Note: Descriptions are shown in the official language in which they were submitted.
-
1 332i~ ~
-1- PC-3147
A LONG LIFE ANODE FOR ELECTROWINNING
The present invention relates to the field of anodes for
electrowinning. More particularly, it rela~es to an improved lead or '~
alloyed lead anode for electrowinning. ~;
5~ BACKGROUND OF THE ART AND PROB~EM
The~anodes used in the electrowinning of copper are ideally
constructed of~materials which are inert or passive in the ;~
el'ectr ~ tic~cell~ Lead anodes are essentially inert in the~
''e1ectrowinning~of~copper. However? in the electrowinning~process,
10~ somé of the lead oxidizes in the~sulfuric acid~electrolyte to form a
lead~sulfate layer OD portions of~the anode exposedito'the sulfuric'
acid electrolyte.~
The~lead su1fate layer forméd~on the anode is hard,~brittle ~-
i~ff~CUit~to~remQVe. Thi8 lead~6ulfate layer adds electrical
15~ re~i~stance~ 1ightly lncreasing the energy required to electrowin ~
copp~r.~ After~tbe~1ead sulfate accumulates on the anode surface for ~'
`a~pe'riod Q~ tlme~, the lead sulfate begins to crack and~flake. The '`' '~:
f1alkes~fi~11 off of~ehe~anode surface,~exposing uncovered lead ~ ~'
~ 1~321~9 ~
-2- PC-3147
anode~ which results in an irregular anode current distribution. The
irregular anode current distribution reduces the practical operating
efficiency by promoting the formation of nodules of copper on the
cathode. The nodules on the cathode grow to subtantially mirror
image the areas in whi~h the lead sulfate has flaked off the anode,
forming on areas across from where the lead sulfate has flaked off.
Additionally, the nodules grow into the ad~acent anodes,
short circuiting the electrowinning process. The nodules also entrap
any impurities dissolved in the electrolyte such as iron, nickel,
cobalt, arsenic, sulfur and lead. ~igh lead levels cause "hot
cracking" or "hot shortness" during the rslling of rod for
wiremaking. This reduces the applications of electrowon copper.
Thus, it is desirable to produce nodule free electrical grade copper,
because electrical grade copper from which wire is formed generally
commands a higher market price than lower grade copper.
The lead anode flaking or corrosion has been minimized by -~
~ alloying the lead with 6 percent antimony. However, despite the 6
- ~ percent antimony, the anode con~inues to oxidize and lead sulfate
continues to flake from the anode surface, interfering with the -
electrowinning process. Another possible suggested solution would be
the use of a truly inert anode, such as a refractory metal coated
with gold, platinum, iridium, rhodium and ruthenium. These anodes, ~;
however, would be prohibitively expensive.
As far as known, a lead anode which effectively resists the
formation of lead sulfate or a cost effectlve substitute for the lead
anode has not been discovered.
SUMMARY OF THE INVENTION
The invention comprises an anode and a process for ~
electrowinning in an electrolytic cell having an aqueous sulfuric ~ -
acid-containing electrolyte. A lead plate member has a lead or lead
alloy outer surface. The outer surface is rough and contains
elevations and depressions. The elevations and depressians are
effective in causing lead sulfate formed by the oxidation of the
rough outer surface of the lead plate member to adhere to the rough
outer surface for reducing flsking of the lead sulfate. A support
~ 3~2159
-3- PC-3147
means suspends the lead plate member in the electrolyte.
Preferably, the outer surface of the plate member also
cont~ins 6 percent antimony for further resistance to lead sulfate -
formation. Ideally, the elevations and depressions are formed by
sandblasting. The depressions in the surface preferably are less
than 1 mm in depth as measured from ad~acent elevations.
BRIEF DESCRIPTION OF THE DRAWING
Figure 1 is a schematic cross sectional side view of an
electrolytic cell illustrating the anodes and cathodes. ~`
Figure 2 is a schematic frontal view of an anode with a
portion of the rough outer surface layer of the anode partially
broken away. ;~
Figure 3 is an enlarged schematic cross sectional view of a ~;
portion of the plate member of the anode of Figure 2.
Figure 4 is an enlarged schematic cross sectional view of
the plate member of the anode of Figure 2 illustrating lead sulfate
adheFing to the rough outer surface of the anode. ~`~
DESCRIPTION OF PREFERRED EMBODIMENT
Referring to Figure 1, the electrolytic cell 10 is filled
with copper containing aqueous electrolyte 12. Aqueous electrolyte
12, containing sulfuric acid, enters inlet 14 and exits overflow
outlet 16. The cell 10 contains anodes 18 and cathodes or blanks 20
suspended in the cell. Tank walls 22 form a rectangularly shaped
cell in which the anodes and cathodes are suspended. The anodes 18
are constructed out of an essentially inert or non-dissolving metal
such as lead or an antimony lead alloy. Oxygen is produced at the
`~ ~ anodes 20 generating 2 bubbles 24. The bubbles 24 interact with a
foaming agent to form a protective foam layer 26. The protective ~-
foam layer 26 helps prevent sulfuric acid from entering the
atmosphere. Skimmer 27 prevents foam 26 from exiting directly
~`~ through overflow outlet 16. The blanks or mandrels 20 are preferably
constructed out of titanium.
,"-''~,.''
1 3~21S9
-4- PC-3147
:
The reaction for the electrowinning of copper involves
reduction of the cupric ion to metallic copper at the csthode and
evolution of gaseous oxygen at the anode.
The lead or lead alloy anode used in the electrowinning
procese ideally remains inert. However, ln the presence of sulfuric
acid the oxidizing potential of the anode is sufficient to oxidize
the outer portion of the lead anode to form a lead sulfate (PbS04)
layer on the anode. After about one year of electrowinning copper, -
the lead sulfate begins to flake off of a 6 percent antimony lead ~ -
anode that lacks the anode surface of the invention.
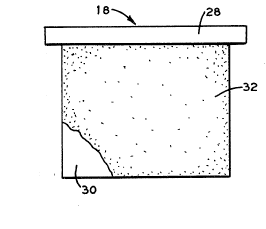
Referring to Figures 2 and 3, the anode 18 is suspended
from support means or crossbar 28. The anode 18 is constructed with ~;~
a plate member 30 having a rough outer surface 32. It has been
discovered that a rough outer surface 32 of a lead anode or an `~
antimony lead anode inhibits the flaking off of lead sulfate. The
anodes produced for use in electrowinning of copper generally have a ;~
~; smooth outer surface. To inhibit the flaking off of lead sulfate the
: smooth surfaces of the anode which are suspended in the electrolyte,
are roughened to produce elevations 34 and depressions 36 (elevations
34 and depressions 36 are only shown in Figure 3). For purposes of
this specification, an elevation 34 is defined as a surface area
above the ayerage height of the rough outer surface 32 and a
depression 36 is defined as a surface area below the average height
of~the rough outer surface 32.
~ Referring to Figure 4, during use of the blank 18, the lead ~-
anode oxitizes to form a~layer of lead sulfate 38. The layer of lead ;~
sulfate adheres to the~elevations 34 and depressions 36, preventing
the flaking of lead sulfate from the anode 18. In test, anodes
; j j treated by this method have functioned without flaking for ove~ twoyears. After two years of use, the lead sulfate continues to form on
the anode, but the layer of lead sulfate adheres to the anode surface
having very little, if any, negatlve effects on the electrowinning
process. The smooth anodes by comparison flake after only one year
of use disrupting ~he electrowinning process.
The anode of the invention provides the several unique
:. ~ .
benefits of: 1) The rough surface anode oxidizes to form a uniform
` ~ layer of lead sulfate which adheres to the roughened lead anode,
. ~ . .
~3~i~;Q
-5- PC-3147
reducing short circuiting of the electrowinning process; 2) The
current between the anode and cathode has an efficient uniform
current distribution; 3) the uniformly deposited copper has a higher
purity, absent the nodules which entrap electrolyte impurities; 4)
the market value of the copper is increased; and 5) the invention
reduces the maintenance costs of cleaning the lead sulfate from the
anode. -
The anodes utiliæed in the experiment were cast lead 6
percent antimony tapered anodes. The anodes had a length of 109 cm,
(91 cm in electrolyte), and a width of 86 cm. The top thickness of
the anode was 2.5 cm and the bottom thickness was 0.8 cm. The anodes -~ -
having a weight of 109 kg were suspended by a suppor~ means
consisting of 1.3 X 6.4 cm copper cross rods.
The blanks utilized in the experiment were titanium
15 mandrels. The blanks had a length of 114.6 cm, (102 cm in the
electrolyte), and a width of 100 cm. The blanks had a thickness of
0.3 cm and a weight of 13 kg. The blanks were suspended by a copper
cross rod. Typical electrolyte composition was as follows~
~- Temperature - cell inlet - 60C
20 Temperature - cell outlet - 65-70C u
Composition - cell inlet - Cu 50-65 gpl
H S04 180-200 gpl
Fe 7-10 gpl ;~
~: .: :~
Ni 8-12 gpl
Co 6-10 gpl
As 2-2.5 gpl
Se~ 0.005 gpl
Te c0-005 gpl
Copper Concentration - cell outlet - Cu 40-50 gpl
H S0 190 210 1 `~
.. ~, - - .-
The flow rate was about 40 liters per minute (lpm) through
; a cell having 67 anodes and 66 titanium cathodes.
To create the rough outer surface the anodes were
sandblasted to form surface elevations and depressions. The surface "~
of the antimony lead anodes was prepared by sandblasting with silica
i3~?,~,~9
-6- PC-3147
sand. The silica sand was sorted with a screen having approximately 12.6 openings
per cm (No. 32 mesh size). The anode was sprayed with a silica sand at about 7.5atmospheres gage (110 psig) through a venturi type nozzle. The nozle had an
intemal diameter of approximately 1.1 cm (7/16 in). The depressions created in the
S relatively soft lead were less than 1 mm in depth as measured from adjacent elevations.
Both previously used anodes having flaking lead sulfate on the surface of
the plate member and new cast or rolled anodes were roughened by sandblasting togreatly improve the anodes useful life. To produce a rough surface on an anode
covered or partially covered with lead sulfate, the lead sulfate must first be
removed. The flaking lead sulfate proved difficult to remove. A wire brush was
ineffective at removing the lead sulfate. However, when anodes havir~g a lead
sulfate layer are sandblasted, the lead sulfate is easily stripped away from the anode,
leaving an anode having a rough and relatively clean surface free of lead sulfate.
lS The surface of a new anode may be produced by several methods, such as rolling
with a rough surface, pressing with a rough surface, casting in a roughened surface
mold, machining the surface or by any other known methods of producing a rough
surface.
The depressions and elevations of the invention proved ef~ective in
preventing the flsking of lead sulfate. I'~ivo years after the introduction of the
roughened test anodes to the cell, there was a slight lead sulfate accumulation on
the sandblasted anodes which adhered tightly to the anode. This layer of soft lead
sulfate which formed on the anode surface had the texture of fine sandpaper and
had no tendency to peel or flake off.
It was also discovered that the rough outer surface anodes produced
more 2 bubbles when f~rst introduced into the cell. As the lead sulfate formed,larger but fewer bubbles formed adjacent to the anodes. The bubble size effects
the formation of the fosm layer on the electrolyte surface which provides acid mist
protection. To prevent disparities in foam production it is recommended that allanodes utilize similar surface roughness when a standard amount of foaming agentis used.
'i X ~
~ ~3~
-7- PC-3147
While in accordance with the provisions of the statute,
there is illustrated and described herein specific embodiments of the
invention, those skilled in the art will understand that changes may : :
be made in the form of the invention covered by the claims and that
certain features of the invention may sometimes be used to advantage
without a corresponding use of the other features.
: ~:
; ,., ~.
,-
' ', ,"'.
~ ~ : -: ~ : .-
!
`.~ :~, " `-~'
'''~'`.'~