Note: Descriptions are shown in the official language in which they were submitted.
132~394
This invention relates to a system for the
destruction of organic waste material and more
particularly it relates to a process for the
destruction of organic waste material which may or may
not contain polyhalogenated waste material, and to
apparatus for carrying out the process.
It is known to destroy halogenated organic waste
material by reduction procedures using, for example,
sodium metal or sodium napthalide. It is also known
to destroy halogenated organic waste material by
oxidation, using, for example, high temperature
incineration. These known processes have certain
limitations or disadvantages in that they can only be
used for particular kinds of waste material.
Moreover, the chemical reagents used for the
destruction are sometimes hazardous to handle and the
- 2 - 1 3 2 4 3 ~ ~
destruction may lead to the formation of highly toxic
by-products.
The dimensions of the problem relating to the
disposal of polyhalogenated liquids, particularly
polychlorinated biphenyls (known generally, and to the
public as PCBs) and associated wastes have been
reviewed in the Environment Canada Economic and
Technical Review Report EPS 3-EC-83-1. Bearing in
mind the suspected carcinogenic nature of PCBs and the
apparent absence of degradation in nature, over
extremely long time periods, the identified quantities
of PCBs presently in use in electrical transformers
and capacitors (Table 7 of the Report), comprising a
mass value of 14.8 million kilograms, conveys some
idea of the scale of the problem.
Furthermore, the accumulation of these materials
in numerous localities, mostly remote from the
existing large-scale incinerators available in the
United States and Canada for disposal purposes further
emphasizes the extreme nature of the problem. Public
awareness to the potential danger of PCBs to public
health also further complicates the situation in
precluding transportation thereof to existing
combustion facilities. The occurrence of certain,
_ 3 - ~32 4394
well-publicized, PCB spills has further exacerbated
this aspect of the problem.
The existing method of disposing of these types
of substance has been by incineration, in large
installations. While claims have been made to the
achievement of very high percentile effectiveness of
disposal by this method, there is concern that the
reports may prove less than valid, on a long-term,
continuing basis. In addition incineration or
oxidation will form highly toxic by-products sl~ch as
chlorinated dioxins if operated outside of exact
optimal temperatures and residence time requirements.
One identified problem, referred to at Page 28 of
the above-noted Report, which contributed to the
demise of the related PCB incinerator, was the
formation of a ring of "agglomerated material" during
incineration. This type of "glop" formation may well
be a characteristic by-product of incineration
systems, and possibly is indicative of partial
recombinations of molecules into ring compounds of a
suspect type. This constitutes a further reason for
providing a changed process.
_ 4 _ ~32 43~ 4
Pyrolysis or starved air thermal destruction or
degradation of solid and liquid organic waste products
of hydrocarbons has previously been hampered by the
formation of tars and polynuclear aromatic
hydrocarbons containing from one to five aromatic
rings. This invention includes the addition of
gaseous red~cing agents, particularly hydrogen, in
concentrations sufficient to saturate or reduce the
moleculeæ produced æuch that polyaromatic structures
are eliminated as by-products of the intended reaction.
In a first embodiment, the invention comprises a
treatment process for organic waste material. The
organic waste material is reduced in the presence of a
gaseous reducing agent and the reduced material is
subseguently subjected to a cooling step in the
presence of water, a condensed liquid agueous material
thus forming. A portion of this aqueous material is
recycled into the cooling step while a major portion
of the cooled reduced material is available for
subsequent recovery or further treatment.
We have found according to a second embodiment of
the invention that organic waste material can be
destroyed efficiently and completely by use of a
process which combines a gas phase chemical reduction
- 5 - 132439~
in a reducing atmosphere at a high temperature
followed by oxidation of the hot reaction mixture from
said chemical reduction in a high temperature
incinerator.
It is possible to subject certain waste materials
to reduction processes and to subsequently recover
reduced products.
It is advantageous to pre-heat waste material to
be reduced before its introduction into an isolated
reduction zone.
It is possible to capture, through the use of a
heat exchanger, energy released during recovery steps
and to use the energy to pre-heat unrecovered
materials to be oxidized in a later step.
It is possible to capture, through the use of a
heat exchanger, energy released during the oxidation
step and to transfer the energy to the waste material
for pre-heating prior to subjecting it to the
reduction process.
According to the invention, we provide a process
for the destruction of organic waste material which
- 6 - 1~2~39~
comprises sub~ecting said waste material to chemical
reduction with a gaseous reducing agent at a
temperature above about 600C and thereafter
subjecting the hot reaction mixture thus obtained to
oxidation with a gaseous oxidizing agent at a
temperature above about 1000C. As a further feature
of the invention, we provide a process for the
destruction of organic waste material which comprises
subjecting the waste material to reduction with a
gaseous reducing agent at a temperature of from about
700C to about 900C, the reduction being effected
over a residence time of from about 0.1 seconds to
about 10 seconds and thereafter subjecting the hot
reaction mixture thus obtained to oxidation with a
gaseous oxidizing agent at a temperature of from about
1000C to about 1400C, the oxidation being effected
over a residence time of from about 1 second to about
4 seconds.
The process of this invention may, for example,
be conveniently carried out by the direct injection of
organic waste material, for example, halogenated
organic waste material in the form of a gas, a liquid,
a pumpable sludge, a fine particulate slurry such as
contaminated sediment/water mixtures, or a pulverized
solid, upwardly into a pressurized reaction vessel, in
- 7 - 1324394
the absence of oxygen. The reaction vessel is heated
and maintained at temperatures above about 60~C,
preferably at a temperature of from about 700C to
about 900C.
Another embodiment incorporates the use of a
reduction vessel of generally circular horizontal
cross-section and having a centrally located ceramic
tube with an outlet of the vessel located above the
aperture of the tube. Waste and a gaseous reducing
agent are introduced with intimate mixing at the upper
perimeter of the vessel. This arrangement provides a
baffling effect. Once introduced into the upper
region of the vessel, gaseous wastes must travel to
the lower region, through the holes of the tube and
upwardly through the tube and aperture in order to
reach the next zone provided by ths apparatus. A
reduction vessel having a tube as a part thereof and
an upper inlet thus provides a greater necessary
travel distance for gaseous waste and a greater vessel
surface area with which such waste may come into
contact. Organic material contained in solid waste
may be volatized when it strikes a hot vessel
surface. In such an embodiment, a flow of water may
be provided through the bottom of the vessel to trap
particulate matter and carry it to a collection tank.
.
-- 8
132439'1
A steady flow of gaseous reducing agent may also be
provided in the lower region of the vessel above the
flow of water to hinder gaseous waste from contact
with water stream.
Waste may be co-injected with a gaseous reducing
agent such as hydrogen, gaseous ammonia, natural gas,
methane, propane or water vapor, or a mixture of such
reducing agents. The reduction may be carried out
with or without the addition of a metal catalyst such
as iron, zinc, tin or nickel in the form of iron
filings or powdered zinc, tin or nickel which may be
co-injected into the vessel to promote the reduction
reaction.
In one aspect, the invention comprises the steps
of co-injecting (on a continuous basis) the waste,
with hydrogen, into a pre-heated reduction vessel,
maintaining the vessel at an internal pressure above
atmospheric, up to one atmosphere above ambient,
without the addition of catalyst materials.
It is also preferable to pre-heat waste material
to be injected into the reduction vessel.
- 9 - 132439~
The reaction vessel can be arranged and the rate
of injection may be adjusted, such that the residence
time of gaseous material in the reduction vessel is
greater than about 0.1 seconds and preferably from
about 5 seconds to about 10 seconds. A particularly
effective residence time in which to effectively
complete the reduction is about 5 seconds.
The reduction may be carried out at a temperature
of from about 600C to about 1100C, preferably within
the range of from about 700C to about 900C and
particularly at a temperature of from about 800C to
about 900C.
The reduction may also be carried out in the
presence of a metal, such as an iron, nickel, zinc or
tin catalyst. The catalyst may be in the form of iron
filings, powdered nickel, powdered zinc or powdered
tin.
The organic waste material may or may not contain
organic compounds such as halogenated biphenyls,
halogenated benzenes, halogenated phenols, halogenated
cycloalkanes, halogenated alkanes, halogenated
alkenes, halogenated dioxins and halogenated
dibenzofurans. For example, the organic waste
lo- 13243~
material may contain commonly used chlorinated organic
compounds such as chlorinated biphenyls, also known as
polychlorinated biphenyls ~PCBs), chlorinated
benzenes, chlorinated phenols, chlorinated
cycloalkanes, chlorinated al~anes, chlorinated
alkenes, chlorinated dioxins and chlorinated
dibenzofurans.
The organic waste material may be in the form of
a liquid, a pumpable sludge, a fine particulate slurry
such as contaminated sediment/water mixtures, or a
pulverized or shredded solid such as contaminated wood
waste or soils. Such waste material may include, for
example, oils containing polychlorinated biphenyls
(PCBs) as waste products from capacitor and
transformer manufacturing processes, or from obsolete
electrical or non-electrical equipment, and products
used in various industries as plasticizers, hydraulics
fluids and lubricants.
The organic waste material may also be such that
it contains non-halogenated organic compounds. It
may, for example, be in the form of shredded or
particulate organic solid material, such as shredded
pathogenic waste material.
32ll33Ll
The reaction vessel for the reduction stage of
the process is lined with suitable chemical and
thermal resistant materials to withstand gaseous
by-products generated, such as hydrogen halides, for
example, hydrogen chloride. It may also be fitted
with a clean-out auger to remove solid debris or
by-products, such a~ metals, metal salts, silicates or
other solid matter, which accumulates in the vessel.
While gaseous reducing agents such as gaseous
ammonia are less costly and also potentially less
explosive than hydrogen, methane or propane, certain
advantages are obtained with these latter reducing
agents. However, while the use of gaseous hydrogen is
preferred for a number of reasons, it is contemplated
that propane may be used if the BTU content of the
waste is potentially too low for proper oxidation to
take place. More preferably, hydrogen is used for the
reduction and propane is added during oxidation.
The use of a reducing vessel and selection of
gaseous hydrogen as the reducing agent has many
beneficial and optimizing effects. Thus, in the case
of one embodiment, a road-mobile system for the
on-site destruction of accumulated PCBs and other
halogenated wastes, the utilization of hydrogen
- 12 - 132~3~
minimizes the necessary size of the reducing vessel
for carrying out a continuous process and the
potential for production of carbon. Reduction of PCBs
or hazardous waste to gaseous fuel further reduces the
necessity of additional fuel and the additional
required combustion air for the fuel, therefore
greatly reducing the size of the secondary incinerator
and overall destruction apparatus.
The safety requirements for the reduction vessel
involve the use of a multiple purge by inert gas, such
as nitrogen, to ensure the absence of oxygen (by way
of air) within the vessel, so as to preclude the
li~elihood of explosion. As the process is generally
proposed to be a continuous process, the purging
requirement becomes proportionately less onerous.
In the second preferred embodiment, having the
reduction vessel directly connected with the
combustor, both of the vessels are initially thus
purged. In view of the desirability of operating the
reduction vessel directly adjacent to, and preferably
in direct connection with a high temperature oxidizing
zone (the combustor), the reduction zone is maintained
at a pressure sufficiently higher than that within the
combustor as to warrant no flow-back of oxidizing
- 13 - 132~
agents, including air, from the combustor, into the
reduction vessel. Furthermore, in certain
embodiments, the arrangement of the reduction zone has
the mixing nozzles, wherein the pressurized reducing
gas mixes intimately with and atomizes the incoming
waste by high velocity impact therewith, located at a
low level within the zone, and a gaseous residence
zone containing reduced gaseous products located
thereabove, so as to isolate the reduction zone from
the outlet to the 02idation zone. This interface zone
between reduction and oxidation may include a ceramic
firestop to prevent flash backs. The hot reduced gas
is introduced into the oxidation zone through a
combustion mantle, combustion nozzles or suitable
apparatus to allow adequate mixing with the combustion
air introduced into the oxidation zone and to allow
optimal positioning of the flame front within the
oxidation chamber.
A further advantage of utilizing hydrogen,
generally in gaseous form, is the capability of
utilizing a jet or jets of hydrogen in impacting
relation with the substances being reduced, so as to
achieve a highly active mixing zone wherein the
as-supplied state of the hydrogen is utilized in order
to optimize the mixing efficiency, and the reduction
process.
- 14 - 1324394
In the case of fluid waste, a jet of the waste
can be impacted by a transversely directed jet of
hydrogen, to effect atomization with intimate mixing,
to promote the chemical effectiveness of the reduction
process.
One embodiment incorporates the use of a radially
inward gas cross-flow nozzle of the CALDYN (TM) type.
The subject system is capable of handling fluid waste
incorporating particles up to one-quarter inch mesh
size therein, and droplets sized down to as small as
forty microns (40) can be economically obtained.
It is to be understood that the reduction may
also be carried out in the presence of water vapor
which does not inhibit the reduction reaction. Thus,
it is poæsible to destroy organic waste material,
sludges or sediments, such as contaminated harbour
sludges or sediments, containing substantial
quantities of water.
The hot reaction mixture from this reduction
procedure will generally be dehalogenated,
hydrogenated or reduced hydrocarbons or substantially
dehalogenated hydrocarbons together with hydrogen
halide, such as hydrogen chloride, water and excess
hydrogen.
- 15 - 1 32 4 3~
The reaction vessel to be used for the reduction
in the second preferred embodiment is vertically
interfaced with a second vessel to be used for the
second, oxidation phase. The hot reaction mixture
from the reduction stage, at a temperature of from
about 600C to about 1000C, and more particularly
from about 800C to about 900C, may be forced through
a short insulated ceramic or refractory lined tube by
convection and the pressure created as a result of
evaporation and volatilization of the injected liquid
or partially liquid waste together with continuous
expansion of the gases as the reduction breakdown
occurs. Excess air or oxygen can be introduced into
the second vessel in such a way as to create a
turbulent flow of hot ga~es of the hot reaction
mixture together with oxygen which will promote the
complete combustion of those gases at a temperature of
above about 1900C. Particularly useful temperatures
are those within the range of about 1000C to about
1500C and especially a temperature of from about
1200C to about 1400C.
The size of the second vessel for the oxidation
procedure may be such that the retention or residence
time of the hot reaction mixture in the combustion
chamber will be from about 1 second to about
- 16 - 13243~4
4 seconds, preferably for a residence time of about
2 seconds, or more. The combustion chamber of the
second vessel may also be lined with suitable material
to withstand the hot acidic gases, such as hydrogen
chloride, which will pass through it. The hot
emission from this second vessel is then rapidly
cooled and scrubbed with water and aqueous alkali such
as sodium hydroxide mist or sodium carbonate in order
to remove and neutralize the acidic gases.
It may be depending upon the waste material, that
it is preferable to recover a substantial portion of
the initially reduced products.
In one embodiment of the invention, products of
the reduction step are cooled in the presence of
aqueous mist injected into the flow path of the
gaseous reduction products. In a subseguent step,
gaseous remains may be further cooled, condensed to a
second liquid and collected before the oxidation step
of any material which still remains in gaseous form.
One initial reduction product, if the starting
waste material contains halogenated organic material,
is a hydrogen halide gas, which will largely
dissociate when dissolved in the initially collected
- 17 - 1324394
aqueous liquid, making it acidic. Such initially
collected acidic liquid may thus be neutralized in the
presence of a base such as sodium bicarbonate, calcium
carbonate, sodium hydroxide or other suitable basic
material.
Depending upon the starting material, and
conditions under which the process is carried out, a
second liquid portion having a generally lower
liquification temperature than the first may be
subsequently collected. If, for example, the starting
waste material contained substantial amounts of
chlorobenzene, a second liquid containing substantial
amounts of organic material including the reduced
product benzene may be collected. In such an
instance, a substantial amount of the second liquid
may also contain water which was not collected as part
of the first liquid. The water, or a large part
thereof, if it forms a separate layer or phase from
the organic material may be separated removed and
recycled for use as aqueous mist in the initial
cooling of products of the reduction step.
The gaseous material remaining after the second
cooling may then be transferred to an oxidation zone.
Material for oxidation is preferably pre-heated before
- 18 - 132~39~
introduction to oxidation conditions. In certain
embodiments, a heat exchange process is used to
capture and transfer energy released or removed during
the second cooling step to aid in the pre-heating of
material to be oxidized. Such a heat exchange process
may be sufficient on its own for the heating step
prior to o~idation.
A demisting step may be used prior to the
pre-heating process in order to ensure that all
condensed materials are removed from the remaining
gaseous material.
In certain embodiments, a heat exchange process
is used to transfer energy liberated during the
oxidation process to the waste pre-heating step prior
to reduction. This transfer is preferably achieved by
heating water, which may form into steam, in a closed
zone. The heated water or steam may be circulated
within its closed zone from the oxidation zone to the
waste pre-heating zone and back again to effect
pre-heating of waste material.
As described previously, it is usually preferable
to introduce the reducing agent and waste through a
nozzle such that they are intimately mi~ed. Where it
- lg 1324394
arises that it is inconvenient to introduce the waste
through a nozzle the waste material may be, as an
alternative, initially introduced to a bath of molten
vitrification material which provides a pre-heating of
the waste before entry into the reduction chamber. An
example of material used in the vitrification of
incinerator ash is molten silica. In such an aspect
of the invention, the waste material to be reduced
vaporizes from the molten material and is subsequently
red~ced by a gaseous reducing agent which is
introduced separately and above the vitriication
material.
The invention is illustrated in principle by, but
not limited to, the following examples:
Example 1
1 Molar equivalent of polychlorinated biphenyl
(Arochlor*1248) was reacted with 22 molar e~uivalents
of hydrogen in a first reaction chamber at a
temperature of 875C and 1 atmosphere gauge during a
reaction period of about 3~ seconds. This reaction
produced 99.9% destruction and the gaseous reaction
mi~ture thus obtained contained hydrogen chloride,
benzene, biphenyl and chlorobenzenes. This gaseous
- 20 - ~324394
reaction mixture was then passed through an
interfacing tube at 8'75C into a second reaction
chamber where oxidation could take place. A 5% excess
of pre-heated air was then mixed with the gaseous
reaction mixture in the second reaction chamber and
oxidation was completed at a temperature of 1000 to
1200C during a residence time of 2 seconds. This
oxidation of the gaseous reaction mixture was
effective in completing oxidation of the remaining
reactants in the mixture.
* TM
Example 2
9 Molar equivalents of monochlorobenzene and
2 molar equivalents of 1,2,4-trichlorobenzene were
reacted with 21 molar equivalents of hydrogen in a
first reaction chamber at a temperature of 925C and
1 atmoæphere pressure during a reaction time of
30 seconds. The reaction produced 99.95%
dehalogenation of the chlorobenzenes. This gaseous
resction mi2ture was then passed through an
interfacing tube at 875C into a second reaction
chamber where oxîdation could take place.
- 21 - 13243~
A 5% excess of pre-heated air was then mixed with
the gaseous reaction mixture in the second reaction
chamber and oxidation was completed at a temperature
of 1000C to 1200C during a residence time of
2 seconds. This oxidation of the gaseous reaction
mixture was effective in completing oxidation of the
remaining reactants in the mixture.
Example 3
1 Molar equivalent of chloroform was reacted with
10 moles of water vapor at 950C at 1 atmosphere of
pressure in a first reaction chamber during a reaction
time of 30 seconds. This reaction caused g9.9%
dehalogenation of chloroform. This gaseous reaction
mixture was then passed through an interfacing tube at
875C into a second reaction chamber where oxidation
could take place. A 5~ excess of pre-heated air was
then mixed with the gaseous reaction mixture in the
second reaction chamber and oxidation was completed at
a temperature of 1000C to 1200C during a residence
time of 2 seconds. This oxidation of the gaseous
reaction mixture was effective in completing oxidation
of the remaining reactants in the mixture.
- 22 - 132 4394
Example 4
A 0.44% solution of hexachlorobenzene in hexane
was passed through a tubular reaction chamber at a
rate of 1.8 millimoles per minute of hexane together
with hydrogen at a rate of 17 millimoles per minute
and water at a rate of 28 millimoles per minute for a
60 minute period. The reactor temperature was
maintained at 1000C at the injection end, 950C in
the middle, and 900C at the exit end, and the average
residence time was 3.3 seconds. The reaction products
were captured in a condensing flask at the reaction
tube exit followed by an XAD7 resin cartridqe. The
flask ana tube contents were analyzed for
hexachlorobenzene and chlorobenzenes, and the
destruction removal efficiency for both
hexachlorobenzene and total chorobenzenes was found to
be 99.999%. The products of the reaction were
methane, hydrogen chloride, benzene, and
chlorobenzenes. The specific chlorobenzenes detected
included 1,2,3,4-tetrachlorobenzene,
pentachlorobenzene and hexachlorobenzene. In other
tests, chlorobenzenes found also included
monochlorobenzene, 1,2-dichlorobenzene,
1,3-dichlorobenzene, 1,4-dichlorobenzene,
1,2,3-trichlorobenzene 1,2,4-trichlorobenzene,
- 23 -
132~39~
1,3,5-trichlorobenzene, 1,2,3,5-tetrachlorobenzene,
and 1,2,4,5-tetrachlorbenzene. Water was recovered
from the products of each reaction in a quantity
similar to that introduced.
The foregoing examples constituted laboratory
feasibility tests, to establish effectiveness and
residence times.
In carrying out the present invention at an
effective production level, it is necessary to provide
a reduction chamber suitably pre-heated to a reaction
sustaining temperature.
In view of the advantages that obtain by use of
hydrogen, as previously set forth, and the imperative
need to provide an effective gaseous purge, the
pre-heating of the subject vessel is preferred by way
of passive heating means, such as electrical heating
elements, as opposed to active heating methods, such
as gas combustion. Owing to the highly acti~e
chemicals generally evolved from the subject process,
the use of protective, chemically resistant vessel
linings is important. This requirement and the use of
radiant heat are not incompatible.
- 24 - 132~394
The use of steam or superheated steam as both a
purge gas and as the pre-heating agent, is
contemplated.
The present invention thus provides a system for
the reduction and subsequent oxidation of organic
waste materials, the system having a reducing vessel
for the chemical reduction of the waste materials
therein, first gas entry means for admitting a purge
gas within the vessel in air purginq relation thereto,
to provide an oxygen-free environment within the
vessel; pre-heating means for raising the temperature
within the vessel above a predetermined minimum
reduction temperature for a predetermined group of the
organic waste material; feed means for feeding the
organic waste material in a feedable form into the
ve6sel within a localized mixing zone therein; fluid
admission nozzle means for admitting a reducing fluid
in directed intimate mixing relation with the waste
material in the mixing zone, whereby the waste
material is effectively reduced to a form including
combustible gaseous components therewith.
The system further provides a combustion chamber
to receive the combustible gaseous products for
combustion therein.
- 25 - 132 4 39~
In one embodiment, the combustion chamber is
superposed over the reduction vessel, to receive the
gases therefrom in upward flowing relation, through a
central passage, equipped with a ceramic fire stop to
prevent flash back. The hot reduced gas is introduced
into the oxidation zone through a combustion mantle,
combustion nozzles or other suitable apparatus to
promote mixing with the combustion air being admitted
to the oxidation zone, and provide optimal positioning
of the flame front within the oxidation chamber.
The system further provides 1ue gas treatment
means to handle the highly acidic f lue gas by way of
an alkaline scrub down, prior to release to the
atmosphere.
A control system, not forming a part of the
present invention, provides an automated control, in
order to monitor and maintain appropriate feed rates
of the process constituents, and safe and suitable
environmental conditions for the respective stages of
~0 the process.
Certain embodiments of the invention are
described, reference being made to the accompanying
drawings, wherein:
- 26 - ~32 439~
Figure l is a schematic elevation, in diametrical
section, of a reduction chamber; combined with a
combustor, shown partly sectioned;
Figure 2 is a scrap view, in diametrical section,
of an atomizing nozzle portion of the reduction
apparatus;
Figure 3 is a diagramatic arrangement of a plant
for carrying out the process;
Figure 4 is an impression of a mobile plant
embodiment: and
Figure 5 is a schematic diaqram of a process
embodiment according to the invention;
Figure 6 is a schematic elevation, in diametrical
section, of a reduction chamber having upper waste
inlets and an inner tube;
Figure 7 is a horizontal cross-section take along
7-7 of Figure 6;
Figure 8 is a schematic of a grit collection
system for use with a reduction chamber; and
- 27 - 132 439~
Figure g is a schematic of an embodiment process
employing a vitrification process in conjunction with
a reduction process.
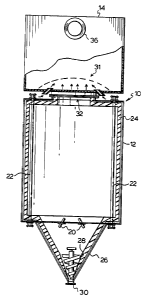
Referring to the embodiment illustrated by Figure
1, the reduction vessel 10 for carrying out a
reduction process has a metal shell 12 and is
substantially free-standing, having a combustion
chamber 14 mounted thereon.
The reduction vessel 10 has one or more inlet
nozzles 20 ~see Fig. 2) for injecting waste, including
pulverized olids, for atomization of the liquid
portion thereof by jets of hydrogen gas through an
annulus of nozzles 27.
The vessel 10 has a bank of radiant electrical
heaters 22 oP known commercial type such as
Carborundum silicon carbide glow bar heaters lining
the walls thereof. Chemically resistant FIBERFRAX
(TM~ ceramic thermal insulation 24 protects the shell
12, while also ensuring a safe working thermal
environment in the locality of the vessel 10.
A bottom portion 26 of shell 12 includes an auger
28, and a sealed outlet 30 whereby cleaning out of
solid inorganic residues can be effected.
- 28 - 132439~
A passage 32 connects vessel 10 with vessel 14
for upward passage therethrough of the reduced gaseous
products. The passage 32 is positioned and sized to
allow the pressure within vessel 10 to be controlled,
in order to ensure a safe, positive pressure
differential between reduction chamber 10 and
combustion chamber 14. A blow-out panel (not shown)
safeguards the vessel 10 against e~plosive over
pressure.
An air supply nozzle and combustion grid
combination 31 ensures safe and stable combustion
within chamber 14.
Referring to Figure 2, the nozzles 20, positioned
as shown in Figure 1, each have a liquid inlet 21 and
a gaseous inlet 23. The gaseous inlet 23 connects, by
way of control valves 201, 203 with respective
pressurized nitrogen and pressurized hydrogen supplies
(not shown), for use in initially purging the combined
vessel 10/14, and for subsequently operating the
chemical reduction process, respectively.
The exhaust opening 36 of chamber 14 connects
with an acidic gas scrubber and centrifugal fan or
particulate removal apparatus 61, particularly in view
of the hydrogen chloride content of the e~haust gases.
- 29 132 43~
Referring to Figure 3, the system 40 is shown
schematically, with reduction chamber 10 supporting
combustion chamber 14. Feed of waste-with~ uid or
liquids such as PCB or PCB-containing sludge is fed by
way of a controllable feed valve 50 to the inlets 21
of the nozzles 20 in chamber 10. The reduced gases
pass by way of passage 32 to combustion chamber 14.
Variable air inlet controls 54 permit regulation of
combustion chamber 14, operating substantially at
atmospheric pressure.
Exhaust gases leave by way of passage 56, passing
through a scrubber system 60 centrifugal fan
particulate removal apparatus 61, and cooling showers,
shown schematically at 62, to leave by stack 65, to
atmosphere. The subject system includes neutralizing
water tank 67, cooling water tank 69, and associated
pumps and controls.
The overall ~ystem does not preclude other types
of scrubbers.
Figure 4 is an artist's impression of the system
as set up as a low-bed trailer 90, having a hydraulic
actuator 92 for positioning the composite
reduction/combustion vessel 10~14.
- 30 - 1 32 4394
It is contemplated that steam from a steam
generator may be utilized, both as the purging agent
for the combined vessel 10/14, and as the pre-heating
agent for the reduction and combustor chambers 10/14.
Process control instrumentation located in
ancilliary trailer 95 provides automated control and
back-up by way of instrumentation and controls (not
shown).
Turning to Figure 5, a process incorporating both
reduced product recovery and water coolant recycling
is illustrated schematically. Waste passes through
pre-heater 100 as indicated by arrows 102 to be
introduced into reduction vessel 104 through a nozzle
107 in which the waste is intimately mixed with a
gaseous reducing agent, preferably hydrogen (H2),
through waste/reducing agent inlet ports 106 in the
upper region of the reduction vessel. As shown in
Figure 6, vessel 104 comprises ceramic tube 108 with
ports 110 located in its lower region. Glow bars 112
heat the vessel. A steady but low flow of gaseous
reducing agent is introduced in the lower region of
the vessel also, at inlet ports 114 to create a
barrier which hinders entry of gaseous waste into
water stream 15~ which is further described below.
- 31 - 132 43~
Gaseous waste materials must travel into the lower
region of the vessel to pass through ports 110 and up
through the interior of tube 108 to proceed to a next
step of the process.
As illustrated for the Figure 5 embodiment,
reduced gaseous waste materials next pass through
retention zone 116 with subsequent introduction of
water. The process illustrated in Figure S is for the
treatment of halogenated organic waste products, a
reduction product of which is hydrogen halide gas.
Water is injected as a mist and hydrogen halide gases
dissolve and dissociate in the water droplets which
condense and collect in tank 118. The dissolved gases
make the water, that is aqueous liquid, acidic. Tank
118 is provided with entry means 120 for controlled
introduction thereinto of an acid neutralization agent
such as a bicarbonate salt, sodium hydroxide, calcium
carbonate, etc. The acid in the aqueous liquid
collected in tank 118 may thus be neutralized.
Uncondensed gases, including both reduction products
and water vapor nest pass through zone 122 into
cooling zone 124. The gases are cooled to a lower
temperature and more gases condense. The cooling
temperature of this zone is maintained within a few
degrees, and usually above the freezing temperature of
- 32 - 1324394
the condensed liquids which collect in vessel 128.
Gases which remain uncondensed flow through zone 128,
through demister 130 which is a baffle to catch any
remaining small droplets or particles. Demister 130
is located so as to permit any matter caught thereby
to flow into vessel 128.
According to this embodiment, the remaining gases
next pass through flame arrest 132 to be oxidized in
the final stages of the process. Propulsion of gases
through the above process steps is aided by fan
located in vicinity 134.
An oxidizing agent, such as air or oxygen is
introduced to mix with the gases after passage through
the flame arrest 132. Gases passing through the
oxidation pre-heating zone located immediately after
the flame arrest 132 are pre-heated prior to oxidation
in oxidation zone 136. Oxidation zone 136 is heated,
as may be re~uired, with an oxidation gas such as
natural gas or propane to promote oxidation of the
remaining gases by the oxygen and the final products
are released through stack 138.
In the Figure 5 embodiment, a heat exchanger 126
is used in the cooling zone 124, the energy released
- 33 ~ 132 4394
being used in conjunction with heat exchanger 135 to
provide heat for pre-heating of the gases in the
oxidation pre-heating zone as indicated by arrow 125.
Further, a closed zone 140 containing water is located
to capture energy released during the oxidation step,
the water being heated, so as to vaporize to steam,
which is used to aid the pre-heating of waste in
pre-heater 100. Movement of water and steam within
zone 140 is indicated by arrows 142.
The process is operated with controlled
throughput, as by control valves 145, and temperatures
such that a maior portion of the acidic gases produced
by the reduction step are collected and neutralized in
tank 118. This may be achieved by maintaining the
temperature in the retention zone 116 in the
neighborhood below the boiling point of water, that
is, between about 70C and about 100C and preferably
about 85C. Some organic reduction products may or
may not also be collected as liquid in tank 118 and
this liquid thus may or may not require further
treatment.
Cooling zone 124 is maintained at a significantly
lower temperature than retention zone 116, in the
neighborhood of and usually above the freezing
~ 34 ~ 1324394
temperature of the liquid being collected in vessel
128, that is up to about 20C and preferably about
5C. As is known to those skilled in the art organic
reduction products such as those which condense and
collect in vessel 128 are readily separable, in large
part, from the aqueous liquid which also condenses.
That is, the liquid collected in vessel 128 will
generally form two layers, a first aqueous layer
comprising mainly water, and a second organic layer
comprising mainly organic reduction products. The
aqueous layer is separated and recycled as indicated
by arrow 146 into retention zone 116. The remaining
organic layer collected in vessel 128 may then be
purified by distillation and/or other techniques to
remove undesired impurities. The product of the
purification may then be used or sold, etc.
It will be appreciated that the step of trapping
acidic gases in the aqueous liquid collected in tank
118 is necessary to the extent that such gases are
produced by reduction of the particular organic waste
being used as feedstock for the process, and such a
step may not be required in which case components of
the ~aseous stream from the reducing vessel would be
directly condensed and cooled to a low temperature
below about 20C.
- 35 - 1324394
Waste material often comprises non-volatile
particulate material which upon entry into vessel 104
at 106 will travel to the bottom of the vessel. Such
material is here referred to as ~grits~ and the
preferred embodiment provides a grits collection step.
As best seen in Figure 8, hydrogen inlet ports,
114 are located in the lower region of vessel lQ4. A
water (H2O) inlet port 150 is located below ths
hydrogen inlet ports. Grits fall into the water
stream 152 and are carried into grit collection tank
154. The level of water in tank 154 is monitored, for
example, with the use of a mercury level switch 156
which automatically activates valve 158 to empty tank
154 when the water level has reached a particular
height. Appropriate safeguards are employed to ensure
that grits clogging the bottom of vessel 104,
malfunction of switch 156, valve 158, etc., may be
detected. The effluent emptied from tank 154 is
appropriately treated and disposed of.
An alternative waste introduction system,
particularly useful for ash or solid waste utilizes a
vitrification process. With reference to Figure 9,
vitrification vessel 170 is provided with electrodes
172, 174, waste inlet port 176, slag outlet port 178,
132~39~
- 36 -
gaseous reducing agent inlets 180, and the interior of
the vessel communicates with retention zone 116
through upper outlet 182. The waste inlet 176 is
provided with rotating valves 184, 186. In use valve
186 is opened to permit waste material 188 to fall
into passage 190 while valve 184 is closed. Valve 186
is then closed, valve 184 is opened and waste material
contained in the passage falls through inlet 176 into
vessel 170. Thus, an "air-lock" system is provided to
preclude large amounts of oxygen from entering the
vessel 170. It will be appreciated that the object of
this valve arrangement is to preclude large explosive
amounts of oxygen from contacting hydrogen within the
vessel and other suitabla ~air-lock~ systems may be
employed.
In use, vitrification material is added to vessel
170 and may or may not be part of the waste being
treated.
An electric current is passed from one electrode
20 to the other via the material which is heated and
vitrifies. Volatile organic material in the
vitrification material is released into the overlying
gaseous reducing atmosphere, usually hydrogen. The
vitrification process itself may be controlled to
provide sufficient heat for the reduction process.
- 37 - 1324394
The description of the foregoing embodiments
illustrate the present invention and the invention is
not limited by it, the invention being limited by the
appended claims.
7439b/1 -38