Note: Descriptions are shown in the official language in which they were submitted.
1 335256
OCCLUSIVE BODY FOR ADMINISTERING A
PHYSIOLOGICAI.LY ACTIVE SUBSTANCE
This invention relates to an occlusive body (such as
a patch, pad or bandage for example) for the transdermal
5 administration of a physiologically active substance (by
attachment to the skin or a buccal membrane for example)
at a controlled rate over an extended period. In U.S
Patent No 3598122 (Zaffaroni) there was disclosed a
bandage for use in the continuous administration of
10 systemically active drugs by absorption through the skin
or oral mucosa.
US 3598122 disclosed a broad range of systemically
active drugs which could be employed, and indicated that
any systemically active drug which is absorbed by the body
15 surface beneath the bandage could be employed. The
substances disclosed as being suitable for incorporation
in the bandage included antimicrobial agents such as
pencillin, tetracycline, oxytetracycline, chlortetra-
cycline, chloramphenicol, and sulfonamides; Sedatives and
20 Hypnotics such as pentabarbital sodium, phenobarbital,
secobarbital sodium, codeine, ( L bromoisoaleryl) urea,
carbromal, and sodium phenobarbital; Psychic Energizers
such as 3-(2-aminoprophyl) indole acetate and 3-(2-
aminobutyl) indole acetate; Tranquilizers such as
25 reserpine, chlorpromazine hydrochloride, and thiopropazate
hyudrochloride; Hormones such as adreno-corticosteroids,
for example, 6 -methyl-prednisolone, cortisone, cortisol,
and triamcinolone; androgenic steroids, for example,
methyltestosterone, and fluoxymesterone, estrogenic
30 steriods, for example, estrone, 17B-estradiol and ethinyl
estradiol; progestational steroids, for example 17
hydroxyprogesterone acetate, medroxyprogesterone acetate,
l9-norprogesterone, and norethindrone; and thyroxine;
Antipyretics such as aspirin, salicylamide, and sodium
35 salicylate; Antispasmodics such as atropine,
methscopolamine bromide, methscopolamine bromide with
phenobarbital; Antimalarials such as the 4-
aminoquinolines, 8-aminoquinolines, and pyrimeth~mine; and
` -
~_ 2 1 335256
Nutritional agents such as vitamins, essential amino
acids, and essential fats.
The bandage comprised a backing member having on
one surface thereof a reservoir cont~ining a systemically
S active drug. The reservoir had a wall distant from the
backing member and permeable to the passage of the drug. A
pressure-sensitive adhesive layer, also permeable to
passage of the drug, was carried by the reservoir. The
drug was in a form acceptable for absorption through the
skin or the mucosa of the mouth. It was explained that the
percutaneous rather than oral route enabled continuous
administration of the drug over a period of time, and for
this purpose fibrous masses and fabrics that merely
absorbed and released drug solutions in a gross and
uncontrollable manner were to be avoided.
It was indicated that the percutaneous route had the
advantage over the oral route of drug administration that
uncertainties in the rate of dosage through the
gastrointestinal tract (depending on the amount and type
of food eaten, for example) were avoided. Also, charge
peaks in the drug concentration in the bloodstream were
thereby avoided.
The materials used to form the reservoir could also
form the membrane, and suitable materials included
organopolysiloxane rubbers, hydrophilic polymers of
monoesters of an olefinic acid such as acrylic acid and
methacrylic acid, polyvinylalcohol, polyvinylacetate,
plasticised nylon, collagen, modified collagen, gelatin,
and waxes such as polyethylene wax. An exemplary bandage
contained megesterol acetate powder within a reservoir of
dimethyl silicone rubber, which was stated to be effective
over a 24 hour period. No liquid was present within the
reservoir.
However US 3598122 did indicate that drugs which in
isolation do not pass through the skin could be dissolved
in absorbable pharmacologically acceptable solvents such
as C2 to C10 alcohols, C5 to C12 hydrocarbons, C4 to C10
aldehydes and ketones, C4 to CFlo esters, ethereal oils,
1 3 3 5 2 5 6 20159-472
halogenated hydrocarbons and mixtures of the above.
Furthermore, U. S. Patent No. 3,598,122 taught that by
varying the composition and thickness of the reservoir wall the
dosage rate per area of bandage can be controlled, since the
reservoir wall acts as a solubility membrane to meter the flow
or diffusion of the drug.
One material disclosed as being suitable for the
reservoir wall was a hydrophilic polymer of an ester of an
olefinic acid.
The percutaneous administration of nicotine by means of
an occlusive pad in a dose approximating that delivered by a
variety of nicotine-containing products was described in U. S.
Patent No. 4,597,961 (Etscorn). A typical pad had a reservoir
defined by a cavity within a backing sheet and filled with 1-4
microlitres of nicotine base. The nicotine in the reservoir was
separated from the skin by a microporous nicotine-permeable
membrane, but no directions were given about what kind of
membrane should be used, nor any directions concerning the
relationship between the membrane and the reservoir materials.
In another pad disclosed: U. S. Patent No. 4,597,961, nicotine
base was absorbed in fibrous or porous material which was held
in an open reservoir in the bandage. In use, the nicotine wicked
from the porous material as it diffused through the skin.
EP-A-186,071 discloses a patch for the transdermal
delivery of timolol comprising a rate-controlling microporous
membrane having an adhesive layer on one major surface thereof
and a reservoir of timolol and carrier material in contact with
3a
1 335256 20159-472
its other major surface. The reservoir comprises an impermeable
backing member which is sealed around its periphery to the
microporous membrane.
The carrier material may be a semi-solid material such
as mineral oil gelled with polyethylene, polyisobutylene,
aluminium stearate, propylene glycol or a fatty acid ester, or
may be a solid such as silicone, acrylic adhesive, and
plasticised polyvinylchloride.
The microporous membrane may be microporous
.. . ..
~i ~
_ 4 l 335256
polypropylene, microporous nylon or microporous
polycarbonate. EP-A-186071 also discloses rate-
controlling membranes of non-microporous material, namely
silicone, ethylene vinyl acetate and polyurethane.
It will be noted that nearly all the membrane
materials and carrier materials disclosed in EP-A-186071
are hydrophobic. Particular membrane material-carrier
material combinations disclosed in EP-A-186071 include:
Carrier material Membrane material
10 gelled mineral oil (microporous polypropylene
(ethylene vinyl acetate
(silicone
(polyurethane
15 gelled mineral oil (polyisobutylene + mineral
(oil
(microporous polypropylene
There is no disclosure of a hydrophobic carrier
material in combination with a hydrophilic membrtane
material, nor is there any disclosure of a hydrophilic
carrier material in combination with a hydrophobic
membrane material.
It is stated in EP-A-186071 that the drug timolol
may be administered by the disclosed patches at "a
controlled low zero order rate" (p. 2 lines 15 and 16).
However an equation given subsequently in the description
indicates that the flux through the membrane (and hence
the rate of transdermal delivery of the timolol) is
proportional to the timolol concentration in the
reservoir. Accordingly it is clear that the rate of
transdermal delivery of timolol from the patches of EP-A-
186071 is zero order (if at all) only over a small
proportion of the dose.
An object of the present invention is to provide a
system for the transdermal delivery of a physiologically
active substance in which the rate of delivery of the
active substance is more nearly constant and/or in which
the rate of delivery of the active substance is
-
5 l 335256 26839-12
substantlally constant over a greater proportlon of the total dose
contained ln the reservolr, ln comparlson wlth systems of the
prlor art.
Unexpectedly, lt has now been dlscovered that the dosage
rate of a physlologlcally actlve substance from a reservolr
through a membrane permeable to that substance may be llnearised
by providing that either of the following condltlons ls satlsfled,
namely: a) said membrane is hydrophobic, the reservoir contents
are hydrophilic and said reservoir contains a hydrophilic wettlng
agent; or b) sald membrane is hydrophilic and sald reservolr
contents are hydrophoblc.
Accordlngly ln one aspect the present lnvention provides
an occlusive body for the transdermal administration of a
physlologlcally actlve substance, said body comprising the
comblnatlon of:
a) an lmpermeable backing;
b) a rate-controlling microporous membrane, said lmpermeable
backing and rate-controlling microporous membrane defining a
cavity therebetween;
c) a llquld materlal, comprlslng sald physlologlcally actlve
substance ln liquld form, conflned between sald lmpermeable
backlng and sald rate-controlllng mlcroporous membrane wlthln sald
cavlty;
d) a vlscous flowable gel materlal conflned between said
lmpermeable backlng and sald rate-controlllng mlcroporous membrane
wlthln sald cavlty for substantially lmmoblllzlng sald llquld
materlal; and
e) means for attachlng the body to the skln, whlch means does
,. ~ .
~.~
5a l 335256 26839-12
not substantlally affect release of said substance through sald
microporous membrane,
said rate-controlling microporous rnembrane being permeable to
and in contact wlth said physlologlcally actlve substance and
wherein either,
i3 the rate-controlling microporous membrane is
hydrophilic and the materlal ln said cavity is hydrophoblc, or
ii) the rate-controlllng microporous mernbrane is
hydrophoblc and sald cavity contalns a hydrophlllc wetting agent,
whereby in use, passage of said physiologically active
substance through said microporous membrane is rate-controlling
and said physiologically active substance is released from said
rate-controlling microporous membrane at a rate that is
substantially constant over a period of hours.
In another aspect the invention provides an occlusive
body for the transdermal administration of a physiologically
active substance, said body comprising the combination of:
a) an impermeable backing;
b) a rate-controlling microporous membrane, said impermeable
backing and rate-controlling microporous membrane defining a
cavity therebetween;
c~ a liquid materlal, comprising said physiologically active
substance in liquld form, conflned between said lmpermeable
backing and said rate-controlling microporous membrane withln said
cavlty; and
d) a viscous flowable gel materlal confined between said
impermeable backing and said rate-controlling microporous membrane
within said cavity for substantially immobilizing said liquid
1 335256 26839-12
materlal,
said rate-controlling microporous membrane being permeable to
and in contact with said physiologically active substance and
wherein either
i) the rate-controlling microporous membrane is
hydrophilic and the material in sald cavity is hydrophobic, or
il) the rate-controlllng microporous membrane is
hydrophobic and said cavity contains a hydrophilic wettlng agent,
wherein said cavity contains Tea Tree Oil.
In yet another aspect the invention provides an
occlusive body for the transdermal administration of a
physiologically active substance, sald body comprising the
combination of:
a) an impermeable backlng;
b) a rate-controlllng permeable membrane capable of
chemically adsorbing and desorbing said physiologically active
substance, said rate-controlling permeable membrane and
impermeable backing defining a cavlty therebetween;
c) liquid l-naterial comprlslng said physiologically active
substance in liquid form confined between .said impermeable backing
and said rate-controlling permeable membrane within said cavity;
d) viscous flowable gel materlal conflned between sald
lmpermeable backlng and sald permeable membrane within said cavlty
for substantlally immobilizing said llquld material; and
e) means for attaching the body to the skin, which means does
not substantially affect release of said substance through sald
permeable membrane,
said membrane and liquid material being selected from the
~ .
~ .~
-
-
5c l 3 3 5 2 5 6 26839-12
group consistlng of:
1) the permeable mlcroporous membrane belng hydrophlllc
and the materlal ln sald cavlty belng hydrophoblc, and
11) the rate-controlllng permeable membrane belng
- hydrophoblc and sald cavlty contalnlng a hydrophlllc wettlng
agent,
whereby, ln use, passage of sald physlologlcally actlve
substance through sald permeable membrane ls rate-controlllng and
sald physlologlcally actlve substance 18 released from sald
permeable membrane at a rate that ls substantlally constant over a
perlod of hours.
In yet another aspect the lnventlon provldes an
occluslve body for the transdermal admlnlstratlon of a
physlologlcally actlve substance, sald occluslve body comprlslng
the comblnatlon of:
a) an lmpermeable backlng;
b) a rate-controlllng permeable membrane capable of
chemlcally adsorblng and desorblng sald physlologlcally actlve
substance, sald rate-controlllng permeable membrane and
lmpermeable backlng deflnlng a cavlty therebetween;
c) llquld materlal comprlslng sald physlologlcally actlve
substance ln llquld form conflned between sald lmpermeable backlng
and sald rate-controlllng mlcroporous membrane wlthln sald cavlty;
and
d) vlscous flowable gel materlal conflned between sald
lmpermeable backlng and sald permeable membrane wlthln sald cavlty
for substantlally lmmoblllzlng sald llquld materlal,
sald membrane and llquld materlal belng selected from the
,, . ~ j
-
-
Sd 1 335256 26839-12
group conslstlng of:
i) the permeable membrane being hydrophilic and the
material in said cavity being hydrophobic; and
ii) the rate-controlling permeable membrane being
hydrophobic and said cavity containing a hydrophllic wetting
agent,
wherein said cavity contains Tea Tree Oil.
In International Journal of Pharmaceutics, 48 ~1988) pp
247 to 254, (published after the priority date of the present
application) C. T. O'Nelll et al dlsclose in vitro arrangements
for transdermally administering timolol base from both hydrophobic
and hydrophilic reservoirs via varlous rate-controlling permeable
membranes, including both hydrophobic and hydrophilic membranes.
Halrless mouse skin is used as a model for human skln. In Flgure
3 of this paper a plot of drug penetration: time is given for a
reservoir containlng 4% sodIum car~oxymethyl-cellulose
(hydrophilic) in combination with three hydrophobic microporous
membranes (Celguard ~Reg. Trade-Mark) 2400, 2402 and 2412
respectively) and two non-
,,
r
~ _ 6 l 335256
*
microporous membranes, namely Silastic and EVA. Theselast two membranes are shown to result in a very low dose
rate but the three Celguard membranes each show a
substantially linear (zero'th order) dose rate over eight
hours and thereby support the teachings of the present
invention.
A preferred embodiment of the present invention is
in the form of an occlusive pad or patch for attachment
to the skin or to a buccal surface to administer a
physiologically active substance transdermally, said patch
comprising an impermeable backing and a membrane defining
therebetween a cavity containing a liquid, characterised
in that either (a) the membrane is of a hydrophobic
microporous polymer and that the liquid in the cavity is
hydrophilic or (b) the membrane is of a hydrophilic
microporous polymer and the material in the cavity is
hydrophobic, so that the active substance is released at a
rate that is substantially constant (e.g. + 20~ preferably
+ 10~ or less) over a period of hours (e.g. 5 hours).
Without wishing to be bound by any theory of the
present invention, it is believed that in use, the
physiologically active substance tends to concentrate near
the permeable membrane as a result of the mutual repulsion
between the hydrophilic (or hydrophobic) membrane and the
hydrophobic (or hydrophilic) material in the reservoir.
This concentration gradient is stabilised by the filler
material and ensures a steady rate of diffusion of the
active substance through the membrane, largely unaffected
by the concentration in the bulk material in the
reservoir.
If the active substance is a solid it may be
dissolved in a solvent which is miscible with the active
substance at least to a degree.
In preferred embodiments in which the membrane is
hydrophobic and the reservoir contents comprise a
hydrophilic wetting agent, the solvent should be
essentially hydrophobic to enable it to pass through the
membrane with the dissolved active substance, but should
tr~m~r~
-
_ 7 1 335256
be sufficiently hydrophilic to mix with the reservoir
material.
It will be noted that the active substance in the
occlusive body of the present invention should be in
liquid form in order to permeate through the membrane. In
some cases the active substance will be liquid at ambient
temperature and optionally may be diluted with a
physiologically compatible solvent in order to control its
rate of diffusion through the membrane. In other cases
the active substance will be solid at ambient temperatures
and a solvent will be essential in order to bring at least
some of the active substance into solution so that it can
diffuse throuqh the membrane. It will be noted that the
invention includes within its scope an occlusive body (as
specified above) in which the active sub~tance is
partially in solution (~ uid form") and partially in
solid form in contact with the solution.
In general, where a solvent is used to dilute the
active substance or to bring a normally solid active
substance into solution, the tendency of the active
substance to be released from solution and to flow through
the membrane will depend on the difference between the
solubility parameters ~ of the active substance and
solvent. The greater the difference in solubility
parameters ~ , the greater the rate of permeation through
the membrane. In J. Soc. Cosmet. Chem., 36 pp. 319 to 333
(September/October 1985) "Using solubility parameters in
cosmetics formulation" - C.D. Vaughan, the solubility
parameters of over 150 materials are listed, the
solubility parameter ~ being defined as follows:
= ~23.7 TB + 0.02 TB2 _ 2950 -1.98~ Ko
MW/density
where: TB = boiling point at one atmosphere (in Kelvin)
Ko = temperature (in Kelvin) at which the density
measurement is taken
MW = molecular weight (in grams), the units of
~ _ 8 1 335256
density being g/cm3, and sub~ect to the provisions that if
the substance is an alcohol then a value of 1.4 is added
to the value obtained from the above formula, if the
substance is an ester then a value of 0.6 is added to the
5 value obtained from the above formula and if the substance
is a ketone having a boiling point above 100C then a
value of 0.5 is added to the value obtained from the above
formula.
The above definition is based upon a widely accepted
10 formula due to J.H. Hildebrand (JACS 38 pp 1442 - 1473
(1916) and is embodied in a computer program for
calculating solubility parameters which is given in the
above-mentioned article by Vaughan. In the present
application, the term n solubility parameter" is to be
15 understood to mean the term in accordance with the above
definition (including the provisos) but if the definition
is not applicable (e.g. because the boiling point of the
substance cannot be measured owing to decomposition), then
any other generally accepted definition may be employed,
20 such as those given on pp 326 and 327 of the above-
mentioned article by Vaughan, for example.
The solubility parameter (as defined above) of a
solvent may be a tentative guide to its suitability for
use with a given active substance whose solubility
25 parameter is known. In general, suitable solventR will
tend to be somewhat hydrophobic, if a hydrophobic membrane
i8 used, or somewhat hydrophilic in the case that a
hydrophilic membrane is used, and will typically have a
solubility parameter J~ of 8 to 11. However, it should
30 be noted that some solvents which do not have a solubility
parameter within this range may be suitable and that other
solvents which do have a solubility parameter within this
range may be unsuitable.
A preferred embodiment of the invention is described
35 below by way of the following non-limiting Example, and
with reference to Figure 1 of the accompanying drawings.
In the accompanying drawings:
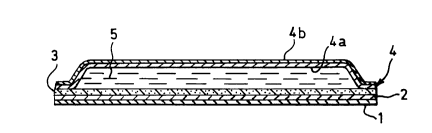
Figure 1 i8 a schematic cross-section of the patch
-
- 9
1 335256
of Example l; and
Figures 2 to 7 are graphs of release against time
for 200 3-minute measurement cycles.
Example 1
5A layer of Dow Corning X7-2910 BIO PSA (Registered
Trade Mark) pressure-sensitive adhesive of 35 um thickness
was formed on a 75 um thick sheet of Akrosil BIO RELEASE
(Registered Trade Mark) release liner by applying a single
coating of a 21 wt% solution in freon of the adhesive and
allowing the coating to dry.
A 3M MSP 80487 hydrophobic microporous polyethylene
membrane of Gurley No. 2856 seconds and thickness 4S.7 um
(1.8 mils) was then laminated onto the coated face of the
release liner.
15A nicotine formulation was made by mi xi ng 4.95 g of
nicotine base (supplied by BDH Ltd) with 0.05 g of Tea
Tree Oil (supplied by De Monchy Ltd). A gel was made
comprising 5 wt% "high substitution" grade methyl
cellulose (supplied by BDH Ltd) in water. All the
nicotine base/Tea Tree Oil mixture was then mixed with 95
ml of the methyl cellulose gel. The resulting formulation
remained in the form of a gel.
Approximately 200 mg of the above nicotine
formulation was then applied in a "blob" to the
microporous polyethylene membrane of the laminate. A
sheet of 3M Scotchpak (Registered Trade Mark) aluminised
polyester backing material (having a depression formed
therein to accommodate the nicotine formulation) was
applied to the microporous membrane of the laminate and
heat sealed thereto around the periphery of the depression
to enclose the nicotine formulation.
The resulting patch had dimensions of approximately
50 mm x 40 mm in the plane of the laminate and contained
approximately 10 mg of nicotine base.
35The patch of Example 1 is shown in Figure 1 which is
a schematic cross-section.
The microprous hydrophobic membrane 3 is shown heat-
sealed around the periphery of its upper face to the
` l--
~ _ lo 1 335256
polyester face 4a of a backing sheet 4, which is provided
on its outer face with an aluminised layer 4b. Nicotine
formulation 5 in the form of a gel is enclosed within a
cloæed body formed by membrane 3 and backing sheet 4 and
tends to permeate through membrane 3 and a layer 2 of
pressure-sensitive adhesive which is coated on the lower
face of the membrane.
Prior to use, such permeation is prevented by
release liner 1. Release liner 1 may be stripped from the
adhesive layer 2 immediately prior to use and the patch
may be adhered to the skin (e.g. of the arm) of a user by
the exposed pressure-sensitive adhesive.
The design for other patches using powdered drugs
such as paracetamol, ephedrine, or fentanyl may be as for
nicotine, except that the drug in an acceptable solvent
may be substituted for nicotine. The adhesive should be
selected to ensure that it does not hinder the passage of
the active ingredients. The solvent should be
sufficiently hydrophobic to pass through the membrane.
Nitroglycerine and other liquid drugs may be
substituted directly for nicotine. Again, it may be
necessary to alter the adhesive specification to ensure
compatibility, and to use a membrane having a different
permeability to ensure an appropriate dosage rate.
The invention is further illustrated by way of
example only in the attached Figures 2-7, which are graphs
of release against time for 200 3-minute measurement
cycles. Figure 2 shows the release of nicotine from a
methyl cellulose reservoir through a microporous
hydrophobic polypropylene membrane, covered with adhesive
into an aqueous buffer (pH 7.4). A nicotine release rate
of about 1.1 mg per square centimetre per hour was
maintained for about 6 hours. Figure 3 shows the same
system without the methyl cellulose and it is observed
that the release of nicotine is rapid. In Figure 4 the
methyl cellulose has been replaced by sodium lauryl
sulphate, and linear nicotine release over about 4 hours
was observed. Figure 5 shows the effect of cetrimide in
-
-- _ 11 1 335256
place of the methyl cellulose, and again a linear nicotine
release over about 4 hours was observed. In Figure 6, the
effect of using Tween 20 (Registered Trade Mark) in place
of methyl cellulose is shown, and release is again linear
and is slower and more sustained than with the cationic
or anionic surfactants. Figure 7 shows the effect of using
Celgard 3401 (Registered Trade Mark) which is a
hydrophilic microporous polypropylene, and it is seen that
nicotine release is exponential, not linear.
In another aspect, the invention provides an
occlusive body for the transdermal administration of a
physiologically active substance, the occlusive body
comprising a reservoir containing said active substance
preferably in liquid form, a wall of the reservoir being
permeable to said active substance and the reservoir
additionally cont~in;ng Tea Tree Oil or a major component
thereof.
Typically the dosage rate (i.e. the rate of passage
of the active substance through the membrane with time)
will vary by + 10% or less (preferably + 5% or less) until
at least 25% (preferably at least 50%) of the active
substance originally in the reservoir has passed through
the membrane.
It is envisaged that the filler material may be a
gel-forming substance which transforms the reservoir
contents to a gel, or it may be a porous material which
absorbs the reservoir contents.
The permeable membrane may, for example, be
microporous, or it may be capable of chemically adsorbing
and desorbing the active substance.
The àctive material to be delivered by the occlusive
body of the invention may be nicotine, which has been used
in the experiments described below. But it is envisaged
that the occlusive patch may be used to deliver other
pharmacologically active substances in an aqueous medium
-which may contain a water- and oil-miscible solvent for
the drug such as ethanol, benzyl alcohol, hexanol, butanol
or alkoxy alkanols of up to C8 (MW = 147) for example.
-
_ 12 l 335256
Drugs which it may be possible to deliver using the
occlusive body include methacin in a quinoline or pyridine
buffer, beta-ionone, fentanyl and pethidine or ephidrine
in an aqueous medium containing a suitable drug solvent.
The solvent may also be an enhancer, or an enhancer having
the required miscibility may be added. Such enhancers may
include oleic acid or other pharmaceutically acceptable
material.
The membrane may optionally be composed of a multi-
ply material. Only the inner layer of such a membraneneeds to be hydrophobic (in the case that the reservoir
contents are hydrophilic) or hydrophilic (in the case that
the reservoir contents are hydrophobic).
It is believed that the greater the difference in
wetting properties between the reservoir material and the
membrane (or the innermost layer of the membrane if
a multi-ply membrane is used), the wider the range of
useful solvents and the more linear the release of the
drug. Accordingly it is desirable to employ either a
strongly hydrophobic or a strongly hydrophilic microporous
membrane, in conjunction with, strongly hydrophilic
reservoir contents and strongly hydrophobic reservoir
contents respectively.
The occlusive body may for example have an outer
layer of an impervious material such as a layered
aluminium foil to prevent seepage or leaching of the
contents of the reservoir, which is further contained by
the membrane as indicated above. The reservoir side of the
membrane may be faced with an area-reducing mesh formed,
for example, by a non-woven fabric or by a perforated
impermeable material such as aluminium foil. Suitable
membrane materials are hydrophobic and microporous, for
example Celgard (Registered Trade Mark) 2500 polypropylene
of thickness 0.025 mm (1 mil) and pore size 0.4-0.04
microns. The face of the membrane distant from the
reservoir is coated with a layer of adhesive of typical
thickness about 30 micrometers, which may be any suitable
dermatologically acceptable pressure sensitive adhesive
-
13 l 335256
that does not react chemically with the reservoir contents
or prevent passage of the active material through the
membrane from being rate-controlling. Thus the active
material should pass reasonably rapidly through the
S adhesive layer, though some retardation may be acceptable
in practice. The adhesive may suitably be an elastomeric
silicone polymer. A protective sheet of release coated
paper or other material will usually cover the adhesive
layer until the pad is to be used.
For use with aqueous media in the reservoir, the
membrane is preferably hydrophobic, in which case the
reservoir contents may be made hydrophilic by addition of
a surface active agent, which may be an anionic surface
active agent e.g. sodium lauryl sulphonate, a cationic
lS surface active agent e.g. cetrimide or a non-ionic surface
active agent such as Tween 20 (Registered Trade Mark).
It is a further subsidiary or preferred object of
the invention to provide an occlusive patch containing a
physiologically active substance in a reservoir, in which
a gel structure of the contents reduces abrupt absorption
of the active substance in the event of sudden failure of
the reservoir and release of the contents onto the skin.
In order to solve that subsidiary problem, the viscosity
of the reservoir contents is desirably high enough that
2S they are resistant to spreading in the event of reservoir
puncture, which is important from the standpoint of
safety. Methyl cellulose in water is an advantageous
material to use because it can perform the dual functions
of surface active agent (to enhance the hydrophilicity of
the reservoir contents) and viscosity modifier or gel
former. When used in association with nicotine, a methyl
cellulose content of about S-6% by weight, is
satisfactory. The proportion of nicotine in the reservoir
material may be less than 2S% by weight of the reservoir
3S contents and desirably from 2 to 10% by weight, preferably
4 to 6%. With this relatively dilute nicotine
concentration, the dose present in each reservoir is more
easily controllable, the product is easier to manufacture,
. -
_ 14 1 335256
and to change to meet the requirements of different patchdesigns.
The nicotine or other pharmacologically active
substance may for example be mixed with up to 2%
(typically about 1% by weight) of oil of Melaleuca
Alterniifolia (Tea Tree Oil) or another bactericide before
being introduced into the gel material to be filled into
the reservoir. The Tea Tree oil may also be mixed with an
adhesive to form a layer covering a face of the membrane
remote from the reservoir as described below. The major
constituents of Tea Tree Oil are 1-terpinen-401 and
terpinene with minor amounts of 1,8 cineole and ~-cymene,
and its properties, together with those of other
Australian essential oils, are described by M.F. Beylier,
Perfumer & Flavorist, 4, 23 (April/May 1979). Tea Tree Oil
may be substituted by other essential oils that possess
antibacterial qualities.
In this invention, the membrane may for example be a
hydrophobic microporous material such as hydrophobic
microporous polypropylene or polyethylene. The reservoir
contents are preferably a wetting agent water based gel
formed, for example, by methyl cellulose. It has been
found experimentally in vitro that the combination of a
hydrophobic microporous polypropylene membrane and a
water-based gel containing about 5% of methyl cellulose
gives a linear or zero order release of other products
such as nicotine, whilst retaining water and solids. The
existence of the desirable zero-order characteristics is
believed to be at least partially independent of the area
of the reservoir. Reservoir contents having about 5~ by
weight nicotine in a high viscosity water-based medium
(e.g. a medium of 5% methyl cellulose content) have given
a linear release of nicotine with time for about 25-50% of
the capacity for nicotine, and a barely discernible curve
of release up to 80% of capacity. In tests carried out in
vitro a steady nicotine loss through the membrane of about
1.5 mg per square centimetre per hour for a period of
typically about 8 hours has been measured, followed by a
1 335256
810w progressive reduction in release rate up to 15 hours.
The desirable linear release properties are retained when
a layer of silicone adhesive such as is desirably used in
an occlusive bandage is applied to the outer face of the
membrane.
Further preferred features are defined in the
dependent claims.
Similar desirable properties are obtained when the
methyl cellulose (high viscosity - BDH 29779) is replaced
by high viscosity VEGUM (R.T. Vanderbilt PLC) which is
another water-based gel that also acts as a wetting agent.
However, linear release properties are not obtained when
the gelling agent in the reservoir is changed to Carbopol
which is not a hydrophilic wetting agent, nor are they
obtained when the membrane is changed to a hydrophilic
grade of microporous polypropylene.
In order to establish that the linear release of
nicotine is not caused by gravity but is a property of the
membrane that is "chromatographic", a sample was enclosed
in a blank and immersed sideways in a buffer solution.
Although there was a risk of a non-meaningful result being
obtained as a result of back-diffusion of the buffer
through the membrane into the reservoir, a straight line
release with time was in fact obtained.
In order to establish that the gel material within
the reservoir was not passing through the membrane with
the nicotine, a mixture of nicotine and methyl cellulose
gel containing 5~ by weight of nicotine was assayed for
nicotine content which was found to be about 5% in a 10 mg
sample. After about 2 mg of nicotine had released into an
aqueous medium in an in vitro experiment, the contents of
the reservoir were sampled and assayed. The reduction in
the nicotine-gel ratio corresponded to the amount of
nicotine released from the reservoir and was inconsistent
with the simultaneous release of other materials including
water therefrom. It is believed that the non-passage of
the gel material through the pores of the microporous
membrane results from the hydrophilic nature of the gel
w
-
16 l 335256
material combined with the hydrophobic nature of the
membrane.
It will normally be expedient to dissolve the
physiologically active substance in an appropriate
pharmaceutically acceptable vehicle, which will carry the
active substance through the reservoir membrane.
Furthermore, it will normally be convenient to employ a
hydrophobic membrane and hydrophilic reservoir contents.
Typically, the most useful hydrophilic material will be a
gel-forming surface active agent such as methyl cellulose
mixed with water. This provides the additional function
of immobilising the reservoir contents as noted above.
The rate of delivery of the active substance through
the membrane into the blood stream of the subject can be
varied as follows:
(i) by varying the surface area, the thickness and
the composition of the reservoir membrane;
(ii) by varying the weight ratio of active
substance: vehicle;
20(iii) by varying the hydrophilicity of the reservoir
contents.
Thus the dosage rate can be varied over a wide range
by suitable adjustment of various parameters of the
occlusive body, whilst maint~ining a substantially uniform
dosage rate. However, in order to mi ~ i ri se variations in
dosage rate between different patients owing to variations
in their skin resistance, the permeability of the
reservoir membrane is preferably slightly less than the
permeability of the least permeable skin likely to be
encountered in the use of the invention and may for
example be 75% to 90% of the permeability of the most
resistant skin.