Note: Descriptions are shown in the official language in which they were submitted.
13~463
Novel alkanoDhenones
The invention relates to novel substituted
alkanophenones of general formula
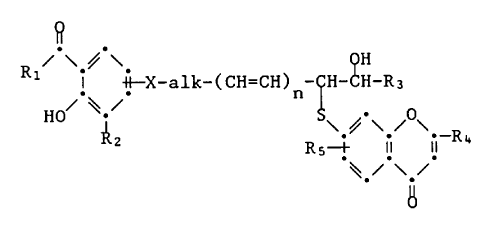
~X-alk-(CH=CH~CH CH R4
in which Rl is lower alkyl or mono-, di- or poly-fluoro-lower
alkyl, R2 is hydrogen, lower alkyl, lower alkenyl or mono-, di- or
poly-fluoro-lower alkyl, X is Cl-C3-alkylene, oxy or thio, alk is
lower alkylene, n is 1 or 2, R3 is phenyl that is unsubstituted or
is substituted by lower alkyl, lower alkoxy, halogen, carboxy,
lower alkoxycarbonyl, amino, N-mono- or N,N-di-lower alkylamino,
carbamoyl, N-mono- or N,N-di-lower alkylcarbamoyl or by
trifluoromethyl, or is lower alkyl, mono-, di- or tri-fluoro-
lower alkyl, carboxy-lower alkyl, lower alkoxycarbonyl-lower
alkyl, carbamoyl-lower alkyl or N-mono- or N,N-di-lower
alkylcarbamoyl-lower alkyl, R4 is carboxy, lower alkoxycarbonyl,
5-tetrazolyl, carbamoyl, N-mono or N,N-di-lower alkylcarbamoyl, or
N-(benzenesulfonyl)-carbamoyl that is unsubstituted or substituted
in the phenyl moiety by lower alkyl, lower alkoxy, halogen or by
trifluoromethyl, and R5 is hydrogen or lower alkyl, and their
salts, to processes for their preparation, to pharmaceutical
preparations containing them as active ingredient, and to their
use as active ingredients in medicaments.
L.~
. .
1340~3
la
The spatial arran~ement shown in the above formula I for
the preferred compounds in which the O atom of the hydroxy group
is in the relative trans-configuration with the S atom is to be
understood as follows: the symbols in the first line lie above
the plane of the drawing and the symbols in the third line
therefore lie below the plane of the drawing (or vice versa),
which for the formula shown corresponds to the opposite
configuration (RS)-(SR), according to the Kahn-Ingold-Prelog
,.:;..,
1340~63
-- 2 --
convention at the carbon atom bonded to the sulphur atom, (C-S-), and
the carbon atom carrying the hydroxy group, (C-OH). When n is 2, the
enantiomers having the S(C-S-), R(C-OH)-configuration and, when n is 1,
the enantiomers having the R(C-S-), S(C-OH)-configuration are espec-
ially preferred. In a vinylene or buta-1,3-dienylene radical repre-
sented by the symbol -(CH=CH~-, the double bond or the double bond
of the butadienylene radical originating from the carbon atom bonded to
the radical alk is preferably, but not necessarily, in the cis-con-
figuration, usually designated (Z), the other double bond then prefer-
ably, but again not necessarily, having the trans-configuration,
usually designated (E).
Unsubstituted or fluorinated lower alkyl is lower alkyl or mono-, di-
or poly-fluoro-lower alkyl.
Etherified or esterified hydroxy is, for example, lower alkoxy or
halogen, respectively.
Unsubstituted or lower alkylated amino is, for example, amino, lower
alkylamino or especially di-lower alkylamino.
Free, esterified or amidated carboxy is carboxy, esterified carboxy,
such as lower alkoxycarbonyl, or amidated carboxy, such as carbamoyl or
N-mono- or N,N-di-lower alkylcarbamoyl or, as R3, N-(benzenesulfonyl)-
carbamoyl that is unsubstituted or substituted, preferably in the
phenyl moiety, by lower alkyl, lower alkoxy and/or by halogen.
N-(benzenesulfonyl)-carbamoyl that is unsubstituted or substituted in
the phenyl moiety as indicated is, for example, unsubstituted or mono-
substituted, preferably in the ortho-position. As a free, esterified or
amidated carboxy substituent of R3, carboxy is especially preferred,
and as R4 esterified carboxy, especially lower alkoxycarbonyl, is
especially preferred.
1340463
Hereinbefore and hereinafter "lower" radicals and compounds are to be
understood as being, for example, those radicals and compounds con-
taining no more than 7 and, unless otherwise indicated, preferably no
more than 4 carbon atoms (C atoms).
Lower alkyl is, for example, Cl-C7alkyl, especially straight-chain
Cl-C4alkyl, such as methyl, ethyl, propyl, isopropyl, butyl or sec.-
butyl, but may also be a branched-chain C1-C4alkyl, such as isobutyl or
tert.-butyl, or a pentyl, hexyl or heptyl radical. Lower alkyl Rl, Rs
and as a substituent of phenyl or N-(benzenesulfonyl)-carbamoyl is
preferably Cl-C4alkyl, for example methyl; lower alkyl R2 is preferably
C2-C5alkyl, for example propyl, and lower alkyl R3 is preferably C3-C7-
alkyl, for example propyl, butyl or pentyl.
Mono-, di- or poly-fluoro-lower alkyl has, for example, up to and
including 5 fluorine atoms and is, for example, mono-, di- or tri-
fluoro-Cl-C7alkyl, especially ~-fluoro- or ~ -trifluoro-Cl-C4alkyl,
such as trifluoromethyl, 2,2,2-trifluoroethyl or 3,3,3-trifluoropropyl.
Fluorinated lower alkyl Rl and as a substituent of phenyl R3 is espec-
ially trifluoromethyl, and fluorinated lower alkyl R3 is preferably
~ -trifluoro-C2-C4alkyl, for example 3,3,3-trifluoropropyl.
Lower alkenyl Rz is, for example, Cz-C4alkenyl, such as vinyl, prop-l-
enyl or especially prop-2-enyl (allyl).
Lower alkylene is, for example, straight-chain Cl-C7alkylene, and in
the case of X especially Cl-C3alkylene, such as methylene or ethylene,
and in the case of alk especially C2-C6alkylene, such as ethylene,
1,3-propylene, 1,4-butylene, also 1,5-pentylene or 1,6-hexylene.
Lower alkoxy is, for example, Cl-C4alkoxy, such as methoxy.
Lower alkoxycarbonyl is, for example, Cl-C4alkoxycarbonyl, such as
methoxy-, ethoxy-, propoxy- or butoxy-carbonyl.
- 4 - 13401~3
Lower alkylamino is, for example, Cl-C4alkylamino, such as methyl-,
ethyl-, propyl- or isopropyl-amino.
Di-lower alkylamino is, for example, di-Cl-C4alkylamino, such as
dimethylamino, diethylamino or N-ethyl-N-methylamino.
N-mono- or N,N-di-lower alkylcarbamoyl is, for example, N-Cl-C4alkyl-
or N,N-di-Cl-C4alkyl-carbamoyl, such as N-methyl-, N-ethyl- or N,N-di-
methyl-carbamoyl.
Halogen is, for example, halogen having an atomic number of up to and
including 35, such as fluorine, chlorine or bromine.
Most of the compounds of formula I can, depending upon their individualcharacter, also be in the form of salts. Those compounds which have
sufficient acidity, such as, especially, those having carboxy, tetra-
zolyl or sulfamoyl groups, can form salts with bases, such as, espec-
ially, inorganic bases, preferably physiologically tolerable alkali
metal salts, especially sodium and potassium salts. However, ammonium
salts with ammonia or physiologically tolerable organic amines, such as
mono-, di- or tri-lower alkylamines, for example diethylamine, mono-,
di- or tri-(hydroxyalkyl)-amines, such as tris(hydroxymethyl)-methyl-
amine, or D-glucosamine, also come into consideration.
The compounds of formula I and their salts exhibit advantageous pharma-cological properties, especially a pronounced leucotriene-antagonism.
For example, in vitro in a concentration range of approximately from
0.001 to 1.0 ~moltl, they inhibit the contraction of a smooth muscle
induced by leucotriene-D4 (LTD4). This so-called LTD4-antagonism is
detected experimentally, for example, as follows: in segments which
have been removed from the ileum of a guinea pig weighing 300-400 g and
which have been incubated in an organ bath in Tyrode's solution at 38~C
and while being gassed with a mixture of 95 % oxygen and 5 ~/0 carbon
dioxide at a load of 1 g, contractions are induced with synthetic
leucotriene D4 (in potassium salt form) and are registered isotonic-
. . . , _ ~
~ 5 ~ 1 ~ 40 4 6 3
ally. The extent of the inhibition by the test compound is detectedafter a preliminary incubation of 2 minutes and is evaluated as IC50,
that is to say the concentration which reduces the test contraction by
50 %. The compounds of formula I also have excellent activity in vivo.
In addition, they have a relatively long duration of action which is a
very significant advantage both specifically and therapeutically. For
example, in an in vivo bronchoconstriction standard test on guinea
pigs, with aerosol administration of a solution containing from 0.0001
to 1 % by weight of the test compound, a marked LTD4-antagonistic
effect was demonstrated. (A description of the test method can be found
in the appendix after the Examples).
Surprisingly, many compounds of formula I also exert a pronounced
inhibitory action on other physiologically important enzyme systems.
For example, the inhibition of phospholipase Az obtained from human
leucocytes was observed in the tested concentration range of approxi-
mately 0.5-50 ~mol/l. (The experimental procedure for this determi-
nation is described in more detail in the appendix after the Examples.)
Likewise, the inhibition of phospholipase C obtained from human
thrombocytes was observed in the tested concentration range of approxi-
mately l-lOO ~mol/l.
Owing to these valuable pharmacological properties, the compounds of
formula I according to the invention can be used therapeutically in all
cases where the action of leucotrienes results in pathological con-
ditions, and alleviate or eliminate these conditions. Accordingly, they
can be used, for example, for the treatment of allergic conditions and
diseases, such as, especially, asthma, but also hay fever and
obstructive pulmonary diseases, including cystic fibrosis. Owing to
their anti-inflammatory activity, they are also suitable as
inflammation-inhibiting agents, especially as external (topical) skin
phlogistatics for the treatment of inflammatory dermatoses of any
origin, as in mild skin irritations, contact dermatitis, exanthemas and
burns, and also as mucous membrane phlogistatics for the treatment of
inflammation of the mucosa, for example of the eyes, nose, lips, mouth
and genital or anal region. They can also be used as sun screens. The
... ..
1340 1~
-- 6 --
high inhibitory effect on various blood factors also points to the
possibility of the therapeutic use of the compounds of formula I where
thrombosis and blood coagulation are indicated.
The invention relates especially to compounds of formula I in which R
is lower alkyl or mono-, di- or poly-fluoro-lower alkyl, R2 is
hydrogen, lower alkyl, lower alkenyl or mono-, di- or poly-fluoro-lower
alkyl, X is lower alkylene, oxy or thio, alk is lower alkylene, R3 is
phenyl that is unsubstituted or is substituted by lower alkyl, lower
alkoxy, halogen, carboxy, lower alkoxycarbonyl, amino, N-mono- or
N,N-di-lower alkylamino, carbamoyl, N-mono- or N,N-di-lower alkyl-
carbamoyl and/or by trifluoromethyl, or is lower alkyl, mono-, di- or
tri-fluoro-lower alkyl, carboxy-lower alkyl, lower alkoxycarbonyl-lower
alkyl, carbamoyl-lower alkyl or N-mono- or N,N-di-lower alkylcarbamoyl-
lower alkyl, R4 is carboxy, lower alkoxycarbonyl, 5-tetrazolyl,
carbamoyl, N-mono- or N,N-di-lower alkylcarbamoyl, or N-(benzene-
sulfonyl)-carbamoyl that is unsubstituted or substituted in the phenyl
moiety by lower alkyl, lower alkoxy, halogen and/or by trifluoromethyl,
and Rs is hydrogen or lower alkyl, and to their salts, especially
pharmaceutically acceptable salts.
The invention relates especially, for example, to those compounds of
formula I in which Rl is lower alkyl, R2 is unsubstituted or
fluorinated lower alkyl or lower alkenyl, and X, R3, R4 and R5 are as
defined above, and to their salts, especially pharmaceutically
acceptable salts.
The invention relates preferably to those compounds in which the group
X is bonded in the para-position to the R1-C(=O) group, that is to say
compounds of formula
. . .
_ 7 - 1 39 0~ ~3
R .
R 1 i ~ \ .
HO/ ~ -alk-~CH=CH~-TH-8H-R3 (Ia),
z\ ~-\ /0\
Rs-+ il il-R4
~~./-\./-
in which Rl, Rz, X, alk, n, R3, R4 and Rs are as defined above, but
preferably Rl is lower alkyl, R2 is unsubstituted or fluorinated lower
alkyl or lower alkenyl, and/or phenyl R3 is preferably substituted as
indicated, and to their salts, especially pharmaceutically acceptable
salts.
The invention relates more especially to compounds of formula I and Ia
in which Rl is Cl-C4alkyl, such as methyl, or ~ -trifluoro-Cl-C4-
alkyl, such as trifluoromethyl, R2 is Cl-C4alkyl, such as propyl,
C2-C4alkenyl, such as alkyl, ~ -trifluoro-Cl-C4alkyl, such as
3,3,3-trifluoropropyl, or secondly hydrogen, X is Cl-C3alkylene, such
as methylene, oxy or thio, alk is straight-chain C2-C6alkylene, such as
ethylene, 1,3-propylene or 1,4-butylene, n is 1 or 2, R3 is phenyl that
is unsubstituted or substituted by Cl-C4alkyl, such as methyl, Cl-C4-
alkoxy, such as methoxy, halogen having an atomic number of up to and
including 35, such as chlorine or bromine, trifluoromethyl, carboxy
and/or by Cl-C4alkoxycarbonyl, such as methoxycarbonyl, or is Cl-Cg-
alkyl, such as propyl or butyl, ~ -trifluoro-C2-Csalkyl, such as
3,3,3-trifluoropropyl or 4,4,4-trifluorobutyl, carboxy-C2-Csalkyl, such
as 3-carboxypropyl or 4-carboxybutyl, or Cl-C4alkoxycarbonyl-C2-Cs-
alkyl, such as 3-methoxycarbonylpropyl or 4-methoxycarbonylbutyl, R4 is
carboxy or N-(benzenesulfonyl)-carbamoyl, and Rs is hydrogen, and when
n is 1, the chain carbon atom bonded to the sulfur atom preferably has
the (R)-configuration and the chain carbon atom bonded to the hydroxy
group preferably has the (S)-configuration or, when n is 2, the chain
carbon atom bonded to the sulfur atom preferably has the (S)-con-
figuration and the chain carbon atom carrying the hydroxy group prefer-
ably has the (R)-configuration and the double bond joined to the
- 8 - 13~ 0 4 63
radical alk is preferably in the cis-configuration and the additional
double bond which may be present is preferably in the trans-con-
figuration, and preferably to those compounds- in which R1 is C1-C4alkyl
and R2, X, R3, R4 and Rs are as defined above and to their salts,
especially pharmaceutically acceptable salts.
The invention relates especially, for example, to compounds of formula
I and Ia in which R1 is C1-C4alkyl, such as methyl, Rz is C1-C4alkyl,
such as propyl, C2-C4alkenyl, such as alkyl, or ~ -trifluoro-C1-C4-
alkyl, such as 3,3,3-trifluoropropyl, X is C1-C3alkylene, such as
methylene, oxy or thio, alk is straight-chain C2-C6alkylene, such as
ethylene, 1,4-butylene or 1,6-hexylene, n is 1 or 2, R3 is a group of
formula -A-R3' in which -A- is C1-C4alkylene, phenylene or a direct
bond and R3' is C1-C4alkyl, such as methyl, trifluoromethyl, carboxy or
C1-C4alkoxycarbonyl, such as methoxycarbonyl, R4 is carboxy or
N-(benzenesulfonyl)-carbamoyl, and Rs is hydrogen, and when n is 1, the
chain carbon atom bonded to the sulfur atom preferably has the (R)-con-
figuration and the chain carbon atom bonded to the hydroxy group
preferably has the (S)-configuration or, when n is 2, the chain carbon
atom bonded to the sulfur atom preferably has the (S)-configuration and
the chain carbon atom carrying the hydroxy group preferably has the
(R)-configuration and the double bond joined to the radical alk is
preferably in the cis-configuration and the additional double bond
which may be present is preferably in the trans-configuration, and to
their salts, especially pharmaceutically acceptable salts.
The invention relates especially to compounds of formula Ia in which R
is C1-C4alkyl, such as methyl, Rz is C1-C4alkyl, such as propyl, X is
oxy, alk is C2-C6alkylene, such as ethylene, 1,3-propylene or
1,4-butylene, n is 1 or preferably 2, R3 is phenyl substituted by
C1-C4alkyl, such as methyl, C1-C4alkoxy, such as methoxy, halogen
having an atomic number of up to and including 35, such as chlorine,
trifluoromethyl or by C1-C4alkoxycarbonyl, such as methoxycarbonyl, or
is C2-Caalkyl, especially C3-Csalkyl, such as propyl or butyl,
-trifluoro-C3-Csalkyl, such as 3,3,3-trifluoropropyl or
4,4,4-trifluorobutyl, or C1-C4alkoxycarbonyl-C1-C4alkyl, such as
1340463
_ 9 _
3-methoxycarbonylpropyl or 4-methoxycarbonylbutyl, R4 is carboxy, and
Rs is hydrogen, and when n is 1, the chain carbon atom bonded to the
sulfur atom preferably has the (R)-configuration and the chain carbon
atom bonded to the hydroxy group preferably has the (S)-configuration
or, when n is 2, the chain carbon atom bonded to the sulfur atom
preferably has the (S)-configuration and the chain carbon atom carrying
the hydroxy group preferably has the (R)-configuration and the double
bond joined to the radical alk is preferably in the cis-configuration
and the additional double bond which may be present is preferably in
the trans-configuration, and to their salts, especially pharma-
ceutically acceptable salts.
The invention relates preferably to compounds of formula Ia in which R
is C1-C4alkyl, such as methyl, R2 is C1-C4alkyl, such as propyl, X is
oxy, alk is C2-C6alkylene, such as ethylene or 1,4-butylene, n is 1 or
preferably 2, R3 is a group of formula -A-R3' in which -A- is C1-C4alk-
ylene, such as ethylene, or phenylene, especially m-phenylene, and R3'
is C1-C4alkyl, such as methyl, trifluoromethyl or C1-C4alkoxycarbonyl,
such as methoxycarbonyl, R4 is carboxy or N-(benzenesulfonyl)-car-
bamoyl, and Rs is hydrogen, and when n is 1, the chain carbon atom
bonded to the sulfur atom preferably has the (R)-configuration and the
chain carbon atom bonded to the hydroxy group preferably has the
(S)-configuration or, when n is 2, the chain carbon atom bonded to the
sulfur atom preferably has the (S)-configuration and the chain carbon
atom carrying the hydroxy group preferably has the (R)-configuration
and the double bond joined to the radical alk is preferably in the cis-
configuration and the additional double bond which may be present is
preferably in the trans-configuration, and to their salts, especially
pharmaceutically acceptable salts.
The invention relates more especially to compounds of formula Ia in
which R1 is C1-C4alkyl, such as methyl, R2 is C1-C4alkyl, such as
propyl, X is oxy, alk is C2-Csalkylene, such as ethylene, 1,3-propylene
or 1,4-butylene, n is 2, R3 is phenyl substituted, especially in the
meta-position, by C1-C4alkyl, such as methyl, trifluoromethyl or C1-C4-
alkoxycarbonyl, such as methoxycarbonyl, or is C3-Csalkyl, such as
1340~i)3
-- ~o --
propyl or butyl, ~ -trifluoro-C3-Csalkyl, such as 3,3,3-trifluoro-
propyl or 4,4,4-trifluorobutyl, or Cl-C4alkoxycarbonyl-C2-C4alkyl, such
as 3-methoxycarbonylpropyi or 4-methoxycarbonylbutyl, R4 is carboxy,
and Rs is hydrogen, the chain carbon atom bonded to the sulfur atom
preferably has the (S)-configuration and the chain carbon atom carrying
the hydroxy group preferably has the (R)-configuration and the double
bond joined to the radical alk is preferably in the cis-configuration
and the other additional double bond is preferably in the trans-con-
figuration, and to their salts, especially pharmaceutically acceptable
salts.
The invention relates specifically to the compounds of formula I
mentioned in the Examples and to their salts, especially pharma-
ceutically acceptable salts.
The process according to the invention for the preparation of compoundsof formula I and their salts is based on methods known per se and is
carried out as follows:
an epoxide of formula
~ .
i *-X-alk~CH=CH) -C~ - CH-R3 (II),
HO/ ~-/
R2
in which Rl, Rz, X, alk, n, A and R3 are as defined above, is reacted
with a thiol of formula
HS\ ~-\ /0\ /R4
Rs-+ i! i! (III),
~./ \./
in which R4 and Rs are as defined above, or with a salt thereof, and,
if desired, a compound obtainable in accordance with the process is
converted into a different compound of formula I, a stereoisomeric
mixture obtainable in accordance with the process is separated into the
components and/or a free compound obtainable in accordance with the
1340~63
process is converted into a salt, or a salt obtainable in accordance
with the process is converted into the free compound or into a
different salt.
In the reaction of epoxides II with thiols III, the configuration at
the carbon atom bonding with the thio group is reversed and the
configuration at the carbon atom carrying the hydroxy group is
retained. In order to obtain the preferred compounds having the
opposite configuration at these two carbon atoms, it is therefore
preferable to use the corresponding trans-epoxides II as starting
materials. Starting from R,R-epoxides II there are obtained compounds I
having the S(C-S-), R(C-OH)-configuration, and starting from
S,S-epoxides II there are obtained compounds I having the R(C-S-),
S(C-OH)-configuration. The reaction is effected under conditions known
per se at a temperature of from approximately -20~C to approximately
+50~C, preferably at room temperature, that is to say from 18~C to
25~C, and especially in a basic medium, for example in the presence of
an amine, especially a tertiary aliphatic, arylaliphatic or saturated
heterocyclic amine, such as a trialkylamine (for example triethylamine
or ethyldiisopropylamine), a dialkylbenzylamine (for example
N,N-dimethylbenzylamine), an N,N-dialkylaniline (for example
N,N-dimethylaniline) or N-methyl- or N-ethyl-piperidine or
N,N'-dimethylpiperazine. The reaction is usually carried out in an
inert organic solvent, such as a lower alkanol, for example methanol or
ethanol.
In a preferred form, components II and III in which R4 is esterified
carboxy or tetrazolyl and R3 is as defined above and is, for example,
esterified carboxy or unsubstituted or fluorinated lower alkyl are used
as starting materials and R4 is hydrolysed (optionally selectively) to
carboxy, which is then converted, if desired, into amidated carboxy.
Starting materials for the process according to the invention are
either known per se or can be obtained in a manner known per se by
known analogy processes.
13404~3
- 12 -
The epoxide of the above-defined formula II used as starting material
can be prepared especially by means of the same processes as those used
in the synthesis of leucotrie.nes. In a typical general method of
synthesis for compounds II in which n is 1, for example, an aldehyde of
formula
O=CH-R3 (IV),
in which A and R3 are as defined above, is used as starting material, a
free carboxy group R3 which may be present being protected in the form
of an ester, for example a lower alkyl ester. This compound is
condensed with formylmethylenetriphenylphosphorane (or an equivalent
reagent), the corresponding trans-3-R3-prop-2-enal of formula
CH~ R3 (V)
being formed. This compound is then epoxidised in a manner known E~er
se, preferably under weakly alkaline conditions (for example in the
presence of alkali metal carbonates), with aqueous hydrogen peroxide,
to produce a trans-, that is to say 2(RS),3(RS)-epoxy-3-R3-propanal of
formula
O=C~ / \ / (VI).
The epoxyaldehyde VI can then be reacted to form the corresponding
epoxide II in which R4 is esterified carboxy and n is 1 by condensation
with a phosphonium halide
R .
Rl ~ *-X-alk-CH2-P(C6Hs)3Hal (VII),
HO/ ~-/
R2
in which Rl, R2 and alk are as defined above and Hal is a halogen atom,
preferably bromine, and with a base, for example sodium amide, in
tetrahydrofuran.
- 13 - 1 34 04 63
Compounds VII are prepared especially by reaction of a corresponding
compound of formula
~ .
R1 i *-X-alk-CH 2 -Hal (VIII)
HO/ ~-/
Rz
with triphenylphosphine in customary manner. Compounds VIII in which X
is oxy or thio are obtained, for example, by condensing with one
another corresponding compounds of formulae
~ .
i *-XH (IX) and Hal-alk-CH2-Hal (X),
HO/ ~-/
in customary manner.
In another method of preparing compounds II, trans-3-R3-prop-2-enol of
formula
CH~ R3 (XI),
in which R3 is as defined above, but free carboxy as a substituent of
R3 is preferably in an ester form, is epoxidised, for example, by means
of tert.-butyl hydroperoxide in the presence of titanium tetraiso-
propanolate and a D- or L-tartaric acid di-lower alkyl ester, and when
a D-tartaric acid ester is used there is obtained predominantly
2R,3R-epoxy-3-R3-propanol XIIa, and when an L-tartaric acid ester is
used there is obtained predominantly the corresponding 2S,3S-epoxy-3-
R3-propanol XIIb
HOCH2\ ~0 ~H HOCHz\ ~0~ ~H
~- ~\ (XIIa) and ~C C (XIIb).
H R3 H \R3
- 14 - 1 3 4 0 ~ 6 3
This compound is then oxidised, for example by treatment with oxalyl
chloride/dimethyl sulfoxide and then with triethylamine, to the
corresponding epoxyaldehyde VI which can then be reacted with the
corresponding phosphonium salt VII to form the corresponding epoxide II
in which R3 is esterified carboxy and n is 1.
In that reaction there are obtained predominantly epoxides II in which
the double bond has the preferred cis-stereoconfiguration. If a
D-tartaric acid ester is used, then, as mentioned above, there are
obtained predominantly compounds II in which the epoxy group has the
R,R-configuration, or S,S-enantiomers when the reaction is carried out
in the presence of L-tartaric acid esters.
For the preparation of epoxides II in which n is 2, for example the
epoxy alcohol XIIa or XIIb is first converted by treatment with
N,N'-dicyclohexylcarbodiimide and dimethyl sulfoxide in the presence of
trifluoroacetic acid and pyridine and then with triphenylphosphoran-
ylideneacetaldehyde into the corresponding 4R,5R- or 4S,5S-4,5-epoxy-
5-R3-pent-2-enal of formula XIIIa or XIIIb, respectively
O=HC-CH=CH\ O ~H O=HC-CH=CH\ ~0~ H
~C - C\ (XIIIa) or " ( b)
which is then reacted further with the phosphonium halide VII to form
the corresponding epoxide II in which n is 2. It is preferable to
obtain those epoxides in which the double bond joined to the radical
alk has cis-stereo-configuration and the double bond joined to the
oxirane ring has trans-stereo-configuration.
Compounds obtainable in accordance with the process can, if desired, beconverted into other compounds of formula I.
For example, esterified or amidated carboxy groups can be hydrolysed tofree hydroxy, preferably under basic conditions, for example in the
presence of sodium hydroxide solution, and preferably in a water-
miscible organic solvent, such as tetrahydrofuran, dioxane or a lower
alkanol, such as methanol or ethanol. Starting from compounds I in
13404~3
which R4 is esterified carboxy, such as lower alkoxycarbonyl, and R3
contains such a group as substituent, the hydrolysis can be controlled
in such a manner that selectively only R4 or both R4 and the lower
alkoxycarbonyl substituent of R3 are hydrolysed to carboxy. If an
equimolar sodium hydroxide solution is used and mild reaction con-
ditions are chosen, for example stirring at room temperature for about
0.5 to 2 hours, virtually only alkoxycarbonyl R4 is hydrolysed, whilst
under extreme conditions, for example with prolonged reaction periods
or with heating, both R4 and the alkoxycarbonyl group in R3 are
hydrolysed to carboxy.
Conversely, carboxy R4 and a carboxy substituent of R3 can be
esterified in customary manner.
Furthermore, free or esterified carboxy R4 and such a group as a sub-
stituent of R3 can be amidated in customary manner, for example by
treatment with ammonia or with a mono- or di-lower alkylamine. For
example, carboxy R4 can be converted in customary manner, for example
in the presence of a carbodiimide salt, for example N-ethyl-N'-(3-di-
methylaminopropyl)-carbodiimide hydrochloride, and 4-dimethylamino-
pyridine, with an unsubstituted or substituted benzenesulfonamide into
the corresponding N-benzenesulfamidoylcarbamoyl groups.
Of course, it is also possible to separate resulting diastereoisomeric
mixtures into the individual components on the basis of the different
physical properties of the components and/or to separate resulting
mixtures of enantiomers into the individual enantiomers according to
customary racemate separation processes.
If individual diastereoisomers are desired, then advantageously an
individual diastereoisomer of a starting material can be used at any
stage or one diastereoisomer can be formed preferentially from a
starting material in diastereoisomer form by means of stereoselective
reaction conditions or optically active reagents, or racemic
13~04~3
- 16 -
diastereoisomeric mixtures can be separated into the individual
diastereoisomers by physical separation methods, optionally using
optically ac.ive auxiliaries.
From the stereochemical standpoint, however, both the condensation
according to the invention of components II and III and the preparation
of the starting materials are preferably carried out using starting
materials that are uniform in stereo-configuration in each case, where
possible carrying out the reactions stereoselectively, for example by
the use of configuratively uniform, optically active reagents and/or
auxiliaries, and isolating configuratively uniform products from
reaction mixtures immediately after the reaction. For example, in the
preparation of the unsaturated starting materials, cis- and
trans-isomers which may be formed are separated from one another
immediately, for which purpose the customary physical separation
methods, such as, especially, chromatography, are suitable. In the main
reaction there is used especially the stereoisomeric epoxide II having
the stereoconfiguration of the double bond(s) that is preferred in the
end product and in racemic form (which is often formed in the variant
of the epoxidisation of the compound V with hydrogen peroxide) or
preferably in the form of an individual diastereoisomer in which the
configuration at the oxirane carbon atom making a bond with the S atom
is opposite to the configuration at the (C-S-) carbon atom preferred in
the end product I.
Likewise, resulting salts can be converted, for example by treatment
with an acid, into the free acids, and resulting free acids can be
converted by treatment with a base into salts.
As a result of the close relationship between the novel compounds in
free form and in the form of their salts, hereinbefore and hereinafter
the free compounds and their salts should be understood, where
appropriate, as meaning also the corresponding salts and free
compounds, respectively.
- 17 - 13 ~ O ~ ~ 3
The invention relates also to those forms of the process according to
which a compound obtainable as intermediate at any stage of the process
is used as starting material and the remaining steps are carried out or
a starting material is used in the form of a salt or is formed under
the reaction conditions.
The invention relates also to the novel starting materials and
intermediates occurring in the processes according to the invention and
their preliminary stages.
Preferably, the starting materials used and the reaction conditions
chosen are such that the compounds listed above as being especially
preferred are obtained.
The present invention relates also to pharmaceutical preparations and
medicaments that contain one of the compounds of formula I according to
the invention or a pharmaceutically acceptable salt thereof. The
pharmaceutical preparations according to the invention are especially
those which are intended for local administration and especially for
administration by inhalation, for example in the form of an aerosol, a
micronised powder or a fine spray solution, to mammals, especially
humans, and which contain the active ingredient on its own or together
with a pharmaceutically acceptable carrier.
Pharmaceutical preparations for topical and local use are, for example,for the treatment of the skin, lotions and creams which contain a
liquid or semi-solid oil-in-water or water-in-oil emulsion, and
ointments (which preferably contain a preservative). Suitable for the
treatment of the eyes are eye drops which contain the active ingredient
in aqueous or oily solution, and eye ointments which are preferably
manufactured in sterile form. Suitable for the treatment of the nose
are aerosols and sprays (similar to those described below for the
treatment of the respiratory tract), coarse powders which are
administered by rapid inhalation through the nostrils, and especially
nose drops which contain the active ingredient in aqueous or oily
solution; suitable for local treatment of the buccal cavity are
1340~3
- 18 -
lozenges which contain the active ingredient in a mass generally formed
of sugar and gum arabic or tragacanth, to which flavourings may be
added, and pastilles which contain the active ingredient in an inert
mass, for example of gelatine and glycerine or sugar and gum arabic.
Pharmaceutical preparations suitable for administration in the form of
aerosols or sprays are, for example, solutions, suspensions or
emulsions of the compound of formula I according to the invention with
a suitable pharmaceutically acceptable solvent, such as, especially,
ethanol and water, or a mixture of such solvents. They may, as
necessary, contain other pharmaceutical adjuncts, such as non-ionic or
anionic surface-active agents, emulsifiers and stabilisers, and also
active ingredients of other kinds, and especially advantageously they
can be mixed with a propellant gas, such as an inert gas under elevated
pressure or especially with a readily volatile liquid, preferably a
liquid that boils under normal atmospheric pressure below customary
room temperature (for example from approximately -30 to +10~C), such as
an at least partially fluorinated polyhalogenated lower alkane, or a
mixture of such liquids. Such pharmaceutical preparations, which are
used predominantly as intermediates or stock mixtures for the
preparation of the corresponding medicaments in finished form, contain
the active ingredient customarily in a concentration of from
approximately 0.1 to approximately 10 % by weight, especially from
approximately 0.3 to approximately 3 % by weight. For the preparation
of medicaments in finished form, such a pharmaceutical preparation is
introduced into suitable containers, such as flacons and pressurised
bottles, which are provided with a spray device or valve suitable for
such purposes. The valve is preferably constructed in the form of a
metering valve which on operation releases a predetermined amount of
liquid, corresponding to a predetermined dose of the active ingredient.
In the preparation of the finished medicament form, it is also possible
for corresponding amounts of the pharmaceutical preparation in stock
solution form and of the propellant to be introduced separately into
the containers and to be mixed with one another only at that stage. The
dosage of the compound of formula I to be administered and the
frequency of administration depend upon the effectiveness and the
.. . . ... .. ....
- lg - 1340 163
duration of action of each individual compound, upon the severity of
the disease to be treated and its symptoms, and upon the sex, age,
weight and individual responsiveness of the mammal to be treated. On
average, the recommended daily dose of a compound of formula I
according to the invention for a mammal (especially a human) weighing
75 kg might be in the region of from approximately 10 to approximately
500 mg, preferably from approximately 25 to approximately 250 mg, which
can advantageously be administered in several doses per day, as
necessary.
The invention relates also to the use of the active ingredients of
formula I according to the invention for alleviating or eliminating
pathological conditions and/or symptoms of the body of a mammal,
especially of a human, which can be attributed to the action of
leucotrienes and occur especially in asthma. This use or the
corresponding curative method comprises the treatment of the affected
body or part of the body with an anti-allergically effective amount of
a compound of formula I on its own or in the form of a medicament,
especially a pharmaceutical preparation intended for inhalation. The
expression "an anti-allergically effective amount" is to be understood
as being that amount of the active ingredient which is sufficient to
produce a significant inhibition of the contractions caused by
leucotrienes.
The following Examples illustrate the present invention in more detail
but do not limit the scope thereof. All temperatures are given in
degrees Celsius.
~xample 1: (lR,2S)-1-hydroxy-1-(3-trifluoromethylphenyl)-8-(4-acetyl-
3-hydroxy-2-propylphenoxy)-octa-3(E),5(Z)-dien-2-yl-7-thio-
4-oxo-4H-1-benzopyrane-2-carboxylic acid methyl ester
A solution of 0.93 g of (lR,2R)-1,2-epoxy-1-(3-trifluoromethylphenyl)-
8-(4-acetyl-3-hydroxy-2-propylphenoxy)-octa-3(E),5(Z)-diene in 25 ml of
methanol is stirred under argon with 0.80 g of triethylamine and 0.62 g
of 7-mercaptochromone-2-carboxylic acid methyl ester for 20 hours at
room temperature and concentrated by evaporation. The residue is
13404b3
- 20 -
dissolved in ethyl acetate and filtered over silica gel. The filtrate
is washed once with 2N hydrochloric acid and 3 times with brine, dried
over magnesium sulfate and concentrated by evaporation. Purification of
the residue by chromatography on silica gel with hexane/ethyl acetate
(1:1) yields the title compound having a melting point of 62-63~;
[~]D~ (methanol, 0.135 %) = 103 + 7.4~; UV (methanol): ~ (E) = 216
(50,000), 235/sh, 271 (27,940), 285/sh; 325 (12900).
The starting material is prepared, for example, as follows:
a) (2R,3R)-2,3-epoxy-3-(3-trifluoromethylphenyl)-propanol
Under totally anhydrous conditions and an argon atmosphere, a solution
of 4.62 ml of tetraisopropyl orthotitanate in 100 ml of methylene
chloride is cooled to -70~, and 3.2 ml of D(-)-tartaric acid diethyl
ester and 5.45 g of 3-(3-trifluoromethylphenyl)-prop-2(E)-enol in a
small amount of methylene chloride are added. After stirring for
10 minutes at -70~, 21.5 ml of 3-molar tert.-butyl hydroperoxide
solution in toluene are added, the temperature rising to -60~C. The
temperature is allowed to rise to 0~ within a period of 2 hours, and
the resulting yellow solution is poured slowly into a solution of
14.5 g of iron(II) sulfate and 5.8 g of L(+)-tartaric acid in 60 ml of
water (cooling!, exothermic) and the mixture is stirred for 30 minutes
at 5-10~. The aqueous phase is separated off and extracted with ether.
The combined organic extracts are dried over sodium sulfate and concen-
trated by evaporation. The residue is dissolved in 90 ml of ether,
cooled to 0-5~, and a suspension of 2.32 g of sodium hydroxide in 60 ml
of brine is added and the mixture is stirred for 1 hour at 0-5~. The
aqueous phase is separated off and extracted with ether. The combined
ether phases are dried over sodium sulfate and concentrated by
evaporation. The residue is purified by chromatography on silica gel
with hexane/ethyl acetate (3:2). The title compound is thus obtained in
the form of a colourless oil;
IR (CH2Cl2): 3550, 3430, 2950, 2880, 2830, 1310, 1150, 1110, 1050 cm
[~]D~ (methanol, 0.175 %) = 42.3 + 5.7~; Rf = 0.30 (hexane/ethyl
acetate 3:2).
. . ~ . . .
13404~3
- 21 -
b) (4R,5R)-4,5-epoxy-5-(3-trifluoromethylphenyl)-pent-2(E)-enal
A solution of 4.5 g of (2R,3R)-2,3-epoxy-3-(3-trifluoromethylphenyl)-
propanol in 105 ml of dimethyl sulfoxide is stirred under argon with
1.7 ml of pyridine, 0.77 ml of trifluoroacetic acid and 12.75 g of
N,N-dicyclohexylcarbodiimide for 6 hours at room temperature. After the
addition of 8.25 g of formylmethylenetriphenylphosphorane, stirring is
continued for a further 20 hours at room temperature; 320 ml of ethyl
acetate are added and after 10 minutes the mixture is poured onto
320 ml of brine. The resulting suspension is stirred for 5 minutes and
filtered. In the filtrate the aqueous phase is extracted twice with
ethyl acetate. The combined organic phases are washed 3 times with
brine, dried over sodium sulfate and concentrated by evaporation. The
residue is filtered over silica gel with ether/hexane = (4:1). The
filtrate is concentrated by evaporation and the residue is purified by
chromatography on silica gel with hexane/ethyl acetate (3:1). The title
compound is thus obtained in the form of a light-yellow oil;
IR (CHzC12): 2780, 2695, 1670, 1620, 1305, 1145, 1105 cm ; Rf = 0.31
(hexane/ethyl acetate 3:1) [~]D~ (chloroform, 0.245 %) =
144.5 + 4.1~.
c) 3-(4-acetyl-3-hydroxy-2-propylphenoxy)-propyl-triphenylphosphonium
bromide
A solution of 27 g of 3-(4-acetyl-3-hydroxy-2-propylphenoxy)-propyl
bromide in 50 ml of toluene is heated at reflux with 21.85 g of tri-
phenylphosphine for 20 hours. The resulting suspension is cooled to
room temperature; 200 ml of ether are added and the mixture is stirred
for 1 hour. The colourless precipitate is filtered off with suction,
washed with ether and dried. The title compound has a melting point of
211-212~.
d) (lR,2R)-1,2-epoxy-1-(3-trifluoromethylphenyl)-8-(4-acetyl-3-hydroxy-
2-propylphenoxy)-octa-3(E),5(Z)-diene
A suspension of 5.55 g of 3-(4-acetyl-3-hydroxy-2-propylphenoxy)-
propyl-triphenylphosphonium bromide in 80 ml of tetrahydrofuran is
stirred under argon with 0.78 g of NaNHz and 60 mg of potassium tert.-
butanolate for 1 hour at room temperature, then cooled to 0-5~; 1.7 g
1340~3
- 22 -
of (4R,5R)-4,5-epoxy-5-(3-trifluoromethylphenyl)-pent-2(E)-enal in
20 ml of tetrahydrofuran are added within a period of 5 minutes and the
mixture is then stirred for 2 hours at room temperature. The resulting
suspension is poured onto phosphate buffer (pH 7) and extracted with
ether. The combined ether extracts are washed with phosphate buffer
pH 7, dried over sodium sulfate and concentrated by evaporation. The
residue is taken up in hexane/ethyl acetate/triethylamine (24:71:5) and
filtered over silica gel that has been prewashed with that solvent
mixture. The filtrate is concentrated by evaporation and yields the
title compound in the form of a light-yellow oil; Rf = 0.75 (hexane/
ethyl acetate 3:2).
~xample 2: Sodium salt of (lR,2S)-1-hydroxy-1-(3-trifluoromethyl-
phenyl)-8-(4-acetyl-3-hydroxy-2-propylphenoxy)-octa-
3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-l-benzopyrane-2-
carboxylic acid
0.7 g of (lR,2S)-l-hydroxy-1-(3-trifluoromethylphenyl)-8-(4-acetyl-3-
hydroxy-2-propylphenoxy)-octa-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-l-
benzopyrane-2-carboxylic acid methyl ester is dissolved under argon in
20 ml of tetrahydrofuran; 5.1 ml of 0.2N sodium hydroxide solution are
added and the mixture is stirred for 1 hour at room temperature.
Concentration by evaporation and purification of the residue by
chromatography on a "Reversed Phase" silica gel column (for example
Merck Lichroprep~ RP-8) with methanol/water (3:1) yield the title
compound, m.p. 207-209~, [~]D~ (0-54 ~o- methanol) = 96.3 + 1.9~
UV (methanol): ~max (~) = 220 (488840), 235/sh, 267 (25940), 285
(22900), 324/sh.
~xample 3: (lS,2R)-l-hydroxy-1-(3-trifluoromethylphenyl)-8-(4-acetyl-3-
hydroxy-2-propylphenoxy)-octa-3(E),5(Z)-dien-2-yl-7-thio-4-
oxo-4H-l-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from (lS,2S)-
1,2-epoxy-1-(3-trifluoromethylphenyl)-8-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-octa-3(E),5(Z)-diene; m.p. 68-69~.
~he starting material is prepared, for example, as follows:
. ~ .
13~04~3
- 23 -
a) (2S,3S)-2,3-epoxy-3-(3-trifluoromethylphenyl)-propanol
The title compound is prepared as described in Example la) but using
L(+)-tartaric acid diethyl ester; colourless oil; IR (CH2C12): 3590,
3480, 2920, 2870, 1330, 1165, 1125, 1070 cm ; [~]D~ (methanol,
0.175 %) = -41.7 + 5.7~; Rf = 0.34 (hexane/ethyl acetate 1:1).
b) (4S,5S)-4,5-epoxy-5-(3-trifluoromethylphenyl)-pent-2(E)-enal
The title compound is prepared analogously to Example lb) from the
epoxy alcohol according to a); light-yellow oil; IR (CH2Cl2): 2780,
2695, 1670, 1620, 1305, 1145, 1110 cm ; [~]D~ (chloroform,
0.15 %) = -158.0 + 6.7~; Rf = 0.4 (hexane/ethyl acetate 4:1).
c) (lS,2S)-1,2-epoxy-1-(3-trifluoromethylphenyl)-8-(4-acetyl-3-hydroxy-
2-propylphenoxy)-octa-3(E),5(Z)-diene
The title compound is prepared analogously to Example ld) from the
epoxyaldehyde according to b); light-brown oil; Rf = 0.61 (hexane/ethyl
acetate 3:2).
~xample 4: Sodium salt of (lS,2R)-1-hydroxy-1-(3-trifluoromethyl-
phenyl)-8-(4-acetyl-3-hydroxy-2-propylphenoxy)-octa-
3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-1-benzopyrane-2-car-
boxylic acid
The title compound is prepared analogously to Example 2 from the
corresponding methyl ester according to Example 3; m.p. 210-212~,
[~]D~ (MeOH, 0.18 %) = -86.1 + 5.6~
UV (MeOH): ~ (~) = 220 (42820); 235/sh; 267 (26660); 285 (23760); 320
(15800).
~xample 5: (lR,2S)-1-hydroxy-1-(3-trifluoromethylphenyl)-6-(4-acetyl-3-
hydroxy-2-propylphenoxy)-hex-3(Z)-en-2-yl-7-thio-4-oxo-4H-1-
benzopyranecarboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from (lR,2R)-
1,2-epoxy-1-(3-trifluoromethylphenyl)-6-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-hex-3(Z)-ene; light-yellow viscous oil; [~]D~ (MeOH,
0.115 %) = 57.4 + 8.7~;
~ . . .. .
13~04~3
- 24 -
UV (MeOH): ~max(E) = 220/sh; 271 (5280); 285/sh; 320 (2800).
The starting material is p-epared, for example, as follows:
a) (2S,3R)-2,3-epoxy-3-(3-trifluoromethylphenyl)-propanal
A solution of 1.1 g of oxalyl chloride in 15 ml of methylene chloride
is cooled to -65-70~ under argon and within a period of 2 minutes 1.5 g
of dimethyl sulfoxide in 5 ml of methylene dichloride are added. After
stirring for 10 minutes at -65-70~, 1.7 g of (2R,3R)-2,3-epoxy-3-(3-
trifluoromethylphenyl)-propanol in 15 ml of methylene chloride are
added dropwise. After stirring for a further 30 minutes, 4 g of tri-
ethylamine are added dropwise, the temperature rising to -40~. The
temperature is allowed to rise to 0~ and the reaction mixture is poured
onto phosphate buffer (pH 8). The organic phase is separated off and
the aqueous phase is extracted with methylene chloride. The combined
organic extracts are washed twice with brine, dried over sodium sulfate
and concentrated by evaporation. Chromatography of the residue on
silica gel with hexane/ethyl acetate (7:3) yields the title compound in
the form of a colourless oil; IR (methylene chloride): 2820, 1730,
1330, 1165, 1125, 1070 cm . Rf = 0.36 (hexane/ethyl acetate 3:2).
[~]D~ (chloroform, 0.20 %) = -17.5 + 5~.
b) (lR,2R)-1,2-epoxy-1-(3-trifluoromethylphenyl)-6-(4-acetyl-3-hydroxy-
2-propylphenoxy)-hex-3(Z)-ene.
The title compound is prepared analogously to Example ld) from the
epoxyaldehyde according to a); light-yellow oil; Rf = 0.69 (hexane/
ethyl acetate 3:2).
~xample 6: Sodium salt of (lR,2S)-1-hydroxy-1-(3-trifluoromethyl-
phenyl)-6-(4-acetyl-3-hydroxy-2-propylphenoxy)-hex-3(Z)-en-
2-yl-7-thio-4-oxo-4H-1-benzopyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the
corresponding methyl ester according to Example 5; m.p. 222-224~;
[~]D~ (MeOH, 0.135 %) = 62.2 + 7.4~.
UV (MeOH): ~max(E) = 267 (22060); 285 (21140); 318/sh.
~ -
13~0~b3
- 25 -
Example 7: (lS,2R)-1-hydroxy-1-(3-trifluoromethylphenyl)-6-(4-acetyl-
3-hydroxy-2-propylphenoxy)-hex-3(Z)-en-2-yl-7-thio-4-oxo-
4H-1-benzopyranecarboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from (lS,2S)-
1,2-epoxy-1-(3-trifluoromethylphenyl)-6-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-hex-3(Z)-ene; colourless powder having a melting point of
69-71~.
The starting material is prepared, for example, as follows:
a) (2R,3S)-2,3-epoxy-3-(3-trifluoromethylphenyl)-propanal
The title compound is prepared analogously to Example 5a) from
(2S,3S)-2,3-epoxy-3-(3-trifluoromethylphenyl)-propanol. Light-yellow
liquid; IR (CH2Cl2): 2820, 1730, 1330, 1165, 1130, 1070 cm ; [~]D~
(chloroform, 0.20 ~/0) = o.o + 5~; [~]20 (chloroform, 0.20 %) =
3 6 5
475.0 + 5.0~; Rf = 0.44 (hexane/ethyl acetate 7:3).
b) (1S,2S)-1,2-epoxy-1-(3-trifluoromethylphenyl)-6-(4-acetyl-3-hydroxy-
2-propylphenoxy)-hex-3(Z)-ene
The title compound is prepared analogously to Example ld) from the
corresponding epoxyaldehyde according to a). Light-yellow oil; Rf =
0.43 (hexane/ethyl acetate 3:2).
Example 8: Sodium salt of (1S,2R)-1-hydroxy-1-(3-trifluoromethyl-
phenyl)-6-(4-acetyl-3-hydroxy-2-propylphenoxy)-hex-3(Z)-en-
2-yl-7-thio-4-oxo-4H-1-benzopyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the
corresponding methyl ester according to Example 7; m.p. 239-241~;
[~]D~ (methanol, 0.15 %) = -60.7 + 6.7~;
UV (methanol): ~ (~) = 216 (44080); 268 (22520); 285 (21640); 320/sh.
max
Example 9: (lR,2S)-1-hydroxy-1-(3-methylphenyl)-8-(4-acetyl-3-hydroxy-
2-propylphenoxy)-octa-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-
1-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from (lR,2R)-
1,2-epoxy-1-(3-methylphenyl)-8-(4-acetyl-3-hydroxy-2-propylphenoxy)-
13~0463
- 26 -
octa-3(E),5(Z)-diene; light-yellow powder having a melting point of
71-72~; [~]D~ = 81.7 + 8.7~ (MeOH, 0.115 %); UV (MeOH): ~ (E) = 217
(53680); 235/sh; 271 (29120); 285/sh; 325 (13200).
The starting material is prepared, for example, as follows:
a) (2R,3R)-2,3-epoxy-3-(3-methylphenyl)-propanol
The title compound is prepared analogously to Example la) from
3-(3-methylphenyl)-prop-2(E)-enol; colourless, viscous oil; IR
(CHzCl2): 3560, 3410, 2880, 2830, 1590, 1050 cm ; Rf = 0.39
(hexane/ethyl acetate 1:1); [~]D~ = 38.9 + 5.3~ (chloroform, 0.19 %).
b) (4R,5R)-4,5-epoxy-5-(3-methylphenyl)-pent-2(E)-enal
The title compound is prepared analogously to Example lb) from the
epoxy alcohol according to a); light-yellow powder, m.p. 60-61~;
IR (CH2Cl2): 2920, 2820, 2740, 1690, 1640, 1610, 1155, 1130 cm
Rf = 0.34 (hexane/ethyl acetate 4:1).
c) (lR,2R)-1,2-epoxy-1-(3-methylphenyl)-8-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-octa-3(E),5(Z)-diene
The title compound is prepared analogously to Example ld) from the
corresponding epoxyaldehyde according to a); light-yellow oil;
Rf = 0.63 (hexane/ethyl acetate 3:2).
~xample 10: Sodium salt of (lR,2S)-1-hydroxy-1-(3-methylphenyl)-8-(4-
acetyl-3-hydroxy-2-propylphenoxy)-octa-3(E),5(Z)-dien-2-
yl-7-thio-4-oxo-4H-1-benzopyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester according to Example 9; beige powder having a
melting point of 217~ (decomp.); [~]D~ (methanol, 0.15 %) =
71.3 + 6.7~;
UV (methanol): ~ (~) = 219 (51520); 234/sh; 267 (26460); 284 (23280); - max
~22/sh.
.
134~46;~
- 27 -
~xample 11: (lS~2R)-l-hydroxy-1-(3-methylphenyl)-8-(4-acetyl-3-hydroxy-
2-propylphenoxy)-octa-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-
l-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from (lS,2S)-
1,2-epoxy-1-(m-tolyl)-8-(4-acetyl-3-hydroxy-2-propylphenoxy)-octa-
3(E),5(Z)-diene; [~]D~ (MeOH, 0.148 %) = -75.7 + 6.8~; UV (MeOH):
(E) = 217 (51760); 240/sh; 271 (27860); 290/sh; 328 (12600).
max
The starting material is prepared, for example, as follows:
a) (2S,3S)-2,3-epoxy-3-(3-methylphenyl)-propanol
The title compound is prepared analogously to Example la) from
3-(3-methylphenyl)-prop-2(E)-enol but using L(+)-tartaric acid diethyl
ester; colourless oil; IR (CH2C12): 3550, 3470, 2940, 2880, 2830, 1590,
1050 cm 1; Rf = 0.31 (hexane/ethyl acetate 3:2).
b) (4S,5S)-4,5-epoxy-5-(3-methylphenyl)-pent-2(E)-enal
The title compound is prepared analogously to Example lb) from the
epoxy alcohol according to a); light-yellow oil; IR (CH2Clz): 2920,
2820, 2740, 1690, 1640, 1610, 1155, 1130, 1085 cm ; Rf = 0.29
(hexane/ethyl acetate 4:1).
c) (lS,2S)-1,2-epoxy-1-(3-methylphenyl)-8-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-octa-3(E),5(Z)-diene
The title compound is prepared analogously to Example ld) from the
epoxyaldehyde according to b); light-yellow oil; R = 0.51
(hexane/ethyl acetate 7:3).
~xample 12: Sodium salt of (lS,2R)-l-hydroxy-1-(3-methylphenyl)-8-(4-
acetyl-3-hydroxy-2-propylphenoxy)-octa-3(E),5(Z)-dien-2-
yl-7-thio-4-oxo-4H-l-benzopyrane-2-carboxylic acid
The title compound is prepared analogously to Example 1 from the
corresponding methyl ester according to Example 11; m.p. 197-198~;
[~]D~ (0-14 %~ methanol) = -72.1 + 7.1~; UV (MeOH): ~ (E) = 218
(50700); 235/sh; 267 (25780); 285 (22600); 321 (15000).
~ 1340 i~3
Example 13: (lR~2s)-l-hydroxy-l-(3-methylphenyl)-6-(4-acetyl-3-hydr
2-propylphenoxy)-hex-3(Z)-en-2-yl-7-thio-4-oxo-4H-1-benzo-
pyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(lR,2R)-1,2-epoxy-1-(3-tolyl)-6-(4-acetyl-3-hydroxy-2-propylphenoxy)-
hex-3(Z)-ene; colourless powder having a melting point of 136-138~;
[~]D~ = 28.1 + 7.4~ (methanol, 0.135 %); UV (methanol): ~ (~) = 271
(26020); 282/sh; 323 (13700).
The starting material is prepared, for example, as follows:
a) (2S,3R)-2,3-epoxy-3-(3-methylphenyl)-propanal
The title compound is prepared analogously to Example 5a) from
(2R,3R)-2,3-epoxy-3-(3-methylphenyl)-propanol; light-yellow oil.
IR (methylene chloride): 2920, 2820, 1730, 1610, 1140, 1070 cm
Rf = 0.49 (hexane/ethyl acetate 3:2).
b) (lR,2R)-1,2-epoxy-1-(3-methylphenyl)-6-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-hex-3(Z)-ene
The title compound is prepared analogously to Example ld) from the
corresponding epoxyaldehyde according to a); light-yellow oil;
Rf = 0.73 (hexane/ethyl acetate 3:2).
Example 14: Sodium salt of (lR,2S)-1-hydroxy-1-(3-methylphenyl)-6-(4-
acetyl-3-hydroxy-2-propylphenoxy)-hex-3(Z)-en-2-yl-7-thio-
4-oxo-4H-1-benzopyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester according to Example 13; beige powder having a
melting point of 238-240~; [~]D~ (methanol 0.135 %) = 31.1 + 7.4~;
UV (methanol): ~max(~) = 268 (21800); 285 (20860); 322/sh.
Example 15: (lR,2S)-l-hydroxy-1-(3-methoxycarbonylphenyl)-8-(4-acetyl-
3-hydroxy-2-propylphenoxy)-octa-3(E),5(Z)-dien-2-yl-7-thio-
4-oxo-4H-l-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(lR,2R)-1,2-epoxy-1-(3-methoxycarbonylphenyl)-8-(4-acetyl-3-hydroxy-2-
13~0~63
- 29 -
propylphenoxy)-octa-3(E),5(Z)-diene; [~]D~ (methanol, 0.14 %) =
27.9 + 7.1~;
UV (methanol): ~max(~) = 221 (53560); 234/sh; 270 (28980); 285/sh; 326
(13860).
The starting material is prepared, for example, as follows:
a) (2R,3R)-2,3-epoxy-3-(3-methoxycarbonylphenyl)-propanol
The title compound is prepared analogously to Example la) from
(E)-3-methoxycarbonylcinnamic alcohol; slightly yellowish oil; Rf = 0.3
(hexane/ethyl acetate 1:1).
b) (4R,5R)-4,5-epoxy-5-(3-methoxycarbonylphenyl)-pent-2(E)-enal
The title compound is prepared analogously to Example lb) from the
corresponding epoxy alcohol; light-yellow oil; Rf = 0.35 (hexane/ethyl
acetate 3:2).
c) (lR,2R)-1,2-epoxy-(3-methoxycarbonylphenyl)-8-(4-acetyl-3-hydroxy-
2-propylphenoxy)-octa-3(E),5(Z)-diene
The title compound is prepared analogously to Example ld) from the
corresponding epoxyaldehyde according to b); viscous yellow oil;
Rf = 0.50 (hexane/ethyl acetate 3:2).
Example 16: Sodium salt of (lR,2S)-l-hydroxy-1-(3-methoxycarbonyl-
phenyl)-8-(4-acetyl-3-hydroxy-2-propylphenoxy)-octa-
- 3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-l-benzopyrane-2-
carboxylic acid
The title compound is prepared analogously to Example 2 from (lR,2S)-l-
hydroxy-l-(3-methoxycarbonylphenyl)-8-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-octa-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-l-benzopyrane-2-
carboxylic acid methyl ester according to Example 15; light-brown
powder having a melting point of 181~ (decomp.); [~]D~ (methanol,
0.15 %) = 32.0 + 6.7~; UV (methanol): ~ (~) = 222 (51600); 232/sh; 267
(24160); 284 (22940); 320/sh; 400/sh.
134~ 163
- 30 -
Example 17: (1S,2R)-l-hydroxy-1-(3-methoxycarbonylphenyl)-8-(4-acetyl-
3-hydroxy-2-propylphenoxy)-octa-3(E),5(Z)-dien-2-yl-7-thio-
4-oxo-4H-1-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from the
(lS,2S)-1,2-epoxy-1-(3-methoxycarbonylphenyl)-8-(4-acetyl-3-hydroxy-2-
propylphenoxy)-octa-3(E),5(Z)-diene; m.p. 77-78~ (colourless powder);
[~]20 (methanol, 0.15 ~0) = -52.7 + 6.7~; UV (methanol): ~ (E) = 221
D max
~57420); 235/sh; 271 (29980); 2881sh; 326 (14320).
~he starting material is prepared, for example, as follows:
a) (2S,3S)-2,3-epoxy-3-(3-methoxycarbonylphenyl)-propanol
The title compound is prepared analogously to Example la) from
(E)-3-methoxycarbonylcinnamic alcohol but using L(+)-tartaric acid
diethyl ester; colourless oil; Rf = 0.48 (hexane/ethyl acetate 1:1).
b) (4S,5S)-4,5-epoxy-5-(3-methoxycarbonylphenyl)-pent-2-(E)-enal
The title compound is prepared analogously to Example lb) from the
corresponding epoxy alcohol; colourless oil;
IR (methylene chloride): 3050, 2990, 2945, 2820, 2730, 1725, 1695,
1640, 1290, 1255 cm 1; Rf = 0.34 (hexane/ethyl acetate 3:2).
c) (1S,2S)-1,2-epoxy-1-(3-methoxycarbonylphenyl)-8-(4-acetyl-3-
hydroxy-2-propylphenoxy)-octa-3(E),5(Z)-diene
The title compound is prepared analogously to Example ld) from the
corresponding epoxyaldehyde according to b); light-yellow oil;
Rf = 0.54 (hexane/ethyl acetate 3:2).
~xample 18: Sodium salt of (1S,2R)-1-hydroxy-1-(3-methoxycarbonyl-
phenyl)-8-(4-acetyl-3-hydroxy-2-propylphenoxy)-octa-
3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-1-benzopyrane-2-car-
boxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester according to Example 17; beige powder having a
melting point of 174-176~; [~]D~ (methanol, 0.155 %) = -80.6 + 6.5~;
- 31 - 1 3 4 0 4 b 3
UV (methanol): ~max(~) = 223 (59300); 235 sh; 267 (27300); 284 (24800);321 (16200).
~xample 19: (lR,2S)-1-hydroxy-1-(3-methoxycarbonylphenyl)-6-(4-acetyl-
3-hydroxy-2-propylphenoxy)-hex-3(Z)-en-2-yl-7-thio-4-oxo-
4H-1-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(lR,2R)-1,2-epoxy-1-(3-methoxycarbonylphenyl)-6-(4-acetyl-3-hydroxy-
2-propylphenoxy)-hex-3(Z)-ene; colourless oil; [~]D~ (methanol,
0.11 %) = 24.5 + 9.1~;
~V (methanol): ~ (~) = 270 (22400); 322 (12000).
max
~he starting material is prepared, for example, as follows:
a) (25,3R)-2,3-epoxy-3-(3-methoxycarbonylphenyl)-propanal
The title compound is prepared analogously to Example 5a) from
(2R,3R)-2,3-epoxy-3-(3-methoxycarbonylphenyl)-propanol; light-yellow
oil; IR (methylene chloride): 2950, 2820, 1725, 1610, 1590, 1430, 1290,
1255 cm ; Rf = 0.33 (hexane/ethyl acetate 3:2).
b) (lR,2R)-1,2-epoxy-1-(3-methoxycarbonylphenyl)-6-(4-acetyl-3-
hydroxy-2-propylphenoxy)-hex-3(Z)-ene
The title compound is prepared analogously to Example ld) from the
epoxyaldehyde according to a); light-yellow oil; Rf = 0.39
(hexane/ethyl acetate 3:2).
~xample 20: Sodium salt of (lR,25)-1-hydroxy-1-(3-methoxycarbonyl-
phenyl)-6-(4-acetyl-3-hydroxy-2-propylphenoxy)-hex-3(Z)-
en-2-yl-7-thio-4-oxo-4H-1-benzopyrane-2-carboxylic acid
(A), and disodium salt of (lR,25)-1-hydroxy-1-(3-carboxy-
phenyl)-6-(4-acetyl-3-hydroxy-2-propylphenoxy)-hex-3(Z)-en-
2-yl-7-thio-4-oxo-4H-1-benzopyrane-2-carboxylic acid (B)
A solution of 0.5 g of (lR,25)-1-hydroxy-1-(3-methoxycarbonylphenyl)-6-
(4-acetyl-3-hydroxy-2-propylphenoxy)-hex-3(Z)-en-2-yl-7-thio-4-oxo-4H-
1-benzopyrane-2-carboxylic acid methyl ester according to Example 19 in
20 ml of tetrahydrofuran is stirred under argon for 40 hours with
.. ...
13~04~3
- 32 -
7.5 ml of 0.2N sodium hydroxide solution at room temperature and is
then concentrated by evaporation. The residue is purified by chromato-
graphy on Lichroprep~ RP-8, Merck, with methanol/water (3:1) and yields
in fractions 2-5 title compound B; m.p. 262-264~; [~]D~ (methanol,
0.105 ~0) = 17.1 + 9.5~; UV (methanol): ~ ax(E) = 268 (19680); 284
(19060); 325/sh; and in fractions 8-12 title compound A; m.p. 155~
(decomp.); [~]D~ (methanol, 0.12 %) = 22.5 + 8.3~;
UV (methanol): ~max(E) = 268 (26200); 286 (29220); 325/sh.
~xample 21: (lS,2R)-l-hydroxy-1-(3-methoxycarbonylphenyl)-6-(4-acetyl-
3-hydroxy-2-propylphenoxy)-hex-3(Z)-en-2-yl-7-thio-4-oxo-
4H-l-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(lS,2S)-1,2-epoxy-1-(3-carboxymethylphenyl)-6-(4-acetyl-3-hydroxy-2-
propylphenoxy)-hex-3(Z)-ene; colourless oil which hardens in the
refrigerator, m.p. 90-91~; [~]D~ = -41.5 + 7.7~ (0.13 % methanol);
UV (methanol): ~ (E) = 271 (25120); 322 (13280).
~he starting material is prepared, for example, as follows:
a) (2R,3S)-2,3-epoxy-3-(3-methoxycarbonylphenyl)-propanal
The title compound is prepared analogously to Example 5 from
(2S,3S)-2,3-epoxy-3-(3-methoxycarbonylphenyl)-propanol; colourless oil;
IR (methylene chloride): 2910, 2780, 1705, 1590, 1570, 1415, 1270,
1235 cm ; Rf = 0.36 (hexane/ethyl acetate 3:2).
b) (lS,2S)-1,2-epoxy-1-(3-methoxycarbonylphenyl)-6-(4-acetyl-3-hydroxy-
2-propylphenoxy)-hex-3(Z)-ene
The title compound is prepared analogously to Example ld) from the
corresponding epoxyaldehyde according to a); colourless oil, not
characterised in more detail.
~xample 22: Sodium salt of (lS,2R)-l-hydroxy-1-(3-methoxycarbonyl-
phenyl)-6-(4-acetyl-3-hydroxy-2-propylphenoxy)-hex-3(Z)-
en-2-yl-7-thio-4-oxo-4H-l-benzopyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the
13~0~6~
- 33 -
corresponding methyl ester according to Example 21; beige powder having
a melting point of 208-210~; [~]D~ (methanol, 0.12 %) = -41.7 + 8.3~;
UV (methanol): ~max(~) = 268 (21060); 285 (21540); 325/sh.
~xample 23: (4R,5S)-l,l,l-trifluoro-4-hydroxy-11-(4-acetyl-3-hydroxy-
2-propylphenoxy)-undeca6(E),8(Z)-dien-5-yl-7-thio-4-oxo-
4H-l-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(4R,5R)-4,5-epoxy-1,1,1-trifluoro-11-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-undeca-6(E),8(Z)-diene; beige powder having a melting point of
64-66~, [~]D~ (methanol, 0.15 Y0) = 147.3 + 6.7~. UV (methanol):
~ (E) = 220 (48960); 270 (28500); 283/sh; 325 (13400).
~he starting material is prepared, for example, as follows:
a) (2R,3R)-2,3-epoxy-6,6,6-trifluoro-hexanol
The title compound is prepared analogously to Example la) from
6,6,6-trifluoro-hex-2(E)-enol; colourless oil;
[~]D~ (chloroform, 0.17 %) = 35.9 + 5.9~; IR (methylene chloride):
3550, 3420, 2950, 2890, 2830, 1125 cm
b) (4R,5R)-4,5-epoxy-8,8,8-trifluoro-oct-2(E)-enal
The title compound is prepared analogously to Example lb) from the
corresponding epoxy alcohol according to a); light-yellow oil;
IR (CHzCl2): 3050, 2980, 2930, 2820, 2730, 1695, 1645, 1450, 1150,
1100 cm ; Rf = 0.41 (hexane/ethyl acetate = 3:2).
c) (4R,5R)-4,5-epoxy-1,1,1-trifluoro-11-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-undeca-6(E),8(Z)-diene
The title compound is prepared analogously to Example ld) from the
epoxyaldehyde according to b); light-yellow oil; Rf = 0.48
(hexane/ethyl acetate 3:2).
- 34 - 13~ 0 4 ~3
~xample 24: Sodium salt of (4R,5S)-1,1,1-trifluoro-4-hydroxY-11-(4-
acetyl-3-hydroxy-2-propylphenoxy)-undeca-6(E),8(Z)-dien-5-
yl-7-~hio-4-oxo-4H-1-benzopyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester according to Example 23; beige powder having a
melting point of 198-200~; [~]D~ (methanol, 0.15 %) = 127.3 + 6.7~;
W (methanol): ~ (~) = 221 (48820); 230/sh; 267 (25440); 285 (23080);
322/sh.
~xample 25: (4S,5R)-1,1,1-trifluoro-4-hydroxy-11-(4-acetyl-3-hydroxy-
2-propylphenoxy)-undeca-6(E),8(Z)-dien-5-yl-7-thio-4-oxo-
4H-1-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(4S,5S)-4,5-epoxy-1,1,1-trifluoro-11-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-undeca-6(E),8(Z)-diene; colourless powder having a melting
point of 69-71~; [~]D~ = -153.3 + 6.7~ (methanol, 0.15 %).
UV (methanol): ~ (~) = 220 (49560); 235/sh; 270 (29220); 285/sh; 325
(12990).
~he starting material is prepared, for example, as follows:
a) (2S,3S)-2,3-epoxy-6,6,6-trifluoro-hexanol
The title compound is prepared analogously to Example la) from
6,6,6-trifluoro-hex-2(E)-enol but using L(+)-tartaric acid diethyl
ester; colourless oil; [~]D~ (chloroform, 0.17 %) = -25.9 + 5.9~;
IR (methylene chloride): 3550, 3430, 2940, 2880, 2830, 1125 cm
b) (4S,5S)-4,5-epoxy-8,8,8-trifluoro-oct-2(E)-enal
The title compound is prepared analogously to Example lb) from the
corresponding epoxy alcohol according to a); light-yellow oil;
IR (methylene chloride): 3050, 2990, 2930, 2820, 2730, 1695, 1645,
1450, 1150, 1100 cm ; Rf = 0.36 (hexane/ethyl acetate 3:2).
, .. . ... ~ . . _ ....
_ 35 _ 13404~3
c) (4S,5S)-4,5-epoxy-1,1,1-trifluoro-11-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-undeca-6(E),8(Z)-diene
The title compound is prepared analogously to Example ld) from the
corresponding epoxyaldehyde according to b); light-yellow oil.
~xample 26: Sodium salt of (4S,5R)-1,1,1-trifluoro-4-hydroxy-11-(4-
acetyl-3-hydroxy-2-propylphenoxy)-undeca-6(E),8(Z)-dien-
5-yl-7-thio-4-oxo-4H-1-benzopyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester according to Example 25; beige powder having a
melting point of 201-203~; [~]D~ (methanol, 0.15 %) ~ -117.3 + 6.7~;
UV (methanol): ~max(~) = 222 (48320); 233/sh; 267 (26440); 285 (24440);
330/sh.
~xample 27: (4R,5S)-1,1,1-trifluoro-4-hydroxy-9-(4-acetyl-3-hydroxy-2-
propylphenoxy)-non-6(Z)-en-5-yl-7-thio-4-oxo-4H-1-benzo-
pyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(4R,5R)-4,5-epoxy-1,1,1-trifluoro-9-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-non-6(Z)-ene; colourless, viscous oil; IR (methylene
chloride): 3530, 2920, 2890, 2820, 1720, 1640, 1605, 1585 cm
~he starting material is prepared, for example, as follows:
a) (2S,3R)-2,3-epoxy-6,6,6-trifluoro-hexanal
The title compound is prepared analogously to Example 5a) from
(2R,3R)-2,3-epoxy-6,6,6-trifluoro-hexanol; colourless liquid having a
boiling point of 80-81~/26 mbar.; [~]D~ (chloroform, 0.15 %) =
-10.7 + 6.7~.
b) (4R,5R)-4,5-epoxy-1,1,1-trifluoro-9-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-non-6(Z)-ene
The title compound is prepared analogously to Example ld) from the
epoxyaldehyde according to a); light-yellow oil.
1340 i6~
~ 36 -
Example 28: Sodium salt of (4R,5S)-l,l,l-trifluoro-4-hydroxy-9-(4-
acetyl-3-hydroxy-2-propylphenoxy)-non-6(Z)-en-5-yl-7-thio-
4-oxo-4H-l-benzopyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester according to Example 27; light-yellow powder
having a melting point of 204-206~; [~]D~ (methanol, 0.15 %) =
42.7 + 6.7~;
~V (methanol): ~ (~) = 218 (36920); 268 (20280); 285 (20180); 325/sh.
max
~xample 29: (5R,6S)-5-hydroxy-12-(4-acetyl-3-hydroxy-2-propylphenoxy)-
dodec-7(Z)-en-6-yl-7-thio-4-oxo-4H-l-benzopyrane-2-car-
boxylic acid methyl ester
The title compound is prepared analogously to Example 1 from (5R,6R)-
5,6-epoxy-12-(4-acetyl-3-hydroxy-2-propylphenoxy)-dodec-7(Z)-ene;
light-yellow oil; Rf = 0.37 (hexane/ethyl acetate 1:1).
~he starting material is prepared, for example, as follows:
a) 5-(4-acetyl-3-hydroxy-2-propylphenoxy)-pentyl-triphenylphosphonium
bromide
The title compound is prepared as in Example lc) from
5-(4-acetyl-3-hydroxy-2-propylphenoxy)-pentyl bromide; colourless
crystals having a melting point of 82-85~.
b) (2S,3R)-2,3-epoxy-heptanal
The title compound is prepared analogously to Example 5a) from
(2R,3R)-2,3-epoxy-heptanol; [~]D~ = ~99 4 + 0.1~.
c) (5R,6R)-5,6-epoxy-12-(4-acetyl-3-hydroxy-2-propylphenoxy)-dodec-
7(Z)-ene
The title compound is prepared analogously to Example ld) from the
epoxyaldehyde according to a), light-yellow oil.
.. . . ..
13~0463
- 37 -
Example 30: Sodium salt of (5R,6S)-5-hydroxy-12-(4-acetYl-3-hYdroxY-
2-propylphenoxy)-dodec-7(Z)-en-6-yl-7-thio-4-oxo-4H~l~
benzopyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester according to Example 29; m.p. 192-194~; [~]D~
(methanol, 0.125 %) = +5.6 + 8.0~; UV (methanol): ~ (E) = 218
(38000); 268 (22040); 285 (21120); 325 sh.
Example 31: (5S,6R)-5-hydroxy-12-(4-acetyl-3-hydroxy-2-propylphenoxy)-
dodec-7(Z)-en-6-yl-7-thio-4-oxo-4H-l-benzopyrane-2-car-
boxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(5S,6S)-5,6-epoxy-12-(4-acetyl-3-hydroxy-2-propylphenoxy)-dodec-7(Z)-
ene; light-yellow oil; Rf = 0.41 (hexane/ethyl acetate 1:1).
The starting material is prepared, for example, as follows:
a) (2R,3S)-2,3-epoxy-heptanal
The title compound is prepared analogously to Example 5a) from
(2S,3S)-2,3-epoxy-heptanol; [~]D~ = +104.3 + 0.4~.
b) (5S,6S)-5,6-epoxy-12-(4-acetyl-3-hydroxy-2-propylphenoxy)-dodec-
7(Z)-ene
The title compound is prepared analogously to Example ld) from the
epoxyaldehyde according to a); light-yellow oil, Rf = 0.42 (hexane/
ethyl acetate 3:2).
Example 32: Sodium salt of (5S,6R)-5-hydroxy-12-(4-acetyl-3-hydroxy-2-
propylphenoxy)-dodec-7(Z)-en-6-yl-7-thio-4-oxo-4H-l-benzo-
pyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the
corresponding methyl ester according to Example 31; m.p. 192-194~;
[~]D~ (methanol, 0.145 %) = 0 + 6.9~;
UV (methanol): ~max(~) = 218 (37060); 268 (21760); 286 (20520); 325 sh.
.. .. . .. ... ... .. . . ..... ..
1340~3
- 38 -
Example 33: (4R,5S)-l,l,l-trifluoro-4-hydroxy-11-(4-acetyl-3-hydroxy-2-
propylphenoxy)-undec-6(Z)-en-5-yl-7-thio-4-oxo-4H-l-benzo-
pyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(4R,5R)-4,5-epoxy-1,1,1-trifluoro-11-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-undec-6(Z)-ene, light-yellow oil; Rf = 0.47 (hexane/ethyl
acetate 1:1).
The starting material is prepared, for example, analogously to
Example ld) from (2S,3R)-2,3-epoxy-6,6,6-trifluoro-hexanal; light-
yellow oil.
~xample 34: Sodium salt of (4R,5S)-1,1,1-trifluoro-4-hydroxy-11-(4-
acetyl-3-hydroxy-2-propylphenoxy)-undec-6(Z)-en-5-yl-7-
thio-4-oxo-4H-l-benzopyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester according to Example 33; m.p. 193-195~; [~]D~
(methanol, 0.135 %) = +16.3 + 7.4~; UV (MeOH): ~ (E) = 218 (37380);
267 (21380); 286 (21020); 325/sh.
~xample 35: (5R,6S)-5-hydroxy-10-(4-acetyl-3-hydroxy-2-propylphenoxy)-
dec-7(Z)-en-6-yl-7-thio-4-oxo-4H-l-benzopyrane-2-carboxylic
acid methyl ester
The title compound is prepared analogously to Example 1 from
(5R,6R)-5,6-epoxy-10-(4-acetyl-3-hydroxy-2-propylphenoxy)-dec-7(Z)-ene,
light-yellow oil; [~]D~ (methanol, 0.135 %) = +48.9 + 7.4~;
~V (methanol): ~ (E) = 216 (38140); 271 (24040); 285 sh, 322 (12700).
max
The starting material is prepared, for example, analogously to
Example ld) from (2S,3R)-2,3-epoxy-heptanal; light-yellow oil. Rf =
0.45 (hexane/ethyl acetate = 7:3).
~xample 36: (5R,6S)-5-hydroxy-10-(4-acetyl-3-hydroxy-2-propylphenoxy)-
dec-7(Z)-en-6-yl-7-thio-4-oxo-4H-l-benzopyrane-2-carboxylic
acid
The title compound is prepared analogously to Example 2 from the
. .
-
- 39 - 1 3 4 0 4 ~3
corresponding methyl ester according to Example 35 and is converted
into the free acid with hydrochloric acid; m.p. 58-60~; [~]D~
(methanol, 0.130 %) = +36.2 + 7.7~;
UV (methanol): ~max(E) = 218 (35240); 269 (20760); 283 (19800); 330 sh.
Example 37: (5S,6R)-5-hydroxy-10-(4-acetyl-3-hydroxy-2-propylphenoxy)-
dec-7(Z)-en-6-yl-7-thio-4-oxo-4H-l-benzopyrane-2-carboxylic
acid methyl ester
The title compound is prepared analogously to Example 1 from
(5S,6S)-5,6-epoxy-10-(4-acetyl-3-hydroxy-2-propylphenoxy)-dec-7(Z)-ene,
light-yellow oil; [~]D~ (methanol, 0.115 %) = -50.4 + 8.7~;
UV (MeOH): ~ (~) = 217 (38000); 271 (24100); 285 sh, 321 (12800).
max
The starting material is prepared, for example, analogously to Example
ld) from (2R,3S)-2,3-epoxy-heptanal; light-brown oil; Rf = 0.52
(hexane/ethyl acetate 7:3).
Example 38: Sodium salt of (5S,6R)-5-hydroxy-10-(4-acetyl-3-hydroxy-2-
propylphenoxy)-dec-7(Z)-en-6-yl-7-thio-4-oxo-4H-l-benzo-
pyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester according to Example 37; m.p. 224-226~, [~]D~
(methanol, 0.145 %) = -29.0 + 6.9~; UV (methanol): ~ (~) = 219
max
(38760); 268 (21880); 285 (21260); 325 sh.
Example 39: (5S,6R)-5-hydroxy-10-(4-acetyl-3-hydroxy-2-propylphenoxy)-
dec-7(Z)-en-6-yl-7-thio-4-oxo-4H-l-benzopyrane-2-(N-
benzenesulfonamidyl)-carboxamide
A solution of 0.20 g of (5S,6R)-5-hydroxy-10-(4-acetyl-3-hydroxy-2-
propylphenoxy)-dec-7(Z)-en-6-yl-7-thio-4-oxo-4H-l-benzopyrane-2-car-
boxylic acid in 10 ml of methylene chloride is stirred under argon at
room temperature with 60 mg of benzenesulfonamide, 44 mg of 4-dimethyl-
aminopyridine and 70 mg of N-ethyl-N'-(3-dimethylaminopropyl)-carbo-
diimide hydrochloride for 24 hours. The resulting solution is diluted
with 30 ml of methylene chloride, washed twice with lN hydrochloric
acid and twice with brine, dried over magnesium sulfate and concen-
.. . ... .
13~0~3
- 40 -
trated by evaporation. Chromatography of the residue on silica gel with
methylene chloride/methanol (9:1) yields the title compound having a
melting point of 140-142~.
~xample 40: (4RS,5SR)-l-methoxycarbonyl-4-hydroxy-9-(4-acetyl-3-
hydroxy-2-propylphenoxy)-non-6(Z)-en-5-yl-7-thio-4-oxo-4H-
l-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(4RS,5RS)-4,5-epoxy-1-methoxycarbonyl-9-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-non-6(Z)-ene; colourless oil; [~]D~ (methanol, 0.125 %) =
0.0 + 8.0~;
UV (methanol): ~ (~) = 270 (24000); 340 (13200).
The starting material is prepared, for example, analogously to
Example ld) from 5,6-epoxy-6-formylhexanoic acid methyl ester; light-
yellow oil; Rf = 0.35 (hexane/ethyl acetate 3:2).
~xample 41: Disodium salt of (4RS,5SR)-l-carboxy-4-hydroxy-9-(4-acetyl-
3-hydroxy-2-propylphenoxy)-non-6(Z)-en-5-yl-7-thio-4-oxo-
4H-l-benzopyrane-2-carboxylic acid
0.24 g of the methyl ester according to Example 40 is dissolved under
argon in 15 ml of tetrahydrofuran; 3.8 ml of 0.2N sodium hydroxide
solution are added and the mixture is stirred for 20 hours at room
temperature. Concentration by evaporation and purification of the
residue by chromatography on a "Reversed Phase" silica gel column (for
example Merck Lichroprep~ RP-8) with methanol/water (7:3) yield the
title compound having a melting point of 248-250~ (decomp.); UV
(methanol): ~max(~) = 218 (33900); 268 (18580); 284 (18240); 330 sh.
~xample 42: (lR,2S)-1-hydroxy-1-(3-trifluoromethylphenyl)-10-(4-acetyl-
3-hydroxy-2-propylphenoxy)-deca-3(E),5(Z)-dien-2-yl-7-thio-
4-oxo-4H-l-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from (lR,2R)-
1,2-epoxy-1-(3-trifluoromethylphenyl)-10-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-deca-3(E),5(Z)-diene; light-yellow oil. [~]D~ (CHCl3,
0.363 %) = 46.6 + 2.8~;
~ ., .
- 41 - 1 3~ 04 ~3
UV (CHC13): ~max(E) = 270 (26500); 285 (24240); 322 (15200).
The starting material is prepared, for example, as follows:
a) (lR,2R)-1,2-epoxy-1-(3-trifluoromethylphenyl)-10-(4-acetyl-3-
hydroxy-2-propylphenoxy)-deca-3(E),5(Z)-diene.
The title compound is prepared analogously to Example ld) from
5-(4-acetyl-3-hydroxy-2-propylphenoxy)-pentyltriphenylphosphonium
bromide (Example 29a) and
(4R,5R)-4,5-epoxy-(3-trifluoromethylphenyl)-pent-2(E)-enal (Example
lb); light-brown oil; [~]D~ (CHCl3, 0.224 %) = 70.8 + 10~; Rf = 0.50
(hexane/ethyl acetate = 1:1); IR (CH2Cl2): 2960, 2930, 2865, 1735,
1625, 1330, 1125 cm
~xample 43: Sodium salt of (lR,2S)-1-hydroxy-1-(3-trifluoromethyl-
phenyl)-10-(4-acetyl-3-hydroxy-2-propylphenoxy)-deca-
3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-1-benzopyrane-2-car-
boxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester (Example 42); m.p. 217-219~; [~]D~ (MeOH,
0.160 %) = 145.6 + 6.3~;
UV (MeOH): ~ (~) = 220 (50480), 230 (sh), 267 (26240), 284 (23000),
320 sh.
~xample 44: (lR,2S)-1-hydroxy-1-(3-methylphenyl)-10-(4-acetyl-3-
hydroxy-2-propylphenoxy)-deca-3(E),5(Z)-dien-2-yl-7-thio-
4-oxo-4H-1-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(lR,2R)-1,2-epoxy-1-(3-methylphenyl)-10-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-deca-3(E),5(Z)-diene; m.p. 59-60~; [~]D~ (CHCl3, 0.163 %) =
31.9 + 6.1~; UV (CHCl3): ~ (~) = 241 (31420); 286 (23060).
The starting material is prepared, for example, as follows:
- 42 - 13 1 0 ~ 63
a) (1R,2R)-1,2-epoxy-1-(3-methylphenyl)-10-(4-acetyl-3-hydroxy-2-
propylphenoxy)-deca-3(E),5(Z)-diene
The title compound is preparad analogously to Example ld) from 5-(4-
acetyl-3-hydroxy-2-propylphenoxy)-pentyltriphenylphosphonium bromide
(Example 29a) and (4R,5R)-4,5-epoxy-5-(3-methylphenyl)-pent-2(E)-enal
(Example 9b); light-yellow oil; [~]D~ (CHCl3, 0.273 %) = 118.7 +
3.7~. Rf = 0.62 (hexane/ethyl acetate = 3:2).
~xample 45: Sodium salt of (lR,2S)-1-hydroxy-1-(3-methylphenyl)-10-(4-
acetyl-3-hydroxy-2-propylphenoxy)-deca-3(E),5(Z)-dien-2-yl-
7-thio-4-oxo-4H-1-benzopyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester (Example 44); m.p. 208-210~; [~]D~ (MeOH,
0.30 ~O) = 50.7 + 3.3~.
UV (MeOH): ~ (E) = 219 (52420), 230 (sh), 267 (26620), 285 (23520),
325 (sh).
~xample 46: (15,2R)-1-hydroxy-1-(3-trifluoromethylphenyl)-10-(4-acetyl-
3-hydroxy-2-propylphenoxy)-deca-3(E),5(Z)-dien-2-yl-7-thio-
4-oxo-4H-1-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(15,25)-1,2-epoxy-1-(3-trifluoromethylphenyl)-10-(4-acetyl-3-hydroxy-
2-propylphenoxy)-deca-3(E),5(Z)-diene; viscous mass; Rf = 0.48
(hexane/ethyl acetate = 1:1).
~he starting material is prepared, for example, as follows:
a) (15,25)-1,2-epoxy-1-(3-trifluoromethylphenyl)-10-(4-acetyl-3-
hydroxy-2-propylphenoxy)-deca-3(E),5(Z)-diene
The title compound is prepared analogously to Example ld) from
5-(4-acetyl-3-hydroxy-2-propylphenoxy)-pentyltriphenylphosphonium
bromide (Example 29a) and (45,55)-4,5-epoxy-5-(3-trifluoromethyl-
phenyl)-pent-2(E)-enal (Example 3b); light-yellow oil; [~]D~ (chloro-
form, 0.454 % = -86.8 + 2.2~; Rf = 0.46 (hexane/ethyl acetate = 1:1).
IR (methylene chloride): 2960, 2930, 1730, 1625, 1330, 1130 cm 1.
1340463
- 43 -
~xample 47: Sodium salt of (lS,2R)-1-hydroxy-1-(3-trifluoromethyl-
phenyl)-10-(4-acetyl-3-hydroxy-2-propylphenoxy)-deca-
3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-1-benzopyrane-2-car-
boxylic acid
The title co.pound is prepared analogously to Example 2 from the corre-
sponding methyl ester (Example 46); m.p. 168-170~; [~]D~ (methanol,
0.150 %) = -66.7 + 6.7~;
UV (MeOH): ~ (E) = 220 (49640), 230 (sh), 266 (25640), 285 (22140),
320 (sh).
~xample 48: (lR,2S)-1-hydroxy-1-(3-trifluoromethylphenyl)-8-[4-acetyl-
3-hydroxy-2-(3,3,3-trifluoropropyl)-phenoxy]-octa-
3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-1-benzopyrane-2-car-
boxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(lR,2R)-1,2-epoxy-1-(3-trifluoromethylphenyl)-8-[4-acetyl-3-hydroxy-
2-(3,3,3-trifluoropropyl)-phenoxy]-octa-3(E),5(Z)-diene; light-yellow
oil; Rf = 0.34 (hexane/ethyl acetate = 1:1).
~he starting material is prepared, for example, as follows:
a) (lR,2R)-1,2-epoxy-1-(3-trifluoromethylphenyl)-8-[4-acetyl-3-
hydroxy-2-(3,3,3-trifluoropropyl)-phenoxy]-octa-3(E),5(Z)-diene
The title compound is prepared analogously to Example ld) from
3-[4-acetyl-3-hydroxy-2-(3,3,3-trifluoropropyl)-phenoxy]-propyltri-
phenylphosphonium bromide and (4R,5R)-4,5-epoxy-5-(3-trifluoromethyl-
phenyl)-pent-2(E)-enal (Example lb); light-yellow oil; [~]D~ (chloro-
form, 0.406 %) = -58.6 + 2.5~; Rf = 0.45 (hexane/ethyl acetate = 7:3).
IR (methylene chloride): 2945, 1670, 1610, 1310, 1055 cm
b) 3-[4-acetyl-3-hydroxy-2-(3,3,3-trifluoropropyl)-phenoxy]-propyl-
triphenylphosphonium bromide
The title compound is prepared analogously to Example 1c) from
3-[4-acetyl-3-hydroxy-2-(3,3,3-trifluoropropyl)-phenoxy]-propyl
bromide. M.p. 184-185~.
~.
13~0~b3
- 44 -
Example 49: Sodium salt of (lR,2S)-l-hydroxy-1-(3-trifluoromethyl-
phenyl)-8-[4-acetyl-3-hydroxy-2-(3,3,3-trifluoropropyl)-
phenoxy]-octa-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-l-benzo-
pyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester (Example 48); m.p. 238-240~; [~]D~ (methanol,
0.150 ~O) = 208 + 6.6~;
UV (MeOH): ~ (~) = 216 (50040), 230 (sh), 267 (28960), 280 (sh), 320
(16020).
Example 50: (lR,2S)-1-hydroxy-1-(3-methylphenyl)-8-[4-acetyl-3-hydroxy-
2-(3,3,3-trifluoropropyl)-phenoxy]-octa-3(E),5(Z)-dien-2-
yl-7-thio-4-oxo-4H-1-benzopyrane-2-carboxylic acid methyl
ester
The title compound is prepared analogously to Example 1 from
(lR,2R)-1,2-epoxy-1-(3-methylphenyl)-8-[4-acetyl-3-hydroxy-2-(3,3,3-
trifluoropropyl)-phenoxy]-octa-3(E),5(Z)-diene; light-yellow, viscous
oil; Rf = 0.41 (hexane/ethyl acetate = 1:1). [~]D~ (chloroform,
0.155 %) = 32.3 + 6.5~.
UV (chloroform): ~max(~) = 271 (32320), 318 (16100).
The starting material is prepared, for example, as follows:
a) (lR,2R)-1,2-epoxy-1-(3-methylphenyl)-8-[4-acetyl-3-hydroxy-2-(3,3,3-
trifluoropropyl)-phenoxy]-octa-3(E),5(Z)-diene
The title compound is prepared analogously to Example ld) from
3-[4-acetyl-3-hydroxy-2-(3,3,3-trifluoropropyl)-phenoxy]-propyltri-
phenylphosphonium bromide (Example 48b) and (4R,5R)-4,5-epoxy-5-(3-
methylphenyl)-pent-2(E)-enal (Example 9b), light-yellow oil; Rf = 0.38
(hexane/ethyl acetate = 3:2).
Example 51: Sodium salt of (lR,2S)-l-hydroxy-1-(3-methylphenyl)-8-[4-
acetyl-3-hydroxy-2-(3,3,3-trifluoropropyl)-phenoxy]-octa-
3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-l-benzopyrane-2-
carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
- 13404~
- 45 -
sponding methyl ester (Example 50); m.p. 233-235~; [~]D~ (methanol,
0.195 %) = 69.7 + 5.1~;
UV (MeOH): ~ (E) = 213 (52320), 230 (sh), 267 (29040), 280 (sh), 320
(16000).
~xample 52: (lR~2s)-l-hydroxy-l-(2-trifluoromethylphenyl)-8-(4-acetyl-
3-hydroxy-2-propylphenoxy)-octa-3(E),5(Z)-dien-2-yl-7-thio-
4-oxo-4H-1-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(lR,2R)-1,2-epoxy-1-(2-trifluoromethylphenyl)-8-(4-acetyl-3-hydroxy-2-
propylphenoxy)-octa-3(E),5(Z)-diene; m.p. 66-68~; Rf = 0.23 (hexane/
ethyl acetate - 3:2). [~]D~ (methanol, 0.150 ~/ O) = 22.0 + 6.7~;
UV (MeOH): ~ (E) = 216 (50000), 238 (sh), 271 (27860), 285 (sh), 324
(13900).
~he starting material is prepared, for example, as follows:
a) (lR,2R)-1,2-epoxy-1-(2-trifluoromethylphenyl)-8-(4-acetyl-3-hydroxy-
2-propylphenoxy)-octa-3(E),5(Z)-diene
The title compound is prepared analogously to Example ld) from
3-(4-acetyl-3-hydroxy-2-propylphenoxy)-propyltriphenylphosphonium
bromide (Example 1c) and (4R,5R)-4,5-epoxy-5-(2-trifluoromethylphenyl)-
pent-2(E)-enal; light-brown oil; [~]D~ (chloroform, 0.207 %) =
-5.8 + 4.8
Rf = 0.25 (hexane/ethyl acetate = 4:1).
b) (4R,5R)-4,5-epoxy-5-(2-trifluoromethylphenyl)-pent-2(E)-enal
The title compound is prepared analogously to Example lb) from
(2R,3R)-2,3-epoxy-3-(2-trifluoromethylphenyl)-propanol; yellow
crystals; Rf = 0.36 (hexane/ethyl acetate = 4:1). [~]D~ (methanol,
0.165 ~O) = 23.0 + 6.1;
UV (MeOH): ~max(E) = 216 (14400), 235 (17240).
I340 Ib3
- 46 -
c) (2R,3R)-2,3-epoxy-3-(2-trifluoromethylphenyl)-propanol
The title compound is prepared analogously to Example la) from
3-(2-trifluoromethylphenyl)-prop-2(E)-enol; colourless crystals;
Rf = 0.38 (hexane/ethyl acetate = 3:2);
IR (methylene chloride): 3600, 3050, 2990, 2920, 2870, 1610, 1585,
1320, 1170, 1125 cm
~xample 53: Sodium salt of (lR,2S)-1-hydroxy-1-(2-trifluoromethyl-
phenyl)-8-(4-acetyl-3-hydroxy-2-propylphenoxy)-octa-
3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-1-benzopyrane-2-car-
boxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester (Example 52); m.p. 155-157~; [~]D~ (methanol,
0.180 %) = 12.8 + 5.6~;
UV (MeOH): ~ (E) = 219 (48400), 230 (sh), 266 (25480), 284 (22540),
325 (sh).
~xample 54: (lS,2R)-1-hydroxy-1-(2-trifluoromethylphenyl)-8-(4-acetyl-
3-hydroxy-2-propylphenoxy)-octa-3(E),5(Z)-dien-2-yl-7-thio-
4-oxo-4H-1-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(lS,2S)-1,2-epoxy-1-(2-trifluoromethylphenyl)-8-(4-acetyl-3-hydroxy-
2-propylphenoxy)-octa-3(E),5(Z)-diene; m.p. 71-73~; Rf = 0.25
(hexane/ethyl acetate = 3:2).
[~]D~ (methanol, 0.170 %) = -27.1 + 5.9~;
UV (MeOH): ~ (~) = 216 (51040), 235 (sh), 271 (28140), 285 (sh), 324
(13500).
~he starting material is prepared, for example, as follows:
a) (lS,2S)-1,2-epoxy-1-(2-trifluoromethylphenyl)-8-(4-acetyl-3-hydroxy-
2-propylphenoxy)-octa-3(E),5(Z)-diene
The title compound is prepared analogously to Example ld) from
3-(4-acetyl-3-hydroxy-2-propylphenoxy)-propyl-triphenylphosphonium
1310463
- 47 -
bromide (Example 1c) and (4S,5S)-4,5-epoxy-5-(2-trifluoromethylphenyl)-
pent-2(E)-enal; reddish oil; [~]D~ (chloroform, 0.207 %) = 5.4 +
4.8~; Rf = 0.29 (hexane/ethyl acetate = 4:1).
b) (4S,5S)-4,5-epoxy-5-(2-trifluoromethylphenyl)-pent-2(E)-enal
The title compound is prepared analogously to Example lb) from
(2S,3S)-2,3-epoxy-3-(2-trifluoromethylphenyl)-propanol; yellow
crystals; Rf = 0.38 (hexane/ethyl acetate = 4:1); [~]D~ (methanol,
0.180 %) = -25.0 + 5.6~;
~V (MeOH): ~ (E) = 215 (13960), 236 (17060).
max
c) (2S,3S)-2,3-epoxy-3-(2-trifluoromethylphenyl)-propanol
The title compound is prepared analogously to Example la) from
3-(2-trifluoromethylphenyl)-prop-2(E)-enol; colourless crystals;
Rf = 0.35 (hexane/ethyl acetate = 3:2).
IR (methylene chloride): 3600, 3050, 2990, 2920, 2870, 1610, 1585,
1320, 1170, 1125 cm
~xample 55: Sodium salt of (lS,2R)-1-hydroxy-1-(2-trifluoromethyl-
phenyl)-8-(4-acetyl-3-hydroxy-2-propylphenoxy)-octa-
3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-1-benzopyrane-2-
carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester (Example 54); m.p. 182-184~; [~]D~ (methanol,
0.205 %) = -16.6 + 4.9~;
UV (MeOH): ~ (~) = 219 (47680), 235 (sh), 266 (24960), 284 (22100),
330 (sh).
~xample 56: (lR,2S)-1-hydroxy-1-(4-trifluoromethylphenyl)-8-(4-acetyl-
3-hydroxy-2-propylphenoxy)-octa-3(E),5(Z)-dien-2-yl-7-thio-
4-oxo-4H-1-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(lR,2R)-1,2-epoxy-1-(4-trifluoromethylphenyl)-8-(4-acetyl-3-hydroxy-2-
propylphenoxy)-octa-3(E),5(Z)-diene; m.p. 68-70~; Rf = 0.16 (hexane/
ethyl acetate = 3:2). [~]D~ (methanol, 0.155 %) = 110.3 + 6.5~;
13~0~3
- 48 -
UV (MeOH): ~ (E) = 217 (52880), 236 (sh), 270 (28880), 285 (sh), 326
(13700)-
The starting material is prepared, for example, as follows:
a) (lR,2R)-1,2-epoxy-1-(4-trifluoromethylphenyl)-8-(4-acetyl-3-hydroxy-
2-propylphenoxy)-octa-3(E),5(Z)-diene
The title compound is prepared analogously to Example ld) from
3-(4-acetyl-3-hydroxy-2-propylphenoxy)-propyltriphenylphosphonium
bromide (Example lc) and (4R,5R)-4,5-epoxy-5-(4-trifluoromethylphenyl)-
pent-2(E)-enal; yellow oil; [~]D~ (chloroform, 0.220 ~O) = 6.5 + 4.5~;
Rf = 0.35 (hexane/ethyl acetate = 4:1).
b) (4R,5R)-4,5-epoxy-5-(4-trifluoromethylphenyl)-pent-2(E)-enal
The title compound is prepared analogously to Example lb) from
(2R,3R)-2,3-epoxy-3-(4-trifluoromethylphenyl)-propanol; yellow
crystals; m.p. 67-70~; Rf = 0.24 (hexane/ethyl acetate = 4:1). [~]D~
(methanol, 0.150 %) = 171.3 + 6.7~; UV (MeOH): ~ (E) = 237 (19660).
c) (2R,3R)-2,3-epoxy-3-(4-trifluoromethylphenyl)-propanol
The title compound is prepared analogously to Example la) from
3-(4-trifluoromethylphenyl)-prop-2(E)-enol; colourless crystals;
Rf = 0.25 (hexane/ethyl acetate = 3:2).
IR (methylene chloride): 3550, 3010, 2950, 2880, 2830, 1605, 1310,
1150, 1110, 1050 cm
~xample 57: Sodium salt of (lR,2S)-1-hydroxy-1-(4-trifluoromethyl-
phenyl)-8-(4-acetyl-3-hydroxy-2-propylphenoxy)-octa-
3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-l-benzopyrane-2-
carboxylic acid
The title compound is prepared analogously to Example 2 from the
corresponding methyl ester (Example 56);
m.p. 229-231~; [~]D~ (methanol, 0.160 %) = 118.8 + 6.3~;
UV (MeOH): ~ (E) = 220 (53060), 235 (sh), 267 (27280), 284 (23880),
320 (16300).
~ 49 ~ ~3 40 463
~xample 58: (lS,2R)-1-hydroxy-1-(4-trifluoromethylphenyl)-8-(4-acetyl-
3-hydroxy-3-propylphenoxy)-octa-3(E)75(Z)-dien-2-yl-7-thio-
4-oxo-4H-l-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(lS~2s)-l~2-epoxy-l-(4-trifluoromethylphenyl)-8-(4-acetyl-3-hydroxy-2
propylphenoxy)-octa-3(E),5(Z)-diene; m.p. 67-69~; Rf = 0.13 (hexane/
ethyl acetate = 3:2); [~]D~ (methanol, 0.155 %) = -109.7 + 6.5~;
UV (MeOH): ~ (E) = 217 (52640), 235 (sh), 270 (28660), 285 (sh), 326
(13680).
~he starting material is prepared, for example, as follows:
a) (lS,2S)-1,2-epoxy-1-(4-trifluoromethylphenyl)-8-(4-acetyl-3-hydroxy-
2-propylphenoxy)-octa-3(E),5(Z)-diene
The title compound is prepared analogously to Example ld) from
3-(4-acetyl-3-hydroxy-2-propylphenoxy)-propyl-triphenylphosphonium
bromide (Example 1c) and (4S,5S)-4,5-epoxy-5-(4-trifluoromethylphenyl)-
pent-2(E)-enal; reddish oil; [~]D~ (chloroform, 0.199 %) =
-5.4 + 5.0~; Rf = 0.23 (hexane/ethyl acetate = 4:1).
b) (4S,5S)-4,5-epoxy-5-(4-trifluoromethylphenyl)-pent-2(E)-enal
The title compound is prepared analogously to Example lb) from
(2S,3S)-2,3-epoxy-3-(4-trifluoromethylphenyl)-propanol; yellow
crystals; m.p. 67-69~. Rf = 0.18 (hexane/ethyl acetate = 4:1). [~]D~
(methanol, 0.150 %) = -180.0 + 6.7~; UV (MeOH): ~ (~) = 236 (19740).
c) (2S,3S)-2,3-epoxy-3-(4-trifluoromethylphenyl)-propanol
The title compound is prepared analogously to Example la) from
3-(4-trifluoromethylphenyl)-prop-2(E)-enol; colourless crystals;
Rf = 0.23 (hexane/ethyl acetate = 3:2).
IR (methylene chloride): 3550, 3010, 2950, 2880, 2830, 1605, 1310,
1150, 1110, 1050 cm
1340463
- 50 -
Example 59: Sodium salt of (lS,2R)-l-hydroxy-1-(4-trifluoromethyl-
phenyl)-8-(4-acetyl-3-hydroxy-2-propylphenoxy)-octa-
3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-l-benzopyrane-2-
carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester (Example 58); m.p. 228-230~; [~]D~ (methanol,
0.175 %) = -99.4 + 5.7~;
~V (MeOH): ~ (E) = 220 (49800), 235 (sh), 267 (25320), 284 (22200),
max
~20 (15600).
~xample 60: (lR,2S)-1-hydroxy-1-(3-trifluoromethylphenyl)-8-(4-acetyl-
3-hydroxy-2-propylphenoxy)-octa-3(Z)-en-2-yl-7-thio-4-oxo-
4H-1-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(lR,2R)-1,2-epoxy-1-(3-trifluoromethylphenyl)-8-(4-acetyl-3-hydroxy-2-
propylphenoxy)-octa-3(Z)-ene; colourless oil; Rf = 0.41 (hexane/ethyl
acetate = 1 1); [~]D~ (chloroform, 0.150 ~O) = 46.7 + 6.7~; UV (MeOH):
(E) = 271 (22760), 288 (20060), 270 (28880), 324 (14460).
max
The starting material is prepared, for example, as follows:
a) (lR,2R)-1,2-epoxy-1-(3-trifluoromethylphenyl)-8-(4-acetyl-3-hydroxy-
2-propylphenoxy)-octa-3(Z)-ene
The title compound is prepared analogously to Example ld) from
3-(4-acetyl-3-hydroxy-2-propylphenoxy)-pentyl-triphenylphosphonium
bromide (Example 29a) and (2S,3R)-2,3-epoxy-3-(3-trifluoromethyl-
phenyl)-propanal (Example 5a); light-yellow oil; [~]D~ (chloroform,
0.221 %) = 25.3 + 4.5~; Rf = 0.56 (hexane/ethyl acetate = 3:2).
~xample 61: Sodium salt of (lR,2S)-l-hydroxy-1-(3-trifluoromethyl-
phenyl)-8-(4-acetyl-3-hydroxy-2-propylphenoxy)-octa-3(Z)-
en-2-yl-7-thio-4-oxo-4H-1-benzopyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester (Example 60); m.p. 231-233~; [~]D~ (methanol,
0.190 %) = 51.1 + 5.3~;
UV (MeOH): ~ (E) = 215 (42320), 267 (22220), 286 (20800), 320 (sh).
- 51 - 1 3~ 0 4 ~3
~xample 62: (lR,2S)-1-hydroxy-1-(3-trifluoromethylphenyl)-9-(4-acetyl-
3-hydroxy-2-propylphenoxy)-nona-3(E),5(Z)-dien-2-yl-7-thic-
4-oxo-4H-1-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(lR,2R)-1,2-epoxy-1-(3-trifluoromethylphenyl)-9-(4-acetyl-3-hydroxy-2-
propylphenoxy)-nona-3(E),5(Z)-diene; light-yellow viscous oil; [~]D~
(chloroform, 0.155 %) = 44.5 + 6.5~; Rf = 0.50 (hexane/ethyl acetate =
1 : 1 ) ;
UV (CHCl3): ~max(E) = 270 (26800), 284 (23100), 323 (14480).
~he starting material is prepared, for example, as follows:
a) 3-(4-acetyl-3-hydroxy-2-propylphenoxy)-butyl-triphenylphosphonium
bromide
The title compound is prepared analogously to Example 1c) from
3-(4-acetyl-3-hydroxy-2-propylphenoxy)-butyl bromide; m.p. 167-169~.
b) (lR,2R)-1,2-epoxy-1-(3-trifluoromethylphenyl)-9-(4-acetyl-3-hydroxy-
2-propylphenoxy)-nona-3(E),5(Z)-diene
The title compound is prepared analogously to Example ld) from
5-(4-acetyl-3-hydroxy-2-propylphenoxy)-butyl-triphenylphosphonium
bromide and (4R,5R)-4,5-epoxy-5-(3-trifluoromethylphenyl)-pent-2(E)-
enal (Example lb); light-yellow oil; [~]D~ (chloroform, 0.308 %) =
40.7 + 3.2~; Rf = 0.70 (hexane/ethyl acetate = 3:2); IR (methylene
chloride): 2950, 2860, 1625, 1325, 1120 cm
~xample 63: Sodium salt of (lR,2S)-1-hydroxy-1-(3-trifluoromethyl-
phenyl)-9-(4-acetyl-3-hydroxy-2-propylphenoxy)-nona-
3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-1-benzopyrane-2-
carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester (Example 62); m.p. 204-206~; [~]D~ (chloroform,
0.289 %) = 8.0 + 3 5~; [~]D~ = 55.5 + 6.5~ (methanol, 0.155 %);
~V (methanol): ~ (~) = 219 (49320), 232 (sh), 266 (25800), 285
max
~22060), 330 (sh).
, . . ~
- 52 - 13 4 0 ~ ~ 3
~xample 64: (lR,2S)-1-hydroxy-1-(3-trifluoromethylphenyl)-11-(4-acetyl-
3-hydroxy-2-propylphenoxy)-undeca-3(E),5(Z)-dien-2-yl-7-
thio-4-oxo-4H-1-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(lR,2R)-1,2-epoxy-1-(3-trifluoromethylphenyl)-11-(4-acetyl-3-hydroxy-2-
propylphenoxy)-undeca-3(E),5(Z)-diene; light-yellow viscous oil;
[~]D~ (chloroform, 0.160 %) = 48.1 + 6.3~; Rf = 0.50 (hexane/ethyl
acetate = 1:1).
~ (CHCl3): ~ (~) = 270 (27120), 286 (23300), 323 (14740).
max
~he starting material is prepared, for example, as follows:
a) 3-(4-acetyl-3-hydroxy-2-propylphenoxy)-hexyl-triphenylphosphonium
bromide
The title compound is prepared analogously to Example 1c) from
3-(4-acetyl-3-hydroxy-2-propylphenoxy)-hexyl bromide; the compound
crystallises very slowly.
b) (lR,2R)-1,2-epoxy-1-(3-trifluoromethylphenyl)-11-(4-acetyl-3-
hydroxy-2-propylphenoxy)-undeca-3(E),5(Z)-diene
The title compound is prepared analogously to Example ld from
5-(4-acetyl-3-hydroxy-2-propylphenoxy)-hexyl-triphenylphosphonium
bromide and (4R,5R)-4,5-epoxy-5-(3-trifluoromethylphenyl)-pent-2(E)-
enal (Example lb); light-yellow oil; [~]D~ (chloroform, 0.450 %) =
41.1 + 2.2~; Rf = 0.66 (hexane/ethyl acetate = 3:2); IR (methylene
chloride): 2960, 2860, 1625, 1325, 1120 cm
~xample 65: Sodium salt of (lR,25)-1-hydroxy-1-(3-trifluoromethyl-
phenyl)-11-(4-acetyl-3-hydroxy-2-propylphenoxy)-undeca-
3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-1-benzopyrane-2-
carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester (Example 64); m.p. 216-218~; [~]D~ (chloroform,
0.258 %) = 34.9 + 3 9~; [~]D~ = 73.8 + 6.3~ (methanol, 0.160 %);
_ 53 _ 13 40~6 3
UV (methanol): ~m (E) = 219 (50140), 232 (sh), 266 (26120), 286
(22460), 320 (15600).
~xample 66: (lR,2S)-1-hydroxy-1-phenyl-8-(4-acetYl-3-hydroxY-2-propyl~
phenoxy)-octa-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-1-benzo-
pyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(lR,2R)-1,2-epoxy-1-phenyl-8-(4-acetyl-3-hydroxy-2-propylphenoxy)-octa-
3(E),5(Z)-diene; light-yellow foam; Rf = 0.41 (hexane/ethyl acetate =
1:1); [~]D~ = 47.3 + 2.6~ (chloroform, 0.385 %).
~he starting material is prepared, for example, as follows:
a) (lR,2R)-1,2-epoxy-1-phenyl-8-(4-acetyl-3-hydroxy-2-propylphenoxy)-
octa-3(E),5(Z)-diene
The title compound is prepared analogously to Example ld) from
3-(4-acetyl-3-hydroxy-2-propylphenoxy)-propyl-triphenylphosphonium
bromide (Example lc) and (4R,5R)-4,5-epoxy-5-phenyl-pent-2(E)-enal;
light-yellow oil; Rf = 0.60 (hexane/ethyl acetate = 3:2).
b) (4R,5R)-4,5-epoxy-5-phenyl-pent-2(E)-enal
The title compound is prepared analogously to Example lb) from
(2R,3R)-2,3-epoxy-3-phenyl-propanol; yellow oil which crystallises on
standing; Rf = 0.38 (hexane/ethyl acetate = 3:2); [~]D~ = 185 + 5.0~
(chloroform, 0.200 %).
c) (2R,3R)-2,3-epoxy-3-phenyl-propanol
The title compound is prepared analogously to Example la) from
3-phenyl-prop-2(E)-enol; colourless oil which crystallises at low
temperature. Rf = 0.49 (hexane/ethyl acetate = 1:1); IR (methylene
chloride): 3590, 3040, 2980, 2920, 2870, 1605, 1080, 1070 cm 1.
[~]D~ (chloroform, 0.279 %) = 47.7 + 3.6~.
.. , ... . ~ ....
- 54 - 1 3 4 o 4~
~xample 67: Sodium salt of (lR,2S)-1-hydroxy-1-phenyl-8-(4-acetyl-3-
hydroxy-2-propylphenoxy)-octa-3(E),5(Z)-dien-2-yl-7-thio-
4-oxo-4H-1-benzopyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester (Example 66); m.p. 219-221~; [~]D~ (chloroform,
~.160 %) = 103.1 + 6.3~; UV (methanol): ~ (~) = 221 (51180), 232
max
~sh), 267 (27040), 284 (23840), 321 (16200); UV (chloroform): ~
max
~74 (26080), 286 (sh), 330 (sh).
~xample 68: (lR,2S)-1-hydroxy-1-(3-trifluoromethylphenyl)-8-(3-acetyl-
4-hydroxy-5-propylphenoxy)-octa-3(E),5(Z)-dien-2-yl-7-thio-
4-oxo-4H-1-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(1R,2R)-1,2-epoxy-1-(3-trifluoromethylphenyl)-8-(3-acetyl-4-hydroxy-5-
propylphenoxy)-octa-3(E),5(Z)-diene; Rf = 0.21 (hexane/ethyl acetate,
3:2).
~he starting material is prepared, for example, as follows:
a) 3-(3-acetyl-4-hydroxy-5-propylphenoxy)-propyl bromide
6.2 g of potassium carbonate and 0.5 g of potassium iodide are added to
a solution of 5.8 g of 2,5-dihydroxy-3-propyl-acetophenone and 6.1 ml
of 1,3-dibromopropane in 60 ml of methyl ethyl ketone. The reaction
mixture is heated at reflux for 24 hours, and then poured onto 300 ml
of ice-water, rendered acidic with hydrochloric acid and extracted with
dichloromethane (3 x 150 ml). The combined extracts are washed with
50 ml of water and dried over sodium sulfate. After filtration and
concentration by evaporation in vacuo, the residue is chromatographed
on 400 g of silica gel with dichloromethane. In the first fraction the
title compound is eluted which, after concentration by evaporation, is
obtained in the form of light-yellow crystals having a melting point of
69-70~.
13~4~3
- 55 -
b) 3-(3-acetyl-4-hydroxy-5-propylphenoxy)-propyl-triphenylphosphonium
bromide
The title compound is prepared analogously to Example lc) from 3-(3-
acetyl-4-hydroxy-5-propylphenoxy)-propyl bromide and triphenylphos-
phine; m.p. 103-105~.
c) (lR,2R)-1,2-epoxy-1-(3-trifluoromethylphenyl)-8-(3-acetyl-4-hydroxy-
5-propylphenoxy)-octa-3(E),5(Z)-diene
The title compound is prepared analogously to Example ld) from 3-(3-
acetyl-4-hydroxy-5-propylphenoxy)-propyl-triphenylphosphonium bromide
and (4R,5R)-4,5-epoxy-5-(3-trifluoromethylphenyl)-pent-2(E)-enal;
Rf = 0.54 (hexane/ethyl acetate 2:1).
Example 69: Sodium salt of (lR,2S)-l-hydroxy-1-(3-trifluoromethyl-
phenyl)-8-(3-acetyl-4-hydroxy-5-propylphenoxy)-octa-
3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-l-benzopyrane-2-
carboxylic acid
The title compound is prepared analogously to Example 2 from (lR,2S)-
l-hydroxy-l-(3-trifluoromethylphenyl)-8-(3-acetyl-4-hydroxy-5-propyl-
phenoxy)-octa-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-l-benzopyrane-2-
carboxylic acid methyl ester; yellow foam; lH-NMR (CD30D): ~ = 7.91,
7.76-7.56, 7.50, 7.36, 7.05, 6.82, 6.41, 6.00, 5.75, 5.47, 5.12, 4.45,
3.78, 2.65-2.35, 1.88, 1.58, 0.94 ppm.
Example 70: (lR,2S)-l-hydroxy-1-(3-chlorophenyl)-8-(4-acetyl-3-hydroxy-
- 2-propylphenoxy)-octa-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-
l-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from (lR,2R)-
1,2-epoxy-1-(3-chlorophenyl)-8-(4-acetyl-3-hydroxy-2-propylphenoxy)-
octa-3(E),5(Z)-diene; m.p. 76-77~; [~]D~ (chloroform, 0.215 %) =
51.2 + 4.7~;
UV (chloroform): ~ (E) = 271 (28160), 285 (sh), 321 (15380).
max
The starting material is prepared, for example, as follows:
. .. .. , . . . . . _ . ... . ..
1340~63
- 56 -
a) (lR,2R)-1,2-epoxy-1-(3-chlorophenyl)-8-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-octa-3(E),5(Z)-diene
The title compound is prepared analogously to Example ld) from 3-(4-
acetyl-3-hydroxy-2-propylphenoxy)-propyl-triphenylphosphonium bromide
(Example lc) and (4R,5R)-4,5-epoxy-5-(3-chlorophenyl)-pent-2(E)-enal;
light-yellow oil; [~]D~ (chloroform, 0.541 %) = 63.9 + 1.8~.
b) (4R,5R)-4,5-epoxy-5-(3-chlorophenyl)-pent-2(E)-enal
The title compound is prepared analogously to Example lb) from
(2R,3R)-2,3-epoxy-3-(3-chlorophenyl)-propanol; dark-yellow oil;
Rf = 0.23 (hexane/ethyl acetate = 4:1).
[~]20 (chloroform, 0.30 %) = 184.7 _
c) (2R,3R)-2,3-epoxy-3-(3-chlorophenyl)-propanol
The title compound is prepared analogously to Example la) from 3-(3-
chlorophenyl)-prop-2(E)-enol; light-yellow oil; Rf = 0.22 (hexane/ethyl
acetate = 7:3). IR (methylene chloride): 3580, 3040, 2980, 2910, 2860,
1600, 1570, 1070 cm 1 [~]D~ (chloroform, 0.334 %) = 47.3 + 3.0~.
~xample 71: Sodium salt of (lR,2S)-1-hydroxy-1-(3-chlorophenyl)-8-(4-
acetyl-3-hydroxy-2-propylphenoxy)-octa-3(E),5(Z)-dien-2-
yl-7-thio-4-oxo-4H-1-benzopyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester (Example 70); m.p. 217-219~; [~]D~ = (methanol,
0.160 %) = 107.5 + 6.3~;
UV (methanol): ~max(E) = 219 (55060), 235 (sh), 267 (27340), 284
(23920), 320 (16500).
~xample 72: (lR,2S)-l-hydroxy-1-(3-chlorophenyl)-10-(4-acetyl-3-
hydroxy-2-propylphenoxy)-deca-3(E),5(Z)-dien-2-yl-7-thio-
4-oxo-4H-1-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(lR,2R)-1,2-epoxy-1-(3-chlorophenyl)-10-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-deca-3(E),5(Z)-diene; m.p. 61-62~. [~]D~ (chloroform,
0.170 %) = 52.9 + 5.9~;
~V (chloroform): ~ (E) = 271 (27120), 286 (24800), 322 (15400).
max
1340~3
- 57 -
The starting material is prepared, for example, as follows:
a) (lR,2R)-1,2-epoxy-1-(3-chlorophenyl)-10-(4-acetyl-3-hydroxy-2-
propylphenoxy)-deca-3(E),5(Z)-diene
The title compound is prepared analogously to Example ld) from
3-(4-acetyl-3-hydroxy-2-propylphenoxy)-pentyl-triphenylphosphonium
bromide (Example 29a) and (4R,5R)-4,5-epoxy-5-(3-chlorophenyl)-pent-
2(E)-enal (Example 70b); light-yellow oil; [~]D~ (chloroform,
0.391 %) = 61.4 + 2.5~.
~xample 73: Sodium salt of (lR,2S)-l-hydroxy-1-(3-chlorophenyl)-10-
(4-acetyl-3-hydroxy-2-propylphenoxy)-deca-3(E),5(Z)-dien-
2-yl-7-thio-4-oxo-4H-l-benzopyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester (Example 72); m.p. 204-206~; [~]D~ (methanol,
0.205 %) = 58.5 + 4.9~;
~V (MeOH): ~ (~) = 218 (27940), 267 (13140), 285 (11600), 320 (sh).
max
~xample 74: (lR,2S)-l-hydroxy-1-(3-methoxyphenyl)-8-(4-acetyl-3-
hydroxy-2-propylphenoxy)-octa-3(E),5(Z)-dien-2-yl-7-thio-
4-oxo-4H-l-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from (lR,2R)-
1,2-epoxy-1-(3-methoxyphenyl)-8-(4-acetyl-3-hydroxy-2-propylphenoxy)-
octa-3(E),5(Z)-diene; m.p. 65-67~; UV (chloroform): ~ (E) = 271
(30360), 285 (sh), 323 (16600).
~he starting material is prepared, for example, as follows:
a) (lR,2R)-1,2-epoxy-1-(3-methoxyphenyl)-8-(4-acetyl-3-hydroxy-2-
propylphenoxy)-octa-3(E),5(Z)-diene
The title compound is prepared analogously to Example ld) from 3-(4-
acetyl-3-hydroxy-2-propylphenoxy)-propyl-triphenylphosphonium bromide
(Example lc) and (4R,5R)-4,5-epoxy-5-(3-methoxyphenyl)-pent-2(E)-enal;
light-yellow oil; [~]D~ (chloroform, 0.315 %) = 78.4 + 3.2.
- 58 - 13 '~ 0463
b) (4R,5R)-4,5-epoxy-5-(3-methoxyphenyl)-pent-2(E)-enal
The title compound is prepared analogously to Example lb) from (2R,3R)-
2,3-epoxy-3-(3-methoxyphenyl)-propanol; yellow oil; Rf = 0.41
(hexane/ethyl acetate = 3:2);
[~]D~ (chloroform, 0.567 %) = 168.9 + 1.8~.
c) (2R,3R)-2,3-epoxy-3-(3-methoxyphenyl)-propanol
The title compound is prepared analogously to Example la) from 3-(3-
methoxyphenyl)-prop-2(E)-enol; light-yellow oil; Rf = 0.31
(hexane/ethyl acetate = 3:2). IR (methylene chloride): 3550, 2890,
1580, 1565, 1465, 1445, 1130 cm
~xample 75: Sodium salt of (lR,2S)-1-hydroxy-1-(3-methoxyphenyl)-8-
(4-acetyl-3-hydroxy-2-propylphenoxy)-octa-3(E),5(Z)-dien-
2-yl-7-thio-4-oxo-4H-1-benzopyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester (Example 74); m.p. 206-208~; [~]D~ (methanol,
0.293 %) = 99.7 + 3.4~;
UV (methanol): ~ (~) = 221 (57840), 235 (sh), 268 (29360), 282
(26400), 320 (16800).
~xample 76: (lR,2S)-1-hydroxy-1-(3-methoxyphenyl)-10-(4-acetyl-3-
hydroxy-2-propylphenoxy)-deca-3(E),5(Z)-dien-2-yl-7-thio-
4-oxo-4H-1-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from (lR,2R)-
1,2-epoxy-1-(3-methoxyphenyl)-10-(4-acetyl-3-hydroxy-2-propylphenoxy)-
deca-3(E),5(Z)-diene; m.p. 53~ (partly sublimes); UV (chloroform):
~ (~) = 272 (29920), 285 (sh), 323 (15540); [~]D~ (chloroform,
0.180 %) = 44.4 + 5.6~.
~he starting material is prepared, for example, as follows:
a) (lR,2R)-1,2-epoxy-1-(3-methoxyphenyl)-10-(4-acetyl-3-hydroxy-2-
propylphenoxy)-deca-3(E),5(Z)-diene
The title compound is prepared analogously to Example ld) from 3-(4-
acetyl-3-hydroxy-2-propylphenoxy)-pentyl-triphenylphosphonium bromide
13~04~3
- 59 -
(Example 29a) and (4R,5R)-4,5-epoxy-5-(3-methoxyphenyl)-pent-2(E)-enal
(Example 74b); light-yellow oil; [~]D~ (chloroform, 0.375 %) =
72.5 + 2.7.
~xample 77: Sodium salt of (lR,2S)-1-hydroxy-1-(3-methoxyphenyl)-10-
(4-acetyl-3-hydroxy-2-propylphenoxy)-deca-3(E),5(Z)-dien-
2-yl-7-thio-4-oxo-4H-1-benzopyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the
corresponding methyl ester (Example 76); m.p. 187-188~; [~]D~
(methanol, 0.150 %) = 72.0 + 6.7~;
UV (methanol): ~max(~) = 222 (55780), 268 (27820), 282 (25200), 321
(16200).
~xample 78: (lR,2S)-1-hydroxy-1-(3-trifluoromethylphenyl)-8-(4-tri-
fluoroacetyl-3-hydroxyphenoxy)-octa-3(E),5(Z)-dien-2-yl-
7-thio-4-oxo-4H-1-benzopyrane-2-carboxylic acid methyl
ester
The title compound is prepared analogously to Example 1 from
(lR,2R)-1,2-epoxy-(3-trifluoromethylphenyl)-8-(4-trifluoroacetyl-3-
hydroxyphenoxy)-octa-3(E),5(Z)-diene;
Rf = 0.23 (hexane/ethyl acetate 3:2).
~he starting material is prepared, for example, as follows:
a) 3-(4-trifluoroacetyl-3-hydroxyphenoxy)-propyl bromide
The title compound is prepared analogously to Example 68a) from
2,4-dihydroxytrifluoroacetophenone and is obtained in the form of a
light-yellow oil; IR (dichloromethane): 3150, 2940, 1645, 1625, 1380,
1210, 1150, 1125, 1020, 940 cm
b) 3-(4-trifluoroacetyl-3-hydroxyphenoxy)-propyl-triphenylphosphonium
bromide
The title compound is prepared analogously to Example 1c) from 3-(4-
trifluoroacetyl-3-hydroxyphenoxy)-propyl bromide and triphenylphos-
phine; m.p. 125-130~C.
1340~3
- 60 -
c) (lR,2R)-1,2-epoxy-1-(3-trifluoromethylphenyl)-8-(4-trifluoroacetyl-
3-hydroxyphenoxy)-octa-3(E),5(Z)-diene
The title compound is prepared analogously to Example ld) from
3-(4-trifluoroacetyl-3-hydroxyphenoxy)-propyl-triphenylphosphonium
bromide and (4R,5R)-4,5-epoxy-5-(3-trifluoromethylphenyl)-pent-2(E)-
enal; Rf = 0.56 (hexane/ethyl acetate 2:1).
~xample 79: Sodium salt of (lR,2S)-1-hydroxy-1-(3-trifluoromethyl-
phenyl)-8-(4-trifluoroacetyl-3-hydroxyphenoxy)-octa-
3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-1-benzopyrane-2-
carboxylic acid
10 ml of O.lN aqueous sodium hydroxide solution are added to a
solution, cooled to 10~C, of 708 mg of the methyl ester of the title
compound (see Example 78) in 25 ml of tetrahydrofuran. The reaction
mixture is stirred at room temperature for 24 hours, freed of solvent
in vacuo and the residue is taken up in water. After filtration until
clear, the solution is lyophilised. The title compound is obtained in
the form of an olive-green amorphous powder. 1H-NMR (CD30D): + = 7.94,
7.78-7.46, 7.36, 6.90, 6.36, 6.04, 5.74, 5.42, 5.12, 4.42, 3.86 ppm.
~xample 80: (lR,2S)-1-hydroxy-1-(3-trifluoromethylphenyl)-8-(4-tri-
fluoroacetyl-3-hydroxy-2-propylphenoxy)-octa-3(E),5(Z)-
dien-2-yl-7-thio-4-oxo-4H-1-benzopyrane-2-carboxylic acid
methyl ester
The title compound is prepared analogously to Example 1 from
(lR,2R)-1,2-epoxy-1-(3-trifluoromethylphenyl)-8-(4-trifluoroacetyl-3-
hydroxy-2-propylphenoxy)-octa-3(E),5(Z)-diene; Rf = 0.18 (toluene/ethyl
acetate 5:1).
~he starting material is prepared, for example, as follows:
a) 3-(4-trifluoroacetyl-3-hydroxy-2-propylphenoxy)-propyl bromide
The title compound is prepared analogously to Example 68a) from 2,4-
dihydroxy-3-propyl-trifluoroacetophenone and is obtained in the form of
a yellow oil; IR (dichloromethane): 3150, 2950, 2860, 1640, 1620, 1500,
1295, 1210, 1150, 1120, 1070 cm
13~0~63
- 61 -
b) 3-(4-trifluoroacetyl-3-hydroxy-2-propylphenoxy)-propyl-triphenyl-
phosphonium bromide
The title compound is prepared analogously to Example lc) from 3-(4-
trifluoroacetyl-3-hydroxy-2-propylphenoxy)-propyl bromide and tri-
phenylphosphine; m.p. 170-190~C.
c) (lR,2R)-1,2-epoxy-1-(3-trifluoromethylphenyl)-8-(4-trifluoroacetyl-
3-hydroxy-2-propylphenoxy)-octa-3(E),5(Z)-diene
The title compound is prepared analogously to Example ld) from 3-(4-
trifluoroacetyl-3-hydroxy-2-propylphenoxy)-propyl-triphenylphosphonium
bromide and (4R,5R)-4,5-epoxy-5-(3-trifluoromethylphenyl)-pent-2(E)-
enal; Rf = 0.55 (hexane/ethyl acetate 2:1).
~xample 81: Sodium salt of (lR,2S)-l-hydroxy-1-(3-trifluoromethyl-
phenyl)-8-(4-trifluoroacetyl-3-hydroxy-2-propylphenoxy)-
octa-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-l-benzopyrane-2-
carboxylic acid
6.6 ml of O.lN aqueous sodium hydroxide solution are added to a
solution, cooled to 10~C, of 500 mg of the methyl ester of the title
compound (see Example 80) in 20 ml of tetrahydrofuran. The reaction
mixture is stirred for 1 hour at 10~C, freed of tetrahydrofuran in
vacuo and the solution that remains is lyophilised. The title compound
is obtained in the form of a yellowish-green amorphous powder; lH-NMR
(CD30D): ~ = 7.93, 7.77-7.60, 7.50, 7.36, 6.92, 6.43, 6.04, 5.73, 5.47,
5.13, 4.47, 4.00, 2.50, 2.38, 0.72 ppm.
~xample 82: (lR,2S)-l-hydroxy-1-(3-trifluoromethylphenyl)-8-(4-acetyl-
3-hydroxy-2-propylphenylthio)-octa-3(E),5(Z)-dien-2-yl-7-
thio-4-oxo-4H-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from (lR,2S)-
1,2-epoxy-1-(3-trifluoromethylphenyl)-8-(4-acetyl-3-hydroxy-2-propyl-
phenylthio)-octa-3(E),5(Z)-diene; Rf = 0.19 (hexane/ethyl acetate 3:2).
The starting material is prepared, for example, as follows:
- 62 - 13 4 0 4 ~ 3
a) 3-(4-acetyl-3-hydroxy-2-propylphenylthio)-propyl bromide
The title compound is prepared analogously to Example 68a) from
2-hydroxy-4-mercapto-acetophenone; light-yellow oil.
b) 3-(4-acetyl-3-hydroxy-2-propylphenylthio)-propyl-triphenylphos-
phonium bromide
The title compound is prepared analogously to Example 1 from
3-(4-acetyl-3-hydroxy-2-propylphenylthio)-propyl bromide and tri-
phenylphosphine.
c) (lR,2S)-1,2-epoxy-1-(3-trifluoromethylphenyl)-8-(4-acetyl-3-hydroxy-
2-propylphenylthio)-octa-3(E),5(Z)-diene
The title compound is prepared analogously to Example ld) from
3-(4-acetyl-3-hydroxy-2-propylphenylthio)-propyl-triphenylphosphonium
bromide and (4R,5R)-4,5-epoxy-5-(3-trifluoromethylphenyl)-pent-2(E)-
enal.
~xample 83: Sodium salt of (lR,2S)-l-hydroxy-1-(3-trifluoromethyl-
phenyl)-8-(4-acetyl-3-hydroxy-2-propylphenylthio)-octa-
3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-benzopyrane-2-
carboxylic acid
The title compound is prepared analogously to Example 2 from the
corresponding methyl ester (Example 82).
~xample 84: (lR,lS)-l-hydroxy-1-(3-trifluoromethylphenyl)-10-(4-acetyl-
3-hydroxy-2-propylphenylthio)-deca-3(E),5(Z)-dien-2-yl-7-
thio-4-oxo-4H-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from (lR,2S)-
1,2-epoxy-1-(3-trifluoromethylphenyl)-10-(4-acetyl-3-hydroxy-2-propyl-
phenylthio)-deca-3(E),5(Z)-diene; Rf = 0.17 (hexane/ethyl acetate 3:2).
~he starting material can be prepared, for example, as follows:
a) 5-(4-acetyl-3-hydroxy-2-propylphenylthio)-pentyl bromide
The title compound is prepared analogously to Example 68a) from
2-hydroxy-4-mercapto-acetophenone; light-yellow oil.
13~0463
- 63 -
b) 5-(4-acetyl-3-hydroxy-2-propylphenylthio)-pentyl-triphenylphos-
phonium bromide
The title compound is prepared analogously to Example 1 from
5-(4-acetyl-3-hydroxy-2-propylphenylthio)-pentyl bromide and triphenyl-
phosphine.
c) (lR,lS)-1,2-epoxy-1-(3-trifluoromethylphenyl)-10-(4-acetyl-3-
hydroxy-2-propylphenylthio)-deca-3(E),5(Z)-diene
The title compound is prepared analogously to Example ld) from
5-(4-acetyl-3-hydroxy-2-propylphenylthio)-pentyl-triphenylphosphonium
bromide and (4R,5R)-4,5-epoxy-5-(3-trifluoromethylphenyl)-pent-2(E)-
enol.
~xample 85: Sodium salt of (lR,lS)-l-hydroxy-1-(3-trifluoromethyl-
phenyl)-10-(4-acetyl-3-hydroxy-2-propylphenylthio)-deca-
3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-benzopyrane-2-car-
boxylic acid
The title compound is prepared analogously to Example 2 from the
corresponding methyl ester (Example 84).
~xample 86: (5R,6S)-l,l,l-trifluoro-5-hydroxy-12-(4-acetyl-3-hydroxy-
2-propylphenoxy)-dodeca-7(E),9(Z)-dien-6-yl-7-thio-4-oxo-
4H-l-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from (5R,6R)-
5,6-epoxy-1,1,1-trifluoro-12-(4-acetyl-3-hydroxy-2-propylphenoxy)-
dodeca-7(E),9(Z)-diene; light-yellow foam; Rf = 0.24 (hexanetethyl
acetate = 3:2).
~he starting material is prepared, for example, as follows:
a) (2R,3R)-2,3-epoxy-7,7,7-trifluoro-heptanol
The title compound is prepared analogously to Example la) from 7,7,7-
trifluoro-hept-2(E)-enol; light-yellow oil;
Rf = 0.38 (hexane/ethyl acetate = 3:2). [~]D~ (chloroform,
.
- 64 - 1340 4 6 3
0.490 %) = 15.3 + 2.0~. IR (methylene chloride): 3550, 3430, 2900,
1180, 1125 cm
b) (4R,5R)-4,5-epoxy-9,9,9-trifluoro-non-2(E)-enal
The title compound is prepared analogously to Example lb) from (2R,3R)-
2,3-epoxy-7,7,7-trifluoro-heptanol; oil, which crystallises in the
refrigerator. Rf = 0.63 (hexane/ethyl acetate = 1:1). [~]D~ (chloro-
form, 0.210 %) = 19.5 + 4.8~.
c) (5R,6R)-5,6-epoxy-1,1,1-trifluoro-12-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-dodeca-7(E),9(Z)-diene
The title compound is prepared analogously to Example ld) from
3-(4-acetyl-3-hydroxy-2-propylphenoxy)-propyltriphenylphosphonium
bromide (Example 1c) and (4R,5R)-4,5-epoxy-9,9,9-trifluoro-non-2(E)-
enal; yellow oil; Rf = 0.56 (hexane/ethyl acetate = 3:2).
~xample 87: Sodium salt of (5R,6S)-1,1,1-trifluoro-5-hydroxy-12-(4-
acetyl-3-hydroxy-2-propylphenoxy)-dodeca-7(E),9(Z)-dien-
6-yl-7-thio-4-oxo-4H-1-benzopyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester (Example 86); m.p. 190-191~; [~]D~ (methanol,
0.268 %) = 105.2 + 3.7~;
UV (methanol): ~max(E) = 222 (48800), 235 (sh), 267 (25920), 285
(22920), 320 (16000).
~xample 88: (4R,5S)-1,1,1-trifluoro-4-hydroxy-13-(4-acetyl-3-hydroxy-2-
propylphenoxy)-trideca-6(E),8(Z)-dien-5-yl-7-thio-4-oxo-
4H-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from (4R,5R)-
4,5-epoxy-1,1,1-trifluoro-13-(4-acetyl-3-hydroxy-2-propylphenoxy)-
trideca-6(E),8(Z)-diene; yellow oil.
The starting material is prepared, for example, as follows:
1340463
- 65 -
a) (4R,5R)-4,5-epoxy-8,8,8-trifluoro-oct-2(E)-enal
The title compound is prepared analogously to Example lb) from
(2R,3R)-2,3-epoxy-6,6,6-trifluoro-hexanol; light-yellow oil which
crystallises in the refrigerator; Rf = 0.53 (hexanetethyl acetate =
3:2). [~]D~ (chloroform, 0.290 %) = 21.7 + 3.4~. IR (methylene
chloride): 3050, 2980, 2930, 2810, 2730, 1690, 1640, 1145 cm 1.
b) (4R,5R)-4,5-epoxy-1,1,1-trifluoro-13-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-trideca-6(E),8(Z)-diene
The title compound is prepared analogously to Example ld) from 3-(4-
acetyl-3-hydroxy-2-propylphenoxy)-pentyl-triphenylphosphonium bromide
(Example 29a) and (4R,5R)-4,5-epoxy-8,8,8-trifluoro-oct-2(E)-enal;
yellow oil; Rf = 0.69 (hexane/ethyl acetate = 3:2).
~xample 89: Sodium salt of (4R,5R)-1,1,1-trifluoro-4-hydroxy-13-(4-
acetyl-3-hydroxy-2-propylphenoxy)-trideca-6(E),8(Z)-dien-
5-yl-7-thio-4-oxo-4H-1-benzopyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester (Example 88); m.p. 150-152~; [~]D~ (methanol,
0.265 %) = 72.5 + 3.8~;
UV (methanol): ~max(E) = 221 (44680), 231 (sh), 266 (22560), 285
(20560), 330 (sh).
~xample 90: (4R,5S)-1,1,1-trifluoro-4-hydroxy-13-(4-acetyl-3-hydroxy-
2-propylphenoxy)-dodeca-6(E),8(Z)-dien-5-yl-7-thio-4-oxo-
4H-1-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from (4R,5R)-
4,5-epoxy-1,1,1-trifluoro-12-(4-acetyl-3-hydroxy-2-propylphenoxy)-
dodeca-6(E),8(Z)-diene; yellow oil; Rf = 0.31 (hexane/ethyl acetate =
3:2).
~he starting material is prepared, for example, as follows:
a) (4R,5R)-4,5-epoxy-1,1,1-trifluoro-12-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-dodeca-6(E),8(Z)-diene
The title compound is prepared analogously to Example ld) from
1340~
- 66 -
3-(4-acetyl-3-hydroxy-2-propylphenoxy)-butyl-triphenylphosphonium
bromide (Example 62b) and (4R,5R)-4,5-epoxy-8,8,8-trifluoro-oct-2(E)-
enal (Example 86b); yellow oil; Rf = 0.64 (hexane/ethyl acetate = 3:2).
~xample 91: Sodium salt of (4R,5S)-1,1,1-trifluoro-4-hydroxy-12-(4-
acetyl-3-hydroxy-2-propylphenoxy)-dodeca-6(E),8(Z)-dien-
5-yl-7-thio-4-oxo-4H-1-benzopyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester (Example 90); m.p. 180-182~; [~]D~ (methanol,
0.292 %) = 77.1 + 3.4~;
~V (methanol): ~ (~) = 222 (47480), 231 (sh), 267 (24840), 285
max
~22120), 321 (15800).
~xample 92: (5R,6S)-5-hydroxy-12-(4-acetyl-3-hydroxy-2-propylphenoxy)-
dodeca-7(E),9(Z)-dien-5-yl-7-thio-4-oxo-4H-1-benzopyrane-
2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from (5R,6R)-
5,6-epoxy-12-(4-acetyl-3-hydroxy-2-propylphenoxy)-dodeca-7(E),9(Z)-
diene; light-yellow foam; Rf = 0.43 (hexane/ethyl acetate = 1:1);
[~]D~ (methanol, 0.150 %) = 22.0 + 6.7~; IR (methylene chloride):
3580, 2950, 1745, 1655, 1625, 1600 cm
~he starting material is prepared, for example, as follows:
a) (4R,5R)-4,5-epoxy-non-2(E)-enal
The title compound is prepared analogously to Example lb) from
(2R,3R)-2,3-epoxy-heptanol; yellow oil; Rf = 0.29 (hexane/ethyl acetate
= 4:1); [~]D~ (chloroform, 0.390 %) = 21.3 + 2.6~; IR (methylene
chloride): 2950, 2920, 2860, 1690, 1640, 1100, 970 cm
b) (5R,6R)-5,6-epoxy-12-(4-acetyl-3-hydroxy-2-propylphenoxy)-dodeca-
7(E),9(Z)-diene
The title compound is prepared analogously to Example ld) from
3-(4-acetyl-3-hydroxy-2-propylphenoxy)-propyl-triphenylphosphonium
bromide (Example 1c) and (4R,5R)-4,5-epoxy-non-2(E)-enal; light-yellow
oil; [~]D~ (chloroform, 0.650 %) = 23.7 + 1.5~.
13~0i63
- 67 -
~xample 93: Sodium salt of (5R,6S)-5-hydroxy-12-(4-acetyl-3-hydroxy-
2-propylphenoxy)-dodeca-7(E),9(Z)-dien-6-yl-7-thio-4-oxo-
4H-l-benzopyrane-3-carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester (Example 92); m.p. 205-207~; [~]D~ (methanol,
0.278 %) = 115.1 + 3.6~;
UV (methanol): ~m (E) = 222 (50960), 232 (sh), 267 (27400), 285
(24000), 321 (16400).
~xample 94: (5R,6S)-5-hydroxy-12-(4-acetyl-3-hydroxy-2-propylphenoxy)-
dodeca-7(E),9(Z)-dien-6-yl-7-thio-4-oxo-4H-l-benzopyrane-
2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(5S,6S)-5,6-epoxy-12-(4-acetyl-3-hydroxy-2-propylphenoxy)-dodeca-
7(E),9(Z)-diene; colourless foam; [~]D~ (methanol, 0.260 %) =
136.2 + 3.8~;
W (methanol): ~m (E) = 221 (48040), 271 (28320), 327 (13200).
~he starting material is prepared, for example, as follows:
a) (4S,5S)-4,5-epoxy-non-2(E)-enal
The title compound is prepared analogously to Example lb) from
(2S,3S)-2,3-epoxy-heptanol; yellow oil; Rf = 0.27 (hexane/ethyl
acetate = 5:1); [~]D~ (chloroform, 0.325 %) = 23.1 + 3.0~.
b) (5S,6S)-5,6-epoxy-12-(4-acetyl-3-hydroxy-2-propylphenoxy)-dodeca-
7(E),9(Z)-diene
The title compound is prepared analogously to Example ld) from
3-(4-acetyl-3-hydroxy-2-propylphenoxy)-propyltriphenylphosphonium
bromide (Example lc) and (4S,5S)-4,5-epoxy-non-2(E)-enal; light-yellow
oil; [~]D~ (chloroform, 0.600 %) = 24.8 + 1.6~.
.
l~iO463
- 68 -
~xample 95: Sodium salt of (5S,6R)-5-hydroxy-12-(4-acetyl-3-hydroxy-2-
propylphenoxy)-dodeca-7(E),9(Z)-dien-6-yl-7-thio-4-oxo-4H-
l-benzopyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester (Example 94); m.p. 204-206~; [~]D~ (methanol,
0.570 %) = 121.1 + 1.8~;
UV (methanol): ~max(E) = 222 (51240), 235 (sh), 267 (27360), 284
(21400), 320 (16400).
~xample 96: (5R,6S)-5-hydroxy-14-(4-acetyl-3-hydroxy-2-propylphenoxy)-
tetradeca-7(E),9(Z)-dien-6-yl-7-thio-4-oxo-4H-1-benzo-
pyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example l from
(5R,6R)-5,6-epoxy-14-(4-acetyl-3-hydroxy-2-propylphenoxy)-tetradeca-
7(E),9(Z)-diene; light-yellow viscous oil; [~]D~ (chloroform,
0.424 %) = 66.5 + 2.4~;
~V (chloroform): ~ (E) = 270 (28560), 288 (sh), 325 (15240).
max
~he starting material is prepared, for example, as follows:
a) (5R,6R)-5,6-epoxy-14-(4-acetyl-3-hydroxy-2-propylphenoxy)-tetradeca-
7(E),9(Z)-diene
The title compound is prepared analogously to Example ld) from
3-(4-acetyl-3-hydroxy-2-propylphenoxy)-pentyl-triphenylphosphonium
bromide (Example 29a) and (4R,5R)-4,5-epoxy-non-2(E)-enal
(Example 92a); light-yellow oil; [~]D~ (chloroform, 0.441 %) =
20.6 + 2.3~.
~xample 97: Sodium salt of (5R,6S)-5-hydroxy-14-(4-acetyl-3-hydroxy-2-
propylphenoxy)-tetradeca-7(E),9(Z)-dien-6-yl-7-thio-4-oxo-
4H-1-benzopyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester (Example 96); m.p. 193-195~; [~]D~ (methanol,
0.284 ~0) = 71.1 + 3.5~.
UV (methanol): ~max(E) = 222 (49320), 232 (sh), 267 (25520), 286
(22680), 321 (16000).
- 69 - 13 40~ 3
~xample 98: (5S,6R)-5-hydroxy-14-(4-acetyl-3-hydroxy-2-propylphenoxy)-
tetradeca-7(E),9(Z)-dien-6-yl-7-.hio-4-oxo-4H-l-benzo-
pyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(5S,6S)-5,6-epoxy-14-(4-acetyl-3-hydroxy-2-propylphenoxy)-tetradeca-
7(E),9(Z)-diene; light-yellow viscous mass; [~]D~ (chloroform,
0.463 %) = 79.0 + 2.2~;
UV (chloroform): ~max(~) = 270 (27120), 288 (sh), 326 (14960)-
~he starting material is prepared, for example, as follows:
a) (5S,6S)-5,6-epoxy-14-(4-acetyl-3-hydroxy-2-propylphenoxy)-tetradeca-
7(E),9(Z)-diene
The title compound is prepared analogously to Example ld) from
3-(4-acetyl-3-hydroxy-2-propylphenoxy)-pentyl-triphenylphosphonium
bromide (Example 29a) and (4S,5S)-4,5-epoxy-non-2(E)-enal
(Example 94a); light-yellow oil; [~]D~ (chloroform, 0.472 %) =
18.8 + 2.1~.
~xample 99: Sodium salt of (5S,6R)-5-hydroxy-14-(4-acetyl-3-hydroxy-2-
propylphenoxy)-tetradeca-7(E),9(Z)-dien-6-yl-7-thio-4-oxo-
4H-l-benzopyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester (Example 98); m.p. 193-195~; [~]D~ (methanol,
0.296 %) = 65.9 + 3.4~.
UV (methanol): ~max(~) = 222 (49760), 232 (sh), 267 (25800), 285
(22520), 320 (16000).
~xample 100: (5R,6S)-l,l,l-trifluoro-5-hydroxy-14-(4-acetyl-3-hydroxy-
2-propylphenoxy)-tetradeca-7(E),9(Z)-dien-6-yl-7-thio-4-
oxo-4H-1-benzopyrane-2-carboxylic acid methyl ester
The title compound is prepared analogously to Example 1 from
(5R,6R)-5,6-epoxy-1,1,1-trifluoro-14-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-tetradeca-7(E),9(Z)-diene; light-yellow oil; Rf = 0.32
(hexane/ethyl acetate = 3:2).
_ 70 _ 1 3 4 0463
The starting material is prepared, for example, as follows:
a) (5R,6R)-5,6-epoxy-1,1,1-trifluoro-14-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-tetradeca-7(E),9(Z)-diene
The title compound is prepared analogously to Example ld) from
3-(4-acetyl-3-hydroxy-2-propylphenoxy)-pentyl-triphenylphosphonium
bromide (Example 29a) and (4R,5R)-4,5-epoxy-9,9,9-trifluoro-2(E)-enal
(Example 86a); yellow oil; Rf = 0.72 (hexane/ethyl acetate = 3:2).
~xample 101: Sodium salt of (5R,6S)-1,1,1-trifluoro-5-hydroxy-14-(4-
acetyl-3-hydroxy-2-propylphenoxy)-tetradeca-7(E),9(Z)-
dien-6-yl-7-thio-4-oxo-4H-1-benzopyrane-2-carboxylic acid
The title compound is prepared analogously to Example 2 from the corre-
sponding methyl ester (Example 100);
~V (methanol): ~ (~) = 222 (48800), 235 (sh), 267 (25920), 285
max
~22920), 320 (16000).
Example 102: In a manner analogous to that described in Examples 1
to 77 it is also possible to prepare:
the sodium salt of (lR,2S)-1-hydroxy-1-phenyl-11-(4-acetyl-3-hydroxy-
2-propylphenoxy)-undeca-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-1-benzo-
pyrane-2-carboxylic acid;
the sodium salt of (lR,2S)-1-hydroxy-1-phenyl-10-(4-acetyl-3-hydroxy-
2-propylphenoxy)-deca-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-1-benzo-
pyrane-2-carboxylic acid;
the sodium salt of (lR,2S)-1-hydroxy-1-phenyl-9-(4-acetyl-3-hydroxy-2-
propylphenoxy)-nona-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-1-benzopyrane-
2-carboxylic acid;
the sodium salt of (lR,2S)-1-hydroxy-1-(3-chlorophenyl)-9-(4-acetyl-3-
hydroxy-2-propylphenoxy)-nona-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-1-
benzopyrane-2-carboxylic acid;
. . .
1340A~3
- 71 -
the sodium salt of (lR,2S)-l-hydroxy-1-(3-chlorophenyl)-11-(4-acetyl-3-
hydroxy-2-propylphenoxy)-undeca-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-l-
benzopyrane-2-carboxylic acid;
the sodium salt of (lR,2S)-l-hydroxy-1-(3-fluorophenyl)-8-(4-acetyl-3-
hydroxy-2-propylphenoxy)-octa-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-l-
benzopyrane-2-carboxylic acid;
the sodium salt of (lR,2S)-l-hydroxy-1-(3-fluorophenyl)-9-(4-acetyl-3-
hydroxy-2-propylphenoxy)-nona-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-l-
benzopyrane-2-carboxylic acid;
the sodium salt of (lR,2S)-l-hydroxy-1-(3-fluorophenyl)-10-(4-acetyl-
3-hydroxy-2-propylphenoxy)-deca-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-l-
benzopyrane-2-carboxylic acid;
the sodium salt of (lR,2S)-l-hydroxy-1-(3-fluorophenyl)-11-(4-acetyl-3-
hydroxy-2-propylphenoxy)-undeca-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-l-
benzopyrane-2-carboxylic acid;
the sodium salt of (lR,2S)-l-hydroxy-1-(3-methoxyphenyl)-9-(4-acetyl-3-
hydroxy-2-propylphenoxy)-nona-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-l-
benzopyrane-2-carboxylic acid;
the sodium salt of (lR,2S)-l-hydroxy-1-(3-methoxyphenyl)-11-(4-acetyl-
3-hydroxy-2-propylphenoxy)-undeca-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-
l-benzopyrane-2-carboxylic acid;
the disodium salt of (lR,2S)-l-hydroxy-1-(3-carboxyphenyl)-8-(4-acetyl-
3-hydroxy-2-propylphenoxy)-octa-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-l-
benzopyrane-2-carboxylic acid;
the disodium salt of (lR,2S)-l-hydroxy-1-(3-carboxyphenyl)-9-(4-acetyl-
3-hydroxy-2-propylphenoxy)-nona-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-l-
benzopyrane-2-carboxylic acid;
1340463
the disodium salt of (lR,2S)-l-hydroxy-1-(3-carboxyphenyl)-10-(4-
acetyl-3-hydroxy-2-propylphenoxy)-deca-3(E),5(Z)-dien-2-yl-7-thio-4-
oxo-4H-l-benzopyrane-2-carboxylic acid;
the disodium salt of (lR,2S)-l-hydroxy-1-(3-carboxyphenyl)-11-(4-
acetyl-3-hydroxy-2-propylphenoxy)-undeca-3(E),5(Z)-dien-2-yl-7-thio-
4-oxo-4H-l-benzopyrane-2-carboxylic acid;
the sodium salt of (lR,2S)-l-hydroxy-1-(3-methoxycarbonylphenyl)-9-(4-
acetyl-3-hydroxy-2-propylphenoxy)-nona-3(E),5(Z)-dien-2-yl-7-thio-4-
oxo-4H-l-benzopyrane-2-carboxylic acid;
the sodium salt of (lR,2S)-l-hydroxy-1-(3-methoxycarbonylphenyl)-10-(4-
acetyl-3-hydroxy-2-propylphenoxy)-deca-3(E),5(Z)-dien-2-yl-7-thio-4-
oxo-4H-l-benzopyrane-2-carboxylic acid;
the sodium salt of (lR,2S)-l-hydroxy-1-(3-methoxycarbonylphenyl)-11-
(4-acetyl-3-hydroxy-2-propylphenoxy)-undeca-3(E),5(Z)-dien-2-yl-7-
thio-4-oxo-4H-l-benzopyrane-2-carboxylic acid;
the sodium salt of (lR,2S)-l-hydroxy-1-(3,4-dichlorophenyl)-10-(4-
acetyl-3-hydroxy-2-propylphenoxy)-deca-3(E),5(Z)-dien-2-yl-7-thio-4-
oxo-4H-l-benzopyrane-2-carboxylic acid;
the sodium salt of (lR,2S)-l-hydroxy-1-(2,4-dichlorophenyl)-10-(4-
acetyl-3-hydroxy-2-propylphenoxy)-deca-3(E),5(Z)-dien-2-yl-7-thio-4-
oxo-4H-l-benzopyrane-2-carboxylic acid;
the sodium salt of (lR,2S)-l-hydroxy-1-(3,4-dimethoxyphenyl)-10-(4-
acetyl-3-hydroxy-2-propylphenoxy)-deca-3(E),5(Z)-dien-2-yl-7-thio-4-
oxo-4H-l-benzopyrane-2-carboxylic acid;
the sodium salt of (lR,2S)-l-hydroxy-1-(2,4-dimethoxyphenyl)-10-(4-
acetyl-3-hydroxy-2-propylphenoxy)-deca-3(E),5(Z)-dien-2-yl-7-thio-4-
oxo-4H-l-benzopyrane-2-carboxylic acid;
1340463
the sodium salt of (lR,2S)-1-hydroxy-1-(2,4-dimethylphenyl)-10-(4-
acetyl-3-hydroxy-2-propylphenoxy)-deca-3(E),5(Z)-dien-2-yl-7-thio-4-
oxo-4H-l-benzopyrane-2-carboxylic acid;
the sodium salt of (lR,2S)-l-hydroxy-1-(3-dimethylaminophenyl)-10-(4-
acetyl-3-hydroxy-2-propylphenoxy)-deca-3(E),5(Z)-dien-2-yl-7-thio-4-
oxo-4H-l-benzopyrane-2-carboxylic acid.
Example 103: In a manner analogous to that described in Examples 1
to 101 it is also possible to prepare the following compounds:
the sodium salt of (lR,2S)-l-hydroxy-1-(3-bromophenyl)-8-(4-acetyl-3-
hydroxy-2-propylphenoxy)-octa-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-l-
benzopyrane-2-carboxylic acid;
the sodium salt of (lR,2S)-l-hydroxy-1-(3-bromophenyl)-9-(4-acetyl-3-
hydroxy-2-propylphenoxy)-nona-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-l-
benzopyrane-2-carboxylic acid;
the sodium salt of (lR,2S)-1-hydroxy-1-(3-bromophenyl)-10-(4-acetyl-3-
hydroxy-2-propylphenoxy)-deca-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-l-
benzopyrane-2-carboxylic acid;
the sodium salt of (lR,2S)-1-hydroxy-1-(3-bromophenyl)-11-(4-acetyl-3-
hydroxy-2-propylphenoxy)-undeca-3(E),5(Z)-dien-2-yl-7-thio-4-oxo-4H-l-
benzopyrane-2-carboxylic acid;
the sodium salt of (5R,6S)-1,1,1-trifluoro-5-hydroxy-13-(4-acetyl-3-
hydroxy-2-propylphenoxy)-trideca-7(E),9(Z)-dien-6-yl-7-thio-4-oxo-4H-
l-benzopyrane-2-carboxylic acid;
the sodium salt of (5R,6S)-1,1,1-trifluoro-5-hydroxy-15-(4-acetyl-3-
hydroxy-2-propylphenoxy)-pentadeca-7(E),9(Z)-dien-6-yl-7-thio-4-oxo-
4H-l-benzopyrane-2-carboxylic acid;
13~0463
the sodium salt of (5R,65)-5-hydroxy-13-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-trideca-7(E)~9(z)-dien-6-yl-7-thio-4-oxo-4H-l-benzopyrane-2
carboxylic acid;
the sodium salt of (5R,6S)-5-hydroxy-15-(4-acetyl-3-hydroxy-2-propyl-
phenoxy)-pentadeca-7(E),9(Z)-dien-6-yl-7-thio-4-oxo-4H-1-benzopyrane-
2-carboxylic acid;
the disodium salt of (4R,5S)-1-carboxy-4-hydroxy-11-(4-acetyl-3-
hydroxy-2-propylphenoxy)-undeca-6(E),8(Z)-dien-6-yl-7-thio-4-oxo-4H-1-
benzopyrane-2-carboxylic acid;
the disodium salt of (4R,5S)-1-carboxy-4-hydroxy-12-(4-acetyl-3-
hydroxy-2-propylphenoxy)-dodeca-6(E),8(Z)-dien-5-yl-7-thio-4-oxo-4H-
1-benzopyrane-2-carboxylic acid;
the disodium salt of (4R,5S)-1-carboxy-4-hydroxy-13-(4-acetyl-3-
hydroxy-2-propylphenoxy)-trideca-6(E),8(Z)-dien-5-yl-7-thio-4-oxo-4H-
1-benzopyrane-2-carboxylic acid;
the disodium salt of (4R,5S)-1-carboxy-4-hydroxy-14-(4-acetyl-3-
hydroxy-2-propylphenoxy)-tetradeca-6(E),8(Z)-dien-5-yl-7-thio-4-oxo-
4H-1-benzopyrane-2-carboxylic acid.
Examples of pharmaceutical preparations
and corresponding finished medicament forms
In the following the term "active ingredient" is to be understood as
being a compound of formula I according to the invention, especially a
compound described as a product in Examples 1 to 9, for example the
sodium salt of (lR,2S)-1-hydroxy-1-(3-trifluoromethylphenyl)-8-(4-
acetyl-3-hydroxy-2-propylphenoxy)-octa-3(E),5(Z)-dien-2-yl-7-thio-4-
oxo-4H-1-benzopyrane-2-carboxylic acid.
13404~3
Example A: An inhalation suspension, containing propellant and forming
a solid aerosol, containing 0.1 % by weight active ingredient
Composition: % by weight
active ingredient, micronised 0.1
sorbitan trioleate 0.5
Propellant A
(trichlorotrifluoroethane) 4.4
Propellant B
(dichlorodifluoromethane and 15.0
1,2-dichlorotetrafluoroethane) 80.0
Preparation: In the absence of moisture, the active ingredient is
suspended in trichlorotrifluoroethane using a customary homogeniser and
with the addition of the sorbitan trioleate, the suspension is
introduced into an aerosol container provided with a metering valve;
the container is sealed and filled up with propellant B under pressure.
Example B: An approximately 2 % aqueous solution, suitable for
inhalation, of an active ingredient in the form of its sodium or
potassium salt.
Composition:
active ingredient (K or Na salt)2000 mg
disodium salt of
ethylenediaminetetraacetic acid10 mg
benzalkonium chloride 10 mg
water, freshly distilled ad 100 ml
Preparation: The active ingredient is dissolved in approximately 60 ml
of freshly distilled water, and the stabiliser (disodium salt of
ethylenediaminetetraacetic acid) and the preservative (benzalkonium
chloride) are added. When all the components have completely dissolved,
the resulting solution is made up to 100 ml and introduced into small
.
13404G3
- 76 -
pressurised bottles which are then sealed in gas-tight manner. The
propellant is added, as required, in gaseous form under pressure or in
liquid form.
.
1340463
- 77 -
APPENDIX - PHARMACOLOGICAL TEST METHODS
Bronchoconstriction test on guinea pigs (in vivo, aerosol):
Male guinea pigs weighing 400-700 g are anaesthetised intraperitoneallywith 1.4 g/kg of urethane and a polyethylene cannula is inserted into
the jugular vein. A second polyethylene cannula is inserted into the
trachea. The pressure in the oesophagus is recorded by means of a
cannula that is inserted into the oesophagus and that is connected to a
Statham pressure transducer. The animal is placed in an airtight
plexiglass chamber which is connected to a Fleisch's tube No. 000 and a
Validyne transducer MP 45-1. This arrangement is used to measure the
flow.
After the surgical preparation of the test animals, a certain period oftime is allowed to elapse to enable the pulmonary functions to
stabilise. The test compound is then administered in accordance with
the following procedure: The test animals are exposed for one minute to
a 1 % aerosol solution of the test compound (weight/volume) or to
distilled water (for control purposes). For all the test compounds that
are administered by inhalation, a Monaghan ultrasound spray apparatus
(model 670) of which the particle size varies between 1 and 8 microns,
the majority being 3 microns, is used.
Aqueous solutions are freshly prepared each time and are introduced
into the chamber of the spray device using an On-stream drug vial. The
spray mist produced is administered to the experimental animals via a
glass chamber of 65 ml capacity which is connected to the trachea by a
cannula. When the treatment period has elapsed, LTD4 (0.3 ~g/ml) is
administered over a period of 2 minutes using a second Monaghan
ultrasound spray device (model 670) and via a similar glass chamber.
1340463
..
- 78 -
The reduction in compliance in the third minute after the LTD4 admini-
stration is read off and the average value of three animals is compared
with the average value of three control animals and the percentage
inhibition of compliance is calculated in accordance with the following
formula:
(100 - compliance preparation) x 100
% inhibition = 100 -
(100 - compliance control)
If different concentrations of active ingredient are tested, the
percentage inhibition for each concentration is recorded, the log
concentration on the abscissa being plotted against the percentage
inhibition on the ordinate. The ICso is then determined by linear
regression analysis.
In vitro test to determine the inhibition of phospholipase Az obtained
from human leucocytes
Neutrophilic polymorpho-nuclear human leucocytes are isolated from
"Buffy coats" by multi-step fractional sedimentation and are deep-
frozen. The phospholipase A2 is extracted from the cell suspension by
homogenisation with the addition of ice-cold 0.36N H2SO4 in 2N NaCl and
the supernatant obtained after centrifugation at 10,000 x g is dialysed
against sodium acetate buffer pH 4.5.
In order to determine the enzyme activity, enzyme (10-30 ~g of protein)
is incubated at 37~ for 1 hour in O.lM tris/HCl buffer pH 7 with the
addition of 1 mmol of CaClz and substrate consisting of phospholipids
(2 ~m) of Escherichia coli radioactively labelled biosynthetically with
14 C-oleic acid. The reaction is stopped by the addition of Dole reagent
(isopropanol/heptane/lN HzSO4 40:10:1, v/v) and the 14 C-oleic acid
freed selectively by phospholipase A2 is extracted. Substrate extracted
therewith is completely removed by filtration of the extract through a
column of silica gel. The determination Of 14 C-oleic acid in the eluate
is effected by radiometry.
1340~3
- 79 -
In order to detect an inhibitory action of test substances on phospho-
lipase A2, the test substances are added to the incubation mixture in
the form of solutions in water, dimethyl sulfoxide (final concentration
in the batch up to 5 ~~, v/v) or ethanol (final concentration in the
batch up to 2.5 %, v/v). The degree of action of the test substances is
expressed by the ICso~ that is to say the concentration which effects
inhibition of 50 % of the control activity. The ICso is determined
graphically by plotting the percentage inhibition on the ordinate
against the log of the concentration (~m) on the abscissa.
Under the described test conditions mepacrin inhibits phospholipase A2
with an ICso of 1600 ~m.
In vitro test to determine the inhibition of phospholipase C obtained
from human thrombocytes
Human thrombocytes are obtained from "Buffy coats" by fractional
centrifugation and are then deep-frozen. The phospholipase C is freed
by ultrasound treatment of the cell suspension and after ultracentri-
fugation (150,000 x g for 1 hour) is present in soluble form in the
supernatant.
In order to determine the enzyme activity, enzyme (20-100 ~g of
protein) is incubated at 37~ for 5 minutes in 0.025M tris/maleate
buffer pH 6 with the addition of 0.2 mmol of CaClz and 0.02 mmol of
radioactively labelled substrate, phosphatidyl-[14C]-inositol. The
reaction is stopped by shaking with CHCl3/CH30H 2:1 (v/v), in the
course of which unused substrate is extracted into the organic phase,
while the reaction product, 14 C-inositol phosphate, remains in the
aqueous phase and can be measured by radiometry of an aliquot.
In order to detect an inhibitory action of test substances on
phospholipase C, the test substances are added to the incubation
mixture in the form of solutions in water, dimethyl sulfoxide (final
concentration in the batch up to 5 %, v/v) or ethanol (final concen-
13404~
- 80 -
tration in the batch up to 2.5 %, v/v). The degree of action of the
test substances is expressed by the ICso~ that is to say the concen-
tration which effects inhibition of 50 % of the control activity. The
ICso is determined graphically by plotting the percentage inhibition on
the ordinate against the log of the concentration (~m) on the abscissa.
Under the described test conditions mepacrin inhibits phospholipase C
with an ICso of 20 ~m.