Note: Descriptions are shown in the official language in which they were submitted.
4114S/1037A
~ 3383 1 9
- 1 - 17501
TITLE OF THE INVENTION
A NEW CYCLOSPORIN DERIVATIVE WITH MODIFIED
n 8-AMINO ACID"
BACKGROUND OF THE INVENTION
Immunoregulatory abnormalities have been
shown to exist in a wide variety of "autoimmune" and
chronic inflammatory diseases, including systemic
lupus erythematosis, chronic rheumatoid arthritis,
type 1 diabetes mellitus, inflammatory bowel disease,
biliary cirrhosis, uveitis, multiple schlerosis and
other disorders such as crohns disease, ulcerative
colitis, bullous pemphigoid, sarcoidosis, psoriasis,
ichthyosis, and graves ophthalmopathy. Although the
underlying pathogenesis of each of these conditions
may be quite different, they have in common the
appearance of a variety of autoantibodies and
self-reactive lymphocytes. Such self-reactivity may
be due, in part, to a loss of the homeostatic
controls under which the normal immune system
operates.
4114S/1037A - 2 - 1 3383 1 917501
Similarly, following a bone-marrow or an
organ transplantation, the host lymphocytes recognize
the foreign tissue antigens and begin to produce
antibodies which lead to graft rejection.
The end result of an autoimmune or a
rejection process is tissue destruction caused by
inflammatory cells and the mediators they release.
Antiinflammatory agents such as NSAID's and
corticosteroids act principally by blocking the
effect or secretion of these mediators but do nothing
to modify the immunologic basis of the disease. On
the other hand, cytotoxic agents such as cyclophos-
phamide, act in such a nonspecific fashion that both
the normal and autoimmune responses are shut off.
Indeed, patients treated with such nonspecific
immunosuppressive agents are as likely to succumb
from infection as they are from their autoimmune
disease.
The cyclosporins are a family of
immunosupressive compounds isolated from fermentation
broths of various fungal species including
Tolypocladium inflatum and Cylindrocarpon lucidum.
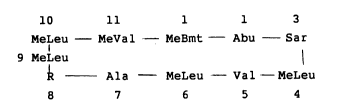
The generic structure of the class of
cyclosporins has been established as a cyclic peptide
which contains 11 amino acids.
For example, the structure of cyclosporin A
was established as a cyclic peptide containing several
methylated amino acids and at position 8 this amino
acid is D-alanine which has been considered important
for the biological activity of cyclosporin.
1 3383 1 9
4114S/1037A - 3 - 17501
10 11 1 1 3
MeL~u - MeVal MeBmt Abu - Sar
9 MeL~u
D-Ala Ala - MeLeu - Val - MeLeu
8 7 6 5 4
Bmt = (4R)-4-[(E)-2-butenyl]-4-methyl-L-
threonine-
Me = Methyl
Abu = a-Aminobutyric acid
Val = Valine
Ala = Alanine
MeLeu = N-methyl Leucine
MeVal = Methyl valine
Sar = Sarcosine
Generally a cyclosporin such as cyclosporin
A is not cytotoxic or myelotoxic. It does not
inhibit migration of monocyctes nor does it inhibit
granulocytes and macrophage action. Its action is
specific and leaves most established immune responses
intact. However, it is nephrotoxic and is known to
cause the following undesirable side effects:
(1) abnormal liver function;
(2) hirsutism;
(3) gum hypertrophy;
(4) tremor;
(S) neurotoxicity;
(6) hyperaesthesia; and
(7) gastrointestinal discomfort.
1 3383 1 9
4114S/1037A - 4 - 17501
Accordingly, an object of the present
invention is to provide a less nephrotoxic new
cyclosporin derivative which will (1) restore the
balance of the help-and-suppression mechanism of the
immune system by acting at an earlier point than the
anti-inflammatory agents and (2) induce specific
long-term transplantation tolerance through a
suppressor cell circuit without increasing the body's
susceptibility to infection.
Another object of the present invention is
to provide pharmaceutical compositions for
admin ~tering to a patient in need of the treatment
the active immunosuppressive agent of the present
invention.
Still a further object of this invention is
to provide a method of controlling graft rejection,
autoimmune and chronic inflammatory diseases by
administering a sufficient amount of the novel
immunosuppressive agent in a mammalian species in
need of such treatment.
Finally, it is the object of this invention
to provide processes for the biosynthesis and
isolation of the active compound.
DETAILED DESCRIPTION OF THE INVENTION
A. Scope of the Invention
This invention relates to cyclosporin
derivatives having 3-fluoro-alanines at the 8-
position:
1 3383 1 9
4114S/1037A - 5 - 17501
10 11 1 1 3
MeLeu - MeVal MeBmt - Abu ~- Sar
9 Me_eu
R Ala MeLeu - Val ~ MeLeu
8 7 6 5 4
wherein R is 3-fluoro-D-alanine or 2-deutero-3-fluoro-
D-alanine. The 3-fluoro-alanine derivatives
exhibited immunosuppressive properties similar to
cyclosporin A but with lower nephrotoxicity.
B. Biosynthesis Methodology
The modified cyclosporin of this invention
has been prepared according to the following
fermentation procedure.
EXAMPLE 1
Preparation of 8-(2-deutero-3-fluoro-D-alanine)-
cyclosporin A
Culture: Tolypocladium inflatum MF5080, NRRL-8044
Media: Slant Medium A g/L
Malt Ext. 20.0
Yeast Ext. 4.0
Agar 20.0
Seed Medium B
Malt Ext. 70.0
Glucose 50.0
1 3383 1 9
4114S/1037A - 6 - 17501
Culture Medium C
Glucose 40.0
Caseinpeptone 10.0
MgS04 7H20 0 . 5
K 2 4 2.0
NaNO3 3.0
KCl o.5
FeSO4-7H2O 0.01
A lyophile tube was aseptically opened and
grown in seed medium B (20 ml in a 250 ml 3-baffle
Erlenmeyer flask) for 4 days on a rotary shaker (220
rpm) at 27C.
This seed was then used to inoculate slants
(medium A) for future studies. The slants were
incubated at 27C for 14 days after which time they
were stored at 4C until used.
The spores were washed from an entire slant
with 5 ml of medium C and used to inoculate a
preculture flask (50 ml medium C in a 250 ml
Erlenmeyer flask). This preculture was incubated for
5 days at 27C.
Five ml of the preculture was used to
inoculate the production medium (50 ml of medium C
and 5 mg/ml of 2-deutero-3-fluoro-D-alanine in a 250
ml Erlenmeyer flask). The filter sterilized
2-deutero-3-fluoro-D-alanine was added (S mg/ml,
final concentration) post-sterilization and prior to
inoculation. Forty-four flasks containing a total of
2.2 liters of production medium were incubated 14 to
21 days with agitation (220 rpm) at 27C. Following
incubation, the fermentation broths were extracted by
procedures described below in item C.
1 3383 1 9
4114S/1037A - 7 - 17501
EXAMPLE 2
Preparation of 8-(3-fluoro-alanine)cyclosporin
Following essentially the same procedures as
described in Example 1 except that the preculture was
used to inoculate a production medium of a total
volumn of 400 ml containing 5 mg/ml of 3-fluoro-
alanine instead of 2-deutero-3-fluoro-alanine, there
was obtained the fermentation broth which was
extracted by the procedures described below in item C.
C. Extraction Methodology
a. The cells were removed from the broth by
centrifugation.
b. The clarified broth was extracted 3 times
each with 25 ml portions of methylene
chloride.
c. The cells were extracted 3 times each with
25 ml portions of acetone.
d. The methylene chloride and acetone extracts
were pooled and taken to dryness under
vacuum.
e. The residue was solubilized with methanol,
dried with anhydrous Na2SO4, filtered
and taken to dryness under vacuum.
f. The samples were submitted for HPLC analysis
to determine and isolate the cyclosporin
derivatives.
D. HPLC Analysis
Example 1 - 8-(2-deutero-3-fluoro-alanine)cyclo-
sporin A
1 3383 1 9
4114S/1037A - 8 - 17501
Crude extracts were assayed by HPLC chromatography
using the following chromatographic system.
Solvent: 80/20 v:v acetonitrile:water
Flow rate: 0.6 mL/min
Column: DuPont Zorbax ODS 4.6mmx25cm
maintaned at 60 C
Detector: LDC Spectromonitor III, 210nm 0.05
AUFS
Integrator: Spectra-Physics SP4100 Computing
Integrator
The concentration of the desired 2-deutero-3-fluro-D-
alanine analog of cyclosporin A which has a retention
time equal to 92% of the retention time of
20 cyclosporin A, were calculated by dividing the
observed area counts by the area counts/mcg of
cyclosporin A obtained from an external standard of a
known concentration of cyclosporin A.
25 The extraction residues from four fermentations,
representing 2.2L of broth, were combined in 75ml of
methanol and assayed by HPLC chromatography. The
sample which contained 43.lmg of crude 8-(2-deutro-3-
fluoro-D-alanine)-cyclosporin A was labeled A.
*Trade mark
~,
1 33 83 1 9
4114S/1037A - 9 - 17501
Sample A was concentrated to a slightly oily
residue. The residue was taken up in 6ml of 1:1 v:v
methylene chloride: methanol. The solution was then
chromatographed on a 200ml column of Pharmacia LH-20,
previously equilibriated with methanol. The
chromatography was carried out with methanol at a
flow rate of 5ml/min collecting one eight ml fraction
followed by 40x5 ml fractions. Fractions 22 through
26 were combined and labeled B, volume 25ml.
Sample B contained 33.7mg of 8-(2-deutero-3-fluoro-D-
alanine)-cyclosporin-A by HPLC analysis.
Sample B was concentrated to dryness and the residue
taken up in 1 ml of methanol. This solution was
subjected to preparative HPLC chromatography on a
DuPont Zorbax ODS column 2.lx25cm maintained at 60C
using a solvent system of 80/20 v:v acetonitrile:water
at a flow rate of l0ml/min. The effluent stream was
monitored at 210nm using a Gil~on~Model 116 U.V.
detector equipped with a 0.05mm path length cell and
a setting of 0.32 AUFS. The U.V. signal was
monitored with a Spectra-Physics SP4100 computing
integrator and 15 fractions were collected bàsed on
the ultra-violet trace. Fraction 9 was labeled C.
Fraction 10 was concentrated to dryness and the
residue labeled D.
Sample D was dissolved in 0.5 ml of methanol. This
solution was subjected to preparative HPLC
chromatography on a DuPont Zorbax ODS column 2.lx25cm
maintained at 60C using a solvent systems of B0/20
v:v acetonitrile:water at a flow rate of 10mL/min.
*Trade mark
~`` B ~
~,
1 3383 1 9
4114S/1037A - 10 - 17501
The effluent stream was monitored at 226nm using an
LDC Spectromonitor II equipped with a lmm path length
cell and a setting of 0.32 AUFS. The U.V. signal was
monitored with a Spectra-Physics SP4100 computing
integrator and 10 fractions were collected based on
the ultra-violet trace. Fractions 4 and 5 were
selected and combined with Sample C, volume 35 ml,
and was labeled E. Sample E contained 20.1 mg of
8-(2-deutero-3-fluoro-D-alanine)-cyclosporin A with
an ultra-violet purity of >99~ at 226nm by HPLC
analysis. Sample E was concentrated to dryness under
high vacuum to yield 20.2 mg of 8-(2-deutero-3-
fluoro-D-alanine)-cyclosporin A.
Example 2 - 8-(3-fluoro-D-alanine)-cyclosporin A
The extraction residue from one 400ml fermentation
was taken up in lml of methylene chloride and the
solution chromatographed on a 40ml column of
Pharmacia LH-20 previously equilibriated with
methanol. The chromatography was carried out with
methanol at a flow rate of 2ml/min., collecting one
ten ml fraction followed by 30xlml fractions.
Fractions 16 through 27 were selected and combined,
based on HPLC analysis. The combined fractions were
concentrated to dryness and the residue labeled F.
Sample F was taken up in 250 ml of methanol and
subjected to preparative HPLC chromatography on a
DuPont ~orbax ODS column 0.94x25cm maintained at
60C., Chromatography was carried out with a solvent
system of 80:20 v:v actonitrile:water at a flow rate
of 2ml/min. The effuent stream was monitored at
1 3383 1 9
4114S/1037A - 11 - 17501
220nm using an LDC Spectromonitor II equipped with a
lmm path length cell and a setting 1.28 AUFS. The
ulta-violet signal was monitored using a Spectra-
Physics SP4100 computing integrator and eleven
fractions were collected based on the ultra-violet
trace. Fraction 7 contained 3.25mg of 8-(3-fluoro-D-
alanine)-cyclosporin-A with an ultra-violet purity of
>99% at 210nm by HPLC analysis. Fraction 7 was
concentrated to dryness under high vacuum to yield
3.3mg of 8-(3-fluoro-D-alanine)-cyclosporin A.
E. Physical Characterization of 8-(2-deutero-3-
fluoro-D-alanine)-cyclosporin A
Mass spectrum: (M+H)+, m/2 1221, 19 mass units
up from the value (1202) was found for cyclo-
sporin A, and is consistent with the substitution
of an alanine residue in cyclosporin A by
2-deutero-3-fluoro-D-alanine.
'H NMR Spectrum: The 'H NMR data established the
incorporation of the 2-deutero-3-fluoro-D-alanine
at the 8-position.
13C NMR Spectrum:
The spectrum was recorded at 100 MHZ in CDC13
on a Varian XL-400 spectrometer at ambient room
temperature. Chemical shifts are shown relative
to TMS at zero ppm using the solvent peak at 77.0
ppm as reference: 10.0, 16.1, 17.0, 18.1, 18.5,
18.8, 20.0, 20.5, 21.2, 21.8, 22.0, 23.6(2x),
23.9(2x), 24.0, 24.2, 24.5, 24.9, 25Ø 25.6,
~?
~ *Trade mark
1 3383 1 9
4114S/1037A - 12 - 17501
29.3, 29.88, 29.94, 29.96, 31.2, 31.4, 35.8,
36.0, 36.2, 37.5, 39.2, 39.6, 40.6, 48.75(2x),
48.83, 50.4, 55.3, 55.45, 55.49. 57.6, 57.9,
59.0, 74.8. 81.9d, (J=177.2Hz)*, 126,2, 129.5,
169.8, 169.9(2x), 170.9d*, 170.1, 171.1, 171.53,
171.56, 173.4, 173.59, 173.61 ppm.
* These resonances of the 3-fluoro-2-deutero-D-
alanine residue are observed as doublets due to
coupling with the 19F nucleas. The 13C NMR
data of 61 carbons is consistent with the
molecular formula C62HlogDNllO12
assumption that the a-carbon of the 3-fluoro-
2-deutero-D-alanine residue carrying the deuterium
atom is not observed.
F. Utility of the compounds within the scope of the
invention
This invention also relates to a method of
treatment for patients suffering from graft rejection
after transplantation, autoimmune or chronic inflamma-
tory diseases which involves the administration of a
compound of formula (I) as the active constituent.
For the treatment of these conditions and
diseases a compound of formula (I) may be
administered orally, topically, parenterally, by
inhalation spray or rectally in dosage unit
formulations containing conventional non-toxic
pharmaceutically acceptable carriers, adjuvants and
vehicles. The term parenteral as used herein
includes subcutaneous injections, intravenous,
intramuscular, intrasternal injection or infusion
techniques. In addition to the treatment of warm-
1 3383 1 9
4114S/1037A - 13 - 17501
blooded animals such as horses, cattle, sheep, dogs,
cats, etc., the compounds of the invention are
effective in the treatment of humans.
The pharmaceutical compositions containing
the active ingredient may be in a form suitable for
oral use, for example, as tablets, troches, lozenges,
aqueous or oily suspensions, dispersible powders or
granules, emulsions, hard or soft capsules, or syrups
or elixirs. Compositions intended for oral use may
be prepared according to any method known to the art
for the manufacture of pharmaceutical compositions
and such compositions may contain one or more agents
selected from the group consisting of sweetening
agents, flavoring agents, coloring agents and
preserving agents in order to provide pharma-
ceutically elegant and palatable preparation.
Tablets containing the active ingredient in admixture
with non-toxic pharmaceutically acceptable excipients
may also be manufactured by known methods. The
excipients used may be for example, (1) inert diluents
such as calcium carbonate, sodium carbonate, lactose,
calcium phosphate or sodium phosphate; (2) granulating
and disintegrating agents such as corn starch, or
alginic acid; (3) binding agents such as starch,
gelatin or acacia, and (4) lubricating agents such as
magnesium stearate, stearic acid or talc. The
tablets may be uncoated or they may be coated by
known techniques to delay disintegration and
absorption in the gastrointestinal tract and thereby
provide a sustained action over a longer period. For
example, a time delay material such as glyceryl
monostearate or glyceryl distearate may be employed.
They may also be coated by the techniques described
1 3383 1 9
4114S/1037A - 14 - 17501
in the U.S. Patents 4,256,108; 4,160,452; and
4,265,874 to form osmotic therapeutic tablets for
controlled release.
In some cases, formulations for oral use may
be in the form of hard gelatin capsules wherein the
active ingredient is mixed with an inert solid
diluent, for example, calcium carbonate, calcium
phosphate or kaolin. They may also be in the form of
soft gelatin capsules wherein the active ingredient
is mixed with water or an oil medium, for example
peanut oil, liquid paraffin, or olive oil.
Aqueous suspensions normally contain the
active materials in admixture with excipients
suitable for the manufacture of aqueous suspensions.
Such excipients may be
(1) suspending agents such as sodium
carboxymethylcellulose, methyl-
cellulose, hydroxypropylmethyl-
cellulose, sodium alginate, polyvinyl-
pyrrolidone, gum tragacanth and gum
acacia;
(2) dispersing or wetting agents which
may be
(a) a naturally-occurring phosphatide
such as lecithin,
(b) a condensation product of an
alkylene oxide with a fatty acid,
for example, polyoxyethylene
stearate,
(c) a condensation product of ethylene
oxide with a long chain aliphatic
alcohol, for example, heptadeca-
ethyleneoxycetanol,
1 3383 1 9
4114S/1037A - 15 - 17501
(d) a condensation product of ethylene
oxide with a partial ester derived
from a fatty acid and a hexitol
such as polyoxyethylene sorbitol
monooleate, or
(e) a condensation product of ethylene
oxide with a partial ester derived
from a fatty acid and a hexitol
anhydride, for example polyoxy-
ethylene sorbitan monooleate.
The aqueous suspensions may also contain one
or more preservatives, for example, ethyl or n-propyl
p-hydroxybenzoate; one or more coloring agents; one
or more flavoring agents; and one or more sweetening
agents such as sucrose or saccharin.
Oily suspension may be formulated by
suspending the active ingredient in a vegetable oil,
for example arachis oil, olive oil, sesame oil or
coconut oil, or in a mineral oil such as liquid
paraffin. The oily suspensions may contain a
thickening agent, for example beeswax, hard paraffin
or cetyl alcohol. Sweetening agents and flavoring
agents may be added to provide a palatable oral
preparation. These compositions may be preserved by
the addition of an antioxidant such as ascorbic acid.
Dispersible powders and granules are suitable
for the preparation of an aqueous suspension. They
provide the active ingredient in admixture with a
dispersing or wetting agent, a suspending agent and
one or more preservatives. Suitable dispersing or
wetting agents and suspending agents are exemplified
by those already mentioned above. Additional excipi-
ents, for example, those sweetening, flavoring and
coloring agents described above may also be present.
1 3383 1 9
4114S/1037A - 16 - 17501
The pharmaceutical compositions of the
invention may also be in the form of oil-in-water
emulsions. The oily phase may be a vegetable oil
such as olive oil or arachis oils, or a mineral oil
such as liquid paraffin or a mixture thereof.
Suitable emulsifying agents may be (1) naturally-
occurring gums such as gum acacia and gum tragacanth,
(2) naturally-occurring phosphatides such as soy bean
and lecithin, (3) esters or partial esters derived
from fatty acids and hexitol anhydrides, for example,
sorbitan monooleate, (4) condensation products of
said partial esters with ethylene oxide, for example,
polyoxyethylen-~ sorbitan monooleate. The emulsions
may also contain sweetening and flavoring agents.
Syrups and elixirs may be formulated with
sweetening agents, for example, glycerol, propylene
glycol, sorbitol or sucrose. Such formulations may
also contain a demulcent, a preservative and
flavoring and coloring agents.
The pharmaceutical compositions may be in
the form of a sterile injectable aqueous or
oleagenous suspension. This suspension may be
formulated according to known methods using those
suitable dispersing or wetting agents and suspending
agents which have been mentioned above. The sterile
injectable preparation may also be a sterile
injectable solution or suspension in a non-toxic
parenterally-acceptable diluent or solvent, for
example as a solution in 1,3-butane diol. Among the
acceptable vehicles and solvents that may be employed
are water, Ringer's solution and isotonic sodium
chloride solution. In addition, sterile, fixed oils
` -
1 3383 1 9
4114S/1037A - 17 - 17501
are conventionally employed as a solvent or suspend-
ing medium. For this purpose any bland fixed oil may
be employed including synthetic mono- or diglycer-
ides. In addition, fatty acids such as oleic acid
find use in the preparation of injectables.
A compound of (I) may also be administered
in the form of suppositories for rectal administra-
tion of the drug. These compositions can be prepared
by mixing the drug with a suitable non-irritating
excipient which is solid at ordinary temperatures but
liquid at the rectal temperature and will therefore
melt in the rectum to release the drug. Such
materials are cocoa butter and polyethylene glycols.
For topical use, creams, ointments, jellies,
solutions or suspensions, etc., containing the
immunoregulants are employed.
The amount of active ingredient that may be
combined with the carrier materials to produce a
single dosage form will vary depending upon the host
treated and the particular mode of administration.
For example, a formulation intended for the oral
administration of humans may contain a therapeutically
sufficient amount of active agent compounded with an
appropriate and convenient amount of carrier material
which may vary from about 5 to about 95 percent of
the total composition.
The specific dose level for any particular
patient will depend upon a variety of factors
including the activity of the specific compound
employed, the age, body weight, general health, sex,
diet, time of administration, route of
administration, rate of excretion, drug combination
and the severity of the particular disease undergoing
therapy.
-
1 3383 1 9
4114S/1037A - 18 - 17501
G. Biological evidence in support of utility of the
compounds within the scope of the invention
It has been found that the compounds of
formula (I) have immunosuppressive activities and are
thereby useful in the treatment of various
~autoimmune" and chronic inflammatory diseases. They
may also be useful in the prevention of graft
rejection or rejection of "donor" organs in
transplantation operations. The following tables
illustrate and support the utility of the compounds
of the present invention:
In this in vitro nephrotoxicity assay, 8-(2-deutero-3-
fluoro-D-alanine)-cyclosporin A was less toxic than
cyclosporin A over the relatively narrow dose range
at which solubility permitted testing.
In Vitro Nephrotoxicity Assay
A sample of 2-deutero-3-fluoro-D-alanine was
evaluated in the in vitro nephrotoxicity assay which
utilizes freshly prepared proximal tubules from
rabbit as the target tissue, and measures changes in
H-leucine incorporation as a parameter of
toxicity. The purpose of the assay was to determine
the toxicity of the test compounds relative to
cyclosporin A. Previous validation of the assay
using cephalosporin antibiotics has shown that this
assay can accurately predict relative, inherent toxic
potential at the cell level. The only assumption
that needs to be made is that pharmacokinetic/drug
distribution parameters are not substantially
different. The utility of the methodology was
further demonstrated in a comparison of in vivo and
1 3383 1 9
4114S/1037A - 19 - 17501
_ vitro data on thienamycin analogs which showed
that in vitro assay is at least 90% accurate in
predicting in vivo nephrotoxicity relative to a
reference compound.
Suspensions of tubules were exposed to the
test compound at appropriate concentrations for a
total of 23 hours, with a 3H-leucine pulse being
given during the last 3 hours of exposure. Incorpora-
tion of leucine was determined per ug protein; the
specific activity of each test point was graphed as a
percent of control specific activity. In this
experiment, we decided to dose with levels of drug
which appeared to be out of solution in order to
achieve the highest possible dose (assuming that some
of the compound would dissolve and equilibrate during
the 23 hours exposure). As can be seen in the
following table, compound 8-(2-deutero-3-fluoro-D-
alanine)cyclosporin A was less toxic than cyclosporin
A at dose 30 ug/ml:
Compound Dosage Mean Specific Activity
(+ standard error)
Cyclosporin A 30~g/ml 75.1 (10.1)
8-(2-deutero-3- ~ 92.0 (0.3)
fluoro-D-
alanine)-cyclo-
sporin A
Based on the data over the limited range
tested, and assuming that pharmacokinetic factors are
equivalent, it is expected that 8-(2-deutero-3-fluoro-
D-alanine)-cyclosporin A to be less nephrotoxic in
animals than is cyclosporin A.