Note: Descriptions are shown in the official language in which they were submitted.
~ 13376~3
..
. `
.
METHOD AND MICROORGANISM FOR THE
PRODUCTION OF FAERIEFUNGIN
BACKGROUND OF THE INVENTION
(1) Field of the Invention
The present invention relates to a novel
carbonyl pentaene macrolide composition, referred to as
"faeriefungin", and the compounds contained therein as
produced by a unique strain of Streptomyces which are
antimicrobial, nematicidal and insecticidal. In
particular, the present invention relates to faeriefungin
produced by Streptomyces griseus var. autotrophicus var.
10 nov ATCC 53668.
(2) Prior Art
The prior art has described carbonyl pentaene
macrolide mixtures having the molecular formulae C36HsgOlo
and C37H60Olo which are stereoisomers of the faeriefungin
15 of the present invention. However, the prior art
stereoisomeric forms have a limited ability to inhibit
microorganisms, have significant cytotoxicity, and are
unstable at room temperature.
The stereoisomer "mycoticin" from Streptomyces
20 ruber has been described by Burke, R. et al in Journal of
Investigative Dermatology 23: 163-168 (1954). Mycoticin
was active in vitro against yeasts and molds, was rapidly
inactivated and relatively highly cytotoxic. It produced
hemolysis in blood agar medium. The molecular and
25 structural formulae for mycoticin were accurately
determined much later by Wassermann, et al, J. of American
Chemical Society 89, 6 (1967) and Wassermann, et al.,
Chemical Communications, 1634 (1970) and detailed
analytical data was presented. The structural formulae
.. ~
1337643
~ were as follows:
O ~;~
~23 ~
R ~ Oll Ol1 O~ O~ OH O
IA,R=H
B,R=CH3
where R is H- or CH3- and referred to as "A" and "B",
respectively. It is uncertain whether the pentaene
macrolides being produced in the Wassermann et al studies
were the same as those of Burke et al because of
differences in the data.
Flavofungin is described by Bekesi, Nature, Vol.
181: 908 (1958). It was inactive against bacteria.
Schneider, et al, Russian Journal (1967) compare related
pentaene macrolide compositions. The mycoticin
(mycothicin) from Streptomyces ruber is indicated to be a 1
to 1 mixture of A and B. Flavofungin is described as a 9:1
mixture of A to B. Antiviral activity is described.
Vetlugina, L. A., et al, Russian Journal (1974) set forth
further analytical data including chromatographic spectra.
Bognar et al, Tetrahedron Letters 7, 471-474 (1970)
concludes that mycoticin and flavofungin contain the same
compounds in different proportions. The ratio of A to B in
flavofungin is set forth as 9:1. Bognar et al., J.C.S.
Perkin - I 1848 (1972) describe flavofungin and mycoticin.
Analytical data is presented.
Summaries of the properties of flavofungin and
mycoticin are set forth in Antibiotics Vol. II Koryyoski et
al, American Society for Microbiology (1978) and in
Kirk-Othmer 3, pages 21-47 (1978).
Objects
It is an object of the present invention to
provide a novel pentaene macrolide composition containing
compounds having molecular formulae which are the same as
flavofungin and mycoticin but with different stereoisomeric
--` 13376~3
--3--
structures, stabilities and antimicrobial properties.
Further it is an object of the present invention to provide
a broad spectrum antimicrobial composition which inhibits
certain virus, fungi and bacteria. Further still, it is an
object of the present invention to provide an antimicrobial
composition from a unique microbial strain. These and
other objects will become increasingly apparent by
reference to the following description and the drawings.
In the Drawings
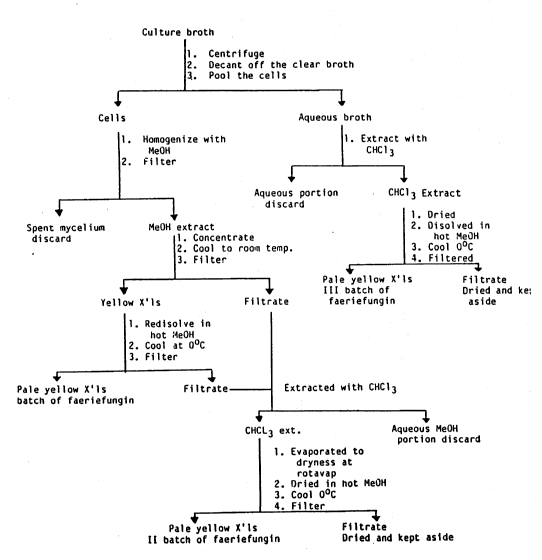
Figure 1 is a flow chart showing the method for
isolation of faeriefungin using methanol (MeOH) or
trichloromethane (CHC13).
Figure 2 shows an x-ray diffraction pattern for
the novel pentaen,e macrolide composition of the present
invention, referred to as faeriefungin (FUNGIN.RD), wherein
the ratio of A to B by weight is about 1 to 1.
Figure 3 shows an x-ray diffraction pattern for
the prior art mycoticin (MYCON.R~; MYC) wherein the ratio
of A to B is 1 to 1.
General Description
The present invention relates to a carbonyl
pentaene macrolide compound from a composition referred to
as faeriefungin having a structural formula
O =~
R ~ Ol~ Oll O~ O~ OH Ol~
wherein R is selected from hydrogen (A) and methyl (B) and
all double bonds are trans and having negative optical
rotation and an x-ray diffraction pattern showing 28
crystalline peaks at a d-spacing between 1.5408 and 43.9165
Angstroms for a 1 to 1 molar ratio of A to B. The d values
are shown in Table 4.
Further the present invention relates to a
carbonyl pentaene macrolide composition referred to as
faeriefungin characterized by a melting point of 209-212C
1337643
-4-
- with partial decomposition, by a molecular formulae
selected from the group consisting of C36HsgOlo and
C37H60Olo and mixtures thereof and positive ion fast atom
bombardment mass spectrometry indicated molecular ions of
651.4017 for C36HsgOlo and 665.4237 for C37H61Olo, a
negative optical rotation and an x-ray diffraction pattern
as shown in Figure ~ or a 1 to 1 molar mixture.
Further still the present invention relates to a
composition which comprises Streptomyces griseus var.
autotrophicus var. nov ATCC 53668 and a synthetic culture
medium which maintains the Streptomyces griseus.
The present invention also relates to a method
for producing a carbonyl pentaene macrolide referred to as
faeriefungin which comprises growing Streptomyces griseus
var. autotrophicus var. nov ATCC 53668 mycelia in an
aqueous growth medium containing sources of carbon,
nitrogen and inorganic substances to produce the carbonyl
pentaene macrolide having a negative optical rotation
pattern and an x-ray diffraction pattern as shown in Figure
~ 2
The present invention also relates to a method
for inhibiting the growth of a microorganism which
comprises exposing the microorganism to an effective amount
of a carbonyl pentaene microlide compound from a
composition referred to as faeriefungin having a structural
formula:
O U'
~16
R ~ OH Ol~ OH OH O~ Ol~
wherein R is selected from hydrogen (A) and methyl (B)
and all double bonds are trans and having a negative
optical rotation and an x-ray diffraction pattern with
peaks at a d-spacing between 1.5408 and 43.9165 Angstroms
for a 1 to 1 molar ratio of A to B.
1337~43
Further the present invention relates to a mixed
carbonyl pentaene macrolide composition referred to as
faeriefungin for inhibiting the growth of microorganisms
characterized by a melting point of 209-212C with partial
decomposition, by molecular formulae of C36HsgOlo and
C37H60Olo and mixtures thereof and fast atom bombardment
mass spectrometry indicated molecular ions of 651.4017 for
C36HsgOlo and 665.4237 for C37H61Olo, by a negative optical
rotation and by an x-ray diffraction pattern as shown in
Figure ~1n a carrier in an amount between about 1 and 100
micrograms per ml or per gram.
In the present specification, the word "carrier"
means a pharmaceutically acceptable non-toxic substance
that when mixed with the active ingredient or ingredients
renders it suitable for administration. The expression
preferably excludes water and low-molecular weight organic
solvents commonly used in chemical synthesis, except in the
presence of other phramaceutically necessary ingredients
such as salts in correct quantitites to render the
composition isotonic, buffers, surfactants, coloring and
flavoring agents, and preservatives. Examples of suitable
solid and liquid diluents and carriers are the following:
water containing buffering agents which can be rendered
isotonic by the addition of glucose or salts; non-toxic
organic solvents; such as paraffins, vegetable oils;
alcohols; glycols; natural ground rock (for example
kaolins, aluminas, talc or chalk); synthetic rock powders
(for example highly dispersed silica or silicates); and
sugars.
Oral administration can be effected utilizing
solid and liquid dosage unit forms such as powders,
tablets, dragees, capsules, granulates, suspensions,
solutions and the like. Where appropriate, dosage unit
formulations for oral administration can be
microencapsulated to prolong or sustain the release as for
example by coating or embedding particulate material in
polymers, wax or the like.
' ` 1337643
-6-
Parenteral administration can be effected
utilizing liquid dosage unit forms such as sterile
solutions and suspensions intended for subcutaneous,
intramuscular or intravenous injection. These are prepared
by suspending or dissolving a measured amount of the
compound in a nontoxic liquid vehicle suitable for
injection such as an aqueous or oleaginous medium and
sterilizing the suspension or solution. Stabilizers,
preservatives and emulsifiers can also be added. -~
Generally the parenteral dosage will be from
0.01 to 50 mg/kg, preferably from 0.1 to 10 mg/kg, of body
weight per day, and the oral dosage form will be from 0.1
to 500 mg/kg, preferably 0.5 to 100 mg/kg, of body weight
per day.
Streptomyces griseus var. autotrophicus var. nov
has been deposited under the Budapest Treaty with the
American Type Culture Collection as ATCC 53668. This
strain produces the composition referred to herein as
"faeriefungin" .
SPECIFIC DESCRIPTION
Example 1
Faeriefungin was isolated from the mycelium of
Streptomyces griseus var. autotrophicus var. nov, ATCC
53668 (also known as MSU SM008). This strain was isolated
from a soil sample collected from a fairy ring from a grass
lawn. The composition was produced by growth in bacto
peptone, glucose, Brer Rabbit-Green~ Label-molasses,
5:10:20 grams per liter in distilled water (A-9 medium)
under the following conditions. Smaller batches of
cultures were grown in two liter baffle-bottomed Erlenmeyer
flasks containing 400 ml medium, placed on a rotary shaker
at 100-200 rpm at 26C for 5 to 7 days. Larger batches
were grown in a 130 liter fermentor containing 100 liter of
A-9 medium, aerated at 100 L/minute and stirred at 100 rpm
at 26C for 5 days. Streptomyces griseus var.
autotrophicus var. nov produced approximately 1.0 g/L or
more of faeriefungin over a 7-12 day period in A-9 medium.
1337643
-7-
A. Microbiological Aspects:
1. Isolation and Growth. ATCC 53668 was
isolated from a soil sample collected from the center of a
fairy ring. The soil was suspended in sterile
physiological saline and serial dilutions were plated on
various isolation media. The colony of ~this strain was
picked up from a Czapeck agar plate (sucrose 20.0g, NaNO3
3.0g, K2HPO4 1.0g, MgSO4 7H2O 0.5g, KCl 0.5g, FeSO4 7H2O
0.01g, bacto agar 15.0g, distilled water 1 liter). The
microorganism grows well at room temperature ~25C) on most
of the laboratory media. On YM~ agar (yeast extract, malt
extract, glucose, agar; 4:10:4:18 grams per liter in
distilled water), it produced slightly wrinkled colonies
that were yellowish orange with abundant aerial hyphae at
the periphery. The growth was powdery on N.Z. Amine-A
(NZ amine-A 3 g in 1 liter distilled water) agar and
leathery on nutrient agar (Difco, Detroit, Michigan).
Older colonies developed cracks typical of Nocardia
autotrophica. During the microscopic examination, the
aerial as welI as substrate hyphae appeared straight with
branchings at right angles. Spirals, sporangia, spore
chain or endospores were not seen. The microorganism
decomposed adenine, tyrosine, hypoxanthine, xanthine, and
casein. It produced acid with adonitol, cellobiose,
glucose, galactose, inositol, lactose, maltose, mannitol,
melibiose, a-methyl-D-glucoside, raffinose, trehlose, and
xylose. Acid production was not observed with arabinose,
erythritol, melezitose, rhamnose, and sorbitol.
Although the colonial morphology of ATCC 53668
was similar to that of _. autotrophica, its physiological
characteristics were closer to those of Streptomyces
griseus. Consideration of these two major traits warranted
recognition of this strain as a new variety of S. griseus.
We adopted the nomenclature, S. griseus var. autotrophicus
var. nov.
~ 13376~
B. Isolation, Purification and Chemical Characterization:
The isolation and purification of faeriefungin
is as set forth in Figure 1.
As can be seen from Figure 1, the culture is
grown in broth and then the cells are harvested. The
cells are treated with ~ethanol and the broth is treated
with trichloromethane. The crystals (X'ls) of faeriefungin
are separated by cooling from the methanol twice and then
the filtrate is extracted with trichloromethane. The
crystals can be combined. Thus as can be seen,
faeriefungin is both intra- and extra-cellular.
1. Basic Physical Data:
Melting Point: 209-212C with partial
decomposition.
Solubility: Faeriefungin is sparingly
soluble in methanol, fairly
soluble in
chloroform-methanol
mixtures, partially soluble
in water, completely soluble
in DMSO at room temperature.
Previously reported pentaene
macrolides are soluble in
methanol at the same amount
and temperature at which
faeriefungin was studied.
Stability: Pure faeriefungin is stable
at room temperature and
under laboratory artificial
. 30 light conditions, whereas
previously reported pentaene
macrolides are unstable
under those conditions.
2. Spectral Data: MeOH
a. W Spectra. A Max (meOH) = 262, 363.5,
no base shift by addition of 5% KOH in
MeOH. UV spectra of peracetate of
' . 1337643
- faeriefungin has the ~ollowing ~Max
values: 210, 261, 363.5; with previously
studied acetates of pentaene macrolides,
the max 363.5 resolved into 3 peaks.
Faeriefungin acetate did not.
b. Mass Spectra: Faeriefungin (homologous
mixtures of C36Hs8Olo and C37H60H10)
M+650, FAB, High Resolution, Peak
Matching, 651.4017 (C36HsgOlo, M+ + H).
M+664, FAB, High Resolution, Peak
Matching, 665.4237 (C37H71Olo~ M + H)-
c. IR-Spectra:
Max 3400 (OH, 1695 (lactone C=O), 1610
(C=C), 1570 (conjugated C=C) cm~
d. 13C-NMR:
Natural abundance 13C-NMR indicated
resonances for a lactone carbonyl,
olefinic carbons, carbon bearing
secondary hydroxyl groups, carbon
attached to oxygen which is part of the
lactone ring, methyl groups, methylene
groups, and methyne groups.
e. lH-NMR:
Faeriefungin contains five adjacent and
one separate C=C bonds. All of the
double bonds are trans, contains two
secondary (2) methyl groups and
contains eight secondary (2) -OH
groups, which was confirmed by
acetylation.
Faeriefungin was a mixture of 13, 15, 17, 19,
21, 23, 25, 27 octahydroxy-31-isopropyl-14,
30-dimethyl-hentriaconta -2,4,6,8,10, 28-hexen-31-olide (A)
and 13, 15, 17, 21, 23, 25, 27 octahydroxy-14,
30-dimethyl-31-S-butyl
hentriaconta-2,4,6,8,10,28-hexene-31-olide (B).
..
- 133764:3
--10--
The stereochemistry of the -OH and alkyl
substituents on the macrolide ring is not known.
Faeriefungin is a 1:1 molar mixture of C36HsgOlo and
C37H60Ol0-
Table 1 shows à comparison of the properties of
faeriefungin, mycoticin and flavofungin.
Table 1
Faeriefungin Mycoticin Flavofungin
Appearance pale yellow yellow yellow
needles crystals crystals
Stability stable, when stored Decomposes Decomposes
at 25C in solid
form
Converts to new Unknown Unknown
compound in dioxane,
chloroform, methanol,
dichloromethane,
pyridine when kept
in light. No change
in the dark.
m.p. 210-213 220-222 210
charring at 310C (decomposes) (decomposes)
UV (E~%cm) 363.5 (965), 364 (948), 363(8%)
259.5 (71) 262 (79)
363.5 (~=63413),
259.5 (~=4649.54)
IR (KBr) 3375, 3000, 2950 3400, 1695 3400, 2945,
2925, 2900, 2850, 1610, 1710, 1680,
1678, 1605, 1560, 1570 cm~l* 1232, 1122,
1420, 1350, 1300 1020, 840 cm~l*
MF C36H58Olo (50%) C36H58H10 C36H58Olo
(50%) (90%)
C37H6010 (50%) C37H60Ol0 C37H60O10
(S0%) (10%)
M. wt 650 and 664 650 and 664 650 and 664
- Example 2
The specific rotations for the compositions
produced by two Streptomyces strains, ATCC 3348 of Burke et
al and Wassermann et al and ATCC 53668, in A-9 medium and
the medium of Burke et al (1954) were determined. The [~]
~` ~` 1337643
- values were for a 0.2~ solutions in pyridine and
dioxane and 0.15~ in MeOH. The results are shown in Table
2.
Table 2
Compound Pyridine Dioxane MeOH
t=O t=10 t=O t=10 t=O t=10
FF -70.5 -70.5 -40.5 -52.5 -34.0 -35.33
Mycoticin - - +63.5 +81.7
(Burke et al)
MYC-N -74.5 -74.5 -58.0 -58.0 -44.66 -42.0
Flavofungin -85-90 - - - -47.51
(from literature)
MYC -72.0 -73.5 -50.0 -51.5 -38.66 -38.66
(from literature)
FF-II -46.0 -54.0
t = time in minutes.
FF = Faerifungin, produced by ATCC 53668 in A-9 medium
(1 to 1 A to B).
MYC-N = Compound produced by ATCC 3348 in A-9 medium (9:1
A to B).
MYC = Compound produced by ATCC 3348 in 'Burke's' medium
(supposed to be mycoticin (1 to 1 A to B)).
FF-II = Compound produced by ATCC 53668 in 'Burke's'
medium.
Flavofungin = (9 to 1 A to B).
ATCC 3348 is Streptomyces ruber, the original mycoticin A
and B producing organism. Burke's medium was the original
medium used to produce mycoticin A and B, [a]~5
(c=0.48%, dioxane) = +63.5, by ATCC 3348. Streptomyces
ruber ATCC 3348 no longer produced mycoticin A and B in the
original Burke's medium. ATCC strain 3348 produced a
90:10% composition of a polyene macrolide (MYC-N) in A-9
medium with molecular formulae (MF): C36H5810 (90%) and
C37H60Olo (10%). This was confirmed by C13-NMR and MS.
1337643
- -12-
~ Streptomyces griseus var. autotrophicus ATCC 53668,
produced a 50:50 (1:1) mixture of faeriefungin (FF) with MF
C36H5810 and C37H6010- MYC (literature) had a specific
rotation different from FF where the molar proportions of A
to B were about equal; however, this was not considered to
clearly differentiate FF from MYC.
Bognar et al confirmed the specific rotation
change of mycoticin found by Burke et al in dioxane with
time. The ta]~5 value in dioxane changed from +63.5 to
+81.7 in 9.5 minutes and reached zero value at 15 minutes.
The final rotation at t = ~, was -18.2. Flavofungin did
not show this effect, possibly because of the high
proportion of A to B (9:1). Faeriefungin showed some
change in rotation with different solvents, but retained
the same negative value obtained after t = 10 minutes. It
appears that flavofungin and mycoticin (MYC-N or MYC)
differ only in the proportions of A to B. Faeriefungin
(FF) appears to be a different stereoisomeric composition.
Example 3
Circular dichroism (CD) studies of
octa-acetylmycoticin and -flavofungin in dioxane were made
and compared to the CD values for faeriefungin in Table 3.
Table 3(1)
Octa-acetyl Octa-acetyl Octa-acetyl
flavofungin faeriefungin mycoticin
A l~ )~ l~ A ~
208 +2.34 347 -11.3 210 +0.85
224 -2.34 363 -19.2 224 -2.04
260 -5.19 385 -11.9 260 -3.73
343 -2.51 faeriefungin 345 -1.86
371 -2.18 387 -10.2 362 -2.04
389 -2.51 366 - 9.7 380 -1.86
347 -10.2
(1) No CD transitions were observed in the 260 to 240 nm
region for faeriefungin octa-acetate or faeriefungin.
~- 1337643
-12a-
,
Table 3(a)
Circular dichroism (CD) of faeriefungin and mycoticin-N in
MeOH.
Faeriefungin Mycoticin-N
A ~ ~ ~
380-14.6 377 -11.4
374-16.3 372 -11.4
368-11.8 370 -16.6
362-13.5 365 -15.8
360 -13.5 360 -15.0
354 -10.1 355 -13.4
349 -10.1 348 - 7.1
344 - 9.6 339 - 3.9
The CD curves of the octa-acetate of mycoticin
and flavofungin were similar in shape, but quantitatively
different as shown in Table 3 (Bognar et al., and Brown et
al., J. C. S. Perkin I, 1848, 1972). The octa-acetyl
faeriefungin produced a CD curve different from mycoticin
and flavofungin octa-acetates. It is different both
quantitatively and in shape. Table 3(a) shows that
faeriefungin and mycoticin N have different CD values. The
results show that faeriefungin contains significantly
different stereoisomers. The structures are different from
those characterized by Schreiber, S. L. et al in
Tetrahedron Letters 28, 6001-6004 (1987) and 28, 6005-6008
(1987) for mycoticin A and B produced in the manner of
Wasserman et al (1967 and 1970). Schreiber, S. L. et al
indicated that there were 64 possible stereoisomers.
~ ` 1337643
- -13- ~
- Example 4
The x-ray diffraction patterns ~or faeriefungin
and mycoticin (MYC-N) were determined as shown in Figures 2
and 3. Faeriefungin had 28 discernible crystalline peaks
whereas mycoticin (MYC-N) had 14 as shown in Tables 4 and 5.
Only about 6 of the peaks are common. The results show
that faeriefungin contains significantly different
stereoisomers.
Table 4
10 Summarized X-ray Diffraction Pattern for Faeriefungin
PEAKS
Listed DI file name : FUNGIN.DI
Raw data file name : FUNGIN.RD
Sample identification : fungin
Date of measurement : 9-DEC-87
Generator settings : 35 kV, 20 mA
Step size, count time : 0.020 deg, 1.00 s
Angle range (2theta) : 2.010 - 59.990 deg
Al, A2 wavelengths : 1.54056, 1.54435 Ang
Range in d-spacing : 1.5408 - 43.9165 Ang
Monochromator used : Yes
Maximum peak counts : 19404. cts, 19404. cps
Peak Angle Tip width Peak Backg D spac I/Imax Type Qual
no (deg) (deg) (cts) (cts) (Ang) (%) Al A2 0t
15.4325 0.1271. 46. 16.2542 0.36 X X0.93
26.4100 0.0819404. 55. 13.7775 100.00 XX 31.62
36.g675 0.14279. 66.12.6763 1.44 X X5.62
49.5950 0.12734. 69.9.2101 3.78 X X10.23
510.7275 0.1656. 72.8.2402 0.29 X X0.85
611.2600 0.14681. 74.7.8517 3.51 X X11.75
712.0900 0.1667. 77.7.3144 0.35 X X1.58
813.3125 0.1234. 69.6.6454 0.17 X X1.29
914.8850 0.2053. 64.5.9467 0.27 X X1.07
1015.3300 0.1655. 72.5.7751 0.28 X X0.87
1115.8300 0.2059. 72.5.5938 0.31 X X1.20
1216.8775 0.201901. 94.5.2489 9.80 X X32.36
1317.7950 0.20142. 102.4.9802 0.73 X X1.55
1418.1700 0.32117. 102.4.8783 0.60 X X1.51
1518.8125 0.10146. 110.4.7131 0.75 X X1.29
1619.8175 0.22784. 130.4.4763 4.04 X X17.38
1720.8150 0.16172. 135.4.2640 0.88 X X1.05
1821.1850 0.16185. 142.4.1903 0.95 X X0.83
1921.5100 0.12156. 151.4.1278 0.81 X X1.02
2022.5600 0.12524. 204.3.9380 2.70 X X3.63
2124.0625 0.201384. 204.3.6954 7.13 X X24.55
2224.8900 0.2494. 204.3.5744 0.48 X X1.66
2328.2900 0.1696. 193.3.1520 0.49 X X0.98
2430.4700 0.1662. 193.2.9313 0.32 X X0.83
2534.0125 0.2018. 161.2.6337 ~.10 X X0.85
~ 1 3 3 7 6 4 3
.
, -14-
- 2639.9900 0.9616. 121. 2.2527 0.08 X X 0.83
2743.0925 1.2814. 128. 2.0974 0.07 X X 0.89
2849.2900 0.9610. 125. 1.8472 0.05 X X 0.81
28 peaks identified
28 crystalline
0 amorphous
All listed
Table 5
PEAKS
Listed DI file name : MYCON.DI
Raw data file name : MYCON.RD
Sample identification : mycon
Date of measurement : 9-DEC-87
Generator settings : 35 kV, 20 mA
Step size, count time : 0.020 deg, 1.00 s
Angle range (2theta) : 2.010 - 59.990 deg
Al, A2 wavelengs : 1.54056, 1.54435 Ang
Range in d-spacing : 1.5408 - 43.9165 Ang
Monochromator used : Yes
Maximum peak counts : 1156. cts, 1156. cps
Peak Angle Tip width Peak Backg D spac IJImax Type Qual
no (deg) (deg) (cts) (cts) (Ang1 (~) Al A2 0t
16.4075 0.121156. 69. 13.7829 100.00 X X 4.07
26.9625 0.10303. 74.12.685426.19 X X 4.47
38.2575 0.16106. 67.10.69879.18 X X 1.82
410.3525 0.2037. 62.8.53783.22 X X 0.78
510.8200 0.2877. 62.8.17006.70 X X 1.62
612.1875 0.1237. 79.7.25623.22 X X 1.86
714.1025 0.2421. 71.6.27481.83 X X 0.78
816.7425 0.5685. 81.5.29097.32 X X 4.37
917.9225 0.2450. 92.4.94514.36 X X 0.91
1019.9275 0.80161. 137.4.451813.95 X X 9.77
1121.3850 0.2461. 159.4.15165.26 X X 1.07
1222.8475 0.24119. 177.3.889110.28 X X 0.91
1324.8600 0.4810. 243.3.57860.89 X X 1.48
1426.0400 0.9610. 213.3.41900.89 X X 1.91
14 peaks identified
14 crystalline
0 amorphous
All listed
Example 5
Table 6 shows comparisons of minimum inhibitory
concentrations (CMIC) of faeriefungin and other anti~ungal
agents in ug/ml.
'~. 1337643
-L5-
- Table 6
Substance Organisms
Candida Aspergillus Microsporum Trichophyton
albicans fumigatuscanis rubrum
Faeriefungin5.5 3.2 3.2 3.2
Amphotericin B 3.2 80 16 16
Nystatin 3.2 >100 >100 >100
Pimaricin 5.5 16 16 16
Ketoconazole0.5-500 1-500 ~ 5.5-80 5.5-80
Miconazole 1-50 8-500 16 80
Clotrimazole1-50 1-500 16 80
5-flurocytosine* 0.5-500 50-100 >500 >500
*This data is based upon the literature. The
prior art antifungal agents are listed by their generic
names.
Example 6
In order to confirm the data from the
literature, the c~ltures were grown in 500 ml baffled
bottom Erlenmeyer~flasks, each containing 100ml of the
liquid A-9 medium. The inoculated flasks were placed on a
rotary shaker at 200 r.p.m., 26C. The pH and the
antifungal activities were monitored daily up to a period
of 14 days. The antifungal activities were determined by
spreading evenly a thick suspension (200 x 10 cells/ml) of
the spores or yeast-like cells of the test species on
Emmons (neopeptone, glucose, bacto agar; 10:20:18 grams per
liter in distilled water) agar in 100 mm Petri dishes and
placing 25 ul of the culture broth on the surface. The
test culture was incubated at 26C for 2 days. A clear
zone of inhibition was indicative of antifungal activity.
Production of the antifungal compound could be detected
after 3 days, peaked on 8th day and declined after 11th day.
During this period, pH of the medium ranged between 6 and
8.
~
~rade~ a J~lR
1337643 :;
-16-
- Larger batches were grown in 2 L flasks
containing 400 ml of A-9 medium. After 8 days of growth at
26C (shaker speed: days 1-3, 100 r.p.m.; days 4-5, 150
r.p.m.; days 6-8, 200 r.p.m.) the culture broth was
centrifuged and the cake was extracted in methanol as shown
in Figure 1. The purified substance(s) was dissolved in
DMSO and known amounts are bioassayed against Candida
albicans, Aspergillus flavus and Fusarium species.
Comparable amounts of nystatyin, pimaricin and amphotericin
B also dissolved in DMSO were tested simultaneously.
Faeriefungin was more active than a Amphotericin B against
_. fumigatus, it was slightly less active than pimaricin,
but better than nyst~atin. Against C. albicans, this
compound had similar activity as amphot~ricin B. Against
Fusarium species, Faeriefungin was better than any of the
three antibiotics tested. Faeriefungin also has a low MIC
against a broad spectrum of fungi.
Table 7 shows the minimum inhibitory
concentra~ions (MIC) in ug/ml of faeriefungin, amphotericin
B and nystatin against various bacteria and fungi.
~ 13376~3
-17
Table 7
Organisms Faeriefungin Amphotericin B Nystatin
FUNGI:
Aspe~gillus fumigatus 3.2 80 80
A. :_avus 3.2 80 80
Can~ da albicans 5.5 3.2 16
_. trophicalis ~ 5.5 5.5 16
Microsporum canis ~ 3.2 16 >100
Trichophyton rubrum 3.2 16 >100
Alternaria solani 3.2 >100 >100
~usar_um oxysporum 3.2 >100 >100
'u-a~ um moniliforme 3.2 >100 >100
'y'h:.um ultimum 12.0 >100 >100
~h a_ophora graminicola 12.0 >100 >100
~eptosphaeria korrae 12.0 >100 >100
BACTERIA:
~'scherichi-- coli not active not active not active
~roteus vu_garis not active not active not active
'. m.rabil_s not active not active not active
20 Pseu~omonas aeruginosa not active not active not active
Staphylococcus aureus 16.0not active not active
S. saprophyticus 64.0not active not active
Streptococcus bovis 16.0not active not active
S. agalac iae 16.0not active not active
S. faecal s 16.0not active not active
S. pneumoniae 16.0not active not active
S. pyogenes 16.0not active not active
S. sanguis 16.0not active not active
Strep ococcus group C 16.0not active not active
Strep-ococcus group D 16.0not active not active
`eiseria gonorrhaeae 16-24not active not active
. meningitidis 32-64>128 not active
. c:nerea 32-64>128 not active
. f_ava 64>128 not active
. mucosa 64-128>128 not active
. subflavanot active not active not active
. sicca64 >128 not active
. per__avanot active not active not active
aemop~ lus influenzae64-128 >128 not active
~cenetob~cter calesnot active not active ~ not active
Branhame_la catarrhalis 64 >128 not active
Klebsiel_a pneumoniae not active not active not active
As can be seen faeriefungin is active against a number of
strains of bacteria whereas the prior art compositions are
not.
1337643
~ .
-- -18-
- Tables 8 and 9 show the reported activities for
flavofungin and mycoticin
Table 8
Inhibitory Conc
ug/ml
Fungi
Asperg ' us clavatus 15
Pen c_ __um chrysogenum 8
?en c __ um novum hybr d 8
,~en c __ um sp (two s rains) ' 10-20
copu_ar opsis sp 15
Cepha_osporium sp 15
~olosporium apiospermum 15
le_min hosporium sp 10
Tr cho~eccum roseum 15
Mastigocladium sp 10
Yeast and Yeast-Like Fungi
Cane ~a ~lbicans (three strains) 4-5
Can~_~a ~rusez 6
20 Cane ~a ropicalis 15
Sacc~aromyces cerevisiae (three strains) 3-20
Sacclaromyces niger 2
Cryp ococcus neoformans 2
Toru'a utilis 10
25 Han-enula anomala 10
Rho~otorula sp 2
Torulopsis pulcherrima 2
Pathogenic Fungi
Tr'cloply on mentagrophytes 20
30 Tr clop~y on tonsuran (two strains) 8-30
Tr_cloply on rubrum (,wo strains) 10
Tr c~op~y on gypseum ,two strains) 20
Tr c~op~y o~ sulfureium 8
Ep_eermoply on ~aufmann-Wolf (three strains) 15-20
35 Ep eermoply on nguinule 10
crosporum can_s 8
cro~porum gypseum 8
~cho~ on quinckeanum 8
Cera _nomyces 10
40 Phia'ophora verrucosa 20
Histaplasma capsula um 10
Sporo_richum schenk i 10
'ormo~eudrum compac um 8
~ocar~ia asteroides 20
45 ~eotr chum sp 20
Burke et al, the Journal of Investigative Dermatology, 23,
163 (1954)
~ 1337643
.
-19-
Table 9
Inhibitory Concentration
Organismmcg/ml, No. Hours on Agar
Cryptococcus neoformans ATCC 10226 6-8
Candida albicans ATCC 10261 6-10
Blastomyces dermatitidis ATCC 10225 2-4
Histoplasma capsulatum ATCC 10220 1-2
Sporotrichum schenckii ATCC 10213 1-3
Hormodendrum pedrosoi ATCC 9475 7-10
Coccidioides immitis Conant 2150 2-3
Trichophyton mentagrophytes ATCC 9972 5-7
Trichophyton mentagrophytes Harvard 16 6-9
Trichophyton rubrum ATCC 9806 3-5
Microsporum audonini ATCC 10216 2-3
Microsporum canis ATCC 9084 4-7
Microsporum gypseum Harvard 22 8-.0
Bacillus subtilis >100
Escherichia coli >100
Staphylococcus aureus >100
Mycobacterium phlei >100
Streptococcus hemolyticus >100
Salmonella typhi >100
Leishmania donovani >100
Tryphanosoma tropica >100
Endamoeba histolytica >100
Table 10 shows a summary of the biological
activity of faeriefungin, mycoticin and flavofungin.
Table 10
Faeriefungin Mycoticin Flavofungin
Biological Antifungal Antifungal Antifungal
Activity Antibacterial Not anti- Not anti-
bacterial bacterial -
Antiviral Not anti- Antiviral
viral
13~7643
- 20
Antiviral activity of carbonyl pentaene
macrolides except faeriefungin has been studied on
influenza A and B virus, variolavaccine virus and Rous
sarcoma virus by M. A. Schneider et al (Schneider, et al.,
Russian Journal, 1967). It was reported that flavofungin
was the most active against the above viruses.
Example 7
Table 11 shows the toxicity of the faeriefungin
to human erythrocytes.
Table 11
Concentration Faeriefungin Amphotericin B
100 ug/ml Toxic Toxic
20 ug/ml Toxic Toxic
4 ug/ml Non-toxic Toxic
0.8 ug/ml Non-toxic Non-toxic
As can be seen, faeriefungin is non-toxic at low
levels in vitro.
Example 8
Broths of Streptomyces ATCC 53668 containing
faeriefungin also showed a highly toxic rapid response
(greater than 50% kill within 2 hours) on mosquito (Aedes
aegypti) larvae. However, pure mycoticin (MYC-N) was only
moderately toxic to mosquito larvae (35% mortality at 100
ppm for 24 hours), poss-ibly indicating that another
component produced by ATCC 3348, may be the primary
insecticidal moiety. Broths were moderately toxic (rapid
to slow) to cultures of the nematode Panagrellus redivivus.
The biosynthesis of mycoticins A and B has been
studied using labelled acetate, propionate, and methionine
by Wassermann, et al., Chem. Communication, 1634 (1970).
The results indicated that the macrolide pentaene ring is
biosynthesized through acetate. The alkyl side chain and
two methyl groups substituents are biosynthesized from the
propionate, i.e., C-14 and C-30 methyl substituents and the
isopropyl (isobutyl) side chain at C-31 (Wassermann, et
- 1337643
.~ :
-21-
al., Chem. Communication, 1634 (1970)). No methionine
uptake was reported.
In the case of faeriefungin, experiments with
13C-labeled acetate indicated incorporation of acetate for
S the ring carbons. Propionate was biosynthesized for C-14
and C-30 methyls. There was no incorporation of either
acetate nor propionate for the C-31 carbon and the alkyl
side chain (isopropyl or iso-butyl) at C-31. The
experiments confirmed that faeriefungin pr,~oduction is
proceeding through a different biosynthetic pathway than
the reported route for the production of mycoticins and
that the compounds are different for this reason.
The faeriefungin can be used to inhibit plant or
animal pathogens. Various well known solid or liquid
carriers for pharmaceutical, veterinary or plant use can be
used. The composition can be applied topically very
effectively.
It is intended that the foregoing Examples are
only illustrative of the present invention and that the
invention be limited only by the hereinafter appended
claims.