Note: Descriptions are shown in the official language in which they were submitted.
TLC 120A
1 ~')3(~4~
BACKGROUND OF THE INVENTION
The present invention relates to the methods and
compositions for the entrapment of compounds in vesicles
composed of salt forms of organic acid derivatives of
alpha-tocopherol (Vitamin E) that are capable of forming
bilayers.
Alpha-tocopherol, to which a hydrophilic moiety such
as a salt form of an organic acid is attached, can be used
to prepare suspensions of multilamellar or small
unilamellar vesicles. These vesicles may be prepared with
or without the use of organic solvents, and they may
entrap, or associate with, water-soluble compounds,
partially water-soluble compounds and water-insoluble
compounds. For convenience, the vesicles of the invention
20 will simply be referred to as "alpha-tocopherol vesicles",
but it must be understood that salt forms of organic acid
derivatives of alpha-tocopherol are always used in the
preparation of the vesicles.
The alpha-tocopherol vesicles described herein are
25 particularly useful for the entrapment of, or association
with, biologically active compounds or pharmaceutical
compounds which can be administered in vivo.
Alternatively the vesicles of the present invention may be
used in vitro. For instance, the alpha-tocopherol
--2--
1 3 ~3n4~
hemisuccinate vesicles described herein may be used ln
vitro in divalent cation-dependent assay systems.
The alpha-tocopherol vesicles of the invention are
liposomes. Liposomes are completely closed bilayer
membranes containing an encapsulated aqueous phase.
Liposomes may be any variety of multilamellar vesicles
(onion-like structures characterized by membrane bilayers
each separated by an aqueous layer) or unilamellar
vesicles (possessing a single membrane bilayer).
Two parameters of liposome preparations are functions
of vesicle size and lipid concentration: (1) Captured
volume, defined as the volume enclosed by a given amount
of lipid, is expressed as units of liters entrapped per
mole of total lipid (1 mol 1). The captured volume
depends upon the number of lamellae and the radius of the
liposomes, which in turn is affected by the lipid
composition of the vesicles and the ionic composition of
the medium. (2) Encapsulation efficiency is defined as
the fraction of the initial aqueous phase sequestered by
the bilayers. (See Deamer and Uster, 1983, Liposome
Preparation: Methods and Mechanisms, in Liposomes, ed. M.
Ostro, Marcel Dekker, Inc., NY, pp. 27-51.
The original method for liposome preparation [Bangham
et al., J. Mol. Biol. 13:228 (1965)] involved suspending
phospholipids in an organic solvent which was then
evaporated to dryness, leaving a waxy deposit of
phospholipid on the reaction vessel. Then an appropriate
amount of aqueous phase was added, the mixture was allowed
to "swell", and the resulting liposomes which consisted of
multilamellar vesicles (hereinafter referred to as MLVs)
were dispersed by mechanical means. The structure of the
resulting membrane bilayer is such that the hydrophobic
(non-polar) "tails" of the lipid orient toward the center
of the bilayer, while the hydrophilic (polar) "heads"
~3~ 1 3 ~3n4q
orient toward the aqueous phase. This technique provided
the basis for the development of the small sonicated
unilamellar vesicles (hereinafter referred to as SUVs)
described by Papahadjopoulos and Miller [Biochim. BioDhvs.
5 Acta. 135:624 (1967)].
An effort to increase the encapsulation efficiency
involved first forming liposome precursors or micelles,
i.e., vesicles containing an aqueous phase surrounded by a
monolayer of lipid molecules oriented so that the polar
10 head groups are directed toward the aqueous phase.
Liposome precursors are formed by adding the aqueous
solution to be encapsulated to a solution of polar lipid
in an organic solvent and sonicating. The liposome
precursors are then emulsified in a second aqueous phase
15 in the presence of excess lipid and evaporated. The
resultant liposomes, consisting of an aqueous phase
encapsulated by a lipid bilayer are dispersed in aqueous
phase (see U.S. Patent No. 4,224,179 issued September 23,
1980 to M. Schneider).
In another attempt to maximize the encapsulation
efficiency, Paphadjapoulos (U.S. Patent No. 4,235,871
issued November 25, 1980) describes a "reverse-~hase
evaporation process" for making oligolamellar lipid
vesicles also known as reverse-phase evaporation vesicles
(hereinafter referred to as REVs). According to this
procedure, the aqueous material to be encapsulated is
added to a mixture of polar lipid in an organic solvent.
Then a homogeneous water-in-oil type of emulsion is formed
and the organic solvent is evaporated until a gel is
30 formed. The gel is then converted to a suspension by
dispersing the gel-like mixture in an aqueous media. The
REVs produced consist mostly of unilamellar vesicles
(large unilamellar vesicles, or LUVs) and some
oligolamellar vesicles which are characterized by only a
35 few concentric bilayers with a large internal aqueous
space.
4 1 3 ~,3049
Liposomes can also be prepared in the form of: (a)
stable plurilamellar vesicles (SPLVs) accordin~ to the
procedures set forth in Lenk et. al., U.S. Patent
4,522,803, (b) monophasic vesicles (MPVs) according to the
procedures of Fountain et. al., U.S. Patent 4,588,578 and
(c) freeze and thawed multilamellar vesicles (FAT~LVs)
according to the procedures of Bally et. al., Canadian
patent application No. 520,029 filed October 7, 1986.
Liposomes can be dehydrated and rehydrated; see
Janoff et al, "Dehydrated Liposomes, n PCT application
Serial No. 8601103, published February 27, 1986~
15 Much has been written regarding the possibilities of using
liposomes for drug delivery systems. See, for example,
the disclosures in U.S. Patent No. 3,993,754 issued on
November 23, 1976, to Yueh-Erh Rahman and El zabeth A.
Cerny, and U.S. Patent No. 4,145,410 issued on March 20,
1979, to Barry D. Sears. In a liposome drug delivery
system the medicament is entrapped during liposome
formation and then administered to the patient to be
treated. The medicament may be soluble in water or in a
non-polar solvent. Typical of such disclosures are U.S.
25 Patent NO. 4,235,871 issued November 25, 1980, to
Papahadjapoulos and Szoka and U.S. Patent No. 4,224,179,
issued September 23, 1980 to M. Schneider. When preparina
liposomes for use in vivo it would be advantageous (1) to
eliminate the necessity of using organic solvents during
30 the preparation of liposomes; and (2) to maximize the
encapsulation efficiency and captured volume so that a
greater volume and concentration of the entrapped material
can be delivered per dose.
SUMMARY OF THE INVENTION 1 3 ~ 3 0 4 ~
The present invention involves methods and
compositions for the entrapment and administration of
various compounds in vesicles, the bilayers of which
comprise salt forms of organic acid derivatives of
alpha-tocopherol. The vesicles of the present invention
are particularly useful for the administration of the
entrapped compound ln vivo, in which a case a
biocompatible salt form of an organic acid derivative of
D-alpha-tocopherol should be used to prepare the
vesicles. In fact for in vivo administration, the
tris(hydroxymethyl) aminomethane salt (tris-salt) form of
organic acid derivatives of alpha- tocopherol are
particularly useful as the vesicle bilayer component.
Such derivatives may be the ester or hemiester of succinic
acid, and the vesicles may entrap bioactive agents such as
but not limited to hormones, antifungal agents and
antiglaucoma agents. The vesicles may be administered by
various routes including, but not limited to, topically,
parenterally, orally and vaginally.
The method for preparing the alpha-tocopherol
vesicles involves adding to an aqueous buffer a salt form
of an organic acid derivative of alpha-tocopherol capable
of forming closed bilayers in an amount sufficient to form
completely closed bilayers which entrap an aqueous
compartment. A suspension of vesicles is formed by
shaking the mixture. The formation of vesicles is
facilitated if the aqueous buffer also contains the
counterion of the salt in solution. Furthermore, if the
dissociated salt form of the organic acid derivative of
alpha-tocopherol is negatively charged at neutral pH, the
aqueous buffer should be essentially free of divalent
cations. Similarly, when the dissociated salt form of the
organic acid derivative of alpha-tocopherol is positively
35 charged at neutral pH, the aqueous buffer should be
-6- ~ 3 ~30 4
essentially free of multivalent anions. The application
of energy of the suspension, e.g., sonication, will
convert multilamellar vesicles to unilamellar vesicles.
To entrap a water-soluble compound, a partially
water-soluble compound or a water-insoluble compound in
the alpha-tocopherol vesicles of the present invention, a
number of approaches are possible. Compounds which either
partition into the alpha-tocopherol bilayers (e.g.,
water-insoluble compounds) or water-soluble compounds may
be added to the aqueous phase before formation of the
vesicles to entrap the agent within the vesicles during
formation. Alternatively, compounds which are
water-insoluble or lipid soluble may be added to the
suspension of vesicles after the vesicles are formed, in
15 which case the compound partitions into the
alpha-tocopherol bilayers. In another embodiment, a
water-insoluble compound and the salt-form of an or~anic
acid derivative of alpha-tocopherol may be added to an
organic solvent so that both are solubilized
(co-solubilized). The organic solvent may then be
evaporated, leaving a film containing a homogeneous
distribution of the water-insoluble compound and the
alpha-tocopherol derivative. Alpha-tocopherol vesicles
entrapping the water-insoluble compounds are formed when
25 an aqueous buffer is added to the film with agitation.
Such vesicles may then be sonicated, forming unilamellar
vesicles.
The alpha-tocopherol vesicles of the present
invention are particularly advantageous when used to
30 entrap water-insoluble bioactive agents or those that are
sparingly soluble in water. ThiS enables the
administration in vivo of water-insoluble bioactive agents
such as drugs. Furthermore, it allows for the
administration in vivo of higher concentrations of the
35 water-insoluble compounds, because it allows alteration of
--7--
1 3S3049
the dose:volume ratio. The alpha-tocopherol vesicles of
the present invention offer similar advantages when used
to entrap water-soluble bioactive agents. The
alpha-tocopherol vesicles may be derivatized forming
esters or hemiesters of organic acids, and these organic
acid derivatives may further be salt forms of bioactive
agents, as with pilocarpine. The vesicles of the present
invention may also be used in diagnostic essays in vitro.
The present invention includes compositions
comprising salt forms of organic acid derivatives of
alpha-tocopherol, particularly those including bioactive
agents. The salt form can be derived from an ionizable
bioactive agent. In the case of pilocarpine
alpha-tocopherol vesicles, the pilocarpine salt form of
the organic acid derivative of alpha-tocopherol is used;
preferably in a mole ratio of 1:1.
Also embraced by the present invention are
compositions comprising a salt form of an organic acid
derivative of a sterol and a salt form of an organic acid
20 derivative of alpha-tocopherol. The composition can
additionally contain a bioactive agent. The salt form of
the organic acid derivative of either the sterol or
alpha-tocopherol, or both can include an ionizable
bioactive agent. Such bioactive agents include, but are
-- 25 not limited to, polypeptides such as the immunosuppressive
agent cyclosporin A. These compositions can be used to
form liposome vesicles.
The present invention affords a number of advantages
in that the alpha-tocopherol vesicles:
(1) are formed easily and rapidly;
(2) have high encapsulation efficiencies compared to
phospholipid MLVs;
-8- 1 3~3304q
(3) do not require the use of organic solvents for
their preparation (although the alpha-tocopherol
vesicles of the present invention can be
prepared using organic solvents);
(4) have high captured volumes; and
(5) can entrap a bioactive or pharmaceutical aqent
which, when administered in vivo, is released
and metabolized. The fate of the entrapped
agent in vivo, depends upon the mode of
administration.
The alpha-tocopherol vesicles of the present
invention may further be used in a two-step process in
which a material to be entrapped is first solubilized by
incorporation into alpha- tocopherol vesicles, and then
the alpha-tocopherol vesicles containing the entrapped
material are themselves incorporated into conventional
liposomes.
BRIEF DESCRIPTION OF THE DRAWING
The present invention may be more readily understood
20 by reference to the following figures, wherein
FIGS. 1 and 2 are graphical representations of the
effects of constituent concentrations on the formation of
complex vesicles containing alpha-tocopherol hemisuccinate
Tris ~alt, cholesterol hemisuccinate Tris salt and
25 associated cyclosporin and 70C and 60C,
respectively;
FIG. 3 is a graphical representation of the effects
of constituent concentrations on the formation of complex
vesicles containing alpha-tocopherol hemisuccinate Tris
30 salt, cholesterol hemisuccinate Tris salt and associated
miconazole;
1 3 ~3n~9
g
FIG. 4 is a graphical representation of the effects of
free and vesicle-entrapped bovine growth hormone on the
growth of hypophysectomized rats, with growth measured as a
change in weight shown as a function of time.
FIG. 5 illustrates a continuous size reduction
apparatus according to the invention.
DETAILED DESCRIPTION OF THE INVENTION
Alpha-tocopherol vesicles can be used for the
entrapment of water-soluble, partially water-soluble or
water-insoluble compounds in the vesicles, the bilayers of
which comprise salt forms of organic acid derivatives of
alpha-tocopherol that are capable of forming ciosed bi-
layers. Accordingly, the alpha-tocopherol vesicles of the
present invention can be prepared to (1) entrap a water-
soluble compound in the aqueous compartment; (2) entrap a
water-insoluble compound which partitions into the vesicle
bilayers; or (3) entrap both a water-soluble compound and a
water-insoluble compound in one vesicle preparation.
Any salt form of an organic acid derivative of alpha-
tocopherol which is capable of forming completely closed
bilayers in aqueous solutions similar to liposomes may be
used in the practice of the invention. The suitability of
a particular salt-form of an organic acid derivative of
alpha-tocopherol depends upon its ability to sequester a
water-soluble compound so that the compound is not in
contact with the outside environment.
To determine definitively that entrapment within the
aqueous compartment of a vesicle has occurred, the follow-
ing criteria for liposomes, which may be applied by ana-
logy, have been established [See Sessa and Weissmann,
Biol., Chem. 245:3295 (1970)]: (a) there must be a clear
separation of free from sequestered compound by gel
filtration; (b) there must be no hydrophobic or charge-
charge interaction between the outermost vesicle
-lo- 1 3~3049
bilayer and the entrapped compound since this may result
in a failure to achieve separation of the free compound
from the vesicles by molecular sieving, thereby
artificially increasing the apparent sequestration
efficiency. To eliminate this possibility it must be
shown that the water-soluble compound added to a
suspension of previously formed vesicles does not coelute
with the vesicles; (c) disruption of gel-filtered vesicles
by use of detergents or other membrane perturbants must
induce a shift in the gel filtration pattern of the
sequestered molecule from a position coincident with the
vesicle peak to one that coelutes with the free molecule.
Organic acids which can be used to derivatize the
alpha-tocopherol include but are not limited to the
carboxylic acids, dicarboxylic acids, polycarboxylic
acids, hydroxy acids, amino acids and polyamino acids.
Such derivatives may be esters or hemiesters. Because the
salt forms increase the water solubility of organic acids,
any organic acid may be used to derivatize the
alpha-tocopherol; however an advantage may be obtained if
the organic acid moiety itself is water soluble. Such
water-soluble organic acid moieties include but are not
limited to water-soluble aliphatic carboxylic acids such
as acetic, propionic, butyric, valeric acids and the like
(N.B., up to four-carbon acids are miscible with water;
- the five-carbon free acid is partly soluble and the longer
chain free acids are virtually insoluble); water-soluble
aliphatic dicarboxylic acids such as malonic, succinic,
glutaric, adipic, pimelic, maleic and the like (N.B., the
30 shorter chains are appreciably more soluble in water;
borderline solubility in water occurs at C6 to C7);
and water-soluble aromatic dicarboxylic acids such as
hemimellitic, trimesic, succinimide, and the like;
polycarboxylic acids; water-soluble hydroxy acids such as
35 glycolic, lactic, madelic, glyceric, malic, tartaric,
citric, and the like (N.B., alpha-hydroxy acids containing
1 3~`3(~4~
a branched chain attached to the alpha-carbon of the
carbonyl group would be less susceptible to hydrolysis
and, therefore, advantageous in the practice of the
present invention): and any of the amino acids and
polyamino acids.
The salt forms of the derivatized alpha-tocopherol
can be prepared by dissolving both the organic acid
derivative of the alpha-tocopherol and the counterion of
the salt (e.g., the free base of the salt) in an
appropriate volatile solvent, and removing the solvent by
evaporation or a similar technique leaving a residue which
consists of the salt form of the organic acid derivative
of alpha-tocopherol. Counterions that may be used
include, but are not limited to, tris,
2-amino-2-methyl-1-3-propanediol, 2-aminoethanol, bis-tris
propane, triethanolamine, and the like to form the
corresponding salt. In fact, the free base of an
ionizable bioactive agent such as miconazole free base and
the like may be used as the counterion.
Generally a 1:1 molar ratio of the free base of the
bioactive agent and the dicarboxylic acid derivative of
alpha-tocopherol are employed to prepare the corresponding
bioactive agent salt form. An organic solvent which
dissolves both starting materials are those such as
25 methanol, ethanol, chloroform, methylene chloride,
dimethylformamide and dimethylsulfomide. The starting
materials are added to the solvent, preferably at about
20-50C, more preferably about 20-30C. Following
reaction, the resulting bioactive agent salt can be
isolated by solvent removal under reduced pressure,
evaporation, crystalization, or other methods known in the
art. The preferred dicarboxylic acid derivative is that
of succinic acid. The preferred alpha-tocopherol is
D-alpha-tocopherol.
-12-
1 3 '`3n4 q
When the bioactive agent free base is pilocarpine and
the dicarboxylic acid is succinic acid, mole ratios
ranging from 1:0.5 to 1:1 pilocarpine = D-alpha-tocopherol
may be used; most preferably equi-molar amounts of the
starting materials are dissolved in a polar organic
solvent such as chloroform or methylene chloride. For
methylene chloride, the starting materials are preferably
added at about 30-60C, more preferably about 40-60C,
most preferably about 55C, and heated to reflux. After
reaction is complete, the solvent is removed under reduced
pressure to obtain the product which is the pilocarpine
salt of alpha-tocopherol hemisuccinate.
The free base of antifungal agents such as
miconazole, terconazole, econazole, isoconazole,
tioconazole, bifonazole, clotrimazole, ketoconazole,
butaconazole, itraconazole, oxiconazole, fenticonazole,
mystatin, naftifine, amphotericin B, zinoconazole and
ciclopirox olamine, preferably miconazole or terconazole
may be employed as the ionizable bioactive agent.
The alpha-tocopherol vesicles of the present
invention may be prepared by adding to an aqueous phase a
salt form of an organic acid derivative of
alpha-tocopherol so that the derivatized alpha-tocopherol
is present in an amount sufficient to form vesicles (i.e.,
25 completely closed bilayers containing an entrapped aqueous
compartment). The preparation is then shaken until a
milky suspension of vesicles, generally multilamellar, is
formed. In the preferred embodiment, the aqueous phase
should contain the salt in solution to facilitate vesicle
30 formation. Furthermore, if the dissociated salt form of
the organic acid derivative of the alpha-tocopherol is
negatively charged at neutral pH, the aqueous buffer
should be essentially free of multivalent cations.
Similarly, when the dissociated salt form of the orqanic
-13- ~ 3 ~
acid derivative is positively charged at neutral pH, the
aqueous buffer should be essentially free of multivalent
anions.
The vesicles of the invention may be used as
multilamellar vesicles, or size-reduced using a number of
techniques known in the art, such as filtration or
sonication. Vesicles may also be size reduced using the
VET procedure (vesicle extrusion technique) as described
in Hope et al., BBA Vol. 812, 1985, pp. 55-65, and in
Cullis et al., PCT publication WO 86/00238, published
January 16, 1986 entitled "Extrusion Technique for
Producing Unilamellar Vesicles". Another
technique for sizing vesicles is the CSR procedure
(continuous size reduction) whereby vesicles are
continuously extruded through a filter unit by a pump.
In complete contrast to reported methods fo.
multilamellar vesicle formation [e.g., phospholipid
vesicles or the cholesterol liposomes of Brockerhoff and
Ramsammy, Biochim. Biophys. Acta. 691:227 (1982)], the
method for the formation of the alpna-tocopherol
multilamellar vesicles of the present invention does not
re~uire the use of organic solvents. Furthermore, unlike
the me~hod of Brockerhoff and Ramsammy sonication is not
necessary to form multilamellar vesicles. Sonication of
the milky suspension of the alpha-tocopherol multilamellar
vesicles of the present invention, or the use of a French
press (SLM-Aminco, Urbana, ILL.) followed by sonication,
may be used however to convert the milky suspension of
multilamellar alpha-tocopherol vesicle~ to a clear
suspension of unilamellar vesicles. Often, use of the
French press without sonication results in unilamellar
veslcles .
As previously explained, the tris-salt form of any
organic acid derivative of alpha-tocopherol may be
-14-
1 3 ~ 3 0 -t 3
advantageously used in the practice of the present
invention. For example, the tris-salt form of an
alpha-tocopherol hemi-dicarboxylic acid such as an
alpha-tocopherol hemisuccinate or a mixture of
hemisuccinates are particularly useful for forming the
vesicle bilayers of the alpha-tocopherol vesicles to be
administered in vivo. For instance, when usinq
alpha-tocopherol hemisuccinate, about 5 to 700 micromoles
of the tris-salt form may be added to about 5.0 ml of
aqueous buffer containing Tris-HC1 (tris(hydroxymethyl)-
aminomethane hydrocholoride) to form vesicles. In this
case, the aqueous buffer should be essentially free of
divalent cations.
According to the present invention, the entrapment or
association of water-soluble compounds, water-insoluble
compounds, or sparingly soluble compounds in liposomes
composed of the salt form of organic acid derivatives of
alpha-tocopherol may be accomplished in a number of ways:
(l) A water-insoluble compound can be added to a
suspension of alpha-tocopherol vesicles (either
multilamellar or unilamellar), which were prepared as
described above using an appropriate salt form of an
organic acid derivative of alpha-tocopherol. The compound
is entrapped in the vesicles because it partitions into
25 the alpha-tocopherol bilayers. This embodiment may be
conveniently carried out as follows: the water-insoluble
compound may be dissolved in an appropriate organic
solvent which is then evaporated, leaving a film or
residue of the compound. When an aqueous suspension of
30 previously formed alpha-tocopherol vesicles is added to
the residue, the residue will be entrapped in the bilayers
of the vesicles. In the preferred embodiment unilamellar
vesicles should be used. If multilamellar vesicles are
used instead, the water-insoluble compound may be
-15- 1 3 S3 n4 q
entrapped only in the outermost bilayers of the vesicles,
leaving the innermost bilayers unaltered with a wasteful
use of derivatized alpha-tocopherol.
(2) Both a water-insoluble compound and the salt
form of an organic acid derivative of alpha-tocopherol can
be co-solubilized in an organic solvent which is then
evaporated, leaving a film comprising a homogeneous
distribution of the water-insoluble compound and the
alpha-tocopherol derivative. A suspension of
alpha-tocopherol vesicles containing the entrapped
compound is formed when an aqueous phase is added to the
film with shaking. Multilamellar vesicles may be
converted to unilamellar vesicles as previously described.
(3) A water-soluble compound or a water-insoluble
15 compound can be entrapped in the alpha-tocopherol vesicles
by adding the compound to the aqueous phase which is used
in the preparation of the vesicles; i.e., the compound can
be added to the aqueous phase before or simultaneously
with the addition of the salt form of an organic acid
derivative of alpha-tocopherol. In this case, a
water-insoluble compound becomes entrapped when it
partitions into the bilayers during vesicles formation;
whereas a water-soluble compound becomes entrapped in the
aqueous compartment of the vesicles. In either case, the
25 multilamellar vesicles can be converted to unilamellar
vesicles as previously described.
(4) If the bioactive agent is ionizable, the free
base of the bioactive agent may be used as the counterion
to prepare the salt form of the organic acid derivative of
30 alpha- tocopherol. The resulting composition can have
enhanced solubility or stability. Furthermore, the
alpha-tocopherol vesicles may be prepared by any of the
methods previously described herein using the bioactive
agent-salt form of the organic acid derivative of
-
-16-
1 3 ~30~'t
alpha-tocopherol. For example, the free base of
antifungal agents such as miconazole, terconazole,
econazole, isoconazole, tioconazole, bifonazole,
clotrimazole, ketoconazole, butaconazole, itraconazole,
oxiconazole, fenticonazole, nystatin, naftifine,
amphotericine B, zinoconazole and ciclopirox olamine,
preferably miconazole or terconazole, may be used to make
the salt derivatives in one embodiment of the present
invention. Also, the free base of pilocarpine may be used
to make the salt derivatives in one embodiment of the
present invention. Liposomes using the pilocarpine salt
derivative of alpha-tocopherol hemisuccinate may be made
by adding an aqueous solution to the dried salt form of
the pilocarpine alpha-tocopherol hemisuccinate at a
temperature of 30-40C and agitating the suspension.
Examples of aqueous solutions that may be used are water
for injection, USP; and any of the following alone or in
combination: 0.01% (w/v) benzylkonium chloride, 0.025-0.1
(w/v) sorbic acid EDTA (ethylenediaminetetraacetic acid),
1.4% (w/v) polyvinyl alcohol, 0.1% (w/v) benzylkonium
chloride, and 0.05-0.50% (w/v) hydroxypropyl
methylcellulose. Other ionizable bioactive agents named
herein may also be employed.
The compositions of the present invention, in
addition to salt forms of organic acid derivatives of
-- alpha-tocopherol, may contain bioactive agents entrapped
within or between the bilayers of the vesicle, or
alternatively the bioactive agent may be in association
with the bilayers. Such an association may result in the
bioactive agent being located on the exterior of the
vesicle.
The compositions of the present invention may be used
for ocular administration in the treatment of ocular
afflictions such as glaucoma. In such applications the
compositions may be administered by ocular delivery
-
-17- 1 3 ~3n~Y
systems known in the art such as eye droppers or
applicators. The compositions can further contain
mucomimetics such as hyaluronic acid, chondroitin sulfate,
hydroxypropyl methylcellulose, or polyvinyl alcohol; and
preservatives, such as sorbic acid EDTA or benzylkonium
chloride, in the above-named percentages, and the usual
quantities of diluents and/or carrier materials.
For administration to humans in the treatment of
ocular afflictions such as glaucoma, the prescribing
physician will ultimately determine the appropriate dose
for a given human subject, and this can be expected to
vary according to the age, weight, and response of the
individual as well as the nature and severity of the
patient's symptoms. Typically, ocular dosages of the
compositions will be in the range of 25-50ul of a 4%
pilocarpine solution administered 1-2 times daily for an
average adult patient in a suitable pharmaceutically
acceptable diluent or carrier. These figures are
illustrative only, however, and in some cases it may be
necessary to use dosages outside these limits.
Using any of the four methods described above, both a
water-soluble compound and water-insoluble compound may be
entrapped in one alpha-tocopherol vesicle preparation.
According to the methods described above for the
25 entrapment of water-insoluble compounds using the alpha-
tocopherol vesicles of the present invention, it is not
required that the vesicles remain intact once a
water-insoluble compound partitions into the bilayers. In
fact, once the compound partitions into the bilayers, the
30 vesicles may be disturbed or disrupted leading to the
leakage or release of aqueous entrapped compounds.
Although these "leaky" vesicles could be used to deliver
the entrapped water-insoluble compound, they should not be
used to encapsulate or deliver a water-soluble substance.
-18- l 3 s304q
According to one embodiment of the present invention,
liposomes can be prepared using the tris-salt form of
alpha-tocopherol hemisuccinate as follows: about 1 to 400
mg of the tris-salt form of alpha-tocopherol hemisuccinate
is added per ml of aqueous buffer containing 0.01 M
Tris-HCl, 0.14 M NaCl. The mixture is shaken and a milky
suspension of alpha-tocopherol hemisuccinate vesicles
forms. The vesicles may be pelleted by centrifugation and
washed repeatedly with aqueous buffer. Suspension of
alpha-tocopherol hemisuccinate multilamellar vesicles
(AHS-MLVs) may be sonicated to form alpha-tocopherol
hemisuccinate small unilamellar vesicles (AHS-SUVs). The
vesicles are unstable in the presence of divalent cations;
i.e., upon exposure to divalent cations the entrapped
aqueous compartment and water-soluble compounds are
released. ThuS, the aqueous medium used in the
preparation or during storage of the vesicles should be
essentially free of divalent cations.
The compounds which are entrapped according to the
method of the present invention may be used in various
ways. For example, if the compound is a bioactive agent,
the alpha- tocopherol vesicle-entrapped compound may be
administered in vivo. This facilitates the in vivo
delivery of bioactive agents which are normally insoluble
or sparingly soluble in aqueous solutions. Entrapment in
vesicles composed of the salt form of organic acid
derivatives of alpha-tocopherol enables ease in the
administration of such insoluble compounds at a higher
dose: volume ratio. In fact, the alpha-tocopherol
vesicles of the present invention are particularly
advantageously used in vivo because the vesicles may be
used to entrap one or more bioactive agents for delivery
in vivo. Furthermore, the vesicles of the present
invention offer an advantage over conventional lipid
35 vesicles or liposomes when used in vivo because they can
be prepared without using organic solvents.
--19--
1 3'`3()4q
Compounds which are bioactive agents can be entrapped
within the alpha-tocopherol vesicles of the present
invention. Such compounds include but are not limited to
antibacterial compounds such as gentamycin, antiviral
agents such as rifampacin, antifungal compounds such as
amphotericin B, anti-parasitic compounds such as antimony
derivatives, tumoricidal compounds such as adriamycin,
anti-metabolites, peptides, proteins such as albumin,
toxins such as diptheriatoxin, enzymes such as catalase,
polypeptides such as cyclosporin A, hormones such as
estrogen, hormone antagonists, neurotransmitters such as
acetylcholine, neurotransmitter antagonists, glycoproteins
such as hyaluronic acid, lipoproteins such as
alpha-lipoprotein, immunoglobulins such as IgG,
immunomodulators such as interferon or interleuken,
vasodilators, dyes such as Arsenazo III, radiolabels such
as 14C, radio-opaque compounds such as 90Te,
fluorescent compounds such as carboxy fluorscein, receptor
binding molecules such as estrogen receptor protein, anti-
inflammatories such as indomethacin, antiglaucoma agentssuch as pilocarpine, mydriatic compounds, local
anesthetics such as lidocaine, narcotics such as codeine,
vitamins such as alpha-tocopherol, nucleic acids such as
thymine, polynucleotides such as RNA polymers,
25 psychoactive or anxiolytic agents such as diazepam, mono-
.- di- and polysaccharides, etc. A few of the many specific
compounds that can be entrapped are pilocarpine, a
polypeptide growth hormone such as human growth hormone,
bovine growth hormone and porcine growth hormone,
30 indomethacin, diazepam, alpha- tocopherol itself and
tylosin. Antifungal compounds include miconazole,
terconazole, econazole,isoconazole, tioconazole,
bifonazole, clotrimazole, ketoconazole, butaconazole,
itraconazole, oxiconazole, fenticonazole, nystatin,
35 naftifine, amphotericin B, zinoconazole and ciclopirox
olamine, preferably miconazole or terconazole. The
~ 3 ~3049
-20-
entrapment of two or more co~pounds sim~ltaneously ~ay be
espec~ally desirable where such compounds prcduce
complementary or synergistic effects. The amounts of
drugs administered in liposomes will generally ~e the same
as with the free drug; however, the frequency of dosing
may be reduced.
The alpha-tocopherol vesicle-entrapped or -associated
agent may be administered in vivo by an suitable route
including but not limited to: parenteral inoculation or
injection (e.s., intravenous, intraperitoneal,
intramuscular, subcutaneous, intra-aural, intra-mammary,
and the like), topical application (e.~., on areas such as
eyes, skin, in ears or on af.lictions such as wounds and
burns), and by absorption through epithelial or
mucocutaneous linings (e.g., nasal, oral, vaginal, rectal,
gastrointestinal mucosa, and the like).
In another example of their use, the alpha-tocopherol
vesicle-entrapped compounds may be incorporated into a
broad range of materials including but not limited to
lipid vesicles or liposomes, gels, oils, emulsions and the
like. For instance, the suspension containing the
entrapped compound may be added to the aqueous phase as an
ingredient in any type of liposome preparation (e.g.,
phospholipid SP~Vs, MPVs, FATMLVs, MLVs, SUVs, LUVs, REVs,
2~ and others). This allows the entrapment of the
water-insoluble compound in the phospholipid liposomes.
The alpha-tocopherol vesicles of the present
invention can be used advantageou~ly in conjunction with
vesicles of the salt form of an organic acid derivative of
30 a sterol, such as cholesterol hemisuccinate tris salt
vesicles, as described in PCT publication WO 85/04578
published October 24, 1985 entitled "Steroidal Liposomes"
Such steroidal
-21- 1 3 ~3i~l49
liposomes form bilayer vesicles, may be unilamellar or
multilamellar, and can entrap compounds that are bioactive
agents.
Generally any sterol which can be modified by the
5 attachment of an organic acid may be used in the practice
of the present invention. For example, such sterols
include, but are not limited to, cholesterol, vitamin D,
phytosterols (including but not limited to sitosterol,
campesterol, stigmasterol, and the like), steroid
10 hormones, and the like.
Organic acids which can be used to derivatize the
sterols include those that may be used to derivatize
alpha-tocopherol, as discussed herein before.
The organic acid can be linked to an hydroxyl group
15 of the sterol via an ester or an ether bond using
conventional methods (see, for example, U.S. Patent No.
3,859,047; U.S. Patent No. 4,040,784; U.S. Patent No.
4,042,330; U.S. Patent No. 4,183,847; and U.S. Patent No.
4,189,400). The salt forms of the derivatized sterols can
20 be prepared by dissolving both the organic acid derivative
of the sterol and the counterion of the salt (e.g., the
free base of the salt) in an appropriate volatile solvent,
and removing the solvent by evaporation or a similar
technique leaving a residue which consists of the salt
25 form of the organic acid derivative of the sterol.
Counterions that may be used include, but are not limited
to, tris, 2-amino-2-methyl-1,3-propanediol,
2-aminoethanol, bis-tris propane, triethanolamine, and the
like to form the corresponding salt. In fact, the free
30 base of an ionizable bioactive agent such as miconazole
free base and the like may be used as the counterion.
Thus, the bioactive agent can be used as a counterion.
-22- l 3 S3 '$q
Where an organic acid derivative of a sterol is used
in conjunction with alpha-tocopherol in the vesicles of
the invention, any ratio of the sterol to alpha-tocopherol
may be used from 0:100 to 100:0 mole %.
The alpha-tocopherol vesicles of the present
invention can also be used in conjunction with traditional
liposomes to solubilize a variety of materials. The
material may first be incorporated into alpha-tocopherol
vesicles and, thus entrapped, into the liposomes.
Alternatively, the material to be solubilized may be
present at the onset with all of the materials needed to
make both kinds of vesicles.
Other uses, depending upon the particular properties
of the preparation, may be envisioned by those skilled in
the art. For example, because of their divalent cation
sensitivity, the alpha-tocopherol hemisuccinate vesicles
of the present invention may be made to entrap indicator
dyes which are divalent cation sensitive for use in
colorimetric diagnostic assays in vitro.
The following examples are given for purposes of
illustration and not by way of limiting the scope of the
invention.
EXAMPLE 1
PREPARATION OF THE TRIS SALT OF
ALPHA-TOCOPHEROL HEMISUCCINATE
Five grams of alpha-tocopherol hydrogen succinate
(Sigma Chemical Co., St. Louis, MO) were dissolved in 100
ml of diethyl ether. Tris base (Fisher, Fair Lawn, NJ)
(1.14 g) dissolved in about 5 ml of water was then added
30 in 0.5 ml portions to the ether solution while stirring or
-23- 1 3 ~3 nl~9
shaking. The solution was rotoevaporated to dryness and
then further dried under high vacuum, to produce the title
compound as a gummy, yellow residue.
EXAMPLE 2
PREPARATION OF THE TRIS SALT OF
ALPHA-TOCOPHEROL HEMISUCCINATE
To a solution of 10 g of D-alpha-tocopherol acid
succinate in 100 ml of methylene chloride at 25C in a
500 ml round bottom flask was added 2.28 g of
tris(hydroxymethyl)aminomethane in 3 ml of hot water while
vortexing. The reaction mixture was rotated on a rotary
evaporator while being kept a 55C with a constant
temperature bath for 15 minutes.
The solvent was removed under reduced pressure and
the resulting material was frozen for 24 hours. The
material was removed, ground with a mortar and pestle, and
the excess solvent was removed in vacuo for 24 hours. The
resulting title compound (4.8 g) was stored in a tiqhtly
sealed glass jar and protected from light.
20Alternatively, the material was frozen for 48 hours.
In another preparation, the solvent was removed by
lyophylization.
EXAMPLE 3
ENTRAPMENT OF ARSENAZO III IN
25ALPHA-TOCOPHEROL VESICLES
One hundred milligrams of alpha-tocopherol
hemisuccinate Tris salt, prepared as described above, were
added to 1 ml of a solution containing 0.01 M Tris-HCl,
0.14 M NaCl and 4.5 mM Arsenazo III, all at pH 7.3, and
-24- 1 3~30-t'~
the suspension was subjected to vortex mixing in the
presence of 3 mm glass beads. The alpha-tocopherol
hemisuccinate Tris salt vesicles that formed were pelleted
by centrifugation at 10,000 x g for 15 minutes, after
5 which the pellet was washed and recentrifuqed 3 times in
10 ml of a solution containing 0.01 M tris-HCl and 0.14 M
NaCl, pH 7.3. The resulting pellet was red in color due
to the entrapment of Argenazo III. The degree of
entrapment was estimated to be 30 percent.
EXAMPLE 4
SOLUBILIZATION OF PREGNANOLONE
Fifty milligrams of alpha-tocopherol hemisuccinate
tris salt, 50 mg of cholesterol hemisuccinate Tris salt
and 20 mg of 5 beta-Pregnan-3 -ol-20-one (Kabi Vitrum,
15 Stockholm, Sweden) were added to an excess amount of
methanol and dried under vacuum in a round bottom flask.
A resulting film was then resuspended in 1.0 ml of 10 mM
Tris-HCl buffer with 140 mM NaCl, pH 7.4 with shaking in
the presence of glass beads until a stiff gel formed. The
20 viscosity of the gel was reduced by extensive sonication
in a Branson E-module (40 KHz) 5-gallon water bath
sonicator, to yield 0.2-0.4 micron diameter vesicles.
The cholesterol hemisuccinate Tris salt had been
prepared as follows. Cholesterol hydrogen succinate (50.3
25 g, 0.11 moles) from ICN, Cleveland, Ohio, was dissolved in
1.5 liters of diethyl either. Tris base (12.1 g, 0.1
moles) from Fisher, Fairlawn, NJ, was dissolved in 30 ml
of water. The Tris solution was then added to the
cholesterol solution, and the resulting solution was
30 rotoevaporated to a milky wet residue. The milky residue
was freeze dried for 12 hours, after which the cholesterol
hemisuccinate Tris salt product was recrystallized three
times from about 5-liter volumes of boiling ethyl acetate.
-25-
1 3~3rJ49
The boiling ethyl acetate solution was filtered hot
and cooled to room temperature. A gel-like cholesterol
hemisuccinate Tris salt appeared which was filtered
through a 100 ml scintered glass funnel, and the ethyl
acetate was removed. Initial removal of the solvent was
by squeezing. In another preparation, initial solvent
removal was performed by mechanical compression. Further
solvent removal was carried out under a 0.1 mm Hg vacuum
for 12 hours, at which time a silver-dollar sized 23 g
disc of hard, brittle white material was evident.
The white disc was pulverized in a mortar and pestle,
and the last traces of ethyl acetate were removed by
heating the material to 50C and applying a 0.1 mm Hg
vacuum. Fifty five milligrams of the resulting powder
15 were suspended in 1.0 ml of 0.01 M Tris-HCl buffer with
0.14 M NaCl, pH 7.4. A milky suspension developed which
was sonicated in the bath sonicator, to form a clear
cholesterol hemisuccinate Tris salt vesicle solution.
EXAMPLE 5
SOLUBILIZATION OF CYCLOSPORIN A
Cyclosporin A (Sandoz, Inc., East Hanover, NJ),
cholesterol hemisuccinate Tris salt and alpha-tocopherol
hemisuccinate Tris salt were dissolved in methanol in the
relative proportions indicated in Table 1.
Aliquots of the solutions sufficient to make 0.25 ml
volumes of the final aqueous suspensions were dried to
thin films in 13 x 100 mm test tubes by placing the tubes .
in 500 ml round-bottom flasks in the 70C water bath and
removing the solvent by rotary evaporation. The films
30 thus obtained were rehydrated by the addition of 0.25 ml
-26-
1 3-~304't
of 0.01 M Tris-HCl buffer with 0.14 M NaCl, pH 7.3, with
vortex mixing of the suspensions in the presence of qlass
beads.
The presence or absence of Cyclosporin A crystals
upon microscopic examination under the various conditions
listed in Table 1 was noted, with the results shown in
Figure 1. As shown in Figure 1, when the concentration of
cholesterol hemisuccinate tris salt and/or
alpha-tocopherol hemisuccinate tris salt were too low,
Cyclosporin A crystals were observed.
The run was repeated using a 60C water bath and
the results are shown in FIG. 2.
EXAMPLE 6
SOLUBILIZATION OF MICONAZOLE
Stock solutions of miconazole free base (MCZ), the
tris salt of cholesterol hemisuccinate (CHS) and the tris
salt of D-alpha-tocopherol hemisuccinate (THS) were
prepared as 50mM solutions in absolute ethanol.
Appropriate voluems of the stock solutions were
pipetted to 50 ml round bottom flasks. Each flask
contained a total of 0.1 mmoles of material in the
relative proportions indicated in Table 2.
The solvent was removed by rotary evaporation at
60C. The material was then resuspended in 1.0 ml of
.15 M NaCl with 0.01 M Tris-HCL at pH 7.4.
Preparations were examined microscopically for
evidence of miconazole crystals and macroscopically for
evidence of phase separation over a period of two weeks.
Preparations were judged to be acceptable if neither
crystals or phase separation were observed (see FIG. 3).
-27-
13..3049
EXAMPLE 7
SUSTAINED DELIVERY OF BOVINE GROWTH
HORMONE TO HYPOPHYSECTOMIZED RATS
To demonstrate the solubilizing ability and slow
release characteristics of the invention, two types of
vesicles were prepared with which bovine growth hormone
(BGH) was associated. One type of vesicle contained ega
phosphatidyl choline (EPC, or lecithin) and
alpha-tocopherol hemisuccinate Tris salt associated with
BGH. The other vesicles contained the same components
and, additionally, egg phosphatidyl ethanolamine (EPE).
Vesicles lacking EPC were prepared as follows. A
solvent phase was prepared by removing the solvent from a
chloroform solution containing 400 mg of EPC (Sigma
Chemical Co., St. Louis, MO) by rotoevaporation at
37C. The residue was then dissolved in 5 ml of diethyl
ether. An aqueous phase containing BGH in association
with alpha-tocopherol vesicles was prepared by adding 25
mg of alpha-tocopherol hemisuccinate to 1 ml of 0.01 M
Tr is-HCl with 0.14 M NaCl, pH 7.4. An opaque suspension
developed which was clarified by sonication as described
above until an optical density of between 0.3 and 0.6 was
obtained at 500 nm. A 0.3 reading is preferable.
Twenty-eight milligrams of powdered BGH (Eli Lilly,
Indianapolis, IN) were added to 0.3 ml of the vesicle
preparation by vortexing with brief sonication to disperse
the powder. A milky suspension developed which showed no
evidence of intact powder. This aqueous phase suspension
was added dropwise to the solvent phase, wherein, if
undisturbed, the droplets would have sunk to produce an
opaque bottom layer.
With the addition of the aqueous phase to the solvent
phase, however, the formation of stable plurilamellar
-
-28- 1 3 ~304q
vesicles [SPLVs, Lenk et al., U.S. Patent 4,522,803] was
promoted by sonicating the aqueous droplets in ether phase
while passing a stream of nitrogen over the vessel. The
solvent evaporated, leaving a paste which was rehydrated
in 10 ml of the above Tris-HCl buffer containing a final
concentration of 10 mM CaC12. The calcium enhanced the
tight pelleting of the liposomes upon centrifugation by
neutralizing the charge imparted by the alpha-tocopherol
hemisuccinate Tris salt. Rehydration was facilitated by
gentle vortex mixing.
The resulting SPLVs were pelleted by centrifugation
for 10 minutes at 10,000 x g, and the pellets from 4
batches were pooled and washed twice more in the
Tris-HCl/calcium buffer. The supernatant fluid was
decanted, leaving a viscous pellet, approximately 0.5 ml
aliquots of which were used for the study below.
Vesicles additionally containing EPE were prepared by
combining chloroform solutions containing 1.54 g EPC and
52.8 mg EPE (Avanti Polar Lipids, Inc., Birmingham, AL) in
a round-bottom flask and removing the chloroform by
rotoevaporation at 37C. The resulting dry lipid film
was dissolved in 20 ml of diethyl ether, and an aqueous
phase containing BGH in association with alpha-tocopherol
hemisuccinate Tris salt vesicles was added.
The aqueous phase was prepared by solubilizing 112 mg
of BGH in 1.2 ml of 25 mg/ml alpha-tocopherol
hemisuccinate Tris salt in 0.01 M Tris-HCl buffer with
0.14 M NaCl, pH 7.4 The alpha-tocopherol suspension had
previously been passed through a French Pressure Cell
Press (SLM INstruments, Inc., Urbana, IL) at 40,000 pounds
per square inch pressure to produce an optical density at
550 nm of between 0.3 and 0.6.
By adding the aqueous phase to the ether phase with
sonication under nitrogen as described above, a viscous
-
1 3~3~)4'`,
SPLV paste was produced that was rehydrated with 20 ml of
buffer containing 0.01 M Tris, 0.14 M NaCl and 10 mM
CaC12, pH 7.4. The rehydration was carried out while
vortex mixing in the presence of glass beads. The
5 resulting liposomes were washed three times with a total
of 20 ml of the above buffer, with centrifugation at
10,000 rpm for 45 minutes in a Backman J2-21 centrifuge,
using a JA-14 rotor. Approximately 0.5 ml aliquots of the
viscous pellet that resulted were used for the study below.
The slow-release capability of the vesicles was
examined in 25-day old female hypophysectomized rats from
Charles River Breeding Laboratories, Inc., Wilmington,
MA. The animals were weighed upon arrival and placed on a
596 glucose diet for 2 days, after which they were switched
15 to water and rat chow ad libitum. The animals were
weighed at 32 and 39 days of age, and those gaining more
than 10 grams weight during the interval were rejected as
incompletely hypophysectomized. Groups of eight animals
were then injected intramuscularly (I.M.) or
20 subcutaneously (S.C.) with either free BGH or with BGH
entrapped in one of the vesicle preparations. Animals
receiving free BGH were injected daily S.C., while those
receiving vesicle-associated BGH were injected only once
on day 0 with about 9.8 mg of associated hormone (assuming
25 70% association during preparation of the vesicles) for
the EPCjEPE formulation or 5.6 mg (assuming 40%
association for the EPC formulation), by the routes shown
in Figure 4. Control animals were left untreated. The
data represent the average weight change values for the 8
30 animals of each group.
In Figure 4, the abbreviations shown are as follows:
(1) EPC:EPC/ -THS-BGH S.C. means the bovine growth hormone
associated with vesicles containing egg
phosphatidylcholine and ethanolamine and alpha-tocopherol
35 hemisuccinate Tris salt was administered subcutaneously;
(2) EPC/ -THS-BGH S.C. means that bovine growth hormone
-30-
1 3 ~3n4q
associated with vesicles containing egg phosphatidyl
choline and alpha-tocopherol hemisuccinate Tris salt (but
lacking egg phosphatidyl ethanolamine) was administered
subcutaneously; and (3) EPC/ -THS-BGH I.M. is the same as
(2) but with intramuscular administration.
As shown in Figure 4, untreated control animals
showed no significant weight gain over the course of the
experiment. Strong growth was produced by free BGH, but
the administration of the free hormone was carried out
daily. Growth stimulation caused by the various
associated BGH-vesicle preparations was somewhat lower but
remarkable in view of the fact that only a single
injection was made of these preparations. The best
performance was seen in the case of BGH associated with
15 EPC:EPE/ THS vesicles administered S.C.
EXAMPLE 8
ALPHA-TOCOPHEROL HEMISUCCINATE
TRIS SALT ENTRAPMENT OF SOLUTE
Various amounts of the tris salt of
20 D-alpha-tocopherol hemisuccinate (nthe Tris salt") powder
shown in Table 3 were added in a round bottom flask to 5.0
ml quantities of 0.01 M Tris/0.14 M NaCl buffer (pH 7.4)
to which had been added 5 microliters of 51Cr. The
suspensions were vortexed for two minutes to dissolve the
25 tris salt and allowed to sit undisturbed for two hours, to
form MLVs. Meanwhile, Thomas Scientific Spectrapor 12,000
MWCO 1/4 n dialysis tubing was cut into seven inch lengths
and boiled in two changes of distilled water for one
hour. The bags were tied at one end, and 1.0 ml from each
30 MLV prep was pipetted into bags. Bags were closed at the
top with plastic fasteners and counted for radioactivity i
a TMAnalytic model 1191 gamma counter for one minute.
i
-31-
~ 3 ~304~
Each bag was then dialyzed in 100 ml of Tris/NzCl buffer
while stirring. After six hours, the dialysate was
changed to fresh buffer. Dialysis continued for twenty
hours. Bags were then counted for radioactivity as above
and percent 51Cr entrapped was calculated as follows:
no. averaged counts post-dialysis X 100 = % 51Cr.
Entrapped
no. averaged counts pre-dialysis
Captured volume (solute) values were calcualted as
follows: counts from three trials per sample amount of
the tris salt were averaged and from the calculated %
entrapment (above) and known microliter of sample volume
(5.0 ml = 5000 1), the l/mol 51Cr per micromole of
lipid was calculated as follows:
(~ entrapped)(total microliter volume)
micromoles of the tris salt
The results are shown in Table 3.
EXAMPLE 9
PILOCARPINE - ALPHA TOCOPHEROL HEMISUCCINATE
Five grams of pilocarpine base were added to a
weighed 500ml round bottom flask and stopper, and the
total mass in grams recorded. D-alpha-tocopherol acid
succinate (12.75 g, corresponding to a 1:1 M ratio of
pilocarpine:D-alpha- tocopherol) was added to the flask
and the contents again weighed. Methylene chloride (50
ml) was added and the flask agitated to dissolve the
solids and the flask again weighed. The flask was placed
on a rotary evaporator in a water bath at 55C and
rotated for 30 minutes (no vacuum applied). After 30
-32- 1 3 -`3049
minutes, the flask and contents were again weighed, then
rotary evaporated with vacuum at 55C. The weight of
the flask was recorded every 30 minutes thereafter until
two successive weighings were within 0.lq. The
preparation was then cooled to room temperature (25C),
stoppered, and stored at 4C.
EXAMPLE 10
PILOCARPINE - ALPHA-TOCOPHEROL HEMISUCCINATE
The materials and procedures of Example 9 were
followed using a 5.0 liter round bottom flask and stopper,
30 g of pilocarpine base, 76.5 g of D-alpha-tocopherol
acid succinate (corresponding to a 1:1 M ratio of
pilocarpine:D-alpha- tocopherol), and 300 ml of methylene
chloride.
EXAMPLE 11
PILOCARPINE - ALPHA-TOCOPHEROL HEMISUCCINATE
LIPOSOME PREPARATION
The 500 ml round bottom flask containing the product
of Example 9 above was placed on a rotary evaporator; with
the water bath temperature set at 55C. The salt was
warmed for 30 minutes, then the water bath temperature
reduced to 35C. An aqueous solution of 0.1% (w/v)
sorbic acid 0.1% (w/v) sodium EDTA dihydrate (92 ml) was
added and the suspension vortically mixed. The final
25 volume was adjusted to 125 ml with additional aqueous
phase. The resulting liposomes were processed by CSR
(Continuous Size Reduction), a process and device for the
continuous processing of large volumes of liposomes to
produce size-reduced liposomes having a uniform average
30 mean diameter.
1 3';30~9
-33-
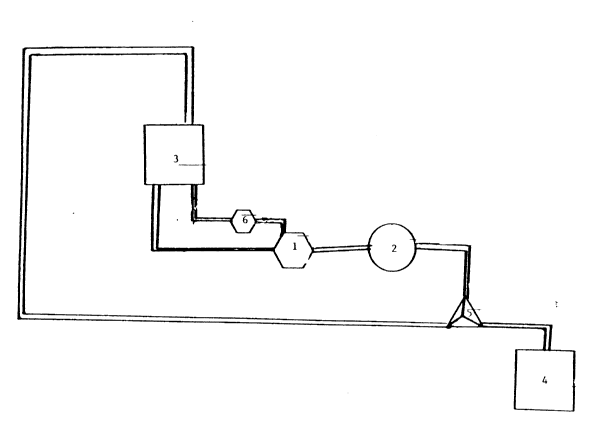
Fig. 5 depicts a continuous size reduction apparatus.
This has (a) a high pressure piston pump 1, (b) an in-line
filter element 2 having a pre-determined pore size, (c) a
reservoir 3 for holding the feed stock (liposome suspen-
sion), and (d) a reservoir 4 for collection of the pro-
cessed feed stock. The system may also include a valving
unit 5 to direct the flow of material either back into the
feed vessel for recycling or into the process collection
vessel. The feed stock may be introduced to the pump by
any usual means including but not limited to drawing into
the pump head by the piston action of the pump head itself,
and/or external pumping of the feed stock by an external
device shown at 6. The pump head then provides the energy
for circulation of the feed stock through the filter unit.
In the above example, the liposomes were passed 10
times through a stainless steel filter having a nominal
pore size of 500 nm.
The above procedure was also performed using the
aqueous phase compositions according to Table 4.
EXAMPLE 12
SIZING STUDY OF PILOCARPINE
ALPHA-TOCOPHEROL HEMISUCCINATE VESICLE
The preparation of Example 11 was examined using
freeze-fracture electron microscopy. The results show a
population of mainly unilamellar liposomes having a size
range of about 30-225 nm. Vesicles produced as in Example
11 were also measured using quasi-elastic light scatterin~
1 3 ~304,
-34-
EXAMPLE 13
PILOCARPINE - ALPHA-TOCOPHEROL HEMISUCCINATE
The materials and procedures of Example 11 were
followed using the VET400 method (vesicle extrusion
technique) of size reduction through two 400 nm pore-size
Nucleopore filters. The VET 400 method is described in
Cullis et al., PCT publication WO 86/00238, pub]ish~d
January 16, 1986 entitl~d "Extrusion Technique for
producing Unilamellar Vesicles".
The above procedure was performed using 0.1% (w,~v)
sorbic acid with 0.1% (w/v) EDTA as the aqueous phase.
EXAMPLE 14
PILOCARPINE - ALPHA-TOCOPHEROL HEMISUCCINATE
COMBINED BATCH LIPOSOME PREPARATION
The materials and procedures of Example '1 were
followed anZ sized batches combined for a to'al vclume of
750 ml. Liposomes were then processed as in Exam-le lI.
The above procedure was also performed using the
aqueous phase compositions according to Table 6.
EXAMPLE 15
PILOCARPINE - ALPHA-TOCOPHEROL HEMISUCCINATE
LIPOSOME PREPARATION
The 5 liter round bottom flask containing the product
25 of Example 10 above was placed on a rotary evaporator;
with the water bath temperature set at 55C. The salt
was warmed for 30 minutes, then the water bath temperature
-35- 1 3 ~ 3 ~ 4 9
was reduced to 35C. An aqueous solution of 0.01% (w/v)
sorbic acid with 0.01% (w/v) disodium EDTA dihydrate (550
ml) was added and the suspension mixed for 1-1.5 hours
using an agitator blade. The final volume was adjusted to
750 ml with additional aqueous phase. The resulting
liposomes were processed by CSR (Continuous Size
Reduction); passing the liposomes 10 times through a
stainless steel filter having a nominal pore size of 500
nm.
The above procedure was also performed using the
aqueous phase according to Table 7.
EXAMPLE 16
PILOCARPINE - ALPHA-TOCOPHEROL HEMISUCCINATE
The procedures and materials of Example 11 were
followed, using a pilocarpine: D-alpha-tocopherol mole
ratio of 1:0.5, corresponding to 1.0 g pilocarpine and
1.28 g D-alpha-tocopherol. Liposomes were formed
according to the procedures of Example 12, wherein the
aqueous phase added was Water For Injection, USP, to a
final concentration of 4% pilocarpine. Results for the
viscosity of the above liposome solution are listed in
Table 8.
The above procedure was repeated using an aqueous
solution of 0.1% (w/v) sorbic acid with 0.1% (w/v)
25 disodium EDTA dihydrate in Water For Injection, USP.
EXAMPLE 17
The procedure and materials of Example 16 were
followed, using a pilocarpine:D-alpha-tocopherol mole
ratio of 1:2, corresponding to 1.0g pilocarpine and 5.1 g
30 D-alpha- tocopherol. Liposomes were formed according to
, .
-36- 1 3 S304~
the procedures of Example 12, wherein the aqueous phase
added was Water for Injection, USP, to a final
concentration of 4% pilocarpine. Viscosity results for
the above liposome solution are listed in Table 8.
The above procedure was repeated using an aqueous
solution of 0.1% (w/v) sorbic acid with 0.1% (w/v)
disodium EDTA dihydrate in Water For Injection, USP.
EXAMPLE 18
The procedures and materials of Example 16 were
followed, using a pilocarpine:D-alpha-tocopherol mole
ratio of 1:4, corresponding to 1.0 g pilocarpine and 10.2
g D-alpha- tocopherol. Liposomes were formed according to
the procedures of example 12, wherein the aqueous phase
added was Water For Injection, USP, to a final
15 concentration of 4% pilocarpine. Viscosity results for
the above liposome solution are listed in Table 8.
The above procedure was repeated using an aqueous
solution of 0.1% (w/v) sorbic acid with 0.1% (w/v)
disodium EDTA dihydrate in Water For Injection, USP.
-
_37- 73s30~q
TABLE 1
Solubilization of Cyclosporin A
Mole Percent
Cyclosporin A CHSa THsb Total moles/mlC
0 168
18.8 56.2 168
37.5 37.5 168
56.2 56.2 168
0 75 168
0 144.5
21.3 63.7 144.5
42.5 42.5 144.5
63.7 21.3 144.5
0 85 144.5
3 97 0 130
3 24.2 72.8 130
3 48.5 48.5 130
3 72.8 24.2 130
3 0 97 130
63 63 63 189
180
158
a CHS is cholesterol hemisuccinate Tris salt.
b THS is alpha-tocopherol hemisuccinate Tris salt.
c The figures shown are the total number of moles of
Cyclosporin A + CHS + THS in the final aqueous solution.
-38-
1 3 '30~'t
TABLE 2
Proportions (mole percent) of miconazole, cholesterol
hemisuccinate (tris salt), and D-alpha-tocopherol
hemisuccinate (tris salt) in the preparations (Total
micromoles = 0.10)
Cholesterol D-Alpha-Tocopherol
Miconazole Hemisuccinate Hemisuccinate
(Free Base) (Tris Salt) (Tris Salt)
0 .95
.238 .713
.475 .475
.713 .713
.9S0 0
0 85
21.25 63.75
42.5 42.5
63.75 21.25
0
0 75
18.7 56.3
37.5 37.5
56.3 18.7
0
0 65
16.25 48.75
32.50 32.50
48.75 16.25
0
0 55
13.75 41.25
27.50 27.50
41.25 13.75
0
0 45
11.25 33.75
22.50 22.50
33.75 11.25
0
--3 9 -
~ 3 s304q
TABLE 3
Alpha-Tocopherol Hemisuccinate Tris Salt
moles mg Entrapment /mole
Efficiency (%)
15.0 9.89 9.41+ 31.37+
0.79 2.63
40.0 26.36 12.50+ 15.63+
3.36- 4.20
65.8 43.37 12.84+ 9.76+
1.21 0.92
100.0 65.91 15.59+ 7.79_
4.06 2.03
175.0 115.34 17.15+ 4.90+
3.49 1.00
263 . 2 173 .4 8 25. 01+ 4 . 75+
0.80 0.15
526.4 34 6. 95 39. 03+ 3. 71+
0.41 0.04
658. 0 4 33 . 69 5o7 7527+ o 8557+
-40-
1 3:~304q
TABLE 4
Preparation Aqueous Phase Composition % (w/v)
BAKSorbic Acid *EDTA PVA MPMC
2 0.000.10 0.10 1.40 0.00
3 0.100.00 0.10 0.00 0.00
4 0.00 0.10 0.10 0.00 0.50
-41-
1 3 ~30~ ~
TABLE 5
MEASUREMENT OF PILOCARPINE - THS VESICLES
USING QELS PROCEDURE
Pilo-THS:Artifical Tears Diameter (nm)
neat 205
4:1 202
1:11 206
1:10 233
-42-
IJ'')(J/1')
TABLE 6
Preparation Aqueous Phase Composition % (w/v)
BAK Sorbic Acid *EDTA PVA ~PMC
1 0.00 0.10 0.10 0.00 0.50
2 0.00 0.67 0.67 0.00 0.00
3 0.00 0.10 0.10 1.40 0.00
4 0.00 0.05 0.05 0.00 0.00
0.00 0.025 0.025 0.00 0.00
6 0.00 0.025 0.025 0.00 0.50
7 0.00 0.05 0.05 0.00 0.50
--4 3--
1 ~3s04q
TABLE 7
Preparation Aqueous Phase Composition ~ (w/v)
BAK Sorbic ACid *EDTA PVA HPMC
0.00 0.01 0.01 0.00 0.05
2 0.00 0.05 0.05 1.4 0.00
3 0.00 0.01 0.01 1.4 0.00
4 0.00 0.05 0.05 0.00 0.00
0.00 0.05 0.05 0.00 0.00
6 0.00 0.025 0.025 0.00 0. 50
7 0.00 0.05 0.05 0.00 0.50
-44-
1 3 ~304q
TABLE 8
EFFECT OF VARIANCE OF MOLE RATIO OF
PILOCARPINE-BASE: D-ALPHA-TOCOPHEROL
ON VISCOSITY OF LIPOSOME PREPARATION
Mole Ratio Result
WFI Sorbic Acid EDTA
1:0.5 viscous dispersed & turbid
1:1 dispersed & turbid dispersed & turbid
1:2 not dispersible not dispersible
1:4 not dispersible not dispersible
-