Note: Descriptions are shown in the official language in which they were submitted.
1 33752~
RAN 4212/54
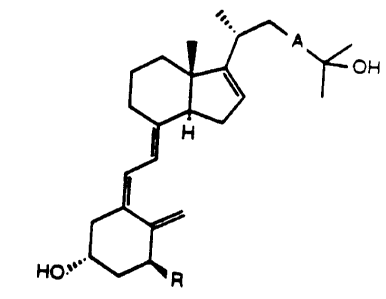
The invention relates to compounds of the focmula
~ A ~ I
H0` R
wherein R is hydrogen or hydroxy, and ~ is -C-C-,
-CH=CH- with E-configuration or -CH2-CHz-,
to pharmaceutical compositions comprising one, two or more
compounds of formula I, and to the use of said compounds for
the manufacture of such compositions useful in the treatment
of hyperproliferative skin diseases, such as psoriasis, and
for the treatment of neoplastic diseases, such as leukemia.
Examples of Cl 4-alkyl groups as referred to below,
are methyl, ethyl, propyl, isopropyl, butyl and t-butyl.
Examples of aryl-Cl 4-alkyl groups are benzyl, phenethyl
and phenylpropyl. Examples of aryl groups are phenyl and
p-tolyl. Halogen denotes bromine, chlorine, fluocine or
iodine.
Compounds of formula I of the invention are compounds
- 2 _ 1 337 529
to F as defined below:
A: 1,25-dihydroxy-16-dehydrocholecalciferol;
B: 25-hydroxy-16-dehydrocholecalciferol:
C: 1,25-dihydroxy-16,23E-bisdehydrocholecalciferol:
D: 25-hydroxy-16,23E-bisdehydrocholecalciferol:
E: 1,25-dihydroxy-16-dehyro-23-didehydrocholecalciferol: and
F: 25-hydroxy-16-dehydro-23-didehydrocholecalciferol:
among which the 1,25-dihydroxylated compounds A, C and E are
preferred.
The compounds of formulae la and Ib (encompassed by
formula I) can be prepared as described in the Schemes 1, 2
and 3 by reacting a corresponding compound of formula I
containing instead of the two or three hydroxy groups two o~
three protected hydroxy groups of the formula
-OSi(Rl,R2,R3)
wherein Rl and R3 are Cl 4-alkyl and R2 is
Cl_4-alkyl, aryl or aryl-Cl_4-alkyl,
with an agent capable of removing the protecting groups.
~ - 3 - ~ 33~ 529
Scheme 1
=~
H
O ~l
~CS~ 2 ~ R~
~ ~c~ ~ I R2-5 a~ ~
R~ R~ R~
lV~
IVb
C ~ A '~
HO-`` ~CH HO `~
wherein A is as described above and RL and R3 are
independently Cl 4-alkyl and R2 is inde~endently
Cl 4-alkyl. aryl, or aryl-Cl_4-alkyl.
_ 4 _ 1 337529
Scheme 2
~ ~OH
~/
Lo
'~.,~
~CH ~ I OH
O Vl HO Vll
. "'~0~
~'1~ 2 Ç~
o H R3 Vlll
llb
..
~CS~--R2
lla
wherein Rl, R2 and R3 are as described above.
_ - 5 - 1 337 52 9
Scheme 3
~ orS si--R2
R. ~/ X R~
R~ \~
IX
~ f ~ r ~ 2
~ I
R ~ ~?/ ~
R2--SiO H
H Xll
HO
~" ~r
H Xlll
I
~" ~`l R~
~ I 05;_R2
~ lle
o H
wherein Rl, R2 and R3 are as described above, X is
chlorine, bromine or iodine and Ts is tosyl.
~ - 6 - 1337529
The intermediates of formula II, encompassing those of
formulae IIa, lIb and IIc, are novel and are part of the
invention.
In Scheme l, the compound of formula II is converted to
a compound of formula IVa or IVb by reaction with the
corresponding compound of formula
~ POPh2
R2 SlO` ~ ~ III
R3
where Ph is phenyl: R4 is H or -OSi(Rl, R2, R3),
and Rl, R2 and R3 are as described above.
The reaction is carried out at -60 to 90C, preferably
-75C, in a polar, aprotic, organic solvent, e.g. dry ether.
or preferably dry tetrahydrofuran (THF), in the presence of
a strong base, such as an alkyl lithium, e.g. butyl lithium.
The protecting groups of a compound of formula IVa or
IVb are removed by reaction with a fluorine salt, e.g.
tetrabutylammonium fluoride in a polar, organic solvent,
e.g. ether or preferably THF, to yield a corresponding
compound of formula la or Ib.
In Scheme 2, the compound of formula V is oxidized to
the compound of formula VI by treatment with an oxidizing
agent, e.g. 2,2'-bipyridinium chlorochromate or preferably,
pyridinium chlorochromate, in an aprotic, organic solvent
such as methylene chloride.
- 7 - 1 337 52q
The compound of formula VI is converted to a compound of
formula IIb, by reaction with, e.g., a (trialkylsilyl)-
imidazole such as (trimethylsilyl)imidazole, in an aprotic
organic solvent such as THF, or preferably, methylene
chloride.
The compound of formula V may also be partially
hydrogenated to the compound of formula VII by reaction with
a reducing agent, e.g. lithium aluminium hydride, preferably
in the presence of an alkali metal alkoxide, e.g. sodium
methoxide, in an aprotic organic solvent e.g. ether, or
preferably THF, at reflux temperature (about 68C for THF),
for about 10-20 hours.
The resulting compound of formula VII is oxidized to the
compound of formula VIII by treatment with an oxidizing
agent as described above for the oxidation of V to VI.
The compound of formula VIII is converted to a compound
of formula IIa, by reaction with a (trialkylsilyl)imidazole.
as described above for the conversion of VI to IIb.
In Scheme 3, the compound of formula X is reacted in
ether, preferably THF, at reflux temperature with magnesium.
The resulting Grignard solution is treated with cuprous
iodide and then the compound of formula IX is added.
The resulting compound of formula XI is reacted with a
fluoride salt, e.g. tetrabutylammonium fluoride in ether or
preferably THF.
The obtained compound of formula XII may be oxidized as
described above for the oxidation of V to VI.
- 8 - 1 33752~
The resulting compound of formula XIII is converted to a
compound of formula IIc, by reaction with a (trialkylsilyl)-
imidazole as described above for the conversion of VI to IIb.
To prepare a compound of formula IX, the compound of
focmula
" ~ OH
~ XIV
HO
(Tetrahedron 40, 1984,2283) can be reacted with a tosylating
agent, such as a p-toluenesulfonyl halide, e.g. the
chloride, in an organic base, e.g. collidine or preferably
pyridine. The resulting compound of formula
~ OTs
XV
HO
is then converted to a compound of formula IX by reaction of
a trialkylsilyl chloride, e.g. trimethylsilyl chloride, in
the presence of imidazole and in an aprotic organic solvent,
e.g. T~IF or methylene chloride.
To prepare a compound of formula X, a compound of focmula
X-CH2CH2COCH3 XVI
~ - 9 - 1 33752q
can be converted to a compound of formula
X-CH2CH2C(CH3)2-OH XVII
wherein X is as above, by ceaction with a methyl Grignard
eeagent such as methylmagnesium bromide in ether. The
compound of formula XVII is converted to a compound of
formula X, by reaction with a trialkylsilyl chloride, as
described above for converting XV to IX.
For preparing the compound of formula V, the compound of
formula XV above is reacted with a cyanide forming agent,
e.g. sodium cyanide, in an a~rotic organic solvent, e.g.
dimethyl sulfoxide (DMS0), at a temperature between 80 and
100C for l to 5 hours to give a compound of formula
- CN
~ XVIII
HO
This is converted to the compound of formula
/~ CHO
> XIX
HO
by reaction with a reducing agent, e.g. diisobutylaluminium
hydride, followed by hydrolysis with, e.g. a mineral acid,
-- lo - 1 337529
such as hydrochloric acid. The reduction is conducted in an
aprotic organic solvent, e.g. methylene chloride, at about
-10 to 10C for about 20 to 90 minutes. The compound of
formula XIX is converted to the compound of formula
H
4`c--Br
~> Ir XX
~--
H0
by reaction with a mixture of triphenylphosphine, carbon
tetrabromide and zinc dust, in an aprotic organic solvent.
e.g. methylene chloride, for about 1 to 30 hours.
The compound of formula XX is converted to the compound
of formula
~ XXI
H0
by reaction with a strong base, e.g. butyllithium, in a
polar aprotic solvent, e.g. THF, at about -80 to -70C, for
about 1 to 3 hours. The compound of formula XXI is converted
to the compound of formula
11 1 337529
~ H
~ XXII
H
Me3 SiO
by reaction with (trimethylsilyl)imidazole in an aprotic
organic solvent, e.g. THF or methylene chloride. This
compound is converted to the compound of formula
' ~
OH XXIII
~ H
Me3 SiO
by reaction with a strong base, e.g. butyllithium and then
with acetone. The reaction is conducted in an aprotic
organic solvent, e.g. THF at about -80 to -60C. The
compound of formula XXIII is deprotected to give the
compound of formula ~ (in Scheme 2) by reaction with a
fluorine salt, e.g. tetrabutylammonium fluoride in an
organic solvent, e.g. ether or THF.
The compound of formula I stimulate differentiation and
decrease proliferation of human keratinocytes. Accordingly,
they are useful as agents in the treatment of hyper-
proliferative skin disorders, such as psoriasis, basal cell
carcinomas, disorders or keratinization and keratosis. The
compound of formula I are also useful as agents in the
treatment of neoplastic diseases, such as leukemia.
- L2 - 1 33752q
The activity of compounds of formula I as agents for the
treatment of hyperproliferative skin diseases can be
demonstrated e.g. by test procedures known in the art, such
as set forth in The Society for Investigative Dermatology
1986, 709-714. The effects of the compounds A to F above on
the morphologic differentiation of cultured human
keratinocytes compared to the effect of L,25-dihydroxy-
cholecalciferol (compound X) are expressed in the Tables 1
to 4 below, as number (xlO ) of human keratinocytes in
culture. A compound which induces the differentiation of
basal cells to squamous and envelope cells is useful as an
agent in the treatment of skin diseases characterized by
disorders of keratinization, such as psoriasis.
Table 1
Compound Dose Number of cells (xlO ):
(M)
total basal squamous envelope
Control 133i5118i4 15il 18i2
X lO_lo 122i4103i2 l9i2 23il
-8 112i689i2 23+4 30~3
lo-6 95i764i6 31+1 34+2
A lO_lo 132i8115i7 17il 27+2
-8 128ilO106i8 22~2 33+2
lo-6 lOli771i5 30i2 39~2
B lO_lo 133i6115i5 18il 25+1
-8 131i4lO9i2 22i2 2gi2
lo-6 104i474i3 30il 33il
- 13 _ 1 33752q
Table 2
Compound Dose Number of cells (xlO ):
(M)
total basal squamous envelope
Control 123i7 105i6 18il 74i7
X 10-1 116i9 95i8 21il 91i4
10-8lOlilO 75i8 26i2 122ill
1o-6 83i5 57i4 26il 146il6
C 10-1 117i4 92i2 25i2 103i6
10-8 108i3 80i2 28il 128+3
10-6 80i7 54i6 26+1 153il
D 10-1113i7 93i6 20il 104ilO
10-8 llli7 86+3 25i2 128i5
1o-6 94i3 68il 26i2 144i7
Table 3
Compound Dose Number of cells (xlO ):
(M)
total basal squamous envelope
Control 108ilO 93i8 15i2 88i8
X lo-lo106i7 86i6 18il lOOi9
10-8 84i8 61i5 23i3 122+8
10-6 73i7 51i5 22i2 142ill
E }o~l 86i4 63i2 23i2 114i5
10-8 82i3 53i2 29il 141i5
10-6 78i3 41il 27i2 147+4
3o F lO_lo103i5 81i3 22i2 103i4
10-8 97i3 67i2 29il 121+6
10-6 84i4 55i2 29il 137i7
The activity of compounds of formula I as agents for the
treatment of hyperproliferative skin diseases can also be
demonstrated by determining the number of human keratino--
cytes grown and the number of envelopes formed, as well as
the number squamous carcinoma cell lines (SCC-L15) grown in
1 337529
- 14 -
cultures, in the presence of said compounds. The results are
given in the Tables 4 and 5:
Table 4
Treatment Dose Number of Number of
(M) keratinocytes envelopes
(x104) (x102)
Control 189.49+22.3 858.28+185.70
(0.1% ethanol)
Compound E lo~l2 187.36~15.33 1136.63i 383.66
10-1 175.34+10.19 1444.87+ 312.47
1o~8 145.79il5.66 2113.62ilO49.33
41.95i 7.53 1916.83 887.66
Control 148.73il6.23 2193.7i 921.9
(0.1% ethanol)
Compound ~ lo~l2 114.91ilO.95 1662.2i 420.1
10-1 130.37i24.32 3973.8i 126.99
10 8 120.67il6.87 7235.2i 55.5
10 6 109.22il5.87 8323.5i 157.6
- 15 - 1337529
Table 5
Treatment Dose Number of
(M) cell lines (SCC-15)
(xlO
Control 7.35+1.75
Compound E lo~l2 6.98il.68
5.89+1.58
10 8 5.76il.53
lo~6 0.40iO.98
Compound A 10 0.49iO.13
The above results show that the compounds of foemula I
induce differentiation of skin cells and, accordingly, are
useful in the treatment of hyperproliferative disorders of
the skin, such as psoriasis.
In order to demonstrate the activity of the compounds of
formula I as agents for the treatment of neoplastic
diseases, the anti-proliferative (AP) and differentiation-
-inducing (DI) effects of the compounds A to F and human
promyelocytic HL-60 tumor cells were evaluated. In Table 6
the AP effect is given in percent reduction of cell number
and in concentration ID50 f the compound which reduced
the cells number by 50%. The DI effect is expressed as the
percentage of differentiated cells and as the concentration
ED50 of the compounds which induced a 50% differentiation
of the cells.
- 16 - 1337529
Table 6
Concentration % Reduction ID50 Differen- ED50
(xlO M) in cell number (x 10 M) tiated (xlO M
cells(%)
Compound X
0.01 6 3
0.1 5 11
1 16 2 19 2
66 68
100 84 98
COmPound A
0.01 10 3
0.1 33 16
1 84 0.2 92 0.2
97
100 85 98
Compound B
0.1 10 5
1 8 4
14 35 6 32
100 82 93
1000 9S 95
- 17 - 1 337 52~
Compound C
0.01 18 3
0.1 20 19
1 81 0.3 92 0.3
97
100 86 99
Compound D
0.1 12
1 12 2
17 150 17 200
100 46 31
looo 95 97
Compound E
0.01 6 9
0.1 59 50
1 80 0.0796 0.1
81 98
Compound F
0.1 13 4
1 10 12
8 70 21 70
100 58 55
1000 95 91
These data indicate that each of the compounds in
~uestion restrains the proliferation of human promyelocytic
cells, in vitro, even though they are not toxic to the
cells. Furthermore, the cells differentiate toward a more
mature phenotype at the same doses which inhibit
prolifecation. From these results it can be seen that each
- 18 - 1 337 52q
of the compounds tested is useful as an agent in the
treatment of neoplastic diseases, such as leukemia.
The compounds of formula I can be administered orally
for the treatment of neoplastic diseases or for the
tceatment of hyperproliferative skin diseases, to
warmblooded animals, which need such treatment, e.g. to an
adult human, in dosage that are in the range of about 0.1 to
10 ~g per day.
For the treatment of hyperproliferative skin diseases
the compounds of formula I can also be administered
topically to warmblooded animals which need such treatment,
in dosage of about 1 to L000 ~g per gram of topical
formulation per day.
Oral dosage forms comprising compounds of formula I may
be incorporated e.g. in capsules or tablets with
pharmaceutically acceptable carriers. Examples of such
carrier materials which may be incorporated into capsules
are binders, such as gum tragacanth or gelatin: excipients,
such as dicalcium phosphate; disintegrating agents, such as
corn starch; lubricants, such as magnesium stearate;
sweetening agents, such as sucrose; flavoring agents, such
as peppermint. Tablets may be coated with shellac, sugar or
both. A syrup or elixir may contain a sweetening agent,
methyl and propyl parabens as preservatives, a dye and a
flavoring agent.
Topical dosage forms comprising compounds of formula I
include ointments and creams encompassing formulations
having oleaginous, adsorbable, water soluble and
emulsion-type bases, such as lanolin and polyethylene
glycols. Topical dosage forms also comprise gels, lotions,
powders and aerosols. The topical compositions can also be
employed in the treatment of inflammations of the skin or of
mucous membranes, e.g. the mucous lining of the mouth or
- 19 - I 33752q
lower colon.
Lotions, i.e. liquid preparations varying from simple
solutions to aqueous of hydroalcoholic preparations
containing finely divided substances, can contain suspending
or dispersing agents, such as cellulose derivatives, e.g.
ethyl or methyl cellulose; gelatin or gums, which
incorporate the active ingredient in a vehicle made up of
water, alcohol or glycerin. Gels are semi-solid preparations
made by gelling a solution or suspension of the active
ingredient in a carrier vehicle. The vehicles, which can be
hydrous or anhydrous, are gelled using a gelling agent, e.g.
carboxy polymethylene, and neutralized to a proper gel
consistency with the use of alkalies, e.g. sodium hydroxide,
or amines, such as polyethylenecocoamine.
Example 1
a) A mixture of 3.24 g of [l(R~),3aR~-(3a~,4,7aa)]-
3a,4,5,6,7,7a-hexahydro-4-hydroxy-~,7a-dimethyl-3H-indene--
l-ethanol, 30 ml of pyridine and 3.51 g of p-toluenesulfonyl
chloride is stirred at 0 for 18 hr. After addition of ice
and dilution with water, the mixture is extracted with
methylene chloride. The organic phase is washed with lN
H2SO4, saturated NaHCO3, then dried and evaporated.
The residue is chromatographed on silica gel with ethyl
acetate-hexane (1:1:5) to afford 4.61 g (82%) of
[l(R~),3aR~-(3a~,4a,7aa)]-3a,4,5,6,7,7a-hexahydro-
4-hydroxy-~,7a-dimethyl-3H-indene-l-ethanol -4-methyl-
benzenesulfonate, [a]D + 31.9 (c 0.53, CHC13).
b) To a solution of 4.61 g of the product of a) in 22 ml ofDMSO is added 1.10 g of sodium cyanide and the mixture is
heated at 90C for 2 hours. After cooling to room
temperature, the mixture is pumped to remove the solvent,
then diluted with water. The mixture is extracted with
ether. The organic phase is washed with saturated brine,
- 20 _ 1 33 75 29
dried and evaporated. The residue is chromatographed on
silica gel with methylene chloride-hexane-ethyl acetate
(86:7:7) to give 2.52 g (91~) of [l(R*),3aR*-
(3a~,4a,7aa)]-3a,4,5,6,7,7a-hexahydro-4-hydroxy-~,7a-
dimethyl-3H-indene-l-propanenitrile, [a]D + 29.2
(c 0.65, CHC13).
c) To a mixture of 6.85 ml of diisobutylaluminium hydride
in hexane and 5.2 ml of methylene chloride at -6C is added
a solution of 0.430 g of the product of b) in 10 ml of
methylene chloride. The mixture is stirred at -6C for 55
minutes. After addition of saturated ammonium chloride. the
mixture is hydrolyzed with 3N HCl-ether (2:1). The aqueous
layer is extracted with ether. The organic layers are washed
with saturated brine, dried and evaporated. The residue is
chromatographed on silica gel with ethyl acetate-hexane
(1:2) to afford 260 mg (60%) [l(R*),3aR*-(3a~,4a,7aa)]-
-3a,4,5,6,7,7a-hexahydro-4-hydroxy-~,7a-dimethyl-3H-indene-
-l-propanal, [a]D + 43.1 (c 0.32, CHC13).
d) A mixture of 1.77 g of triphenylphosphine, 2.23 g of
carbon tetrabromide, 441 mg of Zn dust and 23 ml of
methylene chloride is stirred at 25C for 31 hours. To this
mixture is added a solution of 0.430 g of the product of c)
in 38 ml of methylene chloride and the mixture is stirred
for 18 hours. The mixture is diluted with pentane and
insoluble material is filtered off. The insoluble fraction
is dissolved in methylene chloride and the solution is again
diluted with pentane. After filtration, the combined
~iltrates are evaporated. The residue is purified on silica
gel with 1:4 ethyl acetate-hexane tO give 0.490 g (67%) of
[l(R*),3aR*-(3a~,4a,7aa)]-1-(4,4-dibromo- l-methyl
-3-butenyl)-3a,4,5,6,7,7a-hexahydro-7a-methyl-3H-
indene-4-ol, [a]D + 14.4 (c 0.55, CHC13).
e) To a solution of 0.680 g of the product of d) in 3L ml
of THF at -75C are added dropwise 3.77 ml of 1.6M solution
- 1 33752~
of butyllithium in hexane. The mixture is stirred at -75C
for 1 hour and at 25C for 1 hr. After addition of saturated
brine, the mixture is diluted with saturated aqueous
NaHC03 and extracted with ether. The organic phase is
washed with saturated brine, dried and evaporated. The
residue is chromatographed on silica gel with ethyl
acetate-hexane (1:4) to afford 0.350 g (89%) of [l(R*),3aR~-
(3aB,4a,7aa)]-3a,4,5,6,7,7a-hexahydro -7a-methyl-1-
(l-methyl-3-butynyl)-3H-inden-4-ol, [a]D + 30.7
(c 0.42, CHC13).
f) To a solution of 1.29 g of the product of e) in 80 ml of
methylene chloride are added 3.59 g of l-(trimethylsilyl)-
imidazole. The mixture is stirred at 25C for 3 hours. After
adding 40 ml of water and stirring for 20 minutes, the
mixture is extracted with ethyl acetate. The organic phase
is washed with water and saturated brine, dried and
evaporated. The residue is purified on silica gel with ethyl
acetate-hexane (1:15) to give 1.70 g (99%) of [l(R*),3aR*-
(3aB,4a,7aa)]-3a,4,5,6,7,7a-hexahydro -7a-methyl-1-
(l-methyl-3-butynyl)-4-[(trimethylsilyl)oxy] -3H-indene,
[a]2 + 39.7O (c 0.30, CHC13).
g) To a solution of 1.70 g of the product of f) in 48 ml of
THF at -75C are added dropwise 6.01 ml of 1.6M butyllithium
in hexane. After stirring for 40 minutes, 3.05 ml of acetone
are added and the mixture is stirred at --75C for Z0 minutes
and at 25C for 75 minutes. After addition of 40 ml of a 1:1
mixture of 2M KHCO3 and lM potassium sodium tartrate, the
mixture is stirred for 20 minutes and then extracted with
ethyl acetate. The organic phase is washed with saturated
brine, dried and evaporated. The residue is chromatographed
on silica gel with ethyl acetate hexane (1:5) to give 1.62 g
(89~) of [l(R*),3aR*-(3aB,4a,7aa)]-6-(3a,
4,5,6,7,7a-hexahydro-7a-methyl--4-[(tcimethylsilyl)oxy]-
3H-inden-l-yl)-2-methyl--3-heptyn-1-ol, [a]2 + 39.7O
- 22 - ~ 337 529
(c 0.30, CHC13).
h) To a solution of 1.62 g of the product of g) in 53 ml of
THF are added 15.5 ml of lM tetrabutylammonium fluoride in
THF. The mixture is stirred for 50 minutes. After dilution
with half saturated NaHC03, the mixture is evaporated to
remove most of the solvent and extracted with ethyl acetate.
The organic phase is washed with half saturated brine, dried
and evaporated. The residue is chromatographed on silica gel
with ethyl acetate-hexane (1:1) to give 1.17 g (82%) of
[l(R*),3aR*-(3aB,4a,7aa)]-3a,4,5,6,7,7a-hexahydro
-1-(1,5-dimethyl-5-hydroxy-3-hexynyl)-7a-methyl-3H-inden-4-ol,
m.p. 105-107.
i) To a solution of 0.720 g of the product of h) in 44 ml
of methylene chloride are added 1.59 g of sodium acetate and
3.18 g of 2,2'-bipyridinium chlorochromate. The mixture is
stirred for 2 hours. Additional 1.59 g of 2,2~-bipyridinium
chlorochromate are then added and the stirring continued for
2 hours. Then, after the addition of 6 ml of 2-propanol, the
mixture is diluted with water and extracted with ether-ethyl
acetate (1:1). The organic phase is washed with water, lN
H2S04, saturated NaHCO3 and saturated brine. After
drying, the solution is evaporated and the residue chroma-
tographed on silica gel with ethyl acetate-hexane (1:1) to
give 0.560 g (78%) of [l(R*),3aR*-(3aB,7aa)]-
-3,3a,5,6,7,7a-hexahydro-1-(5-hydroxy-1,5-dimethyl-
-3-hexynyl)-7a-methyl-4H-inden-4-one, [a]D +35.3 (c
0.36, CHC13).
j) To a solution of 0.552 g of the product of i) in 70 ml
of methylene chloride are added 2.00 g of l-(trimethyl-
silyl)imidazole. After stirring for 17 hours and addition of
22 ml of water, the mixture is extracted with ethyl acetate.
The organic phase is washed with water and saturated brine,
then dried and evaporated. The residue is chcomatographed on
silica gel with ethyl acetate-hexane (1:4) to give 0.693 g
- 23 - 1 33 7 52~
(99%) of [l(R~),3aR~-(3a~,7aa)]-3,3a,5,6,7,7a-hexahydro
-1-(1,5-dimethyl-5-[(teimethylsilyl)oxy]-3-hexynyl)
-7a-methyl-4H-inden-4-one, [a~D + 29.5 (c 0.20,
CHC13).
k) To a solution of 2.00 g of ~3S-(lZ,3a,5~)]-
[2-[3,5-bis[[(l,l-dimethyl)dimethylsilyl]oxy~-2-methylene-
cyclohexylidene]ethyl]diphenylphosphine oxide in 45 ml of
THF at -75C are added dropwise 1.87 ml of 1.6M butyllithi~m
in hexane. After stirring for 6 minutes a solution of
0.693 g of the product of j) in 26 ml of THF are added
dropwise. After stirring at -75C for 70 minutes and
addition of a 1:1 mixture of lM potassium sodium tartrate
- and 2M KHC03, the mixture is extracted with ethyl acetate.
The organic phase is washed with saturated brine, dried and
evaporated. The residue is chromatographed on silica gel
with ethyl acetate-hexane (1:15) to give 1.23 g (87%) of
(la,3~,5Z,7E)-1,3-bis[[l,l-dimethylethyl)dimethylsilyl]
-oxy-25-[(trimethylsilyl)oxy]-9,~0-secocholesta-5,7,10(19),16-
tetraene-3-yne, ~a]D + 47.1 (c 0.2L, CHC13).
1) To a solution of 0.228 g of the product of k) in 11 ml
of THF are added 1.92 ml of lM tetrabutylammonium fluoride
in THF. The mixture is stirred for 16 hours. After dilution
with water, the mixture is extracted with ethyl acetate. The
organic phase is washed with half saturated brine and
saturated brine, dried and evaporated. The residue is
purified on silica gel with ethyl acetate-hexane (3:1) to
afford 0.126 g (96%) of 1,25-dihydroxy-16-dehydro-23-dide-
hydeocholecalciferol, [a]D + 21.5+ (c 0.20, MeOH).
E~ 2
a) As described in Example lk), but starting from 0.343 g
of [5S-(lZ)]-[2-[5-[[(1,1-dimethylethyl)dimethylsilyl]oxy]
-2-methylenecyclohexylidene]ethyl]diphenylphosphine oxide
and 0.186 g of the product of Example Lj), thece were
,
- 24 - 1 33 75 29
obtained 0.205 g (80%) of (3~,5Z,7E)-3-[[(L,l-dimethyl-
ethyl)dimethylsilyl]oxy]-25-[(trimethylsilyl)oxy]
-9,10-secocholesta-5,7,10(19),16- tetraen-23-yne, MS m/e 580
(M ).
b) By treating 0.248 g of the product of a) as described in
Example 11), there were obtained 0.153 g (91%) of
25-hydroxy-16-dehydro-23-didehydrocholecalciferol,
[a]D +99.6 (c 0.25), MeOH).
Example 3
a) To a mixture of 0.146 g of lithium aluminum hydride,
0.211 g of sodium methoxide and 6.5 ml of THF at 0C is
added dropwise a solution of 0.180 g of the product of
Example lh) in 13 ml of THF. The mixture is heated at 68C
for 16 hours and recooled at 0C. ~fter dilution with 13 ml
of ether and addition of 0.30 ml of water and 0.26 ml of 10
aqueous NaOH, the mixture is stirred at room temperature for
1 hour and filtered. The solids are triturated with ether
and filtered. The combined filtrates are evaporated and
chromatographed on silica gel with ethyl acetate-hexane
(1:2) to give 0.179 g (99%) of [l(R~),1(3E),3a~,4,-
7aa)]-(3a,4,5,6,7,7a-hexahydro--1-(5-hydroxy-1,5-dimethyl-
-3-hexenyl)-7a-methyl-lH-inden-4-ol, [a]D +11.5~ (c
0.33, CHC13).
b) To a solution of 0.120 g of the product of a) in 10 ml
of methylene chloride are added 0.500 g of pyridinium
dichromate and 25 mg of pyridinium p-toluenesulfonate. The
mixture is stirred for 135 minutes. ~fter addition of 40 ml
of ether, the mixture is sticred for 5 minutes and filtered.
The solids are triturated with ether and filtered. The
combined filtrates are washed with saturated aqueous
CUSO4, water, half saturated aqueous NaHCO3 and
saturated brine. The organic phase is dried and evaporated.
The residue is chromatographed on silica gel with 35% ethyl
- 25 - 1 337 529
acetate-hexane to give 90 mg (76%) of [l(R~),1(3E),(3a~,
7a)]-3,3a,5,6,7,7a-hexahydro-1-(5 -hydroxy-1,5-
dimethyl-3-hexenyl)-7a-methyl-4H-inden-4-one,
~a]D +30.6 (c 0.17, CHC13).
c) By treating 0.099 g of the product of b) as desccibed in
Example li), there are obtained 0.111 g (89%) of
[l(R~),1(3E),(3a~,7aa)]-3,3a,5,6,7,7a-hexahydro-L-(1,5-
-dimethyl-5-[(trimethylsilyl)oxy]-3-hexenyl)-7a-methyl-
-4H-inden-4-one, [a]D +26.4 (c 0.22, CHC13).
d) As described in Example lk), starting from 0.265 g of
[3S-(lZ,3a,5~)]-[2-[3,5-bis[[(l,l -dimethyl)dimethyl-
silyl]oxy] -2-methylenecyclohexylidene]ethyl]diphenyl-
phosphine oxide and 0.095 g of the product of c), there areobtained 0.162 g (83%) of (lB,3a,5Z,7E,23E)-1,3-bis[[(l,l
-dimethylethyl)dimethylsilyl]oxy]-25-[(trimethylsilyl)oxy]
-9,10-secocholesta-5,7,10(19),16,23-pentaene, MS m/e 712
(M ).
e) By treating 0.159 g of the product of d) as in Example
11), there are obtained 0.077 g (84) of 1,25-dihydroxy-
16,23E-bisdehydrocholecalciferol, []24 +46.5 (c
0.20, MeOH).
Example 4
a) As described in Example lk), starting from 0.225 g of
[5S-(lZ)]-[2-[5-[[(1,1-dimethylethyl)dimethylsilyl]oxy]
-2-methylenecyclohexylidene]ethyl]diphenylphosphine oxide
and 0.110 g of the product of Example 3c), there are
obtained 0.150 g (81%) of (3~,5Z,7E,23E)-3-[[(1,1-
dimethylethyl)-dimethylsilyl]oxy] -25-[(t~imethylsilyl)-
oxy]-9,10-secocholesta -5,7,10(19),16,23 pentaene,
[a]D +68.3 (c 0.18, CHC13).
- 26 _ 1 33 7529
b) By treating 0.144 g of the product of a) as described in
Example 11), there are obtained 0.076 g (78%) of
25-hydroxy-16,23E-bisdehydrocholecalciferol,
[a]22 +6Z 5 (c 0 20 MeOH)
Example 5
a) To a solution of 6.25 g of ethyl 3-bromopropionate in
28 ml of THF at -20C are added 28.8 ml of 2.8M
methylmagnesium bromide in ether. The mixture is stirred at
room temperature for 170 minutes. After addition of 15 ml of
saturated aqueous ammonium chloride and of 42 ml of lN HCl,
the organic phase is separated and the aqueous phase
extracted with ether. The organic extracts are washed with
saturated brine, dried and evaporated. The residue is
chromatographed on silica gel with 30% ethyl acetate-hexane
to give 2.57 g (45%) of 4-bromo-2-methyl-2-butanol, MS m/e
151 (M -CH3).
b) To a solution of 2.56 g of 4-bromo-2-methyl-2-butanol
and 4.86 g of imidazole in 15 ml of N,N-dimethylformamide at
0C are added 6.48 g of chlorotriethylsilane. The mixture is
stirred at room temperature for 200 minutes. After adding
ice, the mixture is diluted with water and extracted with
pentane. The organic phase is washed with water and
saturated brine, dried and evaporated. The residue is
chromatogrpahed on silica gel with pentane to give 4.02 g
(93%) of (3-bromo-1,1-dimethylpropoxy)triethylsilane, MS m/e
265 (M -CH3).
c) To a solution of 0.930 g of [l(R~),3aR~(3a~,4a,
7aa)]-3a,4,5,6,7,7a-hexahydro -4-hydroxy-~,7a-dimethyl-
3H-indene-l-ethanol 4-methyl-benzenesulfonate and 1.10 g of
imidazole in 73 ml of methylene chloride at 0C are added
0.580 g of chlorotriethylsilane. The mixture is stirred at
room temperature for 1.5 houcs. After adding ice, the
mixture is diluted with water and stirred foc 20 minutes.
- 27 _ 1 337 52~
The organic layer is extracted with methylene chloride. The
extracts are washed with water, lN N2SO4, satucated
aqueous NaHC03 and saturated bcine. After drying and
evaporation, the residue is purified on silica gel with
ethyl acetate-hexane (l:S) to afford 1.22 g (100%) of
[l(R*),3aR*-(3aB,4a,7aa)]-3a,4,5,6,7,7a-hexahydro
-4-[(triethylsilyl)oxy]-~,7a-dimethyl-3H-indene-l-ethanol
4-methylbenze sulfonate, [a]D +46.1 (c 0.3L,
CHC13).
d) To a solution of 3.08 g of (3-bromo-1,1-dimethyl-
propoxy)triethylsilane in 31 ml of THF are added 0.282 g of
magnesium. The mi-xture is heated at 68C for 3.5 hours. Then
a mixture of 0.686 g of cuprous iodide and the above
mentioned Grignard solution are stirred at 3C for 30
minutes. To this is added a solution of 1.02 g of the
product of c) and the mixture is stirred at room tempecature
for 40 minutes. After adding a mixture of ice and water, the
mixture is extracted with ether. The organic phase is washed
with lN H2S04 and saturated aqueous NaHC03, deied and
evaporated. The residue is chromatographed on silica gel
with ethyl acetate-hexane (l:lS) to afford 1.80 g of
[l(R*),3aR*-(3a~,4a,7aa)]- 3a,4,5,6,7,7a-hexahydro-
-1-[1,5-dimethyl-5-[(triethylsilyl)- oxy]hexyl]-
-4-[(triethylsilyl)oxy]-7a-methyl-3H-indene, MS m/e 479
(M -Et).
e) To a solution of 1.60 g of the product of d) in 5 ml of
THF are added 2.00 ml of lM tetrabutylammonium fluoride in
THF. The mixture is heated at 68C for 50 minutes. After
cooling to coom temperature, the mixture is diluted with
water and extracted with methylene chloride. The organic
phase is washed with brine, dried and evaporated. The
residue is purified on silica gel with ethyl acetate-hexane
- 35 (1:1) to afford 0.420 g (79%) of [l(R*),3aR*-(3a~,4aa,
7aa)]-3a,4,5,6,-7,7a-hexahydro-4-hydroxy-a,a-- F, 7a-
tetramethyl-lH-indene-l-pentanol, [a]D =~12.0
- 28 - 1 337 52~
(c 0.25, CHC13).
f) To a solution of 0.210 g of the product of e) in 18 ml
of methylene chloride are added 0.870 g of pyridinium
dichromate and 44 mg of pyridinium p-toluenesulfonate. The
mixture is stirred for 175 minutes. After addition of 50 ml
of ether, the mixture is stirred for 5 minutes and filtered.
The solids are washed with saturated aqueous CUSO4, water,
half saturated a~ueous NaHCO3 and saturated brine. The
organic phase is dried and evaporated. The residue is
chromatographed on silica gel with 35% ethyl acetate-hexane
to give 0.175 g (84%) of [L(R*),3aR*--(3a~,7aa)]-
3,3a,5,6,7,7a-hexahydro-1-(5-hydroxy-1,5-dimethylhexyl)-
7a-methyl-4H-inden--4-one, ~a]D +28.2 (c 0.22,
cHc13).
g) By treating 0.168 g of the product of f) as described in
Example lj), there are obtained 0.211 g (100%) of
[l(R*),3aR*-(3a~,7aa)]-3,3a,5,6,7,7a-hexahydro-1-(1,5-
dimethyl-5-[(trimethylsilyl)oxy~hexyl)--7a-methyl-4H-inden-
4-one, [a]2 +21.9 (c 0.27, CHC13).
h) As described in Example Lk), starting from 0.581 g of
[3S-(lZ,3a,5B)]-[2-[3,5-bis[[(l,l-dimethyl)dimethylsilyl]-
oxy]-2-methylenecyclohexylidene]ethyl]diphenyl phosphine
oxide and 0.210 g of the product of g), there are obtained
0.358 g (83%) of (la,3~,5Z,7E)-1,3-bis[[(l,l-dimethyl-
ethyl)dimethylsilyl]oxy]-25-[(trimethylsilyl)oxy]-9,10-
secocholesta--5,7,10(19),16-tetraene, MS m/e 714 (M ).
i) Treating 0.350 g of the product of h) as described in
Example 11), there are obtained 0.168 g (83%) of
1,25-dihydroxy-16-dehydrocholecalciferol,
[a]D +40 0 (c 0.17, MeOH).
- 29 _ 1 337 529
Example 6
a) As described in Example lk) staeting from 0.383 g of
[5S-(lZ)]-[2-[5-[[(1,1-dimethylethyl)dimethylsilyl]oxy]--
2-methylenecyclohexylidene]ethyl]diphenylphosphine oxide and
0.188 g of the product of Example 5g), there are obtained
0.245 g (78%) of (3a,5Z,7E)-3-[[(1,1-dimethylethyl)-
dimethylsilyl]oxy]-25-[(trimethylsilyl)oxy]-9,10-seco-
cholesta-5,7,10(19),16-tetraene, []D +67.5
lo (c 0-20, CHC13).
b) Treating 0.239 g of the product of a) as described in
Example 11) affords 0.135 g (83%) of Z5-hydroxy-16-dehydro-
cholecalciferol, [a]D +75.4 (c =.13, MeOH).
The following Example A and B illustrate the composi-
tion of soft gelatine capsules for oral administration and
of a topical cream:
Example A
. mq/capsule
Compound E 0.000L-0.010
Butylated hydroxytoluene 0.016
25 Butylated hydroxyanisole 0.016
Fractionated coconut oil 160.0
_ 30 _ 1337529
Example B
mq/q of cream
Com~pound E O.OOl-l.0
5 Cetyl alcohol l.5
Stearyl alcohol 2.5
Soebitan monostearate 2.0
Glyceryl monostearate and
polyoxyethylene glycol stearate 4.0
10 P0lysorbate 60 l.0
Mineral oil 4.0
Propylene glycol 5.0
Propylparaben 0.05
Butylated hydroxyanisole 0.05
15 Sorbitol solution 2.0
Edetate disodium O.Ol
Methylparaben 0.18
Distilled water q.s. to lO0 g