Note: Descriptions are shown in the official language in which they were submitted.
1335783
SAMPLE PAD ASSAY INITIATION DEVICE
The field of the subject invention concerns
diagnostic assay strips permitting visual measurement
from any fluid and providing for red blood cell free
fluid from blood.
The ability to measure a wide variety of
physiologically active compounds, both naturally
occurring and synthetic, has become of increasing
importance, both as an adjunct to diagnosis and
therapy. While for the most part, assays of
physiological fluids and drugs have required clinical
laboratory determinations, there is an increasing
awareness of the importance of being able to carry out
assay determinations in the doctor's office and in the
home. To be able to perform an assay in a doctor's
office or home requires that an assay have a simple
protocol and be relatively free of sensitivity to small
changes in the conditions under which the assay is
carried out. Importantly, inaccurate measurements of
133~7~3
reagents and sample should whenever feasible be
avoided. Numerous systems have been developed in
efforts to try to address the various problems
associated with analysis outside of the clinical
laboratory. There is, nevertheless, a continuing
interest in providing improved and alternative methods
to those which are presently generally available.
Exemplary of this situation is the need today
to be able to determine cholesterol levels or low or
high density lipoprotein levels in blood. There is a
clearly established relationship between total blood
cholesterol (mainly LDL fraction) and coronary artery
disease (Journal of the American Medical Association
(1985) 253:2080-2086). New guidelines have been
established for adults over 20 years of age to identify
risk groups associated with blood cholesterol level.
These levels are as follows: <200 mg/dl is a desirable
blood cholesterol; 239 mg/dl is borderline high blood
cholesterol; ~240 mg/dl is high blood cholesterol.
Cholesterol levels can be controlled by both
diet and cholesterol lowering drugs. The key is to
identify those individuals at risk. Being able to
monitor one's own cholesterol at home for those indivi-
duals at risk will provide a significant tool in
monitoring cholesterol levels and reducing the
potential for heart disease. The measuring of other
naturally occurring compounds of physiologic importance
and synthetic drugs is also of great interest. For
example, therapeutic dosage monitoring, drugs of abuse,
iodothyronines, alcohol, cytokines, as well as numerous
other haptens and antigens are monitored. Also of
interest are microorganisms ~-HCG for ectopic births,
antibodies associated with disease, and the like.
In many of the assays it will be necessary to
provide a blood sample free of red blood cells to a
measurement strip. Furthermore, for home use or use by
non-technical individuals, it will be desirable that
~ 40545-32 1~35783
the volume of sample applied to a measurement strip
permit a relatively broad range of sample volume, which
is then accurately metered to the measuring strip. Any
such device must be relatively simple, provide
reproducible results, and be in a form which allows it to
be joined to a measurement strip in a fixed or removable
manner.
Relevant Literature
Demacker, et al., Clin. Chem. (1983) 29:19161922
reports the evaluation of cholesterol assay kits.
Studies associated with enzyme assays include Gochman and
Schmitz, Clin. Chem. (1971) 17:12; Paul, The Enzymes
(1963) 8:227-274; Current Status of Blood Cholesterol
Measurement in Clinical Laboratories in the United
States: A Report from the Laboratory St~n~rdization
Panel of the National Cholesterol Education Program
(1988) 34(1):193-201; and U.S. Patent Nos. 4,391,904;
4,366,241; 4,168,146; 4,435,504; 4,533,629; 4,540,659,
and references cited therein. See also, Zuk, et al.,
Clin. Chem. (1985) 31:1144.
German Patent No. 22 22 951 describes a filter
assembly cont~;n;ng chemical reagents for removing cells
from blood and measuring CPK.
In a first aspect the invention is an analyte
measuring device which cooperates with a signal producing
system for producing a detectable signal in a detection
zone, said device comprising a housing comprising a well
adjacent to one end of said housing, a flow path for
holding in position two bibulous strips spaced apart in
tandem relationship to define a space, and proximal to
said well an interspace for receiving a slide mech~n;s~;
an orifice for receiving a sample displaced from and
substantially aligned with the space between said two
bibulous strips; a slide element in said interspace
4 1335783
comprising (1) a sample receiving element positioned (i.)
under said orifice when said slide element is in a first
position, and (ii.) positioned in said space, when said
slide is in a second position; and means for releasing a
liquid into said well when said slide is moved from said
first position to said second position; and first and
second bibulous strips in said flow path, said first
strip extending from said well to said space to transport
liquid from said well to said sample receiving element
and said second strip comprising said detection zone and
extending from said space toward the end opposite from
said well.
In a further aspect the invention is a method for
detecting the presence of an analyte in a sample
employing a signal producing system having a plurality of
reagents being distributed between being bound non-
diffusibly to a surface or free in solution, said signal
producing system with said analyte producing a detectable
signal in a detection zone on a measuring strip employed
as a said surface, said method comprising applying said
sample to a sample receiving element capable of moving
from a first position to a second position, said second
position in liquid transferring relationship with said
measuring strip; moving said sample receiving element to
said second position; transporting fluid to said sample
receiving element for transporting at least one member of
said signal producing system from said sample receiving
element to said measuring strip; and allowing said fluid
to wick through said measuring strip with formation of a
detectable boundary at a distance from said sample
receiving element in relation to the amount of analyte in
said sample.
The invention is illustrated in the drawings in
which:
i~
~ 13357~3
4a
Figure 1 is a diagrammatic plan view of the base
plate and slide o a device according to the subject
invention;
Figure 2a is a diagrammatic plan view of an
inter~^~;~te plate which covers the base plate;
Figure 2b is a diagrammatic plan view of the same
cover plate which is inverted to show the underside.
Figure 3 is a plan view of an assembled device;
Figure 4 is an alternative design of the subject
device with;
Figure 4a being a diagrammatic plan view of the base
plate and slide;
Figure 4b being a diagrammatic plan view of the
cover plate.
Methods and apparatus are provided for the
measurement of an analyte employing a continuous flow
path, which has a sample receiving region as an internal
region, which is brought into contact with the adjacent
two regions to serve as a bridge. Prior to the addition
of sample to the sample receiving region, the sample
receiving region is inhibited from transport of fluid to
complete the path. After receiving sample, the sample
receiving region acts as a bridge between first and
second portions of the path, where the first portion
serves to transport fluid to the sample receiving region
and the second portion is the measuring region,
optionally joined to the sample receiving region by a
mixing region.
5 1335783
Various techniques may be employed for
organizing the flow path, inhibiting transport by the
sample region, and providing for flow of a reagent
solution through the sample region into the measuring
region. In addition, various chemistries may be
employed, using different types of reagent systems to
produce the desired signal. The assay may be
qualitative or quantitative. With agglutination
assays, qualitative results may be obtained. For
quantitative, the extent of the border of the signal
from the sample region indicates the amount of analyte
in the sample.
The flow path is primarily divided into three
parts. The first part is a bi bulous short element
which serves by capillary action to wick or transport
reagent solution to the sample receiving element. For
the most part, the transport element will be a bibulous
strip which absorbs a hydrophilic liquid and allows for
transport of the reagents contained in the reagent
solution, without chromatographing the components of
the reagent solution.
The sample receiving element serves a
plurality of functions. The sample receiving element
receives a sample and may, for the most part, have one
or more members of a signal producing system present on
the sample. Generally, the members will be non-
diffusively bound, so as to be retained on the sample
receiving element, although in other situations,
diffusively bound members of the signal producing
system may be present. The sample receiving element
also serves as a bridge for transferring the reagent
solution through the sample receiving element to the
measuring element. For the most part, prior to the
time that the transport element serves to transport
reagent solution to the sample receiving element, the
sample receiving element will be inhibited from acting
as a bridging element between the transport element and
6 13357~3
the measuring element. After receiving the sample, the
sample element is then permitted to be a bridging
element which allows for the flow of the reagent
solution through the sample element and into the
measuring element.
The measuring element will be an extended
member, which allows for flow of the reagent solution
through the measuring element, by means of capillary
action. The measuring element will have one or more
members of the signal producing system present on the
measuring element, where the height or distance of the
signal board, the distance from the sample receiving
element to the signal front, will be related to the
amount of analyte in the sample and on the sample
receiving member. Alternately, appearance of a signal
at a predetermined area can indicate the presence or
absence of an analyte. Gold sol assays are
illustrative.
By appropriate choice of members of the signal
producing system for a quantitative assay, visually
observable color fronts may be obtained, fluorescent
signals, or the like. In addition, the measuring
element may have one or more additional regions between
the measuring region, and the sample receiving element.
These regions may serve to control the dynamic range of
the assay, to provide a delay before the elements of
the signal producing system move into the measuring
region to allow mixing or a reaction to occur, and the
like. By providing for a reaction in this region with
a member of the signal producing system, the dynamic
range of the assay will be changed in the measuring
region. Since for many analytes there may be a
threshold value which is of interest and values below
the threshold are not of interest, one could provide a
sufficient amount of reagent in a threshold contact
region to react with a member of the signal producing
member, so that the threshold value becomes the zero or
7 133~783
low value observed in the measuring zone. The reaction
may be as a result of specific binding pair member
complex formation, chemical reactions involving
transformation of a chemical reactant to a product, or
the like. The mixing region will usually be a bibulous
member which serves to transport the liquid medium from
the sample receiving element to the measuring zone or
threshold zone.
Various techniques may be employed for
inhibiting fluid flow to and from the sample receiving
element to the other elements involved in the flow
path. Of particular interest is the use of a slide
which can be moved from a first position, where the
sample receiving element receives the sample, to a
second position where the sample receiving element
serves as a bridge between the two other elements of
the flow path. The slide therefore prevents sample
spreading to the other elements of the flow path,
before it is time to carry out the assay. The path of
the sample receiving element, in moving from the site
at which the sample is received to the site where it is
in the flow path, may provide for means for removing
excess sample from the sample receiving element. Such
means provides for a quantitative measure of the amount
of sample received by the sample receiving element.
Thus, by having a region in the path of the slide which
is narrowed, so as to remove unabsorbed sample medium,
without significantly squeezing the sample receiving
element, the amount of sample absorbed by the sample
receiving element can be relatively accurately
reproduced. The narrowing may be as a result of a
convexity, such as a rod in relief, a roller, or any
convenient scraping means. The narrowing of the path
should provide a space about equal to or slightly less
than the wet thickness of the sample receiving element.
The slide, therefore, not only serves to move the
sample receiving element, but also to meter the amount
8 133~7g3
of fluid absorbed by the sample receiving element.
The slide may also serve an additional
function in releasing the reagent solution. In
providing for a self-contained device, it is desirable
that the device include the reagent solution. In order
to prevent evaporation, the reagent solution may be
packaged in a sealed pouch situated in a well. The
slide can be provided with an arm which scores the
pouch, so as to open the pouch and release the fluid.
By having one end of the transferring element in the
well, the transferring element will transport the
medium to the sample pad. Therefore, in moving the
slide, one may move the sample receiving element,
monitor the amount of fluid associated with the sample
receiving element, as well as release the reagent
solution for development of the assay.
The subject method may be employed in any
situation where a fixed amount of a substance is
involved, which can be transferred to the sample
receiving element for measurement and ultimately an
interaction occurs with another compound to produce a
detectable boundary. These types of assays may be
illustrated by ELISA assays, EMIT assays, sandwich
assays, CEDIA assays, agglutination assays, or the
like.
Depending upon the protocol, the sample
receiving element to which the sample is added may be
prepared in a variety of ways. It may be untreated,
impregnated with buffer, or provide one or more
reagents of a signal-producing system. A variety of
sophisticated reagents, protocols or regimens can be
devised based on a limited amount of material migrating
to produce a boundary in proportion to the amount of
analyte present. Examples of protocols would include
particles having first and second ligands, where the
first ligand competes with analyte for receptor bound
to a surface. After carrying out the competition for a
9 1335783
limited amount of receptor between analyte and
particle, an aliquot of the assay medium is transferred
to the sample receiving element and the particle
transported with effluent through the measurement
zone. By having receptor for the second ligand in the
measurement zone, the particle boundary will be defined
by the number of particles added to the pad. By having
colored particles, charcoal particles, magnetic
particles, particles coated with gold and selenium
salts, dyes, dye-polymer conjugates, proteins with high
visible extinction coefficients, e.g., phycobili-
proteins, or the like, the boundary will be readily
defined.
Any technique which allows for binding of a
detectable entity in proportion to an analyte of
interest may be employed. These may include cleavage
of a bond to release the entity, where the bond to the
entity is not cleavable when the entity is bound to a
receptor, binding to a support which inhibits migration
of the entity in proportion to the amount of analyte in
a sample, or the like. The entity may be a particle as
described above, an enzyme which catalyzes the
production of a detectable product, or the like.
Of particular interest is where a product is
produced on the sample receiving element which provides
for a detectable boundary. For example, where the
analyte is a substrate, the sample receiving element
may be impregnated with the appropriate enzyme or
enzymes to provide for a product. Normally, the enzyme
product will react, either directly or indirectly, with
a compound which is fixed in the assay measurement
zone. This may be exemplified by cholesterol, glucose,
or the like, which reacts with an oxidase to provide an
oxidizing species. The oxidizing species may then
react with the bound compound or a mobile compound
which reacts with the bound compound, to produce a
lO 13357~3
detectable boundary. Illustrative of this situation
would be the hydrolysis of serum cholesterol ester by
cholesterol esterase (EC:3.1.1.13) and subsequent
oxidation of cholesterol by cholesterol oxidase
(EC:1.1.3.6) to produce a stoichiometrically identical
amount of H2O2. This H2O2 is formed at the sample
receiving element and combines with horseradish
peroxidase (HRP) which is in the mobile phase. The
HRP-H2O2 reacts with a bound substrate to produce a
detectable boundary.
Depending upon the assay, other reagents may
also be present. For example, detergents find use
where a lipophilic analyte in blood is involved, where
the lipophilic analyte binds to proteins present in the
blood. This may be illustrated by cholesterol which
binds to proteins, as for example in very low, low, and
high density lipoproteins. Thus, detergents such as
non-ionic, anionic, or cationic detergents may be
employed. Of particular interest are polyoxyalkylenes,
ethoxylated alkylphenols, octylphenoxypolyethoxy-
ethanol, octylphenol-ethylene oxide condensates and
polyoxyethylene lauryl ethers, or anionic detergents,
such as bile acids, e.g., sodium cholate and sodium
taurocholate. In addition, various sticking agents or
adhesives may be employed, such as gum arabic. Also of
interest will be proteins which are substantially non-
interfering, which may include gelatin, casein, serum
albumin, or gamma globulins. In addition, the reagent
pad may include preservatives, such as sucrose,
polyvinyl alcohol, polyvinyl pyrrolidone, dextran or
sodium azide. Finally, a buffered solution will
normally be employed for impregnating the sample
receiving element, where any convenient buffer may be
employed, generally a substantially dilute buffer,
which may include phosphate, tris, MOPS, borate,
carbonate, or the like. Usually, the buffered solution
will be at a pH in the range of about 4 to 9. The
ll 1335783
buffer concentration will generally be from about 10 to
500 mM.
In the case of the cholesterol assay as
illustrative of other assays, the impregnating solution
will have from about 2 to 100 units/ml of the two
enzymes, cholesterol esterase and cholesterol
oxidase. The detergents will be in total weight from
about 0.1 to 5 weight percent of the medium, while in
the case of mixtures the weight of the non-ionic
detergents may be from about 10 to 90%, usually from
about 25 to 75 weight percent of the total detergent
mixture. The binding agents or adhesives will
generally be in the range of about 0.2 to 10, more
usually from about 1 to 5 weight percent of the
medium. A preservative or hydrogen bonding agent may
be present in from about 1 to 20 weight percent, more
usually from about 2 to 10 weight percent. The
remaining additives will generally be present in total
amount of less than about 10 weight percent, more
usually of less than about 5 weight percent. The
remaining composition may be water, non-reactive
ingredients, excipients, extenders, and the like.
In the case of thyroxine, various reagents may
be used to release the polyiodothyronines from binding
protein, e.g., thyroxine binding globulin.
Illustrative reagents include sodium hydroxide,
tetrachlorothyronine salicylate, 8-amino-1-
naphthalenesulfonic acid" 2-hydroxy-4-
methoxybenzophenone-5-sulfonic acid, etc. (see EPA 0
133 464).
Any analyte may be determined by using a
variety of different protocols. For example, by
employing conjugates of an epitopically cross-reactive
compound and an enzyme such as horseradish peroxidase,
where anti-analyte receptors, particularly antibodies
are uniformaly bound in the measurement zone. In
addition, an oxidase is also uniformally bound in the
12 133~783
measurement zoneO The presence of analyte in the
sample will provide for competition between analyte and
enzyme conjugate for available antibody. The more
analyte present, the further the limited amount of
conjugate will progress. By employing as the reagent
solution a reactant which in combination with the
oxidase, which produces hydrogen peroxide, and a leuco
dye, which forms a colored dye upon a horseradish
peroxidase catalyzed reaction between hydrogen peroxide
and the leuco dye, the color front will be related to
the amount of analyte in the sample. For example,
glucose oxidase may be used with glucose, urea oxidase
with urea, and the like.
Different protocols may be used for different
analytes. For theophylline, for example, the same
reagents may be employed as described in Zuk, et al.,
supra. The measuring region has antitheophylline
antibodies. The wicking solution comprises
theophylline-HRP conjugate in PBS and glucose oxidase
or the glucose oxidase may be in the measuring
region. After wicking the sample, the measurement
region may be flooded with substrate, e.g., 4-chloro-1-
naphthol and glucose in PBS.
For a thyroxine (T4) assay, the sample is
contacted with a thyroxine releasing reagent, which may
be present on the membranes for separating the red
blood cells. The measurement region would comprise
anti-T4 antibodies. The wicking solution and developer
would be analogous to the theophylline assay,
substituting theophylline with T4 in the conjugate. In
the same way, other hapten analytes could be assayed.
The assay is carried out by impregnating a
sample receiving element, usually a pad, which serves
as a bridge between the other flow path elements
positioned in tandem juxtaposition along their long
axes. Thus the two elements define one long flow path,
usually comprised of two differently sized bibulous
~ 13 1335783
strips with a separation between the two strips, where
the sample receiving element may act as a bridge to allow
for fluid flow between the two strips.
Where blood is the sample, the sample receiving
element is positioned under a red blood cell removing
filtering device. The blood sample will normally be one
or a series of small drops, generally having a total
volume under about 100 yL, more usually from about 10-50
yL. The layers through which the sample flows will
usually include a mesh layer, a first membrane, and a
second membrane cooperating with the first membrane to
ensure the substantially complete removal of any
interfering cells from the blood sample. The first
cellular separation member is used to reduce the
concentration of red and white blood cells received by
the second filtration member. By lowering the red blood
cell content from about 10 to 90~, usually from about 30
to 90% of the original red blood cell content, with the
first membrane member, the second membrane member is able
to efficiently and accurately remove at least
substantially all of the red blood cells from the blood
sample. Since the first membrane acts as a coarse
separation means, the first membrane may take any of a
wide variety of forms.
Various packings or sieving depth filters may be
employed, such as glass fibers, cellulose filters treated
with red blood cell capture reagents, glass fiber
filters, or synthetic fiber filters. Glass fiber filters
are available from such manufacturers as Whatman,
Schleicher and Schuell, MSI, and Pall. The glass fiber
filters are further characterized by a glass fiber
diameter in the range of about 0.5-9 y, and a density of
about 50 to 150 g/m2. The glass fiber filters may be
illustrated by S&S Glass 30~, Whatman GFD~, and S&S 3662~.
Other coarse separation membranes may include
cellulosic membranes, e.g., filter paper, to which red
~Trademarks
~f 14 ~33~78~
blood cell binding proteins or agglutination agents
immobilized. Such proteins may include lectins,
antibodies specific for RBC surface membrane proteins,
thrombin, ion exchange agents, etc. The preparation of
such filters by conjugating proteins or other agents to
cellulose is well known. Cellulose may be activated in a
wide variety of ways employing carbo~;;m;de, carbonyl
diimidazole, cyanogen bromide, chloroacetic acid, where
the acid may then be activated with carbodiimide, or the
like. The literature is replete with examples of binding
of proteins to cellulosic membranes for a variety of
reasons, which techniques may be employed here.
Alternatively, multiple layers of coarse separation
membranes may be employed.
With the two membranes, immediately beneath the
first membrane will be the second membrane, which will be
in fluid receiving relationship with the first membrane,
either in contact with the first membrane or in close
proximity thereto. Generally, the spacing between the
first and second membranes will not exceed a distance
which inhibits fluid flow, so that fluid readily flows
from the first to the second membrane. The non-
asymmetric membranes which are employed will be those in
the medium porosity range, having an average porosity in
the range of about 0.65 ~ to 7 ~, preferably about 1 to 5
~, where the pores may or may not be of substantially
uniform diameter through the membrane. By contrast,
where an asymmetric membrane is employed, that is the
diameter of the pores vary from one surface to the other,
desirably the membrane will have a minimum porosity not
less than about 0.4 ~, preferably not less than about
0.45 ~, and the m~;mllm porosity will generally not
exceed about 40 ~, more usually not exceed about 20 ~.
Illustrative microporous membranes which may find use
include Filterite~ polysulfone asymmetric, 20 y - .45 ~,
Sartorious~ cellulose acetate, 1.2 ~, Nucleooore~, etc.
~Trademarks
-
~ .
1335783
-
The choice of the second membrane is important,
since the amount of red blood cell lysis is dependent on
a number of factors. Depending on the size of the pores,
the amount of lysis will greatly vary. Since lysis
results in release of colored cell components, which
interfere with detection of the border in the measuring
strip and act to decompose hydrogen peroxide, merely
removing cells is insufficient. A further consideration
is the pressure differential across the membranes.
Again, the appropriate choice of membranes will affect
the pressure drop and forces acting on the cells, where
the pressure differential can affect the stability of the
cells.
Thus, the two membranes serve to act together to
efficiently and accurately remove red blood cells from
the blood sample with little, if any, hemolysis, so as to
provide a plasma or serum sample which may be accurately
analyzed without interference from hemolytic products,
such as heme.
The sample receiving element will be immediately
beneath the red blood cell removing membranes and in
fluid receiving relationship with the membranes. The
sample receiving element will normally be a bibulous
member able to absorb the fluid. Various bibulous
materials may be used, such as cellulosic materlals,
e.g., paper, or the like. The sample receiving element
will usually be of a size in the range of 5 to 50 mm2
surface area and a thickness in the range of about .1 to
2mm, having a volume capacity of from about 1 to 30 ~1.
The sample receiving element may be round, square,
rectangular, quadrilateral or polygonal, depending on the
manner in which it is to be used to act as a bridge for
the other members of the flow path. For further
characterization see C~n~ n patent application Serial
No. 600,099, filed May 18, 1989.
The assay is carried out by impregnating a sample
receiving element which serves as a bridge
,,7 = ~
16 1335~3
between two bibulous members positioned in tandem
juxtaposition along their long axes to define a flow
path. Thus the two strips define one long strip with a
separation between the two strips, where the sample
receiving element can act as a bridqe to allow fluid
flow between the two strips. A first bibulous transfer
member serves to receive the transport and reagent
solution, which may or may not have reaction
components, depending upon the assay. The first
bibulous transfer member, transfers the fluid to the
sample receiving element. The sample receiving element
receives the transport and reagent fluid from the first
bibulous transfer member and serves as a bridge to
transfer the reagent fluid to the assay measurement
region.
The sample is prevented from interacting with
the two bibulous members when sample is transferred to
the sample receiving element. Besides the slide
mechanism, other separation means may be employed.
These separation means will usually comprise an inert
non-porous film, which blocks transfer from the sample
receiving element to the bibulous members of the flow
path. The amount of sample accepted by the sample
receiving element and involved in the assay medium may
be controlled by providing for transfer of fluid beyond
the amount saturating the sample receiving element
through a non-wetting screen into an absorbent layer.
After addition of the sample to the sample receiving
element, and an incubation of up to about 30 minutes,
the porous non-wetting material and absorbent layer are
removed, leaving the sample receiving element as the
sole repository of sample for the assay. Where a
wiping film is employed it will be removed upon
saturation of the sample receiving element.
The entire flow path may have a length of
about 25 to 200 mm, more usually from about 50 to
.
17 133~783
150 mm, preferably about 100 mm. About 25 to 90% of
the length of the flow path will be the measurement
region comprising the quantitation zone, optionally a
mixing zone and/or a threshold value zone. The mixing
and or threshold value zone will generally be from
about 5 to 35% of the flow path. The strips which
provide for flow of fluid to and from the sample
receiving element may be of the same or different
length and will generally be from about 5 to 25 mm,
more usually about 10 to 20% each of the length of the
flow path. The upstream strips may be part of the
mesurement region strip, or an independent entity.
Alternatively, this strip may be used to control the
threshold value. The sample receiving element will
generally be from about 1 to 10%, more usually from
about 2 to 8% of the length of the flow path; the
longer the flow path, the larger the sample recéiving
element may normally be. The width of the strips may
be varied widely, usually being at least about 2 mm and
not more than about 10 mm, preferably from about 3 to
7 mm. The two strips will usually each overlap the
reactant pad by at least about 0.2 mm and not more than
about 2 mm, usually about 1 mm, being primarily a
matter of convenience, so long as the two strips are
not in direct fluid communication.
Any convenient material may be used for the
various bibulous parts of the assay strips forming the
flow path. Usually, the thickness of the bibulous
components will be in the range of about 0.05 to
2.0 mm, more usually 0.15 to 0.75 mm. A wide variety
of bibulous supports may be employed, particularly
cellulosic supports, such as chromatography paper,
silica on a support, alumina on a support, and
polymeric membranes such as nitrocellulose and nylon.
The characteristics of the bibulous material employed
for the measurement region or zone include the need in
many instances to covalently or irreversibly bind an
18 133~7~
indicator molecule to the support, that the color
developed should be clear and sharp, and that the fluid
should be capable of flowing at a convenient rate
through the bibulous members.
Of particular interest is an assay device
which is self-contained and only requires the sample
for carrying out the assay. The device may serve as a
one-step diagnostic test device using a disposable
cassette format. The device may be fabricated of three
individual injection molded parts into which various
components of the assay system are associated. These
include the filtration medium designed to separate
plasma from whole blood, means for metering a precise
sample volume, a slide to transfer the sample receiving
element to the transfer and measurement elements and to
release a transport and reagent solution. The
transport solution initiates capillary migration
through the flow path resulting in the development of a
detectable boundary related to the amount of analyte in
the sample.
For further understanding of the subject
invention, the drawings will now be considered. The
invention may be fabricated from three injection molded
parts or by any other convenient process. The parts
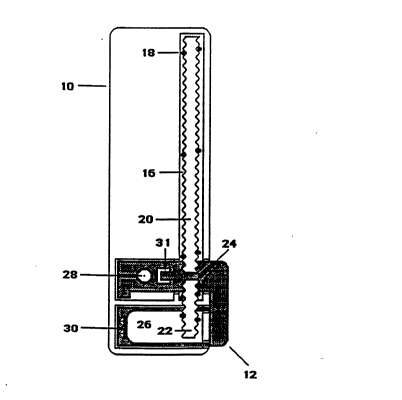
comprise a base plate 10, a slide 12 and a cover plate
14, as shown in Figs. 1 and 2. The base plate 10
consists of a cutout to accept the slide 12, a slot 16
with locating pins 18 into which the quantitation strip
20 and bibulous strip 22 are precisely positioned,
maintaining about a 2 mm gap 24 between them, and a
well 26 designed to capture the released transport
solution, e.g., wicking buffer.
The slide 12 consist of a vented receptor site
28 into which the reagent pad is inserted, an arm 30
with dual shearing designed to facilitate the release
of the transport solution from a pouch which is housed
in well 32 of cover plate 14, and a snap 31 to lock the
l9 1~3~78~
slide in place, once pulled. The cover plate 14
consists of a well 32, which houses a sealed foil pouch
(not shown) containing the transport solution. The
cover plate has an orifice 34 for the introduction of
the sample. Underneath orifice 34 are filters 36, for
separating cells from blood samples. The filtration
system may comprise dual glass fiber disks and a final
filtration membrane in order to deliver cell free
plasma to the sample receiving element. The cover
plate also comprises the squeegee metering bar 38,
which serves to control the volume of sample absorbed
by the sample receiving element, as well as a viewing
slot 40. At the top of the viewing slot 40 is an
indicator hole 42, which changes color when the test is
complete to inform the user that a reading may be
taken~
The final assembly is depicted in Fig. 3,
where the assembled device is obtained by introducing
the slide 12 into base plate 10, positioning transfer
strip 22 and measurement strip 20 at their appropriate
sites, introducing the transport solution pouch into
well 32, assembling the cover plate and base plate and
then sealing, conveniently by sonic welding, the base
plate and the cover plate. This procedure locates the
sample receiving site of the slide directly beneath the
filtration media of the cover plate, as well as
locating the shearing points of the slide beneath the
foiled sealed pouch located in the cover plate.
A modified device is shown in Fig. 4, where an
additional liquid reagent is supplied for flooding the
measurement strip.
~ The device comprises a base plate lOa and a
cover plate 14a, as well as slide 62. The sample is
introduced at sample receiving site 50. Cover plate
14a comprises well 52 which may include one or more
pouches with the same or different reagent solutions.
Also involved with the movement of the slide is
1335783
squeegee 54, which serves to control the volume
absorbed by the sample receiving element.
Additional to the subject device is a
developer pouch well 56 which houses a pouch which has
a reagent solution for developing the detectable
signal. When the reagent is released from the pouch,
the reagent may enter slot 16a through channels 57 and
57a, so as to flood the slot and substantially wet the
measurement strip 58. The slide 62 includes the sample
receiving element 60 as well as slide snaplock 64 and
dual shearing points 65 for shearing the pouch housed
in well 52. A second slide 66 serves to open the pouch
in well 56 to release the developer solution. A viewing
window 68 is superimposed over slot 16a, so as to view
measurement strip 58.
In order to carry out a cholesterol measure-
ment, the user lances his or her finger and applies a
hanging drop of blood to the application site, which is
a white central well with a red border. When the white
center is no longer visible, a sufficient amount of
blood has been applied. The user then waits about 30
seconds to 2 min or more to allow adequate filtration
and recovery of plasma onto the sample receiving pad.
Impregnated on the sample receiving pad is are
cholesterol esterase, cholesterol oxidase and
detergents in sufficient amount to react with all of
the cholesterol ester and cholesterol and to disperse
the cholesterol. The slide is then pulled until it
snaps into place. At this point the reagent pad
containing the plasma sample has been metered by the
squeegee metering bar and is brought into contact and
fluid transferring relationship with the transfer strip
and the measurement strip over the two mm gap. The
shearing points of the slide have also pierced the foil
seal of the pouch in the well of the cover plate,
releasing the transport solution into the receiving
well in the base plate. The transport solution is 0.1-
~ 21 13357~3
2 ml of an aqueous buffer which may contain horseradishperoxidase in sufficient amount to insure rapid reaction
of the hydrogen peroxide and dye. This begins the
wicking of the strip assembly which washes the sample
from the sample receiving element onto the measurement
strip. The measurement strip is impregnated with a
peroxidase substrate, particularly a modified N, N-
dimethylaniline. (See C~n~; an patent application
600,099 filed May 18, 1989). The reaction of the
reagents results in a colored region with a defined
boundary, thereby giving the user a precise reading of
the cholesterol level. This reading is made when the
color indicator site above the viewing slot shows the
test is complete. Normally, it will take about 15 min
for the assay to be complete, reading the peak of a blue
area in the viewing slot.
Therapeutic dosage monitoring assays may be
performed in accordance with the subject invention
employing commercially available devices. The
immunochromatographic strip from a theophylline
Acculevel~ device was removed from the plastic cassette.
The strip was affixed to a 10 mil acetate plastic backing
with 3M~ 415 double stick adhesive tape so that the dye
band on top of the strip faced upwards and the top of the
strip was flush with the end of the plastic backing. At
the low~er end of the strip, an 11 x 5 mm section of S&S
470~ paper to serve as a mixing zone was positioned on the
backing to provide a 1 mm overlap with the bottom of the
strip and a 1 mm overlap with a 7 x 5 mm section of
Whatman 3lET~ paper also adhered to the backing to serve
as a sample site. An additional 11 x 5 mm section of S&S
470 paper was adhered below the 3lET paper with a 1 mm
overlap to the 3lET paper and ending at the terminus of
the plastic backing. To interrupt wicking between the
31ET paper and the two 470 sections, 3 x 10 mm pieces of
Mylar~ were positioned between the 1 mm overlap points.
- ~Trademarks
'I
133~78~
22
The Acculevel~ control solution was diluted
l:lO with pH 7.0 phosphate buffer. Solutions of 0.5
~g/mL and l.O ~g/mL theophylline in phosphate buffer
were gravimetrically prepared. The samples were
applied to the 31ET paper section of the strip with a
micropipet. In all cases, lO ~1 of sample was applied.
After application of the sample, the Mylar pieces were
removed and the strips placed in the Acculevel~ wicking
buffer (solution 1) with the lower 470 section dipped
in the solution. The solution wicked through the lower
470 section, throught the 31ET paper (sample site),
through the mixing zone, and finally through the
immunochromatographic strip. The wicking was allowed
to continue until the dye level at the top of the strip
smeared.
Immediately after completion of the wicking,
the strip was removed from the wicking solution and the
immunochromatographic paper was immersed in Acculevel0
developer (solution 2). A color band of good quality
and rocket front developed over several minutes. When
the color development was complete, the strip was
removed from the developer and the migration height of
the color band was measured from the bottom of the
strip to the color band front (apex).
RESULTS MIGRATION HEIGHT
l/lO Acculevel0 control 2.90 ~g/ml 39 mm
.5 ~g/mL theophylline solution 33 mm
30 l.O ~g/mL theophylline solution 40 mm
Creating a 2-point curve with the .5 and l.O
~g/mL solutions allowed for interpolation of the l:lO
Acculevel~ control. A value of O.9 ~g/mL was
indicated. This value falls within the control range
0.83-1.23 ~g/ml.
In some instances, the sample receiving
~ 23 I3~783
element may include reagents which react with the
analyte. If insufficient time for reaction is provided
by the time the transport solution reaches the sample
receiving element, then an incubation period of from 1
to 30 min. may be employed before the slide is moved to
bring the sample receiving element into position in the
flow path.
It is evident from the above results, that a
simple self-contained device is provided which can be
used without any technical experience. ~he user only
requires the need of placing a sample at the appro-
priate position and then moving one or more slides at
appropriate times. Reading can be done visually,
although the reading may also be done by instrumen-
tation if desired. Thus, a wide variety of analytesmay be determined by untrained individuals in various
settings, where a quantitative result can be obtained.
~ Although the foregoing invention has been
described in some detail by way of illustration and
example for purposes of clarity of understanding, it
will be readily apparent to those of ordinary skill in
the art in light of the teachings of this invention
that certain changes and modifications may be made
thereto without departing from the spirit or scope of
the appended claims.