Note: Descriptions are shown in the official language in which they were submitted.
- ~ 3 ~
- 1 - RR325
ELECTRICAL WIRE AND CABLE
_
This invention relates to electrical wire and
cables.
In certain fields where wire and cables are used,
for example in military or mass transit applications,
it is desired to use cables which are capable of func-
tioning for a period of time during a fire without
shorting or otherwise failing. These cables have been
called circuit integrity cables or signal integrity
~cables depending on their use. The previously proposed
cables have generally used the principle that the indi-
vidual conductors should be separated from one another
by mica tapes, by large volumes of packing materials,
by relatively thick layers of silicone insulation or by
combinations thereof in order to prevent the formation
of short circuits during a fire. There is therefore a
need for a cable that will retain its integrity for a
period of time when subjected to a fire but which is
relatively small and lightweight and which is relati-
vely inexpensive to manufactureO ~`
According to the present invention, there is pro-
vided an electrical wire which comprises a metallic
electrical conductor, an insulating mineral layer
'~
~ 9~
- 2 - RK325
electrolytically formed on the conductor from chemi-
cally delaminated weathered mica, and a silicone
polymer layer located on the mineral layer.
It is known that several 2:1 layer phyllosilicate
minerals form interlayer complexes with a wide range of
charged and uncharged species of both organic and
inorganic origins e.g. alkylammonium ions, amino acids
and amino acid cations. The inclusion of inter-
callating species between the layers of the
macrocrystal usually results in changes to the basal
spacing which can be measured by X-ray diffraction
techniques. Under certain circumstances an additional
swelling can take place whereby further intercallation,
by a wide range of polar and non-polar solvents,
occurs. In special cases the degree of expansion can
be so extensive as to produce 'gel-like' samples. The
application of mild mechanical action to these exten-
sively swollen systems can lead to the production of
colloidal dispersions of the mineral on a dispersing
solvent, this process being known as "chemical
delamination".
This effect can be particularly apparent in a
range of mica-type complexes containing n-alkylammonium
ions, with water as a dispersing solvent. Whether
additional interlayer expansion occurs depends on the
layer change density separating successive layers on
the mineral and the length of the alkyl chain of the
associated intercallants~
Minerals with a surface charge density in the
range of 0.5 to 0.9, saturated with certain short chain
n-alkylammonium ions e.g. n-propyl, n-butyl and
~9~
- 3 - RR325
isoamyl, behave exceptionally well in that they show
extensive interlayer swelling in water. Crystals which
show this type of behaviour can increase in volume by
up to, and sometimes more than, 30 times their oriyinal
volume and remain coherent and 'gel-like'. Interstrat-
ified minerals containing mixed layers of exchangeable
and non-exchangeable cations can be partially saturated
with short chain alkylammonium ions and subsequently
treated with water to swell 'macroscopically' only part
of the layered structure.
In either case mild mechanical shear will delami-
nate the swollen crystals alony the macroscopically
swollen cleavage plane where interlayer forces are
minimised. This action can be used to produce a
colloidal dispexsion of thin high aspect ratio plate-
lets. In the case whereby the starting mineral is of a
homogenous nature the composition of the colloid will
be consistent~ However, if mixed layer minerals are
used then there can be a wide variation of platelet
composition and characteristics throughout the
colloidal dispersion. Fractionation techniques,
including sedimentation, can be used to isolate com-
ponents of the dispersion which exhibit different che-
mical and physical characteristics from each other and
from the parent mineral.
The term "weathered mica" is used herein to
describe the weathering products of natural mica and
includes minerals comprising vermiculite or minerals of
a mixed layer type containing vermiculite layers as a
major constituent. It includes any hydratable, layer
latticed, expandable silicate structure, and primarily
the three layer micas. The layers usually have a
- ~319~
~ 4 - RK325
thickness of about 10 Angstrom units with the main ele-
mental constituents being magnesium, aluminium, silicon
and oxygen. It may be formed by replac~ment of non-
exchangeable cations, e.g. potassium ions, by
exchangeable cations, e.g. sodium or magnesium ions, in
mica. Such replacement will normally occur through
weathering of mica, but the term includes materials
formed by other methods of cation exchange, e.g. by
hydrothermal action or some synthetic micas. The term
includes materials such as vermiculites and smectites
in which there has been complete replacement of the
non-exchangeable cations, and any intermediate
materials such as formed by partial replacement of the
non-exchangeable cations, provided, as explained below,
that it is possible to form a colloidal dispersion from
the material. The use of a weathered mica instead of
unweathered mica has the advantage that the cohesion of
the resulting mineral layer is much larger than that of
a deposited mica layer with the result that it is then
~possible to handle the wire more easily during manufac-
ture and use, and in addition, much higher electrolytic
deposition rates can be achieved with lower deposition
voltages.
Preferably the weathered mica is a mineral of a
mixed layer type that contains mica layers interspersed
with other layers that are formed by weathering. The
weathered layers may comprise any hydratable, layer
latticed, expandable silicate structure, e.g. hydro-
biotite and hydrophlogopite layers, and preferably
hydrophlogopite II layers although other layers may
instead be present. The hydratable layers may comprise
a major part of the original mineral although it is
preferred for the major part (by weight) to be formed
from unweathered mica layers.
~ 3 ~
_ 5 _ RK325
Thus, the mineral used according to the invention
may be regarded as formed from platelets that have a
micaceous, or predominantly micaceous interior, and a
surface that is formed from a hydrated silicate layer.
The platelets preferably have an average thickness of
not more than 500 Angstroms, more preferably not more
than 30~ Angstroms, especially not more than 200
Angstroms and most especially not more than 100
Angstroms, but preferably at least 20 Angstroms, more
preferably at least 40 and especially at least 60
Angstroms.
The wire will normally be provided with an outer
protective layer or jacket which will protect the
weathered mica layer from mechanical abuse during
handling and which is preferably also electrically
insulating so that it can provide further electrical
insulation during normal operation. The protecting and
insulating layer will normally be a polymeric layer
which is formed on the coated conductor by an extrusion
process although in some cases it may be preferred to
apply the insulation by a tape wrapping process for
example in the case of polytetrafluoroethylene or cer-
tain polyimides. In other cases however, for exaMple
in the case of electric motor windings or transformer
windings, where very thin, high temperature wire is
required, it is possible to dispense with the polymeric
insulation altogether.
The wire according to the invention may be manu-
factured in a particularly simple manner by passing an
elongate electrical conductor through a dispersion of
chemically delaminated weathered mica and applying an
electrical potential to the conductor in order to depo-
``` ~3~9~
270~5-168
sit reconstituted weathered mica (hereinafter referred to simply
as the "mineral") onto the conductor and dryiny the conductor and
the mineral layer so formed. After the mineral layer has been
dried, the silicone layer may be formed on the coated conductor by
any appropriate method, e.g. by extrusion or dip-coating, and then
curing the silicone layer so formed.
The wea~hered mica dispersion may be formed by treating
the weathered mica ore consecutively with an aqueous solution of
an alkali metal e.g. a sodium salt, and especially sodium
chloride, and an aqueous solution of a further salt, e.g. an
organo substituted ammonium salt such as an n-butyl ammonium salt,
in order to swell the ore for example as described in British
Patent No. 1,065,385. After the ore has been swelled to a number
of times its original size in water, it is delaminated for example
by means of a mill, a mixer, an ultrasonic agitator or other
suitable device to form the majority of the expanded mineral into
a colloidal dispersion. The colloidal dispersion so formed can be
fractionated by sedimentation into several cuts. With a mineral
such as vermiculite or other very highly weathered systems, as one
moves from the 'fines' to the more coarse fractions the degree of
hydration decreases through successive layers, the K2O content
increases and the x-ray diffraction pattern moves closer to
resembling the parent mineral. When partially weathered micas are
used a distinctive increasing micaceous component can be easily
identified and as one moves to the coarse unprocessable -fraction
of the mineral its x-ray diffraction pattern, TGA trace and
elemental composition distinctly identifies it as
~ 3 ~
_ 7 _ RR325
pure mica. In the latter case it is possible to form a
dispersion of predominantly micaceous lamellae by
selecting the appropriate fractions of the colloid i.e.
by discarding the coarse mica fraction and the highly
hydrated vermiculitised fines. It is therefore
possible to generate a dispersion of mica-like plate-
lets as identified by XRD, TGA and elemental anaylsis
by utilising the chemical exchangeability of ver-
miculite interlayers on partially weathered interstra-
tified layered minerals.
In a typical process, the dispersion is permitted
to stand for between 1 and 60 minutes, preferably 5 to
20 minutes, and the top fraction decanted to supply the
working colloid. In many instances where partially
weathered mica is employed, it will not be possible for
all the mineral to be brought into suspension since the
weathering process does not occur uniformly throughout
the mineral, and the greater the degree of weathering
or cationic replacement, the greater the proportion of
mineral that can be dispersed. The particle size range
of the decanted fraction typically is between l and 250
um, preferably between 1 and 100 ,umO Preferably the
suspension has a concentration of at least 0.5 and
especially at least 1% by weight although lower con-
centrations may be used provided that the concentration
is not so low that flocculation occurs. The maximum
concentration is preferably 8% and especially 4~ by
weight, beyond which the relatively high viscosity of
the suspension may lead to unreproduceable coatings.
The conditions that are employed to form the suspension
will depend among other things on the particular type
of mineral that is employed. The preferred method of
forming the weathered mica dispersion is described in
~ 3 ~
:
27065-lG8
our copending Canadian patent application No. 571,~94 entitled
"Mineral" and filed on July 8, 1988.
In order to coat the conductor, it is passed
continuously through a bath containing the mineral suspension
while being electrically connected as an anode with respect to a
cathode that is immersed in the suspension, so ~hat the weathered
mica platelets are reconstituted electrolytically on the conductor
in the form of a gelatinous coating. The fact that the coating is
gelatinous and therefore electrically conductive means that it is
not self-limiting in terms of the coating thickness and therefore
enables relatively thick coatings to be formed. The plating
voltage will depend on a number of factors including the residence
time of the conductor in the bath, the desired coating thickness,
the electrode geometry, the bath concentration and the presence or
otherwise of other species, especially ionic species, in the bath.
The plating voltage will normally be at least 5V, more preferably
at least lOV and especially at least 20V since lower voltages
usually require very long residence times in the bath in order to
achieve an acceptable coating thickness. The voltage employed is
usually not more than 200V and especially not more than lOOV since
higher voltages may lead to the production of irregular coatings
and poor concentricity of the coating layer, to oxidation of the
anode or electrolysis of the bath water and hence a poorly adhered
coating. Such plating voltages will usually correspond to a
current density of 0.1 to 6 mA mm
After the coated wire has left the bath, and pre-
o ~
_ g _ R~325
ferably before being contacted by any rollers or otherparts of the equipment, the coating is dried in order
to remove residual water from the gel. This may be
achieved by hauling the coated wire through a hot-air
column or a column heated by infrared sources or hot
filaments. Additional columns may be used if desired.
The wire may then be hauled off for final use or to be
provided with an outer protective insulation. The
orientation of the platelets in a direction parallel to
the underlying conductor means that relatively rapid
drying methods can be used to collapse the gel to leave
an integral, self-supporting inorganic layer.
The silicone polymers used for forming the sili-
cone polymer layer are preferably elastomeric and
adapted for coating conductors by extrusion or dip-
coating. It is preferred to use elastomers rather than
solvent based resins because the resin will impregnate
the mineral layer at least to some extent which will
normally require a long drying period during manufac-
ture of the wire. In addition it has been found that
the use of a silicone elastomer layer will improve the
fire performance of the wire as described below.
Suitable forms of silicone polymer from which
silicone elastomers may be derived include polymers of
which at least some of the repeating units are derived
~rom unsubstituted or substituted alkyl siloxanes, for
example, dimethyl siloxane, methyl ethyl siloxane,
methyl vinyl siloxane, 3,3,3-trifluoropropyl methyl
siloxane, polydimethyl siloxane, dimethyl siloxane/-
methyl vinyl siloxane co-polymers, fluoro silicones,
e.g. those derived from 3,3,3-trifluoropropyl siloxane.
The silicone polymer may be, for example, a homopolymer
.
~ 3 ~
- 10 - RR325
or a copolymer of one or more of the above siloxanes,
and is advantageously polydimethyl siloxane or a copo-
lymer of dimethyl siloxane with up to 5% by weight of
methyl vinyl siloxane. Silicone modified EPDM, such as
~Royaltherm (available from Uniroyal) and room tem-
perature vulcanising silicones are also suitable
materials.
The silicone elastomer may, if desired, contain
fillers, for example reinforcing fillers, flame retar-
dants, extending fillers, pigments, and mixtures
thereof. For example, suitable fillers include diato-
maceous earth and iron oxide. It will be appreciated
that such fillers may be used in addition to a rein-
forcing filler such as silica that is added to silicone
polymer to form the silicone elastomer.
Other materials such as antioxidants, U V stabili-
sers, thermal stabilisers, extending silicone oils,
plasticisers and cross-linking agents, may be included.
We have found that improvements in the mechanical
performance of the wire may be achieved if a binder is
incorporated in the mineral coating which can improve
processability of the mineral-clad conductor. Thus,
according to one preferred aspect of the invention, a
binder is incorporated in the mineral dispersion and is
deposited on the conductor along with the mineral in
order to improve the processability of the clad conduc-
tor. The material chosen for the binder should be
inert, i.e. it should not corrode the conductor metal
or react with the mineral coating and preferably it
improves the bonding of the mineral layer to the con-
ductor metal. It should also be electrophoretically
*tr.ccf~ r~
1 3 ~
~ RR325
mobile and non-flocculating. The binder may be disper-
sible in the medium that is used to form the mineral
suspension (water), for example it may comprise a
water-dispersed latex, e.g. a styrene/butadiene/car-
boxylic acid latex, a vinyl pyridine/styrene/butadiene
latex, a polyvinyl acetate emulsion, an acrylic copo-
lymer emulsion or an aqueous silicone emulsion. It is
preferred to use binders in the form of emulsions
because they may be dried quickly with only a few
seconds residence time in the drying tower, whereas
with aqueous solutions much longer drying times are
necessary, and, if drying is forced, bubbles may be
formed in the mineral layer that will cause imperfec-
tions in the resulting dried layer. In addition at
least some binders that are hydrophobic have the advan-
tage that they can prevent or reduce the uptake of
moisture by the mineral layer after it has been dried.
This is particularly useful where the weathered mica
has a relatively high degree of cationic replacement,
i.e. where it contains a relatively high degree of ver-
miculite, so that undesired exfoliation of the mineral
layer when subjected to a fire can be eliminated. The
binder is preferably non-curable since curable binders
do not significantly improve the performance of the
wira and will normally reduce the speed at which the
wire can be manufactured.
We have observed that the presence of a polymeric
binder usually has a detrimental effect on the electri-
cal resistance of the mineral layer, usually during the
irst one or two minutes that the wire is subjected to
a fire, after which the effect becomes insignificant,
with the result that any wires that have been tested
for circuit integrity performance at reasonably high
~ 12 - RK325
voltages e.g. 200V, will either fail within the first
minute or two or will survive for a number of hours at
the test temperature. It is believed that the reduc-
tion in resistance of the wire is due to carbonisation
of the binder as the temperature rises and/or to the
generation of gaseous conductive species from the
binder or any other organic components in the cable,
and that this effect rapidly dies away as the carbon so
formed is oxidized. However, the detrimental effect on
the resistance caused by most of the binders may
usually be ameliorated by the presence of the thin
silicone layer. It is believed that the silicone layer
acts as some form of electrical and/or mechanical
barrier which prevents the char from the binder forming
an electrical short circuit. Thus, for the first
minute or so of the test, the electrical performance of
the wire is usually dominated by that of the silicone
layer. By the time the silicone layer has ashed, the
char from the binder will normally have completely oxi-
dized away and will no longer have any effect on the
wire performance. Thus, according to another aspect,
the invention provides a flame resistant electrical
wire which comprises a metallic electrical conductor
and electrical insulation which comprises an insulating
mineral layer that is formed from weatherad mica and
contains an organic binder, and, located on the mineral
layer, a layer of a material that will provide a tem-
porary barrier when the wire is subjected to a fire
which will reduce or eliminate the detrimental effect
of char formed from the binder on the electrical
resistance of the wire insulation.
The binder is preferably used in quantities in the
range of from 5 to 30~, and especially from lO to 25%
~3~9!1~3~
- 13 - RK325
by weight based on the weight of the weathered mica.
The use of smaller quantities may not sufficiently
improve the processability of the conductor and/or may
not improve the adhesion of the mineral layer to the
metal conductor adequately while the use of larger
quantities of binder may lead to the generation of too
much char for the silicone layer to mask. Also, it is
preferable not to use binders such as neoprene that
generate large quantities of char. Preferably the
binder has a carbonaceous char residue of not more than
15%, more preferably not more than 10% and especially
not more than 5%.
The char residue can be measured by the method
known as thermogravimetric analysis, or TGA, in which a
sample of the binder is heated in nitrogen or other
inert atmosphere at a defined rate, e.g. 10C per
minute to a defined temperature and the residual
weight, which is composed of char, i5 recorded. The
char residue is simply the quantity of this residual
char expressed as a percentage of the initial polymer
after having taken into account any non polymeric vola-
tile or non-volatile components. The char residue
values quoted above are defined as having been measured
at 850C.
As stated above, an outer protective layer, pre-
ferably a polymeric insulating layer, may be provided
in order to protect the underlying mineral layer from
mechanical abuse and in order to prGvide the required
insulating and dielectric properties during normal use.
Examples of polymers that may be used to form the outer
layer include olefin homopolymers and copolymers of
olefins with other olefins and with other monomers eOg.
:~ 3 ~
- 14 - R~325
vinyl esters, alkyl acrylates and alkyl alkacrylates,
e.g. low, medium and high density polyethylene, linear
low density polyethylene and ethylene alpha-olefin
copolymers, ethylene/propylene rubber, ethylene vinyl
acetate, ethylene ethyl acrylate and ethylene acrylic
acid copolymers, and styrene/butadiene/styrene,
styrene/ ethylene/butadiene/styrene block copolymers
and hydrogenated versions of these block copolymers. A
particularly preferred class of low charring polymers
is the polyamides. Preferred polyamides include the
nylons e.g. nylon 46, nylon 6, nylon 7~ nylon 66, nylon
~10, nylon 611, nylon 612, nylon 11 and nylon 12 and
aliphatic/aromatic polyamides, polyamides based on the
condensation of terephthalic acid with trimethylhexa-
methylene diamine (preferably containing a mixture of
2,2,4- and 2,4,4-trimethylhexamethylene diamine
isomers), polyamides formed from the condensation of
one or more bisaminomethylnorbornane isomers with one
or more aliphatic, cycloaliphatic or aromatic
dicarboxylic acids e.g. terephthalic acid and
optionally including one or more amino acid or lactam
e.g. ~-caprolactam comonomers, polyamides based on
units derived from laurinlactam, isophthalic acid and
bis-~4-amino-3-methylcyclohexyl) methane, polyamides
based on the condensation of 2,2-bis-(p-aminocyclo-
hexyl) propane with adipic and azeleic acids, and
polyamides based on the condensation of trans cyclo-
hexane-1,4-dicarboxylic acid with the trimethylhexa-
methylene diamine isomers mentioned above. Other
aliphatic polymers that may be used include polyesters
e.g. polyalkylene terephthalate and especially poly-
tetramethylene terephthalate, and cycloaliphatic
diol/terephthalic acid copolymers e.g. copolymers of
terephthalate and isophthalate units with 1,4-cyclo-
:~ 3 ~
- 15 ~ RR325
hexanedimethyloxy units, polyethers e.g. polybutylene
ether copolymers, and especially polyether esters such
as those having polytetramethylene ether and
poly(tetramethylene terephthalate) blocks; aliphatic
ionomers e.g. those based on metal salts of ethylene
(meth)acrylic acid copolymers or sulphonated olefins
such as sulphonated EPDM, and the like. Preferred
aliphatic polymers include polyethylene, polybutylene
terephthalate, ionomers based on metal salts of
methacrylated polyethylene, acrylic elastomers e.g.
those based on ethyl acrylate, n-butyl acrylate or
alkoxy-substituted ethyl or n-butyl acrylate polymers
containing a cure site monomer and optionally ethylene
comonomer, and block copolymers having long chain ester
units of the general formula:
O O
-OGO-C-R-C-
and short-chain ester units of the formula
O O
Il ll
-ODO-C-R-C-
in which G is a divalent radical remaining after
the removal of terminal hydroxyl groups from a
polyalk~lene oxide) glycol, preferably a poly (C2
to C4 alkylene oxide) having a molecular weight of
about ~00 to 6000; R is a divalent radical
remaining after removal of carboxyl groups from at
least one dicarboxylic acid having a molecular
weight of less than about 300; and D is a divalent
13~9t~
- 16 - R~325
radical remaining after removal of hydroxyl groups
from at least one diol having a molecular weight
less than 250.
Preferred copolyesters are the polyether ester
polymers derived from terephthalic acid,
polytetramethylene ether glycol and 1,4-butane
diol. These are random block copolymers having
crystalline hard blocks with the repeating unit:
( cH2 ) 4 o-3~8-
and amorphous, elastomeric polytetramethylene
ether terephthalate soft blocks of repeating unit
{ O(CH2)4-~-O C ~ COI_
n
having a molecular weight of about 600 to 3000,
i.e. n = 6 to 40.
Other preferred aliphatic polymers include those
based on polyether and polyamide blocks, especially the
so called a "polyether-ester amide block copolymersl' of
repeating unit:
-C-A-C-O-B-O-
tl 11
O O
wherein A represents a polyamide sequence of average
molecular weight in the range of from 300 to 15,000,
~3~0~
27065-1~8
pre~erably from 800 to 5000; and B represents a linear or branched
polyoxyalkylene sequence o~ average molecular weight in the range
of from 200 to 6000, preferably from 400 to 3000.
Pre~exably the polyamide sequence is ~ormed frorn
alpha,omega-aminocarboxcylic acids, lactams or
diamine/dicarboxylic acid combinatlons having C4 to C14 carbon
chains, and the polyoxyalkylene sequence is based on ethylene
glycol, propylene glycol and/or tetramethylene glycol, and the
polyoxyalkylene sequence constitu~es from 5 to 85%, especially
1~ from 10 to 50% of the total block copolymer by weight. These
polymers and their preparation are described in UK Patent
Specifications Nos. 1,473,972, 1,532,930, 1,555,644, 2,005,283A
and 2,011,450A.
The polymers may be used alone or in blends with one
another or with other polymers and may contain fillers e.g. silica
and metal oxides e.g. treated and untreated metal oxide ~lame
retardants such as hydrated alumina and titania. The polymers may
be used in single wall constructions or in multiple wall
constructions e.g. as described in British Patent Application No.
~0 2,128,394A. The polymers may be uncrosslinked or may be
crosslinked for example by chemical crosslinking agents or by
electron or gamma irradiation, in order to improve their
mechanical properties and to reduce flowing when heated. They ma~
also contain other material.s e.g. antioxidants, stabilizexs,
crosslinking promotors, processing aids and the like. In some
cases polymer insulation or at least the inner wall of the
insulation may he substantially halogen-
11 3~0~
- 18 - R~325
free. In addition, it has been found that certain
halogen~containing polymers may generate electrically
conductive species during a fire and so cause the wire
to fail prematurely. In those cases the insulation
preferably contains not more than 5~ by weight halo-
gens, especially not more than 1% by weight halogens
and most especially not more than 0.1% by weight halo-
gens. Howevex, in other cases, for example in the case
of airframe wire where high temperature ratings are
desirable, it may be appropriate for the outer wall or
primary jacket of the insulation to include a halogen-
ated polymer. One class of halogenated polymer that is
particularly useful is the fluorinated polymers, pre-
ferably those containing at least 10%, more preferably
at least 25~ fluorine by weigh~. The fluorinated
polymer may be a single fluorine containing polymer or
a mixture of polymers one or more of which contains
fluorine. The fluorinated polymers are usually homo-or
copolymers of one or more fluorinated, often per-
fluorinated, olefinically unsaturated monomers or copo-
lymers of such a comonomer with a non-fluorinated
olefin. The fluorinated polymer preferably has a
melting point of at least 150C, often at least 250C
and often up to 350C, and a viscosity (before any
crosslinking) of less than 104 Pa.s at a temperature of
not more than 60C above its melting point. Preferr~d
fluorinated polymers are homo- or copolymers of tetra-
fluoroethylene, vinylidine fluoride or hexafluoro
ethylene, and especially ethylene/tetrafluoroethylene
copolymers e.g. containing 35 to 60~ ethylene, 35 to
60~ tetrafluoroethylene by mole and up to 10~ by mole
of other comonomers, polyvinylidine fluoride, copoly-
mers of vinylidine fluoride with hexafluoropropylene,
tetrafluoroethylene and/or hexafluoroisobutylene, poly-
~ 1319 ~
27065-168
hexafluoropropylene~ and copolymers of hexafluoropropylene and
tetrafluoroethylene. Alternatively Cl-C~ perfluroalkoxy
substituted perfluoroethylene homopolymers and copolymers with the
above fluorinated polymers may he used.
In addition, the polymeric insulation, or the inner
layer of any polymeric insulation, pre~erably has a carbonaceous
char residue of not more than 15% by weight as determined by
thermogravimetric analysis. Such wires are ~he subject of our
copending Canadian patent application ~o. 571r48g enti~led
`'Electrical Wire'` filed on July 8, 1988.
The wire according to the invention may be formed using
most commonly available electrical conductor materials such as
unplated copper and copper that has been plated with ~in, silver
or chromium. In addition, if desired the conductor may be coated
with an electrically conductive refractory layer, for example as
described in European Patent Applica~ion No. l9Q,888, published on
August 13, 1986.
One embodiment of a wire in accordance with the present
invention and a method of manufacturing it will now be described
by way of example with reference to the accompanying drawing, in
which:
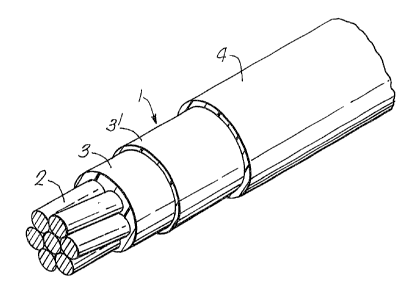
Figure 1 is an isometric view of part of a wire in
accordance with the invention with the thicknesses of the layers
of insulation exaggerated for the sake of clarity; and
lg
:L 3 ~
- 20 - RK325
Figure 2 is a schematic view of apparatus for
forming the wire of figure l; and
Figures 3a to c are graphical respresentations
showing the effect of a binder and a silicone
layer on the circuit integrity performance of
the wires.
Referring to the accompanying drawings, an
electrical wire 1 comprises a 22 ~WG seven strand
copper conductor 2 which has been coated with a 50
micrometre thick layer 3 of a partially weathered mica,
a 50 micrometre thic~ silicone polymer layer 3' and
followed by a 0~15mm thick extruded layer of polymeric
insulation 4 based on a blend of polytetramethylene
terephthalate and a polytetramethylene ether
terephthalate/polytetramethylene terephthalate block
copolymer.
~ The wire may be formed by means of the apparatus
shown schematically in figure 2. In this apparatus the
conductor 2 is fed into a bath 5 that contains a
colloidal suspension of the weathered mica and binder,
the suspension being fed from a supply bath 5', and
agitated in order to maintain uniform mixing of the
dispersion. The conductor passes down into the bath,
around a roller 6 and then vertically upwards as it
leaves the bath. A hollow tube 7 is positioned around
the part of the conductor that leaves the bath and a
hollow electrode 8 is located inside the hollow tube 7
so that the weathered mica is deposited on the rising
part of the conductor. This prevents the mineral
coating so formed being damaged as the conductor is
passed around roller 6.
.
~31~
- 21 - RK325
After the coated conductor leaves the bath it
passes through a drying tower 8 about 1.5 metres in
length that is heated by a counter current of warm air
so that the top of the drying tower is at a temperature
of about 200C while the bottom is at about 1~0C.
After the mineral coating has dried the coated conduc-
tor is passed through a coating pot 10 that contains a
silicone polymer. After a layer of silicone polymer is
applied to the wire, it is passed through a further
warm air drying tower 11 arranged to ha~e a temperature
of about 130C at the top and 90C at the bottom.
When the silicone layer has been applied and dried
the wire may then be spooled to await the provision of
an insulating top-coat or a top-coat may be provided
in line for example by means of an extruder 12.
The feed rate of the conductor 2 to the coating
apparatus will depend on the thickness of the intended
coating, the electrophoresis potential and the con-
centration of the weathered mica in the bath. Feed
rates in the range of from 2 to 20, and especially 5 to
10 metres per minute are preferred although increases
in the feed rate should be possible, for example by
increasir.g the dimensions of the bath in order to main-
tain the same residence time with higher conductor
speeds.
Figures 3a to 3c show the effect of both the
binder and the silicone layer on the electrical perfor-
mance of the wire insulation. In each case a 1 metre
long twisted pair of wires was heated to 900C in a gas
flame, and the electrical resistance between the wires
was recorded, and is shown along the ordinate, as a
~ 3~ ~ !1 (3 ~1.
~ 22 RR325
function of time since the heating commenced, shown
along the abscissa.
Figure 3a shows the performance of wires insulated
only by means of a 25 micrometre thick layer of
weathered mica that contained no binder. The
resistance fell when the wire was heated to a value
slightly below 107 ohms in about 60 seconds, and
remained at that level until the end of the test.
Although this insulating layer had satisfactory
electrical performance, it had inadequate mechanical
performance and could not be manufactured at economic
wire and cable processing rates.
Figure 3b shows the performance of wires in which
the mineral layer contains 15~ by weight of a styrene
butadiene styrene block copolymer binder. The mechani-
cal properties were excellent and the wire could easily
be mechanically handled through wire and cable pro-
cessing operations at rates of up to 50m minute~l. In
this case the electrical resistance of the wire fell to
a value of about 105 ohms after 30 seconds, whereupon
the resistance rose 510wly until it reached about 107
ohms after 150 to 200 seconds and remained at this
level until the test was terminated. The resistance
drop to 1050hms would greatly restrict the voltage
range to which such a wire could be specified.
Figure 3c shows the performance of the wires of
~igure 3b with an additional 50 micrometre layer of a
silicone elastomer to give a total thickness of 75
micrometres. The resistance falls to slightly over
107 ohms at 100 seconds after commencement of the test
and remains at that level until the test is terminated.
~3:~9~3~
270~5-168
Thus the deleterious effect of the organic binder i~ completely
xemoved. The mechanical performance of the insulation was good,
the limits being determined by the streng~h of the silicone layer.
The wire could easily be provided with a ~urther layer of
polymeric insulation.
The following Examples illus~ra~e the invention:
In all the ~xamples the worklng colloid that was used
for coating the conductor was formed as follows: 800 grams of a
weathered mica in accordance with our co-pending Canadian patent
application No. 571,506 entitled "Wire" filed on July 8, 198g, was
washed with boiling water for about 30 minutes and the resulting
liquid was decanted to remove the clay fraction. The mineral was
then refluxed for 4 to 24 hours in saturated sodium chloride
solution to replace the exchangeable cations with sodium ions.
This was then washed with distilled or deionised water to remove
excess sodium chloride until no further chloride ions could be
observed by ~esting with silver nitrate. The material was then
refluxed for 4 to 24 hours with molar n butyl ammonium chloride
solution followed by further washing with distilled or deionised
water until no chloride ions could be detected wi~h silver
chloride.
The swollen material was then worked in a Greaves mixer
for 20 minutes ~o shear the mineral and was allowed to stand for
20 minutes to sediment the unprocessed mineral. The ~op fraction
was used as the working colloid.
23
~ 3 ~
- 2~ - RR3~5
Example 1
A colloid having 4% by weight weathered mica and
15% by weight carboxylated styrene-butadiene-styrene
rubber based on the weight of the weathered mica, was
used as the plating bath. A 20 AWG wire was passed
through a 40 cm long bath of the colloid at a speed of
metres minute~l while the weathered mica was
electrophoretically deposited on the conductor at a
~.2V plating voltage and a 165 mA current. The coated
wire was then passed through a drying tower as shown in
the drawing to form a mineral layer of 30 micrometre
dry thicknessO The wire was then passed through a bath
of a two part silicone (KEl204 ex Shinetsu) and cured
again as shown in the drawing to form a 50 micrometre
thick silicone layer. Thereafter a lO0 micrometre
thick single wall insulation formed from low density
polyethylene containing 8% by weight decabro~odiphenyl
ether and 4~ antimony trioxide flame retardant was
extruded onto the wire.
The wire was tested for circuit integrity by
twisting three wires together and connecting each wire
to one phase of a three phase power supply, and then
heating the wire to 900C for a test period of three
hours in accordance IEC 331. The wire was able to sup-
port 300V phase-to-phase for the entire test at 900C
without failing (i.e. without blowing a 3A fuse).
~ 3 ~
- 25 RR325
Examples 2 to 5
Example 1 was repeated with the exception that the
following binders were used.
Example 2 polyvinyl acetate
Example 3 acrylic copolymer emulsion
Example 4 polyvinylidine chloride
Example 5 vinylpyridine terminated
styrene-butadiene-styrene
rubber
The wire was tested as described in Example 1 and
in each case the wires were able to support 300V phase-
to-phase at 900C for 3 hours.
Example 6
Example 1 was repeated with the exception that the
silicone layer was formed from an extended polydimethyl
siloxane based formulation.
The silicone composition was room-temperature
extruded onto the coated conductor to give a 75 to 100
micrometre thick layer and was vulcanised in a tube
furnace at 300C (20.5 second residence time).
The wire was tested as described in Example 1 and
supported 440V phase-to-phase for 3 hours at 900C.
~ 3 ~
- 26 - RK325
Example 7
Example 6 was repeated with the exception that the
plating voltage of the deposition bath was 15.5V
(300mA) which gave a mineral layer thickness of 40
micrometres.
The wire supported 440V phase-to-phase for 3 hours
at 900C~
Example 8
Example 1 was repeated with the exception that the
silicone used was a dip-coated solventless silicone
tsylgard 184) applied to a thickness of 70 micrometres.
The wire supported 300V phase-to-phase for 3 hours at
900C.
Example 9
Example 1 was repeated with the exception that the
low density polyethylene insulation was replaced with a
100 micrometre thick layer comprising:
~ 3 ~
- 27 - RR325
parts by weight
polybutylene terephthalate (PBT) 80
Surlyn ionom~r 20
decabromodiphenyl ether 8
antimony trioxide 4
Irganox 1010 2
triallyl isocyanurate cross- 5
linking promotor
The wire supported 300V phase-to phase for 3 hours
at 900C.
Example 10
Example 9 was repeated with the exception that the
PBT/Surlyn layer contained no flame retardant
(decabromodiphenyl ether/Sb203) and that an additional
polymeric layer of thickness 100 micrometres was pro-
vided on top of the PBT/Surlyn layer. The additional
layer had the composition:
parts by wei~
polybutylene terephthalate (PBT) 70
polybutylene terephthalate - 30
polybutylene ether tereph-
thalate block copolymer
ethylene bis-tetrabromo- 10
phthalimide
antimony trioxide 4
magnesium hydroxide 20
1 3 ~
- 28 - RR325
The wire supported 300V phase-to-phase for 3 hours
at 900C.
Example 11
Example 7 was repeated with the exception that the
low density polyethylene insulation was replaced by the
additional layer of Example 10. The wire supported
440V phase-to-phase for 3 hours at 900C.
Example 12
Example 6 was repeated with the exception that the
low density polyethylene insulation was replaced with a
100 micrometre thick layer of un-flame retarded high
density polyethylene. The wire supported 300V phase-
to-phase for 3 hours at 900C.
Example 13
Example 1 was repeated with the exception that the
binder used was a vinyl acetate/ethylene copolymer, the
plating voltage was 12.5V and current 422 mA, the line
speed was 10 metres minute~l and the silicone layer and
polymer insulation had the compositions shown below:
~ 3 11 ~
- 29 - RR325
Silicone composition
Parts by_weight
polydimethyl siloxane 61.2
fume silica 22.3
ground silica 6.8
fumed titania 3.4
Iron oxide 3.4
peroxide 2.4
thermal stabiliser -
(cerium hydrate) 0.5
elemental platinum 0.005
Insulation Parts by weight
polybutylene terephthalate 43O5
butylene terephthalate/
polybutylene oxide
- terephthalate copolymer 15.8
polycarbodimide 2.8
decabromo diphenyl ether 9.5
antimony trioxide 3.8
244-26 3 9
antioxidant (Irganox 1010) 1.9
magnesium hydroxide 18.8
The silicone layer had a thickness of lOt) um and
the polymer layer had a thickness of 125 um. The wire
was tested as described in Example 1 and was able to
support 440V t3A) phase-to-phase for the entire test at
900C.
- l3~a~.
_ 30 _ RR325
Example 14
Example 13 was repeated with the exception that
the polymer insulation had the composition:
Composition Parts by weight
polybutylene terephthalate 43.5
butylene terephthalate/
polybutylene 15.6
oxide terephthalate copolymer15.8
polycarbodimide 2.8
decabromodiphenyl ether 9.5
antimony trioxide 3~8
244-2~ 3.~
antioxidant (Irganox 1010) 1.9
magnesium hydroxide 18.8
The plating voltage was 11.5V and current was 365
mA. The mineral layer has a thickness of 25 um and the
silicone layer had a thickness of 125 um.
The wire was able to support 440V (3A) phase-to-
phase for the entire test (3 hours) at 900C.