Note: Descriptions are shown in the official language in which they were submitted.
- 1 - 27169-147
OF 52-794 1330993
Cyclic amine derivotlve~ ;
This inventlon relates to certa:ln novel cyclic
amlne derivatlve~, to pharmaceutical compos1tion~
con~alning them and to proce~se~s for .their preparation. -:~
Canadian Patent Nb. n ,086,726 describes, inter alia~ ;the c~mpound
of formula
~ :
~ ~ ~CH2CH2CH2- N - CH2CH2 ~ 0CH3
and the physiologic~lly ~ccept~ble acid aadition :~
salts thereof, which has valuable pharmacological
propertie~, namely a mild hypotensive activlty
and, more particuIarly, a selective heart rate ''` '~
lowering aotlvlty.
Surpri~ingly, it has now:been found that certain ; ~
new cycllc ~mine d~rivatives have even more valuable ; ~ ~;
pharmacological propertie~, particularly a hypo~ensive
ef~ect ~nd ~ long-l~sting heart rate lowering actlvlty ...
and the effect of reduclng ~he 2 requlrement of
~he heart.
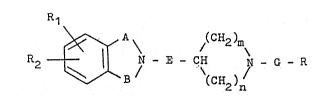
Thus in one n~pect the present lnventlon provlde~
a compound o~ formula tIl ~.
~ ~ A \ ~CH2 ~
R2 - _ ¦ N - E - CH ~ - G - R ~ .
~3 / (C )n *
~, ' ~'~ "
`
-- 2 --
(wherein 1330~93
A represents a -CH2-, -CH2-CH2- or -CH=CH- group;
B represents a -CH2-, -CH2-CH2-, or -CO- group
or a -CH2CO- group of which the carbonyl carbon is attached :~.
to the nitrogen atom;
E represents a straight-chained Cl_3 alkylene group
optionally substituted by a C1 3 alkyl group;
G represents straight-chained Cl 6 alkylene group
optionally substituted by a Cl_3 alkyl group, or
a group -G'-G"- (where G' represents a straight .
chained C2 5 alkylene group optionally substituted
by a Cl 3 alkyl group and G" is attached to an :
aromatic or heteromatic group R and represents
an oxa, thia, sulphinyl or sulphonyl group or an ~ :
imino group optionally substituted by a Cl 3 alkyl .
group);
Rl represents a hydrogen or halogen atom or a trifluoro-
methyl, nitro, amino, Cl 3 alkylamino, di(Cl 3 alkyl)amino, ~ :
Cl 3 alkyl, hydroxy, Cl 3 alkoxy or phenyl(Cl 3 alkoxy) ;~ ~ ;
group and
R2 represents a hydrogen or halogen atom or a hydroxy,
Cl 3 alkoxy, phenyl(Cl 3 alkoxy) or Cl 3 alkyl group,~ .
or
Rl and R2 together represent a Cl 2 alkylenedioxy
group;
m is any one of the integers from 1 to 6 and
n is any one of the integers from O to 3, and the
sum of n and m is 3, 4, 5 or 6; and
' :~
R represents
' ~
1 3 3 ~3 r9 9 3
-- 3
a ring carbon- or ring nitrogen-attached cyclic
group comprising a 5- or 6-membered heteroaromatic ~ :
ring optionally carrying a fused benzene ring, .
wherein said heteroaromatic ring contains one
oxygen, sulphur or n~trogen atom, two nitro~en
atoms or one nitrogen atom and one oxygen or sulphur
atom, wherein the carbon structure of said cyclic ~:
group optionally is mono- or disubstituted by substituents :
selected from halogen atoms and Cl_3 alkyl, hydroxy,
Cl_3 alkoxy, phenyl(Cl_3alkoxy), phenyl, dimethoxyphenyl,
nitro, amino, acetylamino, carbamoylamino, N-(Cl_3 :: :
alkyl)-carbamoylamino, hydroxymethyl, mercapto, ::~
Cl_3 alkylmercapto, Cl 3 alkylsulphinyl, Cl 3 alkylsulphonyl,
Cl 3 alkylsulphonyloxy, Cl 3 alkylsulphonylamino, ;~
(Cl 3 alkoxy)carbonylmethoxy, carboxymethoxy and
(Cl 3 alkoxy)methyl groups or optionally is substituted ;~
by a methylenedioxy or ethylenedioxy group, wherein
any imino group in said heteroaromatic ring optionally
is substituted by a Cl 3 alkyl, phenyl(Cl 3 alkyl)
or phenyl group, wherein where said cyclic ~roup comprises
an indolyl group it additionally optionally is
substituted by a methylamino, dimethylamino, methoxy,
aoetoxy, trifluoromethyl, trichloromethyl, carboxy, `~
methoxycarbonyl, ethoxycarbonyl, cyano, cyclohexyl,
trimethoxyphenyl, trifluorophenyl, trichlorophenyl,
tribromophenyl or dihaloaminophenyl group or by ~ ::
a benzyl, benzyloxy or benzylamino group optionally :~
mono-, di- or trisubstituted in the phenyl ring of the
benzyl nucleus by methoxy or methyl groups, ,~
or a naphthyl group optionally substituted by a
Cl 2 alkylenedioxy group or optionally mono- or :.
disubstituted by ~ubstituents selected from halogen
atoms and Cl_3 alkyl, hydroxy, Cl_3 alkoxy, Cl_3
alkylsulphonyloxy, nitro, amino and Cl_3 alkanoylamino ~: :
groups, :;
or a benzyloxy or 4,5,6,7-tetrahydro-benzo[b]thienyl
group,
-? . 1 3 3 0 .~ 9 3
, ................................................................... .
-- 4
or, if B represents a -CH2- or -Co- group, R may
also represent a phenyl qroup optionally substituted by a
Cl 2 alkylenedioxy group or by a halogen atom or
by a Cl 3 alkyl, hydroxy, Cl 3 alkoxy, phenyl(C1 3
alkoxy), nitro, amino, Cl_3 allcanoylamino, Cl_3
alkylsulphonylamino, bis(Cl_3 alkylsulphonyl)amino, ~ -
Cl 3 alkylsulphonyloxy, trifluoromethyl, trifluoromethoxy
or trifluoromethylsulphonyloxy group, or disubstituted
by substituents selected from halogen atoms and
Cl 3 alkyl and Cl 3 alkoxy groups, or a tri(Cl 3alkoxy)~
phenyl group, a tetra(Cl_3 alkyl)phenyl group or a ~-
dihaloaminophenyl group)
or an enantiomer, diastereomer, N-oxide or acid ~-
addition salt thereof.
The compounds of the invention may be in salt form,
e.g. in the form of the physiologically acceptable
salts of the compounds of formula I with organic
or inorganic acids. Suitable acids for reaction
include, for example, hydrochloric, hydrobromic,
sulphuric, phosphoric, tartaric, acetic, lactic, `
furmaric, citric, maleic and succinic acids.
Where moieties in the structure of formula I may
carry two or more optional substituents then unless
otherwise stated these may of course be identical
.
or different. m
:~
Examples of the definitions of the groups given
hereinbefore include:
for Rl: hydrogen, fluorine, chlorine and bromine
atoms, and mèthyl, ethyl, n-propyl, isopropyl,
trifluoromethyl, hydroxy, methoxy, ethoxy, n-propoxy,
isopropoxy, nitro, amino, methylamino, ethylamino,
n-propylamino, isopropylamino, dimethylamino, diethylamino,
di-n-propylamino, diisopropylamino, N-methyl-ethylamino,
N-methyl-n-propylamino, N-methyl-isopropylamino,
N-ethyl-n-propylamino, benzyloxy, l-phenylethoxY~ ~ -
133~3
l-phenylpropoxy, 2-phenylethoxy and 3-phenylpropoxy
groups,
for R2: hydrogen, chlorine and bromine atoms, and
methyl, ethyl, n-propyl, isopropyl, hydroxy, methoxy,
ethoxy, n-propoxy, isopropoxy, benzyloxy, l-phenylethoxy, -
2-phenylethoxy, 2-phenylpropoxy and 3-phenylpropoxy
groups,
for Rl together with R2: methylenedioxy and ethylene-
dioxy groups,
for E: methylene, ethylene, n-propylene, ethylidene,
n-propylidene, n-butylidene, 2-methyl-n-propylidene,
l-methyl-ethylene, l-ethyl-ethylene, 2-methyl-ethylene,
2-ethyl-ethylene, l-n-propyl-ethylene, l-methyl- `~
n-propylene, 2-methyl-n-propylene, 3-methyl-n-propylene,
l-ethyl-n-propylene, 2-n-propyl-n-propylene and
3-ethyl-n-propylene groups (n.b. the alkylene group
is attached at its l-position to the nitrogen),
for G: methylene, ethylidene, n-propylidene, n- `
butylidene, 2-methyl-propylidene, ethylene, l-methyl-
ethylene, l-ethyl-ethylene, l-propyl-ethylene,
2-methyl-ethylene, 2-ethyl-ethylene, n-propylene,
n-butylene, n-pentylene, n-hexylene, l-methyl-n-
propylene, l-methyl-n-butylene, l-methyl-n-pentylene,
l-ethyl-n-propylene, 2-ethyl-n-propylene, l-ethvl-
n-butylene, ethyleneoxy, n-propyleneoxy, n-butyleneoxy,
ethylenethio, n-propylenethio, n-butylenethio, ~`
ethylenesulphinyl, ethylenesulphonyl, n-propylene- ~ `
sulphinyl, n-propylenesulphonyl, n-butylenesulphinyl,
ethyleneamino, n-propyleneamino, n-butyleneamino,
N-methyl-ethyleneamino, N-methyl-n-propyleneamino,
N-ethyl-n-propyleneamino, N-isopropyl-n-propyleneamino
and N-methyl-n-butyleneamino groups (n.b. the alkylene `
groups are attached at their l-positions to the `
nitrogen atom of the saturated ring),
~ . .
3 3 ~
-- 6 --
for R: pyrrol-2-yl, pyrrol-3~yl, N-methyl-pyrrol-
2-yl, N-methyl-pyrrol-3-yl, ll2-aimethyl-pyrr
3-yl, 2,5-dimethyl-pyrrol-3-yl, fur-2-yl, fur-3- ~ :
yl, 5-methyl-fur-2-yl, 2-methyl-fur-3-yl, 5-nitro-
fur-2-yl, 5-methoxymethyl-fur-2-yl, benzorb]fur-
2-yl, benzolb]fur-3-yl, 7-methylbenzo[b]fur-3-yl, :
2-methoxy-benzo[b]fur-3-yl, 3-methoxy-benzo[b]fur-
2-yl, 4-methoxy-benzo r b]fur-3-yl, 5-methoxy-benzo[b]fur-
3-yl, 6-methoxy-benzo[b]fur-3-yl, 7-methoxy-benzo[b]fur- ~ :
3-yl, 5-methoxy-3-phenyl-benzo[b]fur-2-yl, 3-methyl- ~:
5-methoxy-benzo[b]fur-2-yl, thien-2-yl, thien-3-
yl, 5-methyl-thien-2-yl, 2~methyl-thien-3-yl, 3- ~ -~
methyl-thien-2-yl, 2,5-dimethyl-thien-3-yl, 4,5,6,7-
tetrahydro-benzo[b]thien-3-yl, 4,5,6,7-tetrahydro- ~: ~
benzo[b]thien-2-yl, 5-chloro-thien-2-yl, 5-bromo- ~; . `
thien-2-yl, 5-phenyl-thien-2-yl, 2-phenyI-thien- ~:
3-yl, benzo[b]thien-2-yl, benzo[b]thien-3-yl, 2,5-
dimethyl-benzo[b]thien-3-yl, 5-methyl-benzo[b]thien- ~ :
3-yl, 6-methyl-benzo[b]thien-3-yl, 5-chloro benzo[b]thien-
2-yl, 5-bromo-benzotb]thien-3-yl, 6-hydroxy-benzo[b]thien-
3-yl, 7-hydroxy-benzorb]thien-3-yl, 5-hydroxy-benzorb]thien-
2-yl, 6-hydroxy-benzo~b]thien-2-yl, 7-hydroxy-benzo[b]thien~
2-yl, 6-methanesulphonyloxy-benzo[b~thien-3-yl,
3-methoxy-benzo[b]thien-2-yl, 4-methoxy-benzo[b]thien- ~:
2-yl, 5-methoxy-benzo[b]thien-2-yl, 6-methoxy-benzo[b]thien-
2-yl, 7-methoxy-benzo[b]thien-2-yl, 2~-methoxy-benzo[b]thien-
3-yl, benzo~b]thien-4-yl, benzo[b]thien-5-yl, benzo[b]thien-
6-yl, benzo[b]thien-7-yl, 4-methoxy-benzo[b]thien-
3-yl, 5-methoxy-benzo[b]thien-3-yl, 6-methoxy-benzo[b]thien-
3-yl, 7-methoxy-benzo[b]thien 3-yl, 5,6-dimethoxy~
benzolb]thien-3-yl, 5,6-methyienedioxy-benzo[b]thien-
3-yl, 6-ethoxy-benzorb]thien-3-yl, 6-propoxy-benzo[b]thien- :~
3-yl, 6-isopropoxy-benzo[b]thien-3-yl, 6-mercapto-
benzo[b]thien-3-yl, 6-methylmercapto-benzo[b]thien-
3-yl, 6-methylsulphinyl-benzo[b]thien-3-yl, 6-methylsulphonyl-
benzo[b]thien-3-yl, 6-methylsulphonyloxy-benzo[b]thien-
3-yl, 6-methoxycarbonylmethoxy-benzo[b]thien-3-
. ~:, .
~33~3
_ 7 --
yl, 6-ethoxycarbonylmethoxy-benzo[b]thien-3-yl,
6-carboxymethoxy-benzo[bJthien-3-yl, 6-amino-benzo[b]thien-
3-yl, 6-methylamino-benzo[b]thien-3-yl, 6-dimethylamino~
benzo[b]thien-3-yl, 6-diethylamino-benzorb]thien-
3-yl, 6-acetamino-benzo[b]thien-3-yl, 6-methylsulphonylamino-
benzo[b]thien-3-yl, pyrazol-l--yl, pyrazol-3-yl,
3,5-dimethyl-pyrazol-1-yl, 1,5-dimethyl-pyrazol-
3-yl, imidazol-l-yl, imidazol 2-yl, imidazol-4~5)- ;~:
yl, l-methyl-imidazol-4-yl, l~benzyl-imidazol-4-
yl, 5-nitro-2-methyl-imidazol~-1-yl, 2-(3,4-dim~thoxy- :~
phenyl)-imidazol-4(5)-yl, benzo[d]imidazol-l-yl,
2-benæyl-benzo[d]imidazol-1-yl, benzo[d]imidazol-
2-yL, imidazo[l,2-a]pyrid-3-yl, oxazol-4-yl, oxazol-
S-yl, isoxazol-3-yl, 3-methyl-isoxazol-5-yl, 5- ~ :
methyl-isoxazol-3-yl, 3,5-dimethyl-isoxazol-4-yl,
4-methyl-thiazol-5-yl, benzo[d]oxazol-2-yl, benzotd]isoxazol-
3-yl, benzo[d]thiazol-2-yl, 5-ethoxy-benzo[d]thiazol-
2-yl, benzo[d]isothiazol-3-yl, benzo[d]pyrazol-
l-yl! benzo[d]pyrazol-3-yl, pyrid-2-yl, pyrid-3-
yl, pyrid-4-yl, pyrid-3-yl-N-oxide, 6-methyl-pyrid-
2-yl, 4-nitro-pyrid-2-yl, 4-amino-pyrid-2-yl, 4-
acetylamino-pyrid-2-yl, 4-carbamoylamino-pyrid-
2-yl, 4-N-methyl-carbamoylamino-pyrid-2-yl, 2-chloro- ~ .
pyrid-3-yl, 2-chloro-pyrid-4-yl, 6-chloro-pyrid- : :
2-yl, 6-hydroxymethyl-pyrid-2-yl, quinol-2-yl,
isoquinol-l-yl, 2-methyl-quinol-4-yl, 7-methyl-
quinol-2-yl, 4-chloro-quinol-2-yl, 6,7-dimethoxy-
quinol-4-yl, 6,7-dimethoxy-isoquinol-4-yl, 6,7-
dimethoxy-isoquinol-4-yl-N-oxide, indol-~-yl, indol- .
3-yl, 5,7-dibromo-6-cyano-indol-3-yl, 5,7-dichloro- .
6-aminocarbonyl-indol-3-yl, 4,6-dibromo-5-aminocarbonyl- .
indol-3-yl, 5,7-dibromo-6-methoxy-indol-3-yl, 5,6- ~ ~ -
dihydroxy-indol-3-yl, 4-dimethylamino-indol-3-yl, ~ ~.
4-methoxy-6-dimethylamino-indol-3-yl, 4-methyl-
6-dimethylamino-indol-3-yl, 5-acetoxy-6-dimethylamino-
indol-3-yl, 5-acetamido-7-dimethylamino-indol-3-
yl, 5-dimethylaminocarbonyl-indol-3-yl, 5-carboxy- :~
~` ~330~9~ ~
-- 8 --
indol-3-yl, 5-acetamido-indol-3-yl, 4-nitro-5-acetamido-
indol-3-yl, 5-acetamido-6-nitro-indol-3-yl, 5-nitro-
6-acetamido-indol-3-yl, 6-acetamido-7-nitro-indol~
3-yl, 4-chloro-5 acetamido-indol-3-yl, 5-acetamido-
6-chloro-indol-3-yl, 5-chloro-6-acetamido-indol~
3-yl, 6-acetamido-7-chloro-indol-3-yl, 5-dimethylamino-
indol-3-yl, 6-dimethylamino-indol-3-yl, 7-dimethylamino-
indol-3-yl, 5-dimethylamino-6-chloro-indol-3-yl,
5-chloro-6-dimethylamino-indol-3-yl, 7-benzyl-indol~ -
3-yl, 4-(3,4,5-trimethoxy-benzyl)-indol-3-yl, 5-
(3,4,5-trimethoxy-benzyl)-indol-3-yl, 6-(3,4,5-
trimethoxy-benzyl)-indol-3-yl, 7-(3,4,5-trimethoxy-
benzyl)-indol-3-yl, 4-trifluoromethyl-indol-3-yl, :
4-trichloromethyl-indol-3-yl, 4,5-methylenedioxy- -~
indol-3-yl, 4,5-dimethoxy-indol-3-yl, 4,5-dimethyl-
indol-3-yl, 5-methyl-6-methoxy-indol-3-yl, 4-methyl-
5-methoxy-indol-3-yl, 5-trifluorot"ethyl-indol-3-
yl, 5-methoxy-7-bromo-indol-3-yl, 5-bromo-7-methoxy-
indol-3-yl, 5-bromo-indol-3-yl, 5-chloro-indol- ~:
3-yl, 5-(3,4,5-trimethoxy-benzyloxy)-indol-3-yl,
6-(3,4,5-trimethoxy-benzyloxy)-indol-3-yl, 5-(3,4,5-
trimethoxy-benzylamino)-indol-3-yl, 6-(3,4,5-trimethoxy- ~;
benzylamino)-indol-3-yl, 5-(3,5-dichloro-4-amino-
benzyloxy)-indol-3-yl, 6-(3,5-dibromo-4-amino-benzyloxy)-
indol-3-yl, 5-(3,5-dichloro-4-amino-benzylamino)-
indol-3-yl, 6-(3,5-dibromo-4-amino-benzylamino)- :~
indol-3-yl, 5-benzyl-indol-3-yl, 4-benzyl-indol- ~:
3-yl, 6-benzyl-indol-3-yl, 5,6,7-trimethoxy-indol-
3-yl, 5,6,7-trimethyl-indol-3-yl, 5-nitro-indol- ~:
3-yl, 4,6-dichloro-5-amino-indol-3-yl, 4,6-dichloro-
5-nitro-indol-3-yl, 5,7-dibromo-6-amino-indol-3- .
yl, 5,7-dibromo~6-nitro-indol-3-yl, 4,6-dichloro-
5-methoxy-indol-3-yl, 5,6-dimethoxy-indol-3-yl,
5,7-dimethoxy-indol-3-yl, 6,7-dimethoxy-indol-3-
yl, 4,7-dimethoxy-indol-3-yl, 5,6-methylenedioxy- : -
indol-3-yl, 6,7-methylenedioxy-indol-3-yl, 5,6-
dimethyl-indol-3-yl, 5,7-dimethyl-indol-3-yl, 6,7-
` ` 1330~93
g
dimethyl-indol-3-yl, 4,7-dimethyl-indol-3-yl, 6
methoxy-7-methyl-indol-3-yl, 4,6-dibromo-5-carboxy-
inaol-3-yl~ 5,7-dichloro-6-carboxy-indol-3-yl,
5-ethoxycarbonyl-indol-3-yl, 6-ethoxycarbonyl-indol- ~ ~ -
3-yl, 4,6-dibromo-5-ethoxycarbonyl-indol-3-yl,
5,7-dichloro-6-ethoxycarbonyl-indol-3-yl, 5-cyano- ~
indol-3-yl, 6-cyano-indol-3-yl, 4-cyano-indol-3- :
yl, 4-hydroxy-indol-3-yl, 7-hydroxy-indol-3-yl,
5-phenyl-indol-3-yl, 6-phenyl-indol-3-yl, 5-(3,4,5-
tribromo-phenyl)-indol-3-yl, 6-(3,4,5-trimethoxy-
phenyl)-indol-3-yl, 5-(4-trifluoromethyl-phenyl)-
indol-3-yl, 6-(3,5-difluoro-4-amino-phenyl)-indol- .
3-yl, 4-phenyl-indol-3-yl, 5-cyclohexyl-indol-3- . .
yl, 5-(2-methoxy-benzyl)-indol-3-yl, 6-(2-methoxy-
phenyl)-indol-3-yl, 6-(2,4-dimethyl-benzyl) indol-
3-yl, 5-(2,4-dimethoxy-benzyl)-indol-3-yl, 7-(2-
methoxy-benzyloxy)-indol-3-yl, 4-(2,4-dimethyl-
benzyloxy)-indol-3-yl, 5-(2,4-dimethoxy-benzyloxy)-
indol-3-yl, 6-(2-methoxy-benzylamino)-indolyl-3,
5-(2-methoxy-4-methyl-benzylamino)-indol-3-yl,
4-(2,4-dimethoxy-benzylamino)-indol-3-yl, 5,7-dibromo-
6-cyano-indol-2-yl, 5,7-dichloro-6-aminocarbonyl-
indol-2-yl, 4,6-dibromo-5-aminocarbonyl-indol-2- .
yl, 5,7-dibromo-6-methoxy-indol-2-yl, 5,6-dihydroxy- `.
indol-2-yl, 4-dimethylamino-indol-2-yl, 4-methoxy- ~ :
6-dimethylamino-indol-2-yl, 4-methyl-6-dimethylamino-
indol-2-yl, 5-acetoxy-6-dimethylamino-indol-2-yl,
5-acetamido-7-dimethylamino-indol-2-yl, 5-dimethylamino- -~
carbonyl-indol-2-yl, 5-carboxy-indol-2-yl, 5-acetamido-
indol-2-yl, 4-nitro-5-acetamido-indol-2-yl, 5-acetamido-
6-nitro-indol-2-yl, 5-nitro-6-acetamido-indol-2
yl, 6-acetamido-7-nitro-indol-2-yl, 4-chloro-5~
acetamido-indol-2-yl, 5-acetamido-6-chloro-indol-
2-yl, 5-chloro-6-acetamido-indol-2-yl, 6-acetamido~
7-chloro-indol-2-yl, 5-dimethylamino-indol-2-yl,
6-dimethylamino-indol-2-yl, 7-dimethylamino-indol- :~
2-yl, 5-dimethylamino-6-chloro-indol-2-yl, 5-chloro- ~.
~3309~ -
~ 10 -- ,
6-dimethylamino-indol-2 yl, 7-benzyl-indol-2-yl,
4-~3,4,5-trimethoxy-benzyl)-indol-2-yl, 5-(3,4,5-
trimethoxy-benzyl~ indol-2-yl, 6-(3,4,5-trimethoxy-
benzyl)-indol-2-yl, 7-(3,4,5-trimethoxy-benzyl)-
indol-2-yl, 4-trifluoromethyl-indol-2 yl, 4-trichloromethyl-
indol-2-yl, 4,5-methylenedioxy-indol-2-yl, 4,5-
dimethoxy-indol-2-yl, 4,5-dimethyl-indol-2-yl, ~ :
5-methyl-6-methoxy-indol-2-yl, 4-methyl-5-methoxy-
indol-2-yl, 5-trifluoromethyl-~indol-2-yl, 5-methoxy-
7-bromo-indol-2-yl, 5-bromo-7~-methoxy-indol-2-yl,
5-bromo-indol-2-yl, 5-chloro-indol-2-yl, 5-(3~4,5-
trimethoxy-benzyloxy~-indol-2--yl, 6-(3,4,5-trimethoxy-
benzyloxy)-indol-2-yl, 5-(3,4,5-trimethoxy-benzylamino)-
indol-2-yl, 6-~3,4,5-trimethoxy-benzylamino)-indol-
2-yl, 5-(3,5-dichloro-4-aminobenzyloxy)-indol-2-
yl, 6-(3,5-dibromo-4-amino-benzyloxy)-indol-2-yl,
5-(3,5-dichloro-4-amino-benzylamino)-indol-2-yl,
6-(3,5-dibromo-4-amino-benzylamino)-indol-2-yl,
5-benzyl-indol-2-yl, 4-benzyl-indol-2-yl, 6-benzyl- ~ ~.
indol-2-yl, 5,6,7-trimethoxy-indol-2-yl, 5,6,7- .
trimethyl-indol-2-yl, 5-nitro-indol-2-yl, 4,6-dichloro-
5-amino-indol-2-yl, 4,6-dichloro-5-nitro-indol-
2-yl, 5,7-dibromo-6-amino-indol-2-yl, 5,7-dibromo- - ~ :
6-nitro-indol-2-yl, 4,6-dichloro-5-methoxy-indol- .
2-yl, 5,6-dimethoxy-indol-2-yl, 5,7-dimethoxy-indol-
2-yl, 6,7-dimethoxy-indol-2-yl, 4,7-dimethoxy-indol-
2-yl, 5,6-methylenedioxy-indol-2-yl, 6,7-methylenedioxy-
indol-2-yl, 5,6-dimethyl-indol-2-yl, 5,7-dimethyl- .
indol-2-yl, 6,7-dimethyl-indol-2-yl, 4,7-dimethyl-
indol-2-yl, 6-methoxy-7-methyl-indol-2-yl, 4,6-
dibromo-5-carboxy-indol-2-yl, 5,7-dichloro-6-carboxy-
indol-2-yl, 5-ethoxycarbonyl-indol-2-yl, 6-ethoxycarbonyl-
indol-2-yl, 4,6-dibromo-5-ethoxycarbonyl-indol-
2-yl, 5,7-dichloro-6-ethoxycarbonyl-indol-2-yl,
5-cyano-indolyl-2, 6-cyano-indol-2-yl, 4-cyano- . ~. :
indol-2-yl, 4-hydroxy-indol-2-yl, 7-hydroxy-indol-
2-yl, 5-phenyl-indol-2-yl, 6-phenyl-indol-2-yl, '~
`' '
~330993
-- 11 --
5-(3,4,5-trlbromo-phenyl)~indol-2-yl, 6-(3,4,5-
trimethoxy-phenyl)-indol-2-yl, 5-(4~trifluoromethyl-
phenyl)-indol-2-yl, 6-(3,5-difluoro-4-amino-phenyl)-
indol-2-yl, 4-phenyl-indol-2-yl, 5-cyclohexyl-indol- :
2~yl, 5-(2-methoxy-benzyl)-indol-2-yl, 6-(2-methoxy-
phenyl)-indol-2-yl, 6-(2,4-dimethyl-benzyl)-indol-
2-yl, 5-(2,4-dimethoxy-benzyl)-indol-2-yl, 7-(2-
methoxy-benzyloxy)-indol-2-yl, 4-(2,4-dimethyl-
benzyloxy)-indol-2-yl, 5-(2,4-dimethoxy-benzyloxy-
indol-2-yl, 6-(2-methoxy-benzylamino)-inaol-2
5-(2-methoxy-4-methyl-benzylamino)-indol-2-yl,
4-(2,4-dimethoxy-benzyIamino)-indol-2-yl, phenyl,
4-methyl-phenyl, 3-methyl-phenyl, 2-methyl-phenyl,
4-ethyl-phenyl, 4-isopropyl-phenyl, 4-cyano-phenyl,
4-trifluoromethyl-phenyl, 2-methoxy-phenyl, 3-methoxy- ~
phenyl, 4-methoxy-phenyl, 2-ethoxy-phenyl, 4-nitro- ..
phenyl, 2-nitro-phenyl, 4-methylamino-phenyl, 4-
ethylamino-phenyl, 4-n-propylaminophenyl, 4-dimethylamino-
phenyl, 4-diethylamino-phenyl, 4-di-n-propylamino-
phenyl, 4-N-ethyl-methylamino-phenyl, 4-formylamino- .~ :
phenyl, 4-acetamido-phenyli 4-propionyl-amino-phenyl, .
3,4-methylenedioxy-phenyl, 3,4-ethylenedioxy-phenyl,
3,4-dimethoxy~phenyl, 3,5-dimethoxy-phenyl, 2,6-
dimethoxy-phenyl, 2,4-dimethoxy-phenyl, 3,4-diethoxyphenyl,
3,4-dimethyl-phenyl, 3,5-dimethyl-phenyl, 2,6-dimethyl- :~:h~
phenyl, 2,4-dimethyl-phenyl, 3,4-diethyl-phenyl,
2,4-dichloro-phenyl, 2,4-dibromo-phenyl, 3-methyl-
4-methoxy-phenyl, 3-methoxy-4-methyl-phenyl, 3-
chloro-4-methyl-phenyl, 3-bromo-4-methyl-phenyl,
3-chloro-4-methoxy-phenyl, 3-bromo-4-methoxy-phenyl,
3-methyl-4-chloro-phenyl, 3-methyl-4-bromo-phenyl,
3-methoxy-4-chloro-phenyl, 3-methoxy-4-bromo-phenyl,
benzyloxy, naphth-l-yl, naphth-2-yl, 2-methyl-naphth- ~::
l-yl, 6-methoxy-naphth-2-yl, 7-methoxy-naphth-2- ~ :
yl, 5-methyl-6-methoxy-naphth-2-yl, 5,6-dimethoxy- ~
naphth-2-yl, 5,6-dichloro-naphth-2-yl, 5,6-dimethoxy- -~ ~ :
naphth-l-yl, 5,6-dichloro-naphth-1-yl, 6-methoxy- ;
~` ~33a~t~
- 12 -
naphth-l-yl, 5-methyl-6-methoxy-naphth-1-yl, 6-
nitro-naphth-l-yl, 6 nitro~naphth-2-yl, 6-methoxy-
5-nitro-naphth-1-yl, 6-methoxy-5-nitro-naphth-2- ~ :
yl, 6-amino-naphth-2-yl, 4-methoxy-naphth-1-yl,
5,6-diethoxy-naphth-2-yl, 5,6-di-n-propoxy-naphth-
2-yl, 6-chloro-naphth-1-yl, 6-chloro-naphth-2-yl,
6-~hloro-7-nitro-naphth-2-yl, 6-chloro-7-amino-
naphth-2-yl, 6-chloro-7-acetamido-naphth-2-yl,
5,6-methylenedioxy-naphth-2-yl, 5-chloro-6-methoxy-
naphth-2-yl, 6-ethyl-naphth-2-yl, 6-methylmercapto-
naphth-2-yl and 6-isopropyl-naphth-2-yl groups.
The following are compounds which fall within the :~
scope of the present invention and are listed by ;~
way of example~
.. ,..: ~ -
2-[(N-(2-rnaphth-2-yl)-ethyl)-hexahydro-aæepin~
3-yl)-methyl~-6,7-dimethoxy-1,2,3,4-tetrahydro-
isoquinoline, : - :
,. . :- .
2-[ (N-(2- (naphth-2-yl)-ethyl)-azacyclooct-3-yl)-
methyl~-6,7-dimethoxy-1,2,3,4-tetrahydro-isoquinoline, ~- -
2-[(N- (2-(naphth-1-yl)-ethyl)-hexahydro-azepin-
3-yl)-methyl]-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro- ~ :
isoquinoline,
2-[(N-(2-(naphth-1-yl)-ethyl)-azacyclooct-3-yl)- ;
methyl~-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline,
''''~: ~':~
2-[(N-(2-methyl-naphth~l-yl)-methyl)-pyrrolid-3- :
yl)-methyl]-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline,
' :. .:::,:
2-[3- (N- ( 2-methyl-naphth-1-yl)-methyl)-piperid-
3-yl)-propyl]-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinolinle,
:
L 3 3 ~ ~ 9 3
- 13 -
2-[(N-t2-t5-methyl-6-methoxy-naphth-2-yl)-ethyl~-
pyrrolid-3-yl)-methyl]-6,7-dimethoxy-1,2,3,4-tetrahydro-
isoquinoline,
2-[(N-(2-(5-methyl-6-methoxy-naphth-2-yl)-ethyl)-
azacyclooct-3-yl)-methyl~-6,7-dimethoxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline,
2-[(N-(2-~6-methoxy-naphth-2-yl)-ethyl)-hexahydro-
azepin-3-yl)-methyl]-6,7-dimet:hoxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline,
2-[(N-(2-(6-methoxy-naphth-2-yl)-ethyl)-azacyclooct- :
3-yl)-methyl]-6,7-dimethoxy~l-oxo-1,2,3,4-tetrahydro-
isoquinoline, ~ -~
2-[3-(N-(2-(6-methoxy-naphth-2-yl)-ethyl)-pyrid-
3-yl)-propyl]-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro- .
isoquinoline,
2-[(N-(4-(naphthyl-2-oxy)-butyl)-azacyclooct-3-
yl)-methyl]-6,7-dimethoxy-1,2,3,4-tetrahydro-isoquinoline,
2-[2-(N-(4-(naphthyl-2-oxy)-butyl)-piperid-2-yl)- :~
ethyl]~6,7-dimethoxy-1-oxo-1~2,3,4~tetrahydro-isoquinoline,
-: ~,
2-[(N-(3-(naphthyl-2-oxy)-propyl)-azacyclooct-3-
yl)-methyl]-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline,
2-[(N-(3-(naphth-2-yl)-propyl)-piperid-3-yl)-methyl]-
6,7-dimethoxy~1,2,3,4-tetrahydro-isoquinoline,
2-[(N-(3-(naphth-2-yl)-propyl)-azacyclooct-3-yl)-
methyl]-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinolin~e, ;
. .,
330~93
- 14 -
2-[~N-(2-(naphth-2-yl~-ethyl~-piperid-3-yl~-methyl]-
6,7-methylenedioxy-1,2,3,4-tetrahydro-isoquinoline,
2-[(N-(2-(naphth~2-yl~-ethyl)-azacyclooct-3-yl)-
methyl~-6,7-methylenedioxy-1,2,3,4-tetrahydro-isoquinoline,
2-[(N-(2-methyl-naphth-1-yl)-methyl)-pyrrolid-3- ~ ``;
yl)-methyl]-6,7-methylenedioxy-1-oxo-1,2,3,4-tetrahydro- ~,;
isoquinoline,
2-[(N-(2-methyl-naphth-1-yl~-methyl)-piperid-3- ~: ~
yl)-methyl]-6,7-methylenedioxy-1,2,3,4 tetrahydro- ~
isoquinoline, -- .
2-[(N-(2-methyl-naphth-1-yl)-methyl)-azacyclooct- . ... ..
3-yl)-methyl]-6,7-methylenedioxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline,
~ ,: . . ~
2-[(N-(2-(5-methyl-6~methoxy-naphth-2-yl)-ethyl~
azacyclooct-3-yl)-methyl]-6,7-methylenedioxy-1-
oxo-1,2,3,4-tetrahydro-isoquinoline,
2-[2-(N-(2-(S-methyl-6-methoxy-naphth-2-yl)-ethyl)-
piperid-3-yl)-methyl]-6,7-methylenedioxy-1-oxo-
1,2,3,4-tetrahydro-isoquinoline,
~.. .:.
2- r (N-~2-~6-methoxy-naphth-2-yl)-ethyl)-piperid-
3-yl)-methyl~-6,7-methylenedioxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline,
2-[(N-(4-(naphthyl-2-oxy)-butyl)-pyrrolid-3-yl)-
methyl~-6,7-methylenedioxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline,
2-[(N-(4-(naphthyl-2-oxy)-butyI)-piperid-3-yl)- ~ :
methyl]-6,7-methylenedioxy-1-oxo~ ,3,4-tetrahydro-
isoquinoline,
'..", ~
133~3
- 15 -
2- [ ~N- ( 4-(naphthyl-2-oxy~-butyl)-hexahydro-azepin-
3-yl)-methyl]-6,7-methylenedioxy-1,2,3,4-tetrahydro- ~.:
isoquinoline,
2-[(N-(2-(naphth-2-yl)-ethyl)-pyrrolid-3-yl)-methyl]- ~: .
6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-isoquinoline, ~.
.
2-[ tN- (2-(naphth-2-yl)-ethyl)-piperid-3-yl)-methyl]-
6,7-dimethyl-1,2,3,4-tetrahydro-isoquinoline, - ~ .
2-[(N-(2-(naphth-2-yl)-e~hyl)-azacyclooct-3-yl)-
methyl]-6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-isoquinoline,
2-[(N-(2-(naphth-2-yl)-ethyl)-azacyclooct-3-yl)~
methyl]-6,7-dimethyl-1,2,3,4-tetrahydro-isoquinoline,
2-[(N-(2-(naphth-1-yl)-ethyl)-pyrrolid-3-yl)-methyl]-
6,7-dimethyl-1,2,3,4-tetrahydro-isoquinoline,
2-[(N-(2-(naphth-1-yl)-ethyl)-hexahydro-azepin~
3-yl)-methyl]-6,7-dimethyl-1,2,3,4-tetrahydro-isoquinoline,
2-[3-~N-(2-(naphth-1-yl)-ethyl)-piperid 3-yl)-propyl]-
6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-isoquinoline, ;~
2-[(N-(2-methyl-naphth-1-yl)-methyl)-piperid-3-
yl)-methyl]-6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-
isoquinoline,
2-[(N-(2-methyl-naphth-1-yl)-methyl)-piperid-3~
yl)-methyl]-6,7-dimethyl-1,2,3,4-tetrahydro-isoquinoline,
2-[(N-(2-methyl-naphth-1-yl)-methyl)-azacyclooct-
3-yl)-methyl.]-6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-
isoquinoline,
~` 1 33~93
. . .
- 16 -
2-[2-(N-(2-methyl-naphth-1-yl)-methyl)-piperid- `
2-yl)-ethyl]-6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro- :~:
isoquinoline, :~
2-[(N-(2-(5-methyl-6-methoxy-naphth-2-yl)-ethyl)-
piperid-3-yl)-methyl]-6,7-dimethyl-1-oxo-1,2,3,4- ~:
tetrahydro-isoquinoline, : :
. :..: , .::
2-[(N-(2-(5-methyl-6-methoxy-nlaphth-2-yl)-ethyl)- `
hexahydro-azepin-3-yl)-meth~1]-6,7-dimethyl-1-oxo~
1,2,3,4-tetrahydro-i~oquinolinle,
2-[(N-(2-(5-methyl-6-methoxy-naphth-2-yl)-ethyl)-
azacyclooct-3-yl)-methyl~-6,7-dimethyl-1-oxo-1,2,3,4-
tetrahydro-isoquinoline,
2-[(N-(2-(5-methyl-6-methoxy-naphth-2-yl)-ethyl~
azacyclooct-3-yl)-methyl]-6,7-dimethyl- 1,2,3,4-
tetrahydro-isoquinoline,
, ~ ' '':
2-[(N-(2-(6-methoxy-naphth-2-yl)-ethyl)-pyrrolid-
3-yl)-methyl]-6,7-dimethyl-1,2,3,4-tetrahydro-isoquinoline, ~ ~ .
2- r (N-(2-(6-methoxy-naphth-2-yl)-ethyl)-piperid-
3-yl)-methyl]-6,7-dimethyl-1,2,3,4-tetrahydro-isoguinoline,
.' ' :.'
2-r(N-(2-(6-methoxy-naphth-2-yl)-ethyl)-hexahydro- ~:~
azepin-3-yL)-methyl]-1,2,3,4-tetrahydro-isoquinoline, ~ ~ ;
'
2-t2-(N-(2-(6-methoxy-naphth-2 yl)-ethyl)-piperid-
2-yl)-ethyl]-6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro- ~ : :
isoquinoline,
2-[(N-(4-(naphthyl-2-oxy)-butyl)-piperid-3-yl)-
methyl]-6~7-dimethyl-1-oxo-1,2,3,4-tetrahydro-isoquinoline,
: '
~` ~3309~3
- 17 -
2-[(N-(4-(naphthyl-2-oxy)-butyl)-hexahydro-azepin-
3-yl)-methyl~-6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro~
isoquinoline,
2- [ (N- ( 4- ( naphthyl-2-oxy)-butyl~-azacyclooct-3- .
yl)-methyll-6,7-dimethyl-1,2,3,4-tetrahydro-isoquinoline,
2-[(~-(3-(6-methoxy-naphthyl-2--oxy)-propyl)-hexahydro-
azepin-3-yl~-methylJ-6,7-dimethyl-1-oxo-1,2,3,4- '~
tetrahydro-isoquinoline,
2-[(N-(3-(6-methoxy-naphthyl-2-oxy)-propyl)-azaayclooct-
3-yl)-methyl]-6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-
isoquinol1ne,
2-[(N-(3-(5,6-dimethoxy-naphthyl-2-oxy)-propyl)~
azacyclooct-3-yl)-methyl]-6,7-dimethyl-1-oxo-1,2,3,4-
tetrahydro-isoquinoline,
2-r(N-(3-(naph~h-2-yl)-propyl)-azacyclooct-3-yl)- ~:-
methyl]-6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-isoquinoline,
3-[(N-(3-(pyrid-3-yl)-propyl)-pyrrolid-3-yl)-methyl]-
7,8-methylenedioxy-2-oxo-1,3,4,5-~etrahydro-2H- :~
3-benzazepine, ~ `
3-r(N-(pyrid-3 yl-methyl)-pyrrolid-3-yl)-methyl]-
7,8-methylenedioxy-1,3,4,5-tetrahydro-2H-3-benzazepine, -
""~
3-[(N-(3-(pyrid-4-yl)-propyl)-pyrrolid-3-yl)-methyl]-
7,8-dimethyl-1,3,4,5-tetrahydro-2H-3-benzazepine, :
.
2-[(N-(pyrid-3-yl-methyl)-pyrrolid-3-yl)-methyl]-
6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-isoquinoline,
2-[(N-(3-(pyrid-3-yl)-propyl)-pyrrolid-3-yl)-methyl]-
5,6-methylenedioxy-1-oxo-1,3-dihydro-isoindole,
'~' ' , l3~a~3
- 18 -
2-[(N-~3-(pyrid-4-yl)-propyl)-pyrrolid~3-yl)-methyl]-
5,6-methylenedioxy-1,3-dihydro-isoindole, -
2-[ ~N-(2- (6-methyl-pyrid-2-yl)--ethyl)-pyrrolid-
3-yl~-methyl]-5,6-methylenedio~y-1-oxo-1,3-dihydro-
isoindole,
2-[(N-~ 2-(6-methyl-pyrid-2-yl)-ethyl)-pyrrolid-
3-yl)-methyll-5,6-methylenedioKy-1,3-dihydro-isoindole,
2-[ (N- (pyrid-3-yl-methyl)-pyrrolid-3-yl)-methyl]-
5,6-dimethyl-1,3-dihydro-isoindole, ~-
: .' " ':
3-[(N- ( 3-(pyrid-3-yl)-propyl)-pyrrolid-3-yl)-methyl]- : .
7,8-dimethoxy-1,3,4,5-tetrahydro-2H-3-benzazepine, ~
'"'~ '' "- ' :~
2-[(N-(3-(pyrid-4-yl)-propyl)-pyrrolid-3-yl)-methyl]- : . -
5,6-dimethoxy-1,3-dihydro-isoindole,
2-[(N-(pyrid-3-yl-methyl)-pyrrolid-3-yl)-methyl]-
5,6-dimethoxy-1,3-dihydro-isoindole,
2-r(N-(pyrid-3-yl-methyl)-pyrrolid-3-yl)-methyl]- ~ ~.
5,6-dimethoxy-1-oxo-1,3-dihydro-isoindole,
2-[(N-(2-(6-methyl-pyrid-2-yl~-ethyl)-pyrrolid-
3-yl)-methyl]-6,7-dimethoxy-1,2,3,4-tetrahydro-
isoquinoline, ;~
2-[(N-(2-(6-methyl-pyrid-2-yl)-ethyl)-pyrrolid-
3-yl)-methyl]-5,6-dimethoxy-1,3-dihydro-isoindole, -;~
2-~(N-(2-(6-methyl-pyrid-2-yl)-ethyl)-pyrrolid-
3-yl)-methyl]-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro- -~
isoquinolinle, :~
'' '';~''''~ ~:
-~ ~330~9~
- 19 - ,~
2-[(N-(2-(6-methyl-pyrid-2-yl)-ethyl)-pyrrolid-
3-yl)-methyl]-5,6-dimethoxy-1-oxo-1,3-dihydro-isoindole,
2-[2-(N-(3-(pyrid-4-yl)-propyl)-piperid-2-yl)-ethy~ 5
6,7-dimethoxy-1,2,3,4-tetrahydxo-isoquinoline,
2-~2-(N-(3-(pyrid-4-yl)-propyl)-piperid-2-yl)-ethyl]-
6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-isoquinoline, ~ :
, :,
3-[(N-(2-(6,7-dimethoxy-i soqu i !nol- 4 -y 1 ) -ethyll-
piperid-2-yl)-methylJ-7,8-dimethyl-2-oxo-1,3,4,5- .
tetrahydro-2H-3-benzazepine, :-
2-[(N-(pyrid-4-yl-methyl)-piperid-3-yl)-methyl]- ::~
6,7-dimethyl-1,2,3,4-tetrahydro-isoquinoline,
2-[(N-(2-(6,7-dimethoxy-isoquinol-4-yl)-ethyl)-
piperid-3-yl)-methyl]-5,6-methylenedioxy-1-oxo-
1,3-dihydro-isoindole,
2-12-(N-(3-(pyrid-4-yl)-propyl)-piperid-2-yl)-ethyl]-
5,6-methylenedioxy-1,3-dihydro-isoindole,
~ .
2-[(N-(3~(pyrid-3-yl)-propyl)-hexahydro-azepin-
3-yl)-methyl]-6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-
isoquinoline,
2-[(N-(3-(pyrid-3-yl)-propyl)-hexahydro-azepin-
3-yl)-methyl]-6,7-dimethyl-1,2,3,4-tetrahydro-isoquinoline,
2-[(N-(2-(6-methyl-pyrid-2-yl)-ethyl)-hexahydro~
azepin-3-yl)-methyl]-5,6-dimethoxy-1,3-dihydro-
isoindole,
2-[(N-(2-~6-~methyl-pyrid-2-yl)-ethyl)-hexahydro- ;
azepin-3-yl~-methyl~-5,6-dimethoxy-1-oxo-1,3-dihydro- :
isoindole,
.
~ . . ., ~ - , , . ~ . - .,
`~ ~ 3 3 ~
- 20 -
3-r(N-(3-(5-hydroxy-indol-3-yl~-propyl)-pyrrolid-
3-yl)-methyl]-7,8-dimethyl-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine,
2-[ (N- (3-(5-hydroxy-indol-3-yl)-propyl)-pyrrolid-
3-yl)-methyl]-6,7-methylenedioxy-1,2,3,4-tetrahydro-
iso~uinoline, ~ .
3-1(N-(3-(5-methoxy-indol-3-yl)-propyl)-pyrrolid~
3-yl~-methyl]-7,8-dimethyl-1,3,4,5-tetrahydro-2H~
3-benzazepine,
2-[(N-(3-(5-methoxy-indol-3-yl)-propyl)-pyrrolid- ~ ~
3-yl)-methyl]-5,6-methylenedioxy-1,3-dihydro-isoindole, -
2-[(N-(3-(5-benzyloxy-indol-3-yl)-propyl)-pyrrolid- ~-
3-yl)-methyl~-6,7-methylenedioxy-1-oxo-1,2,3,4- ~: ;
tetrahydro-isoquinoline,
-:...::
2-[(N-(3-(5-benzyloxy-indol-3-yl)-propyl)-pyrrolid- ;~
3-yl)-methyl]-5,6-methylenedioxy-1-oxo-1,3-dihydro-
isoindole, ;
3-[(N-(3-(N-methyl-indol-3-yl)-propyl)-pyrrolid-
3-yl)-methyl]-7,8-methylenedioxy-1,3,4,5-tetrahydro-
2H-3-benzazepine,
2-[(N-(3-(N-methyl-indol-3-yl)-propyl)-pyrrolid-
3-yl)-methyl]-6,7-dimethyl-1,2,3,4-tetrahydro-isoquinoline,
2-[(N-(3-(indol-3-yl)-propyl)-pyrrolid-3-yl)-methyl]-
5,6-dimethyl-1,3-dihydro-isoindole,
3-r(N-(3-(indol-3-yl)-propyl)-pyrrolid-3-yl)-methyl]-
7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine,
- . .
',~ `i'
~ ~330~93
- 21 -
2-[(N-(3-(5-hydroxy-indol-3-yl)-propyl~-pyrrolid-
3-yl)-methyl]-5,6-dimethyl-1-oxo-1,3-dihydro-isoindole,
2-[(N-(3-(5-hydroxy-indol-3-yl)-propyl)-pyrrolid-
3-yl)-methyl]-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro~
isoquinoline,
3-[(N-(3-(5-methoxy-indol-3-yl)-propyl)-pyrrolid-
3-yl)-methyl~-7,8-dimethoxy-1,3,4,5-tetrahydro-
2~-3-benzazepine,
2-[(N-(3-(5-methoxy-indol-3-yl)-propyl)-pyrrolid-
3-yl)-methyl]-5,6-dimethoxy-1-oxo-1,3-dihydro-isoindole,
2-[rN-(3-(5-benzyloxy-indol-3-yl)-propyl)-pyrrolid-
3-yl)-methyl]-6,7-dimethoxy-1,2,3,4-tetrahydro-
isoquinoLine, .
2-[(N-(3-(5-benzyloxy-indol-3-yl)-propyl)-pyrrolid-
: 3-yl~-methyl]-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline,
~:''-,
2-[(N-(3-(N-methyI-indol-3-yl)-propyl)-pyrrolid-
3-yl)-methyl]-5,6-dimethyl-1-oxo-1,3-dihydro-isoindole,
.
2-[(N-(3-(N-methyl-indol-3-yl)-propyl)-pyrrolid-
3-yl)-methyl]-5,6-dimethoxy-1,3-dihydro-isoindole,
2-[(N-(2-(3,4-dimethoxy-phenyl)-ethyl)-hexahydro-
azepin-3-yl)-methyl]-6,7-methylenedioxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline, ::
:
2-[(N-(3-(3,4-methylenedioxy-phenoxy)-propyl)-hexahydro-
azepin-3-yl)-methyl]-6,7-methylenedioxy-1-oxo-1,2,3,4- .
tetrahydro-:Lsoquinoline,
'~
- ~233~3
2-[(N-(2-(4-trifluoromethoxy-phenyl)-ethyl~-piperid-
3-yl)-methyl]-5,6-dime~hoxy-1-oxo-1,3-dihydro-isoindole, :
2-[(N-(2-(4-trifluoromethyl-phenyl)-ethyl~-piperid-
3-yl)-methyl]-5,6-dimethoxy-1-oxo-1,3-dihydro-isoindole,
2-t (N- (2- ( 3,4-dichloro-phenyl)-ethyl)-piperid-3-
yl)-methyl]-5/6-dimethoxy-1-oxo-1,3-dihydro-isoindole,
2-[(N-(2-~3,4,5-trimethoxy-phenyl~-ethyl)-piperid- ~ ~
3-yl)-methyl]-5,6-dimethoxy-1-oxo-1,3-dihydro-isoindole, ::
2-[(N-(2-(4-methoxy-phenyl~-ethyl)-piperid-3-yl)- . :
methyl~-5,6-dimethoxy-1-oxo-1,3-dihydro-isoindole, : ~-
2-[(N-~2-(3-methyl-phenyl)-ethyl)-piperid-3-yl)-
methyl]-5,6-dimethoxy-1-oxo-1,3-dihydro-isoindole,
2-[(N-(2-(3,4-dimethyl-phenyl)-ethyl)-piperid-3-
yl)-methyl]-5,6-dimethoxy-1-oxo-1,3-dihydro-isoindole,
2-t(N-(2-(2,3,4,5-tetramethyl-phenyl)-ethyl)-piperid-
3-yl)-methyl]-5,6-dimethoxy-1-oxo-1,3-dihydro-isoindole,
2-[~N-(2-(4-benzyloxy-phenyl)-ethyl)-piperid-3
yl)-methyl]-5,6-dimethoxy-1-oxo-1,3-dihydro-isoindole, -`~
-r (N-(2-(4-hydroxy-phenyl)-ethyl)-piperid-3-yl)-
methyl]-5,6-dimethoxy-1-oxo-1,3-dihydro-isoindole,
2-[( N- ( 2-(4-methanesulphonyloxy-phenyl)-ethyl)~
piperid-3-yl)-methyl]-5,6-dimethoxy-1-oxo-1,3-dihydro-
isoindole, :; ;.
-,-
2-[ (N- ~2-~4--trifluoromethanesulphonyloxy-phenyl)-
ethyl)-piperid-3-yl)-methyl]-5,6-dimethoxy-1-oxo- ; -~
1,3-dihydro--isoindole,
' ` '.,. ," ~
:: ~
-- 23 --
2- [ (N- ~ 2- ~ 4-methanesulphonylamino-phenyl)-ethyl~-
piperid-3-yl)-methyl~-5,6-dimethoxy-1-oxo-1,3-dihydro-
i soinaole,
2-[ (N- (2- (4-dimethanesulphonylamino-phenyl)-ethyl)-
piperid-3-yl)-methyl~-5,6-dimethoxy-1-oxo-1,3-dihydro-
isoindole,
2-~(N-(3-(4-bromo-phenyl)-propyl)-piperid-3-yl)~
methyl~-5,6-dimethoxy-1-oxo-1,3-dihydro-isoindole,
2-r(N-(3-(4-methoxy-phenyl)-propyl)-piperid-3-yl)-
methyl]-5,6-dimethoxy-1-oxo-1,3-dihydro-isoindole,
2- r (N- (3-(3-methoxy-phenyl)-propyl)-piperid-3-yl)-
methyl]-5,6-dimethoxy-1-oxo-1,3-dihydro-isoindole,
2-[(N-(3-(3,4-dimethoxy-phenyl)-propyl)-piperid- ~ -
3-yl)-methyl]-5,6-dimethoxy-1-oxo-1,3-dihydro-isoindole,
2-[(N-(4-(4-methoxy-phenyl)-butyl)-piperid-3-yl)-
methyl]-5,6-dimethoxy-1-oxo-1,3-dihydro-isoindole,
2- r (N-(3-(4-amino-3,5-dibromo-phenoxy)-propyl)~
piperid-3-yl)-methyl]-5,6-dimethoxy-1-oxo-1,3-dihydro-
isoindole, -;
3-[(N-(2-(2,5-dimethyl-thien-3-yl)-ethyl)-piperid-
3-yl)-methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine,
3-t(N-(2-(5-methoxy-benzo[b]thienyl-3)-ethyl)-piperid-
3-yl)-methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-te~rahydro-
2H-3-benzazepine,
3-[(N-(2-(6--methoxy-benzo[b]thienyl-3)-ethyl)-piperid-
3-yl)-methy]L]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro- :
2H-3-benzaz~?pine,
~ ~330~
- 24 -
3-[(N-(2-(6-bromo-benzo[b]thienyl-3~-ethyl~-piperid-
3-yl~-methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine, ~ ~
3-r tN- (2-(benzo[b]thienyl-2~-ethyl)-piperid-3-yl)- .:
methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro- .
2H-3-benzazepine, .
3-[(N-(2-(benzo[b]furyi-3~-ethyl)-piperid-3-yl)- :
methyl]-7,8~dimethoxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine, ~ :
': ~,.. :'
3-[(N-(2-(2,5-dimethyl-thien-3-yl)-ethyl)-piperid-
3-yl)-me~hyl]-7,8-dimethyl-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine,
3-[(N-(2-(5-methoxy-benzo[b]thienyl-3)-ethyl)-piperidy-
3-yl)-methyl3-7,8-dimethyl-2-oxo-1,3,4,5-tetrahydro- :~
2H-3-benzazepine,
3-~(N-(2-(6-methoxy-benzorb]thienyl-3)-ethyl)-piperid-
3-yl)-methyl]-7,8-dimethyl-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine,
3-[(N-(2-(6-bromo-benzo[b]thienyl-3)-ethyl)-piperid- :~
3-yl)-methyl]-7,8-dimethyl-2-oxo-1,3,4,5-tetrahydro-
2~-3-benzazepine,
3-r(N-(2-(benzo[b]thienyl-2)-ethyl)-piperid-3-y~
methyl]-7,8-dimethyl-2-oxo-1,3,4,5-tetrahydro-2H- : .
3-benzazepine, :~.
3-~(N-(2-(benzo[b3furyl-3)-ethyl)-piperid-3-yl)- ;
methyl~-7,8--dimethyl-2-oxo-1,3,4,5-tetrahydro-2H-
3-benzazepine,
~'~:' . '
'
. - 25~ -3 3 ~ ~ 9 3
3-[(N-~2-~2,5-dimethyl-thien-3-yl~-ethyl)-piperid-
3-yl)-methyL]-7,8-methylenedioxy-2-oxo-1,3,4,5-
tetrahydro-2H-3-benzazepine,
3-[(N-(2-(5-methoxy-benzo[b]thienyl-3)-ethyl)-piperid-
3-yl)-methyl]-7,8-methylenedioxy-2-oxo-1,3,4,5-
tetrahydro-2H-3-benzazepine,
3-[(N-(2-(6-methoxy-benzo[b]thienyl-3)-ethyl~-piperid~
3-yl)-methyl]-7,8-methylenedioxy-2-oxo-1,3,4,5- - :
tetrahydro-2H-3-benzazepine,
3-[(N-(2-(6-bromo-benzo[b]thienyl-3)ethyl)-piperid-
3-yl)-methyl]-7,8-methylenedioxy-2-oxo-1,3,4,5-
tetrahydro-2H-3-benzazepine,
3-[(N-(2-(benzo[b]thienyl-2)-ethyl)-piperid-3-yl)- ~ ~ :
methyl[-7,8-methylenedioxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine,
.: "
3-[(N-(2-(benzo[b]furyl-3)-ethyl)-piperid-3-yl)-
methyl]-7,8-methylenedioxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine,
3-[(N-~3 (2,5-dimethyl-thien-3-yl)-propyl)-piperid-
3-yl)-methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine,
3-[(N-(3-(5-methoxy-benzo[b]thienyl-3)-propyl)-
piperid-3-yl)-methyl]-7,8-dimethoxy-2-oxo-1,3,4,5- ` :~
tetrahydro-2H-3-benzazepine, ;
` '',`
3-[(N-(3-(6-methoxy-benzo[b]thienyl-3)-propyl)-
piperid-3-yl)-methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-
~etrahydro-2H-3-benzazeplne,
~,.
:':
~33~9~3 ~
- 26 ~
3-[(N-(3-(6-bromo~benzo[b]thienyl-3)-propyl~-piperid-
3-yl) methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine,
3-[(N-t3-(benzo[b]thienyl-2)-propyl)-piperid-3-
yl) methyl~-7,8-aimethoxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine, ~ ~-
3-t (N- (3-(benzo[b]furyl-3)-propyl)-piperid-3-yl)
methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro~
2H-3-benzazepine,
3-[(N-(3-(2,5-dimethyl-thien-3-yl)-propyl)-piperid-
3-yl)-methyl]-7,8-dimethyl-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine,
.
:.~-
3-~tN-(3-(6-methoxy-benzo~b]thienyl-3)-propyl)-
piperid-3-yl)-methyl]-7,8-dimethyl-2-oxo-1,3,4,5-
tetrahydro-2H-3-benzazepine,
3-~(N-~3-(benzorb]thienyl-2)-propyl)-piperid-3-
yl)-methyl]-7,8-dimethyl-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine, .-
3-[(N-(3-(benzo[b]furyl-3)-propyl)-piperid-3-yl)-
methyl~-7,8-dimethyl-2-oxo-1,3,4,5-tetrahydro-2H-
3-benzazepine,
- : ~
3-~(N-(3-(2,5-dimethyl-thien-3-yl)-propyl)-piperid- ;~
3-yl)-methyl]-7,8-methylenedioxy-2-oxo-1,3,4,5- :
tetrahydro-2H-3-benzazepine,
.: - ;
: 3-~(N-(3-(6-methoxy-benzo~b]thienyl-3)-propyl)-
piperid-3-yl)-methyl]-7,8-methylenedioxy-2-oxo-
1,3,4,S-tetrahydro-2H-3-benzazepine, ~.
:
-~233a9~3
3-[ (N- (3-(benzo[b]thienyl-2)-propyl)-piperid-3-
yl)-methyl]-7,8-methylenedioxy-2-oxo-1,3,4,5-tetrahydro- : .
2H-3-benzazepine,
3-[(N-(3-(benzo~b]furyl-3)-propyl)-piperid-3-yl)-
methyl]-7,8-methylenedioxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine,
3-[(~-(2-(thien-2-yl)-ethyl)-piperid-3-yl)-methyl]-
7,8-dimethyl-2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine,
3-[(N-(2-(benzolb]thienyl-31-ethyl)-piperid-3-yl)-
methyl~-7,8-dimethyl-2-oxo-1,3,4,5-tetrahydro-2H- :-:
3-benzazepine,
3-[(N-(2-(benzo[b]furyl-2)-ethyl)-piperid-3-yl)-
methyl]-7,8-dimethyl-2-oxo-1,3,4,5-tetrahydro-2H-
3-benzazepine, ~ :
.,
3-[(N-(2-(thien-2-yl)-ethyl)-piperid-3-yl)-methyl]-
7,8-methylenedioxy-2-oxo-1,3,4,5-tetrahydro-2H-
3-benzazepine,
3-[(N-(2-(benzorb]thienyl-3)-ethyl)-piperid-3-yl)-
methyl]-7,8-methylenedioxy-2-oxo-1,3,4,5-tetrahydro- '~ `'t~Jy~,
2H-3-benzazepina, ::
:
: 3-[(N-(2-(benzo[b]furyl-2)-ethyl)-piperid-3-yl)- : -
methyl]-7,8-methylenedioxy-2-oxo-1,3,4,5-tetrahydro- ~-~
2~-3-benzazepine,
3-[(N-(2-(thien-2-yl)-ethyl)-hexahydro-azepin-3-
yl1 methyl]-7,8-dimethyl-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine, ;~
3-[(N-(2-(benzo[b]thienyl-3)-ethyl)-hexahydro-azepin-
3-yl)-methyl]-7,8-dimethyl-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine, ~:
~ ' ,
~.33a~93 ::
--28 -
3-(N-(2-(benzo[b]furyL-2~-ethyl)-hexahydro-azepin-
3-yl)-methyl]-7,8-dimethyl-2-oxo-1,3,4,5 tetrahydro-
2~-3-benzazepine,
3-[ (N- ~2-~thien-2-yl)-ethyl)-hexahydro-azepin-3-
yl)-methyl]-7,8-methylenedioxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine,
3-[(N-(2-(benzo[b]thienyl-3)-ethyl)-hexahydro-azepin-
3-yl)-methyl]-7,8-methylenedioxy-2-oxo-1,3,4,5-
tetrahydro-2H-3-benzazepine,
3-~(N-(2-(benzo~b]furyl-2)-ethyl)-hexahydro-azepin-
3-yl)-methyl]-7,8-methylenedioxy-2-oxo-1,3,4,5-
tetrahydro-2H-3-benzazepine,
3-[ (N- (2-(thien-2-yl)-ethyl)-pyrrolid-3-yl)-methyl]-
7,8-dimethyl-2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine,
3-[(~-(2-(benzorb~thienyl-3)-ethyl)-pyrrolid-3
yl)-methyl]-7,8-dimethyl-2-oxo-1,3,4,5-tetrahydro- ::::
2H-3-benzazepine,
3-l(N-(2-(benzo~b]furyl-2)-athyl)-pyrrolid-3-yl)- ~ .
methyl]-7,8-dimethyl-2-oxo-1,3,4,5~tetrahydro-2H- -~
3-benzazepine,
3-[(N-(2-(thien-2-yl)-ethyl)-pyrrolid-3-yl)-methyl]-
7,8-methylenedioxy-2-oxo-1,3,4,5-tetrahydro-2H-
3-benzazepine,
3-r(N-(2-(b~nzo[b]thienyl-3)-ethyl)-pyrrolid-3-
yl)-methyl]--7,8-methylenedioxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine,
3-[(~-(2-~bienzo[b]furyl-2)-ethyl)-pyrrolid-3-yl)-
methyl]-7,8-methylenedioxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzaz,epine,
,: .
~33~3
- 29 -
3-[ (N- (2-~thien-2-yl)-ethyl)-pyrrolid-3-yl)-methyl]- ~
7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine, ::
3-[ (N- (2-(benzo[b]thienyl-3)-ethyl)-pyrrolid-3-
yl)-methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine,
3-[(N-(2-(benzo[b]furyl-2)-ethyl)-pyrrolid-3-yl)-
methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine,
3-[(N-(2-(thien-2-yl~-ethyl)-hexahydro-azepin-3- - -
yl)-methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro- :
2H-3-benzazepine,
3-[(N-(2-(benzolb]thienyl-3)-ethyl)-hexahydro-azepin-
3-yl)-methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine,
~ ~r'~
3-r(N-(2-(benzo[b]furyl-2)-ethyl)-hexahydro-azepin-
3-yl)-methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro- ..
2H-3-benzazepine,
3-[(N-(4-(thien-2-yl)-butyl)-piperid-3-yl)-methyl]- ~.
7,:8-dimethyl-2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine,
~'
3-[(N-14-(benzo[b]thienyl-3)-butyl)-piperid-3-yl)-
methyl]-7,8-dimethyl-2-oxo-1,3,4,5-tetrahydro-2H-
3-benzazepine,
3-[(N-(4-(benzolb]furyl-2)-butyl)-piperid-3-yl)-
methyl]-7,B-:dimethyl-2-oxo-1,3,4,5-tetrahydro-2H-
3-benzazepine, : :
3-[(N-(5-(thien-2-yl)-pentyl)-piperid-3-yl)-methyl]-
7,8-dimethyl-2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine,
. ':'.~;.'
' ''' .' ; ' :~.'
~33~9~
- 30 -
3-[(N-~5-tbenzorb]thienyl-3)-pentyl)-piperid-3-
yl)-methyl]-7,8-dimethyl-2-oxo-1,3,4,5 tetrahydro- ;~
2H-3-benzazepine,
3-[(N-(5-(benzo[b]~uryl-2)-pentyl)-piperid-3-yl)~
methyl]-7,8-dimethyl-2-oxo-1,3,4,5-tetrahydro-2H-
3-benzazepine, .
3-[(N-(4-(thien-2-yl)-butyl)-piperid-3-yl)-methyl]-
7,8-methylenedioxy-2-oxo-1,3,4,5-tetrahydro-2H-
3-benzazepine,
3-[(N-(4-tbenzo[b]thienyl-3)-butyl)-piperid-3-yl)-
methyl]-7,8-methylenedioxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine,
3-r (N-(4-(benzo[b]furyl-2)-butyl)-piper~d-3-y~
methyl]-7,8-methylenedioxy-2-oxo-1~3,4,5-tetrahydro-
2H-3-benzazepine,
,
3-[(N-(S-(thienyl-2)-pentyl)-piperid-3-yl)-methyl]-
7,8-methylenedioxy-2-oxo-1,3,4,5-tetrahydro-2H- ~:
3-benzazepine,
3-t(N-(5-(benzo[b]thienyl-3)-pentyL)-piperid-3- :~
yl)-methyl]-7,8-methylenedioxy-2-oxo-1,3,4,5 tetrahydro-
2H-3-benzazepine,
3-[(N-(5-(benzo~b]furyl-2)-pentyl)-piperid-3-yl)-
methyl]-7,8-methylenedioxy-2-oxo-1,3,4,5-tetrahydro-
, . , :
2H-3-benzazepine,
-~ .
3-[(N-(4-(benzo[b]thienyl-3)-butyl)-piperid-3-yl)- : :
methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro- . .-
2H-3-benzazepine,
..,, ~:
`'. ~"':"
' ~ .'~' ~ '.
~33~3
. - 31 -
3-[ (N- (4-~benzo[b]furyl-2~-butyl~-piperid-3-yl)-
methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine,
3-r(N-(5-(benzo[b]thienyl-3)-pentyl)-piperid-3-
yl)-methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine,
3-[(N-(5-(benzo[b]furyl-2)-pentyl)-piperidyl-3)-
methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-
2~-3-benzazepine, and : :~
the enantiomers, diastereomers, N-oxides and acid :
addition salts thereof, more particularly, for
pharmaceutical use, the physiologically acceptable . -
acid addition salts thereof.
Preferred compounds according to the invention
include those of general formula I wherein
A, B, m and n are as hereinbefore defined,
E represents a straight-chained Cl_3 alkylene group, ~;
G represents a straiqht-chained Cl 6 alkylene group
or a group -G'-G"- wherein G' represents :
a straight chained C2 5 alkylene group and G" represents
an oxa, methylimino or ethylimino group, . ~ .
~:
Rl represents a methyl or methoxy group and
R2 represents a methyl or methoxy group, or R
and R2 together represent a methylenedioxy group, .:~
and ..
'`''. "' -'~
R represents an optionally methyl-substituted furyl,
thienyl, pyridyl, benzo[b]furyl or benzo[b]thienyl
` ~ ~33~3
.,~,
- 32 -
qroup, a benzo[b]thienyl group substituted by a : : ::
halogen atom or by a methoxy or methanesuLphonyloxy
group, an indolyl or N-methylindolyl group optionally
substituted by a hydroxy, methoxy or benzyloxy -~
group, a dimethyl-thienyl or dimethoxy-isoquinolyl
group, a naphthyl group optionally mono- or disubstituted
by methyl or methoxy groups wherein the substituents ~ :
may be the same or different, or if B represents
a -CH2- or -CO- group, R may also represent a phenyl
group optionally substituted by a methylenedioxy ~ :~
group, a phenyl group mono- or disubstituted by
chlorine or bromine atoms or methyl or methoxy groups ::
wherein the substituents may be the same or different, ~ :
a phenyl group substituted by a hydroxy, benzyloxy,
methanesulphonyloxy, trifluoromethanesulphonyloxy,
trifluoromethyl, trifluoromethoxy, nitro, am;no,
acetamido, methanesulphonylamino or bis(methanesulphonyl~
amino group, or a trimethoxyphenyl, tetramethylphenyl ~ ~ :
or dihaloaminophenyl group, ~::
~ '
and the enantiomers, diastereomers, N-oxides and
acid addition salts thereof.
Particularly preferred compounds of the invention
include those of formula Ia : :
":' ' ':
R1 ~ A \ /(CH2)m\ ~ ;~
R2 B / \(CH ) /
.. .
wherein
Rl~ R2, R, A, B, E, G, m and n are as hereinbefore
defined,
.,~.. :, .
~` ~33a9~
- 33 -
and the enantiomers, diastereomers, N-oxides and
acid addition salts thereof, more particularly,
for pharmaceutical use, the physiologically acceptable
acid addition salts thereof. :
Especially preferred compounds of the invention
include those of formula Ia above wherein
A represents a -CH2CH2- group, -
B represents a -CH2-, -CH2-CH2-, -CO- or -CH2CO- . ~ :~
group, .
E represents a methylene or ethylene group,
G represents a straight-chained C2 4 alkylene group . ~:~
or a straight-chained C2 3 alkyleneoxy group,
Rl represents a methoxy group and ~ .
R2 represents a methoxy group or Rl and R2 together
represent a methylenedioxy group,
m represents any one of the integers from 2 to 4,
::'. ~:'
n represents the integer 1, and
R represents a naphth-2-yl, 6-methoxy-naphth-2- .
yl, 5-methyl-6-methoxy-naphth-2-yl, thien-2-yl,
benzo[b]furyl-2 or benzo~b]thienyl-3 group or,
if B represents a -CH2- or -CO- group, a 4-methoxyphenyl
or 3,4-dimethoxyphenyl group, ;.
and the enantiomers, diastereomers, N-oxides and
physiologically acceptable acid addition salts
thereof.
`-`i ` ~3~09~3
- 34 -
The following are particularly preferred compounds~
3-[(N-(2-(naphth-2-yl)-ethyl)-piperid-3-yl)-methyl~
7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine,
3-[(N-(2-(5-methyl-6-methoxy-naphth-2-yl)-ethyl)- :.
piperid-3-yl)-methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-
tetrahydro-2H-3-benzazepine,
3-~2-(N-(2-(6-methoxy-naphth-2-yl)-e~hyl~-piperid-
2-yl)-ethyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro~
2H-3-benzazepine, -. :
:
2-[(N-(2-(6-methoxy-naphth-2-yl)-ethyl)-heXahydro- ~ `
azepin-3-yl)-methyl]-6,7-methylenedioxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline,
~:
2-r(~-(2-(naphth-2-yl)-ethyl)-hexahydro-azepin- .
3-yl)-methyl]-6,7-methylenedioxy-1-oxo-1,2,3,4- . ~.
tetrahydro-isoquinoline,
. :.
2-[(N-(2-(3,4-dimethoxy-phenyl)-e~hyl)-piperid-
3-yl)-methyl]-6,7-dimethyl-1,2,3,4-tetrahydro-isoquinoline,
2-r(N-(2-(3,4-dimethoxy-phenyl)-ethyl)-piperid-
3-yl)-methyl]-6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-
isoquinoline,
::, ~ ,
2-[(N-(3-(4-methoxy-phenoxy)-propyl)-piperid-3-
yl)-methyl]-6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro- -~
isoquinoline,
3-[(N-(4-(thien-2-yl)-butyl)-piperid-3-yl)-methyl]- ` - ~:
7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-2H-3-benzazeplne,
3-[(N-(2-(benzo[b]furyl-2)-ethyl)-piperid-3-yl)-
methyl]-7,B-dimethoxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine,
,~
~ 35 - ~33~3
3-[(~-~2-~benzo[b]thienyl-3)-ethyl)-piperid-3-yl~-
methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro- -
2H-3-benzazepine,
2-[ (N- (3-(6-methoxy-naphthyl-oxy)-propyl)-pyrrolid-
3-yl)-methyl]-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro- :
isoquinoline,
2-[(N-(2-(6-methoxy-naphth-2-yl)-ethyl)-hexahydro- ~:
azepin-3-yl)-methyl~-6,7-methylenedioxy-1-oxo-1,2,3,4- ~:
tetrahydro-i~oquinoline,
3-[(N-(2-(4-methoxy-phenyl)-ethyl)-hexahydro-azepin-
3-yl)-methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine, . -
3-[(N-(2-(3~4-dimethoxy-phenyl)-ethyl)-hexahyaro-
: azepin-3-yl)-methyl]-7,8-methylenedioxy-2-oxo-1,3,4,5-
tetrahydro-2H-3-benzazepine, and
the enantiomers, diastereomers and acid addition
salts thereof.
'., ,.,"" '. -
The following compounds are especially highly preferred: `` .;.;
3-[(N-(2-(naphth-2-yl)-ethyl)-piperid-3-yl)-methyl~- . `
N : 7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine, .
2-[(N-(3-~4-methoxy-phenoxy)-propyl)-piperid-3-
yl)-methyl]-6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-
isoquinoline,
: . ,., :.~,~ ~,,
3-[(N-(4-~thienyl-2)-butyl)-piperid-3-yl)-methyl~
7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine,
2-[(N-t3-~6-methoxy-naphthyl-2-oxy)-propyl)-pyrrolid-
3-yl)-methyl]-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro- ~ .
isoquinoline,
. . . .
: `'
~ 3~ o ~
2-[(N-(2-(6-methoxy-naphth-2-yl)-ethyl)-hexahydro- :~
azepin-3-yl)-methyl]-6,7-methylenedioxy-l-oxo-l,2,3,4-
tetrahydro-isoquinoline,
3-[(N-(2-(4-methoxy-phenyl)-ethyl)-hexahydro-azepin- :
3-yl~-methyl]-7,8-dimethoxy-2-oxo-l,3,4,5-tetrahydro-
2H-3-benzazepine, : .
3-[(N-(2-(3,4-dimethoxy-phenyl)-ethyl)-hexahydro-
azepin-3-yl)-methyl]-7,8-methylenedioxy-2-oxo-l,3,4,5-
tetrahydro-2H-3-benzazepine, and .
the enantiomers, diastereomers and acid addition .
salts thereof.
In a further aspect ~he present invention provides
a process for preparing the compounds of the invention,
said process comprising a~ least one of the following :::
steps~
a) reacting a compound of formula II .
R1
~1 \ / \ (II)
R2 ~ l ~ / \(CH ) / `~
(wherein
Rl, R2, A, B, E, m and n are as hereinbefore defined) -~
with a compound of formula III .~
..:
Zl ~ G~ - R
wherein
R is as hereinbefore defined and
f~
_ 37 _ ~330~3
Zl represents a nucleophilic leaving group such
as a halogen atom or a sulphonyloxy group, e.g.
a chlorine, bromine or iodine atom, a methanesulphonyloxy,
p-toluenesulphonyloxy or ethoxysulphonyloxy group,
or a hydroxy group and G"' is a group G as hereinbefore
defined or Zl represents an oxygen atom and G'l'
represents a straight chained Cl 6 alkanylylidene
group optionally substituted by a Cl 3 alkyl group
or a group -G""G"- where G" i~; as hereinbefore defined
and G"" represents a straight--chained C2 5 alkanylylidene ; ~ ~
group optionally substituted by a Cl_3 alkyl group; .
. ., -.:
b) reacting a compound of formula IV
R1 .' .. "- '. ':,
R2 ~ ~ \ - H (IV)
(wherein
Rl, R2, A and B are as hereinbefore defined) with
a compound of formula V
Z2 ~ E - CH N - G - R ~V) ~ :
(CH2 )n
'': ,'~ . ' '~ ' '
wherein
R, E, G, m and n are as hereinbefore defined and
~ :'
.:
..
`~ ~330~
- 38 - 27169-147
Z2 represent~ a nucleophlllc leaving group such
as a halogen atom or a su~phonyloxy group, e.g.
a chlorine, bromine or 1odine atom or a methane~ulphonyl-
oxy, p-toluene~ulphonyloxy or ethoxysulphonyloxy
group;
c1 (to prepare compounas of formula I wherein B
repre~ent~ a -CH2- or -CH2CH2- group) ~.
redu~ing ~ compound o~ formulD VI
R
R2 ~ \ / \
wherein
R, Rl, R2, E, G, m and n are as hereinbefore
defined A' represents a -CH2-, -CH2CH2-, -CH=CH- or -cO-
group, and
Bl represent~ a -CO- group or a -C~2CO- group of :~
which the carbonyl carbon i8 attached to the nltrogen
atom;
d~ ~to prepare compounds of formula I whereln A
represent~ a -CH2- group and B repre~ents a -CO-
or -CH2CO- group)
reducing a compound of formula VII
r.. t .
~ CO~ CH2)~
, ~
~33~993
39 ~ 27169-1~7
lwhereln
R, Rl, R2, E, G, m and n are deflned a~ hereinbe~ore and ~. .
82 represents a -CO- group or a -C~2CO- group of
which the carbonyl caebon is attached to the nitrogen ~-
atom) w1th na~cent hydrogen
e~ ~to prep~re compounds of formula I wherein G
is a group a3 de~lned for G hereinbefore, with
the exceptlon o~ the group~ corlta~ning a sulphur
atom or ~ sulphinyl or sulphonyl group, and wherein ;~
A represent~ a -CH2-CH2- group and ~ rePresents
a methylene, carbonyl or -CH2-CO- group)
hydrogenating a compound of ~ormula VI~I
R1 . ,~
2 ~ / CNz)
wherein
R, Rl, R2, a, E, m ~nd n are as hereinbe~ore de~ined, -~
Gl i~ a gro~p G a~ hereinbefore defined wlth the
provl~o that it does not represent a group containing
a sulphur atom or d sulphinyl or sulphonyl group, :~
and
B3 represents a -CH2- , -CO- or -CH2-CO- group; . ~:
f~ reduclng a resulting compound of formula
I wherein R contains a nitro group to yield a corre~ponding ~:
amino compound of formula Is
~:
g) acylating a resultlng compound of formula
I wherein R contains sn amino group to yield a
:.
~ ' . , .
_ 40 _ 1~ 3
corresponding alkanoylamino compound o~ ~ormula
I;
h~ resolving a resulting compound of formula ~
I lnto its diastereomers or enantiomers; and ~ ~-
i) converting a compound of formula I into an -~
acid addition salt thereof or a salt of a compound `~
of formula I into the free base. -~ -~
: .. :
In general, the process of the invention may conveniently
be carried out in a solvent or solvent mixture.
In reaction step (a~, if Zl represents a nucleophilic
leaving group, the reaction is convenientLy carried
out in a solvent or mixture of solvents ~such as
acetone, diethylether, methylformamide, dimethylformamide,
dimethylsulfoxide, benzene, chlorobenzene, tetrahydrofuran,
benzene/tetrahydrofuran and dioxan) or in an excess
of the compounds of formulae II and/or III used
and optionally in the presence of an acid binding
agent (e.g. an alkoxide such as potassium tert.butoxide, ;
an alkali metal hydroxide such as sodium or potassium
hydroxide, an alkali metal carbonate such as potassium
carbonate, an alkali metal amide such as sodium
amide, an alkali metal hydride such as sodium hydride,
a tertiary organic base such as triethylamine or
pyridine, twhich may simultaneousIy be used as ~;
solvent)) or a reaction accelerator such as potassium
iodide, depending on the reactivity of the nucleophilically
exchangeable group, conveniently at temperatures
of between O and 150C, preferably at temperatures ~
of between 50 and 120C, e.g. at the boiling temperature
of the solvent used. However the reaction may
also be carried out without a solvent. It is,
however, particularly advantageous to perform the ~
~"':
'::
:
~33~3
- 41 -
reaction in the presence of a tertiary organic ~ ~
base or an excess of the amine o formula II used. ~ ~-
If Zl represents a hydroxy group, the reaction -
of step (a) is preferably carried out in a solvent
such as methanol, ethanol, tetrahydrofuran, dioxan,
ethyl acetate or glacial acetic acid, with hydrogen
in the presence of a hydrogenation catalyst such
as platinum, palladium/charcoal or Raney nickel
under a hydrogen pressure of from 2 to 10 bar,
preferably 5 bar, and at temperatures of between
20 and 120C, preferably at temperatures between
50 and lOO~C.
If Zl represents an oxygen atom, the reaction of
step (a) is preferably carried out in a solvent
such as methanol, ethanol, tetrahydrofuran, dioxan,
ethyl acetate or glacial acetic acid, with hydrogen ~
in the presence of a hydrogenation catalyst such -
as platinum, palladium/charcoal or Raney nickel ~ -~
under a hydrogen pressure of from 2 to lO bar, ~ -
preferably 5 bar, and at temperatures of between
20 and 120C, preferably at temperatures of between
50 and 100C, or in the presence of a complex metal -
hydride such as sodium cyanoborohydride in a solvent
such as methanol, ethanol, tetrahydrofuran, dioxan ~ ;
. . .
or acetonitrile at temperatures of between 0 and
50C, but preferably at ambient temperature.
The reaction of step (b) is preferably carried
out in a solvent or mixture of solvents such as
methylformamide, dimethylformamide, dimethylsulphoxide,
benzene, chlorobenzene, tetrahydrofuran, benzene/tetra~
hydrofuran or dioxan in the presence of an acid-
binding agent, e.g. an alkoxide such as potassium ;~ ~
tert.butoxide, an alkali metal hydroxide such as ;
i33~9~
- 42 -
sodium or potassium hydroxide, an alkali metal
carbonate such as potassium carbonate an alkali
metal amide such as sodium amide or an alkali metal
hydride such as sodium hydride, conveniently at
temperatures of between 0 and 150C, preferably
at temperatures of between 0 and 50C.
The reduction of step (c) is preferably carried
out with a metal hydride such as li~hium aluminium
hydride or diborane or a complex of borane and
a thioether, e.g. with borane--dimethylsulphide ~
complex, in a solvent such as diethylether or tetra- -
hydrofuran, at temperatures of between 0 and 80C,
but preferably at temperatures between 10 and 45C.
The reduction of step (d) is conveniently carried
out in a suitable solvent such as glacial acetic -~
acid, glacial acetic acid/water or glacial acetic
acid/ethanol with nascent hydrogen, e.g. in the
presence of zinc/glacial ace~ic acid, tin/hydrochloric~ `
acid or tin dichloride/hydrochloric acid at temperatures
between 20 and 150C, but preferably at the boiling
temperature of the reaction mixture, e.g. at temperatures
between 80 and 100C.
'~:
The hydrogenation of step (e) is conveniently carried
out in a solvent or mixture of solven~s such as
methanol, ethanol, ethyl acetate or glacial acetic
acid with catalytically activated hydrogen, e.g.
with hydrogen in the presence of platinum or palladium/~
charcoal, under a hydrogen pressure of from 1 to
7 bar, but preferably from 3 to 5 bar, and at temperatures
of between 0 and 75C, but preferably at temperatures
between 20 and 50C.
~.
3 3 a ~ 9 ~
- 43 ~
In the reactions described above, any reactive
groups present such as hydroxy, amino, alkylamino
or imino groups may be protected during the reaction
by means of conventional protecting groups which
are cleaved off again after the reaction.
For example, suitable protecting groups for a hydroxy
group include trimethylsilyl, acetyl, benzoyl,
benzyl and tetrahydropyranyl groups and suitable
protecting groups for an amino, alkylamino or imino
group include acetyl, benzoyl, ethoxycarbonyl and
benzyl groups. ;
The optional subsequent cleaving of a protecting
group is preferably carried out hydrolytically
in an aqueous solvent, e.g. in water, isopropanol/water, --
tetrahydrofuran/water or dioxan/water, in the presence
of an acid such as hydrochloric or sulphuric acid ~-
or in the presence of an alkali metal base such
as sodium hydroxide or potassium hydroxide at temperatures
between 0 and 100C, preferably at the boiling
temperature of the reaction mixture. However, ;; ;~
a benzyl group is preferably split off by hydrogenolysis,
e.g. with hydrogen in the presence of a catalyst
such as palladium/charcoal in a solvent such as
methanol, ethanol, ethyl acetate or glacial acetic
acid, optionally with the addition of an acid such
as hydrochloric acid at temperatares between 0
and 50C, but preferably at ambient temperature,
under a hydrogen pressure of from 1 to 7 bar, preferably
from 3 to 5 bar.
The reductlon of ~he nitro compound in step (f)
is preferab:Ly carried out in a solvent such as
water, water/ethanol, methanol, glacial acetic
acid, ethyl acetate or dimethylformamide, conveniently
with hydrogen in the presence of a hydrogenation `
1 ~ 3 ~
- 44 -
catalyst such as Raney nickel, platinum or palladium/char-
coal, with metals such as iron, tin or zinc in
the presence of an acid, with salts such as iron(II)-
sulphate, tin~II) chloride or sodium dithionite
or with hydrazine in the presence of Raney nickel
at temperatures of between 0 and 50C, but preferably
at ambient temperature.
The acylation of step (g) is conveniently carried
out in a solvent such as methylene chloride, chloroform,
carbon tetrachloride, ether, tetrahydrofuran, dioxan,
benzene, toluene, acetonitrile or dimethylformamide,
preferably with a reactive derivative of the acid,
for example with acetyl chloride, acetic anhydride
or propionic anhydride, and optionally in the presence
of an inorganic base such as sodium carbonate or
a tertiary organic base such as triethylamine or
pyridine, which may simultaneously serve as solvent,
at temperatures of between -25C and 100C, but
preferably at temperatures of between -10C and
the boiling temperature of the solvent used.
Since they have at least one chiral centre, the -
compounds of formula I obtained can be resolved
by conventional methods into their diastereomers,
for example by column chromatography, and into
their enantiomers, for example by column chromatography
on a chiral phase or by crystallisation with optically
active acids, e.g. with D- or L-monomethyl tartaric
acid, D- or L-diacetyl tartaric acid, D- or L-tartaric
acid, D- or L-lactic acid or D- or L-camphoric
acid.
The compounds of formula I obtained may also be
converted into the acid addition salts thereof,
and particularIy for pharmaceutical use into the
physiologically acceptable acid addition salts
` ~ :
_ 45 _ ~33~93 :~
thereof with inorganic or organic acids. Suitable
acids include, for example, hydrochloric, hydrobromic,
sulphuric, phosphoric, acetic, lactic, citric,
tartaric, succinic, maleic ancl fumaric acids.
The compounds of formulae Il to VIII used as starting
materials are known from the literature in some
cases or may be obtained using methods known Per
se.
, .
Thus, for example, a starting compound of formula
II may be obtained by alkylation of a corresponding ;
imino compound of formula IV with a cyclic amine
protected at the N atom by a conventional prot~cting
groupl said cyclic amine being substituted in the ;
carbon structure by an alkyl group which is in i`~
turn substituted in the terminal position by a
nucleophilic leaving group, and subsequently cleaving
the protecting group used. The cyclic amine required
for this reaction may be obtained by converting
a corresponding cyclic amine substituted by a hydroxyalkyl
group into a suitable halogen- or sulphonic acid
ester derivative thereofl and the imino compound
of formula IV required for this reaction may be
obtained by cyclising a corresponding compound,
e.g. by cyclising a compound of formula IX
R1 ., ~ ~:
: R2 ~ N CH2CH \ (IX) ~
,',', ' "'',
''', '"''~'''
''' "' ,'';'~
- ~6 - ~ ~;3
or of formula X
R1 ~: :
R2 ~ 1l N~ - COCH2Cl (X)
/ ,,.
2 2
optionally followed by catalytic hydrogenation
and/or reduction of the carbonyl group, for example
with sodium borohydride/glacial acetic acid ~see
EP-A-7070, ~P-A-65229 and EP-A-109639).
A compound of formula V used as starting material ~ ~-
may be obtained by N-alkylation of a corresponding
~ ,
~ :cyclic amine, substituted in the carbon~structure
:~:: by a hydroxyalkyl group, with a corresponding compound:~
or with a corresponding l,h~-dihaloalkane and subsequent
: reaction with a corresponding HO, SH or HN compound
and if necessary subsequent ox;idation, a hydroxyalkyl
,
compound thus obtained then being:converted into
~:: its reactive halogen or sulphonic acid ester derivatives.
; : ,, :
~ A compound of formulae VIt VII:or:~VIII used as ~
..
: starting material may preferably be obtained by
reacting a corresponding haIogen~compound with
a corresponding amine; optionally followed by the
: spli~tting off of protecting groups used to protect
: amino groups.
: . ,;, ~:
.: -
: ~ . ., ~.
~y~
- 47 _ 133~,~9~
As already mentioned hereinbefore, the new compounds :
of formula I and the physiologically acceptable
acid addition salts thereof with inorganic or organic
acids have valuable pharmacological properties, .
particularly a hypotensive effect and an especially
long-lasting heart rate lowering effect and the :
effect of reducing the 2 reauirement of the heart, .~-
with only minor side-effects on the central nervous
system.
" ::
For example, the following compounds~
, .,: : .....
A = 3-[(N-(2-(naphth-2-yl)-ethyl)-piperid-3-yl)-
methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine-hydrochloride, ~ ~:
B = 3-[(N-(2-(5-methyl-6-methoxy-naphth-2-yl)-ethyl)-
piperid-3-yl)-methyl]-7,8-dimethoxy-2-oxo-1,3,4,5- -~
tetrahydro-2H-3-benzazepine-hydrochloride,
C = 3-[2-(N-(2-(6-methoxy-naphth-2-yl)-ethyl~-piperid-
2-yl)-ethyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine-hydrochloride, ;~
,.: '..,.:, '.'~'
: ~ . - : .
.: :~:, " ~
' ;' ~ ' ~
'~"'~ `'
' ~.
~ `, '
~ 48 - L33~
D = 2-[(N-(2-(6-methoxy-naphth-2-yl)-ethyl)-hexahydro-
azepin-3-yl)-methyl]-6,7-methylenedioxy-1-oxo- ~ .
1,2,3,4-tetrahydro-isoquinoline-hydrochloride,
: ~ . .. .
E = 2-[(N-(2-(naphth-2-yl)-ethyl)-hexahydro-azepin~
3-yl)-methyl]-6,7-methylenedioxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline-hydrobromide,
F = 2-[(N-(2-(3,4-dimethoxy-phenyl)-ethyl)-piperid-
3-yl)-methyl] 6,7-dimethyl-1,2,3,4-tetrahydro-
isoquinoline-dihydrochloride,
.
~: G = 2-[(N-(2-(3,4-dimethoxy-phenyl)-ethyl)-piperid- :
~:~ 3-yl)-methyl]-6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-
isoquinoline-hydrochloride,
: H = 2-[(N-(3-(4-methoxy-phenoxy)-propyl)-piperid~
3-yl)-methyl]-6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-
isoquinoIine-hydrochloride,
I = 3-[(N-(4-(thienyl-2)-butyl)-piperid-2-yl)-methyl]-
: 7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-2H-3-
benzazepine-hydrochloride,
~ '""
- ~ 33~9~
- 49 -
K = 3-[(N-(2-(benzo[b~furyl-2)-ethyl~-piperid-3-
yl)-methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine-hydrochloride and ~
L = 3-[(N-f~-(benzo[b]thienyl-3)-ethyl~-piperid- ~-:
3-yl)-methyl]-7~8-aimethoxy-2-oxo-l~3~4~5-tetrahydro- ~:
2H-3-benzazepine-hydrochloride
were tested for their biological properties as
follows:
Effect on heart rate in rats
. ~, . .
The activity of the test substances on the heart
rate was investigated, for each dosage, on 2 rats
with an average weight of 250-300 g. The rats :~ ~d~-
were anaesthetised with pentobarbital (50 mg/kg -~
i.p. and 20 mg/kg s.c.). The test substances
were injected in aqueous solution into the jugular -~ :
vein (0.1 ml/100 9). . .
The blood pressure was measured using a cannula
inserted in a carotid artery and the heart rate
was recorded from an ECG (second or third derivation)
derived with needle electrodes. The heart rate
of the animals in the control period was between ;~
350 and 400 beats per minute (b/min). .
The results obtained are set forth in the following
.
Table~
_ 50 - 133~3
Substance Dosage Lowering of heart Lowering of blood
rate in b/m after pressure in mmHg
after ~ -
[mg/kg] 5 min. 20 min. 5 min. 20 min.
A 5.0 - 218 - 259 - 46 - 32
B 5.0 - 188 - 218 - 22 - 15
C 5.0 - 236 - 194 - 40 - 31
D 5.0 - 173 - 123 - 21 - 16
E 5.0 - 169 - 150 - 30 - 16
F 5.0 - 150 - 120 - 49 - 27
G 5.0 - 207 - 101 - 57 - 8
H 5.0 - 190 - 180 - 57 - 33
I 5.0 - 320 - 253 - 65 - 32
K 2.5 - 138 - 156 - 36
L 2.5 - 110 - 163 - 40 - 24
~ ` ~
. `:
- 51 - 133~
When administered in therapeutic doses the compounds ~ -
prepared according to the invention show no toxic
side effects of any kind. Thus, for example when
administered intravenously to mice, even in a high
dosage of 20 mg/kq, substances A and L showed no
toxic side effects apart from a slight sedation.
~ ~ '
In view of their pharmacological properties, the
compounds according to the invlention are suitable
for the treatment of sinus tachycardia of various
origins and for the prevention and treatment of ;~
ischaemic heart disease.
, ~,: . , : : .
The dosage required to achieve this effect is conveniently
from 0.01 to 0.2 mg/kg of body weight, preferably ; ~ Y
from 0.03 to 0.15 mg/kg of body weight, once or
twice a day. The compounds of formula I and the
:
: ~ .
' :, '
2 - ~33~
physiologically acceptahle acid addition salts
thereof with inorganic or organic acids may be incorporated,
optionally combined with other active substances,
with one or more inert conventional carriers and/or
diluents, e.g. with corn starch, lactose, glucose,
microcrystalline cellulose, magnesium stearate, ` ~-
polyvinylpyrrolidone, citric acia, tartaric acid,
water, water/ethanol, water/glycerol, water/sorbitol,
water/polyethyleneglycol, propyleneglycol, carboxymethyl~
cellulose or fatty substances such as hard at
or suitable mixtures thereof, to produce conventional
galenic preparations such as tablets, coated tablets,
capsules, powders, suspensians, drops, ampoules, -
syrups or suppositories.
Thus, in a further aspect the invention provides -~
a pharmaceutical composition comprising a compound
of formula I, or a physiologically acceptable acid
addition salt thereof, together with at least one
pharmaceutical carrier or excipient.
In a still further aspect, the invention provides
a method of treatment of the human or non-human body
to combat sinus tachycardia or ischaemic heart disease, `
said method comprising administering to said body a
compound of formula I or a physiologically acceptable
salt thereof.
In a yet further aspect the present invention provides
the use of a compound of formula I or a physiologically
acceptable salt thereof, for the preparation of
a therapeutic agent for use in the treatment of
sinus tachycardla or ischaemic heart disease.
The following Examples are intended to illustrate
the invention without restricting its scope in any
way.
:-
Unless otherwise stated, all ratios, parts and percentages
given herein are by weight.
' :'. . '
'..'., ''.".:' ."~
~, '.~ : ~`,'', ",` ~, '.? !?`
- 53 L33~993
ExamPle A
2-~Piperid-3-yl)-meth~1]-6~7-dimethoxy-1-oxo-1~2~3~4-
tetrahydro-isoquinoline
~ :
a) N-BenzYl-3-(hydrox~methyl~-piperidine
A mixture of 40O3 g (0.35 mol) of 3-(hydroxymethyl~
piperidine, 97.4 ml ~0.70 mol) of triethylamine
and 40.3 ml (0.35 mol) of benzyl chloride is heated
to 95C within 30 minutes and kept at this temperature
for 2 hours. The reaction mixture is cooled down
and dissolved in a mixture of 2 molar sodium hydroxide
solution and ethyl acetate. The organic phase ;~
is washed with water, separated off, dried over
magnesium sulphate and evaporated down in vacuo. -~
Yield: 57.2 g (79.6% of theory~
Rf value: 0.45 (aluminium oxide, neutral, eluant:
3% ethanol in methylene chloride).
b) N-Benzyl-3-(benzenesulphonyloxYmethyl)-piperidine
A mixture of 6.8 g ~0.033 mol) of N-benzyl-3-(hydroxy-
methyl)-piperidine, 6.7 ml (0.~52 mol) of benzenesulphonic
acid chloride, 50 ml of 20% aqueou~ sodium hydroxide
solution, 100 ml of toluene and 1 spatula tip of
tetrabutyl ammonium bromide is stirred for 3 hours
at ambient temperature. The mixture is then diluted
witn 250 ml of ethyl acetate and the organic phase
is washed with water. The organic phase is separated
off, dried over magnesium sulphate and evaporated
to dryness in vacuo. The residue is purified over ~ :~
200 g of silica gel (0.063 - 0.2 mm) with methylene
chloride and then with increasing amounts of ethanol
(up to 5%).
Yield: 9.4 g (92% of theory),
Rf value: 0.5 (silica gel, eluant: 5% ethanol in
methylene chloride). ~ ;
. .
, .
133~93
- 54 -
c~ 2-[(N-Benzy~piperid-3-yl)-methyll-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro-isoguinoline
5.2 g (0.025 mol) of 6,7-dimethoxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline are di~;solved in 70 ml
of dimethylsulphoxide and 3.1 cl ~0.025 mol) of
potassium tert.butoxide are added with stirring.
After half an hour, 9.3 9 (0.0275 mol) of N-benzyl-
3-(benzenesulphonyloxymethyl)-piperidine in 20 ml
of dimethylsulphoxide are added to the resulting
potassium salt suspension and the mixture is stirred
for 2 hours at 40~C. It is then poured onto ice
water and extracted three times, each time with
120 ml of ethyl acetate. The combined organic
phases are washed with water, dried over magnesium ~;;r.
sulphate and evaporated down in vacuo. The residue
obtained is purified over 200 g of silica gel (0.063 -
0.2 mm) with methylene chloride and then with increasing
amounts of ethanol (up to 2%). ;
Yield: 7.7 g (78.5% of theory),
R value: 0.5 (silica gel, eluant: 5% ethanol in
methylene chloride and 1 drop of ammonia).
d) 2-[tPiPerid-3-yl)-methyl]-6,7-dimethoxy-1-oxo-
1,2,3,4-tetrahYdro-isoquinoline
9.4 g (0.0238 mol) of 2-[(N-benzyl-piperid-3-yl)-
methyl]-~,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline are hydrogenated in 200 ml of methanol
in the presence of 2 g of 20% palladium hydroxide/charcoal
for 3 hours at ambient temperature under 5 bar
of hydrogen. The catalyst is then removed by suction
filtering and the filtrate is evaporated to dryness ~;~
in vacuo.
Yield: 7.2 g (99~ of theory),
Rf value: O.lS (silica gel, eluant: 10% ethanol
in methylene chloride and 1 drop of ammonia).
- , , "- .
~' ': ., ".
'"'"'`''.."'.'"-" "
: : :
~ 55 1 3 ~ a.~ ~3
Example B
".
3-Chloromethyl-N-[3-(naphthyl-2-oxY)-propyl~-Piperidine
a) 3-(HydroxYmethYl)-N-[3-(naphthY1-2-oxy)-propY~
piperidine ~ ;
A mixture of 11.5 g (0.2 mol) o~ 3-hydroxymethyl-
piperidine and 11 g of 2-(3-chloropropoxy1-naphthalene
is heated to 120C for 1 hour. The residue is ~ -~
purified over silica gel (0.063 - 0.2 mm) with -
ethyl acetate/ethanol/ammonia = 90:10:1.
Yield: 11.5 g (76.6~ of theory~
Melting point: 99-101C.
b) 3-Chloromethyl-N-[3-(naPhthyl-2-oxy)-propyl]-
~iperidine
- .
A solution of 1.5 9 (5 mmol) of 3-(hydroxymethyl)-
N-~3-(naphthyl-2-oxy)-propyl]-piperidine in 25 ml
of chloroform is mixed with 1.5 ml of thionyl chloride
and refluxed for 1~ hours. The mixture is evaporated ~ ~
to dryness ln vacuo. The residue is taken up in - -
methylene chloride, washed with water, 2 molar
sodium hydroxide solution and again with water.
After the methylene chloride phase has been dried
over magnesium sulphate it is evaporated down in vacuo.
Yield: 1.4 g (87.5% of theory),
Rf value: 0.8 (silica gel, eluant: ethyl acetate/ethanol/
ammonia = 90:40:2).
Example C
2-[(Hexahydro-azepin-3-yl)-methyl]-6,7-methylenedioxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline ;~
a) N-Benzyl--caprolactam ~ ~ -
''''. ~,
- 56 - i~3~3
33.9 g (0.3 mol) of caprolactam are dissolved in
250 ml of dimethylsulphoxide and 37 g (0.33 mol~
of potassium tert.butoxide are added with stirring.
The reaction temperature rises to 60C. It is -
stirred for half an hour at 60C and then 35 ml
(0.3 mol) of benzyl bromide are added dropwise. -
After another 2~ hours at 60C it is poured onto
1 litre of ice water and extr~cted three times
with ethyl acetate. The orgamic phases are combined,
washed with water, dried over magnesium sulphate
and evaporated down in vacuo.
Yield: 60.3 g (99% of theory),
Rf value: 0.6 (silica gel, eluant: 5% ethanol in
methylene chloride). -~
. .
b) l-Benzyl-caprolactam-3 carboxylic acid
::
180 ml of 2.6 molar butyllithium solution in n- -
hexane are added at -60C, with stirring and under
nitrogen, to 33.9 g = 47.1 ml (0.33 mol) of diisopropyl-
amine in 450 ml of absolute ether. Tben, while
cooling is continued, 48.8 g (0.24 mol) of N-benzyl-
caprolactam dissolved in 150 ml of absolute ether
are added dropwise. After stirring for 10 minutes,
the cooling bath is removed and carbon dioxide
is piped in for 15 minutes. The reaction mixture
is poured onto ice, the ethereal phase is separated
off and extracted twice with 2 molar sodium hydroxide
solution. The aqueous-alcoholic phases are combined, -
extracted with ether, acidified with concentrated ~
hydrochloric acid and extracted twice with methylene ~ `
chloride. The combined methylene chloride phases ~-
are dried over magnesium sulphate and the solvent
is distilled off in vacuo.
Yield: 15.7 g (26.5~ of theory),
IR spectrum (methylene chloride): 1735 and 1600 cm ~CO).
~3~993 ~
~ ,,
- 57 -
c) l-Benzyl-3-h5~droxYmethYl-hexahYdro-aZePine
14.8 g t0.06 mol) of 1-benzyl-caprolastam-3-carboxylic
acid dissolved in 300 ml of absolute tetrahydrofuran
are added drop~ise to 6.84 g (0.28 mol) of lithium
aluminium hydride in 300 ml of absolute tetrahydrofuran.
Then the mixture is refluxed for 6 hours, then
6.8 ml of water, 6.8 ml of 2 molar sodium hydroxide
solution and 21 ml of water are added whilst cooling
with ice water. The precipitate is suction filtered,
washed with tetrahydrofuran and the filtrate is
evaporated down in vacuo. ~he residue is purified
by column chromatography over aluminium oxide N
(activity II, eluant: methylene chloride~
Yield: 8.4 g (63.8% of theory),
IR spectrum (methylene chloride): 3620 cm 1 ~OH~
d) l-BenzY1-3-(4-toluenesulphonYloxymeth~l)-hexahydro-
azepine
, ~
15 9 (0.0684 mol) of 1-benzyl-3-hydroxymethyl-hexahydro-
azepine are dissolved in 150 ml of pyridine and
14.3 9 tn.o75 mol) of p-toluenesulphonic acid chloride
are added with stirring and the resulting mixture
is stirred for 1 hour at ambient temperature.
It is evaporated down in vacuo, taken up in methylene
chloride and washed with 2 molar sodium hydroxide
solution and water. After drying over magnesium
sulphate the organic phase is evaporated down in
vacuo
Yield- 23.3 9 t91.3~ of theory), `;~
Rf value: 0.45 (silica gel, eluant: 5~ ethanol
in methylene chloride).
e) 2-[(N-Benz~l-hexahydro-azepin-3-yl)-methyl]-
6,7-methylenedioxy-1-oxo-1,2 L3,4-tetrahydro~
isoquinoline ~`
- 58 - 133~9~3
7.4 g ~0.0387 mol) of 6,7-methyl0nedioxy-l-oxo-
1,2,3,4-tetrahydro-isoquinoline are dissolved in i r ;~
100 ml of dimethylsulphoxide, 4.5 g tO.04 mol)
of potassium tert.butoxide are added and the mixture
is stirred for half an hour at ambient temperature.
Then 16.8 9 (0.044 mol) of 1-benzyl-3-~4-toluenesulphonyl-
oxymethyl)-hexahydro-azepine are added and the
mixture is stirred for 3 hours at ambient temperature.
The reaction mixture is dissolved in ethyl acetate
and extracted twice with water. The organic phase
is dried over magnesium sulphate and evaporated
down in vacuo. The residue is purified over aluminium ~-
oxide N (activity II, eluant: methylene chloride,
methylene chloride + 2% ethanol).
Yield: 3.0 g (19.6~ of theory),
Rf value: 0.6 (silica gel, eluant: 5% ethanol in
methylene chloride~.
f) 2-[(HexahYdro-azepin-3~ methyl]-6,7-methylenedioxy- -
l-oxo-1,2,3,4-tetrahYdro-isoquinoline
6.7 g (0.017 mol) of 2-r(N-benzyl-hexahydro-azepin-
3-yl)-methyl]-6 r 7-methylenedioxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline are hydrogenated in 250 ml
of methanol in the presence of 2 g of 20~ palladium
hydroxide/charcoal for 4 hours at ambient temperature
under 5 bar of hydrogen. The catalyst is then
removed by suction filtering and the filtrate is ~ `
evaporated down ~o dryness in vacuo. The residue
is crystallised from acetone. ~ ~-
Yield: 4.9 g (95% of theory), ;~
M.p.: 267-269C.
Example D
~ . . .
3-Chloromethyl-N-[3-(naphthyl-2-oxy)-propyl~-hexahydro-
azepine
', ' ~.
' ~ "
'' '
~'' ',-'
~ 1 3 ~
-- 59 --
a) 3-Hydroxymethyl-hexahydro-azepine
16.6 g (0.0757 mol) of l-benzyl-3-hyaroxymeth
hexahydro-azepine are hydrogenated in 500 ml of
methanol in the presence of 16.6 g of 20~ palladium
hydroxide/charcoal for 2 hours at ambient temperature
under 5 bar of hydrogen. The catalyst is then
removed by suction filtering and the filtrate is
evaporated down _ vacuo.
Yield: 8 g (81.8~ of theory),
Rf value: 0.5 (silica gel, eluant: methylene chloride/-
ethanol/ammonia = 5:4:1).
b) 3-HYdrox~methYl-N-[3-rnaphthyl-2-oxy)-~ropyl]
hexahydro-azePine-hydrochloride
A mixture of 7.8 g (0.06 mol) of 3-hydroxymethyl-
hexahydro-azepine and 6.7 g (0.03 mol) of 2-(3-
chloropropoxy)-naphtbalene is heated for 1 hour
to 120~C. The reaction mixture is purified over
silica gel 10.063 - 0.2 mm) with ethyl acetate/ethanol/
ammonia 90:10:1.
Yield: 2.3 g (24.3% of theory),
Melting point: 127-129C. ;~
c) 3-Chloromethyl-N-[3-(naPh~ yl-2-oxy)-pr
hexahydro-azepine
- ..
2.4 g (6.86 mmol) of 3-hydroxymethyl-N-[3-(naphthyl-
2-oxy)-propyl~-hexahydro-azepine hydrochloride
dissolved in 30 ml of chloroform are mixed with
5 ml of thionyl chloride and refluxed for 1 hour.
The mixture is evaporated to dryness in vacuo. ~`~
The residue is dissolved in methylene chloride,
and extracted with water, 2 molar sodium hydroxide
solution ancl again with water. The methylene chloride ;`~
phase is dried over magnesium sulphate and evaporated
down in vacuo.
- 60 - ~3~ 3
Yield: 1.1 g ~48.5~ o theory~,
Rf value: 0.55 (silica gel, eluant: 5% ethanol
in methylene chloride).
Example E
2-[(Pyrrolid-3-yl)-methyl]-6,7-dimethoxy-1-oxo-
1,?,3,4-~etrahydro-isoquinoline
-
a) N-Benzyl-2-pyrroliaone
~ .
14.4 9 (0.33 mol) of 50% sodium hydride dispersion
in oil is added in batches to 25.5 g (0.3 mol) ~ ;
of 2-pyrrolidone in 300 ml of absolute dimethylsulphoxide.
The mixture is then stirred for 5 hours at 40 to
50C and, at 25 to 30C, 56.4 g = 39.2 ml (0.33 mol) :
of benzyl bromide are added dropwise. After stirring
for 10 hours at ambient temperature the reaction
mixture is dissolved in S00 ml of ethyl acetate
and extracted several times with water. The organic
phase is separated off, dried over magnesium sulphate
and the solvent is eliminated in vacuo. The residue -~ `
obtained is purified over 900 g of aluminium oxide ~-
(neutral, activity II) with methylene chloride
and 0.1% ethanol.
Yield: 35.6 g (67.7~ of theory),
Rf value: 0.77 ~aluminium oxide, neutral, eluant:
5% ethanol in methylene chloride).
b) N-Benzyl-2-pyrrolidone-3-carboxylic acid
lS0 ml of 1.6 molar butyllithium solution in n-
hexane are added with stirring and under nitrogen
at -60C to 28.3 g = 39.3 ml ~0.28 mol) of diisopropyl-
amine in 400 ml of absolute ether. 35.1 g ~0.2 mol)
of N-benzyl--2-pyrrolidone dissolved in lS0 ml of
absolute ether are added dropwise thereto at -60C.
" ~
~33~3
- 61 -
The cooling bath is removed and dry carbon dioxide
is introduced for 15 minutes. After stirring for
10 minutes, the mixture is poured onto ice, the
organic phase is separated off and extracted twice
with 2 molar sodium hydroxide solution. The combined
aqueous phases are extracted once with ether and
then acidified with concentrated hydrochloric acid
whilst being cooled. The aqueous phase is extracted
twice with methylene chloride and ater the organic
phase has been dried over magnesium sulphate it
is evapora~ed down in vacuo.
Yield: 35 g (79.8~ of theory),
Rf value: 0.42 (silica gel, eluant: 5~ ethanol
in methylene chloride).
c) N-Benzyl-3-hydroxymethyl-pyrrolidine
35 g (0.16 mol) of N-benzyl-2-pyrrolidone-3-carboxylic
acid dissolved in 250 ml of absolute tetrahydrofuran
are added dropwise, with stirring to 12.2 g (0.32 mol)
of lithium aluminium hydride in 350 ml of absolute
tetrahydrofuran. After refluxing for 6 hours,
18.2 ml of water, 12.2 ml of 15% aqueous sodium
hydroxide solution and 36.6 ml of water are added
whilst cooling with ice water. The precipitate
formed is suction filtered and washed with tetrahydrofuran.
The combined filtrates are evaporated down i vacuo
and the residue obtained is purified over 900 9
of aluminium oxide (neutral, activity II) with
methylene chloride and then with increasing amounts
of ethanol (up to 2~
Yield: 16 g (52.3% of theory),
Rf value: 0.42 (aluminium oxide, neutral, eluant:
5% ethanol in methylene chloride).
d) 3-(Benzenesulphonyloxymethyl)-l-benzyl-pyrrolidine
'~ ~
- 62 - 133~
40 ml of 20~ sodium hydroxide solution are added
dropwise, for a period of 1 hour, to a mixture
of 3.8 g (20 mmol) of N-benzyl-3-hydroxymethyl-
pyrrolidine and 3.7 ml (24 mmol) of benzenesulphonic
acid chloride. 150 ml of toluene are added, the
organic phase is washed with water, dried over
magnesium sulphate and evaporated to dryness in
vacuo.
Yield: 5.9 g (89.4% of theory), ;~
Rf value: 0.4 (silica gel, eluant: 5% ethanol in
methylene chloride).
. . .:. :-
-~ . . :. :.
e) 2-[N-(Benzyl-pyrrolid-3-yl)-methyl~-6,7-dimethoXV- -~
l-oxo-1,2,3,4-tetrahydro-isoquinoline - ~ ~
~...-, ~.. ..
3.3 g (15.9 mmol) of 6,7-dimethoxy-1-oxo-1,2,3,4
tetrahydro-isoquinoline are dissolved in 50 ml
of dimethylsulphoxide and combined with 2 q (17.5 mmol)
of potassium tert.butoxide with stirring at ambient
temperature. After half an hour, 5.8 g (17.5 mmol)
of 3-(benzenesulphonyloxymethyl)-1-benzyl-pyrrolidine
in 10 ml of dimethylsulphoxide are added to the -
resulting potassium salt suspension and the mixture
is stirred for 3 hours at 60C. It is poured onto
ice water and extracted with ethyl acetate. The
combined organic phases are washed with water and -
dried over magnesium sulphate. The organic phase ~ ;~
is evaporated down in vacuo and the residue obtained
is purified over 350 9 of aluminium oxide N (activity II) `~
with methylene chloride and then wi~h increasing
.
quantities of ethanol (up to 1%).
Yield: 3.3 g (54.4% of theory),
Rf value: 0.74 raluminium oxide N, eluant: 5% ethanol
in methylene chloride).
f) 2-~(Pyrrolid-3-yl)-methyl]-6,7-dimethoxy-1-oxo-
1,2,3,4-tetrahYdro-isoquinoline
1 3 ~ r9 ~3
.
- 63 -
3.2 9 (8.4 mmol) of 2-[(N-benzyl-pyrrolid~3-yl)-
methyl]-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline are dissolved in 250 ml of methanol
and hydrogenated in the presence of 1 g of 20%
palladium hydroxide/charcoal for 3 hours at ambient
temperature under 5 bar of hydrogen. The catalyst
is then removed by suction filtering and the filtrate
is evaporated to dryness in vacuo. The residue
is purified over 150 g of silica gel (0.063 - 0.2 mm)
with methylene chloride~ethanol/ammonia = 6:1:0.5.
Yield: 0.9 g (37.5~ of theory),
Rf value: 0.6 (silica gel, eluant: methylene chloride/-
ethanol/ammonia = 5:4:1).
Example F
3-(p-ToluenesulphonYloxymethyl)-N-[2-~6-methoxy- ;~
naphth-2-yl)-ethyl]-pyrrolidine
.
a) 3-Hydroxymethyl-pyrrolidine ;~
14 g (0.073 mol) of N-benzyl-3-hydroxymethyl-pyrrolidine
are hydrogenated for 7 hours at 50C and under
5 bar in 300 ml of methanol and in the presence
of 1.5 g of 20% palladium hydroxide/activated charcoal.
The catalyst is then removed by suction filtering
and the filtrate is evaporated down in vacuo.
Yield: 7.3 g (99% of theory3,
Mass spectrum: molecular peak 101.
b) 3-HYdroxYmethyl-N-[2-(6-methoxy-naPhth-2-yl)
ethyl]-pyrrolidine
A mixture of 3.6 g (29.4 mmol) of 3-hydroxymethyl-
pyrrolidine and 4.7 g (14.7 mmol) of 2-(2-bromoethyl3-
6-methoxy-naphthalene is heated to 120C for 2
hours. The reaction mixture is purified over 200 g
" ~ ~ . . . ; ,. . ~ .
- 64 -
of silica gel (0.063 - 0.2 mm) with methylene chloride
and then with increasing amounts of ethanol (up ~ -
to 5%).
Yield: 3.48 g (82.5% of theory), -~
Melting point: 121-123C.
.:
c) 3~ ToluenesulphonYloxymethyl)-N-[2-(6-methoxy- -~
naphth-2-yl)-eth~l]-pyrrolidine
0.8 g (2.8 mmol) of 3-hydroxymethyl-N-[2-(6-methoxy-
naphth-2-yl)-ethyl]-pyrrolidine are dissolved in ~ -
10 ml of pyridine and 1.2 g (6.3 mmol) of p-toluene-
sulphonic acid chloride are added with stirring.
After 2 hours at ambient temperature the mixture
is evaporated to dryness in vacuo. The residue
is dissolved in methylene chloride, washed with
2 molar sodium hydroxide solution and water. The ~-
organic phase is then dried over magnesium sulphate
and evaporated down in vacuo. The residue is purified
over 150 g of silica gel (0.063 - 0.2 mm) with
ethyl acetate and then with increasing amounts ;
of ethanol. `
Yield: 0.6 g (48.8~ of theory),
Rf value: 0.45 (~ilica gel, eluant: 5% ethanol
in methylene chloride). ~ ~
, ~ . .
' ': . '
~ . r~
- 65 -
Example G ~ 3 3 ~ 9 9 ~
~.
2-[(Azacyclooctyl-3)-methyl]-6!7-dimethoxy-1-oxo-
1,2,3,4-tetrahYdro-isoquinoline
a) l-Benzyl-2-oxo-azacyclooctane ~ ;
25 9 (0.196 mol) of 2-azacyclooctanone are dissolved
in 150 ml of dimethylsulphoxide and combined, with
stirring, with 24.2 g (0.216 mol3 of potassium
tert.butoxide and stirred for half an hour at 40C.
Then 24 ml (0.2 mol) of benzylbromide are added
dropwise over a period of a quarter of an hour,
during which time the temperature rises to 80C.
The mixture is stirred for 2 hours, during which
the reaction temperature falls back to ambient ~;
temperature. The reaction mixture is poured onto
1 litre of ice water and extracted 4 times, each
time with 150 ml of ethyl acetate. The combined -
organic phase is washed with water, dried over
magnesium sulphate and evaporated down in vacuo.
Yield: 42.7 9 (100% of theory),
Rf value: 0.55 (silica gel, eluant: 5% ethanol
in methylene chloride).
~ .. . .
b) l-Benzyl-azacylooctane-2-oxo-3-carboxylic acid
:: ~
147 ml of 1.6 molar butyl lithium solution in n- ~;-r .
hexane are added dropwise at -60C, with stirring
and under nitrogen, to 26.7 9 = 38.4 ml (0.26 mol)
of diisopropylamine in 250 ml of absolute ether.
Then at -60C, 42.7 g (0.196 mol) of 1-benzyl-2-
oxo-azacyclooctane in 100 ml of ahsolute ether
were added dropwise thereto. After 10 minutes,
dry carbon dl~oxide was introduced for 20 minutes. ;
The reaction mixture is poured onto ice, the ethereal
phase is separated off and extracted twice with
13~93
.. `~.`. . ;~
- 66 -
2 molar sodium hydroxide solution. The combined ;
aqueous phases are extracted once with ether and
then acidified with concentrated hydrochloric acid,
whilst being cooled. The mixture was extracted ~ -
3 times with methylene chloride, and the methylene
chloride phase is dried over magnesium sulphate ~
and evaporated down in vacuo. -
Yield: 25.9 g ~50.6% of theory),
Rf value: O.lS (aLuminium oxide, eluant: 5% ethanol
in methylene chloride~
c) l-Benzyl-3-hydroxYmethyl-azacyclooctane
,
53.6 g (0.205 mol) of 1-benzyl-2-oxo-azacyclooctane-
3-carboxylic acid dissolved in 100 ml of absolute
tetrahydrofuran is added dropwise with stirring
to 22.7 g (0.6 mol) of lithium aluminium hydride `~
in 800 ml of absolute ether. After refluxing for
half an hour, the mixture is combined with 28.4 ml
of water, 19 ml of 15% sodium hydroxide solution
and 57 ml of water, whilst cooling with ice water.
The precipitate formed is suction filtered and
washed with tetrahydrofuran. The combined filtrates
are evaporated down in vacuo and the residue obtained ~ ;
is purified over 700 g of aluminium oxide (neutral,
activity II) with 1% ethanol in methylene chloride. -~
Yield: 9.3 g (19.6% of theory),
Rf value: 0.5 (silica gel~ eluant: 5% ethanol in
methylene chloride). ;- ~-
d) l-Benzyl-3-chloromethvl-azacYclooctane
9.3 g (39.8 mmol) of l-benzyl-3-hydroxymethyl-azacyclo
octane are combined in 30 ml of pyridine with 10 ml ~ ;
(79.6 mmol3 of benzenesulphonic acid chloride and
stirred for 2~ hours at ambient temperature. The
reaction mixture is evaporated down in vacuo.
~33~993
- 67 -
The residue remaining is dissolved in 150 ml of
methylene chloride, then washed with 2N sodium
hydroxide solution and water. ~he organic phase
is dried over magnesium sulphate, evaporated to
dryness and purified over 150 g of silica gel (0.063-
0.2 mm) with methylene chloride.
Yield: 3.5 g (35~ of theory)
Rf value: 0.75 (silica gel, eluant: 5% ethanol
in methylene chloride).
. ,
e) 2-[~N-Benzyl-azacyclooct-3-~ methyl]-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline
2.3 g (11.1 mmol) of 6,7-dimethoxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline are dissolved in 40 ml
of dimethylsulphoxide and 1.33 g (12.2 mmol) of
potassium tert.butoxide are added with stirring.
After half an hour, 3.5 g (9.4 mmol) of l-benzyl-
3-chloromethyl-azacyclooctane in 40 ml of dimethylsulphoxide
are added to the resulting potassium salt suspension
and the mixture is stirred for 2~ hours at 120C. ;~
It is poured onto ice water and extracted 3 times,
each time with 50 ml of ethyl acetate. The combined
organic phases are washed with water, dried over
magnesium sulphate and evaporated down in vacuo.
The residue obtained is purified over 150 g of
silica gel (0.063-0.2 mm) with 1% ethanol in methylene
chloride.
.::
Yield: 1 9 (25.1~ of theory),
Rf value: 0.5 (silica gel, eluant: 2% ethanol in ~ -
methylene chloride).
.
f) 2-~(Azacyclooct-3-yl)-methyl]-6,7-dimethoxy- ~ ~
l-oxo~1,2,3,4-tetrahydro-isoquinoline ,
0.85 9 (2 mmol) of 2-[(N-benzyl-azacyclooctyl-3)-
methyl]-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro- ~
.:
~ ~3~993
- 68 -
isoquinoline are hydrogenated in 50 ml o methanol
in the presence of 0.85 g of 20~ palladium hydroxide/char~
coal for 4l hours at ambient temperature under
5 bar of hydrogen. The catalyst is then removed
by suction filtering and the f;ltrate is evaporated
to dryness.
Yield: 0.5g (74.6% of theory),
Rf value: 0.45 (silica gel, eluant: 25~ ethanol
in methylene chloride and 1 drop of ammonia)
ExamPle H
l-Chloro-3-1N-(4-methoxy-phenyl)-methylamino]-propane
:
10 g (0.073 mol) of N-methyl-4-methoxy-aniline
are dissolved in 50 ml of dimethylsulphoxide and
9 g (0.08 mol) of potassium tert.butoxide are added
with stirring. After haLf an hour, 10 ml of 1~
bromo-3-chloropropane are added and the mixture
is stirred for 3 hours at ambient temperature. `
It is poured onto ice water, extracted with ethyl ~ ~
acetate, the organic phase is ~ashed with water, ~ ~ ;
dried over sodium sulphate and evaporated to dryness.
The residue is purified over silica gel (0.063 - 0.2 mm)
with methylene chloride.
Yield: 9.1 g (58.3% of theory),
Rf value: 0.55 (silica gel, eluant: methylethyl
ketone/xylene = 1:6)
Calculated: C 61.82H 7.55 N 6.55 Cl 16.59
Found: 61.71 7.88 6.69 16.24.
.. ::
Example I -~
2-[(N-(3-(Pyrid-3-yl)-propvl)-piperid-3-yl)-methYl]-
5,6-methylenedloxy-phthalimide ~ ;
~';.'"'''.`''''`''~'''''
..~',': ' ' ',- "'.
13~0~93
- 69 -
2.1 g ~0.011 mol) of 5,6-methylenedioxy-phthalimide
are dissolved in 100 ml of dimethylsulphoxide and
1.25 g (0.012 mol) of potassium tert.butoxide are
added with stirring. The potassium salt is precipitated.
It is stirred for a further ~ hour at ambient temperature, ;-
a solution of 2.5 g (0.01 mol) of 3-chloromethyl-
N- (3-(pyrid-3 yl)-propyl)-piperidine in 20 ml of
dimethylsulphoxide is added and the mixture is
heated to 120C for 8 hours. It is poured onto
ice water, extracted three times, each time with
150 ml of ethyl acetate, and after drying over
magnesium sulphate, the organic phase is evaporated ;~
down in vacuo. The residue is purified over 200 g
of silica gel (0.063 - 0.2 mm) with ethyl acetate/ethanol/
~ammonia = 80:10:0.5.
Yield: 3 g (74% of theory),
Calc. (2 x HCl) C 55.42 H 5.86 N 8.43Cl 14.22
Found: 55.28 6.06 8.2614.64
Example K
2-[N-(3-Chloropropyl)-piperid-3-yl-methYl]-6,7-
dimethoxy-l-oxo-1,2,3,4-tetrahydro-isoc~noline
3 g (Q.01 mol) of 2-[(piperid-3-yl)-methyl]-6,7-
dimethoxy-l-oxo-1,2,3,4-tetrahydro-isoquinoline
are dissolved in 50 ml of dimethylsulphoxide and
1.3 g (0.011 mol) of potassium tert.butoxide are
added with stirring. After half an hour, 3 mI
of l-bromo-3-chloro-propane are added and the mixture
is stirred for 1 hour at ambient temperature.
It is pourecl into ice water, extracted with ethyl
acetate, and the organic phase is washed with water,
dried over sodium sulphate and evaporated to dryness
in vacuo. ;
Yield: 2.7 g (71% of theory),
Rf value: 0065 (silica gel, eluant: ethyl acetate/ethanol/
ammon~a = 50:45:5).
.' ,. ' '.'.
` ~ 13~ 3
- 70 -
Example 1
2-[(N-(3-(Naphth-2-vl)-proP~ piperid-3-yl)-meth
6,7-dimethoxv-1-oxo-1,2,3,4-tetrahydro-isoquinoline-
hydrochloride ~-
A mixture of 1 g (3.2 mmol) of 2-(piperid-3-yl-
methyl)-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline, 5 ml of dimethylsulphoxide, 0.5 g
(0.36 mmol) of potassium carbonate and 0.75 g (3.66 mmol)
of 2-(3-chloropropyl)-naphthalene is heated to
120C for 3 hours. The reaction mixture is poured
onto ice water and extracted three times, each
time with 50 ml of ethyl acetate. The combined
organic phases are washed with 2 molar sodium hydroxide
solution and water, dried ~ver magnesium sulphate,
evaporated down ln vacuo and the residue obtained
is purified over silica gel (0.063 - 0.2 mm) with
1% ethanol in methylene chloride. The hydrochloride
is precipitated from a solution in acetone with
ethereal hydrochloric acid and recrystallised from
acetone.
Yield: 0.74 g (44% of theory),
Melting point: 179-181C
Calculated: C 70.77 H 7.37 N 5.50 Cl 6.96
Found: 70.47 7.40 5.47 7.06. ~-~
Example 2
2-[(N-(3-(NaphthY1-2-oxy) -pro~yl)-piperid-3-yl)-
methyl]-6,7-dimethyl-1-oxo-1,2,3,4-tetrahYdro-isoquinoline- ~ -
..... ...
hydrochloride-monohydrate ;~
1.58 g (9 mmol) of 6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-
isoquinoline are dissolved in 30 ml of dimethylsulphoxide
and 1.1 g (9.9 mmol) of potassium tert.butoxide
are added with stirring. After 1 hour, a solution
~, ,~. ..:
' ' "' ' ' ' ~ ~
3 3 ~ 9 9 D j~
- 71 -
of 2.9 g (9.1 mmol) of 3-chloromethyl-N-[3-(naphthyl-
2-oxy)-propyl]-piperidine in 10 ml of dimethylsulphoxide
is added and the reaction mixture is stirred for
16 hours at 80C. It is then poured onto ice water,
extracted 3 times, each time with 50 ml of ethyl
acetate, the organic phase is washed with water/
dried over magnesium sulphate and, after evaporation,
purified on silica gel (0.63 - 0.2 mm~ with ethyl
acetate/ethanol/ammonia = 95:5:0.5. The hydrochloride
is obtained as a hydrate from a solution in acetone
using ethereal hydrochloric acid.
Yield: 2 g ~45.1% of theory),
Melting point: 152-154C
Calculated: C 70.50 H 7.69 N 5.49 Cl 6.93
Found: 70.31 7.52 5.49 7.10
Example 3
2-[(N-~3-~Naphth-2-yl)-propyl)-piperid-3-yl)-methYl]-
6,7-dimethyl-1,2,3,4-tetrahYdro-isoquinoline-dihYdro-
chloride
0.8 g (18 mmol) of 2-[~N-(3-(naphth-2-yl)-propyl)-
piperid-3-yl)-methyl]-6,7-dimethyl-1-oxo-1,2,3,4-
tetrahydro-isoquinoline are dissolved in 10 ml
of absolute tetrahydrofuran and 20 ml of absolute
ether, 70 mg (18 mmol) of lithium aluminium hydride
are added and the mixture is refluxed for 1 hour.
The reaction mixture is decomposed by the addition
of 5 ml of satura~ed aqueous sodium sulphate solution,
filtered to remove the sodium sulphate precipitated
and washed with tetrahydrofuran. The filtrate
is dried over magnesium sulphate and after evaporation,
purified over 150 g of silica gel (0.063 - 0.2 mm)
with ethyl acetate/ethanol/ammonia = 90:10:0.05.
The hydrochloride is precipitated from a solution
in acetone with ethereal hydrochloric acid.
i, :., .,<,~ t~jt~,
` ` 133~3
- 72 -
Yield: 0.49 9 ~54.4~ o~ theory),
Melting point: 148-150C
Calculated: C 69.61 H 8.18 N 5.41 Cl 13.70
Found: 69.46 8.32 5.26 14.17
Example 4
... ..
2-[(N-(3-(Pyrid-3-yl)-propyl)-piperid-3-yl)-meth_l]-
5,6-methylenedioxy-1-oxo-1,3-dihydro-isoindole-
dih~drochloride
2.4 9 (S.9 mmol) of 2-[(N-(3-(pyrid- 3-yl)-propyl)-
piperid-3-yl)-methyl]-5,6-methylenedioxy-phthalimide
are dissolved in S0 ml of glacial acetic acid and
refluxed for 5 hours. At intervals of 1 hour,
1 9 batches of zinc powder are added. After the
reaction time has ended, the mixture is suction
filtered and evaporated with ethanol. The residue
is dissolved in methylene chloride, extracted with
concentrated ammonia, dried over magnesium sulphate
and after evaporat;on in vacuo purified over 150 g
of silica gel (0.063 - 0.2 mm) ethyl acetate/ethanol/-
ammonia = 90:10:0.2. The hydrochloride is precipitated
from a solu~ion in acetone. ;~
Yield: 2.05 9 (75~ of theory),
Melting point: 165-167C
Calculated: C 59.22 H 6.27N 9.00Cl 15.20 -~
Found: 59.03 6.45 8.85 15.06
.:."". ",,. ,~
ExamPle 5
3-~(N-~3-~Pyrid-3-yl)-propyl)-pyrrolid-3-Yl)-methyl]-
7,8-d~methoxy-2-oxo-1,3,4,5-tetrahydro-2H-3-benzoazepine~
dihydrochloride
1.1 9 (2.6 mmol) of 3-[(N-(3-(pyrid-3-yl)-propyl)-
pyrrolid-3-yl)-methyl]-7,8-dimethoxy-2-oxo-1,3-
`~ ~3~93
-73- 27169-147
dihydro-2H-3-benzazepine, dissolved in 50 ml of ethanol, are ~ ~
hydrogenated in the presence of 1 g oE 10~ palladium on activated ~ -
charcoal at 80C and under 5 bar of hydrogen for 2 hours. Then
the catalyst is removed by suction filtering and, after being
evaporated down in vacuo, ~he filtratle is purified over 100 g of
silica gel (0.063 - 0.2 mm) with ethyl acetate/ethanol/ammonla =
80:40:1. The hydrochloride is precipitated from a solution in
acetone.
Yield: 0.37 g (33% of theory),
Meltlng point: 96-98C
Calculated: C 60.42 H 7.11 N 8.46 C1 14.28
Found: 60.35 7.468. 4314 . 58
Example 6
3-l(N-( 3- ( Indol-3-Yl)-propyl) -hexahydro-azePin-
3-vl)-methvll-7,8-dimethoxy-1,3,4,5-tetrahvdro-
2H-3-benzazepine-dihvdrochloride-monohydrate
Prepared from 3-[(N-(3-(indol-3-yl)-propyl)-hexahydro-
azepin-3-yl)-methyl]-7,8-dimethoxy-2-oxo-1, 3, 4, 5-
tetrahydro-2H-3-benzazepine and lithium aluminium
hydride in tetrahydrofuran and diethylether analogously
to Example 3.
Yield: 53% of theory, ' ~
Melting point: 158-160C
Calculated: C 63.58 H 8.00N 7.41 C1 12.51
Found: 63.41 8.18 7.36 12.23
~,. ~. ~
:
~ ~L33~9~3
2 7 1 6 9 1 ~L 7
~ 74 -
' ~ ~
.
Example 7
.
2-t(N-~2-~4-Amino-phenyl)-ethyl)-piperld-3-yl)-
methyl]-5,6-dlmethoxY-l-oxo-1,3-dihydro-isoindole- i
dlhyarochloriae
2.1 g t4.78 mmol) of 2-[~N-~2-14-nitro-phenyl)-
ethyl~-piperld-3-yl~-methyl]-5,6-dlmethoxy-1-oxo-
1,3-dlhydro-l~oindole are dissolved in 50 ml of
glaclal acetic acid and 0.4 ml ~8.22 mmol) of hydrazine
hydrate and 1 spatula ~lp of Raney nickel are added
with ~tirring. The addition of 0.2 ml of hydrazine ~ -
hydrate ~nd 1 spatula tlp of Raney nickel i~ repeated
3 time~ ~t interval~ of 1 hour. The cat~lyst i8 .
removed by ~uction filtering, the residue is wa~hed `~
with methanol, the filtrate is dried with magne~ium ~ ~
~ulphate, evaporated down in vacuo and the re~idue ~ `
obtained i9 puriied over aluminium oxide ~neutral, - ~
actlvlty II~ with methylene chloride and then wlth ~ -
inareasing quantitles of ethanol.
yield: 1.8 9 ~91. 8% of theory),
1 9 18 di~olved ln ac~tone and the dlhydrochloride
precipltated with ethereal hydrochlorlc ~cid.
Yleld: 1.02 9 (86.4~ of theory based on the base),
Melting point: 232-235DC
Calculated:C 59.72 H 6.89 N 8.71 Cl 14.69
Found:59.54 7.08 8 56 14.45
,
ExamPle 8 ~
; ~ . .
2-ttN-~2-(4_Acetamino PhenYl)-ethyl)-plperid-3~
Yl)-methyll~5r6-dimethoxy-l-oxo-l~3-dihydro-lsoindole ~i
'.:;
133~
- 75 -
819 mg (2 mmol) of 2-[(N-(2-~4-amino-phenyl~-ethyl)-
piperid-3-yl)-methyll-5,6-dimethoxy-1-oxo-1,3-dihydro- ~ -
isoindole are mixed with 10 ml of methylene chloride
and after the addition of 0.3 ml ~2.2 mmol) of
triethylamine, 0.16 ml ~2.2 mmol) of acetyl chloride
are added dropwise. The reaction temperature rises
to 30C. The mixture is stirred for half an hour
at ambient temperature, extracted twice with water,
the organic phase is dried over magnesium sulphate
and evaporated down in vacuo. The residue is crystallised
from acetone.
Yield: 660 mg (73.2% of theory),
M.p.: 195-196C
Calculated: C 69.16 ~1 7.37 N 9.31
Found: 69.33 7.11 9.16
ExamPle 9
3-[(N-~3-(FurYl-2)-propyl~-piPerid-3-yl)-methYl~-
7,8-dimethoxy-2-oxo-1,3,4,5-tetrahYdro-2H-3-benzazepine~
hydrochloride
3.2 g (0.010 mol) of 3-[(piperid-3-yl)-methyl]-
7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine
are hydrogenated in 100 ml of absolute ethanol
in the presence of 1.3 g (0.010 mol) of 3-(furyl-2)-
propanal and 1 9 of Raney nickel at 80C for 2
days under 5 bar. The catalyst is removed by suction -
filtering, the filtrate is evaporated down and
purified over a silica gel column with methylene
chloride/methanol as eluant. The hydrochloride
is precipitated with e~hereal hydrochloric acid
and crystallised from acetone.
Yield: 0.50 g (11% of theory~
Melting point: 204-206C
Calculated: C 64.85 H 7.62 N 6.05 Cl 7.66
Found: 64.88 7.76 5.93 7.55
-
^; ~L330~3
- 76 -
Rf value: 0.69 (silica gel; methylene chloride/methanol =
10:1; ammonia/atmosphere~
::
Example 10
2-~(N-(3~(3-Methylphenoxy)-prop~l)-piperid-3-yl)- ~
methyl]-6,7-dimethoxy-1,2,3,4-tetrahydro-isoquinoline- ~ ~;
dihydrochloride -
Prepared from 2-1(N-t3-(3-methylphenoxy~-propyl)-
piperid-3-yl)-methyl]-6,7-dimethoxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline and lithium aluminium hydride
in diethylether and tetrahydrofuran analogously -~
to Example 3.
Yield 92.9% of theory, ;~
Melting point: 100-103C ~-
Calculated: C 61.23 H 7.99 N 5.29 Cl 13.39
Found: 61.21 8.13 5.10 13.15
Example 11
2-[(N-(3-(NaPhthYl-2-oxy)-propyl)-pyrrolid-3-yl)-
methyl]-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline~hydrochloride
Prepared from 6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline and 3-chloromethyl-N-[3-(naphthyl-
2-oxy)-propyl]-pyrrolidine analogously to Example 2.
Yield: 22% of theory, ~
Melting point: 78-80C ~ -
Calc. (x H2O): C 65.83 H 7.04 N 5.29 Cl 6.70 ~
Found: 65.79 7.00 5.03 6.99 ~;
~ ~33~3
-- 77 --
Example 12 ~-
2-[(N-(3-(NaPhthyl-2-oxY~eropYl)-pyrrolid-3-Yl)-
methYl]-6~7-methYlenedioxy-l-oxo-l~2l3~4-tetrahydr
isoquinoline-hydrochloride
. :
Prepared from 6,7-methylenedioxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline and 3-chloromethyl-N-E3-
(naphthyl-2-oxy)-propyl~-pyrrolidine analogously ~~
to Example 2
Yield: 53% of theory,
Melting point: 78-80C
Calc. (x H2O): C 65.56H 6.48N 5.46 Cl 6.91
Found: 65.44 6.32 5.38 7.13
Example 13 -
2-[(N-(2-(Naphth-2-yl)-ethYl)-p~rrolid-3-yl)-methyl]- It~
6,7-dimethoxy-1,2,3,4-tetrahydro-isoquinoline-hYdrochloride'~':
Prepared from 2-r(N-(2-(naphth-2-yl)-ethyl)-pyrrolid-
3-yl)-methyl]-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline and lithium aluminium hydride in tetrahydro-
furan and ether analogously to Example 3.
Yield: 66.2% of theory,
Melting point: 239-241C
Calc. (x H2O): C 64.48 H 7.44 N 5.37 Cl 13.91
Found: 64.30 7.34 5.52 13.69
~: ;
Example 14
2-[(N-((2-~Methyl-naphth-l-yl)-methyl)-hexahydro-
azepin-3-yl)-methyl]-6,7-dimethoxy-1,2,3,4-tetrahYdro-
isoquinoline-hydrochloride
Prepared from 2-[(N-((2-(methyl-naphth-1-yl)-methyl)-
hexahydro-a~epin-3-yl)-methyl]-6,7-dimethoxy-1-
.. .. - ~..-:
,. :' ~,
133~993
- 78 -
oxo-1,2,3,4-tetrahydro-isoquinoline and lithium
aluminium hydride in tetrahydrofuran and ether
analogously to Example 3.
Yield: 73.8~ of theory,
Melting point: 182-184C
Calc. ~x H~O): C 65.56 H 7.70 N 5.09 Cl 12.90
Found: 65.52 7.57 5.32 12.72
Example 15
.. ~ ,:,. : .:
2-[(N-(4-(NaphthYl-2-oxy)-butyl)-pyrrolid-3-yl)-
methYl]-6l7-dimethyl-l-oxo-l~2l3~4-tetrahydro-isoquinoline
.
hYdrochloride
Prepared from 2-(pyrrolid-3-yl-methyl)-6,7-dimethyl-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and 2-(4- ~ ~
bromo-butyloxy)-naphthalene analogously to Example 1 -
Yield: 30% of theory,
Melting point: 133-136C
Calculated: C 67.02 H 6.92 N 5.21 Cl 14.86
Found: 67.26 7.03 5.36 14.89
Example 16
~. ...
2-[(N-(2-Methyl-naphth-l-yl)-methyl)-pyrrolid-3- :.
yl)-methYl]-6~7-dimethyl-l-oxo-l~2~3r4-tetrahydro-
isoquinoline-hYdrochloride r
Prepared from 2-(pyrrolid-3-yl-methyl)-6,7-dimethyl-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and l-chloromethyl-
2-methyl-naphthalene analogously to Example 1.
Yield: 32.5~ of theory,
Melting point: 142-144C
Calculated: C 69.34 H 7.69 N 5.77 Cl 7.37
Found: 69.59 7.63 5.72 7.89
Example 17
`
33~93
,9
2-[(N-(2-t5-MethyL-6-methoxy-naphth-2-yl~-ethYl~-
pyrrolid-3-yl)-methyl]-6~7-dimethyl-l-oxo-l~2~3~4
tetrahydro-isoquinoline-hydrobromide
Prepared from 2-[N-(pyrrolid-3-yl-methyl)]-6,7-
aimethyl-l-oxo-1,2,3,4-tetrahydro-isoquinoline
and 2-t2-bromoethyl)-5-methyl-6-methoxy-naphthalene -~
analogously to Example 1.
Yield: 19% of theory,
Melting point: 230-232C
Calculated: C 67.02 H 6.93 N 5.21 Br 14.86 ;~
Found: 67.10 7.12 5.33 15.01
ExamPle 18
.
2-[~N-(2-(Naphth-2-Yl)-ethyl)-p ~rolid-3-yl)-methyl]-
6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-isoquinoline-
hydrochloride
Prepared from 2-(pyrrolid-3-yl-methyl)-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and 2-(2-
bromoethyl)-naphthalene analogously to Example 1.
Yield: 7.8~ of theory,
Melting point: 219-221C -
Calc. (x H2O): C 67.39 H 7.07 N 5.61 Cl 7.10
Found: 67.21 7.23 5.57 7.63
Example 19
2-[(N-~2-(6-Methoxy-naphth-2-yl)-ethyl)-pYrrolid-
3-yl)-methyl]-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahYdro- -
isoquinoline-hydrochloride
Prepared from 2-((pyrrolid-3-yl)-methyl)-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and 2-(2-
bromoethyl)-6-methoxy-naphthalene analogously to
Example 1.
'"'~;"..'' " ..
: ~ ... .. .
1 ~0~3 ~ Y
- 80
Yield: 39.2~ of theory,
Melting point: 224-226c
Calc. (x H2O): C 65.84 H 7.05N 5.29 Cl 7.05
Found: 66.08 7.1~ 5.39 6.77
:,
Example 20
.
2-L(N-(3-~Naphth-2-yl)-propyl)-piperid-3-yl)-methyl~
6,7-dimethyl 1-oxo-1,2,3,4-tet.rahydro-isoquinoline-
hydrochloride
Prepared from 6,7-dimethyl-1-oxo~1,2,3,4-tetrahydro-
isoquinoline and 3-chloromethyl-N-[3-(naphth-2- -
yl)-propyl]-piperidine analogously to Example 2.
Yield: 40.4~ of theory,
Melting point: 185-187C
Calculated: C 75.52 H 7.82 N 5.87 Cl 7.43
Found: 75.39 7.85 5.82 7.52
Example 21
2-[2(N-t3-(Naphthyl-2-oxy~-propvl~-piperid-2-yl)-
ethylJ-6~7-methylenedioxy-l-oxo-1~2~3~4-tetrahYdr
isoquinoline-hydrochloride
Prepared from 2-(2-(piperid-2-yl)-ethyl)-6,7-methylenedioxy-
l-oxo-1,2,3,4-tetrahydro-iso~uinoline and 2-t3-chloropropoxy)-
naphthalene analogously to Example 1.
Yield: 21.2% of theory,
Meltinq point: 85-87C
Calc. (x H2O): C 66.59 H 6.89N 5.17 Cl 6.53
Found: 66.77 6.98 4.95 6.74
Example 22
2-[(N-(3-(Naph~hyl-2-oxy)-propyl)-piperid-3-yl)- ~ ~-
methyl]-6,7-dimethyl-1,2,3,4-tetrahydro-isoquinoline-
dihydrochloride
` 1~3~3
- 81 -
Prepared from 2-[(N-(3-(naphthyl-2-oxy~-propyl~
piperid-3-yl~-methyl]-6,7-dimethyl-1-oxo-1,2,3,4-
tetrahydro-isoquinoline and lithium aluminium hydride
in tetrahydrofuran/ether analogously to Example 3.
Yield: 53% of theory,
Melting point: 133-135C i r
Calc. (x HzO) C 68.30 H 6.87 N 5.31 Cl 13.44
Found: 68.05 6.85 5.23 13.03
Example 23
2-[(N-(3-(Naphthyl-2-ox~-propyl~-piperid-3-yl~-
methyl]-6~7-methylenedioxy-1~2~3,4-tetrahYdro-iso~uinoline
dihvdrochloride
~ ~.
Prepared from 2-[(N-(3-(naphthyl-2-oxy~-propyl~
piperid-3-yl~-methyl]-6,7-methylenedioxy-1-oxo-
1,2,3,4-tetrahydro-isoquinoline and lithium aluminium
hydride in tetrahydrofuran/ether analogously to
Example 3.
Yield: 44.7% of theory,
Melting point: 130-132C
Calc. (x H2O): C 63.62 H 6.62 N 5.11 Cl 12.95
Found: 63.49 6.86 4.97 12.64
Exam~le 24
2-[(N-(~2-Methyl-naphth-l-yl)-methYl)-piperid-3- -
Yl~-methyl]-6,7-dimethoxy-1,2,3,4-tetrahydro-isoquinoline-
dihydrochloride
.... , ., ~., : ~
Prepared from 2-[(N-((2-methyl-naphth-1-yl)-methyl)-
piperid-3-yl)-methyl]-6,7-dimethoxy-1-oxo-1,2,3,4- ~ `
tetrahydro-isoquinoline and lithium aluminium hydride
in tetrahydrofuran/ether analogously to Example 3.
~ield: 80.5% of theory,
Melting point: 210-212C
~ -'
~.'.' ~' ~ '
33a~
- 82 -
Calc. (x H2O): C 65~05 H 7.53 N 5.23
Found: 65.23 7.78 5.03
Example 25
2-[2-(N-(2-(6-Methoxy-naphth-2-yl)-ethyl)-piperid-
2-yl)-ethyl]-6,7-methylenedioxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline-hYdrochloride
~' `'
Prepared from 2-[2-(piperid-2-yl)-ethyl]-6,7-methylene-
dioxy-l-oxo-1,2,3,4-tetrahydro-isoquinoline and
2-~2-bromo-ethyl)-6-methoxy-naphthalene analogously
to Example 1.
Yield: 27.6~ of theory,
Melting point: 112-117C
Calc. (x ~ H2O): C 67.74 H 6.82 N 5.26 Cl 6.65
Found: 67.54 6.73 5.47 6.86
. . :
Example 26
2-[~N-(2-(Naphth-l-yl)-ethyl)-piperid-3-yl)-methyl]-
6,7-dimethoxy-1-oxo-1,2,3,4-tetrahYdro-isoq~inoline-
hYdrochloride
Prepared from 2-[(piperid-3-yl)-methyl]-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and 1-(2-
benzene-sulphonyloxy-ethyl)-naphthalene analogously
to Example 1.
Yield: 26.9~ of theory, -~ -
Melting point: 220-225~C
Calculated: C 67.89 H 7.27 N 5.46 Cl 6.91
Found: 67.75 6.92 5.56 7.00
: :'
: . '
133~3 Y;~
-- ~3 --
Example 27
2-~N- ( 2-(Naphth-2-yl)-ethyl)-piperid-3-yl)-methy~
6,7-methylenedioxy-1-oxo-1~2,3,4-tetrahydro-isoquinoline-
hydrochloride
.
Prepared from 2-[(piperid-3-yl)-methyll-6,7-methylenedioxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and 2-(2-
benzene-sulphonyloxy-ethyl)-naphthalene analogously
to Example 1.
Yield: 27.9% of theory,
Melting point: 128-130C
Calc. (x H2O): C 67.66 H 6.69 N 5.63 Cl 7.13
Found: 67.64 6.70 5.76 7.35
Example 28 ;~
~. .
2-[ (N- (2-(5-MethYl-6-methoxy-naphth-2-yl)-ethyl)-
piperid-3-yl)-methyl]-6~7-methylenedi
1,2,3,4-tetrahydro-isoquinoline-hvdrochloride -
Prepared from 2-[(piperid-3-yl)-methyl]-6,7-methylenedioxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and 2-(2-
bromoethyl)-5-methyl-6-methoxy-naphthalene analogously
to Example 1.
Yield: 51.2% of theory, -~
Melting point: 128-131C
Calculated: C 68.88 H 6.74 N 5.34 Cl 6.77
Found: 68.90 6.61 5.30 7.05 -
Example 29
2-[(N-((2-Methyl-naphth-1-yl)-methyl)-piperid-3-
Yl)-methyl]-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline-hydrochloride
Prepared from 2-[(piperid-3-yl)-methyl]-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and l-chloromethyl- ~
: :
:. ~
- 8~ -
2-methyl-naphthalene analogously to Example 1.
Yield: 57.9% of theory,
Melting point: 212-214C
Calc. ~x 2 H20~: C 67.63H 7.63N 5.44 Cl 6.88
Found: 67.46 7.56 5.54 6.67
Example 30
2-[(N-(4-(Naphthyl-2~oxy)-butyl)-~iperid-3-yl)-
methY-l]-6~7-dimethoxy-l-oxo-l r 2,3,4-tetrahydro-
isoquinoline-hydrochloride
Prepared from 2-[(piperid-3-yl)-methyl]-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and 2-(4-
bromo-butoxy)-naphthalene analogously to Example 1. ~;-
Yield: 46.3~ of theory,
Melting point: 80-84C
Calc. (x H2O): C 66.83~ 7.41 N 5.03 Cl 6.36
Found: 66.79 7.22 4.90 6.64
~ . ~.....
Example 31 -~
'"~` . .~' .' ~
2-[(N-(2-(6-MethoxY-naphth-2-yl)-ethYl)-piperid-
3-Yl)-methYl]-6~7-dimethoxy-l-oxo-l~2~3r4-tetrahydr
isoquinoline-hydrochloride
Prepared from 2-1(piperid-3-yl)-methyl]-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and 2-(2-
bromo-ethyl)-6-methoxy-naphthalene analogously
to Example 1.
Yield: 22.8~ of theory,
Melting point: 80-85C -~
Calc.: (x H2O x HCl x CH3COCH3): C 64.01 H 7.65 N 4.52 Cl 5.72
Found: 64.26 7.70 4.62 5.49
'
'~ ` 133~3 - 85 -
Example 32
2-[3-(N-(2-(6-Methoxy-naPhth-2-yl)-ethyl)-piperid
3-yl)-Propyl]-6,7-methylenedioxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline
Prepared from 2-[3-(piperid-3--yl)-propyl~-6,7-methylenedioxy-
l-oxo-l~2~3~4-tetrahyaro-isoqllinoline and 2-t2-
bromo-ethyl)-6-methoxy-naphthalene analogously
to Example 1.
Yield: 36.8% of theory,
Melting point~ 118-121C
Calculated: C 74.37 H 7.25 N 5.60
Found: 74.60 7.43 5.65
Example 33
. .... ~ ~ .:
2-[3-(N-(2-(Naphth-l-yl)-ethyl)-piperid-3-yl)-propyl]-
6,7-methylenedioxy-1-oxo-1,2,3t4-tetrahydro-isoquinoline- ,~
hydrochloride
Prepared from 2-[3-(piperid-3-yl)-propyl]-6,7-methylenedioxy-
l-oxo-1~2,3,4-tetrahydro-isoquinoline and 1-(2
benzenesulphonyloxy-ethyl)-naphthalene analogou~ly
to Example 1.
Yield: 20.8% of theory,
Melting point: 195-197C
Calculated: C 71.07 H 6.95 N 5.53 Cl 6.99
Found: 71.30 6.95 5.65 6.80
Example 34
: . ~
2-[2-(N-(2-(5-Methvl-6-methoxy-naphth-2-ylt-ethyl)-
piperid-2-yl)-ethyl]-6,7-dimethoxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline-hydrochloride
Prepared from 2-[2-(piperid-2-yl)-ethyl]-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and 2-(2-
~ 1~3~3
- 86 -
bromo-ethyl)-5-methyl-6-methoxy-naphthalene analogously
to Example 1.
Yield: 33.6% of theory,
Melting point: 95-100C
Calc. (x 1 H2O~: C 68.38 H 7.52 N 4.98 Cl 6.30
Found: 68.14 7.43 4.92 6.77
'' .,.
ExamPle 35
2-[(N-(3-(Naphthy1-2-oxy)-Propyl)-piperid-3-Yl)-
methyl]-6,7-methylenedioxy-1-oxo-1,2,3,4-tetrahydro~
isoquinoline-hydrochloride - ~ -
Prepared from 6,7-methylenedioxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline and 3-chloromethyl-N-[3-
(naphthyl-2-oxy)-propyl]-piperidine analogously
to Example 2.
Yield: 27.3% of theory,
Melting point: 104-106C
Calc. (x H2O): C 66.09 H 6.69 N 5.31 Cl 6.72
found: 66.19 6.34 5.24 7.22
Example 36
2-[rN-(3-(Naphth 1-2-oxy)-propyl)-piperid-3-yl)-
methYl~-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahYdro-
isoquinoline-hydrochloride
Prepared from 6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline and 3-chloromethyl-N-[3-(naphthyl-
2-oxy)-propyl]-piperidine analogously to Example 2.
Yield: 28.6% of theory,
Melting point: 191-193C
Calc. (x H2O): C 66.3~ H 7.23 N S.lS Cl 6.53
Found: 66.59 7.19 5.03 6.65
Example 37 ~
,,
~3~
- 87 -
2-[(N-(3-(Naphthyl-2-oxy~-propyl)-hexahydro-azepin-
3-Yl)-methyl]-6,7-dimethox~ oxo-l,2,3,4-tetrahydro-
isoquinoline-hy~rochloride
'.. ".
Prepared from 2-[(hexahydro-azepin-3-yl)-methyl]-
6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-isoquinoline
and 2-(3-chloropropoxy)-naphthalene analogously
to Example 1.
Yield: 16.1~ of theory,
Melting point: 86-88C
Calc. (x H20): C 66.83H 7.42 N 5.03 Cl 6.36
Found: 66.90 7.40 5.26 6.37
: .: . ' .: ':
Example 38
2-~(N-~3-(NaPhthyl2-oxy)-propyl)-hexahydro-azepin-
3-yl)-methyl]-6,7-dimethyl-1-oxo-1,2,3,4-tetrahYdro-
isoquinoline-hydrochloride
Prepared from 6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-
isoguinoline and 3-chloromethyl-N-E3-(naphthyl~
2-oxy)-propyl]-hexahydro-azepine analogously to
Example 2.
.:.
Yield: 22.5% of theory,
Melting point: 191-193C
Calculated: C 73.42 H 7.75N 5.52Cl 6.99 `~
Found: 73.37 7.67 5.52 7.12
Example 39
2-[(N-(2-(5-MethYl-6-methoxy-naphth-2-yl)-ethYl)- -
hexahydro-azepin-3-yl)-methyl]-6,7-dimethoxy-1,2,3,4-
tetrahydro-isoquinoline-dihydrochloride
Prepared frc~m 2-[(N-(2-(5-methyl-6-methoxy-naphth-
2-yl)-ethyl)-hexahydro-azepin-3-yl)-methyl]-6,7-
dimethoxy-l-oxo-1,2,3,4-tetrahydro-isoquinoline
~ ~ ~3~9~3
; - 88 -
and lithium aluminium hydride in tetrahydrofuran
analogously to Example 3.
Yield: 77.6~ of theory,
Melting point: 170-172C -~
Calc. (x H2O): C 64.96 H 7.49 N 4.73 Cl 11.98
Found: 65.11 7.62 4.95 11.84
Example 40
2-[(N-(2-(5-Methyl-6-methoxY-naphth-2-yl)-ethYl)-
hexahydro-azepin-3-yl)-methYl~-6,7-dimethoxY-l-
~:~ : :-
oxo-1,2,3,4-tetrahydro-isoquinoline
Prepared from 2-[(hexahydro-a2epin-3-yl)-methyl~
6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-isoquinoline
and 2-(2-bromoethyl)-5-methyl-6-methoxy-naphthalene
analogously to Example 1.
Yield: 43.5% of theory, - ~-
Melting point: 125-127C
Calculated: C 74.39 H 7.80 N 5.42 -~
Found: 74.31 7.82 5.3S
Example 41
~' ~
2-[(N-(2-Methyl-naphth-l-yl)-methyl)-hexahydro- ~
azepin-3-yl)-methyll-6,7-dimethoxy-1-oxo-1,2,3,4- :-
. .
tetrahydro-isoquinoline-hydrochloride
':
Prepared from 2-[(hexahydro-azepin-3-yl)-methyl]-
6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-isoquinoline ~ -
and l-chloromethyl-2-methyl-naphthalene analogously
to Example 1.
Yield: 67.5% of theory,
Melting point: 128-130C
Calc. (x 2 H2O) C 66.10 H 7.58 N 5.13 Cl 6.50
Found: 66.24 7.44 5.23 6.85
133~99~
- 89 -
E x amp l e 4 2
2-[ tN- t4-~NaphthY1-2-oxY)-butyl)-hexahydro-azepin-
3-Yl)-methyl]-6~7-dimethoxY-l-oxo-1,2,3,4-tetrahydro- ,'.
isoquinoline-hydrochloride
Prepared from 2-[~hexahydro-azepin-3-yl)-methyl]-
6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-isoquinoline -
and 2-(4~bromo-butyloxy)-naphthalene analogously
to Example 1.
Yield: 26% of theory, -~
Melting point: 192-194C
Calculated: C 69.22H 7.80 N 5.04 Cl 6.38
: --: -
Found:70.01 7.70 5.15 6.48
:~ :
Example 43 ~;
- ~ .
2-[(N-(2-t~aphth-1-yl)-ethyl)-hexahydro-azepin-
3-Yl)-methyl]-6,7-methylenedioxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline-hydrochloride
Prepared from 2-1(hexahydro-azepin-3-yl)-methyl]-
6,7-methylenedioxy-1-oxo-1,2,3,4-tetrahydro-isoquinoline
and 2-~2-benzenesulphonyloxy-ethyl)-naphthalene
analogously to Example 1.
Yield: 15.4% of theory,
Melting oint: 236-238~C
Calc. (x ~ H2O):C 69.40 H 6.82N 5.58 Cl 7.06
Found: 69.07 6.74 6.13 7.29
,, .
Example 44
~ ~ .
2-[(N-(2-(Naphth-2-yl)-ethyl3-hexahydro-azepin~
3-~1)-methyl]-6,7-methYlenedioxy-l-oxo-1,2,3,4- -
tetrahydro-lsoquinoline-hydrobromide `
Prepared from 2-[(hexahydro-azepin-3-yl)-methyl]-
6,7-methylenedioxy-1-oxo-1,2,3,4-tetrahydro-isoquinoline
~ '
-~ ~33~33
- 9 0 -- ~ ~
and 2-(2-bromo-ethyl)-naphthalene analogously to ;
Example 1.
Melting point: 100-102C
Yield: 31.6~ of theory,
Calculated: C 64.80 H 6.18 N 5.21 Br 14.86
Found: 65.02 6.07 5.39 14.78
Example 45
: ~:
2-[~N-(2-(6-MethoxY-naphth-2-yl)-ethyl)-hexahydro-
azepin-3-yl)-methYl~-6,7-methylenedioxY-l-oxo-1,2,3,4-
tetrahYdro-lsoquinoline-hYdrochloride ,~ :~
Prepared from 2-[(hexahydro-azepin-3-yl)-methyl]-
6,7-methylenedioxy-1-oxo-1,2,3,4-tetrahydro-isoquinoline
and 2-(2-bromo-ethyl)-6-methoxy naphthalene analogously
to Example 1.
Yield: 34.1% of theory,
Melting point: 147-149C
Calc. (x H2O): C 68.62 H 7.10 N 5.33 Cl 6.75
Found: 68.88 6.98 5.41 6.78
Example 46 ~ ;
2-[(N-(2-~5-MethYl-6-methoxy-naphth-2-yl)-ethYl)- ~--
hexahydro-azepin-3-yl)-methyl]~6,7-methylenedioxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline-hYdrochloride
:
Prepared from 2-[(hexahydro-azepin-3-yl)-methyl]-
6,7-methylenedioxy-1-oxo-1,2,3,4-tetrahydro-isoquinoline
and 2-(2-bromo-ethyl)-5-methyl-6-methoxy-naphthalene -
analogously to Example 1.
Yield: 36~1~ of theory, ~ ;
Melting point: 112-114C
Calc- (x H2O): C 67.07 H 7.08 N 5.04 Cl 6.38
Found: 67.13 7.15 4.97 6.56
' ,
1 3 3 ~ 9 9 3
- 91 -
Example 47
2~(N-(3-(4-Methoxy-phenoxyj-propyl)-piperid-3-
yl~-methYl]-6,7-dimethoxy-1,2,3,4-tetrahYdro-isoquinoline-
dihydrochloride
Prepared from 2-~(N-(3-(4-methoxy-phenoxy)-propyl)-
piperid-3-yl)-methyl]-6,7-dimethoxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline and lithium aluminium hydride
in tetrahydrofuran analogously to Example 3.
Yield: 88.5% of theory,
Melting point: 189-191C -
Calculated: C 59.44 H 7.76 N 5.13 Cl 12.99
Found: 59.55 7.99 5.12 12.61
Example 48
~: ~: ', .
2-L~N-12-(3,4-Dimethoxy-phenyl)-ethYl)-piPerid- : '' ,,:
3-yl)-methYl]-6~7-dimethoxy-l~2~3~4-tetrahydro-
.
isoquinoline-dihydrochloride ~
~'' . " '
Prepared from 2~[(N-(2-(3,4-dimethoxy-phenyl)-ethyl)-
piperid-3-yl)-methyl]-6,7-dimethoxy-1-oxo-1,2,3,4- ;
tetrahydro-isoquinoline and lithium aluminium hydride
in tetrahydrofuran analogously to Example 3. ~-~
Yield: 92.8% of theory,
Melting point: 175-176~C -~
Calculated: C 60.66 ~ 7.35 N 5.33 Cl 13.49
: : :
Found:60.58 7.56 5.32 13.22 ;~ ~
ExamPle 49 ~ ;
' ~
2-[(N-(3-~3-Methoxy-phenoxy)-propyl)-piperid-3-
yl)-methyl]-6,7-methylenedioxy-1~2~3~4-tetrahYdro- i~
isoquinoline-dihydrochloride ''
Prepared from 2-~(N-(3-(3-methoxy-phenoxy~-propyl)-
~: '~ ''
~ ~., ", .. .
,t
~33a993
- 92 -
piperid-3-yl)-methyl]-6,7-methylenedioxy-l~oxo-
1,2,3,4-tetrahydro-isoquinoline and lithium aluminium
hydride in tetrahydrofuran analogously to Example
3.
Yield: 96.6~ of theory,
Melting point: 178-181C
Calculated: C 59.08 H 7.62 N 5.30 Cl 14.31
Found: 58.90 7.50 5.40 14.15
~,
Example 50
2-[(N-(3-(3-Methoxy-phenoxy)-propyl)-piperid-3-
Yl)-methyl]-6~7-methylenedioxy-lt2r3~4-tetrahydr
isoquinoline-dihydrochloride
Prepared from 2-[(N-~3-(3-methoxy-phenoxy)-propyl)-
piperid-3-yl)-methyl]-6,7-methylenedioxy-1-oxo-
1,2,3,4-tetrahydro-isoquinoline and lithium aluminium
hydride in tetrahydrofuran analogously to Example
' : :
Yield: 94.5% of theory,
Melting point: 169-171C
Calculated: C 57.03 H 7.36 N 5.11 Cl 13.86
Found: 56.91 7.26 5.15 13.68
Example 51
2-L(N-(2-(3,4-Dimethoxy-phenyl)-ethyl)-piperid-
3-Yl) -methyl]-6,7-dimeth~l-1,?,3,4-tetrahydro-isoquinoline-
dihydrochloride
Prepared from 2-[(N-(2-(3,4-dimethoxy-phenyl)-ethyl)-
piperid-3-yl)-methyl]-6,7-dimethyl-1-oxo-1,2,3,4-
tetrahydro-isoquinoline and lithium aluminium hydride
in tetrahydrofuran analogously to Example 3.
Yield: 91.3~ of theory,
Melting point: 140-142C
~ ~ '
133~
- 93 -
Calculated:C 62.00H 8.34 N 5.27Cl 13.44
Found: 61.858.27 5.31 13.33
Example 52
2-r(N-(3-(4-Methoxy-phenoxy)-propyl)-piperid-3-
yl)-methyl]-6,7-dimethyl-1,2,3,4-tetrahydro-isoquinoline-
dihydrochloride
~ , ,
Prepared from 2-[tN-(3-(4-methoxy-phenoxy)-propyl)-
piperid-3-yl)-methyl]-6,7-dimethyl-1-oxo-1,2,3,4-
tetrahydro-isoquinoline and lithium aluminium hyaride
in tetrahydrofuran analogously to Example 3.
Yield: 88.3~ of theory,
Melting point: 170-172C
Calculated: C 63.14 H 8.24 N 5.65 Cl 14.31
Found: 63.09 8.33 5.8214.02
Example 53
2-[(N-~2-(3,4-Dimethoxy-Phenyl)-ethyl)-piperid-
3-yl)-methyl]-6,7-methylenedioxY-1,2,3,4-tetrahYdro- -~
isoquinoline-dihydrochloride
Prepared from 2-[(N-(3-(3,4-dimethoxy-phenyl)-ethyl)-
piperid-3-yl)-methyl]-6,7-methylenedioxy-1-oxo-
1,2,3,4-tetrahydro-isoquinoline and lithium aluminium
hydride in tetrahydrofuran analogously to Example
3.
Yield: 93.3% of theory,
Melting point: 150-154C
Calculated: C 60.01 H 7.34 N 5.39 Cl 13.64
Found: 59.96 7.41 5.2513.43
'. '.'~",~-'' ~'
-~ ~330993
- 94 -
Example 54
2-[(N-t3-(4-MethoxY-phenoxY)-propyl)-piperid-3-
Yl)-methyl]-6~7-dimethoxy-l~2~3~4-tetrahydro-isoquinoline
dihydrochloride
Prepared from 2-[(N-(3-(4-methoxy-phenoxy~-propyl)-
piperid-3-yl-3)-methyl]-6,7-dimethoxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline and lithium aluminium hydride
in tetrahydrofuran analogously to Example 3.
Yield: 95.3~ of theory,
Melting point: 182-185C
Calculated: C 61.05 H 7.09 N 5.48 Cl 13.86
Found: 61.10 6.95 5.68 13.55
Exam~le 55
2-[2-(~-(3-(3,4-Methylenedioxy-phenoxy)-ProPYl)-
piPerid-3-yl)-ethyl]-6~7-dimethoxy-l-oxo-l~2~3~4-
tetrahydro-isoquinoline-hydrochloride ' ;:~.
Prepared from 2-[~piperid-3-yl)-ethyl]-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and l-chloro-
3-(3,4-methylenedioxy-phenoxy)-propane analogously -~ ~
to Example 1. ~ ~-
Yield: 35.5~ of theory,
Melting point: 97-100C
Calculated: C 59.09 H 7.28 N 4.62 Cl 6.65
Found: 58.97 7.36 4.66 6.52
:
Example 56
2-[2-~N-(2-(3,4-Dimethoxy-phenyl)-ethyl)-piE~erid-
3-yl)-ethyl]-6,7-d~methoxy-1-oxo-1,2,3,4-tetrahYdro-
isoquinoline-hydrochloride ~ `
Prepared from 2-[(piperid-3-yl)-ethyl~-6,7-dimethoxy~
l-oxo-1,2,3,4-tetrahydro-isoquinoline and l-bromo-
r 'tfr~
~L33~993 : ~
- 95 -
2-(3,4-dimethoxy-phenyl~-ethane analogously to
Example 1. ~-
Yield: 35.9~ of theory,
Melting point: 103-105C
Calculated: C 62.60 H 7.79 N 5.21 Cl 6.83
Found: 62.41 7.82 5~09 7.19
~xample 57
2-[3-(N-(2-(3~4-Dimethoxy-phenyl)-ethyl)-piperid~
3-yl)-Propyl]-6~7-dimethoxy-l-oxo-l~2~3~4-tetrahydr
isoquinoline-hYdrochloride
Prepared from 2-[3-~piperid-3-yl)-propyl]-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and l-bromo-
2-(3,4-dimethoxy-phenyl)-ethane analogously to
Example 1.
Yield: 32.6% of theory,
Melting point: 102-106C
Calculated: C 63.20 H 7.86 N 5.08 Cl 6.43
Found: 63.39 7.90 4.86 6.13
Example 58
2-~3-!N-~3-(3,4-MethYlenedioxy-phenoxy ? -propyl)-
:: :
pi~erid-3-yl)-propyl]-6~7-dimethoxy-1-oxo-1~2~3~4-
tetrahydro-isoquinoline-hydrochloride
Prepared from 2-~3-(piperid-3-yl)-propyl]-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and l-chloro-
3-(3,4-methylenedioxy-phenoxy)-propane analogously ~-
to Example 1.
~ield: 29.7% of theory, ~ -
Melting point: 97-100C
Calculated: C 61.63H 7.31N 4.96Cl 6.47
Found: 61.94 7.46 5.16 6.48
...-
33~93
- 96 -
Example 59
2-[~N-(3,4-Dimethoxy-benzyl)-piperid-3-yl)-methyl~-
6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-isoquinoline-
hydrochloride
Prepared from 2-(piperid-3-yl-methyl)-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and 3,4-dimethoxy-
benzylbromide analogously to Exampl~
Yield: 53.3% of theory,
Melting point: 127-132C ~ ;
Calculated: C 63.58 H 7.18 N 5.70 Cl 7.22
Found: 63.30 7.22 5.52 7.14
_xample 60
2-[(N-(3-(4-Methoxy-phenyl)-propyl)-piperid-3-yl)-
methyl]-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahy~_o-
isoquinoline-hYdrochloride
.
Prepared from 2-(p~perid-3-yl-methyl~-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and l-bromo-
3-(4-methoxyphenyl~-propane analogously to Example 1.
Yield: 42% of theory,
Melting point: 229-231C
Calculated: C 66.31 H 7.63 N 5.73 Cl 7.25
Found: 66.27 7.64 5.65 7.33
Example 61 - - -
2-12-(N-(3-(3-Methyl-phenoxy)-propYl)-piperid-2-
yl)-ethyl]-6,7-dimethoxy-1-oxo-1,2~3,4-tetrahydro-
isoquinoline-hydrochloride
Prepared from 2-[2-~piperid-2-yl)-ethyll-6,7-dimethoxy- ~ ,:"'yr,~
l-oxo-1,2,3~4-tetrahydro-isoquinoline and l-chloro-
3-~3-methyl--phenoxy)-propane analogously to Example 1.
~ 133~93
- 97 -
Yield: 52.4% of theory,
Melting point: 142-144C
Calculated: C 66.85 H 7.81 N 5.57 Cl 7.05
Found: 66.73 7.68 5O53 6.94
Example 62
2-[2-(N-(2-(3,4-~imethoxy-pheny~)-e~hyl)-eiperid- '~
2-yl)-ethyl]-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahYdro-
iso~uinoline-hydrochloride
Prepared from 2-[2-(piperid-2-yl)-ethyl]-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and l~bromo-
2-(3,4-dimethoxy-phenyl)-ethane analogously to
Example 1.
Yield: 47.3% of theory,
Melting point: 150-155C
Calculated: C 64.72 ~ 7.68 N 5.39 Cl 6.82
Found: 64 40 7.83 5.27 6.90 ~ -~
Example 63 ~ ;r.
2-[2-(N-(3-Benzyloxy-propyl)-piperid-2-yl)-ethyl]~
6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-isoquinoline-
hydrochloride
Prepared from 2-r2-(piperid-2-yl)-ethyl]-6,7-dimethoxy- ;-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and l-chloro-
3-benzyloxy-propane analogously to Example 1. ~ ;
Yield: 56.3~ of theory,
Melting point: 116-120C
Calculated: C 66.85 H 7.81 N 5.57 Cl 7.05 ~-
Found: 66.60 7.75 5.25 7.25
~.. , .." , """~,, .,., . ,~;,,~.
~` ` ~ 33~93 ~
- 98 -
Example 64
2-~2-(N-(4-~4-Methoxy-phenyl)-butyl)-p~eerid-2-
yl)-ethyl]-6~7-dimethoxy-l-oxo-1,2~3~4-tetrahYdr
isoquinoline-hYdrochloride
Prepared from 2-[2-(piperid-2-yl)-ethyl]-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and l-bromo-
4-(4-methoxy-phenyl)-butane analogously to Example 1.
Yield: 42.8~ of theory,
Melting point: 107-112C
Calculated: C 67.36 ~ 7.99 N 5.42 Cl 6.86
Found: 67.16 8.055.35 7.34
Example 65
2-[2-(N-(3-(3,5-Dimethoxy-phenoxy)-propyl)-piperid-
2-yl)-ethyl]-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahYdro-
isoquinoline-hydrochloride ;;
Prepared from 2-[2-(piperid-2-yl)-ethyl]-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and l-chloro-
3-(3,5-dimethoxy-phenoxy)-propane analogously to
Example 1.
Yield: 56.3% of theory,
Melting point: 127-132C
Calculated: C 61.41 H 7.64 N 5.10 Cl 6.46
Found:61.56 7.65 5.28 6.89
:
Example 66
. :: ,
2-[2-(N-~3-~3,4-Methylenedioxy-phenoxy)-propyl)-
piperid-2-yl)-ethyl]-6,7-dimethoxv-1-oxo-1,2,3,4-
tetrahydro-isoquinoline-hydrochloride
Prepared from 2-[2-~piperid-2-yl)-ethyl]-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and l-chloro-
.
~ _. 133~93
~ 99 ~ 27169-147
3-~3,4-methylenedioxy-phenoxy)-propane analogously
to Example 1.
Yield: 49~ of theory,
~elting point: 118-120C
Calculated: C 63.09 H 7 . 00 N 5.26 Cl 6.65
Found: 62090 7.04 5.46 6.79
Example 67
2-[(N-~3-~3~5-Dimethoxy-phenoxY)-propyl)-piperld- ;`-~
3-Yl)-methyl~-6,7-dimethoxv-1-oxo-1,2 ! 3,~-tetrahydro~
iso~uinoline-hydrochloride ~
Prepared from 2-l~piperid-3-yl)-methyll-6,7-dimethoxy- ~ rJ
l-oxo-1,2,3,4-tetrahydro-l~oquinoline and l-chloro-
3-(3,5-dimethoxy-phenoxy)-propane analogou~ly to ;~
Example 1.
Yleld: 37.5% of theoey,
Melting point: 98-102C
Caloula~ed: C 62.85H 7.35N 5.24Cl 6.63
Found: 62.81 7.41 5.10 6.75 ;~;
Example 68 -~
,,
2-l(N-~3-~3,!4-MethyIenedioxy-phenyl)-propyl)-piperid-
3-yl)-methYll-6,7-dlmethoxy-1-oxo-1,2,3,4-tetrahydro~
i~oquinollne-hydrochlorlde
Prep~red from 2-l~plperld-2-yl)-metkyl]-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and l-chloro-
3-(3,4-methylenedioxy-phenyl)-propane analoqously
to Example l.
Yield: 50% of theory,
Melting point: 236-238C
Calculate~d: C 64.46 H 7.01 N 5~57 Cl 7.04
Found: 64.30 6.97 5.59 7.08
,
,,,, ., . ~ .
L3~99~
- 100 -
OG 52-794
Examp~e 69
2-[(N-(3-(3,4-MethYlenedioxy-phenoxy~-propyl)-piperid-
3-Yl)-methyll-6l7-dimethoxy-l-oxo-lr2~3~4-tetrahydr
isoquinoline-hydrochloride
: '
Prepared from 2-[(piperid-2-yl)-methyl]-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and l-chloro-
3-(3,4-methylenedioxy-phenoxy)-propane analogously
to Example 1.
Yield: 46.2~ of theory,
Melting pointo 149-153C
Calculated: C 60.38 H 6.94 N 5.21 Cl 6.60
Found: 60.30 6.93 5.29 6.37
Example 70
2-[(N-(3-(2~6-Dimethyl-phenoxy~-propyl)-piperid- ~ ~;
3-yl)-methYl]-6~7-dimethoxy-l-oxo-l~2~3~4-t-etrahydro- ~ /Yi'~
isoquinoline-hydrochloride
Prepared from 2-[(piperid-3-yl)-methyl]-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and l-chloro-
3-(2,6-dimethyl-phenoxy)-propane analogously to
Example 1.
Yield: 53.3% of theory,
Melting point: 131-135C
Calculated: C 66.85 H 7.81 N 5.57 Cl 7.05 ~ : -
.
Found: 66.88 7.95 5.59 6.85 ~`
.`".'''.'.'.. '. .:.
` ~33~9~`3
- 101 -
ExamPle 71
2-[(N-(4-(2,4-Dichloro-phenoxy~-butyl)-piperid-
3-yl)-methyl]-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline-hydrochloride -
-
Prepared from 2-[(piperid-3-yl)-methyl]-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro~isoquinoline and l-chloro-
4-(2,4-dichloro-phenoxy)-butane analogously to ;~
Example 1.
Yield: 54.1% of theory,
Melting point: 125-128C --~ -
Calculated: C 58.12 H 6.32 N 5.02 Cl 19.06
Found: 58.21 6.38 5.08 18.85
Example 72
2-[(N-(2-(3,4-Dimethoxy-phenyl)-ethyl)-E~perid-
3-yl)-mathYl]-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahYdro-
isoquinoline-hYdrochloride ~"~
Prepared from 2-[(piperid-3-yl)-methyl]-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and l-bromo-
2-(3,4-dimethoxy-phenyl)-ethane analogously to
Example 1.
Yield: 57.5% of theory,
Melting point: 118-121C
Calculated: C 64.21 H 7.38 N 5.55
Found: 64.18 7.36 5.19
Example 73
2-[(N-(3-(3!4-Dimethoxy-phenoxy~-~ropyl)-piperid-
3-yl)-methyl]-6,7-dimethoxv-1-oxo-1,2,3,4-tetrahydro-
isoquinoline-hydrochloride
.: .
Prepared from 2-[(piperid~3-yl)-methyl]-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and l-chloro-
~ .
~'" ~ ' '"~'
, ` , ~ lb ~ - ,
133099~
3-(3,4-dimethoxy-phenoxy)-propane analogously to
Example 1.
Yield: 62.5~ of theory,
Melting point: 112-115C ~
Calculated: C 60.80 H 7.47 N 5.24 ~:
Found: 60.65 7.69 5.27
Example 74
2-[(N-(3-(3~4-Dimethoxy-phenoxy)-propyl)-~iperid-3-Y1)-
methyl]-6~7-methylenedioxy-1-oxo-1,2~3,4-tetrah~dro~
isoquinoline-hydrochloride ~ ~ -
Prepared from 2-[(piperid-3-yl)-methyl]-6,7-methylenedioxy-
. .~.^r ~ 4;~
1-oxo-1,2,3,4-tetrahydro-isoquinoline and l-chloro-
3-(3,4-dimethoxy-phenoxy)-propane analogously to
Example 1.
Yield: 60~ of theory, ~ -
Melting point: 97-100C
Calculated: C 60.38 H 6.94 N 5.40 Cl 6.60
Found: 60.20 6.97 5.21 6.83
Example 75
. -
2-[(N-(2-(4-Methoxy-Phenyl)-ethyl)-piperid-3-Yl)-
methyl]-6,7-methYlenedioxY-l-oxo-1,2,3,4-tetrahYdro-
isoquinoline-hYdrochloride ,
'~
Prepared from 2-[(piperid-3-yl)-methyl]-6,7-methylenedioxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and l-chloro-
2-(4-methoxy-phenyl)-ethane analogously to Example 1.
Yield: 71.4~ of theory,
Melting point: 195-197C
Calculated:C 62.94H 6.97 N 5.87 Cl 7.73
Found: 62.90 6.98 5.68 8.04
'.','"''"'' ~ '. ''` ~
Example 76 ~ ~
, ~ . .
' ' ~' ''': "
: : ~ . .:
~` 133~
- 103 - ~
2-[(N-(2-(3,4-DimethoxY-phenyl)-ethyl)-piPerid- , r.
3-Yl)-methyl]-6~7-dimethyl-l-oxo-l~2~3~4-tetrahydr
isoquinoline-hydrochloride
.: . .:
Prepared from 2-[(piperid-3-yl)-methyl]-6,7-dimethyl-
l-oxo-1,2,3,4-te~rahydro-isoquinoline and l-bromo-
2-(3,4-dimethoxy-phenyl)-ethane analogously to
Example 1.
Yield: 41.9% of theory,
Melting point: 132-134C
Calculated: C 63.57 H 8.10 N 5.49 Cl 6.95
Found: 63.70 8.26 5.45 7.13
Example 77
'., : .:~
2-[tN-(3-(4-Methoxy-phenoxy)-propyl)-piperid-3-
yl)-methY-]-6~7-dimethyl-l-oxo-l~2~3~4-~etrahydr
isoquinoline-hYdrochloride
Prepared from 2-[(piperid-3-yl)~methyl]-6,7-dimethyl-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and 1-chloro-
3-(4-methoxy-phenoxy~-propane analogously to Example 1.
Yield: 57.8% of theory,
Melting point: 144-146C
Calculated:C 68.55H 7.88N 5.92Cl 7.49
Found: 68.45 7.80 6.11 7.33
,
Example 78
: ,
2-[(N-(3-(3-Methoxy-phenoxy)-Propyl)-piperid-3-
yl)-methyl]-6,7-methylenedioxy-1-oxo-1,2,3,4-tetrahYdro-
isoquinoline-hYdrochloride
Prepared from 2-[(piperid-3-yl)-methyl]-6,7-methylenedioxy-
1-oxo-1,2,3,4-tetrahydro-isoquinoline and 1-chloro-
3-(3-methoxy-phenoxy)-propane analogously to Example 1.
Yield: 32.5% of theory, ~
Melting point: 142-145C `~ ~'
~ ~33~993
- 104 -
Calculated:C 60.58H 6.95 N 5.52Cl 6.99
Found: 60.426.92 5.50 7.18
Example 79
2-[(N-(3-(3-MethYl-phenoxY~-propyL)-piperid-3-y~
methyl~-6,7-methylenedioxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline-hydrochloride
Prepared from 2-[(piperid-3-yl)-methyl]-6,7-methylenedioxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and 3-(3-
methyl-phenoxy)-l-chloro-propane analogously to
Example 1.
Yield: 31.6~ of theory,
Melting point: 178-180C
Calculated: C 63.59 H 6.97 N 5.70 Cl 7.22 ~ -~
Found: 63.59 6.92 5.86 7.50
. .
Example 80
2-[(N-(3-(4-Methoxy-~-methyl-phenylamino)-Propyl)-
i~erid-3-Yl)-methYll-6,7-dimethoxy-1-oxo-1,2,3,4-
tetrahYdro-isoquinoIine-dihydrochloride
Prepared from 2-[(piperid-3-yl)-methyl]-6,7-dimethoxy- ~-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and l-chloro-
3-(4-methoxy-N-methyl-phenylamino)-propane analogously ~ ;
to Example 1.
Yield: 52.5~ of theory,
Melting point: 180-183C
Calculated:C 60.64H 7.45N 7.58 Cl 12.79 -;~
Found: 60.50 7.35 7.56 12.87
.. .. ~;
''~ ~ "'' -"'., "..'
~: ,
~33~99~
- 105 -
Example 81
2-[ tN-(3- t4-MethoxY-~henoxY)-propY1~-Pyrrolid-3-Yl)-
methyll-6,7-dimethoxy-1-oxo-1,2,3 4-tetrahYdro-
isoquinoline-hydrochloride
.
Prepared from 6 7-dimethoxy-1-oxo-1,2 3 4-tetrahydro-
isoquinoline and N-(3-(4-methoxy-phenoxy)-propyl~
3-benzenesulphonyloxymethyl-pyrrolidine analogously
to Example 2.
Yield: 84.4~ of theory,
Melting point: 142-144C
Calculated: C 63.60 H 7.18 N 5.71 Cl 7.22
Found: 63.75 7.12 5.64 7.32
;,, ~,
Example 82
2-[(N-(3-(6-Methoxy-naphthyl-2-oxy)-propyl)-pyrrolid- `
3-yl)-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahYdro-isoquinoline
Prepared from 6,7-dimethoxy-1-oxo-1,2 3,4-tetrahydro-
isoquinoline and 3-(p-toluenesulphonyloxymethyl)-
N-(6-methoxy-naphthyl-2-oxy)-pyrrolidine analogously
to Example 2.
Yield: 47% of theory,
Melting pointo 142-144C
Calculated: C 71.41 H 7.19 N 5.55
Found: 71.14 7.16 5.53
Example 83
2-1(N-~3-(4-Methoxy-phenoxy)-Prop~ pyrrolid-3-
Yl)-methyl]-6~7-dimethoxy-l~2~3~4-tetrahydro-isoquinoline
dihYdrochloride
Prepared from 2-[(N-(3-(4-methoxy-phenoxy)-propyl)-
pyrrolid-3-yl)-methyl]-6,7-dimethoxy-1-oxo-1,2,3 4-
tetrahydro-isoquinoline and lithium aluminium hydride
in tetrahydrofuran analogously to Example 3.
~330~3
- 106 -
Yield: 90.9~ of theory,
Melting point: 248-250C
Calculated: C 60.81 H 7.46 N 5.46 Cl 13.81
Found: 60.79 7.61 5.48 13.84 -
Exam~le 84
2-[(N-(3-(4-Methoxy-phenoxy)-propYl)-pyrrolid-3-
Yl)-methyl]-6,7-dimeth~l-1-oxo-1,2,3,4-tetrahyaro-
isoquinoline-hYdrochloride
Prepared from 6,7-dimethyl-1-oxo-1,2,3,4-tetrahyaro-
isoquinoline and N-[3-(4-methoxy-phenoxy)-propyl]-
3-benzenesulphonyloxymethyl-pyrrolidine analogously
to Example 2.
Yield: 60.5% of theory,
Melting point: 118-121C
Calculated: C 68.03 H 7.69 N 6.10 Cl 7.72
Found: 67.90 7.71 6.04 7.90
Exam~le 85
'', '".'.'.''''`.'`,"~'
2-[(N-(2-(3,4-Dimethoxy-phenyl)-ethyl)-pyrrolid-
3-Yl)-methyl]-6~7-dimethoxy-l-oxo-l~2~3~4-tetrahydr
isoquinoline-hydrochloride
Prepared ~rom 6,7~dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline and N-[2-(3,4-dimethoxy-phenoxy)-ethyl]- - - -
3-benzenesulphonyloxymethyl-pyrro7idine analogously ~-~
to Example 2.
Yield: 56.7~ of theory,
Melting point: 116-118C
Calculated: C 63.60 H 7.19 N 5.71 Cl 7.22 ;~
Found: 63.82 7.32 5.60 7.66
~'.
',. ::, ,. -.
~ ` ` 133~993
- 107 -
F.xample 86
2-[(N-(3-(4-MethoxY-~henoxy)-propyl)-pyrrolid-3-
~,)-methyl]-6~7-dimethyl-1,2,3,4-tetrahYdro-isoquinoline
dihyarochloride
.
Prepared from 2-[N-(3-(4-methoxy-phenoxy)-propyl)-
pyrrolid-3-yl)-methyl]-6,7-dimethyl-1-oxo-1,2,3,4-
tetrahydro-isoquinoline and lil:hium aluminium hydride
in tetrahydrofuran analogously to Example 3.
Yield: 90.9% of theory,
Melting point: 243-2465C
CalculatedoC 64.85H 7.95N 5.82Cl 14.73
Found: 64.88 7.92 5.63 14.80
.
Example 87
: . .'"
2-[(N-(2-(3,4-Dimethoxy-phenyl)-ethYl)-pyrrolid-
3-Yl)-methy~]-6,7-dimethoxy-1,2~3,4-tetrahYdro-
isoquinoline-dihydrochloride
Prepared from 2-t(N-(2-(3,4-dimethoxy-phenyl)-ethyl)-
pyrrolid-3-yl)-methyl]-6,7-dimethoxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline and lithium aluminium hydride
in tetrahydrofuran analogously to Example 3. i~
Yield: 92.9% of theory,
Melting point: 240-242C
Calculated: C 60.81 H 7.46 N 5.46 Cl 13.81
Found: 60.64 7.61 5.31 13.50 -
Example 88
2-[(N-(3-(Pyrid-4-yl)-prop~l)-pyrrolid-3-yl)-methyl]-
5,6-dimethyl-1-oxo-1,3-dihydro-isoindole-dihydrochloride-
semihYdrate ~
:: .
Prepared from 2-[(N-(3-pyrid-4-yl)-propyl)-pyrrolid-
3-yl)-methyl]-5,6-dimethyl-phthalimide and zinc/gl2cial
acetic acid analogously to Example 4.
- I0~ 3 3 ~ 9 9 3
Yield: 65~ of theory,
Melting point: 119-122C
Calculated: C 62.02 H 7.24 N 9.43 Cl 15.92
Found: 62.25 7.47 9.39 15.90
Example 89
2-[3-(N-(3-(PYrid-3-yl) -~ropYl) -PiPerid-3-yl)-propy-l]
5~6-dimethoxY-l-oxo-1~3-dihYdr--isoindole-dihydrochloride
monohYdrate
Prepared from 2-[3-(N-(3-tpyrid-3-yl)-propyl)-piperid-
3-yl)-propyl]-5,6-dimethoxy-phthalimide and zinc/glacial
acetic acid analogously to Example 4.
Yield: 72% of theory, '~
Melting point: 118-121C
Calculated: C 59.08 H 7.43 N 7.95 Cl 13.41
Found: 59.02 7.23 7.12 13.27
Example 90
2-~(N-(3-(Pyrid-3-Yl)-propyl)-Pi~erid-3-yl)-methyl]- -` ;
5,6-dimethoxy-1-oxo-1,3-dihydro-isoindole-dihydrochloride-
monohYdrate
Prepared fro~ 2-[(N-t3-tpyrid-3-yl)-propyl)-piperld-
3-yl)-methyl]-5,6-dimethoxy-phthalimide and zinc/glacial ;~
acetic acid analogously to Example 4.
Yield: 42% of theorY,
Melting point: 91-96C
Calculated: C 59.08H 7.43N 7.95Cl 13.41 ~
Found: 59.02 7.23 7.12 13.27 ;
ExamPle 91
2-[(N-t2-t6,7-Dimethoxy-isoquinol~4-yl)-ethy~
piperid-3-yl)-methyl]-5,6-dimethoxy-1-oxo-1,3-dihYdro- ,,'~
isoindole
~3~93
-- 109 --
Prepared from 2-[(N-(2-~6,7-dimethoxy-isoquinol-
4-yl)-ethyl)-piperid~3-yl)-methyl]-5,6-dimethoxy-
phthaLimide and zinc/glacial acetic acid analogously
to Example 4.
Yield: 64% of theory,
Melting point: 85-88C
Calculated: C 65.39H 7.19 N 7.88
Found~ 65.16 7.27 7.S3
Example 92
. .
2-[2-(N-(3-(Pyrid-4-yl)-propyl)-piperid-2-yl~-ethYl]-
5,6-dimethyl-1-oxo-lL3-dihydro isoindole ~-
Prepared from 2-[2-(N-(3-(pyrid-4-yl)-propyl)-piperid-
2-yl)-ethyll-5,6-dimethyl-phthalimide and zinc/glacial
acetic acid analogously to Example 4. -~
Yield: 73% of theory,
Melting point: 103-104C
Calculated: C 76.68 H 8.49 N 10.73
Found: 76.57 8.54 10.60
ExamE~e 93 ~:
2-[(N-(3-(Pyrid-4-yl)-propyl)-pyrrolid-3-Yl)-methyl]-
5~6-dimethyl-1~3-dihydro-isoindole-trihYdrochloride
semihYdrate
.- ..
Prepared from 2-[lN-~3-(pyrid-4-yl)-propyl)-pyrrolid-
3-yl)-methyl]-5,6-dimethyl-1-oxo-1,3-dihydro-isoindole
and lithium aluminium hydride in tetrahydrofuran/ether
analogously to Example 3.
Yield: 68% of theory,
Melting range: 118-127C (amorphous)
Calculated: C 59.03 H 7.54 N 8.98 Cl 22.73
Found: 58.93 7.48 8.84 22.92
Example 94
~" ` 13309~3
-- 110 --
2-[2-(N-(3-(PYrid-4-yl)-Propyl)-piperid-2-yl)-ethYl]-
5,6-dimethyl-1,3-dihydro-isoindole
Prepared from 2- E 2-(N-(3-(pyrid-4-yl)-propyl)-piperid-
2-yl)-ethyl]-5,6-dimethyl-1-oxo-1,3-dihydro-isoindole
and lithium aluminium hydride in tetrahydrofuran/ether ~ ` .
analogously to Example 3.
Yield: 70% of theory, ~ -:
Melting range: 135-148C (amorphous)
Calculated: C 54.59 H 8.24 N 7~63 Cl 19.33
Found: 54.48 8.26 7.51 19.60 ~ . :
ExamPle-95
2-[(N-(Pyrid-4-yl-methyl)-~perid-3-~Yl)-methyl]- ~;
5,6-dimethoxY-1,3-dihydro-isoindole-trihydrochloride-
trihYdrate
Prepared from 2-[(N-(pyrid-4-yl-methyl)-piperid- - ~ ~-
3-yl)-methyl]-5,6-dimethoxy-phthalimide and lithium
a~uminium hydride in ether analogously to Example
3.
Yield: 68% of theory, : :-~
Melting range: 176-189C (amorphous)
Calculated: C 49.76H 7.21 N 7.91 Cl 20.03 ~.
Found:49.93 7.12 8.00 20.44 ~.
~: ' ' `
- :
:...; ~.
33~3
Example_96
:, '
2-[(N-(2-(6,7-Dimethoxy-isoquinol-4-yl_-ethy~-
piperid-3-yl) -methyl]-5~6-dimethYl-l~3-dihydro-
isoindole-dihydrochloride-semihydrate ~-
Prepared from 2-[(N-(2-(6,7-dimethoxy-isoquinol-
4-yl)-ethyl)-piperid-3-yl)-met:hyl]-5,6-dimethyl-
l-oxo-1,3-dihydro-isoindole and lithium aluminium
hydride in tetrahydrofuran/ether analogously to
Example 3.
Yield: 22.7% of theory,
Melting range: 222-236C (amorphous~
Calculated: C 62.22 H 7.20 N 7.50Cl 15.83
Found: 62.01 7.64 7.08 15.79 - -~
~ ;.' ~'' ~'
Example 97
2-[(N-(Pyrid-4-yl-methYl)-piperid-3-yl)-methyl]-
5,6-methylenedioxy-1,3-dihyd_o-isoindole-trihydrochloride-
semihydrate
: . .
Prepared from 2-[(N-(pyrid-4-yl-methyl)-piperid-
3-yl)-methyl]-5,6-methylenedioxy-phthalimide and
lithium aluminium hydride in tetrahydrofuran/ether
analogously to Example 3. ;
Yield: 55% of theory
Melting range: 215-225C (amorphous)
Calculated: C 53.68 H 6.22 N 8.94Cl 22.63
Found: 53.60 6.45 8.65 22.28
Example 98
~ ~
2-[(N-(3-(Pyrid-4-yl)-propyl)-pyrrolid-3-yl)-methyl]-
6,7-dimethyl-1,2,3,4-tetrahydro-isoquinoline-trihydro- ;
chloride-monohydrate
33~93
- 112 -
Prepared from 2-[(N-(3-(pyrid-4-yl)-propyl)-pyrrolid-
3-yl)-methyl]-6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-
isoquinoline and lithium aluminium hydride in tetrahydro-
furan/ether analogously to Example 3.
Yield: 63~ of theory, ~ -~
Melting point: 254-256C ~ ~
Calculated: C 58.71 H 7.80 N 8.56 Cl 21.66 -
Found: 58.53 7.72 8.25 21.53
ExamPle 99
2-[!N-(PYrid-4-Yl-methyl)-piPerid- 3 yl)-methYl]~
6,7-dimethoxy-1,2,3,4-tetrahydro-isoquinoline-trihydro-
chloride-dihYdrate -
Prepared from 2-[(N-~pyrid-4-yl-methyl)-piperid~
3-yl)-methyl]-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro- ~ -`
isoquinoline and lithium aluminium hydride in tetrahydro-
furan/ether analogously to Example 3. ~ -
Yield: 63~ of theory,
Melting range: 158-169C lamorphous)
Calculated: C 52.42 H 7.27 N 7.97Cl 20.18
Found: 52.55 7.49 7.57 20.25
Example 100
2-[2-(N-(3-(Pyrid-4-yl)-propyl)-piperid-2-Yl)-ethyl]-
6,7-methYlenedioxy-1,2,3,4-tetrahydro-isoquinoline-
trihYdrochloride-trihydrate
Prepared from 2-r2-(N-(3-(pyrid-4-yl)-propyl)-piperid-
2-yl~-ethyl]-6,7-methylenedioxy-1-oxo-1,2,3,4-tetrahydro- `-~
isoquinolin~ and lithium aluminium hydride in tetrahydro-
furan/ether analogously to Example 3.
Yield: 90~ of theory, `
Melting range: 108-119C (amorphous)
Calculatad: C 52.58 H 7.41 N 7.36Cl 18.62
Found: 52.56 7.25 7.38 19.49 ~
- ': ,
`-```` . `` ` ` ~ 33~3
.
- 113 -
Example 101 ~ ~ -
2-~(N-(3-~Pyrid-3-yl)-Pro~yl)-piperid-3-Yl)-methYl]-
6,7-methylenedioxy-l,2,3,4-tetrahydro-isoqUinoline-
dihyarochloride-monohy-drate
Prepared from 2-[(N-(3-~pyrid-3-yl)-propyl)-piperid~
3-yl)-methyl]-6,7-methylenedioxy-1-oxo-1,2,3,4~
tetrahydro-isoquinoline and lithium aluminium hydride
in tetrahydrofuran/ether analogously to Example
3.
Yield: 72~ of theory,
Melting range: 126-138C (amorphous)
Calculated: C 59.50 H 7.28 N 8.67 Cl 14.64
Found: 59.57 7.29 8.49 14.51 ~ ~
- ' ~,
Example 102 ~;
2-[~N-(3-(Pyrid-3-yl)-propyl)-piperid-3-Yl)-methyl]-
6~7-dimethyL-1~2~3~4-tetrahydro-isoquinoline-trihydro-
chloride
Prepared from 2-[(N-(3-(pyrid-3-yl)-propyl)-piperid-
3-yl)-methyl]-6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-
isoquinoline and lithium aluminium hydride in tetrahydro-
furan/ether analogously to Example 3.
Yield: 59~ of theory,
Melting range: 138-154C (amorphous)
Calculated: C 61.66 H 7.86 N 8.62 Cl 21.a4
Found: 61.53 8.00 8.64 21.35
~,
Example 103
2-~(N-(3-(Pyrid-4-Yl)-propyl)-p~_rolid-3-yl)-methyl]-
6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-isoquinoline-
dihydrochloride-monohydrate
33a~s3 .:
- 114 -
Prepared from 6,7-dimethyl 1-oxo-1,2,3,4-tetrahydro-
isoquinoline and 3-chloromethyl-N-[3-(pyrid-4-yl)- ~ ~-
propyl]-pyrrolidine analogously to Example 2.
Yield: 39~ of theory, ;
Melting range: 74-86C (amorphous)
Calculated: C 61.53 H 7.53 N 8.97 C1 15.13
Found: 61.42 7.62 8.83 15.05
Example 104
2-[~N-(3-(Pyrid-3-Yl)-~ropyl)-piperid-3-yl)-methYl]- -~
6 r 7-dimethoxY-1-oxo-1,2,3,4-tet_ahYdro-isoquinoline-
dihYdrochloride-monohYdrate
Prepared from 6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline and 3-(benzenesulphonyloxy-
methyl-N-[3-(pyrid-3-yl)-propyl]piperidine in dimethylsulphoxide
with potassium tert.butoxide analogously to Example 2.
Yield: 45% o theory,
Melting range: 140-148C (amorphous)
Calc. (x 2 HC1 x H2O): C 58.36 H 7.25N 8.16 Cl 13.78
Found: 58.35 7.32 8.04 13.65
Example 105
..;.^, ~ ~ .
2-E3-(N-(3-(Pyrid-4-yl)-propyl)-piperid-3-yl)-propyl]
6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-isoquinoline-
dihydrochloride-dihydrate
Prepared from 2-[3-(piperid-3-yl)-propyl]-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and 4-(3-
chloropropyl)-pyridine analogously to Example 1.
Yield: 48% of theory,
Melting range: 85-96C (amorph)
Calculated:C 57.84H 7.73N 7.49Cl 12.65
Found: 57.71 7.91 7.35 13.04
Example 106
" .:
: ~
~` ~
` 13~9~
- 115 -
2-[3-tN-t2-~6,7-Dimethoxy-isoquinol-4-yl~-ethYl)-
pi~erid-3-yl)-propyl]-6,7-dimethoxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline-dihYdrochloride-monohYdrate
,
Prepared from 2-[3-(pyrid-3-yl)-propyl]-6,7-dimethoxy-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and 4-(2-
chloroethyl1-6,7-dimethoxy-isoquinoline analogously
to Example 1.
Yield: 34% of theory,
Melting range: 162-171C (amorphous)
Calculated: C 60.17 H 7.10 N 6.57 Cl 11.10
Found: 59.85 7.00 6.86 11.04
ExamPle 107
2-[(~-53-(Pyrid-3-yl)-propyl)-piperid-3-yl)-methYl]-
6,7-methylenedioxy-1-oxo-1,2,3,4-tetrahydro-isoquinoline-
hydrochlorîde ~ -
Prepared from 6,7-methylenedioxy-1-oxo-1,2,3,4- ~ ~
tetrahydro-isoquinoline and 3-chloromethyl-N-[3- ~;
(pyrid-3-yl)-propyl]-piperidine analogously to
Example 2.
Yield: 60~ of theory,
Melting range: 194-196C (amorphous)
Calculated: C 64.92 H 6.81 N 9.46 Cl 7.98
Found: 64.91 6.95 9.67 7.80
Example 108
~'~
2-[(N-(Pyrid-4-Yl-methyl)-piperid-3~yl)-methYl]-
6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-isoquinoline-
dihydrochloride-monohydrate
'
Prepared from 6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline in dimethylsulphoxide with potassium
tert.butoxide and 3-chloromethyl-N-[pyrid-4-
yl-methyl]-piperidine analogously to Example 2.
~33~3
- 116 -
Yield: 41% of theory, ~ -
Melting range: 142-158C (amorphous)
Calculated: C 56.78 H 6.83 N 8.63Cl 14.58 ~
Found: 56.45 6.59 8.66 14.62 ~-
ExamPle 109 :.
2-[(N-(2-(6,7-Dimethoxy-isoquinol-4-yl)-ethyl)-
piperid-3-yl)-meth~1]-6,7-dimethyl-1-oxo-1,2,3,4-
tetrahYdro-isoquino-line-dihydrochloride
Prepared from 6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-
isoquinoline in dimethylsulphoxide with potassium
tert.butoxide and 3-chloromethyl-N-[2-(6,7-dimethoxy-
isoquinol-4-yl)-ethyl]-piperidine analogously to
Example 2.
Yield: 62% of theory, ~-~
Melting range: 148-162C ~amorphous)
Calculated: C 64.27 H 7.01 N 7.49Cl 12.65
Found: 64.11 7.20 7.59 12.89
Example 110
2-L2-(N=~3-(PYrid- 4-Yl) -~ropYl)-~iperid-2-yl)-ethyl]-
6~7-methylenedioxy-l-oxo-1~2,3~4-tetrahYdro-isoquinoline- ~ -~
dihYdrochloride-dihydrate
Prepared from 6,7-methylenedioxy-1-oxo-1,2,3,4- -~
tetrahydro-isoquinoline and 2-(2-chloroethyl)-N-
r3-(pyrid-4-yl)-propyl]-piperidine analogously
... ..
to Example 2.
Melting range: 115-128C (amorphous)
Calculated: C 56.59 H 7.03 N 7.92 Cl 13.37
Found: ~ 56.61 6.90 7.8413.41
` ` 133~93
- 1~7 -
Example 111
2-[ (N- (3-tPyrid-3-~1)-propyl~-piperid-3-yl)-methyl]-
6,7 dimethyl-1-oxo-1,2,3,4-tetrahYdro-isoquinoline-
dihYdrochloride-dihydrate
Prepared from 6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-
isoquinoline in dimethylsulphoxide with potassium
tert.butoxide and 3-chloromethyl-N-[3-~pyrid-3-
yl)-propyl]-piperidine analogously to Example 2.
Yield: 62% of theory,
Melting range: 118-127C (amorphous~
Calculated: C 60.00 H 7.85 N 8.39 Cl 14.16
Found: 60.24 8.07 8.36 14.62
Example 112
2-~(N-(3-(Pyrid-3-yl)-propyl)-pyrrolid-3-yl)-methyl]-
6,7-methYlenedioxy-l-oxo-1,2,3,4-tetrahydro-isoquinoline-
dih~drochloride
Prepared from 6,7-methylenedioxy-1-oxo-1,2,3,4
tetrahydro-isoquinoline and 3-chloromethyl-N-[3-
(pyrid-3-yl)-propyl]-pyrrolidine analogously to
Example 2.
Yield: 26% of theory,
Melting range: 102-113C (amorphous)
Calculated: C 59.22 H 6.27 N 9.01 Cl 15.20 ~ ;~
Found: 59.27 6.49 8.92 14.48 ;~
::
Example 113
2-[(N-~3-(Pyrid-4-yl)-propyl)-pyrrolid-3-yl)-methYl]-
7,8-methylenedioxy-2-oxo-1,3-dihydro-2H-3-benzazePine-
dihydro~hloride
Prepared from 7,8-methylenedic~xy-2-oxo-1,3-dihydro-
2H-3-benzaze~pine and 3-chloromethyl-N-[3-~pyrid-4-
' ~` .", ~"~.
~l3309~
- 118 -
yl)-propyl]-pyrrolidine analogously to Example
2.
Yield: 58~ of theory, - ~
Melting range: 132-141C (amorphous) ~ -
Calculated: C 60.25 H 6.11 N 8.78Cl 14.82
Founa: 60.00 6.40 8.52 14.56
.`
Example 114
2-[(N-(3-(Pyrid-3-yl)-propvl)-piperid-3-Yl)-methyl]- `~
7,8-dimethoxY-2-oxo-1,3-dihydro-2H-3-benzazepine-
....
dihydrochloride-dihydrate
Prepared from 7,8-dimethoxy 2-oxo~1,3-dihydro-2H-3-
benzazepine and 3-chloromethyl-N-[3-(pyrid-3-yl)-
propyl]-piperidine analogously to Example 2.
Yield: 67% of theory,
Melting range: 128-134C (amorphous)
Calculated: C 57.34 H 7.21 N 7.71Cl 13.02
Found: 57.80 7.37 7.92 13.06
Example llS
3-[(N-(3-(Pyrid-3-yl)-proPYl)-piperid-3-Yl)-methYl]
7,8-dimethyl-2-oxo-1,3-dihydro-2H-3-benzazepine~
dihydrochloride-dihydrate -
Prepared from 7,8-dimethyl-2-oxo-1,3-dihydro-2H-3-
benzazepine and 3-chloromethyl-N-[3-(pyrid-3-yl)- ~ `
propyl]-piperldine analogously to Example 2.
Yield: 94% of theory,
Melting range: 77-86~C (amorphousi
Calculated:C 60.92H 7.67N 8~19Cl 13.83
Found: 60.75 7.60 8.37 13.72
.'' ' :
~'` ~,.
~.. ~ ,.
~'` ': ' ,~:
~ 133~93
- 119 -
Example 116
3-[2-(N-(3-(Pyrld-4-Yl)-propyl)-piperid-2-yl)-ethYl~-
7,8-dimethyl-2-oxo-1,3-dihYdro-2H-3-benzazepine-
dihydrochloride
Prepared from 7,8-dimethyl-2-oxo-1,3-dihydro-2H-3-
benzazepine and 2-(2-chloroethyl)-N-[3-(pyrid-4-
yl)-propyl]-piperidine analogously to Example 2.
Yield: 94% of theory,
Melting range: 118-130C ~amorphou~)
Calculated: C 66.11 H 7.60 N 8.56 Cl 14.45
Found: 65.92 7.86 8.33 14.09
Example 117
,
3-[(N-(2-~6,7-Dimethoxy-isoquinol-4-yl)-ethyl)-
piperid-3-Yl)-methyl]-7,8-dimethoxy-2-oxo-1,3-dihydro-
2H-3-benzazepine-monohydrate
Prepared from 7,8-dimethoxy-2-oxo-1,3-dihydro-2
3-benzazepine and 3-chloromethyl-N-[2-(6,7-dimethoxy-
isoquinoL-4-yl)-ethyL]-piperidine analogously to
Example 2.
Yield: 69% of theory, -
Melting range: 85-96C (amorphous) ~ -
Calculated: C 67.73 H 7.15 N 7.64
Found: 67.96 7.19 7.75
Example 118
3-[3-(N-(3-(Pyrid-3-yl)-propyl)-piperid-3-yl)-propyl]-
7,8-dimethoxy-2-oxo-1,3-dihydro-2H-3-benzazePine-
dihydrochloride-monohYdrate
Prepared from 7,8-dimethoxy-2-oxo-1,3-dihydro-2H-
3-benzazepine and 3-(3-chloropropyl)-N-[3-(pyrid-
3-yl)-propyl]-piperidine analogously to Example
2.
-
L33~39~
- 120 -
~ield: 72~ of theory,
Melting range: 94-106~ (amorphous)
Calculated: C 60.64 H 7.45 N 7.57 Cl 12.78
Found: 60.80 7.44 7.46 12.59
Example 119
3-[2-(N-(3-~Pyrid-4-yl)-propyl)-piperid-2-yl)-ethyl]-
7,8-methylened_ oxY-2-oxo-l, 3-dihydro-2~-3-benzazepine-
dihydrochloride-dihy~t~
Prepared from 7,8-methylenedioxy-2-oxo-1,3-dihydro-
2~-3-benzazepine and 2-(2-chloroethyl)-N-13-(pyrid-
4-yl)-propyll-piperidine in dimethylsulphoxide
and potassium tert.butoxide analogously to Example
2.
Yield: 67% of theory, ~ ~
Melting range: 148-161C (amorphous) - ~ ~ -
Calculated: C 57.56 H 6.87 N 7.74 Cl 13.07
Found: 57.72 7.03 7.61 13.62
Example 120
3-[(N-(Pyrid-4-yl-methyl)-piperid-3-yl)-methyl]-
7,8-dimethyl-2-oxo-1,3-dihYdro-2H-3-benzazepine
dihYdrochloride-monohydrate
Prepared from 7,8-dimethoxy-2-oxo-1,3-dihydro-2H-
3-benzazepine and 3-chloromethyl-N-[pyrid-4-
yl-methyl]-piperidine in dimethylsulphoxide and
potassium tert.butoxide analogously to Example
Yield: 75~ of theory,
Melting range: 113-127C ramorphous)
Calculated: C 61.79H 7.13 ~ 9.01 Cl 15.20
Found:61.55 7.32 9.04 15.11
.' ` ~' .
.
:
,'' I A '
~L33~3
- 121 -
Example 121
3-[(N- ( 3-(Pyrid-4-yl)-propyl)-pyrrolid-3-yl~-methY1~-
7~8-methylenedioxy-2-oxo-l~3r4~5-tetrahyaro-2H
3-benzazepine-dihydrochloride-monohydrate
Prepared from 3-[~N-(3-(pyrid-4-yl)-propyl)-pyrrolid-
3-yl)-methyl]-7,8-methylenedioxy-2-oxo-1,3-dihydro- ~-
2H-3H-benzazepine and 10~ palladium/charcoal under
a hydrogen pressure of 5 bar at ambient temperature
analogously to Example 5.
Yield: 71% of theory,
Melting range: 95-106C (amorphous)
Calculated: C 57.83 H 6.67 N 8.43Cl 14.22
Found: 57.67 6.82 8.27 14.05
Example 122
3-[2-(N-(3-~Pyrid-4-yl)-propyl)-piperid-2-yl)-ethYl~
7,8-dimethyl-2-oxo-1,3,4,5-tetrahYdro-2H-3-benzazepine- -
dihydrochloride-semihydrate
~ . '
Prepared from 3-[2-(N-(3-tpyrid-4-yl)-propyl)-piperid-
2-yl)-ethyl]-7,8-dimethyl-2-oxo-1,3-dihydro-2H-
3-benzazepine and 10% palladium/charcoal under
a hydrogen pressure of 5 bar at 80C analogously
to Example 5. ;~
Yield: 73% of theory,
Melting point: 236-238C
Calculated: C 64.65 H 8.04 N 8.37 Cl 14.14
Found: 64.91 8.02 8.2513.92
^: ~ 133~3
..
..
- 122 -
Example 123
3-[3-(N-t2-(2-Methyl-pYrid-6-yl)-ethyl)-piperid-
3-yl)-propyl]-7,8-dimethoxY-2-oxo-l~3~4~5-tetrahydr
2H-3-benzazepine-dihydrochlori;de-monohYdrate -~
Prepared from 3-[3-(piperid-3-yl)-propyl)]-7,8-
dimethoxy-2-oxo~1,3,4,5-tetrahydro-2H-3-benzazepine
and 2-(benzenesulphonyloxy-ethyl)-6-methyl-pyridine
in dimethylsulphoxide with potassium carbonate
at 120C analogously to Example 1.
Yield: 29% of theory,
Melting range: 105-113C (amorphous)
Calculated: C 60.42 H 7.78 N 7.55
Found: 60.68 7.50 7.42
Exam~le 124
3-[(N-(3-(Pyrid-3-yl)-pro~yl)-piperid-3-yl)-methyl]-
7,8-dimethoxy-2-oxo-1,3,4,5-tetrahYdro~2H-3-benzaze~e-
dihydrochloride
, ~ .
Prepared from 3-[(N-(3-(pyrid-3-yl)-propyl)-piperid-
3-yl)-methyl]-7,8-dimethoxy-2-oxo-1,3-dihydro-2H-
3-benzazepine by hydrogenation at 5 bar hydrogen
pressure in ethanol with 10~ palladium charcoal
at 70C analogously to Example 5.
Yield: 52% of theory,
Melting range: 113-122C (amorphous)
Calculated: C 61.17 H 7.30 N 8.23
Found: 61.31 7.50 8.28
,
- `` . ` ~L33~3
.
- 123 -
Example 125
3-[(N-~2-(6,7-Dimethoxy-isoauinol-4-yl~-ethyl)-
piperid-3-yl)-methYl]-7,8-dimethoxy-2-oxo-1,3,4,5-
tetrahYdro-2H-3-benzazePine x ~ H20
Prepared from 3-[(N-(2-(6,7-dimethoxy-isoquinol-
4 yl)-ethyl)-piperid-3-yl)-methyl]-7,8-dimethoxy-
2-oxo-1,3-dihydro-2H-3-benzazepine by hydrogenation
at 5 bar hydrogen pressure with 10% palladium/charcoal
in ethanol at 70C analogously to Example 5.
Yield: 50~ of theory, -
Melting range: 82-86C (amorphous)
Calculated: C 66.40 H 7.55 N 7.48
Found: 66.26 7.50 7.59 ~ ;
Example 126
3-[3-(N-~3-(Pyrid-3-yl)-propyl~-piperid-3-Y1)-propyl]- ~ ~ -
7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine-
dihydrochloride-dihydrate
'` ' ~':
Prepared from 3-~3-(N-(3-~pyrid-3-yl)-propyl)-piperid-
3-yl)-propyl]-7,8-dimethoxy-2-oxo-1,3-dihydro-2H~
3-benzazepine by hydrogenation at 5 bar hydrogen
pressure with 10% palladium/charcoal in ethanol
at 70C analogously to Example 5.
Yield: 64~ of theory,
Melting range: 106-115C (amorphous) ~ -~
Calculated: C 58.53 H 7.89 N 7.31 Cl 12.34
Found: 58.46 7.61 7.14 12.57
~ '
: ::
~; ` i33~93
- 124 -
Example 127
3-l(N-(3-(Pyrid-3-y~-eropyl)-piperid-3-yl)-methyl]-
7,8-methylenedioxy-2-oxo-1,3,4,5-tetrah~dro-2H-
3-benzazepine-dihydrochloride-monohydrate
Prepared from 3-[(N-(3 (pyrid-3-yl~-propyl~-piperid- ::
3-yl)-methyl]-7,8-methylenedioxy-2-oxo-1,3-dihydro-
2H-3-benzazepine at 5 bar hydrogen pressure with
10% palladium/charcoal in ethanol at 80C analogously
to Example 5.
Yield: 81% of theory,
Melting range: 126-138C (amorphous)
CalculatedsC 58.58 H 6.88 N 8.19 Cl 13.83
Found:58.43 7.00 7.85 13.71 .
Example 128
..
, ~ : ::
3-[2-(N-(3-(Pyrid-4-yl)-propyl)-piperid-2-Yl)-ethyl]-
7,8-methy~enedioxy-2-oxo-1,3,4,5-tetrahydro-2H-
3-benzazepine-dihydrochloride-monohydrate
- "'
Prepared from 3-[2-(N-(3-(pyrid-4-yl)-propyl)-piperid-
2-yl)-ethyl]-7,8-methylenedioxy-2-oxo-1,3-dihydro- :~
2H-3-benzazepine at 5 bar hydrogen pressure with
10% palladiumjcharcoal in ethanol at 80C analogously ::
to Example 5.
Yield: 74% of theory,
Melting range: 132-146C (amorphous) :-:
Calculated: C 59.31 H 7.08 N 7.98 Cl 13.46
.
Found: 59.18 7.41 7.80 13.25
.
~ ~ 3 3 ~
- 125 -
Example 129
3-[(N-(3=(Pyrid-3-yl)-Propyl)-piperid-3 Yl~-methyll-
7 ! 8-dimethyl-2-oxo-1,3,4,5-tetrahydro-2H-3-benzaze~ine-
dihydrochloride
Prepared from 3-[(N-~ 3-~pyrid-3-yl)-propyl~-piperid-
3-yl)-methyll-7,8-dimethyl-2-oxo~1,3-dihydro-2H- ~:
3-benzazepine and 5 bar hydrogen pressure with
10~ palladium/charcoal in ethanol at 80C analogously
to Example 5.
Yield: 54% of theory,
Melting range: 92-105C (amorphou~
Calculated: C 62.90 H 7.92 N 8.46Cl 14.28
Found: 63.19 7.90 8.45 14.30
Example 130 ~;!r-~
3-[( N- ( 3-(Pyrid-4-yl)-propyl)-pyrrolid-3-yl)-methyl~-7,8-
methylenedioxy-1,3,4,5-tetrahydro-2H-3-benzazepine-
trihydrochloride-monohydrate
Prepared from 3-[(N-(3-(pyrid-4-yl) propyl)-pyrrolid-
3-yl)-methyl]-7,8-methylenedioxy-2-oxo-1,3,4,5-
tetrahydro-2H-3-benzazepine and lithium aluminium
hydride in tetrahydrofuran/ether analogously to
Example 3.
Yield: 73% of theory,
Melting range: 96-108C (amorphous~
Calculated: C 55.53 H 6.97 N 8.06Cl 20.42
Found: 55.06 7.28 7.77 20.07 ;
Example 131
3-[(N-(3-tPyrid-3-yl)-propyl)-piperid-3-yl)-methyl~
7,8-dimethoxy-1,3,4,5-tetrahYdro-2H-3-benzazepine-
dihydrochloride
`''`~'`~'''''''
r~ ; ' `
` ~33~993
- 126 -
Prepared from 3-[(N-(3-(pyrid-3-yl)-propyl~-piperid-
3-yl)-methyl~-7,8-dimethoxy-2-oxo-],3,4,5-tetrahydro-
2H-3-benzazepine and lithium aluminium hydride
in tetrahydrofuran/ether analogously to Example 3.
Yield: 71~ of theory, ;~
Mielting point: 208-210C
Calculated: C 62.89 H 7.91 N 8.46 Cl 14.28
Found: 62.70 7.53 8.2214.50
Example 132
3-[2-(N-~3-(PYrid-4-yl)-propYl)-piperid-2-yl)-ethYl]-
7,8-methylenedioxy-1,3,4,5-tetrahydro-2H-3-benzazepine-
trihydrochloride
Prepared from 3-[2-(N-(3-(pyrid-4-yl)-propyl)-piperid-
2-yl)-ethyl]-7,8-methylenedioxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine and lithium aluminium hydride
in tetrahydrofuran/ether analogously to Example 3.
~ield: 63% of theory,
Melting range: 123-136C (amorphous)
Calculated: C 58.81 ~ 7.21 N 7.91 Cl 20.00
Found: 58.51 7.41 7.92 19.86
Example 133
3-~(N-(3-(Pyrid-3-yl)-propyl)-piperid-3-yl)-methyl]-
7,8-methylenedioxy-1,3,4,5-tetrahydro-2H-3-benzazepine-
trihydrochloride-semihydrate ;
Prepared from 3~[(N-(3-(pyrid-3-yl)-propyl)-piperid-
3-yl)-methyl]-7,8-methylenedioxy-2-oxo-1,3,4,5-
tetrahydro-2H-3-benzazepine and lithium aluminium
hydride in tetrahydrofuran/ether analogously to
Example 3. `
Yield: 75~ of theory,
Melting range: 126-138C (amorphous)
Calculated: C 57.09 H 7.10 N 7.99Cl 20.22
` ~330993
Found: 57.05 7.32 8.05 20.37
Example 134
. .
3-[2-(N-~3-~PYrid-4-yl)-pro~yl~-PiPerid-2-yl)-ethyl]-
7,8-dimethyl-1,3,4,5-tetrahydro-2H-3-benzazepin~
_ihydrochloride
Prepared from 3-[2-(N-(3-(pyrid-4-yl~-propyl~-piperid-
2-yl~-ethyl]-7,8-dimethyl-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine and lithium aluminium hydride "'~ `'5'~
in tetrahydrofuran/ether analogously to Example
3.
Yield: 92~ of theory,
Melting point: 180-182C
Calculated: C 62.96 H 8.22 N 8.16 Cl 20.65
Found: 63.00 8.29 8.1620.34
Example 135
~ .
3-r(N-(3-(Pyrid-3-yl)-propyl)-piperid-3 yl)-methYl]-
7,8-dimethyl-1,3,4,5-tetrahydro-2H-3-benzazepine-
trihydrochloride
Prepared from 3-[(N-(3-(pyrid-3-yl)-propyl)-piperid-
3-yl)-methyl]-7,8-dimethyl-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine and lithium aluminium hydride -
in tetrahydrofuran/ether analogously to Example
3. -
Yield: 81% of theory,
Melting range: 184-196C (amorphous~
Calcuiated: C 60.17 H 8.16 N 8.10Cl 20.49 -
Found: 60.28 8.25 8.00 20.39
Example 136
.``'', '~:". '`'
3-L2-(N ~2-(6-MethoxY-naphth-2-yl)-ethyl)-piperid-
2-yl)-ethyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine-hydrochloride
~ ~33~93 ~
~. ~
- 128 - ~-
Prepared from 3-[2-(piperid-2-yl)-ethyl]-7,8-dimethoxy- -~
2-oxo~1,3,4,5-tetrahydro-2H-3-benzazepine and 2- -~
(6-methoxy-naphth-2-yl)-ethyl bromide analogously
to Example 1.
Yield: 20~ of theory,
Melting point: 158-160C
Calculated:C 69.48H 7.47 N 5.06Cl 6.41
Found: 69.40 7.56 5.17 6.62 ~
,.:
Example 137 ~
~ .'' :~`
3-[2-(N-~2-~5-~ethyl-6-methoxy-n~hth-2-yl)-ethyl)-
piperid-2-Yl)-ethyl]-7,8-dimethoxy-2-oxo-1,3,4,5-
tetrahydro-2H-3-benzazepine-hydrochloride
': ~
Prepared from 3-[2-(piperid-2-yl)-ethyl]-7,8-dimethoxy- ~ ~ -
2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine and 2-
(5-methyl-6-methoxy-naphth-2-yl)-ethyl bromide
analogously to Example 1.
Yield: 28~ of theory,
Melting point: 140-143C
Calculated: C 69.88 H 7.64 N 4.94 Cl 6.25
- Found: 69.06 7.574.846.44 ~
~: ` :, ' Example 138
3-[2-(N-(2-(NaphthYl-l-oxy)-et~yl)-piperid-2-yl)-
ethyl]-7,8-dimethoxy-2-oxo-1,3j4,5-tetrahyaro-2H-
3-benzazepine-hydrochloride
Prepared from 3-[2-(piperid-2-yl)-ethyl]-7,8-dimethoxy-
2-oxo-1,3,4,5-tetrahydro-2H-3-beniazepine and 2-
. -. . .
(naphthyl-l-oxy)-ethyl bromide analogously to Example 1.
Yield: 27~ of theory,
Melting pOillt: 146-148C ~-
. . . -
Calculated: C 69.06 ~ 7.29 N 5.20 Cl 6.58
Found: 69.00 7.075.31 6.68
" . ~33~3
:~ .
- 129 -
Example 139
3-[ 2- (N- ~2- (Naphth-l-Yl~ -ethYl)-piperid-2-yl)-ethyl]-
7,8-dimethoxv-2-oxo-1,3,4,5-tetrahydro-2H-3-benZazepine- y;~
hydrochloride
Prepared from 3-[2-(piperid-2-yl)-ethyl]-7,8-di~ethoxy-
2-oxo-1,3,4,5-tetrahydro-2~-3-benzazepine and 2- -
(naphth-l-yl)-ethyl bromide analogously to Example 1.
Yield: 24% of theory,
Melting point: 148-150C
Calculated: C 71.18 H 7.51 N 5.36 Cl 6.78
Found: 70.92 7.44 5.57 7.06
- .
Example 140
3-[2-(N-~4-(Naphthyl-2-oxy)-butyl)-~e_rid-2-yl)-
ethyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-2H-
benzazepine-hydrochloride
Prepared from 3-[2-(piperid-2-yl)-ethyl]-7,8-dimethoxy-
2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine and 4-
(naphthyl-2-oxy)-butyl bromide analogously to Example 1.
Yield: 39% of theory,
Melting point: 112-114C
Calculated: C 69.88 H17.64 N 4.64 Cl 6.25
Found: 69.69 7.5a 4.82 6.52
Example 141 -
3-[2-(N-(2-(Naphth-2-yl)-ethyl)-piperid-2-yl)-ethyl]- ;
7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-2H-3-benZazepine- - -
hydrochloride
Prepared from 3-[2-(piperid-2-yl)-ethyl]-7,8-dimethoxy-
2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine and 2-
(naphth-2-y:L)-ethyl bromide analogously to Example 1
Yield: 41~ of theory,
~` ~330~93
- 130 -
Melting point: 120-122C ~
Calculated: C 71.l8 H 7.51 N 5.36 C1 6.78 ~-
Found: 71.10 7.31 5.40 7.05 ~-
Example_142
3-[2-(N-((2-Methyl-naphth-l-yl)-methyl~-piperid- -~
2-yl?-ethyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine-hydrochloride
Prepared from 3-[2-(piperid-2-yl)-ethyl]-7,8-dimethoxy-
2-oxo-1,3,4,5- etrahydro-2H-3-benzazepine and 1-
chloromethyl-2-methyl-naphthalene analogously to
Example 1.
Yield: 24~ of theory,
Melting point: 144-146C
Calculated: C 71.18 H 7.51 N 5.36 Cl 6.78
Found: 70.93 7.38 5.48 6.89
"~ ' ' '~
Example 143
3-[(N-(2-(Naphth~2-Yl)-ethyl)-hexahydro-azepin-
3-Yl)-methyl]-7~8-dimethoxy-2-oxo-l~3~4~s-tetrahydr
2H-3-benzazepine-hydrochloride
Prepared from 3-[thexahydro-azepin-3-yl)-methyl]-
7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine
and 2-(naphth-2-ylj-ethyl bromide analogously to
Example 1.
Yield: 58~ of theory,
Melting point: 204-205C
Calculated: C 71.18 H 7.52 N 5.36 Cl 6.78
Found: 71.41 7.51 5.35 6.50
~` 133~93
- 131 -
Example 144
3-[(N-t2-(Naphth-2-Yl)-ethyl)-~iperid-3-yl)-methYl]-
7,8-dimethoxy-2-oxo-1,3,4,5-tetrahYdro-2H-3-benzazepine- - -
hydrochloride
Prepared from 3-[(piperid-3-yl)-methyl]-7,8-dimethoxy-
2-oxo-1,3,4,5-tetrahydro~2H-3-benzazepine and 2-
~naphth-2-yl)-ethylbromide analogously to Example
1.
Yield: 35% of theory,
Melting point: 239-240C
Calculated: C 70.78 H 7.33 N 5.50 Cl 6.96
Found: 7Q.70 7.10 5.46 7.15
. '':
Example 145
3-[(N-((NaPhth-2-yl)-methyl)-piperid-3-yl)-methyl]-
7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-2H-3-benzazePine ~-
Prepared from 3-[~piperid-3-yl)-methyl]-7,8-dimethoxy-
2-oxo-1,3,4,5-tetrahydro-2H 3-benzazepine and 2-
bromomethyl-naphthalene analogously to Example -;
Yield: 27% of theory,
Melting point: 176-177C ~ ~
Calculated: C 75.95 H 7.47 N 6.11 P - ~ -
Found: 76.11 7.28 6.10
Example 146
3--[(N-(4-(NaphthYl-2-oxy)-butyl)-pipe~id-3-Yl)-
methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine-hydrochloride
Prepared from 3-E(piperid-3-yl)-methyl]-7,8-dimethoxy- -
2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine and 4- -
~naphthyl-2-oxy)-butylbromide analogously to Example 1. ~
. .
~330993
- 132 -
Yield: 24% of theory,
Melting point: 196-197C
Calculated: C 69.48 H 7.47 N 5.06 Cl 6.4l
Found: 69.30 7.36 4.99 6.56
Example 147
3-[(N-(4-(Naphth-l-yl)-ethyl)-piperid-3-yl)-methyl]-
7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine-
hYdrochloride
Prepared from 3-[(piperid-3-yl)-methyl]-7,8-dimethoxy-
2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine and 2-
(naphth-l-yl)-ethyl bromide analogously to Example 1.
Yield: 18~ of theory,
Melting point: 230-231C
Calculated: C 70.78 H 7.33 N 5.50 Cl 6.96 -~-
Found: 70.71 7.07 5.67 6.99
Example 148
3-[(N-(2-(Naphthyl-l-oxy)-ethyl)-piperid-3-yl)~
methY1~-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahYdro-
2H-3-benzazepine-hydrochloride
~ :.. . : .
Prepared from 3-[(piperid-3-yl)-methyl]-7,8-dimethoxy-
2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine and 2-
(naphthyl-l-oxy)-ethyl bromide analogously to Example 1.
Yield: 40% of theory, ~-
Melting point: 214-215C
Calculated: C 68.62 H 7.10 N 5.34 Cl 6.75
Found: 68.40 7.10 5.21 6.77
Example 149
: '
3-r(N-((2-Methyl-naphth-l-yl)-methyl)-piperid-3-
yl)-methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine-hydrochloride
` -`` 1330~93
- l33 -
Prepared from 3-[(piperid-3-yl~-methyl]-7,8-dimethoxy-
2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine and 1-
chloromethyl-2-methyl-naphthalene analogously to
Example 1.
Yield: 67% of theory,
Melting point: 242-243C
Calculated: C 70.78 H 7.33 N 5.50 Cl 6.96
Found: 70.50 7.22 5.34 6.89 ~`
Example 150 ~ ~
' '' '` .'
3-[(N-(2-(6-Methoxy-naphth-2-yl)-ethyl)-piperid-
3-Yl)-methyl]-7~8-dimethoxy-2-oxo-l~3r4~5-tetrahydr
2H-3-benzazepine-hydrochloride -~
Prepared from 3-[~piperid-3-yl)-methyl]-7,8-dimethoxy- ~--
2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine and 2- ~ ~ -
(6-methoxy-naphth-2-yl)-ethyl bromide analogously
to Example 1. -~
Yield: 50% of theory,
Melting point: 156-157~C
Calculated: C 74.07 H 7.62 N 5.57
Found: 73.90 7.55 5.64
Example 151
3-[(N-(2-(5-Methyl-6-methoxy-na~hth-2-yl)-ethyl)-
piPerid-3-yl)-methyl]-7,8-dimethoxy-2-~xo-1,3,4,5-
tetrahydro-2H-3-benzaze~ine-_ydrochloride `~
." ,~. .. .. ..
Prepared from 3-[(piperid-3-yl)-methyl]-7,8-dimethoxy- ~``
2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine and 2- `
(5-methyl-6-methoxy-naphth-2-yl)-ethyl bromide
analogously to Example 1.
Yield: 53% of theory,
Melting pOillt: 240-241C
Calculated: C 69.48 H 7.47 N 5.06 Cl 6.41
Found: 69.58 7.48 5.00 6.54
-` ~33~33
- 134 -
Example 152
2-[N-(2-(3,4-Dimethoxy-phenYl)-ethyl)-piperid-3-
vl)-methyl]-5,6-dimethoxy-1-oxo-1,3-dihYdro-isoindole-
hydrochloride
Prepared from 2-[(piperid-3-yl)-methyl]-5,6-dimethoxy-
1,3-dihydro-isoindole and 2-t3,4-dimethoxy-phenyl)-
ethyl bromide analogously to Example 1. -~
Yield: 68% of theory,
Melting point: 225-226C
Calculated: C 63.60 H 7.18 N 5.20 Cl 7.22
Found: 63.61 7.30 5.70 7.44 -~
Example 153 ~ -
2-[(N-(2-(6-Methoxy-naphth-2-yl)-ethyl)-piperid-
3-yl)-methyl]-5~6-dimethoxy-l-oxo-1~3-dihYdro-isoindole-
hydrochloride ~ -
Prepared from 2-[(piperid-3-yl)-methyl]-5,6-dimethoxy-
l-oxo-1,3-dihydro-isoindole and 2-(6-methoxy-naphth- ~ ~x~
2-yl)-ethyl bromide analogously to Example 1. ~ -
Yield: 75% of theory,
Melting point: 234-236C
Calculated: C 68.16 ~ 6.90 N 5.48 Cl 6.94 -~
Found: 68.10 7.10 5.39 7.10
Example 154 ! ' ~ '
2-[(N-(2-(Naphthyl-l-oxy)-ethyl)-piperid-3-yl)-
methyl]-5,6-dimethoxy-1-oxo-I,3-dihydro-isoindole-
hYdrochloride
' ~ .. .
Prepared from 2-[(piperid-3-yl)-methyl]-5,6-dimethoxy-
l-oxo-1,3-dihydro-isoindole and 2-(naphthyl-1-oxy)-
ethyl bromide analogously to Example 1.
Yield: 60~ of theory,
~33~993
. ~ .
,
- 135 -
Melting point: 150-152C
Calculated: C 67.66 H 6.69 N 5.63 Cl 7.13
Found: 67.50 6.76 5.74 7.54 ~ -
Example 155
2-[(N-(2-(4-Methyl-phenyl)-ethyl)-piperid-3-yl)-
methyl]-5,6-dimethoxy-1-oxo-1,3-dihydro~isoindole
Prepared from 2-r~piperid-3-yl~-methyll-5,6-dimethoxy-
l-oxo-isoindole and 2-(4-methyl-phenyl)-ethyl bromide
analogously to Example 1. -~
. .
Yield: 57% of theo~y, -~
Melting point: 134-136C
Calculated: C 73.50 H 7.90 N 6.86
Found: 73.40 8.04 7.06
Example 156
2-[(N-(2-(3-Methoxy-phenYl)-ethYl)-pi~erid-3-Yl)-
methyl] 5,6-dimethoxy-1-oxo-1,3-dihydro-isoindole- -~
hydrochloride
Prepared from 2-r(piperid-3-yl)-methyl]-5,6-dimethoxy-
l-oxo-isoindole and 2-(3-methoxy-phenyl)-ethyl
bromide analogously to Example 1.
Yield: 54% of theory,
Melting point: 226-228C -~
Calculated: C 65.28 H 7.01 N 6.09 Cl 7.71
Found: 65.30 7.37 5.91 7.61
Example 157
.
2-[(N-(2-(S-Methyl-6-methoxy-naphth-2-yl)-ethyl)-
... ~;
piperid-3-yl)-methyl]-5,6-dimethoxy-1-oxo-1,3-dihydro-
isoindole-hydrochloride
^ 133~93
- 136 -
Prepared from 2-[(piperid-3-yl)-methyl]-5,6-dimethoxy-
1-oxo-1,3-dihydro-isoindole and 2-(5-methyl-6-methoxy-
naphth-2-yl~-ethyl bromide analogously to Example 1. -
Yield: 38~ of theory, ~-
Melting point: 214-216C
Calculated: C 68.62 H 7.10 N 5.33 Cl 6.75
Found: 68.94 7.23 4.98 6.61
Example 158
2-[(N~(2-(4-Nitro-phenyl)-ethyl)-piperid-3-yl)-
methyl]-5,6-dimethoxy-1-oxo-1,3-dihYdro-isoindole- -
hYdrochloride ~ -
. - ~ :
Prepared from 2-[(piperid-3-yl)-methyl~-5,6-dimethoxy-
l-oxo-1,3-dihydro-isoindole and 2-(4-nitro-phenyl)-
ethyl bromide analogously to Example 1.
Yield: 79~ of theory,
Melting point: 215-218C
Calculated: C 60.56 H 6.35 N 8.83 Cl 7.45
Found: 60.41 6.26 8.84 7.62
Example 159
3-[rN-(Z-(Thien-2-yl)-ethYl)-piperid-3-yl)-methyl]-
7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-2H-3-benzazePine-
hydrochloride
::
Prepared from 3-[(piperid-3-yl)-methyl]-7,8-dimethoxy~
2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine and 2-
(2-bromo-ethyl)-thiophene analogously to Example 1.
Yield: 43% of theory,
Melting point: 232-236C
Calculated- C 61.99 H 7.15 N 6.02 Cl 7.62 S 6.89
Found: 61.90 7.06 5.78 7.96 6.84 ~-~
, . . :
Example 160 ~-
-; ~3~993
,~
- 137 -
3-[ (N- t2-(Thien-3-yl)-ethyl)-piperid-3-yl)-methyl~
7~8-dimethoxy-2-oxo-1,3~4~5-tetrahydro-2H-3-benzazepine- ~-
.
hydrochloride -
Prepared from 3-[(piperid-3-yl~-methyl]-7,8-dimethoxy-
2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine and 3-
t2-bromo-ethyl)-thiophene analogously to Example 1.
Yield: 36~ of theory,
Melting point: sinters at 75-80~, melts at 225-230C
Calculated: C 61.99 H 7.15 N 6.02 Cl 7.62 S 6.89
Found: 62.00 7.08 5.98 8.43 6.62
ExamPle 161
. .
3-[(N-(4-(Thien-2-yl)-butyl)-piperid-3-yl)-methyl]-
.
7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-2H-3-benzazePine- -
hydrochloride
'~ ":
Prepared from 3-[(piperid-3-yl)-methyl]-7,8-dimethoxy-
2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine and 2- -~
(4-bromo-butyL)-thiophene analogously to Example 1. `
Yield: 68% of theory,
Melting range: 190-196C
Calculated: C 63.33 H 7.56 N 5.68 Cl 7.19 S 6.50
Found: 63.18 7.72 5.72 7.29 6.59
Example 162 ~ -
3-[(N-(2-(Benzo[b]fur-2-yl)-ethyl)-piperid-3-Yl)-
methyl]-7,8-dimethoxy-2-oxo-1,2,3,4-tetrahYdro-
2H-3-benzazepine-hydrochloride ~ `
: ~
Prepared from 3-[(piperid-3-yl)-methyl]-7,8-dimethoxy- ;~
2-oxo-1,3,4,5-tetrahydro-2~-3-benzazepine and 2-
(2-bromo-ethyl)-benzo[b]furan analogously to Example 1.
Yield: 22% of theory,
Melting point: above 216C (decomp.)
Calculated: C 67.39 H 7.07 N 5.61
: ~ ,
33~3
- 138 -
Found: 67.14 7.36 5.53
Example 163
3-[(N-(2-(Benzo[~]thien-3-yl)-lethylj-piperid-3-
Yl)-methyl]-7~8-dimethoxy-2-oxo-l~3~4~5-tetrahydro- ;~
2H-3-benzazepine-hydrochloride
Prepared from 3-[(piperid-3-yl~-methyl]-7,8-dimethoxy-
2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine and 3-
(2-bromo-ethyl)-benzo[b]thiophene analogously to -~
Example 1.
Yield: 73~ of theory, ;~
Melting range: 70-75C (decomp.)
Calculated: C 65.29 H 6.85 N 5.44
Found: 65.10 6.87 5.73 --
Example 164
3-[(N-(2-(4-Methoxy-benzo[b]thien-3-yl)-ethyl)-
piperid-3-yl)-methYl]-7,8-dimethoxy-2-oxo-1,3,4~5
tetrahYdro-2H-3-benzazepine-hydrochloride
~ ~ , '" ?. ~ .
Prepared from 3-[(piperid-3-yl)-methyl]-7,8-dimethoxy-
2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine and 3-
(2-chloroethyl)-4-methoxy-benzo[b]thiophene analogously
to Example 1.
Yield: 25% of theory,
Mel~ing range: 85-105 (decomp.)
Calculated: C 63.96 H 6.84N 5.14Cl 6.50S 5.88
Found: 63.95 6.85 4.99 6.53 5.75
Example 165
3-[(N-(2-(6--Methylsulphonyloxy-benzo[b]thien-3- ~ ~;
yl)-ethyl)-piperid-3-yl)-methyl]-7~8-dimethoxy-
2-oxo 1,3,4~5-tetrahydro-2H-3-benzazepine-hydrochlori~de
~33~993 ~
- 139 - -
Prepared from 3-[(piperid-3-yl~-methyl]-7,8-dimethoxy-
2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine and 3~
[2-(methylsulphonyloxy~-ethyl]-6-methylsulphonyloxy-
benzo~b]thiophene analogously to Example 1.
Yield: 55% of theory,
Melting point: 90C tdecomp.)
~alculated: C 57~18 H 6.12N 4.60Cl 5.82S 10.53
Found: 57.25 6.14 4.50 5.97 10.36
Example 166
,
3-[~N-(5-rThien-2-yl)-pentyl)-eiperid-3-yl)-methyl]-
7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine~
. :., ~
hydrochloride ~ ~
. .
Prepared from 3-[(piperid-3-yl)-methyl]-7,8-dimethoxy-
2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine and 2-
(5-methylsulphonyloxy-pentyl)-thiophene analogously
to Example 1.
Yield: 39~ of theory,
Melting point: 177C
Calculated: C 63.95H 7.75N 5.52Cl 6.99S 6.32
Found: 63.70 7.92 5.40 7.24 6.62
Example 167
:
3-rtN-(2-(Fur-2-Yl)-ethYl)-piperid-3-yl)-methyl]-
7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine-
hydrochloride
Prepared from 3-[(piperid-3-yl)-methyl]-7,8-dimethoxy-
2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine and 2-
(2-methylsulphonyloxy-ethyl)-furan analogously
to Example 1.
Yield: 44% of theory,
Melting range: 205-215C
Calculated: C 64.20 H 7.41 N 6.24 Cl 7.90
Found: 64.007.45 6.00 7.80
`~` ` 133~3
- 140 -
Exam~e 168
3-[(N-(3-(Fur-2-yl)-propY1)-piperid-3-yl)-methyl-
7~8-dimethoxy-2-oxo-1~3~4~5-tetrahydro-2H-3-benzazePine
hvdrochloride
'
Prepared from 3-[~piperid-3-yl)-methyl]-7,8-dimethoxy-
2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine and 3-
(fur-2-yl)-propionaldehyde anaLogously to Example 9.
~ield: 11% of theory,
Melting point: 201-206C
Calculated: C 64.85 H 7.62 N 6.05 Cl 7.66
Found: 64.88 7.76 5.93 7.55
Example 169
3-[(N-(6-(Thien-2-yl)-hexyl)-piperid-3-yl)-methyl]-
7,8-dimethoxy-2-oxo-1,3,4,5-tetrahYdro-2H-3-benzazePine-
hydrochloride
Prepared from 3-[(piperid-3-yl)-methyl]-7,8-dimethoxy-
2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine and 2-
(6-methylsulphonyloxy-hexyl)-thiophene analogously
to Example 1. -~
Yield: 27~ of theory,
Melting point: 160C
CaLculated: C 64.39H 8.10N 5.40 Cl 6.79
Found: 64.55 7.90 5.23 7.00
Example 170
3-~(N-(3-(Indol-3-yl)-propyl)-piperid-3-yl)-methyl]-
7,8-dimethoxy-2-oxo-1,3,4,5-~etrahydro-2~-3-benzaze~ine-
hydrochloride
Prepared from 3-[(piperid-3-yl)-methyl]-7,8-dimethoxy- ~ ~
2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine and 3- ~ -
!3-methylsulphonyloxy-propyl)-indole analogously
to Example 1.
,
~ ~33~93
- 141 -
Yield: 19% of theory,
Melting point: greater than 80C (decomp.
Calculated: C 60.42 H 6.30 N 6.45
Found: 60.32 6.57 6.67 ~;
Example 171
~.. '
3-[(N-(2-tIndol-3-yl)-ethYl)-piperid-3-yl)-methyl~-
7r8-aimethoxy-2-oxo-l~3~4~s-tetrahydro-2H 3-benzazepine-
hydrochloride
Prepared from 3-[~piperid-3-yl)-methyl]-7,8-dimethoxy-
2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine and 3- ;~
(2-methylsulphonyloxy-ethyl)-indole analogously
to Example 1.
Yield: 23% of theory,
Melting point: greater than 80C (decomp.)
Calculated: C 65.53 H 6.42 N 7.69
Found: 65.33 6.55 7.80
2-[(N-(3-(Naphthyl-2-oxy)-propyl)-hexahYdro-azepin- ~-
3-yl)-methYl]-6~7-methylenedioxy-l-oxo-l~2~3~4-tetra
hydro-isoquinoline
Prepared from 6,7-methylenedioxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline and 3-chloromethyl-[N-(3-
naphthyl-2-oxy)-propyl]-hexahydro-azepine analogously ;
to Example 2.
Yield: 21% of theory,
Melting point: 74-76C
Calc. (x 2 H2O): C 64.45H 7.02N 5.01Cl 6.34 -
Found: 64.32 7.20 5.28 6.44 :~
Example 173
,: ,. '
2-[(N-(3-(Naphth-2-Yl)-propyl)-p~rrolid-3-yl) -methYlL-
6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-isoquinoline
33~993
,
- 142 -
Prepared from 6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-
isoquinoline and 3-(p-toluenesulphonyloxymethyl)-
N-[3-(naphth-2-yl)-propyl]-pyrrolidine analogously
to Example 2.
Yield: 20% of theory,
Melting point: 72-76C
Calc. (x H2O): C 72.41H 7.75N 5.82Cl 7.36
Found: 72.27 7.85 5.70 7.96
Example 174
.
2-[(N-(3-(5;6-DimethoxY-naphthyl-2-oxy)-propyl)-
~yrrolid-3-Yl)-meth~l]-6~7-dimethoxy-l-oxo-l~2~3t4
tetrahYdro-isoquinoline-hydrochloride
Prepared from 6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline and 3-(p-toluenesulphonyloxymethyl)-
N-[3-(5,6-dimethoxy-naphthyl-2-oxy)-propyl]-pyrrolidine
analogously to Example 2.
Yield: 9.5~ of theory,
Melting point: 60-63C
Calc. (x H2O): C 63.20H 7.01N 4.75Cl 6.02
Found: 63.40 7.04 4.49 6.38 ~ ~
: .
Example 175
2-[(N-(3-(5,6-Dimethoxy-naphthyl-2-oxy~-E~ropyl)-
hexahydro-azepin-3-yl)-methyl]-6,7-dimethoxy-1-
oxo-1,2,3,4-tetrahydro-isoquinoline-hydrochloride
Prepared from 6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline and 3-(p-toluenesulphonyloxymethyl)-
N-[3-(5,6-dimethoxy-naphthyl-2-oxy)-propyl~-hexahydro-
azepine analogously to Example 2. ~
Yield: ~3.2~ of theory, ~--
Melting point: 199-201C
Cal~ulated: C 66.15 H 7.23 N 4.67 Cl 5.92
Found: 65.99 7.00 4.44 6.02
,'. ~:
33~993
- 143 -
Example 176
2-[~N-(3-(5,6-DimethoxY-na~hthyl-2-oxy)-propyl)-
hexahydro-azepin-3-yl)-methY1]-6 ! 7-dimethoxy-1,2,3,4-
tetrahydro-lsoquinoline~dihydrochloride ~;
Prepared from 2-~ tN-(3- (5,6-dimethoxy-naphthyl-
2-oxy)-propyl)-hexahydro-azepin-3-yl)-methyl]-6,7-
dimethoxy-l-oxo-l, 2, 3, 4-tetrahyaro-isoquinoline
and lithium aluminium hydride in tetrahydrofuran/ether
analogously to Example 3.
Yield: 86.3~ of theory,
Melting point: 114-116C
Calculated: C 61.97 H 7.56 N 4 . 38 Cl 11.08 -
Found: 62.05 7.65 4.06 10.84
Example 177
2-~(N-(2-(4-Methoxy-phenyl)-ethYl)-pyrrolid-3-yl)-
methyl]-6,7-methYlenedioxy-1,2~3,4-tetrahydro-isoquinoline~
dihydrochloride
Prepared from 2-[(N-(2-(4-methoxy-phenyl)-ethyl)- -~
pyrrolid-3-yl)-methyl]-6,7-methylenedioxy-1-oxo-
1,2,3,4-tetrahydro-isoquinoline and lithium aluminium
hydride/tetrahydrofuran analogously to Example
3.
Yield: 86.7~ of theory, ~-
Melting point: 213-215C
Calculated: C 60.49 H 6.98 N;5.87 Cl 14.88
Found: 60.59 6.96 5.84 14.98
Example 178
2-[tN-(3~(3~4-Dimethoxy-phenoxy)-propyl)-pyrrolid
3-yl)-methyl]-6,7~methylenedioxy-1-oxo-1,2,3,4-
tetrahYdro-isoquinoline-hydrochloride
.
:
~33~9~ _
- 14~ - 27169-1~7
Prepared from l-oxo~1,2,3,4-tetrahydro-6,7-methylenedioxy-
~oquinoline and N-[3-~3,4-dimethoxy-phenoxy)-propyl]-
3-benzo~ulphonyloxymethyl-pyrrol~dine analogou ly
to Example 2.
Yield: 57.4% of theory,
Meltlng point: 108-110C
Calculated: C 59.76 ~ 6.74 M 5.35 Cl 7.02
Found: 60.07 6~87 5.23 7.61
'
~xamplq 179
2-[~N-~2-(4-Methoxy-phenyl~-ethYl) pYrrolid-3-yl)-
methyl]-6,7-methylenedioxy-1-oxo-1,2,3,4-tetrahYdro-
isoquinoline-hydrochlorlae
- ~ ~
Prepared from l-oxo-1,2,3,4-tetrahydro-6,7-methyl~ne-
dloxy-i30quinoline ~nd N-[2-(4-methoxyphenyl)-ethyl~-
3-benzenesulphonyloxymethyl-pyrrolldine analogously
to Example 2.
Yield: 54.7~ of theory,
Melting point: 105-108C
Calculated: C 62.26 R 6~74 N 6.05 Cl 7.97
Found:62.34 6.74 5.88 8.05
~ -
-. , ...,~,
:. , .
:,
~`'~;', ' ' ~;,'
`~ ~33~3
.
- 145 -
Example 181
3-[ (N- (2- ~6-~ethyl-pyrid-2-yl)-ethyl)-pyrrolid-
3-yl~-methyll-7,8-dimethyl 1,3,4,5-tetrahydro-2H-
3-benzazepine x 2.5 ~Cl x H2O
Prepared from 3-[~N-(2-(6-methyl-pyrid-2-yl)-ethyl)~
pyrrolid-3-yl)-methyl]-7,8-dimethyl-2-oxo-1,3,4,5-
tetrahydro-2H-3-benzazepine and lithium aluminium
hydride in diethylether and tetrahydrofuran analogously
to Example 3.
Yield: 50% of theory,
Melting point: 80-91C amorphous
Calculated: C 61.68 H 8.18 N 8.63 Cl 18.21
Found: 61.55 8.36 8.44 18.10
Fxample 182
3-[(N-(2-(6-Methyl-pyrid-2-yl)-ethyl)-pyrrolid-
3-yl)-methyl]-7,8-di_ethyl-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine-dihvdrochloride-semihydrate
Prepared from 3-[(N-(2-(6-methyl-pyrid-2-yl)-ethyl)-
pyrrolid-3-yl)-methyl]-7,8-dimethyl-2-oxo-1,3-dihydro-
2H-3-benzazepine with 10~ palladium/charcoal at -~
5 bar hydrogen and at 80C in ethanol analogously
to Example 5.
YieId: 92~ of theory,
Melting point: 86-94C amorphous
Calculated:C 63.41H 7.66N 8.87Cl 14.97
Found: 63.52 7.14 8.81 14.94
~ ' :` :' .,`, .
Example 183
~ .
2-[(N-(3-(6-Methoxy-naphthyl-2-oxy)-propyl)-pyrrolid-
3-yl)-methyl]-6,7-dimethyl-1-oxo-1,2,3,4~tetrahydro-
isoquinoline
'
33~993
- 146 -
Prepared from 6,7-dimethoxy-1-oxo-1,2, 3, 4-tetrahydro-
isoquinoline and 3-(p-toluenesulphonyloxymethyl)-
N-[3-~6-methoxy-naphthyl-2-oxy)-propyl]-pyrrolidine
analogously to Example 2.
Yield: 44.7~ of theory,
Melting point: 102-104C
Calculated: C 76.24 H 7.68 N 5.93
Found: 75.90 7.62 5.94
Example 1_4 -
2-[(N-(3-(6-Methoxy-naph~hyl-2-oxy)-propyl)-~yrrolid
3-Yl) -methyl]-6,7-dimethoxy-1,2,3,4-tetrahydro-
isoquinoline
Prepared from 2-[N-(3-(6-methoxy-naphthyl-2-oxy~
propyl)-pyrrolid-3-yl)-me~hyl]-6,7-dimethoxy-1-
oxo-1,2,3,4-tetrahydro-isoquinoline and l~thium
aluminium hydride in ~etrahydrofuran and ether
analogously to Example 3.
Yield: 68% of theory, -
Melting point: 106-108C
Calculated: C 73.44 H 7.82 N 5.71
Found: 73.26 7.72 5.81
Example 185
3-[(N-(3-~Indol-3-~l)-propyl)-pyrrolid-3-yl)-methyl]-
7,8-methylenedioxy-2-oxo-1,3,4,5-tetrahydro-2H-
3-benzazepine-monohydrate
Prepared from 3-[(N-~3-(indol-3-yl)-propyl)-pyrrolid-
3-yl)-methyl]-7,8-methylenedioxy-2-oxo-1,3-dihydro-
2H-3-benzazepine with 20% palladium/charcoal at
5 bar hydrogen in ethanol at 80C anaLogously to
Example 5.
Yield: 40% of theory,
Melting ranc1e: 67-74C
` " 133~993
- 147 -
Calculated:C 69.95H 7.17N 9.06
Found: 70.00 7.03 8.97
Example l86
~ '
3-[(N-(2-(6-Methyl-~Yrid-2-yl~-ethyl~-pyrrolid-3-
yl~-methyl]-7,8-dimethyl-2-oxo-1,3-dihydro-2H-3-
benzazepine-dihydrochloride-semihydrate
Prepared from 7,8-dimethyl-2-oxo-1,3-dihydro-2H-
3-benzazepine and 3-chloromethyl-N-[2-(6-methyl-
pyrid-2-yl)-ethyl]-pyrrolidine analogously to Example 2.
Yield: 81% of theory,
Melting point: 86-98C amorphous
Calculated: C 63.68 H 7.27 N 8.91 Cl 15.04
Found: 63.39 7.43 8.87 14.93 ~-
:-. .. ~
Example 187
2- r (N-~3-(3-MethYlphenoxy)propyl)-piperid-3-yl)-
methYl]-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro- ~ `
isoquinoline-hYdrochloride -~
' .'.:
Prepared from 6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline and N-[3-(3-methylphenoxy?-propyl]~
3-benzenesulphonyloxymethyl-piperidine analogously
to Example 2.
Yield: 75.6% of theory,
Melting Point: 106-109C amorphous -
Calculated: C 63.95 H 7.75 N 5.52 Cl 7.25
Found: 64.66 7.91 5.48 7.28
Example 188
2-[lN-(2-15-Methyl-6-methoxy-na~hth-2-yl)-pyrrolid-
3-Yl)-methyl]-6,7-methylenedioxy-1-oxo-1,2,3,4-
.
tetrahydro-isoquinoline-hYdrochloride
~. '
~ 33~93
- 1~8 -
Prepared from 6,7-methylenedioxy-1-oxo--1,2,3,4-
tetrahydro-iso~uinoline and 3-(p-toluenesulphonyloxy-
methyl)-N-[2-t5-methyl-6-methoxy-naphth-2-yl~-ethyl]-
pyrrolidine analogousLy to Example 2.
YieLd: 27.1~ of theory,
Melting point: 249-251~C
Calculated: C 68.43 H 6.53 N 5.50 Cl 6.96
Found: 68.47 6.66 5.30 7.16
ExamPle 189
2-[~N-(3-(Naphth-2-yl)-propyl)-pyrrolid-3-Y1)-methyl]-
6,7-dimethoxy-1-oxo-isoquinoline-hYdrochloride
Prepared from 6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline and 3-(p-toluenesulphonyloxymethyl)-
N-[3-~naphth-2-yl) propyl]-pyrrolidine analogously
to Example 2.
Yield: 20.2% of theory,
Melting point: 84-86C
Calculated: C 67.88H 7.26N 5.46Cl 6.91
Found: 67.86 7.40 5.40 7.17
Example 190
,~
2-[(N-(3-(3,4-DimethoxY-phenoxy)-propyl)-pyrrolid-
3-Yl)-methYl]-6,7-dimethoxy-1,2,3,4-tetrahYdro-
isoquinoline-dihydrochloride
. : :
Prepared from 2-r(N-[3-(3,4-dimet4Oxy-phenoxy)- ~-
propyl)-pyrrolid-3-yl)-methyl]-6,7-dimethoxy-1-
oxo-1,2,3,4-tetrahydro-isoquinoline and lithium
aluminium hydride in diethyl ether and tetrahydrofuran
analogously to Example 3.
Yield: 92.3% of theory,
Melting point: 220-221C
Calculated: C 59.20 H 6.88 N 5.31 Cl 13.44
Found: 59.28 6.97 5.20 13.44
: "
.` 1330~g3
- 149 -
Example 191
~ .
2-[(N-(2-(3,4-DimethoxYphenYl)-ethY1)-pyrrolid-
3-~1)-methyl]-6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-
iso~uinoline
Prepared from 6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro~
isoquinoline and N-[2-(3,4-dimethoxyphenyl)-ethyl]-
3-benzenesulphonyloxymethyl-pyrrolidine analoqo~sly
to Example 2.
Yield: 70~ of theory, - -
Melting point: 168-170C
Calculated: C 73.90 H 8.11N 6.63 -~
Found: 73.96 8.11 6.55
Example 192
~ : -
2-[ (N- (3- (4-Methoxyphenoxy)-Propyl)-piperid-3-yl)- -
methYl~-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahvdro-
isoquinoline-hydrochloride
Prepared from 6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline and N-[3-(4-methoxyphenoxy)-propyl]- -
3-benzenesulphonyloxymethyl-piperidine analogously
to Example 2.
Yield: 78.7~ of theory,
Melting point: 98-102C
Calculated: C 63.08~ 7.51N 5.3SCl 7.02
Found: 62.87 7.69 5.16 7.28
:
Example 193
2-[(N-(3-(3-MethoxyphenoxY)-propY1)-piperid-3-yl)-
methyl]-6,7--dimethoxy-1-oxo-1,2,3,4-tetrahYdro-
.,
isoquinoline-hydrochloride
: :,: :
Prepared from 6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline and N-[3-(3-methoxyphenoxy)-propyl]- -
.,. ~,
:. :
133~9~
- 150 -
3-benzenesulphonyloxymethyl-piperidine analogously
to Example 2.
Yield: 87% of theory,
Melting point: 103-105C
Calculated: C 64.21 H 7.38 N 5.55 Cl 7.02
Found: 64.00 7.55 5.37 7.12
Example 194
2-[(N-(3-(3-Methoxyphenoxy)-~ropyl)-piperid-3-yl)- -~
methyl]-6,7-dimethoxy-1,2,3,4-t:etrahydro-isoquinoline~
dihydrochloride ;~
.'1~4V` '
Prepared from 2-[tN-(3-(3-methoxyphenoxy)-propyl)-
piperid-3-yl)-methyl]-6,7-dimethoxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline and lithium aluminium hydride
in diethylether and tetrahydrofuran analogously
to Example 3.
Yield: 95.4~ of theory,
Melting point: 170-173C
Calculated: C 60.43 H 7.70 N S.22 Cl 13.21
Found: 60.50 7.71 4.91 12.97
Example 195
2-1(N-t2-(3,4-Dimethoxye__ny~)-ethyl)-pyrrolid-
3-Yl)-methyl]-6,7-dimethyl-1,2,3,4-tetrahydro-isoquinoline-
dihYdrochloride ~ -~
: .
Prepared from 2-[(N-(2-(3,4-dimethoxyphenyl)-ethyl)- -
pyrrolid-3-yl)-methyl]-6,7-dimethyl-1-oxo-1,2,3,4-
tetrahydro-isoquinoline and lithium aluminium hydride
in diethylether and tetrahydrofuran analogously
to Example 3.
Yield: 94.1% of theory,
Melting point: 260-262C
Calculated: C 64.85 H 7.96 N 5.82 Cl 14.73
Found: 64.60 8.11 S.91 14.67
'`'-, ' . ' ,'':"'
~33~9~
- 151
~xample 196
~ ;-
2-[~N-t3-(6-Methoxy-naphthyl-2-oX~)-proPyl)-azaCYclo- ~
oct-3-yl)-methYl~-6~7-dimethox~-1-oxo-1,2,3,4-tetrahydro- ~ -
isoquinoline-hydrochloride
Prepared from 2-[(azacyclooct-3-yl)-methyl]-6,7-
dimethoxy-l-oxo-1,2,3,4-tetrahydro-isoquinoline
and 2-(3-chloropropoxy)-6-methoxy-naphthalene analogously
to Example 1.
Yield: 24.4% of theory,
Melting point: 176-178C
Calculated: C 67.96 H 7.43 N 4.80 Cl 6.08
Found: 67.74 7.29 4.71 6.23
Example 197
.
3-[(N-(3-(Indol-3-yl)-propyl)-pyrrolid-3-yl)-methyl]
7,8-dimethyl-2-oxo-1,3,4,5-tetrahydro-2H-3-benzazepine-
hydrochloride ~ ~
.: ' ' :'
Prepared from 3-l(pyrrolid-3-yl)-methyl]-7,8-dimethyl-
2-oxo-1,2,3,4-tetrahydro-2H-3-benzazepine and 3-
(3-benzenesulphonyloxy-propyl)-indole analogously
to Example 1.
Yield: 62% of theory,
Melting point: 106-108C
Calculated:C 69.47H 7.91N 8.68Cl 7.32
Found: 69.57 7.80 8.67 8.51
. -
',' ' .' . '~
:~
133~3
- 152 -
Example 198
3-[(N-(3~(6-Methoxy-naphthyl-2-oxy)-propyl)-hexahvdro- ;~
a~ein-3-yl3-methYl]-7,8-dimethoxy-1,2,3,4-tetrahYdro-
2H-3-benzazepine
., ,r ;~i"~
Prepared from 3-[(N-(3-(6-methoxy-naphthyl-2-oxy3-
propyl)-hexahydro-azepin-3-yl)~-methyl~-7,8-dimethoxy-
1,2,3,4-tetrahydro-2-oxo-2H-3-benzazepine and lithium
aLuminium hydride in tetrahydrofuran/ether analogously
to Example 3.
Yield: 26.8~ of theory,
Melting point: 98-100C
Calc. (x H20): C 71.97 H 8.42 N 5.09
Found: 72.07 8.23 5.10
. .''
Example 199
3-~(N-(3-(6-Methoxv-naphthyl-2-oxy~-propyl3-hexahydro-
azepin-3-yl)-methYl~-l,2,3,4-tetrahydro-2-oxo-2H- -
3-benzazepine
Prepared from 3-[thexahydro-azepin-3-yl)-methyl]-
1,2,3,4-tetrahydro-2-oxo-2H-3-benzazepine and 2- ~;
(3-chloropropoxy)-6-methoxy-naphthalene analogously
to Example 1.
Yield: 45% of theory,
Melting point: 109-111C
Calculated:C 72.50H 7.74N 5.12
Found: 72.35 7.68 4.93 - -
: -~.,.:. :,.
Example 200 ;~
~ -.~: .:: :.:
,:.'- ~. .: ,:
2-~(N-(3-(-Methoxy-naphthyl-2-oxy)-pro~l)-hexahydro- -~ ~-
azepin-3-yl)-methYl]-1,3-dihydro-5,6-dimethoxy~
isoindole
33~93
- 153 -
Prepared from 2-[(N-(3-(6-methoxy-naphthyl-2-oxy)-
propyl)-hexahydro-azepin-3-yl)-methyl~-5,6-dimethoxy-
phthalimide and lithium aluminium hydride in teteahydro-
furan/ether analogously to Example 3.
Yield: 49.2% of theory, ~;
Melting point: 104-146C
Calculated: C 73.78 H 7.99 N 5.55
Found: 73.63 7.99 5.39
Exam~ 201
: .
3-[~N-(3-(Indol-3-yl~-propyl)-pyrrolid-3-Yl)-methYl]-
7,8-dimethyl-1,3,4,5-tetrahydro-2H-3-benzazepine- ~
dihydrochloride-monohydrate ~ -
Prepared from 3-[~N-~3-(indol-3-yl)-propyl)-pyrrolid-
3-yl)-methyl]-7,8-dimethyl-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine and lithium aluminium hydride
in tetrahydrofuran/ether analogously to Example 3.
Yield: 65~ of theory,
Melting point: 168-170C
Calculated: C 66.39 H 8.16 N 8.29 Cl 13.99
Found: 66.29 8.44 8.08 14.08
Example 202
3- UN-(3-(6-Methoxy-naphthyl-2-oxy)-propyl)-hexahydro- ~ - -
azepin-3-yl)-methyl]-1,3,4,5-tetrahYdro-7,8-methylenedioxy- ;~
2H-3-benzazePine ;;
Prepared from 3-[~N-(3-(6-methoxy-naphthyl-2-oxy)-
propyl)-hexahydro-azepin-3-yl)-methyl~-1,3,4,5~
tetrahydro-7,8-methylenedioxy-2-oxo-2H-3-benzazepine
and lithium aluminium hydride/tetrahydrofuran/ether ~d
analogously to Example 3.
Yield: 25% of theory,
Melting poin~: 109-111C
Calculated: C 74.39 H 7.80 N 5.42
Found: 74.27 7.94 5.43
.
~33~93
--154
Example 203
3-[ tN- ( 3-(6-Methoxy-naphthYl-2-oxy)-propYl)-hexahydro-
azepin-3-yl)-methYl]-1,3,4,5-tetrahYdro-7,8-methylenedioxy-
2-oxo-2H-3-benzazepine-hYdroch~oride
Prepared from 3-[(hexahydro-azepin-3-yl)-methyl]-
1,3,4,5-tetrahydro-7,8-methylenedioxy-2~oxo-2H-
3-benzazepine and 2-(3-chloropropoxy)-6-methoxy-
naphthalene analogously to Exasnple 1.
Yield: 68.1~ of theory,
Melting range: 104-108C
Calc. (x 2 H2O): C 65.46 ~I 7.38 N 4.79 Cl 6.06
Found: 65.60 7.25 5.01 6.43
,
Example 204
2-[(N-(3-(6-Methoxy-naphthyl-2~oxy)-propyl)-hexahydro-
azepin-3-yl~-methyl]-6,7-methylenedioxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline-hydrochloride
Prepared from 2-r(hexahydro-azepin-3-yl)-methyl]-
6,7-methylenedioxy-1-oxo-1,2,3,4-tetrahydro-isoquinoline
and 2-(3-chloro-propoxy)-6-methoxy-naphthalene
analogously to Example 1.
Yield: 18.2~ of theory, ~
Melting point: 65-67C `-
Calc. (x H2O): C 65.19H 6.88 N 4~90 Cl 6.20
Found: 65.20 6.75 4.82 6.54
Example 205 ;
2-[(N-(3-(6-Methoxy-naphthyl-2-ox~)-~ropyl)-piperid-
3-Yl)-methyl]-6~7-methylenedioxy-l-oxo-l~2~3~4
tetrahYdro-isoquinoline
. .
Prepared from 6,7-methylenedioxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline and 3-(benzenesulphonyloxYmethyl)-
:::, '. :"
~33~93
- 155 -
N-[3-(6-methoxy-naphthyl-2-oxy)-propyl]-piperidine
analogously to ExampLe 2.
Yield: 5.3% of theory,
Melting point: 144-146C
Calculated: C 71.69 H 6.82 N 5.57
Found: 71.52 6.62 5.46
... .
Example 206
2-[~N-~3-(6-Methoxy-naphthyl-2-oxy)-~ropyl)-hexahYdro-
azepin-3-yl)-methyl]-5,6-2imeth 1-1,3-dihy~ro-1-
oxo-isoindole
.
Prepared from 2-[(N-(3-(6-methoxy-naphthyl-2-oxy)-
propyl)-hexahydro-azepin-3-yl)-methyl]-5,6-dimethyl-
phthalimide and æinc/glacial acetic acid analogously
to Example 4. ~ ~ ~
Yield: 18.2~ of theory, --~ -
Meltinq point: 232-234C
Calc. (x acetone): C 74.97 H 8.14 N 5.14 ~ -
Found: 74.96 7.90 5.30
Example 207
:~' ~''. . :,,:.
2-[(N-(3-(6-Methoxy-naphthyl-2-oxy)-propyl)-hexahydro~
azepin-3-yl)-methyl]-6,7-dimethyl-1-oxo-1,2,3,4-
. : . . .
~ tetrahYdro-isoquinoline-hydrochloride
..: . ~ . .
Prepared from 2-[(hexahydro-azepin-3-yl)-methyl]-
6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-isoquinoline
and 2-(3-chloro-propoxy)-6-methoxy-naphthalene
analogously to Example 1.
Yield: 17.3~ of theory, ~-
Melting range: 68-73C
Calc. (x 2 H2O): C 67.06 H 7.93 N 4.46 Cl 6.66
Found: 67.05 7.73 4.88 6.18
'
Example 208
~ ` ` 133~9~
-- 156 --
2- [ (N- ( 3- (6-MethoxY-naEhthyl-2-oxy)-propyl~-piperid-
3-Yl)-methyl]-6,7-dimethox~l-oxo-l,2,3,4-tetrahydro-
isoquinoline
Prepared from 6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline and 3-(benzenesulphonyloxymethyl)-
N-[3-(6-methoxy-naphthyl-2-oxy)-propyl~-piperidine
analogously to Example 2.
Yield: 12.9% of theory,
Melting point: 124-126C
Calculated: C 71.79 H 7.38N 5.40 ;
Found: 71.83 7.33 5.21
Example 209 .
3-[(N-(3-(5~6-Dimethoxy-naphthyl-2-oxy)-propYl)-
hexahydro-azepin-3-yl)-methyl]-1,3,4,5-tetrahYdro-
7,8-dimethoxy-2H-3-benzazepine -~
. .,, :,. .....
Prepared from 3-r(N-(3-(5,6-dimethoxy-naphthyl- Y~
2-oxy)-propyl)-hexahydro-azepin-3-yl)-methyl]-1,3,4,5-
tetrahydro-7,8-dimethoxy-2-oxo-2H-3-benzazepine
and lithium aluminium hydride/tetrahydrofuran/ether
analogously to Example 3.
Yield: 29.1% of theory,
Melting point: 94-96C
Calculated: C 72.57H 8.24N 4.98
Founù: 72.56 9.35 4.94
. . .
~33~ 3
- 157 -
Example 210
2 - [ (N- ( 3-(6-Methoxy-naPhthyl-2--oxy)-propyl)-hexahydro-
azepin-3-yl)-methy~ 3-aihydro-s~6-dimeth
l-oxo-isoindole
Prepared from 2-[ ( N- (3-(6-methoxy-naphthyl-2-oxy)-
propyl)-hexahydro-azepin-3-yl)~methyl]-5,6-dimethoxy-
phthalimide and zinc/glacial acetic acid analogously
to Example 4.
Yield: 20.5~ of theory,
Melting point: 265-267C
Calc. (x 2 H2O): C 67.13H 7.63 N 5.05
Found: 67.11 7.64 5.07
Example 211
3-[(N-t3-(5,6-Dimethoxy~naphthyl-2-oxy)-propyl)-
hexahydro-azepin-3-_1)-methyl]-1,3,4,5-tetrahydro-
7,8-dimethoxy-2-oxo-2H-3-benzazepine
Prepared from 3-[(hexahydro-azepin-3-yl)-methyl]-
1,3,4,5-tetrahydro-7,8-dimethoxy-2-oxo-2H-3-benzazepine
and 2-(3-chloropropoxy)-5,6-dimethoxy-naphthalene
analogously to Example 1.
Yield: 52.4% of theory, ~
Melting point: 129-131C ~ -
Calculated: C 70.81 H 7.69 N 4.86
Found: 70.66 7.84 4.63
Example 212
2-[(N-(3-(5,6-Dimethoxy-naphthyl-2-oxy~-propyl)-
hexahydro-azepin-3-yl~-methyl]-5,6-dimethoxy-1,3-
dihydro-isoindole-dihydrochloride
Prepared from 2-[(N-(3-(5,6-dimethoxy-naphthyl-
2-oxy)-propyl)-hexahydro-azepin-3-yl)-methyl]-S,6-
. .
3~93
- 158 -
dimethoxy-phthalimide and lithium aluminium hydride
in tetrahydrofuran/ether analogously to Example 3.
Yield: 48.2% of theory,
Melting range: 172-177C
Calc. tx H2O): C 61.43 H 7.41 N 4.47 Cl 11.33 ~ ~
Found: 61.50 7.7:L 4.59 11.45 ~ -
Example 213
',':"'';' " ' ~,',"'
2-[~N-(3-(5,6-Dimethoxy-naphthyl-2-oxy)-propyl)-
hexahydro~azepin-3-yl)-methyl]-5,6-dimethoxy-l
dihydro-l-oxo-isoindole-hydrochloride
Prepared fr~m 2-[(N-(3-(5,6-dimethoxy-naphthyl- ~ - -
2-oxy)-propyl)-hexahydro-azepin-3-yl)-methyl]-5,6-
dimethoxy-phthalimide and zinc/glacial acetic acid ~ -
analogously to Example 4.
Yield: 36.4% of theory,
Melting range: 215-223~C
Calc. (x H2O): C 61.87 H 7.30 N 4.50 Cl 5.70
Found: 62.08 7.15 4.55 5.80
~''-':~''
Exam~le 214 ~ ~
2-[(N-(3-(Indol-3-yl)-propyl)-pyrrolid-3-yl)-methyl]- ~ -
5,6-dimethoxy-lr3-dihydro-isoindole-dihydrochloride- '~
monohydrate ;
Prepared from 2-1(N-(3-(indol-3-yl)-propyl)-pyrrolid-
3-yl)-methyl]-5,6-dimethoxy-phthalimide and lithium
aluminium hydride in tetrahydrofuran analogously
to Example 3. ;
~ield: 87% of theory,
Melting point: 262-264C
CalcuLated: C 61.17 H 7.30N 8.23 Cl 13.89 ;~
Found: 61.34 7.267.92 13.90
~ :.
~L 3 3 ~
- 159 -
Example 215
3-[ (N- (3-(5,6-DimethoxY-naphthyl-2-oxy)-propYl)-
hexahydro-azepin-3-yl)-methyl]-1,3,4,5-tetrahydro-
7,8-dimethyl-2H-3-benzazepine-dihydrochloride
Prepared from 3-[(N-(3-(5,6-dimethoxy-naphthyl-
2-oxy)-propyl)-hexahydro-azepin-3-yl)-methyl]-1,3,4,5-
tetrahydro-7,8-dimethyl-2-oxo-2~-3-benzazepine
and lithium aluminium hydride/tetrahydrofuran/ether
analogously to Example 3.
~ield: 76.9~ of theory,
Melting range: 138-144C ;~
Calculated: C 67.64 H 8.01 N 4.64 Cl 11.74
Found: 67.73 8.26 4.4711.50
Example 216
3-[(N-(3-(5,6-Dimethoxy-naphthyl-2-ox~)-propyl)-
hexahydro-azepin-3-yl)-methyl]-1,3,4,5-tetrahydro-
7,8-dimethyl-2-oxo-2H-3-benzazePine-hydrochloride
Prepared from 3-[(hexahydro-azepin-3-yl)-methyl]-
1,3,4,5-tetrahydro-7,8-dimethyl-2-oxo-2H-3-benzazepine
and 2-(3-chloro-propoxy)-5,6-dimethoxy-naphthalene
analogously t~ Example 1. ~
Yield: 47.5% of theory, -
Melting range: gO-96C
Calc. (x H2O): C 68.15H 7.90N 4.67Cl 5.91
Found: 67.93 7.94 5.056.09
~ . .
~ ~33~9~3
- 160
Example 217
2-[~N-(3-~5,6-Dimethoxy-naphth~1-2-oxy)-Propyl)-
piperid-3-yl)-methyl~-6,7-methylenedioxy-1-oxo- ~':~,.. ,::,:
1,2,3,4-tetrahydro-isoquinolinle-hYdroc-hloride
Prepared from 2-[(piperid-3-yl)-methyl]-6,7-methylenedioxy~
l-oxo-1,2,3,4-tetrahydro-isoquinoline and 2-(3-
chloropropoxy)-5,6-dimethoxy-naphthalene analogously
to Example 1.
Yield: 22.5~ of theory,
Melting range: 93-98C
Calc. (x H2O): C 63.42H 6.69N 4.77 Cl 6.03 -~
Found: 63.59 6.80 4.70 6.28
ExamPle 218 ~
. . :-
2-~(N (3-(5,6-DimethoxY-naphthyl-2-oxy)-propy~
pyrrolid-3-yl)-methyl]-6,7-methylenedioxy-1-oxo-
1,2,3,4-tetrahydro-isoquinoline-hydrochloride
Prepared from 2-[(pyrrolid-3-yl)-methyl]-6,7-methylene- ~ ;
dioxy-l-oxo-1,2,3,4-tetrahydro-isoquinoline and
2-(3-chloropropoxy)-5,6-dimethoxy-naphthalene analogously --
to Example 1.
Yield: 22.5% of theory,
Melting point: 87-90C
Calc. (x H2O): C 62.87 H 6.50 N 4.88 Cl 6.18
Found: 62.72 6.38 4.93 6.30
Example 219
2-[(~-(3-~5,6-Dimethoxy-naphth~1-2-oxY)-propYl)-
piperid-3-yl)-methyl]-6~7-dimethyl-1-oxo-1,2,3,4-
tetrahydro-isoquinoline-hydrochloride ~;~
Prepared from 2-[~piperid-3-yl)-methyl]-6,7-dimethyl-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and 2-(3-
133~3 -
- 161 -
chloropropoxy)-5,6-dimethoxy-naphthalene analogously
to Example 1.
~ield: 21.3~ of theory,
Melting range: 72-78C
Calc. (x ~2) C 67.30 H 7.59 N 4.90 Cl 6.21
Found: 67.44 7.74 5.06 6.53
Example 220
2-[(N-(3-(5,6-DimethoxY-naphthy~2-oxy)-propYl)~
hexahydro-azepin-3-yl)-methYl]-6,7-dimethyl-1-oxo-
1,2,3,4-tetrahydro-isoquinoline-hydrochloride
Prepared from 2-[(hexahydro-azepin-3-yl)-methyl]-
6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-isoquinoline
and 2-(3-chloropropoxy)~5,6-dimethoxy-naphthalene
analogously to Example 1.
Yield 12~ of theory, !
Melting range: 84-90C
Calc. (x H20): C 67.73 H 7.73 N 4.78 Cl 6.06
Found: 67.64 7.81 4.94 6.20
Example 221
2-[(N-(2-(4-MethoxY-phenyl)-ethyl)-hexahydro-azepin-
3-Yl)-methyl]-6,7-methylenedioxy-1-oxo-1,2,3,4
tetrahYdro-isoduinoline-hvdrochloride
Prepared from 6,7-methylenedioxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline and N-r2-~4-methoxy-phenyl)-
ethyl]-3-chloromethyl-hexahydro-azepine analogously
to Example 2.
Yield: 46.7% of theory,
Melting point: 101-105C
Calculated: C 62.44 H 7.26 N 5.60 Cl 7.50
Found: 62.62 7.27 5.48 7.66
Exam~e 222
` ''` 1~309~
- 1 6 2
2-[ (N- (3-(4-Methoxy-phenoxy)-propYl)-hexahydro-
azepin-3-yl)-methyl]-6,7-methylenediox~ oxo-1,2,3,4-
tetrahyaro-isoquinoline-hydrochloride
Prepared from 6,7-methylenedioxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline and N-[3-(4-methoxy-phenoxy)-
propyl]-3-chloromethyl-hexahydro-azepine analogously
to Example 2.
Yield: 43.8~ of theory,
Melting point: 123-126C
Calculated: C 62.23 H 7.16 N 5.37 Cl 7.05
Found: 62.42 7.34 5.30 6.94
: :', ' - "
Example 223 ~;
2-1~N-(3-(4-Methoxy-phenoxy)-propyl)-hexahydro-
azepin-3-yl~-methyl~-6~7-dimethyl-1-oxo-1~2~3~4-
tetrahydro-isoquinoline-hydrochloride ~
. -
Prepared from 6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-
isoquinoline and N-13-(4-methoxy-phenoxy)-propyl]-
3-chloromethyl-hexahydro-azepine analogously to
Example 2.
Yield: 42~ of theory,
Melting point: 172-173C
Calculated: C 69.04 H 8.07 N 5.75 Cl 7.28
Found: 69.06 8.25 5.57 7.39
Example 224
2-[(N-~3-(4-Methoxy-phenoxy)-propyl)-hexahydro- ~ ;
azepin-3-yl)-methyl]-6,7-dimethoxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline-hydrochloride
Prepared from 6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-
isoquinoline and N-[3-(4-methoxy-phenoxy)-propyl~-
3-chloromethyl-hexahydro-azepine analogously
to Example 2. ~
.:
3 ~ 3
- 163 -
Yield: 63.3~ of theory,
Melting range: 115-120C
Calculated: C 62.58 H 7.67 N 5.2l Cl 6.83
Found: 62.44 7.68 4.91 6.81
Example 225
2-~ (2-~4-Methoxy-phenyl)-etllyl)-hexahYdro-azepin-
3-yl)-methYl]-6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline-hydrochloride
Prepared from 6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline and N-[2-(4-methoxy-phenyl)-ethyl]-
3-chloromethyl-hexahydro-azepine analogously to
Example 2.
Yield: 51.6~ of theory,
Melting point: 110-113C
Calculated: C 61.75 H 7.87 N 5.28 Cl 7.25
Found: 61.84 8.02 5.16 7.22 ;
Example 226 -
2-[(N-(2-(3,4-Dimethoxy-phenyl)-ethyl)-hexahydro-
azepin-3-yl)-methy~_-6,7-dimethoxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline-hydrochloride
Prepared from 6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline and N-[2-(3,4-dimethoxy-phenyl)-ethyl]-
3-chloromethyl-hexahydro-azepine analogously to
Example 2.
~ield: 45.5% of theory,
Melting point: 107-111C
Calculated: C 62.61 H 7.69 N 5.21 Cl 6.60
Found: 62.78 8.00 5.00 6.43
:
- 164 - 27169-147
Example 227
2-[~N-(2-(3~4-DlmethoxY-pheny~-3ethyl)-hexahydr
azepln-3-vl~-methy~J-6,7-dimethvl-1-oxo-1,2,3,4-
tetrahydro-l~oquinoline-hYdrochlorlde
Prepared from 6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro- -
isoquinollne and N-[2-~3,4-dimethoxy-phenyll-ethyl]-
3-chloromethyl-hexahydro-azepine analogou~ly to .
Example 2.
Yiel~: 44.8% of theory,
Meltlng point: 162-163C
Calculated:C 66.57~ 8.18N 5.54Cl 7.02
Found- 66.67 8.47 5.35 7.11
' '`~'' ~ ~'
"- . .....
'"'
! 1Y
r~
-165- 2716g-147
ExamPle 234
1330~3 ~
2-~(N-(3-(3-MethYl-PhenoxY)-proPyl)-hexahvdro-azepln
3-Yl)-methyll-6,7-methYlenedioxY-l-oxo-l~2~3~4
tetrahydro-isoquinoline-hYdrochloride
Prepared from 6,7-methylenedioxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline and N-[3-(3-methyl-phenoxy)-
propyl]-3-chloromethyl-hexahydro-azepine analogously -- :
to Example 2. ~ ~
' ' '' ~ ~
~ ~330~3 ~
- 166 - 27169-147
Yleld: 46.83 of theory, : :
~eltlng po~nt: 159-161~C
Calculated: C 65.37 H 7.31 N 5.64 Cl 7.28
Found: 65.44 7.40 5.397.23
Example 235
2-[~N-~3-(3-Methyl-phenoxy)-ProPyl)-hexahy~ro-az~Pin~
.......
3-Yl?-methY1]-6,7-dlmethoxy-1-oxo-1,2,3~ tetrahYdro- '~ :~
i~oqulnollne-hydrochlorlde ~. :
':
Prepared rom 6,7-dlmethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline and N-[3-~3-methoxy-phenoxy)-propyl]-
3-chloromethyl-hexahydro-azepine analogoudly to
Example 2.
Yield: 55.2~ of th~ory, ;~
Melting point: 107-111C
Calculated:C 65.67H 7.87N 5.47Cl 7.05 ~ :
Found: 65~47 8.00 5.50 6.97
-: ' . ~.,- ;,
~`,'''',' ,'. '`.','".
~:'::,
:
f~` ~ 3 3 ~
-167- 27169-147
2-~(N-~3-f3,4-MQthyl~nedloxy-phenoxy)-propyl)-hexshyaeo-
azepln-3-yl)-methyl1-6, 7-a lmethYl-l-oxo-t,2,3,4-
tetrahYdro-l~oqulnollne-hydrochlorlde
Pr~pared ~rom 6,7-dlmethyl-l-oxo-l,2,3,4-tetr~hydro- :
i~oqulnollne ~nd N-~3-~3,4-methylenedloxy-phenoxy)-
propyl]-3-ohlorom~thyl-hexahydro-azepin~ analogou~ly :~
to ~xample 2.
Ylelds 40.6~ of theory,
Meltlng polnt: 123-126C :
Calaulatedl C 64.78 ~ 7.57 N 5.39 :Cl 6.B3
~: Foundt 64.~8 7.59 5.29 7.18
:
. . ~,
~,
"
- . . ~
~!
" ~ .
. " `''
'' ~,
^
168~ 3 ~ 9~3
Example 237
'' ~, ' '
2-~(N-(3-(3,4-Dimethoxy-PhenoxY)-propyl)-hexahYdro-
azepin-3-yl)-methyl]-6,7-dimethoxv-1-oxo-1,2,3,4-
tetrahydro-isoquinoline-hYdroclhloride
Prepared from 6,7-dimethoxy-1-oxo-1,2,3,4-tetrahydro-
isoquinoline and N-[3-(3,4-dimethoxy-phenoxy)-propyl]-
3-chloromethyl-hexahydro-azepine analogously to
Example 2.
Yield: 30.6~ of theory,
Melting point: 101-105C
Calculated: C 62.61 ~ 7.59 N 5.01 Cl 6.46
Found: 62.56 7.76 4.94 6.39 -~
Example 238
2-[(N-(2-(3,4-Dimethoxy-phenYl)-ethY1~-hexahydro-
azepin-3-Yl)-methYl]-5,6-dimethoxY-l-oxo-1,3-dihYdro-
isoindole-h~drochloride
Prepared from 2-[(N-(2-(3,4-dimethoxy-phenyl)-ethyl)-
hexahydro-azepin-3-yl)-methyl]-5,6-dimethoxy-
phthalimide and zinc/glacial acetic acid analogously
to Example 4.
Yield: 41.8% of theory,
Melting point: 150-151C
Calculated: C 61.99 H 7.57 N 5.35 Cl 6.57
Found: 61.89 7.48 5.17 6.52
Example 239
2-~(N-(3-(3-Methyl-phenoxY)-propyl)-hexahydro-azePin-
.~ . ~ . ..
3-yl)-methyl]-5,6-dimethoxy-1-oxo-1,3-dihydro-isoindole-
hydrochloride
Prepared from 2-[(N-(3-(3-methyl-phenoxy)-propyl)-
hexahydro-azepin-3-yl)-methyl]-5,6-dimethoxy-phthalimide
and zinc/glacial acetic acid analogously to Example 4.
` ~ ~ 133~93
- 169 -
Yield: 88.9% of theory,
Melting point: 95-100C
Calculated: C 63.95 H 7.75N 5.52Cl 7.25
Found: 64.09 7.645.357.37
Example 240
2-[~N-~3-(4-Methoxy-phenoxy)-propyl?-hexahYdro-
azepin-3-yl)-methyl]-5,6-dimethoxy-1-oxo-1,3-dihydro-
isoindole-hYdrochloride
Prepared from 2-[(N-(3-(4-methoxy-phenoxy)-propyl)- ~ ~
hexahydro-azepin-3-yl)-methyl]-5,6-dimethoxy-phthalimide `
and zinc/glacial aceti~ acid analogously to Example 4.
Yield: 87.1~ of theory,
Melting point: 107-112C
Calculated: C 61.99 H 7.51 N 5.35 Cl 7.02
Found: 62.07 7.375.287.31
Example 241
2-~N-(3-(3,4-Methylenedioxy-phenoxy)-propyl)-hexahyar
azepin-3-Yl)-methyl]-5~6-dimethoxy-1-oxo-1,3-dihydro-
isoindole-hyd~rochloride `~
Prepared from 2-[(N-(3-(3,4-methylenedioxy-phenoxy)-
propyl)-hexahydro-azepin-3-yl)-methyl]-5,6-dimethoxy-
phthalimide and zinc/glacial acetic acid analogously
to Example 4.
Yield: 59~ of theory,
Melting point: 104-107C
Calculated: C 60.38 H 6.94 N 5.20 Cl 7.31
Found: 60.40 7.154.957.25
~ .
133~3
-- 170 -
Example 2 4 2
2 - ~ ( N- ( 3-~3,4-Methylenedioxy-phenoxy)-propyl)-hexahydro-
azepin-3-yl)-methyl]-5,6-dimethoxy-l~oxo-1,3-dihydro-
isoindole-hydrochloride
Prepared from 2-[(N-(3-(3,4-methylenedioxy-phenoxy)-
propyl)-hexahydro-azepin-3-yl)-methyl]-5,6-dimethoxy-
phthalimide and zinc/glacial acetic acid analogo~sly
to Example 4.
Yield: 79.4% of theory,
Melting point: 112-116C
Calculated: C 62.23 H 7.15 N 5.37 Cl 7.05
Found: 62.16 7.25 4.96 7.03
Example 243 -
3- E (N-(3 (Pyrid-3-yl)-propyl)-hexahydro-aze~in-
3-Yl)-methyl]-7~8-dimethoxy-2-oxo-l~3-dihydro-2H
3-benzazePine-dibydrochloride-dihYdrate .. ~-
Prepared from 7,8-dimethoxy-2-oxo-1,3-dihydro-2
3-benzazepine and N-[3-(pyrid-3-yl)-propyl]-3-chloromethyl-
hexahydro~azepine in dimethylsulphoxide with potassium
tert.butoxide analogously to Example 2.
Yield: 86~ of theory, ~ ~
Melting point: 106-10&C ~ ;
Calculated: C 58.05 H 7.40 N 7.52 Cl 12.69
Found: 57.94 7.55 7.75 12.42
Example 244
.: .
3-[(N-(3-(Pyrid-3-yl)-propyl)-hexahYdro-azepin-
3-yl)-methYl]-7,8-dimethoxY-2-oxo-1,3l4,5-tetrahYdro-
2H-3-benzazepine-dihydrochloride-dibydrate
Prepared from 3-[(N-(3-(pyrid-3-yl)-propyl)-hexahydro-
azepin-3-yl)-methyl]-7,8-dimethoxy-2-oxo-1,3-dihydro-
`-~ ` 133~93
- 171 -
2H-3-benzazepine and 5 bar hydrogen in the presence
of 10% palladium on charcoal in dimethylformamide
at 80C analogously to Example 5.
Yield: 43% of theory,
Melting point: 119-121C
Calculated: C 57.85 H 7.73 N 7.49 Cl 12.65
Found: 57.74 7.79 7.23 12.50
Example 245
3-[(N-(3-(Pyrid-3-yl)-ProE~yl)-hexahydro-azepin
3-yl)-methyl]-7,8-dimethoxy-1,3,4,5-tetrahydro-
2H-3-benzazepine-trihydrochloride-monohydrate
Prepared from 3-[(N-(3-(pyrid-3-yl)-propyl)-hexahydro-
azepin-3-yl)-methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-
tetrahydro-2H-3-benæazepine and lithium aluminium
hydride in tetrahydrofuran and diethylether analogously
to Example 3. ~ ~-
Yield: 82% of theory, ;- ~-~
Melting point: 139-141C
Calculated: C 57.39 H 7.85N 7.43Cl 18.82 ~
Found: 57.42 8.15 7.56 19.04 ~ ~ -
Example 246 ~
, .
2-[(N-(Pyrid-3-Yl-methyl)-pyrrolid-3-yl)-methyl]-
6,7-methylenedioxy-1-oxo-1,2,3,4-tetrahy~ro-isoquinoline-
dihydrochloride-dihYdrate
Prepared from 6,7-methylenedioxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline and 3-chloromethyl-N-[(pyrid-
3-yl)-methyl]-pyrrolidine and dimethylsulphoxide
with potassium tert.butoxide analogously to Example 2.
Yield: 46% of theory,
Melting pOillt: 91-93C
Calculated: C 53.17H 6.16N 8.85Cl 14.95
Found: 53.31 5.93 8.71 15.01
.` '~:'''- . ,`
`- 133~9~
- 172 -
Example 247
2-[(N-(Pvrid-3-yl-methvl)-pYrrolid-3-yl~-methyl]-
6~7-meth~leneaioxy-l~2~3~4-tetrahvdro-isoquinoline
rihyarochloride - 1.5 x hydrat.e
Prepared from 2-~(N-(pyrid-3-yl-methyl)-pyrrolid~
3-yl~-methyl]-6,7-methylenedioxy-1-oxo-1,2,3,4-
tetrahydro-isoquinoline and lithium aluminium hydride
in tetrahydrofuran and diethylether analogously
to Example 3.
Yield: 67% of theory,
Melting point: 178-181C
Calculated: C 51.70 H 6.41 N 8.61 Cl 21.80
Found: 51.63 6.62 8.45 21.07
- ~:
Example 248
3-[(N-(2-(6-Methyl-pyrid-2-yl)-ethyl)-hexahydro-
azepin-3-yl)-methv1]-7,8-dimethoxy-2-oxo-1,3-dihydro- ~;
2H-3-benzazepine-dihydrochloride-dihydrate
Prepared from 7,8-dimethoxy-2-oxo-1,3-dihydro-2H-
3-benzazepine and N-[2-(6-methyl-pyrid-2-yl)-ethyl]-
3-chloromethyl-hexahydro-azepine in dimethylsulphoxide
with pota~sium ter butoxide analosously to Example 2.
Yield: 63% of theory,
MeIting point: 134-136C
Calculated:C 58.05H 7.40N 7.52Cl 12.69
Found: 58.15 7.60 7.45 12.45
~.
Example 249 ~
. ,
3-~(N-(2-(6-Methyl-pyrid-2-vl)-ethyl)-pyrrolid-
3-yl)-methy~]-7,8-dimethoxy-2-oxo-1,3-dihydro-2H-
3-benzazepine-dihydrochloride x 1.5 hydrate
, ~:
:. ' '
~ ::
` ` L33~3
- 173 -
Prepared from 7,8-dimethoxy-2-oxo-1,3-dihydro-2H-
3-benzazepine and 3-chloromethyl-N-[2-(6-methyl-
pyrid-2-yl)-ethyl]-pyrrolidine in dimethylsulphoxide
with potassium tert.butoxide analogously to Example 2.
Yield: 61~ of theory,
Melting point: 78-80C
Calculated: C 57.58H 6.96N 8.06Cl 13.60
Found: 57.40 7.18 8.24 13. 39
.:,
Exam~le 250
3-[(N-~2-~6-Methyl-pYrid-2-yl)-ethyl)-hexahYdro-
azePin-3-yl)-methyL]-7,8-dimethoxy-2-oxo-1,3,4,5-
tetrahydro-2H-3-benzazepine-dihydrochloride x 2.5 -
hydrate
Prepared from 3-[(N-(2-(6-methyl-pyrid-2-yl)-ethyl)-
hexahydro-azepin-3-yl)-methyl]-7,8-dimethoxy-2-
oxo-1,3-dihydro-2H-3-benzazepine and 5 bar hydrogen ~ -
in the presence of palladium/charcoal and dimethyl-
formamide analogously to Example 5.
Yield: 34% of theory,
Melting point: 112-114C
Calculated: C 56.93 H 7.78 N 7.38 Cl 12.45
Found: 56.83 8.04 7O43 12.26
Example 251
3-r(N-~2-(6-Methyl-pyrid-2-yl~-ethyl)-hexahydro-
azePin-3-yl)-methyl]-7,8-dimethoxY-1,3,4,5-tetrahydro-
2H-3-benzazepine-trihydrochloride-dihydrate
Prepared ~rom 3-[(N-(2-(6-methyl-pyrid-2-yl)-ethyl)- ~-
hexahydro-azepin-3-yl)~methyl]-7,8-dimethoxy-2-
oxo-1,3,4,5-tetrahydro-2H-3-benzazepine and lithium
aluminium hydride in tetrahydrofuran and diethylether
analogously to Example 3.
Yield: 74~ of theory, ~ ~
'-'.' ,: ~'
''.''.'''''''`''''';'.~''
`,-` 133~3
.. ... .
- 174 -
Melting point: 148-150C ~ ;~
Calculated: C 55.61 H 7.95 N 7.20 Cl 18.24
Found: 55.72 8.10 7.03 18.00
Example 252
3-[~N-(2-(6-MethYl-pyrid-2-yl)-ethyl)-pyrrolid~
3-yl) methyl]-7,8-dimethoxY-2-oxo-1,3~4,5-tetrahYdro-
2H-3-benzazepine-dihvdrochloride-dihydrate
Prepared from 3-r(N-(2-(6-methyl-pyrid-2-yl~-ethyl)-
pyrrolid-3-yl)-methyl]-7,8-dimethoxy~2-oxo-1,3-
dihydro-2H-3-benzazepine and palladium/charcoal
in dimethylformamide at 50C and 6 bar hydrogen
pressure analogously to Example 5.
Yield: 28% of theory, -
Melting point: 87-90C
Calculated: C 56.38H 7.38N 7.89Cl 13.31
Found: 56.33 7.53 8.09 13.43
Example 253
3-[~N-(2-~6-Meth~l-pyrid-2-yl)-ethyl)-pyrrolid-
3-Yl)-methyl]-7~8 dimethoxy-1~3~4~5-tetrahyaro- ~ -
2H-3-benzaze~ine-trihydrochloride-dihydrate
Prepared from 7 t 8-dimethoxy-1,3,4,5-tetrahydro-
2H-3-benzazepin~ and N-[2-~6-methyl-pyrid-2-yl)-
ethyl]-3-~benzenesulphonyloxymethyl)-pyrrolidine
in dimethylformamide and triethylamine analogously
to Example 2.
Yield: 42% of theory,
Melting point: 225-227C
Calculated: C 54.10 H 7.62N 7.57Cl 19.16 -
Found: 54.18 7.557~5119.30 m
Example 254
133~3
, . .
- 175 -
2-[(N-(3-(Indol-3-yl)-propyl~-piperid-3-yl~-methyl]
5,6-dimethoxy-1-oxo-1,3-dih~dro-isoindole x 1.5
benzenesul~onate-trihydrate ~ .
Prepared from 2-[(piperid-3-yl)-methyl]-5,6-dimethoxy-
l-oxo-1,3-dihydro-isoindole and 3-(3-benzenesulphonyloxy-
propyl)-indole in dimethylformamide and triethylamine
analogously to Example 1.
Yield: 45% of theory,
Melting point: 87-89C
Calculated: C 58.51H 6.54N 5.68
Found: 58.66 6.43 5.34
Example 255
, :' ':
2-[ ~N- (3- ~Indol-3-yl)-pro~l)-piperid-3-yl)-methyl]
6,7-dimethyl 1-oxo-1,2,3,4-tetrahydro-iso~uinoline-
benzenesulphonate-dihydrate
., '
Prepared from 2-[(piperid-3-yl)-methyl]-6,7-dimethyl- ~:
l-oxo-1,2,3,4-tetrahydro-isoquinoline and 3-(3-
benzenesulphonyloxypropyl)-indole in dimethylformamide
and triethylamine analogously to Example 1.
Yield: 94% of theory,
Melting point: 117-119C
Calculated: C 65.46 H 7.27 N 6.73
Found: 65.51 6.91 6.72 -:
Example 256 -:~:~
,., "
3-[(N-(3-(Indol-3-Yl)-propyl)-pyrrolid-3-yl)-methYl]-
7,8-dimethoxy-2-oxo-1,3,4,5-tetrahYdro-2H-3-benzazepine-
hydrochloride-monohydrate~
. :..'`
Prepared from 3-[(N-(3-(indol-3-yl)-propyl)-pyrrolid-
3-yl)-methyl~-7,8-dimethoxy-2-oxo-1,3-dihydro-2H-
3-benzazepine and 5 bar hydrogen in the presence
of palladiumjcharcoal in dimethylformamide at 80C
analogously to Example 5.
3~9~3
- 176 - ~
.
Yield: 58% of theory, - - --
Melting point: 128-130C
Calculated: C 65.16 H 7.42 N 8.14 Cl 6.87
Found: 65.12 7.55 8.11 7.06
Example 257
.:
3-[(N-(3-(Indol-3-Yl)-propyl)-hexahydro-azepin-
3-Yl)-methyl]-7~8-dimethoxY-2-oxo-1~3~4~5-tetrahYdr
2H-3-benzazepine-monohydrate
Prepared from 3-[(hexahydro-azepin-3-yl~-methyl]~
7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-2H-3-benza~epine
and 3-(3-benzenesulphonyloxy-propyl)-indole in
dimethylformamide and triethylamine analogously
to Example 1. -
Yield: 49% of theory, -~-~
Melting point: 56-58C
Calculated: C 70.98H 8.14N 8.27 ;
Found: 71.08 8.10 8.16
Example 258
3-[(N-(3-(Indol-3-yl)-propyl)-hexahydro-azepin-
3-yl)-methyl]-7,8-methylenedioxy-1,3,4,5-tetrahydro-
2H-3-benzazepine-dihy~ochloride-monohydrate
Prepared from 3-~(N-(3-(indol 3-yl)-propyl)-hexahydro-
azepin-3-yl)-methyl]-7,8-methylenedioxy-2-oxo 1,3,4,5- -
tetrahydro-2H-3-benzazepine and lithium aluminium
hydride in diethylether and tetrahydrofuran analogously
to Example 3.
Yield: 56% of theory,
Meîting point: 155-158C
Calculated: C 62.26 H 7.50 N 7.63 Cl 12.88
Found: 62.31 7.82 7.40 12.89
Example 259 ;
~':
~l33~3
- 177 - ~-
3-r (N- (3- (Indol-3-yl)-propyl)-pyrrolid-3-yl)-meth
7,8-dimethoxy-1,3,4,5-tetrahYdro-2H-3-benzazePine-
dihydrochloride-monohydrate
Prepared ~rom 3-[(N-t3-~indol-3-yl)-propyl)-pyrrolid-
3-yl)-methyl]-7,8-dimethoxy-2-oxo-1,3,4,5-tetrahydro-
2H-3-benzazepine and lithium aluminium hydride
in diethylether and tetrahydrofuran analogously
to Example 3.
Yield: 60~ of theory,
Melting point: 125-128C
Calculated: C 62.44 H 7.67 N 7.80 Cl 13.16 -
Found: 62.35 7.87 7.59 13.67
Example 260
:: -
2-[(N-(3-(Indol-3-yl)-propyl)-piperid-3-yl)-methYl]-
6,7-dimethvl-1,2,3,4-tetrahydro-isoquinoline-dihydro-
chloride-monohydrate
Prepared from 2-[(N-(3-(indol-3-yl)-propyl)-piperid-
3-yl)-methyl]-6,7-dimethyl-l~oxo-1,2,3,4-tetrahydro-
isoquinoline and lithium aluminium hydride in diethylether ~-
and tetrahydrofuran analogously to Example 3. ;-
Yield: 90% of theory,
Melting point: 198-201C ; ;
Calculated: C 66.38 H 8.16 N 8.29 Cl 14.00
Found: 66.29 8.21 8.46 13.82 ;
Example 261
3-[(N-(3-(Indol-3-yl)-propyl)-hexahydro-azepin-
3-Yl)-methyl]-7~8-methylenedioxy-2-oxo-l~3~4~5
tetrahydro-2H-3-benzazepine-monohydrate
Prepared from 3-[(hexahydro-azepin-3-yl)-methyl~
7,8-methylenedioxy-2-oxo-1,3,4,5-tetrahydro-2H-
3-benzazepine and 3-(3-benzenesulphonyloxy-propyl)-
indole analogously to Example 1.
~33~9~3
- 178 -
Yield: 61.5% of theory, -
Melting point: 62-64C
Calculated: C 70.84H 7.59N 8.55
Found: 70.73 7.59 8.42
,: .
Example 262
2-[(N-(3-(Indol-3-yl)-propyl)-pyrr-olid-3-yl)-methyl]-
6,7-dimethYl-1,2,3,4-tetrahYdro-isoquinoline-dihYdro-
chloride-semihYdrate
.
Prepared from 2-E(N-(3-(indol-3-yl)-propyl)-pyrrolid-
3-yl~-methyl]-6,7-dimethyl-1-oxo-1,2,3,4-tetrahydro-
isoquinoline and lithium aluminium hydride in ether
analogously to Example 3.
Yield: 86~ of theory,
Melting point: 143-145C
Calculated: C 67.06 H 7.92 N 8.69 Cl 14.66
Found: 66.92 8.07 8.48 14.87
Example 263
, ' -. - ~ - ' ':
2-[(N-(3-(Indol-3-yl)-propyl)-pyrrol _-3-Yl)-methYl~
6,7-dimethyl-1-oxo--1,2,3,4-tetrahYdro-iso~uinoline-
hydrochloride x 1.5 hYdrate
Prepared from 2-[(pyrrolid-3-yl)-methyl]-6,7-dimethyl-
l-oxo-1,2,3,4-tetrahydro-isoquinoline and 3-(3
benzenesulphonyloxy-propyl)-indole analogously
to Example 1.
Yield: 67% of theory,
Melting point: 117-120C
Calculated: C 67.69 H 7.78 N 8.77 Cl 7.40
Found: 67.62 7.80 8.72 7,93
.:.
i33~93
-17~- 271~9-147
Exam~le 264
3-[(N-l3-(Indol-3-~l)-Pro~yl)-~rrolid-3-Yl)-methYll-
7,8-methylenedioxy-1,3,4,S-tetrahYdro-2H-3-benzazepine-
dihvdrochloride 1.5 x hydrate
Prepared from 7,8-methylenedioxy-1,3,4,5-tetrahydro-
2H-3-benzazepine and 3-(benzenesulphonyloxymethyl)-
N-[3-~indol-3-yl)-propyl]-pyrrolidine analogously
to Example 2.
Yield:. 75% of theory, ..
Melting point: 191-193C
: Calculated,C 61.01H 7.21N 7.90 Cl 13.34 ~
Found:60.90 7.27 7.85 13.70 ~ :
'. '-,.' ..~:. :-.
~.~ '; -"''
: "~
~ ' ~ ',
-` ~33~93
- 180 -
~xample I
Tablets containing 7.5 m~ of 3-[(N-(2-(naphth-2-
yl)-ethyl~-piperid-3-Yl)-methYl]-7,8-dimethoxv-
2-oxo-1,3,4,5-tetrahYdro-2H-3-benzazePine-hYdrochloride
Composition:
1 tablet contains:
Active substance 7.5 mg
Corn starch 59.5 mg
Lactose 48.0 mg
Polyvinylpyrrolidone 4.0 mg
Magnesium stearate 1.0 mq ~ -
120.0 mg
Method of preParation
The active substance, corn starch, lactose and
polyvinylpyrrolidone are mixed together and moistened
with water. The moist mixture is pushed through
a screen with a mesh size of 1.5 mm and dried at
about 45C. The dry granulate is passed through
a 1.0 mm mesh screen and mixed with magnesium stearate.
The final mixture is compressed in a tablet press
with dies 7 mm in diameter provided with a dividing
notch to form tablets each weighing 120 mg.
Example II
. . . .
Coated tablets containinq 5 mg of 3-[~N-(2-(naphth~
2-yI)-ethyl)-piperid-3-yl)-methyl]-7~8-dimethoxy-
2-oxo-1,3,4,S-tetr~ydro-2H-3-benzazeE~ine-hYdrochloride ~ ~ -
1 tablet core contains~
Active substance 5.0 mg
Corn starch 41.5 mg
Lactose 30.0 mg
Polyvinylpyrrolidone 3.0 mg
Magnesium stearate 0.5 mg
80.0 mg
` i^~ 133~3
- 181 -
Method of Preparation
The active substance, corn starch, lactose and
polyvinylpyrrolidone are throughly mixed and moistened
with water. The moist mass is forced througb a
1 mm screen, dried at about 45C and then the granulate
is passed through the same screen. After magnesium
stearate has been adaed~ convex tablet cores with r
a diameter of 6 mm are compressed in a tablet making
machine. The tablet cores thus produced are coated ~-
in known manner with a coating consisting essentially
of sugar and talc. The fin$shed coated tablets
are polished with wax and the weight of the final
coated tablet is 130 mg.
Example III
Ampoules containing 5 mg of 3-~(N-(2-(naphth-2-yl)-
i~erid-3-Yl)-methYl]-7~8-dimethoxY-2-oxo-l~3~4~5
tetrahydro-2H-3-benzaze~in-2-one-hydrochloride
1 ampoule contains:
Active substance 5.0 mg
Sorbitol 50.0 mg
Water for injections ad 2.0 ml
: - ~....
Method of Preparation
. ~.
In a suitable mixing vessel the active substance
is dissolved in water for injec~ions and the solution
is made isotonic with sorbitol,
After being filtered through a diaphragm filter -~
the solution is transferred under a current of
N2 into purified and æterilized ampoules and auto-
claved for 20 minutes in a jet of steam.
::
Example IV
:
~` ^` 133~93
- 182 -
Suppositories containinq_10 mg of 3-[(N-(2-(naphth- -
2 Yl)-ethyl)-piperid-3-Yl)-methyll-7r8-dimethoxy-
2-oxo-1,3,4,5-tetrahYdro-2H-3-benzazepine-hydrochloride
1 suppository contains:
Active substance ~ OoO10 g
Hard fat te.g. witepsol H 19 and W 45) 1.690 q
1.700 g
Method of PreParation
The hard fat is melted. At 38C the ground active
substance is homogeneously dispersed in the melt.
It is cooled to 35C and poured into slightly chilled
suppository moulds.
Example V
DroPs solution containing 10 mg of 3-[(N-(2-(naphth-
2-yl)-ethyl)-pi~erid-3-yl)-methyl]-7,8-dimethoxy-
2-oxo-1,3,4,5-tetrahydro-2H-3-benzaze~ine-h~drochloride -
100 ml of solution contain:
Active substance 0.2 g
Hydroxyethylcellulose 0.15g
Tartaric acid 0.1 g
Sorbitol solution with 70%
dry matter 30.0 g ;
Glycerol 10.0 g
Benzoic acid 0.15g
Dist. water ad 100 ml
Method of Preparation
The distilled water is heated to 70C. The hydroxyethyl-
cellulose, benzoic acid and tartaric acid are dissolved
therein with stirring. The mixture is cooled to
ambient temperature and the glycerol and sorbitol
solution are added with stirring. At ambient temperature
~fRde ~C,fk
::~
, ,, . , , ., ` , . . .
^ 13~993
- 183 -
the active substance is added and stirred until
completely dissolved. Th0 syr~p is then evac~ated
of any air with stirring.
:.~' , . ~, ..
',' ,'".'," ",'i,'' ..
' ~ ' ''
~ . . ~ ,,
: ~',''';',
,
.
',.~,.'.':
:. .:
'~