Note: Descriptions are shown in the official language in which they were submitted.
; ~ 337682
MINIMUM P~OCEDURE ~YSl~
FOR TH~ DET~RM~NATION OF ANA~YTES
Field of the Invention
The present invantion ~elates to a test de~ic~ and method
for the colorimetric d~termination of chemical and
biochemical compon~n~s (analytes) in aqueous fluids,
particularly whole blood. In one preferred embodiment it
concerns-a test device and method for colorimetrically
measuring the concentration of glucose in whola blood.
Backg~ound of the Invention
Th~ quantification of chemical and biochemical components
in ~olored aqueous fluids, in particular colored
biologisal fluids such as whole blood and urine and
hiclogical fluid derivatives such as ~lood serum and blood
~5 plasma, is of ever-increasin~ importance. Important
applications e~ist in medical diagnosis and treatment and
in the quantification of e~posure to therapeutic drugs,
intoxic2nts, hazardous chemicals and the like. In some
instances, the amounts of materials being deterrnined are
eitAer so miniscule--in the range of a micro~ram or less
per deciliter--or so diffi.cult to precisely determine that
the apparatus employed is complicated and useful only to
skilled laboratory personnel. In this case the resu?ts
are generally not available fQr some hours or days after
~s sampling. In other instances, there is often an emphasis
on the a~ility of lay operators to perform the test
routinely, quickly and reproducibly outside a laboratory
setting with rapid or immediate information display.
1 337 682
One common medical test is the measurement of blood
glucose levels by diabetics. Current teaching counsels
diabetic patients to measure their blood qlucose level
from two to seven times a day depending on the nature and
5 severity of their individual cases. Based on the observed
pattern in the measured glucose levels, the patient and
physician together make adjustments in diet, esercise and
insulin intake to better manage the disease. Clearly,
this information should be available to the patient
10 immediately.
Currently a method widely used in the United States
employs a test article of the type described in U.S.
Patent 3,298,789 issued January 17, 1967 to Mast. In this
15 method a sample of fresh, whole blood (typically 20-40
is placed on an ethylcellulose-coated reagent pad
containing an enzyme system having glucose osidase and
perosidase activity. The enzyme system reacts with
glucose and releases hydrogen peroside. The pad also
20 contains an indicator which reacts with the hydrogen
peroside in the presence of peroxidase to give a color
proportional in intensity to the sample's glucose level.
Another popular blood glucose test method employs similar
25 chemistry but in place of the ethylcellulose-coated pad
employs a water-resistant film through which the enzymes
and indicator are dispersed. This type of system is
disclosed in United States Patent 3,630,957 issued
December 28, 1971 to Rey et al.
In both cases the sample is allowed to remain in contact
with the reagent pad for a specified time (typically one
minute). Then in the first case the blood sample is
washed off with a stream of water while in the second case
35 it is wiped off the film. The reagent pad or film is then
LFS-3
~ 337682
-- 3 --
blotted dry and evaluated. The evaluation is made either
by comparing color qenerated with a color chart or by
placinq the pad or film in a diffuse reflectance
instrument to read a color intensity value.
While the above methods have been used in qlucose
monitorinq for years, they do have certain limitations.
The sample size required is rather larqe for a finger
stick test and is difficult to achieve for some people
whose capillary blood does not e~press readily.
In addition, these methods share a limitation with other
simple lay-operator colorimetric determinations in that
their result is based on an absolute color reading which
is in turn related to the absolute extent of reaction
between the sample and the test reagents. The fact that
the sample must be washed or wiped off the reagent pad
after the timed reaction interval requires that the user
be ready at the end of the timed interval and wipe or
apply a wash stream at the required time. The fact that
the reaction is stopped by removing the sample leads to
some uncertainty in the result, especially in the hands of
the home user. Overwashing can give low results and
underwashinq can give high results.
Another problem that often e~ists in simple lay-operator
colorimetric determinations is the necessity for
initiatinq a timinq sequence when blood is applied to a
reagent pad. A user will typically have conducted a
finger stick to obtain a blood sample and will then be
required to simultaneously apply the blood from the finger
to a reagent pad while initiating a timing circuit with
his or her other hand, thereby requiring the use of both
hands simultaneously. This is particularly difficult
since it is often necessary to insure that the timing
I ~3768~
circuit is started only when blood is applied to the
reagent pad. All of the prior art methods require
additional manipulations or additional circuitry to
achieve this result. Accordingly, simplification of this
aspect of reflectance reading instruments is desirable.
The presence of red blood cells or other colored
components often interferes with the measurements of these
absolute values, thereby calling for esclusion of red
blood cells in these two prior methods as they are most
widely practiced. In the device of United States patent
3,298,789 an ethyl cellulose membrane prevents red blood
cells from entering the reagent pad. Similarly, the
water-resistant film of United States patent 3,630,957
prevents red blood cells from entering the pad. In both
cases the rinse or wipe also acts to remove these
potentially interfering red blood cells prior to
measurement.
Accordingly, there remains a need for a system of
detecting analytes in colored liquids, such as blood, that
does not require removal of escess liquid from a
reflectance strip from which a reflectance reading is
being obtained.
SummarY of the Invention
Novel methods, compositions and apparatus are provided for
diagnostic assays comprising a hydrophilic porous matris
containing a signal producing system and a reflectance
measuring apparatus which is activated upon a change in
reflectance of the matris when fluid penetrates the
matris. The method comprises adding the sample, typically
whole blood, to the matris which filters out large
particles, such as red blood cells, typically with the
S-3
1 337682
-- 5
matrix present in the apparatus. The signal-producing
system produces a product which further changes the
reflectance of the matrix, which change can be related
to the presence of an analyte in a sample.
Exemplary of the diagnostic assay system is the
determination of glucose in the whole blood, where the
determination is made without interference from the
blood and without a complicated protocol subject to
use error.
More specifically, the present invention relates to a
no-wipe whole blood glucose test strip for measuring
glucose in an unmeasured whole blood sample, said test
strip being adapted for use in a reflectance reading
apparatus capable of measuring reflectance at two5 different wavelengths, said test strip comprising:
a porous, hydrophilic matrix, said matrix
having a sample receiving surface adapted to receive
said whole blood sample on one side of the matrix and
a testing surface from which diffuse reflected light0 is measurable from the other side of the matrix, said
testing surface being opposite to said sample
receiving surface,
said matrix being substantially reflective in
the absence of applied sample,
said matrix containing openings of a size
sufficient to allow the flow of at least a portion of
said blood sample through the matrix from said sample-
receiving surface to said testing surface,
said matrix comprising reagent means for
chemically reacting with glucose to create a change in
reflectance in the presence of optically visible
hemoglobin observable from the testing surface which
change is indicative of the concentration of glucose
~337682
~ - 5a -
present in said sample, said reagent means comprising
glucose oxidase, peroxidase, and a dye precursor
forming a chromophore exhibiting absorbance at a first
wavelength which is proportional to the concentration
of glucose and exhibiting substantially no absorbance
at a second wavelength at which hemoglobin absorbs
light.
Brief DescriPtion of the Drawinqs
The present invention can be more readily understood
by reference to the following detailed description
when read in conjunction with the attached drawings,
wherein:
Figure 1 is a perspective view of one embodiment of a
test device containing the reaction pad to which the
fluid being analyzed is applied;
Figure 2 is a block diagram schematic of an apparatus
that can be employed in the practice of the invention;
Figure 3 is a perspective view of a preferred
embodiment of the test device of the present invention
emplaced within a measuring system;
Figure 4 is an enlarged plan view of a preferred
embodiment of the test device of the present invention
emplaced within a measuring system;
Figure 5 is a graph plotting a second order correction
to eliminate errors due to chromatography effects
during the use of the present invention;
1 ~37682
-- 6
Figures 6a and 6b are scatterqrams of glucose values as
measured by a preferred embodiment of the present
invention (called the single wavelength MPX system)
plotted against Yellow Springs Instruments (YSI) glucose
values; and
Figures 7a, 7b, 7c and 7d are scattergrams of glucose
values as measured by a second preferred embodiment of the
present invention (called the double wavelength MPX
system) plotted against Yellow Springs Instruments (YSI)
glucose values.
Detaile~ Description of the Invention
The Reagent Element
The subject invention provides an improved rapid and
simple methodology employing reliable and easy to operate
apparatus for the determination of analytes such as
glucose, particularly involving an enzyme substrate which
results in the production of hydrogen peroside as an
enzyme product. The method involves applying to a porous
matrix a small volume of whole blood, sufficient to
saturate the matris. It is to be noted that the present
system is capable of determining glucose levels from
optical readings of whole blood samples. Separation of
plasma from blood in the sample is unnecessary, and the
present invention avoids the requirement of this step. In
addition, this system is capable of performing accurate
readings as long as only a small volume saturates the
matris of the test strip. Above this threshold, the
reading is volume independent.
Bound to the matris are one or more reagents of a signal
producing system, which results in the production of a
FS-3
1 337682
.
-- 7 --
product resulting in an initial change in the amount of -
reflectance of the matris. The matri2 is typically
present in a reflectance-measuring apparatus when blood is
applied. The liquid sample penetrates the matris,
resulting in an initial change in reflectance at the
measurement surface. A reading is then taken at one or
more times after the initial change in reflectance to
relate the further change in reflectance at the
measurement surface or in the matris as a result of
formation of the reaction product to the amount of analyte
in the sample.
For measurements in blood, particularly glucose
measurements, whole blood is typically used as the assay
medium. The matris contains an osidase enzyme which
produces hydrogen peroside. Also contained in the matris
will be a second enzyme, particularly a pero~idase, and a
dye system which produces a light-absorbing product in
conjunction with the perosidase. The light-absorbing
product changes the reflectance signal of the matrix
system. With whole blood, readings are taken at two
different wavelenqths, with the reading at one wavelength
used to subtract out background interference caused by
hematocrit, blood 02ygenation, and other variables which
may affect the result. Thus, the present invention is
capable of analyzing samples of whole blood.
A reagent element is employed which comprises the matrix
and the members of the signal producing system contained
within the matri~. The reagent element may include other
components for particular applications. The method
requires applying a small volume of blood, which typically
has not been subject to prior treatment (other than
optional treatment with an anticoagulant), to the matrix.
Timing of the measurement is activated or initialized by
S-3
1 337682
-- 8 --
the apparatus' automatically detecting a change in
reflectance of the matris when fluid penetrates the
matris. The change in reflectance over a predetermined
time period as a result of formation of reaction product
is then related to the amount of analyte in a sample. The
intensity of the light source used to analyze the sample
is, of course, also carefully monitored and regulated, to
insure the repeatability of the measurement.
The first component of the present invention to be
considered is a reagent element, conveniently in the shape
of a pad, comprising an inert porous matris and the
component or components of a siqnal-producing system,
which system is capable of reacting with an analyte to
produce a light-absorbing reaction product, impregnated
into the pores of the porous matris. The signal-producing
system does not significantly impede the flow of liquid
through the matris.
In order to assist in reading reflectance, it is preferred
that the matris have at least one side which is
substantially smooth and flat. Typically, the matris will
be formed into a thin sheet with at least one smooth, flat
side. In use, the liquid sample being analyzed is applied
to one side of the sheet whereby any assay compound
present passes through the reagent element by means of
capillary, wicking, gravity flow and/or diffusion
actions. The components of the signal producing system
present in the matris will ~eact to give a light absorbing
reaction product. Incident light impinges upon the
reagent element at a location other than the location to
which the sample is applied. Light is thus reflected from
the surface of the element as diffuse reflected light.
This diffuse light is collected and measured, for esample
by the detector of a reflectance spectrophotometer. The
,FS-3
1 337682
amount of reflected light will be related to the amount of
analyte in the sample, usually being an inverse function
of the amount of analyte in the sample.
The Matris
Each of the components necessary for producing the reagent
element will be described in turn. The first component is
the matris itself.
The matris will be a hydrophilic porous matris to which
reagents may be covalently or noncovalently bound. The
matris will allow for the flow of an aqueous medium
through the matris. It will also allow for binding of
protein compositions to the matri~ without significantly
adversely affecting the biological activity of the
protein, e.g., enzymatic activity of an enzyme. To the
estent that proteins are to be covalently bound, the
matris will have active sites for covalent bonding or may
be activated by means known to the art. The composition
of the matris will be reflective and will be of sufficient
thickness to permit the formation of a light-absorbing dye
in the void volume or on the surface to substantially
affect the reflectance from the matris. The matris may be
of a uniform composition or a coating on a substrate
providing the necessary structure and physical properties.
The matris will usually not deform on wetting, thus
retaining its original conformation and size. The matris
will have a defined absorbance, so that the volume which
is absorbed can be calibrated within reasonable limits,
variations usually being maintained below about 50%
preferably not greater than 10%. The matris will have
sufficient wet strength to allow for routine manufacture.
The matris will permit non-covalently bound reagents to be
LFS-3
1 337682
-- 10 --
relatively uniformly distributed on the surface of the
matris.
As esemplary of matris surfaces are polyamides,
S particularly with samples involving whole blood. The
polyamides are conveniently condensation polymers of
monomers of from 4 to 8 carbon atoms, where the monomers
are lactams or combinations of diamines and di-carbosylic
acids. Other polymeric compositions having comparable
properties may also find use. The polyamide compositions
may be modified to introduce other functional groups which
provide for charged structures, so that the surfaces of
the matris may be neutral, positive or negative, as well
as neutral, basic or acidic. Preferred surfaces are
positively charged. It has been determined that this
positive charge enhances both stability and shelf-life.
When used with whole blood, the porous matris preferably
has pores with an average diameter in the range of from
about 0.1 to 2.0~m, more preferably from about 0.6 to
1.0~m. When the porous matri~ contains pores having an
average diameter of about 0.8~m, the sample of blood will
not cause a chromatographic effect. That is, the blood
sample will not seek out the edges of the circular
matris. Rather, the blood remains seated within all the
pores of the matri~ and provides for a uniform readability
of the entire matri~. In addition, this pore size
- masimizes the non-blotting effect of the blood. That is,
the pore size is both adequately filled, but not
overfilled, so that the hematocrit level of blood will not
cause the sample to require blotting prior to reading of
the sample. Also, it has been found that pores of this
size are optimal when shelf-life and stability are taken
into consideration.
,-3
1 337682
11
A preferred manner of preparing the porous material is to
cast the hydrophilic polymer onto a core of non-woven
fibers. The core fibers can be any fibrous material that
produce the described integrity and strength, such as
polyesters and polyamides. The reagent that will form the
light-absorbing reaction product, which is discussed later
in detail, is present within the pores of the matris but
does not block the matris so that the liquid portion of
the assay medium, e.g. blood, being analyzed can flow
through the pores of the matris, while particles, such as
erythrocytes, are held at the surface.
The matris is substantially reflective so that it gives a
diffu$e reflectance without the use of a reflective
backing. Preferably at least 25%, more preferably at
least 50%, of the incident light applied to the matris is
reflected and emitted as diffuse reflectance. A matris of
less than about 0.5mm thickness is usually employed, with
from about O.Olmm to about 0.3mm being preferred. A
thickness of from about O.lmm to about 0.2mm is most
preferred, particularly for a nylon matris.
Typically, the matris will be attached to a holder in
order to give it physical form and rigidity, although this
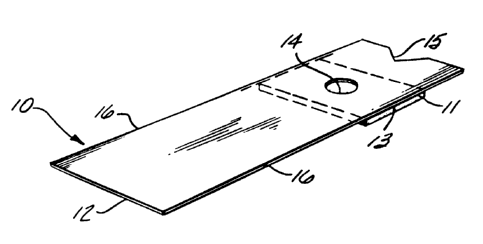
may not be necessary. Figure 1 shows one embodiment of
the invention in which there is a strip 10 having a thin
hydrophilic matris pad 11 is positioned at one end of a
plastic holder or handle 12 by means of an adhesive 13
which directly and firmly attaches the reagent pad 11 to
the handle 12. A hole 14 is present in the plastic holder
12 in the area to which reagent pad 11 is attached so that
sample can be applied to one side of the reagent pad and
light reflected from the other side.
A liquid sample to be tested is applied to pad 11.
1 ~37682
Generally, with blood being e~emplary of a sample being
tested, the reagent pad will be on the order of about
10mm2 to 100mm2 in surface area, especially 10mm2 to
50mm2 in area (or having a diameter of about 2mm to
about 10mm), which is normally a volume that 5-10
microliters of sample will more than saturate. Of course,
once saturation is reached at above the threshold of about
5-10 microliters, no other requirement of blood amount is
necessary.
Diffuse reflectance measurements in the prior art have
typically been taken using a reflective backing attached
to or placed behind the matri~. No such backing is needed
or will normally be present during the practice of the
present invention, either as part o the reagent element
or the reflectance apparatus.
As can be seen from figure 1, the support holds reagent
pad 11 so that a sample can be applied to one side of the
reagent pad 11 while light reflectance is measured from
the side of the reagent pad 11 opposite the location where
sample is applied.
Figure 2 shows a system in which the reagent is applied to
the side with the hole 14 in the backing handle 12 while
light is reflected and measured on the other side of the
reagent pad 11. Other structures than the one depicted
may be employed. The pad 11 may take various shapes and
forms, subject to the limitations provided herein. The
pad 11 will be accessible on at least one surface and
usually two surfaces.
The hydrophilic layer (reagent element) may be attached to
the support by any convenient means, e.g., a holder, clamp
or adhesives; however, in the preferred method it is
FS-3
- 1 33768~
- 13 -
bonded to the backing. The bonding can be done with any
non-reactive adhesive, by a thermal method in which the
backing surface is melted enough to entrap some of the
material used for the hydrophilic layer, or by microwave
or ultrasonic bonding methods which likewise fuse the
hydrophilic sample pads to the backing. It is important
that the bonding be such as to not itself interfere
substantially with the diffuse reflectance measurements or
the reaction being measured, although this is unlikely to
occur as no adhesive need be present at the location where
the reading is taken. For esample, an adhesive 13 can be
applied to the backing strip 12 followed first by punching
hole 14 into the combined strip and adhesive and then
applying reagent pad 11 to the adhesive in the vicinity of
hole 14 so that the peripheral portion of the reagent pad
attaches to the backing strip.
The Chemical Reagents
Any signal producing system may be employed that is
- capable of reacting with the analyte in the sample to
produce (either directly or indirectly) a compound that is
characteristically absorptive at a wavelength other than a
wavelength at which the assay medium substantially absorbs.
Polyamide matrices are particularly useful for carrying
out reactions in which a substrate (analyte) reacts with
an osygen-utilizing osidase enzyme in such a manner that a
product is produced that further reacts with a dye
intermediate to either directly or indirectly form a dye
which absorbs in a predetermined wavelength range. For
esample, an osidase enzyme can osidize a substrate and
produce hydrogen peroside as a reaction product. The
hydrogen peroside can then react with a dye intermediate
or precursor, in a catalysed or uncatalyzed reaction, to
LFS-3
1 337b~
produce an osidized form of the intermediate or
precursor. This osidized material may produce the colored
product or react with a second precursor to form the final
dye.
Nonlimiting esamples of analyses and typical reagents
include the following materials shown in the following
list:
1 ~37682
- 15 -
Analyte and Sample TYpe Reaqents
Glucose in blood, serum, Glucose Osidase, Perosi-
urine or other biological dase and an Osygen
fluids, wine, fruit juices Acceptor
5 or other colored aqueous
fluids. Whole blood is a Osygen Acceptors include:
particularly preferred O-dianisidine (1)
sample type, as separation O-toluidine
is time-consuming and O-tolidine (1)
10 impractical with home use. Benzidine (1)
2,2'-Azinodi-(3-ethylbenz-
thiazoline sulphonic
acid-(6)) (1)
3-Methyl-2-benzothiazoli-
none hydrazone plus N,N-
dimethylaniline (1)
Phenyl plus 4-aminophena-
zone (1)
Sulfonated 2,4-dichloro-
phenol plus 4-amino-
phenazone (2)
3-Methyl-2-benzothiazoli-
none hydrazone plus 3-
(dimethylamino)benzoic
acid (3)
2-Methosy-4-allyl phenol
(4)
4-Aminoantipyrene-
dimethylaniline (5)
~1) As reported Clinical ChemistrY, Richterich and
Columbo, p. 367 and references cited therein.
(2) Analyst, 97, (1972) 142-5.
(3) Anal. Biochem., 105, (1980) 389-397.
(4) Anal. Biochem., 79, (1977) 597-601.
;FS-3
il 337,~
- 16 -
(5) Cli~ica ~h~mica Acta, 75, (1977) 387-391.
The An alysis Method
The analysis method of this invention relies on a change
in absorbance, as measured by diffuse reflectance, which
is dependent upon the amount of analyte present in a
sample being tested. This change may b~ determined by
measuring the change in the absorbance of the test sample
betw~en two or more points in tims.
The first step of the assay to be considered will be
application of the sample to the matri~. In practice, an
analysis could be carried out as follows: First a sample
of a~ueous fluid containing an analyte is obtained. Blood
may be obtained by a finger stick, for e~ample. An e~cess
over threshold matris saturation in the area where
reflectance will be measured (i.e., about 5-10
microliters) o this fluid is applied to the reagent
element or elements of the test device. Simultaneous
starting of a timer is not required (as is commonly
required in the prior art), as will become clear below,
due to the initialization procedure practiced by the
present invention. Excess fluid can be removed, such as
by light blotting, but such removal is also not required.
The test device is typically mounted in an instrument for
reading light absorbance, e.g., color intensity by
reflectance, prior to application of the sample.
Absorbance is measured at certain points in time after
application of the sample. Absorbance refers in this
application not only to light within the visual wavelength
range but also outside the visual wavelength range, such
as infrared and ultraviolet radiation. From these
measurements of absorbance a rate of color development can
... ~ . .. ,.. , . . , , . , . , . ., . , ~ ~ . , ,
13~ 6B2~
- 17 -
be calibrated in terms of analyte level.
The Measurinq Instrument
A suitable instrument, such as a diffuse reflectance
spectrophotometer with appropriate software, can be made
to automatically read reflectance at certain points in
time, calculate rate of reflectance change, and, using
calibration factors, output the level of analyte in the
aqueous fluid. Such a device is schematically shown in
Figure 2 wherein a test device of the invention comprising
backing 12 to which reagent pad 11 is affised is shown.
Light source 5, for esample a high intensity light
emitting diode (LED) projects a beam of light onto the
reaqent pad. A substantial portion (at least 25%,
preferably at least 35%, and more preferably at least 50%,
in the absence of reaction product) of this light is
diffusively reflected from the reagent pad and is detected
by light detector 6, for e~ample a phototransistor that
produces an output current proportional to the light it
receives .
Light source 5 and/or detector 6 can be adapted to
generate or respond to a particular wavelength light, if
desired. The output of detector 6 is passed to amplifier
7, for e~ample, a linear integrated circuit which converts
the phototransistor current to a voltage. The output of
amplifier 7 can be fed to track and hold circuit 8. This
is a combination linear/digital integrated circuit which
tracks or follows the analog voltage from amplifier 7 and,
upon command from microprocessor 20, locks or holds the
voltage at its level at that time.
Analog-to-digital converter 19 takes the analog voltage
from track and hold circuit 8 and converts it to, for
1 337682
- 18 -
esample, a twelve-bit binary digital number upon command
of microprocessor 20. Microprocessor 20 can be a digital
integrated circuit. It serves the following control
functions: 1) timing for the entire system; 2) reading of
the output of analog/digital converter 19; 3) together
with program and data memory 21, storing data
corresponding to the reflectance measured at specified
time intervals; 4) calculating analyte levels from the
stored reflectances; and 5) outputting analyte
concentration data to display 22. Memory 21 can be a
digital integrated circuit which stores data and the
microprocessor operating program. Reporting device 22 can
take various hard copy and soft copy forms. Usually it is
a visual display, such as a liquid crystal (LCD) or LED
display, but it can also be a tape printer, audible
signal, or the like. The instrument also can include a
start-stop switch and can provide an audible or visible
time output to indicate times for applying samples, taking
readings etc., if desired.
Reflectance Switchinq
In the present invention, the reflectance circuit itself
can be used to initiate timing by measuring a drop in
reflectance that occurs when the aqueous portion of the
suspension solution applied to the reagent pad (e.g.,
blood) migrates to the surface at which reflectance is
being measured. Typically, the measuring device is turned
on in a ~ready~ mode in which re-flectance readings are
automatically made at closely spaced intervals ~typically
about 0.2 seconds) from the typically off-white,
substantially dry, unreacted reagent strip. The initial
measurement is typically made prior to penetration of the
matris by fluid being analyzed but can be made after the
fluid has been applied to a location on the reagent
S-3
f
1 337682
-- 19 --
element other than where reflectance is being measured.
The reflectance value is evaluated by the microprocessor,
typically by storing successive values in memory and then
comparing each value with the initial unreacted value.
When the aqueous solution penetrates the reagent matris,
the drop in reflectance signals the start of the measuring
time interval. Drops in reflectance of 5-50% can be used
to initiate timing, typically a drop of about 10~. In
this simple way there is esact synchronization of assay
medium reaching the surface from which measurements are
taken and initiation of the sequence of readings, with no
re~uirement of activity by the user.
Althouqh the total systems described in this application
are particularly directed to the use of polyamide matrices
and particularly to the use of such matrices in
determining the concentration of various sugars, such as
glucose, and other materials of biological origin, there
is no need to limit the reflectance switching aspect of
the invention to such matrices. For esample, the matris
used with reflectance switching may be formed from any
water-insoluble hydrophilic material and any other type of
reflectance assay.
Particular Application to Glucose AssaY
A particular esample with regard to detecting glucose in
the presence of red blood cells will now be given in order
that greater detail and particular advantage can be
pointed out. Although this represents a preferred
embodiment of the present invention, the invention is not
limited to the detection of glucose in blood.
The use of polyamide surfaces to form the reagent element
provides a number of desirable characteristics in the
` 1 337682
- 20 -
present invention. These are that the reagent element is
hydrophilic (i.~., takes up reagent and sample readily),
does not deform on wetting (so as to ~rovide a flat
surface for reflectance reading), is compatible with
enzymes (in order to impart good shelf stability), takes
up a limited sample volume per unit volume of membrane
(necessary in order to demonstrate an e~tended dynamic
range of measurements), and shows sufficient wet strength
to allsw for routine manufacture.
In a typical configuration, the method is carrier out
using an apparatus consisting of a plastic holder and the
reagent element (the matrix having the signal producing
system impregnated therein.) The preferred matri~ for use
in preparing the reagent element is a nylon
microfiltration membrane, particularly membranes made from
nylon-6~ cast on a core of non-woven polyester fibers.
Numerous nylon microfiltration membranes of this class are
produced commercially by the Pall UItrafin~ Filtration
Corporation, having average pore sizes from 0.1 to 3.0
microns. These materials shown mechanical strength and
- fle~ibility, dimensional stability upon e~posure to water,
- and rapid wetting.
Many variations in specific chemical structure of the
nylon are possible. These include unfunctionalized
nylon-66 with charged end groups (sold under the trademark
ULTRAPO~E by Pall Ultrafine Filtration Corporation,
~PallW~. Positive charges predominate below pH 6 while
negative charges predominate above pH 6. In other
membranes the nylon is functionalized before the membrane
is formed to give membranes with different properties.
Nylons functionalized with carboxy groups are neg~ ively
charged over a wide pH range (sold as CAR80~YVYN~ by
; 35 Pall). Nylons can also be functionalized with a high
n
L~ .
~ .
1 337682
,
- 21 -
density of positively charged groups on its surface,
typically quaternary amine groups, so that they display
little variation in charge over a wide pH range (sold as
POSIDYNE by Pall). Such materials are particularly well
suited for the practice of the pressnt invention.
It has been found that keeping the p~ of the solution
below 4.8 will help stabilize the enzym~s in solution.
The most efficient level of stability has been found at pH
4Ø This results in shelf life at room temperature of
12-18 months. Consequently, a strip with positively
charged io~s is most desirable.
~ ' :
It is also possible to use membranes havinq reactive
functional groups desi~ned for covalent iTMobilization of
proteins (sold as BIODYNE IMMUNO AFFINITY membranes by
Pall). Such materials can be used to covalently attach
proteins, e.g. enzymes, used as reagents. Although all of
these materials are usable, nylon having a high density of
positively char~ed groups on its surface provide the best
stability of reagents when formulated into a dry reagent
pad. Unfunctionalized nylon gives the nest best stability
with the carbo~ylated nylons ne~t best.
, ' .
Desirable results can be obtained with pore sizes ranging
from about 0.2-2.0~m, preferably about 0.5-1.2y~, and
most prefera~ly about 0.8~m, when used with whole blood.
The form of the handle on which the reagent element is
assembled is relatively unimportant as long as the handle
allows access to one side of the reagent element by sample
and to the other side of the reagent element by incident
light whose reflectance is being measured. The handle
- also aids in inserting the reagent element into the
absorbance measuring device so that it registers with the
'~
.'~ .
.. .. .. .. . . .... .. . . . . .. . .
1 337682
- 22 -
optical system. One esample of a suitable handle is a
mylar or other plastic strip to which a transfer adhesive
such as 3M 465 or Y9460 transfer adhesive has been
applied. A hole is pl~nche~ into the plastic through the
transfer adhesive. A reagent element, typically in the
form of a thin pad, either containing reagents or to which
reagents will later be added, is then applied to the
handle by means of the transfer adhesive so that it is
firmly attached to the handle in the area surrounding the
hole that has been punched through the handle and the
transfer adhesive.
Such a device is illustrated in Figure 1, which shows a
strip 10 having a reagent pad 11 attached to a Mylar
handle 12 by means of adhesive 13. Hole 14 allows access
of the sample or incident light to one side of reagent pad
11 while access to the other side of the reagent pad is
unrestricted. All dimensions of the reagent pad and
handle can be selected so that the reagent pad fits
securely into a reflectance-reading instrument in prosimal
location to a light source and a reflected-light
detector. Generally, dimensions of the hole are in the
range of about 2-lOmm diameter, and that of the width of
the handle about 15mm. A 5mm diameter hole 14 in the
reagent strip shown in Figure 1 works quite
satisfactorily. Naturally, there is no particular limit
on the minimum diameter of such a hole, although diameters
of at least 2mm are preferred for ease of manufacture,
sample application, and light reflectance reading.
As further seen in Figures 3 and 4, the strip 10 can be
optimally guided into a slot 50 on scanning machine 60.
This is accomplished by placing a notch 15 in the strip 10
at about the midpoint of the top of strip 10. In so
doing, the strip 10, when guided through sides 55 of slot
LFS-3
1 337682
- 23 -
50, will arrive repeatably at the same location, to assure
high assurance in test results. Such repeatability is
accomplished by moving the notch 15 against post 65. The
strip 10 will pivot around the post 65 at the notch 15, so
that the edges 16 of the strip will fit within the sides
55 of the slot 50. This, of course, also repeatably
aligns the hole 14 over the test center 80 comprising
multiple LEDs 5 in the scanning machine 60. This insures
that the hole 14 containing a blood sample will have
uniform dosage of incident light for analysis.
Although a number of dyes could be used as indicators, the
choice will depend upon the nature of the sample. It is
necessary to select a dye having an absorbance at a
wavelength different from the wavelength at which red
blood cells absorb light, with whole blood as the assay
medium, or other contaminants in the solution being
analyzed with other assay media. The MBTH-DMAB dye couple
(3-methyl-2-benzothiazolinone hydrazone hydrochloride and
3-dimethylaminobenzoic acid), although being previously
described as suitable for color development for perosidase
labels in enzyme immunoassays, has never been used in a
commercial glucose measuring reagent. This dye couple
gives greater dynamic range and shows improved enzymatic
stability as compared to traditional dyes used for glucose
measurement, such as benzidine derivatives. This
enzymatic stability also makes the-MBTH-DMAB dye couple
especially desirable in order to insure longer shelf life
of the test strips. Furthermore, the MBTH-DMAB dye couple
is not carcinogenic, a characteristic of most benzidine
derivatives.
Another dye couple that can be used in the measurement of
glucose is the AAP-CTA (4-aminoantipyrene and chromotropic
acid) couple. Although this couple does not provide as
1 337682
.
- 24 -
broad a dynamic range as MBTH-DMAB, it is stable and
suitable for use in the practice of the present invention
when measuring glucose. Again, the AAP-CTA dye couple
provides an espanded dynamic range and greater enzymatic
activity stability than the more widely used benzidine
dyes.
The use of the MBTH-DMAB couple allows for correction of
hematocrit and degree of osygenation of blood with a
single correction factor. The more typically used
benzidine dyes do not permit such a correction. The
MBTH-DMAB dye forms a chromophore that absorbs at
approsimately 635nm but not to any significant estent at
700nm. Slight variations in measuring wavelengths (+
about lOnm) are permitted. At 700nm both hematocrie and
degree of osygenation can be measured by measuring blood
color. Furthermore, light emitting diodes (LED) are
commercially available for both 635nm and 700nm
measurements, thereby simplifying mass-production of a
device. By using the preferred membrane pore size
described above and the subject reagent formulation, both
hematocrit and oxygenation behavior can be corrected by
measuring at the single 700nm wavelength.
Two additional conditions were found to provide particular
stability and long shelf life for a glucose
osidase/peroxidase formulation on a polyamide matrix.
Storage is enhanced at a pH in the range of 3.8 to 5.0
preferably about 3.8 to 4.3, most preferably about 4Ø
Similarly, unexpectedly good storage and stability was
found with misture of a concentrated buffer system to the
the reagents found in the matris. The most effective
buffer was found to be a 10~ weight citrate buffer, with
concentrations from about 5-15% being effective. These
are weight/volume percentages of the solution in which the
- 1 337682
- 25 -
reagents are applied to the matris. Other buffers can be
used on the same molar basis. Greatest stability was
achieved using a low pH, preferably about pH 4, an
MBTH-DMAB dye system, and a high enzyme concentration of
S approsimately 500-1000 M/ml of application solution. As
previously indicated, such strips prepared using these
parameters result in shelf life of about 12-18 months.
.
In preparing the MBTH-DMAB reagent and the enzyme system
that forms the remainder of the signal producing system,
it is not necessary to maintain esact volumes and ratios
although the suggested values below give good results.
Reagents are readily absorbed by the matris pad when the
glucose osidase is present in a solution at about 27-54%
by volume, the perosidase is present at a concentration of
about 2.7-5.4mg/ml, MBTH is present at a concentration of
about 4-8mg/ml, and DMAB is present at a concentration of
about 8-16mg/ml. The DMAB-MBTH weight ratio is preferably
maintained in the range of 1:1 to 4:1, preferably about
1.5:1 to 2.5:1, most preferably about 2:1.
The basic manufacturing techniques for the reagent element
are, once established, straightforward. The membrane
itself is strong and stable, particularly when a nylon
membrane of the preferred embodiment is selected. Only
two solutions are necessary for applying reagent, and
these solutions are both readily formulated and stable.
The first generally contains the dye components and the
second generally contains the enzymes. When using ~he
MBTH-DMAB dye couple, for esample, the individual dyes are
dissolved in an aqueous organic solvent, typically a 1:1
misture of acetonitrile and water. The matris is dipped
into the solution, escess liquid is removed by blotting,
and the matris is then dried, typically at 50C-60C for
10-20 minutes. The matris containing the dyes is then
,-3
- 2!337682
dipped into an aqueous solution containing the enzymes. A
typical formulation would contain the perosidase and
glucose osidase enzymes as well as any desired buffer,
preservative, stabilizer, or the like. The matri~ is then
blotted to remove escess liquid and dried as before. A
typical formulation for the glucose reagent is as follows:
DYe dip
Combine:
40 mg MBTH,
80 mg DMAB,
5 ml acetonitrile, and
5 ml water.
Stir until all solids are dissolved and pour onto a glass
plate or other flat surface. Dip a piece of Posidyne
membrane (Pall Co.), blot off escess liquid, and dry at
56C for 15 minutes.
Enzyme di~
Combine:
6 ml water,
10 mg EDTA, disodium salt,
200 mg Sigma Poly Pep~, low viscosity,
0.668 g sodium citrate,
0.523 g citric acid,
2.0 ml 6 wt~ GAF Gantrez~ AN-139 dissolved in water
30 mg horseradish perosidase, 100 units/mg, and
3.0 ml glucose osidase, 2000 units~ml.
Stir until all solids are dissolved and pour onto a glass
plate or other flat surface. Dip a piece of membrane
previously impregnated with dyes, blot off escess liquid,
and dry at 56C for 15 minutes.
The electronic apparatus used to make the reflectance
readings minimally contains a light source, a reflected
1 337682
- 27 -
light detector, an amplifier, an analog to digital
converter, a microprocessor with memory and program, and a
display device, as seen in Figure 2.
The light source typically consists of a light emitting
diode (LED). Although it is possible to use a
polychromatic light source and a light detector capable of
measuring at two different wavelengths, a preferred
apparatus would contain two LED sources or a single diode
capable of emitting two distinct wavelengths of light.
Commercially available LEDs producing the wavelengths of
light described as being preferred in the present
specification include a Hewlett Packard HLMP-1340 with an
emission masimum at 635nm and a Hewlett Packard QEMT-1045
with a narrow-band emission masimum at 700nm. Suitable
commercially available light detectors include a
Ha~m~matsu 5874-18R and a Litroni~ BPX-65.
Although other methods of taking measurements are
feasible, the following method has provided the desired
results. Readings are taken by the photodetector at
specified intervals after timing is initiated. The 635nm
LED is powered only during a brief measuring time span
that begins approsimately 20 seconds after the start time
as indicated by the previously described reflectance
switching system. If this reading indicates that a high
level of glucose is present in the sample, a 30-second
reading is taken and used in the final calculation in
order to improve accuracy. Typically, high levels are
considered to begin at about 250 mg/dl. The background is
corrected with a 700nm reading taken about 15 seconds
after the start of the measurement period. The reading
from the photodetector is integrated over the interval
while the appropriate LED is activated, which is typically
less than one second. The raw reflectance readings are
~S-3
` 1337682
- 28 -
then used for calculations performed by the microprocessor
after the signal has been amplified and converted to a
digital signal. Numerous microprocessors can be used to
carry out the calculation. An AIM65 single-board
microcomputer manufactured by Rockwell International has
proven to be satisfactory.
The present methods and apparatuses allow a very simple
procedure with minimum operational steps on the part of
the user. In use, the reagent strip 10 is placed in the
detector so that the hole 14 in the strip 10 registers
with the optics of the detecting system. The
above-described notch 15/post 65 system, as seen in
Figures 4 and 5 works nicely to accomplish such
alignment. A removable cap or other cover 90 is placed
over the optics and strip to shield the assembly from
ambient light. This is done to enhance reading of the
strip 10. While the initilization process can begin in
light, direct sunlight or high intensity room light tends
to inhibit results. The cap 90 insures that direct light
- does not hit the reagent strip 10. The cap 90 need not be
light-tight, only enough to protect the strip 10 from
direct light.
The measurement sequence is then initiated by pressing a
button on the measuring apparatus that activates the
microcomputer to take a measurement of reflected light
from the unreacted reagent pad, called an Rdry reading.
The cap 90 is then removed and a drop of blood is applied
to the reagent strip 10, typically while the reagent strip
10 is registered with the optics and the reading device.
It is preferred that the reagent strip be left in register
with the optics in order to minimize handling. The cap 90
is then closed.
- 1 337682
- 29 -
The instrument is capable of sensing the application of
blood or other sample by a decrease in the reflectance
when the sample passes through the matris and reflected
light is measured on the opposite side. The decrease in
reflectance initiates a timing sequence which is described
in detail at other locations in this specification. The
cap 90 should be replaced within 15 seconds of sample
application, although this time may vary depending on the
type of sample being measured.
Results are typically displayed at appro~imately 30
seconds after blood application when a blood glucose
sample is being measured, although a 20 second reaction is
permissible for glucose samples having a concentration of
glucose of less than 250mg/dl. If other samples are being
measured, suitable times for displaying the result may
differ and can be readily determined from the
characteristics of the reagent/sample selected.
A particularly accurate evaluation of glucose level (or
any other analyte being measured) can be made using the
background current, i.e., the current from the photo
detector with power on but with no light reflected from
the reagent pad, in order to make a background
correction. It has been demonstrated that over a 2-3
month period that this value does not change for a
particular instrument prepared according to the preferred
embodiments of this specification, and it is possible to
program this background reading into the computer memory
as a constant.
With a slight modification of the procedure, however, this
value can be measured (or normalized) with each analysis
for more accurate results. Each LED is turned on prior to
3S placement of the blood sample on the reagent strip 10 but
1 337682
- - 30 -
with the reagent strip 10 in place. A reflectance value
of the strip lO is then measured, with the reagent strip
lO in place and the light protective cap 90 closed. If
this measurement is different than the original
measurement of the reflectance value, power to the LED is
increased so that the reflectance will be the same. This
insures that the measurement of blood glucose content is
beinq made on the same repeatable scale for each blood
glucose reading.
The reason for instituting this method is twofold. First,
the intensity of liqht emitting diodes will vary greatly
from LED to LED, even when all the measuring LEDs are
new. Second, the LED efficiency will vary with both
temperature and the life of the LED. With this method,
results are repeatable on the same scale.
The raw data necessary for calculating a result in a
glucose assay are a background current reported as
background reflectance, Rb, as described above; a
reading of the unreacted test strip, Rdry, which is
about 95% opaque to light and is also described above; and
an end point measurement. Using the preferred embodiments
described herein, the end point is not particularly stable
and must be precisely timed from the initial application
of blood. However, the meter as described herein performs
this timing automatically. For glucose concentrations
below 250mg/dl, a suitably stable end point is reached in
20 seconds, and a final reflectance, R2~, is taken. For
glucose concentrations up to 450mg/dl, a 30-second
reflectance reading, R30, is adequate. Although the
system described herein displays good differentiation up
to 800mg/dl of glucose, the measurement is somewhat noisy
and inaccurate above 450mg/dl, although not so great as to
cause a significant problem. Longer reaction times should
~FS-3
1 337682
- 31 -
provide more suitable readings for the higher levels of
glucose concentration.
The 700nm reflectance reading for the dual wavelength
measurement is typically taken at 15 seconds (R15). By
this time blood will have completely saturated the reagent
pad. Beyond 15 seconds the dye reaction continues to take
place and is sensed, to a small part, by a 700nm reading.
Accordingly, since dye absorption by the 700nm signal is a
disadvantage, readings beyond 15 seconds are ignored in
the calculations.
The raw data described above are used to calculate
parameters proportional to glucose concentration which can
be more easily visualized than reflectance measurements.
A logarithmic transformation of reflectance analogous to
the relationship between absorbance and analyte
concentration observed in transmission spectroscopy
(Beer's Law) can be used if desired. A simplification of
the Kubelka-Monk equations, derived specifically for
reflectance spectroscopy, have proven particularly
useful. In this derivation K/S is related to analyte
concentration with K/S defined by Equation 1.
K/S-t = (1 - R*t)2/(2 s R*t) (1)
R*t is the reflectivity taken at a particular end point
time, t, and is the absorbed fraction of the incident
light beam described by Equation 2, where Rt is the end
point reflectance, R20 or R30.
R*t = (Rt ~ Rb)/(Rdry b (2)
R*t varies from 0 for no reflected light (Rb) to 1 for
total reflected light (Rdry). The use of reflectivity
- 1 337682
- 32 _
in the calculations greatly simplifies meter design as a
highly stable source and a detection circuit become
unnecessary since these components are monitored with each
Rdry and Rb measurement.
s
For a single wavelength reading K/S can be calculated at
20 seconds (K/S-20) or 30 seconds (K/S-30). The
calibration curves relating these parameters to YSI
(Yellow Springs Instruments) glucose measurements can be
precisely described by the third order polynomial equation
outlined in Equation 3.
YSI = aO + al(K/S) ~ a2(K/S) + a3(K/S) (3)
The coefficients for these polynomials are listed in Table
1.
TABLE 1.
Coefficients for Third Order Polynomial Fit of Single
Wavelength Calibration Curves
R/S-20 K/S-30
aO -55.75 -55.25
al 0.1632 0.1334
a2 -5.765 s 10-5 -2.241 ~ 10-5
a3 2.58 s 10-8 1.20 s 10-8
The single chemical species being measured in the
preferred embodiments is the MBTH-DMAB inamine dye and the
comple~ matris being analyzed is whole blood distributed
on a 0.8~ Posidyne~ membrane. A review entitled
~Application of Near Infra Red Spectrophotometry to the
Nondestructive Analysis of Foods: A Review of
Esperimental Results~, CRC Critical Reviews in Food
~S-3
1 337682
- 33 -
Science and Nutrition, 18(3) 203-30 (1983), describes the
use of instruments based on the measurement of an optical
density difference ~OD (~a~~b) where OD~a is
the optical density of the wavelength corresponding to the
absorption masimum of a component to be determined and
OD~b is the optical density at a wavelength where the
same component does not absorb significantly.
The algorithm for dual wavelength measurement is by
necessity more comples than for single wavelength
measurement but is much more powerful. The first order
correction applied by the 700nm reading is a simple
subtraction of background color due to blood. In order to
make this correction, a relationship between absorbance at
635nm and 700nm due to blood color can be and was
determined by measuring blood samples with 0 mg/dl glucose
over a wide range of blood color. The color range was
constructed by varying hematocrit, and fairly linear
relationships were observed. From these lines the K/S-15
at 700nm was normalized to give equivalence to the K/S-30
at 635nm. This relationship is reported in Equation 4,
where K/S-15n is the normalized K/S-15 at 700nm.
K/S-15n = (K/S-15 s 1.54) - 0.133 (4)
Note that the equivalence of the normalized 700nm signal
and the 635nm signal is only true at zero glucose. The
espressions from which the calibration curves were derived
are defined by Equations 5 and 6.
K/S-20/15 = (K/S-20) - (K/S-15n) (5)
K/S-30/15 = (K/S-30) - (K/S-15n) (6)
These curves are best fit by fourth-order polynomial
LFS-3
1 337682
- 34 _
equations similar to Equation 3 to which a fourth-order
term in X/S is added. The computer-fit coefficients for
these equations are listed in Table 2.
TABLE 2.
Coefficients for Fourth-Order Polynomial Fit of
Dual Wavelength Calibration Curves
K/S-20/15 K/~-30/15
aO -0.1388 1.099
al 0.}064 0.05235
a2 6.259 s 10-5 1.229 s 10-4
a3 -6.12 s 10-8 -5.83 ~ 10-8
a4 3.21 s 10-11 1.30 ~ 10-11
A second order correction to eliminate errors due to
chromatography effects has also been developed. Low
hematocrit samples have characteristically low 700nm
readings compared to higher hematocrit samples with the
same 635nm reading. When the ratio of (K/S-30)/(K/S-15)
i5 plotted versus K/S-30 over a wide range of hematocrits
and glucose concentrations, the resulting line on the
graph indicates the border between samples which display
chromatography effects (above the curve) and those that do
not (below the curve). The K/S-30 for the samples above
the curve are corrected by elevating the reading to
correspond to a point on the curve with the same
(K/S-30)/(K/S-15), as demonstrated by the correction made
in Fig. 5.
The correction factors reported above were tailored to fit
a single instrument and a limited number of reagent
preparations. The algorithm can be optimized for an
individual instrument and reagent in the same manner that
-- - 1 337~82
- - 35 -
is described above.
In summary, the system of the present invention minimizes
operator actions and provides numerous advantages over
prior art reflectance-reading methods. When compared to
prior methods for determining glucose in blood, for
e2ample, there are several apparent advantages. First,
the amount of sample required to saturate the thin reagent
pad is small (typically 5-10 microliters), and is of
course, volume independent once the threshold volume of
blood is supplied to the reagent pad. Second, operator
time required is only that necessary to apply the sample
to the thin hydrophilic layer and close the cover
(typically 4-7 seconds). Third, no simultaneous timing
start is required. Fourth, whole blood can be used. The
method does not require any separation or utilization of
red-cell-free samples and likewise can be used with other
deeply colored samples. Fifth, via the reflectance
reading and normalization techniques applied in the
present invention the system provides reliable, accurate,
repeatable readings for the lifetime of the scanning
system.
Several unobvious advantages arise as a result of the
practice of the present invention with whole blood.
Normally, aqueous solutions (like blood) will penetrate a
hydrophilic membrane to give a liquid layer on the
opposite side of the membrane, a surface that is then not
suited for a reflectance measurement. It has been
discovered, however, that blood, apparently because of
interactions of red blood cells and proteins in the blood
with the matri~, will wet the polyamide matris without
having an e~cess liquid penetrate the porous matri~ to
interfere with the reflectance reading on the opposite
side of the matri~.
- I 3376~2
- 36 -
Furthermore, the thin membranes used in the present
invention would be espected when wet to transmit light and
return only a weak signal to the reflectance measuring
device. Prior teachings have generally indicated that a
reflective layer is necessary behind the matris in order
to reflect sufficient light. In other cases a white pad
has been placed behind the reagent pad prior to color
measurement. In the present case, neither a reflective
layer nor a white pad is required. In fact, the invention
is typically carried out with a light-absorbing surface
behind the reagent element when incident light is impinged
upon the matris. This is accomplished using a light
absorbing surface behind the reagent element, coupled with
measuring reflectance at two different wavelengths. It
allows acceptable reflectance measurements to be obtained
without removal of escess liquid from the matris, thereby
eliminating a step typically required by previous
teachings.
The invention now being generally described, the same will
be better understood by reference to the following
specific esamples which are presented for purposes of
illustration only and are not to be considered limiting of
the invention unless so specified.
Example I
Reproducibility:
One male blood sample (having a hematocrit level of 45)
was used to collect the reproducibility data using-the
presently preferred embodiment of the system, called the
MPX system. The results are set forth in Tables 3-5.
TABLE 3. 1 ~ 316 8 2
Reproducibility of a Single Wavelength System
Averaqe (mg/dl) *~.D. (ma/dl) %C.V.*~
***YSI(mg/dl) 20sec. 30sec. 20sec. 30sec. 20sec. 30sec.
23.1 23.0 2.1 2.04 9.1 9.0
53.3 53.2 3.19 3.32 6.0 6.3
101 101 101 3.0 3.3 3.0 3.0
326 326.6 327 13.3 9.8 4.1 3.0
501 503 17.1 3.4
690 675 28 4.15
810 813 37 4.5
* S.D. = Standard Deviation
** % C.V. = Covariance (measured by percentage)
*** YSI = Yellow Spring Instrument Glucose reading
TABLE 4.
Reproducibility of a Dual Wavelength System
Average (mg/dl) S.D. (mq/dl) %C.V.
YSI(mq/dl) 20sec. 30sec.20sec. 30sec. 20sec. 30sec.
27 1.34 1.55 5.4 5.7
57.42.58 2.62 4.7 4.6
101 101 101.52.55 2.18 2.5 2.1
326 332 330 15.0 7.1 4.5 2.1
5~1 505 21.3 4.2
690 687 22.8 3.3
810 817 30.4 3.7
6~ ~
- 38 -
TABLE 5.
.
Reproducibility of a 3.Omm Diameter Aperture
% C.V.
YSI (mg/dl) 4.7mm 3.Omm
55-100 4.8 4.9
300 3.0 5.0
600 3.8 5.5
avg. 3.9 5.1
The blood was divided into aliquots and spiked with
glucose across a range of 25-800mg/dl. Twenty
determinations were made at each glucose test level from
strips taken at random from a 500 strip sample (Lot
FJ4-49B). The results of this study lead to the following
conclusions:
1. Sinale vs. Dual Wavelenath: The average covariance
for the 30-second dual result was 3.7% vs. 4.8% for
the 30-second single wavelength result, an improvement
of 23% across a glucose range of 25-810 mg/dl. There
was a 33% improvement in covariance in the 25-326
mg/dl glucose range. Here the covariance decreased
from 5.4% to 3.6%, a significant improvement in the
most used range. The 20-second dual wavelength
measurement gave similar improvements in covariance
compared to the single wavelength measurement in the
25-326 mg/dl range (Tables 3 and 4).
2. Dual Wavelength, 20 vs. 30-second Result: The average
covariance for a 20-second result in the 25-100 mg/dl
range is nearly identical to the 30-second reading,
4.2% vs. 4.1%. However, at 326 mg/dl the 30-second
reading has a covariance of 2.1% and the 20-second
S-3
- 1 ~37682
- 39 -
result a covariance of 4.5%. As was seen in the
X~S-20 response curve, the slope begins to decrease
sharply above 2S0 mg/dl. This lead to poor
reproducibility at glucose levels greater than 300 for
the 20-second result. From this reproducibility data
the cutoff for the 20-second result i5 somewhere
between 100 and 326 mg/dl. A cutoff of 250 mg~dl was
later determined from the results of the recovery
study set forth in E~ample II.
3. Aperture Size: A smaller optics aperture size, 3.0mm,
was investigated. Initial e~perimentation using a 10
replicate, hand-dipped disk sample did show improved
covariances with the 3.Omm aperture, apparently
because of easier registration with the system
optics. However, when machine-made roll membrane was
used, the average (Table 5) of the larger aperture,
4.7mm, was 3.9% vs. an average covariance for the
3.0mm aperture of 5.1%. This 30% increase was
probably due to the uneven surface of the roll
membrane lot as discussed below.
E~amPle II
RecoverY:
For comparison of the present preferred method called MPX
against a typical prior art method using a Yellow Springs
Instrument Model 23A glucose analyzer manufactured by
Yellow Springs Instrument Co., Yellow Springs, Ohio (YSI),
blood from 36 donors was tested. The donors were divided
equally between males and females and ranged in hematocrit
from 35 to 55~. The blood samples were used within 30
hours of collection, with lithium heparin as the
anti-coagulant. Each blood sample was divided into
S-3
1 337~2
- 40 -
aliquots and spiked with glucose to qive lS2 samples in
the range of 0-700 mg/dl glucose. Each sample was tested
in duplicate for a total of 304 data points.
Response curves were constructed for the appropriate
equation (see Tables 1 and 2). These MPX glucose values
were then plotted vs. the YSI values to give scattergrams,
as seen in Figures 6a and 6b for the Single Wavelength
System, and Figures 7a through 7d for the Dual Wavelength
System.
C~parison of MPX SYstems: For both the 20-second and
30-second measurement times there is visually more scatter
in the single-wavelength scatterqrams than the
dual-wavelength scattergrams. The 20-second reading
becomes very scattered above 250 mg/dl but the 30-second
measurement does not have wide scatter until the glucose
level is greater than 500 mg/dl.
These scattergrams were quantitated by determining the
deviations from YSI at various glucose ranges. The
following results were obtained.
TABLE 6.
Accuracy of MPX System from Recovery Data
MPX Measurement *S.D.(mg/dl) C.V. for Range**(%)
Wavelenqth Time ~sec.) 0-50 50-250 250-450
Single 20 +5.6 7.2 14.5
Single 30 +6.9 7.1 8.8 10.2
Dual 20 +2.3 5.3 12.8
Dual 30 +2.19 5.5 5.8 8.4
S-3
7 ~
- 41 -
- Standard Deviation
~* = These are inter-method covariances
Note that:
a. The dual wavelength system gave results that
ranged 30% lower than the single wavelength
system.
b. The single wavelength system, from 0-50 mg/dl,
showed a Standard Deviation of +6-7 mg/dl
whereas the Standard Deviation for a dual
wavelength measurement was only +2.2 mg/dl.
c. The cutoff for a 30-second MPX measurement is
250 mg/dl. ~or the 50-250 mg/dl range both the
20- and 30-second measurements gave similar
inter-method covariances (approsimately 7% for
single wavelength, 5.5% for dual wavelength).
- However, in the 250-450 mg/dl range inter-method
covariances more than double for the 20-second
reading to 14.5% for the single and 12.8% for
the dual wavelength.
d. The 30-second reading was unusable above 450
mg/dl for both the single and dual wavelength
measurement (covariances of 10.2% and 8.4%).
In conclusion, the two MPX systems gave optimum
quantitation in the 0-4S0 mg/dl range.
1. MPX System -- 30 Second Dual Wavelength: This dual
wavelength system gave a 30-second measurement time
with a 95% confidence limit (defined as the
probability of a measurement being within +2 Standard
Deviation of the YSI reading) of 11.3% covariance for
S-3
)
1~3~6B~
- 42 -
the range from 50-450 mgidl (Table 7) and +4.4
mg/dl (Standard Deviation) for 0-50 mg/dl.
2. MPX System -- 30/20 Second Dual Wavelength: This
dual wavelength system gave a 20-second measurement
time in the 0-250 mg/dl range and a 30-second time
for the 250-450 range. The 95% confidence limits are
nearly identical to the MPX 30 Second Dual Wavelength
system (Table 7), 11.1% covariance for 50-450 mg/dl
and ~4.6 mg/dl (Standard Deviation) for 0-50 mg/dl.
TAB$E 7.
Comparison of 95% Confidence Limits or the MPX System,
GlucoScan Plus and Accu-Chek bG~*** Reagent Strips
Measuring
Range MPX Single Wavelenqth MPX Dual Wavelength
mq/dl 20 sec. 30 sec. 20 sec. 30 sec.
0-50 11.2 mg/dl 13.8 mg/dl 4.6 mg/dl 4.4 mg/dl
50-250 14.4 14.2 10.6 11.0
250-450 - 17.6 - 11.6
25 77-405 GlucoScan Plus (Dre~ler Clinical) 15.9%
77-405 Accu-Chek bG (Drexler Clinical) 10.7%
50-450 MPX System 20/30 Sec. Dual Hybrid 11.1%
50-450 MPX System 30 Sec. Dual Wavelength 11.3
**~* Confidence limits for MPX were from the YSI. The
confidence limits for GlucoScan Plus and Accu-Chek bG
were from the regression equation vs. YSI which
eliminates bias due to small differences in
calibration.
LFS-3
I 337682
- 43 -
~ample III
Stability:
Most of the bench-scale work carried out in optimizing
stability was completed using hand-dipped 0.8~ Posidyne~
membrane disks. The specific dye/enzyme formulation was
set forth previously.
1. Room Temperature Stability: This study attempted to
chart any change in response of the 0.8~ Posidyne~
membrane reagent stored at 18C-20C over silica gel
desiccant. After 2.S months there was no noticeable
change as measured by the response of a room
temperature sample vs. the response of a sample
stored at 5C. Each measurement represented a
glucose range of 0-450 mg/dl.
2. Stability at 37C: Stability study using the same
reagent as the room temperature study was carried
out. The differences in glucose values of reagent
stressed at 37C vs. room temperature reagent, for
strips stressed with and without adhesive, was
plotted over time. Although the data was noisy, due
to the poor reproducibility of handmade strips, the
stability was escellent for strips whether they were
stressed with or without adhesive.
3. StabiLity at 56C: Eight 5-day to 6-day stability
studies were carried out using different preparations
of a similar formulation on disk membrane (Table 8).
For the low glucose test level (80-100 mg/dl) the
average glucose value dropped upon stressing by 3.4%
with the highest drop being 9.55%. At the high test
level (280-320 mg/dl) the glucose reading declined by
LFS-3
1 337682
- 44 -
an average of 3.4%, the largest decline being 10.0~.
TABLE 8.
Stability of pH , 4.0, .8~ Posidyne~ Disk Reagent
Formulation Stressed for 5 Days to 6 Days at 56C
% Difference (56C vs. Room TemPerature Sample)
Sample No. YSI (80-100 ma/dl)YSI (280-320 mg/dl)
FJ22B -6.25 +5.4
FJ27A -4.0 -5.14
FJ28B -2.4 -5.3
FJ30H -9.55 -10.0
FJ31C +4.43 -1.24
FJ36 -3.2 -8.5
FJ48B* -3.0 0.0
GM48As -3.0 -2.5
Average of 8 -3.4 -3.4
s These two samples contained twice the normal
concentration of enzyme and dye.
A study of the 56C stressing of this membrane over a
l9-day period showed no major difference for strips
stressed with or without adhesive. In both cases the
l9-day decline in glucose value was less than 15% at
low test levels (80-100 mg/dl) and also at 300
mg/dl.
Another 56C study using hand-dipped 0.8~ Posidyne~
membrane with twice the normal concentration of
enzyme and dye was completed. Two separate
preparations of the same formulation were made up and
LFS-3
i~37682
- 45 -
the stability measured over a 14-day period. The average
results of the two studies were compared. Changes were
within +10% over the 14-day period at both the high and
low glucose test level.
E~am~le IV
SamPle Size:
The sample size requirements for the MPX System are
demonstrated in Table 9.
TA8LE 9.
Effect of Sample Size on MPX System Measurements
15 SamPle Size (~1) Dual Wavelength Single Wavelength
YSI z 56
3 41 50 39 31 40 31 42 30 19 30
4 44 49 49 49 48 41 45 44 45 44
54 48 49 51 50 50 49 48 49 49
48 48 50 47 48 54 53 56 55 54
49 49 49 50 49 55 57 58 60 58
YSI = 360
3 301 260 276 286 280 274 232 244 260 252
4 383 378 367 341 367 361 356 342 318 344
398 402 382 370 388 378 387 366 351 370
364 362 378 368 368 356 358 379 369 366
375 370 380 378 376 380 382 389 385 384
The volumes reported in the table were transferred to the
reagent pad 10 shown in Figure 1 using a micropipet. When
blood from a finger stick is applied to a strip the total
LFS-3
)
1~37682 - -- - -
- 46 -
sample cannot be transferred. Therefore, the~volumes
-reported here do not represent the total sample size needed
to be squeezed from the finger for the analysis. A 3~1
sample is the minimum necessary completely cover the reagent
pad circle. This does not provide enough sample to
completely saturate the reagent pad and the MPX System,
whether single or dual wavelength, gives low results. A
4~1 sample barely saturates the reagent pad, while a 5~1
sample is clearly adequate. A 10~1 sample is a large shiny
drop and a 20~1 sample is a very large drop and is only
likely to be used when blood from a pipet is used for
sampling.
At the lower glucose concentration the single wavelength
result has some dependence on sample size, which is
completely eliminated using the dual wavelength
measurement. Although this dependence with the single
wavelength might be considered acceptable, it is clearly
undesirable.
Example V
ReProducibi litY:
Esperimental measurements described above were always run in
replicate, usually 2, 3 or 4 determinations per data point.
These sets have always shown close agreement even for
samples with estreme hematocrits or extreme osygen levels.
covariances were well below 5~. It appears, therefore, that
reproducibility is very good to escellent.
The subject invention provides for many commercially or have
been described in the literature. The protocols are simple
and require little technical skill and are relatively free
of operator error. The assays can be carried out rapidly.
.FS-3
~37~
- 47 -
They use inespensive and relatively harmless reagents,
important considerations for materials employed in the
home. The user obtains results which can be understood and
used in conjunction with maintenance therapy. In addition,
the rea~ents have long shelf lives, so that the results
obtained will be reliable for long periods of time. The
equipment is simple and reliable and substantially automatic.
All patents and other publications specifically identified
in this specification are indicative of the level of skill
of those of ordinary skill in the art to which this
invention pertains and are herein individually incorporated
by reference to the same e~tent as would occur if each
reference were specifically and individually incorporated by
reference.
The invention now being fully described, it will be apparent
to one of ordinary skill in the art that many modifications
and changes can be made thereto without departing from the
spirit or scope of the invention as defined in the following
claims.
~FS-3