Note: Descriptions are shown in the official language in which they were submitted.
-` 1 32 1 080
Title of the Invention
Flow Injection Analysis Method and Apparatus thereof
Backqround of the Invention
This invention relates to a method of quantitative
analysis by which a chemical component of a substance is
quantitatively determined and also to an apparatus thereof,
and more particularly it relates to an improved Slow injec-
tion analysis (hereinafter, simply called as FIA) and impro-
ved detection members in an apparatus employed for said FIA 10
method.
Further more particularly, this invention relates to a
technical method of analysis in which a sample solution (in
other words, a carrier solution containing a sample) and a
reagent solution are flown respectively at a trace amount in
an anticorrosive tube such as a tube made from Teflon and
- ~
are reacted therein, their reaction product is measured
,
within a flow cell by means of its physical or chemical
characteristics such as done generally in a spectrophotomet-
ric method, and chemical component or components to be 20
analyzed in the sample are thus determined, and the inven-
tion also relates to improvements of an apparatus which can
advantageously be employed in such analysis method.
In general, as wet chemical analysis method, there are
gravimetric analysis, volumetric analysis, spectrophotomet-
ric analysis and so on.
In the gravimetric analysis, chemical components to be
'~
* trade mark
,.-
~r- . . - , :, ,
,:: : : ~ -- :
.
.
1 32 1 080
analyzed shall be compl~tely of their 100% separated by
filtration to the insoluble precipitates, which are in turn
weighed for their quantitative determlnation. And, also in
the volumetric analysis and the spectrophotometric analysis,
a solution containing sample components to be analyzed shall
be reacted completely with a reagent solution by 100%.
Hence, it is prerequisite in the spectrophotometric
method that the reaction proceeds quantitatively without
being accompanied by any side reaction. It shall be noted
also that similarly to other wet chemical analyses in a 10
spectrophotometric method too, a component to be analyzed
has to be changed completely of its 100% to a colored com-
pound, absorbance of which is measured for a quantitative
determination. Thus, the prerequisite basic principle
common to a quantitative analysis is that the determination
has to be made completely as nearly as possible to its 100%.
On the other hand, this invention of FIA method has
been made with the following fundamental design conceptions,
that is, (1) to ailow a reaction rate of FIA to come as
nearly as possible to 100%, (2) to keep the reaction condi- 20
tions constant, and thereby to obtain a constant reaction
state which is close to 100% even if the reaction rate can
not be absolutely 100%, and (3) to save the amount of~
reagents by using a fine tube and a special pump.
However, this principle can not easily be attained in the
conventional FIA. Because, a reagent solution and a sample
solution stay within the tube only for a short period of
, .
-
- '
1 32 1 080
time and consequently they are subjected to a reactlon only
for a short period of time. This results that in almost all
analysis operations, their reactions have not been completed
by 1oo% but are on the way to completion, whereby perfect
determination can hardly be expected by a conventional FIA.
The FIA method is generally practiced by flowing a
reagent solution in a fine anticorrosive tube preferably
made from Teflon, injecting into the flow a sample solution
of several tens to several hundreds ~ , obtaining a reaction
compound by the reaction of said sample and reagent within 10
the tube, and subjecting said compound to the measurement
within a flow cell for the determination of a component in
the sample to be analyzed by means of physical or chemical
characteristics of the reaction compound. In this respect,
an apparatus used in the FIA method resembles to the one
used in liquid chromatography.
However, the FIA method intends to analyze only a
single component in a multicomponent homogeneous liquid
phase, while a liquid chromatography is to make a separate
analysis of each component in a multicomponent homogeneous 20
liquid phase.
Since the FIA method which is the subject of this
invention differs from a liquid chromatography method basi-
cally and noticeably as mentioned above, following remarks
have to be taken into consideration in carrying out said
method successfully.
(a) Sample and reagent solutions have to be mixed up
' ' ' ~
1 32 1 080
thoroughly arld
(b) A single component is to be determined exclusively
with high sensitivity and without interference of other
components.
In order to observe the remarks, this invention is also
provide a specific flow cell.
Brief SummarY of the Invention
In view of the above, this invention is to provide an
application engineering about assembly of flow lines, embo-
died herein as a method and an apparatus which have been
15improved with the following features for solving the afore-
mentioned drawbacks accompanied to a conventional FIA method
and consequently for ascertaining more precise determination
through the flow lines.
Such features are:
(I) Sample and reagent solutions are supplied
alternately to each other and respectively at a trace amount
so that within a fine reaction tube, both solutions can make
liquid-to-liquid contacts with wider reaction surface areas,
whereby they are thoroughly mixed up, resulting in the
improvement of a reaction rate, and
(II) Flow rates of the reagent and sample solu-
tions as well as a reaction temperature thereof are made
constant, whereby a reaction rate is also made constant.
~,,
,
1 32 1 080
More particularly, the flow injection analysis
method according to the invention as claimed hereinafter is
characterized in that a sample solution and a reagent
solution are fed through flow lines which are independent to
each other and each of which has an inner diameter of 0.25-
1.0 mm, and subsequently they are injectedly supplied to a
mixing flow line alternately and respectively at a prede-
termined amount of 1.25-2.0 ~e per injection batch, at least
some part of the mixing flow line being accommodated in a
thermostat bath, an end of said mixing flow line being
connected to an spectrophotometric flow cell which is in
turn connected at its outlet to a back pressure coil for the
adjustment of an inner pressure exerting within the flow
lines.
4a
'
" 1 32 1 080
In said method, a sample solution and a reagent solu-
tion pass throuqh flow lines which are independent to each
other, and then, they are injectedly supplied to a mixing
flow line alternately to each other and respectively at a
predetermined trace amount, whereby they are mixed and
reacted thoroughly.
And, when required, at least a part of said mixing flow
line is passed through a thermostat bath so that the line is
kept at a constant temperature, whereby reaction rate
achieved thereby can also be made constant. And, in addi- 10
tion, there is connected at a back portion of a flow cell a
back pressure coil so that bubbles are prevented to form
thereat and a smooth flow can be obtained with few irregular
pulsation.
While it is preferable that an injection supply amount
of the sample and reagent solutions are as small as possib-
le, said amount is apparently subject to a flow rate accura-
cy controlled by a supply pump, viscosities of the reagent
and sample solutions, diametric accuracy of a reaction tube,
..
and others. Nevertheless, the most appropriate range of 20
said amount and an inner diameter of flow lines can be
selected as follows:
To wit, it has been found that when an inner tubular
diameter of flow lines is less than 0.25mm, frictional
resistance between inner walls of the tubes and solution
flows becomes too high, inner pressure in the tubes becomes
also disadvantageously high, and efficient mixing of sample
,
.
:
1 32 1 08~
and reagent solutions can hardly be obtained. On the
contrary, when the inner diameter exceeds 1.0mm, flow resis-
tance within the tubes lowers, and concurrently inner
pressure lowers also, whereby sample and reagent solutions
are consumed at an amount more than those required for
reasonable analyses and whereby costs for analyses become
high, although the manufacture and handling of an apparatus
including the tubes become easier.
Preferable tubular diameters thus selected above can
accordingly confine a preferable range of flow rates through 10
the tubes. In practice, when two solutions are injected
into a mixing and reaction line alternately to each other
and respectively at a precisely trace amount so that reac-
tion rates thereof can be kept high and constant, extremely
accurate supply of the solutions can hardly be attained if
an injection supply amount per a batch or at each time is
less than 1.25 ,ue, and on the contrary, if said amount
exceeds 20,u~, the reagent and sample solutions would insuf-
ficiently be mixed.
Therefore, in this invention, inner diameters of these 20
flow lines for the sample solution and for the reagent
solution which are independent to each other, and an inner
diameter O'L the mixing flow line shall preferably be within
a ~ange of 0.25-1.0mm, while an amount of the solutions
which are injectedly supplied into the mixing flow line
alternately shall preferably be 1.25-20~ at each injection
batch.
1 32 1 0~0
Secondly, the spectrophotometric flow cell provided to
a detecting member in this invention is briefed below.
When an inner diameter of the mixing flow line which is
followed by the above-mentioned flow cell is selected as
0.25-1.Omm, an optical path diameter of the flow cell has to
be expanded largely than that of the mixing flow line, that
is, as much as a range of 1.5-2.5mm, while its o~tical path
length shall preferably be 10-50mm.
Brief DescriPtion of the Drawinq 10
In the accompanying drawing which illustrates preferred
embodiments of this invention;
Fig. 1 is a view showing a flow linè in the method made
in accordance with this invention,
Fig. 2 is an explanatory view showing states of liquid
phases within a mixing flow line or a reaction coil,
Fig. 3 is a sectional view of an example of flow cell
made in accordance with this invention, and
Fig. 4 is a side view of said flow cell shown in Fig.
3. 20
Detaild Description of the Invention
Now, this invention is described more in detail by way
of the following examples:
Example 1.
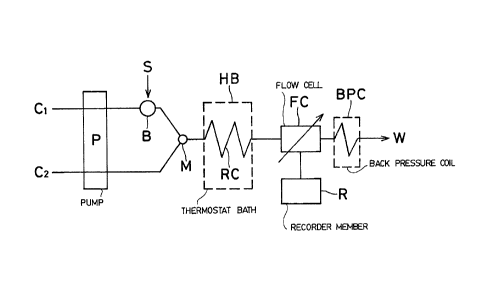
With reference to Figs. 1 and 2, in this invention, a
sample solution C1' and a reagent solution C2' are injected-
,
1 32 1 0~0
ly supplied, through a flow line C1 and another flow line C2which are independent to each other, into a mixing flo~ line
alternately to each other and respectively at a trace amount
as shown in Fig. 2 so that the solutions may have larger
contact sur~aces for efficient mixing thereof.
In order to achieve such alternate injection supplies
of the solutions, a pump P used in this invention method
shall preferably be those of a non-pulsation double plunger
type, one of which plungers for the sample solution flow
line C1 and another of which plungers for the reagent solu- 10
tion flow line C2 are not synchronized to each other, and
injection supply amount made by which is controlled to be
about s,uQ per a stroke. This injection amount is correspon-
dent to a volume of about 25mm length of a Teflon tube of
0.5mm in inner diameter. And, in order to obtain a constant
rate of flow without pulsation, it is recommendable to
employ a phase different double plunger type pump having,
for example a stroke length of 1mm, a stroke delivery of
about 5,uQ, and a plunger diameter of about 2-3 mm. The
letter B in Fig. 1 represents a hexagonal injection valve 20
which is located in thesample solution line C1 and by which
a sample is supplied under pressure into a carrier solution
flown in the line C1. RC represents a reaction coil in
which the two solutions are mixed and reacted. It is prefe-
rable, as shown in the drawing, to accommodate the reaction
coil RC within a thermostat bath HB so that a reaction
temperature of the solutions C1' and C2' ~ay be kept
1 32 1 08~)
constant wnere~v reaction r~te thereo~ is raised high and
kept constant.
The solutions which have been reacted thoroughly in the
reaction coil RC of a mixing rlow line system, are sent to a
flow cell FC where they are subjected to a measurement by an
spectrophotometer and others to obtain a measured value
which is in turn recorded by a recorder member R. And, the
solutions which have been measured are brought outside of
the lines as a waste solution, by means of a back pressure
coil BPC of an inner diameter of 0.20-0.50mm for example. 10
This waste solution W is discharged only after it has been
treated so as not to bring about any water pollution. The
above-mentioned back pressure coil BPC works also to prevent
the solutions throughout the flow lines from producing
bubbles, and as well to obtain a stable constant rate of
flow without pulsation. While it is observed sometimes to
employ a pelister type pump in order to keep solutions
within the flow lines under a condition without pulsations,
this type of pumps are inferior to plunger type pumps with
respect to their endurance. Hence, it is preferable to 20
employ plunger type pumps as explained above.
It shall be noted that features specific to this inven-
tion could be applicable not only to flow injection analyses
but also to other flow line analyses.
Example 2.
In this example, an example of flow cell which is made
in accordance with this invention and could advantageously
1 32 1 080
be employed in a detectLng member of the present FIA appara-
tus, is described in more detail with reference to Figs. 3
and ~.
In the FIA apparatus which uses, as shown by Fig. 1,
the flow cell made in accordance with this invention, the
solution C1' with the sample S and the reagent solution C2'
are supplied by the non-pulsation double plunger type pump P
to the reaction coil RC via a mixing joint M alternately and
respectively at a trace amount from the flow lines C1 and C2
which are independent to each other. The solutions are 10
mixed and reacted thoroughly in the reaction coil RC, and
then sent to the flow cell FC which constitutes a detecting
member and in which the component to be analyzed is detected
and measured. The values measured thus are recorded by the
recorder R, while the solutions which have been subjected to
the measurement pass through the back pressure coil BPC, and
then discharged outside of the system as the waste W. In
this instance, at least a part of the mixing flow line which
is designated as the reaction coil in Fig. 1 is passed
through the thermostat bath HB so that a reaction rate in 20
the mixing flow line can be raised high and also constant.
The flow cell FC which constitutes a detecting or
measuring member is consisted of, as best shown in Fig. 3, a
tubular body 1 made from brass to which there is insertedly
fitted a cylindrical body 2 made from Teflon and having at
its central axis an optical path channel 3. To the both
free ends of the brass tubular body 1, there are fitted by
1 0
1321080
screws 5 discal frames 4 made from brass and having at their
centers optical path holes 4a, ~iameter of which is equal to
the optical path channel 3.
Numeral 6 indicates transparent sheets of glass which
are fitted to the both ends of the optical path channel 3.
The glass sheets 6 which are closely fitted to the cylindri-
cal body 2 by means of the frames 4 through spacers 7,
prevent solutions to be determined from leaking therefrom.
The reacted solutions sent from the reacti~on coil RC
enter into the optical path channel 3 through an inlet 8 of 10
the flow cell FC and one end of said channel. When they are
subjected to an spectrophotometric measurement, they come
out from an outlet 9 and are discharged as the waste W after
having passed the back pressure coil BPC.
In the following, an optical path diameter and an
optical path length of the optical path channel 3 which is
provided in the flow cell made in accordance with this
invention, is explained.
The flow cells of different optical path diameters were
made, while their optical path length was made constant, 20
viz., 1Omm and the inner diameter of the mixing flow line
was made 0.5mm.
A 10-5M picric acid solution was flown through the line
C1 of the FIA apparatus of Fig. 1 as a sample solution
thereof, while a pure water through the line C2. They were
measured of their absorbance (aAbs) at 4,000A by the flow
cell, showing the result of about 0.05. An output of the
:
. .,. ~-:
1 32 1 0~0
spectrophotometer emploved ln t.ie ~easurement was
100mV/Abs, r~hlle ranges recorded bv the recorder R were 10,
5, 2, and lmv.
The above experimental results, that is, relations of
different optical path diameters and S/N ratios are given in
the following Table 1.
Table 1.
No. Optical Path o.P. length (mm) Volume (,uQ) S/N ratio
Diameter(mm) 10
1 1.0 10 8 1
2 1.5 10 18 5
3 2.0 10 31 10
4 2.5 10 50 20
5 3.0 10 71 20
6 4.0 10 130 2G
7 5.0 10 20~ 20
As readily be understood from the above Table 1, larger
the optical path diameter is, better the S/N ratio becomes, 20
resulting in improving electrical sensitivity of the flow
cell. However, it is noticed that when the optical path
diameter exceeds 2.5mm, sensitivities were raised up as
optical volumes increased sharply, while strays also
increase accordingly and S/N ratios thereby tend to saturate.
On the contrary, when the optical path diameter was less
than 1.5 mm, an optical volume remarkably decreased, resul-
..
1 321 080
ting in making it difficult to conduct such analyses whichare of high sensitivity and free from other interferences.
Another series of e.Yperiments were made with respect to
an optical path length of the flow cell, in which an inner
diameter of the mixing flow line was made 0.5mm, and an
optical path diameter of flow cells were made constant as
1.5mm, while their optical path lengths were made different.
The sample solution in the line C1, the solution in the line
C2, and other experimental conditions were same to the above
experiments of the Table 1. 10
Absorbances (~Abs) at 4,000A were measured as shown in the
following Table.
Table 2.
_
No. Optical Path Cell O.P. ~Abs aAbs
Length (mm) Volume (JuQ) Diameter (mm) ratio
_
1 5 9 1.5 0.0250 0.50
2 10 18 1.5 0.0504 1.00
3 20 35 1.5 0.102 2.02
4 50 88 1.5 0.255 5.06 20
5 100 _150 1.5 0.520 10.30
As shown in the above Table 2, it becomes known that
longer the optical path length is, greater the absorbance
ratio becomes. This means that the more the optical path
length is, the more precisely a solution even of a low
chromaticity can be measured. In other words, when the
1 32 1 080
o?~ical ~ath length is sufficientl~ long, even a ~ery trace
amount of a component in a sampLe solution can be determined
with a high sensitivity. However, it is noticed that "hen
the optical path length exceeds 50mm in the determination of
a solution of a high concentration, absorbance thereof satu-
rates whereby a difference of chromaticity can hardly be
obtained. On the contrary, when it is less than 1Omm,
chromaticity can not precisely be measured.
Therefore, an optical path length of the flow cell
shall preferably be within a range of 10-50mm. 10
In view of the explanation made above with respect to
this invention, various remarkable effects obtained by it
are enumerated below.
(I) This invention can provide an extremely high
reaction rate which can indefinitely be close to 100%~ And
in addition, since the reaction rate can regularly be kept
constant, extremely high precise analyses can be made prom-
ptly and can avoid the use of excessive amount of reagents,
whereby the analyses become very economic.
(II) When the flow cell made in accordance with this 20
invention is employed, an entrance optical value in case of
its optical path diameter being 2mm for example, will be
four times of that of a conventional liquid chromatographic
flow cell of an optical path diameter of 1mm, whereby inter-
ferences by other components which are not to be analyzed
can be well avoided, whereby S/N ratios are greatly improved
and a component to be analyzed can accordingly be determined
14
1321080
determined ~ith a high sensitivity.
(III) Compared to inner diameters of the mixing and
reaction line in accordance with this invention, viz., 0.25-
1.0mm, an optical path diameter of the flow cell is as large
as 0.5-2.5mm, whereby the solution to be analyzed is positi-
vely urged to disperse evenly when it reaches the flow cell.
(IV) In addition, the invention has other advantageous
effects such as the improved detecting sensitivity the flow
cell has attained on account of its optical path length
being selected as 10-50mm. 10
-'
: . .