Note: Descriptions are shown in the official language in which they were submitted.
1 3 J 6 1 2 7
-3-
B~CKGROUND OF THE INVENTION
A major use of natural benzaldehyde is as an ingredient in
"natural" cherry flavor and other flavors for augmenting or
enhancing the aroma or taste of consumable materials including
foodstuff~, chewing gums, medicinal products, toothpastes,
chewing tobacco, smoking tob~cco and smoking tobacco articles.
A ma~or use of natural acetaldehyde is as an ingredient
ln "natural" orange flavor and other flavors for augmenting
or enhancing the aroma or taste of consumable materials including
foodstuffs, chewing gums, medicinal products, toothpastes and
chewlng tobacco.
Natural benzaldehyde has been used in natural cherry flavors
in the form of an apricot kernel derivative as is taught in
U.S. Letters Patent 1,416,128 issued on May 16, 1922. An
undesirable feature of the known processes for preparing
natural benzaldehyde from apricot kernels or reground press
cake i8 that along with the benzaldehyde, toxic hydrocyanic
acid is produced which must be separated completely from the
benzaldehyde and from the re~t of the oil prior to use. Other
techniques for producing natural benzaldehyde are known but
these techniques produce it in such yields as to cause the
resulting process to be uneconomical. For example, Hockenhull,
et al, Biochem. J., 50, 605-9, (1952) (Title: "Oxidation of
Phenylacetic Acid by Penicillium chrysogenum")discloses
i production of benzaldehyde starting with phenylacetic acid
through either benzyl alcohol or mandelic acid via the
sequences:
P~.~H,.COO~
P~ LO cit
+O +O
-~o
~.C9~0~ P~.C~OH.COO~
b~cohol m-ntelic ~id
~ ~ - CO,
, ., --CO,
.P~.CHO~ Ph.CO.COO~
'IJhr~ ~id,
~ I
1 3361 27
! 4
Towers, et al, Can. J. Zool. 1972, 50(7), 1047-50
. ("Defensive secretion:biosynthesis of hydrogen cyanide and
benzaldehyde'from phenylalanine by a millipedeU) discloses a
biosynthetic pathway for the production of benzaldehyde from
dietary phenylalanine in Oxidus gracilis, thusly:
O
~0 C=-~--
O
HCI~/ + ~lJ~ <.
Halpin, et al, Biochemistry, 1981, Volume 20, pages
~1525-1533 (Title: "Carbon-13 Nuclear Magnetic Resonance
Studies of Mandelate Metabolism in Whole Bacterial Cells and in
Isolated, in Vivo Cross-Linked Enzyme Complexes") discloses the
:~ biochemical pathway from mandelate ion to benzaldehyde, thusly:
~ 'eJAte ~ (L)~ te
X~ ~ ~ d~yL~se
~13~ At~
~8~ ~
~,7~l~de/
~,li_o ~
i~
1 336 1 27
--5--
Reeves, et al, TAPPI 48(2), pages 121-5, (1965) (Title:
"Reaction Products Formed Upon the Alkaline Peroxide Oxidation
of Lignin-Re~ated Model Compounds") discloses the effect of
;.alkaline hydrogen pero~ide oxidation on cinnamaldehyde whereby
the cinnamaldehyde is split at the double bond with the
formation of the corresponding benzaldehyde and benzoic acid
', according to the reaction:
,,., O
t ~ 11202 ~ ~7
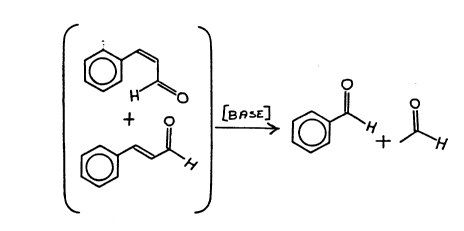
At page 124, column 1, paragraph 1, Reeves~ et al theorizes
that a "reverse aldol reaction" is not responsible for the
formation of vertraldehyde due to the fact that acetaldehyde
the other product of the potential "reverse aldol reacton"
was not obtained. Therefore, our discovery of the "retro-aldol"
reaction taking place, to wit:
~ ~ ~sE]
1 336 1 27
--6
was unexpected and unobvious. The "retro-aldol" reaction, to wit:
.
~~~ [Bl3sE~ ~ Jl~
indeed, took place due to the different reaction conditions
from those proposed and set forth in Reeves, et al; different
insofar as temperature of reaction and time of reaction are
concerned; longer times of reaction and higher temperatures of
reaction being the conditions in our "retro-aldol" reaction as
opposed to shorter times of reaction and lower temperatures
insofar as the Reeves, et al reaction is concerned.
In our own invention, no reagents other than base and
naturally occurring cinnamaldehyde and solvent are utilized
to carry out the "retro-aldol" reaction of our invention, to wit:
~o rB~S~]> ~
-7- 1 3361 27
The process of our invention thus gives rise to unobvious,
unexpected and advantageous results and represents an advance
in the art i~ the production of "natural n benzaldehyde taken
alone or in combination with natural cinnamaldehyde; and, further,
in the production of ~natural acetaldehvde.-
~ -8- 1 3361 27
SU~-D~ARY OF ~HE INVENTION
Our invèntion is directed to the production of "natural"
benzaldehyde and/or "natural n acetaldehyde taken alone or in
admixture with "natural" c~nn~ ~ldehyde according to a reaction
where "natural" cinnamaldehyde is subject to a "retro-aldol"
reaction, thusly:
t8~5E] ~ 1
The cinnamaldehyde reactant may occur in either the "cis" form
having the structure:
~0
, and/or the "trans" form having the structure:
O .. , ~, .. . .
1 3361 27
g
The cinnamaldehyde may be in recovered form from natural sour-
ces as by distillation or extraction or the cinnamaldehyde may
exist in its natural state immediately prior to the reaction,
thusly:
(i) Oil of Cinnamon Ceylon;
(ii) Ceylon cinnamon Bark (Cinnamomum zeylanicum Nees
ex Blume (fam.La~raceae)):
(iii) The Rark of Saigon cinnA ~n (Cinnamomum loureirii
Nees (fam.Lauraceae):
(iv) The Bark of Cassia cinnamon (ex Cinnamomum cassia
(Nees)) Nees ex Blume (fam. Lauraceae):
(v) The Bark of Saigon cin~A -n :
(vi) Oil of cinnamon Bark Ceylon;
(vii) "Quills" from Ceylon cinnamon ( including
"fines","Barcelona" and "Hamburg"):
(viii) Ceylon cinnamon quillings and featherings:
(ix) Ceylon cinn~ -n chips:
(x) Ceylon Cinna ~ n bark oil:
(xi) Oil of cinnamon Seychelles:
(xii) Oil of cinnamon Madagascar:
(xiii) Leaves of Cassia;
(xiv) Cassia Bark (Cassia lignea)in ground or powdered
form;
(xv) Oil of CaQsia.
Thus, the cinn al~ehyde source may be treated with a base such
a~ sodium bicarbonate, potassium bicarbonate, sodium carbonate,
potassium carbonate, lithium carbonate, lithium bicarbonate,
magnesium hydroxide, calcium hydroxide, calcium carbonate,
proline having the structure:
O
~OH
i 1 3361 27
--10--
choline having the structure:
[0~]~
, f~O
or a natural choline source such as natural lecithin having the
~structuro:
rH2 - O R
;H - O - ~ - R'
CHz - O - - O - CH2 - CH2 - N - CH3
~ a CH3
in the presence of base (wherein the residues:
//o C//
R \~,
represent palmitoyl, stearoyl, oleyl, linoleyl, linolenyl and
C20-C22 acid residues) rexamples of naturally occurring lecithin
are soybean lecithin (reference: "Soybeans, Volume II, (Inter-
science Publishing Company, New York, 1951), pages 593-647 and
natural phosphatide lecithi~ whereby a "retro-aldol" reaction
1 3361 27
--11--
takes place, thusly: ,
~ ~B-9sE] ~
A requirement of our invention is that no other reagents be
present which would cause the reaction to give rise to a
composition containing benzaldehyde or acetaldehyde which cannot
be described as ~natural". Thus, the use of substances such as
hydrogen peroxide and/or sodium hydroxide in the reaction mass
would give rise to a material not contemplated within the scope
of our invention.
Thus, our invention specifically is intended to exclude
processes such as those of the prior art, for example, Reeves, et
al, TAPPI, 48(2), 121-5, (1965) which discloses the reaction:
O
t ~ l~z 02 ' U
1 3 3 6 1 27
-12-
The reaction of our invention, to wit:
.
~ ~ I
[~ t8~9sE] ~
may be carried out in a standard reaction vessel preferably at
reflux conditions (preferably when the cinnamaldehyde-bearing
reactant is in the liquid phase, e.g., cinnr ~n oil or cassia oil);
or it may be carried out in solid-liauid phase reaction e~uipment,
e.g., ~Soxhlet~-type equipment (preferably when the cinnA~ldehyde-
bearing reactant is in the solid phase). Thus, the reaction of
our invention may be carried out in a ~Soxhlet" extraction
vessel with the actual reaction taking place in the "Soxhlet"
thimble as more specifically described, infra, or the reaction
of our invention may be carried out in a "Soxhlet~ extraction
vessel with the actual reaction taking place in the reboiler
flask or vessel. The case where the reaction takes place in
the "Soxhlet" thimble occurs when, for example, pulverized
cinn; ~r bark of one of the above types is intimately admixed
with lime or magnesium hydroxide or the like and the resulting
solid mixture is placed in the "Soxhlet" thimble.
In any case, the reaction may take place in the presence
of (i) Cl-C5 alcohols, (ii) water, or (iii) aqueous mixtures
of Cl-C5 alcohols and water. Examples of Cl-C5 alcohols are
methanol, ethyl alcohol, isopropyl alcohol, n-propyl alcohol,
n-butanol, secondary butanol, tertiary butanol, n-amyl alcohol, ``
t-amyl alcohol and isobutanol. The weight ratio of alcohol:water
when an alcoholic solution is used, may vary, and is preferably
from about 6 parts alcohol:4 parts water (by wei~ht) up to about
1 part alcohol:about 10 parts water (by weight).
1 3361 27
~ -13-
The reaction is carried out at temperatures such that acet-
aldehyde and benzaldehyde are removed from the reaction mass as
;they are formed thereby flavoring the "retro-aldol" reaction.
Hence, temperatures substantially greater than the boiling point
of acetaldehyde are to be used. The boiling point of acetaldehyde
is 21C at atmospheric pressure. Pressures o~ from about 0.2
atmospheres up to about 10 atmospheres may be used in carryin~
;out this reaction. Thus, for example, refluxing water at 1 atmo-
sphere gives rise to a reaction temperature of about 90C whereas
refluxing 50:50 ethanol:water at atmospheric pressure gives rise
to a reaction temperature of about 80C. The reaction temperature
may thus vary from about 40C up to about 150C. The reaction
pressure may thus vary from about 0.2 atmospheres up to about 10
- atmospheres. The reaction time may vary from about 5 hours up to
about 80 hours. The longer the reaction time, the greater the
degree of "completion" of the reaction (giving rise to a greater
ratio of benzaldehyde:cinnamaldehyde in the final product). The
shorter the period of reaction time the higher the temperature
required in order to substantially Ncomplete~ the reaction (whereby
the weight percent of benzaldehyde in the reaction mass is greater
than about 40%).
I Thus, within the meaning of our specification, the term
"completion" of reaction means the formation in the reaction
~ mass of at least a 10% yield of "natural" benzaldehyde and a
~- 10% yield of acetaldehyde up to about a 95~ yield of "natural"
benzaldehyde and a 95% yield of Nnatural" acetaldehyde. Carrying
out our process in order to yield less than 10% of benzaldehyde
;or acetaldehyde or greater than 95% yield of benzaldehyde or
acetaldehyde becomes uneconomical and is not contempiated within
the scope of our invention.
,j
When using as a source of c;nnAmaldehyde one or both of
the compounds having the structures:
1,
O
~ and ~
1 3J61 27
-14-
cinni o~ oil or oil of cassia oil, the cinnamon oil or oil of
cassia is ad~ixed with water or a Cl-C5 alcohol or a mixture of
water and a Cl-C5 lower alkanol as well as the base, e.~.,
sodium carbonate, sodium bicarbonate, potassium carbonate,
potassium bicarbonate, lithium carbonate, lithium bicarbonate,
calcium hydroxide, calcium carbonate, magnesium hydroxide,
magnesium carbonate, proline having the structure:
O
~OH
choline having the structure:
[O H]
f~O J
f ~
or a lecithin-base mixture with the lecithin having the structure:
rH2 - 0~ - R
O
CH - O - C - R'
CH2 O _ I! O CH2 - CH2 - N - CH3
1 a CH3
-15-1 3361 27
wherein the moieties:
// and //
~\ ' C~
R `~
~ , ~ ~
are defined, supra, the reaction mixture is then refluxed or
heated for a period of between about 5 hours and about 8~ hours.
During the reaction, it is desirable to remove the benzal-
dehyde-rich reaction product as it is formed. Hence, the
benzaldehyde-rich reaction product may be removed overhead
through a packed vertical reflux column connected to a cooling
heat exchanger as illustrated in Figures 7A, 7B or 7C, infra.
The product thus obtained exists in two phases; an upper aqueous
phase and a lower more dense benzaldehyde-rich phase which can
be separated from each other using a phase splitters or the
benzaldehyde-rich phase is separated from the aaueous phase, for
example, by solvent extraction using such solvents as diethyl
ether, dimethyl ether, hydrocarbons or methylene dichloride,
and the benzaldehyde-rich phase may then be fractionally
distilled. The acetaldehyde may be separated from the benzal-
dehyde by means of the use of a high efficiency fractionation
column and cooling heat exchanger. As will be seen in Figure 7A,
the acetaldehyde may be separated by trapping said acetaldehyde
in a n cold trap".
, ~
-16- 1 336 1 27
Thus, at the end of the reaction or at the end of the desired
time period for proceeding with the reaction, the "natural"
benzaldehyde~and "natural" acetaldehvde are fractionally distilled
yielding mixtures rich in natural benzaldehyde and/or acetaldehyde.
The benzaldehyde-rich fraction also may contain a considerable
proportion of unreacted cinnamaldehyde. This resulting product
may, if desired, be again fractionally distilled in order to
enrich the benzaldehyde stream. From a practical standpoint such
a mixture of cinnamaldehyde and benzaldehyde produced accordina
to the first fractional distillation is usually adequate for use
in food flavors, for example, or in tobacco flavors, for example.
Normally, but not necessarily, the acetaldehyde is prepared
free of aro~atic aldehydeq for use in food flavors.
From a practical standpoint, the mixtures of acetaldehyde,
benzaldehyde and cinnA~aldehyde thus produced have unobvious,
unexpected and advantageous properties for augmenting or enhancing
the aroma or taste of consumable materials includin~ but not
limited to foodstuffq, chewing gums, medicinal products, tooth-
pastes, chewing tobaccos, smoking tobacco and smoking tobacco
articles, particularly almond, orange and cherry flavored food-
stuffs and medicinal products.
The range of mole ratio of base to cinnamaldehyde (contained
in the c;nnA~ldehyde-Dearinq natural substance, e.g., cassia oil,
ci nni ~ bark, cinnamon leaf and the like) may vary from about
0.1:1 up to about 4:1. This mole ratio is based upon the
following:
(a) Whether the reaction is carried out on a solid
containing the cinnamaldehyde such as pulverized
cinnamon bark or in admixture with a base such
as magnesium hydroxide (in which case the higher
end of the range of mole ratios is applicable);
or whether the reaction is a liquid phase reaction
carried out in the presence of a base such as
choline, proline or aqueous sodium bicarbonate
with cinnA~Qn oil and water, alcohol or an aaueous
alcohol mixture (in which case the mole ratio
of base:cinnamaldehyde is at the lower end of
the above-mentioned range;
1 3361 27
-17-
i
(b) The nature of the total ener~y input to the reaction;
ba$ed upon heat input and time of reaction as well as
temperature and pressure of reaction (thus, a relatively
long time of reaction, e.g., 80 hours, will give rise
to a higher ~yield" of benzaldehyde and acetaldehyde
and a lower concentration of cinnamaldehyde in the
reaction product). Depending upon the flavorist's
requirements, it may be desirable to create an
ultimate composition containing, for example, greater
than 80% benzaldehyde or a 50:50 mixture of
benzaldehyde and cinnamaldehyde or substantially
pure acetaldehyde (having present therewith minor
quantities of other low boiling components such as
crotonaldehyde and acetic acid); and
(c) The particle size (where applicable) of the solid
source of c jnni -ldehyde, e.g., pulverized cinnamon
bark or pulverized high cinnamaldehyde-containing
cinni- -n leaf. A small particle size will give
rise to a faster conversion of cinnamaldehyde
(contained in the solid cinnamaldehyde-bearing
source) to benzaldehyde and acetaldehyde.
In all cases, our invention is capable of yielding in a controll-
able fashion desired ratios of benzaldehyde and acetaldehyde
to cinnamaldehyde depending upon the reaction conditions employed.
~ he reaction product containing the cinni ~ldehyde,
benzaldehyde and acetaldehyde produced according to the
reaction:
~ l~fiSE~
~' l i
i
I, 1 3 3 6 1 2 7
-18-
may be considered as a "natural" product, This "natural" product
may be used ~as is~ or it may preferably be physically purified
by such meth~ds as fractional distillation and/or preparative
chromatography. The resulting ~atural~ products will have
novel utilities in au~menting or enhancinn the aroma or taste
of consumable materials including but not limited to foodstuffs,
chewing gums, medicinal product~, toothpastes, chewing tobaccos
and smoking tobaccos particularly cherry flavored, orange
fla~ored, almond flavored foodstuffs and medicinal products.
Accordingly, for example, compositions of matter containing mole
ratios of from about 10:90 up to about 99.9:0.1 of benzal-
dehyde:cinnamaldehyde in their natural state prepared according
to the reaction:
+ o > ~11+ lh
may be utilized in such consumable materials, e.g., foodstuffs
as, for example, macaroon cookies, maraschino cherries, cherry
flavored be~erages such as carbonated cherry drinks, and the like.
Furthermore, substantially pure acetaldehyde containing
minor amounts of impurities may be utilized in such consumable
materials, e.g., foodstuffs such as oranqe drinks.
. I .
- i,,, ~
1 336 1 2 7
., --19--
Collectively, these aforementioned benzaldehyde, cinnamal-
dehyde and acetaldehyde-containing products of our invention are
hereinafter called "aldehyde-containing compositions".
The novel products of our invention may be utilized in
foodstuffs and be~erages in an amount of from about 0.5 ppm up
to about 3~ by weight of the resulting foodstuff or beverage.
The materials can be used in such high percentages because of
the manner in which they are produced; that is, free of any
nitrile-containing substances as would be present if the
aldehyde-containing products were produced from such materials
as apricot kernels.
As used herein, the term "foodstuff" includes both solid
and liquid ingestible materials which usually do, but need not,
have nutritional value. Thus, foodstuffs includes soups,
convenience foods, beverages, dairy products, candies,
vegetable cereals, soft drinks, snacks and the like.
As used herein, the term "medicinal products" includes both
solids and liquid~ which are ingestible non-toxic materials
which have medicinal value such as cough syrups, 'cough drops
and chewing medicinal tablets.
, The term "chewing gum" is intended herein to be a foodstuff
composition comprising a substantially water-insoluble,
chewable plastic gum base such as chicle, or substitutes
therefor, including jelutong, guttakay rubber or certain
comestible natural or synthetic resins or waxes. Incorporated
I with the gum base in admixture therewith may be plasticizers or
! softening agents, e.g., glycerine and a flavoring composition
j which incorporates one or more of the aldehyde-containing
co~positionsof our in~ention and, in addition, sweetenlng
agents which may be sugars, including sucrose or dextrose and/or
artificial sweeteners suc~ as cyclamates or saccharin. Other
optional ingredients may be present.
1 3361 27
., .
-20-
The term "augment" in its various forms is used herein to
mean the supplying, modifying or imparting of a flavor or aroma
characteristi~c note or nuance to an otherwise bland, relatively
tasteless or non-odorous substance or modifying an existing
flavor or aroma characteristic where the natural flavor is
deficient in some regard, or supplementing the existing flavor
or aroma impression to modify its quality, character, taste or
aroma.
,
The term "enhance" is used herein to mean the intensification
of a flavor or aroma characteristic or note without the modifi-
cation of the quality thereof. Thus, ~enhancement" of a flavor
or aroma means that the enhancement agent does not add any
additional flavor note or nuance.
Substances suitable for use herein as co-ingredients or
flavoring adjuvants are well known in the art for such use,
being extensively described in the relevant literature. It is
required that any such material be "ingestibly acceptable" and
thus non-toxic or otherwise non-deleterious, particularly from
an organoleptic standpoint whereby the ultimate flavor and/or
aroma of the consumable material used does not cause the
consumable material to have unacceptable aroma and taste
nuances.
-~ It is a further requirement that such material be
organoleptically compatible with the foodstuff with which it is
used so that the flavor and aroma nuances of such material,
taken together with the flavor and aroma nuances of the
foodstuff (as a whole) give rise to a harmoniously
aesthetically pleasing aroma and taste profile. Such material,
in general, may be characterized as flavoring adjuvants or
vehicles comprising broadly, stabilizers, thickeners, surface
active agents, conditioners, otner flavorants and flavor
intensifiers.
Stabilizer compounds include preservatives, e.g., sodium
chloride: antioxidants, e.g., calcium and sodium ascorbate,
ascorbic acid, butylated hydroxyanisole (mixture of 2- and
3-tertiary-butyl-4-hydroxyanisole), butylated hydroxy toluene
(2,6-di-tertiary-butyl-4-methyl phenol), propyl gallate and the
like and sequestrants, e.g., citric acid.
1 33 6 1 27
-21-
Thickener compounds include carriers, binders, protective
colloids, ~uspending agents, emulsifiers and the like, e.g.,
agar agar, carrageenan, cellulose and cellulose derivatives
such as carboxymethyl cellulose and methyl cellulose; natural
and synthetic gums such as gum arabic, gum tragacanth, gelatin,
proteinaceous materials; lipids, carbohydrates;
starches,pectins and emulsif,iers, e.g., mono- and diglycerides
of fatty acids, skim milk powder, hexoses, pentoses,
disaccharide~, e.g., ~ucrose, corn syrup and the like.
Surface active agents include emulsifying agents, e.g.,
fatty acids such as capric acid, caprylic acid, palmitic acid,
myristic acid and the like, mono- and diglycerides of fatty
acids, lecithin, defoaming and flavor dispersing agents such as
sorbitan monostearate, potas~ium stearate, hydrogenated tallow
alcohol and the liXe.
Conditioners include compounds such a~ bleaching and
maturing agents, e.g., benzoyl peroxide, calcium peroxide,
hydrogen peroxide and the like, starch modifiers such as
peracetic acid, sodium chlorite, sodium hypochlorite, propylene
oxide, succinic anhydride and the like, buffers and
neutralizing agents, e.g., sodium acetate ammonium bicarbonate,
ammonium phosphate, citric acid, lactic acid, vinegar and the
,~ jlike, colorants, e.g., carminic acid, cochineal, tumeric and
curcumin and the like, firming agents such as aluminum sodium
sulfate, calcium chloride and calcium glyconate, texturizers,
anti-caking agents, e.g., aluminum calcium sulfate and tribasic
calcium phosphate, enzymes, yeast foods, e.g., calcium lactate
and calcium sulfate, nutrient supplements, e.g., iron salts
such as ferric phosphate, ferrous gluconate and the like,
riboflavin, vitamins, zinc sources such as zinc chloride, zinc
sulfate and the like.
Other flavorants and flavor intensifiers include organic
acids, e.g., acetic acid, formic acid, 2-hexenoic acid, benzoic
acid, n-butyric acid, caproic acid, caprylic acid, cinnamic
acid, isobutyric acid, isovaleric acid, alpha-methyl-butyric
acid, propionic acid, valeric acid, 2-methyl-2-pentenoic acid,
- j ~
!
i 1 336 1 27
-22-
and 2-methyl-cis-3-pentenoic acid: ketones and aldehydes other
than the aldehydes of the aldehyde-containing compositions of our
invention, e~g., acetophenone~ acetone~-acet~1 methyl
carbinol, acrolein, n-butanal, crotonal, diacetyl,
beta,beta-dimethyl-acrolein, n-hexanal, 2-hexanal,
cis-3-hexenal, 2-heptenal, 4-(p-hydroxyphenyl)-2-butanone,
alpha-ionone, beta-ionone, 2.methyl-3-butanone, 2-pentanone,
-pentenal and propanal; alcohols such as l-butanol, benzyl
alcohol, l-borneol, trans-2-buten-1-ol, ethanol, geraniol,
l-hexanol, 2-heptanol, trans-2-hexenol-1, cis-3-hexen-1-ol,
3-methyl-3-buten-1-ol, l-pentenol, 1-penten-3-ol, p-hydroxy-
phenyl-2-ethanol, isoamyl alcohol, isofenchyl alcohol,
phenyl-2-ethanol, alpha-terpineol, cis-terpineol hydrate,
esters, such as butyl acetate ethyl acetate, ethyl aceto-
acetate; ethyl benzoate, ethyl butyrate, ethyl cinnamate, ethyl
crotonate, ethyl formate, ethyl isobutyrate, ethyl isovalerate,
ethyl alpha-methyl-butyrate, ethyl propionate, ethyl sali-
cylate, trans-2-hexenyl acetate, hexyl acetate, 2-hexenyl
butyrate, hexyl butyrate, isoamyl acetate, isopropyl butyrate,
methyl acetate, methyl butyrate, methyl caproate, methyl
isobutyrate, methyl-2-methyl-butyrate, propyl acetate, amyl
acetate, amyl butyrate, benzyl salicylate, dimethyl anthran-
ilate, ethyl methylphenylglycidate ethyl succinate isobutyl
cinnamate and terpenyl acetate; essential oils such as jasmin
absolute, rose absolute, orris absolute, lemon essential oil,
Bulgarian rose, yara yara, natural raspberry oil and vanilla;
lactones, sulfides, e.g., methyl sulfide and other materials
such as maltol, acetoin and acetals (e.g., l,l-diethoxyethane,
l,l-dimethoxyethane and dimethoxymethane~ ~-
The specific flavoring adjuvant selected for use may beeither solid or liquid depending upon the desired physical form
of the ultimate product, i.e., foodstuff, whether simulated or
natural, and should, in any event, be capable of providing an
environment in which the cyclic chemical compounds can be
dispersed or admixed to provide a homogeneous medium. In
addition, selection of one or more flavoring adjuvants, as well
as the quantities thereof will depend upon the precise
organoleptic character desired in the finished product. Thus,
1 336 1 27
-23-
in the case of flavoring compositions, ingredient selection
;will vary in accordance with the foodstuff to which the flavor
and aroma are,to be imparted. In contradistinction, in the
preparation of solid products, e.g., simulated foodstuffs,
ingredients capable of providing normally solid compo6itions .
should be ~elected such as various cellulose derivatives.
As will be appreciated by those skilled in the art, the
-amount of aldehyde-containing composition of our invention employed
in a particular instance can vary over a relatively wide range
whereby its desired organoleptic effects (having reference to
the nature of the product) are achieved. Thus, correspondingly
greater amounts would be necessary in those instances wherein
the ultimate food composition to be flavored is relatively
bland to the taste, whereas relatively minor quantities may
suffice for purposes of enhancing the composition merely
deficient in natural flavor or aroma. The primary requirement
is that the amount selected (to be effective) be sufficient to
augment or enhance the organoleptic characteristics of the
parent composition (whether foodstuff per se or flavoring
composition).
The use of insufficient quantities of aldehyde-containing
composition of our invention, will, of course, substantially
vitiate any possibility of obtainlng the desired results while
excess quantities prove needlessly costly and in extreme cases,
may disrupt the flavor-aroma balance, thus proving self-defeating.
Accordingly, the terminology "effective amount" and ~sufficient
amount" is to be accorded a significance in the context of the
present invention consistent with the obtention of desired
flavoring effects.
Thus, and with respect to ultimate food composition, it is
~found that quantities of aldehyde-ccntaining composition
of our invention ranging from a small but effective amount,
e,g., 0.5 ppm up to 3% by weight based on total composition
are suitable as stated, supra. Concentrations in excess of the
maximum quantity stated are not normally recommended, since
f
1 3361 27
-24-
they fail to provide cl ~nsurate enhancement of organoleptic
properties. ,In those instances where the aldehyde-containing
composition of our invention is added to the foodstuff as an
integral component of a flavoring composition, it is, of course,
essential that the total quantity of flavoring composition
employed be sufficient to yi~ld an effective amount of aldehyde-
containing composition.
Food flavoring compositions prepared in accordance with the
present invention preferably contain the aldehyde-containing
composition of our invention ranging from about 0.1~ up to about
100% by weight based on the total weight of said flavorin~
composition.
The compositions described herein can be prepared according
to conventional techniques well known as typified by cake
batters and fruit drinks and can be formulated by merely
admixing the involved ingredients within the proportions stated
in a suitable blender to obtain the desired consistency,
homogeneity of dispersion, etc. Alternatively, flavoring
compositions in the form of particulate solids can be
convenlently prepared by mixing the aldehyde-containing
composition of our invention with, for example, ~um arabic,
gum tragacanth, carrageenan and the like, and thereafter
spray-drying the resultant mixture whereby to obtain the
particulate solid product. Pre-prepared flavor mixes in powder
form, e.g., a fruit flavored powdered mix, are obtained by mixing
the dried solid components, e.g., starch, sugar and the like
and aldehyde-containing composition in a dry blender until
the requisite degree of uniformity is achieved.
. ~
The novel aldehyde composition-containing substances
produced according to the novel process of our invention may
be used "as is" as stated, supra, or may be used in con~unction
with other flavor adjuvants including but not limited to:
, .' !
1 3J61 27
-25-
Heliotropin~
Terpinenol-4;
Anisaldehyde;
Phenyl acètaldehyde;
Benzyl formate;
Benzyl acetate;
Cis-3-hexenyl benzoate:
Methyl Hexanoate;
Hexanal;
Eucalyptol,
Eugenol;
Ethyl acetate;
Ethyl butyrate;
Turpentine gum oil;
Limonene;
Gum camphor:
Isobornyl acetate;
Borneol;
Cuminic aldehyde;
Furfural;
Methyl cinnamate;
Cassia oil;
Vanillin;
Maltol;
Parahydroxybenzylacetone;
Dimethyl ~ulfide;
Alpha-ionone;
Acetic acid;
Isobutyl acetate;
Acetone;
Butyric acid;
Formic acid;
Valeric acid;
Amyl acetate;
Amyl butyrate;
Anethol.
Benzyl salicylate;
Diacetyl;
Dimethyl anthranilate;
Ethyl methylphenylglycidate,
t 33 6 1 27
-26-
~thyl succinate;
Ethyl valerate;
Geraniol,
Cis-3-hexen-1-ol;
2-Hexenyl acetate;
2-Hexenyl butyrate;
Hexyl butyrate;
4-(p-Hydroxyphenyl)-2-butanone:
Beta-ionone;
Isobutyl cinnamate;
Jasmine;
Lemon essential oil;
Methyl butyrate:
Methyl capronate;
Methyl disulfide;
Methyl p-naphthyl ketone;
Orri~ butter;
Ro~e absolute;
Terpenyl acetate;
Gamma-undecalactone;
Vanilla;
Alcohol;
Oil of Cubeb;
Phellandrene;
Beta-phellandrene;
Oil of Coriander;
Oil of-Pimento Leaf;
Oil of Patchouli;
Alpha-Pinene;
Beta-Pinene;
Beta-caryophyllene;
Dihydrocarveol;
Piperonal;
Piperine;
Chavicine;
Piperidine;
c l ~
27 1 336 1 27
Oil of 31ack Pepper;
Black Pepper Oleoresin;
Caps icum,
Oil of Nutmeg;
Cardamon Oil;
Clove Oil;
SpeA ; nt Oil; and
Oil of Peppermint.
An additional aspect of our invention provides an organo-
leptically improved smoking tobacco product and additives
therefor, as well as methods of making the same which overcome
specific problems heretofore encountered in which specific
desired sweet and fruity flavor characteristics of natural
tobacco are created or enhanced and may be readily controlled
and maintained at the desired uniform level regardless of
variations in the tobacco components of the blend.
This invention further provides improved tobacco additives
and methods whereby variouæ desirable sweet and fruity
flavoring characteristics may be imparted to smoking tobacco
products and may be readily varied and controlled to produce
the desired uniform flavoring characteristics.
In carrying out this aspect of our invention, we add to
smoking tobacco materials or a suitable substitute therefor
(e.g., dried lettuce leaves) or we add to filters for smoking
~tobacco articles (e.g., cellulose acetate filters) an aroma and
flavor additive containing as an active ingredient the
aldehyde-contA~n~ng composition of our invention which ls
the benzaldehyde/c1nni ~ldehyde composition.
In addition to the benzaldehyde/cinnamaldehyde composition
of our invention other flavoring and aroma additives may be
added to the smoking tobacco material or substituted therefor
either separately or in admixture with the benzaldehyde/cinna-
maldehyde composition of our invention as follows:
1 3361 27
-28-
I. SYNTHETIC MATERIALS
Beta-ethyl-cinnamaldehyde:
Eugenol;
Dipentene;
Beta-Damascenone;
Maltol;
Ethyl maltol;
Delta undecalactone;
Delta decalactone;
Amyl acetate:
Ethyl butyrate;
Ethyl valerate:
I Ethyl acetate;
:! 2-Hexenol;
1,2-Methyl-5-isopropyl-1,3-nonadiene-8-one;
2,6-Dimethyl-2,6-undecadiene-10-one;
2-Methyl-5-isopropyl acetophenone;
2-Hydroxy-2,5,5,8a-tetramethyl-1-(2-hydroxyethyl)-
decahydronaphthalene;
Dodecahydro-3a,6,6,9a-tetramethyl naptho-[2,1-b]-furan;
4-Hydroxy hexanoic acid, gamma lactone;
Polyisoprenoid hydrocarbons defined in Example V of
U.S. Patent No. 3,589,372 issued on June 29, 1971.
II. NATURAL OILS
Celery iseed oil;
Coffee extract;
Bergamot Oil;
Cocoa extract;
Nutmeg oil:
Origanum oil;
.''i
1 33 6 1 27
29
An aroma and flavoring concentrate containing the benzal-
dehyde/cinnamaldehyde composition of our invention and, if
,desired, one~or more of the above indicated additional flavor-
ing materials may be added to the smoking tobacco material, to
the filter or to the leaf or paper wrapper. The smoking
tobacco material may be shredded, cured, cased and blended
tobacco material or reconsti~uted tobacco material or tobacco
-cubstitutents (e.g., lettuce leaves) or mixtures thereof. The
proportions of flavoring additives may be varied in accordance
with taste but insofar as enhancement or the imparting of
natural and/or sweet notes, we have found that satisfactory
results are obtained if the proportion by weight of the sum
total of the benzaldehyde/cinnamaldehyde composition of our
invention to smoking tobacco material i8 between S and 100 ppm
(0.0005-0.01%) of the active ingredients to the smoking tobacco
material. We have further found that satisfactory results are
obtained if the proportion by weight of the sum total of
benzaldehyde/cinnamaldehyde composition of our invention used
to flavoring material is between 50 and 1000 ppm (0.005-0.1%).
Any convenient method for incorporating the benzal-
dehyde/cinnamaldehyde composition of our invention in the
tobacco product may be employed. Thus, the benzal-
dehyde/cinnamaldehyde composition of our invention taken alone
; or along with other flavoring additives may be dissolved in a
suitable solvent such as ethanol, pentane, diethyl ether and~or
other volatile organic solvents and the resulting solution may
either be spread on the cured, cased and blended tobacco
material or the tobacco material may be dipped into such
solution. Under certain circumstances, a solution of the
benzaldehyde/cinnamaldehyde composition of our invention taken
alone or taken further together with other flavoring additives
as set forth above, may be applied by means of a suitable
! applicator such as a brush or roller on the paper or leaf
wrapper for the smoking product, or it may be applied to the
- ;filter by either spraying, or dipping or coating.
Furthermore, it will be apparent that only a portion of the
tobacco or substituted therefor need be treated and the thus
treated tobacco may be blended with other tobaccos before the
ultimate tobacco product is formed.
~ l
1 3 3 6 1 2 7
-30-
!
; In such cases, the tobacco treated may have the
benzaldehyde/cinnamaldehyde composition of our invention in
excess of the`amounts or concentrations above indicated so that
when blended with other tobaccos, the final product will have
the percentage within the indicated range.
In accordance with one specific example of our invention,
; an aged, cured and shredded domestic burley tobacco is spread
with a 20% ethyl alcohol solution of a mixture containing 75~
benzaldehyde and 25~ cinnamaldehyde prepared by carrying out a
~reaction in a "Soxhlet" apparatus of the type set forth in
Figure 4 using an Mg(OH)2 catalyst. The amount of
benzaldehyde/cinnamaldehyde composition is 20 ppm on a dry
basis. Thereafter, the alcohol is removed by evaporation and
the tobacco is manufactured into cigarettes by the usual
techniques. The cigarette when treated as indicated has a
desired and pleasing sweet and fruity aroma with faint
aesthetically pleasing cherry nuances which is detectable in
the main and side streams when the cigarette is s ked. The
aroma is described as being sweeter~ rich~ less harsh~ more
tobacco-like and having fruity notes.
While our invention is particularly useful in the
manufacture of ~moking tobacco, such as cigarette tobacco,
cigar tobacco and pipe tobacco, other tobacco products formed
from sheeted tobacco dust or fines may also be used. Likewise,
the benzaldehyde/cinnar-ldehyde compositions of our invention
can be incorporated with materials such as filter tip
materials, seam paste, packaging materials and the like which
are used along with tobacco to form a product adapted for
smoking. Furthermore, the benzaldehyde/cinnamaldehyde
compositions of our invention can be added to certain tobacco
substitutes of natural or synthetic origin (e.g., dried lettuce
leaves) and, accordingly, by the term "tobacco" as used
throughout this specification is meant any composition intended
for human con~umption by smoking or otherwise. whether composed
of tobacco plant parts or substitute materials or both.
1 1 336 1 27
-31-
, .
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is the GC-IR spectrum for the reaction product of
Example I containing benzaldehyde and cinnamaldehyde.
Figure 2 is a GC-IR spectrum for the distillation residue
of Example I containing cinnamaldehyde and benzaldehyde.
Figure 3 is a GC-IR spectrum for the reaction product of
Example II containing benzaldehyde and cinnamaldehyde
(Conditions: Carbowax column programmed at 7S-225~C at 3C per
~inute~.
Figure 4 is a cut-away cross sectional elevation view of a
Soxh;et apparatus used for carrying out the reaction:
~ ~.
~ tE~SE]> ~ 1
when the cin~a~-ldehyde is present in a solid material such as
pulverized cinna~on bark and when the basic catalyst is a solid
such as ~agnesium hydroxide or calcium hydroxide.
i l
*Trade Mark
il 1336127
-32-
Figure 5 is a simplified Soxhlet reaction apparatus fitting
for carrying out the reactions
~ tB-9~E]
~ ^ ~
-- . 1 3361 27
! 33
: Figure 6 is a diagram of a solid-liquid phase reaction
apparatus useful in carryiny out the retro-aldol reaction, to
wit:
~ t8~9~']> ~ 1
when the cinnamaldehyde having the structure:
~0
o
or a mixture thereof is in existence in a natural solid
.material such as cinnamon bark.
- 34 -
1 33 6 1 2 7
Figure 7A`is a diagram of a liquid-liquid phase reaction and
recovery apparatus for carrying out the reaction:
~ tB~9X]~
and recovering the natural benzaldehyde-containinq composition
and the natural acetaldehyde-containing composition of our
~invention (as employed iD Example VI, infra).
Figure 7B is a diagram of a section of the apparatus of
Figure 7A showing the magnetic coil-actuated recovery-return
mechanis~ of the apparatus useful in the practice of our invention.
. 13~6127
-35-
Figure 7C is a diagram of a continuous liquid-liauid phase
reaction-recovery apparatus for carrying the reaction:
!
~ .
t l ~
and recovering the natural benzaldehyde-containing composition
and natural acetaldehyde-containing composition of our invention.
Figure 8 i8 the GLC profile of the reaction product
produced according to Example VI containing benzaldehyde and
cinnamaldehyde.
Figure 9 is the GLC profile of a first distillation product
of the reaction product of Example VI rich in benzaldehyde.
Figure 10 is the GLC profile of a second distillation
product of the reaction product of Example VI rich in
benzaldehyde.
Figure 11 is the GLC profile of a third distillation
product of the reaction product of Example VI rich in
benzaldehyde.
Figure 12 is a total ion current spectrum of a GC-MS analysis
of acetaldehyde-rich product recovered in cold trap 231 of the
apparatus of Pigure 7A.
1 3~61 27
-36-
DETAILED DESCRIPTION OF THE DRAWINGS
Figure 1 is the GC-IR spectrum for the crude reaction
product of Example I. The peak indicated by reference numeral
10 is the peak for benzaldehyde in the reaction product. The
peak indicated by reference numeral 11 i8 the peak for cinna-
maldehyde having the structures:
.
~ ~nd ~ ~
!
Figure 2 is the GC-IR spectrum for the distil'lation residue
of Example I containing benzaldehyde and cinnamaldehyde. The
;peak indicated by reference numeral 20 is the peak for benzal-
dehyde. The peak indicated by reference numeral 21 is the peak
for the unreacted cin~ ldehyde having the structure:
o
~ and ~
Figure 3 is the GC-IR spectrum for the crude reaction
product of Example II. The peak indicated by reference numeral
30 is the peak for benzaldehyde. The peak indicated by
reference numeral 31 is the peak for cinnamaldehyde.
11 ~
, . - ,
!
,.,~ i
-
_37_ 1 336 1 27
.
The apparatus of Figure 4 (the Soxhlet reaction apparatus)
~is used to effect the reaction: !
~o ~
when the cinnamaldehyde is present in such solid materials as
pulverized cinnamon bark. .~.
The mixture of cinnamaldehyde-bearing material (e.g.,
pulverzied cinna ~n bark ceylon) and solid natural base, (for
example, limestone or lecithin) 46 is placed in a porous
thimble 45 (the thus-filled porous thimble is placed in the
inner tube 42 of the Soxhlet apparatus). The apparatus is then
fitted to a bolt-head flask 41 containing water, a Cl-C5 alkanol
or a mixture of water and a Cl-C5 lower alkanol, e,g., methanol,
ethanol, isopropanol, n-propar.ol, n-butanol, isobutanol, t-butanol,
t-amyl alc~hol or n-amyl a1cohol and to reflux condenser 57
having a cooling jacket 54 fitted with cooling liguid inlet tube 55
surrounding a condenser surface 53. The reflux condenser havinq
outlet 56 is tightly fitted via stopper 52 to the inner tube 42
of the ~Saxhlet~ apparatus. The solvent, the water, the alkanol
Dr the water-alkanol mixture is boiled at location 40 in flask 41.
The vapor passes up through the tube 44 and is condensed by
condenser 57 and the condensed solvent falls from ~53 through
opening 56 into the thimble 45 and slowly fills thè body of the
apparatus 47. When the water or Cl-C5 alkanol or the water Cl-C5
~ower alkanol mixture contacts the mixture of pulverized cinnamal-
dehyde-bearing material and solid base (e.g., Mg(OH)2 in thimble 45,
a retro-aldol reaction is effected, thusly:
~ { ~ ~
- - 1 3361 27
. -38-
~ r ~ ~ 1
,
The result of this reaction is the formation of a water-
cinnamaldehyde-benzaldehyde-acetaldehyde mixture or a water-
cinn; aldehyde-benzaldehyde-acetaldehyde Cl-C5 lower alkanol
mi~ture. The solid-liquid reaction mass residence time in the
thimble must be sufficient to allow a final yield of benzaldehyde
and acetaldehyde in amounts of 10% or more.
When the mixture reaches the top of tube 43 , it siphons
over through tube 43 into flask 41 and thus effects removal of
~that portion of the reaction product which is "extracted" in
thimble 45. The process i~ repeated automatically as the
reaction proceeds in thimble 45, that is, the retro-aldol
reaction, to wit:
~ r ,E~
~ J
proceeds in thimble _ . The resulting "natural" benzaldehyde
~ay be isolated as by fractional distillation.
1 3361 27
39
In place of the solid base, e.g., Hq(OH)2 at location 46,
a lecithin-base mixture (lecithin is a chloline precursor having
the structure:
!
~H2 - - R
O
CH - O - ~ - R'
1 ~ CH3
~H2 ~ - - O - ~H2 - CH2 - N - CH3
~a ~H3
wherein the moietie~:
/ O and / O
~ ~\ C\
R ,p,
ha~e been defined, supra) may be added at location 40 ~ith the
reaction ta~ing place at location 40 rather than at location 46
or a natural proline or choline embedded in an inert polymer
haYing micropores such as microporous polyethylene may be
admixed with the cinnamaldehyde-bearing solid, e.g., the
pulverized clnn~o~ bark at location 46.
In the case of the reaction taking place at location 46,
the siphone tube 43 has an outlet into the flask 41 at 51
wherein the reaction product containing large amounts of ben-
zaldehyde together with water, alkanol or water/alkanol mixture
is passed through the opening 51 of siphon tube 43 and then
through the opening of the Soxhlet apparatus into flask 41.
,
1 33 6 1 27
-40-
The Soxhlet apparatus is firmly in place in a vapor-tiqht manner
as a result of the placement of tube 49 in tiahtly-fitting
stopper 48 located in the neck of flask 41 at location 50.
In the case of the reaction taking place at location 41,
the siphon tube 43 has an outlet into the flask 41 at 51 wherein
!I extracted cinnamaldehyde together with water, alkanol or
!water/alkanol mixture is passed through the opening 51 of
siphon tube 43 and then through the opening of the Soxhlet
apparatus into flask 41. The Soxhlet apparatus is firmly in
place in a vapor-tight manner as a result of the placement of
tube 49 in tightly-fitting stopper 48 located in the neck of
flask 41 at location -50.
In place of Soxhlet apparatus and tube 42, the retro-aldol
reaction can take place in an apparatus of the nature of Figure 5.
Referring now to Figure 5, the solid cinnamaldehyde-con-
taining material, for example, pulverized cinnamon bark may be
placed on a sintered glass disc 70 of Figure 5 and the entire
apparatus may be fitted onto a reaction vessel which is also
fitted with a distillation apparatus~ Hot Cl-C5 alkanol or
hot alkanol-water mixture or hot water may be added through
, opening 73 into tube 71 slowly passedthe pul~erized cinnmal-
dehyde-containing material resting on sintered alass disc 70.
The water, Cl-C5 alkanol or the water/Cl-C5 lower alkanol
! mixture may be admixed with a base such as proline, choline,
sodium bicarbonate~ potassium bicarbonate, sodium carbonate,
sodium bicarbonate, lithium carbonate or lithium bicarbonate
or a mixture of lecithin and base. In the alternative, the
cinn~ ~ldehyde-yielding material may be admixed with solid
, base (e.g., Mg(OH)2 or Ca(OH)2) while restina on sintered glass
j disc 70. The entire apparatus is fitted at 72 into a flask
haYing fitted thereto a di~tillation apparatus. As the
benzaldehyde-rich and acetaldehyde-rich reaction mixture passes
through disc 70 through opening 74 into the flask it may be
simultaneously distilled or it may be recycyled if it contains
an excessive amount of cinnamaldehyde that has not reacted and
if it is desired to create e more enriched benzaldehyde-cont-
aining product.
1336127
:
-41-
Figure 6 is a schematic diagram of a isolid-liauid phase
reaction apparatus which can be used to carry out the retro-aldol
reaction of our invention, to wit:
~o O
~ ~8
Set forth in Figure 6 is a solid-liquid retro-aldol ~- -~
reaction apparatus which is specifically described in United
!States Lettes Patent No. 1,636,550~
: . ,Specifically, in Figure 6,
the numeral 2001 designates a holder for particular~zed
- cinnamaldehyde-bearing solid, for example, particularized
cin~a -- bark or cinna n~ leaf which contains a large quantity
of cinnamaldehyde having the structures:
.
G
~0 ~
ç
1 3361 27
i
-42-
.
taken alone o`r mixed with a solid basic catalyst (e.g., Ca(OH)2
or Mg(OH)2 which is shown at 2002 in the drawin~s. Arranged
below the holder is a vaporizing apparatus for reaction solvent,
~e.g., water a Cl-C5 lower alkanol or a mixture of Cl-C5 lower
'lalkanol and water (such as a 50:50 mixture of ethanol and
water, which apparatus consists preferably of a closed container
2003 arranged in a heating bath vessel Z004 whlch may be
a hot oil bath. Heat may be applied to vessel 2004 either by
gas flame, steam coils located in the vessel, solar energy or
any other suitable means. Connected with the holder 2001 is a
condenser 2005. The condenser 2005 may be of an~ suitable
construction. It is shown as consisting of a vessel provided
with two interior headers 2006 and 2007 having a plurality of
condensing tubes 2008. The space between the headers is
supplied with a cooling fluid by means, for example, of a cold
water inlet pipe 2009. 2010 is an outlet pipe for coolinq fluid
which fluid may, if desired, be artifically cooled before beinq
introduced into the condenser to the extent necessary to completely
condense the vaporized solvent to a temoerature of 60-80C, for
example, although this temperature will necessarily vary with
the pressure in the holder.
Reference numeral 2011 indicates a pipe for conductin~ the
vaporfzed water of lower alkanol or mixture of water and lower
al~anol from ve~sel 2003 into the upper portion of the holder
2001. Reference numeral 2012 indicates a pipe leading from the
lower portion of the holder to the vessel 2003, preferably. It
i i9 desirable to form pipe 2-012 with an upward bend 2013 whereby
I the water or lower alkanol or mixture of water and lower alkanol
j will be accumulated in the holder to a certain level, that is
to say, above the body of reaction mass, that is, the pulverized
c~nn; -ldehyde-bearing solid materials such as pulverized
cinnamon bark Ceylon intimately admixed with solid basic catalyst,
e~g., Mg(OH)2 or Ca(OH)2 before beinq discharged to vessel 2003.
When the outflow from the holder is started, it is continued
siphon~cally until the holder is emptied of liquid so that the
action is intermittent. The solid-liauid reaction mass residence
time in the thimble must be sufficient to allow a final yield
of benzaldehyde and acetaldehyde in a~ounts of 10% or more.
~i t
1 3361 27
,
-43-
An evacuating mechanism is provided for maintaining a constant
I sub-atmospheric pressure in the holder, condenser and vaporizing
i vessel 2003. For example, a vacuum pump 2014 may be connected
by pipe 2015 to the top of the condenser 2005. The method of
the retro-aldol reaction applied to the treatment of the
cinnamaldehyde-bearing solid, e.g., pulverized cinn~ -n bark
or pulverized cinn ~n leaf, and using the apparatus as above
described is as follows:
The pulverized cinnamaldehyde-bearing solid, e.g., cinnamon
bark Ceylon is comminuted and placed in the holder 2001. At a
1:1 mole ratio (for example) the solid basic catalyst, e.g.,
Mg(OH)2 or MgO or CaO or Ca(OH)2 is added to the pulverized
cinnamaldehyde-bearing material (the mole ratio is based on the
cinnamaldehyde determined to be in the pulverized cinnamaldehyde-
bearing material) and allowed to stand under water, alcohol or
an aqueous alcohol mixture such as a 50:50 mixture of ethyl
alcohol and water for a period of time (e.g., 30-40 hours).
The water, alkanol or aqueous alcohol mixture may be used in an
amount approximating 40-60~ by volume of the pulverized
cinn~ ~lAehyde-bearing material, e.g., cinnamon bark Ceylon.
After the pulverized cinnamaldehyde-bearing solid, e.q.,
c~nn. cn bark hag been macerated, in this Anner, as long as
necess~ry, a volume of water, alcohol or aqueous alcohol, e.g.,
50:50 ethanol:water preferably equal to at least the volumetric
contents of the holder 2001 is placed in vessel 2003 and the
water or alcohol-water mixture in vessel 2004 is heated to a
temperature in the range of 80-100C (e.g., 85C, for example,
when a 50:50 mixture of ethanol and water is present) to bring
about vaporization of the alcohol mixture. At the same time,
the vacuum pump 2014 is started. The pump may be operated so as
to maintain a constant vacuum in the apparatus of from approxi-
mately 250 mm/Hg pressure up to approximately 750 mm/Hg pressure.
44 1 3761 27
The vaporized solvent passes from vessel 2003 through pipe
!~ 2011-into the ~pace 2016 above the material 2002 in holder and
into the conden~er 2005. ColDing in contact with the water
li cooled tubes 2008, the vapor is condensed and i8 refluxed upon
'! the pulverized cinnamaldehyde-bearing material (e.g., cinnamon
bark) treated. As ~oon as the~level of the liquid in the
holder ri~es above the upper bend of siphon 2013, the solvent
admixed with benzaldehyde~acetaldehyde and cinnamaldehyde is
drawn from the bottom of the holder and discharged into vessel2003
, by the siphoning action described. ~he ~raporization of the-solvent
and its condensation and precip~tation on the pulverized
cinnamaldehyde-bearing material, (e.g. ~ c~nn~ -n bark~ -basic
catalyst mixture (e.g., Mg(OH)2 is cor.tinuous so that the
extracting operation may be carried on as long as may be
necessary in order to remove the reaction product, that is, the
high benzaldehyde and acetaldehyde-containing reaction product
from the pulverized cinnA~aldehyde-bearing materlal~ (e.a., cinna~non
bark) to the extent desired. Ordinarily, the vaporization and
condensation of the solvent will not keep pace with its
discharge through the siphon 90 that the operation of the
apparatus so far as withdrawal of the solvent and extraction is
concerned, will be intermittent. That is, a certain amount of
the solvent will collect and remain in contact for a time with
,the pulverized cinna -ldehyde-bearing material (e.g.,
pulverized cinnA ,n bark or pulverized cinn;~ n leaf) and then
will be discharged, the holder being practically emptied of
liquid before the siphoning action is stopped.
* * * * *
Figure 7A is a schematic diagram of a liquid-liquid phase
reaction-product recovery apparatus which can be used to carry
out the retro-aldol reaction of our invention, to wit:
1 3361 27
, . ,
-45-
~ tE~ E]>
;j ~ ~
,
Set forth in Figure 7A is a liquid-liquid retro-aldol reaction-
product recovery apparatus which is composed of a reaction vessel
169 attached to a packed refluxing column 181 containing packing
(e.g., Raschig Rings or Berle Saddles) 182 up to level 183, which,
in turn, is connected to the condenser/vapor line/product recovery-
return system (hereinafter referred to as the "CVPRR system. The
"CYPR" system consists of vapor line 185 containing the-~ eter
or temperature gauge 186 connected back into the main column
through llne 188~ at the very top of the column is condenser 199
surrounded by cooling liquid in ~acket 202 with the cooling liquid
entering at 201 and exiting at 203. Fixed ~unnel 187 is located
below condenser 199 which has opening 200 leading into fixed
funnel 187. Liquid from fixed funnel 187 is directed into movable
funnel 189 which is caused to be moved by means of magnet 193
operated using magnetic coil 191 using electric timer 192. Movable
funnel 189 can cause llquid to be directed back onto packing
surface 183 through space 184 or the liquid may be directed into
tube 194 through opening 190. Hence, according to the way the
electric timer is set~ condensed liquid may intermittently be
directed back into the packing or into recovery tube 194 past
valve 197 through tube 205 past valve 207 through tube 216 and
opening 217 into separatory funnel 218. Material having a higher
vapor pressure such as acetald~hyde proceeds through tube 206
past valve 208 passed ~T~ joint 211 through valve 210 and tube 226
through opening 227 into cold trap 231 wherein the substantially
pure acetaldehvde containing minor impurities is collected
(shown by reference numeral 230).
f ~
1 3361 27
-46-
. . .
In carrying the reaction:
~ ' O
~ t8~']~
a liquid-bearing cinnamaldehyde substance, e.g., cassia oil or
cinni -n oil or natural solvent-containing cassia oil or
cinnamon oil l is placed in reaction vessel 169. Simultaneously,
or subsequently base, e.g., sodium carbonate or sodium bicarbonate
or proline or choline is placed in reaction vessel 169 with
stirring by stirrer 173 powered by stirrer motor 175 through
shaft 174. Simultaneously, a nitrogen blanket is maintained
over the stirred reaction mass using nitroqen gas pumped in
through opening 176 into the reaction vessel 169 at orifice 177.
Reaction mass 170 may also contain water or a Cl-C5 lower alkanol
such as ethyl alcohol or a mixture of water and a Cl-C5 lower
alkanol. Heating mantle 171 containing heating elements 172 is
energized while the stirrer motor is in operation causing the
reaction mixture 170 to undergo a reaction whereby a mixture of
ci~n~ ~ldehyde, acetaldehyde and benzaldehyde together with either
of the Cl-C5 alkanol solvent or the Cl-C5 alkanols solvent/water
mixture or water is vaporized through opening 180, and reaction
flask neck 179 into packed column 181 containing packing 182 and
having a packinq surface at 183. The vapor is partially condensed
in the packing 182 and the condensed material returns through the
packing back into the reaction flask for subse~uent reaction.
1 33 6 1 27
-47-
Simultaneously, part of the vapor proceeds through vapor tube 185
passed thermometer or temperature gauge 186 through tube 188
back into the column and onto condenser 199. With valve 197
open with respect to tube 198, highly volatile mixture containing
acetaldehyde proceeds passed the condenser 199 throu~h tube 198
passed tube 205 throuqh tube 206 (with valves 208 and 210 "open")
through tube 209 and through tube 226 into cold trap 231 through
opening 227. Thus, substantially pure acetaldehyde is collected
at 230 using dry ice trap 228 containing dry ice at location 229.
Less volatile condensate (e.g., a mixture rich in cinnA~ldehyde
and benzaldehyde and containing smaller amounts of acetaldehyde
is condensed at 199 and the condensate passes back through opening
200 into fixed funnel 187. The condensate then proceeds into
movable funnel 189 wherein part of the condensate is returned
through space 184 into packing 182 and then back into the
reactor 169 for subse~uent reaction and part of the condensate
is directed into tube 194 through opening 190 intermittently
as a result of the setting of electric timer 192 which operates
magnetic coil 191 which actuates magnet 193 causing movable
funnel 189 to move laterally; at one point in the ti~.e interval
causing fluid to enter opening 190 and at another point in the
time interval causing fluid to enter the packed column 181
through packinq 182. Hence with valve 197 open with respect
to tube 194-196, benzaldehyde/cinnamaldehyde reaction product
passes through the HU~ tube 195 past valve 197 through tube 205
past valve 207 (in open position) through tube -216 through
opening 217 into separatory funnel- 218 wherein water or mixture
of water and alkanol or alkanol separates out~ The benzal-
dehydefci~ ldehyde mixture is located at location 220 and thewater or water~alkanol mixture of alkanol is at location 219
separated at phase separation location 221. When the separatory
funnel fills, valve 222 is opened permittin~ benzaldehyde/cinna-
maldehyde mixture 200 to proceed into product container 224 at
location 225.
~1 1 3 3 6 1 2 7
-48-
When valve 207 is open, simultaneouslv acetaldehyde vaPors
not condensin~ may still pass through tube 206 with valve 208
and valve 210 open and valve 212 and 213 closed with the
acetaldehyde condensing in cold trap 231 coo~ed by dry ice 229
in container 228 otherwise vapors are vented to the atmosphere
if valve 210 is closed and va~ves 208, 212 and 213 are open with
'the acetaldehyde passing through tube 206 and past tube 214.
In addition, other vapors may pass through tube 215 through
tube 213 into the open atmosphere.
Referring to Figure 7B, Figure 7B shows magnetic coil 191
in the vicinity of magnet 193 whereby movable funnel 189 may be
moved so that the funnel may be positioned to direct liauid
coming into same from funnel 187 either into tube 190 for
recovery purposes or back onto packed column 181 (on packina 182)
for recycle purposes.
Referring to Figure 7C, Fia,ure 7C is a "continuous apparatus"
version of the batch type apparatus of Figure 7A.
In actuality, Figure 7C is a schematic diagram of a continuous
liquid-liquid phase reaction-product recovery apparatus which can
be used to carry out the retro-aldol reaction of our invention,
to wit:
~ '] ~ 1
-49-
1 3361 27
Set forth in Figure 7C is a liquid-liquid retro-aldol
reaction-product recovery apparatus which is composed of a
reaction vessel 304 attached to a packed refluxing column 305 -~ ~
containing packing 306 which, in turn, is connected to a cooling
heat exchanger 321 containing heat exchange tubes 322 cooled
using cooling liquid entering at 323 and exitina at 324, which,
in turn, is connected to product recovery and recycle system
composed of lines 325, 327,two-way valve 326, line 328, valve 329,.
pump 330 and line 331 and receiver 332.
In carrying out the reaction:
~o O
~ ~ >~/+~h
~ J
.. i
a liquid-bearing cinnA~Aldehyde substance, e.~., cassia oil or
C~ nn- -n oil or natural solvent-containing cassia oil or
ci n~ _r oil 310 contained in container 309 is pumped through
line 313 past valve 314 using pump 315 through line 316 into
reactor 304. Simultaneously, or subsequently, base 312 such as
aqueous sodium bicarbonate contained in holding tank 311 is
pumped through line 317 past valve 318 using pump 319 throuqh
line 320 into reactor 304 at location 300. The resulting mixture
300 which may also contain a Cl-C5 lower alkanol such as ethyl
alcohol and/or water is heated to reflux and refluxed in packed
column 305 having packing 306 (e.~., Raschig Rings or Berle
Saddles) while being stirred by stirrer 303. The refluxing
substance is continued to be refluxed in packed column 305
having packing 306 until analysis indicates that a desired
;amount of benzaldehyde and acetaldehyde has been formed in the
reaction mass 300 whereupon the heat input into reactor 304
1 3361 27
-50-
is increased whereby a significant portion of the reacting material
is distilled overhead through heat exchanger 321 cooled using
cooling liauid entering at 323 and exiting at 324. The resultin,q
;condensed material is passed through line 325 passed reflux
valve 326 through line 328 past valve 329 using pump 330 through
line 331 into receiving vessel 332. A portion of the condensed
material may be refluxed back into the reactor 304 past reflux
valve 326 through line 327 through pipe 308 back into the packed
column 305 containing packing 306 and then back into the reactor
304. In receiver 332, the lower phase is benzaldehyde and
acetaldehyde-rich (indicated by reference numeral 334) and the
uppér phase is solvent-rich (e.q., water and/or lower alkanol),
reference numeral 333. The benza _ehyde and acetaldehyde-rich
phase is then pumped into opening 338 through line 335 using
pump 336 past valve 337 through line 339 into distillation
column 340 at location 341 where overhead acetaldehyde-rich
material is distilled through line 342 past reflux valve 343
through line 345 past valve 346 using pump 347 through line 348
into receiver 350, the acetaldehyde-rich material being indicated
by reference numeral 349. The bottoms which are benzaldehyde
and cinn~ ildehyde-rich are removed through line 351 past return
valve 352 through line 354 past valve 355 using pump 356 through
line 357 into receiver 358 with the benzaldehyde/cinnamaldehyde-
rich phase indicated by reference numeral 3S9. With regard to
distillation column 340, line 344 is the reflux line for the
acetaldehyde-rich phase and line 353 is the reboiler line for
the benzaldehyde/cinn; aldehyde-rich phase.
- The benzaldehyde/cinnamaldehyde-rich phase 359 may then be
redistilled in distillation column 365 by passing the contents
of receiver 358 through line 360 past valve 361 using pum~ 362
passed line 363 into distillation column 365 at location 364.
Overhead distillate rich in benzaldehyde is then removed throuah
line 366 past reflux valve 367 through line 370 past valve 368
using pump 369 into receiver 371, the benzaldehyde-rich material
being indicated by reference numeral 37~. The bottoms which are
cinni i~ldehyde-rich are removed through line 373 past return valve
3,4 through line 378 using pump 377 past valve 376 into receiver
379, the cinn~ ehyde-rich phase indicated by reference numeral
380. The bottoms return line is indicated by reference numeral 375.
-51- 1 336 1 27
The aforementioned batch apparatus is used in the practice
of Example VIt infra.
j Figure 8 is the GLC profile for the reaction product of
Example VI wherein the reaction: !
+~o H ~
takes place. The peak indicated by reference numeral 800 is the
peak for benzaldehyde. The peak indicated by reference numeral
810 is the peak for the cinnamaldehyde.
Figure 9 is the GLC profile for a first distillation
product of the reaction product of Example VI rich in
benzaldehyde. The peak indicated by reference numeral 900 is
the peak for benzaldehyde.
Figure 10 is the GLC profile of a second distillation
product of the reaction product of Example VI rich in
benzaldehyde. The peak indicated by reference numeral 101 is
the peak for benzaldehyde.
Figure 11 is the GLC profile of a third distillation
product of the reaction product of Example VI rich in
benzaldehyde. The peak indicated by reference numeral 111 is
the pea~ for the benzaldehyde.
1 3361 27
-52- ~
Figure 12 is the total ion current spectrum of a GC-MS
analysis of the acetaldehyde-rich material condensed in the
~cold trap" 231 as indicated by reference numeral 230 on Figure 7A.
The peak indicated by reference numeral 120 is the peak for
acetaldehyde. The shoulder indicated by reference numeral 121
is for ethyl alcohol. The peik indicated by reference numeral 122
is the peak for acetic acid. The peak indicated by reference
numeral 123 is the peak for crotonaldehyde. The peak indicated
by reference numeral 124 is the peak for benzaldehyde.
.
In further illustration of this invention the following
examples are given. The instant invention should not be
- ,limited to these examples but is only limited by the scope of
the claims as set forth, infra.
1 33 6 1 27
-53-
EXAMPLE I
PREPARATION OF NATURAL BENZALDEHYDE-CINNAMALDEHYDE MIXTURE
Reaction:
~0 ~OH
~ H;~,o ~
~ J ~ 3
Into a 250 ml, three neck flask is placed 10 grams cassia
oil, 50 ml ethanol (95% foodgrade), 50 ml distilled water and 2
grams of L-proline (natural). Boiling chips are added and a
water-washed stream of nitrogen is past over the reaction mass
to help prevent oxidation of the formed benzaldehyde. The
mixture is heated to reflux and refluxed for a period of 18
hours at atmospheric pressure (82C).
The resulting product contains 40% benzaldehyde and 60
cinnamaldehyde.
I Figure 1 is the GC-IR spectrum for the resulting product.
The peak indicated by reference numeral 10 is the peak for the
benzaldehyde reaction product. The peak indicated by reference
I numeral 11 is the peak for the unreacted cinnamaldehyde.
I!
The resulting material is fractionally distilled. The
'bottoms at the end of the fractional distillation are analyzed.
Figure 2 is the GC-IR spectrum for the bottoms in the
distillation pot. The peak indicated by reference numeral 20
is the peak for benzaldehyde. The peak indicated by reference
numeral 21 is the peak for the cinnamaldehyde.
1 336 1 27
-54-
EXAMPLE II
PREPARATION OF
NATURAL CINNAMALDEHYDE AND NATURAL BENZALDEHYDE
Reaction: ~
Aqueous
Sodium
Carbonat~
~ + ~C~
J
Into a three neck flask equipped with stirrer, thermometer
and reflux condenser is placed 10 grams of cassia oil and 100
ml of a 3% aqueous sodium carbonate solution. Boiling chips
are added and a water-washed stream of nitrosen is passed over
the reaction mass to help prevent oxidation of the formed
benzaldehyde. The mixture is heated to reflux and refluxed for
a period of 7 hours (100C). The resulting macerial contains
70% benzaldehyde and 30% cinnamaldehyde.
Figure 3 is the GC-IR spectrum for the crude reaction
mass. The peak indicated by reference numeral 3,0 is the peak
for benzaldehyde. The peak indicated by reference numeral 31
is the peak for the cinnamaldehyde. (Conditions: Carbowax
column programmed at 75-225C at 3C per minute).
!;
,i l
1 3361 27
I ~ 1
-55-
EXAMPLE III
'
At the rate of 3% to two separate samples of natural cherry
liquer the product of Example I and the product of Example II
are added. In each of the cases the resulting cherry liqueur
has a more natural, more aesthetically pleasing rich, ripe
cherry aroma and taste nuance remeniscent of natural cherry
flavor. A bench panel of five members not associated with the
inventive entity of the instant application unanimously prefers
the cherry liquer containing the products of Examples I and II
to the products not containing such materials.
EXA~IPLE IV
Each of the cherry liqueurs produced,L~ Example III is in-
timately admixed with carbonated Perrier ~ water at the
weight ratio of 50:50 (Perrier ~ water;cherry liquer), The
resulting "carbonated" beverage haQ an excellent, natural
cherry aroma and taste. A bench panel of five members prefers
the "resulting cherry soda" to a similar cherry soda produced
without the use of the products of Examples I or II.
1 3J 6 1 27
-56-
EXAMPLE V
A cherry fruit puree is produced (for the purpose of adding
to an unflavored yogurt). At the level of 0.1%, each of the
products of Examples I and II i8 added to separate samples of
the cherry puree. At the rate of 10% each of the cherry puree
samples is added to unflavored yogurt and intimately admixed
therewith. A bench panel of five members not associated with
the inventive entity of the instant application unanimously
prefers the cherry flavored yogurt containing the products of
~Examples I and II to the same product not containing such
materials.
1 33 6 1 27
! -57-
; EXAMPLE VI
PREPARATION OF
NATURAL BENZALDEHYDE-CINNAMALDEHYDE MrxTuRE
AND NATURAL ACETALDEHYDE C~MPOSITION
Reaction: .
~i
~0 ~ O
Ho > [~+lh
Into a reaction vessel in the apparatus as set forth in
Figure 7A, equipped with stirrer, thermometer and reflux packed
column fitted with overhead condenser to which are connected
receivers for benzaldehyde-rich materials and acetaldehyde-rich
materials as ~pecified, supra, are placed 1 liter of water,
50 grams cassia oil and 20 ml of a 45% solution of choline base
in methyl alcohol.
The reaction mass is heated to reflux and maintained at
reflux for a period of 0.5 hours, at which point in time,
20 ml of a 45% solution of choline base in methanol is added.
The reaction mass is continued to be refluxed for a period of
4 hours, slowly removing the methanol from the system through
the overhead condenser with the reflux temperature rising from
65 to 99C. At the end of the 4 hour period, 300 ml water is added
to the reaction mass. The reaction mass is then refluxed for a
period of 8 hours. At the end of the 8 hour refluxing period,
additional heat is imparted to the reaction vessel whereby the
reaction product begins to be distilled usin~ the overhead
~ - ~c~
1 335 1 27
-58-
condenser 199 and the controlled reaction product recovery
apparatus shown in Figures 7A and 7B into (i) receiver 218
where the benzaldehyde-rich fraction 220 is collected and
tii) cold tràp 231 where the acetaldehyde-rich material 230 is
condensed and collected.
¦ The original cassia oil utilized contained 88% cinnamal-
dehyde.
The yield of benzaldehyde based on 88% cinnamaldehyde-con-
taining cassia oil iR 65%. The third distillation fraction
contained a ratio of benzaldehyde:cinnamaldehyde of 13:1.
Figure 8 is the GLC profile of the reaction product prior
to the first distillation. The peak indicated by reference
numeral 800 is the peak for benzaldehyde. The peak indicated
by reference numeral 810 is the peak for cinnamaldehyde.
Figure 9 i8 the GLC profile for the first distillation of
the benzaldehyde-rich phase 89. The peak indicated by
reference numeral 900 is the peak for ben~aldehyde.
Figure 10 iq the GLC profile for the second distillation of
the lower phase benzaldehyde-rich product. The peak indicated
by reference numeral 101 is the peak for benzaldehyde.
v
Figure 11 is the GLC profile for the third distillation of
the benzaldehyde-rich phase. The peak indicated by reference
numeral 111 is the peak for benzaldehyde.
Figure 12 is the total ion current spectrum of a GC-~S
analysis of the acetaldehyde-rich composition containing minor
impurities 230 trapped in cold trap 231 of the aPparatus of
Figure 7A. The peak indicated by reference numeral 120 is the
peak for the acetaldehyde. The shoulder indicated by reference
numeral 121 is for ethyl alcohol. The peak indicated by
;reference numeral 122 is the peak for acetic acid. The peak
indicated by reference numeral 123 is the peak for croton-
aldehyde. The peak indicated by reference numeral 124 is the
peak for benzaldehyde,
1 3 3 6 1 2 7
-59-
EXAMPLE VII
The following sweet cherry flavor formulation is prepared:
Ingredients Parts by Weight
Allyl isovalerate..,...,,,,,~,,,,,, lS.0
Amyl butyrate.... ..,,..-,,,.,,,~, 200.0
Anisic aldehyde.. ,,.,.......,......... 37.0
Anisyl acetate~.,,.,,,,~,,..,,,, 25.0
Anisyl butyrate..............,........ 12.0
Anisyl propionate.. ~.~,..... .,........ 12.0
Benzyl acetate..... ,..,..... ..,.~..... 50.0
Third distillation product
of the reaction product
of Example VI ~identified ..... ,,.4~658.0
by the GLC profile of
Figure II)
Eugenol~.. ~...... ~....,.. .,... ,,,.... 7.0
Cyclohexyl cinnamate..,......... 5.0
Cyclohexyl formate...,.,.,,,,,,.,,, 8.0
Ethyl acetate,...........,,,.. ,.. , 680.0
Ethyl butyrate....,.,,,,,,,,.,...... 152.0
Ethyl methylglycidate~....,,..,..., 100.0
Rhodinol,,,,,,,~.. ~,,,,,,,. 60.0
Beta-ionone.~,~... ,,.... ,.. ,,,,., 4.0
Jasmine absolute,,,,,,,,,.,,,,.,,,, 13.0
Citral.. ,,,.,.,..,,,,,,,,,,,,...... 1.0
Maltol (5% in ethanol)..,,,,,.. , 1.0
Orris butter.......... ,...... ,,.,.,,.... 30.0
Orris resinoid.................. 160.0
Rhodinyl formate...... ,,,.,.. .,......... 1.0
Rhodinyl isovalerate......... ..,........ 12.0
Para-Toluic aldehyde.. ~.. , 500-0
Vanillin ..~................... 400.0
Propylene glycoL .,,..,.~ ,,, 2,920.0
, .
- Total 10,000.0
1 3J61 27
-60-
The resulting flavor is compared with the same flavor
produced usi~g a mixture of bitter almond essential oil and
extracted Ceylon cinn c~ cinna~aldehyde in a combined amount
of 4658.0 parts by weight (grams). The cherry flavor containinq
the third distillation product of the reaction product of Example
VI is unanimously preferred b~ a bench panel of five members
independent of the inventive entity of the instant patent
application due to the more natural nature of the overall flavor.
The natural cherry nuances imparted using the third distillation
product of the reaction product of Example VI give rise to un-
expected, unobvious and advantageous properties of the resulting
cherry flavor formulation.
~' .
, I
i
!
!!
i
1 3361 27
61--
EXAMPLE VIII
A. Powder ~lavor Formulation
Twenty grams of the flavor composition of Example VII is
emulsified in a ~olution containing 300 grams gum acacia and
700 grams of water. the emulsion i8 spray dried with a Bowen
Lab Model Drier utilizing 260 c.f.m. of air with an inlet
temperature of 500F and an outlet temperature of 200F and a
wheel speed of 50,000 rpm.
B. Sustained Release Flavor
The fol~lowing mixture is prepared:
Ingredients Parts by Weight
Liquid cherry flavor .,............... 20
composition of
Example VII
Propylene glycoL...................... 9
Cab-O-Sil(~) M-5
(brand of silica produced , .
by the Cabot Corp. o f ............ 5
125 High Street, Boston,
~ss. 02110)
Physical properties:
Surface area:............ 200 m2/gm
Nominal particle size:... 0.012 microns
Density:................. 2.3 lbs/cu.ft.
The Cab-O-Sil~ is dispersed in the liquid cherry flavor
composition of Example VII with vigorous stirring thereby
resulting in a viscous liquid. Seventy-one parts by weight of
the powder flavor composition of Part A, supra, is then blended
into said viscous liquid with stirring at 25C for a period of
30 minutes resl~ltinq in a dry, free-flowing sustained release
flavor powder.
*Trade Mark
1 3 3 6 1 2 7
-62-
EXAMPL~ IX
Ten parts by weight of 50 Bloom pigskin gelatin is added to
ninety parts by weisht of water at a temperature of 150F. The
mixture is agitated until the gelatin is completely dissolved
and the solution is cooled to 120F. Twenty parts by weight of
the liquid flavor composition of Example VII is added to the
colution which is then homogenized to form an emulsion having a
particle size typically in the range of 5-40 microns. The
material is kept at 120F under which conditions the gelatin
will not gel.
Coacervation is induced by adding slowly and uniformly,
forty parts by weight of a 20% aqueous solution of sodium
sulphate. During coacervation of gelatin, molecuLes are
deposited uniformly about each oil droplet as a nucleus.
Gelation is effected by pouring the heated coacervate
mixture into 1,000 parts by weight of a 7% aqueous solution of
sodium sulphate at 65F. The resulting gelled coacervate may
be filtered and washed with water at temperatures below the
melting point of gelation, to remove the salt.
Hardening of the filter cake, in this example, is effected
by washing with 200 parts by weight of 37% solution of
formaldehyde in water. The cake is then washed to remove the
residual formaldehyde.
j
1 3361 27
-63-
!
EXAMPLE X
CHEWING GUM
One hundred parts by weight of chicle are mixed with four
parts by weight of the flavor prepared in accordance with
Example VIII, Part B. Three hundred parts of sucrose and one
hundred parts of corn syrup are added. Mixing i8 effected in a
ribbon blender with jacketed side walls of the type
manufactured by the Baker Perkins Co.
The resultant chewing gum blend is then manufactured into
strips one inch in width and O.l inches in thickness. The
strips are cut into lengths of three inches each. On chewing,
the chewing gum has a pleasant, long-lasting natural cherry
flavor.
EXAMPLE XI
One hundred parts by weight of chicle are mixed with
eighteen parts by weight of the flavor prepared in accordance
with ~xample IX. Three hundred parts of sucrose and one
hundred parts of corn syrup are then added. Mixing is~effected
in a ribbon blender with jacketed side walls of the type
manufactured by the Baker Perkins Co.
The resultant chewing gum blend is then manufactured into
strips one inch in width and 0.1 inches in thickness. The
strips are cut into lengths of 3" each. On chewing, the
chewing gum has a pleasant, long-lasting natural cherry flavor.
1 3361 27
-64-
~XAMPLE XII
TOOTHPASTE FORMULATION
.
i~ The following separate groups of ingredients are prepared:
Parts by Weight Ingredients
Group "A"
30.200...................... Glycerine
15.325...................... Distilled water
0.100...................... Sodium benzoate
0.125...................... Saccharin sodium
0.400...................... Stannous fluoride
Group "B"
12.500...................... Calcium carbonate
37.200...................... Dicalcium phosphate
(dihydrate)
Group "C"
2.000...................... Sodium n-lauroyl
sarcosinate
(foaming agent)
Group "D"
1.200...................... Flavor material
of Example VIII,
Part B
100.000...................... (Total)
Procedure:
1. The ingredients in Group "A" are stirred and heated in a
steam jacketed kettle to 160F.
2. Stirring is continued for an additional three to five
minutes to for~ a homogeneous gel.
~3. The powders of Group "B" are added to the gel,
while mixing until a homogeneous paste is formed.
'4. With stirring, the flavor of "D" is added and
lastly, the sodiu~ n-lauroyl sarcosinate.
! 5. The resultant slurry is then blended for one
hour. The completed paste is then transferred
to a three roller mill and then homogenized, and
finally tubed.
The resulting toothpaste, when used in a normal
toothbrushing procedure, yields a pleasant, sweet, cherry
flavor of constant strons intensity throughout said procedure
(1-1.5 minuteJ).
- ` .
1336127
-65-
EXAMPLE XIII
CHEWABLE VITAMIN TABLETS
The flavor material produced according to the process of
Example VIII, Part B, is added to a chewable vitamin tablet
formulation at a rate of 10 gm/kg which chewable vitamin tablet
formulation is prepared as f~ollows:
In a Hobart Mixer, the following materials are blended to
homogeneity:
Ingredients Gms/1000 Tablets
Vitamin C (ascorbic acid
as ascorbic acid-sodium
ascorbate mixture 1~ ........... 70.000
Vitamin Bl (thiamine
mononitrate) as Rocoat ~
thiamine mononitrate 33-1/3%
(Hoffman LaRoche) ....................... 4.000
¦Vit~mi ~2 (riboflavin) as
¦Rocoat ~ riboflavin 33-1/3%¦............ 5.000
Vitamin B6 (pyridoxine
hydrochloride) as Rocoat
pyridoxine hydrochloride
33-1/3% .........4.000
¦Niacinamdie as Rocoat ~ ¦
niacinamide 33-1/3% ¦....... ~.......... 33.000
Calcium pantothenate................... 11.000
¦Vitamin ~12 (cyanocobalami~¦
! las Merck 0.1% in qelatin ............. 3.500
Vitamin E (dl-alpha topcopheryl
acetate) as dry Vitamin E
acetate 33-1/3% Roche ................. 6.600
d-Biotin............................... 0.044
Certified lake color................... 5.000
¦ Flavor of Example VIII, Part B......... as indicated above
Sweetener sodium saccharin............. 1.000
Magnesium stearate lubricant........... 10.000
Mannitol q.s. to make.................. 500.000
*Trade Marks
1 3361 27
-66-
Preliminary tablets are prepared by slugging with flatfaced
punches and grinding the slugs to 14 mesh. 13.5 Grams dry
Vitamin A acetate and 0.6 grams Vitamin D are then added as
beadlets. The, entire blend i8 then compressed using concave
! punches at 0.5 grams each.
I
Chewing of the resultant tablets yields a pleasant, long-
lasting, consistently strong,.cherry flavor for a period of 12
minutes.
t 3361 27
-67-
EXAMPLE XIV
CHEWING TOBACCO
Onto 100 pounds of tobacco for chewing (85% Wisconsin lead
and 15% Pennsylvania lead) the following casing is sprayed at a
rate of 30%:
Ingredients Parts by Weight
Corn syrup.......................,~ 60.0
Licorice........................ ,.~ 10.0
Glycerine............................... 20.0
Fig juice...................... ,.,,, 4.6
~ Prune juice............................. 5.0
- ~lavor material of
Example ~III of
Part B ~ ,. .................... ~,. 0~4
The resultant product is redried to a moisture content of
20%. On chewing, this tobacco has an excellent substantially
consistent, long-lasting, sweet, cherry nuance (20 minutes) in
conjunction with the main fruity tobacco note,
~ l i
1 3361 27
-68-
B AMPLE XV
! ` ` F~AVOR~D FOODSTUFF
2.25 Ounces of a coconut macaroon mix distributed by Drake
Bakeries, Division of Borden,~Inc. of Columbus, Ohio 43215 is
intimately admixed at the le~e of 20 ppm with the benzaldehyde/cin-
namaldehyde mixture (second distlllation product) prepared
according to Example VI.
"
The coconut macaroon composition contains corn syrup,
coconut, sugar and egg white.
,; .
The coconut macaroon composition is then baked at 325F at
atmospheric pressure for a period of 20 minutes. The resultant
coconut macaroon cookies have an excellent ~natural coconut~
notes with intense almond nuances not present in the cookies
without the composition of Example ~I.
When the composition of Example VI is replaced with the
compositions of Examples I or Ii, a similar'hatural coconut"
~l sr' nuance is created.
l -69- 1 3~6 1 27
, I .
EXAMPLE XVI
TOBACCO FLAVOR FORMULATION AND TOBACCO
A tobacco mixture is produced by admixing the following
materials:
Ingredients ~ Part~ by Weight
Bright............................. ~ 40.1
Burley............................. ,,,, 24.9
Maryland........................... ,,,, 1.1
Turkish......................... ,.. ..,, 11.6
Stem ~flue-cured)................ ,,,,, 14.2
Glycerine.......................... ,,,, 2.8
Water................................... 5~3
Cigarettes having cellulose acetate filters are prepared
from this tobacco:
The following flavor formulation is prepared:
; Ingredient~ Parts by Weight
Ethyl butyrate.................... ,.~., .05
Ethyl valerate.................. ~..... .05
" Maltol.......................... ,.... 2.00
Cocoa extract....................... 26.00
! 'Coffee extract.................... ,,. 10.00
Ethyl alcohol (95% aqueous)........... 20.00
Water................................. 41.90
The above-stated tobacco flavor formulation is applied at
the rate of 0.1% to all of the cigarettes produced using the
above tobacco formulaton. One-third of the cigarettes are then
treated in the tobacco section thereof with 5 ppm of the
benzaldehyde/ci~n~r-ldehyde mixture produced by the third
distillation of Example VI. One-third of the cigarettes are
treated onthe cellulose acetate filter with 1 microliter of a
0.1~ ethanol solution of the cinnamaldehyde/benzaldehyde
mixture of the third distillation of Example VI.
i
1 336 1 27
, ! 70
Il
The above-stated tobacco formulation is applied at the rate
of 0.1 % to all of the cigarettes produced using the above
tobacco formulation. One-third of the cigarettes are then
, treated in the tobacco section thereof with 5 ppm of benzal-
dehyde/ci~n~r~ldehyde mixture produced by the third distil-
lation of Example VI. One-third of the cigarettes are treated
on the cellulose acetate filter with 1 microliter of a 0.1%
ethanol solution of the cinnamaldehyde/benzaldehyde mixture of
the third-distillation of Example VI.
',
, The control cigarettes not containing the mixture of ben-
zaldehyde and cinnamaldehyde produced according to the process
of Example VI and the experimental cigarettes which do contain
the mixture of benzaldehyde and cinnamaldehyde produced accord-
ing to the process of Example VI are evaluated by three-way
comparison, and the results are as follows:
In aroma, the cigarettes containing the benzaldehyde and
cinnamaldehyde of Example VI in the tobacco or in the
filter have been found to be sweeter and fruitier with
faint aesthetically pleasing cherry nuances.
In ~moke flavor, the cigarettes containing the benzaldehyde
and cinnA aldehyde mixture are more aromatic, more sweet,
;j,fruitier and slightly less harsh in the mouth and throat. In
addition, those cigarettes containing the benzaldehyde and
cinnamaldehyde mixture of Example VI in the tobacco give rise
to a fruity nuance in the taste and aroma on smoking.
~ o l ~
1 3361 27
-71-
EXAMPLE XVII
! APPLE FLAVOR FORMULATION
The following basic apple flavor formulation is prepared:
; Ingredients . Parts by Weight
Amyl acetate............................ 1.0
Gamma decalactone.................................... 1.5
Caproic acid.......... ~.............................. 1.5
n-Hexyl acetate........... ........................... 2.5
Coriander Oil............. ........................... o,5
n-Hexyl iso-butyrate...... ........................... 2.5
n-Hexanal................. ........................... 5.0
Ethyl isovalerate......... ........................... 5.0
cis-3-~eYer~ol .. - ... - .................. - - .. - .. - 18.0
Ethyl-2-methvl butyrate............. 18.0
trans-2- ~eYe~A ~ 18.0
Apple Fusel Oil..................... 26.0
~altol.............................. 0.5
95% food grade ethanol~...,........ 100.0
:~ .
.
This basic apple flavor is compared, in water, with and
without the addition of natural acetaldehyde prepared according
to Example VI at the rate of 6 ppm and at the rate of 10 ppm in
water. The flavor with the addition of the natural acetaldehyde
composition has a fresh apple ~uice character with light fruity
1 3J61 27
! -72-
i
topnotes. Both notes are missin~ in the flaYor that does not
contain the natural acetaldehyde composition of Example ~I~ For
,this reason, the flavor with the natural acetaldehyde composition
of Example VI is preferred unanimously by a three-member bench
panel.
1 33~ 1 ~ 7
-73-
_.
EXAMPLE XV I I I
A. POWDER FLA~OR ~ORMULATION
20 Grams of the flavor formulation of Example IV is emulsi-
fied in a solution containing 300 g ~um acacia and 700 g water,
The emulsion is spray-dried with a Bowen Lab Model Drter utilizing
260 c.f.m. of air with an inlet temperature of 500F., an outlet
temperature of 200F and a wheel speed of 50,000 rpm.
B. SUSTAINED RELEASE FLAVOR
; The following mixture is prepared:
Ingredients Parts by Weight
~iquid Apple Flavor of ;
Example rv 1.................... 20.00
Propylene Glycol.................. 9.00
Cab-O-Sil~-5
~rand of Silica produced by
the Cabot Corporation of ... 5.00
125 High Street, Boston,
Mass. 02110:
(Physical Properties:
Surface Area:...--.-------- 200 m2/qm
~ Nominal particle size:----- 0.012 microns
! Density:........ -.-------- 2.3 lbs/cu.ft.)
, ~ .,
~he Cab-O-Sil is dispersed in the liquid apple flavor composition
of Example XVII with vigorous stirrinq, thereby resulting in a
iscous liquid. 71 Parts by weight of the powder flavor
composition of Part 1, suPra, is then blended into the said
viscous liquid, with stirring at 25C for a period of 30 minutes,
resulting in a dry, free flowin-g sustained release powder.
1~36127'
-74-
BAMPLE ~:rX
,;
10 parts by weight of 50 Bloom pigskin gelatin ifi added
to 90 parts by weight of water at a temperature of 150F. The
mixture is agitated until the gelatin is completely dissolved
and the solution is cooled to 120F. 20 parts by weight of the
liquid apple flavor composition of ExampleXVII is added to the
solution which is then homogenized to form an emulsion having
particle size typically in the range of 5-40 microns. This
material is kept at 120F under which conditions, the gelatin
will not jell.
Coacervation is induced by adding slowly and uniformly,
40 parts by weight of a 20% aqueous solution of sodium sulphate.
During coacervation the gelatin molecules are deposited uni-
formly about each oil droplet as a nucleus.
Gelation is effected by pouring the heated coacervate
mixture into 1,000 parts by weight of 7% aqueous solution
of sodium sulphate at 65F. The resulting jelled coacervate
may be filtered and washed with water at temperatures below
the melting point of gelatin, to remove the salt.
r
Hardening of the filtered cake, in this example, is
effected by washing with 200 parts by weight of 37~ solution
of formaldehyde in water. ~he ca~e is then washed to remove
residual formal~ehyde.
1 33~
. .
--75--
EXAMPLE XX
- , CHEWING GUM
100 parts by weight of chicle are mixed with 4 parts
by weight of the flavor prepared in accordance with EXampleXVIII~).
300 Parts of sucrose and 100 parts of corn syrup are added.
Mixing is effected in a ribbon blender with jacketed walls
of the type manufactured by the Baker Perkins Co.
The resultant chewing gum blend is then manufactured
into strips 1 inch in width and 0.1 inches in thickness.
The strips are cut into lengths of 3 inches each. On chewing,
the chewing gum has a pleasant, long lasting aople flavor.
133~l27
-76-
EXAMPLE XXI
CHEWING G~
100 Parts by weight of chicle are mixed with 18 parts
by weiqht of the flavor prepared in accordance with ExampleXIX.
300 Parts of sucrose and 100 parts of corn syrup are then
added. Mixing is effected in a ribbon blender with jacketed
walls of the type manufactured by the ~aker Perkins Co.
The resultant chewing gum blend is then manufactured
into strips 1 inch in width and 0.1 inches in thickness. The
strips are cut into lengths of 3 inches each. On chewing,
the chewing gum has a pleasant, long lasting apple flavor.
13361~1
"
!
EXAMPLE XXII
i TOO~HPASTE FORMULATION
The following separate groups of ingredients are pre-
pared:
Parts by Weight Ingredient
_ _ _ _
Group ~A~
30.200 .... ,.. ,...................... ..Glycerine
15.325 .... ,......................... ..Distilled Water
.100 ......... .................... ..Sodium Benzoate
.125 ......... .................... ..Saccharin Sodium
.400 .......... .................... ..Stannous Fluoride
Group ~
12.500 .......... .................... ..Calcium Carbonate
37.200 ....... ,...................... ..Dicalcium Phosphate
(Dihydrate)
~roup ~C~
2.000 .... ,......................... , Sodium N-Lauroyl
Sarcosinate (foaming
. agent)
Group ~D~
1.200 .... ....,............ ,........ ..Flavor Material of
Example XvI~I(B)
100.00 - TO~AL
1 ~3~
-78-
1,
Procedure: `
. The inqredients in Group ~A" are stirred and heated in a
steam jacketed kettle to 160F.
2. Stirring is continued for an additional three to five minutes
to form a homogeneous gel.
3. The powders of Group "B" are added to the gel, while
mixing, until a homogeneous paste is formed.
4. With stirring, the flavor of "D" is added and lastly
the sodium-n-lauroyl sarcosinate.
5. The resultant slurry is then blended for one hour.
The completed paste is then transferred to a three
roller mill and then homogenized, and finally tubes.
The resulting toothpaste, when used in a normal tooth-
brushing procedure yields a pleasant apple flavor, of constant
strong intensity throughout said procedure (1-1.5 minutes).
133612~
-79-
EX~PLE XXIII
CHE~'ABLE VIm~IN TABLE~S
The flavor materi~ produced according to the process of
Example XVIII(B) is added to a Chewable Vitamin Tablet Formulation
at a rate of 10 gm/Kg, which Chewable Vitamin Tablet Formulation
is prepared as follows:
In a Hobart Mixer, the followin~ materials are blended
to homogeneity:
Ingredients Gms/1000 Tablets
Vitamin C (ascorbid acid)
as ascorbic acid-sodium
ascorbate mixture 1:1 ..................... 70.11
Vitamin Bl ~thiamine mononitrate)
as Rocoat thiamine mononitrate
33 1/3~ (Hoffman La Roche) ................. 4.0
jVitamin ~ (rl~oslavln) as
¦ Rocoat ~ riboflavin 33 1/3~¦.............. 5.0
vltamin ~6 ~pyrlaoxlne nydroc~lorlde)'
as Rocoat ~ pyridoxine hvdrochloride
33 1/3% ~. 4.0
¦Niacinamide as Rocoat ~ niacinamide¦
33 1/3~ 1.... 33.0
Calcium pantothenate......................... 11.5
¦Vitamin 812 (cya~ocob~lamin) asl
~Merck 0.1~ in gelatin ¦..................... 3.5
IVitamin E (dl-alpha to~opheryl acetate)l
¦ as dry Vitamin E acetate 33 1/3~ ¦ 6.6
d-Biotin.................................... 0.044
Flavor of Example ~VIII(~)........... (as indicated abovei
Certified lake color......................... 5.0
Sweetener - sodium saccharin................. 1.0
Magnesium stearate lubricant................. 10.0
Mannitol q.-. to make-------.-.............. 500.0
~Trade Mark
13~61 27
-80-
Preliminary tablets are prepared by Slugging with flat-
faced punches and grinding the slugs to 14 mesh. 13.5 g dry
Vitamin A Acetate and 0.6 g Vitamin D are then added as beadlets.
The entire blend is then compressed using concave punches at
0.5 g each.
Chewing of the resultant tablets yields a pleasant, long-
lasting, consistently strong apple flavor for a period of 12
minutes.