Note: Descriptions are shown in the official language in which they were submitted.
_- 1 3 3 8 2 4 0
61211-919D
This is a division of appllcation Serial No. 592,604 filed
March 2, 1989 entitled "ULTRASOUND BRAIN LESIONING SYSTEM"
Backqround of the Invention
The present invention relates ln general to brain
lesioning methods and apparata. More particularly, the present
invention relates to a cornbination system for brain lesioning
which initlally uses a separate visualization system for site
localization. The data from the visualization system is
digitized and translated by computer into linear and rotary
positioning means for positioning the ultrasound transducer
which is used to create volume lesions. The separate
visualization system may be a CT or MRI scan or may be
ultrasonic imaging. The required output in the depicted
configuration is a transparency of the imaged tumor or other
volume to be lesioned which can then be translated into a
computer by use of a digitizing tablet.
Traditionally, the selected method for treatment of
brain tumors and related disorders was to first take and
process an X-ray filrn of the brain and from that film roughly
determine the size, shape and location of the tumor. The next
step was to surgically remove as much of the tumor as possible.
As technology has advanced, X-ray usage has yielded to other
visualization methods, such as ultrasound, CT scan techniques
and MRI utilization. The surgical procedures have expanded to
cryoknives and gamma knives. Radon seeds have been irnplanted
and ionlzing radiation used. Each of these approaches has met
with some success but not without their share of adverse side
effects, including incomplete treatment.
1 338240
Any cutting procedure is risky, especially in the area
of the brain, in that the procedure may result in the
incomplete removal of the tumor tissue, the excess removal
of healthy tissue or both. Ionizing radiation creates a
cumulative effect of the dosage to the other, surrounding
brain tissue. These concerns and their attendant problems
are addressed and solved by the use of ultrasound to
produce volume lesions in the brain. As is well known,
the noninvasive nature of ultrasound provides a safe and
convenient means of treatment by selection of a suitable
dosage to produce volume lesions.
The success of any ultrasound approach depends on a
number of factors. Not only must the dosage (intensity
and time) be controlled, but the alignment of the beam,
spot size and completeness of the treatment over the full
volume of the tumor or other selected tissues are
critical. A multiplicity of individual focal lesions can
be used to produce ultrasonically generated specific size
and shape volume lesions. It is necessary to know the
level of ultrasonic intensity at the focal site below
which no proliferating damage will occur outside the
primary lesioning site. An added concern with the
treatment of brain tumors with ultrasound is the risk of
"skimming" of the ultrasound beam by the edge of the bony
opening in the skull. Finally, since ultrasound is not a
"sighted" treatment technique, the physician needs to have
some means of determining the exact location of the tumor,
its size and its shape.
The number of engineering and anatomical concerns over
the use of ultrasound for treatment of brain tumors has
1 33824Q
meant that over the years there has been very little
interest in producing volume lesions in the brain for the
elimination of tumors. The inability to deal with these
engineering and anatomical concerns has meant that a
valuable treatment option has not been adequately
utilized. There is no doubt that noninvasive ultrasound
is preferred over the surgeon's scalpel. What has been
missing and what is provided by the present invention is a
means to translate the tumor location and shape
determination data from a reliable imaging technique such
as ultrasound, CT scan or MRI into computer-controlled
linear and rotary drive means for the ultrasound
transducer. By digitizing the ultrasound, CT scan or MRI
data, alignment of the focused ultrasound beam can be
precise and the dosage determined so as to be able to use
ultrasound to produce volume lesions in the brain for the
treatment of tumors. The present invention provides a
number of unique and valuable structures which cooperate
to provide an apparatus which is both extremely accurate
and precise and which avoids the prior art problems.
f.-. .
~?
1 338240 61211-919D
summarY of the Inventlon
In accordance wlth the present lnventlon there ls
provlded an ultrasound treatrnent transducer assembly for
dlrectlng a focused ultrasound beam at an anatomical slte, sald
transducer assembly comprlslng:
an acoustlc focuslng lens havlng a concave front surface
and a substantlally flat back surface;
a substantlali~y flat plezoeléctrlc transducer plate
dlsposed ln spaced relatlon to sald focuslng lens and havlng a
rear surface and a front surface whlch ls dlsposed at a flxed
dlstance of separatlon wlth respect to,the back surface of sald
acoustlc focuslng lens~
an acoustlc coupllng medium disposed between the back
surface of sald focuslng lens and the front surface of said
transducer plate;
flrst pressurlzlng rneans contactlng sald acoustlc coupllng
medlum for malntalnlng sald acoustlc coupllng rnedium between
sald transducer plate and said focusing lens at a desired
pressure; and
alr pressure means contactlng the rear surface of said
transducer plate for applylng alr pressure agalnst sald rear
surface, whereln the pressure applled against said rear surface
by sald alr pressure means ls hlgher than the deslred pressure
on the front surface of sald transducer plate due to said
acoustlc coupllng medlum to malntain the spaced relatlon
between sald front surface of sald transducer plate and sald
back surface of sald lens.
An apparatus for treatment of brain tumors or other
selected braln t1ssues accordlng to one embodiment of the
present lnventlon lncludes a skull flxatlon apparatus which
~lncorporates a plurallty of allgnment spheres, a movable
translatlon assembly whlch lncludes a preclsion ball for
1 338240
61211-919D
providlng llnear and rotary positloning data by way of a cup
whlch is adapted to flt over the plurality of spheres, a llnear
encoder whlch cooperatlvely interfaces with the precision ball
for deriving X, ~ and Z linear positioning data, a pair of
rotary encoders whlch cooperatively interface wlth the
precision ball for deriving rotary positioning data, digitizing
means for deriving skull fixatlon coordinates and tumor data
frorn an image transparency produced by a suitable imaging or
scan technlque, computer means which cooperatlvely interface
with the digitizing means for retrieving and storing skull
fixation coordlnates and tumor data from the imaglng or scanner
means, and wherein the cornputer means is operatively coupled to
the linear encoder and to the palr of rotary encoders for
receiving linear and rotary positioning data and which is
operable to automatically move the transducer in both llnear
and rotary directions such that the focused beam of ultrasound
from the transducer ls able to be dlrected at the locations of
the braln tumors.
One obiect of the present invention is to provide an
improved method and apparatus for generatlng volume leslons in
the brain by focused ultrasound.
Related ob~ects and advantages of the present
invention will be apparent from the following description.
1 338240
Brief Description of the Drawings
FIG. 1 is a diagrammatic illustration of the main
component parts and their relationship to one another
according to a typical embodiment of the present invention.
FIG. lA is a diagrammatic illustration of the patient
positioned for initial imaging.
FIG. 2 is a diagrammatic illustration of positional
data translating means comprising a portion of the present
invention.
FIG. 2A is a perspective view of a skull fixation
device with reference landmarks attached for spatial
coordinates.
FIG. 3 is a front elevation, diagrammatic illustration
in full section of the transducer design comprising a
portion of the present invention.
FIG. 4 is a diagrammatic illustration of the focused
ultrasound beam as directed into the brain tumor.
FIG. 5 is a diagrammatic illustration of the skull
opening and centralizing of the ultrasound beam which is
directed by the present invention.
1 338240
Description of the Preferred Embodiment
For the purposes of promoting an understanding of the
principles of the invention, reference will now be made to
the embodiment illustrated in the drawings and specific
language will be used to describe the same. It will
nevertheless be understood that no limitation of the scope
of the invention is thereby intended, such alterations and
further modifications in the illustrated device, and such
further applications of the principles of the invention as
illustrated therein being contemplated as would normally
occur to one skilled in the art to which the invention
relates.
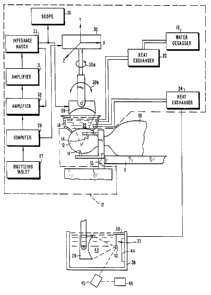
Referring to FIG. 1, there is a diagrammatic
illustration of the main portions of the present
invention. The patient 10 is shown lying on table 11. As
illustrated, the patient shown is lying on his side but it
should be understood that the system permits any patient
position, including even a sitting position. The skull 12
is shown attached to rigid skull fixation apparatus 13.
Metal pins 14 are driven into the skull 12 for rigidly
fixing the skull in position as is well known in the art.
Although only two pins 14 are illustrated, a third pin is
employed on somewhat equal radial spacing so as to
suitably support the skull.
Prior to placing the patient in position for treatment
by ultrasound irradiation apparatus 17, the patient has
been subjected to some form of imaging technique as shown
in FIG. lA in order to generate brain site identification
and localization data which will include the size, shape
and location of any tumors and landmarks which establish a
1 333240
precise frame of reference and orientation for the
patient. Imager 15 is representative of the suitable
forms of imaging for the present invention, including
ultrasound, CT scan or MRI with lines 15a and 15b being
representative of information transmission lines for the
image data and lines 15c being representative of the
transmitted and/or received image signals. Regardless of
the technique, external landmarks must be provided for
precisely locating the skull features and tumors spatially
relative to the fixation apparatus 13. The patient still
with apparatus 13 attached is then placed into the
ultrasound irradiation apparatus 17. As will be explained
in greater detail hereinafter, a coordinate transformation
is made from the coordinates in brain space as derived
from the imaging data to brain space relative to the
ultrasound irradiation apparatus for the computer
controlled, automatic guiding and positioning of the
ultrasound transducer, it is thus important for apparatus
13 to remain fixed in position relative to the position of
apparatus 17.
It is to be understood that while a number of
significant advantages in the treatment of brain tumors
are derived by the use of ultrasound, ultrasound is not a
"sighted~ technique and thus some means must be provided
in order to precisely identify the location of the brain
tumors or other brain sites as well as their size and
geometry. Since these particular features and data can be
derived from ultrasonic imaging or CT or MRI scans, one of
those techniques is initially applied for subsequent use
in practicing the present invention. However, simply
- 1 338240
having this data, whether in the form of transparencies or
data signals derived from these scans is not suficient
unless coordinate landmarks are provided on the
transparency or as part of the image data signals so that
they can be used as a point or frame of reference for the
ultrasound transducer. The translating of this imaging
data from the scan transparency into the mechanical drive
for the transducer is critical to the success of the
present invention. However, if transparencies are not
used, the image data signal is input directly into the
computer. The referenced landmarks may take various forms
but they need to have a specific, fixed and known position
relative to fixation apparatus 13. The dimensional
(coordinate) relationship can be programmed into the
computer control to assist in position identification.
Additionally, these landmarks must be visible on the image
transparency, if that form of data entry is used, so that
their position can be digitized and input into the
computer.
Once the patient is in place, a Steridrape cloth with
a hole large enough to outline the edge of the scalp area
of the ultrasound or radiation field is attached to the
patient's scalp. The cloth is then drawn up through an
open bottom in waterbath support 1~ so that this plastic
liner forms a water-tight container from the surface of
the scalp to the top of the waterbath support. This
waterbath support is actually supported above the
patient's scalp and does not rest on the scalp.
A water degasser 19 (shown as a boiling unit) provides
hot, degassed water through heat exchanger 20. The water
~a ~e ~ 9
1 338240
output through heat exchanger 20 is controlled to the
normal body temperature as waterbath support 18 is filled
with degassed water 22. Heat exchanger 24 provides
circulating water through coils and waterbath support 18
in order to maintain a fixed temperature for the degassed
water 22. It should also be noted that the patient as
illustrated has a lateral skull section removed which is
done surgically at a previous time and the ultrasound
irradiation is conducted through the hole in the scalp.
The generated transparencies, which will be the form
of data entry described for the preferred embodiment, from
either the ultrasonic imaging or by CT or MRI scans (and
which include reference landmarks or benchmarks) are
placed on digitizing tablet 27 which is back-illuminated.
The digitizing tablet with either a light pen or similar
data pick up and transferring means, such as a cursor,
enables the physician or technician to locate the
reference landmarks and to outline the various tumor
areas. Also digitized is the bony opening in the skull so
that its geometric center can be established. As should
be well known by those of ordinary skill in the art, with
a CT scan a plurality of transparencies are taken due to
the multiple "slices" through the brain which must be
derived in order to provide three-dimensional, volume
information. Thus, this digitizing step must be repeated
for all of the CT transparencies for the particular
patient such that when completed, there is total geometry
data of the entirety of all tumors or other brain sites
and those tumors are positioned relative to the referenced
landmarks. All essential features of the brain sites,
1 338240
skull opening, reference landmarks are scanned on
digitizing tablet 27 and all of this information is stored
in computer 28.
From the transparencies which are outlined and
transferred from digitizing tablet 27 to computer 28, all
of the dimensional information is provided to the
formulation for computing the voltage drive levels to all
sites in order to give the necessary sound intensity at
the selected brain sites. Since the brain volume selected
for ablation is in general of a complex shape, this
complexity is treated by a multiplicity of individual
ultrasound lesions. Different ultrasonic frequencies are
generally required for varying brain tissue depths, which
requires careful determination of the upper sound
intensity for the tissue depth to avoid proliferating
damage beyond the primary lesioning site. The focal
position for each of these individual ablation sites is
selected by the computer 28 through preprogramming, and
the transducer focus is automatically brought to the
appropriate sites. The transducer 29 is provided with
five degrees of freedom. There are three orthogonal
motions through a normal XYZ coordinate system 30 and two
rotational motions as illustrated by the arrows of
rotation 30a and 30b.
The programming of computer 28 has been arranged so
that for each individually selected lesion site, the
central ray of the ultrasonic beam from transducer 29
passes generally through the geometric center of the skull
opening so that beam "skimming" at the skull bone edge is
avoided.
11
- 1 338240
Power is provided to the transducer 29 from power
amplifier 31. Amplifier 31 is driven by a low-level
amplifier 32 with its frequency source. Impedance
matching network 33 couples the amplifier 31 to transducer
29. Once the required acoustic output from transducer 29
is decided, the driving voltage to transducer 29 is
computed by way of computer 28 which in turn sets the
input in amplifier 32 so that the required voltage is
set. Once set, the feedback loop from matching network 33
to amplifier 32 maintains this fixed voltage level. As an
additional check on the voltage drive level to transducer
29, an additional absolute voltage readout is provided
through oscilloscope 36.
Periodic monitoring of the sound output from
transducer 29 is provided by the radiation force method.
A bifilar suspension 37 mounted to tank 38 by support 39
suspends a stainless steel ball 40 in a temperature
controlled and degassed volume of water 43 which is
contained within tank 38. This water temperature is
maintained by unit 24. The transducer 29 is brought into
tank 38 by placing tank 38 in support 17 (without the
patient in place). Coordinate system 30 (XYZ coordinates
of linear motion) is a mechanical drive structure which is
capable of moving transducer 29 so that the focal position
of the generated ultrasound beam from transducer 29
impinges on the stainless steel ball 40.
Sound-absorbing material 44 which is mounted in tank
38 serves to suppress standing waves in tank 38 during the
calibration procedure. Deflec~ion of the stainless steel
ball for a specified set of drive voltages into transducer
12
1 338240
29 is recorded by the use of an optical telescope 45.
Telescope 45 is mounted outside but attached to tank 38.
Horizontal motion displacement of the stainless steel ball
is recorded on micrometer 46. This ball deflection is
directly related to the sound output intensity at the
focal position in the sound field and this particular
method is believed to be a primary standard for measuring
sound field intensity.
Referring to FIGS. 2 and 2A, the apparatus for
transformation of coordinates to the irradiation apparatus
is illustrated. This transformation is accomplished by
the use of a precision joystick apparatus 48 which is
attached to the back of the housing for focusing
transducer 29. This joystick apparatus includes member 49
which can be linearly translated by way of precision ball
50. Universal angular motion of member 49 is provided
directly through precision ball 50. Attached to member 49
is cup 51 which includes a hemispherical concave surface
that is designed and sized to fit precisely over each of
the balls 52 (see FIG. 2A) which are added to and
supported on skull fixation apparatus 13 by corresponding
upright members 53. Each combination of member 53 and
ball 52 is detachable but fits into precision holes which
are bored into apparatus 13. As is appropriate for
complete alignment, three ball and member combinations are
added to skull fixation apparatus 13. The use of these
three assemblies and the interlocking of each with cup 51
permits the complete transformation of coordinates into
computer 28.
13
-
The patient is positioned within33p8par4a~us 13 and the
alignment balls 52 are attached. Further apparatus 13 is
rigidly affixed relative to the position of apparatus 17
at a known position such that as the cup 51 is moved into
position over each ball 52, the position coordinates are
derived relative to apparatus 17. As should be
understood, each component used in this procedure has
specific dimensions and points of attachment, all of which
are known and programmed into the computer. The
extrapolation is sequential, but not complicated. The
free space coordinates of the alignment balls is derived
relative to the movement of transducer 29. These balls
have a fixed position (known) relative to fixation
apparatus 13 and apparatus has a fixed position (known)
relative to the brain tumor(s).
It should be noted that member 49 electrically
interfaces with linear encoder 56 and two rotary encoders
(potentiometers) 57 and 58, which are used to obtain
rotation degrees. The two rotary encoders are positioned
at right angles to each other. The coordinate translating
system of FIGS. 2 and 2A builds upon the image data and
landmarks previously digitized. This earlier derived
information provides dimensional data and space
coordinates regarding the size and shape of the brain
tumor(s) and their location relative to the skull fixation
apparatus 13. What is missing is the positional data of
the ultrasound irradiation apparatus 17 relative to
apparatus 13. Once the ball 52 and member 53 assemblies
are installed into their precise locations on the fixation
apparatus these become the frame of reference for the
14
transducer positioning. 1 3 3 8 2 4 0
The X, Y and Z coordinates and the two degrees of
rotary freedom for the transducer at each position when
the cup 51 is placed over each ball 5Z, once input into
the computer, enables the transducer to be moved by
computer control to each tumor position for volume
lesioning. A key point to be noted is that no precise
alignment is required in using this system. All that
needs to be done is to position cup 51 in the proximity of
each ball 52 so that the cup can be drawn over each ball
for establishing the coordinates. This sequence of steps
is repeated for a total of three sets of data for the
complete transformation and while the system is extremely
precise, it does not require any precise alignment.
Referring to FIG. 3, transducer 29 is illustrated in
greater detail including several unique features which are
provided in order for a stable acoustic output to be
obtained at all preselected driving levels. These driving
levels are required in order to produce controlled focal
brain lesions in deep brain sites. In order to achieve
this necessary objective, it is necessary to have a stable
sound-producing source such as generally circular (disc)
quartz plate 61 which is used in this particular
embodiment. The quartz plate 61 is able to be maintained
flat and parallel to generally circular, plano-concave
lens 62 by the structure which will be described
hereinafter. Lens 62 is a hard anodized aluminum lens
with an elliptic concave surface for minimizing the
half-intensity lenyth of the beam at the focus. In order
to maintain flatness and parallelism of plate 61 and lens
1 338240
62 with a fixed spacing distance therebetween, the
aluminum flat side of the lens is precisely machine flat
with at least one l/8-inch diameter rod 63 machined on the
surface to extend a distance above the lens surface equal
to a 1/4 wave length in the silicone oil 65 in space 66.
A suitable silicone oil for this application is Dow
Corning 710 fluid.
In order to maintain this 1/4 wave length spacing to
within plus or minus 0.0001 inches, it is required that
the outer peripheral lip 62a of aluminum lens 62 provide
unanodized surfaces (flat top and bottom surfaces and
outer edge surface) which rest directly in contact with
the flat machined surface of housing 64 and end plate
64a. Housing 64 includes an inwardly and upwardly
directed lip 64b, of an annular ring configuration, whose
underside abuts against the top surface of lip 62a and
whose top surface supports plate 61. The height of this
lip is precisely machined since it is the means to fix the
1/4 wave length separation between the plate 61 and lens
62. Rod 63 provides center stabilizing for the plate due
to its span between peripheral edge supports and the
pressure differential between the top and bottom surfaces
of the quartz plate. The space 66 between the plate 61
and lens 62 (the 1/4 wave length spacing) is filled with
silicone oil 65 which is freely exchanged through radially
open channels in lip 64b. Gasket 64c seals the oil in
space 66.
One gold-plated and polished electrode (not shown) is
electrically connected to quartz plate 61 and rests in
direct contact with the top machined surface of lip 64b
16
1 338240
and provides the electrical ground contact for the quartz
plate.
In order to keep plate 61 in pressure contact with
housing 64, a flat, flexible compression gasket 71 is
firmly pressed against plate 61 through metal member 72.
ln order to provide electrical contact for power to plate
61 another foil electrode 73 fabricated of an approximate
.001 thick soft metal foil (gold, brass, silver) extends
part-way under compression gasket 71, while the remainder
of gasket 71 acts as a seal for the silicone oil. The
power and ground electrodes on plate 61 do not extend to
the edge of plate 61 and the silicone oil provides
insulation around the edge. The foil electrode 73 is
attached to metal member 72 with a series of metal screws
74.
To provide RF power to drive quartz plate 61 a coaxial
cable 79, with a metal outer jacket 80 is used. Jacket 80
is cut and peeled back so as to place the cut, free end of
jacket 80 between plates 81 and 82. Plates 81 and 82 are
clamped together using mechanical fasteners in order to
establish an electrical ground on jacket 80. The coaxial
cable has an end plug 84 which side pressure contacts
plate (metal member) 72 through a central hole. Space 85
is an air space so that the quartz plate 61 is not back
acoustically loaded thereby directing all its acoustic
output through the interspace 66 and lens 62 into the
fluid which is in front of lens 62. To insure flatness of
quartz plate 61 and parallelism with the flat surface of
lens 62, the air space 85 and all other air spaces in the
transducer housing 64 are pressurized through tube 86 into
17
1 338240
element 87. This air pressure holds quartz plate 61
against machined rod 63 to maintain the necessary
parallelism. Pressure is applied from source 88.
In order to maintain a positive differential pressure
in space 85 relative to the pressure in interspace 66,
flow communication is provided from interspace 66 via flow
access channels 89 into column 90 and well 91. These
areas are all silicone oil filled and in pressure
equilibrium is a thin flexible diaphragm 92 which covers
well 91. Above diaphragm 92, the air space 93 is
exhausted through flexible tubing 94 and rigid tube 95 to
the outside atmosphere.
A further feature to suppress cavitation in the oil in
space 65 between the quartz plate 61 and lens 62 when the
system is run at the highest acoustic output power is
provided by pressure system 96 providing
greater-than-atmospheric pressure to space 93. Typically
this pressure will be that which prevents any cavitation
in space 65 (of the order of 40-50 pounds per square
inch). This pressure in space 93 is readily transmitted
through diaphragm 92 to the silicone oil 65 in well 91 and
hence through column 90 into space 66. The pressure
provided by source 88 is in the order of 2 pounds per
square inch higher than the pressure in system 96 in order
to keep plate 61 flat and held against lens 62 through rod
63.
Element 99 in the transducer assembly is an insulating
member to which element 72 is bolted by screw(s) 100.-
Gasket 101 keeps the silicone oil contained in column 90
from reaching the coaxial cable 79. Metal plate 82 is
18
~_ 1 338240
bolted to housing 64 around the outer periphery of plate
82. Oil is kept in column 90 and well 91 by the use of
O-ring seal 103 positioned between housing 64 and plate 82
and by gasket 105. Member 106 is bolted and sealed to
plate 82. Top metal plate 107 is bolted by screws 108 to
housing 64 and sealed thereto through O-rings 109. Metal
tube 95 is sealed to element 87 through seal 110. The
coaxial cable 79 is water-tight and sealed to top plate
107 through member 111 and O-ring 112.
In order to accomplish the task of producing lesions
of any complex size or shape in the human brain with
intense focused ultrasound it is necessary to provide for
ultrasound dosage conditions which produce individual
focal lesions (from which the complex volume can be
generated), which do not compromise brain tissue outside
the intended focal lesions side and permit subsequent
individual focal lesions in a contiguous manner. To do
this in both gray and white matter and abnormal brain
tissue, it is necessary to inhibit the production of
microbubble formation at the primary focal site so that
there can be no vascular dispersion of such microbubbles
away from the primary focal site which microbubbles could
initiate off primary site lesion production and hemorrhage
due to ultrasound passage through microbubble comprised
tissue.
In order to accomplish this task while being able to
accomplish primary site lesions, it is necessary to derive
these sound intensities as a function of frequency which
will not produce microbubbles at the primary lesion site.
For a 1 MHz sound frequency (a frequency necessary to
19
_ 1 33~240
achieve deep penetration into the human brain), the
primary site sound intensity which can be used for volume
lesions generated from a multiplicity of single-site
lesions delivered in time sequence of the order of 1-5
minutes, must not exceed 300 watts per square centimeter.
At this intensity and for lower intensities, gray and
white matter lesions on a multiplicity of individual
contiguous sites can be produced without undesirable side
effects (microbubbles) or proliferation of damage outside
the lesioning site.
For lesions of less distance from the cortical
surface, as the ultrasonic frequency can be increased
above 1 MHz and the primary site sound intensity can be
proportionately increased without producing microbubbles
or damage beyond the primary site. At the higher
frequencies, the penetration capability in brain tissue is
diminished. For instance, at 4 MHz frequency which is the
upper frequency which can be considered for more
superficial brain lesion production of the order of 3 cm
below the cortical surface, the intensity which will not
lead to microbubble formation approximately 1200 watts per
square centimeter. At these intensity limits, the time-on
period of sound irradiation at each individual site can be
extended to as many seconds as is needed to ablate the
tissue at the focal site without microbubble formation.
Thus, for frequencies between 1 MHz and 4 MHz, the safe
upper sound intensity will fall between 300 w/cm2 and
1200 w/cm2, the intensity value increasing as the
frequency increases.
1 33~24~
-
In order to constrict the individual lesion sites so
that the boundaries of desired volume lesions can be
constrained, the transducer configuration used will give a
half intensity length at the lesion focal region in the
order of 15 mm at 1 MHz operating frequency. This length
of half intensity is consistent with the necessity of
constraining lesions in the human brain so that the
extending of individual lesions into white matter (white
matter is more sensitive than gray matter) can also be
constrained.
Referring to FIGS. 4 and 5, the details of the
ultrasound being focused and central beam axis are shown
with respect to the skull bone opening, the brain volume
site to be lesioned and the tissue depth which is
described in order to compute the tissue attenuation from
the scalp surface to the brain volume selected to be
lesioned.
Skull 116 with skull hole outline 117 is constructed
(digitized) in computer 28 along with brain tissue volume
118 and scalp and muscle tissue 119. Prior information on
individual brain lesion~120 dimensions along with
preselection of the pattern in which the individual
lesions 120 are to be produced, spacing of individual
lesions to give the desired overlap in tissue boundary
patterns are processed in the computer. Individual
lesions 120 within volume 118 are irradiated along the
central axis 121 of the beam with the axis programmed to
pass through the skull opening 117 at the geometric center
124. As the transducer changes orientation by computer
control, the central axis will shift, but the focal point
21
1 338240
of the beam will be at the desired lesion site and the
central axis will be close to the center 124.
Each individual lesion 120 within volume 118 has its
distance computed along the transducer beam axis starting
at the scalp surface 125, proceeding through the scalp and
muscle 119 tdistance A) through the brain tissue 126
(distance B). The scalp and muscle average attenuation
coefficient and the brain attenuation coefficient along
with dimensions A and B are involved in the computation of
total acoustic beam loss. From these computations, the
necessary driving voltage to transducer 29 is provided
automatically for each individual lesion site 120 in brain
tissue volume 118.
It is also possible in some cases to apply the above
system and technique to the production of focal lesions in
the brain through the intact scalp muscle and skull bone.
In this circumstance, the attenuation factor for the skull
must be entered as an additional attenuation and the
central beam axis of transducer 29 held within plus or
minus five degrees perpendicular to the skull surface on
its path to each individual lesion site 120. Although
this system is specifically designed for the brain, it can
be used in the transcutaneous mode to produce lesions and
other appropriate body tissues.
While the invention has been illustrated and described
in detail in the drawings and foregoing description, the
same is to be considered as illustrative and not
restrictive in character, it being understood that only
the preferred embodiment has been shown and described and
that all changes and modifications that come within the
spirit of the invention are desired to be protected.
22