Note: Descriptions are shown in the official language in which they were submitted.
^` 1~268~
ETALL~C l;'AlY~Y ACID PI~LLETS
~CXGROUND OF THE INVENTION
Field of the Inventlo~
The present inventlon relates to powder met~lllc
salts of ~llphntlc monoc~rboxyllc acld~, for ex~mple,
commerclal stearic acid, ~nd partlcularly to a modlfied
powder form and method of manuf~cture.
Description of the Prior Art
Powder metallic salts of commercial stearic acid,
commonly ~nown a8 metallic ~te~rates, which qenerally
consl~ts of 50~ to 75~ by weight ~tearie aeid
(octodecano~c acld), 25~ to 50~ by weight palmitic acid
(hexadecanole acld), and ~m~ll amount~, l.e. generally
less than 2~ by weight, other C~-C~ allphatie ~aturated
~nd uns~turated monocarboxylle aeid~, ~re used a~
~dditive~ ln a varlety of consumer product~. ~or
ex~mple, aluminum ~te~rate ~ used a8 a defoamer for oll
well drilling mud~, a thlckening agent for crnyon~, a
gelling agent for greases and oils, and a su~pen~ion
agent in palnts and lnk~. Calcium ~teara~e 18 used a~ a
eecond~ry stablllzer, mold release agent and lubricant in
rlqld PVC~ a neutralizer of resldual cntalyst~ in
polyethylene ~nd polypropylene, and an antlcaklng a~ent
ln food appllcatlon~. Magnesium stearate i8 used a~ a
mold rele~se agent ln powder~ and tablet~ for co~etlc
~nd pharmaceutlc~l appllc~tlon~, ~ dlspersant ~nd
~ '
~ 1~268~9
.
antlcaklng agent for flre extlngulsher powder mlxtures,
and a lubrlcant and proce~sing ald ln acrylonltrlle-
butadiene-~tyrene ~ABS) polymers. Zlnc stearate 18 used
as a proce6slng and lubrlcant ald for crystal and impact
S polystyrene, a d~sperslng agent in plgment blending, and
a lubrlcant and mold release agent in polyethylene. Lead
stearate 18 used as a stabil~zer for PvC.
In many applicatlons, a flnely dlvided or ~mall
partlcle slze, such as an average partlcle slze ln the
range from 2 to 200 microns, 18 needed for the proper
blendlng and functlonlng of the metalllc stearate. In
other appllcatlons, an average partlcle ~lze up to 100
mesh (0.2 mm) can be used. Dry powder metalllc
stearates, after manufacture, are transported and handled
ln bulk fonm, for example, by tank car or truck or ~ay be
placed ln bags whlch are then handled and transported to
the user. At the user, the bulk metalllc tearate,
elther from storaqe facllltle~ or bag~ further
handled and placed ln hoppers to mlxer~ and blender~.
Durlng thl~ handllng and transport, the flne powdery
metalllc ~tearate materlal 18 sub~ect to produclng du~t
whlch 1~ hazardou~. For e~ample, metalllc ~tearate duat,
lf lgnlted, can re~ult ln an exploslon cau~ln~ property
d~mage and serlou~ ln~ury and death to personnel.
The prlor art dl~closes addlnq 25~ by welght mlneral
oll to the powder to ~uppres~ du~t formatlon. Howe~er,
such quantlty of oll dllute~ the powder and can often
render the powder unsultable for lt~ lntended purpo~e.
$U~MARy OP_~Hg INVgN~ION
It ha~ been dlscovered that powder metalllc aalt~ of
allphatlc monocarbo~yllc aclds can be pelletlzed to a~old
the formatlon of dust durlng transport and handlln~ when
fro~ O.S to 20~ by welght llquld or pllable ~olld blnder
msterlal 1~ blended w~th the powder prlor to passlng the
' ~' ' . ~
:- - . ;
, , , . , ~ ,
~ 326859 ,
mlxture through a pellet m~ll. When ~uch powder pellets
are used, by blending the met~lllc stear~te~ in
conventlonal blendlng and mlxlng equlpment, the pellets
readily dlslntegrate to their powder form, and thus
S additlonal grlnding equipment i8 not necessary to return
the pellets to a powder form prior to use. Furthermore,
careful selection of the partlcular blnding material
avoids alterlng the properties of the end product or the
effectlveness of the powder in it~ lntended function.
An ob~ect of the invention is to eliminate explosion
hazards produced in handlinq and transport of powder
metalllc salts of allphatlc monocarboxyl~c acids.
One advantage of the lnvention is that expenslve
dust handllng facllltles for preventing hazardous dust
15 condltlons of powder metallic salts of allphatlc
monocarboxyllc aclds 18 el~lnated.
One feature of the inventlon l~ that a variety of
materlals are sultable for asslstlng ln the blndlng of
the powdery metalllc salts of allphatlc monocarboxyllc
20 aclds ~o that a particular materlal may be selected based
upon Ita belng relatl ely inert and non-altering to the
end u~e or consumer product.
Other ob~ect~, advantage~ and features of the
invention wlll be app~rent from the following descrlption
25 of the preferred embod$ment talcen in con~unct~on with the
accompanylng drawlnSI wherein~
BRI~P DESCRIPTION O~ THB DRAWING
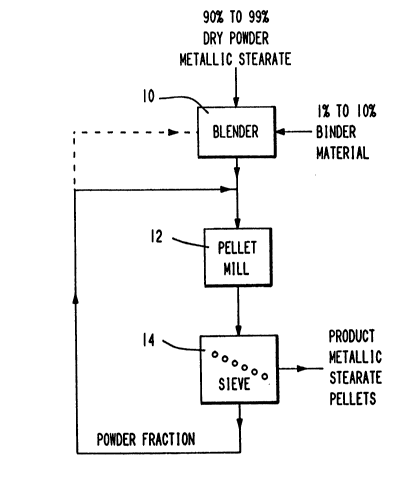
The drnwlng is a flow diagram illustratinq a process
for manufacturlng powder pellets of metalllc salts of
30 aliphatic monocarboxylic acids in accord~nce wlth the
invention.
D~:RIPTION OF T~B PREFEMBD l~lBODIMBNT
~ illustrated ln the accompanying drawing, powder
pellets of metall~c salt~ of ~liphatic monoc~rboxylic
- .
.
. , - -.: . . ~ .
~ , , ' .,: :
,.: . . .,' . ' . :
.
,. : -
4 1~268~9
acids are produced in accordance with the invention by
blending the dry powder and from 0.5 to 20~ by weight
binder material in a blender 10 and passing the blended
material through a pellet mill 12 to form product powder
pellets suitable for handling and transport with
substantially reduced risk for generating hazardous dust
conditions.
The dry powder metallic salt is any powder metallic
salt of an aliphatic monocarboxylic acid which is used
commercially, is handled and transported in bulk form, and
tends to generate hazardous dust conditions during the
handling and transport. Generally, calcium, magnesium,
aluminum, zinc and lead salts of aliphatic monocarboxylic
acids having from 12 to 22 carbon atoms are such powders.
Examples are commercial metallic stearates including
calcium, magnesium, aluminum, zinc, and lead stearates
produced by conventional precipitation and fusion processes
from commercial stearic acids which generally consist of
50% to 75% by weight stearic acid ~octadecanoic acid), 25%
to 50% by weight palmitic acid (hexadecanoic acid), and
small amounts, i.e. generally less than 2% by weight, other
C12-C22 aliphatic saturated and unsaturated
monocarboxylic acids. The dry powder metallic salt is
generally in the range from 80% to 99.5% by weight of the
material being added to the blender 10. Preferably, the
dry powder metallic salt i8 in the range from 90% to 99% by
weight of the material in the powder pellets, and most
preferably, in the range from 96% to 98% by weight of the
material in the powder pellets.
Commercial powder metallic stearates generally contain
relatively little residual unreacted fatty acid, i.e., less
than 1%, so that the powder material cannot be pelletized
at ordinary ambient temperature by mechanical
pelletizing equipment. Pellets made from dry powder
.. . , ~
.. . .
,, . , ~ ; ~ ; . ,
. i,. . . , , : , :
.. . . .
, ; , : : . .: ~
13268~9
, .
metallic stearates w~thout the addition ~f the binder
material have little strength and inte~rity, and will
revert back to their dusty, powdery form under normal
minimal forces exerted on the pellets during handling and
transport.
The binder material is a liquid or pliable solid
material which can be readily blended with the metallic
stearate without altering the end use properties of the
metallic stearate including the dispersal and mixing of the
fine powder in a consumer or intermediate product.
Generally, suitable binder materials such as mineral oils,
plasticizers, etc. or low melting waxes, fats, fatty acids,
silicone oils, etc. Examples of suitable mineral oils with
a viscosity in the range from 7 cst to 70 cst at 40C;
mineral oils with a viscosity in the range of 7 to 30 cst
are known as light mineral oils. Examples of suitable
plasticizers include bis(2-ethylhexyl)phthalate,
bis(2-ethylhexyl)adipate, epoxidized soybean oil,
epoxidized linseed oil, and diethylene glycol
dipelargonate. Examples of suitable waxes include waxes,
such as paraffin wax and bayberry wax, having a melting
point below 60 C. Examples of suitable fatty acids
include stearic acid, palmitic acid, oleic acid, lauric
acid, and myristic acid; and suitable fats include those
formed from such acids. Examples of suitable silicone
fluids include polydimethylsiloxanes having viscosities in
the range from 10 to 10,000 cst, copolymers of
dimethysiloxane and diphenylsiloxane, and copolymers of
dimethylsiloxane and methylphenylsiloxane. The binder
material is generally in the range from 0.5% to 20% by
weight of the material being added to the blender 10.
Preferably the binder material is in the range from 1% to
10% by weight of the material forming the pellets, and
~ . : . . : : . :
,: ~ , :
132685.g
moet preferably, ln the range from 2~ to ~- by welght of
the materlal ln the pellet~
The blender 10 18 a commerc$al blender app~ratus ~-
suitable for blending dry powdery metalllc salts of fatty
acld~ wlth llquld or pllable solld materlal~ ln
sufflclently large batch or contlnuou~ flow technlque~
sultable to produce the des~red quantlty of product The
blender 1~ operated ln a manner to produce a homogeneou~
mlxture of the blnder materlal and dry powder metalllc
salt
The mlxed powder and binder materlal from the
blender 10 18 then fed to a pellet mlll 12 whlch 1~ also
a conventlonal pellet mlll selected for produclng
throughput at a rate sufflclent to handle the dealred
lS quantlty of product
The output of the pellet mlll 1~ then passed througb
a ~leve 14 where the product metalllc powder pellet~ are
separated from a re~ldual powder fractlon So~e of the
materlal pa~-lng through the pellet ~111 wlll not be
adequately pelletlzed or 1~ ub~ect to dl-lntegratlon
upon lmplnglng upon the leve 1~ 8uch materlal 1- then
recycled back to the feed hopper or entrance of the
pellet mlll 12 for further proce~slng Alternatlvely,
the powder fractlon from the ~leve 1~, or a portlon
thereof, can be recycled back to the blender 10 a- ~hown
ln the da~hed llne
In accordance with the present lnventlon lt 1-
dl~covered that powder metalllc salt- of C~.-C~.
allphatlc monocarboxyllc aclds ~an be formed lnto pellet-
when blnder ~aterlal~ are blended wlth the powder and thereaultlng blended materlal 1~ pas~ed through a pell-t
alll. The blendlng and pelletl~lng l~ performed at
te~perature~ well below the meltlng polnt of the powder
metalllc alt to a~old fu-lon of the powder. 8uch
. ~ .
.
.
1~26~9
pellet~ substantlally elinlnate the rlsk of produclng
hazardous dust conditlons during handllng and tr~naport
of the bulk powder material. When the powder pellet~ are
eubsequently pa3sed to commerclal blender~ or mixlng
equlpment ln the formatlon of consumer products, the
powder pellet~ readlly dl~integrate, due to the much
greater stress applied by such blendlng and mlxlng
equipment, 80 that the powder 18 dlsper6ed and ~ixed in
the same manner aJ lf the powder material had not been
formed lnto pellet~.
E%amDle 1
Mlneral oll, ERVOL 11ght, vlsco~lty 24 to 26 cst at
40 C, whlte mlneral oll, Witco Chemlcal, 40 g wa~ added
gradually to 2000 g Calclum Stearate HP Granular,
Malllnc~rodt, ~nc., belng blended ln laboratory rlbbon
mlxer model O-A, Paul O. Abbe, Inc. The mixture wa~
allowed to blend for about 20 minute~. The dry blend
thus produced wa~ tran~ferred to the hopper o a
laboratory pellet mlll model CL-3, Callfornla Pellet M111
Company. The pellet mlll wa~ e~ulpped with ~n L89 alloy
~teel l/~ x 1/2~ dle and ~a~ operatlnq at about 250 rp~.
Dry blended calclum ~tearate/mlneral oll wa~ then fed
from the hopper into the dle cavlty. Product wa~
collected a~ lt emerged out of the die. A product mlx
contalnlng pellet~ and powder wa~ collected. It wa~
~leved on a ~.S. ~tandard ~leve No. l0 separatlng the
pellet~ from the powder fr~ction. The fractions were by
weight 56~ pellets and 46~ powder. The powder fr~ctlon
wa8 passed through the mlll for a second time resultlng
3~ ln a total yield by welght of 7q~ pellets and 26~ powder.
Pellet~ made ln accordance wlth ~xample l were
substltuted for powder calclum stearate ln the
manufacture of a polyethylene re~ln. The pellets readlly
d~s~ntegrated ln the blendlng apparatus and formed a
$- * Trade-mark
~ . .
~ 8~9 - -
` I
homogeneous mlxture of llnear low denslty polyelylene
whlch wa~ equal to that normally formed using
unpelletlzed powder calclum stearate
ExamDle 2
S In the manner de~crlbed ln Example 1, unmodlfled
Calclum Stearate HP Granul~r was fed to the pellet mlll
and the product collected It conslsted of 100~ powdery
materlal Pa~sinq the product for a second tlme and then
a third t~me through the pellet mill d~d not alter the
phyelcal form of the product collected whlch remalned
about 100~ powder
ExamDle 3
In the manner descrlbed ln ~xample 1, ~,000 g
Calclum Stearate Standard, Malllnckrodt, Inc was blended
15 wlth ~0 g 300 Pla~tlc~ Oll, vl~co~lty ~8-C0 c~t at ~0 C,
Wltco Chemical After pelletizlng the blend and ~levlng
the product, the following fractlon~ by welght were
collectedt pellet~ 6~, powder 36
exam~
In the mann~r descrlbed ln Example 1, ~,000 g
Calclu~ ~tearate Standard wa~ blended wlth 160 g 300
Pla~tlc~ O11 After pelletl~lng th- blend and ~levlng
the product, th followlng fractlon~ by welght were
collectedt psllet~ 9~ 5-, powder 5 5
~xamDle 5
Alumlnu~ Stearate A~603,~Malllnc~rodt, Inc contaln~
about 3 5~ of unreacted fatty acld The materlal wa~ fed
to the pellet mlll de~cr~bed ln ~xample 1 and a mlxture
contalnlng by welght ~6~ pellet~ and 5~ powder w~-
collected.
~xample ~
Alumlnu~ 8tearate AX603, 550 g, wa- blended wlth
11 0 g ~lneral oll The blend wa- then pelletlzed a-
de~crlbed ln Bxa~ple 1. A mlxture cont~lnlng by welght
. .
- 13268~9~
65~ pellets and 35% powder was collected. Improvement ln
feeding r~te, without ~amming the mill, a8 well ~ ln
level of pellets produced ~esultad from the incorporstion
of mlneral oll into the aluminum ~tenrate.
S The integrity of the pellets produced in the above
Example~ 1-6 was ascertained by determlnin~ percentage of
attritlon, i.e. powder produced, ln a 50 g sample tumbled
at 30 rpm mlnutes aR shown in the following Table I.
Te~ts were run using a PHARMA TEST lnstrument type PTFR
D6452 manufactured ln Hainbury, West Germany. Following
the 30 m~nutes test perlod the sample was transferred to
a U.S. standard sleve No. 16. The fractlon of samp1e
pa~sing through the ~creen was determlned. The higher
that fractlon, the wea~er the pellets. The followlng
resu1t~ were obta~ned~
TABLE ~
Pellets Produced %
in Example ~ttrlLQn
1 10.5
3 19.7
4 4.1
3.~
6 2.3
Crush strength of alumlnum stearate pellets produced
ln Example~ 5 and 6 was determlned uslng 8 Schleuniger-2E
lnstrument manufactured in Switzerland by Dr. R.
Schleunlger ~ Co. a8 ~hown ln the followlng Table II.
The results are glve~ ln ~P unlts where 1 RP ~ 2.248 lbs.
TABLE I~
~ellets from ~xample Crush Strenoth. RP
5 2.13
C 2.53
Slnce m~ny modlflcatlon~, varlatlon~ and changes ln
detall may be made to the a~ove described embodlment
wlthout departlng fro~ the scope and splrit of the
`- 132~8~g ~
lnvention, lt 18 lntended that all matter described ln
the foregoing description ~nd shown ln the accompanylng
drawing be lnterpreted as only illustrative of the
$nvent$on.
.
.
.
-