Note: Descriptions are shown in the official language in which they were submitted.
-2- 1 335265
~ The present invention is concerned with a process
,it for the production of a specifically bindable protein
substance bound to an insoluble carrier material,
~9 ; especially for use in a heterogenous analysis process
according to the immunoassay principle.
For the determination of a specifically bindable
substance, there are frequently used processes according
to the immunoassay principle. One of the components of
a pair of substances specifically bindable with one
another is thereby reacted with the receptor specific
for it, which is labelled in known manner. The
conjugate of these two substances can then also be
reacted with a receptor which is specific for the
conjugate or one of the two parts of the conjugate.
There are many variations for these immunological
processes. It is thereby advantageous when one of the
receptors is present bound to a solid phase. This
simplifies the separation of reaction components
present bound and non-bound. For the determination of
the specifically bindable substance, there is then
measured the amount of labelled reaction component
bound ~o the solid phase or of the labelled reaction
component present in the solution and placed in known
manner in relationship to the amount of reaction
component to be determined.
As solid phases in the case of the ~immunological
processes, there are usually used synthetic resin test
.. ~
~'
_3_ l 335265
tubes or microtitre plates, on the inner surface of
which is fixed the reaction component, or spheres on
the outer surface of which is fixed the reaction
component. These synthetic resin test tubes, microtitre
plates or spheres usually consist of a relatively inert
synthetic resin material so that the binding of the
reaction component gives rise to difficulties. Further-
more, the binding of the specific reaction componen~ to
the particular surface must take place in such a way
that it does not lose the ability for the specific
binding of the substance specifically bindable therewith.
For this reason, the binding of the reaction component
to the solid phase is usually adsorptive. Therefore,
it has already been suggested to bring about the fixing
of the reaction component to the solid phase via a
coupling agent which brings about the binding. Care
must ~hereby also be taken that the binding of the
reaction component to the coupling agent does not
destroy the specifically reacting region of the molecule
or that the reaction component is bound in such a
manner that its reactive position away from the solid
phase faces the binding component. Furthermore, it is
suggested in Federal Republic of Germany Patent
Specification No. 25 33 701, in order to achieve a better
binding, to cross-link the individual immunologically
active proteins and then to adsorb on polystyrene
spheres. A further possibility which is mentioned in
l 335265
--4--
this literature reference is simultaneously to cross-link
an inert protein with the protein with immunological
properties so that there results a cross-linked product
of inert and active protein which is again adsorbed on
to polystyrene spheres. However, depending upon the
chosen reaction conditions, this type of cross-linking
results in varying and non-reproducible cross-linkings
with varying proportions of non-cross-linked as well as
insolubilised protein. Furthermore, due to the varying
degree of cross-linking, products result with differing
binding properties. A similar process is described in
European Patent Specification No. 0,122,209 which also
displays the same disadvantages. Thus, all of these
known processes are still not satisfactory, still do not
lead to an optimum adhesion of the specifically bindable
substance and are of little suitability for the
reproducible production of coated solid phases.
In addition, a further problem is that the anti-
body bound to the solid phase must, as a rule, be
different for various tests. Therefore, for each test,
a specific coated solid phase must be made available,
which is very laborious. Since, furthermore, it is not
ensured that each antibody immobilised in known manner
remains bindable, because non-specific bindings often
also take place, and since, furthermore, the dissolving
off rate in the case of the previously known processes
is very high, the antibody must be used in great excess.
.
.
~5~ 1 335265
.) Nevertheless, the number of binding places is limited.
This results in an increased tendency to disturbance.
~,
This is disadvantageous since antibodies are difficult
and expensive to produce.
Therefore, it is an object of the present
invention to provide a process which reproducibly
improves the adhesion of the specifically bindable
substance to the solid phase and, furthermore, to
provide a process with which a solid phase matrix can
be produced which can be used universally for all
immunoassays. Since, in the case of many immunological
processes, working is carried out with the addition of
detergents in order to avoid turbidities and non-
specific bindings, it is also an object of the present
invention to improve the adhesion to such an extent
that, even in the presence of detergents, the bound,
specifically bindable substance is not dissolved off.
Thus, according to the present invention, there
is provided a process for the production of a
specifically bindable protein substance bound to an
insoluble carrier material. especially for use in a
heterogenous analysis process according to the immuno-
assay principle, wherein a protein with a molecular
weight of more than about 200,000, which has a plurality
of components Pl of a specific binding pair, is first
bound to an insoluble carrier material and subsequently
cross-linked with a polymer which contains or consists
-6- 1 335265
of a plurality of the other component P2 of the
specific binding pair via a specific binding of Pl with
P2, the polymer thereby having not only binding points
for Pl but also for an immunological complex to be
determined.
With this process, a solid phase matrix can be
produced which can be used for all kinds of immunoassays,
for example sandwich tests or competitive tests in a one-
step or two-step process. For the sandwich test in the
one-step process, the polymer of the component P2 can,
for example, provide the binding points on to which the
complex to be determined is then immobilised. During
the determination process, a sample which contains the
substance to be determined is reacted with a labelled
receptor, as well as an unlabelled receptor which is
bindable with the polymer. The complex formed from the
substance to be determined, labelled receptor and
receptor with binding points for the polymer then binds,
on the basis of the specific bindability, to the polymer
so that, in ~his way, the whole complex is immobilised.
After separa~ion of the phases, the labelling can then
be measured in one of the two phases.
If the sandwich test is carried out in the two-
step process, a solid phase matrix can be used in which
an unlabelled receptor is bound in the polymer. The
sample and the labelled receptor are then incubated in
the presence of this solid phase matrix. After
.
1 335265
--7--
separation of the phases, the labelling bound to the
solid phase can then be measured.
In the case of carrying out a competitive ~est,
sample and labelled sample analogue then compete for an
unlabelled receptor. For the test variant of a one-
step process, in this case a polymer is used to which
the unlabelled receptor is bound. For ~he other variant
of the two-step process, the unlabelled receptor is
bound during the test in competition with the labelled
receptor so that, in this case, a polymer is then used
which has binding points for these receptors.
As protein, it is preferred to use one which is
more hydrophobic than the components Pl and P2. Soluble
proteins are especially preferred with a molecular
15 weight of more than about 200,000 to about 20,000,000
and which are possibly obtained from proteins with a
molecular weight of 10,000 to 700,000.
In an especially preferred embodiment, a
conjugate is first prepared from a soluble protein
20 with a molecular weight above about 200,000 and a
component Pl of a specifically binding pair.
The binding of the component Pl to the protein
takes place in known manner, appropriate coupling
methods being described, for example, by Ishikawa in
25 J. Immunoassay, 4, 209-327/1983. Either functional
groups of the component Pl which are suitable for a
binding with the protein are thereby used or, if
-
-8- ~ 335265
. suitable functional groups are not present, these are
introduced into the Pl molecule. Thus, for example,
in the case of using biotin as Pl, the N-hydroxy-
succinimidyl derivative thereof can be bound to the
protein by reaction with the amino groups of the
protein. Other suitable forms of derivatisation are
well known and do not need to be explained here.
Appropriate binding components which can be used
as Pl and P2 include, for example, biotin-avidin,
biotin-streptavidin, antigen-antibody, hapten-antibody
and protein A-immune-~-globulin. By antibodies there
are thereby to be understood monoclonal and polyclonal
complete antibodies and antibody fragments. If
protein A is used as Pl or P2, then in the immunoassay
only the receptor which is to be bound to the solid
phase should be a complete antibody, whereas as
labelled receptor there should be used a Fab or F(ab')2
fragment in order not to bring about a non-specific
binding of the labelled receptor with the solid phase
which would lead to a falsification of the result.
For the choice of soluble proteins suitable
according to the present invention, the molecular
weight, as well as the hydrophobicity must be determined
in comparison with the corresponding value for the
specifically bindable substances. The molecular weight
is determined by methods which are known in the art.
A comparison of the hydrophobicity between soluble
-9- l 335265
- proteins and specifically bindable substances can also
take place according to well-known methods. Appropriate
methods include, for example, a comparison of
- the fluorescence extinction after binding to coloured
material (Biochem. Biophys. Acta, 624, 13-20/1980);
- the elution behaviour in the case of hydrophobic
chromatography (Biochem. Biophys. Acta, 576, 269-279/
1979);
- the surface ~ension (Biochem. Biophys. Acta, 670,
64-73/1981);
- the retention times in the case of hydrophobic inter-
action chromatography (HIC) (Angew. Chemie, 98,
530-548/1986; J. Chromat., 296, 107-114/1984; Anal.
Biochem., 137, 464-472/1984).
A comparison of the hydrophobicity of substances
which are suitable according to the present invention
is to be found in Sep. Sci. Technol., 14, 305-317/1979.
According to this, the hydrophobicity increases, for
example, in the following sequence:
a2-macroglobulin (M.W. 820,000)
bovine serum albumin/human serum albumin (M.W. 70,000)
egg albumin
a2HS-glycoprotein (M.W. 49,000)
~lc/~lA~glbulin
immunoglobulin (M.W. 150,000)
transferrin (M.W. 90,000).
Thus, if an immunoglobulin is used as specifically
3 7~
.
-lo- 1 335265
~- bindable substance, then, for example, human serum
albumin or a2HS-glycoprotein are not suitable as soluble
proteins in the meaning of the present invention without
further pre-treatment.
Both proteins must here be subjected not only to a
hydrophobing but also to an increasing of the molecular
weight. In the case of transferrin, a cross-linking
suffices and in the case of a2-macroglobulin a hydro-
phobing is sufficient.
Proteins which are suitable for coupling with
immunoglobulin as specific bindable substance without
pre-treatment include, for example, ~-lipoproteins
(M.W. about 3.2 mio) and a2-lipoprotein (M.W. about 5 to
20 mio).
The hydrophobing can take place, for example by
the use of heat, treatment with acids, denaturing agents
and/or chaotropic ions and/or by chemical coupling with
a hydrophobic compound.
The increasing of the molecular weight can take
place, for example, by the use of heat, treatment with
acids, denaturing agents and/or chaotropic ions and/or
by cross-linking with a bi- or polyfunctional protein
reagent.
A protein which is not sufficiently hydrophobic
:75 or the molecular weight of which is not sufficiently
high is treated until a protein polymer is obtained
with a molecular weight of 200,000 or more. It is
-11- 1 335265
especially preferred to use a protein polymer which has
a molecular weight of 500,000 to 20 mio.
If the protein is to be cross-linked, a hydro-
phobing can take place before, during or after the cross-
linking. However, the hydrophobing cannot be carriedout in the presence of the specifically bindable sub-
stance when the specifically bindable substance is a
protein.
For hydrophobing by heating, there is usually
employed a temperature of from 40 to 95C. over a period
of time of from 1 minute to 10 hours, such as is
described, for example, in Biochem. Biophys. Acta, 624,
13-20/1980.
For the treatment with acids, there can be used,
for example, acetic acid, propionic acid, lactic acid
or hydrochloric acid. Usual concentrations are from
1 to 100 mMole/litre in the case of periods of action
of from 10 minutes to 16 hours.
For the treatment with chaotropic ions, there can
be used, for example, thiocyanates, iodides, fluorides,
bromides, perchlorates and sulphates. As denaturing
agents, there can be used, for example, guanidine hydro-
chloride or urea. Concentrations of from 10 mMole/litre
to 6 mole/litre are here usually employed.
For derivatisation with hydrophobic compounds,
there are preferably used soluble fatty acids, lipoids
in low or high molecular weight form, as well as
-12- ~ 33~5
` synthetic polymers, such as polypropylene glycol or
soluble co-polymers of polystyrene. The derivatisation
takes place according to well-known methods.
The cross-linking via bi- or polyfunctional
S compounds is carried out with known protein binding
agents. These are compounds which mostly carry two
functional groups which can be the same or different
and which react via these functional groups with
functional groups of proteins. Compounds are preferably
used which consist of an alkyl chain on the ends of which
are present succinimide, maleinimide and/or aldehyde
groups.
The protein is then cross-linked in known manner
with the bi- or polyfunctional compound by reacting the
soluble protein and the bi- or polyfunctional compound.
For the hydrophobing and/or cross-linking, there
are preferably used proteins with a molecular weight of
from lO,000 to 700,000, bovine serum albumin, lipase
or immune-y-globulin being used especially preferably. -
The protein or protein conjugate prepared in this
way is then bound to an insoluble carrier material. The
binding thereby takes place via the protein and is, as
a rule, adsorptive. As carrier materials, there can be
used the solid phases which are normally employed, for
~5 example Luran, glass, titanium dioxide, polystyrene,Y-activated polystyrene, polystyrene-acrylonitrile co-
polymers, paper and/or polyolefins. Before the further
* trade-mark
-13- l 33~6~
` treatment, the carrier material can be physically or
chemically pre-treated. Thus, for example, a synthetic
resin surface can be pre-swollen or activated in some
other known way. As a rule, the carrier material is
present in the form of test tubes, microtitre plates or
spheroids but other forms are, however, also possible.
Subsequently, the protein having a plurality of
components Pl which is bound to the carrier material is
cross-linked with a polymer. This polymer has a
plurality of components P2. It can consist either only
of P2 or of a mixture of P2 and other components. The
polymer has not only binding points for Pl which are
provided by P2 but, furthermore, also binding points
for an immunological complex to be determined which, in
the following, is referred to as receptor. As
receptors, there are thereby used specifically bindable
substances, especially either specifically bindable
complete antibodies which can be polyclonal or mono-
clonal, antibody fragments thereof and conjugates of
antibodies or antibody fragments with haptens or
antigens, as well as haptens and antigens. The binding
points of the receptor can either also be provided by
P2 or by another component of the polymer.
The individual components P2 can be bound with
one another via homo- or hetero-, bi- or polyvalent
linkers. The cross-linking is preferably carried out
with bivalent linkers since these make possible an
.
-14- l 335265
easier control of the degree of polymerisation. How-
ever, polyvalent linkers can also be used. As linkers,
there can be used those compounds which have reactive
groups which are able to react in aqueous solution
with the functional groups of the specifically bindable
components with the formation of a covalent bond. A
large number of bifunctional and polyfunctional linkers
suitable for this purpose is known. Typical examples
for homo- or heterobifunctional and trifunctional
linkers which are well suited in the scope of the
present invention are set out in the following Table 1:
-15- 1 335265
` Table
abbreviation chemical designation
SPDP N-succinimidyl 3-(2-pyridylthio)-
propionate
5 EADB ethyl 4-azidophenyl-1,4-dithiobutyrimid-
ate hydrochloride
FNPA 4-fluoro-3-nitrophenylazide
HSAB N-hydroxysuccinimidyl 4-azidobenzoate
MABI methyl 4-azidobenzoimidate hydrochloride
10 MBS -maleimidobenzoyl N-hydroxysuccinimide
ester
NHS-ASA N-hydroxysuccinimidyl 4-azidosalicylic
acid
MHS maleimidohexanoyl N-hydroxysuccinimide
ester
:15 PNP-DTP p-nitrophenyl 2-diazo-3,3,3-trifluoro-
propionate
SADP N-succinimidyl (4-azidophenyl)-1,3'-
dithiopropionate
SAND sulphosuccinimidyl 2-(m-azido-o-nitro-
benzamido)-ethyl 1,3'-dithiopropionate
SANPAH N-succinimidyl 6-(4'-azido-2'-nitro-
phenylamino)-hexanoate
SASD sulphosuccinimidyl 2-(p-azidosalicyl-
amido)-ethyl-1,3'-dithiopropionate
25 SIAB N-succinimidyl (4-iodoacetyl)-amino-
benzoate
SMCC succinimidyl 4-(N-maleinimidoethyl)-
cyclohexane-l-carboxylate
SMPB succinimidyl 4-(p-maleimidophenyl)-
butyrate
DSS disuccinimidyl suberate
DMS dimethyl suberimidate
Traut's 2-iminothiolane + 2,4,6-trichloro-s-
reagent triazine
35 SAMBA s'-acetylmercaptosuccinic anhydride
1 335265
-16-
For carrying out the cross-linking, a solution of
the component P2 is mixed with the linker molecules
under conditions which lead directly to the cross-
linking. In this case, the extent of the cross-linking
is controlled by the amount of linker added.
In a further preferred embodiment, the binding
component P2 is cross-linked with appropriate bindable
components which are inert with regard to Pl and to the
complex to be determined. For this purpose, there can
]0 be used, for example, a soluble protein such as was
defined hereinbefore, especially bovine serum albumin
or human serum albumin. ~
The heterogenous cross-linking can, for exarnple,
take place in such a manner that not only the protein
material used as "inert component" but also the
specifically bindable component P2 are provided with an
activated bindable group and subsequently reacted. In
this way, there is obtained a cross-linked polymer which
contains a sufficient number of bindable components P2.
The binding of the component P2 to the protein must, of
course, thereby take place in such a manner that neither
the specific bindability with the component Pl is
impaired nor is the specific binding point for the
complex to be determined blocked.
In a further preferred embodiment of the process
according to the present invention, the binding
component P2 is cross-linked with other components which
-17- 1 335265
have specific binding points for the receptor. This
embodiment is specifically used when P2 only has one
specific binding point for the binding with Pl. The
other component then provides the bindin~ point for
the receptor to be bound. As other components, there
can be used substances which have specific binding
points, especially componen~s of a specifically-binding
pair such as are defined hereinbefore.
The two specifically binding components Pl and P2
and possibly the other components are preferably used
in such a ratio that P2 and possibly the other components
are present in large excess in comparison with their
binding components. In this way, very many binding
points are provided for the complex to be immobilised.
On the other hand, the binding to the carrier material
is very stable since, even in the case of the dissolving
off of some components Pl from the protein, the binding
by the cross-linking of the second component is still
present.
~0 The solid phase matrix produced according to the
present invention is used for determinations according
to the immunoassay principle. It can be used not only
for the variants of the sandwich test but also for the
variants of the competitive test. There are many
variants for these determinations. For example, the
sample which contains the substance to be determined
can thereby be reacted with a receptor which carries a
1 335265
-18-
, labelling and at least one further receptor to which
is bound a substance specifically bindable with the
polymer of the specifically binding component P2. The
complex to be determined, preferably one of the bound
receptors, thus has a point which is bindable with the
polymer. This binding point can be identical to or
different from that of Pl. This reaction can already
take place in a test tube coated with the matrix
according to the present invention or in an appropriately
coated microtitre plate. However, it is also possible
to add the solid phase matrix, for example in the form
of spheroids, only after the incubation. In the case
of contact with the solid phase matrix, the complex of
substance to be bound, labelled receptor and receptor
conjugated with specifically binding substance then
binds to the polymer which, in turn, is bound via Pl
and the protein to the carrier. In this way, the complex
to be determined can be immobilised.
The present invention also provides a solid phase
matrlx, wherein it consists of an insoluble carrier
material to which is bound a protein with a molecular
weight of over about 200,000 which has a plurality of
components Pl of a specific binding pair and the
protein is cross-linked with a polymer of a plurality
of the other components P2 of the specific binding
pair, the polymer having not only binding points for
Pl but also for an immunological complex to be
determined.
-19- 1 33526~
For carrying out immunoassays, a solid phase
matrix is especially preferred in which the protein is
a conjugate of a soluble protein with a molecular
weight of from 200,000 to 20,000,000 and of a plurality
of biotin, avidin or strep~avidin molecules. Further-
more, a solid phase matrix is preferably used in which
the polymer consists of biotin, avidin or streptavidin
molecules. The polymer is preferably formed from
biotin, avidin or streptavidin and a hydrophobed
protein.
According to the present invention, a universal
matrix is provided, as well as a process for the
production thereof, which can be used in all known
immunoassays.
This solid phase matrix is independent of the
nature of the receptors used. Furthermore, it is
characterised by a high stability.
The following Examples are given for the purpose
of illustrating the present invention, with reference
to the accompanying drawings, in which:
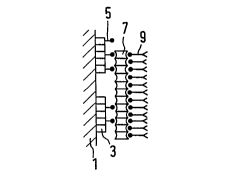
Fig. 1 shows two embodiments of the solid phase matrix
according to the present invention:
a) shows one embodiment of the solid phase matrix
according to the present invention. On to a
solid phase 1 is adsorbed a conjugate of a
protein 3 with one component 5 of a specific
binding pair Pl. On 5 is bound a polymer 7
1 335265
-20-
which consists of the other component P2 of the
specific binding pair. The polymer consists of
homogeneous cross-linked identical molecules.
In the case of carrying out an immunoassay,
antibodies 9, which are conjugated with Pl, bind
to this polymer.
b) Shows a further embodiment of the solid phase
matrix according to the present invention. Here,
on a solid phase 1 is adsorbed a conjugate of a
protein 3 and a component 5 of a specific binding
pair Pl. On 5 is bound a polymer 7. This polymer
consists of the other component of the specific
binding pair P2, as well as a receptor R. In the
carrying out of an immunoassay, on this polymer
bind antibodies 9 which are conjugated with a
substance bindable with the receptor R.
Fig. 2 shows a diagram in which are plotted calibration
curves for different coated test tubes. The
curves of this diagram have the following meanings:
X : Two-component matrix consisting of thermo-
BSA-biotin and homogeneously cross-linked
streptavidin; Luran test tubes;
: Two-component matrix consisting of thermo-
BSA-biotin and thermo-BSA-streptavidin; Luran
test tubes
+ : BSA-streptavidin on y-irradiated polystyrene
test tubes
1 33526~
-21-
: Homogeneously cross-linked streptavidin on
irradiated polystyrene test tubes
: Homogeneously cross-linked streptavidin on
Luran test tubes.
The individual curves were thereby obtained with
the following test tubes:
X : Luran test tubes coated with a two-component matrix
consisting of thermo-BSA-biotin and homogeneously
cross-linked streptavidin
0 ~: Luran test tubes coated with a two-component matrix
consisting of thermo-BSA-biotin and thermo-BSA-
streptavidin
+ : polystyrene test tubes Y-irradiated and coated with
BSA-streptavidin
:15 0 : polystyrene test tubes ~-irradiated and coated wi~h
homogeneously cross-linked streptavidin
: Luran test tubes coated with homogeneously cross-
linked strep~avidin.
Fig. 3 shows one embodiment of the solid phase matrix
according to the present invention. In this
embodiment, on a carrier material 1 is adsorpt-
ively bound a first component 3 which has a
plurality o components of a specific binding
pair. A second cornponent 5, which contains a
plurality of the other component o the specific
binding system, is bound via the specific bindings
7 of the two components to the irst component 3,
B
~. ..~
-22- ~ 3
cross-linked polymers of considerable size
thereby resulting on the carrier material 1.
Example 1.
la) Production of thermo-bovine serum albumin-biotin.
1 g. Bovine serum albumin (BSA) is dissolved in
50 ml. 50 mM potassium phosphate (pH 7.8). While
stirring, 1.9 ml. D-biotinyl-Y-aminocapronic acid N-
hydroxysuccinimide ester (NHS-X-biotin; Boehringer
Mannheim GmbH) in dimethyl sulphoxide (20 mg./ml.) are
added dropwise thereto. Subsequently, the reaction
mixture is incubated for 3 hours at 25C. After react-
ing, it is dialysed overnight at 4C. against a 50 fold
volume of 20 mM potassium phosphate (pH 7.0). The
retentate is mixed with the same volume of 20 mM
potassium phosphate/200 mM sodium chloride (pH 7.0),
heated to 70C. and incubated for 4 hours at this
temperature, with careful stirring. Subsequently, the
solution is cooled to ambient temperature and filtered.
The filtrate is dialysed overnight at 4C. against a
,70 50 fold volume of 2 mM potassium phosphate (pH 7.0) and
subsequently lyophilised. The product obtained is
adsorbed on the solid phase and represents, in the
matrix according to the present invention, the component
Pl bound to the soluble protein.
lb) Activation of streptavidin with maleimidohexanoyl
N-hydroxysuccinimide ester.
30 mg. Streptavidin are dissolved in 3 ml. 30 mM
-23- 1 33~65
potassium phosphate/100 mM sodium chloride (pH 7.1)
and tempered to 25C. While stirring, 0.15 ml.
maleinimidohexanoyl N-hydroxysuccinimide ester (MHS)
(Boehringer Mannheim GmbH) in dimethyl sulphoxide
(10 mg./ml.) is added dropwise thereto. After a
reaction time of 1 hour at 25C., the solution is
cooled in an ice-bath. Subsequently, it is dialysed
twice at 4C. against 1 litre 50 mM potassium phosphate/
100 mM sodium chloride (pH 5.0)
lc Activation of strepavidin with S-acetylmercapto-
succinic anhydride.
30 mg. Strepavidin are dissolved in 3 ml. 100 mM
potassium phosphate (pH 7.8) and tempered to 25C.
While stirring, 0.175 ml. S-acetylmercapto-succinic
anhydride (SAMBA) in dimethyl sulphoxide (10 mg./ml.)
is added dropwise thereto. After a reaction time of
3 hours at 25C., it is dialysed at 4C. against
1 litre 50 mM potassium phosphate/2 mM ethylenediamine-
tetraacetic acid (EDTA) (pH 6.5).
ld) Homogeneous cross-linking of streptavidin.
3 ml. of a solution of activated SAMBA-streptavidin
(10 mg./ml.) (preparation according to Example lc)) are
tempered to 25C. and mixed with 50 ~1. lM hydroxylamine
(pH 6.5). After 30 minutes at 25C., dilution is carried
out by the addition of 15 ml. 50 mM potassium phosphate/
100 mM sodium chloride/l mM EDTA (pH 6.5). The homogen-
eous cross-linking of the streptavidin is started by the
-24- 1 3~
addition of 3 ml. activated MHS-streptavidin (10 mg./ml.)
- (preparation according to Example lb)). After a
reaction time of 2 hours at 25C. with careful
stirring, the reaction is terminated by the addition
of 0.2 ml. 100 mM cysteine hydrochloride. After an
incubation time of 30 minutes at 25C., the pH value
of the solution is adjusted to 7.5 by the addition of
lM dipotassium hydrogen phosphate. After the addition
of 0.2 ml. 500 mM iodoacetamide, the reaction mixture
is incubated for a further hour at 25C. Subsequently,
it is dialysed twice at 4C. against 3 litres 50 mM
potassium phosphate/100 mM sodium chloride (pH 7.5).
After the dialysis, the conjugate is concentrated in
an ultrafiltration cell.
The homogeneously cross-linked streptavidin can
either be used directly or after gel filtration
(Superose*6 prep. grade; Pharmacia, Sweden) and renewed
concentration for adsorption to the solid phase. In
the matrix according to the present invention, it
represents the cross-linked component P2.
Example 2.
Thermo-BSA is produced as described in Example
la) but here the biotinylation is omitted.
68 mg. Thermo-BSA are dissolved in 2 ml. O.lM
potassium phosphate (pH 7.8) and slowly mixed with
0.38 ml. SAMBA (10 mg./ml. in dimethyl sulphoxide).
After a reaction time of 3.5 hours at 25C., it is
* trade-mark
-!
-25- ~ 33~65
dialysed twice at 4C. against l litre 50 mM potassium
phosphate (pH 6.5).
Heterogeneously cross-linked streptavidin is
.! produced for use as P2 in a matrix according to the
present invention. The heterogeneous cross-linking of
streptavidin with thermo-BSA takes place analogously
to the homogeneous cross-linking described in Example
ld). 60 mg. activated MHS-streptavidin (production
according to Example lb)) are thereby reacted with
68 mg. activated SAMBA-thermo-BSA (see above). The
reaction product is purified by gel filtration
(Superose 6 prep. grade) and concentrated in an ultra-
filtration cell. The product obtained is subsequently
lyophilised. The product can be used as P2.
Example 3.
Loading of Luran*(polystyrene-acrylonitrile co-polymer)
or of ~-irradiated polystyrene test tubes.
The products obtained according to Examples 1 and
2 are dissolved in 50 mM potassium phosphate (pH 7.4)
to give a concentration of lO ~g./ml. Into each test
tube to be loaded there is then placed 1.5 ml. of a
solution of the thermo-BSA-biotin conjugate produced
according to Example la) and first loaded for 3 to 5
hours. Subsequently, after complete sucking out, there
is introduced into the test tubes 1.5 ml. of a solution
of homogeneously cross-linked streptavidin according to
Example 1 or of heterogeneously cross-linked
* Trade Mark
_~. .
-
1 335265
-26-
streptavidin according to Example 2 and incubated
overnight at ambient temperature. Thereafter, the
test tubes are completely emptied and used for the
corresponding tests.
For comparison, test tubes are only loaded with
cross-linked streptavidin according to Example 1 or 2
or a conjugate of streptavidin and thermo-BSA obtained
according to Example 7, without pre-loading with
biotinylated protein.
Example 4.
Determination of the binding capacity of test tubes
produced according to Example 3.
The test tubes loaded with the different strept-
avidin polymers according to the present invention, as
well as the comparison test tubes, are incubated with
1 ml. of a solution of biotinylated horse radish
peroxidase (biotin-POD, Sigma) (10 mU/ml. in 50 mM
potassium phosphate/0.5% BSA (pH 7.4)) for 45 minutes
at ambient temperature. The test tubes are then
emptied and washed twice with double distilled water.
Subsequently, the detection reaction takes place with
the use of ABTS ~ (ammonium salt of 2,2'-azino-di-(3-
ethylbenzothiazoline-6-sulphonic acid) for 30 minutes
at ambient temperature. The measurement is carried out
photometrically at 405 nm. The binding capacity (Bica)
is determined via a displacement curve. For this
purpose, to the biotin-POD solution are added
1 335265
-27~
increasing concentrations (0 to 15 or 0 to 200 ng./ml.,
respectively) of D-biotin (Sigma). The binding
capacity is then calculated from the curves obtained
by plotting the individual values from the semi-maximum
extinction.
Example 5.
The stabili~y of the surface adhesion of the
matrix coated with protein and biotin and, in each case,
a streptavidin polymer is tested by incubation of the
loaded test tubes with 1.5 ml. of a detergent-
containing dissolving-off buffer (0.2% Tween 20 in
50 mM potassium phosphate (pH 7.0)). After an
incubation time of 1 hour at ambient temperature, for
the determination of the amount of conjugate dissolved
off, in each case 1 ml. is transferred from the test
tube into a test tube coated with a thermo-BSA-biotin
(preparation according to Example la)). Parallel
thereto, for the production of a calibration curve, to
thermo-BSA-biotin test tubes is added 1 ml. of
dissolving-off buffer which contains increasing con-
centrations of streptavidin. After an incubation time
of 1 hour at ambient temperature, the test tubes are
completely emptied and mixed with 1 ml. of a biotin-POD
solution (100 mU/ml. in 50 mM potassium phosphate (pH
7.0)). After a further incubation of 30 minutes at
ambient temperature, the tubes are emptied and subse-
quently washed three times with double distilled water.
B * Trade Mark
.. ... i~
1 335265
-28-
The amount of bound biotin-POD is proportional to
the amount of conjugate dissolved off from the test
tube wall and is determined photometrically by the
` substrate reaction with ABTS (lncubation for 1 hour
at ambient temperature). On the basis of the
calibration curve, there is quantified the amount of
conjugate dissolved off and designated the dissolved-
off biotin binding capacity.
The following Table 2 shows, for various Luran
and Y-irradiated polystyrene test tubes, the biotin
binding capacities determined according to Example 4
and the desorption of the conjugates determined accord-
ing to Example 5. The biotin binding capacity (and
thus the binding capacity for biotinylated antibodies)
of the homogeneously cross-linked specific binding
component bound to the solid phase (comparison) (in this
example poly-streptavidin) is distinctly greater than
that of the heterogenously cross-linked specific binding
component bound to the solid phase (comparison) (in this
example BSA-streptavidin; preparation analogously to
Example le) from activated SAMBA-BSA and activated MHS-
streptavidin). As the dissolving off data show, the
cross-linked specific binding component P2 can, however,
then only be applied with high binding capacity and
desired secure wall adhesion when previously a loading
with pre-cross-linked protein, which contains covalently
bound the specific binding component Pl, i.e. biotin,
1 335265
-29
is reacted in situ. The influence of the binding
capacities and solid phase dissolving off of the
various conjugates on the sensitivity of a function
test carried out with the use of detergents is des-
cribed in more detail in Example 6.
Table 2
coating BSA-SA pSA pSA T-BSA-biotinl T-BSA-biotin
(comp- (comp- (comp- + 2 2 +
arison) arison) arison) T-BSA-SA pSA
(according to (according to
the the
invention) invention)
test tube r-PS Y-PS Luran Luran Luran
material
loading con-
centration 10 10 10 10 /82 10 /82
(~g./ml.)
L5 biotin-
Bica 7.5 58 60 15 120
(ng)
desorption
(Bi-Bica) 0.017 0.267 9.3 0.005 0.015
(ng)
% Bi-Bica 0 3 0 5 15.5 0.03 0.01
Abbreviations:
BSA-SA = bovine serum albumin-streptavidin conjugate
pSA = polymeric homogeneously cross-linked streptavidin
T-BSA-biotin or -SA = thermo-BSA-biotin or -streptavidin
y-PS = irradiated polystyrene test tubes
biotin-Bica or Bi-Bica = biotin binding capacity.
1 335265
-30-
Example 6.
The test tubes obtained according to Example 5
are used in a TSH tes~.
Reagents:
Reagent 1 tantibody incubation solution)
16 mMole/litre phosphate buffer (pH 6.9)
1.5 ~g./ml. biotinylated monoclonal antibody aginst
TSH (ECACC 87122201) (the biotinylation
took place according to J.A.C.S., 100,
3585-3590/1978 with biotin by reaction
with N-hydroxysuccinimide-biotin in a
ratio of 10:1).
Reagen~ 2 (antibody-POD conjugate solution)
36 mMole/litre phosphate buffer (pH 6.9)
2.0 U/ml. conjugate of POD and monoclonal anti-
bodies against TSH (ECACC 87122202).
Reagent 3 (substrate chromogen solution)
100 mMole/litre phosphate-citrate buffer (pH 4.4)
3.2 mMole/litre sodium perborate
1.9 mMole/litre ABTS (diammonium salt of 2,2'-
azino-di-(3-ethylbenzthiazoline-6-
sulphonic acid).
As solid phases, there are used test tubes which
have been coated with different matrices as described in
Example 3. In these test tubes are placed 0.2 ml. of
sample (TSH standard), 0.9 ml. Reagent 1 and 0.1 ml.
Reagent 2 and incubated for 2 hours at ambient
~ 335265
-31-
temperature. Subsequently, the test tubes are com-
pletely emptied and washed three times with water. The
POD activity bound to the test tube walls is then
determined, a~ter adding l ml. of ~eagent 3 and
incubating for 1 hour, by measuring the extinction at
405 nm. The intensity of the colour reaction is
proportional to the TSH concentration of the standard.
The results obtained are shown in Fig. 2 of the
accompanying drawings.
As Fig. 2 shows, the gradient of the calibration
curve (and thus the sensitivity of the test) increases
distinctly in the case of using detergent-containing
incubation buffer froril test tubes which are loaded with
a single-component matrix to test tubes which are
loaded with the two-co;nponent matrix according to the
present invention. Furthermore, the greatest
sensitivity is achieved by the us~ of the two-corllponent
matrix which, as component B, contains a homogeneously
cross-linked binding component (here polymaric strept-
avidin).Example 7.
A matrix is produced in which, on the solid phase,
thermo-BSA is adsorbed to which streptavidin is bound
as component Pl. On to the streptavidin is then
coupled hornogeneously cross-linked, biotinylated
protein A as component P2.
-32- l 335265
a) Preparation of thermo-BSA streptavidin.
.~ The preparation takes place as described in
~xample le).
b) Preparation of llomogeneously cross-linked,
biotinylated protein A.
50 mg. protein A (Boehringer Mannheim GmbH) are
dissolved in 5 ml. 30 mM potassium pllosphate (pH 7.1)
and mixed with a lO fold molar excess of NHS-X-biotin
(dissolved in an amount of 10 mg./ml. in dimethyl
sulphoxide). After an incubation period of l hour at
25C., the reaction mixture is dialysed overnight at
4C. against 10 litres 50 mM potassiurn pnosphate (pH
8.0). The retentate is subsequently concentrated in
an ultrafiltration cell to a concentration of 50 mg.
biotin-protein A/ml.
The concentrated solution of biotin-protein A is
warmed to 25C. Subsequently, while carefully stirring,
50 ~1. of a disuccinimidyl suberate solution (DSS,
Pierce; 7 mg./ml. dioxan) are added thereto. The
cross-linking is monitored by IIPLC on a TSK 3000 gel
filtration column (LKB). At intervals of 1 hour, in
each case 50 ~1. of the DSS solution are added thereto
until the peak of the monomeric protein A has been
reduced to less than 10% of its initial size. There-
after, the further cross-linking is stopped by the
addition of 50 ~1. lM ethanolamine (pH 8.0). Incub-
ation is carried out overnight at 4C. and subsequently
1 3 3 5 2 6 5
-33-
dialysis is carried out twice against 2 litres 2mM
~, potassium phosphate (pH 7.5). The separation of the
monomeric protein A takes place by gel filtration on
..
Superose 12 prep. grade. The homogeneously cross-
linked product is collected and concentrated in an
ultrafiltration cell. Test tubes are then loaded
according to Exa,-nple 3 with these two components.
Example 8.
A matrix is prepared consisting of a conjugate
of thermo-BSA with mouse F ~fragments as Pl and a
homogeneously cross-linked polyclonal anti-mouse-Fc
antibody from sheep as P2.
a) Preparation of thermo-BSA-mouse Fc~fragment.
The Fc~fragments are prepared by papain cleavage
of r.louse immunoglobulins G and separation of the Fab
fragments by ion exchange chromatography on DE-52-
cellulose according to conventional processes.
Activated MHS-Fc~fragment is prepared analogously
to the preparation of activated MHS-streptavidin (see
Example lb)). Activated SAMBA-thermo-BSA is prepared
as described in Example le). The conjugate of 6~ mg.
activated SAMBA-thermo-BSA with 10 mg. activated MHS-Fc~
fragment takes plac2 in the same way as the preparation
of heterogeneously cross-linked streptavidin (see
Example le)). The reaction product is purified by gel
filtration on Superose 6 prep. grade, concentrated in
an ultrafiltration cell and subsequently lyophilised.
1 335265
-34-
b) Preparation of homogeneously cross-linked anti-
, mouse-Fc~antibodies.
50 mg. Anti-mouse-Fc~antibodies are dissolved in
1 ml. 50 mM potassium phosphate (pII 8.0) and warmed to
25C. Analogously to the preparation of homogeneously
cross-linked protein A (see Example 2b)), at intervals
of 1 hour there are, in eacl~ case~ added 50 ~1. of a
DSS solution (7 Mg./ml. in dioxan) until in the HPLC
analysis on a TSK 3000 gel filtration column, the peak
of the ~ono~eric IgGs has dropped to 10% o~ its initial
size. Subsequently, as described in Example 7b ), the
reaction is stopped with ethanolamine and then dialysed.
The monomeric IgG is, as described in Example 7b),
separated off by gel filtration. The cross-linked IgG
is possibly concentrated by ultrafiltration. Altern-
atively, the hornogeneously cross-linked product can be
prepared by activation of the antibody witl~ MHS and
SAiviBA (preparation analogously to Examples lb) and lc))
and subsequent cross-linking (analogously to Example
ld)).
Test tubes are loaded according to Exa.nple 3 with
these products obtained according to a) and b).
Exa~ple 9.
A matrix is prepared consisting of a thermo-~SA-
digitoxigenin conjugate as Pl and homogeneously cross-
linked anti-digoxin-antibody from sheep as P2.
* trade-mark
. .
1 335265
-35-
a) Preparation of thermo-BSA-digitoxigenin.
Thermo-BSA is prepared as described in Example la).
68 mg. Thermo-BSA are dissolved in 6.8 ml. 50 m~i
, potassium phosphate/100 mM sodiurn chloride (pH 8.5) and
warmed ~o 25C. I~hile stirring, 2.86 rng. digitoxigenin-
3-succinimidyl hydroxysuccinimide ester in 0.68 ml.
dioxan are added thereto. After a reaction time of 3
hours at 25C., dialysis is carried out twice against
1 litre 2 mM potassium phosphate (pH 7.2). Subsequently,
the reaction product is concentrated in an ultra-
filtration cell.
b) Preparation of a homogeneously cross-linked anti-
digoxin antibody.
The preparation of the homogeneously cross-linked
anti-digoxin antibody takes place in the same way as
described for the preparation of the homogeneously
cross-linked anti--.nouse-Fc~antibody (Example 8b)).
Subsequently, test tubes are successively loaded
with solutions of the two products.
Digitoxigenin-labelled antibodies are used in the
immunoassay. The preparation of such labelled anti-
bodies takes place analogously to Example 9a) with
digitoxigenin-3-succinimidyl hydroxysuccinir.lide ester.
-36- 1 335265
The Patent Specifications referred to
herein are more fully identified below.
Federal Republic of Germany Patent
Specification (Offenlegungsschrift)
2533701, filed July 28, 1975,
published (laid open) February 12, 1976,
W.F. Barg, assigned to American Cyanamid
Co .
European Patent Specification 0,122,209,
L0 published October 17, 1984, M. Delaage et
al, assigned to Immunotech S.A.