Note: Descriptions are shown in the official language in which they were submitted.
r ~ 1 ~ 1333226
HOECHST AKTIENGESELLSCHAFT HOE 88/F 064 Dr. LO/gm
Description
Liquid herbicidal agents
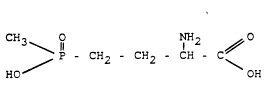
US Patent 4,168,963 discloses that compounds of the formula
CH3 O NH2
H I
/ P - CH2 - CH2 - CH - C ~
HO OH
and their derivatives (I) possess a good and broad activity
against weeds of many botanical families. The compounds
I contain an asymmetric carbon atom. Formula I encompas-
ses all stereoisomers (D and L form), in particular the
biologically active L enantiomer. The ammonium salt of
these compounds (both L form and racemate) is particularly
important.
The compounds are suitable for the non-selective control
~ of undesired plant growth, for example on agricultural
cropped areas, in viticulture, in orchards and oil palm
plantations, and on industrial terrain and rail tracks.
They are usually formulated as aqueous solutions.
Furthermore, it has been disclosed that the activity of
herbicides can be improved in many cases by the addition
of surface-active agents (cf. German Offenlegungsschriften
2,725,8Z3 and 2,554,232). (C12-C1g)-Fatty alcohol poly-
glycol ether and alkylphenol polyglycol ether are used
particularly frequently for this purpose. EP-A 0,048,436
shows that coconut fat alkylbenzyldimethylammonium
chloride or C12-C1g-alkyl polyglycol ether sulfates
enhance the action of I compared with the fatty alcohol
polyglycol ethers and alkylphenol polyglycol ethers which
were included in the comparison test. However, the water-
containing liquid formulations of I are only stable when
polar solvents, such as, for example, dimethylformamide,
- 2 - 1333226
N-methylpyrrolidone or ethylene glycol monomethyl ether
are added. Otherwise, phase separation occurs in the
formulation to give phases which are enriched in active
substance and relatively poor in surfactants and phases
which are relatively poor in active substances and en-
riched in surfactants.
Furthermore, it has emerged that low-temperature stab-
ility of the formulations is often insufficient for
practical requirements. Even though active substance or
surfactant only precipitate below freezing point between
O and -10C, problems may still occur when preparations
which are stored under conditions where they may be ex-
posed to frost are drawn off from larger containers into
small casks. Thus, for example, the large casks have to
be stored in the warmth for a relative long time so that
active substance and surfactant redissolve and the formu-
lation can be drawn off homogeneously into small packages.
Moreover, the preparations should contain no, or the smal-
lest possible amounts, of organic solvents due to theflammability and potential hazard for the user. It is
also important to improve rain resistance of the formu-
lations which contain compounds of the formula I or de-
rivatives thereof as active substance since these active
substances are water-soluble and are taken up by the plants
via the leaf surface. Above all in tropical areas, there
is thus the danger of the active substance being washed
away from the leaf surface due to rain starting after the
application and thus becoming inefficient. Additions of
adhesives, as they are used in wettable powders for im-
proving rain resistance, for example polyvinyl alcohols,
polyv;nylpyrrolidones, polyv;nyl acrylates, polyvinyl
acetates, hydroxyethylcelluloses, carbethoxyethylcellu-
loses, methylcelluloses, dextrins, hydrolysed peptides,
heteropolysaccharides, ligninsulfonates, cation-active
compounds or mineral oils were shown to be ineffective in
experiments.
From the practical point of view, in particular the fol-
lowing requirements have thus to be made for the liquid
_ 3 _ 1333226
formulations of compounds of the formula I:
a) high low-temperature stability
- b) better herbicidal action compared with the known
formulations
c) good rain resistance and
d) amount of organic solvents added as small as possible.
Surprisingly, it has been found that formulations of the
abovementioned active substances, which exhibit these im-
proved properties, can be obtained by using certain sur-
factants.
The present invention thus relates to liquid herbicidal
agents containing a compound of the formula
CH3 \ 1 lH2
/ ~ ~ CH2 ~ CH2 ~ CH - COOH
HO
in the form of the racemate or of the L enantiomer,
lower alkyl esters thereof or salts thereof with acids or
bases (I) in combination with surfactants in the form of
a) (C12-C1g)-alkyldimethyl-, (C10-C1g)-fatty acid
amidopropyldimethyl- or (C10-C1g)-fatty acid amido
ethyldimethylamine oxides or
b) the betaine of coconut alkyldimethylaminoacetic acid
or of coconut alkylaminopropionic acid or
c) (C12-C1g)-alkanesulfonates and mixtures thereof
with (C10-C1g)-fatty alcohol polyglycol ether
sulfosuccinic acid monoesters or (C10-C1g)-fatty
alcohol polyglycol ether sulfates or
d) (C12-C1g)-alkylsulfosuccinic acid monoesters or
(C10-C1g)-fatty alcohol polyglycol ether sulfosuccinic
acid monoesters and (C10-C1g)-fatty alcohol polyglycol
ether sulfosuccinic acid esters and their mixtures with
(C10-C1g)-fatty alcohol polyglycol ether sulfates or
e) (C12-C20)-~-olefinsulfonates and mixtures thereof
with tC10-Clg)-fatty alcohol polyglycol ether sulfates,
_ 4 _ 1333226
(C10-C1g)-fatty alcohol polyglycol ether sulfosuccinic
acid monoesters or (C12-C1g)-alkylsulfosuccinic acid
monoesters, sulfonates which can be used being alkali
metal salts, ammonium salts, alkaline earth metal salts
or substituted alkyl- or alkanolamine salts of the
corresponding sulfonic or sulfuric acids.
Surfactants which are preferably used are (C12-C1g)-
alkyldimethylamine oxides, (C12-C1g)-alkanesulfonates,
(C12-C1g)-alkylsulfosuccinic acid monoesters, here
in particular isodecylsulfosuccinic acid monoester, and
(C12-C20)-~-olefinsulfonates and the mixtures of these
compounds with (C10-C1g)-fatty alcohol polyglycol ether
sulfates.
A further preferred embodiment of the agents according
to the invention comprises, besides 5-40 % by weight of
a compound I, the 0.5-8-fold proportion by weight of the
surfactants according to the invention and 0-20 % by
weight of a water-miscible polar solvent, for example
methylglycol, propyleneglycol monomethyl ether, PEG 200,
isopropanol, DMF or NMP.
The ready formulation contains further surface-active sub-
stances in amounts of in particular between 4-15 % by
weight.
The agents according to the invention contain 5-40 % by
weight of an active substance of the formula I in the
form of an aqueous solution and 0.5-8 parts of the sur-
factants according to the invention per part of active
substance. Further surface-act;ve agents for improving
the wettability, adhesives and binders, urea or inorganic
salts such as for example ammonium sulfate, water-soluble
solvents and defoamers may additionally be present. These
surfactants can also be advantageously employed in combin-
ation formulations of a compound I with other herbicidal
active substances, for example simazin, terbutylazin,
diuron, monolinuron, metolachlor, chlortoluron, oxyfluorfen,
1333226
bifenox, imazethapyr, chlorimuron-ethyl, sulfonyl ureas,
for example sulfometuron, metsulfuron, and they can en-
hance the action of I.
Alternatively, the surfactants can be added prior to ap-
plication directLy to the slurry of the active substance
solution of I or to the mixed formulations with the herbi-
cides mentioned.
The agents according to the invention are present as so-
lutions, in mixtures ~ith water-insoluble active substances,
for example the abovementioned triazine active substances
and urea herbicide active substances, as suspension con-
centrates in which the insoluble active substances are
present in the solid phase, and the compound I and the
surfactants according to the invention in the aqueous
liquid phase. Active substances of low melting point or
liquid active substances, such as metolachlor, are prepared
with a compound I and the surfactants in the form of a
stable emulsion in which the compound I and the surfactants
according to the invention are present in the aqueous
phase and the vater-insoluble liquid or the active sub-
stance, dissolved in organic solvents, is present in the
"oily" liquid phase, where the organic solvents themselves
should not be water-soluble.
Mixed formulations of this type can be prepared in many
ways. On the one hand, a procedure can be followed in
~hich individual components are prepared separately in
the form of individual dispersions and solutions, and
these are then mixed using a colloid mill. Like~ise, it
;s possible to gr;nd the act;ve substances of the finely-
disperse phase together and to add the active substance
solution to this mixed dispersion. In principle, it is
also possible to process all active substances in one run
to give the desired mixed formulation.
The combination formulations prepared in this manner are
storage-stable, show virtually no chemical changes and
1333226
are simple to apply for use.
The agents according to the invention are applied fol-
lowing dilution in water. Suitable active substances of
the compound I are in particular those compounds which
are described in US Patent 4,168,963 or which can be pre-
pared accordingly, for example (3-amino-3-carboxypropyl)-
methylphosphinic acid (phosphinothricin), its hydro-
chloride, monosodium salt, disodium salt, monopotassium
salt, dipotassium salt, monocalcium salt, ammonium salt,
NH3(CH3) salt, NHz(CH3)2 salt, NH(CH3)3 salt,
NH(CH3)2(C2H40H) or NH2(CH3)(C2H4OH) salt, or it~
methyl ester, ethyl ester, propyl ester or butyl ester.
To prepare the agents according to the invention, the ac-
tive substance is dissolved in water, the calculated
amount of action-enhancing surface-active agent and if
desired further customary auxiliaries, such as solubiliz-
ers (propylene glycol monomethyl ether, glycols, poly-
glycols, block polymers, DMF, N-methylpyrrolidone and the
like), other surfactants, colorants or defoamers (for
example silicones, polyethylene polypropylene glycols,
soaps and the like), are added and the batch is mixed
intimately.
The surfactants according to the invention and the other
customary formulation auxiliaries are described, for ex-
ample, in:
Watkins, "Handbook of Insecticide Dust Diluents and
Carriers", 2nd Ed., Darland Books, Caldwell N.J.; H.v.Olphen,
"Introduction to Clay Colloid Chemistry", 2nd Ed., J.
Wiley & Sons, N.Y.: Marschen, "Solvents Guide", 2nd Ed.,
Interscience, N.Y. 1950; McCutcheon's, "Detergents and
Emulsifiers Annual", MC Publ. Corp., Ridgewood N.J.;
Sisley and Wood, "Encyclopedia of Surface Active Agents",
Chem. Publ. Co. Inc. N.Y. 1964; Schonfeldt, "Grenzflàchen-
aktive Athylenoxidaddukte" [Surface-active ethylene oxide
adducts], Wiss. Verlagsgesell., Stuttgart 1976; Winnacker-
Kuchler, "Chemische Technologie" [Chemical Technology],
7 1333226
Yolume 7, C. Hauser Ver~ag Munchen, 4th Edition 1986.
Examples of the surfactants according to the invention
which may be mentioned are:
- (C12-C1g)-alkyldimethylamine oxides: Alkamox L ~
(Alkaril Chemicals), Aromox DMM CD-N~ (Akzo Chemie),
Genaminox~ (Hoechst AG), Nissan Unisafe ALM (Nippon
Oil & Fats Co.)
- Fatty acid amidopropyldimethylamine oxides: Alkamox CAP
(Alkaril Chem.) Rewominox B 204~ (Rewo Chem. ~erke),
Steinapon AM B13~ (Rewo Chem. ~erke)
- Betaines of, for example, coconut alkyldimethylamino-
acetic acid: ALkateric B ~ (Alkaril Chemicals),
Armorteric I ~ (Akzo~ or coconut alkylaminopropionic
acid: Alkateric AP-CW (Alkaril Chemica~ls), Amphoteric
B4~ (Zschimmer & Schwarz)
- Isodecylsulfosuccinic acid ester: Netzer IS~ (Hoechst AG)
- Sulfosuccinic acid esters based~on fatty alcohol poly-
gly~ol ethers are: Texapon SB 3~(Henkel KG), Setacin
103~ (Zschimmer & Schwarz), Genopur SB 312 ~
(Hoe~hst AG), Elfanol 616~ (Akzo Chem.), Konacool-
L400~ (Toko Chem. Ind. Co. Ltd.)
- (C12-C1g)-alkanesulfonate: Hosta~ur SA ~ (Hoechst AG)
- a-Olefinsulfonates: Elfanol OS46~ (Akzo Chemie),
Hostapur OS45~ (Hoechst AG)
- Fatty alcohol polyglycol ether sulfates: Genapol LR ~,
Genapol LRC~, (Hoechst AG), Gezavon LL 2 ~ (Zimmerli
AG), Texapon ASV~, Texapon Na~, Texapon M (Henkel KG)
- (C12-C1g)-alkylsulfates: Texapon K 12~ (Henkel KG)
The following examples serve to illustrate the invention:
- 8 - 1333226
a~ ~
,
~ C
-- .,, o ~
~ X ., ~, ~ aJ
Z O _ ~ n I _
c~ Q~ ~ ~
n o
c ~ ~ o ~ c
E ~ E
'~ ~ C~ c~ ~ , - _
~n ~ _ ~ O -- C E
~ ~ -- ~ E ~
' '-- >` a~ ~ ~ ~ ~ .1~ _ J
' a, x c-- ~ _ _
V ~ 5 N
~ a ~ ~ ~ ~- ~n
J ~ E ~-- C
~ C ,_ ,C O ,~0 ~E E
O -- E ~ E -- ~ ~ -- _ . c ~
x ~ _ ~ ~ O ~~l ~ -- O --
o -- ~-- o ~ n ~ _ E
~ -- Q ~ a cn ~ -- ~
~ o ~ I CI C -- ,~ -
~ ! . -- ~-- o ~ O -- ~ n
~ -- 1'- 1 C ~ C ~ _ _
O ~ ~ ~ c ~ _
Q ~ ~ E -- E QJ a~ o
c _ ~ _ ~ ~ -- c~ c~
-- E ~ ~ , ~, ~ ~ v
O _ ~ _ _ ~ _-- O c ~ ~
S _c~ O ~n_ ~.7 ~ o
-- ~ ~ ~ _ I~ cc ~n ~ ~- c C
cn ~ ~ ~ '~ O_ I ~_ c .-- ._
~ ~ ~ - I ~ O O c~ ~ o ~ c~
O I ~ ~ v _ I ~ ~ v
O O I r~aJ ~ ~-- ~ O Q
J ~ z c~m ~ ~n~S ~n ~~~ ~ m
~ ~o
V~ O ~ ~ ~ u~ o o~ or~l O c
~ N N Nc~v ~I~J
a~
C ' ' ' ~ .
S S S S S S
~n , c Q Q Q ~ ~ ~ Q ~ Q ~ Q
~o ~ O O O ~ ~ ~ O ~ O ~ O
~ ~v ~C---- ~ O O O O ~ O L O
cn ~ _ ~ ~c~ ~ ~ ' '~l ' ~_ -- ~ ~
- O ~ ~ ~ ~ ~ ~
O O O ---- ~-- G -- O _ O --
3 3 3 ~ 3 ~ 3 ~ 3
.,
~ ~ o ~ r~ o 1~ ~o ~ ~ ~ o
O 3
.,
a
.,
r ~ rv
'~ ~
r ~: ~n
o
.,
~ C
_ O C
tn -- O
n
, O
C_ ' ,
CJ
VJ
10'~ O CO
Z C_ f~
- 8a - 133322~
aJ
., ~
aJ
~,
L~
o o
. o
D u~
V) --__-- ___ _ _ _
~ n D D D n D D I D n D
'~ E ~ ~ v v ~ ~ ~ ~, ,,,.~,
L~ aJ V) V) V) V) V) V) V) V) V) V)
L ~
V)
J
E
E O
~ _
o ~ ~ V) ~ ~ S~
L~ 3 o o o o 3 o ~ o _ o
L~ o ____ ___~ _o _ _I
,, L~ L~ L~ L) L~ V~ U V) L~
3 ' ~ '~ v L ~ V V L ~ _ L ~ L --~ O L--
V) O OOOO OOC LOO 0 ~0
D -- r~ O C ~ a~ o
v) D l l l l l l l '~ l l l ~ I
._ ~
t- ~ + >.
L~ ~ '''' ''' ' ~_~
~1 ~ L ~ 0 ~ 0
o o ~ aJ a~ ~ ~ aJ ~ a o o c, ~
O -------- ------ _~___
O ~ aJ .IJ L,~ L~ ~L~ L~ L~ L~ LJ + L~ L~
O
E ~ v~
. _ L,>~ L L _ L L _ L ~ _ _ L
o,-- o v ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~
D. O __ _ _ _ _ _ _ _ _ _ _
(~ . ~r (~J L~ L~ L~L ~ L~ L. U L 1 L ~ L~ L
L~
c
-
~J C
~_ O
V) _ C
O~ ~ O
(~ V)
EaJ--, '--
O_ ~
v n n~ ~J
. C
O ~IJ ~(~J ~) ~' L~ ~
~~ Z ~ _ ~
1333226
C U
C_,~ ~ C~
C~ C~
~ ~ ~ C C C ~ . . ~
Z Z Z Z Z . . Z C C Z
~ ~ ~ 0 C~ ~ C ~ .
Z Z Z ~ 2 ` ~ ` ` '~ '~ C~ ~ '~ '~
a, ~ ~ ~ a, ~ ~ ~ ~~ c~ c~
' ' ~ ' ~ ~ ~ ~ O O ~ V)
~ ~ ~ ~ ~ ~ ` I
cn cr~ ~ ~ ~ cn ~ ~1 ~ ~ ~ ~ ~ ~ = ~ ~ ~
C~ ~ ~ cn ~ ~ ~ u cnv) cn v~ cn ~ =
~ O r) O cn V ~ cn
Z -- -- -- -- ~ ~ - Z aJ QJ
Z ~ ~ ~ ~ ~ O ~ O ~ ~ O O O ~ O ~ O O O ~ ~ O
OJ ~ (~ C ~ ~ Z Z >~ Z ~ ~
~11v c -- -- -- -- -- -- -- , -- -- -- c -- c -- -- -- >~
vtl~ O C C C vn C 0~ tJ tJ v~ ~) O'v') O ~) 0~ vn _ _
tl~~ ~ -- _ -- >~ ~-- ~ t~ v~ >~ tV tV ~ tV ~ ~ >~ t~ ~ ~ v~
_ tJ t~ t_~ _ t~ _ _ ~ _ v v J v _ _ _ Z ~ ~ ~ ~ >~
- t~ t~ tJ O t_~ O ~ ~n o ~ o D~ O -- O ~ O O O _ -- O
Q ~ Q O ~ C C Q C Q ~ ~ Q r~ Q O O C
~,~n~n ~ ~n ~n ~n ~n ~-- ~ o o O D ~ L J Z Q Q
c ---- _ O O O ~ O -- -- ~ -- ~ ~ -- ~ -- -- ~V ~ -- ~
tv>~ ~ ~ ~ ~ O ~ O ~ ~ O -- -- o _~ o o v o z o ~ o _ _ O
~, v ~ _-- _ s-- s ~n ~n ~ ~ ~ ' ~ t~ q ~ ' L '
v _ _ _ ~ ~ ~ o ~ O--_ O ~n ~n o ~^ O-- O ~J ~ ~ ~V ~ ~ L O
t~t~ t~ ~n ~n ~n ~ n tJ ~ ~ t~ tv tV V cu 1_~ tt~l t_l o tl~ ~ t_ t ~ c~ O t~ O tlJ U
_ _ _ _ _ _ ~ V _ C ~ ~ ! . ~ I _ C I ~ tv ~ ~ ~ t~ Z tJ ~ ~
tV ~~v tv :~ ~ ~ tt~ ~ tt~ t~ t~ -- t~ v t~J tX~ t~l _ ttJ ~n ~ n D~
~ t_~ tJ tJ ~ ~ 8 g ~ v v ~ v ~ tJ ~ tJ ~ ~ o >~ ~ t-- ~ >~
tJ I I I ~ O ~ V ~ n ~n ~-- -- ~-- JJ I ~ ~ I ~ ~ . ~ >~ tv ~ ~ v
ttO t~J t,~ t~ t~ -- ~ O O ~ O ~ r~ tJ ~ tJ ~_ ~
.- . ~ n n ~ n ~ o I I tr~ I D~ ~ t~J tt~ C~ ttl~S tV ~n v tJ ttJ
~L t~ tv ~ D~ U_
tto
L^. L^.
. L~^.
O O ~J O O ~ t V ~ ~J O ~J tv O L . U~ O O ~ J t~ L .
~ ~ s u u o ~ ~
uu u >~ ~ O u ~
U ~ ~ _ ~ Q ~ v _ ~ Q Q vq v~ ~ ~ vq
-- C ~ ~~~ O ~ ~ vq v~ O O V ;~ ~
GJ O O ~_ O O ~ ~ UJ . O
~ ~ -- s ~, s s z ~ ~ ~ ~ s ,
v)-- ~.~ ~ ~ ~ ~
~ O -- O --O ~-- ~-- O O O O O O O O -- O
o n 3 ~ 3 ~ 3 3 f~ 3 r~J
~_ L^
~V o ~ o o o ~ o o
r, ~v u~ ~v L^. ~ ~ ~J ~r ~ ~ L^. ~ L^. ~
rq
~ ~ o o o O cv ~ O O v~ v~ v~
V ~ ~J `^J(~J .^J -- ~ ~AJ t~ _ ~ ~ ~ ~ ~ ~
C ~n
~ ^J ~, ~ L^. ~ r- a~ v~ O
O O v
~I Z
1333226
>~
~,
-
n u~
~: D I ~ ~ Q.D ~') g D n ~ Q
~ O ~ ~ ~ __ ~ ~ ~ ~ ~._
O J OO O OO OO O OO J
O ~ ---- -- ~ n _------ _ O
O ~ V V V ~ V ~ V V ~ V V
-- O I O O O O O O O O O O O
~_ ~ C ~
O O O
._ >, , ._
a~ ~ ~ _ _ _ _ _, ~ _ _ _ _ _ _ _ _
C a~
~n u " 1'7
'. ~
--~
-
1333226
-- 10 --
"
Z Z Z Z Z ~ Z Z Z Z Z Z U~
., ~ t
v~ ~ ~ ~ ~ ~ ~ ~ V ~ ~ ~ E
_ _ ~ ~ ~ _ _ _ _ _ _ _ _
U) Ul Ul U~ Ul ~ ~J ~~J Ul U1 Ul U1 Ul Ul
. _ ~ U) O
z aJ z ~ za, z ~ ~ ' ~ ~~ ~ Z Z~ ~ ~ ~ ~ _ U~
O U~
a~ O ~a~o ~ o a~o o E E -- ~ O ~ a~O O o o o ~ C
V ~ ~~ ~ ~ ~ O ~ ~ ~ IJ ~ 3 ~ O J _
C ~ O C -- C-- C -- -- ~ ~ ~-- _ c C -- -- -- _ _ ~ ~. a. =
o cn ~ o cn o cn o cn cn r~ _ J C cn o o cn cn cn cn cn u _ c o
>~ -- ~ ~ ~ ~ ~~ ~-- -- cn - ~ "cn aJ
o ~ o ~ o -- o o _ ~ o ~ o o o o o .
~ Q ~ -~ Q -~ Q ~ Q Q-r - o ~ Q '` ~ Q Q Q Q Q
'~ rr~ I~1 ~ Q ul ~r _ ~, O
, _ J _J _ _ _ o J ,J J J J J >~ ~ O _
~ ~ O O O O ~ ~ J ~ O O O O O O ~ Q >~ ~ ~
o -- ~ s ~ o-- cr
o ~ _ o _o _ o o _ _ ~~ o -- -- o o O O O ~ C O ~ ._
cn ~ u n~ ~ o ~ Ul L ~ ~ ~ n o _
. _ ", ~ ,, . ~
~ ~ C ~. rLI ~ vI I ~ ~ ~ ~ ~ O
~ L~ c_~ v ~ ~ ~ ~
a, ~ o ~ ~1 '
J
o~ ~oo ~O ~ O O O ~ ~ ~ ~ O r~ o o o
V~
.,,, O
U S ~ S S O S S S ~ ~ U
O Vl
. ~ ~ E
v cn Q Q Q Q ~ ~
3 . ~ c ~ 3 3 3 3 cl ILJ
_ c s s Q Q Q Q S S S S S Q Q O Q Q S ~Ll O ,,, ~- S E
--O O O O O -- -- -- --. -- O O O O O ~ E
cn O ~3 ~ ~ ~ ~ ~~ 3 3 3 3 3 ~ ~ ~ ~ C
O ~O _
C~ O ~O ~ C
. . . . v
c ~ O O C?` ~ C~` O ~ ~ O O O ~ O` O -- ~ U
3 E
_~ J J ~ ,~ c~ ~ u C
O O O ~ ~ O ' ' ~ O O ~ ~--
D O ~ ~
U ~ C _
" ~ a~ o c,
_ _ U~ ~
>~ C
~n s ~ ~
~ ~ V t_
E ~ r~J Ut ~
~ _ D E
O -- 11 ~ ~ O
u~ cn
O ~ 11
00cl~ O~ ~ o i~ 1~0 C~ O r~
. 1l cn ~.
~, ~ ..
c . ~ . _
z _ u c~
Z
13 3 3 2~ 6
- o
. o
U~
~~ QD ~1~ n D ~ n D D ~ D n
o--OOOO OOO O OOOOO
oO-------- ------ _ _____
OOOOO OOO O OOOOO
N
O O
~J ~ _
o ~~ ~ aq~''~ ~ aJ ~ o q ~ a~ a aJ
d~ ~ 1-- ------ J
O ' ~ J~L~~ ~ O ~
I~)
E ~n
7 :~tlJ
~J~____ ____ _ _ ____
~ O
., _
~ ~O ~N ~`J ~ `O 1~- 00
O O O ,~
~ ~ Z
- 11 - 133322~
B) HioLogical exa-ples
Example 1
Barley plants which have been grown in the greenhouse
were sprayed in the 3-leaf stage with formulations diluted
in water (active substance: phosphinothricin ammonium
salt) with the active substance concentrations indicated
in Table 2. The comparison agent mentioned in Table 1
acted as the control. The plants were scored after 17
days. The damage (action) is expressed as a percentage.
The results are represented in Table 2.
TabLe 2
Greenhouse experiment on barley
Action as a percentage 17 days after treatment
Water application rate: 300 l/ha
Formulation from Table 1 Dosage : g of active substance/ha
No. 31.2562.5 125250 500
comparison agent - 7 67 87 88
1 - 25 80 96 100
2 - 25 78 94 99
3 - 20 75 93 98
4 - 25 75 95 99
- 20 78 96 100
34 22 75 94 99
82 95100
Example 2
In a field experiment, oilseed rape, field bean and white
goosefoot were sprayed in the 3-5 leaf stage with the
formulations according to the invention which had been
diluted in water. THe water application rate was 300 l/ha.
The plants were scored 13 days after the treatment. The
- 12 - 1333226
damage is expressed as a percentage. The active substance
concentration and the experimental results are represented
in Table 3.
Table 3
Field experiments on oilseed rape, field bean and white
goosefoot
Action as a percentage 13 days after treatment
Formulation from Table 1 Dosage in kg of active substance/ha
No. Oilseed Field White
rape bean goosefoot
0.5 1.0 0.5 1.0 0.5 1.0
Comparison agent 73 85 91 99 88 90
23 75 90 96 100 93 99
24 80 90 95 99 92 93
82 90 95 100 94 97
26 78 94 95 99 93 95
27 80 95 96 99 92 95
29 82 92 95 98 90 96
81 92 96 99 92 96
31 80 94 99 100 92 96
32 92 96 98 100 96 96
Exa~ple 3
In order to determine the rain resistance of the formu-
lations according to the invention, barley plants ~hichhad been grown in the greenhouse were sprayed in the 3-
leaf stage with solutions of the various formulations
(water application rate 300 l/ha). Some of the plants
were exposed to artifical overhead irrigation about
3 hours after this treatment, approximately 10 mm of
artificial rain being applied. The activity (damage to
the plant) was scored 19 days after this treatment.
The active compound concentrations and the experimental
results are represented in Table 4.
- 13- 1333226
c~ L~ L'~
O G C~
-
O L~l L~) ~ L~
.-
C
O
O ~ O O O ~ n L' o o c
O ~ ._
_ ~ C L'~ -- L' C: C ^ ~^ L" L-~
r~ _
Cl
L
O ~ ~ ~ O C
E
1~ E
r O
L
O X o U'1 ~ ~ O O
C C ~ C~ C O
C ~ ~ _ _
._ C~
C ~
~ O L'~
C E '~ c C 1~ ~ 2
r~ ~ C._ ~
a~ E L
~ L
O ~ I~
a, ~ c ~ ~ n C ~J ~ C
L L O
3 ~
L._
-- u ~ c ~ o ~ -- o c o L
.0
0~ L
C _O O' ~
O ~ _ O L~ _
C ~ ~
E _'J C O
~ r~ U ~ ~
'` U
~ ~ ~ ~n ~ c
X o Q _ ~ ~ : : :
~ ~ _,
E --
O C
~n c ~n L 8~ ~ ~
._ ~ ~ _ ~ ~ L
~J o ~ ~ ~
L~ C ~ C ~ E
C ~1 0 -- O C ~ O
L ._ O ,_
n~ ~ o ~ ~
o _ L C _l
E C~
~ ~-- ~ O ~
o o O ~ ,_, ~ ~ ~ ~r ~r
- 14 - 1333226
Exa-pLe 4
In order to test the formulations according to the in-
vention for rain resistance, an experiment with Paspalum
S conjugatum, a perennial Graminea, was set up in an olive-
tree plantation. After the plants had been sprayed with
solutions of the various formulations, some of the plants
were exposed to artificial overhead irrigation, an arti-
ficial rain of an amount of precipitation of approx. 20 mm
being applied. The activity (damage to the plants) was
scored Z weeks after this treatment. The active substance
concentrations and experimental results are represented
in Table 5.
TabLe S
Field experiment on Paspalum conjugatum
Action as a percentage 2 weeks after treatment
Overhead irrigation 4 hours after 20 mm's treatment
Formulation from Table 1 Without overhead With overhead
No. irrigation irrigation
9 of a i /ha 400 500 400 500
Comparison
agent 85 95 20 30
33 90 98 75 82
g of a i /ha 200 250 200 250
41 95 99 80 85