Note: Descriptions are shown in the official language in which they were submitted.
~ l3~02a
This is a divisional application of copending
application 562,531, filed Màrch 25, 1988.
The present invention relates to synthetic peptide
antigens, the sequences of which correspond to regions of
HIV-l proteins, and their use as diagnostic reagents to
detect the presence of antibodies to HIV-l. The peptides may
also be useful as immunogens in compositions to elicit the
production of antibodies against HIV-l.
HIV-l (human immunodeficiency virus-l) is the name given
to a group of highly related viruses which have been
identified as the primary etiologic agent of the acquired
immunodeficiency syndrome (AIDS) in humans. HIV-l which is
also known as HTLV-III, LAV and ARV, is a major worldwide
health problem. Since HIV-l was first identified as the
etiologic agent of AIDS, substantial progress has been made
~ 3 8 0 2 8 27125-890/8699
in studies on the virus per se and mechanisms by which the
virus causes disease and in the development of diagnostic
tests to detect exposure to the virus or infection.
Methods for detecting HIV-l infection, in general,
measure exposure to the virus by detecting and quantifying
antibodies to HIV-l antigens in blood, sera, and blood-
derived products. Such methods are used to aid diagnosis of
AIDS and ARC (AIDS-Related Complex) and to screen blood and
blood products for previous exposure to HIV-l.
The diagnosis of HIV-l infections and screening
of blood for exposure to HIV-l is generally performed by
enzyme-linked immunosorbent assay (ELISA) techniques in order
to detect the presence of antibodies to immunogenic compo-
nents of HIV-l in a test sample. Other methods involve the
use of Western blotting techniques to detect HIV-l specific
antibodies in test samples (Schorr et al., N. Enql. J. Med.
(19851 313:384-385). In general, almost any known immuno-
assay, such as radioimmunoassays, can be adapted, by use of
specific reagents, for the detection of HIV-l and antibodies
thereto.
The source of antigens-in the first generation of
these assays has generally been antigenic proteins obtained
from HIV-l produced by human helper T-lymphoblastoid cell
lines, such as H9 (Popovic et al., Science (1984) 224:497-
500; Gallo et al. Science (1984) 224:500-503). Even though
these first generation ELISA tests for HIV-l are quite
sensitive and specific, the use of antigens obtained from
live virus preparations has several significant drawbacks.
The production of HIV-l per se in continuous cell
lines must be performed in high risk (P3 containment)
1 3 3 8 0 2 8 27125-890/8699
laboratories due to the danger to investigators who may
become adversely exposed to the virus. In addition, there
are clearly false negative and false positive results that
have been obtained with ELISA tests using whole virus
antigens. Western blot analyses, using electroblotted whole
virus antigen, provide greater specificity but are more
laborious and time-consuming then ELISA tests (Schorr et al.,
su~ra). Furthermore, since H9 and other HIV-l producing
cells are human cell lines, viral antigens preparation
obtained from these cell lines, unless exhaustively purified,
may be contaminated with normal cellular antigens, such as
HLA antigens, which could produce false positive reactions in
an ELISA test (Kuhnl et al., Lancet I (1985): 1222-1223;
~unter et al., Lancet II (1985): 397.)
Exhaustive purification of viral antigens from
cell lines can also conceivably destroy immunogenicity of
immunologically important proteins or otherwise inactivate
antigens, thereby producing reagents that result in false
negative reactions. In addition, false negative reactions
using antigens obtained from intact virus preparations may
occur because of steric hinderance whereby antibodies to one
viral antigen cannot react with their specific antigen
because the reaction is blocked by the presence of other
viral antigens and antibodies in the reaction mixture.
Second generation ELISA tests to detect BIV-l
infection have employed immunologically important viral
proteins that have been produced by cloning portions of the
BIV-l genome in bacteria. The viraL etiologic agent of AIDS
(i.e.~ those viruses previusly named ~TLV-III, LAV and ARV)
from a variety of sources has been isolated, cloned and the
1 338028
nucleotide sequence determined (Popovic et al., Science
(1984) 224: 497; Gallo et al., Science (1984) 224: 500:
SChllphAch et al., Science (1984) 224: 503; Shaw et al.,
Science (1984) 226: 1165; Barre-Sinoussi et al., Science
(1983) 220: 868; Levy et al., Science (1984) 225: 840; Ratner
et al., Nature (1985) 313: 277; Muesing et al., Nature (1985)
313: 450; Wain-H~h~on et al., Cell (1985) 40:9; Sanchez-
Pescador et al., Science (1985) 227: 484; Shaw et al.,
Adv. Intern. Med. (1984) 30: 1).
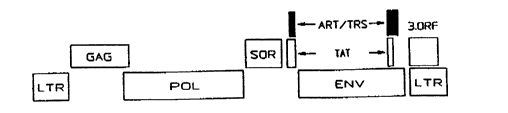
HIV-l is a relatively complex retrovirus containing at
least seven genes. The viral structural genes designated
gag, pol and env respectively code for the viral core
proteins, reverse transcriptase, and the viral glycoproteins
of the viral envelope. The other genes shown in Fig. 1 are
accessory genes involved in viral replication. The gag and
env genes encode polyproteins, i.e., the proteins synthesized
from each of these genes are post translationally cleaved
into several smaller proteins. Previous studies have shown
that the proteins coded by the gag and especially the env
regions of the HIY-l are genome immunological important,
since antiho~ies to the products of the gag and env genes are
found in the sera of AIDS and ARC patients.
The env gene encodes a glycoprotein (gpl60) with an
apparent molecular weight (Mr) of about 160,000 daltons which
is post synthetically cleaved into two glycoproteins, gpl20
and gp41, of Mr 120,000 and Mr 41,000 respectively.
1 338028
Gly~u~G~ein gpl20 is apparently the external protein of the
viral envelope, while gp41 appears to be a transmembrane
protein. Both gpl20 and gp41 are immunogenic, with
antihoAies to both proteins readily detectable in AIDS and
ARC patient sera.
- 4a -
27125-890/8699
1 338028
Because antibodies to gpl20 and gpl60 in sera of AIDS and ARC
patients, as well as asymptomatic individuals infected with
the virus, are neutralizing, i.e., inhibit binding of the
virus, gpl20, gpl60 or portions thereof are candidates for a
subunit vaccine. The trAn- - hrane glycoprotein gp41 is the
HIV-l antiqen most consistently recognized by antibodies in
AIDS and ARC patient sera (Allar. et al., Science (1985) 228:
1091-1094; Barin et al., Science (1985) 228: 1094-1096). In
addition, antibodies in patient sera also recognize epitopes
of the viral core proteins encoded by the gag qene.
Immunologically important HIV-l antigens for use in
diagnosis and as potential vaccine compositions have been
prepared by cloning portions of the HIV-l genome in various
expression systems such as bacteria, yeast or vaccinia (See,
e.g., Cabradilla et al., Biotechnoloqy (1986)4: 128-133;
Chang et al., Biotechnoloqy (1985) 3: 905-909; Putney et al.,
Science (1986) 234: 1392-1395; Kieny et al. Biotechnoloqy
(1986) 4: 790-795). HIV-l antigens produced by recombinant
DNA methods, however, must still be exhaustively purified to
avoid false positive reactions in the ELISA due to any
antibody reactivity to antigens of the expression system
which may contaminate the HIV-l an~igen preparation. Also,
denaturation of HIV-l antigens during purification may
destroy important antigen activity.
For example, Chang et al., supra and Cabridilla
et al., supra produced portions of gp41 by recombinant DNA
techniques which were then used to detect the presence of
antibodies in sera of AIDS/ARC patients by ELISA. Both
studies reported false negative reactions in 2/132 sera
(Chang et al) and 2/127 sera (Cabradilla et al.). While the
2~125-890/8699
~ 33~028
studies documented a significant improvement in the percent-
age of false negatives and false positives over ELISA tests
based on human cell line-derived antigens, because of the
nature of AIDS, a diagnosis that is as close to 100% accurate
as possible is desirable and other reagents have been devel-
oped to try to achieve this.
Protein antigens contain a number of epitopes or
antigenic determinants which are the regions of the proteins
which comprise the binding sites for specific antibodies. In
general, proteins antigens contain between 5 to 10 epitopes,
each of which containing a sequence of 6 to 8 amino acids.
Epitopes can be either continuous, in which the 6 to 8 amino
acids are present in sequence, or discontinuous, in which the
amino acids that form the epitope are brought together by the
three dimensional folding of the protein. Even though an
epitope constitutes only a relatively few amino acids, its
reactivity with an antibody is influenced by the amino acids
in the protein which surround the epitope.
Studies aimed at mapping antigenic sites or
epitopes of proteins have been aided by the use of synthetic
peptides corresponding to various regions of the proteins
of interest (See e.g., Lerner et al., in The Bioloqy of
Immunoloqical Disease: A Hospital Practice Book, (1983) Dixon
and Fisher, eds., pp. 331-338; Lerner, Adv. Immunol. (1984)
36: 1). $n addition to their usefulness in epitope mapping
studies, synthetic peptides, if encompassing major antigenic
determinants of a protein, have potential as vaccines and
diagnostic reagents. Synthetic pept~ide antigens have several
advantages in regard to specific antibody production and
reactivity. The sequence of the synthesized peptide can be
27125-890/8699
~ ~38~
selected from the amino acid sequence as actually determined
by amino acid sequencing of a protein or predicted from the
DNA sequence coding for the protein. The use of specific
synthetic peptides eliminate the need for using the full-
length protein in the production of or assay for specificantibodies. Furthermore, the solid phase peptide synthetic
techniques of Merrifield and cowcrkers allow for essentially
unlimited quantities of the peptide of interest to be chemi-
cally produced. ~See, e.g., Erickson and Merrifield in The
Proteins, 3rd Edit. (1976), Yol. 2, Academic Press, New York,
Chapter 3). The availability of automated peptide synthesi-
zers has further advanced such techniques.
Several publications have presented data showing
immunologic reactivity of selected synthetic peptides corres-
ponding to antigenic proteins of ~IV-l. In one study, a
peptide having the amino acid sequence Tyr-Asp-Arg-Pro-Glu-
Gly-Ile-Glu-Glu-Glu-Gly-Gly-Glu-Arg-Asp-Arg-Asp-Arg-Ser-Gly-
Cys which corresponds to amino acid residues 735-752 of 8IV-l
was synthesized. (Kennedy et al., Science ~1986) 231:1556-
1559). This peptide which is a portion of gp41 was used toimmunize rabbits in an attempt to elicit a neutralizing
antibody response to HIV-l. Furthermore, several sera from
- AIDS patients known to contain anti-gp41 antibodies were
weakly reactive with this peptide, thus indicating that this
peptide contains at least one epitope recognized, to some
extent, by antibodies to native gpl60/gp41.
Recent work has been directed at providing synthet-
ic peptides for AIDS diagnostics, in addition to developing
vaccine compositions. In one study (Wang et al., Proc. Natl.
Acad. Sci. (1986) 83:6159-6163), a 21 amino acid peptide
t 3 3 8 0 28 27125-890/8699
called SM284 with the amino acid sequence Arg-Ile-Leu-Ala-
Val-Glu-Arg-Tyr-Leu-Lys-Asp-Gln-Gln-Leu-Leù-Gly-Ile-Trp-Gly-
Cys-Ser was synthesized. This peptide, which corresponds to
amino acids 586-606 of gpl60 and comprises an antigenic
segment of gp41, was reactive with HIV-l positive sera from
AIDS/ARC patients by ELISA and Western blotting. The SM284
peptide compared favorably with ~IV-l-derived proteins in
detecting HIV-l antibodies in an ELISA. For example, the
ELISA using SM284 detected antibodies in serum samples from
96.5% of AIDS/ARC patients and 34.6% of healthy asymptomatic
high risk individuals. There were no false positives in 387
sera from control individuals.
Serological and chemical analysis of the SM284
peptide and related peptides sho~ed that certain amino acids
were apparently more important than others in the antibody-
antiqen interaction of the peptide. Deletion of residues
Arg-l, Ile-2 and Lys-10 from the peptide significantly
reduced or destroyed serologic reactivity. Deletion of the
Try-Gly-Cys-Ser residues at the carboxy terminal of the
peptide, on the other hand, resulted in moderate loss of
seroloqic activity, indicating that the amino terminal
portion of the peptide is apparently more important than the
carboxy terminal portion for immunologic reactivity.
U.S. Patent No. 4,629,783 issued December 16, 1986
to Cosand provides several immunologically reactive synthetic
peptides corresponding to regions of HIV-l proteins for
detection of AIDS and AIDS-related disease. Of particular
interest is peptide V (39) having the sequence Arg-Ile-Leu-
Ala-Val-Glu-Arg-Tyr-Leu-Lys-Asp-Gln-Gln-Leu-Leu-Gly-Ile-Trp-
Gly-Cys-Ser-Gly-Lys-Leu-Ile-Cys-X, where X is OH or NH2,
27125-890/8699
~ 1 338028
which corresponds to a portion of gp41 encoded by base pairs
(bp) 7516-7593 of the HIV-l genome. Peptide V (39) reacted
with 23/24 (95.8%) serum samples from patients with confirmed
HIV-l infections.
S SUMMARY OF THE INVENTION
In accordance with the present ir.vention, several
novel synthetic peptides corresponding to antigenic HIV-l
proteins are provided which are useful and superior in highly
selective diagnostic methods for detecting HIV-l infections.
Novel synthetic peptide antigens corresponding to
glycoprotein gp41 encoded by the HIV-l env gene and the
protein products encoded by the HIV-l gag gene have now been
found. These peptides are useful for diagnosing AIDS in
suspected individuals and in methods for screening for
exposure to HIV-l in blood and blood-derived products with a
high degree of reliability and very few false results.
The peptides can be used in methods of detecting
antibodies to HIV-l in samples. The methods involve contact-
ing the sample with the peptide antigens under conditions
which allow an immunological complex to form between the
peptide and any HIV-l specific antibodies in the sample.
Measuring complex formation by suitable detection means
indicates the presence or absence of antibodies to HIV-l in
the sample.
The novel peptides may also be used as immunogens
in vaccine compositions for immunization against HIV-l
infection or for the production in animals of HIV-l specific
antibodies against HIV-l antigens.
1 338028 27125-890/8699
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. l is a schematic representation of the genes
of HIV-l.
Fig. 2 is a schematic representation of gp41 of
HIV-l depicting the overlapping peptides synthesized in
accordance with the invention and a comparison with prior art
peptides corresponding to gp41.
Fig. 3 is a histogram showing ELISA values obtained
using gp41A5 as antigen.
DESCRIPTION OF THE INVENTION
The present invention provides a number of peptides
of 15 to 27 amino acids in length corresponding to regions of
- the protein product of the entire HIV-l gag gene and to gp41
of HIV-l which have been synthesized and tested for immuno-
reactivity to HIY-l positive serum samples obtained from the
United States, United Kingdom, Israel, Africa and Sweden.
The novel peptides are useful in tests to diagnose HIV-l
infection or prior exposure to the virus and as immunogens in
compositions to elicit the production in animals and man of
antibodies against HIV-l. Peptides corresponding to gag
proteins and in particular gp41 were selected and synthesized
because these HIV-l proteins demonstrate little strain to
strain variation characteristic of other immunologically
important HIV-l antigenic proteins such as gpl20. The
peptides encompassed by the invention comprise oligopeptides
having amino acid sequences containi-ng therein sequences
which comprise continuous (linear) epitopes reactive with
HIV-l specific antibodies.
--10--
27125-890/8699
1 338028
The invention thus encompasses a group of immuno-
logically reactive peptides and functionally equivalent
variants thereof which do not significantly affect the
antigenic properties of the peptide correspondinq to gp41
encoded by the env gene of HIV-l (Fig. 1). The regions of
gp41 corresponding to each peptide described in detail below
is shown in Fig. 2. The peptides were all synthesized by
known solid phase peptide synthesis techniques (see e.g.,
- Merrifield and Barany, The Pe~tides: Analvsis, Synthesis,
Bioloqy (1980), Vol. 1, Gross and Meinenhofer, eds., Academic
Press, New York, Chap. 1). The synthesis also allows for one
or two amino acids not corresponding to the original protein
sequence to be added to the NH2- or COOH-terminus of the
peptides. Such extra amino acids are useful for coupling the
peptides to each other, to a large carrier protein or to a
support. Amino acids that are useful for these purposes
include tyrosine, lysine, glutamic acid, aspartic acid,
cysteine and derivatives thereof. Additional protein modifi-
cation techniques may be used, e.g., NH2-acetylation or
COO~-terminal amidation, to provide additional means for
coupling the peptides to another protein or peptide molecule
or to a support.
The novel peptides corresponding to gp41 are set
forth below:
qp4lA5
X-Asp-Gln-Gln-Leu-Leu-Gly-Ile-Trp-Gly-Cys-Ser-Gly-Lys-Leu-
Ile-Cys-Thr-Thr-Ala-Val-Pro-Trp-Asn~Y-Z, wherein X is either
a H of the~amino terminal N~2 group of the pepti'de or an
additional amino acid bonded to the amino terminal, NH2 group
1 3 3 8 0 2 8 27125-890/8699
of the peptide, the additional amino acid being selected to
facilitate coupling the peptide to a carrier protein; Y is
absent or Cys; and Z is OH or NH2.
Peptide gp41A5, which corresponds to amino acids
596-618 encoded by about bp 7563 through 7632 of the HIV-l
genome corresponding to the env gene, (Wang et al., supra
numbering), is a particularly preferred embodiment of the
present invention. Peptide gp41A5 in which X is H2, Y is Cys
and Z is OH is especially preferred.
ap41CT4
The peptide gp41CT4 which corresponds to the region
of the gp41 protein encoded by about bp 8173 through 8238 of
the HIV-l genome, has the formula: X-Ala-Leu-Lys-Tyr-Trp-
Trp-Asn-Leu-Leu-Gln-Tyr-Trp-Ser-Gln-Glu-Leu-Lys-Asn-Ser-Ala-
Val-Ser-Y-Z, wherein X, Y, and Z have the same definitions as
above.
qp41CT3
The peptide gp41CT3M which corresponds to the
region of gp41 encoded by about bp 8220 through 8280 of the
HIV-l env gene, has the formula: X-Lys-Asn-Ser-Ala-Val-Ser-
Leu-Leu-Asn-Ala-Thr-Ala-Ile-Ala-Val-Ala-Glu-Gly-Thr-Asp-Y-Z,
wherein X,Y and Z have the same definitions as`above.
qp4lBl
The peptide gp41Bl which corresponds to the region
of gp41 encoded by about bp 7614 through 7686 of the HIV-l
env gene has the formula: X-Thr-Ala-Val-Pro-Trp-Asn-Ala-
Ser-Trp-Ser-Asn-Lys-Ser-Leu-Glu-Gln-Ile-Trp-Asn-Asn-Met-Thr-
-12-
~ 3 3 8 0 ~ 8 27125-890/8699
Trp-Met-Y-Z, wherein X,Y and Z have the same definitions as
above.
qp4 lB3
The peptide qp41B3, which corresponds to the region
of gp41 encoded by about bp7705 through 7773 of the ~IV-l
genome has the formula: X-Ile-Asn-Asn-Tyr-Thr-Ser-Leu-Ile-
His-Ser-Leu-Ile-Glu-Glu-Ser-Gln-Asn-Gln-Gln-Glu-Lys-Asn-Glu-
Y-Z, wherein X,Y and Z have the same definitions as above.
The invention further encompasses several immuno-
logically reactive peptides and functionally equivalentvariants thereof corresponding to regions o the protein
products encoded by the gag gene of ~IV-l (Fig. 1). These
novel peptides are set forth as follows:
GAG 2
The peptide GAG 2, which corresponds to the region
of the gag gene product encoded by about bp 779 through 840
of the gag gene of ~IV-l, has the formula: X-Pro-Arg-Thr-
Leu-Asn-Ala-Trp-Val-Lys-Val-Val-Glu-Glu-Lys-Ala-Phe-Ser-Pro-
Glu-Val-Y-Z, wherein X,Y and Z have the same definitions as
above.
GAG 3
The peptide GAG 3, which corresponds to the region
of the gaq gene protein product encoded by about bp 810
through 870 of the gag gene, has the formula: X-Val-Glu-
Glu-Lys-Ala-Phe-Ser-Pro-Glu-Val-Ile-Pro-Met-Phe-Ser-Ala-Leu-
Ser-Glu-Gly-Y-Z, wherein X,Y and Z have the same definitions
as above.
- ! 3 3 8 0 2 8 27125-890/8699
GAG 14
The peptide GAG 14, which corresponds to the region
of the gag gene product encoded by about bp 1368 through 1430
of the HIV-l gag gene, has the formula: X-Glu-Met-Met-Thr-
Ala-Cys-Gln-Gly-Val-Gly-Gly-Pro-Gly-His-Lys-Ala-Arg-Val-Leu-
Ala-Glu-Y-Z, wherein X, Y and Z have the same definitions as
above.
P17-D
The peptide P17-D, which corresponds to the region
of the gag gene protein product encoded by about bp 495
through 560 of the HIV-l gag gene, has the ~ormula: X-Ser-
Glu-Gly-Cys-Arg-Gln-Ile-Leu-Gly-Gln-Leu-Gln-Pro-Ser-Leu-Gln-
Thr-Gly-Ser-Glu-Glu-Leu-Y-Z, wherein X, Y and Z have the same
definitions as above.
P17-F
The peptide P17-F, which corresponds to the region
of the gag gene protein product encoded by about bp 588
through 647 of the HIV-l gag gene, has the formula: X-Leu-
Tyr-Cys-Val-His-Gln-Arg-Ile-Glu-Ile-Lys-Asp-Thr-Lys-Glu-Ala-
Leu-Asp-Lys-Ile-Y-Z, wherein X,Y and Z have the same defini-
tions as above.
The peptides can be used in methods for detection
of antibodies to HIV-l or HIV-l associated antigens. Pref-
erably the methods which use the peptides to detect the
presence of HIV-l specific antibodies in the sample involve
contacting the sample with at least one of the peptides under
conditions which allow the formation of an immunological
complex between the peptide antigen and any antibodies to
1 338028
HIV-l that may be present in the sample. The formation of
an immunological complex if any, indicating the presence
of antibodies to HIV-l in the sample, is then detected and
measured by suitable means.
Such methods include, inter alia, homogeneous and
heterogeneous binding immunoassays, such as radioimmunoassays
(RIA), ELISA and Western blot analyses. Further, the assay
protocols using the novel peptides allow for competitive and
non-competitive binding studies to be performed.
- 10 ~he peptides may be labeled (signal-generating) or
unlabeled depending on the type of assay used. Labels which
may be coupled to the peptides are those known in the art and
include inter alia enzymes, radionuclides, fluorogenic and
chromogenic substrates, cofactors, biotin/avidin, colloidal
gold, and magnetic particles. Modification of the novel
peptides, allows for coupling by known means to carrier
proteins or peptides or to known supports, for example,
polystyrene or polyvinyl microtiter plates, glass tubes or
glass beads and chromatographic supports, such as paper,
cellulose and cellulose derivates, and silica.
Preferred assay techniques, especially for large-
scale clinical screening of patient sera and blood and
blood-derived products are ELISA and Western blot techniques,
ELISA tests being particularly preferred. The ELISA tests
employing the peptides described above are based on those
currently in use with human cell-derived or recombinant DNA-
derived ~IV-l proteins or portions thereof as antigens.
For use as reagents in these assays,-.the peptides are conve-
niently bonded to the inside surface of microtiter wells.
The peptides may be directly bonded to the microtiter well.
1 3 3 8 '~ 2 8 27125-890/8699
It has been found, however, that maximum binding of the
peptides to the wells can be accomplished by pretreating the
wells with polylysine prior to the addition of the peptides.
Additionally, the novel peptides may be covalently attached
by known means to a carrier protein, such as BSA, with the
resulting conjugate being used to coat the wells. Generally
the peptides were used in a concentration of between 10 to
100 ~g/ml for coating, although some peptides required as
much as 500 ~g/ml for the assay to be successful.
Samples are then added to the peptide coated wells
where an immunological complex forms if antibodies to HIV-l
are present in the sample. A signal generating means may be
added to aid detection of complex formation. A detectable
signal is produced if ~IV-l specific antibodies are present
in the sample.
The invention is further illustrated by the follow-
ing specific examples which are not intended in any way to
limit the scope of the invention.
Example 1
An Applied ~iosystems peptide-synthesizer Model 430
A, was utilized for the synthesis of all of the peptides.
Each synthesis used a p-methylbenzylhydrylamine solid phase
support resin (Peptides International, Louisville, KY). The
peptides were synthesized according to the Users Manual for
25 PePtide SYnthesizer Model 430A, Applied Liosystems, 1986.
All amino acids for use in synthesis contained
t-butylcarbonyl groups (t-Boc) protecting the ~-NH2 group and
were obtained from Novabiochem A~, Switzerland. Amino acids
with reactive side chain groups contained additional protec-
tive groups to prevent unwanted and undesirable side chain
-16-
27125-890/8699
1 -j38()~8
reactions. The individual protected amino acids used in
synthesizing all of the peptides are set ~orth in Table 1.
27125-890/8699
t 33a:~:2~
Table 1
Amino Acids Used in the Synthesis of PePtides
Boc-Ala-OH
Boc-Arg (Tos)-OH
Boc-Asn-OH
Boc-Asp 'OBzl)-OH
Boc-Cys pMeOBzl)-Oh
80c-Glu ~OBzl)-OH
Boc-Gln-OH
Boc-Gly-OH
Boc-His(Tos)-OH
Boc-Ile-OH-1/2 H20
Boc-Leu-OH-H O
- Boc-Lys (2-C~-Z)-OH (cryst.)
Boc-Met-OH
Boc-Phe-OH
Boc-Pro-OH
Boc-Ser(Bzl)-OH-DCHA
Boc-Thr (Bzl)-OH
Boc-Trp (Formyl)-OH
Boc-Tyr(2-Br-Z)-OH
Boc-Val-OH
Tos = Tosyl or p-Toluene sulfonic acid
oBzl = Benzyloxy
pMeo8zl = p-Methylbenzyloxy
2-Cl-Z = Carbobenzoxy chloride
2-Br-Z = Carbobenzoxybromide
-18-
27125-890/8699
1 338G28
After completion of a particular synthesis, the
protecting groups were removed from the synthesized peptide
and the peptide cleaved from the solid support resin by
treatment at 0C with anhydrous hydrofluoric acid (HF)
combining 10% anisole and 10% di~ethylsulfide as scavenging
agents. 2% thiocresol was also added for cysteine-containing
peptides. After cleavage, the ~F in the sample was purged
under a stream of N2, with removal of any residual HF accom-
plished by subjecting the sample to a vacuum at 0C. The
peptides were extracted from the resin by treatment with
trifluoracetic acid ($EA) which ~as then removed by evapo-
ration at room temperature. Following TFA removal, the
peptides were precipitated and washed with anhydrous ether.
Prior to use in specific assays, the peptides can
be further purified, if desired, by reverse phase high
performance liquid chromatography (HPLC). A particularly
suited column for such purification is the reverse-phase
Vydek~C-18 column using a water (TFA) - acetonitrile (TFA)
gradient to elute the peptides.
Example 2
Peptide gp41A5 having the amino acid sequence Asp-
Gln-Gln-Leu-Leu-Gly-Ile-Trp-Gly-Cys-Ser-Gly-Lys-Leu-Ile-Cys-
Thr-Thr-Ala-Val-Pro-Trp-Asn-Cys-0~ was synthesized as des-
cribed in Example 1 and used in an ELISA to measure its
immunologic reactvity.
Polylysine at a concentration of 1 mg/ml was
added to the microtiter plates and allowed to incubate for
30 minutes. The polylysine was then~discarded and peptide
gp41A5 was added to the wells of the plate in a concentration
- 30 of from 10 to 100 ~g/ml for coating. After the peptide
--19--
~ 338028
incubated in the well for a length of time sufficient to
allow the peptide to be bonded to the well, the peptide
solution was removed and a solution of glutaraldehyde, which
stabilizes the peptide attachment to the wells, was added for
15 minutes. The glutaraldehyde solution was then removed,
the wells washed with buffer, and a mixture of glycine and
bovine serum albumin (BSA) was added which served to block
unbound sites in the wells and minimize non-specific binding
of antibodies during the ELISA test per se. After a final
washing step, the plates were ready to use. The prepared
peptide-coated microtiter plates could be stored for several
months without any decrease in antigenic activity of peptide
gp41A5 coated on the wells.
A convenient variation of known ELISA methods was used
with the microtiter plates prepared as above. Serum samples
from individuals which had been diluted 1:50 in PBS
(phosphate buffered saline) containing 0.05% polyoxyethylene-
sorbitan monolaurate (Tween 20) and 1% BSA were added to
each well and allowed to incubate for 90 minutes at 37C in a
humidified atmosphere. The diluted serum samples were then
removed from the plates and the wells washed three times with
PBS containing 0.05% Tween 20. A conjugated antihuman Ig
antibody was then added to the wells and allowed to incubate
for 90 minutes. The conjugated antibody was produced in a
goat or rabbit and was specific for human IgG, IgM,
immunoglobulin light chains, or combinations thereof.
Preferably, alkaline-phosphatase conjugated antihuman IgG
(from Dakopatts) diluted 1:500 for use in PBS containing
0.05% Tween 20 and 1% BSA was used in the ELISA. After the
conjugate had incubated a sufficient length of time to react
-20-
* Trade mark
1 33ao28
with bound human antibodies, the plates were washed three
times as above. In order to detect antibodies to HIV-l in
the human serum that react with the peptide gp4lAS used as
the antigen, (i.e. positive reactions), a chromogenic
substrate alkaline phosphatase substrate (Sigma Cat. No. 104
tablets) dissolved in a NA carbonate/MgCl buffer and adjusted
to a concentration of 1 ~g/ml which was cleaved by the enzyme
attached to the antihuman Ig to yield a colored product was
added. After incubation for approximately 40 minutes at room
temperature, positive reactions indicated the presence of
antibodies in the sample reactive with the antigen. A yellow
to orange to reddish-brown color in each well indicating a
positive reaction, was read in a spectrophotometer at 405 nm
to quantify the reaction. Spectrophotometric readings were
adjusted to correct for background reactions.
Fig. 3 is a histogram showing antibody values obtained
following four separate ELISA determinations using gp41A5 as
antigen. 101 true positive (by Western blot analysis) sera
from AIDS and ARC patients and 179 true negative sera were
reacted in the ELISA with gp4lA5. The mean cutoff for
positive reactions was determined to be O.D.405 = 0.376.
Fig. 3 shows clearly that gp41A5 gave a positive reaction
with 100% (101/101) of true positive sera and 0% (0/179) of
true negative sera.
Example 3
For purposes of comparison with novel peptides the known
peptide V (39) of Cosand was synthesized as described in
Example 1 in accordance with its amino acid sequence
disclosed in U.S. Patent No. 4,629,783. The relative
location of this peptide in gp41 is shown in Fig. 2
* Trade mark - 21 -
1 3 3 ~ 0 2 8 27l2s-89o/8699
which also provides the relative sequence location of gp41A5
and other subject peptides corresponding to gp41 of HIV-l.
Cosand reported that peptide V (39) reacted with 23/24
(95.8%) confirmed positive sera ti-e-, one false negative
reaction). The peptide was used in the ELISA described in
Example 2 with the substitution of peptide V(39) for gp41A5.
Peptide (V)(39) produced 221/233 (94.8%) positive
reactions with known HIV-l positive serum samples. Twelve
false negative reactions were seen as shown in Table 2. In
addition peptide V(39~ gave a faise positive reaction in one
of 102 (0.98%) confirmed negative sera. Serum samples were
confirmed as positive and negative by Western blot analysis
with HIV-l proteins. All false positive and negative reac-
tions were corroborated by Western blot analysis.
It is further seen in Pig. 2, that gp41A5 has a
partial overlap in amino acid sequence with peptide SM284 of
Wanq et al., Proc. Natl. Acad. Sci. (1986) 83:6159-6163, and
peptide V (39) of Cosand. Peptide gp41A5 thus contains the
amino acid sequence of the carboYy terminal portion of the
two known peptides plus an additional sequence corresponding
to a region of gp41 to the carbo~y terminal side of peptides
SM284 and V(39). As discussed previously, Wang et al. con-
sidered the carboxy terminal portion of peptide SM284 to be
less important immunologically when compared to the amino
terminal portion of peptide SM284. Contrary to Wang et al.,
extending the permutation of gp41A5 to the carboxy terminus
of gp41, however, resulted in a peptide having excellent
antigenic properties for detection of AIDS-specific anti-
bodies in human sera. This peptide shows both a higher
~ 27125-890/8699
1 338028
degree of reactivity and greater specificity in ELISA and
Western blots than both peptides V(39) and SM284.
Table 2 also clearly shows that gp41A5 reacts with
all 233 (100%) confirmed positive HIV-l sera. These results
were obtained from the same 233 sera tested against peptide
V(39) which yielded 12 false negative reactions. The mean cut
off values for a positive reaction in the ELISA tests was an
O.D.405 of about 0.3. Any reading below 0.3 was considered a
negative reaction. It is readily apparent that the serum
samples numbered 7, 19, 40, 44, 48, 60, 101, 482, 511, 324,
375 and 393 clearly show no reactivity with peptide V(39) but
superior reactivity with gp41A5.
Table 3 provides a further comparison of the supe-
rior immunologic reactivity of gp41A5 compared with peptide
V(39) of Cosand. Thirty-eight of the 233 serum samples
presented in Table 2 were confirmed as positive by Western
blot analysis. These positive sera plus 8 confirmed negative
serum samples (by Western blot) were reacted with peptides
gp41A5 and V(39) in parallel ELISA tests with poqitive
reactions being anything greater than an O.D.405 of about
0.3. In addition, a non-HIV-l antiqen (negative antigen) was
used a a control. It is readily apparent that gp41A5 pro-
duced no false negative or false positive reactions. Thus,
gp41A5 surprisingly and unexpectedly gives ELISA results that
are clearly 100% accurate. The diagnostic and screening
capabilities of assays using gp41A5 as antigen are clearly
superior to currently available assay using peptides V(39)
and SM284 or any other ~IV-l antigen;-
~ 3 3 8 () ~ ~ 27125-890/8699
TABLE 2
~mmunologic Reactivity Determined By ELISA
Between ~IV-l Antibodies In 233 Confirmed
Positive Serum Samples And Synthetic Peptide
5Antigens Corresponding To Regions Of gp41
Antigen
Serum Sample No. V (39) gp41A5
4 0.436 2.809
7 0.137 1~412
12 0.336 2.222
13 0.836 2.890
17 0.885 1.527
18 1.336 2.711
19 0.289 2.922
23 1.706 2.822
24 0.485 2.010
0.484 2.635
26 1.443 2.600
27 0.810 2.029
29 1.399 2.459
1.889 2.500
31 0.737 2.343
32 1.244 2.902
33 1.332 2.871
0.938 2.714
37 1.087 2.859
38 1.028 2.378
39 1.362 2.529
0.227 1.495
41 1.282 2.692
42 0.731 2.289
43 0.522 1.978
44 1.199 2.443
1.096 1.844
46 1.456 2.796
47 0.665 2.636
48 0.195 2.592
49 1.532 2.809
51 0.712 2.579
53 1.217 2.714
54 0.602 2.423
1.444 2.567
56 0.832 2.511
57 1.164 2.468
58 1.516 2.232
59 1.919 2.726
0.219 1.399
78 0.489 1.826
79 0.927 2.247
101 0.205. 0.863
482 0.120 1.316
511 0.248 1.036
130 0.346 1.741
134 1.256 2.425
137 1.095 2.413
140 0.952 2.364
27125-890/8699
1 338û28
~ `
TABLE 2 (Continued)
Immunologic Reactivity Determined By ELISA
Between HIV-l Antibodies In 233 Confirmed
- Positive Serum Samples And Synthetic Peptide
Antigens Corresponding To Regions Of gp41
Antiqen
Serum Sample No. V (39) gp41A5
225 1.464 2.548
232 1.877 2.654
243 0.644 2.397
272 1.968 2.767
273 1.404 2.660
274 2.037 2.821
275 1.786 2.609
276 1.900 2.745
277 1.106 2.243
278 1.203 2.354
279 0.555 2.579
283 1.293 2.570
285 0.437 1.977
286 1.017 2.108
287 0.79B 1.845
288 0.875 2.569
289 1.544 2.572
291 0.728 2.561
292 0.690 2.032
293 1.096 2.466
294 0.861 2.228
295 1.631 2.696
297 1.598 2.764
298 1.204 2.636
300 1.854 2.636
301 0.796 2.431
302 1.065 2.623
303 0.733 2.381
305 1.375 2,686
306 1.745 2.696
307 1.239 2.383
308 1.142 2.687
312 1.291 2.553
313 1.100 2.152
314 0.456 1.266
317 0.669 1.051
318 1.329 1.616
319 1.255 2.008
320 1.024 1.941
321 1.493 2.347
322 1.092 1.850
323 1.292 2.077
324 0.281 1.299
330 0.725- 1.483
332 0.683 1.385
333 1.620 1.901
334 0.923 1.579
336 1.309 1.951
337 1.258 2.166
340 1.194 1.689
27125-890/8699
~. 338028
TABLE 2 ~continued)
Immunologic Reactivity Determined By ELISA
Between HIV-l Antibodies In 233 Confirmed
Positive Serum Samples And Synthetic Peptide
Antigens Corresponding To Regions Of gp41
Antiqen
Serum Sample No. V (39) gp41AS
341 1.290 1.763
357 1.138 1.754
358 0.738 1.733
359 0.599 1.033
360 0.528 0.840
- 363 0.711 1.291
365 1.390 1.963
366 0.821 1.682
368 0.665 1.417
371 1.183 1.585
372 1.075 1.593
373 0.799 1.711
374 1.046 1.650
375 0.280 0.957
377 1.065 1.480
378 1.261 1.644
379 0.610 1.242
380 1.205 1.763
381 1.321 1.645
383 0.552 1.146
384 0.620 1.029
385 0.973 1.618
386 1.687 1.646
387 0.695 1.189
388 0.928 1.396
389 0.938 1.544
390 0.679 1.190
391 0.709 1.250
392 0.629 1.014
393 0.195 0.874
394 0.622 1.244
395 0.799 1.404
396 0.736 1.416
398 0.766 1.647
400 1.210 1.621
401 0.482 1.073
402 0.614 1.302
403 0.498 1.145
406 1.570 1.600
407 0.742 1.186
408 0.398 0.801
411 1.154 1.603
412 1.209 0.864
413 0.557-. 1.220
414 1.170 1.787
416 0.526 1.326
417 0.473 1.201
419 1.020 1.592
420 0.637 0.944
422 0.742 1.479
27125-890/8699
t 33~g
-TABLE 2 (Continued)
Immunologic Reactivity Determined 8y ELISA
Between HIV-l Antibodies In 233 Confirmed
Positive Serum Samples And Synthetic Peptide
Antigens Corresponding To Regions Of gp41
Antiqen
Serum Sample No. V (39) qp41A5
426 0.921 1.381
427 0.548 1.116
428 0.355 1.184
429 0.381 1.051
431 0.542 1.198
432 1.192 1,633
433 1.402 1.793
434 0.637 1.196
~ 435 1.017 1.951
436 1.155 1.512
q37 0.465 1.408
438 0.517 0.572
439 0.483 0.422
440 1.345 1.380
441 0.618 1.238
442 0.660 1.088
443 0.638 1.288
444 0.604 0.737
445 1.187 1.462
446 1.731 0.662
447 2.737 2.913
448 2.150 2.936
449 1.580 2.901
450 2.287 2.956
451 1.645 2.777
452 1.741 2.636
453 1.998 2.922
454 1.756 2.860
455 2.590 3.010
456 1.022 2.936
457 1.055 2.724
458 1.706 2.868
459 1.133 2.849
460 1.611 2.636
461 1.809 2.881
462 1.417 2.788
463 1.034 1.536
464 2.027 2.936
477 0.586 1.843
478 0.651 1.783
480 0.678 1.554
510 0.807 2.180
512 1.583 2.670
513 1.592 -- 2.796
514 1.922 2.812
515 2.102 2.769
516 1.434 1.918
517 0.369 0.791
518 1.143 2.498
520 0.593 1.923
-27-
~ 3 3 8 0 2 8 27125-890/8699
~ABLE 2 ~Continued)
Immunologic Reactivity Determined By ELISA
Between HIV-l Antibodies In 233 Confirmed
Positive Serum Samples And Synthetic Peptide
Antigens Corresponding To Regions Of gp41
Antiqen
Serum Sample No. V (39) gp41A5
521 2.418 2.831
522 1.107 2.493
523 0.509 1.043
524 0.825 2.621
s 525 0.829 1.993
526 1.456 2.612
527 2.311 2.849
528 0.679 2.026
~ 529 0.530 2.631
530 1.655 2.745
531 1.177 2.739
532 0.384 1.617
533 1.085 2.698
534 1.852 2.834
535 1.412 2.?44
536 0.350 1.093
537 o.gos 2.764
538 1.147 2.590
539 0.219 0.407
540 0.643 2.644
-28-
~ 338U~
27125-890/8699
7125-890/8699
TABLE 3
ELISA Tests Comparing Specificity And
Selectivity of Synthetic Peptide Antigens
5) Antiqen
ERUM Negative
AMPLE NO. WB gp41A5 V (39) antigen**
4 + 2.809* 0.436* 0.084*
7 + 1.412 0.137 0.066
12 + 2.222 0.336 0.087
19 + 2.922 0.289 0.071
+ 2.635 0.484 0.071
31 + 2.343 0.737 0.101
+ 1.495 0.227 0.090
42 + 2.289 0.731 0.140
43 + 1.978 0.522 0.062
44 + 2.443 1.199 0.085
48 + 2.592 0.195 0.073
+ 1.399 0.219 0.084
78 + 1.826 0.489 0.219
101 + 0.863 0.205 0.072
482 + 1.316 0.120 0.100
511 + 1.036 0.248 0.067
140 + 2.369 0.952 U.081
277 + 2.243 1.106 0.260
285 + 1.977 0.437 0.057
298 + 2.636 1.204 O.Oq6
324 + 1.299 0.281 0.057
375 + 0.957 0.280 0.066
393 + 0.874 0.195 0.067
416 + 1.326 0.526 0.082
428 + 1.184 0.355 0.142
446 + 0.612 1.731 0.215
457 + 2.72q 1.055 0.205
477 + 1.843 0.586 O.lOq
478 + 1.783 0.651 0.069
517 + 0.791 0.369 0.029
520 + 1.923 0.593 0.088
523 + 1.093 0.509 0.080
524 + 2.621 0.825 0.056
525 + 1.993 0.809 0.067
532 + 1.617 0.38q 0.082
536 + 1.093 0.350 0.086
539 + 0.407 0.219 0.051
540 + 2.64q 0.643 0.056
39508 - 0.15q 0.153 0.087
39509 - 0.191 0.166 0.043
39510 - 0.156 0.085 0.044
t39511 - 0.241 0.177 0.028
39512 - 0.101 0.144 0.093
39513 - 0.178 0.123 0.048
39514 - 0.101 0.118 0.051
39515 - 0.086 0.252 0.063
* Spectrophotometric readings, O.D.405
** Non HIV-l Antigen
-29-
1 3 3 8 0 2 8 27125-890/8699
Cut off = Mean O.D.405 of Negative Sera + 3xSD
Cut off A5 = 0.151 + 3x0.053 = 0.310 (O.D.405)
Cut off V(39) = 0.15~ + 3x0.050 = 0.302 (O.D.405)
WB = Western Blot using HIV-l antigens
. . .
-30-
27125-890/8699
t 33802~
Example 4
A peptide A5-Trunc(a) missing the carboxyl terminal
portion of gp41A5 (see Fig. 2) ~as also synthesized as
described in Example 1 and used in ELISA tests as described
in Example 2 but substituting peptide A5-Trunc(a~ for gp41A5
to measure antigenic reactivity. It is readily seen from the
ELISA results presented in ~able 4 that the carboxy terminal
portion of gp41A5 is clearly required for the high degree of
reactivity and specificity of this peptide.
1 ~ 3 8 0 2 8 27125-890/8699
TABL~ 4
Reactivity Of Antibodies in Serum Samples
With Synthetic Peptide Antigens Corresponding
to HIV-l Antigens
Antiqen
SERUM Negative
SAMPLE NO. WB gp4lA5 V (39) A5-Tru~cta) antigen
4 + 2.809* 0.436~ 0.905* 0.084*
1 + 1.412 0.137 0.164 0.066
12 + 2.222 0.336 0.583 0.087
19 + 2.922 0.289 0.850 0.071
+ 2.635 0.484 0.532 0.071
15 31 + 2.343 0.73~ 0.444 0.101
+ 1.495 0.227 0.547 0.090
42 + 2.289 0.731 0.420 0.140
43 + 1.978 0.522 0.144 0.062
44 + 2.443 1.199 0.406 0.085
20 48 + 2.592 0.195 0.601 0.073
+ 1.399 0.219 0.098 0.084
78 + 1.826 0.489 0.485 0.219
~ 101 + 0.863 0.20; 0.187 0.072
482 + 1.316 0.120 0.605 0.100
25511 + 1.036 0.248 0.139 0.067
140 + 2.369 0.952 0.318 0.081
277 + 2.243 1.106 0.307 0.260
285 + 1.977 0.437 0.553 0.057
298 + 2.636 1.204 0.229 0.046
30324 + 1.299 0.28i 0.363 0.057
375 + 0.957 0.280 0.631 0.066
393 + 0.874 0.195 0.254 0.067
416 + 1.326 0.526 0.166 0.082
428 + 1.184 0.355 0.303 0.142
35446 + 0.612 1.731 0.310 0.215
457 + 2.724 1.055 0.326 0.205
477 + 1.843 0.586 0.672 0.104
478 + 1.783 0.651 0.386 0.069
517 + 0.791 0.369 0.125 0.029
40520 ~ 1.923 0.593 0.990 0.088
523 + 1.093 0.509 0.272 0.080
524 + 2.621 0.825 0.557 0.056
525 + 1.993 0.809 0.485 0.067
532 + 1.617 0.384 0.432 0.082
4S536 + 1.093 0.350 0.532 0.086
539 + 0.407 0.219 0.147 0.051
540 + 2.644 0.643 0.334 0.056
39508 - 0.154 0.153 0.209 0.087
39509 - 0.191 0.166 0.179 0.043
5039510 - 0.156 0.085 0.160 0.044
39511 - 0.241 0.117 0.151 0.028
39512 - 0.101 0.144 0.156 0.093
39513 - 0.178 0.123 0.131 0.048
39514 - 0.101 0.118 0.130 0.051
5539515 - 0.086 0.252 0.216 0.063
Cut off = Mean O.D. of Negative Sera + 3xSD
Cut off A5 = 0.151 t~x0.053 = 0.314
Cut off V139) = 0.152 + 3xO.050 = 0.302
Cut off A5-Trunc(a) = 0.166 + 3x0.033 = 0.265
WB = Western Blot
1 33~028
27125-890/8699
* Spectrophotometric reading, O.D.405
** Non-HIV-l Antigen
-33-
1 3 3 8 0 2 8 27125-890/8699
Example 5
Peptide gp41CT4 having the amino acid sequence set
forth above was synthesized as in Example 1 and was tested in
the ELISA described in Example 2 (substituting gp41CT4 for
gp41A5) against 105 HIV-l positive sera (all of which reacted
with gp41A5). The peptide, at a concentration of between 10
and 100 ~g/ml was coated onto microtiter plates as described
in Example 2. As shown in Table 5, this peptide reacted with
68/105 (64.8%) positive sera. No false positive reactions
were seen when this peptide was tested against 28 confirmed
negative sera.
Example 6
Peptide gp41CT3 having the amino acid sequence set
forth above was synthesized as in Example 1 and coated onto
microtiter plates as described in Example 2 (substituting
gp41CT3 for gp41A5). As shown in Table 5, gp41CT3 reacted
with 38/80 (48.8~) of the confirmed positive serum samples in
the ELISA assay described in Examples 2 and 3. The peptide
- produced only one false positive reaction when tested against
20 confirmed negative sera
Example 7
Peptide gp41Bl synthesized as in Example 1 at a
concentration of from 10 to 100 ~g/ml was coated into micro-
titer plates as described above and used in the ELISA
described in Example 2 (substituting gp41Bl for gp41A5).
This peptide reacted with 45/95 ~47.4%) of the confirmed
HIV-l positive serum samples (Table 5). No false positive
reactions were seen with two negative sera.
~ 3 3 ~ ~ 2 ~ 27125-890/8699
ExamPle 8
Peptide qp41B3 synthesized as described in
Example 1 was coated at a concentration of from 10 to
100 ~g/ml onto microtiter plates. It yielded positive
reactions with 22/32 (68.8%) of the confirmed positive HIV-l
serum samples in the ELISA described in Examples 2 and 3
~Table 5). No false positive reactions were seen with two
confirmed neqative sera.
~ 3 3 8 ~ 2 ~ 27125-890/8699
TABLE 5
Reactivity Of Synthetic Peptides Corresponding
To Regions Of HIV-l gp41 With Antibodies Against
HIV-l In Confirmed Positive Sera
s
Peptide tFiq. 21
Serum A5 A4 81 B3 B4 CT3 CT4
+ -- + + + + (+)
102 + + + - + (+
3 + + + + + + +
4 + + + + + +
S + + + + -- + (+)
6 + + + + + _ +
157 + +
8 + + + + (+) _ +
g + + + + ~+) +
+ + + (+) + _ _
11 + + -- _ +
2012 + + - + +
13 + + + + + + +
14 + + + + t+) +
+ -- + _ _ _ +
16 + + +
2517 + + +
18 +
19 + + +
+ ~ + +
21 + + + + - +
3022 + - + - +
23 + _ +
24 + +
+ _ _ +
26 + - - +
3527 + _ _ +
28 + ( )
29 + _ +
+
31 + + +
4032 + +
33 + + +
34 + + (+) + _ + +
+ _ + +
36 + + + + +
4537 + + + +
38 + _ + +
39 + (+
+ (+
41 +
5042 + + +
43 + _ +
44 + . + +
+ (+) + +
46 +
5547 + + + +
48 +
49 + + +
+ + _ + + + +
-36-
1 3 3 8 0 2 8 27125-890/8699
TABLE S (Continued)
Reactivity Of Synthetic Peptides Corresponding
To Regions Of HIV-l gp41 With Antibodies Against
HIV-l In Confirmed Positive Sera
Peptide ~Fig. 2)
Serum A5 A4 Bl B3 B4 CT3 CT4
51 +
52 +
53 +
54 +
+
56 +
57 +
58 +
59 +
+
61 +
62 +
63 +
64 +
+
66 +
67 +
68 +
69 +
+
71 +
72 +
73 +
74 +
25 75 +
76 +
77 +
78 +
79 +
30 80 +
81 +
82 +
83 +
84 +
35 85 +
86 +
87 +
88 +
89 +
40 90 +
91 +
92 +
93 +
94 +
95 +
96 +
97 +
98 +
99 +
100 +
101 +
-37-
27125-890/8699
1 33802~
- - TABLE 5 tContinued~
Reactivity Of Synthetic Peptides Corresponding
To Regions Of HIV-l gp41 With Antibodies Against
HIV-l In Confirmed Positive Sera
PePtide tFiq. 2)
Serum A5 A4 Bl B3 B4 CT3 CT4
102 +
103 + ~ +
104 + + +
105 + - +
5 106 + - +
107 + + +
108 +
- 109 +
110 +
-
- = negative reaction
~+)= low positive reaction
+ = strong positive reaction
-38-
1 3 3 8 0 2 8 27125-890/8699
Example 9
Peptides GAG2 and GAG3 and GAG 14 were synthesized
as described in Example 1. Each peptide was coated at a
concentration of from 10 to 100 yg/ml onto microtiter plates
and used in the ELISA described in Example 2, but substitut-
ing GAG 2, GAG 3, or GAG 14 for gp41A5 as the antiqen.
Peptide GAG 2 was also assayed against HIV-2 positive serum
samples. The results o~ the assays are presented in Table 6.
-39-
TA~LE 6
Immunologic Reactivity Of Peptide Antigens
Corres~ondina To HIV-1 Gaq Gene Product
Serum Source
Confirmed Pos. (HIV-l) Confirmed Pos. (HIV-2) Confirmed Neg. Sera
No, Reactive/No. Tested ~ No. Reactive/No. Tested ~ No, Reactive/No, Tested
Antigen
GAG 2 9/19 (47.4) 2/2 (100) 0/4
GAG 3 4/5 (80) N.D. 0/2
GAG 1-4 10/10 (lO0) N.D. 0/2
N.D. 5 not done
.;
r~
a~
O :~ U / 0 0 ~
1 338028
.~, .
Example 10
Peptides P17-D and P17-F were synthesized as
described in Example 1 and each peptide was coated at a
concentration of 500 ~g/ml onto microtiter plates and used in
the ELISA described in Example 2, but substituting P17-D or
P17-F for gp41A5 as the antigen. These peptides were also
assayed against HIV-2 positive serum samples. The results
are presented in Table 7.
HIV-2, a retrovirus related to, but not identical
with, HIV-l, has been recently isolated from West African
patients with AIDS who tested negative in ELISA tests using
HIV-l antigens.
It can be seen from the data in Tables 6 and 7 of
Examples 9 and 10 that peptides GAG 2, P17-D and P17-F each
contain epitopes recognized by antibodies to both HIV-l and
H~V-2. Thus these three peptides may be useful in diagnos-
tics and vaccine compositions for both HIV-l and HIV-2
infections.
TABLE 7
Immunologic Reactivity Of Antibodies In Serum Samples
From HIV-1 and HIV-2 Patients With Peptides Corresponding
To HlV-l gaq Gene Products
Antigen Confirmed Pos. (HIV-l) Confirmed Pos. (HIV-2) Confirmed Neg.
Tested No, Reactive/No. Tested (~1 No. Reactive/No. Tested (~1 No. Reactive/No.
P17-D 4/4 (100) 2t2 (100) 0/2
P17-F 5/5 (100) 1/1+ 0/2
+ weakly reactive
,.
~;-
o
O~
~ 338028
27125-890/8699
It is evident from the foregoinq results that the
novel synthetic peptides described herein which correspond-
ing to the regions of immunologically important proteins of
HIV-l, e.g., gp41 and gag gene products provide a superior,
sensitive, and selective assay for the presence of antibodies
to HIV-l.