Note: Descriptions are shown in the official language in which they were submitted.
" ` 133~70
O.Z. 0~50/38936
Pulverulent, water-d;spersable carotenoid formulat;ons
~ ~ . . .
and the;r preparation
The present invention relates to the convers;on
of carotenoids to a finely divided, pulverulent form,
S the carotenoid being dissolved in an edible o;l and the
oil solut;on being in the form of very small oil drops.
This formulation is used in particular for coloring food
and animal feeds.
The carotenoids constit~te a group of colored
pigments which have a yellow to red hue, occur ~idely in
nature and impart a typical color to many foods. The
most important members of this class of substances are
B-carotene, ~-apo-8'-carotenal, canthaxanthine and cit-
ranaxathine. These substances ~hich can be synthesized
are important colorants for both the food and animal
feeds industry and pharmaceutical technology, for exam-
ple as substitutes for synthetic colorants, and are of `;
interest, for example, because of their provitamin A
~; activity. -
Virtual~y all carotenoids are insoluble ir, water
and also have only sl;ght solubility in fats and oils.
This limited solubility and the high sensitivity to
oxidation prevent the relatively coarse-particled pro~
ducts obtained in the synthesis from being used directly
for coloring food or animal feeds, since only a low
color yield can be achieved and the substances in coarsely
crystalline form are poorly absorbed. These effects w-hich
are disadvantageous when the carotenoids are used in
practice are particularly evident in an aqueous medium,
::
since ~ost of them are completely insoluble therein.
Various methods have been described for improving
the color yields and increasing the absorbability, all ~ ~;
of which aim at reducing the crystallite size of the
active ingredients and bringing it to a particle size
range smaller than 10 ~m. For example, according to
Chimia 21 (1967), 329, ~-carotene together with edible
oil can be milled to a particle size of from 2 to S ~m
"
".
.
.,~ .,"
` 133~7~
- Z - o.Z~ 0050/38936 '
under a nitrogen atmosphere in a colloid mill. Accord-
ing to Food Technol. 12 (1958), 527, the coating oil
simultaneously protects the active ingredient from ox;-
dation. A suspension obtained in this manner and con-
taining up to 20 or 30~ of active ingredient can success-
; fully be used for coloring fats and oils since the low
'solubility is nevertheless sufficient to dissolve the
crystals at the low concentration usually employed.
~hile oily or fatty systems can thus readily be
colored ~ith the pure crystalline substances, aqueous
systems'are virtually impossibLe to color with the sa;d
substances. Fur'thermore, because most of the carotenoids
are completely ;nsoluble in ~ater, the pure carotenoids
ix~d'~ith the food or ~ith the animal feed are very
poorly ut;lized by the human or animal organism. The
carotenoids have very limited solubility even in organic
solvents, such as alcohols and alkanes.
The desired coloring ind absorptibn properties ;~
can only be achieved via the very finely divided state.
20 A desirable partic~le size is smaller than 1 ~m, ~hich ~;
can be achieved by milling only with damage to the active
ingredient, if at all. ``~
Processes which represent a certain amount of
progress in comparison are known processes in which the
Z5 active ingredient is dissolved in a water-immisc;ble sol-
vent, preferably a`chlorohydrocarbon, such as chloroform '~
o~ methyle'ne chloride,'the solution is emulsified by
homogenization in a gelatine/sugar solution, and finally
.:. . ..
the solvent is'str;pped off from the emulsion, the active
30 ingredient being liberated in finely crystalline form. '''~
This process is described in Chimia 21 (1967), 329, German ~`
Published Application DAS 1,211,911 and German Laid-Open
Application DOS 2,534,091. A finely divided powder is
~ then obtained from the resulting suspension by removal of
;~ 35 ~ater.
Ho~ever, the abovementioned process has the dis-
advantage that chlorohydrocarbons have to be used in
"` ~ 133~27~
- 3 - O.Z. 0050/38936
order to achieve a suffic;ently high concentrat;on of
active ingred;ent ;n the emulsion phase. Complete re-
moval of the chlorohydrocarbons, wh;ch ;s necessary for ~ ;
toxicological reasons, is technically d;ff;cuLt to achieve.
These disadvantages were overcome by the process of
European Patent 65,193, in which a carotenoid is dissolved
in a volatile, water-miscible organic solvent at from 50 to
Z00C, if necessary under superatmospheric pressure, in
less than 1tl seconds, the carotenoid is immediately precipi-
tated in colloidal form from the resulting molecular disperse
solution by rapid mixing with an aqueous solution of a swel-
lable colloid at from 0 to 50C, and the resulting dispers;on
is freed from the solvent and the dispersing med;um in a
~ conventional manner. The mean size of the sol;d carotenoid
; 15 particles produced by this method is l3ss than 0.3 ~m.
In another process (cf. U.S. Patent 2,861,891 and
Austrian Patent 202,273), a water-dispersable carotenoid
formulation is obtained by preparing, at fro~ 100 to 160C,
a supersaturated solution of the carotenoid in an edible
oil which is li~uid at about 20-40C, emulsifying this super-
saturated solution in an aqueous, gelatinous material, and
converting the emulsion in a conventional manner to small
dry particles. ~ -
~; If the dry po~der is redispersed in warm water, ;~
a cloudy, orange yellow e0ulsion is formed again. This
can be used, for example, for coloiring food. Important
. . .
performance characteristics of these colorant preparations
are solubility, hue, color strength, opacity and stab;lity -
in the mediun used (R.H. 3unnell, ~. Driscoll and I.C.
aauernfeind~ Food Technol. XII t1958), 1-81). ~`
However, a spectrophotometric investigation of
the finely divided carotenoid formulations produced by
the stated prior art shows that, particularly at fairly .k~J''.i'~'
high concentrations, for example 3 2% of dry powder, the
extinction values at the band maximum of the tinctorially
effective absorption bands in the visible spectral range
are only SOX of the maximum values achievable in a true
133~70
- 4 - o.Z. 0050/38936
solution. From the economic point of view, this is a
considerable d;sadvantage since only 50% of the poten-
tial color strength of a carotenoid can be utilized, for
example for coloring a food, and the dose of the color-
S ant therefore has to be doubled in order to obtain thedesired coLor strength. It is also known that the hue
of a food colored with carotenoids is determined to a
great extent by the particle s;ze and the physical state ~-
(solid, solution). With the prior art products, the
range of potential differences in the hue of a selected
carotenoid is far from being exhausted. It is further- ;
more known that the biological absorption of water-insoluble
act;ve ingredients, for example after oral administration,
is greatly influenced by the particle size and the physi-
cal state. The carotenoid preparations produced accord-
ing to the prior art therefore do not satisfy the pre- ~; ;
condition for optimum biological absorption. -~
It is an object of the present invention to pro-
vide a process which makes it possible to prepare caroten-~
20 oid formulations which do not have the stated disadvan- - ~`
tages.
~; ~e have found that this object is achieved, ac-
cording to the invention, if the carotenoid is rapidly
dissolved in a volatile, water-miscible, organic so-lven~
at from 50 to 240C, preferably from 150 to 200C, to-
gether with from 1.5 to 20 times the weight, based on the
carotenoid, of an edible oil~ and an emulsifier, under
atmospheric or superatmospheric pressure, the hydrophilic
solvent component is transferred to the aqueous phase~ ~ ~
30 froM the resulting molecular disperse solution by rapid --
mixing with an aqueous solution of a protective colloid
at from û to 50C, the hydrophobic oil phase which con-
tains the carotenoid in solution being formed as the
~icrodisperse phase, and the resulting two-phase mixture
is freed from the solvent and the water in a conventional
manner.
The novel procedure utililes ehe fact that the ;~
~ ;;; .,' '',
:~ .
.
'~' ,
1330270
~ 5 - O.Z. 0050~38936
solubility of the carotenoids in a solution of an edible
oil ;n water-miscible solvents, which is very low at low
temperatures increases substantia~ly with increasing tem-
perature, but, despite the high temPeratures~ a short
S residence time at elevated temperatures substantially
prevents isomerization which affects the hue, the color
strength and the biological activity. However, this was
in contradiction to the fact that the isomer;zation which
usually occurs on heating in hot oil is itself used to
1û inhibit recrystallizat;on in the drops of the emulsified
o;l phase when the temperature is reduced, the said re-
crystallization having an adverse effect on the hue and
the color strength of the desired end product. lt was
therefore surprising that, in spite of suppress;ng the
tendency to isomerizatio~ at elevated temperatures, and
after cool;ng, the product obtained by the novel pro-
cedure contains the caroteno;d, spectrophotometrlcally
detectable, as a molecular disperse, suPersaturated oil
solution stabilized to recrystallization, in the form
of submicroscoPic oil particles, and, compared w;th prior
art products, has up to 100% greater color strength coupled
with a shift in the hue to previously unaccessible ranges. -
The photochemical stability of the hydrosols prepared by - ~-
. .
the novel process is super;or to that of the products pre-
pared according to the prior art by a factor of more than
5.
The carotenoids which can be used for carrying
out the invention are the known, obtainable, natural or
synthetic members of this class of compounds, which can
3û be used as color-imparting materials, for example carot-
ene, lycopene, bixine, zeaxanthine, cryptoxanthine, cit-
ranaxanehine~ luteine, canthaxanthine, astaxanthine,~
apo-4'-carotenal, 3-apo-8'-carotenal, 3-apo-12'-carotenal,
3-apo-8'-carotenic acid and esters of hydroxyl-contain-
ing and carboxyl-containing members of this group, for
example the lower alkyl esters and preferably the methyl
and ethyl esters. The compounds which have been readily
6 ~ 7 ~ z ooSo/38936
available industrially to date, such as B-carotene, can- -
thaxanthine, B-apo-8'-carotenal and ~~apo-8'-carotenates,
are part;cularly preferred.
Water-miscible, heat-stable, volatile solvents
contain;ng only carbon, hydrogen and oxygen, eg. alcohols,
ethers, esters, ketones and acetals, are particularly suit-
able for carry;ng out the novel process. Ethanal, n-propanol,
isopropanol, butane-1,2-diol 1-methyl ether, propane-1,2- ~-
diol 1-n-propyl ether and acetone are preferably used.
In general, it is advantageous to use solvents
which are not less than 10% water-miscible, have a boil-
ing point of less than 200C and/or contain less than 10
carbon atoms.
Suitable edible oils are oils which are liquid
15 at from Z0 to 40C. Examples are vegetable oils, such ~
as corn oil, coconut oil, sesame oil, peanut oil, soybean ~ ~ ;
oil or co~tonseed oil. Peanut oil is particularly pre~
ferred. Other suitable oils or fats are shortening,
beef dripping and butter fat. The edible oils are gener~
aLly used ;n an amount of from 1.5 to 20, preferably from
3 to 8, times the we;ght of the carotenoid, and the total
oil content of the carotenoid formulation should not ex~
ceed 60% by weight if it is intended to prepare a dry
powder.
Su;table protective colloids are any conventional -~
protective colloids perm;tted ;n food and an;mal feeds,
examples are gelat;ne, starch, dextr;n, dextran, pect;n,
gum arabic, case;n, caseinate, whole milk, skimmed milk,
milk powder or mixtures of these. Ho~ever, it ;s also
possible to use polyvinyl alcohol, polyvinylpyrrolidone,
methylcellulose, carboxymethylcellulose, hydroxypropyl-
cellulose and alginates. For further details, reference
may be made to R.A. Morton, Fast Soluble Vitamins, Intern.
Encyclopedia of Food and Nutr;tion, Vol. 9, Pergamon
35 Press, 1970, pages 128-131~ To increase the mechanical
stability of the end product, it is advantageous to add
to the colloid a plasticizer, such as sugar or sugar
.: .
~ ~.
" 133~0
~ 7 O.Z. 0050/38936
alcohols, eg. sucrose, glucos~, lactose, invert sugar,
sorbitol, mannitol or glycerol. M;nor amounts of methyl
esters or propyl esters of p-hydroxybenzoic acid, sorbic
acid and Na benzoate may also be added as preservatives.
The ratio of protective colloid, pLasticizer and
oil to carotenoid is in general chosen so that the result-
ing end product conta;ns from 0.5 to 10, preferabLy from
2 to 5, % by we;ght of carotenoid, from 5 to 50% by
we;ght of an edible oil, from 10 to 50% by we;ght of a pro-
tective collo;d and from 20 to 70% by weight of a plas-
ticizer, all percentages being based on the dry we;ght
of the powder, as well as m;nor amounts of a stabilizer~
The mean particle size of the oil phase present ;n the ~
powder and supersaturated molecular disperse carotenoid ~-
15 is less than 0.3 ~m, and the half width of the size ~ ~;
distribution is less than 50%. The product contains
virtually no oil particles having a particle size greater
than 1 ~m.
To increase the stability of the active ingredient
to oxidative degradation, ;t is advantageous to add stabil~
izers, such as ~-tocopherol, lecithin, tert-butylhydroxy-
toluene, tert-butylhydroxyanisole, ethoxyquine or ascorbyl
palmitate.
They can be added to either the aqueous phase or
the solvent phase but are preferably dissolved together
with the colorants and the oil, in the solvent phase.
~ The novel process gives a viscous liquid which
; has a deep coloration and from which the solvent can be
removed in a conventional manner depending on the boil-
ing point, for example by d;stillation, under atmospheric
or reduced pressure, or by extraction with a water-
.
immiscible solvent. Preferably, however, the solvent is
removed together with the water by spray drying or spray
granulation.
The dry powder obtained can be redissolved in
water with uniform fine distribution of the active in-
gredient in the particle size range < 0.5 ~m. The ~
, ','.'' ~.' '
., .. ~...
:, . . .::
.. :
. .-: : :::
.:
- ".'- ' ''.
"' ' ~
8 I 3 3 0 ~ ~ ~ Z . 0050,38936
photochemical stability test shows that the resulting
hydrosol of the act;ve ingredient is extremely stable
despite being finely d;vided.
If necessary, the microdisperse oil phase super-
saturated with carotenoid can also be brought to a suitable
pH and then flocculated together with the protective colloid -~
and thus converted to a form from which the solvent and
a major part of the dispersing medium can be removed in
a simple manner by filtration or centr;fuging~ The co-
acervate thus obtained is then further dried in a conven~
tional manner and converted to granules.
Specif;cally, the novel process ;s carr;ed out
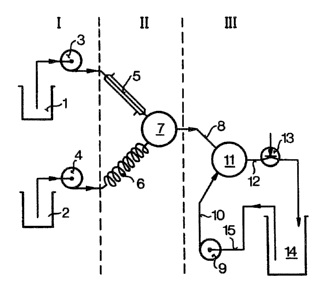
as follo~s, for example using an apparatus as shown
schematically in F;g. 1.
15The apparatus is divided into parts I, II and ~-~
III. Part II is the high temperature sect;on, while in
parts I and III the temperatures are less than 50C.
In vessel (1), a suspension of the carotenoid to-
gether with the oil in the selected solvent in a concen-
20 tration of from 2 to 20% by ~eight, based on the mixture, `
with or ~ithout the addition of from û.1 to 1û% by weight
of stabilizers, is initially taken. Vessel (2) conta;ns
the solvent without admixed carotenoid. The suspensions
of active ingredient and the solvent are fed to the mix~
25 ing chamber t7) via the pumps (3) and (4) respectively; ;~
the mixing ratio can be predetermined by choosing the
particular deLivery of the pumps, and is selected so that, ~9
depending on the solvent and the residence time, the
resulting carotenoid concentration in the mixing chamber
30 is from 0.5 to 10~ by weight, based on the solutlon. The -
volume of the mixing chamber (7) is such that the resi-
dence time in (7) is preferably less than 1 second at
the selected delivery of the pumps (3) and (4).
3efore entering the mixing chamber, the solvent
is brought to the desired temperature by means o~ the
heat exchanger (6), while the oil-containing suspension
of active ingredient is kept at belo~ 50C by feed;ng it
,.. 133~70
- 9 - O~Z. 0050/38936
via the thermally insulated l;ne t5). As a result of
turbulent mi~ing in (7) at from 50 to 24~C, preferably
fro~ 150 to 200C, the active ingredient goes into solu- ~ ~
tion, and, after a short res;dence time, preferably less ~ -
than 1 second, the result;ng solution passes via (8) into
the second mixing chamber (11), in which, by admixing an
aqueous protective colloid/plasticizer solution via pump
(9) and feed line (10), the molecular disperse carotenoid
solution is divided into a two-phase mixture with forma-
10 tion of a microdisperse oil phase containing the active ~
ingredient in supersaturated solution and a homogeneous, ~ `
aqueous phase containing the water-miscible solvent. The
microdisperse two-phase mixture is then discharged via
line (12) and the pressure rel;ef valve and fed to the
stock vessel (14). To obta;n a very high concentration of
active ingredient, the dispersion can be circulated via
the suction line (15).
If the pressure relief valve (13) is set at above .
one bar, it is even possible to use solvents at tempera- ~-
tures above their boiling point (under atmospheric pres-
sure) in the novel process. ~ -
A pulverulent preparation can be obtained from
the dispersion in a conventional manner, for example as; -
described in German Laid-Open Application DOS 2,534,091,
by spray drying or by spray cooling or by coating the
particles, separation and drying in a fluidized bed. ;~
For spray drying, the dispersion is either first
`~ freed from the solvent by distillation, preferably under
reduced pressure, or by extraction with a water-immiscible
solvent, or the entire mixture is spray-dried and water
` and solvent are stripped off together in the spray tower
in this manner.
The carotenoid powder is obtained in either dry
or free-flowing form at the bottom of the spray tower. `~ -
35 In some cases, it may be advantageous additionally to :
carry out complete drying in a fluidized bed. Instead ~ `~
of preparing the powder formulation by spray drying, it
~: .'. ` .'~
- . . , -. ~ : ~::
, :,, , ..',,
,' ,'": `:',' ,'
I ~ 3 V 2 70~ z . ooso/38936
is also possible to use any other methods to convert the
caroteno;ds already f;nely d;str;buted ;n the water/o;l/
solvent dispers;on into powder form.
A known and equally su;table method comprises,
S for example, emulsify;ng the dispersion freed from the
solvent with liquid paraffin, cooling the mixture, separat- -~
ing the liquid paraffin from the coated carotenoid par~
~icles, washing the result;ng carotenoid prepara~ion
with naphtha and drying the preparation in a fluidized ;
bed.
In the novel procedure, it was particularly sur~
prising that the use of the stated ~ater-misc;ble sol-
vents mixed ~ith an edible oil which may additionally
contain emulsifiers, such as ascorbyl palmitate, mono-
and diglycerides, esters of monoglycerides with aceticacid, citric acid, lactic acid or diacetyltartaric acid,
polyglycerol fatty acid esters, sorbitan fatty acid es- -
ters, propylene glycol fatty ac;d esters, stearoyl 2-
lactylates or lecithin, permits the preparation of highly
supersaturated solutions in uhich, in spite of suppres-
sion of the trans-cis isomerization, no recrystallization
of the carotenoid takes place ~ithin the submicroscopic
oil drops supersaturated with active ingredient in the
microdisperse oil phase, after the phase separation in-
duced by turbulent mixing with the aqueous protectivecolloid solut;on, even during removal of the volatile
solvent, for example by distillation or spray dry;ng,
and after cool;ng.
It is furthermore surpr;sing that admixing of the
solvent-containing o;l solut;on of the caroteno;ds with
the aqueous protective collo;d solution induces phase ;~
separat;on dur;ng wh;ch the disperse oil phase ;s ob-
ta;ned in the form of extremely small part;cles, as can-
; not be obtained by mechan;cal homogenizat;on. This f;nely
dispersed state of the oil phase supersaturated w;th ac~
tive ingredient is also ma;nta;ned dur;ng removal of the
volat;le solvent, for example by spray drying. It is
: .: :
. ~.: :::-:
:::: . .
:' ~'
,
13~27~
0.Z. 0050/38936
easily Possible to obta`in preparations in which the major
fraction in the o;l phase has a particle size of 0.2 ~m,
without part;cles of active ingred;ent larger than 1 ~m -~`
simultaneously being present. The absorption spectrum
5 of such carotenoid preparations shows the band form and ~--
extinction typical of the molecular disperse solut;on of
a carotenoid in an edibLe oil, even after spray drying
and redissolution in an aqueous medium.
The Examples which follow illus~rate the novel
process.
EXAMPLE 1
S g of 3-trans-carotene are suspended in Z40 9
of a solution of 4 g of ascorbyl palmitate, 5 9 of
a-tocopherol and 20 9 of peanut oil in ;sopropanol, the
` 15 pressure relief valve (13) is set at 25 bar and the said
suspension is mixed in mixing chamber (7) with 360 9 of
isopropanol which has been heated to 225C in heat ex-
changer (6). The suspension is metered at 2 l/h and the~ .
solvent at 3 l/h, and the residence time in mixing cham-
ber (7) is 0.35 second. The molecular disperse solution
formed at 190C is then fed to mixing chamber (11), in
~;~ which turbulent mixing with 4,000 9 of an aqueous solu-
tion of 60 9 of gelatine and 90 9 of sucrose, brought to
pH 9 with lN NaOH, at a metering rate of 27 l/h results
~ 25 in phase separation ~ith formation of a microdisperse oil~ ;
;~ phase which contains the 3-carotene in the form of a `
supersaturated solution. A microdisperse two-phase mix- -
ture having a yellow hue and a temperature of 50C is ob~
tained in collecting vessel (14). ParticLe size analy- `~-
sis by proton correlation spectroscoPy gives a mean par~
t;cle size of the oil phase of 210 nm and a distribution
width of ~40Z.
.. .....
Removal of the solvent under reduced pressure at
50C in a distiLLation apparatus gives a viscous l;quid
~; 35 which can be converted to a stable, water-soLubLe dry
po~der by spray drying. The ~-carotene content of this
dry powder is 2.4% by weight.
,, .
;': ' ~
3~27~ ~
- 12 - O.Z. 0050/38936
Redissolving the dry powder in cold water gives
a yello~ solution in which the oil phase is again present -~
as a microdisperse phase having a particle size of Z20 nm
+30%.
Spectrophotometric investigation of this solution
shows the ~-carotene band form typical of a molecular
disperse solution (Fig. 2a). In contrast, the prior art
gives a product which, for the same concentrat;on of ac-
tive ingredient in aqueous solution, gives an absorption
spectrum sho~ing the typical curve of a ~-carotene solid-
state spectrum (Fig. 2b). The ratio of the extinction
values at the band maxima is 2.1. Hence, the novel pro-
cess g;ves a preparation which has more than twice the
; color strength of a prior art preparation.
In Spiee of the extremely finely divided nature
and the high color strength, the hydrosol exhibits ex-
cellent stability in the photostability test. Under
standardized irradiation conditions, a loss of active
ingredient of 10X is recorded during an irradiation time
; 20 of 270 minutes. In the case of products having a
3-carotene content of Z.4~ and prepared according to the
prior art, a loss of active ingredient of 10% is ob-
served under the same irradiation conditions after only
50 minutes.
EXAMPLE Z
A two-phase mixture is obtained as described in
ExampLe 1, but with the use of 10 9 ~-trans-carotene,- `
8 9 of ascorbyl palmitate and 40 9 of peanut oil; in the -
said mixture, the oil phase supersaturated ~ith ~-carotene -
is in the form of submicroscopic droplets having a mean
; particle size of 249 nm +52%.
The dry powder obtained by spray drying contains ~-
SX of active ingredient and, after redissolution in water, -;~
gives a hydrosol having a mean particle size of 289 nm
35 +54X. Compared with the prior art product, the extinction `~
ratio at the band ~~xinun is 1.8.
,:,:
, :
133~
.
;` - 13 - O.Z. 0050/38936
EXAMPLE 3
A dry powder is obta;ned as descr;bed ;n Example
1, but w;th the use of 5 9 of canthaxanthine; after re- .
d;ssolution ;n water, the said powder gives a hydrosol
having a mean particle size of 191 nm +42%. The ext;nc-
tion at the band maximum at ~ ~ 478 nm is 90% of the
theoretical maximum value, whereas prior art products
give extinct;on values which are no more than 5û~ of the :-
theoretical values.
EXAMPLE 4 . :
A dry powder is obtained as described in Example
;~ 1, but with the use of 60 9 of gum arabic as a protective
~: colloid; after dissolution in water, the said powder gives
a hydrosol having a mean particle size of 359 nm +42%. : :
' ~ :
'
~ . . ,;
.: ,- :.
~ , . . ~:
. ...:
.~":',';, :`
.' ,,.'~' '
` .:',.`-;' ~`
''' ~.':
' -
, :',
!