Note: Descriptions are shown in the official language in which they were submitted.
32 ~ 7~2
THIXOT~OPIC AQUEOUS AU~OMATIC
DISHWAS~ING DETERGENT COMPOSITIONS
S FIELD OF INVENTION
The present invention relates to scented and unscented
liquid automatic dishwashing detergent compositions
having thixo~ropic properties and improved physical
stability and methods for preparing such compositions.
BACKGROUND OF_THE INVENTION
Recent activity has focused on paste-form, gel-like and
thixotropic forms of liquid automatic dishwasher
detergents because of their des`irable adYantages to the
con~umer over conventional powdered forms of detergents.
These advantages include ease of dispensing from the
container, lower volume consumption per wash load
becauRe of higher concentrations of active ingredients,
and long term storage without moisture spoilage~
~he development of liquid automatic dishwasher
detergents has had problems due ~o the requirement that
such formuLations incorporate a number of ingredients
which are generally incomparLble, i.e., they tend to
react with each other pr ior eo use in the dishwasher.
Additionally, a liquid automacic dishwasher detergen~
should also exhibit thixotropic properties, i.e., it
-2- ~321~2
should be highly viscous in a quiescent state and have
relatively high yield values (Bingham Plastic), but when
subjected to a shear stress, such as being squeezed
through a orifice, it should have flow properties
similar to a viscous liquid so it can be easily
dispensed into a dishwasher detergent dispenser cup.
Once inside the dispenser cup it should quickly revert
to the high viscosity/Bingham plastic state.
Another common problem with liquid automatic dishwashing
detergent compositions is that they tend to separate
into substantially solid and liquid phases during the
shelÇ life of the product. Improvement in the phase
stability has been accomplished by additives, however, a
lS drawback of this approach is that any addition to the
detergent composition may adversely affect the
rheological properties of the detergent composition.
Similarly, addition of a fragrance to a liquid automatic
dishwasher detergent to impart a desirable scent has
also been a problem because of its effect on the complex
balance of ingredients within the composition necessary
to produce the de~ired rheology and other physical
characteristic~. For example, the presence of a
chlorine releasing compound would be expected to have
detrimental afect on an oil-type fragrance that is
readily oxidized. In addition, the presence of oil-like
ragrances would be expected to have an adverse effec~
on the phase stability of such compositions because Oc
their tendency to act as defoamers.
SUMMARY AND ADVANTAGES OF ~HE rNVENTION
~he present invention pcovides ?cented and unscented
thixotropic liquid automatic dishwasher detergent
compositions havin~ improved physical stability, as well
as a novel process whereby liquid automatic dishwasher
~3~7~2
--3--
detergent compositions having thixotropic properties can
be produced which are physically stable and not prone to
separation during extended periods of storage.
The presen~ invention also provides a novel process
whereby air is entrained in a thixotropic liquid
automatic dishwasher detergent composition so as to
maintain stability for extended periods of storage.
A further advantage of the present invention is that it
provides a novel process whereby a fragrance is
introduced to a liquid automatic dishwasher detergent
composition to impart a pleasant scent to the
composition without adversely affecting the phase
stability or rheological properties.
The present invention provides a liquid automatic
dishwasher composition having thixotropic properties and
improved long term physical stability and a method for
making such compositions.
The invention provides a thixotropic liquid automatic
dishwasher composition comprising a concentrated
dispersion of solid particles in a liquid phase
cha~acterized in that air bubbles are entrained in the
compo~ition in an amount suf~icient to equilibrate the
~pecific gravity of the liquid phase with the bulk
specific gravity oE the composition, thereby improving
the physical ~tability of said thixotropic dishwashing
composition,
The present invention also provides a scented liquid
automatic dishwasher detergent composition having
thixotropic properties and improved long term physical
stability. According to the ~nvention, specific scen~ed
liquid automatic dishwasher detergent compositions may
contain severa. oc all of t~e Eo_lovilg:
' ~
~4~ ~32~7'~2
(l) alkali metal tripolyphosphates to soften or tie up
hard-water minerals and to emulsi~y and/or peptize soil;
(2) sodium silicate to supply the alkalinity necessary
for effective detergency and to provide protection for
fine china glaze and pattern;
(3) alkali me~al carbonate, generally considered to be
optional, to enhance alkalinity;
(4) a chlorine-releasing agent to aid in the
elimination of soil specks which lead to water spotting;
~5) chlorine bleach stable defoamer to reduce foam,
thereby enhancing machine efficiency and supplying
additional detergency;
(6) chlorine bleach stable surfactant, sometimes
referred to as detergent active material,which is
compatible with the other ingredients and provides for
detergency;
(7) thixotropic thickener in an amount effective to
provide the composition with a thixotropy index of about
2.0 to 10;
(8) caustic, as necessary, to adjust the pH to within
the range of about 10 to 14;
~9) a long chain fatty acid or salt of a long chain
fatty acid as a physical stabilizer in an amount
effective to increase the physical s~ability Gf the
composition;
(lO) fraqrance in an amount effective to impart a
pleasant scent to the composition without adversely
aEEectinq the stability or th.xotro~c propertles ot the
-5- ~32~2
composition;
(11) water in an a~ount ef~ective to avoid destruction
of the desired thixotropic properties: and
(12) air in the form of bubbles having a diameter of
about 5 to about 80 microns in an amount ranging from
about 2~ to 10% by volume, effective to provide the
composition with a bulk specific gravity of about 1.20
to about 1.35 and improve the physical stability of the
composition.
The present invention provides a process for
manufacturing liquid automatic dishwasher detergent
compositions having a ~ulk specific gravity about equal
to the liquid phase specific gravity and exhibiting
improved physical stability and rheological properties,
comprising the steps of:
(a) forming a predispersion mix containing
water, physical stabilizer, defoamer and
surfactant;
(b) forming a thickener premix containing the
predispersion mix of step (a), water and a
thixotropic thickener and mixing the
thickener premix until the thixotropic
thickener is hydrated and deagglomerated;
(c) mixing, preferably high-shear mixing, the
thickener premix ~rom step (b) and
additional water while adding other
desired detergent ingredients to form a
liquid automatic dishwasher detergent
composition, containing about 2 to 10% by
volume air, and
6 ~32~ 1~2
(d) homogeniz.ng the li~uid automacic
dishwasher detergent composition to effect
equilibration of the bulk and liquid phase
specific gravities of the composition.
The process of the present invention may be carried out
under conditions which ensure that the thixotropic
liquid automatic dishwasher composition achieves a
highly stable condition~ It has been found that this
condition is reached when about 2% to about 10% by
volume of air is entrained in the composition and the
bulk specific gravity of the composition is about equal
to the liquid phase specific gravity of the composition.
The present invention also provides a process whereby a
fragrance is added to the liquid automatic dishwasher
detergent composition under conditions and in an amount
so as not to adversely affect the thixotropic properties
or physical stability of the composition. For example,
a process in which the liquid automatic dishwasher
detergent composition from step (d) above i5 first
cooled to a maximum temperature less than about ~5F and
thereafter the liquid automatic dishwasher detergent
composition and the desired fragrance are introduced to
a mixer wherein the fragrance is uniformly dispersed
throughout the final liquid automatic dishwasher
detergent product.
Thus, the present invention provides a process for
combining ingredients in proportions so as to provide a
liquid automatic dishwasher de~ergent product having a.
improved combination of prooerties, particularly
thixotropy and phase stabili~y.
15 DESCPcIPTION OF THE DRAWING .~ND ~RMS
The drawing is an elevational schematic of the preferred
:
.
~ 3 2 ~L rl~ ~; 2
process of the present invention.
The term "bulk specific gravity", as used herein, refers
to the specific gravity of a homogeneous liquid
automatic detergent composition including all required
ingredients. The term "liquid phase specific gravity"
as used herein, refers to the specific gravity, as
measured by conventional techniques, of a deaerated
liquid removed centrifugally from the liquid automatic
detergent composltion, i.e., bulk composition.
The term "thixotropy index" is the ratio of viscosities
measured at 3 rpm and 30 rpm at room temperature after 3
minutes using a Brookfield HATDV II viscometer with a
lS spindle.
DETAILED DESCRIPTION OF THE INVENTION
The present invention is directed to liquid automatic
detergent compositions having thixotropic proper~ies a~
long term phase stability and which may include a
fragrance which does not adversely affect the propert~e~
of the composition. The present invention is also
directed to a process for producing liquid automatic
detergent compo~itions having thixotropic properties an~
improved long term phase stability wherein air is
entrained into the composition in an amount from abou~
2~ to lO~ by volume so as to effect an equilibration o~
the bulk and liquid phase specific gravities of the
composition. Moreover, the present invention~is also
directed to a process for incorporating a fragrance l~tO
liquid automatic detergent compositions without
adversely affecting the rheo1oqicaL properties or 10~5
term phase stability of the composition.
A preferred example of the present invention provides
for a composition which may be scented with fragrance,
-8- ~32~
comprising the following ingredients on a weight basis
unless specified otherwise:
(a) 5 to 35% alkali metal tripolyphosphate;
s
(b) 2.5 to 20~ sodium silicate;
(c) 0 to 9~ alkali metal carbonate;
(d) 0.1 to S~ chlorine bleach stable, water
dispersible organic detergent active
material;
(e) 0.01 to 5% chlorine bleach stable foam
depressant;
(f). chlorine bleach compound in an amount t~
provide about 0.2 to 4% o available
chlorine;
(g) thixotropic thickener in an amount
sufficient to provide the composition
with a thixotropy index of about 2.0 to
10;
(h) alkali metal hydroxide, as necessary, to
adjuet the pH from about 10 to 14;
(i) a long chain fatty acid or lts salt as a : -
physical stabilizer in an amount
effective to increase the phyaical
stability of the composition; ~
(j) (optionally) fragrance in an a~ount
effective to prov~ide a scent and~to dVOi~
destruction of the~desired thixotropy a,~d
physical stability of the composltlon;~
~ ~2~7~
g
(k) water in an amount effective to avoid
destruction of the desired thixotropic
properties; and
(1) air in an amount ranging from about 2~ to
10~ by volume, effective to provide the
composition with a bulk specific gravity
of about 1.20 to about 1.35.
According to the process of the present invention, a
phase stable, thixotropic liquid automatic detergent
composition is produced by entraining air into the
composition so as to effect an equilibration of the
specific gravities of the bulk and liquid phases of the
composition.
It has been found that concentrated dispersions which
contain both liquid and solid phases, such as the liquid
automatic dishwasher detergent compositions, can be
stabilized by dispersing an appropriate amount of air in
the form of micron size bubbles throughout the liquid
phase of the composition. It has also been found that
the air can be dispersed and stabilized as bubbles
throughout the liquid phase by employing a multi-part
stabilizing ~y tem comprising two or more components,
preferably three, categorized generally as, physical
stabilizers, foam depressants or defoamers and
surfactant~. While not wishing to be bound by any
theory to explain how the stabilizing system and air
interact in the liquid automatic dishwasher detergent
compositions, it is believed that the stabilizing
components interact at the airjllquid interface such
that the hydrophobic groups of these components are
oriented towards the~air bubbles ~hlle the hy~rophilic
groups are oriented towards the aqueous~ phase. The
hydrophilic groups, in turn, interact with the solid
particles of the suspension either through hydrogen
:
::
-10- ~3~
bonding or through electrostatic interaction. In other
words, the liquid/air interface consists of the
stabilizing system components and solid particulates
giving rise to a liquid crystalline type structure for
S the interface.
Accordinq to the preferred process of the present
invention, a three-part stabilizin~ system produces a
highly stable liquid automatic dishwasher detergent
composition by stabilizing the micron size air bubbles
throughout the composition such that the bulk specific
gravity of the liquid automatic dishwasher detergent
composition is about equal to the specific gravity of
the liquid phase only, in the liquid automatic
dishwasher detergent composition. It is at this
condition that the liquid automatic dishwasher detergent
composition exhibits high stability, i.e., there is
little or no tendency for phase separation due to
density variations in the composition.
In order to effectively disperse the air throughout the
liquid automatic dishwasher detergent composition it has
be~n discovered that the size of the entrained air
bubbles must be greater than the size of any dispersed
solid particles. The bubble size generally may vary
fro~ about 5 to about ~0 microns and preferably from
about 20 to about 60 microns. Air bubble size can be
controlled, generally, by varying the blade tip speed o~
the dispersers or agitators during the mixing
operations. It has also been found that air entrainment
from about 2 to about 10% by volume produced phase
stable compositions, the preferred range being from
about 4.0 to about 9.0~ by volume, the most preferred
range being from about 6.5 to about 8.5% by volume.
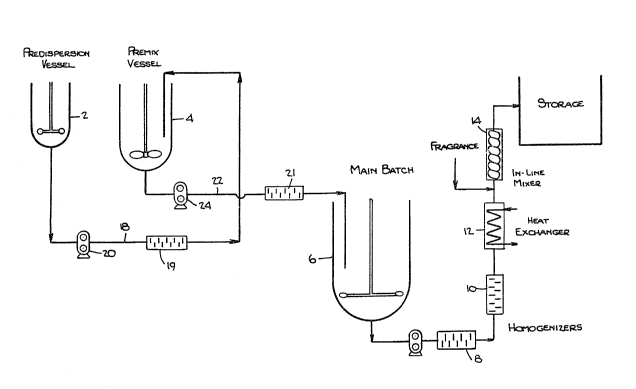
As best seen in the drawing, the prooess of the preses~
invention can be performed in a blending system
2 :1 7 L~ 2
incorporating predispersion vessel 2, premix vessel 4,
main batch vessel 6, homogenizers 8, 10, 19 and 21, heat
exchanger 12, in-line mixer 14 and storage tank 160 As
can be seen from the drawing all vessels are open to the
atmosphere.
A predispersion mix comprising the stabilizing system is
prepared in predispersion vessel 2 then fed to the
premix vessel 4 through line 18 and homogenizer 19 via
pump 20 where it is added to a thixotropic thickener to
prepare a thickener premix. The thickener premix is
then fed to the main batch vessel 6 through line 22 and
homogenizer 21 via pump 24 wherein the remaining
components of the liquid automatic detergent composition
are added.
The detergent composition from vessel 6 is then fed
through homogenizers 8 and 10 and thereafter cooled in
the exchanger 12. If a scented dishwasher detergent
composition is desired, the cooled product is fed
through an in-line static mixer 14 where a fragrance is
added. The liquid dishwasher detergent composition is
then fed to tank 16 where it is stored.
In the preferred process of the present invention, a
liquid detergent predispersion mix is first prepared
including the selected physical stabilizer, foam
depressant and surfactant components of the liquid
automatic dishwasher detergene composition as well as a
portion of the total liquid automatic dishwasher
detergent water content. 3ependlng on the selection o~
stabilizing components, one or more of the components
may initially be solid, requ: ir.g either the addition
heat to form a melt or ~he addition of water to form a
solution or emulsion. The amount of water added to the
predispersion mix should be limited so as to maintain a
highly viscous mix. The predispersion mix is subjected
~3217~
-12-
to mixing, preferably high-shear mixing for about S
minutes during which time the predispersion mix
temperature may exceed 100F. High-shear mixing, as
used herein, is defined in terms o~ shear rates and is
dependent on a number of variables, the most important
being the configuration of the mixing vessel and the
impeller tip speed. For example, the pre-dispersion mix
is prefe.ably high shear mixed in a Myers HSD~ using an
8 inch impeller at an impeller speed of about 4500
ft/min. rhe "high shear" rate at this condition is
approximated to be of the order of 100 sec 1.
The predispersion mixing step may be accomplished in
other conventional milling or high-shear mixin~
equipment, for examp;e, roller mills, colloid mills and
Premier mills.
The predispersion mixing step is followed by a second
mixing step during which a thixotropic thickener, e.g.,
slay, and an additional portion of the total liquid
automatic dishwasher detergent water content is added tC
the predispersion mix to Eorm a thickener premix. The
thickener premix is preferably subjected to low-shear
mixin~ for about 20 minutes during which time the
thickener is hydrated, deagglomerated and dispersed
throughout the thickener premix. Low-shear mixing, as
used herein, is also defined in terms of shear rates a~
as discussed above with respect to high shear is a
~unction of a number of variables including mixing
vessel configuration and impeller tip speed. Equipme~:
suitable for low-shear mixing of the thickener premi.Y
includes conventional paddle blade mixers wherein
average shear rates are on the order of about 10 sec 1
The amount of water added to each of the first two
mixinq steps is somewhat arbitrary within the limits c~
the total water content of the final liquid automatic
132~ 7~2
-13-
dishwasher detergent composition. However, it has been
found that the amount of water added ~o the
predispersion mix should be less than that which
produces an unduly low viscosity and high fluidity
mixture since such a condition would adversely affect
the mixing, particularly under high-shear mixing
conditions.
The second mixing step is followed by a main batch
mixing step during which the thickener premix, the
balance of the total liquid automatic dishwasher
detergent water content and other desired liquid
automatic dishwasher detergent ingredients are mixed,
preferably under high-shear conditions to form 2 main
batch composition. During ~nis mixing step the
remaining liquid automatlc dishwasher detergent
ingredients are preferably added. Shear rates on the
order of 100 sec 1 are achieved during the main batch
mixing step. The remaining liquid dishwasher detergent
inqredients which may be added include the following:
sodium hydroxide, sodium carbonate, silicates, alkali
metal tripolyphosphates, chlorine bleach compounds, and
other suitable ingredients which comprise the desired
liquid automatic dishwasher detergent composition.
Equipment suitable for the high-shear mixing operation
include roller mills, collold mills, Premier mills and
Myers ~SD, among others.
The main batch composition from the hiyh-shear mixing
step is then subjected to a series of coarse and ~ine
homogenizing steps until the solid and liquid phases o
the liquid automatic dishwasher detergent composition
are thoroughly homogenized. ~he homogenizing steps are
carried out under high-shear conditions wherein shear
rates of the order of about 10~ sec 1 is achievedO The
homogenizing step is complete when the bulk specific
gravity of the liquid automa~ic dishwasher detergent
11 3 2 ~
-14-
composition is about equal to ~he specific gravity of
the automatic dishwasher detergent liquid phase only.
Homogenization of the liquid automatic dishwasher
detergent composition may be accomplished in
conventional homogenizers, such as a high speed Dispax~,
available from IKA-Works, Inc.
According to the invention, the dishwasher detergent
composition is preferably subjected to mixing at a rate
and a duration which ensures air entrainment in an
amount of about 2% to about 10% by volume, preferably 4
to 93 and most preferably 6.5 to 8.5% by volume in the
dishwasher composition. In the preferred embodiment of
the invention, the air is entrained into the composition
iS during the high shear mixing of the dishwasher detergent
ingredients. ~owever, according to the invention air
may be introduced to the composition at any point in the
process by conventional means to produce a phase stable
composition.
The presence of a bulk specific gravity about equal to
the liquid phase specific gravity is indicative of air
entrainment and high product stability. Generally, it
has been found that specific gravities within the range
of 1.20 to 1.35 provide a phase stable liquid automatic
dishwasher detergent composition, the preferred specific
gravity being within the range from about 1.26 to about
1.32.
Accordinq to a more preferred embodiment of the
invention, a fragrance is added to the liquid automatic
dishwasher detergent composition subsequent to the
coarse and fine homogenizing s~eps described above. In
this embodiment, the liquid automatic dishwasher
detergent composition is cooled and thereafter the
liquid a~tomatic dishwasher detergent composition and
the desired fragrance are mixed, preferably in a static
~32~7~2
-15-
mixer, to produce a physically stable and uniformly
dispersed liquid automatic dishwasher detergent product.
According to an optional embodiment o~ the invention,
the liquid automatic dishwasher detergent c~mpositio~ is
preferably cooled to a temperature less than about 85F
prior to introduction of a desired fragrance. It has
been found that the addition of the desired fragrance
prior to cooling has a detrimental effect on the
specific gravity of the liquid automatic dishwasher
detergent composition which, in turn, affects the phase
stability of the composition. It has also been found
that introduction of the fragrance prior to
homogenizing, i.e., during the main batch preparation,
has resulted in a product having poor physical
stability, i.e. the liquid automatic dishwasher
detergent composition begins to phase separate almost
immediately upon standing. It has also been found that
fragrance addition in amounts ranging from about 0.01 to
about 0.4% by weight produces a desirable fragrance
without adversely affecting the rheological properties
or phase stability o~ the composition, the preferred
fragrance addition being from about 0.02 to 0.2~ by
weight.
In an alternate embodiment of the present invention, the
liquid and solid components of the thixotropic detergent
composition, a~ described above, are added sequentially
to a high-shear mixer while continuously mixing,` until
all desired ingredients are included. Thereafter, the
deter~ent composition is subjected to high-shear mixing
for about 15 minutes to produce a homogeneous air
entrained thixotropic detergent composition. The high-
shear mixing step is complete when the bulk specific
gravi~y of the composition is about equal to the liquid
phase specific gravity.
:1 3 21 r/ d~ 2
-16-
While the process of the invention has been described
terms of preferred ingredients and amounts, it would be
understood to those skilled in the art that a highly
st~ble thixotropic detergent composition could be
achieved in the absence of one or more of the
ingredients by appropriate adjustment of the remaining
ingredients. For example, it may be possible to
formulate a phase stable composition in the absence of a
foam depressant by minimizing the surfactant level and
increasing the amount of physical stabilizer in the
composition.
The liquid automatic detergent compositions produced by
the process of the present invention generally are
ormulated with the ingredients in the proportions
described in detail below.
Any linear, branched, polymeric or polybasic, saturated
or unsaturated long chain fatty acid may be used as the
physical stabilizer according to the present invention.
The fatty acid is preferably linear and saturated,
having from about 10 to about 22 carbon atoms,
preferably from about 10 to 20 carbon atoms, and most
preferably from about 14 to 18 carbon atoms, inclusive
of the carbon atom of the carboxyl group of the fatty
acid. Mixtures of fatty acids may be used, such as those
derived from natural sources, such as tallow fatty acid,
coco fatty acid, soya fatty acid, etc., or from
synthetic sources available rom industrial
manufacturing processes.
Examples of the fatty acids wnich can be used as
physical stabilizers include, ~or exampIe, decanoic
acid, dodecanoic acid, pal~itic acid, myristic acid,
stearic acid, behenic acid, oleic acid, eicosanoic acid,
tallow fatty acid, coco atty acid, soya fatty acid,
etc. and mixtures of these acids. Behenic acid, stear;c
~3~ 7l~,
acid and mixed ~atty acids are preferred. In liquid
automatic dishwasher detergent compositions, as well as
any other applications where the compositions prepared
in accordance with this invention will or may come into
contact with articles used ~or the handling, storage or
serving of food products or which otherwise may come
into contact with or be consumed by people or animals,
the use of the fatty acids as the physical stabilizing
aqent are of particular advan~age because of their known
low toxicity. For this purpose, the stearic acid and
behenic acid are especially preferred. Another distinct
advantage of the use of the fatty acids as stabilizers
is their lower cost as compared to the fatty acid metal
salts.
The amount of physical stabilizer required to achieve
the desired enhancement of physical stability will
depend on such factors as the nature of the fatty acid,
the nature and amount of the thixotropic agents,
detergent active compounds (surfactants), inorganic
salts, especially tripolyphosphates (TPP) and other
liquid automatic dishwasher detergent ingredlents, as
well as the an~icipated storaqe and shipping conditio~a.
Salts of the above fatty acids may also be used as
physical stabilizers, e.g. alkali, alkaline earth and
polyvalent metal salts. The alkali metal salts include
sodium, potassium and ammonium salts of the fatty aci~,.
The alkaline earth salts include calcium, barium and
strontium salts of the fatty acids. Examples of the
fatty acids from which the polyvalent metal salt
stabilizers can be formed include, for example, decan~lc
acid, dodecanoic acid, palmitic acid, myristic acid,
stearic acid, oleic acid, eicosanoic acid, tallow facty
~5 acid, coco fatty acid, soya fatty acid and mixtures ~f
these ac.ids. Stearic acid and mixed fatty acids are the
preferred fatty acids from which polyvalent metal salc
- ~32~7~2
-18-
stabilizers can be formed.
The preferred polyvalent metals are the metals of Groups
IIA, IIB and IIIB, particularly magnesium, calcium,
aluminum and zinc, although other polyvalent metals,
including those of Groups IIIA, IVA, VA, I3, IVB, VB,
VIB, VIIB and VIII of the Periodic Table of the Elemer.ts
can also be used. Specific examples of such other
polyvalent metals include Ti, Zr, V, Nb, Mn, ~e, Co, Ni,
Cd, Sn, Sb, Bi, etc. As discussed above with respect to
the selection of safe free fatty acids, the metal salt
should also be selected by taking into consideration its
toxicity. For this purpose, the calcium and magnesium
salts are especially preferred because they are
generally recognized as safe .ood additives.
The metal salts described above are generally
commercially available but can also be easily produced,
for example, by saponification of fats and oils, e.g.
animal fat, or by neutralization of free fatty acids
with an hydroxide or oxide of the polyvalent metal e.g.
alumina, alum, etc. Alternatively, metal salts of fatty
acids may be produced by the reaction of a soluble metal
salt with a soluble fatty acid salt.
Calcium stearate, i.e., calcium distearate, magnesium
stearate, i.e., magnesium distearate, aluminum stearate,
i.e., aluminum monostearate, aluminum distearate,
aluminum tristearate and mixtures thereof, and zinc
stearate, i.e., zinc distearate, are the preferred
polyvalent fatty acid salt stabilizers.
The amount of fatty acid or fatty acid salt stabilizers
necessary to achieve the desired enhancement of physica:
stability will depend on such factors as the nature of
the fatty acid or its salt, the nature and amount of t:ze
thixotropic agent, detergent active compound, inorgan~c
~321~
g
salts, especially T~P, and other liquid automatic
dishwasher detergent ingredients, as well as the
anticipated storage and shipping conditions.
Generally, however, it has been found that long term
stability, i.e., absence of phase separation at low and
elevated temperatures, is achieved with the addition of
free fat~y acids or their salts in amounts ranging from
about 0.01 to about 1.0~ by weight, preferably from
about 0.06 to about 0.8 percent and most preferably from
about 0.08 to about 0.4~ by weight. In addition, it has
been found that the free fatty acids are preferable over
their salts primarily because of their ease of
dispersibility.
Alternatively, or in addition to the above physical
stabili2ers, small but effective amounts of polyacrylic
acid polymers and copolymers and their salts may be
added to improve the physical stability of the
compositions. These polymers and their salts are
generally commercially available. Suitable polymers are
the polyacrylic acids and their sodium salts available
from Rohm and Haas as ACRYSOL~ LMW. The proportions of
polymer may be in the range of 0.01 to 3~ depending on
the molecular weight of the polymers, the lower
proportions being more suitable for the higher molecular
weight polymers.
Foam inhibition during the dishwashing cycle is
important to maximize dishwasher efficiency and minimize
destabilizing effects which might occur due to the
presence of excess foam within the washer. Foam may be
sufficiently reduced by suitable selection of the type
and/or amount of detergent active material, the main
foam-producing component. The degree of foam is also
somewhat dependent on the hardness of the wash water in
the machine whereby suitable adjustment of the
1 ~2~ ~L~2
-20-
proportions of water softeners, e.g., alkali metal
tripolyphosphate, may provide the desired degree of Coam
inhibition. However, according to the invention, there
is preferably included a chlorine bleach stable foam
depressant or defoamer as a component of the stabilizing
system. Effective defoamers include the alkyl
phosphonic acid esters of the formula:
1
10 ~0-----P R
OR
and the alkyl acid phosphate esters of the formula:
ll
~0---P ---OR
OR
available, for example, from Hooker (SAP) or from
American Hoechst as Knapsack (LPKn-158J, in which one or
both R groups in each type of ester may represent
independently a Cl2_20 alkyl group. Mixtures of the two
ester types, or any other chlorine bleach stable types,
or mixtures of mono and di-esters of the same type, may
also be employed. The preferred inhibitor according to
the invention is a mixture of mono- and di-C16_18 alkyl
acid phosphate esters such as monostearyl/distearyl acid
pho~phates 1.2/1 tKnapsack LPKnl58). In addition, it is
an advantageous feature of this invention that many of
the stabilizing long chain fatty acids, such as stearic
acid and behenic acid act as supplemental foam killers.
The detergent compositions of the invention generally
contain a foam depressant in an amount from 0 to about
5% by weight, preferably from about 0.01 to about 5.0
and most preferably from about 0.01 to about 0.5~ by
weight. In addition the weight ratio of surfactant t~
foam depressant preferably ranges rom abcut 0:1 to
about 1:1, most preferably from abcut ~:L to about 1:'.
1 3 ~ 2
-21-
The detergent active material, i.e., surfactant selected
for use in the liquid automatic dishwasher detergent
composition of the invention must be stable against
chemical decomposition and oxidation by the strong
active chlorine bleaching agent also present in the
liquid automatic dishwasher detergent composition.
Surfac~ants useful in the present invention are of
either the anionic or non-ionic type or combinations of
the two. Preferred surfactants are mono- or di anionics
containing sulfate, sulfonate or carboxylates, as
amphiphiles. The most preferred surfactants accordlng
to the invention are the linear or branched alkali metal
mono-and/or di-(C8_14)alkyl diphenyl oxide mono and/or
disulfonates, commercially available from Dow Chemica ,
for example as DO~FAXD 3B-2 and DOWFAX~ 2A-l. In
addition, the surfactant should be compatible with the
other ingredients of the composition. Other preferred
surfactants include the primary alkylsulphates,
alkylsulphonates, alkylarylsulphonates, sec. -
alkylsulphates and olefin sulfonate. Examples include
sodium C10-C18 alkanesulphonates such as sodium lauryl
sulfonate, sodium hexadecyl-l-sulphonate and sodium
Cl2-C18 alkylbenzenesulphonates such as sodium
dodecylbenzenesulphonates. The corresponding potassium
salts may also be employed.
Other suitable surfactants or detergents useful herein
inc~ude, the amine oxide surfactants of the structure
R2R NO in which each R represents a lower alkyl group,
for instance, methyl, and ~ represents a long chain
alkyl group having from 8 t3 22 carbon atoms, for
instance a lauryl, myristyl, ~almityl or cetyl group.
Instead of an amine oxide, a cor-esponding surfactant
phosphine oxide R2RlPO or sul?hoxide RRlSO can be
emp~oyed. Betaine surfactants are ~ypically of the
R2R -R'COO-, in which each R represents a lower
1~2~7~
-22-
alkylene group havinq f.om 1 to 5 carbon atoms.
Specific examples o~ the amino oxide sur~actants are
lauryl-dimethylamine oxide, myristyldimethylamine oxide,
the corresponding phosphine oxides and sulphoxides, and
the corresponding betaines, including
dodecyldimethylammonium acetate,
tetradecyldiethylammonium pentanoate,
hexadecyldimetyhylammonium hexanoate and the like. Fo
biodegradability reasons, the alkyl groups in these
surfactants are preferably linear. Detergent
compositions according to the invention may contain fro~
0 to about 5% surfactant by weight, preferably from
about 0.1 to about S~ by weight and most preferably from
about 0.3 to 2.0g by weight.
Thixotropic agents, i.e., thickeners or suspending
agents which produce thixotropic properties in an
aqueous medium, are known in the art. These thixotrop c
agents are water soluble, water dispersible or colloid-
forming, organic or inorganic, and monomeric orpolymeric. They must be stable in the detergent
compositions of the present invention, i.e., stable t~
high alkalinity and chlorine bleach compounds, such as
sodium hypochlorite. The preferred thixotropic agents
are the inorganic, colloid-forming clays of smectite
and/or attapulgite types. These agents are generally
used in amounts of about 0.1 to about 10~ by weight t~
confer the desired thixotropic properties or Bingham
Plastic behavior to the liquid automatic dishwasher
detergent formulations. Other suitable thixotropic
aqents include small but effective amounts of an
aliphatic long chain fatty acid having 8 to 22 carbor.
atoms or the dimers or trimers thereof. These agents
are generally used in amounts ranginq from 0.02 to 0.5`s
by weight. One advantage of the liquid automatlc
dishwasher detergent formulations of the present
invention is that the desired thixotropic properties or
132~L~L~2
-23-
Bingham Plastic behavior can be obtained in the presence
of the aforementioned physical stabilizing system with
lesser amounts of the thixotropic thickeners. For
example, inorganic colloid-Eorming clays of the smect~te
and/or attapulgite types added to the liquid automatic
dishwasher detergent compositions o~ the invention in
` the amount of about 0 to 3~ by weight, preferably 0.2 t~
2.5%, most preferably 0.5 to 2.2% by weight, are
generally sufficient to achieve the desired thixotropic
properties and Bingham Plastic character when used in
combination with the physical stabilizing system.
The smectite clays include montmorillonite (bentonite),
hectorite, saponite, and the like. Montmorillonite
clays are preferred and are available under tradenames
such as Thixogel~ No. 1 and Gel White~ GP, H, etc., from
Georgia ~aolin Company and ECCAGUM~ GP, H, etc., from
Luthern Clay Products. Attapulgite clays include the
materials commercially available under the tradename
Attagelm, i.e. Attagel~ 40, Attagel~ 50 and Attagel~ 150
from Engelhard Minerals and Chemicals Corporation.
Mixtures of smectite and attapulgite types in weight
ratios of 4:1 to 1:5 are also useful herein. Thickening
or suspending agents of the foregoing types are well
known in the art, being described, for example, in U.S.
Patent No. 3,985,668. Abrasives or polishing agents
should be avoided in the liquid automatic dishwasher
detergent compositions as they may mar the surface of
fine dishware, crystal and the like.
As an alternative to the above thixotropic thickeners,
smaLl bùt effective amounts o~ high molecular weight
polyacrylic acid polymers and copolymers and their sal~s
may be added to improve the rheological properties as
well as the physical stability of~the compositions.
These polymers and their saits are generally
~3~ 7'~2
-24-
commercially available and have the formula:
Rl R2
l I
_ C - C --_
l l
R3 COOM
_ n
wherein Rl, R2 and R3 can be the same or dif ferent and
can be hydrogen, Cl-C4 lower alkyl, or combinations
thereof. The value of n is 5 to 2000, preferably 10 to
1500 and most preferably 20 to 1000. M represents
hydrogen, or an alkali metal such as sodium or
potassium, the preferred substitute being sodium. The
preferred Rl, R2 and R3 groups are hydrogen, methyl,
ethyl and propyl. ~referred acrylic acid monomer is one
where Rl to R3 are hydrogen, e.g., acrylic acid, or
where Rl, and R3 are hydrogen and R2 is methyl, e.g.
methyl acrylic acid monomer. Polyacrylic acid
copolymers can include copolymers of, for example,
acrylic acid or methacrylic acid and polycarboxylic acid
anhydride or acid such as succinic anhydride, succinic
acid, maleic acid, maleic anhydride, citric acid and the
like.
Specific polyacrylic acid polymers and their salts which
can be used include ACRYSOL~ acrylic acid polymers from
Rohn and Haas, ALCOPERSE~ 110 from Alco or CARBOPOL from
B.P. Goodrich. A polyacrylic acid copolymers that can
be used i~ SOKALAN~ CP5 available from BASP.
The proportions of the polymer used in the compositions
may range from about O to 3~ depending on the presence
and amount of other stabilizLng components.
The detergent compositions of the present invention may
also contain various inorganic builder materials such as
alkali metal tripolyphospna~es and si11cates.
~2~
--25--
A preferred builder material is sodium tripoly-
phosphate (NaTPP) which serves to soften hard-water
minerals and to emulsify and/or peptize soil. The NaT?P
employed in the liquid automatic dishwasher detergent
5 compositions of the present invention are in a range of
about 5 to about 35% by weight, preferably about 20% to
about 30% by weight. The NaTPP should preferably be
free of heavy metal which tends to decompose or
inactivate the preferred sodium hypochlorite and other
chlorine bleach compounds. The NaTPP may be anhydrous
or hydrated, including the stable hexahydrate with a
degree of hydration of 6 corresponding to about 22~ by
weight of water or more. The NaTPP is available
commercially in the anhydrous or hydrated forms under
the trademarks Thermphos NW~ and Thermphos N~,
respectively. Preferred liquid automatic dishwasher
detergent compositions have been obtained, for example,
when employing a weight ratio of anhydrous to
hexahydrated NaTPP in the range of about 0.5:1 to about
2:1, preferably about 1:1.
In compositions where no or low phosphates are desired,
other functionally equivalent builder materials may be
substituted therefor. For example, 5 to 35%
aluminosilicate zeolite may be employed in the
compositions of the present invention when the sodium
silicate level is increased to 25~ or more.
It is preferred that the liquid automatic dishwasher
detergent compositions of the present invention include
an alkali metal silicate, e.g., sodium silicate, to
provide composition alkalinity as well as protection of
hard surfaces such as fine china glaze and pattern. ~he
silicate component is present in the liquid automatic
dishwasher detergent composition in an amount from 0 to
about 50~ by weight, preferably about 2.5 to about 20%
by weight and mos~ preferably from about 5.0 to about
1 3 ~ 2
-26-
15.0~ by weight. The silicate is generallly added in
the form of an aqueous solution, preferably having a
~a2O:SiO2 ratio of about 1:2.2 to 1:2.8.
A chlorine bleach compound may be employed in the liquid
automatic dishwasher detergent compositions prepared
according ~o the process of the present invention. The
source of the chlorine compcund is preferably an alkali
metal hypochlorite, for example, potassium hypochlorite,
lithuim hypochlorite, calcuim hypochlorite, magnesium
hypochlorite and most preferably sodium hypochlorite.
Other sources of chlorine bleach compounds include
dichloro-isocyanurate, dichloro-dimethyl hydantoin, and
chlorinated TSP, among others. The liquid automatic
dishwasher detergent compositions according to the
invention should contain sufficient chlorine compounds
to provide about 0.2 to 4.0~ by weight, preferably about
0.8 to 1.6~ by weight of available chlorine, as
determined, for example, by acidification of 100 parts
of the composition with excess hydrochloric acid. A
solution containing about 0.2 to about 4.0~ by weight of
sodium hypochlorite contains or provides about the same
percentage of available chlorine.
As an alternative to the chlorine bleach compound~ a
stabilized enzyme system may be employed to provide
proteolytic and amylolytic enzyme cleaning activity to
the dishwasher compositions. The stabilized system
preferably contains 0.5 to 2.0~ wt. enzyme, 1 to 4~ wt.
of a water dispersible proteinaceous material selected
from the grouup consisting of casein and collagen, 0.75
to 2~ wt. of a boron compound and 1.5 to 4% o~ an
alpha-hydroxy carboxylic ac:d.
It is preferred that the pH of the liquid automatic
dishwasher detergent compositions prepared by the
process of the present invention be at least 9.5,
1 3 ~
-27-
preferably about 10 to L4.0 and most preferably about
11.0 to 11.5 ~easured in a 1~ aqueous solution. .he
liquid automatic dishwasher detergent compositions are
adjusted to the desired alkaline level by the addition
of an alkali metal hydroxide, e.g., sodium hydroxide.
Typical concentrations of sodium hydroxide in the liquid
automatic dishwasher detergent compositions range from
about 0 to about 6% by weight, preferably 0 to 3.0~ by
weight. The presence of sodium hydroxide serves the
additional function of neutralizing the phosphate or
phosphonic acid ester.
An alkali metal carbonate, e.g. sodium carbonate, may
also be used in liquid automatic dishwasher detergent
comp_sitions prepared according to the process of the
present invention. The carbonate serves as a buffer ~3
maintain the desired pH level. Typical concentrations
of sodium carbonate in the liquid automatic dishwasher
detergent compositions range from about 0 to 9.0% by
weight, preferably 2 to 9.0% by weight.
~ragrances useful in the present invention must be
stable against chemical decomposition and oxidation b~
the strong active chlorine bleaching agent also preser~
in the campositions. Fragrances useful in the present
invention include those derived from natural sources,
sUch as extract~ of botanical matter, e.g., essential
oils or ~rom synthetic sources available from industrlal
manufacturing processes. Examples o~ bleach-stable
Eragrance materials useful ~or imparting a fragrance to
the dishwasher detergent composition are p-cresol methy
ether, dihydrolimonene epoxide, dodecene-1,2-epoxide and
n-undecyl nitrile, among others. Other examples o
suitable bleach stable ~ragrances are disclosed in ~.S.
Patent No. 3,876,551. It should be understood that the
fragrance selected must be reasonably stable in a bleacn
environment, that is, it should not be easily oxidizec
13~42
-2~-
by the hypochlorite in the detergent composition. This
is important for two reasons, first, the hypochlorite
loss would exceed the limits of acceptability in a
dishwasher detergent product and secondly, the oxidation
of the fragrance would reduce the aromatic
characteristic of the product and in certain cases may
actually result in an unpleasant odor. It has been
found that fragrance addition to the compositions in t~.e
amount oE about 0.01 to 0.40, preferably 0.02 to 0.2% by
weight imparts a desirable fragrance without affecting
the rheological properties or physical stability of the
dishwasher detergent composition.
The amount of water contained in these compositions
should, of course, be sufficient to produce the des red
viscosity and fluidity without adversely affecting the
thixotropic properties of the composition. The proper
amount o water is readily determined by routine
experimentation in any particular instance, and
generally ranges from about 30 to 75~ by weight,
preferably from about 35 to 65% by weight. In addition,
the water is preferably deionized or softened.
In addition to the components described above, the
detergent compositions produced by the process of the
present invention may include small amounts of
additional ingredients~ generally less than 3~ by weight
of hydrotropic agents such as sodium benzene, toluene,
xylene and cumene sulphonates, preservatives, dyestuffs
and pigments and, enzymes, all being stable to chlorine
bleach and high alkalinity. Especially preferred for
coloring are the chlorinated phthalocyanines and
polysulphides of aluminosilicate which pro~ide,
respectively, pleasing green and blue tints. TiO~ may
3S be employed for whitening or neutralizing off-shades.
Especially preferred for eliminating or mini~izins glass
13~1 7L~
--29--
~ilming and spotting are low molecular weight
polyacryLic acid polymers and their salts having
molecular weights in the range of 2,000-10,000,
preferably 4,000 to 6,000. Specific polymers that may
be used are the low molecular weight polyacrylic acids
and their corresponding sodium salts available, for
example, from Rohm and Haas as ACRYSOL~ LMW.
The liquid automatic dishwasher detergent compositions
of this invention are readily employed in a known manner
for washing dishes, kitchen utensils and the like in an
automatic dishwasher provided with a suitable detergent
dispenser, and in an aqueous wash bath containing an
effective amount of the composition. ~hile the
invention has been particularly described in connection
with its application to liquid automatic dishwasher
detergents and methods for making same it will be
readily understood by one of ordinary skill in the art
that the benefits which are obtained by the entrainment
of air in a three-part stabilizing system, namely
increased physical stability of the thixotropic
suspension, will apply e~ually well to other thixotropic
suspensions.
The invention may be put into practice in various ways
and the preferred embodiment will be described to
illustrate the invention with reference to the
accompanying examples.
EXAMPLE 1
A scented thixotropic liquid automatic detergent
composition having the formulation describe~ below, was
prepared using the preferred process of the present
invention as illustrated in the accompanying drawing.
~ 3 ~ 2
-30-
STAGE COMPONENT WEIG~T
Water (Softened)41.44
PREDISPERSION Mono- and di-C16 to Cl8 8.84
acid phosphate ester
(Foam depressant)
(I) Al stearate (Physical 5.52
Stabilizer)
~odium Mono- and didecyl 44.20
disulfonated diphenyl oxide
(Surfactant)
Total 100.00
PREMIX
(II) Water (Softened)82.37
Predispersion (I)10.43
Montmorillonite clay7.20
- Total 100.00
MAIN ~ATCH
20 (III) Water (Softened)25.69
Premix (II) 17.53
Sodium hydroxide (50~ A.I.) 2.42
Sodium carbonate 5.05
Sodium silicate (43.5% A.I) 17.42
NaTPP hydrate 12.12
NaTPP anhydrous 12.12
Sodium hypochlorite (13~ A.I) 7.48
Subtotal 99.83
HOMOGENIZE,
COOL ~ MIX
(IV) Eraqrance 0.17
Total 100.00
According to the preferred process of the invention, a
predispersion mix was prepared in a vessel (2) equipped
with a high speed disperser, e.g., Myers HSD~. The
-31- 1321~2
amount of water included in the predispersion vessel was
limited so that the mixture remained viscous and
susceptible to high shear dispersing. The high shear
dispersing was carried out for about 5 to 10 minutes at
which point the predispersion mix was pumped through a
homogenizer (19) to a premix vessel (4) where the clay
thickener and water were added to the predispersion mix
under low-shear conditions. A paddle blade type mixer,
e.g., baffled crutcher was used in the premix vessel
which mechanically deagglomerated the clay as it was
hydrated. The preparation of the premix generally lasts
for about 20 minutes depending on the mixer speed. The
resultant premix was removed and homogenized in
homogenizer (21), then added with water to the main
batch vessel (6) where it was subjected to high-shear
dispersing using a Myers HSD~. During the high-shear
mixing, the remaining liquid and solid ingredients were
sequentially added to the main batch vessel (6).
As additional ingredients were added, particularly the
solid ingredients, the mixture became more viscious and
the high speed disperser ground the particles to a fine
particle size which, in turn, caused an increase in
temperature, i.e., to about 125F - 150F. The
continuous high shear dispersing also resulted in
entrainment of a substantial portion of atmospheric air
in the main batch vessel (6) which is open to the
atmosphere. The high shear dispersing continued for a
total of about 20 minutes during which visible lumps of
solid material disappeared and the particle size of the
undissolved particles was reduced so that a phase stable
dispersion was formed.
Thereafter, the main batch material was fed through a
series of coarse and fine homogenizers (8 and 10), where
the material was milled at high speeds for relatively
short times to further deagglomerate any remaining
~2~7~
-32-
solids particles. The resultant product was a stable
thixotropic liquid automatic dishwasher detergent
composition including entrained atmospheric air bubbles
having diameters in the range of 5 to 80 microns.
~hen it was desired to add a fragrance to the detergent
composition, as in the present example, the main batch
material was cooled in heat exchanger (12) from the .~ain
batch temperature whicn is generally greater than 100F,
typicallyl 105F to 125~, to a temperature of about
85F or less. The cooled main batch material and
fragrance were then fed through a series of in-line
static mixers (14) and the resultant product was a
stable scented thixotropic liquid automatic detergent
composition.
It has been found that the addition of fragrance to the
composition according to this method avoids an adverse
effect on the rheological properties of the compositio~
or on the long term phase stability of the composition.
The specific gravity, viscosity and phase stability,
i.e., phase separation, of the scented detergent
composition were measured (Example lA). ~or comparison,
a sample of the main batch material (Example lB) was
removed for analysis prior to the fragrance addition.
Specific gravity measurements of the bulk and liquid
phases were made by conventional techniques known to
those skilled in the art. For example, the specific
gravity of tAe bulk composition was determined by
weighing a known volume of the bulk composition and a~
identical volume of water. The ratio of the bulk
composition weight to the weight of the water is ter~ed
the "bulk specific gravity."
The liquid phase specific gravLty was determined by
first loading a sample of the liquid dishwasher
composition into a conventional centrifuge, e.g., Iva.
~3~ ~ 7l~
-33-
Sorvall, then spinning the centrifuge at a speed of
about 2000 rpm to remove a sufficient amount of
supernatant (clear deaerated liquid phase) for weishing.
The centrifugation step requires approximately 1 - 1 '/2
hours to separate a sufficient amount of supernatant for
several measurements. Thereafter, the supernatant
specific gravity was calculated by dividing the weight
of an 8 ml. vial of the supernatant by the weight of an
identical volume of water, the ratio being defined as
the "liquid phase specific qravity."
The viscosity of the compositions were measured using a
Brookfield HATDV II Model II viscometer with a #4
spindle tBrookfield Labs, Stoughton, Mass.). The
viscosity was recorded after the compositions were
sheared for 90 seconds at a shear rate of 20 rpm. The
results are summarized below.
EXAMPLE 1
Property ¦ lA _ I lB
Specific gravit~ (BULK) l1.28 1 1.2~
Specific gravity (LIQUID) ¦1.28 ¦ 1.28
Viscosity (cP) - 1 day after
preparation l5060 I 4760
Viscosity (cP) - 12 weeks
after preparation l5150 1 6350
Separation (~)
- 12 weeks after 1 0 1
preparation
The above data demonstrates that the process of the
present invention produces a thixotropic liquid
automatic detergent composition which is highly stable
and not subject to phase separation after long periods
of storage.
_34_ ~ 3~ 7~
EXAMPLE 2
~he following liquid automatic detergent compositions,
having the formulations described in Table I, were
prepared in a single open mixer according to an
alternative embodiment of the process of the invention.
. TAsLE I
10 Com~onent Example 2A Example 2B
Water 36.90 36.15
Mono- and di- 3.20 3.20
C16t 18 alkyl
acid phosphate
15 ester (Foam
depressant) 5~
Sodium mono- and 0.80 0.80
didecyl disulfonated
diphenyl oxide
20 (Surfactant)
Stearic acid 0.10 0.10
(Physical
Stabilizer)
Montmorillonite clay 1.25 0
Caustic (50S A.I.) 2.40 2.40
Soda ash 5.00 5.00
Silicate (45~A.I.) 17.34 17.34
NaTPP hydrate 12.00 12.00
N~TPP anhyd~ous12.00 12.00
Bleach (11~ A.I.) 9.00 9.00
Acrylic acid 0 2.00
polymer
Air (BALANCE) 0.01 - :0.01
:
TOTAL 100.00 100.00 :
All of the above ingredients~were~mlxed ~n a Prem1er~
.
~32~
-35-
.~ill Mixer at room temperature. In the examples, a ,~
aqueous dispersion of defoamer (LPKn) is initially
prepared by heating and mixing the defoamer ln water
un~il dispersed. Similarly, the surfactant (Dowfax~)
and a physical stabilizer (stearic acid), are heated tO
~orm an emulsion prior to and during addition to the
mixer.
After addition of the surfactant and physical
stabilizer, tne mixture is allowed to cool and the
remaining ingredients were added sequentially in the
order shown in Table I, while subjecting the mixture to
constant high shear mixing in the presence of
atmospheric air.
Upon adding the final ingredient, typically a bleach
compound, the composition is subjected to additional
high shear mixing until atmospheric air in the amount of
about 2% to about 10% i9 entrained in the thixotropic
detergent composition in the ~orm of innumerable bubbles
having a diameter in the range of 5 to 8 microns which
equilibrates the bulk speciEic gravity o the product
with the specific gravity of the deaerated liquid phase.
As seen in the above examples a three component air
stabilizing system, i.e., a physical stabilizer, foam
depre~sant (deoamer) and surfactant is employed in each
composition.
Eaoh of the resulting liquid detergent compositions were
measured for specific gravity, degree of aeration and
phase stability, i.e., phase separation upon standing.
The degree of aeration is calculated as follows:
density of de-aerated product-
degree of aeration = density of aerated product x 'C0
density of de-aerated product
~ 3 ~
-36-
The density of the de-aerated product is determined by
centrifuging the composition to remove all entrained
air, then measuring the density of the centrifuged
composition by conventional means. The results obtair.ed
are summarized below.
EXAMPLE 2
PROPERTY 2A _2s
Specific
gravity(bulk) 1.28 1.29
Specific
gravity(liquid) 1.28 1.28
Degree of
aeration (~) 7.91 7.20
Nature of
separation 0.00~ 0.00*
*Age 8 wks after Sample Preparation
The above data demonstrates that liquid detergent
compositions comprising a three component stabilizing
system according to the present invention exhibit
excellent stability. As shown, the air stabilized
compo~ition of Example 2A has a bulk specific gravity
(1.28 g/cc) identical to the liquid phase specific
gravity (1.28 g/cc) of the composition. Under these
conditions the composition exhibits excellent phase
stability.
Substantially identical results were obtained in the
composition of Example B in rhe absence of a thixotropic
thickener, e.g. clay, where a bulk specific gravity of
1.29 g/cc was achieved, almost identical to the liquid
phase specific gravity of the composition. Example 2B
demonstrates that clay is not required for producing an
acceptable stabilized composition. Such a composition
is further advantageous In that a~l of ies ingredients
~.
.
-37-
are completely water soluble, resulting in superior
spotting and filming performance compared to clay based
thixotropic detergents.
The invention in its broader aspects is not limited to
the specifically described embodiments or example and
departures may be made therefrom without departing ~rom
the principles of the invention and without sacrificing
its chief advantages.