Note: Descriptions are shown in the official language in which they were submitted.
1 330425
BACKGROUND OF THE INVENTION
I . F I EL D OF THE I NVENT I ON
.. ..
This invention relates to a method for increasing the
yield of desired protein products obtainable by the expression
of foreign genes in the baculovirus-cellular expression system
using intermediate DNA modifications in the method, and to
novel recombinant baculoviruses so-produced, particularly
those which express HIV-l specific rev, vif, pol and tat
proteins upon infection of insect cells. The invention also
relates to the utilization of these proteins for the
development of prognostic reagents, diagnostic reagents and
combined subunit vaccine against AIDS.
II. DESCRIPTION OF T~E PRIOR ART
An important goal of recombinant DNA technology, as far
-as it relates to protein engineering, is to provide a gene
expression system which will produce large quantities of
desired gene products and provide protein modifications
similar to those of the naturally occurring proteins.
; Both prokaryotic and eukaryotic cells have been used -~
to express cloned foreign genes and Escherichia coli is the
most commonly used prokaryotic host system for foreign gene
expression. However,jproka~ryotic cells are suitable for
foreign gene expression only if the gene product does not
require post-translational modifications such as glycosyl- :~
ationr phosphorylation or signal peptide cleavage.
Since prokaryotic cells do not possess the appropriate
~ machinery needed for the proper modification of many
,i,
~ eukaryotic proteins, it has been necessary to develop gene
,~
, :. , 1330425
-- 2 --
expression systems using eukaryotes to obtain appropriately
modified gene products. There have been impressive successes
in the expression of foreign genes using eukaryotic hosts
such as yeast, mammalian, plant and insect cells. The impetus
for the develop~ent oE new systems has come mainly from the
need to produce larger quantities of properly modified cloned
gene products.
Advances in the genetics of invertebrate viruses and cells
have allowed the development of viral-cellular systems which
give both a high level of synthesis and complex processing of -
recombinant products. In particular, baculoviruses such as
; Autographa californica nucleopolyhedrosis virus (AcNPV) and
y~ mori nucleopolyhedrosis virus (BmNPV) are extremely
useful helper-independent eukaryotic expression vectors which
are easily engineered. In the case of AcNPV, the system is
based on a cell line established in the late 1970's from
pural ovarian cells of the moth Spodoptera frugiperda. When
infected with baculovirus carrying a foreign gene, these ~;
cells synthesize recombinant products complete with post -
~d translational modifications. In the case of BmNPV, foreign
gene products can be expressed in living insects, namely
silkworms. Both these viral systems are based on the
utilization of the strong promoter of the gene encoding
polyhedrin, the sole component of the crystalline matrix that
acts as a protective shield for viral particles outside their
insect host. The techniques conventionally employed in these
systems are described in detail in U.S. Patent 4,745,051 to
~- Gale E. Smith et al issued on May 17, 1988; Baculovirus
,;: .
~ 3~ A ~
- 3 - ~ 33 o4~5
Vectors for Expression of Foreign Genes, C. Yong Kang,
Advances in Virus Research, Vol. 35, pp 177-192, Academic
Press Ins., 1988; A ~anual of ~ethods for Baculovirus Vectors
and Insect Cell Culture Procedures, Max D. Summers and Gale
E. Smith, May 1987, Texas A & M University; and Baculoviruses
as Gene Expression Vectors, Lois K. Miller, Ann. Rev.
Microbiol. 42, pp 177-199, 1988. This expression system has
been used for the successful production of large quantities
of many different gene products including human fibroblast
interferon, human c-mye protein, human interleukin 2, etc.
Howeverr not all genes under the polyhedrin gene promoter
Z~ express at high levels, e.g. those for HIV-l specific rev,
vif, pol and tat, as mentioned above. Many researchers who
are utilizing the baculovirus expression system have tried
numerous techniques in order to improve the expression levels
of such genes, but without much success (International
Conference on Baculoviruses, Oxford, Great Britain, August 30
September 3, 1988). Accordingly, the products wilich can
`
be success~ully produced by the system to date have been
dependent upon the control mechanism that nature has selected
for high level expression. ;~
~ OBJECTS OF THE INVENTION
'~ An objec~ of the present invention is to provide a method
of genetic engineering which provides high level expression
of genes formerly expressing at only low or intermediate
levels in the baculovirus-cellular expression syste~
- ~ 1 33042-.
Another object of the invention is to provide refined
site-directed mutagenesis methods with synthetic oligno-
nucleotide linkers which can be used to engineer transfer
vectors for the preparation of recombinant haculoviruses
suitable for high level expression of foreign genes in the
baculovirus-cellular expression system.
Yet another object of the invention is to provide
recombinant baculoviruses capable of expressing desired
foreign genes at a high level, particularly the human
immunodeficiency virus genes pol, tat, vif and rev. ;~
SUMMARY OF THE INVENTION
According to one aspect of the invention there is
d, provided a recombinant baculovirus comprising at least a major
part of a polyhedrin gene promoter region; at least a trans-
Z 15 cription termination sequence of a polyhedrin structural gene;
a foreign structural gene having a translation start codon
followed by coding sequences and a translation stop codon,
said foreign gene being located between said promoter region
and said termination sequence: and, immediately upstream of
~; 20 said start codon, at least a part of a putative insect cell
ribosome binding site for the polyhedrin gene effective for at
least partially overcoming resistance of susceptible insect
cells to express said foreign gene at a high level, said part
. of said putative ribosome binding site comprising at least the
final four nucleotides of the sequence 5'-ACCTATAAAT-3'.
~; According to another aspect of the invention there is -
provided a process for producing a recombinant baculovirus:; .
-; containing a foreign gene; said process comprising: providing
~:
C '~
~ .
... : . .. .... .... . .. ...... ,. . .,.,.,., . . . . . . , .. .... . , j.. .. .. ... .. .. . . . .. .. . . .
1 3304 ~'~
. 5
said foreign gene having a translation start codon followed by
coding sequences and a translation stop codon; adding a
nucleotide sequence immediately upstream of said start codon,
said added nucleotide sequence comprising at least a part of a
putative insect cell ribosome binding site for the polyhedrin
gene effective for at least partially overcoming resistance of
susceptible insect cells to express said foreign gene at a
high level, said part of said putative ribosome binding site
comprising at least the final four nucleotides of the sequence
5'-ACCTATAAAT-3'; introducing said foreign gene and added
nucleotide sequence into a baculovirus vector containing at ,
least a major part of a polyhedrin gene promoter region and at
least the transcription termination sequence of a polyhedrin
structural gene in a position and orientation to come under
transcription control of said promoter region; cotransfecting
:~ susceptible insect cells with the resulting baculovirus vector
DNA and wild type baculovirus genomic DNA; and isolating
~: recombinant viruses containing said foreign gene and added
nucleotide sequence.
,
~: 20 The term !l immediately upstream" as used above and
throughout this disclosure means that there are no intervening
~:~ nucleotides between the start' codon (ATG) of the~foreign gene
. and the added putative ribosome binding site.
The term "a major part" of the polyhedrin gene promoter
`~ 25 region means a sufficient part of the region to avoid loss of
the effect of the promoter region during the transcription of
: ......
i~ the foreiqn gene.
~ C
,'
- 6 - 1 3304~5
The purpose of the invention is to increase the yield
of proteins that would otherwise be expressed in low or inter-
~ediate yield in the baculovirus-cellular system. There is
of course no great advantage in using ~he present invention
to produce proteins that are already expressed in high yield.
Although the terms "low", "intermediate" and "high" have not
been Eormally defined in the art, in general it can perhaps
be stated that when the desired protein forms less than about
1% of the total cellular protein the yield is considered to
be low (and the protein is generally not visualized on
polyacrylamide gel stained with Coomassie blue); a yield
betwcen about 1 and 10% of t'ne total cellular protein is
considered to be intermediate; and a yield above 10~, and
preferably 15-50% or more, is considered to be high.
BRIEF DESCRIPTION OF THE DRAWINGS
~,J"
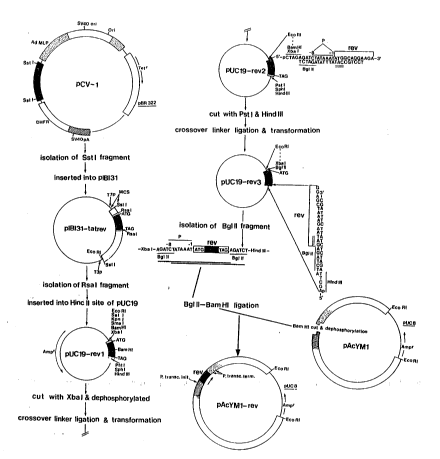
Fig. 1 is a schematic diagram showing a procedure
according to a preEerred embodiment of the invention in which
a modified rev gene of HIV-l is inserted into a pAcYMl vector
to form a transfer vector pAcYMl-rev suitable for forming a
recombinant baculovirus AcNPV-HIVYKrev capable of producing
rev at 'nigh levels;
Fig. 2 shows the time course results of a polyacrylamide
gel electrophoresis of the proteins from cells infected with a
recombinant AcNPV-HIVYKrev virus produced by the procedure of
Fig. 1 and an AcNPV-HIVPKvif virus produced by the procedure
of Fig. 3, showing the expression of rev and vif proteins;
ig. 3 is a schematic diagram showing a preferred
..,~
:'~
,~:
. .
,.:s :.
~ - 7 - 1 3 3 0 4 2 5
procedure for the modiication of the vif gene of HIV-l and
its introduction into a pAcYMl vector to form a transfer vec-
tor pAcYMl-vif suitable for forming a recombinant baculovirus
ACNPV-HIVPKvif capable of producing vif at high levels;
Fig. 4 is a schematic diagram showing a preferred
procedure for modification of the pol gene of HIV-l and
introduction of the modified gene into pACYM1 transfer vector
to form a transfer vector pAcYMl-pol suitable for forming a
recombinant baculovirus AcNPV-HIVYKpol suitable for producing
pol at high levels; and
Fig. 5 shows the time course results of a polyacrylamide
gel electrophoresis of total cellular proteins from cells
infected with recombinant AcNPV-HIVYKpol virus. -
DETAILED DESCRIPTION OF THE INVENTION AND THE PREFERRED
~:~
EMBODIMENTS
, ~ -
In the conventional baculovirus expression system, a
foreign gene is inserted into the baculovirus genome as a
partial or complete replacement for the polyhedrin structural
gene while retaining the polyhedrin gene promoter and a
stretch of the polyhedrin transcription termination signal.
The promoter for the polyhedrin structural gene is allowed
to remain so that i~ exerts a strong influence on the
transcription but, as noted above, some gene products are
nevertheless not produced at a high level.
~ The pre.qent invention is based on the introduction of
i~ a putative insect cell ribosome binding site immediately ~ ;
.~
, ~
1 330~25
- 8 -
upstream of the foreign gene without interveninq sequences
under the polyhedrin gene promoter in the baculovirus
transfer vector. In addition, the invention also involves
the elimination of any non-coding flanking sequences at
preferably both the 3' and 5' ends of the foreign gene using
a uniquely modified crossover linker mutagenesis method.
This modification of the baculoviral vector overcomes any
tendency of the viral-cellular system to resist expression of
the foreiqn gene. -~
The putative insect cell ribosome binding site referred
to in the present invention is the sequence of up to 10 bases
i~nediately upstream of the translation initiation site (ATG)
of the natural polyhedrin structural gene, i.e. the
~ underlined nucleotides in the sequence:
:~ -50 TRANSCRIPTION INITIATION
I
~ (5') TAAATAAGTATTTTACTGTTTTCGT
;! polyhedrin promoter
polyhedrin
AACAGTTTTGTAATAAAAAAACCTATAAAT ATG (3')
.................... - I
Putative Ribosome Binding Site Start Codon
. ~
The sequence is referred to herein as the "putative"
ribosome binding site because there has been as yet no
experimental verification that this sequence, when
`~ transcribed, takes part in ribosome binding.
While this sequence is present immediately upstream of
the polyhedrin structural gene in wild type baculoviruses,
the sequence is partially eliminated and/or displaced
9 1 330425
-`~? upstream of the start codons o foreign genes inserted into
known baculovirus transfer vectors. It has now been found
that the introduction of the putative eibosome binding site
j immediately upstream of the foreign gene start codon without
intervening flanking seauences and/or restriction enzyme
sites overcomes any resistance of the cell to express the
foreign gene at only low or intermediate levels. The entire
putative ribosome binding site sequence need not be intro- ~-
duced and instead merely a part of the seauence that is
effective to improve expression yields can be introduced.
The final part of the sequence appears to be the most
important and must normally be present. It is believed that
as few as the final four nucleotides, 5'-AAAT-3', can improve
expression yields, but at least the final eight nucleotides,
5'-CTATAAAT-3', are normally provided. More preferably,
the added sequence contains the nine nucleotides
(5'-CCTATAAAT-3').
As described in more detail below, the above sequences
are most conveniently introduced, and non-coding se~uences
lanklng the foreign gene are most conveniently eliminated, ~-
by means of a crossover linker mutaqenesis strategy employing
single stranded, or more preferably double stranded,
oligonucleotide linkers. Furthermore, the same strategy is
normally used to remove any non-coding flanking sequences at
the 3'-end of the foreign gene and to add a restriction site
at this end.
" - lo - 1 3 3 0 4 2 5
In general terms, the crossover linker mutagenesis
procedure can be described as follows. The foreign gene is
synthesized or isolated from a suitable DNA or RNA source
(e.g. a commercially available plasmid having suitable
restriction sites bracketing the foreign gene1 and is
inserted into a small plasmid using standard techniques.
If isolated from a natural source, the gene is normally
accompanied by non-coding flanking sequences and, to the
extent possible, these are partially removed by standard
digestion and ligation techniques.
A suitable oligonucleotide linker for upstream
modification of the gene is produced using standard DNA `~-
synthesizing techniques. This linker may be single stranded,
but is more preferably double stranded, especially if it is
desired to introduce a restriction enzyme site in the
linker. If a single stranded linker contains a restriction
site, the efficiency of crossover mutation drops because of
self annealing of the self complementary palindrome ~ -
sequences. The linker, or the primary strand if a double
stranded linker is employed, normally contains a sticky end
restriction site and a different restriction site, e.g. Bam
~I or Bgl IT, immediately upstream of ~and possibly partially
overlapping) the effective putative ribosome binding site
sequence, followed by at least 9 and preferably 12-15 bases
of homology searching sequences which represent the first
NH2-terminal 4-5 amino acids coding sequence of the foreign
;;:~
` 1 3304~5
-- 11
,
gene. It is important to avoid, if possible, any homopoly-
meric sequences in the homology searching sequences since
some DNA molecules contain a stretch of homopolymer. ~nen
the linker is double stranded, the second strand comprises
the complementary sequence except for the missing bases
necessary to form the sticky end restriction site and for
three to five missing bases at the opposite end to form a
single stranded overhang tthe latter being necessary to avoid
blunt end ligation of the linkers during the crossover
mutagenesis).
The plasmid containing the foreign gene is linearized
using a restriction endonuclease digestion which acts on a
restriction site upstream of the foreign gene and the ends of
the linearized plasmid are preferably dephosphorylated to
prevent re-circularization. Alternatively, two restriction
endonuclease digestions can be used to avoid recirculariz-
~ ation. The oligonucleotide linker is ligated by virtue of
::: :
its sticky end restriction site to the linearized plasmid
and the resulting modified structure is introduced into a
':.:
i~ suitable competent cell system, preferably E. coli, by the
standard DNA transfection method. The transfected cells are
capable of deleting unwanted bases flanking the foreign!gene
~ and circularizing the plasmid.
`~ A restriction site is also normally introduced at the 3'
end of the foreign gene and any unwanted non-coding sequences
at the 3' end are preferably deleted by a similar crossover
i~ .
,`. ~ :'
.~ .
:~:
sj ~ ".. ,.-... ... .:
d
1 3 3 0 4 2 5
- 2 -
linker mutagenesis techniaue using a single or double
stranded linker. In this case, the linker comprises a
minimum of 9 to 12 bases of homology searching sequences
corresponding to the final coding sequence of the foreign
gene at the 3' end, followed by the restriction site and a
sticky end of a different restriction site. The plasmid
containing the modified foreign gene resulting from the
previous crossover linker mutagenesis is then linearized at
a site downstream of the 3' end of the foreign gene, the
oligonucleotide linker is ligated and the resulting DNA
structure is transfected into a competent microorganism,
again preferably E. Coli, which deletes the unwanted flanking
sequences, adds a desired restriction enzyme site and
recircularizes the plasmid. -
The modified foreign gene can then be cut out and
inserted into a baculovirus transfer vector from which part
or all of the polyhedrin structural gene has been excised and
which contains a suitable cloning site downstream of the
transcription initiation site of the polyhedrin promoter
region of the vector. Since various baculovirus transfer
vectors containing suitable cloning sites are readily
available, it is advantageous to start with such a known
vector rather than construct a new one specifically for this
invention, although this could be done if desired. The
baculovirus transfer vector employed should preferably have
an intact polyhedrin promoter region ~e.g. pAcYMl or pVL941)
hut those with partial deletions may also be employed,
,: ~
-
1 330425
- 13 -
provided they are still capable of high level transcription.
For example, vectors pAc373, pAcRP6 and pAc610, which start
at the -8 position of the upstream sequences, can be employed
(see the article by C. Yong Rang mentioned above). The two
most efficient transfer vectors appear to be pAcYMl and
pBM030 (available from Drs. Bishop in England and Maeda in
Japan, respectively) which contain all of the upstream
sequences of the polyhedrin gene adjacent to a Bam HI
restriction site (pAcYMl) or a Bgl II restriction site
(pBM030). The baculovirus transfer vector should also
contain the transcription termination codon and preferably
the polyadenylation sequences of the polyhedrin gene.
The vectors are linearized by appropriate restriction
endonuclease digestion followed by phosphatase treatment.
The foreign qene having the modified flanking regions is
inserted into the restriction site of the baculovirus transfer
vector and the orientation of the foreign DNA insert is then
determined by standard restriction endonuclease mapping
and/or DNA sequencing. The resulting baculovirus transfer
vector containing the modified foreign DNA is amplified and
,
purified by standard techniques.
After the foreign gene with the desired upstream putative
ribosome binding sequences has been inserted into the
transfer vector, the construct DNA is cotransfected into
suitable insect cells with purified authentic wild type
baculovirus DNA of the same strain, e.g. by the procedure
as outlined in U.S. Patent 4,745,051 mentioned above.
~ 1 33042~
~ . .
..,
- 14 -
The insect cells are generally employed as a monolayer and,
following infection, are incubated in a suitable culture
medium for a number of days and the supernatant is
harvested. Polyhedrin-negative viruses resulting from
homologous recombination appear as clear plaques in plaque
assay and can be selected by plaque picking. An alternative
approach to this biological assay system is to screen
polyhedrin-negative plaques by nucleic acid hybridization
techniques using the cloned foreign DNA as a hybridization
probe.
The recombinant virus can then be propogated after
successive plaque isolation to exclude wild type viruses
~y isolating a single plaque and amplifying the virus in
monolayer culture in a suitable culture medium. After a few
days of infection, the supernatant can be harvested and used
to infect large numbers of cells in suspension or monolayer
cultures. -
The resulting recombinant virus, which forms a vector for
,:: ~
the expression of the foreign gene, can be used to infect
appropriate insect cells or insects, whereupon the gene is
expressed and the desired protein forms in high yield. If
the gene product is a secretory protein such as IFN, IL-2 or
HBsAg, the infected cells release these proteins after
, ~
synthesis and these can be recovered from the extra cellular
fluid of cultured cells or from the hemolymph of the infected
insects. In contrast, if the protein in nature is phos-
phorylated and anchored in the cell, the expressed gene
products remain in the infected cells and can be recovered
'''~ :
1 330425
- 15 -
from the cells after 2-4 days of infection. For example,
the pol, tat and rev proteins of HIV-l remain in the nucleus
whereas human hepatitis B virus surface antigen (Kang et. al.,
J. Gen. Virol. 68: 2607-2613, 1987) and gp 120 of HIV-l
(Bishop, Oxford, UK - Personal Communication) are secreted
into the extra cellular culture fluid.
The expressed gene products may be analyzed by direct
protein analysis using polyacrylamide gel electrophoresis and
Coomassie blue staining.
As noted above, the method of the invention can be used
with a variety of baculovirus-cellular systems, the preferred
ones being ~utographa californica nuclear polyhedrosis
(particularly the Hl strain used in the later Examples~ which
infects Spodoptera frugiperda cells, and Bombyx mori which
~;- infect silkworm cells. (However, virtually any species or
strain of baculovirus may be employed). Other viruses and
strains include those listed in U.S. Patent 4,745,051 (col.
` 9, lines 21-39).
; The preferred restriction site introduced with the puta-
tive ribosomal binding site is Bam HI, but other restriction ~;
sites which provide compatible cohesive ends (i.e. isoenzyme ~--
; sites) can be employed, e.g. Bcl I, Bgl II, MbO I and XhO II
which all produce the 5'-GATC-3' sequence upon digestion, as
, ~ d~es Bam HI itself. The possibility of using alternative
, ~ restriction sites is convenient when the foreign gene DNA
itself contains internal Bam HI or Bgl II restriction sites.
Instead of using the crossover linker mutagenesis
i ~ strategy for modifying the foreign gene prior to its
i ~
,
~ 16 - 1 330425
introduction into the baculovirus transfer vector, it would
be possible to achieve the same results by ligation of the
linkers to the linearized ~ene-containing plasmid after
deletion of the non-coding flanking sequences by exonuclease
digestion e.g. with Bal 31. However, such a techni~ue is
very imprecise and difficult and, while included within the
scope of the ~resent invention, is not the preferred
technique.
The present invention makes it possible to produce
proteins at high levels of expression and many of these
proteins can then be used for medical purposes such as for
prognostic reagents, diagnostic reagents and combined subunit
vaccines. The rev, vif, pol and tat proteins of HIV-l
produced in this way are particularly useful for the
management of acquired immunodeficiency syndrome (AIDS~, e.g.
by the techniques indicated in the publication entitled
Clinica, Testing for HIV and AIDS, The Next Five Years,
~` George Street Publications Ltd., Richmond, Surrey, UK,
Presently preferred embodiments of the present invention
" :~
2~ are described in the following Examples.
: - -These Examples relate to the preparation of the rev, vif
and pol proteins of HIV-l. However, the tat protein of 8IV-l
has also been produced by similar techniques. The
~ recombinant baculovirus capable of producing the tat protein
,~
(AcNPV-tatYK) has been deposited at the American Type Culture
~; Collection under the terms of the Budapest Treaty and the
deposit is identified by the number ATCC VR 2200.
'~
: ~
' ~
1 3~0425
- 17 -
EXAMPBE l PRODUCTION OF THE rev PROTEIN OF HIV~
A recombinant baculovirus containing the rev structural
gene and the additional sequences required by the present
invention was produced by a procedure as shown in Fig. 1.
The coding sequences of the rev protein were originally
isolated from the Sst l fragment of pCV-l plasmid. The Sst l
fragment was inserted into pIBI31 plasmid. The Rsal fragment
containing the rev coding sequences was isolated and inserted
into the Hinc II site of pUCl9. The resulting pUCl9-rev l
plasmid was then digested with Xba I and dephosphorylated and
a double-stranded crossover linker was ligated to the Xba I
- ~ site of the linearized pUCl9-rev l plasmid.
-`~ A double stranded crossover linker was synthesized using ~-~
standard DNA synthesizing techniques. The first linker
; ~ : :
~ strand comprised an XbaI sequence suitable as a sticky end
`~ (CTAGA), a Bgl II restriction site (AGATCT) (this restriction
site is used because rev gene contains an internal Bam HI
~;~ site), a TATAAAT sequence, and the initial 12 nucleotides of
f '~'~'', ',
the coding sequence of rev (ATGGCAGGAAGA). The second linker
strand comprised the complementary sequences of the first
linker strand but omitting the sequences at one end required
to form the Xba I sticky end and omitting the final 3 -
nucleotides at the opposite end to form a single stranded
tail. The linker strands were then annealed to form the
; following double stranded linker:
Translation initiation
,~ XbaI
5'-pCTAGAGATCTATAAATATGGCAGGAAGA-3'
3'-TCTAGATATTTATACCGTCCT-5'
Bgl II rev
- 18 - 1 3 3 0 4 2 5
The double-stranded crossover linker was ligated to
the Xba I site of the linearized pUCl9-rev 1 plasmid and
the resulting elongated linearized recombinant plasmid was
transfected into competent E. coli cells to carry out a
crossover linker mutagenesis. Ampicillin resistant cells
were selected and cloned, and the resulting pUCl9-rev
2 plasmids containing transformants were isolated.
The plasmid pUCl9-rev 2 contained the desired sequence
upstream of the rev gene but also contained unwanted ;
non-coding sequences downstream of the rev gene and these
were removed by the following technique :
A second double stranded oligonucleotide linker was
synthesized by a standard DNA synthesis technique. The
first strand of this linker comprised the final lS nucleo-
tide sequence of the rev gene including the translation
termination codon, a Bgl II site and a nucleotide for a
Hind III site. The second strand comprised the Hind III
sticky end and the complementary sequences of the first
strand, except for the final three nucleotides. When
,~
annealed, the double stranded linker thus was as follows:
.' ~ ::
;G translation
termination
Bgl II
5'-GGAGCTAAAGAATAGAGATCTA-3'
. 3'-CGATTTCTTATCTCTAGATTCGAp-5'
rev Hind III
;ii,: :
The pUCl9-rev 2 DNA was cut with Pst I and Hind III
`~ ~ without dephosphorylation, the second double stranded
synthetic linker was ligated to the Hind III site and the
;~;~ resulting elongated linearized plasmid was transfected
into competent E. coli cells. The ampicillin resistant
~ , ;:
- 19 - 1 3 3 1)4 ~ 5
cells were selected and cloned. The bacterium recircul-
arized the plasmid and deleted the unwanted downstream
sequences to form plas~id pUCl9-rev 3. This contained
XbaI followed by Bgl II, CTATAAAT (partially overlapping
the Bgl II site and forming the putative ribosome binding
site of S. frugiperda cells) and the entire coding
sequence of rev followed by Bgl II and Hind III.
~, : .
The rev gene-containing sequence was isolated using
Bgl II digestion and was ligated into a baculoviral
transfer vector pAcYMl that had been linearized with
I Bam HI and dephosphorylated, to give a desired vector
,!~
~ pAcYMl-rev.
;
The vector was then used to cotransfect Spodoptera
frugiperda cells together with wild type AcNPV DNA and
polyhedrin-negative recombinant viruses AcNPV-HIVYKrev
were selected and amplified.
AcNPV-HIVYKrev was used to infect S. frugiperda cells
which were harvested 24, 48, 72 and 96 hours after
infection and the recombinant virus infected cellular
proteins were subjected to protein analysis by poly-
acrylamide gel electrophoresis with Coomassie blue
staining as shown in Fig. ~. In the Figure, lane 1 shows
uninfected S. frugiperda cells, lane 2 shows wild type
AcNPV infected cells, lane 3 shows AcNPV-HIVYKrev virus
infected cells and lane 4 shows AcNPV-HIVPKvif virus
. ~
;~ infected cells (pertinent to Example 2). Tne symbol p
denotes the polyhedrin protein, v denotes vif protein and
;~.''.
i~! ~
-~
` 1 3 ~ 5
- 20 -
r denotes rev protein. The M lane shows molecular weight
markers. A band representing the rev protein is clearly
visible indicating a large yield (ca 20~) of this protein.
The recombinant virus AcNPV-HIVYKrev has been
deposited at the American Type Culture Collection under
the terms of the Budapest Treaty and the deposit is
identified by the number ATCC VR 2231.
EXAMPLE 2 PRODUCTION OF vif PROTEIN OF HIV-l
As shown in Figure 3, using techniques similar to
those of Example 1, the vif gene containing the entire
coding sequences was isolated from the plasmid pHXB-2D by
EcoRI digestion (the coding sequence of vif is located
within the EcoRl fragment-mapping unit of 4227-5322 bps-
and approximately 1100 bps were isolated). The EcoRl ~i~
fragment was filled in with Klenow and inserted into the
'~inc II site of pUC19 (pUC19-vif 1).
Using standard DNA synthesizing techniques, the
following double stranded linker was synthesized;
Translation
Initiation
Bam HI
5'-GATCCTATAAATATGGAAAACAGATGG-3'
3'-GATAI'TTATAÇCTTTTGTCT-5'
vif
., ,
This linker contained a Bam HI sticky end at the 5'
end followed with aCCTATAAAT sequence (the putative ribo-
~ ~ some binding site p) with 12 nucleotide coding sequences
;~ of the vif gene. This double stranded linker was used to
~ modify the upstream sequences of the vif gene by cutting
,' ,: ~
; - 21 - l 3 3 0 4 2 5
the plasmid pUCl9-vif 1 with sAM HI and Xbal, ligating the
linker and transforming competent E. coli cells as in
Example l. This resulted in the formation of a recombinant
plasmid pUCl9-vif 2.
To modify the downstream se~uences, the following
oligonucleotide linker was synthesized;
Translation
Term nation
S'-ATGAATGGACACTAGGATCCA-3'
3'-TTACCTGTGATCCTAGGTTCGA-5'
vLf Bam HIHind III
¦ This linker contained a 5'-12 nucleotide overlapping
¦~ sequences of the vif gene which included the translation-
¦ termination signal TAG followed by Bam HI and Hind III
~; sticky end. The 22 nucleotide long complementary sequence
with a Hind III sticky end was used to protect the Bam HI
site.
~;~ The recombinant plasmid pUCl9-vif 2 was cut with Pst l
and Hind III, the double stranded linker was ligated at
the Hind III site and the plasmid was used to transform
competent E. coli cells. This resulted in the deletion of
the 3' non-coding sequences of the vif gene and the
, !
addition of a Bam HI restriction site. The resulting
pUCl9-vif 3 plasmid contained Bam HI sites at either the
putative ribosome binding site (p), the entire coding
~ - sequence of vif including the translation termination
; coding sequence TAG at the end. This Bam HI fragment was
isolated and inserted into the Bam HI site of pAcYMl in
,,~
`
~ .
. ~ r~
--` 1 330425
- 22 -
.'
the correct orientation ~pAcYMI-vif). The pAcYMl-vif DNA
was used to transfect Spodoptera frugiperda cells with
wild type AcNPV DNA to isolate the recombinant baculovirus
AcNPV-HIVPKvif. The recombinant baculovirus, AcNPV-
HIVPKvif, was used to infect Spodoptera frugi~erda cells
to express the vif gene. The AcNPV-HIVPKvif virus infected
cells produced a 26K Dalton protein (v) which represents
at least 30% of the total cellular protein at 96 hours
after infection, as shown in Figure 2.
The AcNPV-HIVPKvif virus has been deposited at The
American Type Culture Collestion under the terms of the
Budapest Treaty and the deposit is identified by the
number ATCC VR 2235.
EXAMPLE 3 PRODUCTION OF T~E pol PROTEIN OF HIV-l
As shown in Fig. 4, using techniques similar to those
of Examples 1 and 2, the pol gene-containing part of the
protease gene at the 5' end followed by the entire coding
sequence of the reverse transcriptase gene and the coding ,~
sequences of the integrase at the 3' end were isolated
from a plasmid pHXB-2D by digesting the plasmid with Bgl
II and Sal I. The Bgl II-Sal I fragment was then inserted
into plasmid pUClB and upstream and downstream sequences
were modified to remove some of the non-coding flanking
sequenc@s. The pUC18 containing the entire coding
sequences of the polymerase gene was digested with Sac I
~: ~
and dephosphorylated.
: ~:
i~ ~
~ ~ .
1 33042~
- 23 -
Using standard DNA synthesizing techniques, the
following double stranded linker was synthesized:
TRANSLATION
INITIATION
Bam HI
S'-CGGATCCTATAAATATGAGTTTCCCAGGA-3'
3'-TCGAGCCTAGGATATTTATACTCAAAGGGT-5'
Sac I prt
This linker contains a Sac I sticky end followed by
Bam HI plus nine nucleotides of the putative ribosome
binding site (partially overlapping with the Bam Hl site)
in front of the initial 15 nucleotides of the protease
(Prt) coding sequence. The 30 nucleotide complementary
sequence starts with the 3' Sac I sticky end which extends
to the fourth nucleotide from the 3' end of the first
strand leaving a three nucleotide single strand tail at
the 3' end.
This double stranded linker was ligated to the
~,
~ linearized plasmid and was used to modify the upstream
: (5') sequences of the pol gene, using the same crossover
linker mutagenesis method as described for the rev gene in
Example 1 and the vif gene in Example 2, to delete some of
the 5' non-coding flanking sequences plus some coding
sequences of the protease gene.
To modify the downstream sequences, the following
~:
~; oligonucleotide linker was synthesized: ;
Translation
Termination
Sph 1
5'-CAGGATGAGGATTAGGATCCGCATG-3'
3'-CTACTCCTAATCCTAGGC-5'
Int. Bam HI
~' :: ,,.',
,.,.
1 330425
- 24 -
This linker contained fifteen nucleotide overlapping
sequences of the integrase gene (Int.) which include the
termination codon of translation followed by Bam HI and an
Sph I sticky end at the 3' end. The 18 nucleotide
complementary sequence was used to protect the Bam HI site.
~ y employing the crossover linker mutagenesis as
described in Examples 1 and 2, the linker was used to
delete the 3' non-coding sequences and to add a Bam HI
restriction site.
The resulting pUC 18-pol plasmid, containing no
non-coding flanking sequences at either end, was digested
with 8am HI and the Bam HI fragment was isolated and
: ~
inserted into the Bam HI site of pAcYMl in the correct -~
orientation to form pAcYMl-pol.
The pAcYMl-pol DNA was used to co-transfect Spodotera
frugiperda (SF9) cells with wild type AcNPV DNA to isolate
a recombinant baculovirus AcNPV-HIVYKpol virus.
The recombinant AcNPV-HIVYKpol virus was used to
infect SF9 cells to express the pol gene. The
~ AcNPV-HIVYKpol virus infected SF9 cells were harvested at
- 48, 72, 96 and 120 hours after infection and the total
~ cellular proteins werelsub~ected to polyacrylamide gel
b~ ~`, electrophoresis with Coomassie blue staining. The results
are~ shown in Fig. 4 in which lane 2 shows the 48 hour
product, lane 3 shows the 72 hour product, lane 4 shows
;~ the 96 hour product and lane 5 shows the 120 hour
product. Lane 6 shows the wild type AcNPV infected SF9
r, ~
- 25 - 1330425
cells with polyhedrin protein (p), lane 7 represents the
uninfected SF9 cells and lane 1 shows the molecular weight
marker .9 .
An approximately 95k Dalton pol protein (as shown with
an arrow) was synthesized and accumulated in virus infected .:
cells, representing approximately 30% of the total
cellular protein.
The AcNPV-HIVYKpol virus has been deposited at the :
American Type Culture Collection under the terms of the
: ~
Budapest Treaty and is identified by the deposit No. ATCC .-
VR 2233.
,` ~
,'`~
,' '.'''~
, ~ ~