Note: Descriptions are shown in the official language in which they were submitted.
1333547
- 1 - PC-3122
METAL COATING OF INORGANIC FIBERS AND SOLID PARTICULATES
The present invention is concerned with metallization of
heat resistant inorganic materials such as fibers and particulates
and, more particularly, with metallization of glass or glass-like
fibers.
BACKGROUND AND PROBLEM
Recent regulations applied on a national and international
scale limiting the EMI (Electromagnetic Interference) levels emitted
by various electronic devices has stimulated development of various
composite materials and coatings to reduce the EMI emissions to the
required levels. The efficiency of EMI absorbing constituents of
such composites improves with the conductivity and with the aspect
ratio of the particles. A number of products have been introduced to
the market for this purpose. They include: nickel plated graphite
fibers, stainless steel fibers, nickel fibers, nickel plated mica,
nickel plated mineral fibers, etc. Generally speaking, the currently
available materials are either expensive or poorly effective. Nickel
coated glass fiber produced in a single operation could be marketed
at a much lower cost and could be therefore used in a wide range of
13335~7
consumer product applications. EMI shielding conductive fibers are
usually sold in chopped bundles joined by a suitable binder or sizing
to el~ ~nAte the environmental hazards of handling loose individual
fibers.
The use of sized glass fibers in structural composites is
well established. Glass rovings from nickel coated fibers can be
used in similar applications, where electrical conductivity (or other
metallic characteristic) of the structural element is required for
some reason, e.g. EMI shielding or rapid curing by induced current.
Glass fibers can also be used for reinforcing light metal
composites and special grade glass has been developed for this
purpose. Wetting of the glass is, however, difficult and is greatly
facilitated by using nickel plated fibers.
Methods of plating separately formed glass fibers as have
been suggested in the past, e.g. electroless plating from aqueous
solution, are complicated by the necessity to apply a protective
sizing on freshly formed glass fibers to protect them from rapid
deterioration. Such sizing has to be removed as completely as
possible immediately prior to electroless plating as any residual
sizing will interfere with the plating process and the quality of the
product. Isolated disclosures of gas plating glass fibers and
assisting the process of gas plating by induction heating such as
appear in U.S. Patent No. 2,867,552 of 1959 to H.J. Homer have been
found to be inoperative.
There is also a need to provide products such as high
quality nickel-coated graphit~ fibers and nickel-coated inorganic and
heat resistant organic particulates at relatively reasonable cost
compared to the present cost of producing such materials.
OBJECT OF THE lNV~NllON
It is an object of the invention to provide an operative,
novel process for gas plating glass fibers with a metal such as
nickel.
It is a further object of the invention to provide an
operative, novel process for gas plating non-metallic fibers and
particulates stable at temperatures at least up to about 300C.
_ 3 _ 1333547 PC-3122
DRAWINGS
Figure 1 of the drawing depicts schematically the process
of the present invention as applied specifically to glass.
Figure 2 of the drawing depicts schematically the process
of the present invention as applied specifically to heat resistant
particulates.
DESCRIPTION OF THE lNV~NllON
A process for coating a non-metallic fiber or particulate
with an endothermically vapor-deposited metal comprising initially
introducing hot fiber or particulate (i.e. the substrate) into an
atmosphere containing thermally decomposable metal compound to
thereby initiate metal deposition on said substrate. Thereafter
the amount of metal on the substrate is increased by maintaining the
substrate in an atmosphere containing decomposable metal compound
while electrically inducing in the initially coated substrate at a
frequency at least in the megahertz (MHz) range sufficient energy to
maintain the metal surface thereof at a temperature sufficiently high
to support decomposition of the metal compound on the surface.
The process of the present invention advantageously employs
as a starting material heat resistant, e.g. an inorganic, non-
metallic fiber. For practical purposes such a fiber can be made of
graphite or can be made of glass. "Glass" is defined for the
purposes of this specification and claims as "an inorganic material
usually comprising oxides which can be cooled from the molten state
to a relatively stable non-crystalline, apparently solid state". As
commonly encountered, "glass" comprises silica, an alkali metal oxide,
an alkaline earth metal oxide and optional amounts of alumina, boron
oxide and other metal oxldes. However, glass can comprise
essentially silica or can be based entirely on oxides such as boron
oxide or phosphorus oxide. Por purposes of this specification and
claims, "fiber" is considered to be an elongated mass of material
having a diameter or major cross sectional dimension of the order of
10-50 micrometers. The fiber is considered to be hot if it is at a
temperature significantly in excess of the temperature of
4- 1333S~7 PC-3l22
decomposition of the decomposable metal compound and up to that
temperature at which the fiber decomposes, sinters or melts.
Specifically with respect to glass, a glass is deemed to be molten
when it has a vlscosity of less than about 100,000 centipoises.
The thermally decomposable metal compound used in
accordance with the present invention is advantageously nickel
carbonyl, Ni(C0)4, which rapidly decomposes into metal and carbon
monoxide under about atmospheric pressure at about 150C. However,
other metal compounds thermally decomposable to metal and a combining
moiety can be used. Materials such as, for example, carbonyls and
nitrosyls, both pure and mixed of nickel, iron, chromium, molybdenum,
tungsten, etc., copper acetyl acetonate, hydrides such as stibine and
arsine, carbonyl halides such as osmium carbonyl bromide, ruthenium
carbonyl chloride, metal alkyls such as trimethyl aluminum, triethyl
tin, etc. can be employed in the present invention. Those skilled in
the art will appreciate that by controlling the thermally
decomposable materials in an atmosphere in contact with hot
substrate, e.g. fiber or particulate, various products such as
complex composite structures, alloys and the like can be produced.
For example, if hot glass fiber is introduced into an atmosphere
containing both nickel and iron carbonyl, an alloy can be deposited
on the glass. An example of the utility of such a product would be
the deposition of a low expansion nickel-iron alloy containing say
36% nickel so as to match reasonably well the thermal expansion of
the metal and the glass. Another example of a useful, complex
composite structure would be introdlf-ing hot glass into an atmosphere
cont~ining nickel carbonyl and, after producing an initial layer of
nickel, introducing the still hot or inductively heated fiber into an
atmosphere containing chromium carbonyl Cr(C0)6 to deposit a layer of
chromium atop the layer of nickel.
It is essential in the process of the present invention
that inductive heating of initially plated fiber be done at MHz
frequencies. It has been discovered that disclosures mentioned
hereinbefore such as those by Homer simply were not operable due to
the fact inductive heating frequencies available to him were too low.
The process of the present invention has been found to be
1333S~7
- 5 - PC-3122
operative using an induction heating coil operating at 13.6 MHz, said
coil and associated electronics being the product of Leco Corporation
and sold for the general purpose of analyzing solids for carbon and
sulfur content. Generally speaking frequencies in excess of about 5
MHz are operative for purposes of the invention.
Considering nickel carbonyl, Ni(C0)4, as a model material
thermally decomposable to metal for use in the present invention,
metal is deposited on glass fibers from the gas phase by virtue of
the endothermic reaction:
Ni(C0)4 Ni + 4C0
It is known that in the presence of a catalyst, e.g. nickel, carbon
monoxide can disproportionate according to the formula:
2C0 C + CO2
The result of these two reactions proceeding simultaneously is
deposited metallic nickel along with some small amount of elemental
carbon. As taught in U.S. Patents Nos. 3,694,186 and 3,820,977 the
amount of carbon codeposited along with nickel can be ;n; ;~ed by
including in the atmosphere cont~ining nickel carbonyl an oxide of
nitrogen such as N20 (nitrous oxide), N0 (nitric oxide), N203
(nitrogen trioxide) or N02 (nitrogen peroxide). Assuming that nickel
carbonyl is present in a minor amount in a carrier gas such as carbon
monoxide, an oxide of nitrogen can be present in the carrier
gas/carbonyl mixture in an amount of about 1 to about 1500 ppm. The
carrier gas is advantageously carbon monoxide but can be any gas
inert with respect to the metal compound decomposition reaction,
e.g. nitrogen or argon. It is important that the gas/carbonyl
mixture be substantially free of oxygen, halides, hydrogen halides,
dust or aerosol particles or other substances which can nucleate
decomposition of nickel carbonyl to form a powder product. Likewise,
the wall of the apparatus in which coating of fiber with metal is
carried out should be cool or washed by gas relatively free of nickel
carbonyl in order to prevent unwanted plating of such walls with
nickel.
13335~7
- 6 - PC-3122
Attention is also directed to the use of substances such as
H2, N0, PF3, PH3, NH3 or halogens to catalyze the decomposition of
iron pentacarbonyl as disclosed in U.S. Patent No. 4,056,386 and to
the use of N0, N203 and N02 for the identical purpose as disclosed in
U.S. Patent No. 3,694,187. The present invention contemplates the
use of these substances in small amounts when plating glass fiber or
any other heat resistant substrate with iron and the use of any
substance capable of catalyzing the decomposition of a compound
thermally decomposable to produce metal when plating glass fiber or
substrate with any metal or combination of metals.
Speaking again particularly with respect to nickel, the
latent heat content of a newly formed glass fiber 10 micrometers in
diameter from a molten glass mass is sufficient to cause the
deposition of up to about 0.2 micrometer thick layer of nickel on the
fiber. In accordance with the present invention, a fiber is provided
with a nickel (or other metal) coating at least about one micrometer
thick, advantageously about 1-3 micrometers thick and even coatings
much thicker. Accordingly for purposes of the present invention to
provide a ini adequate coating of nickel it is necessary to
supply to the fiber by means of induction heating an amount of heat
at least about 5 times the latent heat in a newly formed glass fiber.
Thus the input of heat to the fiber by induction heating is a
significant feature of the present invention.
In order to give those of normal skill in the art a greater
appreciation of the advantages of the invention, reference is made to
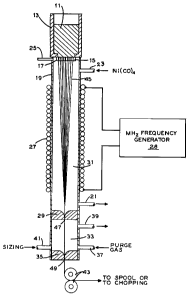
Figure 1 of the drawing which de?icts schematically the process of
the present invention as applied to glass fiber. Referring now
thereto, glass 11 is melted in furnace 13 and forced by head or head
plus applied pressure through holes 15 in spinneret 17. Glass
melting furnace 13 is supported on gas impervious, non-metallic
tubular column 19 made, for example, of silica or other heat
resistant material. Tubular column 19 has ports 21 and 23 for
ingress of gas containing nickel carbonyl and egress of gas depleted
in nickel carbonyl. Tubular column 19 can also be provided with port
25 adjacent spinneret 17 through which a wash gas such as nitrogen or
carbon monoxide can pass to wash the hot, outer surface of spinneret
17 with a gas free of nickel carbonyl and thus to rin; l~e deposition
13335~7
- 7 - PC-3122
of nickel on the outer surface of spinneret 17. Internally cooled
induction coil 27 surrounds tubular column 19 and is connected to MHz
frequency generator 28. Near the bottom of tubular column 19 is
located baffle 29 separating the active plating chamber 31 above
baffle 29 from purge chamber 33 below baffle 29 and above baffle 35.
Purge chamber 33 has inlet 37 and outlet 39 for purge gas and inlet
41 for sizing. Below baffle 35 tubular column 19 ends being
supported in position by means not shown. Pinch rolls 43 are mounted
below the open but baffled end of tubular column 19.
In operation, molten glass 11 from furnace 13 is forced
through holes 15 in spinneret 17 to form tow 45 of several hundred
fibers. Initially these fibers are fed through tubular column 19
through ports 47 and 49 in baffles 29 and 35 respectively and between
pinch rolls 43. Pinch rolls 43 pull on tow 45 so as to produce
fibers of the desired diameter. When the apparatus is operating
satisfactorily to produce the proper glass fiber, plating is
initiated by introduction of gas containing nickel carbonyl into
plating chamber 31. Megahertz frequency generator 28 operating at,
for example, 13.6 MHz is activated energizing coil 27. The quantity
of nickel carbonyl passing into plating chamber 31 in unit time is
controlled vis-a-vis the amount of glass fiber emerging from
spinneret 17 such that a coating of nickel at least about 1
micrometer thick is formed on each of the glass fiber of tow 45. It
is within contemplation of the present invention to provide means,
such as a vibrating means, within plating chamber 31 to enhance the
separation of fibers of tow 45 and thus facilitate metal deposition.
Generally speaking, coil 27 maintains the fibers of tow 45 at a
temperature of at least about 150C and advantageously in the range
of about 180C to about 240C. After the fibers of tow 45 have been
properly coated with nickel, they are sized in purge chamber 33 or at
another down stream location from plating chamber 31. In this
context, "sizing" means coating the fibers with a binder which is
either readily removable or compatible with the ultimate usage of the
fiber. The purpose of "sizing" in this sense is to bind individual
fibers into more or less loosely aggregated flocks so as to
facilitate the handling of chopped fibers and ;n~ ;~e dusting and
atmospheric levitation of individual fibers. For general purposes a
1333547
- 8 - PC-3122
water-removable size could be polyvinyl alcohol applied as an aqueous
aerosol. For ultimate use in organic binder fiystems useful sizes
could be polystyrene, polymethyl methacrylate, stage 2 phenol- or
urea-formaldehyde resin and the like all applied by means of an
aerosol.
Once coated fiber tow 45 is purged of residual nickel
carbonyl and is sized, it exits through port 49 into the open
atmosphere, passes between pinch rolls 43 and is then either spooled
or chopped. As indicated hereinbefore, products of the process of
the present invention have numerous uses including EMI shielding
materials, reinforcing means in resinous and metallic systems,
electrical conductors in fabrics, felts, concretes and the like,
magnetically responsive means in plastic systems, in-situ susceptors
for resin curing and the like.
Graphite fiber can be treated essentially in the same
manner as glass fiber except that graphite as fiber is heated by any
appropriate means in an inert, e.g. argon atmosphere instead of being
formed from a melt as depicted in Figure 1. Graphite fiber exiting
from a heating chamber, like the emerging glass fiber tow 45 in
Figure 1, is initially contacted with gas containing a volatilized,
decomposable, metal compound, e.g. Ni(CO)4, and then subjected to a
megahertz frequency induction field in the presence of an atmosphere
containing a decomposable metal compound.
An apparatus for coating solid particulates, e.g. sand,
alumina, mica, zirconia, tetrafluoroethylene powder, etc. with metal
such as nickel is depicted schematically i~ Figure 2. Referring now
thereto, particulate 53 is fluidized in non-metallic fluid bed 55 by
a gas consisting of carbon monoxide and nickel carbonyl. The gas is
forced through line 57 by pump 59 through porous grate 61. The gas
is not heated except to the extent that it is heated by compression.
Particulate 53 enters fluid bed 55 from hopper 63 by means of sealed
screw auger 65 operated by motor 67. Particulate 53 passes into
standpipe 69 and is heated up to about 300~C or higher (depending on
the nature of particulate 53) by means of electrical resistance
heating jacket 71. Particulate 53 is retained in the vicinity of
jacket 71 for a residence time necessary to achieve the desired
temperature by constriction 73 in standpipe 69. Constriction 73 can
`- 13335~7
- 9 - PC-3122
be of any construction and advantageously is vibratable so as to
meter hot particulate 53 into fluid bed 55. On entering fluid bed 55
hot particulate 53 reacts with nickel carbonyl to form an initial
thin layer of nickel on the surface of the particles. Thereafter
while in residence in fluid bed 55, particulate 53 is heated by
inductive coupling with internally cooled coil 27 energized by
megahertz frequency generator 28 to increase the amount of nickel on
the particle surface. Gases exit from fluid bed 55 through line 75
which in turn leads into cyclone 77 with off-gas line 79. Entrained
solids in exiting gases are returned to standpipe 69 through return
line 81. Product comprising particulate 53 coated with metal, e.g.
nickel, is removed via line 83 into container 85 which can be
isolated by valve 87. In Figure 2, line 83 is depicted as coming off
near the bottom of fluid bed 55 on the premise that particulate 53 is
substantially uniform in particle size and is relatively low density
material such as silica or alumina. A relatively thick deposit of
heavy nickel on such a particle will, on the average, cause such a
particle to be levitated at a higher gas velocity, i.e. near the
bottom of a diverging fluid bed.
While in accordance with the provisions of the statute,
there is illustrated and described herein specific embodiments of the
invention, those skilled in the art will understand that changes may
be made in the form of the invention covered by the claims and that
certain features of the invention may sometimes be used to advantage
without a corresponding use of the other features.