Note: Descriptions are shown in the official language in which they were submitted.
1332782
ANNULAR HEATED FLUIDIZED BED REACTOR
This invention relates to an improved heated _uidized bed reactor,
and a method for heating such reactor. In a preferred embodiment, the present
invention relates to an improved heated fl~ i7ecl bed reactor used in the production
5 of polycrystalline silicon by the pyrolysis of silane containing gases.
A variety of means are known in the art for supplying the necessary
heat to fluidized bed reaction zones. For example, in the pyrolysis of silane
cont~ining gas to silicon, heat can be provided by capacitive heating of the _uidized
bed reaction zone, as discussed in U.S. Patent 4,292,344 to McHale. Other methods
10 of heating such as uniform induction coils, electrical resistance elements and indirect
gas fired heaters have also been used and are disclosed in U.S. Patents 3,012,861 to
Ling and 3,012,862 to Bertrand et al. A suitable heat transfer fluid and inductive or
electrical resistance heaters are also examples of means for directly supplying heat
to the surfaces of conventional fluidized bed reactors. While adequate for the
15 purposes of some fluidized bed applications, these means are not always s~ti~f~r~tory
for other fluidized bed applications because of the nature of the reactions occurring
therein and the heat requirements of the fluidized bed.
For instance, in the production of polycrystalline silicon from silane
containing gases in a fluidized bed reaction zone, conventional means for supplying
2 0 heat to the wall of the _uidized bed are generally lln~ti.~f~ctory. In this example,
silicon particles are suspended in a fluidizing gas stream into which silane containing
gases are introduced. The process conditions are desirably maintained so that the
decomposition of the silane cont~ining gas occurs heterogeneously on the surface of
the silicon particles of the fluidized bed, rather than on the hot wall of the fluidized
2 5 bed. The silicon particles grow and enlarge by the deposit of silicon thereon so that
sufficiently large silicon product particles are produced and removed from a
collection zone below the reaction zone.
Supplying heat to the wall of the fluidized bed reaction zone causes
the temperature of the wall to be higher compared to the temperature of the silicon
3 0 particles. This may result in an undesired deposition of the silicon on the wall of the
reaction zone, in preference to the desired deposition of the silicon onto the surface
of the fluidized silicon particles. In addition to reducing the amount of silicon that
deposits onto the surface of the silicon particles, the deposition of silicon on the
~'
, ~.,,~
_ 2 1332782
reactor walls has the effect of reducing the heat transfer efficiency into the reaction
zone because of the additional layer through which the heat must travel.
Therefore, a need exists for an improved heated fluidized bed reactor,
useful, for example, as an improved heated fluidized bed reactor for the production
of high purity polycrystalline silicon.
The present invention is an improved heated fluidized bed reactor
and a method for supplying heat to the reactor. The improved fluidized bed reactor
includes a reaction vessel containing a peripheral heating zone annulus that
surrounds an inner fluidized bed reaction zone. A heat source supplies heat to
fluidized bed particles contained in the heating zone annulus. The heating zone
laterally confines the particles from the fluidized bed in an incipient fluidized state.
The heating zone annulus includes an upper inlet for the entry of the particles from
the fluidized bed and a lower outlet for introducing heated partides from the heating
zone annulus into the inner reaction zone. Heat is supplied to the inner reaction
zone through the boundary of the inner reaction zone and by the heated partides of
the heating zone annulus that enter the inner reaction zone from the lower outlet of
the heating zone annulus.
In another embodiment, the present invention is a method of
supplying heat to a fluidized bed reaction zone by introducing heated partides from
2 0 a peripheral heating zone annulus to the inner fluidized bed reaction zone. The
peripheral heating zone annulus surrounds the inner reaction zone within a reaction
vessel. The peripheral heating zone annulus indudes an upper inlet for entry of
partides from the fluidized bed and a lower outlet for introducing heated partides
from the heating zone annulus into the inner reaction zone. The particles from the
2 5 fluidized bed in the heating zone annulus are in an incipient fluidi7ed state and are
laterally confined by the inner reaction zone wall and an outer heating zone wall.
Heat is supplied to the particles in the peripheral heating zone annulus through the
outer heating zone wall by a heat source disposed externally to the outer heating
zone wall.
In still another embodiment, the present invention is a method for
producing high purity polycrystalline silicon by pyrolyzing a silane containing gas in
a heated inner fluitli7ed bed reaction zone. Heat is supplied to the inner reaction
zone through the boundary of the inner reaction zone and by heated silicon particles
that have been introduced into the inner reaction zone after passing through a
3 5 peripheral heating zone annulus. The peripheral heating zone annulus is defined by
'
;:
13327~2
the annular space between the wall of the inner reaction zone and an outer heating
zone wall. Heat is supplied to the particles in the peripheral heating zone annulus
by a heating means disposed externally to the outer heating zone wall.
In accordance with the present invention, an improved heated
5 fluidized bed reactor in~ ling a peripheral heating zone annulus is provided. Other
features and advantages of the present invention will be readily apparent from the
following description of certain preferred embodiments thereof, taken in conjunction
with the accompanying drawings. It is to be understood that variations and
modifications may be effected without departing from the spirit and scope of the10 novel concepts of the present invention.
The present invention is described herein with regard to a preferred
embodiment relating to the pyrolysis of silane cont~ining gases to silicon. It is
understood that the present invention is equally applicable to other types of fluidized
bed operations requiring the input of heat. Examples of such operations include
15 catalytic reactions, ion PYrll~nge reactions, separation operations and the like,
wherein a fluidized bed of particles requires the input of heat.
As used herein, the term "heterogeneous decomposition" refers to the
reduction of silane or a halosilane to silicon that occurs in t vo or more phases such
as when the decomposition occurs at a boundary between a gas and a solid phase.
20 The heterogeneous decomposition results in the deposition of silicon on either
silicon particles in the fluidized bed or on the exposed internal surfaces of the
fluidized bed reactor. "Homogeneous decomposition" occurs in a single phase, such
as the gas phase, and produces high surface area silicon powder or dust in the
micron to submicron size range. Generally, for a given temperature, the
,~
~ ~h ~ $ ~
-4-
decomposition of silane and halosilanes will be either heterogeneous and/or homogeneous,
depending on the concentration of the silane and/or halosilane containing gas. Generally, a
low silane and/or halosilane feed concentration is desired to maintain the decomposition of
silane and halosilane containing gases to silicon in a heterogeneous mode. However, a very
low feed concentration of silane and/or halosilane containing gases may not provide a high
production rate of silicon.
The term "silicon seed particle" means those particles of the fluidized bed that range
in particle size from about 50 microns to about 400 microns. These particles grow and
enlarge as silicon is deposited thereon, and are eventually collected as silicon product
particles. "Silicon product particles" describes the silicon seed particles that have grown and
enlarged to a particle size ranging from at least about 400 microns, preferably about 400
microns to about 1300 microns. The silicon product particles segregate in a collection zone
near the bottom of the reaction zone and are removed therefrom by conventional means. The
term "silicon particle" refers to both silicon seed particles and silicon product particles of the
1 5 fluidized bed .
The term "silicon powder" refers to generally micron to submicron, high surface area
silicon resulting from the homogeneous decomposition of the silane and/or halosilane
containing gas.
As used herein, the term "silane containing gas" refers to both silane and/or halosilane
containing gases unless otherwise indicated.
The term "total fluidizing gas" as used herein refers to the combination of silane
containing gas and any other additional carrier gas which is added to the fluidized bed reactor
to aid in the fluidization of the silicon particles and/or to control the reaction rate or heat
transfer.
Polycrystalline silicon may be prepared by introducing a flow of silane containing gas
into a heated fluidized bed of silicon particles suspended in a reaction zone. These silicon
particles are suspended by the upward flow of the silane containing gas and the carrier gas
passing through the reaction zone. The total gas velocity through the reaction zone is
maintained above the minimum fluidization velocity of the silicon particles. The temperature
of the silicon particles in the reaction zone ranges between the decomposition temperature
of the silane containing gas and the melting point temperature of silicon. The silane
containing gas decomposes to form silicon that deposits on the surface of the silicon particles.
As the silicon deposits on the silicon particles, these particles
CA I 332782
enlarge and segregate in a collection zone near the bottom of the fluidized bed. The collected
product particles are recovered from the collection zone by conventional means.
The silane containing gas is introduced into the fluidized bed reaction zone from the
bottom thereof in accordance with conventional practices such as a gas distributor. The
5 silane containing gas may be introduced without dilution or the gas may be diluted with
hydrogen or an inert carrier gas such as argon, helium, or the like. In this gas distribution
zone, the distributor surface is cooled to a temperature ranging from about 200C to about
400C, by cooling water, nitrogen, or the like. Such temperatures are maintained to prevent
the premature decomposition of silane containing gases to silicon and to prevent the
10 deposition on the distributor apparatus.
Any suitable silane containing gas capable of being thermally pyrolized or reduced in
the gas phase to silicon can be used as a feed gas to the fluidized bed. Illustrative of such
gases are silane and the halosilanes of chlorine, bromine, fluorine, and iodine. While the
chlorosilanes, such as trichlorosilane, tetrachlorosilane, and dichlorosilane may be employed,
15 particular advantages are realized through the use of silane. The pyrolysis of silane is slightly
exothermic, goes substantially to completion, is irreversible, and is initiated at a lower
temperature of about 200C when compared to the temperature necessary to pyrolyze
halosilane containing gases and the like. In addition, the silane and its decomposition
products, i.e., silicon and hydrogen, are noncorrosive and nonpolluting. The by-product
20 hydrogen gas generated (1 mole of silane yields 2 moles of hydrogen) may be u sed as a
recycle carrier gas within the reaction system. In comparison, the thermal decomposition of
chlorosilane is a reversible and incomplete reaction that results in the production of reaction
by-products that are corrosive in nature. Accordingly, silane is a preferred gas for use when
the present invention is used to pyrolyze silane containing gas to silicon, although other silane
25 containing gases may be utilized.
The silane containing gas and the carrier gas streams can be introduced into thereaction zone by employing a conventional gas distributor below the reaction zone. The
bottom of the fluidized bed is also where seed particles may be introduced into the fluidizing
gas. The total fluidizing gas velocity through the reaction zone generally ranges from about
30 two to eight times the minimum fluidization velocity necessary to fluidize the particles of
average diameter within the bed. As used herein, the term "average diameter" means one
over the
6 CA 1 332782
summation of the quotients of the weight fraction and particle diameter attributed to the
particular fraction of particles. Preferably, the total fluidizing gas velocity is about four to six
times the minimum fluidization velocity based on the average diameter of the particles in the
fluidized bed. The minimum fluidization velocity may be determined by conventional means
5 known in the art, such as the equation:
~; 3 2 Vo2 ~ Dpp(l-t) V DpgP(o -o)
wherein
V0 = minimum superficial gas velocity for fluidization (cm/s)
Dp = average diameter of particles in the bed (cm)
p = density of fluidization gas (g/cm3)
pp = density of particles (g/cm3)
q~S = sphericity of particles
~ = void fraction in bed of particles at minimum fluidization
absolute viscosity of fluidizing gas (g/cm-s)
g = gravitational acceleration (cm/s2)
The minimum fluidization velocity is a strong function of gas viscosity and gas density, as well
as average particle diameter, particle shape and void fraction. Thus, the minimum fluidization
20 velocity may cover a wide range with small change in these factors.
In the production of polycrystalline silicon in the fluidized bed reactor described herein,
silicon seed particles must be supplied to the fluidized bed reaction zone. In order to supply
replenishing seed particles to the fluidized bed, a small fraction of the product material can
be suitably crushed or ground into small, fine seed-sized particles. These particles may then
25 be reintroduced into the fluidized bed. Upon introduction, the small seed particles become
sites for the deposition of silicon resulting from the silane decomposition. As the silane is
decomposed and the silicon deposited, the particles grow and enlarge in size. The enlarged
silicon product particles segregate in a collection zone near the bottom of the reaction zone.
The silicon product particles are collected by continuously or periodically removing the product
30 particles from the collection zone. The product particles are of sufficient size to be easily
handled without undue contamination of the high purity silicon material. It is to be understood
that the
~hi i J J;~
--7--
particular type of the particles comprising the fluidized bed are not critical to the invention per
se. The particles may be of the types commonly employed in the various fluidized bed
applications known in the art.
The fluidized bed reactor in accordance with a preferred embodiment of the present
5 invention is a generally vertical reaction vessel wherein the desired fluidized bed reaction is
carried out. A preferred reaction is the pyrolysis of silane containing gas to silicon that
deposits on silicon particles in a fluidized bed reaction zone. The fluidized bed reactor in the
context of the present invention includes a peripheral heating zone annulus surrounding an
inner reaction zone. The inner boundary of the heating zone annulus is defined by the inner
10 reaction zone wall that surrounds the fluidized bed of particles. The outermost boundary (i.e.,
outer heating zone wall) of the heating zone annulus is defined by the wall or liners of the
cylindrical reaction vessel. This configuration resembles a small inner cylinder (i.e., inner
reaction zone wall) disposed within a larger outer cylinder (i.e., outer heating zone wall). The
interior of the small inner cylinder defines the reaction zone, with the annular space between
15 the inner and outer cylinder defining the peripheral heating zone annulus. While the cylindrical
vessel and reaction zone are preferred, it is to be understood that any configurations that are
acceptable to fluidized bed operations can be used as long as space is provided for the heating
zone annulus between the reaction vessel wall or liners and the inner reaction zone wall.
The dimensions of the particular reaction vessel and reaction zone are not critical to
20 the practice of the present invention. The particular dimensions will be primarily dependent
upon the economics of design. The reaction zone must not be too narrow or this leads to low
production efficiency; however, it must not be too large or this leads to increased energy
costs associated with heat transfer inefficiencies and bed fluidization difficulties.
In the production of silicon, the ratio of bed height to bed diameter of the reaction zone
25 wherein the silicon particles are suspended by the total fluidizing gas flow ranges from about
1 :1 up to about 10:1, preferably, 1 :1 to about 5:1 . The skillèd artisan will appreciate that the
particular ratio of bed height to bed diameter will primarily be dependent upon the total
fluidizing gas velocity, the silicon seed particle size and the silicon product particle size. The
diameter of the reaction zone preferably ranges from about 15 cm to about 122 cm, and more
30 preferably about 30 cm. The outer cylinder (i.e., outer heating zone wall) that defines the
outer boundary of the peripheral heating zone is preferably
-8- ~A 1332782
concentrically positioned in relationship to the inner cylinder defining the reaction zone. The
length of the outer cylinder is equal to or preferably greater than the length of the inner
cylinder defining the reaction zone. The preferred increased height of the outer cylinder
provides a disengagement zone that allows entrained particles to lose their upward velocity
and gravitate back into the fluidized bed or the heating zone. The diameter of the outer
cylinder preferably ranges from about 18 cm to about 142 cm, most preferably about 41 cm,
to provide a heating zone annulus having a width of about 1 cm to 10 cm, and preferably
about 5 cm.
The peripheral heating zone annulus includes an upper inlet that allows the entry of a
portion of the fluidized particles from the inner fluidized bed. To facilitate the understanding
of the flow of the particles through the heating zone annulus and back into the reaction zone,
consider the present invention wherein the particles in the inner bed and the annular heating
zone are incipiently fluidized. The incipient fluidized state refers to the condition of a bed of
particles that has fluidizing gases passing therethrough at a velocity just above the minimum
fluidization velocity for the bed. Thus, at incipient fluidization, the entire bed and the entire
heating zone annulus consists of a dense phase that will behave like a liquid. In this state,
there will be no convective transport of particles because the bulk densities in both the inner
bed and annular heating zone are the same, and consequently there is no driving force for
circulation .
When the flow of fluidization gas through the inner reaction zone increases, bubbles
will begin to form in the inner bed. This causes the inner bed to expand and therefore have
a lower bulk density than the incipiently fluidized heating zone annulus. In the steady state,
if both the inner bed and the bed of particles in the annular heating zone extend above the
inner reaction zone wall, the top portions of the beds will be sharing the same free space and
therefore the pressure in each bed at the top of the inner reaction zone wall will be the same.
By free space is meant that portion of the reactor between the upper surface of a fluidized
bed and the top of the reactor vessel. At the bottom of the bed, however, the pressure is
higher in the annulus because of the higher bulk density of the material in the annulus. This
results in a pressure gradient across the bottom of the annular heating zone to the bottom of
the inner reaction zone. This pressure drop serves as the driving force for the introduction of
the heated particles from the heating zone annulus to the inner reaction zone.
Although it is possible to maintain the particles in the heating zone annulus in a
nonfluidized state, it is preferred that the particles be in a state of incipient
g CA 1 332782
fluidization because the heat transfer coefficient from the outer heating zone wall to the
incipiently fluidized particles is greater than the heat transfer coefficient from the outer heating
zone wall to nonfluidized particles. Since fluidized particles flow like liquids, the particles in
an incipiently fluidized state are also more easily and uniformly reintroduced from the heating
zone annulus into the reaction zone than are the particles in a nonfluidized state.
The bottom of the peripheral heating zone includes the outlet through which the heated
particles, after passing downwardly through the heating zone, are introduced into the fluidized
bed. As the particles flow downwardly through the heating zone, they pick up heat from a
heating means disposed on the exterior of the outer heating zone wall. The heat is then
transferred to the fluidized bed by the introduction of the heated particles to the reaction zone.
It should be understood that heat is also supplied to the inner reaction zone through the inner
reaction zone wall that surrounds the inner reaction zone because the annulus is hotter than
the reaction zone.
The entrance to the inner bed at the bottom of the heating zone annulus behaves like
an orifice in traditional fluid applications. The mass flow rate through the passage is
proportional to the square root of the pressure drop across the orifice and is proportional to
the area of the opening. The area of the opening should be kept as small as possible,
consistent with good design parameters, in order to minimize the diffusion of silane into the
heating zone annulus. The circulation of the particles between the annulus and the inner bed
can be varied by changing the pressure drop across the entrance orifice or by using other
means such as gas jets. The pressure drop is determined by the degree of fluidization in the
annulus, the higher the degree of fluidization, the smaller the pressure drop and accordingly,
the smaller the material circulation between the inner reaction zone and the heating zone
annulus .
In situations where it is desirable to maintain the free surface of the bed of particles
in the annular heating zone below the top of the inner reaction zone wall, the fluidized
particles from the inner reaction zone will flow over the top of the wall and fall into the
annulus. This condition could also occur after the batch removal of the product particles from
a silane reactor. In either situation, under steady state conditions, the height of the bed in the
annular heating zone will automatically adjust to a position where the mass flow of particles
into the annulus is in equilibrium with the mass flow rate of particles out of the annulus.
When establishing the equilibrium height, consideration should be given to ensuring
-~- CAl 332782
the equilibrium height at least corresponds to the height of the heating means disposed on the
exterior of the outer heating zone wall.
In the particular example of a silane pyrolysis reaction, it is desirable to isolate the
outer heating zone wall from the silane-containing gas in order to prevent silicon from
depositing thereon. This is achieved by fluidizing the particles in the heating zone annulus
with hydrogen or inert carrier gases in the absence of any substantial amount of silane.
Because the resistance to gas flow is greater through the heating zone annulus compared to
the reaction zone, any mixing of gases, after they enter the reactor vessel, is generally a result
of the fluidizing gases from the annular heating zone mixing into the silane containing gas
used to fluidize the particles of the reaction zone. Although certain amounts of silicon may
deposit on the inner reaction zone wall and thus reduce the efficiency of heat transfer from
the heating zone annulus to the reaction zone through the inner reaction zone wall, an
increase in the flow rate of heated silicon particles from the heating zone annulus should
compensate for the loss.
The heating zone is preferably located on the inner periphery of the liners within the
reaction vessel. Heat is supplied to the heating zone by a heating means that, in the pyrolysis
of silane, maintains the temperature of the particles in the heating zone between the thermal
decomposition temperature of the silane containing gas and the melting point temperature of
silicon. Preferably, the temperature ranges between about 200C and about 1400C and
most preferably, between 550C and about 1 000C. The heating means can be any type of
resistive heating, conductive heating, inductive heating and/or other conventional means for
supplying heat to the heating zone through the outer heating zone wall.
The fluidized bed reaction zone is surrounded by the heating zone and occupies the
remaining inner portion of the reaction vessel. The reaction zone is heated by interaction with
the heated particles that have been introduced to the inner reaction zone from the lower outlet
of the peripheral heating zone and by heat supplied from the heating zone through the inner
reaction zone wall. In the pyrolysis of silane, the temperature of the particles in the fluidized
bed reaction zone ranges from about 200C to about 1400C, preferably about 550C to
about 1 000C. The temperature of the silicon particles in the peripheral heating zone annulus
ranges from about 300C to about 1400C, preferably about 550C to about 1000C.Therefore, the present invention provides a heated fluidized bed reactor wherein heat
is supplied to the fluidized bed reaction zone by particles of the
11 133~782
flui~li7e.d bed that have been heated in a peripheral heating zone. In the particular
application relating to the pyrolysis of silane, the heating zone :~nnulns serves to
isolate the outer heating zone wall from silane containing gases that may decompose
thereon and inhibit the heat transfer into the heating zone and, consequently, the
5 inner reaction zone.
The invention is described further, by way of illustration, with
reference to the accompanying drawings, in which:
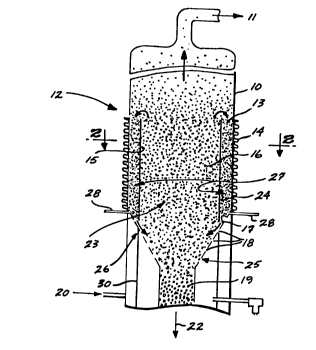
FIGURE 1 illustrates a cross-sectional view of the annular heated
_uidized bed reactor in accordance with a preferred embodiment of the present
10 invention; and
FIGURE 2 illustrates a top view of the annular flui(1i7ed bed reactor
in accordance with a preferred embodiment of the present invention.
Referring to FIGURE 1, silicon particles 16 are fluidized in a reactor
useful for the production of high purity polycrystalline silicon product particles 19 by
15 the pyrolysis of silane containing gas. Silane containing gas in conduit 21 enters the
bottom of the flui~li7ed bed reactor vessel 12 below the portion 25 of the gas
distributor plate that is positioned directly below the _uidized bed reaction zone 23.
Hydrogen gas in line 20 also enters the bottom of the fluidized bed reactor vessel 12
below the portion 26 of the gas distributor plate that is directly below the peripheral
2 0 heating zone annulus 27. The hydrogen gas and silane containing gas entering the
bottom of the fluidized bed reactor vessel 12 are isolated from each other by wall 30,
such that the gases cannot come into contact until after they pass through the gas
distributor plate. The mixing of the hydrogen gas and the silane containing gas
within the _uidized bed reactor 12 above the gas distributor is preferably restricted
25 by positioning the lower end of the inner reaction zone wall 15 as close to the
distributor plate as possible, taking into consideration the gap needed to allow the
heated partides 24 to be reintroduced into the fluidized bed reaction zone 23. The
hydrogen gas passes upwardly through the portion 26 of the gas distributor plate and
fluidizes the particles 24 in the heating zone annulus 27 without a substantial amount
30 of silane-cont~ining gas mixing into the hydrogen gas. The silane containing gas
enters the _uidized bed reaction zone 23 directly below the bed of particles 16 and
preferably passes directly upward to fluidize the bed 23 without mixing into thehydrogen gas. Any mixing that does occur, preferably occurs by hydrogen gas mixing
into the silane containing gas as opposed to the silane containing gas mixing Into the
3 5 hydrogen containing gas. By isolating the outer heating zone wall 10 (e.g., quartz
lla 13327~3~
liner) from the silane containing gas, the amount of silicon that deposits on the heat
transfer surface of the outer heating zone wall 10 is minimi7:ed.
-12- CA 1332782
Heat is supplied to the fluidized bed reaction zone 23 through the inner reaction zone
wall 15 and by silicon particles 16 that have passed through the peripheral heating zone
annulus 27. The peripheral heating zone annulus 27 occupies the space between the inner
reaction zone wall 15 of the fluidized bed reaction zone 23 and the outer heating zone wall
10 of the reaction vessel 12. The peripheral heating zone annulus 27 contains laterally
confined silicon particles 16 which have entered the heating zone annulus 27 through an
upper inlet 13. The silicon particles 16 are in an incipient fluidized state 24 due to the lower
fluidization gas velocity passing through the heating zone 27 compared to the gas velocity in
the reaction zone 23. Heat is supplied to the incipiently fluidized silicon particles 24 by
conductive heat transfer from a heat source 14 through the outer heating zone wall 10. The
incipiently fluidized silicon particles 24 pass downwardly through the peripheral heating zone
annulus 27 where the particles 24 pick up heat from the heating source 14. Near the bottom
of the peripheral heating zone annulus 27 is an outlet 17 for the introduction of the silicon
particles 24 into the fluidized bed reaction zone 23 from the heating zone annulus 27. The
lower outlet 17 of the heating zone annulus may employ some type of driving force such as
pulsed gas jet 28 to promote the introduction of the silicon particle 24 to the fluidized bed
reaction zone 23.
The silane feed gases entering the fluidized bed reaction zone 23 through the
perforated plate 25 are thermally decomposed to silicon and deposit onto the silicon particles
16. The silicon particles 16 enlarge to form silicon product particles 19. The product
particles 19 segregate in a collection zone near the bottom of the fluidized bed reaction zone
23 and are collected as indicated by the arrow 22. By-product hydrogen and other fluidizing
gases that enter the bottom of the bed exit the fluidized bed reaction zone 23 through an
upper outlet 11. The heating zone annulus 27 isolates the outer heating zone wall 10 from
the silane-containing gas and consequently prevents the deposit of silicon thereon, a condition
that reduces the heat transfer efficiency into the heating zone 27. Although silicon may
deposit on the wall 15 of the inner reaction zone 23, thus reducing the heat transfer efficiency
through the wall 15, such reduction has a minimal effect because the primary source of
transferring heat to the reaction zone 23 is the heat introduced by the heated silicon particles
24 that reenter the inner reaction zone 23.
Referring to FIGURE 2, the peripheral heating zone annulus 27 is formed by the
concentrically located wall 15 of the reaction zone 23 and the outer heating
-13- CA l 332782
zone wall 10. Heat is supplied to the heating zone annulus 27 through the outer reaction zone
wall 10 by the heating source 14. Within the annular region between the reaction zone wall
15 and the outer heating zone wall 10 are silicon particles 24 in an incipiently fluidized state.
The silicon particles 24 comprise silicon particles 16 of the reaction zone 23 that have passed
5 through the upper inlet 13 into the heating zone annulus 27 and will eventually be
reintroduced into the reaction zone 23.
It is to be understood that modifications and changes to the preferred embodiment of
the invention herein described and shown can be made without departing from the spirit and
scope of the invention. The following examples are set forth to illustrate the invention,0 however, the examples are not intended as limitations thereof.
Example 1
The following example is conducted in a fluidized bed reactor similar to the oneillustrated in FIGURE 1. The fluidized bed reaction zone is defined by a quartz reaction zone
wall that has a diameter of 30 centimeters. The fluidized bed reaction zone is contained
15 within a stainless steel jacket that has a diameter of 60 centimeters and is surrounded by
insulation 10 cm thick. Kanthal heaters operating at 1200 C are mounted on the interior wall
of the stainless steel jacket and have an inner diameter of about 50 centimeters. Between
the reaction zone wall and the Kanthal heaters is a 0.5 centimeter-thick quartz liner having an
inner diameter of 39.5 cm. The quartz liner isolates the 4.75 cm thick annular heating zone
20 created between the inner reaction zone wall and the quartz liner from the Kanthal heaters.
The fluidized bed reaction zone contains silicon particles ranging in diameter from about 300
to 800 microns. The particles of the fluidized bed reaction zone are fluidized by a gas mixture
of 20 volume percent silane and 80 volume percent hydrogen. The particles that occupy the
annular heating zone are fluidized by 100 volume percent hydrogen. The silane and hydrogen
25 gases enter the fluidized bed reaction zone and the annular heating zone through a
conventional gas distributor that is positioned beneath the reaction zone and annular heating
zone. The hydrogen gas and silane containing gas are introduced below the gas distributor
into separate compartments that prevent the mixing of the hydrogen gas and silane containing
gas. The hydrogen gas, at 773K, enters a compartment below the portion of the gas
30 distributor that is beneath the annular heating zone. The silane containing gas, at 300K,
enters a compartment directly below the portion of the gas distributor that is beneath the
fluidized bed reaction
-14- CA 1 332782
~zone. The superficial velocity of the silane containing as at the gas distributor for the fluidized
bed reaction zone is about 70 centimeters/second, corresponding to the minimum fluidization
velocity for 800 micron particles in the reaction zone. The superficial velocity of the hydrogen
gas at the gas distributor is about 42 cm/sec. The silane gas distributor has a diameter at its
5 large end of about 30 centimeters and a diameter at its small end of about 5 centimeters with
a height of about 5 centimeters. Portions of the stainless steel jacket are held in place by three
flanges having an outer diameter of 92 centimeters and a thickness of 9 centimeters. The
reactor vessel has a height of 263.0 cm and extends 113.0 cm above the top of the fluidized
bed which has a height of 150.0 cm. The pressure of the reactor is maintained at about two
10 atmospheres.
The temperature at the top of the fluidized bed is 923K and the temperature at the
bottom of the fluidized bed is 823K. The temperature of the portion of the distributor cone
through which the silane containing gas passes is maintained at 523K with the ambient
temperature being about 300K. The temperature of the portion of the distributor used to
15 introduce the hydrogen gas into the annulus is about 773K. The temperature of the heating
zone annulus is about 933K. The dense phase of the fluidized bed exhibits a void fraction
of about 0.46 and the annular heating zone exhibits a void fraction of about 0.46. The heat
transfer characteristics of the fluidized bed are summarized below:
Heat Transfer
coefficient between
wall and bed 0.00717 cal/cm2 sec C
Thermal conductivity of
liner 0.0116 cal/cm sec C
Emissivity of liner - 0.60
Thermal conductivity of
insulation 3.45 x 10-5 cal/cm sec C
Heat capacity of silicon 0.168 cal/gramC
The mass flow rate of particles through the heating zone annulus and into the fluidized
bed reaction zone if 11 kg/sec. The downward particle velocity through the annulus is 8.62
35 cm/sec. The total power input into the reactor from the Kanthal heaters is 110 kilowatts using
a height of Kanthal heaters of about 115.0 cm surroundin~q the quartz liner that forms the
outer heating zone wall.
Taking into consideration the heat losses and inputs attributable to the: (1) silane
distributor; (2) product and effluent streams; (3) flanges; (4) radiation
CA I 33278~
-1 5-
from the top and bottom of the bed; (5) insulation; and (6) heat transfer from Kanthal heaters
through the bed walls; and assuming: (1 ) temperature of the fluidized bed reaction zone is
constant everywhere except in the small region at the bottom of the bed; (2) temperature of
the heating zone annulus is constant everywhere; (3) heat transfer through the quartz wall
5 between the reaction zone and annular heating zone is neglected; (4) the temperature of the
quartz liner is uniform in the axial direction; (5) the silane distributor temperature is constant
and heat transfer between the bed and silane distributor is determined by the temperature of
the bed and the temperature of the distributor; (6) the hydrogen distributor temperature is the
same as the hydrogen feed gas; (7) heat transfer between the heaters and liners occurs by
10 radiation only; (8) the silane feed stream enters the distributor at ambient temperature and the
product silicon and effluent gases leave the reactor at the temperature of the bed; (9) the
recirculating silicon particles enter the annular heating zone at the temperature of the bed and
leave the annular heating zone at the temperature of the heating zone annulus; and (10) the
bed and heater radiate as black bodies; the temperature of the fluidized bed is determined to
15 be 650C, clearly suitable for the economic decomposition of silane containing gases to
silicon.
The present invention provides high heat flux to a fluidized bed, such as a fluidized bed
for the pyrolysis of silane to silicon, in a region close to the cooled distributor where a large
amount of heat loss is present. The present invention also has an equally important
20 advantage in a silane pyrolysis reactor in that the deposition of silicon on the heating surface
can be controlled or eliminated by keeping the silane containing feed gas away from the
heated reactor walls. Further, any reduction in heat transfer efficiency resulting from the
deposit of silicon on the inner reaction zone wall is minimized because the primary source of
heat transfer into the inner reaction zone is the introduction of the heated particles of the
25 annular heating zone into the inner reaction zone. The present invention has been described
in relation to various embodiments, including the preferred applications and parameters. One
of ordinary skill after reading the foregoing specification, will be able to effect various
changes, substitutions of equivalents, and other alterations without departing from the broad
concepts disclosed herein. It is therefore intended that the scope of Letters Patent granted
30 hereon will be limited only by the definition contained in the appended claims and equivalents
thereof.