 ~ 3
B. Procedure
In Examples 1, 2 and 3, a specific pH and air flow
were utilized and the extent of cyanide stripping and
recovery was evaluated over time. The air flow passed
from the compressor, through the regulator, the flow
meter, and the first volatilization column, and finally
through the second absorption column. The air flow
exiting the second column passed into a fume hood to
vent unabsorbed cyanide.
Exam~le_l
The ore used in Example 1 was prepared by grinding
kilograms of ore together with 13.5 kilograms of
water ti.e. 65% solids) and 240 grams of Ca(OH)2 (i.e.
9.6 kilograms per ton) for 42 minutes in order to
achieve a particle size distribution of about 85~ of the
ore less than 45 microns in size. Twenty kilograms of
water were added after grinding in order to thin the
slurry. The slurry was ground a total of 3 times.
Makeup water (9.6 kilograms) was added at the completion
of the three grinds and the pH was adjusted to 10.5.
The slurry was leached with cyanide. Initially,
83.5 grams of NaCN as a 5% solution was added. After 2
hours, 33 additional grams of NaCN (5% solution) was
added as the cyanide concentration had dropped. The
total cyanide added to the system was equivalent to 385
parts per million cyanide. During leaching, an air flow
of 1 liter per minute was maintained. The pH and
cyanide concentration of the leach slurry was monitored
hourly. No further additions of NaCN were needed. The
final cyanide concentration was measured at 210 parts
per million. Finally, carbon was added after 16 hours.
~owever, the gold and silver concentrations were not
monitored. After removal of the carbon, the composition
of the barren leachate was measured prior to stripping.
The composition is shown in Table I.
-27-
r~
Table I
Composition of Barren Leachate Befora Stripping
pH 10.3
Alkalinity 475
Ammonia-N
Cyanate 23
Cyanide (Total) 202, 192
Cyanide (WAD) 200, 190
Sulphate 320
Thiocyanate 24
Arsenic 0.8
Copper 3.90
Iron 0.15
Silver 0.06
Zinc 2.10
For each of the six runs of Example 1, 10 liters of
the slurry prepared as described above were placed in
the first volatilization column. Initial samples of the
solution were analyzed for free cyanide (for example, by
ion selective electrcde or by silver nitrate titration),
the weak acid dissociable cyanide (CNWAD - by ASTM
Method C), and pH. For runs 1 and 2 the initial pH was
not adjusted. For runs 3 and 4 the pH was adjusted with
H2SO4 to 8.7. For runs 5 and 6 the pH was adjusted to
7.6.
Ten liters of caustic solution was placed in column
2 (the absorption column). The caustic solution was
prepared by adding sufficient sodium hydroxide pellets
to bring the pH of the solution to about ll to about
11.5.
Air was then introduced into the columns. In runs
1, 3 and 5, the air flow rate was 60 liters per minute
(+20%) and in runs 2, 4 and 6, the air flow rate was 82
-28-
liters per minute (-~20~). Table II summarizes the pH
and air flow rates for each of the runs in Example 1.
Table II
Conditions for Stripping
Run No. 1 2 3 4 5 6
pH 10.5 10.5 8.7 8.7 7.6 7.6
air flow 60 82 60 82 50 82
(l/min)
+20%
The amount of total cyanide (CNT) and Method C
cyanide (CNWAD) was measured both in parts per million
and in milligrams for th~ slurry in column 1 and the
caustic solution in column 2. The results are shown in
Table III.
The first column labeled /'Hours Stripping" lists
the six runs and the time each sample was taken. The
second column labeled "Kilograms in System" is the
kilograms of liquor in the first column. Initially, 10
kilograms of total slurry was added, made up of liquor
and solid tailings. The third and fourth columns list
the CNT and CNWAD measurements in parts per million for
each run at each time period listed. The fifth and
sixth columns list the CNT and CNwAD in milligrams. The
seventh and eighth columns list the same measurements as
in the sixth and seventh columns except they have been
adjusted as to account for the samples which were
removed.
Columns 2 through 8 list measurements taken from
the slurry in column 1. Columns 9 through 14 list
similar measurements which were performed on the caustic
solution in column 2 in order to determine the total
amount of cyanide absorbed. The percent extraction of
CNT and CNWAD are listed in columns 15 and 16.
The percentage extraction of CNT is based on the
total CNT figure for that particular hour and includes
-29-
r~ ~ ~
the adjustments. The extraction percentages are low
because the CN drained from the slurry column is
actually not available for stripping. A caustic sample
was lost in run number 4 and therefore there are no
corresponding numbers. In runs 1 and 2 the milligram
CNWAD analysis was not performed on the slurry.
The 10 liters of initial slurry for runs 3 and 4
required 75 milliliters of a 10 volume percent sulfuric
acid solution to reduce the pH to 8.7. For runs 5 and
6, 115 milliliters of a 10 volume percent H2S04 solution
was added to the 10 liters of slurry to reduce the pH to
7.6.
-30-
7 ~ ~
= l
ll N 0 0~1~ N '`O 0. ¦ O -.:t N I Lrl ~ Yl ~
~ ~ ~ O~ æ N ~ o ¦ ~ ` J ¦ 3 0' æ ~
I ~ _ __
_ U~ Ul 0~ ~ ~0 rJ _ o ~- _ r- ~ I ~ o Y~ I
u7 0~ ~ rJ
~_ _ N N N ~ O` ~i ~ 0~ ~ 0` 0` ~ O
I _ I
OOOOO 0000 I OOOO I QOOO
N N N N N N ~3 N N ¦ N _ _ _ ¦ N _ _ N N
I :_~ __ I _ _
1 0 OOOOO OOOOO OOOOO OOOO OOQO I OOOOO
¦ I-- 0~ 0~ O ~ N t 7 1~ O N O ~ ~ N N _ N ~'?1~ O ¦ N . ~r~ ~
I I_ _____ _____ .___~ __~ ._, I ____
I_ . _ I _
l 2 _ N N 1.'1 M ~ ~ _ O l-'10 ~ O N N 0 æ ~ ~ I o 0 O O O
l I _
C: I _ ~8 ~ _ _ r~ ~ _ N M t~l O t~'l Q O ~ O O ¦ O ~ O O ~ ~1~ ~ N
. _~ I ~_ _ I _ _
U I ~ o 01-) o _ O O ~ O N O _ N o _ ' I O o o o t~
¦ O ~ ~ o~ 0N ~ --N 1'~1 ~ o~ _ _ _ _ _ _ 1~ ~ _ _ _ ~ _ _ _
H Ul
H ~ l ._ ~ ~3 ~-- o o ul N 1 o o _ 0 u o o ~!; N 0 o u~ o ~ o o
~d l ci~ -_o~o~o~ __o~o~eo __o~o~0 __~o~0 _.o~o~0 __c~o~0
1~ I_ _ _ I
~ l 3 ~ o o _ N M eo N N ~ ~ o r` _ 0~ 0 0~ 0 `.0 M M
U~ I ,~8~ _ I
a) l ~ N Ul _ N ~ 0 ~ _ 1~ N o M _ M U~ C~ ~ U, M N M N ~! M _ N N N _ _
~ I ~ ~ _ _ _ _ _ _ _ _ _ _ _ _
~ l _ 3 N M _ 0 ~ O Ul 0~ M C N N N 0 N _ 1~ ~ 0
l 3 _ l
¦ _ ~ 0 ~O ~O 0 `S 1~ g _g o o M N 0~ ~0 ~ N O O O _---- 0 ~ ~0 _I e" t-- _ N _ O ^~ _ _ O _ 0 M ~t N _ _ _ N _ _ _ rJ N _ _ N N _ _
I U~ _ _ I _
N ~ M N N 00 N ~ ~ N O M t--O` N N ~0 N 0 N ~r O `:t 0` N .:t ~0 0~ --v
~J ~ ~ U, `:t ~t _ `O u~ ~ M _ _ 00 _ 0 N _ ~ _ .- ~ O 0 --N _ r
_- ~ ~ ~ ~ 0_
- ~ o 0--00 o NO _ 0 U, ~ ~ $ ~ C~ C~ Mo U, ~t ~ U0 U~
U~ N
~- ~ ~ t~ t~ 1~ ~ c ~ ,~vV~
~Y -- _ o~ 0 r--~o o~ 0 ~--¦ 0~ 011_ ~0 C~ 0 1~ ~.0 O` 0 ~ O u~
=~ _ c o _ N M I ~ O _ N M ~ o _ N M ~ O _ N ~ ~ O _ (N M C O . ._
I In I ~Y e a: ~c el Cl: ~
I _ ~ ~
"
--31--
.,
jf~
Example 2
Following the procedure employed in Example 1, new
tests were run on ore samples. In the first run, the
air flow was 80 liters per minute (+20%). In the second
run, the air flow was 100 liters per minute (+20%). The
compositions before and after the runs are shown in
Table IV.
Table IV
Composltion of Barren Leachate Before and After St~ipping
_'
BEFORE AF~Ef~
. . , ~
Run No. 1 2
A;r F10w l~m~n +20~L UO 100
pH 1D,4 9.7 10.2
alkal ~n~ty 575 170 16g
CNT 213 29.4 24.6
CNWAD 21B 7.4 6,8
hardness 307 2170 2030
S04 360 2525 Z350
SCN 34 37 3a
E.C. ~S/cm 20~C~ 1714
A~ 0.3 O.B 0.7
C~ 123 869 814
Cd c 0.01 < 0.01 < 0.01
Cr 0.02 < 0.02 ~ 0.02
Co 0. 16 0.33 0.30
Cu 4.7 6.0 6.1
Fe 1.3 8.7 6.7
P~ c 0.1 < 0.1 c 0.1
Mn o.O1 0.02 0.02
1~
Ni 0.12 0.43 0.41
Se
Ag O.lS 0.04 0.04
Zn 0.64 0.01 0.06
, . __ .. . .. .. ._
Rea~nt consUIllptiQn to either lower or ra~se pH f~r 10 ~ slurry
final pH B.1 9.7 lO.O
rea~ent 10% v~v H2 SO~ Ca(OH)2 Ca~OH)2
2mount 110 Inl 7, 7 ~ 9 . O g
-33-
~. 3 i ~ P~
The pH of the initial slurry was 8.1. This pH was
achieved by adding 110 milliliters of 10 volume p~rcent
H2SO4 to the 10 liters of slurry. After run number 1,
7.7 grams of Ca~OH)2 was added to the tails to raise the
pH to 9.7. After run number 2, 9.0 grams of Ca(OH~2 was
added to the tails to raise the pH to 10Ø The results
for runs number 1 and 2 in Example 2 are shown in Table
V.
_ _ ~ j~ N 1-1 N 8 7 ~ ~
x _ I ..
0~ O ~ ~ O
L~ wwww
z L~ w=~o
O 1_ ~ ` N ~ N ~ U'l _ Ul In
2 o~ g~ o~ o~ N ~ æ ~
~ ~ U O ~ 00 N ~ N 1.~ N 3
w z ~' ~ W W ~ o N _ - _
~ C aE o o ~ ~ ~ o o ~ N O~
w m _ _ O~ O O c~ _ _ ~ O 00
N U 3 ~ p ~
c 2 _ ~ Sl N N N 1~ N N N--
u 3 ~ O ~ ~O O
_ _ _ o l~ N N _ ~` N N _ _
L ~ _ ~ _ oN 1~ = O N C
---- ~ Nl~ IN ~--
~ V ~ 0 ~ l N N r~ ~ ~ v
_ _ N U
O _ 5 O _ N _ ~; O _ N _ ~ V .~
--35--
$
Example 3
Five runs were performed in order to test the
efficiency of a reactor employing air inlets and a
turbine to create turbulence. The pH in each run was
varied as was the air flow rate. In run number 1, the
pH was 8 and the air flow was 290 liters per minute ~2.9
meters3/meters2 x minute). In run number 2, the pH was
7.8 and the air flow rate was 100 liters per minute (1.0
meters3/meters2 x minute). In run number 3, the pH was
8.2 and the air flow rate was 50 liters per minute (.5
meters3/meters2 x minute). In run number 4, the pH was
7.8 and the air flow rate was 200 liters per minute (2.0
meters3/meters2 x minute). In run number 5, the pH was
8 and the air flow rate was 200 liters per minute. In
runs 1 through 5, 30 liters of solution were tested.
Table VI shows the percent CNWAD remaining after 15, 30,
60, 120 and 180 minutes.
Table VI
Run 1 2 3 4 5
20 Time Percent CNwAD Remaining
- (minutes)
59.6 76.6 96.~ 52.1 66.2
36.5 58.5 92.5 33.3 42.1
27.4 46.3 46.2 20.8 24.8
25 120 22.1 30.3 35.5 12.5 21.1
180 19.2 23.4 33.3 13.5
Example 4
The efficiency of a flotation machine and a dif-
fuser column were tested in runs 1 and 2 of Example 4,
respectively. In run number 1, a flotation machine was
employed with a 40 liter per minute air flow into a 3
liter slurry ~1.4 meters3/meters2 x minute). In run
number 2, a diffuser column was employed with 50 liters
per minute air introduced into a 10 liter slurry (9.4
meters3/meters2 x minute). In both runs 1 and 2, the pH
-36-
~ 3 ~
was 8. The results of these tests are shown in TableVII.
Table VII
Run 1 2
Time Percent CNWAD Remaining
(minutes)
43 76
11 46
120 10 12
180 8 7
Example 5
A continuous pilot plant was used in which five (5)
stirred vessels sealed to the atmosphere and each having
a volume of 200 liters were connected in series with
pipes in and out the top of each vessel. The lead
reactor was connected to a vessel through which tailings
slurry could be introduced. The lead reactor was also
connected to a vessel from which a 10% solution of
sulfuric acid could be added. Arrangement was also made
to introduce sodium cyanide as required into the lead
reactor in order to maintain a desired level of free
cyanide in the slurry being leached. The final reactor
in the series was connected to a sealed aeration basin
having a coarse bubble flexicap defuser in the bottom
region of the basin. The aeration basin was divided
with plywood baffles into five sections. Each plywood
baffle had a hole in the top with a drop pipe to the
bottom of the next section with the pipe sized to the
flow of feed into the basin. Agitation was accomplished
by air flow. The diffuser was connected to a source of
compressed air with a controller which could pr~vide a
range of controlled air flow rates. A transfer line was
connected from the top of the sealed aeration basin to a
fan which was capable of providing a negative pressure
in the aeration basin and conducting the air and
-37-
&r~
hydrogen cyanide mixture from the vapor space above theliquid in the aeration basin. The exit of the fan was
connected to a dilution stacX which diluted the effluent
hydrogen cyanide with air to allow venting. Another
transfer was connected to the lower portion of the
aeration basin to allow removal of tailing slurry and
transfer to a stirred sealed neutralization vessel. A
transfer line into the vessel was used to introduce
sodium hydroxide solution to increase the pH to the
desired level or a batch basis as necessry. A transfer
line allowed removal of the reneutralized tailings
slurry. Results from runs using this procedure are
presented in Table VIII and ~able IX.
Table VIII
Slurry Feed Influent
RateInfluentWAD CN- Air Flow
Run No. 1 3/hr) (pH) (mq/L) m3/m2 min
1 1.7 9.6 230 4.5
2 1.7 9.6 150 4.5
3 2.2 9.6 228 4.6
4 2.2 9.7 228 3.9
1.7 9.7 198 4.4
6 1.8 9.7 195 4.5
7 2.2 9.8 168 2.4
8 2.2 10.0 182 4.5
9 0.5 10.0 207 4.5
0.5 10.0 157 2.8
11 0.5 10.0 198 4.5
12 0.5 10.0 170 4.5
30 13 0.5 10.0 203 4.5
14 0.5 10.0 179 6.2
0.5 10.0 171 8.8
16 0.5 9.9 161 4.5
17 0.5 9.0 176 6.0
-38-
-" ~3~7~
Table VIII (continued)
Total
No. of Aeration Effluent
Slurry Depth Reactors Period WAD CN-
5 Run No. (m) ~In Series (min) ~mq/L)
1 1.3 1 138 67
2 1.3 1 138 43
3 1.3 1 106 67
4 1.3 1 106 67
1.3 3 138 60
6 1.3 3 130 52
7 1.3 3 106 84
B 1.3 5 92 61
~ 1.3 5 312 26
1.3 5 312 28
11 1.3 5 312 23
12 1.3 5 312 22
13 1.3 5 312 23
14 1.3 5 312 16
1.3 3 187 16
16 1.3 5 312 19
17 1.3 5 312 15
Table IX
Complete
Mix Aeration
Influent Air Flux Reactor Period Effluent
CN- PH m3/m2 min Staqe (min) CN-
198 6.0 4.5 1 63 33
2 125 31
3 187 27
4 250 25
312 24
179 8O0 6.2 1 63 21
2 125 20
3 187 17
4 249 18
312 14
171 8.0 8.8 1 63 16
2 125 15
3 187 16
-39- .
~3~{~
Example 6
A continuous pilot plant was used as in Example 5
except the agitator was removed from the final pH
adjustor reactor in the series and aeration basin was
replaced by a packed tower having a diameter of 0.5
meters and a height of 6 meters. The tower was packed
with about 3 meters of either 50 millimeter or 75
millimeter plastic Pall rings. The influent
distribution system consisted of a ceramic multiple weir
trough and a demister. The packing media was supported
by a multiple-beam ceramic gas injector plate. The
results from this configuration are provided in Table X
for 75 mm rings and Table XI for 50 mm rings.
Table X
Slurry Air No. ofAir/
Flow Flow TowerLiquid
Run No. (m3/hr)tm3/hr) PassesRatio
1 2.37 845 1 357
2 2.37 845 1 357
3 1.94 839 1 432
4 2.17 839 1 387
2.54 839 1 330
6 2.10 2126 1 1012
7 2.21 2126 1 962
8 2.33 1484 1 637
2.39 1400 2 586
9 2.36 1615 1 684
2.45 1615 2 659
4.1 2137 1 571
4.0 2137 2 534
11 4.17 2581 1 619
4.0 2581 2 645
-40-
~ 3 ~
Table X ~continued~
Influent Effluent pH of
Run No. WAD CN- WAD CN- Slurry
1 182 3~.6 ---
2 182 24.5 ---
3 156 45.1 ---
4 166.4 22.7 ---
166.4 22.7 ---
6 192.4 15.0 7.9
7 192.4 13.7 ---
8 197.6 18.3 8.0
19.1 5.6 ---
9 223.6 23.9 7.9
22.0 6.0 8.1
174.0 29.0 7.6
25.0 7.0 ---
11 193.0 26.0 7.7
22.0 7.0 ---
Table XI
Slurry Air No. of Air/
Flow Flow Tower Liquid
Run No.(m3/hr) (m3/hr) Passes Ratio
12 3.9 1364 1 349
3.7 1364 2 369
13 5.0 1682 1 336
4.6 1682 2 365
14 4.0 2452 1 ~13
4.1 2452 2 598
15 4.1 1~03 1 342
3.9 1403 2 360
164.18 2389 1 ---
17 4.2 2389 1 ---
-41-
Table XI (continued)
Influent Effluent pH of
Run No. WAD CN- WAD_CN- Slurry
12 165.0 23.0 7.8
13 186.0 25.0 7.7
14 Z13.2 17.5 7.5
202.8 22.9 7.6
16 170.8 14.4 7.9
17 162.9 14.1 ---
While various embodiments of the present invention
have been described in detail, it is apparent that
modifications and adaptations of those embodiments will
occur to those skilled in the art. However, it is to be
expressly understood that such modifications and
adaptations are within the spirit and scope of the
present invention, as set fort~ in the following claims.
-42-