Note: Descriptions are shown in the official language in which they were submitted.
~ `:` 1 1 330951
.... .
,, .~ ,.~ ,. . ~,
PAT~NT ;~::
CIT-~88~
SYNT~TIC PO~YME~ FOR ~NDO~APSUI.AR ~NS RE~AC~M~NT ~-.
:- - ;:.: -: ..
T~CNNICAI. FIIU.D
The present lnvention relates to treatment~ ol~ de~ect~
;~ ~ ln the eye, ~nd, a~ore partlcularly, to the roplacement of
di~eased or otherwlse ~efoctlve eye len~e~
, ................................................................................. ......... .
- ~,,
ACKGROUND ART
Sur~ery on the ~ye l~:b~co~ing Dore co~monplace und
10 sophistlcated ~B new technlgues and devlc~ are :;~eveloped .
to :co~bat i~palred~si~ht or even blin~n~s. ;~One ~uch
; field of ~urgery i8 the:replace~ent of the lens ln th~ eye
whlch~can be necessitated~,:for:exa~ple, by ca~aract devel~
op~ent, wh~oh:opaclfl~s the lens. :~
15: ~ Procedures have be~n d~veloped~for removal of th~
len~ Early procedures have~1nvolvèd:th~ re~oval of the
ens~:and~l-ns~cap-ule (transpar-nt~ ~bran- onc-psulatlng : ~.
the lens) by ~eans of forceps~or suction.~ More recently,
less~ trau~atlc aeans have been devoloped: ouch ~ean~:;ln~
20~ volve ~partloul~tlng ~the l-n-, an exa~pl~ ~of ~hlch ~ 18
: called onlcatlon, ~hlch lnvolve~ ultr~oonlc di~inte~ra~
tion of the len~ by.appllcatlon of hl~h frQquency vlbra- ~a~
tlQn8 thereto. The lens fra~ent~ ar~ then r~oved ~y
pir~tion. :~
25:~ ~ ~Replac~nt of the l~ns to:~vold r~quirln~ the pa~
~:;tlent to ~ear ~pectaclc~ ~ith ~a~lv-~ -nRe3 has ~been '~ ''.'t~
inve~tl~ated. So~e 801UtloD~ hav~ lnoludo~ in~actln~ a
,. ~ 2 1330~51
vi~couo liquld or a sillcone into the ~acant l~ns cap~ule.
Implantation of lntraocular lenses has also been done, but
the l~plant i8 rl~ld and not ~ocu~able and 18 oaslly dls~
lod~ed by shock or vibratlon.
More recently, G. M. Wr1~ht ~nd T. D. Talcott ln U.S.
Patent~ 3~,943; 4,642,~42; and 4,G08,050 have dlsclosed
ln~ectlon by n~edle of a poly~erlc lomposition into the
lens capsule. The poly~eric composl~ion comprl~es a slli~
cone prepolymer, a croos-llnker and a platlnum-based cata-
10 lyst. The composltlon cures ln thle l~n8 capoule to an -:~:
optically clear, ~el-ll~e ~at~rial whlch may accommodate,
or focus, through action of the eye lans mu3cle~.
However, a proble~ with the polymeric composltion
disclosed 18 that a ~eparate h~ating step is required to ;~
15 permit removal of the needle fro~ the eye to lnltiate :~:
polymerlzatlon at the ln~cctlon slte and thus prevent 108s
of polymer therefrom. Further, the tlme of lnltlal cross- :
linking i8 on the order o~ ~everal hour8, whlch lnvolves
lengthy immobillzatlon of the eye to per~lt co~plete cur-
ing.
Thus, what i8 reqUirQd i8 a poly~eric compofiitlon
providlng the advantayes of the prior art while avoidlng
~ost, lf not all, the problem~ assoclated wlth the prlor ~
art approache~. :
:
DISCLOSURE OF INVENTION
In accordance wlth the lnventlon, a ~ynthetlc polymer
is provided for endocapsular lens replac~3ent. The poly-
ner, which is ln~ect~d into the lens capsule after rc~ovalof the l-n~s, co~prlses an oxy~en-stabill~od photocon~ltlve
prepolymer. An 2xa~ple of ~uch a prepolymer comprises a
polyether with urethane llnkages wlth one or both ~nds
capped with a ~unctlonal yroup containin~ at l~a~t one
' ~
,., ,, i ~
r~ ~
" -~ 1 3 3 0 q 5 1 . '~
- 3 -
double bond, such as an acrylate, methacrylate, or a sty~
rene.
The polymerization reaction is initiated by light
using a photoinitiator such as dimethoxyphenylacetophen~
one and other aryl ketones and is ~uenched in the
presence of oxygen. Contrary to the prior art polymers,
the time of curing is on the order of only a minute or
so. The viscosity and thickness of the polymer formed
may be tailored to achieve a desired index of refraction.
In accordance with a first aspect of the present
invention there is provided a method of endocapsular
lens replacement by forming a synthetic polymer in the
lens capsule of an eye after removal of the lens
comprising: (a~ injecting a polymer composition
comprising a photo-sensitive prepolymer and a
photoinitiator into the lens capsule in the absence of
oxygen; (b) exposing the composition to light to form
the synthetic polymer; and (c) curing the polymer within
several minutes.
', ~: ~',.'
BRIEF DESCRIPTION OF THE DRAWINGS ~ ;
Figs. 1-5 are diagrammatic views of a portion of
an eye, showing the procedure of lens removal, followed
by injection of the polymer used in the method of the
invention.
",. : .: ~'~
.:. ~..:
. ~ ,..
,' ~
-: I 33095 1 ~
- 3a ~
BEST_MODES FOR CARRYING OUT THE INVENTION
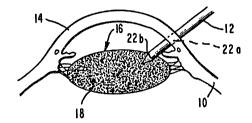
Referring now to the drawings, wherein like
numerals designate like elements throughout, a portion
of an eye 10 is shown, with a probe 12 inserted through
the cornea 14 and the lens capsule 16 into the lens 18.
As shown in FIG. 1, the probe, which advantageously
comprises means for emitting high frequency vibrations, ~:~
has caused ultrasonic disintegration of the lens 18 by a
10 process known as sonication. In FIG. 2, the probe 12, :~
which also includes aspiration means 12', has begun . :~
removal of the denatured protein of the lens, or lens
fragments, 18. In FIG. 3, the process is complete,
leaving behind the empty lens capsule 16. The
15 particular method of disintegrating the lens and e
removing it is immaterial to the practice of the ~ ~:
invention, and thus forms no part of this invention~ :-
n FIG. 4, a needle 20 is inserted into the same
incisions 22a and 22b by the probe 12 and is used to inject a
: ,..
.
~, ~
'~ '' , .
: ,
1 poly~er co~posltlon 2~ lnto the lonl c~p~ull 16. Th~
polymer 18 d~oxy~enated prlor to inJectlon.
Th~ needle 20 18 ~radually wlthdr~wn a~ the polymer
flll~ the capsule 16 and the lncislon 22a throu~h the
cornea 14 18 ~ur~lcally clo~ed. Since the polymer cures
to lt~ S~nal ~tatQ wlthin ~ ~attor of ~lnutos u~in~ ht,
no heatlng ot~p 18 r~qulrQd to cure the poly~cr around the
lnci~lon 22b ln the l~ns capBule 16 to per~lt removal of
the needle 20. The co~pletely filled lens c~p~ule 16 18
depicted ln FIG. 5.
The poly~er composltlon 2~ 1B lnJected lnto the lens
capsule 16 throu~h 31it 22b, ~h~ch 18 typlcally no larger
than 3 ~, and i8 ~ntended to cro~s-link rapldly ~o that
the compositlon, which la lnJectod in a llquid ctate, wlll
not squlrt out the sllt when th~ needle 20 1B with~r~wn.
The poly~er compo3it~0n us~d ln the practlc~ of the
invention co~prises (a) a prepoly~er and (b) a photoinitl-
ator. The prepoly~er 18 preferably one that 18 ~ubstan-
tially llnear, with one, znd preferably both, onds capped
with a functional group h2vin~ at least one olefinlc bond.~amples of ~uch ~unctional yroups lnclude acrylates,
~ethacrylates, ~nd styren-s.
It 15 pre~erred that both ~nds be capped, ln order to
for~ a ~ore ho~ogeneous poly~er. The pre~ence of ~he
double bond p~rmlts olofinlc-type cro~s-llnkln~.
Exa~ples Or ~ultable prspoly~-rs include polypropyl~
~ne ~lycols, polypropylene ~lyC018 wlth polyethylene ~ly-
col unlts, polybutylene ~lycols wlth pcly~thylene ~lycol
unlts, urethanes, and ~llloones such as dl~ethyl sllicone
and ethyl slllcone. The ~olecular wel~ht o~ the prepoly~
~er ~dvantay~ously ranges from about 2,000 to 8,000, al-
thou~h lower or hl~h~r ~olecular wei~ht ~aterlal ~y b~
e~ployed
The photoinitlator co~prises a co~po~ltlon ~hlch
inltlatos poly~erlzation o~ ol~flnlc ~nd ~roups ~n the
..
~``` 1 33()~5i
1 vi~lblo-to-near- W r~on. ~xa~plas oS such photolnltla-
tors, partlcularly sultcd Por c~usin~' cro~s-llnklng of
acrylat~ ~roups, lnclude ac~tophenon2 d~rlvatlvo~, ~uch ~8
~l~ethoxyphenylac~tophenone.
The procodure ~or for~ation ~ld re~oval Or l~n3 ~ra~-
~ents 18 lnvolv~ tho u~e Or a alcro~copa and a l~mp ror
lllumlnatlon of the eye. ~hen lnJectlng the poly~er co~-
posltlon 2~ lnto the lon~ capsulc 16, th~ cros~-llnklng
procedure ~ay be perfor~ed u~ln~ the same la~p ~mployed ln
con~unctlon wlth tho ~lcroscope ~ d by the ~ur~on to
r~move the lens fr~y~ents 18. Thl~ lamp i8 0~ th~ appro-
prlatc wavelen~th and i~ of ~uffic:lent ~nt~nsity to actl-
vate the photolnitiator and b~ln the proces~ of cross-
llnking. The concentratlon of photolnitiator ~8 nor~ally
about 0.25 to 2 wt%.
The time ~or reaction 1~ ordin~rlly 1~8~ than about
five ~lnutes. The obs~rvable physlcal properties do not
seem to chan~e aftcr the ~lrst ~everal ~lnu~ of cross-
l$nklng.
The photoinltiator i8 quenched by the presence Or
oxygen, ~nd ~ccordin~ly, the operation ~ay be done by
contlnuously ~lushln~ the eye wlth an lnert gas, such as
nltrogen or arqon, or wlth saline untll th~ reaction i~
~ubstanti~lly co~plete.
The prepoly~er 18 ~tabil~z~d wlth oxyg~ durln~ stor~
in~ and sample pr~par~tion and do~a~ed in the dark before
ln~ectlon, ~uch ~a wlth a vacuum pU~p, ~nd back-fllled
w1th the lnert ga~. Normal ~t~osphere i8 ~ufflclent to
stablllze the propoly~er durlng ~a~dlln~ and stora~e.
It ~ well-~nown that the eye len~ i8 layerod, llke
that of an onlon, wlth ~ch l~y-r h~vln~ a ~lff~rent re-
fractlve lndex. Th~ poly~erized co~posltlon ~8 ~8~0ntlal-
ly ho~o~en~ou~. ~owever, th~ viscoslty and rofractlv~
lndex o~ the co~positlon i8 a ~unction o~ th~ uncapped
. ~ ~ . . - .. . : . . .............................. . - -. ..
?
~:~` 6 1 330~51 : ~
1 portlon o~ ~he pr~poly~r, and aay b0 csn~gNr~d to pro~
vlde a refractive lndex b~tweon about l.S and 1.6.
Slnce the poly~erlzed composi~ion 24 18 ~n ~ to~er,
th~ ~uscles of the eye 10 ~ay otill p~rfor~ their nor~al
6 ~unction of acco~oda~ion. Further, the ~billty to tallor
the poly~-rlz~d oo~ool~ion ln tor~s Or Qlastici~y, vi~-
co~ity and refractlve lndex ~eans that the lons for~atlon,
when done ln co~Junction wlth the l~onication len~ r~oval,
~ay be perfor~ed ~arly ln the process of cataract for~a-
tlo~.
RXAMPL~S
Polyether ~lycols o~ avera~e ~ol~cular ~l~hts bc-
tween 1,000 and 4,000 were combined throu~h ur~thane llnk-
age~ u~ing dllsocyanates ~uch as lsophorone diisocyanate
(~luka Che~ical Corp., Hauppau~e, NY) or ~ethylene bis (4-
cyclohexyldilsocyanate) (Mobay Ch~tcal Corp., ~itt~bur~h,
PA). The ends were then capped wlth a co~pound contalnin~
a tor~lnal alke~e such as lsocyanatoethyl ~ethacrylate
(Dow Che~ical Co., Mldland, MI) or 2-hydroxyethyl sethac-
rylate (Aldrlch Che~lcal Co., Mllwaukee, WI). Reaction
were catalyzed by a tin co~pound ~uch as ~tannou~ dioctan~
oate.
The resultant pr~polymer ~as a colorless liguid with
vioco~ity ~u~ficie~tly low ~o that lt could be lnJected by
~yrin~e through a 20 gauge nsedle. The ~at~rl~l coul~ be
purified, if nocessary, throu~h various ~nown chro~ato-
~raphic ~thod~. BHT (25 to 100 pp~) ~y b~ added to
~tabillze the poly~er.
She ~oly~er was thorou~hly ~ixed with tb~ ~pproprl~t~
quantity o~ photolnltiator, ~uch ~ 2,2-dl~ethoxy-2 phenyl
acetoph~none ~Aldrlch Che~lcal Co.), tra~s~erred to
~yrln0~, then pumped in vacuo, pro~ct~d ~ro~ llght, gor
: ~, '~ ~. :'
. . -,
1 330q5 1 ::
1 at least 24 hrs. The polymer-filled syringc was ~tored,
protected f rom l~ght, under an lnert atmosphere.
The Table below liQt~ various polymer ~ormulations :
prepared pursuant to the foregoing teachlngs.
: 5
Table.
No. Polyether Dii~ocyanate Cata- End cap; Photoln-
alYcol ~ a linker: a l~st q itiator; X
; ~ 10
1 Voranol* IPDI 0.76 Sn(Oc)2 I~M; 0.29 DMPA; 0.3
(lg65); 8.8 .~
: 2 Voranol* IPDI; 0.96 -do- I~M; 0.28DMPA; 0.25 ::
(1965); 10.7
15 3 Voranol* IPDI; 0.58 -do- I~M; 0.~3 DMPA; 0.3
: (3829); 20.1
4 Voranol* IPDI; 1.1 -do- ~A; 0.69 DMPA; 1.2
(3829); 10.0 ~ :
: 5 PPG-4000~ De ~; 1.~ -do- H~M; 0.75 DMPA; 1.0
11.6
6 PPG-4000~ DeQW; 1.4 -do- HEA; 0.67 -do-
11.6
: 7 PPG-4000~ IPDI; 0.93 -do- HEA; 0.50 -do-
8.9 :~
25 8 PPG-3000;*~: IPDI 1.0 -do- ~EM; 0.31 -do~
9 . 0 ,-~
9 PPG-3000* IPDI; l.0 -do- HEA; 0.27 -do-
9,O ~ -
10 Terethane --- -do- I~M; 7.1 -do-
. 30 (650); 15.0
L~end~
;~ Voranol (1965) - Polypropylene ~lycol ¢nd capped with
; 12X ethylene oxlde; M~ = 1,965 ~ :
* - Trade-marks ~-
"~'~. ':,
` .~
_ 8 l 330951
,
1 Voranol ~3829) - Polypropylene ~lycol end capped with
22% ethylene oxlde; MW = 3,829
PPG-4000 - Polypropylene glycol; MW = ~,000
PPG-3000 - Polypropyl~ne glycol; MW = 3,000
Tcrethane (650) - Polytetramethylene ~lycol; MW = 650
IPDI - Ioophorono dllsocyanate
DesW - Desmodur*W tmethylene bis(4-cyclohexylisocyan-
ate)] .
Sn(Oc)2 - Stannous dloctanoate
IEM - I~ocyanatoethyl ~ethacrylate r' ~:
HEA - 2-Hydroxyethyl acrylate ;
HEM - 2-Hydroxyethyl methacrylate
DMPA - 2,2-Dimethoxy-2-phenylacetophenone
'
Several of the foregolng polymers (Nos. 1, 2, 5, 6,
~) were in~ected into len6 capsules of rabbit;s. Photo ;~
; cross-linking was promoted by the visible light used to
~; illuminate the surgical procedure. The poly~er was -
~; stabilized ln the lens shape within ~ to 2 ~inutes and
reaction wa~ complete withln 5 minutes.
The ~echanism for the photoinitiation apparently
lnvolves homolytic cleavage of the initiator to give two
radicals, which then lnltiate the cross-linking reaction.
Because molecular oxygen reacts with radlcals, deoxy~ena-
tlon of the ~onomer allow~ for rapid cross-linklng.
The re~ultant in situ cross-linked polymer was a
transparent plastlc with variable elasticity, depending on
the degree of cross-link~ng and chemlcal compositlon. ;
Thus, a ~ethod of endocapsular lens replacement by ~`
30 forming a synthetic polymer in the lens capsule of an eye -~
after removal of the len~ has been disclo~ed. The ~ethod
compri~es in~ecting a photosensitlve prepolymer into the --
lens capsule ln the absence of oxygen. It will be clear
to one of ordinary skill in the art that various changes
and modifications of an obvious nature ~ay be ~ade without
''''' " "'
. :
*- Trade-mark
,~ 9 1 330q51
. ;. ,
d~partln~ ~ro~ the splrlt o~ the lnv~ntlon, ~nd ~ uch
chan~os ~nd l~odl~lcation~ ~re con~lder-d to b~ ~ithln th~
~cop~ o~ the lnvontion, ~ de~inod by the~ ~ppend-d c:lal~.
: ~:, : .
~.
-
- -
' ... , .,: .
:~ ~ .' ,', ' '
: ""~
~ ' "' '~