Note: Descriptions are shown in the official language in which they were submitted.
13289~6
METHOD AND APPARATUS FOR CAPACITIVEL~
REGENERATING TIS~;UE AND BONE
Field of the Invention
This invention relates generally to the electric stimulation of tissue and bone
and, more particularly, to the production of a stimulative electric current in the
tissue or bone via a capacitively established electric field.
Background of the Invention
The use of electric current to facilitate the healing of traumatized tissue
and broken or fractured bone has been recognized for some time. The stimulative
effect of such current appears to occur whether the flow of current is induced
naturally, by internal body mechanisms, or artificially, by external sources. While
the natural flow of current produced by electrochemical, myoelectric, and pie~o-electric-like body mechanisms advantageously facilitates healing without external
circuitry, in some instances it is desirable to expedite the recuperative process by
artificially supplementing the natural current flow.
A variety of different techniques have been devised for establishing supple-
mental electric currents in tissue and bone. Briefly, such techniques can be
grouped according to both the type of current developed and the manner in which
the current is established. Considering first the type of current produced, the
current may be characterized as either a direct (DC) current, having an amplitude
that is substantially constant as a function of time, or an alternating (AC) current,
which exhibits a time-dependent amplitude variation. The use of AC current is
preferred because it can be established in a variety of ways, discussed below. DC
current, on the other hand, can be induced only by providing a direct electricalconnection between the tissue or bone and the energy source.
The manner in which the auxiliary stimulating current is induced offers
seversl alternative forms of classification. First, such techniques can be catego-
rized as being either invasive or noninvasive, depending on the connections pr
1328906
vided to the patient. Invasive techniques involve the application of electric
current directly to the site of the trauma or fracture through electrodes
implanted at the site. While this approach minimizes the electric potential
required to generate a particular desired current in the tissue or bone, it also5 involves the expense and risk of infection attendant surgical implantation proce-
dures. As a result, noninvasive procedures, in which the flow of electric current
in the tissue or bone is induced by apparatus external to the patient, are pre-
ferred.
The noninvasive establishment of an AC current in tissue or bone can be
10 further grouped according to the electric principle involved in its production. For
example, a resistive approach involves the conduction of current directly to thepatient through special electrodes coupled to the patient's skin via conductive
gel. This technique has the disadvantage of requiring good electrical contact
between the electrodes and the skin, necessitating the periodic replacement of the
conductive gel on the electrodes.
A second, or inductive, approach employs magnetic fields to establish the
desired AC current in the tissue or bone. Specifically, this approach involves the
application of an electric current to magnetic coils positioned proximate the
patient. The flow of current through the coils produces a magnetic field in the
20 patient's bone or tissue, resulting in the establishment of the desired alternating
therapeutic current. This approach has a number of disadvantages including the
required use of bulky magnetic coils and an energy source having an output whosefrequency and waveform are sufficient to induce the desired stimulating currentsat the patient site. The inductive approach also involves relatively large energy
25 losses attributable to the heating of the coil windings produced by the flow of
current therethrough.
The third technique for noninvasively establishing an alternating current in
the patient's tissue or bone can be referred to as the "capacitive" or electric field
approach. This technique typically employs a pair of electrodes that are placed on
30 opposite sides of the treatment site and are insulated from the patient's skin.
Energy applied to the electrodes establishes an electric field between the elec-trodes, normal to the skin. It is this electric field that induces the alternating
therapeutic current at the treatment site.
While this approach overcomes the difficulties outlined above with respect
35 to the resistive and inductive techniques, the capacitive production of therapeu-
tically effective levels of electric current at the patient treatment site tradi-
-3- 13289Q6
tionally presents several additional problems. For example, the patient~s skin
normally contributes a series capacitive reactance to the equivalent electric
circuit representative of the elements between the electrodes. In addition, a
much larger variable capacitive reactance is exhibited by the insulative, dielectric
5 "gaps" between the electrodes and the patient.
The combined series capacitive reactance of the elements betw~en the
electrodes has been a problem for several reasons. First, this reactive component
seriously attenuates the current flow produced by a given potential applied to the
electrodes. To understand how this energy loss arises, it may be helpful to briefly
review the dynamics of interaction between the stimulating current and the
patient. To be therapeutically effective, the electric current must have a fre-
quency that is low enough to ensure its penetration to the site of the fracture or
trauma. This requirement is imposed because a high-frequency current will con-
centrate near the dermal region of the patient in response to a mechanism known
as the "electromagnetic skin effect." Other, biological, mechanisms that lim;t the
efficacy of relatively high-frequency current also likely exist.
At therapeutically effective frequencies, the peak energy stored electro-
statically in both the electrode-to-skin interface and the epidermis during one
cycle of the alternating voltage potential applied to the electrodes is substantially
larger than the energy absorbed by the tissue and bone being treated. This stored
energy is typically either dissipated within the source or radiated as electro-
magnetic energy, resulting in a system inefficiency or energy loss. As a result,high levels of reactive power are required to establish the desired therapeutic
current.
As an alternative to the use of higher voltages to compensate for energy
losses, an inductive reactance can be employed to produce a resonant circuit that
reduces the energy losses. For example, a series-connected inductor can be used
to recapture the stored energy and apply it to the treatment site during the next
cycle of the alternating voltage applied to the electrodes. As a result, only a
relatively small amount of energy is dissipated and radiated.
Even with energy losses reduced, the capacitive technique of establishing
therapeutic current in tissue and bone still presents several problems. As notedpreviously, the capacitive reactance exhibited by the dielectric gaps between the
electrodes and the patient represents a rather large and variable electrical imped-
ance to the drive circuit. The addition of the inductor to form a resonant circuit
having a high quality factor Q, provides a significant reduction in impedance when
-4- ~328~
the circuit is operated at itS resonant frequency. Because the capacitive portion
of the circuit may vary substantially in response to both the condition of the
patient and movement between the electrodes and patient, when a fixed induc-
tance is employed the circuit can be maintained at resonance only by adjusting the
5 frequency of the driver to correspond to the resonant frequency of the circuit as
it varies with the changing capacitance. Alternatively, a variable inductive
reactance can be used to negate the effect of the changes in capacitance, leaving
the resonant frequency of the circuit unchanged.
In United States Patent No. 4,459,988 (Dugot) a circuit is disclosed that
employs the former technique. Specifically, a portion of the patient positioned
between stimulating electrodes is included in a series resonant circuit incorpora-
ting positive feedback to maintain the frequency of the stimulating signal at the
resonant frequency of the circuit. The Dugot approach, however, suffers from
several disadvantages. First, the disclosed implementation is relatively complexand involves a large number of components. Positive feedback is required to
stabilize the circuit with respect to frequency and automatic gain control is
preferably employed to regulate the amplitude of the signal generator's output. In
addition, the output produced by the circuit may be subject to spurious and mul-tiple high-frequency oscillations decreasing the efficiency of the system. The use
of a variable inductive reactance to maintain a constant resonant frequency in the
presence of capacitive changes disadvantageously requires the use and expense ofsome form of adjustable inductor and feedback to control it.
In light of the preceding remarks, it would be desirable to provide a method
and apparatus for noninvasively establishing a regenerative electric current in
traumatized tissue and broken or fractured bone. It would further be desirable to
employ a capacitive technique of establishing such a current that is simple, does
not require a high-~oltage potential to overcome large variable gap capacitances,
is inherently stable with respect to both frequency and amplitude, and rejects
high-frequency spurious oscillations.
Summary of the Invention
In accordance with this invention, a method and apparatus are provided for
capacitively establishing an alternating electric field between a pair of stimula-
tion electrodes. The electrodes are typically separated by a patient region thatincludes traumatized tissue and/or broken or fractured bone and by dielectric gaps
between the electrodes and the patient. The electric field is designed to produce
an alternating current in the tissue or bone to accelerate healing and is estab-
~5- 132~
lished by a free-running oscillator. Oscillation iS maintained with the aid of aresonator that includes an inductor and the resistive and capacitive components
provided by the patient~ electrodes, and gaps. The oscillator has a simple
construction, designed to operate in an amplitude and frequency-stable manner,
with its frequency of oscillation tracking changes in the resonant frequency of the
resonator attributable to, for example, variations in the capacitance of the dielec-
tric gaps. The oscillator is further constructed to limit the occurrence of spurious
high-frequency oscillations.
In accordance with a particular aspect of this invention, an apparatus is
provided for applying an electric current to a region of a patient through a pair of
electrodes positionable in noncontacting relationship with respect to the patient.
This region of the patient, and any gaps between the patient and the electrodes,exhibits a series capacitance and resistance. The apparatus includes an inductor,
coupled to one of the electrodes to define a resonator in cooperation with the
l 5 series capacitance and resistance. An oscillator is coupled to the inductor to
provide to the resonator a periodic current having a frequency that is substantially
equal to the resonant frequency of the resonator. Finally, an element is included
to limit the occurrence of spurious oscillation frequencies in the periodic current
provided by the oscillator.
In accordance with another aspect of this invention, an apparatus is provided
for establishing an electric field between a pair of electrodes separable by a gap
that exhibits a series capacitance and resistance. The apparatus includes an
inductor connectable in series with one of the electrodes to define a resonant
circuit with the series capacitance and resistance. A low-impedance voltage
source is included to apply a square wave output, shifted in phase by approxi-
mately 180 degrees from the source input, to one of the electrodes. The other one
of the electrodes is coupled to the input of an open-loop operational amplifier
whose output is coupled to the voltage source. The operational amplifier has a
gain sufficient to provide a unity gain for the apparatus and produces a phase shift
sufficient to provide a zero-degree phase shift for the apparatus.
Brief Description of the Drawings
The invention will presently be described in greater detail by way of
example, with reference to the accompanying drawings, wherein:
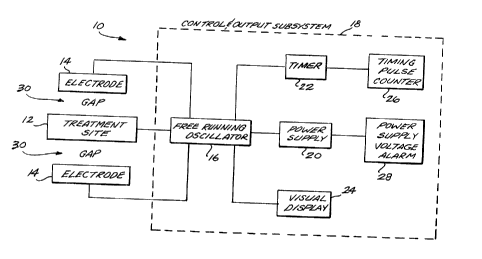
FIGURE 1 is a block diagram of a system, constructed in accordance with
this invention, that capacitively regenerates the tissue and bone of a patient;
-6- 132~Q6
FIGURE 2 is a schematic diagram of a an oscillator circuit, employed by the
system of FIGURE 1, which provides a periodic current to the patient through a
pair of electrodes separated from the patient by gaps;
FIGURE 3 is a more detailed schematic diagram of the embodilnent of the
oscillator circuit shown in FIGURE 2; and
FIGURE 4 is a schematic diagram of a second embodiment of the oscillator
circuit of FIGURE 2.
Detailed Description of the Preferred Embodiment
Referring now to FIGURE 1, a system 10 for facilitating the healing of
traumatized tissue and broken or fractured bone is illustrated. The system 10
establishes an alternating electric current at a treatment site 12, which includes
the bone and tissue for which enhanced regeneration is desired. As discussed in
greater detail below, this therapeutic current is induced by the establishment of
an electric field between a pair of electrodes 14 positioned on opposite sides of
the site 12. A free-running oscillator 16, included in a control and output sub-system 18, provides the energy required to maintain the field between
electrodes 14. Oscillator 16 is powered by a supply 20 and is controllably acti-vated and deactivated in response to a timer 22. A visual display 24, included in
subsystem 18, provides an output indicating that a therapeutic current is flowing
at the treatment site 12. As additional outputs, subsystem 18 includes a timing
pulse counter 26 to provide information concerning the length of time during
which therapeutic current is applied to the site 12 and an alarm 28 that indieates
that supply 20is no longer able to sustain the desired operation of oscillator 16.
While the operation of system 10 is described in greater detail below, its
primary function is to establish a therapeutic current at site 12. In the preferred
arrangement, an alternating therapeutic current of approximately 5 milliamperes
is produced. The frequency of the alternating current is a function of a variety of
factors, including the structure of site 12, electrodes 14, and free-running oscil-
lator 16, as well as the relative position of site 12 and electrodes 14.
While a therapeutic effect is produced by induced currents having a rela-
tively wide range of frequencies, the current's frequency should be sufficientlyhigh to prevent the deleterious electromigration of isotopes or ionic species at the
treatment site 12. On the other hand, because a "conductor" such as site 12
exhibits a "skin effect" at high frequencies that causes current to concentrate
near the surface of the conductor, the frequency must be sufficiently low to
ensure that current is induced, for example, in the fracture of a bone lying well
7 1~2~906
beneath the surface of the patient's skin. It has been found that the establishment
of an alternating current with a nominal frequency of approximately 50 kilohertzprovides the desired therapeutic effect for all normally expected patient and
electrode conditions and ensures that the frequency will remain within an accept-
able range as the oscillator 16 responds to varying patient and electrode condi-tions.
Turning now to a more detailed discllssion of the various components of
system 10, the electrodes 14 are constructed to satisfy a variety of operating
criteria. For example, because the current-inducing electric field is established
normal to the surface of electrodes 14, the area of electrodes 14 directly affects
the cross-sectional area of the treatment site 12 in which a therapeutic current is
produced. In addition, the construction details of electrodes 14, including their
size and the materials employed, will influence the capacitive reactance intro-
duced by the electrodes. In the preferred arrangement, electrodes 14 are circular
plates of conductive elastomer, such as carbon-filled silicone rubber, having a
diameter of 8 cm, a thickness of D.l cm, and a typical capacitance of 50 pF.
As indicated in the block diagram of FIGURE 1, the electrodes 14 are
preferably spaced apart from the treatment site 12 by gaps 30. The gaps 30 can
be physically maintained by the inclusion of a rigid dielectric material betweenthe electrodes 14 and site 12. For example, in a preferred arrangement, the
electrodes 14 are embedded in a cast that surrounds the traumatized tissue and
broken or fractured bones. The gaps 30 then include both the dielectric cast
material and any air gap interposed between electrodes 14 and the treatment
site 12 of the patient. Because the length of the air gaps will likely vary substan-
tially in response to, for example, patient movement, the equivalent capacitive
reactance of the circuit may undergo significant variations. It is these variations
that the free-running oscillator 16 is designed to compensate for, resulting in the
production of an acceptable therapeutic current at the site 12 under all normally
expected conditions.
Turning now to a more detailed discussion of the free-running oscillator 16,
reference is had to FIGURE 2. As shown, the oscillator 16 operates in connectionwith a series resonant circuit or resonator 32 defined by an equivalent site
circuit 34 and an inductor Ll. The equivalent circuit 34 includes a resistive
component R1 and a capacitive component C1 that are representative of site 12,
electrodes 14, and gaps 30. As discussed in greater detail below, the oscillator 16
is constructed to ensure that its operating frequency precisely tracks the changing
-8- 132$~6
resonant frequency of resonator 32. Oscillator 16 is further constructed from a
minimUm of components, providing a stable level of injected current over a
desired frequency range without the introduction of high-frequency spurious
oscillations.
As noted, free-running oscillator 16 is designed to operate resonator 32 at its
resonant frequency. Resonance is, by definition, the condition in which the
impedance of the resonator 32 is purely resistive, causing the voltage and current
at the input of resonator 32 to be in phase. Because the inductive and capacitive
components L1 and C1 do not affect the current flowing through resonator 32 in
this condition, the magnitude of the current is a function only of the voltage
applied to resonator 32 and the resistance of resonator 32.
To ensure that the free-running oscillator 16 operates at the resonant fre-
quency of resonator 32, two conditions characteristic of the stable operation ofany oscillator must be satisfied. First, the closed-loop gain of the circuit defined
by the oscillator 16 and resonator 32 must be equal to unity. Addressing this
condition in greater detail, the circuit shown in FIGURE 2 can be considered to
include a forward network 38 and a feedback network 40. The forward network 38
includes an amplifier 42 that amplifies the output V1 of feedback network 40 by a
complex, frequency-dependent gain A to produce an output voltage V0. The
feedback network 40 includes the resonator 32 and feedback elements 46. The
feedback network 40 is characterized by a complex, frequency-dependent transfer
function ~ and produces the output V1, which is equal to the product of voltage V0
and the transfer function ~.
The loop gain of the circuit in FIGURE 2 is equal to the product of the terms
A and ~. As this loop gain approaches ~1, the ratio of the circuit's output voltage
to its input voltage approaches infinity, allowing the circuit to oscillate or pro-
duce an output even when no input is applied. Both A and ~ are complex, fre-
quency-dependent quantities that can be expressed in polar form as an amplitude
and an angle. Because oscillation can take place only if the vector product of Aand ~ is +1, the product of the amplitudes of A and ~ must be equal to +1, whilethe sum of the angles of A and ~ must equal 0. This latter characteristic defines
the second requirement for oscillation, which is that the loop phase shift must be
equal to 0 or some multiple of 360 degrees.
Discussing the various components of the basic circuit of FIGURE 2 in
greater detail, resonator 32 can be considered to include four elements. As noted
previously, the equivalent circuit 34, representative of site 12, electrodes 14, and
9 ~ 32~91~6
gaps 30, in~ludes a series capacitance C1 and resistance R~. The portion of C1
attributable to the gaps 30 is relatively large and variable, leading to fluctuations
in the resonant frequency of resonator 32. The equivalent serieæ resistance R1
includes the resistance inherent in the living tissue, which is on the order of
100 ohms. This resistance R1 also includes the effective series resistance asso-ciated with the forward network 38 and feedback network 40, as well as the losses
in the series inductor L1. This latter component may be made relatively small bycarefully designing the inductor L1 to provide a high quality factor Q.
As shown in FIGURE 2, resonator 32 also includes a capacitor C2 connected
I0 in series with equivalent circuit 32. Capacitor C2 prevents oscillator 16 from
operating in spurious and multiple high-frequency modes by introducing a high-
frequency roll-off into the feedback network 42. The capacitance (e.g., 10 nF) of
capacitor C2 is t~pically much greater than that of C1. One end of capacitor C2
is coupled to ground, while the other end is connected directly to equivalent
circuit 34 and by feedback to the forward network 38. The series inductor L1 is
included in resonator 32 to reduce the dissipation of energy from the electric field
established between electrodes 14 by receiving most of the stored energy of the
field and returning it during the next cycle of the alternating potential applied to
the electrodes 14. Inductor L1 typically has an inductance on the order of
100 mH.
Turning now to a discussion of the forward network 40, network 40 prefer-
ably includes a source 42 that provides a low-impedance output voltage to reso-
nator 32. The voltage source 42 also preferably operates in a switching mode forenhanced efficiency. A suitable source or amplifier 42 is a complementary,
metal-oxide-semiconductor inverter of the type manufactured by Motorola under
the designations MC14049UB or MC14069UB. Such an amplifier 42 provides a
square wave output and introduces a phase shift of approximately 180 degrees into
the loop at the desired operating frequency of around 50 kilohertz.
Forward network 40 also includes a resistor R2. As noted previously, at
resonance, the capacitive and inductive reactances have a cancellative effect oneach other, exerting no influence on the amplitude of the current flowing through
resonator 32. As a result, the therapeutic current induced at the patient site 12
can be controlled either by altering the voltage applied to resonant circuit 32 or
its series resistance. The resistor R2 is connected in series with the equivalent
resistance R1 of resonator 32 to regulate the therapeutic current to the desiredlevel. Preferably, R2 has a resistance (e.g., 75D ohms) that is sufficient to
~:2~
-10-
increase the total series resistance of the circuit to approximately 1000 ohms. As
will be discussed in greater detail below, with a compact, 9-volt battery for
source 20 and the 4049-type inverter 42 employed in forward network 40, an
oscillator output of approximately 5 volts rms is achieved, providin~ a normallydesired therapeutic current of 5 milliamperes at resonance.
As shown in FIGURE 2, feedback network 40 ;ncludes both the resonator ~2
and feedback elements 46. Feedback elements 46 provide the loop with the
desired unity ~ain and zero phase shift characteristics. While elements 46 couldbe merged with the forward network 38, both in theory and in practice, they are
shown separately in FIGURE 2 to assist in an understanding of the circuit.
As represented in FIGURE 2, the eeedback elements 46 include an amplifier
48 and phase lag 50, which ensure that the complex transfer function ~ of the
feedback network 40 satisfies the loop's unity gain and zero phase shift require-
ments. Specifically, the gain provided by amplifier 48 compensates for both the
gain of amplifier 42 and the attenuation introduced by resonator 32. Regarding
phase shift, the current flowing through the resonator 32 is in phase with the
voltage at the output of the forward network 38 at resonance. This current
produces a voltage across capacitor C2 having a phase that lags that of the output
voltage ~rom amplifier 42 by 90 degrees. Assuming that amplifiers 42 and 48
introduce a phase shift of 270 degrees into the loop, phase lag 50 is required to
provide the remaining 90 degrees of shift. As a result, 360 degrees of phase shift
is produced, allowing stable oscillation.
To ensure that oscillator 16 will oscillate at start-up, the combined gain of
amplifiers 42 and 48 is designed to exceed any voltage attenuation introduced bythe resonator 32 and phase shifter 50. The unity gain condition for stable oscil-
lation of the loop is satisfied by making at least one of the amplifiers 42 or 48
saturable. With a 4049-type device employed for amplifier 42, such saturation isinherent.
FIGURE 3 illustrates, in slightly greater detail, a preferred embodiment of
the system 10 of FIGURE 2. Addressing first the resonator 32, the capacitor C1
of FIGURE 2 has been depicted as two capacitors Cla and C1b in FIGURE 3. The
capacitance of element Cla corresponds to that of the electrodes 14 and gaps 30,while capacitor Clb is representative of site 12.
The series resonant circuit 32 of FIGURE 3 also includes an additional
capacitor C3, having a capacitance on the order of 220 picofarads. Wh;le the
inclusion of this capacitor C3 is not essential, it assures oscillation of system 10,
2 ~ ~ 0 6
regardless of the normal variations of capacitance of element~ Cla and C1b. As
will be appreciated, the series capacitance of elements C1a and C1b may become
very large and even representative of a virtual short circuit when, for example,the patient is perspiring heavily and the electrodes 14 are spaced apart from the
5 patient by only a woven cotton sleeve designed to "wick" perspiration from thesite. As a result of this increase in capacitance, the resonant frequency of
resonator 32 could shift sufficiently to prevent oscillator 16 from achieving a
unity loop gain or zero phase shift and, hence, oscillation. By adding a
capacitor C3 whose capacitance is preferably on the order of four times that of
the nominal value of the series combination of capacitors C1a and Clb, the
resonant frequency of resonator 32 cannot drop to less than approximately one-
half its nominal value and oscillation is assured. It is also preferable to use the
additional capacitor C3 to prevent the possible flow of direct current through the
electrode circuit. Such flow might have adverse consequences through
electrolytic action, especially in the presence of moisture on the electrodes.
FIGURE 3 also includes additional details regarding the construction of the
feedback elements 46. As noted previously, this portion of network 42 is designed
to satisfy the zero phase shift and unity gain requirements of oscillation by intro-
ducing a 90-degree phase lag into the circuit, as well as sufficient gain to over-
come the loss introduced by the resonator 32. Both functions are conveniently
provided by an operational amplifier 52 connected "open-loop" or without feed-
back. The operational amplifier 52 should have a sufficient open-loop gain over
the range of expected operating frequencies to make the oscillator loop gain equal
to unity without depending upon amplifier 42 for gain. A suitable operational
amplifier 52 is provided by any one of the four operational amplifiers included in
the integrated circuit device manufactured by Texas Instruments under the part
designation TLC27M4. This device is available with internal compensation to
provide a 90-degree phase shift over a wide frequency range, including the desired
operating frequency of approximately 50 kilohertz.
The inverting input of the operational amplifier 52 is coupled to a voltage
divider formed by the series combination of resistors R3 and R4. More particu-
larly, one end of resistor R3 is coupled to the supply 20, one end of resistor R4 is
coupled to ground, and the connection of resistors R3 and R4 is coupled to the
inverting input. Because both resistors R3 and R4 have a resistance that is on the
order of 100 kilohms, a voltage equal to approximately one-half the supply 20
voltage Vdd is applied to the inverting input. The inverting input of operational
~ 3 2~:8 ~ Q 6
-12-
amplifier 52 is also coupled to ground by a bypass capacitor C4 having a capaci-tance of 100 nF and the noninverting input is coupled to the resonator 32 at theungrounded side of capacitor C2.
DC negative feedback is introduced into the oscillator circuit by a resistor
R5. Resistor R5 is coupled between the noninverting input of operational ampli-
fier 52 and the output oP the amplifier 42 in forward network 38, as shown in
FIGURE 3. Resistor R5 has a resistance of approxirnately 100 kilohms and is
included to ensure the initiation of oscillation by placing amplifiers 42 and 52within their common-mode ranges at start-up.
Turning finally to a more detailed discussion of the forward network 38, as
discussed previously it preferably employs a complementary metal-oxide-semi-
conductor inverter for amplifier 42. The ampli~ier is driven rail-to-rail at thevoltage Vdd provided by supply 20 to enhance efficiency and is also coupled to abypass capacitor C5 having a capacitance of approximately 100 nF.
As noted previously, resistor R2 is included in the forward network 38 and
has a resistance that is sufficient to provide the desired therapeutic current level
for the particular output produced by amplifier 42. A pair of reverse, parallel-connected light-emitting diodes D1 and D2, such as those manufactured by Texas
Instruments under the part number TIL213-2 are included in series with
resistor R2. The diodes D1 and D2 output an easily observable quantity of light
when energized at the therapeutic current level o~ approximately 5 milliamperes
rms. As a result, the light-emitting diodes D1 and D2 directly indicate the appli-
cation of therapeutically effective current to the site 12, rather than simply
indicating that circuit power is available to establish such a current. This advan-
tageously avoids the production of an output if oscillation ceases due to the
failure of a component or an improper spacing of the electrodes 14.
Because each light-emitting diode D1 and D2 only passes current in one
direction, the reverse, parallel connection of diodes D1 and D2 is employed to
accommodate the alternating current established in the resonant circuit 32. The
forward drop of diodes D1 and D2 is on the order of one volt. As a result, the
effective voltage applied to resonator 32 is reduced, requiring that the resistive
value of resistor R1 be adjusted accordingly to maintain the desired level of
therapeutic current.
As will be appreciated, a number of modifications can be made to the oscil-
lator circuit 16 of FIGURE 3. For example, as shown in FIGURE 49 the active
operational amplifier 52 can be removed from the circuit and a resistor R6 and
-13- ~32$90~
capacitor C6 employed as the feedback elements 46. With capacitor C2 exhibiting
a high reactance, for example, at a eapacitanee of 1 nanofarad, the voltage aeross
eapaeitor C2 will remain relatively high. Thus, even after being attenuated by the
eombination of resistor R6 and eapaeitor C6, the voltage will still be suffieient to
drive amplifier 42. The eombination of resistor R6 and eapaeitor C6 also produees
the desired phase shift of approximately 90 degrees for all frequeneies that areattenuated by r~ughly a tenfold faetor or more.
Another modifieation relates to the use of amplifier 42. While a single
amplifier 42 is shown in the schematic diagram of FIGURE 3, a number of such
elements are normally connected in parallel to achieve an internal series resis-tance that is relatively low in comparison to that of R2. As will be app~eciated,
the amplifier resistance is heavily dependent upon ambient temperature and varies
from unit to unit in a production run. Because the series resistance of the circuit
directly affects current level, the resistance of resistor R2 should always be
dominant if the therapeutic current level is to be accurately maintained.
Turning now to a discussion o~ the remaining elements of control and output
subsystem 18, as noted previously, a 9-volt battery may conveniently be employedfor power supply 20. The use of such a battery not only ensures the absence of
any high-voltage failure modes, it also contributes to the relative portability of
system 10, which may be partieularly desirable when system 10 is mounted to a
patient's east for extended periods of use.
Because the level of therapeutic current induced at site 12 is a function, in
part, of the voltage applied to resonator 32, it is important that supply 20 main-
tains a suffieient voltage for availability to oseillator 16. In this regard, a power
supply voltage output 28 is included to provide an output indieative of the status
of supply 20. For example, a simple direet eurrent voltmeter eould be employed
for output 28 to indieate the voltage available from supply 20. Alternatively,
output 28 eould inelude a eomparator having the battery voltage as one input, a
threshold voltage as another input, and an output eoupled to an audible or visual
alarm when the battery voltage drops below the threshold.
Subsystem 18 also ineludes a timer 22 designed to sequenee oscillator 16 on
and off at desired intervals. More particularly, with empirical studies conducted
to determine the eyeling rate resulting in the produetion of an optimal therapeutic
effeet, timer 22 ean then be set to cyele oscillator 16 on and off at that rate. In
addition, timer 22 can be set to initiate and interrupt this cycled operation atdesired times.
-14- 1328~06
As will be appreciated, various constructions can be employed for timer 22,
depending on the particular operation desired. For example, if an adjustable start
time, stop time, and cycle rate are desired, along with the ability to retain output
information regarding the treatment period, a microprocessor-based timer 22
programmed with an appropriate set of operating instructions may be useful. In
this manner, a timing pulse output 26 can easily be provided, displaying informa-
tion indicative of the number of timing pulses applied to oscillator 16, for analysis
by the physician. Alternatively, output 26 can be a simple counter.
As noted previously, the visual di~play 24 preferably includes a pair of
reverse, parallel-connected, light-emitting diodes D1 and D2, which directly
indicate the establishment of a therapeutic current at the site 12. As will be
appreciated, with timer 22 set to cycle oscillator on for one second and off fortwo seconds, as an example, diodes D1 and D2 will appear to be lit and extin-
guished for corresponding intervals. In a preferred arrangement employing an
appropriately programmed, microprocessor-based timer 22, when a low voltage is
sensed at supply 20, the cycle rate produced by timer 22 can be altered to provide
a change in the display produced by diodes D1 and D2 indicating a low-battery
condition.
The system 10 constructed in the manner described above has a number of
advantages. For example, the system 10 induces the desired therapeutic current
at the patient site 12 in a straightforward manner with relatively few
components. The system 10 is also stable, rejects high-frequency spurious oscil-lations, and produces an output directly indicating the establishment of a thera-
peutic current at the treatment site 12.
Another advantage of system 10 is that it enhances patient safety by
avoiding the application of a high-voltage drive output directly to the
electrodes 14. More particularly, with a constant drive voltage employed, the
current established at the treatment site 12 varies in inverse proportion to thecapacitive reactance of the eguivalent circuit 34. In certain circumstances, forexample, when electrodes 14 come into direct contact with the skin of a heavily
perspiring patient, the capacitive reactance may be negligible, resulting in theproduction of a large and potentially injurious "fault" current.
The disclosed system 10 overcomes this difficulty by providing a drive volt-
age that is limited, in case of direct electrode-to-skin contact, to the relatively
low voltage Vdd of the supply 20, for example, 9 volts. As a result, system 10 has
no high-voltage failure modes. Further, the drive current is limited by resistor
-15- ~328906
R2, which may be a series of several resistors or a single resistor constructed to
have only an open-circuit failure mode. In no circumstance will any failure of an
active component result in an over-current condition hazardous to the patient.
Those skilled in the art will recognize that the embodiments of the invention
5 disclosed herein are exemplary in nature and that various changes can be made
therein without departing from the scope and spirit of the invention. In this
regard, and as was previously mentioned, the invention is readily embodied with
either active or passive components in the feedback network to provide the
desired unity loop gain in zero phase shift. Further, it will be recognized that a
lO variety of active elements can be employed in the forward and feedback net-
works. Because of the above and numerous other variations and modifications
that will occur to those skilled in the art, the following claims should not be
limited to the embodiments illustrated and disclosed herein.