Note: Descriptions are shown in the official language in which they were submitted.
2~1389
-- 1
SPECIFICATION
TITLE OF THE INYENTION
PYRIMIDINEDiONE DERIVATIVE COMPOUNDS, METHOD OF PRODUCING
THE SAME AND ANTIARRYTHMIC AGENTS CONTAINING THE SAME
BACKGROUND OF THE IN~ENTiON
1. Field of the Invention
This invention relates to novel pyrimidinedione
derivatives and acid addition salts thereof, to methods of L'
producing the same and to pharmaceutical agents containing
the same, which are effective for the treatment of cardiac
dysfunctions such as arrhythmia and cardiac insufficiency.
2. Description of the Prior Art
The mechanism of the occurrence of arrhythmia is
camplicated. Abnormalities in stimulation production and
disorders in the conducting system or combinations thereof
are considered to be responsible.
As to disorders in excitation conduction, the re-entry
theory is representative.
One of the conditions of occurrence of arrhyttlmia is
irregularity in thc refractory period in various parts of
the heart. In addition, one-directional block, shortened
refractory period, delay in conduction, the presence of
circus movement are complicatedly involved.
Conventionally, varieties of antiarrythmic agents have
been used for the treatment of arrhythmia.
The antiarrythmic agents are classified into four
2~ 389
groups according to their mode of action.
Namely, E. M. Vaughan Williams (Vaughan Williams E. M.;
"Advances in dtulg research, vol. 9"; ed. by Harper N. J.,
Simmonds A. B., Academic Press, London, 1974; pages 69~
classified the antiarrythmic agents into the following four
groups according to their action against the action
potential of cardiac muscle or against the ionic current
which generates the action potential.
Class 1: Sodium channel depressors
These agents are efficacious ;n repressing a sodium
current. However, these agents have no or only minute effects
on the retention time of the normal action potential and
decrease the maximum rising velocity (YmaX) of the sodium
current. The antiarrythmic agents which belong to this
IS class have a high antiarrythmic activity but at the same
time strongly repress cardiac functions. Careful
consideration is required in administering to patients with
cardiac failure or hypotension.
Class 1l: Beta-blocking agents
The agents in this class, represented by propranolol,
are efficacious in the beta-blocking action and are useful
in treating patients with arrhythmia in which the
sympathetic nerve is involved. However, the care must be
taken for use since these agents have side-effects caused by
the beta-blocking action, such a.s depression of card;ac
fllnctions, induction of bronchial asthmatic attach and
_ 3 _ 20~''1389
hypoglycemic seizures.
Class III: Pharmaceutical agents for prolonging the
retention time of the action current.
These agents are efficacious in remarkably
prolonging the retention time of the action current of
the cardiac muscle and in prolonging an effective
refractory period. Re-entry arrhythmia is considered to
be suppressed by the action of the pharmaceutical agents
of Class III. The medicaments of this Class III include
amiodarone and bretylium. However, all the agents have
severe side effects; therefore, careful consideration is
required for use.
Class IV: Calcium antagonists
These agents control a calcium channel and suppress
arrhythmia due to automatic sthenia of sinoatrial nodes
and to ventricular tachycardia in which atrial nodes are
contained the re-entry cycle.
Among these antiarrythmic agents, pharmaceutical
agents of the Class III type are considered to be
particularly important and the most efficacious, and
known to be effective on ventricular arrhythmia, the most
fatal of all symptoms.
SUMMARY OF THE INVENTION
An object of an aspect of the present invention is
to provide a novel compound which is useful as a Class
III type antiarrythmic agent and to provide a process for
producing the same.
Another object of an aspect of the present invention
is to provide a novel compound which is effective in
improving cardiac dysfunction such as cardiac
insufficiency and a process for the preparation of the
same.
Another object of an aspect of the present invention
is to provide a pharmaceutical agent, which contains the
novel compound as an effective component, for the
treatment of cardiac dysfunctions such as arrythmic and
cardiac insufficiency.
2 0 0 1 3 8 9
Accordingly, an aspect of the invention provides
compounds of the general formula (1) shown below and acid
addition salts thereof, along with their pharmacological
properties. These compounds have pharmacological
characteristics to markedly prolong the retention time of
the action potential of cardiomuscular cells and to
markedly prolong the ventricular refractory period.
Furthermore, the compounds of this invention have a
positive inotropic action and are useful as therapeutic
agents for cardiac insufficiency.
According to another aspect of the invention,
compounds of the invention are represented by Formula
(1):
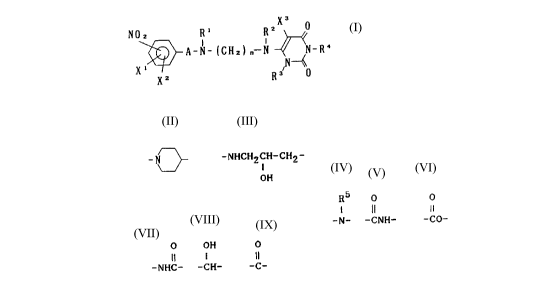
X3
N0z R' RZ ~ 0
X ~ A-N-(CH2) n - N ~ ~N-R4 (1)
X2 R3 ~
in which A represents -(CH2)m-, -B-(CH2)k-,
-D-(CH2)1 ~ -N3 or -NHCH2CH-CH2-;
OH
R5 O O
11 11
B represents an oxygen or a sulfur atom, -N-, -CNH- or -CO-;
O OH O
Il l 11
D represents -NHC-, -CH- or -C-;
R1 and R2 each independently represent a hydrogen atom, a
lower alkyloxycarbonyl group, an unsaturated lower alkyl
group or a lower alkyl group, any one of the hydrogen
atoms of said alkyl groups may be substituted by a group
selected from the group consisting of a hydroxy group; a
.. ~
_ ~ - 5 ~ 2 ~ ~ ~ 3 8 9
lower monoalkylamino group; a lower dialkylamino group; a
lower alkyloxy group; a lower alkanoyloxy group; a
benzoyloxy group; a benzoyloxy group substituted by a
halogen atom or a lower alkyloxy group; a phenyl group; a
phenyl group substituted by a halogen atom or a lower
alkyloxy group; and a lower alkyloxycarbonyl group, or
and R2 may be so linked as to make an alkylene chain and
thus form a heterocyclic structure;
R3 and R4 each independently represent a hydrogen atom, or
a lower alkyl group;
X1 and x2 each independently represent a hydrogen atom,
-CO-R6, a halogen atom, a lower alkyl group, a halogen-
substituted lower alkyl group, a hydroxy group, a lower
alkyloxy group, a lower alkylthio group, a lower
alkyloxycarbonyl group, a carboxy group, a cyano group,
an amino group, a lower alkanoyloxy group, a lower
alkanoylamino group, a lower alkylsulfonamido group, a
lower mono- or di-alkylamino group, a phenyl-substituted
lower alkylamino group or an unsaturated lower alkyloxy
group;
X3 represents a hydrogen atom, a nitro group, a methyl
group or a cyano group; R5 represents a hydrogen atom, a
lower alkanoyl group, a lower alkylsulfonyl group or a
lower alkyl group, or R1 and R5 may be so linked as to
make an alkylene chain and thus form a heterocyclic
structure;
R6 represents a lower alkyl group, a cycloalkyl group or a
phenyl group, said phenyl group may be substituted by
either one or two of groups selected from the group
consisting of a halogen atom, a lower alkyl group, a
hydroxy group and a lower alkyloxy group, or a
heterocyclic ring;
n represents an integral number 2 or 3; m represents an
integral number, O, 1, 2, 3 or 4; k represents an
integral number, 2, 3 or 4; and l represents an integral
number, O, 1, 2, 3 or 4.
2 0 û ~ 3 8 9
-5a-
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
Compounds according to the present invention is
those of the general formula (1) shown below and acid
addition salts thereof. More specifically, the compounds
are described in Examples thereinafter as preferred
embodiments.
N02 A-N-(CH2) n - N ~ -R~ .. ~1
X2 R3' ~
In the formula. A represents -(CH2)m-, -B-(CH2)k-,
-D-(CH2)1-. -N ~ OI -NHCH2CH-CH~-;
OH
~5 O O
11 ll
B represents an oxygen or a sulfur atom, -N-, -CNH- or -CO-;
o OH ~
Il l 11
D represents -NHC-, -CH- or -C-;
Rl and R2 each independently represent a hydrogen atom~ a
lower alkyloxycarbonyl graup, an unsaturated lower alkyl
389
- group or a lower alky1 group, any one of the hydrogen atoms
of said alkyl groups may be substituted by a group selected
from the group consisting of a hydroxy group; a lower
monoalkylamino group; a lower dialkylamino group; a lower
alkyloxy group; a lower alkanoyloxy group; a benzoyloxy
group; a benzoylvxy group substituted by a halogen atom or
a lower alkyloxy group; a phenyl group; a phenyl group
substituted by a halogen atom or a lower alkyloxy group; and
a lower alkyloxycarbonyl group, or Rl and R2 may be so
linked as to form an alkylene chain and thus form a
heterocyclic structure;
R3 and R4 each independently represent a hydrogen atom or a
lower alkyl group;
xl and X~ ea~h independently represent a hydrogen atom,
-CO-Rfi, a halogen atom, a lower alkyl group, a halogen-
substituted lower alkyl group, a hydroxy group, a lower
alkyloxy group, a lower alkylthio group, a lower
alkyloxycarbonyl, groupacarboxylgroup, a cyano group, an amino
group, a lower alkanoyloxy group, a lower alkanoylamino
group. a lower alkylsulfonamido group, a lower mono- or di-
alkylamino group, a phenyl-substituted lower alkylamino
group or an unsaturated lower alkyloxy group;
X3 represents a hydrogen atom, a nitro group, a methyl group
or a cyano group; R5 represents a hydrogen atom, a lower
alkanoyl group, a lower alkylsulfonyl group or a lower alkyl
group, or R1 and R5 may be so linked as to form an alkylene
2~ 89
chain and thus form a heterqcyclic structure;
R6 represents a lower alkyl group, a cycloalkyl group or a
phenyl group, said phenyl group may be substituted by either
one or two of groups independently selected from a
halogen atom, a lower alkyl group, a hydroxy group and a
lower alkyloxy group, or a heterocyclic ring;
n represents an integral number 2 or 3: m represents an
integral number, 0, 1, 2, 3 or 4; k represents an integral
number, 2, 3 or 4; and 1 represents an integral number. 0,
1, 2, 3 or 4.
In the above formula (1), examples of the unsaturated
lower alkyl group include vinyl, allyl and propargyl groups.
Examples of the lower alkyl group include linear- or
branched-alkyl groups having 1 - 5 carbon atoms, such as
methyl, ethyl, propyl, butyl, isopropyl, isobutyl, tertiary-
butyl and secondary-butyl groups.
Examples of the lower alkyl group substituted by a
hydroxyl group include 2-hydroxyethyl, 3-hydroxypropyl, 2-
hydroxyprqpyl and 4-hydroxy-butyl groups.
Examples of the lower alkyl group substituted by a
lower monoalkylamino group include 2-(methylamino)ethyl, 3-
(methylamino)propyl and 2-(ethylamino3ethyl groups.
Examples of the lower alkyl group substituted by a
lower dialkylamino group include 2-(dimethylamino)ethyl, 2-
(diethylamino)ethyl and 3-(dimethylamino)propyl groups.
An example of the lower alkyloxy group is an oxygen
- 8 - ~ 0 ~ ~ ~ 8 ~
atom substituted by a lower alkyl group described above.
Examples of the lower alkanoyloxy group include
acetyloxy, propionyloxy, ~L~loxy~isobutyryloxY and pivaloyloxy
groups.
Examples of the lower alkyl group substituted by a
benzoyloxy group include 2-benzoyloxYethyl~ 3-
benzoyloxypropyl, ~-benzoyloxypropyl and 2-benzoyloxy-1-
methylethyl groups.
Examples of the lower alkyl group substituted by a
phenyl group include benzyl, 2-phenylethyl and 3-
phenylpropyl groups.
An example of the lower alkyl group in the lower
alkyloxycarbonyl group is that identical with the lower
alkyl group described above.
Examples of the halogen atom include fluorine,
chlorine, bromine and iqdine atoms.
No limitation is required of substitution sites in the
halogen-substituted lower alkyl group; the lower alkyl group
substituted by a substituting group selected from the group
consisting of the lower alkyloxy group. the lower
alkanoyloxy group. the benzoyloxy group substituted by a
halogen atom or a lower alkyloxy group, the phenyl group
substituted by a halogen atom or a lower alkyloxy group and
the lower alkyloxycarbonyl group; the benzoyloxy group
substituted by a halogen atom or a lower alkyloxy group; and
the phenyl group substituted bY a halogen atom or a lower
.... .
-
2~ 389
alkyloxy group.
Examples of the lower alkylthio group include sulfur
atoms substituted by the lower alkyl group mentioned above.
Examples of the lower alkanoylamino group include
acetylamino and propionylamino groups.
Examples of the lower alkylsulfonamido group include
methanesulfonamido and ethanesulfonamido groups.
Examples of the mono- or di-lower alkylamino group
include methylamino, ethylamino, dimethylamino and
diethylamino groups.
Examples of the phenyl group-substituted lower
alkylamino group include the above-mentioned alkylamino
groups which are further substituted by phenyl group. No
limitation is required in this substitution.
~xamples of the unsaturated lower alkyloxy group
include vinyloxy, allyloxy and propargyloxy groups.
Examples of the alkyl chain to link Rl and R2 or ~1 and
R5 include ethylene and propylene chains.
Examples of the lower alkanoyl group include formyl,
acetyl, propionyl and butyryl and pivaloyl groups.
Examples of the lower alkylsulfonyl group include
methanesulfonyl and ethanesulfonyl g~oups.
Examples of the cycloalkyl group include cyclopentyl
and cyclohexyl groups.
Examples of the heterocyclic group as ~6 include
pyridyl, pyrazolyl, pyrimidinyl, thienyl, furyl and pyrrolyl
Z~89
gI-O UpS .
No limitation is required of the substitution sites in
the phenyl grqup substituted by a substituting group in R6.
The expression "pharmaceutically acceptable" as used to
describe the pharmaceutically acceptable acid addition salts in the
compounds of the general formula (1) described above means
not to have remarkable side effects or absence of toxicity
and not to reduce the pharmaceutical activities, when
administered to man. These acid addition salts can be
produced by neutralization of the corresponding free bases.
~ xamples of the acids from which these pharmaceutically
acceptalbe salts can be prepared include organic acids or
inorganic acids, such as hydrochloric acid, hydrobromic
acid, phosphoric acid, sulfuric acid, nitric acid,
.
methanesulfonic acid, maleic acid, oxalic acid, malonic
acid, succinic acid, fumaric acid, tartaric acid, citric
acid, lactic acid. and benzenesulfonic acid.
The concrete examples of the compounds of the general
formula (1) include the following compounds.
2~1389
1. I,3-dimethyl-6-[2-~4-nitrqanilino~ethylamino]-2,~-
(lH,3H)-p~rimidinedione
2. 1,3-dimethyl-6-[3-(~-nitroanilino)propylamino]-
2,4(1H,3H)-pyrimidineditne
3. 1,3-dimethyl-6-[4-(4-nitrophenyl)piperazin-1-yl]-
2,4(1H,3H)-pyrimidinedione
. 1,3-dimethyl-fi-[N-ethyl-2-(4-nitroanilino)ethyl-
amino~-2,~(1H,3H')-pyrimidinedione
.~. 1,3-dimethyl-6-[2-(N-meth~ nitroanilino~ethyl-
amino)]-2.~1H.3H'-pyrimidinedione
6. 1,3-dimethyl-6-~ 4-nltrophenyl!homo~ipera~in-
]-yl~-2.4(1H.3HI-pyrimidinedione
7. 1,3-dimethyl-6-~2-(4-nitro~enzylamino~ethyl-
amino]-2,4~1H,3H)-pyrimidinedlone
8. 1,3-dimethyl-6-~3-(~-nitrobenzylamino)Dr.)~yl-
amino)]-2,~(1H,3H)-p~rimidinedione
. 1,3-dimethyl-6-[4-(4-nittlobenzyl)piperazin-
1-yl]-2,~(1H,3H)-pyrimidinedione
10. 1,3-dimethyl-6-[N-propyl-2-;1-nitrobenzylamino)-
ethYlamino~-2.4(lH~3H~-pyrimidinedione
11. 1,3-dimethyl-6-[2-~N-ethvl-4-nitr-)benzylamino)-
ethylamino)~ llH.3H~-pyl-imidinedione
12. 1,3-dimethyl-6-~-[~ 2-hYdro-x~ethy~
nitroben7.ylamino~ethylamino'~-2.~1H.3H!-
pyrimidinedione
13. 1.3-dimethyl-fi-~2-~J--.~-nitrophenvl)ethylamino~-
Z~1389
- 12 -
ethylamino~-2,~(1H,3H)-pyrimidinedione
14. 1,3-dimethyl-6-~3-t2-14-nitrophenyl)eth~laminn~-
propylamino}-2,~(1H,3H)-pyrimidinedione
15. 1,3-dimethy]-6-{N-(2-hydroxyethyl)-2-t2-~4-
nitrophenyl)ethylamino]ethylamino~-2,~(1H,3H)-
pyrimidinedione
16. 1,3-dimethyl-6-~2-tN-ethyl-2-(4-nitrophenYl)-
ethylamino]ethylamino~-2~4(1H.3H!-pyrimidinedione,
17. 1.3-dimethyl-Ç-~2-~N-(2-hydroxyethyl)-2-(~-nitro-
phenyl)ethylamino]ethylamino~-2,4~1H.3H)-
pyrimidinedione
18. 1.3-dimethyl-6-{~-[2-~4-nitl-ophenyl'~ethyl]-
piperazin-l-yl~-2.~(IH.3H)-~yrimidinedione
19 . 1, 3-d i methyl -6- ~4- t2- ( 4-ni tl-ophenyl !ethyl ] -
hnmopiperazin-l-yl~-2?4(1H,~H)-pyrimidinedinne
20. 1,3-dimethyl-6-~2-[N-~'2-acetoxyethyl)-5-l~-nitl(,-
phenyl~ethylamino]ethylamino~-2,~(IH,3H)-
pyrimidinedione
21. 1,3-dimethyl-6-{2-~N-1'3-hydroxypropyl)-2-
~nitrophenyl)ethY]amino]ethylamino'~-2.~(]H.3H!-
pyrimidinedione
22. 1.~-dimethyl-6-~2-~N-~'3-benzoyloxypropyl!-2-(~-
nitrophenyl~ethylamino~ethylamino~-2.~(1H.3H)-
pyrimidinedlone
23. 1.3-dimethyl-6-~-[2-~3-nitro~henvl~eth
piperazin-l-yl ~-2.4~1H.3H!-pYr~ idinedione
2~1389
- 13 -
2~. 1,3-dimethyl-6-{~-~2-~2-nitrophenyl)ethY
piperazin-1-yll-2,1(1H,~H~-pylimidinedione
25. 3-methyl-6-~-t2-(4-nitrophenyl)ethyl]piperazin-
1-yl}-2,4(1H,3H)-pyrimidinedinne
26. 1-methyl-6-~-t2-(~-nitrophenyl)ethyl~piperazin-
I-yl3-2,4(1H,3H~-pyrimidinedione
27. 1,3-diethyl-6-~2-[N-~'2-hydroxvethvl)-2-~4-
nitrophenyl!ethylaminc~ethylaminc,'~ 'lH.3H~-
pyrimidinedione
28. 1.3-diisopropyl-G-~2-rN-~'2-hYdrc,xyethYl!-2-~-
nitrophenyl)ethylaminn]ethylamin~ IH.3H~-
pyrimidinedione
29. 1,3-dimethyl-fi-{2-t3-(4-nitr-)phenyl~propylamino~-
ethylamino~-2~4(1H.3H)-pyrimidinedione
30. 1,3-dimethyl-6-~3-~3-(~-nitroDhenyl)proipylamino]-
propylamino~-2.4(1H.3H)-pyrimidlnedione
31. 1,3-dimethyl-6-{N-methyl-2-t3-(~-nitrophenyl)-
propylamino~ethylamino'~ ('lH.3H)-pyrimidinedione
3~. l,3-dimethyl-~-{~-ethyl-~-~3-~-nitro~henYl)-
propy]amino~ethylamino~ (1H.3H!-pyrimidinedione
33. I,3-dimethyl-fi-i~'-pro~yl-~-t3-~-nitriphenvl)-
propylamino~ethylamin-,~ ]H.3H!-pyrimidinedi-Jne
3~. I.3-dimethyl-fi-~N-~3-hYdro-x~ethVl~-'-t3-~-
nitrophenyl?propylamln~ ethylamincl~ (lH~3H?-
pyrlmidinedion~
3~. I,3-dimethyl-6-~N-~ hYdroxypl~-3py~ - t3- ( ~-
2~1~1389
- 14 -
nitr~iphenyl)propylamino]ethylamino~-7,~IH.3H~-
pylimidinedione
36. I~3-dimethyl-6-{N-(~2-hydroxy-I-methylethyl~-2-
t3-(4-nitrophenyl)prnpylamino~ethylamino~-~7,4-
(IHs3H)-pyrimidinedione
3~. 1,3-dimethyl-6-~N-~2-acetoxyethyl)-2-[3-(~- -
nitrnr3henyl)r3ropylamino~ethylamino'~-2.~-
~IH.3H~-pyrimidinedione
38. 1.3-dimethyl-6-{~-methoxycarbonylmethyl-2-t3-~l-
nitrophenyl~proDylamino~eth~lamln~-2.1~lH.3H!-
pyrimidinedione
39. I,3-dimethyl-6-{~-''2-phenylethyl)-2-~3-'~-nitro-
phenyl)pl~opylamino]ethylamino~-2,4-(1H,3H)-
pyrimidinedi~ne
40. 3,3-dimethyl-6-~2-~N-methy]-3-~-nitrophenyl)-
propylamino]ethylamino~-2,~1H,3H~-pyrimidinedi(lne
~1. 1,3-dimethyl-6-{2-<N'-ethyl-N-t3-~-nitror3henyl!-
propyl]amino>ethylamino~-2,~('IH.3H)-pyrimidinedi-3ne
~'7. 113-dimethyl-6-~2-[N-propyl-3-~4-nitl~oDhenyl~-
propylaminr33ethylamin-3~-2.~1H.3H)--7yrimidinedil~ne
~3. 1.3-dimethyl-6-~- r~- (l-meth~]ethyl!-3-(~-
nitrophenyl)propylamlnolethylamino'~ lHt3H)-
pyrimidinedi-ne
. 1,3-dimethyl-~ -tN-butyl-3-'4-nitrophenyl)-
propylamino~ethvlamin()l-~ '2.~(1H.3H)-
pyrimidinedi-~ne
20~1389
, 5
15. 1,3-dimethyl-fi-~2-t~ tert-butyl~-3(~-nitrnphenyl)-
propylamino]ethylamino~ ,1(1H,3H~-pyrimidinedione
~6. 1,3-dimethyl-6-{2-<N-(2-hydroxyethyl)-N-[3-
~nitrophenyl)pl-cpyl~amino>ethy1amina~-2.4(~H~3H~-
pyrimidinedione
4~ . 1 ,3-dimethyl-6-~ ~-rN-~3-hydl-oxypr~pyl !-3-(~-
nitrophen~l)propylamino]ethylaminll~-2~/1H.3H)-
pyrimidinedione
. 1.3-dimethyl-6-~'2-[N-.~-hv~roxy-l-meth~lethyl)-
3-('4-nitrophenyllplopylamina~ethyl~mino'~
~~1H,3H)-~yrimidinedione
~9. 1.3-dimethyl-6-~-[~-(2-hydroxypropyl--3-('4-
nitrophenyl)pro~ylamino]ethylamino}-2.~(1H.3H'-
pyrimidinedione
5~. 1,3-dimethyl-6-~2-~ -hydroxybutyl)-3-(~-
nitrophenyl)propylamincl~ethylamino'~ lH.3h' -
pyrimidinedione
51. 1,3-dimethyl-6-~ -acetoxyethylj-3-~.l-nitl-o-
pheny]!~ropylamino]ethylamin-J'~-2~1H.3H~-
pyrimidinedi.-,n~
. 1.3-dimethYl-6-~ N-(2-f~llmylc!x'~ t~lyl !-;~-~4-
nitronhenyl~ranylamino]ethylamino~-~J.~(lH.3H)-
pyrimidinedione
~3. 1.~-dimethyl-6-~-[N-(2-~ropionylaxyethyl)-3-'~-
nitrophenvl )pr(pylamina~ethylamincl~-J.~IH,3H!-
pyrimidinedinne
2~1389
- 16 -
.~4. 1 3-dimethyl-6-~-[N-(2-isobutyryldxyethyl3-3 ~-
nitrophenyl~propylamino~ethylamino~-2.4(IH 3H'-
pyrimidinedione
~5. I 3-dimethyl-6-~2-[N-(2-pival~yloxyethyl~ (4-
nitrophenyl)propylaminn]ethylamino~-2 ~(lH.3H~-
pyrimidinedione
56. 1.3-dimethyl-6-~2-~N-~2-acetoxypr~pyl)-3-(4-
nitrophenyl)propylamino3ethylaminc~ .4(lH~3H!
pyrimidinedi-ne
57. 1 3-dimethyl-6-~2-tN-.2-acetoxy-1-methylethyl)-3-
(4-nitrophenyl~propylamino~ethylamino~-2.~(IH.3H)-
pyrimidinedione
58. ] 3-dimethyl-6-~-tN-~2-benzoyloxyethyl)-3-(4-
nitrophenyl)propy1amino]ethylamino~-2 1(1H.~H~-
pyrimidinedione
~9. l,3-d.imethyl-t~-{2-<~ -('4-fluo~ benzovlnxY~-
ethyl]-3-(~-nitrophenyl-pro~ylamincl>ethylamirlol-
~.4'1H,3H)-pvrimidinedion-?
fiO. I~3-dimethYl-fi-~-<N-[2(4-methoxybenzoylf!xy!eth~l3
3- 4-nitl-oDhen~ propylaminl)~ethvlamino~-
2.~(IH.3H~-pylimi~inedi-1ne
61. l.3-dimethyl-fi-~2-<N-~ hlorobenzoyloxv)ethyl ]-
3-(~-nitroDhenyl~pl~cpylamino>ethylamino~-2.4-
~1H 3H)-pyrimid.inedione
G2. I 3-dimethyl-6-~2-<N-[2-(3~-dimethoxybenzoyloxY!-
ethyl~-3-(~-nitrophenyl)prnpylamino>ethylamin-~-
Xg~13~39
17
2,4(1H,3H)-pyrimidinedione
63. 1,3-dimethYl-6-~2-<N-[2-~3~4-dibromobenzoyloxy)-
ethyl~-3-(4-nitrophenyl)propylamino>ethylamino~- -
2,1(1H,3H)-pyrimidinedione
6~. 1,3-dimethyl-6-{2-tN-(2-methoxyethyl)-3-(~-
nitrophenyl)propylamino]ethylamino~-2.4(1H.3Hl-
pyrimidinedione
63 . 1,3-dimethyl-6-~ '-(2-propyloxyethyli-3-~-
nitrophenyl~propylamino]ethylamino'~-2,4~'1H.3H~-
pyrimidinedidne
66. 1,3-dimethyl-6-~-[h~-benzYl-3-(~4-nitJophenYl'
propylamino~ethylamino~-~,4~1H~3H~-
pyrimidinedione
67. 1,3-dimethyl-6-~2-~-(4-methoxybenzyl)-3-(~-nitro-
phenyl~propylamino~ethylamino~-2,4tlH,3H)-
pyrimidinedione
fi8. 1,3-dimethyl-6-~2-[N-(3.1.~-trimethoxybenzyli-3-
(4-nitrophenyl)prflpylamino~ethylamino~-2.4~lH.3H~-
pyrimidined.inne
69. l.3-dimethyl-6-{,-rN-( r -chloroben,vl ! -;i- ~ ~-
nitrophenyl)proDylamindlethylamino'~ lH.3H~-
pyl~imidined~one
7(). 1.3-dimethyl-6-~2-~ henylethyl)-3-/l-
nitrf.~phenyl )prnpylamino]ethylaminn~-2.~(1H.3H)-
pyrimidinedione
71 1,3-dimethyl-6-~2-rN-vinyl-3-~'l-nitl-nphenyl)-
39
propylamino]ethylamino~-2,~(1H.3H)-pyrimidinedione
72. 1~3-dimethyl-6-~2-rN-allYl-3-~4-nitrophenyl !-
propylamino]ethylamino~-2,4(1H,3H)-pyrimidinedione
73. l,3-dimethyl-6-{2-t~-propar~Yl-3-(4-nitrophenyl ! -
propylamino]ethylamino~-2.~1H.3H~-pyrimidinedi~ne
7~ 3-dimethyl-6-~2-[N-etho~ycarb~n~lmethyl-~-(l- -
nitrophenyl)propylamino]ethylamino~-2.~IH.3H)-
p~rimidinedione
5. l,3-dimethYl-~-t2-~N-tert-but~xycal-bonylmethyl-3-
(4-nitrophenyl)propylamino~ethylamino~-~.4(lH.3H~-
pyrinidinedinne
76. 1,3-dimethyl-6-t2-[N-~2-methoxycarbonylethyl)-3-
(~-nitrophenyl~propylamino~ethylamino~-2,411Hl3H)-
.. .
pyrimidinedione
7I. ],3-dimethyl-6-tN-methy]-2-tN-methyl-3-(~-
nitrophenyl~propylamino]ethylaminn~-2,i(1H.3H~-
pyrimidinedione
78. 1,3-dimethyl-6-tN-ethyl-2~N-''2-hydr~xYethyli-
3-(~-nitl-ophenyl3prc~pylamin~1~ethylamino}
~IH,3H3-Dyrimidinedi~-)ne
7~. l.3-dimethyl-6-t2-<~-ethy~ 3-(;~-nitrophenvl)-
pr~lpyl]aminn>ethylamin-!~-2.~;H.3Hl-
pyrimidinedione
80. 1,3-dimethYI-~-~'2-~N-methnxycatbnnvl-3--~-
nitl-ophenYl)Dl-opylamino~ethylamino~-2
(IH,3H)-pylimidinedione
81. 1,3-dimethyl-6-i2-[N-~tert-butoxycarbonyl)-3-
(4-nitrophenyl)propylamino]ethylamino~-~.1-
(IH.3H)-pyrimidinedione
82. l,3-dimethyl-6-~2-<N-~2-('N-methylamino?ethyl]-
3-(4-nitrophenyl)propylamino>ethylaminQ~-2.~-
(lH,3H)-py~imidinedione
83. 1~3-dimethyl-6-~2-~r~-t2-(N.N-dimethyl~mino)ethvl]
3-(4-nitrophenyl')propylamino~ethylamino'~-7.~-
~IH.3H)-pyrimidinedione
8~. 1,3-dimethyl-6-{~-<N-t2-(N.N-diethylamino)-
ethyl3-3-(2-nitrophenyl'~propylamino>ethyl-
amino~-2,4(1H,~H)-pyrimidinedinne
85. 1,3-dimethyl-6-{-7-rN-(2-hydroxyethyl~-3-s7-
nitrophenyl)propylamino~ethylamino'~-2,~1H.3H)-
pyrimidinedione
86. 1,3-dimethyl-6-{~-[3-(~-nitrophenyl)pl-opyl~-
piperazin-l-yl'~-2.~slH.3H)-pyrimidinedinne
8~. l,3-dimeth~rl-6-~-t3-(1-nitroDheny]~pl-op~
homopiperazin-l-yl~-2.~1lH.3H)-pyrimidlnedione
88. 3-methyl-6-~-r~ -nitl-ophenyl~pl-opyll-
~ipera7in-l-vl'~-2.~(1H.3H)-pvrlmidinedlone
89. 1-propyl-6-{2-tN-~2-hydroxyethyl?-3-(~-
nitrophenyl)propylamino]ethylamino~-2.~-
(lH,3H)-pyrimidinedione
9~. 6-~2-tN-ethyl-3-(~-nitrophenyl)propylamino]-
ethylamino~-].3,3-tl-imethyl-'~ ~lH,3H'~-
2~2~-89
pynimidinedione
91. 6-{2-[N-(2-hydroxYethy~ nitrophenyl~-
- proprlamino3eth~1amino~-].3.~-trimethyl-~.4-
(lH.3H)-pyrimidinedione
92. 1.3-dimethy]-6-~?-[~'-(ethyl-3-('4-nitrophenyl!-
proDylamino3ethylamino~-5-nltro-2.~(lH.3H)-
pyrimidinedione
93. 1.3-dimethYl-6-{2-~-(Z-hYdroxyethyl)-3-
~4-nitrophenyl~propylamino]ethylamino~-5-
nitro-2,4(1H,3H~-pyrimidinedione
9~. 1,3-dimethyl-6-{2-~N-~methoxycal-bonylmethyl ! -3- ( 4-
nitrophenyl)propylamino]ethylamino3-5-cyano-
2,~(1H,3H)-pyrimid.inedione
' 95. 1,3-dimethyl-6-f2-~N-~2-hydroxyethyl)-3-(~-
nitrophenyl)propylamino3ethylamino~-v-cyano-
2.~(lH.3H)-pyrimidinedi~ne
96. 1.3-dimethyl-6-~2-~ -nitlophenyl!hutylamin~
ethylamino~-2.~1H.3H)-pyrimidin~dione
g~. 6-(~i-ethyl-~i<~-[~-(4-nitrophenyl)butylam~no3-
ethyl>amino3-1.3-dimethvl-2.~1H.3H,-
pyrimidinedione
98. l.3-dimethyl-6-s~'-(2-hydroxyethYl)-2
~-nitrophen~l)butylamino3ethYlamino~-2.
IlH,3H)-pyrimidinedione
99. l,3-dimethyl-6-~'-methoxycarbonylmethyl-2-
~ -nitrophenyl)~utylamino]ethylamino~-
- 21 -
2.~'1H.3H!-pyrimidinedione
100. 1.3-dim~thvl-~-~2-[N-ethY~ -nitrophen~
butylamino]ethylamino)-~2.i(~1H~3H!-pyl-imidinedione
101. l,3-dimethyl-6-(2-[N-(tert-butyl)-4-(4-nitrQphenyl)-
butylamino~ethylamino~-2~4(lH.3H!-pyl-imidinedinne
102. 1,3-dimethyl-6-~2-[N-i2-hydroxyethyl)-4-(4-
nitrophenyl)butylamino~ethylamino'~-2~4(1H,3H)-
pyrimidinedione
103. 1,3-dimethyl-6-{2-tN-('3-hrdroxypropyl~-4-~4-
nitrophenyl)butylamino]ethylamino~-2,4(1H,3H)-
pyrimidinedione
104. 1,3-dimethyl-6-{2-CN-~'2-acetnxyethyl')-4-(4-
nitrophenyhl'~butylamino]ethylamino~-2.~(1H.3H!-
pyrimidinedione
10~. 1.3-dimethyl-6-~2-[r3-~2-methoxyethy]~-4-(4-
nitrnDhenvl)hutylamino~ethylamino'~-7.4tlH.:~H)-
pyrimidinedione
106. 1~3-dimethyl-6-~2-tN-benzyl-4-(4-nitr-~)phen-il~-
butylamino]ethylamino~-2~4~1H~3H!-
pyrimidinedione
107. 1,3-dimethyl-6-~2-rl~-allyl-4-('4-nitrophenyl)-
butylamino]ethylamino~-2,4<'IH,3H)-
pyrimidinedione
108. 1,3-dimethyl-6-~2-[N-ethoxycarbonylmethyl-4-
(4-nitrophenyl)blltylamino]ethylamino~-2.4-
(IH,3H)-pyrimidinedi-~ne
2001389
- 22 -
109 . I .3-dimethYl-6-~i-methyl-2-tN-methy~
nitr~phenyl!butylamino~ethyl~mino~ .4-
slH~3H)-pyrimidinedione
1l~). 1,3-dimethyl-6-{2-CN-t~-('N.~-d iethylamino~ethyl~-
4-(4-nitrophenyl)butylamino>ethylamino~-2.4(IH~3H)-
pyrimidinedione
111. 1,3-dimethyl-6-{4-t4-(4-nitrophenyl')butyl]-
piperazin-l-yl~-2,4(1H,3H)-pyrimidinedione
112. 6-{2-tN-ethyl-4-14-nitrophenyl)butylamino]-
ethylamino~-1,3,5-tlimethyl-2,4(1H,3H~-
pyrimidinedione
113. 1,3-dimethyl-6-(2-t2-('4-nitrophenoxy!-
ethylamino]ethylamino-~.~(IH.3H)-pyrimidinedione
. l,3-dimethyl-6-~-ethyl-~-t2-(~-nitroiphenoxy~-
ethylamino~ethylamino'~-2.4t;H~3H~-pvl-imidinedinne
11~. 1.3-dimethy]-6-t4-t2-(4-nitl-ophenoxy!ethyl3-
piperazin-1-vl'~-7~/lH.;lHj-pvrimidinedlone
l16. 1.3-dimethyl-6-{2-[3-(~-nltroDhenox~!propYl-
amino]ethylamino~-2~/lH,3H~-pyrimidinedione
11/. 1,3-dimethyl-6-~ thyl- -t3-~4-nitroPhen~xv ! -
propylamino]ethylamino~-2,~l1H,3H~-pyrimidinedione
118. 1,3-dimethyl-6-{2-tN-ethyl-3-(~-nitrophenoxy)-
propylamino]ethylamino~ (lH,3H)-pyrimidinedione
119. 1,3-dimethyl-6-{2-[.N-(2-hydroxyethyl)-3-(4-
nitrophenoxy)~ropYlamino]ethylaminh~-2~ H~3H)
pyrimidinedione
-
Z~1389
-?(~ dimethvl-6-~ J-~c~t~ xY~thyl ~
nitrophen(xy~propylamino~thYlamin("-J ~;IH.1H!-
pyrimidinedione
121. 1.3-dimethyl-6-~N-methyl-2-[N-methyl-3-~-
nitrophenoxy~propylamino]ethylamino~-2.~(IH,3H)-
pyrimidinedione
122. I,3-dimethyl-6-~-t3-~4-nitrophenboxy)proeyl]-
piperazin-1-yl~-2,4(1H,3H)-pyrimidinedione
123. 3-methyl-6-{~-[3-(4-nitrophenoxy)propyl]-
piperazin-1-yl~-2,~(1H.-3H)-pyrimidinedjone
12~. 1,3-dimethyl-6-~4-[3-('3-nitrophenoxy)propyl~-
piperazin-l-yl~-,.4~1H.3H!-~yrimidinedione
125. 1.3-dimethyl-6-~4-~3-~2-nitrophenoxY~propyl]-
piperazin-l-~ -2.~(1H,~ -pyrimldlnedione
I26. 1.3-dimethyl-6-~2-~t-~-nitrophennxy)bllt l~mino3
ethylalr,ino~-c.~(~]H~3H!-pyrilr,idine(iione
127. 1.3-dimethyl-~-~N'-methyl-2~N-methvl-~-
(~-nitrophenoxy!buty]aminc!~ethvlaminc~ 5~(1H.3H~-
pyrimidinedione
128. 1,3-dimethyl-6-{~-~4-(4-nitrophenoxv~butyl~-
piperazin-I-yl~-2,~(IH,3H)-pyrimidinedione
129. ~,3-dimethyl-6-~2-[N-ethyl-2-(4-nitr-)phenylthic~-
ethylamino]ethylamino'~-2,~(IH,3H)-pyrimidinedione
130. 1,3-dimethyl-6-~2-~N-(2-hYdrox~ethyl)-3-l~-
nitrophenylthio)proDylamino]ethylamino~-2.~-
~H..~H~-pyrimidinedione
Z~ 89
- 24 -
131. 1,~-dimethyl-6-i4-[3-~4-nitl-o~henYlthio)DIop~l]-
piperazin-l-yl~-2,~1H.3H'-pyrimidinedinne
132. 1.3-dimethyl-6-{N-methyl-'~-t~i-methvl-~-
(4-nitrophenylthioibutylamino]ethYl~min
2,~( 1H.3H~-pyrimid.inedinne
133. 1,3-dimethy]-6-t~-(4-nitrophenacyl)piperazin-1-y1~- -
2,~(1H,3H)-pyrimidinedi d ne
134. 1,3-dimethyl-6-~-r5-~4-nitrobenzoyl~ethyl]-
Diperazin-1-yl~ (lH,3H)-pYrimidinedione
135. 1,3-dimethyl-6-{N-methyl-5- tN-methYl-4-(4-
nitrophenylthio~butylamino]ethvlaminn~-2.4-
(lH,3H)-pyrimidinedione
136. 1,3-dimethyl-6-{4-t.5-hydroxy-2-(~-nitrophenyl)-
ethyl]piperazin-l-yl~-2,~1H.3H~-pyrimidinedione
13~. 1,3-dimethyl-6-{1-t2--~-nitrnbenzoyl.~xy~ethvl3-
piperazin-l-yl3-2.~(1H~3H~-p~l~imidinedione
138. 1,3-dimethyl-fi-~2-[N-ethvl-~ -nitrQbenzoylnxy)-
propylamino~ethylamin~ .4(~H~3H?-pyl-in,idinedinne
139. 1.3-dimethyl-6-t2-~-ni t ioben~o~laminn)-
ethylaminn~-2.4~1H.;~H ! -p~rimidinedione
140. 1,3-dimethyl-6-{4-[~-~4-nitrobenzoylaminoi-
ethy]~piperazin-1-yl~-2.4('1H.3H)-pyrimidinedione
1~1. 1,3-dimethyl-6-~4-tN-~4-nitroDhenyl)carbamoyl-
methyl~piperazin-1-yl}-2 4(1H,3H)-pyrimidinedione
142. 1,3-dimethyl-fi-{4-t~-(4-nitrophenyl~-
carhamoylethyl~homopipel-azin-1-yl~ (lH.3H!-
ZCli~)~3~9
- 25 -
pvrimidinedione
1~3. 1,3-dimethyl-~ -[3-~-nitroanilino~-2-
hydroxypropyl~piperazin-1-yl~-2.4(1H.3H!-
pyrimidinedione
]44. ~,3-dimethyl-6-~4-<4-tN-(~-nitrophenyl)-
carbamoyl]butyl>piperazin-l-yl~-2,~(lH.3H)- -
pyrimidinedione
145. 1,3-dimethyl-6-~N-methyl-2-[N-methyl-2-(4-
nitroanilino)ethylamino~ethylamino~-2.~(1H,3H)-
pyrimidinedione
146. 1 ,-.~-dimethyl-6-~ lethyl-'~-rN-~2-hyd~ xyet~yl !-
2-~-nitroanillno~ethvlamino]ethylamin
2.4~1H.3H)-pyrimidinedic~ne -
; li7. 1.3-dimethyl-6-~-[~-eth 1-2-~-nitroanilino)-
ethylaminc~ethylamin-,i-2.4~1H.3H!-p.vrimidinedi-,ne
l~x. 1,3-dimethyl-6-~.3-[N-propyl-2-1~-nitroanilino'j-
ethylamino~propylamincl}-2.4~1H.3H~-pyrimidinediclne
1~9. 1,3-dimethyl-6-~-methvl-2-[~'-methyl-2-(N-methyl-
4-nitroanilino)ethy]aminc]ethylamino'~-
- 2,~11H,3H)-pyrimidinedione
15~ 3-dimethyl-6-~N-methYl-3-tN-methyl-2-
(4-nitroanilino')ethylamino~propylamino~-2,~-
(1H,3H)-pyrimidinedione
151. 1,3-dimethyl-6-~2-t.N-ethYl-3-~-nitroanilino)-
propylamino]ethYlamino~-2~ H.;:;Hl-ovl imidinedi-~ne
1.-,2. 1.3-dimethyl-fi-~2-[.N-~2-hdvrox-~ethyl~-;3~
2~3~1389
- 26 -
nitroanilino)propylamino]propylamino~-2.4(1H.3H)-
pyl-imidinedione
1.~3. 1,3-dimethyl-6-{2-tN-methoxycarbonylmethyl-3-
(4-nitroanilino)propylamino~ethYlamino~-2~4(lH~3H!
pyrimidinedione
154. '1,3-dimethyl-6-~4-[3-(4-nitroanilino')propyl]- --
piperazin-l-yl~-2,4('1H,3H)-pyrimidinedione
15~. 1,3-dimethyl-6-{4-t3-(N-methyl-4-nitroanilino~-
propyl]piperazin-1-yl~-2,4(1H,3H~-pyrimidinedione
156. 1,3-dimethyl-6-{4-t3-(N-propyl-4-nitroanilinc~-
propyl]piperazin-l-yl)-~s4(IH,3H)-pyrimid.inedione
1~. 1,3-dimethyl-6-{4-[3-('N-methanesulfonyl-4-nitro-
anilino~propyl]piperazin-l-Yl~-2~4(lH~3H)
pyrimidinedione
1~8. 1.3-dimethyl-6-~4-[3-iN-ethaneslllfonYl-4-
nitroanilinn'.~Dropyl]piperazin-1-vl-~-2.~(1H.3H!-
pyrimidinedione
1~. l,3-dim~thyl-6-~-t3-(N-acetyl-~-nitroanilino!-
propyl]piperazin-1-yl~-~.4~1H.3H)-
pyl-imidinedione
160. I,3-d.imethyl-6-~4-[3-lN-propionyl-~-nitroanilino)-
propyl~piperazin-l-yl~-2,4('1Ht3H3-pyrimidinedic)ne
Ifil. 1,3-dimethyl-6-~2-<[1-(4-nitrophenyl)piperidine-~-
yl ]amino>ethylamino~-2,4('1H,~H)-pyl-imidinedione
162 1,3-dimethyl-6-~.2-t4-(~-nitrophenylJpiperazin-
]-yl ]eth,vlamino~-2,~(1H.3H!-Dyrimidinedione
- 2~13~39
- 27 -
163. 1,3-dimethYl-6-{3-[~ -nitr()Dhenyl)piperazin
yl ]propylamino~-2,4(1H,3H3-pYrimidinedione
164. 1,3-dimethyl-6-~N-(2-hydroxyethyl)-
~(4-nitrophenyl)piperazin-1-yl~-ethylamino~-
2,4(1H,3H)-pyrimidinedione
165. 1,3-dimethyl-6-~N-methyl-2-[4-(~-nitroDhenyl)-
piperazin-l-yl]ethylamino}-2,4(1H,3H)-
pyrimidinedione
166. 1,3-dimethyl-6-{4-[3-(2-acetyl-4-nitrnphenyl)-
propyl]piperazin-1-yl~-2.~]H.3H)-~Yrimidinedic!ne
I67. 1~3-dlmethyl-6-~-tN-eth~ -benz~vl-i-
nitrnphenyl!ethylamino~ethylaminoJ-2~4(1H,3H!-
pyrimidinedione
168. 1,3-dimethyl-6-t4-(3-acetyl-1-nitroiDhenyl~-
piperazin-l-yl]-~2,J~lH,3H~-pyrimidinedione
169. 1,3-dimethyl-6-{4-t1-~-acetyl-~-nitrophenoxy)-
butyl]piperazin-l-yl~-2,i~lH,3H)-~yrimidinedione
170. 1-methyl-6-~4-[3-(2-acetyl-4-nitrophenoxy~-
propyl]piperazin-l-yl~-2,~(1H,3H~-pyrimidinedione
1~1. 1,3-dimethyl-6-{~-<3-t2-nitro-4-( -
pyridinecarbonyl)phennxy]propyl>piperazin-l-
yl~-2.~1H.3H!-pyrimidinedi~ne
172. 1.3-dimethyl-6-(~-[N-~2-hdvrnxyethyl)-3-
~4-benzoyl-2-nitl-~phenoxy~pl(lpyl~min(~
ethylamin~-2.~1H.3H)-Dvrimidinedlnne
173. 1 3-dimethyl-6-~-[3-~ cetYi-~-nitroanilin
2~13~39
- 28 -
propyl]piperazin-l-yl}-2,~(1H.3H)-pyrimidinedione
174. 1,3-dimethyl-6-{~-~3-('2-cyclopentanecarbonyl-4-
nitroanilino)propyl]piperazin-l-yl~-2.~(1H,3H)-
pyrimidinedione
175~ 1,3-dimethyl-6-~4-<3-~2-~2-chlorobenzoyl)-4-
nitroanilino]propyl>piperazin-1-yl~-~.4- -
(IH,3H)-pyrimidinedione
176. 1.3-dimethyl-6-~4-<3-[2-(~-~yridinecarbonyl !-4-
nitroanilino~propyl>piperazin-l-yl~-2~ H.3H)
pyrimidinedione
1~7. 1.3-dimethyl-6-~4-<3-~2-'~-pvridinecarb~nyl)-
4-nitroanilino3propyl>pipel-azin-1-vl~-
2,~1H,3H)-pyrimidinedione
1~8~ 1,3-dimethyl-6-~4-[3-14-acetyl-~-nitroanilino3-
propyl]piperazin-l-yl~-2,~lH.3H)-pYrimidinedione
179. 1,3-dimethyl-6-~4-t3-(4-propanoyl-~-
nitroanilino~propyl]piperazin-l-yl'~-2~-
(1H,3H)-pyrimidinedione
18~. 1,3-dimethyl-6-~1-t3-~4-benzoyl-2-nitroanilino)-
propyl~piperazin-1-yl~-~.1(1H.3H'I-nyl-imidinedione
181. 1,3-dimethyl-6-i~-~3-~'3-acetyl-~-nitroanilino~-
propyl]piperazin-l-yl~ (1H.3H~-~yt~imidinedione
182. 1.3-dimethyl-6-~-[3-~-acetyl-~-nitroanilinoj-
propylamino~ethylaminc~-2.~(1H.3H;-pvrimidinedione
183. l~3-dimethyl-6-~3-~N-'''-h-~dr-lxvethyl)-3-~-
propanoyl-~-nitl-nanilino!nl-nDylamln(~-
2~C)1;~89
- 29 -
pro~ylamino~-2.~1H.3H)-Dyrimidinedione
l84. l.3-dimethyl-6-~4-t3-(2-ben,oyl-~-nitro~nilino~-
propyl]piDerazin-l-yl~-~,4~1H.-3H~-~yrimidinedione
18~. 3-methyl-6-~4-t3-(2-benzoyl-~-ni troani ] ino!-
propyl~piperazin-1-yl~-~,451H.3Hl-pyrimidinedione
J86. 1t3-dimethyl-6-{N-ethy]-2-t3-(~-fol-myl-~-
nitroanilino~propylamino]ethylamino~-2.~1H,3H)-
pyrimidinedicne
187. 1,3-dimethyl-6-{~-[3-~3-fluoro-~-nitrophenoxy~-
propyl~piperazin-l-yl~-2.~(lH~3H!-pyl-imidinedione
188 . I, 3-dimethyl-~-{~-[3-(3-fluoro-~-nitroanilino)-
propy]~piperazin-1-yl~-2,1(1H.3H~-pyrimidinedione
189. 1,3-dimethyl-6-~4-t3-(3,5-difluoro-~-nitroDhenoxy)-
; propyl]piperazin-l-yl~-2,~1H.3H)-pyrimidinedione
190. It3-dimethyl-6-~4-[3-~3.5-difluol-(?-~-nitr-lanilino)-
propyl~niperazin-]-yli-2.~1H.3H~-D~.t~ ]dinedione
191. 1.3-dimethyl-6-~-t3~ -fluorn-~-nltroanilino~-
propyl~piperazin-l-yl~-2.~(lH.~H!-Dyl-imidinedione
19~. 1,3-dimethyl-6-1~-[3-~2-methl-1xy-~-ni troDhenoxy~-
propylamino~ethylamino~-2.~1H.3H.-Dyl-imidinedione
193. 1.3-dimethyl-6-~3-[~-ethyl-3-(3-tl-ifluoromethyl-~-
nitroanilino)propylamino~pl-opylamino~-2~4(lH.3H)
pyrimidinedione
194. ~,3-dimethyl-6-~2-t4-(2-acetyloxy-4-nitro-
phenoxy)butylamino]ethylamino~ (IH,3H)-
pyrimidinedione
2~)1389
- 30 -
195. 1,3-dimethyl-6-{4-~3-(2-dimethylamino-~-
nitroanilino)propyl~homopiperazin-1-yl}-
2,~(1H,3H)-pyrimidinedione
196. ],3-dimethyl-6-~2-[2-(2-diethylamino-4-nit
anilino)ethylamino]ethylamino~-2,4(lH,3H)-
pyrimidinedione
197. 3-methyl-6-~2-[3-~'2-hydroxy-4-nitrophenoxy)-
propylamino~ethylamino~-2~4~1H,3H?-
pyrimidinedione
1~8. l-ethYl-6-i4-[3-(2-brtmo-~-nitroanilino !-
propyl~piperazin-1-yl~ 1H.3H)-
pyrimidinedione
I99. 6-{4-[2-{2-ethyl-~-nitroanilino)ethylamino]-
piperazin-1-yl~-5,~1H,3H)-pyrimidinedione
200. 1,3-dimethyl-6-{~-[N-t3-fllloro-4-nitroiphenyl)-
carbamoylmelthyl~piperazin-l-yl'~-2,4(1H,3H)-
-pyrimidinedione
1. I,3-dimethyl-6-{2-[3-t~-ethoxy-~-nitroDhenylthio~-
propylamino~ethylamino}-2,~(1H.~H ! -pyl- i diminediorle
. I,3-dimethyl-6-i~-t3-'2-ethaneslllfonamldo-4-
nitrophenox~)propyl3piperazin-~-yl'~ -(1H.3H?-
~yrimidinedione
203. 1,3-dimethyl-6-i4-[2-~'3-fluolc!-~-nitrophenYl~-
ethyl]piperazin-l-yI'~ tlH.3H)-pyrimidinedione
20~. 1,3-dimethyl-6-~2-[N-ethyl-3-(3-fluoro-~-
nitrophenyl)propYlamino]ethylamino}-
~
2~8~389
- 31 -
('lH.3H!-pyrimidinedione
205. 1.3-dimethYl-~-f3-~N-~?-hvdr-~xyethyl3-~-~3..~-
dif]uaro-~-nitronhenyl!pl-opylamino~-
propylamino~-2.~lH.3H)-pvrimidinedione
206. l.3-dimethyl-6-~2-rN-(~?-acetoxyethrl~-3-
(2-dimethylamino-~-nitrophenyl)propylamino]-
ethylamino~-2,4(1H,3H)-oyrimidinedione
207. 1,3-dimethyl-6-{2-[N-ethyl-3-~2-ethoxy-~-
nitrnphenyl)propylamino]ethylamino}-2,4-
(lH,3H)-pyrimidinedione
208. 1,3-dimethyl-6-~3- rN- (2-acetyloxrethyl !-
3-(2-ethanesulfonamido-~-nitrophenyl)-
proDylamino]proDylamino~-2.~(lH.3H~-pyrimidinedione
.~
2~9. l,3-dimethyl-6-~ 3-methyl-~-nitrnbenzyl)-
piperazin-1-y]]-2.~(1H.3H~-Dyl-imidinedione
210. 1,3-dimethyl-6-~J-[~ -hy~roxyethv~ /3-
methoxy-~-nitrophen~ pl-oDYl~min~]ethylamino~-
2,4-(lH,3H)-pyrimidinedione
21~. 1.3-dimethyl-6-~2-t.~-(3-ben~oyloxypl-opyl~-3-(2-
ethoxycarbonyl-~-nitrophenyl;propylamino]-
ethylamino~-2,~(~H ! 3H)-pyrimidinedione
212. 1,3-dimethyl-6-~-[3-(2-acetyl-~-nitrophenoxy)-
propyl]piperazin-l-yl~-2,i~H.3H)-prrimidinedione
213. 1,3-dimethyl-6-~-[3-(~-acetyl-2-nitrophenoxy)-
propyl~pipera7jn-1-yl}-2,~(1H,3H~-pyl-imidinedi~ne
21~. 1,3-dimethy~ -[3-~-benzoYl-?-nitrdphenoxyj-
2(~ 9
pl'ODyl ]pipel-azin-l-YI~-2.~' lH.;3H~-plrrimi-linedillne
21~. 1.3-dimethyl-6-i~-[3-13-acetyl-4-nitl-ophen-xy~-
propyl]piperazin-1-vl)-2.4(1H.3H)-pyrimidinedione
216. 1,3-dimethyl-6-~4-[3-~2-benzoy]-4-nitrophenoxy!-
propyl]piperazin-l-yl~-2.~(1H,3H~-pyrimidinedione
217. 1,3-dimethyl-6-~4-<3-[2-(4-bromobenzoyl~-4-
nitrophenoxy]propyl>piperazin-l-yl}-2,4(lH,3H)-
pyrimidinedione
218. 1,3-dimethyl-6-{4-<3-t2-(3-pyrazolylcarbonyl)-4-
nitrophenoxy]propyl>piperazin-l-yl~-2,4(1H.3H)-
pyrimidinedione
2~9. 1,3-dimethy]-6-~-<3-[2-(;!-pyridineCalbonyl !-1-
nitrophenboxy]propyl>piperazin-l-yl~ i lH.3H)-
pyrimidinedione
220. 1,3-dimethyl-fi-~-<3-t2-t3-pvridinecalbon~ -4-
nitrophenoxy~propyl>piperazin-l-yl~-2.~(1H 3H)-
pyrimidinedione
221. l,3-dimethyl-6-~4-<3-tJ-(4-Dyridinecal-bonYli-4-
nitrophenoxy]propyl>piperazin-1-yl~- ,4-
(1H,3H~-pyrimidinedione
222. 1,3-dimethyl-6-~-<3-t2-(2-pyrimidinylcarhnnyl)-
4-nitrophenoxy~propyl>piperazin-1-ylt-2.~ ,3H)-
pyrimidinedione
223. 1,3-dimethyl-6-~-[2-(2-acetyl-4-nitrovhenoxy!-
ethyl]piperazin-1-yl~-2,411H,3H)-pyrimidinedione
22~. 1,3-dimethyl-6-~-t -(2-benzoyl-4-nitloph~noxy~-
26~11389
-- 33 --
ethyl]piDel-azine-l.-vl'~-2.~1,H.3H)-pvl-imi(linedione
22~ . 1 . 3-d i me thyl -fi- ~ ~-< 2- [2- ( 4-bl ~lm~)henzoyl ! -~-
nitrophenoxy~ethyl>piperazin-l-yl~ lH.3H)-
pyrimidinedione
226. 1,3-dimethyl-6-~-[2-(3-acetyl-~-nitrophenyl)-
ethyl~piperazin-l-yl}-2,4(1H,3H)-pyrimidinedione
227. 1,3-dimethyl-6-{4-~2-~2-nitro-4-acetyl)ethyl]-
piperazin-]-yl3-2,4('~H,3H)-pyrimidinedi~ne
228. 1,3-dimethyl-fi-14-t2-('2-nitro-4-benzoyl)-
ethyl~piperazin-1-yl~-2,i(1 Hl 3H3-pyrimidinedione
229. 1,3-dimethyl-6~ 3-~2-(2-hydroxybenzoyl)-4-
nitrophenox~propyl>r3ipe3-azin-l-yl~-2.4( IH. 3H'-
pyrimidinedione
230. 1.3-dimethyl-6-~'4-<3-C2-(2-chlorobenzoyl)-4-
nitrophenoxy]prop~l>piperazin-l-yl~
(lH.3H~-pvrimldinedlone
231. 1,3-dimethYl-b-~ i-[~-nitro-
~pyridinecarbonyl!phenylthic~propyl>plpel-azin-
1-yl}-2,1~1H,3H~-pyrimidinedione
23~. 3-methyl-6-~4-t3-~-nitro-2-benz~ylphen~,xy!-
propyl]piperazin-l-yl~-2.4~1H,3H)-pyrimidinedione
233. 1,3-dimethyl-6-{2-[3-(2-benzoyl-4-nitr~phenoxY~-
propylamino~ethylamino~-2,1(1H,3H)-pyrimidinedione
234. 1,3-dimethYI-G-(2-[N-ethYI-~-14-benzc)YI-2-
nitr~phenoxy)propylamino]ethYlamino-2
1 IH.3H)-pvl~lmidinedione
2~389
- 34 -
23.~. 1.3-dimethyl-6-f4-~ -benzovl-4-nitll-,phenylthi~
ethyl~ipel-azin-l-yl~-2.~1H.3H!-pyrimidinedi~ne
236. 1,3-dimethyl-6-~4-('2-benzoyl-4-nitrophenyl~-
piperazin-]-yl]-2,4(1H,3H)-pyrimidinedione
237. l~3-dimethyl-6-~3-~-nitro-2-(3-pyridinecarbonyl)-
phenylamino]propylamino~-2,4(1H,3H)-pyrimidinedi~ne
238. 1,3-dimethyl-6-[3-(2-benzoyl-4-nitrophenylamino)-
propylamino~-2,4(1H,3H)-pyrimidinedione
239. 1,3-dimethyl-6-t2-~2-benzoyl-4-nitrophenylamino'~-
ethylamino]-5,4(1H,3H!-~yrimidinedione
2~0. 1.3-dimethyl-6-~'2-[1-nitro-2-~3-pyridinecarbonyl)-
phenylamino]ethylamino~-2.4(1H.3H!-pyrilrlidinedione
241. 1.3-dimethyl-6-~ 2-benzoYI-4-nitro~henyl!-
ethyl3piperazin-1-yl~-2.4(1H.3H!-pyrimidinedirne
242. 1,~.j-dimethyl-6-s~-[r'2-ben~oyl-4-nitrophenyl)-
methyl]piperazin-l-yl~-2,4(1H.3H~-pyl~imidinedione
243. 1,3-dimethyl-6-{2-~5'2-benzoyl-4-nitrnphenyl)-
methylamino]ethylamino~-2,~(lH,3H)-pyrimidinedione
24~ -dimethyl-6-{4-~3-('~-benzoyl-~-nitrophenyl;-
propyl]piperazin-1-yl~-2,4(1H.3H)-pyrimidinedione
245. 1,3-dimethyl-6-{4-t2-~'4-benzoyl-2-nitrophenyl)-
ethyl]piperazin-l-yl~-2,4~'1H.3H)-pvlimidinedione
246. 1,3-dimethyl-6-{4-~3-(3-methyl-4-nitro~henyloxYl-
propyl]piperazin-l-yl~-2.4(lH.3H~-pyl-imidinedione
247. 1.3-dimethyl-6-s'~-~3-(~-chloro-~-nitrsJphenvloxv)-
propyl ~piperazin-l-Yl ~-2.4~1H.3H!-D~;rimidlne~ione
-
X(~
?~8. 1,3-dimethyl-6-~4-t3-t:2-chlor~ -nitro-
phenyloxy)propyl]piperazin-l-~l}-2,4~1H.3H~-
pyrimidinedione
249. 1,3-dimethyl-6-~4-t3-(4-methanesulfonamido-~-
nitrophenyloxy)propyl~piperazin-1-yl~-
2,4(1H,3H)-pyrimidinedione
250. 1,3-dimethyl-6-~4-t3-(4-acetamido-2-nitro-
phenyloxy)propyl]piperazin-l-yl~-~.4(1H.3H)-
pyrimidinedione
251. 1,3-dimethyl-6-{4-[3-(2-hYdroxY-~-nitro-
phenyloxy)propyl]piperazin- 1-YI ~ -2 . ~ ( I H. 3H) -
pyrimidinedione
252. 1~3-dimethyl-6-~-[3-(2-allyloxy-5-nitro- ,--
phenyloxy~pl-opyl]piperazin-1-yl3 -? . ~ lH.3H!-
pyrimidinedione
253. 1,3-d.imethyl-6-~4-t3-(~-methylthio- -
nitrophenyloxy:3propyl]piperazin-l-yl~-2,4(1H.3H)-
pyrimidinedione
2~. I,3-dimethyl-6- {~-<3- t2-(~-hYdroxYbenzyl)-4-
nitrophenyloxy]propyl>pipel-azin-1-
~2,4(1H,~H)-pyrimidinedione
25~. ],3-dimethyl-6-~-t3-~3-trifluoromethyl-~-
nitrophenyloxy)propyl]pipera7in-l-yl~
(1H.3H~-pyrimidinedione
?56. 1,3-dimethyl-6-~-[3-/~-m*thoxyearb/ln~JI-~-
nitrophenylxoy!pl-opyl]pipel-aLin-1-yl~-
2~11389
- 36 -
-
~ lH.3H)-pyrimidinedione
257. 1,3-dimethyl-6-~4-~3-(2-carboxy-~-
nitrophenyloxy~propyl~piperazin-I-yl~ IH,3H)-
pyrimidinedione
258. 1,3-dimethyl-6-{4-t3-(2-amino-~-nitrophenylxoy~-
propyl]piperadin-1-yl3-2,~(1H.3H)-pyrimidinedione
259. 1,3-dimethyl-6-{4-[3-(4-methoxycarbonyl-~-
nitrophenyloxy)propyl]piperazin-1-yl~-2.~(1H,3H~-
pyrimidinedione
260. 1.3-dimethyl-6-~4-[3-(2-~yano-~-nitrophenvl--,xyi-
prnpyl]piperazin-l-yl}-2,4(1H,3H)-py,imidinedione
261. 1.3-dimethyl-6-i4-t3-(2-cyano-1-nitrophenyiaminol-
prr1pyl~pioel-azin-l-yl~-2.4(lH.3H!-pyrlmidinedione
2fi2. 1.3-dimethyl-6-{~-[3-52-chloro-~-nitrophenYl-
amino)pl-opyl~piperazin-l-yl~-2.~(1H.3Hi-
pyrimidinedione
263. 1,3-dimethyl-6-~ 3-(2-methoxy-5-nitrophenyloxy)-
propyl]piperazin-l-yl~ 1H,3H3-pyrimidinedione
264. 1,3-dimethyl-6-~-[3-(2-allyloxy-4-nitro-
phenyloxy)propyl~piperazin-]-yl~ IH.3H~-
pyrimidinedione
~265. 1,3-dimethyl-6-~4-{3-(2-hydroxy-4-nitrophenyloxY
propyl ~pipel-azin-l-yl ~ (lH. ~1H3-pyrimidinedione
266. 1,3-dimethYl-6-~4-[3-l2-benzylamino-~-
nitro~henyloxy~pl-oipyl3pipel~azin-l-~ lH.3H~-
D y 1- imidinedione
2~a~1389
- 37 -
267. I,3-dimethyl-6-~ 3-lj-melthoxy-4-nitr-,phenyldxy~-
propyl~piperazin-1-yl~-~,4(1H,~H)-pyrimidinedione
268. 1,3-dimethyl-6-~.~-[3-~2,6-dichloro-~-nitrophe-
nyloxy)propyl]piperazin-]-yl}-2.~(1H,3H~-
pyrimidinedione
2~389
- - 38 -
As shown in the general formula (I) above, compounds
of the present invention have a basic backbone in which the
phenyl and pyrimidinedione parts are linked by a structure
comprising mainly an alkyl chain containing at least two
nitrogen atoms. This basic back bone structure is believed
to be responsible for the pha~maceutical effects.
When the compounds shown by the general formula (1)
above were applied to the fol~owing arrhythmia pathological
models, all the compounds demonstrated efficacy.
I0 Atrial f ibrillation model
Atrial fibrillation model animals were made according
to the method of A. L. Goldberger et al. (lnternational
lournal of Cardiology, 13, 47-55, 1986) by anesthetizing
adult mongrel dogs with pentobarbital sodium (30 mg/kg,
intravenously). Using these atrial fibrillation model
animals, the effects of the compound5 of the present invention
on the a~rial fibrillation model were investigated by
administering the compounds intravenously at a dose of 0.1 -
10 mg/kg. As a result, all Of the compounds according to the
present invention were confirmed to have therapeutic effects
on atrial f ibrillation.
Ventricular tachYcardia model
Adult mongrel dogs were anesthetized with pentobarbital
sodium (30 mg/kg, intravenously). A left thoracotomy was
performed in the fourth intercostal space under artificial
respiration, and the left anterior descending coronary
28~)1389
- 39 -
artery was ligated at the border of the atrial appendage.
The blood was recirculated for 1~0 minutes after the
ligation so that an cardiac infarction lesion was formed to
readily induce tachycardia in each animal.
Thereafter, the ventricular tachycardia model animals
were made by inducing ventricular tachycardia according to
the method of Lynch (~ournal of Cardiovascular Pharmacology,
~, 1132-1141, 1984).
Using these model animals, the compounds of the present
invention were confirmed to have therapeutic effects on
ventricular tachycardia when administered intravenously at a
dose of 0.1 - 3 mg/kg.
The compounds according to the present invention have
marked therapeutic effects on the arrhythmia pathology
model, i.e. atrial fibrillation model and ventricular
tachycardia model; thus they are useful for the treatment
and prevention of arrhythmia.
Furthermore~ the effects of the compounds of the
present invention on cardiac functions were experimentally
investigated and the following results were obtained.
Mongrel dogs (body weights: 8 - 15 kg~ were
anesthetized with pentobarbital sodium (30 mg~kg,
intravenously~. A microsensor catheter was inserted through
the common carotid artery into the left ventricle of each
animal so that primary differential values (dp/dt~ of the
inner pressure of the left ventricle and electrocardiograms
Z~389
- 40 -
were recorded. The compounds of the present invention were
administered intravenously to the dogs ~lmg/kg) and changes
in the dp/dt and electrocardiograms were investigated.
As a result, it was revealed that the compounds of the
present invention significantly increased the values of
dp/dt max and significantly extended QTc on the
electrocardiograms.
Consequently, the compounds according to the present
invention were confirmed to have an antiarrythmic action and
particularly to be useful as Class 111 type antiarrythmic
agents. Furthermore, the significant increase in dp~dt max
demonstrated that the compounds according to the present
invention have a positive inotropic action and accordingly
they are useful as therapeutic agents for cardiac
insufficiency.
As mentioned above, in general, most of patients with
arrhythmia have deficiency in cardiac functions. In the
case where, for example, antiarrythmic agents classified in
Class I or 1l are given to such patients, the greatest care
has to be taken for use because these agents exert more or
less antiarrythmic action as well as a negative inotropic
action ~action to further repress cardiac functions3 (Eivind
S. Platous, Journal of Cardiovascular Pharmacology, 8~3),
459, 1986).
On the contrary, as mentioned above~ the compounds
according to the present invention t-ave a positive inotropic
2Q~1389
- 41 -
action to significantly increase the dp/dt max, as well as
an antiarrythmic action. Accordingly, they are expected to
provide satisfactory results to the patients with arrhythmia,
whose cardiac functions are depressed.
Representative examples of processes for the production
of the compounds of the general formula (1) according to the
present invention will be demonstrated hereinafter; however,
these examples are not to be construed to limit the scope of
the invention
Among the compounds of the general formula (1)
described above, a compound of the following general formula
(2) can be produced according to a method containing the
following step (a).
15NO 2 R ' " X; o
~0- (CH2) K-N- (CH2) n N~' N-R4
x2 R3~ ~
...(2)
[rn the formula (2), ~1 and R2 each independently
represents a hydrogen atom, a lower alkyloxycarbonyl,
unsaturated lower alkyl or lower alkyl group (any one of the
hydrogen atoms of said alkyl groups may be substituted by a
substituting group selected from the group consisting of a
hydroxy, lower monoalkylamino, lower dialkylamino, lower
alkyloxy, lower alkanoyloxy and benzoyloxy groups; a
benzoyloxy group substituted by a halogen atom or a lower
2al~1389
- 42 -
alkyloxy g-;oup; a phenyl graup; a phenyl group substituted
by a halogen atom or a lower alkyloxy group; and a lower
alkyloxycarbonyl group), or Rl and R2 may be so linked as
to make an alkylene chain and thus form a heterocyclic
structure; and
Xl, X2, R2, R3, R4, k and n are defined as in the formula
(1) .
Step (a~:
A compound of the following general formula (9)
NOz
~ -OH (~)
xi ~
XZ
~in which Xl and x2 are defined as in the general formula
(1) above]
and a compound of the following general formula (10
X3 o
H0- (CH2) k-N- (CHz) n--N ~N-R~ (lo
N~
R3! o
rin which R1 is defined as in the general formula (2) above
and R2, R3, R4, X3, n and k are defined as in the general
formula (1) above]
are allowed to react in the presence of a
dehydratecondensing agent in a solution using an appropriate
solvent or in a suspension usin~ an appropriate dispersing
2(~1389
- 43 -
agent (Application of Mitunobu reaction; 0. Mitunabu,
Synthesis, 1-~8, ]981), thereby the compound of the general
formula (2) above being obtained.
The reaction is carried out at or below the reflux
temperature of the solvent or dispersing agent used for the
reaction. For example, the temperature in the range -10 to
80~C is selected.
Examples of the dehydratecondensing agent to be used in
this reaction include various dehydratecondensing agents
used ordinarily for ether bond formation. Among them,
mixed condensing agents of diethylazodicarboxylate and
triphenylphosphine are preferably used.
Any solvent or dispersing agent can be used
for the reaction provided it is inactive to the reaction.
For example, tetrahydrofuran, dimethylformamide, chloroform,
dichloromethane or dioxane can be used.
Next, among the compounds of the general formula (1)
above, a compound of the following general formula (4) can
be produced according to a method containing the following
step (b).
N0z Rl RzX O
~ A''-N-(CHz) n - N ~
rln the formula (4), A" repre.sents -B"-(C~2)k- or -N ~ ,
in which B" represents an oxygen or sulfur atom or
Z0~13~9
-N- ;
R5 represents a hydrogen atom, a lower alkanoyl, lower
alkylsulfonyl or lower alkyl group, or Rl and R5 may be so
linked as to make an alkylene chain and thus form a
heterocyclic structure;
k represents an integral number, 2, 3 or 4; and
Xl, X2, R1, R2, R3, R4, X3 and n are defined as in the
formula ~I) above3.
Step (b):
A compound of the following general formula (11
NOz
~Y ~11)
- X Z
[in which y2 represents a halogen atom and X1 and x2 are
defined as in the general formula (I) above]
and a compound of the following general formula (12)
Rl X3 0
201 1 ~ (12)
HA~-N-(CH2) n - N ~ N-R4
N~
R3~ ~
[in which A is defined as in the general formula (4) above
and Rl, R2, R3, R4, X3 and n are defined as in the general
formula (1) above3
are allowed to react by mixing without a solvent, dissolving
Z~1389
- ~5 -
using an appropriate solvent or suspending using an
appropriate dispersing agent, thereby the compound of the
general formula (4) above being obtained.
The reaction is carried out at a temperature in the
range from room temperature to the reflux temperature of the
reaction mixture. ~or example, a temperature in the range
between 20 - 150~C is preferably selected.
The reaction can be more preferably conducted in the
presence of a base in the reaction mixture.
Any solvent or dispersing agent can be used for the
reaction provided it is inactive to this reaction. For
example, alcohols such as methanol and ethanol,
tetrahydrofuran, dimethylformamide, chloroform,
dichloromethane, dioxane, benzene and dimethylsufonoxide can
be used.
~ urthermore, examples of the base effective to
facilitate this reaction include triethylamine, pyridine,
potassium carbonate, sodium carbonate and sodium hydroxide.
Next, among the compounds of the general formula (1)
above, a compound of the following general formula (3~ can
be produced according to a method containing the following
- step (c).
2a~ 389
- 46 -
NOz A -N- (CHz) n--N ~ON-R (3,
X l x2 R3' ~
[In the formula (3), A' represents -(CH2)m-, -B'-(CH2)~- or
~ , in which B' represents an oxygen or sulfur atom
IR
or -N-;
R5 represents a hydrogen atom, a lower alkanoyl, lower
alkylsulfonyl or lower alkyl group (but does not form a
heterocyclic structure with Rl );
m represents an integral number, 0, 1, 2, 3 or 4;
k represents an integral number~ 2, 3 or 4;
1~ R1 is defined as in the general formula (2) above; and
R2, R3, R4, X1, X2. X3 and n are defined as in the general
formula (1) above.
Step (c):
A compound of the following general formula (13)
NO2
~A -Y ' (13
X ' ~
x2
[in which y1 represents a halogen atom or a substituting
groue that can be an eliminating group in the reaction with
a compound of the general formula (14) helow, Xl and x2 are
2~01~89
defined as in the general f~rmula (I) above, and A' is
defined as in the general formula (3) above]
and a compound of the following general formula ~14)
HN - ( C H 2 ~ n--N ~1~ - R ~ ( 14
N~
R3' ~
[in which Rl is defined as in the general formula ~2~ above
and R2, R3, R4, X3 and n are as defined in the general
formula (1) above]
are allowed to react by mixing without a solvent, dissolving
using an appropriate solvent or suspending using an
appropriate dispersing agent, thereby the compound of the
general formula {3) above being obtained.
The reaction is carried out at a temperature in the
range from room temperature to the reflux temperature of the
reaction mixture. For example. a temperature in the range
between 20 and 170~C is preferably selected.
The reaction can be more preferably conducted in the
presence of a base in the reaction mixture.
Any solvent or dispersing agent can be unlimitedly used
for the reaction provided it is inactive to this reaction.
For example, any of those exemplified in the step (b) above
can be used.
Furthermore, examples of the base effective to
facilitate this reaction include those exemplified in the
- 2(~)1389
- ~8 -
step (b) above.
The compounds of the general formula (1) above can also
be produced according to a method containing the fallowing
step (d):
5A compound of the following general formula (15)
NO2 Rl R2
~A-N- (CH2) n~N~{ (15)
Xl
x2
[in which A, Xl, X2, R1, R2 and n are defined as in the
general formula (1) above~
and a compound of the following general formula (16)
X o
Y3 ~ N-R~ (163
15N~
[in which Y3 is a halogen atom or a substituting group that t
can be an eliminating group in the reaction with the
compound of the general formula (15) above and R3, R4 and X3
are defined as in the general formula (1) above]
are allowed to react by mixing without a solvent, dissolving
using an appropriate solvent or suspending using an
appropriate dispersing agent, thereby the compound of the
general formula (1) above being obtained
The reaction can be earried out at a temperature in the
range from room temperature to the reflux temperature of the
z~al~ss
- 49 -
reaction mixture. For example, a temperature in the range
between 20 and 150~C is preferably selected.
The reaction can be more preferably conducted in the
presence of a base in the reaction mixture.
Any solvent or dispersing agent can be used for the
reaction provided it is inactive to this reaction. For
example, any of those exemplified in the step (b) above can
be used.
Furthermore, examples of the base useful to facilitate
this reaction include those exemplified in the step (b~
above.
Next, among the compounds of the general formula (1)
above, a compound of the following general formula (5) can
be produced according to a method containing the following
step (e~.
~ A-N- (C ~ 2 ) Z- g ~ - Y ~
~In the formula (S), A represents -(CH2)m-, -B-(CH2)k-,
-D-~CH2)1 ~ -N ~ or -NHCH2CH-CH2 ,
OH
F~S
wherein B represents an oxygen or sulfur atom, -N-,
- Z~ 89
- so -
o o o OH ~
Il 11 11 l 11
-CNH- or -CO- and ~ represents -NHC-, -CH- or -C-;
R represents a hydrogen atom, a lower alkyloxycarbonyl.
unsaturated lower alkyl or lower alkyl group (any
one of the hydrogen atoms of said alkyl groups may be
substituted by a substituting group selected from the group
consisting of a hydroxy, lower monoalkylamino. lower
dialkylamino, lower alkyloxy, lower alkanoyloxy and
benzoyloxy groups; a benzoyloxy group substituted by a
halogen atom or a lower alkyloxy group; a phenyl group; a
phenyl group substituted by a halogen atom or a lower
alkyloxy group; and a lower alkyloxycarbonyl group), or may
be so linked with R5 as to make an alkylene chain and thus
. form a heterocyclic structure but not linked with any of
other sites to form heterocyclic structure;
R5 represents a hydrogen atom, a lower alkanoyl, lower
alkylsulfonyl or lower alkyl grou~; and
X1, X2, X3, R3 and R4 are defined as in the formula (1)
above
Step ~e):
A compound of the following general formula (17)
N0z Rl'
~ A-NH
X'
Xz
[in which Xl and x2 are defined as in the general formula
Z0~31389
- 51 -
(1~ above and A and Rl are defined as in the general
formula (5) above]
and a compound of the folIowing general formula (18)
X3
~N ~ N-R~ (18)
N~
R3/ ~
~in which R3, ~4 and X3 are defined as in the general
formula (1~ above]
are allowed to react hy mixing without a solvent, dissolving
using an appropriate solvent or suseending using an
appropriate dispersing agent, thereby the compound of the
general formula (5) above being obtained.
The reaction is carried out at a temperature in the
range from room temperature to the reflux temperature of the
reaction mixture. For example, a temperature in the range
between 20~C and 180~C is preferably selected.
Furthermore, the reaction can be more preferably
conducted in the presence of an acid catalyst in the
reaction mixture.
Any solvent or dispersing agent can be used for the
reaction provided it is inactive to this reaction. For
example, any of those exemplified in the step (b) above can
he used.
Furthermore, examples of the above-mentioned acid
catalyst include p-toluenesulfonic acid and an acidic ion
~Cl 01389
- 52 -
exchange resin (for example, AmberlistR (Rhome and Haas, USA.
e.g., Amberlist 15R)).
Among the compounds of the general formula (I) above, a
compound of the following general formula (7) can also be
produced according to a method containing the following step
~f). I
N0z R'- R2,X 0
X~A-N- (CH2) n--N ~N-R4
X2 R3 ~
tln the formula (7), A represents -(CH2)m-, -B-(CH2)k-,
-1~- (CH2 ) I -, - N~, or -NHCH2C~l-G~2-,
OH
R5
wherein B represents an oxygen or sulfur atom, -N-,
O O O OH O
Il 11 1~ 1 ~I
-CNH- or -CO- and D represents -NHC-, -CH- or -C-;
R1 and R2 each independently represent a hydrogen atom, a
lower alkyloxycarbonyl, unsaturated. Iower alkyl or lower
alkyl group (any one of the hydrogen atoms of said alkyl
gro~lps may be substituted by a substituti-ng group selected
from the group consisting of a hydroxy, lower
monoalkylamino, lower dialkylamino, lower alkyloxy, lower
alkanoyloxy and benzoyloxy groups; a benzoyloxy group
substituted by ~ halogen atom or a lower alkyloxy group; a
2~1)1389
phenyl group; a phenyl group substituted by a halogen atom
or a lower alkyloxy group; and a lower alkyloxycarbonyl
group);
R5 represents a hydrogen atom, a lower alkanoyl, lower
alkylsulfonyl or lower alkyl group; or Rl and R5 may be so
linked as to make an alkylene chain and thus form a
heterocyclic structure; and
Xl, X2, X3, R~, R4, m, k, I and n are defined as in the
general formula ~1) above].
Step (f):
A compound of the general formula (17) above and a
compound of the following general formula (19)
RZ' ~~
Y~- (CHz) n--N ~ N-R~ (lg
lS N~
R3' ~
[in the formula (19), R2 represents a hydrogen atom, a
lower alkyloxycarbonyl, unsatnrated lower alkyl or lower
alkyl group (any one of the hydrogen atoms of said alkyl
groups may be substituted by a substituting group selected
from the group consisting of a hydroxy, lower
monoalkylamino, lower dialkylamino, lower alkrloxy, lower
alkanoyloxy and benzoyloxy groups; a benzoyloxy group
substituted by a halogen atom or a lower alkyloxy group; a
phenyl group; a phenyl group substituted by a halogen atom
or a lower alkyloxy group; and a lower alkyloxycarbonyl
2 ~ Q 1 ~ 8 9
- - 54 -
. _
group~ and is not so linked with Rl' to form any heterocyclic
ring;
R3, R4, X3 and n are defined as in the general formula ~1)
above; and
Y4 represents a halogen atom or a substituting group that
can be an eliminating group in the reaction with a compound
of the general formula (17) above]
are treated in the same manner as in the step (c) above,
thereby the compound of the general formula ~7~ above being
obtained.
Among the compounds of the general formula (1) above~ a
compound of the following general formula (8) can also be
produced according to a method containing the follo~ing step
(g).
NO2 R"-- R2.X o
~A----N- ~CH2) n - N ~ -R4 (8)
( tin the formula (8), A"' represents -(CH2~m-, -B'-'-(CH2)k-,
-D-(CH2)1-. - ~ or -NHCH2CH-CH2 ;
OH
5 ' O
11 11
B"' represents an oxygen or sulfur atom, -N-, -CNH- or -CO-;
25O OH O
~ Il 1 11
D represents -NHC-, -CH- or -C-;
~ .... .
2~)1389
- 55 -
R~ and R2 each independently reprosent a hydrogen atom. a
lower alkyloxycarbonyl, unsaturated lower alkyl or lower
alkyl group (any one of the hydrogen atoms of said alkyl
groups may be replaced by a substituting group selected from
the group consisting of a hydroxy, lower monoalkylamino,
lower dialkylamino, lower alkyloxy, lower alkanoyloxy and
benzoyloxy groups; a benzoyloxy group substituted by a
halogen atom or a lower alkyloxy group; a phenyl group; a
phenyl group substituted by a halogen atom or a lower
1"'
alkyloxy group and a lower alkyloxycarbonyl group~, R
does not link to other sites including R2 or R5 ;
R is defined as in the general formula (3) above; and
X1, X2, X3, A, R3, R4 and n are defined as in the formula
(1) above].
Step (g):
A compound of the following general formula (20
20~A;" N- (CH z ) n--N ~ R- ~20 )
~in w~ich A"' and R2 are defined as in the general formula
(8) above; and X1, X2, X3, R3, R4 and n are defined as in
the general formula (I) above]
and a compound of the following general formula (21)
251''' 4 (21)
[in which Y4 represents a halogen atom or a substituting
2~1389
- 56 -
grollp that can be elîminated in the rea tion with a compound
of the general formula (20) above and R is defined as in
the general formula (8) above]
are treated in the same manner as in the step (c~ above;
thereby the compound of the general formula (8) above being
obtained.
Further, for yl~ y3 and Y4 in the compounds to be used
in the production steps above, examples of the substituting
group that can be an eliminating group include
arylsulfonyloxy group such as p-toluenesulfonyloxy group and
alkylsulfonyloxy group such as methanesulfonyloxy group.
The relationships among Rl, Rl , Rl , Rl , R2, R- , R5
and R5 can be summarized as foLlows:
R may link to R2 or R5 as to form a heterocyclic structure. R
may link only to R5. Rl may link only to R2. R1 , R2 and R5 form
no linkage, i.e., no heterocyclic structure with other sites.
R , R1 , R1 & R1 R2 & R2 and R5 & R5 are synonymously
defined except their above relationshipS.
A compound of the general for~ula (18) above can be produced
according to a method containing the following step (h).
Step (h):
A compound of the general formula (16) above and 2-
aminoethanol are treated in the same manner as in the step
(d) above so that a comPound of the following general
formula (22) can be prePared.
Z(~ 389
.
Xj~J~N R~
HocH2-cH2-NH~N~o (2-~ )
R3
[In the formula (21), X3, ~3 and R~ are defined as in the
general formula (I) above].
The resultant compound is either sulfonated using
methanesulfonyl chloride, p-toluenesulfonyl chloride or the
like, or halogenized using thionyl chloride or phosphorus
tribromide. The compound thus obtained is mixed in the
presence of a base such as sodium hydroxide or sodium
hydride and in a solvent such as acetonitrile, chloroform,
benzene dimethylsulfonoxide and methanol at room temperature
15 or under heating, thereby a compound of the general formula
~18) above being obtained.
A pharmaceutically acceptable acid addition salt of a
compound of the general formula (1) above can be produced by
allowing the compound of the general formula (1) above to
react in w~ter or an organic solvent or the mixture thereof,
for example, with an inorganic or organic acid such as
hydrochloric acid, hydrobromic acid, phosphoric acid,
sulfuric acid, acetic acid, citric acid, maleic acid,
fumaric acid, oxalic acid or methanesulfonic acid.
~hen the compounds of the general formula (1) above
according to the present invention and the acid addition
Z~1389
- 58 -
salts thereof are used as a theraPeUtiC agent to treat
patients with cardiac malfunctions such as arrhythmia and
cardiac insufficiency, the amount and form of doses are
different depending on the properties of the compound of the
present invention to be used as an active ingredient and on
the symptoms of the patients to be treated. ~or example,
the amount in the range from 10 to 1,000 mg/day, preferably lO -
500 mg/day for an adult can be orally administered in forms such as
tablets, granules, powders, suspensions and capsules, or parenterally
in forms of depositories, injections, fluids for infusion, inhalations
or plasters. Daily doses by injection for adults, in particular
phleboclysis, may range from 1 - 1,000 mg, preferably 1 - 300 mg.
General processes for producing pharmaceutical compositions of
the present invention include a method in which the compound o~ the
present invention is dissolved in an appropriate amount in an oil
selected from the group consisting of cotton seed oil, corn oil, peanut
oil, olive oil and the like so as to prepare non-aqueous injections; a
method in which the compound of the present invention is
either suspended or emulsified in water in the presence of
an appropriate surfactant so as to prepare aqueous
injections; or a method in which the compound of the present
invention is prepared in a tablet form ~y adding lactose,
crystallized cellulose~ corn starch or the like and finally
adding magnesium stearate. However, the pharmaceutical
preparations of the present invention can be prepared by any
ordinary method in ~ddition to the methods exemplified above.
2C~1389
- 59 -
Useful antiarrythmic agents, therapeutic agents for
cardiac insufficiency can be provided by the compounds of
the present invention.
The following Examples demanstrate the present
invention more in detail; however, it should be understood
that they are not intended to limit the invention.
X~1389
- 60 -
Example 1
Preparation of 1,3-dimethyl-6-[4-(4-nitrophenyl)pipera-
zin-1-yl3-2,4(1H,3H)-pyrimidinedione (compound 1):
O
02N~F + HNJI~N-CH3
~ 0 (compound 2)-
CH~
> o2N-~-~I4~N-CH3
(compound 1) CH3
In the first place, 0.36 ml of 4-fluoronitrobenzene,
1 g of sodium bicarbonate and 0.5 g of 1,3-dimethyl-6-
(piperazin-1-yl)-2,4(1H,3H)-pyrimidinedione (compound 2)
were added to 5 ml of dimethyl sulfoxide, and reaction was
then performed at 100~C for 3 hours. Afterward, the
reaction mixture was poured into 50 ml of water and was then
extracted with chloroform.
Next, the chloroform extract was washed with water and
was then dried over anhydrous magnesium sulfate, and the
used solvent was distilled off under reduced pressure. The
resulting residue was then purified with a silica gel column
chromatograph (chloroform/methanol = 40:1 in terms of volume
~1389
- 61 _
ratio) in order to obtain 0.44 g of 1,3-dimethyl-6-[4-(4-
nitrophenyl)piperazin-1-yl]-2,4(1H,3H)-pyrimidinedione
(compound 1).
Analytical results of the resulting crystalline
compound 1:
Melting point: 249-250~C
IR V KBrmax (cm~1): 1700, 1660, 1610, 1530
1340, 840
NMR (d6-DMSO), ~ppm: 8.13, 6.98 (dx2, 2Hx2),
5.23 (s, 1H), 3.23, 3.36 (Sx2, 3Hx2), 3.0 (m, 8H)
Values of elemental analysis (as C16H19NsO4)
Calcd. (%): C 55.65; H 5.55; N 20.28
Found (%): C 55.34; H 5.76; N 20.46
Example 2
Preparation of 1,3-dimethyl-6-[4-(4-nitrobenzyl)pipera-
zine-1-yl]-2,4(1H,3H)-pyrimidinedione oxalate (compound 3):
H ~-~~ C H 3 ( compound 2)
,N O
02 N~CH 2 Br (COOH) 2 /CH 3 OH
89
02 N~CH 2 N, N4~N-CH 3 (COOH) 2
(compound 3) C H 3
First, 0.48 g of 4-nitrobenzyl bromide, 0.5 g of
1,3-dimethyl-6-(piperazin -1-yl)-2,4(1H,3H)-pyrimidinedione
(compound 2) and 0.5 ml of triethylamine were suspended in
5 ml of isopropanol, and the resulting suspension was then
heated under reflux for 8 hours. Afterward, the used
solvent was removed from the resulting reaction mixture by
distillation under reduced pressure, and the residue was
dissolved in chloroform and was then washed with water. The
washed organic layer was dried over anhydrous sodium
sulfate. Furthermore, the dried organic layer was subjected
to distillation under reduced pressure so as to remove the
solvent therefrom, and the residue was then purified through
a silica gel column chromatograph (chloroformtmethanol =
50/1 to 20/1 in terms of volume ratio) in order to obtain
0.88 g of 1,3-dimethyl-6-[4-(4-nitrobenzyl)piperazin-1-yl]-
2,4(1H,3H)-pyrimidinedione.
Analytical results of the pyrimidinedione derivative
thus obtained:
NMR (CDCl3), ~ppm: 2.6 (m, 2H), 3,0 (m, 2H), 3.22,
3.34 (Sx2, 3Hx2), 3.36 (S, 2H), 5.14 (S, 1H)
;~01389
7.55, 8.17 (dx2, 2Hx2)
Next, this pyrimidinedione derivative was treated with
an oxalic acid/methanol solution in an ordinary manner,
thereby preparing 1,3-dimethyl-6-[4-(4-nitrobenzyl)pipera-
zin-1-yl]-2,4(1H,3H)-pyrimidinedione oxalate (compound 3).
Analytical results of the crystalline compound 3
thus obtained:
Melting point: 211-212~C (decomposed)
IR y KBrmax (cm~1): 2950, 1720, 1670, 1650
1620, 1520, 1360, 760
Values of elemental analysis [as C17H21N5~4-(C~~H)2]
Calcd. (%): C 50.78; H 5.16; N 15.58
Found (%): C 50.70; H 5.44; N 15.77
Example 3
Preparation of 1,3-dimethyl-6-{2-<N-ethyl-N-[3-(4-
nitrophenyl)propyl]amino>ethylamino~-2,4(1H,3H)-pyrimidine-
dione hydrochloride (compound 4):
~ N-CH3
Cl ~ ~0
Cl-13
HOCH2CH2NH2 CH3~SO2Cl
> >
389
- 64 _
-~ CHa ~ SOa~CH2CH2NH ~ ~ N-CH3 >
(compound 5) CH 3
NaH/DMSO N ~ ~~CHa
> / N~o >
(Compound 6) CH3
1 0
CH2CH3
02N ~ CH2CH2CH2NH HCl/CH30H
(compound 7)
CH2CH3 ~
02N ~ CH2CH2CH2-N-CH2CH2NH y ~ -CH3
(compound 4) CH~
(1) Preparation of 1,3-dimethyl-6-[2-(p-toluenesulfo-
nyloxy)ethylamino]-2,4(1H,3H)-pyrimidinedione (compound 5):
First, 35.0 g of 2-aminoethanol was heated up to 90~C
and then taken out from the oil bath, and 50.0 g of
6-chloro-1,3-dimethyl-2,4(1H,3H)-pyrimidinedione was added
thereto, so that reaction was performed therebetween. In
~0~1389
- 65 -
this case, the addition was carried out at such a velocity
that the reaction temperature was maintained in the range of
90 to 110~C. After completion of the addition, the reaction
mixture was stirred for 10 minutes, and 300 ml of dioxane/-
methanol (which was 10/1 in terms of volume ratio) was thenadded thereto. Afterward, the mixture was allowed to stand
overnight. The resulting crystals were then washed with a
small amount of dioxane, followed by drying to obtain 49.0 g
of white crystals of 1,3-dimethyl-6-(2-hydroxyethylamino)-
2,4(1H,3H)-pyrimidinedione.
Next, 200 ml of a pyridine suspension containing 49.0 g
of the above-mentioned white crystals was cooled to -5~C,
and 40.0 g of p-toluenesulfonyl chloride ~as then added
thereto at such a velocity that the reaction temperature
does not rise up to 5~C or more. In order for the suspen-
sion of the reaction mixture to disappear completely, 51.0 g
of p-toluenesulfonyl chloride was further used.
Moreover, the reaction mixture was then poured into 1.5
liters of ice water containing 70 g of potassium carbonate
and was then allowed to stand overnight. The resulting
crystals were collected by filtration, then washed with
water, and dried under reduced pressure, thereby preparing
50.5 g of light yellow crystals of 1,3-dimethyl-6-[2-(p-
toluenesulfonyloxy)ethylamino]-2,4(1H,3H)-pyrimidinedione
(compound 5).
63689
Analytical results of the crystalline compound 5
thus obtained:
Melting point: 146.0-149.0~C
IR ~ KBrmax (cm~1): 3270, 1682, 1615, 1550,
1480, 1435, 1360, 1190,
1178, 1010, 903, 780
(2) Preparation of 6-(1-aziridinyl)-1,3-dimethyl-
2,4(1H,3H)-pyrimidinedione (compound 6):
To 150 ml of an anhydrous dimethyl sulfoxide solution
containing 47.2 g of the above prepared compound 5 was
slowly added 6.24 g of sodium hydride (60% dispersion in
mineral oil) at room temperature. The resulting liquid
mixture was then stirred vigorously at ~oom temperature for
5 hours and was cooled, and a small amount of water was then
added thereto in order to bring the reaction to an end.
Afterward, this mixture was poured into 1 liter of water
containing 70 g of potassium carbonate and was then
extracted with 200 ml of chloroform three times. The
combined organic layers were then dried over anhydrous
sodium sulfate and was concentrated, and 300 ml of ether was
added to the resulting concentrate. Afterward, the mixture
was allowed to stand overnight.
Light yellow crystals which had been deposited by the
overnight standing were collected by filtration and were
then washed with ether, followed by drying under reduced
389
_ 67 -
pressure to obtain 15.2 g of 6-(1-aziridinyl)-1,3-dimethyl-
2,4(1H,3H)-pyrimidinedione (compound 6).
Analytical results of the crystalline compound 6
thus obtained:
Melting point: 126.0-126.5~C
IR ~ KBrmax (cm~1): 1705, 1650, 1612, 1470, 1440,
1305, 1160, 783, 490
H-NMR (CDCl3), ~ppm: 2.34 (s, 4H), 3.35 (s, 3H),
3.56 (s, 3H), 5.25 (s, 1H)
(3) Preparation of 1,3-dimethyl-6-~2-<N-ethyl-N-[3-(4-
nitrophenyl)propyl]amino>ethylamino}-2,4(1H,3H)pyrimidine-
dione hydrochloride (compound 4):
In 5 ml of chloroform were dissolved 1.28 g of
N-ethyl-N-[3-(4-nitrophenyl)propyl]amine (compound 7) and
1.11 g of the above prepared 6-(1-aziridinyl)-1,3-dimethyl-
2,4(1H,3H)-pyrimidinedione (compound 6), and the resulting
mixture was then concentrated under reduced pressure.
Afterward, 10 mg of Amberlist 15 (made by Rohm & Haas Co.)
was added to the residue (concentrate), and the mixture was
then heated at 80~C for 1 hour.
Next, the resulting reaction mixture was dissolved in
20 ml of ethyl acetate, and the Amberlist was removed
therefrom by filtration. Afterward, 30 ml of n-hexane was
added to the filtrate.
The solution was allowed to stand overnight, and the
20~1389
- 68 _
deposited light yellow crystals were then collected by
filtration. After washing with ether, the crystals were
dried under reduced pressure to obtain 2.20 g of 1,3-
dimethyl-6- 2- N-ethyl-N-[3-(4-nitrophenyl)propyl]amino -
ethylamino -2,4(1H,3H)-pyrimidinedione.
This product was further recrystallized from ethyl
acetate and n-hexane, followed by filtering, washing and
drying in order to obtain 1.85 g of light yellow crystals.
Analytical results of the crystalline pyrimidinedione
derivative thus obtained:
IR ~ KBrmax (cm-1): 3310, 2940, 1692, 1620,
1600, 1545, 1515, 1345,
1205, 770, 755
1H-NMR (CDCl3), ~ppm: 1.07 (t, 3H, J=7.5 Hz),
1.90 (m, 2H), 2.17-3.27 (m, 10H), 3.37 (s, 3H),
3.44 (s, 3H), 4.90 (s, 1H), 5.64 (brs, 1H),
7.47 (m, 2H), 8.30 (m, 2H)
Values of elemental analysis (as C1gH27NsO4)
Calcd. (%): C 58.60; H 6.99; N 17.98
Found (%): C 58.61; H 7.41; N 17.57
Moreover, the light yellow crystals which had been
recrystallized from ethyl acetate and n-hexane were treated
with hydrochloric acid/methanol in a usual manner in order
to obtain an amorphous powder of 1,3-dimethyl-6-{2-~N-ethyl-
N-[3-(4-nitrophenyl)propyl]amino>ethylamino}-2,4(1H,3H)-
- 69 -
pyrimidinedione hydrochloride (compound 4).
Analytical results of the amorphous powder of
the compound 4:
IR V KBrmax (cm~1): 3450, 1705, 1608, 1513, 1345
Values of elemental analysis (as C1gH27NsO4 HCl)
Calcd. (%): C 53.58; H 6.63; N 16.44; Cl 8.33
Found (%): C 53.11; H 6.81; N 16.12; Cl 7.95
Example 4
Preparation of 1,3-dimethyl-6-~2-~N-(2-hydroxyethyl)-N-
[3-(4-nitrophenyl)propyl]amino>ethylamino}-2,4(1H,3H)-
pyrimidinedione fumarate (compound 8):
HOCH2CH2NH2
02N ~ CH2CH2CH20SO2 ~ CH3
' CH2CH20H
> NO2 ~ CH2CH2CH2NH
(compound 9)
~
CH3 ~ S03CH2CH2NH ~ ~ N-CH3
(compound 5) C H3
>
~n ~ ~ ~ 8 9
_ 70 -
(=CHCOOH)2
/ CH 3 OH
CH2CH20H ~
02N ~ CH2CH2CH2NCH2CH2NH ~ N-CH~
N~o (=CHCOOH)2
(compound 8) C H~ -
(1) Preparation of N-(2-hydroxyethyl)-N-l3-(4-nitro-
phenyl)propyl]amine (compound 9):
A mixture of 37.5 g of 3-(4-nitrophenyl)propyl
p-toluene sulfonate, 125 g of aminoethanol and 65 ml of
dioxane was heated with stirring at a temperature of 90 to
100~C for 3 hours. Thereafter, the used solvent was
distilled off from the mixture under reduced pressure, and
the residue was dissolved in 800 ml of chloroform and the
solution was then washed with 1 liter of water. The washed
( organic layer was further washed with a 0.5 N sodium
hydroxide solution and then with water, followed by drying
over anhydrous sodium sulfate. The dried organic layer was
then treated under reduced pressure to distill off the
solvent therefrom, and the residue was recrystallized from
benzene/hexane (which was 2/1 in terms of volume ratio).
The obtained crystals were collected by filtration, washed
and dried, thereby preparing 21 g of N-(2-hydroxyethyl)-N-
.~
~,
~,. -
~ ~ Q 11 ~ 8 ~
[3-(4-nitrophenyl)propyl]amine (compound 9).
Analytical results of the crystalline compound 9
thus obtained:
Melting point: 80.5-81~C
(2) Preparation of 1,3-dimethyl-6-~2-<N-(2-hydroxy-
ethyl)-N-[3-(4-nitrophenyl)propyl]amino)ethylamino}-2,4-
(1H,3H)-pyrimidinedione fumarate (compound 8):
In 350 ml of methanol was dissolved 23.2 g of 1,3-
dimethyl-6-[2-(p-tolllen~r~11fonyloxy)ethylamino]-2,4(lH,3H)-
pyrimidinedione (compound 5) synthesized in Example 3-(1),
and 2.76 g of sodium hydroxide was further added thereto
slowly. The resulting mixture was stirred at a temperature
of S0 to 60~C for 1 hour, and the solvent was then distilled
off from the reaction mixture. Afterward, 120 ml of
chloroform was added to the residue, and insoluble materials
were removed by filtration therefrom. To the filtrate were
added 17 g of N-(2-hydroxyethyl)-N-[3-(4-nitrophenyl)propyl]-
( amine (compound 9) and 0.66 g of p-toluene-sulfonic acid,
and the reaction mixture was then treated under reduced
pressure to distill off the solvent therefrom. The
solvent-free residue was heated and stirred at 80~C for 1
hour and was then dissolved in 480 ml of chloroform, and the
reaction mixture was extracted twice with 300 ml of O.S N
hydrochloric acid. Afterward, potassium carbonate was added
to the extract (hydrochloric acid solution) with the
,,~ .
ZO~)1389
- 72 _
intention of making the latter alkaline, and the liquid
mixture was then stirred at room temperature for 1 hour.
The resulting crystals were then collected by filtration.
The crystals were dried, recrystallized from ethanol,
collected by filtration, washed and dried in order to obtain
22.1 g of 1,3-dimethyl-6-~2-<N-(2-hydroxyethyl)-N-[3-(4-
nitrophenyl)propyl]amino>ethylamino~-2,4(1H,3H)-pyrimidine-
dione.
Analytical results of the crystalline pyrimidinedione
derivative thus obtained:
Melting point: 117.5-118.5~C
H-NMR (CDCl3), ~ppm: 1.86 (m, 2H),
2.48-3.00 (m, 11H), 3.00-3.26 (m, 2H),
3.27 (s, 3H), 3.39 (s, 3H), 3.71 (m, 2H),
4.78 (s, 1H), 6.06 (m, 1H), 7.38 (d, 2H),
8.18 (d, 2H)
This pyrimidinedione derivative was treated with a
fumaric acid/methanol solution in a usual manner to obtain
1,3-dimethyl-6-{2-~N-(2-hydroxyethyl)-N-[3-(4-nitrophenyl)-
propyl]amino)ethylamino}-2,4(1H,3H)-pyrimidinedione fumarate
(compound 8).
Analytical results of the prepared crystalline
compound 8:
Melting point: 152.5-153.5~C
IR ~ KBrmax (cm-1): 3400, 2960, 1700, 1630,
2001:~39
- 73 -
1600, 1555, 1520, 1450, 1355,
1250, 1070, 990, 780
3) Preparation of 1,3-dimethyl-6-~2-(N-(2-hydroxy-
ethyl)-N-[3-(4-nitrophenyl)propyl]amino>ethylamino}-2,4-
(1H,3H)-pyrimidinedione hydrochloride (compund 8'):
1,3-Dimethyl-6-{2-<N-(2-hydroxyethyl)-N-[3-(4-nitro-
phenyl)propyl]amino~ethylamino32,4(1H,3H)-pyrimidinedione
(free from of compound 8) (2.6 g ) was dissolved in 26 ml
methanol under heating and to the resulting solution was
added 2.7 ml 13% (w/w) of HCl/methanol solution drop by
drop, while the temperature of the mixture was maintained
at 40~C.
The resulting mixture was cooled on ice and was left
overnight. Crystals thus formed were collected by filtra-
tion and dried in vacuum; thus, 1,3-dimethyl-6-{2-<N-(2-
hydroxyethyl)-N-[3-(4-nitrophenyl)propyl]amino~ethylamino}-
2,4(1H,3H)-pyrimidinedione hydrochloride (compound 8') was
obtained.
Analytical results of the crystalline 8' thus
obtained:
Melting point: 172-174~C
IR V KBrmax (cm-1): 3230, 1640, 1605, 1540,
1340, 1240
Example 5
Preparation of 1,3-dimethyl-6-{4-[2-(2-nitrophenyl)-
2 ~ Q ~ 3 8 9
- 74 _
ethyl]piperazin-1-yl}-2,4(1H,3H)-pyrimidinedione oxalate
(compound 10):
~ CH2CH20H ~ ~ cH2cH2Br
~2 . 02 (compound 11)
( HN ~ ~ ~ N-CH~
(compound 2) C H~ (COOH) 2 /CH~ON
O
~ CH2CH2 ~ ~ N-CH~
? '-~NO2 N ~ (COOH)2
(compound 10) CH~
(1) Preparation of 2-(2-nitrophenyl)ethyl bromide
(compound 11):
( First, 2.5 ml of 2-(2-nitrophenyl)ethanol and 5.4 ml of
PBr3 were mixed and stirred at 0~C for 30 minutes to perform
reaction therebetween, and the resulting reaction mixture
was diluted with 30 ml of benzene and was then poured into
30 ml of water. Afterward, the separated organic layer~was
collected, dried over anhydrous sodium sulfate, and then
treated under reduced pressure to distill off the solvent
therefrom, thereby preparing 3 g of a crude product,
~ '
;~
Z~1~1389
_ 75 -
2-(2-nitrophenyl)ethyl bromide (compound 11).
(2) Preparation of 1,3-dimethyl-6-{4-[2-(2-nitro-
phenyl)ethyl]piperazin-1-yl}-2,4(1H,3H)-pyrimidinedione
oxalate (compound 10):
To 15 ml of isopropanol were added 3.0 g of the above
prepared compound 11, 3.2 g of 1,3-dimethyl-6-(piperazine-1-
yl)-2,4(1H,3H)-pyrimidinedione (compound 2) and 4.2 ml of
triethylamine, and the resulting mixture was then treated by
the same procedure as in Example 2 in order to obtain 2.2 g
of 1,3-dimethyl-6-{4-[2-(2-nitrophenyl)ethyl]piperazin-1-
yl}-2,4(1H,3H)-pyrimidinedione.
Analytical results of the pyrimidinedione derivative
thus obtained:
NMR (CDCl3), ~ppm: 2.7-3.0 (m, 12H), 3.39 (s, 3H),
3.41 (s, 3H), 5.11 (s, 1H), 7.53 (m, 3H),
7.96 (m, 1H)
Furthermore, this pyrimidinedione derivative was
treated with an oxalic acid/methanol solution in a usual
manner, thereby preparing 1,3-dimethyl-6-{4-[2-(2-nitro-
phenyl)ethyl]piperazin-1-yl}-2,4(1H,3H)-pyrimidinedione
oxalate (compound 10).
Analytical results of the prepared crystalline
compound 10:
Melting point: 212-214~C
Z~1389
- 76 -
Values of elemental analysis
(as C18H23N5o4-(cOOH)2-2H2o)
Calcd. (%): C 50.85; H 5.55; N 14.82
Found (~): C 50.57; H 5.54; N 14.44
IR ~ KBrmax (cm-1): 3050, 2940, 1720, 1680,
1630, 1550, 1380, 770
Example 6
Preparation of 1,3-dimethyl-6-[2-(4-nitroanilino)ethyl-
amino]-2,4(1H,3H)-pyrimidinedione (compound 12):
0
02N ~ F + H2NCH2CH2NH ~ oN-CH3
(Compound 13) C H3
> 02N ~ -NHCH2CH2NH ~ ~ -CH3
(compound 12) C H3N
The same procedure as in Example 1 was repeated with
the exception that 1,3-dimethyl-6-(piperazin-1-yl)-2,4-
(1H,3H)-pyrimidinedione (compound 2) was replaced with
0.45 g of 6-(2-aminoethylamino)-1,3-dimethyl-2,4(1H,3H)-
pyrimidinedione (compound 13), in order to obtain 0.5 g of
1,3-dimethyl-6-[2-(4-nitroanilino)ethylamino]-2,4(1H,3H)-
2~389
pyrimidinedione (compound 12).
Analytical results of the crystalline compound 12
thus obtained:
Melting point: 308~C (decomposed)
IR ~ KBrmax (cm-1): 1690, 1660, 1600, 1550,
1320, 850
NMR (DMSO-d6), ~ppm: 2.9 (m, 2H), 3.1 (m, 2H),
3.29 (s, 3H), 3.38 (s, 3H), 5.09 (s, 1H),
7.04 (d, 2H), 8.09 (d, 2H)
Values of elemental analysis (as C14H17NsO4)
Calcd. (%): C 52.66; H 5.37; N 21.93
Found (%): C 52.55; H 5.34; N 21.82
Example 7
Preparation of 1,3-dimethyl-6-~4-[2-(4-nitrophenyl)-
ethyl]piperazin-1-yl}-2,4(1H,3H)-pyrimidinedione hydro-
chloride (compound 14):
, ~ ~ 021~CH2CH2Br
H ~ N ~ N-CH3 >
/~ 0
CH3
(compound 2)
HCl/CH30H
>
389
- 78 _
-CH2CH2N ~ ~ -CH3
(compound 14) CH3
The same procedure as in Example 2 was repeated with
the exception that 4-nitrobenzyl bromide was replaced with
0.51 g of 4-nitrophenethyl bromide, in order to obtain
1,3-dimethyl-6-{4-[2-(4-nitrophenyl)ethyl]piperazin -1-yl}-
2,4(1H,3H)-pyrimidinedione.
Analytical results of the pyrimidinedione derivative
thus obtained:
NMR (CDCl3), ~ppm: 2.8 (m, 12H), 3.22 (s, 3H),
3.36 (s, 3H), 5.19 (s, 1H), 7.36 (d, 2H),
8.12 (d, 2H)
Moreover, this pyrimidinedione derivative was treated
with a hydrochloric acid/methanol solution in a usual manner
to obtain 1,3-dimethyl-6-{4-[2-(4-nitrophenyl)ethyl]pipera-
zin-1-yl~-2,4(1H,3H)-pyrimidinedione hydrochloride
(compound 14).
Analytical results of the crystalline compound 14
thus obtained:
Melting point: 263-266~C (decomposed)
1389
- 79 -
Values of elemental analysis tas C1gH23NsO4-HCl-0.5H2O)
Calcd. (%): C 51.61; H 6.02; N 16.72; Cl 8.46
Found (%): C 51.78; H 6.28; N 16.93; Cl 8.60
IR V KBrmax (cm~1): 2950, 2500, 1700, 1690,
1630, 1520, 1350, 805
Example 8
Preparation of 1,3-dimethyl-6-[2-(4-nitrobenzylamino)-
ethylamino]-2,4(1H,3H)-pyrimidinedione oxalate
(compound 15):
0
02N ~ -CH2Br + H2N-CH2CH2NH ~ N-CH3
(compound 13) CH3
(COOH)2/CH30H
> >
02N ~ CH2NHCH2CH2NH ~ N-CH3
N ~ n .(COOH)2
(compound 15) C H3 u
The same procedure as in Example 2 was repeated with
the exception that 1,3-dimethyl-6-(piperazin-1-yl)-2,4-
(1H,3H)-pyrimidinedione (compound 2) was replaced with
- X~ 389
- 80 _
0.45 g of 6-t2-aminoethylamino)-1,3-dimethyl-2,4(1H,3H)-
pyrimidinedione (compound 13), in order to obtain 1,3-
dimethyl-6-[2-(4-nitrobenzylamino)ethylamino]-2,4(1H,3H)-
pyrimidinedione.
Analytical results of the prepared pyrimidinedione
derivative:
NMR (CDCl3:DMSO-d6=1:1), ~ppm: 2.9 (m, 2H),
3.2 (m, 2H), 3.30 (s, 3H), 3.38 (s, 3H),
3.60 (m, 2H), 5.07 (s, 1H), 7.42 (d, 2H),
8.06 (d, 2H)
Moreover, this pyrimidinedione derivative was treated
with an oxalic acid/methanol solution in a usual manner to
obtain crystals of 1,3-dimethyl-6-[2-(4-nitrobenzylamino)-
ethylamino]-2,4(1H,3H)-pyrimidinedione oxalate
(compound 15).
Analytical results of the crystalline compound 15
thus obtained:
Melting point: 203-205~C (decomposed)
IR V KBrmax (cm-1): 3150, 2900, 1710, 1650,
1640, 1630, 870
Values of elemental analysis
[as C1sH19NsO4 (COoH)2~o 5H2o]
Calcd. (%): C 47.22; H 5.13; N 16.20
Found (%): C 47.04; H 4.40; N 16.61
1389
- 81 -
Example 9
Preparation of 1,3-dimethyl-6-{2-[2-(4-nitrophenyl)-
ethylamino]ethylamino}-2,4(1H,3H)-pyrimidinedione oxalate
(compound 16):
02N ~ CH2CH2Br_t H2NCH2CH2NH ~ N-CH~
(compound 13)C H~
(COOH)2/CH 3 OH
> >
02N ~ CH2CH2NHCH2CH2NH ~ \N-CH~ -
(COmpound 16) ~N ~ ~ ~(COOH~2
The same procedure as in Example 2 was repeated with
the exception that 4-nitrobenzyl bromide and 1,3-dimethyl-6-
piperazin -1-yl)-2,4-(1H,3H)-pyrimidinedione (compound 2)
were replaced with 0.51 g of 2-(4-nitrophenyl)ethyl bromide
and 0.45 g of 6-(2-aminoethylamino)-1,3-dimethyl-2,4(1H,3H)-
pyrimidinedione (compound 13), respectively, in order to
obtain 1,3-dimethyl-6-{2-[2-(4-nitrophenyl)ethylamino]-
ethylamino3-2,4(1H,3H)-pyrimidinedione.
-- 2~)13~9
- 82 -
Analytical results of the pyrimidinedione derivative
thus obtained:
NMR (CDCl3), ~ppm: 2.7-3.2 (m, 6H), 3.29 (s, 3H),
3.37 (s, 3H), 3.51 (t, 2H), 5.01 (s, 1H),
8.13 (d, 2H), 8.43 (d, 2H)
Furthermore, this pyrimidinedione derivative was
treated with an oxalic acid/methanol solution in a usual
manner, thereby preparing crystals of 1,3-dimethyl-6- 2-[2-
(4-nitrophenyl)ethylamino]ethylamino -2,4(1H,3H)-pyrimidine-
dione oxalate (compound 16).
Analytical results of the crystalline compound 16
thus obtained:
Melting point: 200-202~C (decomposed)
Values of elemental analysis
(as C16H21NsO4-(cOoH)2o2H2o)
Calcd. (%): C 48.43; H 5.42; N 15.69
Found (%): C 48.52; H 5.16; N 16.25
IR KBrmax (cm-1): 3100, 2900, 1710, 1640,
1620, 1560, 1360, 850
Example 10
Preparation of 1,3-dimethyl-6-{N-methyl-2-[N-methyl-3-
(4-nitrophenyl)propylamino]ethylamino}-2,4(1H,3H)-pyrimidine-
dione oxalate (compound 17):
8 9
_ 83 -
O CHaNHCH2CH2NHCH~
C1 ~ _~N-CH~
3 o
CH~
CH~ ~
CH~NHCH2CH2N ~ N-CH~
( N ~ o
(compound 18)C H~
0
O2N ~ CH2CH2CH2B r (COOH)2/CH~OH
CH3 CH3 ~
02N ~ CH2CH2CH2NCH2CH2N ~ N-CH3
~ N ~ o (COOH)2
(compound 17) C H3
(1) Preparation of 1,3-dimethyl-6-{N-methyl-N-~2-
(methylamino)ethyl]amino}-2,4(1H,3H)-pyrimidinedione
(compound 18):
In 200 ml of chloroform were dissolved 50 g of
N,N'-dimethylethylenediamine and 19.8 g of 6-chloro-1,3-
dimethyl-2,4(1H,3H)-pyrimidinedione, and the reaction
mixture was then heated with stirring under reflux for
B
~ . . ..
-
-- 2~1389
- 84 _
5 hours.
The reaction mixture was washed with water, and a
separated organic layer was then dried over anhydrous sodium
sulfate. Furthermore, the dried organic layer was treated
under reduced pressure to distill off the solvent, and ether
was then added to the resulting residue so as to deposit
crystals. The latter were then collected by filtration,
washed and dried in order to obtain 10 g of 1,3-dimethyl-6-
{N-methyl-N-[2-(methylamino)ethyl]amino}-2,4(1H,3H)-pyridine-
dione (compound 18).
Analytical results of the crystalline compound 18
thus obtained:
'! NMR (CDCl3), ~ppm: 2.7 (s, 3H), 2.5-3.3 (m, 4H),
3.25 (s, 3H), 3.3 (s, 3H), 3.33 (s, 3H),
5.25 (s, 1H)
(2) Preparation of 1,3-dimethyl-6-~N-methyl-2-[N-
methyl-3-(4-nitrophenyl)propylamino]ethylamino}-2,4(1H,3H)-
pyrimidinedione oxalate (compound 17):
To 60 ml of isopropanol were added 5.0 g of the above
compound 18, 5.5 g of 3-(4-nitrophenyl)propyl bromide and
6 ml of triethylamine, and the resuting mixture was then
treated by the same procedure as in Example 2 in order to
obtain 8.1 g of 1,3-dimethyl-6-{N-methyl-2-[N-methyl-3-(4-
nitrophenyl)propylamino]ethylamino}-2,4(1H,3H)-pyrimidine-
dione.
~1389
- 85 -
Analytical results of the pyrimidinedione derivative
thus obtained:
NMR (CDC13), ,~ppm: 1.5-2.2 (m, 2H), 2.25 (s, 3H),
2.25-3.2 (m, 8H), 3.3 (s, 3H), 3.35 (s, 3H),
5.25 (s, 1H), 7.3 (d, 2H), 8.15 (d, 2H)
Moreover, this pyrimidinedione derivative was treated
with an oxalic acid/methanol solution in a usual manner to
obtain crystals of 1,3-dimethyl-6-{N-methyl-2-[N-methyl-3-
(4-nitrophenyl)propylamino]ethylamino}-2,4(1 H,3H)-pyrimidine-
10 dione oxalate (compound 17).
Analytical results of the prepared crystalline
compound 17:
Values of elemental analysis
[as C1gH27NsO4-(cOoH)2]
Calcd. (%): C 52.60; H 6.10; N 14.61
Found (%): C 52.41; H 6.33; N 14.21
Example 11
The same procedure as in Example 3-(3) was repeated
with the exception that N-ethyl-N-[3-(4-nitrophenyl)propyl]-
20 amine (compound 7) was replaced with each amino compound in
which the ethyl group portion (R1 ) and the number (m) of
carbon atoms in an alkyl chain of the compound 7 were as
shown in Table 1, in order to obtain compounds 19 to 24.
Physical properties of these compounds are set forth in
~5 Table 1.
-
2~0~89
- 86 -
~ _ _ ~
C~ ~D U~ O
~ ~ ~ O O ~
_z .. .. .. ..
~ _ _ _ _
~ o <s~ U~ ~ ~ ~ ~
:~ ~C .. .. .. ..
_
_
o
.. .. .. ..
O O ~ ~~ CJ',
o
, o o ~C
~ ~ In
0 0-1~ 0-1~ 00 ~ O
o~ ~ ~ ~ ~Z ~Z ~ Z ~ Z ~Z ~ Z
,Z =~ ~ ~ ~ ~~ ~ ~ I
1~ 0 ~ 0 0 00 0~ 0
C O~ C~
Z ~ ~,C~ _ C,,~ _C~ _C,) _ C~ _ O _
-
--- ~~ t-- ~ o oIn ou~ 0 ~ a~
U
-- X' '
'~ ~o oIn o ~ ~ o ~ ~ o
O ~ ~ ~ ~ ~ ~ 0 --.- --~
~ ~~ ~ ~ ~ ~ Q ~ Q
11 ~ _ _
o oo o ~ o ~ o o oo U~
O H~ ~ f'~
~n o o o
U
~ ~n o o
o C ~O ~
I
o In u~If)
. . .
~ l ~
o z -
N
U
S~ -
~ O ~ O
C~ Z
g
- 87 -
Example 12
Preparation of 1,3-dimethyl-6-{2-<N-ethyl-N-[3-(3-
nitrophenyl)propyl]amino>ethylamino}-2,4(1H,3H)-pyrimidine-
dione hydrochloride (compound 25):
C2HsNH2
~ CH2CH2CH20SO2 ~ CH3 >
NOz
CH3 ~ 03CHzCH2NH ~ N-CHs
~N ~ o
(compound 5) C H3
HCl/CH 3 OH
>
~ CH2CH2CH2NCH2CH2NH ~ N-CH3
NO2 /N ~ o HCl
(compound 25) C H3
The same procedure as in Example 4 was repeated with
the exception that 37.5 g of 3-(3-nitrophenyl)propyl
p-toluene sulfonate, 120 g of ethylamine and 188 g of
1,3-dimethyl-6-l2-(p-toluenesulfonyloxy)ethylamino-2,4-
(1H,3H)-pyrimidinedione (compound 5) were used as starting
~0~1389
.
- 88 _
materials, in order to obtain crystals of 1,3-dimethyl-6-{2-
<N-ethyl-N-[3-(3-nitrophenyl)propyl]amino> ethylamino3-
2,4(1 H,3H)-pyrimidinedione.
Analytical results of the crystalline pyrimidinedione
derivative thus obtained:
NMR (CDC13), ,~ppm: 1.00 (t, 3H), 1.6-2.0 (m, 2H),-
2.4-2.8 (m, 8H), 2.9-3.1 (m, 2H), 3.2 (s, 3H),
3.3 (s, 3H), 4.75 (s, 1H), 5.5 (br, 1H),
7.3-7.4 (m, 2H), 7.8-8.0 (m, 2H)
The thus obtained crystals were treated with a
hydrochloric acid/methanol solution in an ordinary manner to
obtain crystals of 1,3-dimethyl-6-{2-(N-ethyl-N-[3-(3-
nitrophenyl)propyl]amino>ethylamino} -2,4(1 H,3H)-pyrimidine-
dione hydrochloride (compound 25).
Analytical results of the crystalline compound 25
thus obtained:
Values of elemental analysis (as C1gH27NsO4-HCl)
Calcd. (%): C 53.58; H 6.63; N 16.44; Cl 8.33
Found (%): C 53.02; H 6.79; N 16.01; Cl 7.98
Example 13
Preparation of 1,3-dimethyl-6-~N-ethyl-N-~2-[4-(4-nitro-
phenyl)butylamino]ethyl>amino~ -2,4(1 H,3H)-pyrimidinedione
oxalate (compound 26):
20~ 89
_ 89 -
~ HOCH2CH2NHC2Hs
Cl ~ N-CH3 >
/N
CH3
C,2 H s ~'
HOCH 2 CH 2 N ~ N-CH 3
/N~o
(compound 27)CH3
~ H
>
1 s ~ N C H 2 G H ~ N ~ON- G H a
~ ~ o (compound 29)
~ CH3
N2H4 H20
>
C~2Hs ~
NH2CH2CH2N ~ /N-CH 3
/N~o
(compound 28) CH 3
- Z6)~ 89
- 90
02N ~ -(CH2)3CH0 NaBH4 (COOH)2/CH3OH
> > . >
C2H5 ~
02N ~ (CH2)4-NHCH2CH2~ ~ N-CH3
(compound 26) C~ ~ ~ ~(CO2H)2
(1) Preparation of 1,3-dimethyl -6-[ N-ethyl-N-( 2-
hydroxyethyl)amino]-2,4(1H,3H)-pyrimidinedione
(compound 27):
Up to 100~C, 11 g of N-ethylaminoethanol was heated,
and while the above temperature was maintained, 10 g of
6-chloro-1,3-dimethyl-2,4(1H,3H)-pyrimidinedione was added
thereto little by little.
The reaction mixture was heated at the same temperature
for 1 hour, and 100 ml of dioxane was then added thereto,
~ollowed by stirring under ice cooling for 1 hour.
The crystals deposited by the above stirring were
removed from the reaction mixture by filtration, and the
filtrate was then concentrated. Afterward, the residue
(concentrate) was purified through a silica gel column
chromatograph (ethyl acetate/methanol = 20/1 in terms of
volume ratio) to obtain 7.2 g of 1,3-dimethyl-6-[N-ethyl-N-
( 2 -hydroxyethyl)amino]-2,4(1H,3H)-pyrimidinedione
-
2~)1389
- 91 -
(compound 27).
Analytical results of the compound 27 thus obtained:
NMR (CDCl3), ,~ppm: 1.15 (t, 3H), 3.0-3.3 (m, 4H),
3.3 (s, 3H), 3.4 (s, 3H), 3.6-3.9 (m, 2H),
5.35 (s, 1H)
(2) Preparation of 1,3-dimethyl-6-[N-ethyl-N-(2-amino-
ethyl)amino]-2,4(1 H,3H)-pyrimidinedione (compound 28):
In 200 ml of tetrahydrofurane were suspended 16.8 g of
the above compound 27, 8.6 g of triphenylphosphine and
11.96 g of phthalimide, and 14.81 g of diethyl azodicarboxy-
late was further added dropwise to the resulting suspension
under ice cooling.
Next, after completion of the addition, the used
solvent was distilled off from the reaction mixture under
reduced pressure, and the residue was then purified through
a silica gel column chromatograph (ethyl acetate) in order
to obtain 22.5 g of 6-[N-ethyl-N-(2-phthaloylaminoethyl)-
amino]-1,3-dimethyl-2,4(1 H,3H)-pyrimidinedione (compound
29). The latter compound was then treated with hydrazine
monohydrate in ethanol in a usual method to remove the
phthaloyl group therefrom, thereby preparing crystals of
1,3-dimethyl-6-[N-ethyl-N-(2-aminoethyl)amino]_2,4(1 H,3H)-
pyrimidinedione (compound 28).
Analytical results of the crystalline compound 28
thus obtained:
25~0~389
- 92 -
NMR (CDCl3:DMSO-d6 = 1:1, v/v), 5ppm: 1.15 (t, 3H),
2.65 (q, 2H), 2.6-3.3 (m, 4H), 3.3 (s, 3H),
3.4 (s, 3H), 4.8 (s, 1H)
(3) Preparation of 1,3-dimethyl-6-{N-ethyl-N-~2-[4-(4-
nitrophenyl)butylamino]ethyl>amino3-2,4(1H,3H)-pyrimidine-
dione oxalate (compound 26):
In 25 ml of ethanol were dissolved 1.58 g of 1,3-
dimethyl-6-[N-ethyl-N-(2-aminoethyl)amino]-2,4(1H,3H)-
pyrimidinedione (compound 28) and 1.35 g of 4-(4-nitro-
phenyl)butanal, and 0.5 g of molecular shieves (3A) (made byJunsei Kagaku Co., Ltd.) was then added to the solution,
followed by stirring at room temperature for 3 hours.
To the resulting reaction mixture was added 0.8 g of
sodium borohydride, and the mixture was then stirred for 2
hours. A small amount of water was added to the mixture,
and the solvent was then distilled off from the mixture
under reduced pressure. Afterward, the resulting residue
was dissolved in chloroform. The chloroform solution was
washed with water, and a separated organic layer was then
dried over anhydous sodium sulfate. The solution was
treated under reduced pressure so as to distill off the
solvent. The resulting residue was purified through a
silica gel column chromatograph (chloroform/methanol = 30/1
in terms of volume), then recrystallized from ethyl acetate,
collected by filtration, washed, and dried in order to
- 236~389
- 93 -
obtain 0.78 g of 1,3-dimethyl-6-{N-ethyl-N- (2-[4-(4-nitro-
phenyl)butylamino]ethyl) amino~ -2,4(1H,3H)-pyrimidinedione.
Analytical results of the obtained crystalline
pyrimidinedione derivative:
NMR (CDCl3), ~ppm: 1.05 (t, 3H), 1.4-1.8 (m, 4H),
2.2-2.8 (m, 8H), 2.9-3.1 (m, 2H), 3.3 (s, 6H),
4.8 (s, 1H), 5.6 (m, 1H), 7.35 (d, 2H),
8.15 (d, 2H)
The thus obtained crystals were treated with an oxalic
10 acid/methanol solution in a usual manner in order to prepare
1,3-dimethyl-6-~N-ethyl-N- (2-[4-(4-nitrophenyl)butylamino]-
ethyl) amino}-2,4(1 H,3H)-pyrimidinedione oxalate
(compound 26).
Analytical results of the crystalline compound 26
thus obtained:
Melting point: 164-166~C
Values of elemental analysis [as C20H2gNsO4~(COOH)2]
Calcd. (%): C 53.54; H 6.33; N 14.19
Found (%): C 53.21; H 6.32; N 13.98
Example 14
Preparation of 1,3-dimethyl-6-[3-(4-nitroanilino)-
propylamino]-2,4(1 H,3H)-pyrimidinedione oxalate
(compound 30):
-
Z~1389
- 94 _
p NH 2 -(CH 2 ) 3 -NH 2
Cl ~ ~N-CH3 >
~ N o
CH3
~ NO2 ~ F
NH2-(CH2~3-NH ~ ~N-CH3
(compound 31) CH3
1 0
(COOH)2/CH30H-
NO2 ~ NH-(CH2~3-NH ~ N-CH 3
(compound 30) C ~ ~ ~(COOH)2
(1) Preparation of 1,3-dimethyl-6-(3-aminopropyl-
amino)-2, 4(1 H,3H)-pyrimidinedione (compound 31):
The same procedure as in Example 10-(1) was repeated
with the exception that N,N'-dimethylethylenediamine was
replaced with 50 g of 1,3-diaminopropane, in order to obtain
9.5 g of 1,3-dimethyl-6-(3-aminopropylamino)-2,4(1H,3H)-
pyrimidinedione (compound 31).
Analytical results of the compound 31 thus obtained:
2~389
NMR (CDCl3), ,~ppm: 1.75-2.10 (m, 2H),
2.75-3.48 (m, 4H), 3.12 (s, 3H), 3.35 (s, 3H),
4.75 (s, 1H)
(2) Preparation of 1,3-dimethyl-6-[3-(4-nitroanilino)-
5 propylamino]-2,4(1 H,3H)-pyrimidinedione oxalate
(compound 30):
The same procedure as in Example 1 was repeated with
the exception that the compound 2 was replaced with 0.5 g of
the above compound 31, in order to obtain 1,3-dimethyl-6-[3-
(4-nitroanilino)propylamino]-2,4(1H,3H)-pyrimidinedione.
The latter compound was further treated with an oxalic
acid/methanol solution in a usual manner, thereby preparing
0.45 g of crystalline 1,3-dimethyl-6-[3-(4-nitroanilino)-
propylamino]-2,4(1 H,3H)-pyrimidinedione oxalate
15 (compound 30).
Analytical results of the crystalline compound 30
thus obtained:
Melting point: 213-21 4~C (decomposed)
IR 1~ KBrmax (cm-1): 2600, 1700, 1640, 1600
1560, 1320, 840
Values of elemental analysis [as C1sH1gNsO4-2(COOH)2]
Calcd. (%): C 50.79; H 5.33; N 18.51
Found (%): C 50.95; H 5.38; N 18.82
Example 15
Preparation of 1,3-dimethyl-6-[3-(4-nitrobenzylamino)-
2(~)1389
_ 96 -
propylamino]-2,4(1H,3H)-pyrimidinedione oxalate
(compound 32):
NH2-(CH2) 3 -NH ~ N-CH 3
(compound 31) /N 0
NO2 ~ -CH2Br (COOH) 2 /CH 3 OH
> >
NO2 ~ CH2NH-(CH2)3-NH ~ N-CH3
(compound 32) CH (COOH)2
To 20 ml of isopropanol were added 1.45 g of 1,3-
dimethyl-6-(3-aminopropylamino)-2,4(1H,3H)-pyrimidinedione
(compound 31) obtained in Example 14-(1), 1.44 g of
p-nitrobenzyl bromide and 1.5 ml of triethylamine, and the
resulting mixture was then treated in the same manner as in
Example 2 in order to obtain crystals of 1,3-dimethyl-6-[3-
(4-nitrobenzylamino)propylamino]-2,4(1H,3H)-pyrimidinedione
oxalate (compound 32).
Analytical results of the crystalline compound 32
thus obtained:
1389
- 97 -
Melting point: 175-178~C
IR y KBrmax (cm-1): 2600, 1690, 1620, 1610
1550, 1350, 850
Values of elemental analysis
[as C16H21N5o4-(cooH)2~3H2o]
Calcd. (%): C 43.99; H 5.95; N 14.25
Found (%): C 43.52; H 6.05; N 14.33
Example 16
The same procedure as in Example 2 was repeated with
the exception that 4-nitrobenzyl bromide was replaced with
0.51 g of 3-(4-nitrophenyl)propyl bromide, in order to
obtain 1,3-dimethyl-6-{4-[3-(4-nitrophenyl)propyl]piperazin-
1-yl}-2,4(1H,3H)-pyrimidinedione oxalate (compound 33).
Furthermore, the same procedure as in Example 2 was
repeated with the exception that 4-nitrobenzyl bromide was
replaced with 0.55 g of 4-(4-nitrophenyl)butyl bromide and
that the oxalic acid/methanol solution was replaced with
hydrochloric acid/methanol, in order to obtain 1,3-dimethyl-
6-{4-[4-(4-nitrophenyl)butyl]piperazin-1-yl}-2,4(1H,3H)-
pyrimidinedione hydrochloride (compound 34).
Physical properties of these compounds were as follows:
(1) 1,3-dimethyl-6-~4-[3-(4-nitrophenyl)propyl]pipera-
zin-1-yl}-2,4(1H,3H)-pyrimidinedione oxalate (compound 33)
20~1389
- 98 -
NO2- ~ -CH2CH2CH2Br + HN N ~ N-CH3
CH3
(compound 2)
(CO2H)2/CH3OH A n ~
> NO2- ~ Nl~o
CH3
(compound 33)
Melting point: 153-156~C (decomposed)
IR V KBrmax (cm~1): 2550, 1680, 1630, 1600
1590, 1510, 1340, 850
Values of elemental analysis
[as C1 gH25N5O4-(cOOH)2-1~5H2O]
Calcd. (%): C 50.00; H 5.99; N 13.88
Found (%): C 50.24; H 5.64; N 13.23
(2) 1,3 -dimethyl -6-{4-[4-(4 -nitrophenyl)butyl]pipera-
zine-1-yl} -2,4(1H,3H)-pyrimidinedione hydrochloride
(compound 3 4)
NO2- ~ CH2cH2cH2cH2Br + HN N- ~ -CH3 >
CH3
(compound 2)
2~1389
99
HCl/CH30H n ~4
> N02-~-CH2CH2CH2CH2NL~Ny N-CH3 HCl
CH3
(compound 34)
Melting point: 202-205.5~C
IR V KBrmax (cm-1): 2920, 2450, 1700, 1650
1615, 1440, 1345, 791, 762, 740'
Values of elemental analysis tas C20H27NsO4 HCl]
Calcd. (%): C 54.85; H 6.44; N 15.99; Cl 8.10
Found (~): C 54.20; H 6.67; N 15.56; Cl 8.95
Example 17
Preparation of tablets containing, as an effective
component, 1,3-dimethyl-6-[4-(4-nitrobenzyl)piperazin-1-yl]-
2,4(1H,3H)-pyrimidinedione oxalate (compound 3) which can be
obtained by the process of Example 2:
With 20 g of corn starch were mixed 1 g of the above
pyrimidinedione derivative oxalate (compound 3) and 123 g of
lactose, and the mixture was further mixed with a solution
prepared by dissolving 5 g of hydroxypropyl cellulose in
100 ml of water, so as to form grains, followed by drying
the grains at 50~C for 4 hours. Afterward, 1 g of magnesium
stearate was added to the dried grains and was then mixed
sufficiently. The mixture was then formed into tablets by
the use of a tableting machine, the weight of each tablet
-
31389
- 100 -
being 150 mg.
Example 18
Preparation of capsules containing, as an effective
component, 1,3-dimethyl-6-~2-<N-ethyl-N-[3-(4-nitrophenyl)-
propyl]amino>ethylamino}-2,4(1H,3H)-pyrimidinedione
hydrochloride (compound 4) which can be obtained by the
process of Example 3:
With 25 g of corn starch were sufficiently mixed 5 g of
the above pyrimidinedione derivative hydrochloride (com-
pound 4) and 120 g of lactose, and hard capsules were filled
with the resulting mixture by the use of a capsule filling
machine to prepare capsules, the content of the mixture in
each capsule being 150 mg.
Example 19
1) Preparation of an injection containing, as an
effective component, 1,3-dimethyl-6-{2-~N-(2-hydroxyethyl-N-
[3-(4-nitrophenyl)propyl]amino>ethylamino}-2,4(1H,3H)-
pyrimidinedione fumarate (compound 8) which can be obtained
by the process of Example 4:
In distilled water for injection were dissolved 20 mg
of the above pyrimidinedione derivative fumarate (compound
8) and 0.85 g of sodium chloride, and the total volume of
the liquid was regulated to be 100 ml, thereby preparing
an injection.
2) Preparation of capsules containing, as an effective
Z(~13~9
- 1 0 1
component, 1,3-dimethyl-6-{2-(N-(2-hydroxyethyl-N-[3-(4-
nitrophenyl)propyl]amino~ethylamino}-2,4(1H,3H)-pyrimidine-
dione hydrochloride (compound 8') which can be otained by
process of Example 4:
With 24 g of corn starch were sufficiently mixed 5 g of
the above pyrimidinedione derivative hydrochloride (compound
8') and 120 g of lactose and the resulting mixture and 1 g
of magnesium stearate were mixed to prepare the final
mixture.
Hard capsules were filled with the resulting final
mixture by the use of a capsule filling machine to prepare
capsules, the content of the mixture in each capsule being
150 mg.
Pharmacological Test 1
(1) Influence on myocardial action potential duration
time (APD7s)
To a hybrid adult dog, 30 mg/kg of pentobarbital was
administered through a vein, and after being anesthetized,
the heart was removed. Afterward, the right ventricular
free- wall of the heart was cut out in a Tyrode solution.
The right ventricular free wall was fixed in an
incubator at 37~C, and a nutritional solution (20 ml of the
Tyrode solution) was refluxed.
In this isolated condition, myocardial action potential
duration times (APD7s) were measured before and after the
26~ 389
- 102 -
administration of the respective compounds prepared in the
above examples in Table 2 and d-sotalol as a control
medicine, and APD75(%) was calculated from the measured
results in accordance with the formula:
APD7s(%) = (B - A)/A x 100
A: APD7s before administration
B: APD7s after administration
Here, APD7s was measured as follows: A field stimula-
- tion of 1 Hz was given to the right ventricular free wall,
and any variation ~fan action potential was depicted on an
oscilloscope via a glass microelectrode (10 to 20 MQ) thrust
into a Purkinje fiber of the free wall and via an amplifier.
Afterward, a waveform on the oscilloscope was analyzed by
the use of a computer, and the ti~c of from a point of the
action potential generation to a point of 75% repolarization
was measured. This measured time was regarded as the
myocardial action potential duration time (APD7s).
Each of the compounds and d-sotalol shown in Table 2
was separately added to the refluxing nutritional solution
(20 ml), and after 20 minutes' incubation, APD7s after the
administration was calculated from the variation of the
myocardial action potential duration time.
Incidentally, this test was carried out in accordance
with a Sato et al's method [H. Sato, K. Hashimoto, Arzneimit-
tel Forschung, 34 (1), 3a, 376-380 (1984)].
2~389
- 103 _
The results are set forth in Table 2.
(2) Influence on ventricular muscle refractory period
Refractory periods were measured in the following
manner before and after each of the compounds and d-sotalol
shown in Table 2 was separately administered to a vein or a
duodenum, and ERP (%; extensibility of refractory period)
was calculated from the measured values:
ERP (~) = (W - Y)/Y x 100
W: Refractory period after administration
Y: Refractory period before administration
To a mongrel adult dog, 30 mgtkg of pentobarbital was
administered intravenously, and afterbeinganesthetized, apair
of silver-silver chloride electrodes separated by 3 mm was
sewn on an opened right ventricule, and electrical stimula-
tion was given at an interval of 400 msec at a duration timeof 4 msec under a current twice as much as the threshold.
Afterward, a small amount of alcohol was injected into a
sinus artery in order toextinguishapacemaker activity, and
the ventricular refractory period (ERP) was measured under
20 ventricule pacing.
That is,each ltraincomprisedlOstimulationshaving
intervals of 400 msec, and an interval between the trains
was usually 400 msec. However, this interval was shortened
10 msec by 10 msec at the time of the refractory period
measurement, and an interval between the trains at the time
2~1389
- 104 -
when reaction to the first stimulation of the traindisappeared
was regarded as the refractory period.
In this case, the electrical stimulation was fed in
accordance with a program by a heart stimulation device
(Diamedical Co., Ltd.; DHM-226-3).
The results are set forth in Table 2.
Table 2 (results of pharmacological test)
Com- APD75 (%) ER;' (%)
pound Dose (~g~ml) Dose mg/kg,i.v.)
No. 0.3 1.0 3.0 10.0 0.10.: 1.0 3.0
1 - - - - 3.63.6 10.3
4 - 11 16 - 5.611.1 16.7 16.7
8 16 22 38 - 010.7 21.4 14.2
- - 2 11 6.76.7 13.3 20
14 18 43 - - 1433.5
- - 6 16 6.312.5 12.5 18.8
16 - - 22 - 6.713.3 13.3
21 - 13 27 37 7 10 14 14
23 - 18 35 39
26 - 20 30 35 6.56.5 6.5 6.5
33 - 17 22 31 14.314.3 17.9 21.4
34 - - 17 25 6 12 12 18
d-sota- 0 3 7.4 15.8 1.76.7 8.7 15.5
lol
- 2~ 389
- 105 -
Toxicity Test 1
Each of the compounds prepared in the above-mentioned
examples was administered into a mouse (ddY strain, male).
In each case, oral administration (p.o.) and intra perito-
neal administration (i.p.) were separately carried out in adose of 300 mg/kg and 250 mg/kg, respectively.
A mortality rate (number of specimens: one group = 2 to
4 mice) of the mice 24 hours after the administration was
calculated, and the results are set forth in Table 3.
Table 3 (results of toxicity test)
CompoundMortality Rate (%)
Number300 mg/kg (p.o.)250 mg/kg (i.p.)
4 0 0
8 0 0
0
22 0 50
23 0
26 0 50
34 100
1389
- 106 -
Example 20
Preparation of 1,3-dimethyl-6-~4-[3-(4-nitrophenoxy)-
propyl]piperazin-1-yl}-2,4(1H,3H)-pyrimidinedione hydro-
chloride (compound 35):
0
HocH2cH2cH2Br + HN N ~ N-CH3
N
CH2
(compound 37)
0
HOCH2CH2CH2N~__,N ~ N-CH3
,N
CH2
(compound 36)
02N ~ OH j HCl/CH30H
2002N ~ OCH2CH2CH2N N ~ N-CH3
(compound 35) CH3
(1) Preparation of 1,3-dimethyl-6-[4-(3-hydroxypropyl)-
piperazin-1-yl]-2, 4(1H,3H)-pyrimidinedione (compound 36):
25To 250 ml of ethanol were added 14.1 g of 1,3-dimethyl-
28~389
-
- 107 _
6-(1-piperazinyl)-2,4(1H,3H)-pyrimidinedione (compound 37),
11.7 g of 3-bromo-1-propanol and 13 g of triethylamine, and
the mixture was then heated under reflux for 20 hours to
perform reaction. After completion of the reaction, the
reaction mixture was concentrated to dryness, and the
residue was then dissolved in 300 ml of chloroform. The
resulting solution was washed with 100 ml of water twice,
and the washed orgnaic layer was then dried over anhydrous
magnesium sulfate. This organic layer was treated under
reduced pressure to distill off the solvent, thereby
obtaining 20.5 g of a composition. Afterward, ether was
added to this composition, followed by crystallizing,
recovering, washing and drying in order to obtain 12.4 g of
1,3-dimethyl-6-[4-(3-hydroxypropyl)piperazin-1-yl]-2,4-
(lH,3H)-pyrimidinedione (compound 36) (yield 69.8%).
Analytical results of the crystalline compound 36
thus obtained:
Melting point: 119-121~C
NMR (CDCl3), ~ppm: 1.8 (dt, 2H), 2.7 (m, 6H),
3.02 (m, 4H), 3.36 (s, 3H), 3.43 (s, 3H),
3.82 (t, 2H), 4.34 (br, 1H), 5.26 (s, 1H)
IR v KBrmax (cm~1): 3380, 3180, 2830, 1695,
1650, 1605, 1440, 1213, 1068, 1000, 921, 760
(2) Preparation of 1,3-dimethyl-6-~4-[3-(4-nitro-
phenoxy)propyl]piperazin-1-yl~-2,4(1H,3H)-pyrimidinedione
389
_ 108 --
hydrochloride (compound 35):
In 15 ml of anhydrous tetrahydrofuran were suspended
1.0 g of the above compound 36, 1.1 g of triphenylphosphine
and 0.57 g of 4-nitrophenol, and 15 ml of an anhydrous
tetrahydrofuran solution containing 0.71 g of diethyl
azodicarboxylate was further added to the resulting
suspension at room temperature.
Next, the resulting reaction mixture was stirred for 10
minutes and then concentrated to dryness, and the residue
was purified through a silica gel column chromatograph
(methanol/ethyl acetate = 1/15 to 1/7 in the terms of volume
ratio) to obtain 1.3 g of 1,3-dimethyl-6-{4-[3-(4-nitro-
phenoxy)propyl]piperazin-1-yl~-2,4(1H,3H)-pyrimidinedione
(yield 80%).
Analytical results of the crystalline pyrimidinedione
derivative thus obtained:
Melting point: 167-170~C
Values of elemental analysis (as C1gH2sN3Os)
Calcd. (%): C 56.57; H 6.25; N 17.36
Found (96): C 56.29; H 6.17; N 17.17
Furthermore, the thus obtained 1,3-dimethyl-6-{4-[3-(4-
nitrophenoxy)propyl]piperazin-1-yl} -2,4(1H,3H)-pyrimidine-
dione was treated with a hydrochloric acid/methanol solution
in a usual manner to obtain 1,3-dimethyl-6-~4-[3-(4-nitro-
phenoxy)propyl]piperazin-1-yl}-2,4(1H,3H)-pyrimidinedione
2~ 89
- 109 -
hydrochloride (compound 35).
Analytical results of the crystalline compound 35
thus obtained:
Melting point: 244-246~C (decomposed)
Example 21
Preparation of 1,3-dimethyl-6-{4-[3-(3-nitrophenoxy)-
propyl]piperazin-1-yl}-2,4(1H,3H)-pyrimidinedione hydro-
chloride (compound 38):
~OH + BrcH2cH2cH2Br
02N
' > ~OCH2CH2CH2Br
0 2 N - (compound 39)
1 5
HNN~N-CH3 HCl/CH30H
~Nff
CH3
(compound 37)
- 2~0~389
- 110 -
~OCH2CH2CH2N N~;~N-CH~
(compound 38) CH 3
(1) Preparation of 3-(3-nitrophenoxy)propyl bromide
(compound 39):
To 100 ml of methyl ethyl ketone were added 13.9 g of
3-nitrophenol, 101 g of 1,3-dibromopropane and 15.2 g of
anhydrous potassium carbonate, and the mixture was then
heated under reflux for 2 hours to perform reaction. After
completion of the reaction, insoluble matters were removed
from the reaction mixture by filtration, and the filtrate
was then concentrated. Next, the resulting concentrate was
dissolved in 300 ml of chloroform, and this chloroform
solution was washed with water. Afterward, the washed
organic layer was then dried over anhydrous magnesium
sulfate. This organic layer was then treated under reduced
pressure to distill off the solvent, thereby obtaining
24.6 g of 3-(3-nitrophenoxy)propyl bromide (compound 39) as
an oily product. This product could be used in the
subsequent reaction without purifying particularly.
(2) Preparation of 1,3-dimethyl-6-~4-[3-(3-nitro-
phenoxy)propyl]piperazin-1-yl}-2,4(1H,3H)-pyrimidinedione
hydrochloride (compound 38):
- 2~01389
- 111 -
In 20 ml of dioxane were dissolved 1.69 g of the above
oily compound 39, 1.12 g of 1,3-dimethyl-6-(1-piperazinyl)-
2,4(1H,3H)-pyrimidinedione (compound 37) and 1 ml of
triethylamine, and the solution was then heated under reflux
for 4 hours to perform reaction. After completion of the
reaction, insoluble matters were removed from the reaction
mixture by filtration, the resulting filtrate was then
concentrated. The residue (concentrate) was dissolved in
chloroform, and the resulting chloroform solution was then
washed with water. Afterward, the water-washed organic
layer was dried over anhydrous magnesium sulfate and then
treated under reduced pressure to distill off the solvent.
Furthermore, the residue was purified through a silica gel
column chromatograph (chloroform/methanol = 100/1 to 25/1 in
terms of volume ratio), and the purified material was then
recrystallized from methanol. Afterward, the crystals were
collected by filtration, washed and dried in order to obtain
1.35 g of 1,3-dimethyl-6-~4-[3-(3-nitrophenoxy)propyl]-
piperazin -1-yl}-2,4(1H,3H)-pyrimidinedione.
Analytical results of the crystalline pyrimidinedione
derivative thus obtained:
Melting point: 164-165~C
NMR(CDC13), ~ppm: 1.8-2.2 (m, 2H), 2.4-2.8 (m, 6H),
2.8-3.8 (m, 4H), 3.34 (s, 3H), 3.42 (s, 3H),
4.15 (t, 2H), 5.25 (s, 1H), 7.1-8.0 (m, 4H)
-- ZC~389
- 112 -
Values of elemental analysis (as C1gH2sNsOs)
Calcd. (~): C 56.57; H 6.25; N 17.36
Found (%): C 56.27; H 6.69; N 17.21
Next, the thus obtained 1,3-dimethyl-6- 4-[3-(3-nitro-
phenoxy)propyl]piperazin-1-yl -2,4(1H,3H)-pyrimidinedione
was treated with a hydrochloric acid/methanol solution to
obtain 1,3-dimethyl-6-~4-[3-(3-nitrophenoxy)propyl]pipera-
zin-1-yl}-2,4(1H,3H)-pyrimidinedione hydrochloride
(compound 38).
10Analytical results of the crystalline compound 38
thus obtained:
IR V KBrmax (cm~1): 1690, 1650, 1525, 1345,
1240, 1200, 1025, 980, 790, 760, 740, 670
Values of elemental analysis (as C1gH2sNsOs HCl MeOH)
15Calcd. (%): C 51.00; H 6.21; N 14.87; Cl 7.53
Found (%): C 50.60; H 6.71; N 14.81; Cl 7.74
Example 22
Preparation of 1,3-dimethyl-6-~4-[2-(4-nitrobenzoyl-
oxy)ethyl]piperazin-1-yl}-2,4(1H,3H)-pyrimidinedione oxalate
(compound 40):
HOCH2CH2N~ N~N-CH3
O
(compound 41) CH~
2(~)1389
- 113 _
02N ~ COCl (COOH)2/CH~OH
0
02N ~ COOCH2CH2N N ~ N-CH3
N ~ . ( COOH)2
(compound 40) / u
CH3
To 5 ml of tetrahydrofuran were added 0. 5 g of
4-nitrobenzoyl chloride, 0.47 g of 1,3-dimethyl-6-[4-(2-
hydroxyethyl)piperazin-1-yl]-2,4(1H,3H)-pyrimidinedione
(compound 41) and 1.5 ml of triethylamine, and the resulting
mixture was stirred at room temperature overnight.
Afterward, the used solvent was distilled off under reduced
pressure, and the residue was then dissolved in chloroform.
The resulting chloroform solution was washed with water,
then dried over anhydrous sodium sulfate, and concentrated
to dryness, thereby obtaining a crude product. The latter
was then purified through a silica gel column chromatograph
(chloroform/methanol = 4û/1 in terms of volume ratio) in
order to obtain 1,3-dimethyl-6-{4-[2-(4-nitrobenzoyloxy)-
ethyl]piperazin-1 -yl3 -2,4(1H,3H)-pyrimidinedione. Next,
this pyrimidinedione derivative was treated with an oxalic
acid/methanol solution in a usual manner to prepare 0.84 g
2 5 of 1,3-dimethyl-6-~4-[2-(4-nitrobenzoyloxy)ethyl]piperazine-
_ 114 -
1-yl}-2,4(1H,3H)-pyrimidinedione oxalate (compound 40).
Analytical results of the crystalline compound 40
thus obtained:
Melting point: 171-173~C (decomposed)
S IR ~ KBrmax (cm 1): 3100, 2550, 1740, 1700,
1660, 1640, 1540, 1360, 850
Values of elemental analysis
~as C1gH25NsO6-(cOoH)2-2H2o]
Calcd. (%): C 46.24; H 5.73; N 12.84
Found (%): C 46.64; H 5.38; N 12.83
Example 23
Preparation of 1,3-dimethyl-6-[4-(4-nitrophenacyl)-
piperazin-1-yl]-2,4(1H,3H)-pyrimidinedione hydrochloride
(compound 42):
HN N~N~CHa
N~
CHa
~_~ Br (compound 37)
02N~COCH~ 2 ~ -
r~
2~1389
- 115 _
02N ~ COCH2N N ~ ~ N-CH3
(compound 43) C H3
HCI/CH30H A ~
- 02N ~ COCH2N N ~ N-CH3
N ~ HCl
(compound 42) C H 3
In 200 ml of chloroform was dissolved 8.25 g of
4-nitroacetophenone, and 20 ml of a chloroform solution
containing 8 g of bromine was added dropwise thereto under
cooling. The resulting reaction mixture was concentrated,
and the residue (concentrate) was recrystallized from
chloroform/ether, collected by filtration, washed, and dried
to obtain 8.03 g of p-nitrophenacyl bromide.
Next, to 150 ml of dioxane were added 4.88 g of the
above prepared p-nitrophenacyl bromide, 4.48 g of 1,3-
dimethyl-6-(1-piperazinyl)-2,4(1H,3H)-pyrimidinedione
(compound 37) and 4.2 ml of triethylamine, and the solution
was heated under reflux for 1 hour. Afterward, the reaction
mixture was cooled to deposit crystals, and the latter were
26:~C)1389
- 116 -
collected by filtration, dissolved in chloroform, washed
with water, and dried (over anhydrous sodium sulfate).
Afterward, the used solvent was distilled off, and methanol
was then added to the residue so as to crystallize it. The
resulting crystals were then collected by filtration,
washed, and dried to obtain 4.02 g of 1,3-dimethyl-6-[4-(4-
nitrophenacyl)piperazin-1-yl]-2,4(1H,3H)-pyrimidinedione
(compound 43).
Analytical results of the crystalline compound 43
thus obtained:
Melting point: 189-192~C
Values of elemental analysis (as C18H21N5~5 2CH30H)
Calcd. (%): C 55.08; H 5.75; N 17.36
Found (%): C 54.86; H 5.44; N 17.62
NMR (CF3COOH), ~ppm: 3.63 (s, 3H), 3.73 (s, 3H),
3.7-4.3 (m, 8H), 5.21 (s, 2H), 6.12 (s, 1H),
8.28 (s, 2H), 8.52 (d, 2H)
Furthermore, 0.9 g of the compound 43 was treated with
a hydrochloric acid/methanol solution in a usual manner to
obtain 0.91 g of 1,3-dimethyl-6-[4-(4-nitrophenacyl)pipera-
zin-1-yl]-2,4(1H,3H)-pyrimidinedione hydrochloride
(compound 42).
Analytical results of the crystalline compound 42
thus obtained:
Melting point: 257-262~C
-
_ 117 -
IR V KBrmax (cm~1): 1685, 1630, 1520, 1340,
1190, 960, 845, 790, 740
Values of elemental analysis
(as C1gH21NsOs-HCl-~CH30H)
Calcd. (%): C 50.52; H 5.50; N 15.92; Cl 8.06
Found (%): C 50.77; H 5.29; N 15.82; Cl 7.61
Example 24
Preparation of 1,3-dimethyl-6-{4-l2-hydroxy-2-(4-
nitrophenyl)ethyl]piperazin-1-yl~-2,4-(1H,3H)-pyrimidine-
dione hydrochloride (compound 44):
O 2 N ~ C O C H 2 N~_JN ~ N-CH 3 >
(compound 43) C/H
LiAlH4 HCl/CH30H
> >
OH O
02N~CHCH2-N, N~N-CH~ ~HCl
(compound 44) CH 3
Under ice cooling, 3.46 g of lithium aluminum hydride
; .. ,
2~ 89
-
- 118 -
was added to 700 ml of a dried tetrahydrofuran suspension
containing 8.12 g of 1,3-dimethyl-6-[4-(4-nitrophenacyl)-
piperazin-1-yl~-2,4-(1H,3H)-pyrimidinedione (compound 43),
and the mixture was then stirred at the same temperature for
30 minutes, followed by stirring at room temperature for
2 hours.
After completion of the stirring operation, 50 ml of
water was added to the reaction mixture under cooling in
order to bring the reaction to an end, and insoluble matters
were removed therefrom by filtration and the filtrate was
then concentrated.
The residue (concentrate) was dissolved in chloroform,
washed with water and dried (over anhydrous sodium sulfate),
and the resulting organic layer was concentrated to a small
amount. Afterward, the concentrate was purified through a
silica gel column chromatograph (chloroform/methanol = 100:1
to 100:2 in terms of volume ratio), thereby preparing 3.7 g
of 1,3-dimethyl-6-{4-[2-hydroxy-2-(4-nitrophenyl)ethyl]-
piperazin-1-yl}-2,4(1H,3H)-pyrimidinedione.
Analytical results of the crystalline pyrimidinedione
derivative thus obtained:
Melting point: 205-207~C
NMR (DMSO-d6), ~ppm: 2.5-2.7 (m, 6H),
2.7-2.95 (m, 4H), 3.06 (s, 3H), 3.20 (s, 3H),
4.79 (t, lH), 7.47 (d, 2H), 8.04 (d, 2H)
~31389
- 119 -
Next, 0.55 g of this pyrimidinedione derivative was
treated with a hydrochloric acid/methanol solution in a
usual manner in order to obtain 0.53 g of 1,3-dimethyl-6-~4-
[2-hydroxy-2-(4-nitrophenyl)ethyl]piperazin-1 -yl3 -2,4-
(1H,3H)-pyrimidinedione hydrochloride (compound 44).
Analytical results of the crystalline compound 44
thus obtained:
Melting point: 250-265~C (gradually colored
and decomposed)
Values of elemental analysis
(as C18H22NsOs-Hcl-4H2O):
Calcd. (%): C 50.23; H 5.74; N 16.27; Cl 8.24
Found (%): C 50.22; H 6.07; N 16.04; Cl 8.20
IR V KBrmax (cm~1): 1700, 1620, 1430, 1340,
1190, 1165, 850, 785
Example 25
Preparation of 1,3-dimethyl-6-{4-[2-(4-nitrobenzoyl-
amino)ethyl]piperazin-1-yl}-2,4(1H,3H)-pyrimidinedione
hydrochloride (compound 45):
NH2CH2CH2Br HBr
NO2 ~COC 1
X~1389
- 120 -
H-N N ~ N-CH3
N
CH3
(compound 37)
~ 02N ~ CONHCHzCH2Br
(compound 46)
HCl/CH30H
>
o2N~CoNHCH2CH2-N~J~4~,N-CH3
(compund 45) / 0
CH~ 2HCl
(1) Preparation of 2-(4-nitrobenzoylamino)ethyl
bromide (compound 46):
In 30 ml of chloroform were added 3 g of 4-nitrobenzoyl
chloride, 3.3 g of 2-aminoethyl bromide hydrobromide and
3.9 ml of pyridine under ice cooling, and they were stirred
at the same temperature for 1 hour. The reaction mixture
was then washed with water, and the resulting organic layer
was concentrated to obtain a crude product of 2-(4-nitro-
benzoylamino)ethyl bromide. Afterward, the latter was
2~389
- 121 -
recrystallized from hexane/ethanol, the resulting crystals
were collected by filtration, washed and dried to prepare
2.9 g of 2-(4-nitrobenzoylamino)ethyl bromide (compound 46).
Analytical results of the crystalline compound 46
thus obtained:
Melting point: 104-108~C
(2) Preparation of 1,3-dimethyl-6-{4-[2-(4-nitroben-
zoylamino)ethyl]piperazin-1-yl}-2,4(1H,3H)-pyrimidinedione
hydrochloride (compound 45):
A mixture of 1.3 g of the compound 46 obtained in the
paragraph (1), 1.8 g of 1,3-dimethyl-6-(1-piperazinyl)-
2,4(1H,3H)pyrimidinedione (compound 37), 1.3 ml of triethyl-
amine and 10 ml of isopropanol was heated under reflux for
3 hours.
Next, the used solvent was distilled off from the
resulting reaction mixture, and water was added to the
reaction mixture, followed by extracting with chloroform.
Furthermore, a chloroform extract was washed with
water, dried over anhydrous sodium sulfate, and concentrated
in order to obtain a crude product. Afterward, the latter
was recrystallized from hexane/ethanol, and the resulting
crystals were collected by filtration, washed and dried in
order to obtain 1.77 g of 1,3-dimethyl-6-{4-[2-(4-nitro-
benzoylamino)ethyl]piperazin-1-yl}-2,4(1H,3H)-pyrimidine-
dione.
389
- 122 -
Analytical results of the crystalline pyrimidinedione
derivative thus obtained:
Melting point: 169-171~C
IR V KBrmax (cm~1): 3000, 2900, 1700, 1640,
1530, 1340, 850, 700
Next, the thus obtained pyrimidinedione derivative
crystals were treated with a hydrochloric acid/methanol
solution in a usual manner to obtain 1,3-dimethyl-6-~4-[2-
(4-nitrobenzoylamino)ethyl]piperazin-1-yl}-2,4(1H,3H)-
pyrimidinedione hydrochloride (compound 45).
Analytical results of the crystalline compound 45
thus obtained:
Melting point: 283-285~C (decomposed)
Values of elemental analysis (as C1gH24N6O5 2HCl):
Calcd. (~): C 46.62; H 5.66; N 17.20; Cl 14.49
Found (~): C 46.45; H 5.79; N 17.01; Cl 14.67
Example 26
Preparation of 1,3-dimethyl-6-{4-[N-(4-nitrophenyl)-
carbamoylmethyl]piperazin-1-yl}-2,4(1H,3H)pyrimidinedione
oxalate (compound 47):
BrCH2COBr
02N~NH2
Z~389
HN N ~ N-CH3
N
CH3
(compound 37)
02N { ~ NHCOCH2Br
(compound 48)
(COOH) 2/CH 3 OH
~
0 2N~NHCOCH 2N~JN~ ~ (COOH)- 2
(compound 47) C H 3
A mixture of 0.8 g of 4-nitroaniline, 0.6 ml of
bromoacetyl bromide, 1.5 g of anhydrous potassium carbonate
and 10 ml of dimethyl sulfoxide was stirred at 100~C for 4
hours, and insoluble matters were removed from the mixture
by filtration while the latter was hot. The filtrate was
cooled to deposit 4-(bromoacetamide)nitrobenzene (compound
48) crystals, and the latter were collected by filtration
and then heated under reflux for 16 hours together with
1.3 g of 1,3-dimethyl-6~ piperazinyl)-2,4(1H,3H)-pyrimi-
dinedione (compound 37), 1.6 g of triethylamine and 15 ml
2~)1389
- 124 -
of isopropanol.
After cooling, the used solvent was distilled off, and
the residue was then dissolved in chloroform. Afterward,
the resulting solution was washed with water and dried over
5 anhydrous sodium sulfate.
Afterward, the solvent was distilled off from the dried
chloroform solution under reduced pressure, and the residue
was purified through a silica gel chromatograph (chloro-
form/methanol = 100/1 to 25/1 in terms of volume ratio) to
obtain 0.85 g of 1,3-dimethyl-6-~4-[N-(4-nitrophenyl)car-
bamoylmethyl]piperazin-1-yl} -2,4(1H,3H)pyrimidinedione.
Analytical results of the crystalline pyrimidine
derivative thus obtained:
NMR (DMSO-d6), ,~ppm: 2.5-2.9 (m, 10H), 3.28 (s, 3H),
3.37 (s, 3H), 5.00 (s, 1H), 7.29 (d, 2H), 8.01 (d, 2H)
Next, this pyrimidine derivative was treated with an
oxalic acid/methanol solution in a usual manner, thereby
preparing 0.81 g of 1,3-dimethyl-6-~4-[N-(4-nitrophenyl)-
carbamoylmethyl]piperazin-1-yl~-2,4(1H,3H)pyrimidinedione
20 oxalate (compound 47).
Analytical results of the crystalline compound 47
thus obtained:
Melting point: 281-283~C (decomposed)
8 9
- 125 -
Values of elemental analysis
(as C18H22N6Os (cOoH)2-H2o)
Calcd. (%): C 47.06; H 5.13; N 16.46
Found (%): C 46.90; H 5.34; N 16.37
Example 27
Preparation of 1,3-dimethyl-6-~4-[3-(4-nitroanilino)-2-
hydroxypropyl]piperazin-1-yl}-2,4(1H,3H)-pyrimidinedione
oxalate (compound 49):
o
ClCH2CH-CH2N N ~ -CH~
b H ~N~o
(Compound 50) CH~
NH3/CH~OH 02N ~ F (COOH)2/CH30H
1s ~ >
02N ~ NHCH2CH-CH2N N ~ N-CH3
OH jN-~o (COOH)2
(compound 49) CH3
In the first place, 20 ml (0.1 g/ml) of an ammonic
methanol solution containing 2.0 g of 1,3-dimethyl-6-~4-(3-
chloro-2-hydroxypropyl)piperazin-1-yl]-2,4(1H,3H)-pyrimidine-
dione (compound 50) was heated at 90~C for 8 hours. The
B
~ . .
Z~1389
- 126 -
reaction mixture was concentrated under reduced pressure,
and the residue (concentrate) was mixed with 0.9 g of
4-nitrofluorobenzene and 1 ml of triethylamine and then
heated at 90~C for 2 hours. The resulting reaction mixture
was poured into 100 ml of water, and the deposited crystals
were then collected by filtration, washed with methanol/-
ether, and dried in order to obtain 2.15 g of yellow
crystalline 1,3-dimethyl-6-{4-[3-(4-nitroanilino)-2-hydroxy-
propyl]piperazin-1-yl}-2,4(1H,3H)-pyrimidinedione.
Furthermore, the yellow crystals were treated with an
oxalic acid/methanol solution in a usual manner to obtain
1,3-dimethyl-6-{4-[3-(4-nitroanilino)-2-hydroxypropyl]pipera-
zin-1-yl}-2,4(1H,3H)-pyrimidinedione oxalate (compound 49).
Analytical results of the crystalline compound 49
thus obtained:
IR ~ KBrmax tcm~1): 3375 (br), 1693, 1640, 1600,
1470 (br), 1308, 1113
Values of elemental analysis [as C19H26N6~5-(C~2H)2]:
Calcd. (~): C 49.60; H 5.55; N 16.53
Found (%): C 50.10; H 5.98; N 16.79
Example 28
Preparation of 1,3-dimethyl-6-{4-[3-(4-nitrophenylthio)-
propyl]piperazin-1-yl 3 -2,4(1H,3H)-pyrimidinedione oxalate
(compound 51):
!20n~389 '
- 127 -
~ BrCH2CH2CH2Br
02N ~ SH
s o2N~scH2cH2cH2Br ?
(compound 52)
0 HN N ~ N-CH~
N ~ . .
CHa
(compound 37) ~ (COOH~2/CH30H~
0
02N ~ SCH2CH2CH2N N ~ N-CHs
N ~ -1.5(COOH)2
(compound 51) ~ u
CHa
(1) Preparation of 3-(4-nitrophenylthio)propyl bromide
(compound 52):
To 50 ml of a 2-butanone solution containing 5.5 g of
4-nitrothiophenol and 28.3 g of 1,3-dibromopropane was added
9.0 g of anhydrous potassium carbonate, and the solution was
then stirred for 30 minutes at room temperature.
Afterward, insoluble matters were removed from the
~'
~ .
26~1389
- 128 -
resulting reaction mixture by filtration, and the filtrate
was then concentrated. The concentrate was dissolved in
chloroform, and the resulting chloroform solution was then
washed with water. Furthermore, the water-washed orgnaic
layer was dried over anhydrous magnesium sulfate and then
treated under reduced pressure to distill off the solvent,
thereby preparing 8.0 g of crystalline 3-(4-nitrophenylthio)-
propyl bromide (compound 52). This product could be used in
the subsequent reaction without purifying particularly.
(2) Preparation of 1,3-dimethyl-6-{4-[3-(4-nitrophenyl
thio)propyl]piperazin-1-yl}-2,4(1H,3H)-pyrimidinedione
oxalate (compound 51):
To 30 ml of ethanol were added 2.0 g of the above
compound 52, 1.68 g of 1,3-dimethyl-6-(1-piperazinyl)-
2,4(1H,3H)-pyrimidinedione (compound 37) and 3 ml of
triethylamine, and the solution was then heated with
stirring under reflux for 3 hours.
The resulting reaction mixture was concentrated to
dryness and then mixed with 100 ml of water. Afterward, the
deposited material was collected by filtration and then
washed with ethanol.
Furthermore, the thus washed deposit was recrystallized
from methanol, collected by filtration, washed and dried to
obtain 2.4 g of 1,3-dimethyl-6-{4-[3-(4-nitrophenylthio)-
propyl]piperazin-1 -yl3 -2,4(1H,3H)-pyrimidinedione.
2C~1389
- 129 _
Analytical results of the resulting crystalline
pyrimidinedione derivative:
Melting point: 145-147~C
In 20 ml of methanol was suspended 2.2 g of the thus
obtained crystals, and 2.0 g of oxalic acid dihydrate was
added thereto, followed by stirring. After complete
dissolution, stirring was further continued for a while.
Afterward, 30 ml of ether was added thereto so as to deposit
crystals, and the latter was collected by filtration,
washed, and dried to obtain 2.0 g of 1,3-dimethyl-6-{4-[3-
(4-nitrophenylthio)propyl]piperazin-1-yl}-2,4(1H,3H)-
pyrimidinedione oxalate (compound 51).
Analytical results of the crystalline compound 51
thus obtained:
Melting point: 164-168~C (decomposed)
Values of elemental analysis
[as C1gH2sNso4s~1.5(cooH)2-H2o]
Calcd. (%): C 46.15; H 5.28; N 12.23; S 5.60
Found (%): C 45.89; H 5.25; N 12.16; S 6.12
IR V KBrmax (cm-1): 3450, 1700, 1641, 1340,
1222, 978, 851, 745, 722
Example 29
Preparation of 1,3-dimethyl-6-{4-[2-(4-nitrophenoxy)-
ethyl]piperazin-1-yl}-2,4(1H,3H)-pyrimidinedione hydro-
chloride (compound 53):
389
- 130
NO2 ~ 0H + HOCH2CH2N N ~ ~ N-CH,
(compound 41 ) C/H
HCl/CH~OH
NO2 ~ OCH2CH2N~_,N ~ N-CH~
~N ~ HCl
(compound 53) C H
To 100 ml of anhydrous tetrahydrofuran were added
5.37 g of 1,3-dimethyl-6-[4-(2-hydroxyethyl)piperazin-1 -yl]-
2,4(1H,3H)-pyrimidinedione (compound 41 ), 3.20 g of
4-nitrophenol and 6.03 g of triphenylphosphine, and the
resulting mixture was treated in the same manner as in
Example 20-(2) to obtain 5.40 g of crystalline 1 ,3-dimethyl-
6-{4-[2-(4-nitrophenoxy)ethyl]piperazin-1 -yl3 -2,4(1 H,3H)-
20 pyrimidinedione.
Analytical results of the crystalline pyrimidinedione
derivative thus obtained:
IR V KBrmax (cm~1 ): 1705, 1663, 1595, 1505,
1340, 1275, 1213, 1180, 1115, 1010, 862, 805, 750
Furthermore, the thus obtained crystals were treated
2G~1389
- 131
with a hydrochloric acid/methanol solution in a usual manner
to obtain 1,3-dimethyl-6- {4-[2-(4-nitrophenoxy)ethyl]pipera-
zin-1-yl~ -2,4(1 H,3H)-pyrimidinedione hydrochloride
(compound 53).
Analytical results of the crystalline compound 53
thus obtained:
IR lz KBrmax (cm-1 ) 1705, 1655, 1595, 1435,
1342, 1267, 1112, 860, 755
Values of elemental analysis
(as C1gH23NsOs-HCl-0.5H2O):
Calcd. (%): C 49.71; H 5.79; N 16.10; Cl 8.15
Found (%): C 49.17; H 6.05; N 16.20; Cl 8.40
Example 30
Preparation of 1,3-dimethyl-6-{4-[4-(4-nitrophenoxy)-
butyl]piperazin-1-yl} -2,4(1 H,3H)-pyrimidinedione hydro-
chloride (compound 54):
HOCH2CH2CH2-CH2Br + HN~N~ _~N-CH3
(compound 37) C/H
02N{~OH HCl/CH30H
~1389
- 132 -
C2N ~ OCH2CH2CH2CH2N N ~ N-CH3
N ~ HCl
(compound 54) C H
The same procedure as in Example 20-(1) was repeated
with the exception that 3-bromo-1-propanol was replaced with
12.9 g of 4-bromo-1-butanol, in order to obtain 13.1 g of
1,3-dimethyl-6-[4-(4-hydroxybutyl)piperazin-1-yl]-2,4-
10 (1H,3H)-pyrimidinedione.
Furthermore, the same procedure as in Example 20-(2)
was repeated with the exception that 1.05 g of this
pyrimidinedione derivative was substituted for 1,3-dimethyl-
6-[4-(3-hydroxypropyl)piperazin-1-yl]-2,4(1H,3H)-pyrimidine-
15 dione (compound 36), in order to prepare 1.2 g of crystalsof 1,3-dimethyl-6-{4-[4-(4-nitrophenoxy)butyl]piperazine-1-
yl}-2,4(1H,3H)-pyrimidinedione hydrochloride (compound 54).
Analytical results of the crystalline compound 54
thus obtained:
Values of elemental analysis (as C20H27NsOs-HCl-0.5H2O)
Calcd. (%): C 51.89; H 6.31; N 15.13; Cl 7.66
Found (%): C 52.01; H 6.24; N 15.41; Cl 7.56
Example 31
Preparation of 1,3-dimethyl-6-{4-[3-(2-nitrophenoxy)-
propyl]piperazin-1-yl} -2.4(1H,3H)-pyrimidinedione hydro-
~ ~ ~L~89
- 133 -
chloride (compound 55):
BrCH2CH2CH2Br
(~OH '
NO2
o
HN N ~ N~CHa
N ~
(compound 37) CH3 HCl/CH30H
1 0
O .
OCH2CH2CH2N N~N-CH3
NO2 ~N ~ HCl
(compound 55) C H 3
The same procedure as in Example 21-(1) and 21-(2) was
repeated with the exception that 3-nitrophenol was replaced
with 13.9 g of 2-nitrophenol, in order to obtain crystals of
1,3-dimethyl-6-{4-[3-(2-nitrophenoxy)propyl]piperazin-1-yl}-
2.4(1H,3H)-pyrimidinedione.
Analytical results of the crystalline pyrimidinedione
derivative thus obtained:
Melting point: 123.5-125~C
Values of elemental analysis (as C1gH2sNsOs)
Calcd. (%): C 56.57; H 6.25; N 17.36
Found (%): C 56.74; H 5.85; N 17.46
-
2~9~1389
- 134
NMR (CDC13), S ppm: 2.03 (m, 2H), 2.66 (m, 6H),
2.98 (m, 4H), 3.32 (s, 3H), 3.40 (s, 3H),
4.11 (t, 2H), 5.22 (s, 1H), 6.9-7.9 (m, 4H)
The thus obtained crystals were treated with a
hydrochloric acid/methanol solution in a usual manner to
obtain 1,3-dimethyl-6- {4-[3-(2-nitrophenoxy)propyl]pipera-
zin-1-yl} -2,4(1H,3H)-pyrimidinedione hydrochloride
(compound 55).
Analytical results of the crystalline compound 55
thus obtained:
Melting point: 251-252~C (decomposed)
Values of elemental analysis (as C1gH2sNsOs-HCl)
Calcd. (%): C 51.88; H 5.96; N 15.92; Cl 8.06
Found (%): C 51.29; H 5.84; N 16.06; Cl 7.58
Example 32
Preparation of 1,3-dimethyl-6-[2-(4-nitrobenzoylamino)-
ethylamino]-2,4(1 H,3H)-pyrimidinedione (compound 56):
02N ~ COCl + H2NCH2CH2NH ~ N~CHg
(compound 57) CH 9
2~ 9
_ 135 --
> 02N~CONHCH2CH2NH~ -CH3
(compound 56) C H
The same procedure as in Example 22 was repeated with
the exception that 1,3-dimethyl-6-[4-(2-hydroxyethyl)pipera-
zin-1-yl]-2,4(1H,3H)-pyrimidinedione (compound 41) was
replaced with 0.35 g of 1,3-dimethyl-6-(2-aminoethylamino)-
2,4(1H,3H)-pyrimidinedione (compound 57), in order to obtain
crystals of 0.49 g of 1,3-dimethyl-6-[2-(4-nitrobenzoyl-
amino)ethylamino]-2,4(1H,3H)-pyrimidinedione (compound 56).
Analytical results of the crystalline compound 56
thus obtained:
Melting point: 284-285~C (decomposed)
IR V KBrmax (cm~1): 1690, 1660, 1640, 1610,
1550, 1360, 860
NMR (DMSO-d6), ~ppm: 3.3 (m, 4H), 3.36 (s, 3H),
3.28 (s, 3H), 4.98 (s, 1H), 8.18 (d, 2H),
8.41 (d, 2H)
Values of elemental analysis (as C1sH17NsOs)
Calcd. (%): C 51.87; H 4.93; N 20.16
Found (%): C 51.89; H 5.19; N 19.73
Example 33
Preparation of tablets containing, as an effective
2001389
- 136 -
component, 1,3-dimethyl-6-{4-[3-(4-nitrophenoxy)propyl]-
piperazin-1-yl}-2,4(1H,3H)-pyrimidine hydrochloride
(compound 35) which can be obtained by the process of
Example 20:
With 20 g of corn starch were mixed 1 g of the above
pyrimidinedione derivative hydrochloride (compound 35) and
123 g of lactose, and the mixture was further mixed with a
solution prepared by dissolving 5 g of hydroxypropyl
cellulose in 100 ml of water, so as to form grains, followed
by drying the grains at 50~C for 4 hours. Afterward, 1 g of
magnesium stearate was added to the dried grains and then
mixed sufficiently. The mixture was then formed into
tablets by the use of a tableting machine, the weight of
each tablet being 150 mg.
Example 34
Preparation of capsules containing, as an effective
component, 1,3-dimethyl-6-{4-[2-(4-nitrobenzoylamino)ethyl]-
piperazin-1-yl}-2,4(1H,3H)-pyrimidinedione hydrochloride
(compound 45) which can be obtained by the process of
Example 25:
With 25 g of corn starch were sufficiently mixed 5 g of
the above pyrimidinedione derivative hydrochloride (compound
45) and 120 g of lactose, and hard capsules were filled with
the resulting mixture by the use of a capsule filling
machine to obtain capsules, the content of the mixture in
2~0~389
- 137 -
each capsule being 150 mg.
Example 35
Preparation of an injection containing, as an effective
component, 1,3-dimethyl-6-{4-[3-(4-nitrophenyl)-2-hydroxy-
propyl]piperazin-1-yl~-2,4(1H,3H)-pyrimidinedione oxalate
(compound 49) which can be obtained by the process of
Example 27:
In distilled water for injection were dissolved 20 mg
of the above pyrimidinedione derivative oxalate (compound
49) and 0.85 g of sodium chloride, and the total volume of
the liquid was regulated to be 100 ml, thereby preparing an
n~ ectlon .
Pharmacological Test 2
Following the same procedure as in Pharmacological
Test 1, APD7s and ERP of the respective compounds prepared
in the above examples in Table 4 were calculated. The
resluts are set forth in Table 4.
Toxicity Test 2
Following the same procedure as in Toxicity Test 1,
toxicity of the respective compounds prepared in the above
examples in Table 5 was tested to calculate a mortality rate
of mice.
Administration was made by oral administration (p.o.)
in an amount of 300 mg/kg of each compound for one mouse.
2()01389
- 138
Table 4 (results of pharmacological test)
Com- APD75 (%) ER' (%)
pound Dose (llg/ml)Dose mg/kg,i.v.)
5 No. 0.3 1.0 3.0 10.0 0.10. 1.0 3.0
- 17 22 30 6 12 15.8 25.3
38 - 15 34 - 0 0 7 14
42 - 2 10 17 4.69.411.7 18.4
10 44 - 5 13 21 0 7.719.9 27.8
47 - 5 11 16
51 - - 33 - 12.612.619.0
53 - 8 22 43 8.82330.1
54 - - 15 26
15 55 - - 18 39 0 0 6.8 13.6
56 - - - - 6.76.76.7
Table 5 (results of toxicity test)
20Compound Number Mortality Rate (%)
0
51 0
54 0
56 50
-
~ 139 ~ 2~1389
Example 36
Preparation of 1,3-dimethyl-6-{4-[3-(4-nitroanilino)-
propyl]piperazin-1-yl}-2,4(1H,3H)-pyrimidinedione hydro-
chloride (compound 58):
O O
NH BrCH2CH2CH2Br ~ N-CH2CH2CH2Br
o
(
o
H ~ ~ N-CH3
N
CHs
(compound 60) hydrazine H2O
H2NCH2CH2CH2N N ~ N-CH~
f (compound 59) /N 0
02N ~ F (compound 58') Hcl/cHsoH~
~ . , .
2~389
- 140 -
02N ~ NHCH2CH2CH2N N ~ N-CH3
(compound 58) / 0 HCl
CH3
(1) Preparation of 1,3-dimethyl-6-[4-(3-aminopropyl)-
piperazin-1-yl]-2,4(1H,3H)-pyrimidinedione (compound 59):
In 100 ml of dimethylformamide were suspended 18.52 g
of potassium phthalimide and 200 g of 1,3-dibromopropane,
and then this suspension was heated with stirring at 120~C
for 6 hours so as to perform reaction. Next, insoluble
matters were removed from the resulting reaction mixture by
filtration, and the filtrate was then concentrated to
dryness under reduced pressure. The residue was washed with
hexane and then recrystallized from ethanol/water, and the
resulting crystals were collected by filtration, washed, and
dried to obtain 13.8 g of N-(3-bromopropyl)phthalimide.
Afterward, 13.0 g of this N-(3-bromopropyl)phthalimide,
10.3 g of 1,3-dimethyl-6-(1-piperazinyl)-2,4(1H,3H)-pyrimi-
dinedione (compound 60) and 20 g of triethylamine were
suspended in 200 ml of dioxane, and the resulting suspension
was refluxed for 6 hours.
Furthermore, insoluble matters were removed from the
reaction mixture by filtration, and the filtrate was then
concentrated to dryness under reduced pressure. The residue
(concentrate) was recrystallized from ethyl
2~)01389
- 141 _
acetate/n-hexane, and the resulting crystals were collected
by filtration, washed, and dried to obtain 12.5 g of
1,3-dimethyl-6-[4-(3-phthaloylaminopropyl)piperazin-1-yl]-
2,4(1H,3H)-pyrimidinedione.
Next, 12.5 g of the thus obtained crystals and 6.0 g of
hydrazine nomohydrate were suspensed in 200 ml of ethanol,
and the suspension was then refluxed for 4 hours. After
cooling, the resulting insoluble matters were removed
therefrom by filtration, and the filtrate was then concen-
trated to dryness under reduced pressure. Furthermore, the
residue (concentrate) was dissolved in water, and dilute
hydrochloric acid was added thereto to adjust a pH to about
3. Insoluble matters which had been formed at this time
were then removed therefrom by filtration, and a great deal
of potassium carbonate was added to the filtrate, followed
by extracting with chloroform. After completion of the
extraction, the resulting organic layer was dried over
anhydrous sodium sulfate and then subjected to a treatment
under reduced pressure so as to distill off the solvent,
thereby obtaining 1,3-dimethyl-6-[4-(3-aminopropyl)pipera-
zin-1-yl]-2,4(1H,3H)-pyrimidinedione (compound 59) as a
colorless syrupy. This product was then allowed to stand,
whereby it crystallized.
(2) Preparation of 1,3-dimethyl-6-~4-[3-(4-nitro-
anilino)propyl]piperazin-1-yl}-2,4(1H,3H)-pyrimidinedione
389
- 142 -
hydrochloride (compound 58):
To 20 ml of dimethyl sulfoxide were added 2.50 g of the
above obtained compound (compound 59) and 1.90 g of
4-nitrofluorobenzene, and the resulting mixture solution was
heated at 80~C for 3 hours. After cooling, the deposited
crystals were collected by filteration, washed, and dried to
obtain 2.75 g of 1,3-dimethyl-6-{4-[3-(4-nitroanilino)-
propyl]piperazin-1-yl}-2,4(1H,3H)-pyrimidinedione
(compound 58').
Analytical results of the crystalline compound 58'
thus obtained:
IR ~ KBrmax (cm-1): 3280, 1635, 1592, 1450,
1425, 1295, 1105, 990, 840
Values of elemental analysis (as C1gH26N6O4):
Calcd. (%): C 56.70; H 6.51; N 20.88
Found (%): C 56.19; H 6.88; N 20.50
Next, the thus prepared compound 58' was treated with a
hydrochloric acid/methanol solution in a usual manner to
prepare 1,3-dimethyl-6-~4-[3-(4-nitroanilino)propyl]pipera-
zin-1-yl}-2,4(1H,3H)-pyrimidinedione hydrochloride
(compound 58).
Analytical results of the crystalline compound 58
thus obtained:
Melting point: 270~C or more
IR V KBrmax (cm-1): 3230, 1645, 1595, 1432,
-
2 ~ 8 9
-- 143 --
1315, 1105, 837, 745
Values of elemental analysis (as C1gH26N6O4-HCl):
Calcd. ~%): C 51.99; H 6.20; N 19.15; Cl 8.08
Found (%): C 52.30; H 6.56; N 18.91; Cl 8.56
Example 37
Preparation of 1,3-dimethyl-6-{2- ([1-(4-nitrophenyl)-
piperidin-4-yl]amino) ethylamino} -2,4(1 H,3H)-pyrimidinedione
oxalate (compound 61 ):
02N~F + HN~}0 ; 02N~N~0
(compound 62)
H2NCHzCHzNH~N-CH~
N~
(compound 63) CH~
(COOH)2/CH30H
o
o2N ~ -N 3 NHCH2CH2NH ~ N-CHJ
N~ (C00H) 2
(compound 61) CH~
(1) Preparation of 1 -(4-nitrophenyl)-4-oxopiperidine
.~
.
2~389
_ 144 -
(compound 62):
In 20 ml of acetonitrile were dissolved 2.8 g of
4-nitrofluorobenzene, 3 g of 4-piperidone hydrochloride and
6.9 ml of triethylamine, and the solution was then heated
under reflux for 6 hours. After cooling, the reaction
mixture was poured into 100 ml of water, and the deposited
crystals were collected by filtration. The crystals were
washed with water and then with ether, and they were
recrystallized from isopropanol/hexane (1/1 in terms of
volume ratio), collected by filtration, washed, and dried in
order to obtain 3.47 g of 1-(4-nitrophenyl)-4-oxopiperidine
(compound 62).
(2) Preparation of 1,3-dimethyl-6-{2-([1-(4-nitro-
phenyl)piperidin-4-yl]amino)ethylamino}-2,4-(1H,3H)-pyrimi-
dinedione oxalate (compound 61):
In 30 ml of methanol were suspended 0.5 g of the aboveprepared compound 62 and 1.8 g of 6-(2-aminoethylamino)-1,3-
dimethyl-2,4(1H,3H)-pyrimidinedione (compound 63), and
0.58 ml of a 4 N HCl/dioxane solution was added dropwise to
the resulting suspension at 0~C, followed by stirring at the
same temperature for 1 hour.
Next, the bulk temperature of the reaction mixture was
maintained at 0~C, and 0.14 g of sodium cyanoborohydride was
added thereto little by little and stirring was then
performed at the same temperature for 3 hours.
2Q~)1389
- 145 -
Then, a small amount of water was added to the reaction
mixture, and methanol was distilled off under reduced
pressure. The resulting residue was dissolved in 0.5 N
hydrochloric acid.
Potassium carbonate was added to this hydrochloric acid
solution to make it alkaline, followed by extracting
with chloroform.
Afterward, the extract (organic layer) was washed with
water and then dried over anhydrous sodium sulfate, and the
used solvent was distilled off under reduced pressure.
Afterward, the residue was then purified through a silica
gel column chromatograph (chloroform/methanol = 50/1 to 25/1
in terms of volume ratio), thereby preparing 0.6 g of
1,3-dimethyl-6-~2-([1-(4-nitrophenyl)piperidin-4-yl]amino~-
ethylamino}-2,4(1H,3H)-pyrimidinedione.
Analytical results of the crystalline pyrimidinedione
derivative thus obtained:
NMR (CDC13) ~ppm: 1.5 (m, 4H), 2.2-3.2 (m, 7H),
3.30 (s, 3H), 3.41 (s, 3H), 4.0 (m, 2H),
4.86 (s, 1H), 6.93 (d, 2H), 8.17 (d, 2H)
Next, this pyrimidinedione derivative was treated with
an oxalic acid/methanol solution in a usual manner to
prepare 0.52 g of 1,3-dimethyl-6-{2-([1-(4-nitrophenyl)-
piperidin-4-yl~amino)ethylamino3-2,4(1H,3H)-pyrimidinedione
oxalate (compound 61).
2001389
- 146 -
Analytical results of the crystalline compound 61
thus obtained:
Melting point: 216-217~C (decomposed)
IR V KBrmax (cm-1): 2500, 1690, 1620, 1600,
1540, 1330, 810
Values of elemental analysis [as C1gH26N604-(COOH)2]:
Calcd. (%): C 51.22; H 5.73; N 17.06
Found (96): C 51.08; H 5.69; N 16.58
Example 38
Preparation of 1,3-dimethyl-6-{2-[4-(4-nitrophenyl)-
piperazin-1 -yl]ethylamino}-2,4(1 H,3H)-pyrimidinedione
oxalate (compound 64):
HN NH
OzN~F ~ OzN~N~ NH
(compound 66)
CH3 /3So3CHzCHzNH~N~CH3
(compound 65) CH~
(COOH)2/CH30H
2~389
- 147 -
-~ 02N ~ N~_,N-CH2CH2NH ~ N-CH3
N ~ (COOH) 2
(compound 64) CH3
(1) Preparation of 1,3-dimethyl-6-[2-(p-toluene-
sulfonyloxy)ethylamino]-2,4(1H,3H)-pyrimidinedione (compound
65):
o
~ ~N-CH~
Cl N O
CH3
HOCH2CH2NH2 CH3 ~ SO2Cl
>
-~ CH3 ~ SO~-CH2CH2NH ~ N-CH3
(compound 65) ~N O
CH3
First, 35 g of 2-aminoethanol was heated up to 90~C,
and it was then taken out of the oil bath. Afterward,
50.0 g of 6-chloro-1,3-dimethyl-2,4(1H,3H)-pyrimidinedione
was added thereto, so that reaction was performed. At this
time, a rate of the addition was such that reaction
temperature was maintained in the range of 90 to 110~C.
2~1389
- 148 -
After completion of the addition, the reaction mixture was
stirred for 10 minutes, and 300 ml of dioxane/methanol (10/1
in terms of volume ratio) was added thereto, followed by
standing onvernight. The resulting crystals were then
5 washed with a small amount of dioxane, and dried to obtain
49.0 g of white crystalline 1,3-dimethyl-6-(2-hydroxyethyl-
amino)-2,4(1 H,3H)-pyrimidinedione.
Next, 200 ml of a pyridine suspension of this white
crystals (49.0 g) was cooled to -5~C, and 40.0 g of
10 p-toluenesulfonyl chloride was added at such a rate that the
reaction temperature did not exceed a level of 5~C.
Furthermore, 51.0 g of p-toluenesulfonyl chloride was
additionally used to completely eliminate the turbidity of
the reaction mixture.
Afterward, the reaction mixture was poured into 1.5
liters of ice water containing 70 g of K2CO3 and then
allowed to stand overnight. The resulting crystals were
collected by filtration, washed with water, and dried under
reduced pressure in order to obtain 50.5 g of light yellow
20 crystalline 1,3-dimethyl-6-[2-(p-toluenesulfonyloxy)ethyl-
amino]-2,4(1H,3H)-pyrimidinedione (compound 65).
Analytical results of the crystalline compound 65
thus obtained:
Melting point: 146.0-149.0~C
IR ~ KBrmax (cm-1): 3270, 1682, 1615, 1550,
2C~01389
- 149
1480, 1435, 1350, 1190, 1178, 1010, 903, 780
(2) Preparation of 1 -(4-nitrophenyl)piperazine
(compound 66):
A mixture of 2.8 g of 4-nitrofluorobenzene, 15 g of
piperazine and 20 ml of acetonitrile was heated under reflux
for 6 hours. After cooling, chloroform was added to the
reaction mixture, and the resulting solution was washed with
water, dried (over anhydrous sodium sulfate), and concen-
trated under reduced pressure to obtain N-(4-nitrophenyl)-
piperazine (compound 66).
(3) Preparation of 1,3-dimethyl-6-{2-[4-(4-nitro-
phenyl)piperazin-1-yl]ethylamino}-2,4(1H,3H)-pyrimidine-
dione oxalate (compound 64):
With 0.6 g of the above N-(4-nitrophenyl)piperazine
(compound 66) was mixed 0.5 g of 1,3-dimethyl-6-[2-(p-
toluenesulfonyloxy)ethylamino]-2,4(1H,3H)-pyrimidinedione
(compound 65), and the mixture was then heated at 80~C for
hour. After cooling, the reaction mixture was diluted with
5 ml of acetonitrile.
Next, the diluted solution was then poured into 20 ml
of a dilute aqueous sodium hydroxide solution, and the
resulting mixture was extracted with chloroform. The
resulting organic layer was washed with water, dried (over
anhydrous sodium sulfate), and concentrated to dryness.
The residue (concentrate) was purified through a silica
2~ 89
- 150 -
gel column chromatograph (chloroform/methanol = 30:1 in
terms of volume ratio) to obtain 1,3-dimethyl-6-~2-[4-(4-
nitrophenyl)piperazin-1-yl]ethylamino~-2,4(1H,3H)-pyrimi-
dinedione.
Analytical results of the pyrimidinedione derivative
thus obtained:
NMR (CDCl3) ~ppm: 2.8-3.8 (m, 12H), 3.36 (s, 3H),
3.41 (s, 3H), 4.86 (s, 1H), 6.87 (d, 2H),
8.16 (d, 2H)
This pyrimidinedione derivative was treated with an
oxalic acid/methanol solution in a usual manner to obtain
0.36 g of 1,3-dimethyl-6-{2-[4-(4-nitrophenyl)piperazine-1-
yl]ethylamino}-2,4(1H,3H)-pyrimidinedione oxalate
(compound 64).
Analytical results of the crystalline compound 6
thus obtained:
IR V KBrmax (cm~1): 1680, 1640, 1600, 1540,
1320, 830
Values of elemental analysis
(as C20H24N6o4-2(cooH)2 4H20)
Calcd. (%): C 43.38; H 5.46; N 12.65
Found (%): C 42.91; H 5.19; N 13.24
Example 39
Preparation of 1,3-dimethyl-6-{N-methyl-2-[N-methyl-2-
(N-methyl-4-nitroanilino)ethylamino]ethylamino}-2,4(1H,3H)-
2~1389
- 151 -
pyrimidinedione oxalate (compound 67):
CH~
CH~NHCH2CH2NCH2CH2NHCH~
02N ~ F
CH3 CH3
02N ~ NCH2CH2NCH2CH2NHCH~
(compound 68)
Cl ~ -CH3
N ~ (COOH) 2 /CH 3 OH
CH3
Cl~3 Cl~3 Cl~3 0
02N~NCI12CH2NCH2CH2N~A:-CH3
N~ - (COOH~ 2
(compound 67) CH 3
(1) Preparation of N,N',N"-trimethyl-N-(4-nitro-
phenyl)diethylenetriamine (compound 68):
In 7 ml of dimethyl sulfoxide were dissolved 1.41 g of
p-fluoronitrobenzene and 14 g of N,N',N"-trimethyldiethylene-
triamine, and the mixture was then stirred at 120~C for 3
2~01389
- - 152 -
hours.
The solvent was then distilled off from the reaction
mixture under reduced pressure, and the residue was then
dissolved in chloroform. This chloroform solution was
washed with a small amount of water, and then dried over
anhydrous sodium sulfate. The used solvent was distilled
off under reduced pressure, so that 2.1 g of N,N',N"-tri-
methyl-N-(4-nitrophenyl)diethylenetriamine (compound 68) was
obtained in a yellow oily state.
(2) Preparation of 1,3-dimethyl-6-{N-methyl-2-[N-
methyl-2-(N-methyl-4-nitroanilino)ethylamino]ethylamino3-
2,4(1H,3H)-pyrimidinedione oxalate (compound 67):
In 10 ml of ethanol were suspended 2.1 g of the above
prepared N,N',N"-trimethyl-N-(4-nitrophenyl)diethylenetri-
amine (compound 68), 1.22 g of 6-chloro-1,3-dimethyl-
2,4(1H,3H)-pyrimidinedione and 2 ml of triethylamine, and
the suspension was then heated under reflux for 1 hour. The
resulting reaction mixture was then concentrated to dryness,
and the residue was purified through a silica gel column
chromatograph (chloroform/methanol = 50/1 to 25/1, v/v) and
then recrystallized from acetone/water (1/1 in terms of
volume ratio), thereby preparing 1.87 g of 1,3-dimethyl-6-
~N-methyl-2-[N-methyl-2-(N-methyl-4-nitroanilino)ethyl-
amino]ethylamino~-2,4(1H,3H)-pyrimidinedione.
Analytical results of the crystals thus obtained:
2~ 389
- 153 -
Melting point: 65~C
NMR (CDCl3), ~ppm: 2.40 (s, 3H), 2.82 (s, 3H),
3.38 (s, 3H), 3.50 (s, 3H), 3.54 (s, 3H),
2.78 (m, 2H), 3.13 (m, 2H), 3.80 (m, 2H),
5.40 (s, 1H), 6.78 (m, 2H), 8.62 (m, 2H)
Values of elemental analysis (as C1gH2gN6O4)
Calcd. (~): C 56.42; H 6.98; N 20.78
Found (~): C 56.63; H 7.31; N 19.98
The thus obtained 1,3-dimethyl-6-{N-methyl-2-[N-methyl-
2-(N-methyl-4-nitroanilino)ethylamino]ethylamino}-2,4-
(1H,3H)-pyrimidinedione was treated with an oxalic acid/-
methanol solution in a usual manner to obtain 1.91 g of
1,3-dimethyl-6-~N-methyl-2-[N-methyl-2-(N-methyl-4-nitro-
anilino)ethylamino]ethylamino}-2,4(1H,3H)-pyrimidinedione
oxalate (compound 67).
Analytical results of the crystalline compound 67
thus obtained:
Melting point: 184-185~C (decomposed)
IR ~ KBrmax (cm-1): 3460, 1700, 1642, 1545,
1480, 1310, 1200, 1100, 833, 722
Values of elemental analysis
(as C1gH26N6O4-(cOoH)2-2H2o)
Calcd. (%): C 50.09; H 6.21; N 16.69
Found (~): C 50.29; H 6.29; N 16.11
~ ~ Q ~ 3 8 ~1
. .
_ 154 -
Example 40
Preparation of 1,3-dimethyl-6-{4-[3-(N-methyl-4-nitro-
anilino)propyl]piperazin-1 -yl3 -2,4(1H,3H)-pyrimidinedione
oxalate (compound 69):
0
02N ~ NHCH2CH2CH2N~_,N ~ -CH3
(compound 58') CH~
CH~I (COOH)2/CH.OH
CH, O
02N ~ NCH2CH2CH2N N ~ -CH3j
~-~ ,N-~3 ~ (COOH)2
(compound 69) C H,
At room temperature, 0.12 g of sodium hydride (60%
( dispersion in mineral oil) was added to a suspension
obtained by suspending 0.1 g of 1,3-dimethyl-6-{4-t3-(4-
nitroanilino)propyl]piperazin-1-yl}-2,4(1H,3H)-pyrimidine-
dione (compound 58') obtained in Example 36 in 15 ml of
anhydrous dimethylformamide, and they were stirred for 30
minutes. Afterward, 0.29 g of methyl iodide was further
added thereto.
This mixture was stirred at room temperature for 10
B
~1389
- 155 -
minutes, and a small amount of methanol and then 50 ml of
chloroform were added thereto. Afterward, the resulting
reaction mixture was poured into 50 ml of water. The
separated organic layer was taken out, washed with water,
and dried (over anhydrous sodium sulfate). The used solvent
was distilled off, and the residue was crystallized from a
methanol/ether solution (1/1 in terms of volume ratio),
collected by filtration, washed, and dried in order to
obtain 0.62 g of 1,3-dimethyl-6-[4-{3-(N-methyl-4-nitro-
anilino)propyl3piperazin -1-yl]-2,4(1H,3H)-pyrimidinedione.
Analytical results of the crystalline pyrimidinedione
derivative thus obtained:
IR V KBrmax (cm~1): 1690, 1640, 1588, 1520,
1480, 1425, 1282, 1205, 1101, 815
NMR (d6-DMSO), Sppm: 1.82 (m, 2H), 2.13-2.73 (m, 6H),
2.73-3.07 (m, 4H), 3.10 (s, 3H), 3.17 (s, 3H),
3.30 (s, 3H), 3.57 (t, 2H), 5.22 (s, 1H),
6.87 (d, 2H), 8.10 (d, 2H)
Values of elemental analysis (as C20H28N6~4 2H2~)
Calcd. (%): C 57.13; H 7.67; N 19.99
Found (~): C 57.49; H 7.02; N 20.16
This pyrimidinedione derivative was treated with an
oxalic acid/methanol solution in a usual manner to obtain
1,3-dimethyl-6-{4-[3-(N-methyl- 4-nitroanilino)propyl]-
piperazin-1-yl}-2,4(1H,3H)-pyrimidinedione oxalate
2 ~ ~ ~ 3 8 ~
_ _ _ 156
(compound 69).
Analytical results of the crystalline compound 69
thus obtained:
Values of elemental analysis
5(as C20H28N6o4~(cooH)2-2H2o)
Calcd. (%): C 51.76; H 6.71; N 16.46
Found (~): C 52.05; H 6.08; N 16.56
Example 41
Preparation of 1,3-dimethyl-6- {4-t3-(N-methansulfonyl-
104-nitroanilino)propyl]piperazin-1-yl}-2,4(1H,3H)-pyrimidine-
dione oxalate (compound 70):
02N~NHCH2CH2CH2N~_N~II-CH,
(compound 58') - CH,
CH,SO,Cl (COOH)2/CH,OH
SO2CH, O
2002N~NCHzCH2CH2N~ N~ ~ (COOH)z
(compound 70) CH ~
In 20 ml of chloroform were dissolved 0.4 g of
1,3-dimethyl-6- {4-[3-(4-nitroanilino)propyl]piperazin-1 -yl3 -
252,4(1H,3H)-pyrimidinedione (compound 58') obtained in
'~
2~01;~89
_ 157 -
Example 36 and 0.4 g of triethylamine, and 0.2 g of
methanesulfonyl chloride was further added to the resulting
solution under ice cooling.
This mixture was allowed to stand overnight at room
temperature, and a small amount of water was added thereto,
followed by stirring at room temperature for 30 minutes.
Furthermore, 20 ml of a dilute aqueous alkaline
solution was added to the reaction mixture, and the
separated chloroform layer was washed with water and then
dried over anhydrous sodium sulfate. Afterward, the dried
chloroform layer was treated under reduced pressure to
distill off the solvent. The resulting residue was purified
through a silica gel column chromatograph (chloroform/-
methanol = 100/1 to 25/1 in terms of volume ratio), thereby
preparing 0.35 g of 1,3-dimethyl-6-{4-[3-(N-methanesulfonyl-
4-nitroanilino)propyl]piperazin-1-yl}-2,4(1H,3H)-pyrimidine-
dione.
This pyrimidinedione derivative was treated with an
oxalic acid/methanol solution in a usual manner to obtain
1,3-dimethyl-6-{4-[3-(N-methanesulfonyl-4-nitroanilino)-
propyl]piperazin -1-yl}-2,4(1H,3H)-pyrimidinedione oxalate
(compound 70).
Analytical results of the crystalline compound 70
thus obtained:
Melting point: 204.0-204.5~C
Z~ 89
_ 158 -
Values of elemental analysis
(as C20H28N6o6s-(cooH)2-2H2o)
Calcd. (%): C 43.56; H 5.65; N 13.85; S 5.29
Found (~): C 43.81; H 5.24; N 13.35; S 5.52
IR ~ KBrmax (cm~1): 1780, 1695, 1630 (br),
1350, 1340, 1205, 1155
Example 42
Preparation of 1,3-dimethyl-6-~2-[N-ethyl-2-(4-nitro-
anilino)ethylamino]ethylamino}-2,4(1H,3H)-pyrimidinedione
oxalate (compound 71):
n2N~ NHCH2CH2NH + CN ~ N-CH3
(compound 72) CH 3
HC1/CH~OH
CH2CH~ O
02N ~ NHCH2CH2NCH2CH2-NH ~ N-CH~
- N~ (CO2H)2
(compound 71) / o
CH3
(1) Preparation of 6-(1-aziridinyl)-1,3-dimethyl-
2,4(1H,3H)-pyrimidinedione (compound 72):
2(~ 89
159
CH9~ SO9-CHZCH2NH~ N-CH9
(compound 6 5) CH~
NaH /DblSO CN~ N-CH3
(COmPOUnd 72) CH3
1 0
TO 150 ml of an anhydrous dimethyl sulfoxide containing
47 2 g of the compound 65 obtained in Example 38-(1) was
slowly added 6. 24 g of sodium hydride (60% dispersion in
mineral oil) at room temperature. This reaction mixture was
stirred vigorously at room temperature for 5 hours, and then
cooled. Afterward, a small amount of water was added
thereto so as to bring reaction to an end. This solution
was poured into 1 liter of water containing 70 g of
potassium carbonate, and then extracted with 200 ml of
chloroform three times. The combined organic layer was
dried over anhydrous sodium sulfate and then concentrated,
and 300 ml of ether was added to the resulting concentrate.
Afterward, the solution thus obtained was allowed to
stand overnight.
Light yellow crystals which had been deposited by the
_ 160 -
overnight standing were collected by filtration, washed with
ether, and dried under reduced pressure in order to obtain
15.2 g of 6-(1-aziridinyl)-1,3-dimethyl-2,4(1H,3H)-pyrimi-
dinedione (compound 72).
Analytical results of the crystalline compound 72
thus obtained:
Melting point: 126.0-126.5~C
IR ~ KBrmax (cm 1): 1705, 1650, 1612, 1470,
1440, 1305, 1150, 783, 490
lH-NMR (CDC13), sppm: 2.34 (s, 4H), 3.35 (s, 3H),
3.56 (s, 3H), 5.25 (s, 3H)
(2) Preparation of 1,3-dimethyl-6-{2-[N-ethyl-2-(4-
nitroanilino)ethylamino]ethylamino)-2,4(1H,3H)-pyrimidine-
dione oxalate (compound 71):
In 5 ml of chloroform were dissolved 1.32 g of
N-ethyl-N'-(4-nitrophenyl)ethylenediamine and 1.12 g of the
above obtained 6-(1-aziridinyl)-1,3-dimethyl-2,4(1H,3H)-
pyrimidinedione (compound 72), and the resulting reaction
mixture was concentrated under reduced pressure. To the
residue (concentrate) was added 10 mg of Amberlist 15 (trade
mark; made by Rohm & Haas Co.), and the mixture was then
heated at 80~C for 1 hour.
Next, the resulting reaction mixture was dissolved in
5 ml of ethyl acetate, and Amberlist 15 was then removed
therefrom by filtration. Afterward, n-hexane was added to
2~ 9
the filtrate.
Furthermore, the resulting solution was allowed to
stand overnight, and deposited crystals were collected by
filtration, washed with n-hexane, and dried under reduced
pressure in order to obtain 1.5 g of 1 ,3-dimethyl-6-{2-[N-
ethyl-2-(4-nitroanilino)ethylamino]ethylamino~ -2,4(1H,3H)-
pyrimidinedione.
Analytical results of the crystalline pyrimidinedione
derivative thus obtained:
1H-NMR (CDC13), ~ppm: 1.12 (t, 3H), 2.52-2.98 (m, 6H),
2.98-3.25 (m, 2H), 3.28 (s, 6H),
3.28-3.48 (m, 2H), 4.78 (s, 1H), 5.27 (m, 1H),
5.45 (m, 1H), 6.59 (d, 2H), 8.08 (d, 2H)
Furthermore, this pyrimidinedione derivative was
treated with an oxalic acid/methanol solution in a usual
manner to obtain 1.4 g of 1,3-dimethyl-6-{2-[N-ethyl-2-(4-
nitroanilino)ethylamino]ethylamino}-2,4(1H,3H)-pyrimidine-
dione oxalate (compound 71 ).
Analytical results of the crystalline compound 71
thus obtained:
Melting point: 175.0-176.5~C
values of elemental analysis (as C18H26N6~4-(C~~H)2)
Calcd. (%): C 50.00; H 5.87; N 17.49
Found (96): C 49.81; H 6.02; N 17.22
2Qal1389
- 162 -
Example 43
Preparation of 1,3-dimethyl-6-{2-[N-ethyl-3-(4-nitro-
anilino)propylamino]ethylamino}-2,4(1H,3H)-pyrimidinedione
hydrochloride (compound 73):
CH2CH3 O
OZN~3~NHCH2CH2CH2NH + C N ~ N-CH3
(compound 72) CH 3
HCl/CH30H
CH2CH3 0
02N ~ NHCH2CH2CH21~lCH2CH2-NH ~ N-CH3
~ i HCl
(compound 73) C/H 3 O
The same procedure as in Example 42-(2) was repeated
with the exception that N-ethyl-N'-(4-nitrophenyl)ethylene-
diamine was replaced with 1.45 g of N-ethyl-N'-(4-nitro-
phenyl)-1,3-propylenediamine as a starting material, in
order to obtain crystals of 1,3-dimethyl-6-~2-[N-methyl-3-
(4-nitroanilino)propylamino]ethylamino}-2,4(1H,3H)-pyrimi-
dinedione.
Analytical results of the crystalline pyrimidinedione
derivative thus obtained:
- 2(~1389
- 163
NMR (DMSO-d6), sppm: 1.05 (t, 3H), 1.6-1.9 (m, 2H),
2.4-2.8 (m, 6H), 3.0-3.4 (m, 4H), 3.1 (s, 3H),
3.25 (s, 3H), 4.65 (s, 1H), 6.4 (br, 1H),
6.6 (d, 2H), 7.2 (br, 1H), 8.0 (d, 2H)
The thus obtained crystals were treated with a
hydrochloric acid/methanol solution in a usual manner to
obtain 1,3-dimethyl-6-{2-[N-ethyl-3-(4-nitroanilino)propyl-
amino]ethylamino} -2,4(1 H,3H)-pyrimidinedione hydrochloride
(compound 73).
Analytical results of the crystalline compound 73
thus obtained:
Values of elemental analysis (as C19H28N6O4-2HCl)
Calcd. (%): C 47.80; H 6.33; N 17.60; Cl 14.85
Found (%): C 47.52; H 6.49; N 17.31; Cl 14.76
Example 44
Preparation of 1,3-dimethyl-6-{N-(2-hydroxyethyl)-2-[4-
(4-nitrophenyl)piperazin-1 -yl]ethylamino} -2,4(1 H,3H)-pyrimi-
dinedione oxalate (compound 74):
~N-CH2CHzOH
02N ~N~JNH
(compound 66)
~n ~ ~ ~ 8 9
_ 164 -
CH2CH20H
-~ 02N ~ N~_N-CH2CH2NH
(compound 75)
~
Cl ~ ~ N-CHs
CH, ~ (COOH)z/CH~OH
(
CH2CH20H
02N ~ N~_JN-CH2CH2N ~ (COOH)2
(compound 74) CH~
(1) Preparation of 1-[2-(2-hydroxyethylamino)ethyl]-4-
(4-nitrophenyl)piperazine (compound 75):
In 20 ml of chloroform was dissolved 4 g of 1-(4-
( nitrophenyl)piperazine (compound 66) and 0.52 ml of
1-(2-hydroxylethyl)aziridine, and the solvent was distilled
off from the resulting reaction mixture under reduced
pressure. To the residue was added 10 mg of Amberlist 15
(trade name; made by Rohm & Haas Co.), and the mixture~was
then heated with stirring at 100~C for 3 hour. Afterward,
the temperature of the reaction mixture was returned to room
temperature. To the reaction mixture was added 20 ml of
. . .
Z~0~389
- 165 _
chloroform, and insoluble matters were then removed
therefrom by filtration. The filtrate was concentrated, and
the resulting residue (concentrate) was purified through a
silica gel column chromatograph (chloroform/methanol = 100/1
to 25/1 in terms of volume ratio), thereby preparing 1 g of
1-[2-(2-hydroxyethylamino)ethyl]-4-(4-nitrophenyl)piperazine
(compound 75).
(2) Preparation of 1,3-dimethyl-6-~N-(2-hydroxyethyl)-
2-[4-(4-nitrophenyl)piperazin-1-yl]ethylamino3-2,4(1H,3H)-
pyrimidinedione oxalate (compound 74):
To 10 ml of ethanol were added 1.0 g of the thus
obtained compound 75, 0.52 g of 6-chloro-1,3-dimethyl-
2,4(1H,3H)-pyrimidined one and 2 ml of triethylamine as
starting materials, and the resulting mixture was treated in
the same procedure as in Example 39-(2) to obtain 0.73 g of
1,3-dimethyl-6-{N-(2-hydroxyethyl)-2-[4-(4-nitrophenyl)-
piperazin-1-yl]ethylamino}-2,4(1H,3H)-pyrimidinedione.
Analytical results of the pyrimidinedione derivative
thus obtained:
NMR (CDC13), ~ppm: 2.65 (m, 6H), 2.9-3.4 (m, 8H),
3.31 (s, 3H), 3.47 (s, 3H), 3.82 (m, 2H),
5.32 (s, 1H), 6.86 (d, 2H), 8.12 (d, 2H)
Then, 0.7 g of this pyrimidinedione derivative was
further treated with an oxalic acid/methanol solution in a
usual manner to obtain crystals of 0.35 g of 1,3-dimethyl-
Z~389
_ 166 -
6-{N-(2-hydroxyethyl)-2-[4-(4-nitrophenyl)piperazin-1-yl]-
ethylamino}-2,4(1H,3H)-pyrimidinedione oxalate
(compound 74).
Analytical results of the crystalline compound 74
thus obtained:
Values of elemental analysis
[as C20H28N6os-1.5(cooH)2-H2o]
Calcd. (%): C 47.18; H 5.68; N 14.35
Found (%): C 47.37; H 5.94; N 14.08
Example 45
Preparation of tablets containing, as an effective
component, 1,3-dimethyl-6-{4-[3-(4-nitroanilino)propyl]-
piperazin-1-ylJ-2,4(1H,3H)-pyrimidinedione hydrochloride
(compound 58) which can be prepared by the process of
Example 36:
With 20 g of corn starch were sufficiently mixed 1 g of
the above pyrimidinedione derivative hydrochloride (compound
58) and 123 g of lactose, and the mixture was further mixed
with a solution prepared by dissolving 5 g of hydroxypropyl
cellulose in 100 ml of water, so as to form grains, followed
by drying the grains at 50~C for 4 hours. Afterward, 1 g of
magnesium stearate was added to the dried grains and then
mixed sufficiently. The mixture was then formed into
tablets by the use of a tableting machine, the weight of
each tablet being 150 mg.
20~1389
- 167 -
Example 46
Preparation of capsules containing, as an effective
component, 1,3-dimethyl-6-~2-~[1-(4-nitrophenyl)piperidin-4-
yl]amino)ethylamino}-2,4(1H,3H)-pyrimidinedione hydro-
chloride (compound 61) which can be prepared by the processof Example 37:
With 25 g of corn starch were sufficiently mixed 5 g of
the above pyrimidinedione derivative hydrochloride (compound
61) and 120 g of lactose, and hard capsules were filled with
the resulting mixture by the use of a capsule filling
machine to prepare capsules, the content of the mixture in
each capsule being 150 mg.
Example 47
Preparation of an injection containing, as an effective
component, 1~3-dimethyl-6-{2-(N-methyl-N-[3-(4-nitroanilino)
propyl]amino)ethylamino}-2,4(1H,3H)-pyrimidinedione
hydrochloride (compound 73) which can be prepared by the
process of Example 43:
In distilled water for injection were dissolved 20 mg
of the above pyrimidinedione derivative hydrochloride
(compound 73) and 0.85 g of sodium chloride, and the total
volume of the liquid was regulated to be 100 ml, thereby
preparing an injection.
Pharmacological Test 3
Following the same procedure as in Pharmacological
389
- 168
Test 1, ADP7s and ERP of the respective compounds prepared
in the above examples in Table 6 were calculated. The
results are set forth in Table 6.
Toxicity Test 3
Following the same procedure as in Toxicity Test 1,
toxicity of the respective compounds prepared in the above
examples in Table 7 was tested to calculate a mortality rate
of mice. The results are set forth in Table 7.
Administration was made by oral administration (p.o.)
in an amount of 300 mg/kg of each compound for one mouse.
Table 6 (results of pharmacological test)
Com- APD75 (%) ER? (%)
pound Dose (lls~ml) Dose mg/kg,i.v.)
15No. 0.31.0 3.0 10.0 0.10. 1.0 3.0
58 - 10 18 28 - - - -
61 - - - - 8.38.3 8.3
64 8 23 32 - 21.428.6 28.6
20 69 - - 11 13 6.76.7 13.3 20.0
71 - 8 15 17 6.77.7 13.4 16.6
73 - 2 12 18
Table 7 (results of toxicity test)
Compound Number Mortality Rate (%)
71 0
2~1389
Example 48:
Preparation of 1,3-dimethyl-6-{4-[3-(2-acetyl-4-
nitrophenoxy)propyl]piperazin-l-yl}-2,4(lH,3H)-
pyrimidinedione oxalate (Compound 76)
N, o BrcHzcHzcHzoH ~ HocHzcH2cH2N N ~,N-CH3
CH3 CH3
0 2 N ~ COCH3
OH(COOH) 2 /CH 3 OH
02N~ocH2CH2CH2N,~N)~N-CH3
3 CH3 ~(COOH)2
(Compound 76)
(1) Preparation of 1,3-dimethyl-6-[4-(3-hydroxypropyl)-
piperazin-l-yl]-2,4(1H,3H)-pyrimidinedione:
14.1 g of 1,3-dimethyl-6-(1-piperazinyl)-
2,4(1H,3H)-pyrimidinedione, 11.7 g of 3-bromo-1-
propanol and 13 g of triethylamine were reacted by
heating them under reflux for 20 hours in 250 m~ of
ethanol. After completion of the reaction, the
reaction mixture was concentrated to dryness and the
residue was dissolved in 300 m~ of chloroform. The
resultant solution was washed twice with 100 m~ of
water. After the washing, the organic layer was dried
2~01389
- 170 -
over anhydrous magnesium sulfate. The organic layer
was heated under reduced pressure, whereby the solvent
was distilled off to obtain 20.5 g of a reaction
product in a crude form. Ether was added to the crude
reaction product to crystallize it. Resulting crystals
were collected by filtration, washed with chilled
ether, and then dried to obtain 12.4 g of 1,3-dimethyl-
6-[4-(3-hydroxypropyl)-piperazin-1-yl]-2,4(lH,3H)-
pyrimidinedione(yield: 69.8%).
Analytical results of crystals of the pyrimidinedione
derivative thus obtained:
Melting point: 119-121~C.
NMR (CDC13), ~ ppm: 1.8 (dt,2H),
2.7(m,6H), 3.02(m,4H),
3.36(s,3H), 3.43(s,3H),
3.82(t,2H), 4.34(br,1H),
5.26(s,1H).
IR vmBax (cm 1): 3380(Br), 3180(s), 2830,
1695, 1650, 1605, 1440,
1213, 1068, 1000, 921,
760.
(2) Preparation of 1,3-dimethyl-6-~4-[3-(2-acetyl-4-
nitrophenoxy)propyl]piperazin-l-yl}-2,4(1H,3H)-
pyrimidinedione oxalate (Compound 76):
2001389
- 171 -
Into a suspension which had been obtained by
mixing 0.6 g of the pyrimidinedione derivative obtained
in the above procedure (l), 0.54 g of 2-hydroxy-5-
nitroacetophenone and 0.69 g of triphenylphosphine in
10 m~ of anhydrous tetrahydrofuran, 0.42 m~ of
diethyl azodicarboxylate was added dropwise under
stirring at room temperature.
Next, the resulting mixture was stirred for 30
minutes at room temperature and then concentrated to
dryness. The residue was purified by chromatography on
a silica gel column (eluent: chloroform/methanol = 50/l
by volume), whereby 0.7 g of 1,3-dimethyl-6-{4-[3-(2-
acetyl-4-nitrophenoxy)propyl]piperazin-l-yl}-2,4(lH,3H)-
pyrimidinedione was obtained as an oily matter.
Analytical results of the thus-obtained pyrimidinedione
derivative:
NMR (CDC13), ~ ppm: 8.47(m,1H),
7.54(m,1H), 6.91(d,1H),
5.23(s,1H), 4.26(m,2H),
3.37(s,3H), 3.30(s,3H),
2.98(m,4H), 2.60(m,6H),
2.63(s,3H), 2.16(m,2H).
Further, the pyrimidinedione derivative was
treated in an oxalic acid/methanol solution by a method
known per se in the art to obtain 0.73 g of 1,3-
dimethyl-6-{4-[3-(2-acetyl-4-nitrophenoxy)propyl]-
Z~6~1389
- 172 -
piperazin-l-yl}-2,4(lH,3H)-pyrimidinedione oxalate
tCompound 76).
Analytical results of Compound 76 thus obtained:
Melting point: 122-124~C (decomposed).
IR vmax (cm 1): 3400, 3020, 2600, 1690,
1640, 1520, 1340, 1280,
1120, 820, 750, 700.
Elemental analysis for C21H27N506 ( 2 2
Calculated (%): C, 48.33; H, 5.82; N, 12.25.
Found (~): C, 48.22; H, 5.69; N, 11.93.
Example 49:
Preparation of 1,3-dimethyl-6-{4-[3-(4-acetyl-2-
nitrophenoxy1propyl]piperazin-1-yl}-2,4(1H,3H)-
pyrimidinedione oxalate (Compound 77):
0
HOCHzCHzCH2N~JN ~ N-CH3
N o
CH3
CH 3 CO~ rNOz
~ OH (COzH)2/CH30H
3CO~OCH2CH2CH2N~JN~N-CH3
CH3 ~(COOH)2
(Compound 77)
Z~ 89
- 173 -
By a procedure similar to Example 48-(2) except
for the use of 4-hydroxy-3-nitroacetophenone in lieu of
2-hydroxy-5-nitroacetophenone, 1,3-dimethyl-6-{4-[3-(4-
acetyl-2-nitrophenoxy)propyl]piperazin-1-yl}-2,4(lH,3H)-
pyrimidinedione oxalate (Compound 77) was obtained.
Analytical results of the pyrimidinedione derivative
(Compound 77) thus obtained:
Melting point: 119-123~C ~decomposed).
IR vKBr (Cm-l~ 3380, 2250, 1700, 1640,
max
1530, 1350, 1020, 760,
700.
Elemental analysis for C21H27N5O6 ( 2 2
Calculated (~): C, 46.86; H, 5.98; N, 11.28.
Found (~): C, 46.79; H, 5.43; N, 11.51.
Example 50:
Preparation of 1,3-dimethyl-6-{4-[3-(4-benzoyl-
2-nitrophenoxy)propyl]piperazin-1-yl}-2,4(lH,3H)-
pyrimidinedione oxalate (Compound 78):
HOCH2CHzCHz~ N ~ ~-CH3
CH3
o
~, N 0 2
OH, (COOH) z/CH30H
2~0~3~39
- 174 -
2cH2cH2N~N~N-CH3
CH3 (COOH)2
(Compound 78)
By a procedure similar to Example 48-(2) except
for the use of 4-hydroxy-3-nitroacetophenone in lieu of
2-hydroxy-5-nitroacetophenone, 1,3-dimethyl-6-{4-[3-(4-
benzoyl-2-nitrophenoxy)propyl]piperazin-1-yl}-
2,4(lH,3H)-pyrimidinedione oxalate (Compound 78) was
obtained.
Analytical results of the pyrimidinedione derivative
(Compound 78) thus obtained:
Melting point: 168-171~C (decomposed).
IR vma (cm 1): 3400, 3050, 1730, 1630,
1540, 1350, 1080, 760,
700.
Y 26 29N5~6 (COOH)2 H2O
Calculated (%): C, 54.63; H, 5.40; N, 11.38.
Found (%): C, 54.99; H, 5.29; N, 11.30.
Example 51:
Preparation of 1,3-dimethyl-6-{4-t3-(3-acetyl-
4-nitrophenoxy)propyl3piperazin-1-yl}-2,4(lH,3H)-
pyrimidinedione oxalate (Compound 79):
2S
2~1389
- 175 -
HOCH2CHzCHzN~ - ~ N-CH3
N o
CH3
CH3CO ~ OH (COOH)z/CH3OH
2N ~ 2 2 2 ~_~ ~ 3
CH3 ~(COOH)2
(Compound 79)
By a procedure similar to Example 48-(2) except
for the use of 5-hydroxy-2-nitroacetophenone in lieu of
2-hydroxy-5-nitroacetophenone, 1,3-dimethyl-6-{4-[3-(3-
acetyl-4-nitrophenoxy)propyl]piperazin-1-yl}-2,4(lH,3H)-
pyrimidinedione oxalate (Compound 79) was obtained.
Analytical results of the pyrimidinedione derivative
(Compound 79) thus obtained:
Melting point: 223-224~C (decomposed).
IR vmax (cm 1): 3440, 3000, 2600, 1730,
1600, 1520, 1350, 1020,
810.
Elemental analysis for C21H27N5O6 ( 2 2
Calculated (%): C, 48.33; H, 5.82; N, 12.25.
Found (%): C, 48.17; H, 5.62; N, 12.05.
Example 52:
1i 389
- 176 -
Preparation of 1,3-dimethyl-6-{4-[3-(2-benzoyl-
4-nitrophenoxy)propyl]piperazin-1-yl}-2,4(1H,3H)-
pyrimidinedione oxalate (Compound 80):
OzN~CI OzN~OH
~=0 ~ ~=0
HOCHz CHz CHzN N ~-CH3
'-- N~- (COOH) z/CH~OH
CH~
02N~oCH2CH2CH2N\JN~N-CH3
~ CH3 ~(COOH) 2
(Compound 80)
(1) Preparation of 2-hydroxy-5-nitrobenzophenone:
In an autoclave, a suspension which had been
obtained by suspending 3 g of 2-chloro-5-nitrobenzo-
phenone and 1.1 g of potassium hydroxide in 30 mQ of
water was heated under stirring at 150~C for 5 hours.
After completion of the heating, the autoclave
was allowed to cool down. The resultant reaction
mixture was added with 30 mQ of water, followed by the
addition of hydrochloric acid to acidify the reaction
mixture. Crystals precipitated upon the acidification
were collected by filtration.
Z~11389
- 177 -
The thus-obtained crystals were recrystallized
from ethanol to obtain 2.3 g of 2-hydroxy-S-nitro-
benzophenone.
Analytical results of the crystals thus obtained:
Melting point: 125-126~C.
NMR (CDC13), ~ ppm: 7.21(m,2H),
7.68(m,4H),
8.41(m,2H),
IR vmax (cm 1): 3040, 1600, 1520, 1330
1280, 1210, 1080, 960,
690.
(2) Preparation of 1,3-dimethyl-6-{4-[3-(2-benzoyl-
4-nitrophenoxy)propyl]piperazin-1-yl}-2,4(lH,3H)-
pyrimidinedione oxalate (Compound 80):
By a procedure similar to Example 48-(2), 0.73 g
of 2-hydroxy-5-nitrobenzophenone obtained in the above
procedure (1), 0.5 g of 1,3-dimethyl-6-[4-(3-hydroxy-
propyl)piperazin-l-yl]-2,4(1H,3H)-pyrimidinedione,
0.58 g of triphenylphosphine and 0.34 m~ of diethyl
azodicarboxylate were reacted in 10 m~ of anhydrous
tetrahydrofuran, whereby 1,3-dimethyl-6-{4-[3-(2-
benzoyl-4-nitrophenoxy)propyl]piperazin-1-yl}-
2,4(1H,3H)-pyrimidinedione was obtained as a syrup-like
matter (0.7 g).
Analytical results of the pyrimidine derivative thus
obtained:
2C~)1389
- 178 -
NMR (CDC13), ~ ppm: 8.36(m,2H),
7.0-7.8(m,6H),
5.14(s,1H),
4.14(t,2H),
3.26(s,3H),
3.34(s,3H),
3.0 (m,4H),
2.2-2.5(m,6H),
l.9(m,2H).
Further, the pyrimidinedione derivative was
treated in an oxalic acid/methanol solution by a method
known E~ se in the art to obtain 0.71 g of 1,3-
dimethyl-6-{4-[3-(2-benzoyl-4-nitrophenoxy)propyl]-
piperazin-l-yl}-2,4(lH,3H)-pyrimidinedione oxalate
(Compound 80).
Analytical results of the pyrimidinedione derivative
(Compound 80) thus obtained:
Melting point: 200-202~C (decomposed).
IR vmax (cm 1): 3450, 2910, 1690, 1650,
1510, 1330, 1280, 1150,
1080, 760, 700.
Elemental analysis for C26H29N5O6-~(COOH)2-~H2O:
Calculated (%): C, 57.85; H, 5.39; N, 12.49.
Found (%): C, 57.71; H, 5.54; N, 12.24.
Example 53:
Preparation of Compounds 87-92:
~ ~i13
- 179 -
By a procedure similar to Example 52-(1) except
for the use of Compounds 81-86 of the following
structural formula (1) - in which Xl means the groups
set out below respectively - in place of 2-chloro-5-
nitrobenzophenone, phenol derivatives of the below-
described structural formula (I') in which Xl varies
respectively were obtained as Compounds 81'-86'.
O2N ~ Cl (I)
~7 X
O2N ~ OH (I')
xl
Compound No. Xl
81, 81' -CO ~ Br
82, 82' -CO ~ NH
83, 83' -CO~
N
84, 84' -CO
85, 85' -CO ~
86, 86' -CO ~ N
2~ 389
- 180 -
Analytical results of Compound 81' thus obtained:
NMR (CDC13), ~ ppm: 8.38(m,2H),
7.70(m,4H),
7.41(m,1H),
Elemental analysis for C13H8NO4Br:
Calculated (%): C, 48.47; H, 2.50; N, 4.35;
Br, 24.81.
Found (%): C, 48.52; H, 2.31; N, 4.45;
Br, 24.42.
Analytical results of Compound 83' thus obtained:
IR vmax (cm 1): 1690, 1560, 1400, 1310,
770, 750.
Analytical results of Compound 84' thus obtained:
IR vmax (cm 1): 1660, 1520, 1340, 1270,
1040, 730.
By a procedure similar to Example 52-(2) except
that the phenol derivatives, i.e, Compounds 81'-86'
were used respectively instead of 2-hydroxy-5-nitro-
benzophenone, pyrimidinedione derivative oxalates
(Compounds 87-92) having the following physical
properties and the following structural formula (II) in
which Xl means the below-described groups respective-
ly were obtained.
O2N ~ lH2CH2CH2N~_~N ~ ~N_cH3 (II)
CH3 ~(COOH)2
2~389
- 181 -
Analytical results of Compound 87 (in which Xl has
the same meaning as in Compound 81):
Melting point: 134-135~C (Decomposed).
IR vmax (cm 1): 2900, 1710, 1640, 1530,
1340, 840, 790.
Elemental analysis for C26H28Ns~6Br-(C~~H)2-3H2~
Calculated (%): C, 46.04; H, 4.97; N, 9.59;
Br, 10.94.
Found (%): C, 46.26; H, 5.16; N, 9.92;
Br, 10.53.
Analytical results of Compound 88 (in which Xl has
the same meaning as in Compound 82):
Melting point: 171-174~C (Decomposed).
IR vmax (cm 1): 3450, 1710, 1640, 1550,
1440, 1350, 1200, 760.
Elemental analysis for C23H27N7O6-2(COOH)2:
Calculated (%): C, 47.86; H, 4.61; N, 14.47;
Found (%): C, 47.38; H, 5.17; N, 14.40.
Analytical results of Compound 89 (in which Xl has
the same meaning as in Compound 83):
Melting point: 149-151~C (Decomposed).
IR vmax (cm 1): 2550, 1700, 1660, 1520,
1340, 850, 800.
e ent n ys s 24H27N7~6 2(COo )2 2 2~
Calculated (%): C, 45.22; H, S.02; N, 13.19;
Found (%): C, 45.60; H, 5.18; N, 13.59.
2~1389
- 182 -
Analytical results of Compound 90 (in which Xl has
the same meaning as in Compound 84):
Melting point: 177-178~C (Decomposed).
IR vmax (cm 1): 2950, 1720, 1650, 1520,
51320, 1260, 860, 760.
Element 1 na ys s 25H28N6~6 2(C )2 2
Calculated (%): C, 48.07; H, 5.01; N, 11.60.
Found (%): C, 48.02; H, 5.05; N, 11.31.
Analytical results of Compound 91 (in which Xl has
the same meaning as in Compound 85):
Melting point: 104-107~C (Decomposed).
IR vKBr (cm~l~ 2700, 1690, 1630, 1540,
max
1340, 1280, 800.
Y 25 28 6 6 ( )2 2
Calculated (%): C, 46.90; H, 5.16; N, 11.32.
Found (%): C, 46.88; H, 5.42; N, 11.38.
Analytical results of Compound 92 (in which Xl has
the same meaning as in Compound 86):
Melting point: Amorphous.
IR vmax (cm 1): 1740, 1690, 1600, 1550,
1350, 1260, 780, 700.
Elemental analysis for C25H28N6O6 ( 2 2
Calculated (%): C, 46.29; H, 4.85; N, 11.89.
Found (%): C, 48.85; H, 4.97; N, 11.44.
Example 54:
Preparation of 1,3-dimethyl-6-{4-[2-(2-acetyl-
-_ - 183 - 2 n ~ 1 ~ 8 9
4-nitrophenoxy)ethyl]piperazin-1-yl}-2,4(1H,3H)-
pyrimidinedione oxalate (Compound 93):
o
HOCH2CH2N NH+ Cl ~N-CH3
N o
CH3
) HOCHzCH2N N ~ ~-CHs
I N
CH3
02N~ COCH3
(COOH)2/CH3OH
3 / O
CH3 ~(COOH)2
(Compound 93)
(1) Preparation of 1~3-dimethyl-6-t4-(2-hydroxyethyl)
( piperazin-l-yl]-2,4(1H,3H)-pyrimidinedione:
2.7 g of 6-chloro-1,3-dimethyl-2,4(lH,3H)-
pyrimidinedione, 4 ml of 1-(2-hydroxyethyl)pipera2ine
and 12 ml of triethylamine were dissolved in 70 ml of
isopropanol. The resultant solution was heated under
reflux for 3 hours.
After completion of the heating, the reaction
mixture was allowed to cool down, the solvent was
removed under reduced pressure from the reaction
.,,
~,...
2(~:)1389
- 184 -
mixture, and the residue was dissolved in 60 m~ of
chloroform. The chloroform solution thus obtained was
washed with water, dried over anhydrous sodium sulfate,
and then concentrated to dryness under reduced pressure
to obtain the reaction product in a crude form.
Next, a mixed solvent of ethanol and ethyl ether
was added to the crude reaction product. Precipitated
crystals were collected by filtration. Those crystals
were then recrystallized from hexane/ethanol to obtain
3.72 g of 1,3-dimethyl-6-[4-(2-hydroxyethyl)piperazin-
l-yl]-2,4(lH,3H)-pyrimidinedione.
Analytical results of the pyrimidinedione derivative
thus obtained:
NMR (CDC13), ~ ppm: 5.21(s,1H),
3.69~t,2H),
3.35(s,3H),
3.26(s,3H),
2.5-3.1(m,10H).
(2) Preparation of 1,3-dimethyl-6-{4-t2-(2-acetyl-4-
nitrophenoxy)ethyl]piperazin-1-yl}-2,4(1H,3H)-
pyrimidinedione oxalate (Compound 93):
0.5 g of the pyrimidinedione derivative obtained
by the above procedure (1), 0.65 g of 2-hydroxy-5-
nitroacetophenone, 0.6 g of triphenylphosphine and
2~1389
- 185 -
0.36 m~ of diethyl azodicarboxylate were reacted in a
similar manner to Example 48-(2) to obtain 0.65 g of
1,3-dimethyl-6-{4-[2-(2-acetyl-4-nitrophenoxy)ethyl]-
piperazin-l-yl}-2,4(1H,3H)-pyrimidinedione.
Analytical results of the pyrimidinedione derivative
thus obtained:
NMR (CDC13), ~ ppm: 8.2-8.6(m,2H),
7.08(m,1H),
5.26(s,1H),
4.35(m,2H),
3.42(s,3H),
3.34(s,3H),
2.72(s,3H),
2.7-3.2(m,10H).
Next, the pyrimidinedione derivative was treated
in an oxalic acid/methanol solution by a method
known Per se in the art to obtain 0.68 g of 1,3-
dimethyl-6- {4-[2-(2-acetyl-4-nitrophenoxy)ethyl]-
piperazin-l-yl}-2,4(lH,3H)-pyrimidinedione oxalate
(Compound 93):
Analytical results of the pyrimidinedione derivative
(Compound 93) thus obtained:
Melting point: 193-195~C (Decomposed~.
IR vKar (cm 1): 3290, 2650, 1720, 1640,
1520, 1340, 1270, 1120,
2~1389
- 186 -
830, 760, 7~0.
Elemental analysis for C20H25N5O6 (C 2 2
Calculated (%): C, 48.98; H, 5.42; N, 12.98.
Found (%): C, 49.02; H, 5.22; N, 12.90.
Example 55:
Preparation of Compounds 99-103:
Using phenol derivatives (Compounds 94-98)
having the below-described structural formula (III) in
which R7, R8 and R9 mean the below-described groups
respectively, pyrimidinedione derivative oxalates
(Compounds 99-103) having the physical properties set
out below and the below-described structural formula
(IV) in which R7, R8 and R9 are different from one
compound to another were obtained respectively in a
similar manner to Example 54-(2).
R7 ~ OH (III)
R8 R9
2~1389
- 187 -
R8 R9 R7
Compound 94 -H -CO ~ -NO2
A
Compound 95 -H -CO ~ Br -NO2
Compound 96-COCH3 -H -NO2
Compound 97 -H -No2 -COCH3
Compound 98 -H -NO2 -CO
~ 2 2 ~ 3
R R CH3 ~ (COOH)2
(IV)
Compound 99 (R7, R8 and R9 are as defined with
respect to Compound 94):
Melting point: 160-162~C (decomposed).
IR vmBax (cm 1): 2530, 1700, 1640, 1520,
1340, 760, 700.
Elemental analysis for C25H27N5O6 ( 2 2
Calculated (%): C, 52.34; H, 5.37; N, 11.30.
Found (%): C, 52.14; H, 5.18; N, 11.42.
- 2~ 89
Compound 100 (R7, R8 and R9 are as defined with
respect to Compound 95):
Melting point: 136-138~C (decomposed).
IR vKBr (cm-l) 2600, 1700, 1600, 1530,
max
1340, 800, 700.
Elemental analysis for C25H26BrN5O6 (C 2 2
Calculated (%): C, 46.43; H, 4.62; N, 10.03;
Br, 11.44.
Found (%): C, 46.64; H, 4.43; N, 10.30;
Br, 11.25.
Compound 101 (R7, R8 and R9 are as defined with
respect to Compound 96):
Melting point: 110~C (amorphous).
IR VKBr (cm-l~ 2990, 2550, 1700, 1630,
max
1520, 1320, 1180, 800.
Y 20 25N5~6 (COOH)2 3H2O
Calculated (%): C, 45.91; H, 5.78; N, 12.17.
Found (%): C, 45.76; H, 5.54; N, 12.07.
Compound 102 (R7, R8 and R9 are as defined with
respect to Compound 97):
Melting point: 139-141~C (decomposed).
IR vKBr (Cm-l~ 3300, 2950, 1700, 1640,
max
1530, 1360, 1270, 800, 760.
Elemental analysis for C20H25N5~6-(COOH)2-H2~
Calculated (%): C, 48.98; H, 5.42; N, 12.98.
Found (%): C, 49.08; H, 5.97; N, 12.97.
2C~01389
- 189 -
Compound 103 (R7, R8 and R9 are as defined with
respect to Compound 98):
Melting point: 133-135~C (decomposed).
IR vKax (cm 1): 3000, 2550, 1740, 1630,
51540, 1340, 1280, 760,
700.
Elemental analysis for C25H27N5O6 ( 2 2
Calculated (~): C, 53.91; H, 5.19; N, 11.64.
Found (%): C, 53.66; H, 4.96; N, 11.86.
Example 56:
Preparation of 1,3-dimethyl-6-{4-<3-t2-(2-
hydroxybenzoyl)-4-nitrophenoxy]propyl>piperazin-
l-yl}-2,4(lH,3H)-pyrimidinedione oxalate
(Compound 104):
~5 O
O2N ~ O-(CH2)3N N ~ -CH3
CH3 ~ (COOH)2
OH
(Compound 104)
(1) Preparation of 2,2'-dihydroxy-5-nitrobenzophenone
(Compound 104'):
~o
~ OH
(Compound 104')
389
-- 190 --
A reaction mixture, which had been obtained by
suspending 3 g of 2,2'-dichloro-5-nitrobenzophenone and
1.1 g of potassium hydroxide in 30 m~ of water, was
heated under stirring at 150~C for S hours in an
autoclave.
After allowing the reaction mixture to cool
down, 30 m~ of water were added to the reaction
mixture. Hydrochloric acid was then added to acidify
the reaction mixture, and precipitated crystals were
collected by filtration.
Those crystals were dried under reduced pressure
to obtain 1.9 g of 2,2'-dihydroxy-S-nitrobenzophenone
(Compound 104'). Those crystals were used in the
following reactions without any further purification.
(2) Preparation of 1,3-dimethyl-6-t4-<3-C2-(2-hydroxy-
benzoyl)-4-nitrophenoxy]propyl>piperazin-1-yl}-
2,4(lH,3H)-pyrimidinedione oxalate (Compound 104):
In a similar manner to Example 48-(2), 0.7S g of
2,2'-dihydroxy-S-nitrobenzophenone obtained in the
above procedure (1), O.S g of 1,3-dimethyl-6-[4-(3-
hydroxypropyl)piperazin-l-yl]-2,4(lH,3H)-pyrimidine-
dione, O.S8 g of triphenylphosphine and 0.34 m~ of
diethyl azodicarboxylate were reacted in 10 m~ of
anhydrous tetrahydrofuran, and the reaction mixture was
treated to obtain 0.6 g of 1,3-dimethyl-6-{4-<3-[2-(2-
hydroxybenzoyl)-4-nitrophenoxy]propyl>piperazin-1-yl}-
Z6~,389
2,4(lH,3H)-pyrimidinedione.
Next, the pyrimidinedione derivative was treated
in an oxalic acid/methanol solution by a method known
E~ se in the art to obtain O.6 g of 1,3-dimethyl-6-
{4-<3-[2-(2-hydroxybenzoyl~-4-nitro-phenoxy]propyl>-
piperazin-l-yl}-2,4tlH,3H)-pyrimidinedione oxalate
(Compound 104):
Analytical results of the pyrimidinedione derivative
(Compound 104) thus obtained:
Melting point: 144-146~C (decomposed).
IR vmBr (cm~l): 3550, 2900, 2550, 1710,
1640, 1530, 1340, 840,
730, 700.
Y 2 6H29N5~7 2 ( COOH ) 2 H20
Calculated (%): C, 49.93; H, 4.89; N, 9.73.
Found (%): C, 49.83; H, 5.07; N, 9.43.
Example 57:
Preparation of 1,3-dimethyl-6-{4-[3-[2-(2-
chlorobenzoyl)-4-nitrophenoxy]propyl]piperazin-
1-yl}-2,4(lH,3H)-pyrimidinedione oxalate
(Compound 105):
~ OCH3 ~ Cl OCH3
02N COCl > 02N ~ CO-
C1
- 192 - ~ 3 8 9
,~,, ~~
) 02N ~ CO
Sr~ ~
HOCH2CH2CH2N N ~ N-CH3
'J N~o (COOH)z/CH30H
CH3
~0
2 ~ ~CH2cH2cH2N N ~ -C~3
lOCO ~ CH3 ~ (C~~H)2
(Compound 105)
(l) Preparation of 3-(2-chlorobenzoyl)-4-methoxynitro-
benzene:
4.0 g of 2-methoxy-5-nitrobenzoyl chloride were
dissolved in 100 ml of anhydrous tetrahydrofuran,
followed by the addition of 2.72 g of 4-(N,N-dimethyl-
amino)pyridine at room temperature. The resultant
mixture was then vigorously stirred for 1 hour.
Added dropwise to the reaction mixture at -10~C
after completion of the stirring were 28 ml of a
Grignard reagent which had been prepared from 3.82 g of
2-chlorobromobenzene and 0.48 g of metal magnesium in
ethyl ether.
After completion of the dropwise addition, the
temperature of the reaction mixture was allowed to
B
- 193 -
gradually rise to room temperature over 3 hours. Water
was then added to the reaction mixture, followed by
concentration to dryness. Next, the residue was
dissolved in 50 mQ of chloroform and the solution thus
obtained was washed with water. The solution was
thereafter washed successively with 50 mQ of a 1 N
aqueous sodium hydroxide solùtion, 50 mQ of 1 N
hydrochloric acid and a saturated NaCl solution.
The thus-washed solution was dried over
anhydrous sodium sulfate. The solvent was then
distilled off, and the residue was was purified by
chromatography on a silica gel column (eluent:
chloroform/hexane = 1/50-1/20, by volume) to obtain
1.55 g of 3-(2-chlorobenzoyl)-4-methoxynitrobenzene.
Analytical results of the compound thus obtained:
NMR (CDC13), ~ ppm: 3.80(s,2H),
6.92-7.75(m,5H),
8.28-8.69(m,2H).
(2) Preparation of 3-(2-chlorobenzoyl)-4-hydroxynitro-
benzene:
1.55 g of 3-(2-chlorobenzoyl)-4-methoxynitro-
benzene obtained in the above procedure (1) were
dissolved in 30 mQ of chloroform, followed by the
addition of 5.31 g of iodotrimethylsilane. The
thus-obtained solution was then heated under reflux and
stirring for 2 hours. The reaction mixture was allowed
2~01389
- 194 -
to cool down, washed with 30 m~ of water, and then
extracted twice with a 1 N aqueous sodium hydroxide
solution.
Hydrochloric acid was added to the alkaline
layer obtained by the above extraction, followed by
extraction with chloroform. The organic layer obtained
by the chloroform extraction was washed with water and
dried over anhydrous sodium sulfate. The solvent was
thereafter distilled off from the organic layer,
thereby obtaining 0.5 g of 3-(2-chlorobenzoyl)-4-
hydroxynitrobenzene in the form of a pale yellow oil.
Analytical results of the pale-yellowish oily compound
thus obtained:
NMR (CDC13), ~ ppm: 7.33(d,1H,J=lO,OHz)
7.46-7.83(m,5H),
8.35(d,1H,J=2.5Hz),
8.50(dd,1H,J=2.5,10.0Hz).
(3) Preparation of 1,3-dimethyl-6-{4-<3-[2-(2-chloro-
benzoyl)-4-nitrophenoxy]propyl>piperazin-1-yl}-
2,4(1H,3H)-pyrimidinedione oxalate (Compound 105):
By a similar procedure to Example 4B-(2) except
for the use of 3-(2-chlorobenzoyl)-4-hydroxynitro-
benzene obtained by the above procedure (2) in place of
2-hydroxy-S-nitroacetophenone, 0.5 g of 1,3-dimethyl-6-
{4-<3-[2-(2-chlorobenzoyl)-4-nitrophenoxy]propyl>-
2~)1389
- 195 -
piperazin-l-yl}-2,4(1H,3H)-pyrimidinedione oxalate
(Compound 105) was obtained as white crystals.
Analytical results of crystals of Compound 105 thus
obtained:
Melting point: Amorphous.
IR vKax (cm 1): 1685, 1650, 1602, 1425,
1335, 1285, 1080,
Y 2 6H2 8ClN506 ( COOH ) 2 H20
Calculated (%): C, 51.74; H, 4.96; N, 10.77;
Cl, 5.45.
Found (%): C, 51.50; H, 5.11; N, 10.97;
Cl, 5.71.
Example 58:
Preparation of 1,3-dimethyl-6-{4-<3-[4-nitro-2-
(2-pyridinecarbonyl)phenylthio]propyl>piperazin-
l-yl}-2,4(lH,3H)-pyrimidinedione oxalate
(Compound 106):
0 2N~Cl OzN /~SCH2CHzCHzCl
~ 0 HSCHzCHzCHzCl ~ 0
'
HN N~N-CH3
- '' N~o
CH3 (COOH) z/Cl~H
2~0~389
- 196 -
O N~SCH2cH2cH2N\ - N~-cH3
~ N CH ~ (COOH)2
(Compound 106)
(1) Preparation of l-chloro-3-[2-(2-pyridinecarbonyl)-
4-nitrophenylthio]propane:
1.58 g of 4-chloro-3-(2-pyridinecarbonyl)nitro-
benzene, 0.66 g of 3-chloro-l-propanethiol and 2 m~ of
triethylamine were dissolved in 5 ml of dimethyl-
sulfoxide. The resultant solution was heated under
stirring at 80~C for 3 hours.
After completion of the heating, the reaction
mixture was concentrated and the concentrate thus
obtained was dissolved in chloroform. The chloroform
solution was washed with water and then dried over
anhydrous sodium sulfate.
Next, the solvent was distilled off from the
thus-dried solution. The residue was purified by
chromatography on a silica gel column (eluent:
chloroform) and then crystallized from a mixed solvent
of hexane and ether to obtain 1.0 g of 1-chloro-3-t2-
(2-pyridinecarbonyl)-4-nitrophenylthio]propane.
Analytical results of crystals of the compound thus
obtained:
Elemental analysis for C15Hl3ClN2O3S:
2001389
- 197 -
Calculated (%): C, 53.49; H, 3.89; N, 8.32,
S, 9.52; Cl, 10.53.
Found (%): C, 53.29; H, 3.79; N, 8.25;
S, 9.35; Cl, 10.37.
(2) Preparation of 1,3-dimethyl-6-{4-<3-t4-nitro-2-
(2-pyridinecarbonyl)phenylthio]propyl>piperazin-
l-yl}-2,4(lH,3H)-pyrimidinedione oxalate
(Compound 10~):
0.93 g of 1-chloro-3-[2-(2-pyridinecarbonyl)-4-
nitrophenylthio]propane, 0.62 g of 1,3-dimethyl-6-
(piperazin-l-yl)-2,4(lH,3H)-pyrimidinedione and 2 m~
of triethylamine were dissolved in 10 ml of dimethyl
sulfoxide, followed by heating under stirring at 120~C
for 4 hours.
After completion of the heating, the reaction
mixture was allowed to cool down, the solvent was
distilled off under reduced pressure from the reaction
mixture, and the residue was dissolved in chloroform.
The thus-obtained solution was washed with water and
then dried over anhydrous sodium sulfate, followed by
purification by chromatography on a silica gel column
(eluent: chloroform/methanol = 50/1, by volumw) to
obtain 0.69 g of 1,3-dimethyl-6-{4-<3-t4-nitro-2-(2-
pyridinecarbonyl)phenylthio~propyl>piperazin-l-yl}-
2,4(1H,3H)-pyrimidinedione in a yellow solid form.
2C~)1389
- 198 -
Analytical results of the pyrimidinedione derivative
thus obtained:
Melting point: 182~C (decomposed).
NMR (CDC13), ~ ppm: 1.89(m,2H), 2.56(m,6H),
3.01(m,4H), 3.36(s,3H),
3.42(s,3H), 3.46(m,2H),
5.28(s,lH),
7.5-8.9(m,7H).
Elemental analysis for C25H28N6O5S-~H2O:
Calculated (%): C, 56.27; H, 5.48; N, 15.75;
Cl, 6.01.
Found (%): C, 56.37; H, 5.36; N, 15.55;
Cl, 5.79.
The pyrimidinedione derivative obtained in the
yellow solid form was treated in an oxalic acid/
methanol solution by a method known E~ se in the
art to obtain 0.62 g of 1,3-dimethyl-6-{4-<3-t4-nitro-
2-(2-pyridinecarbonyl)phenylthio]propyl>piperazin-1-
yl}-2,4(lH,3H)-pyrimidinedione oxalate (Compound 106).
Analytical results of Compound 106 thus obtained:
Melting point: 138~C (decomposed).
IR vKBr (cm~l) 3460, 1693, 1648, 1600,
max
1454, 1435, 1346, 722,
500.
Elemental analysis for C25H28N6O5-(COOH)2 ~H2O
Calculated (%): C, 52.00; H, 5.01; N, 13.48;
2~389
-- 199 --
S, 5.14.
Found (%): C, 51.81; H, 4.86; N, 13.24;
S, 5.06.
Example 59:
Preparation of 3-methyl-6-{4-[3-(4-nitro-2-
~enzoylphenoxy)propyl]piperazin-l-yl}-
2,4(lH,3H)-pyrimidinedione oxalate
(Compound 107):
OzN ~ ocHzcHzcH2-Br HN NH
OzN ~ OCH2CHzCHzN NH
0
o
Cl ~ N-CH3
N~o (COOH)2/CH30H
,
~ 2 N ~O - CH 2 CH 2 CH - N/--\N ~N - CH
CO H/ O
~ ~ ( COOH ) 2
( Compound 10 7 )
2C~1389
- 200 -
(1) Preparation of 1-[3-(2-benzoyl-4-nitrophenoxy)-
propyl]piperazine:
3.5 g of 3-benzoyl-4-(3-bromopropyloxy)nitro-
benzene and 7.8 g of piperazine were dissolved in
5 30 m~ of chloroform. The resultant solution was
heated under reflux for 4 hours.
The resultant reaction mixture was allowed to
cool down, washed with water, and dried over anhydrous
sodium sulfate. The solvent was thereafter distilled
off from the reaction mixture and the residue was
crystallized from hexane/ethanol to obtain 3.5 g of
1-[3-(2-benzoyl-4-nitrophenoxy)propyl]piperazine.
(2) Preparation of 3-methyl-6-{4-[3-(4-nitro-2-benzoyl-
phenoxy)propyl]piperazin-l-yl}-2,4(lH,3H)-pyrimidine-
dione oxalate (Compound 107):
1 g of 1-[3-(2-benzoyl-4-nitrophenoxy)propyl]-
piperazine, 0.34 g of 6-chloro-3-methyl-2,4(lH,3H)-
pyrimidinedione and 0.72 m~ of triethylamine were
dissolved in 3 m~ of 2-propanol, followed by heating
under reflux for 6 hours.
Precipitated crystals were collected by
filtration from the reaction mixture and then
recrystallized from isopropanol to obtain 1.9 g of
3-methyl-6-{4-[3-(4-nitro-2-benzoylphenoxy)propyl]-
piperazin-1-yl}-2,4(1H,3H)-pyrimidinedione.
2~1389
- - 201 -
Analytical results of the pyrimidinedione derivative
thus obtained:
NMR (CDC13), ~ ppm: 8.41(m, 2H),
7.1-7.9(m,6H),
4.77(s,1H), 4.13(m,2H),
3.15(s,3H),
2.0-2.4(m,10H),
1.74(m,2H).
The pyrimidinedione derivative was next treated
in an oxalic acid/methanol solution by a method known
per se in the art to obtain 0.67 g of 3-methyl-6-{4-t3-
(4-nitro-2-benzoylphenoxy)propyl]piperazin-1-yl}-
2,4(1H,3H)-pyrimidinedione oxalate (Compound 107).
Analytical results of Compound 107 thus obtained:
Melting point: 144-146~C (decomposed).
IR vmBax (cm 1): 3360, 2610, 1710, 1660,
1610, 1520, 1350, 1290,
1100, 750, 700.
Y 2 5 H2 7Ns~6 ( COOH ) 2 2 H20:
Calculated (%): C, 52.34; H, 5.37; N, 11.30.
Found (%): C, 51.89; H, 5.04; N, 11.23.
Example 60:
Preparation of 1,3-dimethyl-6-{2-t3-(2-benzoyl-
4-nitrophenoxy)propylamino]ethylamino}-
2,4(lH,3H)-pyrimidinedione oxalate
(Compound 108):
2~1389
-- - 202 -
02N- ~ OH BrCH2CHzCH2Br OzN ~ OCH2CHzCH2-Br
~=0 > ~0
¢I~N - C H 3
NH2CH2CH2NH N~ tCOOH) 2/C~)H
CH3
o
O2N ~ O-cH2cH2cH2NHcH2cH2NH ~ -CH
(COOH)2
(Compound 108)
(1) Preparation of 3-(2-benzoyl-4-nitrophenoxy)propyl
bromide:
1.5 g of 2-hydroxy-5-nitrobenzophenone, 6 ml of
1,3-dibromopropane and 3 g of anhydrous potassium
carbonate were heated and reacted under reflux for 6
hours in 10 m~ of 2-butanone.
After completion of the reaction, the reaction
mixture was allowed to cool down and unnecessary
matters were filtered off. The filtrate was
concentrated to obtain a syrup, which was purified by
chromatography on a silica gel column (eluent:
hexane/ethyl acetate = 4/1, by volume) and then
crystallized form hexane to obtain 1.0 g of 3-(2-
-
2001389
- 203 -
benzoyl-4-nitrophenoxy)propyl bromide.
Analytical results of the propyl bromide derivative
thus obtained:
Melting point: 86~C.
(2) Preparation of 1,3-dimethyl-6-{2-[3-(2-benzoyl-4-
nitrophenoxy)propylamino]ethylamino}-2,4(1H,3H)-pyrimidi
nedione oxalate (Compound 108):
0.77 g of the propyl bromide derivative obtained
in the above procedure (1), 0.5 g of 1,3-dimethyl-6-
(2-aminoethylamino)-2,4(lH,3H)-pyrimidinedione and
0.7 m~ of triethylamine were reacted at 80~C for 4
hours in 10 ml of dimethylformamide. After distilling
off the solvent from the resultant reaction mixture
under reduced pressure, chloroform was added to the
residue, and the solution thus formed was washed with
water, dried and then concentrated to obtain a syrup.
The syrup was purified by chromatography on a
silica gel column (eluent: chloroform/methanol = 40/1,
by volume~ to obtain 0.51 g of 1,3-dimethyl-6-{2-[3-(2-
benzoyl-4-nitrophenoxy)propylamino]ethylamino}-
2,4(lH,3H)-pyrimidinedione.
Analytical results of the pyrimidinedione derivative
thus obtained:
NMR (CDC13), ~ ppm: 7.1-8.1(m,8H),
5.14(s,1H), 4.33(t,2H),
3.22(s,3H), 3.36(s,3H),
- 204 -
2 ~ ~ ~ 3 8 9
2.7-3.2tm,6H),
2.1(m,2H).
The pyrimidinedione derivative was next treated
in an oxalic acid/methanol solution by a method known
Per se in the art to obtain 0.43 g of 1,3-dimethyl-6-
{2-[3-(2-benzoyl-4-nitrophenoxy)propylamino]ethyl-
amino}-2,4(lH,3H)-pyrimidinedione oxalate (Compound
108).
Analytical results of Compound 108 thus obtained:
Melting point: 111-113~C (decomposed).
IR ~max (cm 1): 3050, 1730, 1700, 1640,
1520, 1360, 770, 700.
Elemental analysis for C24H27N5O6-(COOH)2 3H2O
Calculated (%): C, 49.92; H, 5.69; N, 11.20.
Found (%): C, 50.11; H, 5.35; N, 11.00.
Example 61:
Preparation of 1,3-dimethyl-6-{2-[N-ethyl-3-(4-
benzoyl-2-nitrophenoxy)propylamino]ethylamino}-
2,4(lH,3H)-pyrimidinedione oxalate
(Compound 109):
< ~ C ~ OH BrCHzCHzCHzBr
N02
~ -CO ~ OCH2CH2CHzBr cH~cu2~2
N02
r~
2001389
- 205 -
CO~ OCHZcH2cH2NHcHzcH3
NO2
CN~¢~NO (COOH)2/CH30H
3 ~ ~
O CH2CH3 - o
<~C~O-CH2CH2CH2NcH2cH2NH~_cH3
NO2 CH3 ~
~ (COOH)2
(Compound 109)
(1) Preparation of 5-benzoyl-2-(3-bromopropyloxy)-
nitrobenzene:
1.5 g of 4-hydroxy-3-nitrobenzophenone and 1 g
of potassium carbonate were added to 5 ml of methyl
ethyl ketone. The resultant mixture was heated under
stirring for 30 minutes, followed by the addition of
2.0 mQ of dibromopropane. The thus-obtained mixture
was stirred for further 4 hours. After allowing the
reaction mixture to cool down, insoluble matters were
filtered off. The filtrate was then concentrated and
cooled to 0~C. Under those conditions, the reaction
mixture was added with 10 ml of hexane to conduct
sludging. The resultant precipitate of 5-benzoyl-2-
26~1389
- 206 -
(3-bromopropyloxy)nitrobenzene was collected by
filtration.
Analytical results of the precipitate:
NMR (CDC13), ~ ppm: 7.2-8.3(m,8H),
4.40(t,2H), 3.69(t,2H),
2.47(m,2H).
2.0 g of the precipitate of 5-benzoyl-2-(3-
bromopropyloxy)nitrobenzene were employed in the
subsequent reaction without purification.
(2) Preparation of 1,3-dimethyl-6-{2-[N-ethyl-3-(4-
benzoyl-2-nitrophenoxy)propylamino]ethylamino}-
2,4(lH,3H)-pyrimidinedione oxalate ~Compound 109):
1.8 g of the 5-benzoyl-2-(3-bromopropyloxy)-
nitrobenzene precipitate obtained in the above
procedure (1) were reacted with 10 m~ of ethylamine
(70 wt.% of solution in water) at 70~C for 1 hour in
an autoclave. Thereafter, excess ethylamine was
distilled off. The reaction mixture was added with 30
m~ of chloroform and then washed twice with water.
The water-washed chloroform layer was then
concentrated to obtain 2.1 g of 5-benzoyl-2-(3-ethyl-
aminopropyloxy)nitrobenzene as a roughly-purified oily
product.
1.0 g of the roughly-purified oily product,
0.4 g of 6-(1-aziridinyl)-1,3-dimethyl-2,4(1H,3H)-
pyrimidinedione (Compound ~) and 0.05 g of p-toluene-
2~,389
sulfonic acid were reacted at 80~C for 3 hours in a
solventless state. The reaction mixture was directly
subjected to chromatographic purification on a silica
gel column (eluent: CHC13/CH30H = 40/1, by volume),
thereby obtaining 0.61 g of 1,3-dimethyl-6-{2-[N-ethyl-
3-(4-benzoyl-2-nitrophenoxy)propylamino]ethylamino}-
2,4(lH,3H)-pyrimidinedione.
Analytical results of the pyrimidinedione derivative
thus obtained:
NMR (CDC13), ~ ppm: 7.4-8.2(m,8H),
4.99(s,1H), 4.16(t,2H),
3.36, 3.44(s,3H),
2.4-3.0(m,8H),
2.11(m,2H), l.lO(t,3H).
lS The pyrimidinedione derivative was next treated
in an oxalic acid/methanol solution by a method known
per se in the art to obtain O.Sg of 1,3-dimethyl-6-
{2-[N-ethyl-3-(4-benzoyl-2-nitrophenoxy)propylamino]-
ethylamino}-2,4(lH,3H)-pyrimidinedione oxalate
(Compound 109).
Analytical results of Compound 109 thus obtained:
IR vmax (cm 1): 3400, 1690, 1620, 1530,
1360, 1250, 720, 700.
Elemental analysis for C26H31N5O6 ( 2 2
Calculated (%): C, 52.91; H, 5.87; N, 11.02.
Found (%): C, 52.50; H, 5.77; N, 10.92.
2~1389
- 208 -
Example 62:
Preparation of 6-{4-[2-(2-benzoyl-4-nitrophenyl-
thio)ethyl]piperazin-l-yl}-1,3-dimethyl-
2,4(lH,3H)-pyrimidinedione oxalate
5(Compound 110):
02N- ~ Cl HSCH2CH20H 02N ~ -SCH2CH20H
O ~ \~0
0
HN N ~ ~-CH3
'~ N~j (COOH)2/CH30H
CH3
o
2 ~ C~2 2 ~ ~ ~ 3
~ (COOH)2
(Compound 110)
(1) Preparation of 2-(2-hydroxyethylthio)-5-nitrobenzo-
phenone:
2.0 g of 2-chloro-5-nitobenzophenone and 2.0 g
of triethylamine were dissolved in 10 m~ of dimethyl-
sulfoxide, followed by the addition of 0.63 g of
2-mercaptoethanol. The resultant mixture was heated
under stirring at 80~C for 5 hours. The reaction
mixture was poured into water and then extracted with
2~01389
- 209 -
100 m~ of chloroform. After washing the extract with
water and then drying it over anhydrous sodium sulfate,
the solvent was distilled off under reduced pressure to
obtain 2.0 g of 2-(2-hydroxyethylthio)-5-nitrobenzo-
phenone as pale yellow crystals.
Analytical results of the benzophenone derivative thus
obtained:
NMR (CDC13), ~ ppm: 8.16-8.50(m,2H),
7.23-8.06(m,6H),
3.86(t,2H), 3.21(t,2H),
2.63(br.s,lH).
(2) Preparation of 6-{4-[2-(2-benzoyl-4-nitrophenyl-
thio)ethyl]piperazin-l-yl}-1,3-dimethyl-2,4(lH,3H)-
pyrimidinedione oxalate (Compound 110):
2.0 g of 2-(2-hydroxyethylthio)-5-nitrobenzo-
phenone obtained in the above procedure (1) were
dissolved in 20 m~ of chloroform, ~ollowed by the
addition of 1.6 g of triethylamine. Further, 0.91 g of
methanesulfonyl chloride was added at 0~C.
After stirring the resultant mixture at 0~C for
10 minutes, it was stirred at room temperature for
further 16 hours. The reaction mixture thus obtained
was diluted with 80 mQ of chloroform and then poured
into water. The resulting mixture was allowed to
separate into layers. The organic layer was washed
successively with a 1 N aqueous sodium hydroxide
2C~)1389
- 210 -
solution, water and saturated NaCl solution. Next, the
organic layer thus washed was dried over anhydrous
sodium sulfate, and the solvent was distilled off under
reduced pressure to obtain 2.50 g of a mesylate as a
roughly-purified product.
To a solution of the mesylate in 15 ml of
dimethyl sulfoxide, 1.80 g of 1,3-dimethyl-6-(1-
piperazinyl)-2,4(lH,3H)-pyrimidinedione were added.
The resultant mixture was heated at 80~C for 6 hours.
The thus-obtained reaction mixture was poured into 100
m~ of water in which 2.0 g of potassium carbonate were
contained, and was then extracted twice with 50 m~
portions of chloroform. The extracts were combined
together, washed with water and then dried over
anhydrous sodium sulfate. Thereafter, the solvent was
distilled off under reduced pressure. The residue was
purified by chromatography on a silica gel column
(eluent: chloroform/methanol = 50/1 - 25/1, by volume),
thereby obtaining 2.0 g of 6-{4-t2-(2-benzoyl-4-
nitrophenylthio)ethyl]piperazin-l-yl}-1,3-dimethyl-
2,4(lH,3H)-pyrimidinedione as pale yellow crystals.
Analytical results of the pyrimidinedione derivative
thus obtained:
NMR (CDCl3), ~ ppm: 8.27-8.54(m,2H),
7.44-8.07(m,6H),
5.28(s,lH), 3.39(s,3H),
20~)1389
- - 211 -
3.35(s,3H),
2.44-3.27(m,12H).
The pyrimidinedione derivative was next treated
in an oxalic acid/methanol solution by a method known
per se in the art to obtain 6-{4-[2-(2-benzoyl-4-
nitrophenylthio)ethyl]piperazin-l-yl}-1,3-dimethyl-
2,4(1H,3H)-pyrimidinedione ~Compound 110) as white
crystals.
Analytical results of Compound 110 thus obtained:
Melting point: 201.0-202.5~C.
Elemental analysis for C25H27N5O4S-(COOH)2:
Calculated (%): C, 55.57; H, 5.01; N, 12.00;
S, 5.49.
Found (%): C, 55.51; H, 5.55; N, 12.16;
S, 5.38.
Example 63:
Production of tablets containing as an effective
ingredient 1,3-dimethyl-6-{4-t3-(4-benzoyl-2-
nitrophenoxy)propyl]piperazin-l-yl}-2,4-(lH,3H)-
pyrimidinedione oxalate (Compound 78) available
by the process of Example 50:
1 g of the pyrimidinedione derivative oxalate
(Compound 78), 123 g of lactose and 20 g of corn starch
were finely mixed. Using a solution of S g of
hydroxypropylcellulose in 100 m~ of water, the
resultant mixture was granulated. The resultant
z~ 389
- 212 -
particles were dried at 50~C for 4 hours and then
mixed thoroughly with 1 g of magnesium stearate. The
thus-prepared mixture was then compressed into tablets,
each containing 150 mg, by a tablet machine.
Example 64: -
Production of capsules containing as an
effective ingredient 1,3-dimethyl-6-{4-[3-(2-
benzoyl-4-nitrophenoxy)propyl]piperazin-1-yl}-
2,4-llH,3H)-pyrimidinedione oxalate (Compound
811 available by the process of Example 52:
5 g o~ the pyrimidinedione derivative oxalate
(Compound 81), 120 g of lactose and 25 g of corn starch
were finely mixed. The resulting mixture was filled
into hard capsules, each containing 150 mg, by a
capsule filling machine.
Example 65:
Production of injection containing as an
effective ingredient 1,3-dimethyl-6-{4-[3-(2-
chlorobenzoyl-4-nitrophenoxy]propyl]piperazin-1-
yl}-2,4-(lH,3H)-pyrimidinedione oxalate
(Compound 105) available by the process of
Example 57:
20 mg of the pyrimidinedione derivative oxalate
(Compound 105) and 0.85 g of sodium chloride were
26~C)1389
- 213 -
weighed. They were dissolved in distilled water for
injection to give a total volume of 100 m~, thereby
preparing a formulation suitable for injection.
Pharmacological Test 4:
Similarly to Pharmacological Test 1, the ADP75
and ERP of each of the compounds shown in Table 8 and
obtained in the corresponding examples described above
were determined. The results are summarized in Table
8.
Table 8
Result of Pharmacological Test
75 (~) ERP (~)
Compound Dose (~g/m~) Dose (mg/kg, i.v.)
No.
1.0 3.0 10.0 0.1 0.3 1.03.0
76 16.0 21.0 - 2.4 4.3 12.616.5
78 19.0 42.0 - 6.7 13.3 20 26.7
19.0 30.0 34.0 2.6 9.7 21.9
89 19.0 30.0 - 5.6 11.1 11.1
22.0 31.0 - 20.5 20.5 14.3
99 9.0 22.0 29.0 9.8 14.7 23.1
102 7 20.0 - - - - -
104 - 16.0 25.0
105 11.0 23.0 28.0 0 0 6.36.3
Toxicity Test 4:
Similarly to Toxicity Test 1, the toxicity of
each of the compounds shown in Table 9 and obtained in
Z~301;~89
- 214 -
the corresponding examples described above was tested
to determine the mortality rate of mice. The results
are summarized in Table 9.
Incidentally, the administration of each
compound was conducted orally (p.o.) at a dose of
300 mg/Kg.
Table 9
Compound No. Mortality rate (%)
77 0
78 0
0
87 0
100 o
103 0
105 o
Example 66:
Preparation of 1,3-dimethyl-6-{4-~3-(2-benzoyl-
4-nitroanilino)propyl]piperazin-1-yl}-
2,4(lH,3H)-pyrimidinedione oxalate
(Compound 111):
O O
25 1 ~ ~NK + BrCHzCH2CHz8r ~ ~ ~NCH2CH2CH2Br
O O
2~1389
- 215 -
HN N ~ N-CH3
N~o ~NCH2CHzCH2N~N ~Y-CH
O CH3
NH2CHzCH2CH2N N ~ ~-CH302N ~ CO
CH3
(COOH)2/CH30H
>
~o
O2N ~ N~CH2CH2CH2N N ~ N-CH3
CO CH3 (COOH)2
(Compound 111)
(1) Preparation of 1,3-dimethyl-6-{4-(3-aminopropyl)-
piperazin-l-yl}-2,4(lH,3H)-pyrimidinedione (Compound
158):
A suspension of 18.52 g of potassium phthalimide
and 200 g of 1,3-dibromopropane in 100 ml of
dimethylformamide was heated under stirring at 120~C
for 6 hours to react them. Insoluble matters were then
filtered off from the reaction mixture and the filtrate
was then concentrated to dryness under reduced
pressure. The residue was washed with hexane and then
2001~89
- - 216 -
recrystallized from ethanol-water. Resulting crystals
were collected by filtration, washed and then dried to
obtain 13.8 g of N-(3-bromopropyl)phthalimide.
A suspension which had been obtained by
suspending 13.0 g of the N-(3-bromopropyl)phthalimide,
10.3 g of 1,3-dimethyl-6-(1-piperazinyl)-2,4-(lH,3H)-
pyrimidinedione (Compound 157) and 20 g of triethyl-
amine in 200 m~ of dioxane was heated under reflux for
6 hours.
Further, insoluble matters were filtered off
from the reaction mixture and the filtrate was
concentrated to dryness under reduced pressure. The
residue (dry concentrate) was recrystallized from ethyl
acetate/n-hexane. Resultant crystals were collected by
filtration, washed and then dried, thereby obtaining
12.5 g of 1,3-dimethyl-6-t4-(3-phthaloylaminopropyl)-
piperazin-l-yl]-2,4(lH,3H)-pyrimidinedione.
Next, a suspension of 12.5 g of those crystals
and 6.0 g of hydrazine hydrate in 200 m~ of ethanol
was heated under reflux for 4 hours. After allowing
the suspension to cool down, resultant insoluble
matters were filtered off. The filtrate was
concentrated to dryness under reduced pressure. The
residue (dry concentrate) was then dissolved in water,
to which dilute hydrochloric acid was added to adjust
the pH to about 3. Insoluble matters formed by the pH
2~:;L38~3
- 217 -
adjustment were filtered off. After adding a large
amount of potassium carbonate to the filtrate, the
resultant mixture was extracted with chloroform.
Subsequent to the completion of the extraction, the
resulting organic layer was dried over anhydrous sodium
sulfate and then heated under reduced pressure to
distill off the solvent, whereby 6.80 g of 1,3-
dimethyl-6-[4-(3-aminopropyl)piperazin-1-yl]-
2,4(lH,3H)-pyrimidinedione (Compound 158) were obtained
as a colorless syrupy substance. The syrupy substance
was crystallized when allowed to stand overnight.
(2) Preparation of 1,3-dimethyl-6-t4-[3-(2-benzoyl-4-
nitroanilino)propyl~piperazin-l-yl}-2,4(lH,3H)-
pyrimidinedione oxalate (Compound 111):
0.9 g of 1,3-dimethyl-6-[4-(3-aminopropyl)-
piperazin-l-yl]-2,4(lH,3H)-pyrimidinedione and 1.0 g of
2-chloro-5-nitrobenzophenone were added to 10 m~ of
dimethyl sulfoxide. The resultant mixture was heated
at 110~C for 20 hours. After allowing the reaction
mixture to cool down, the reàction mixture was poured
into a saturated aqueous solution of sodium bicarbonate
and the resulting mixture was extracted with
chloroform.
An organic layer (chloroform layer) obtained by
the extraction was separated, washed with water, dried
over anhydrous sodium sulfate, and then concentrated to
-
2~389
- - 218 -
dryness. The residue was purified by chromatography on
a silica gel column (eluent: chloroform/methanol = 50/1
- 20/1, by volume), thereby obtaining 0.95 g of 1,3-
dimethyl-6-{4-t3-(2-benzoyl-4-nitroanilino)propyl]-
piperazin-1-yl}-2,4(lH,3H)-pyrimidinedione as a yellow
oily substance.
Analytical results of the oily substance of the
pyrimidinedione derivative thus obtained:
NMR (CDC13), ~ ppm: 2.04(m,2H), 2.65(m,6H),
3.04(m,4H), 3,36(s,3H),
3.42(s,3H), 3.57(t,2H),
5.30(s,1H), 6.90(d,1H),
7.69(s,5H),
8.35(dd,lH), 8.95(d,lH),
9.50(s,1H).
Further, the oily substance was treated in an
oxalic acid/methanol solution by a method known per
se in the art to obtain 1.0 g of 1,3- dimethyl-6-{4-
[3-(2-benzoyl-4-nitroanilino)propyl]-piperazin-1-yl}-
2,4(1H,3H)-pyrimidinedione oxalate (Compound 111).
Analytical results of Compound 111 thus obtained:
Melting point: Amorphous.
Elemental analysis for C26H30N6O5-(COOH)2:
Calculated (%): C, 56.37; H, 5.41; N, 14.09.
Found (%): C, 56.92; H, 5.40; N, 14.20.
Example 67:
8~
Preparation of Compounds 120-127:
sy a similar procedure to Example 66-(2) except
for the use Compounds 112-119 having the below-
described structural formula (I) - in which R7, R8 and
R9 mean the below-described groups respectively - in
place of 2-chloro-5-nitrobenzophenone, pyrimidinedione
derivative oxalates having the physical properties set
out below and the below-described structural formula
(II) in which R7, R8 and R9 are different from one
compound to another were obtained as Compounds 120-127,
respectively.
R8 ~ Cl (I)
R7 R9
2~1389
- 220 -
R9 R8 R7
Compound 112-COCH3 -No2 -H
Compound 113-CO ~ -NO2 -H
Compound 114-CO ~ -NO2 -H
Compound 115-CO ~ -NO2 -H
Compound 116-CO ~ N -NO2 -H
Compound 117 -No2 -COCH3 -H
Compound 118 -NO2 -CO ~ -H
Compound 119 -H -NO2 -COCH3
R8 ~ NHCH2CH2CH2N N ~ N-CH3 (II)
R7 R9 C/H3 .(COOH)2
-
~ 2~1389
- 221 -
Compound 120 (R7, R8 and R9 are as defined with
respect to Compound 112):
Melting point: 135-137~C (decomposed).
IR ~KBr (cm-l) 3300, 2550, 1720, 1630,
max
1560, 1320, 1250, 800,
710.
enta n lysis for C21H28N6O5 (COOH)2 2H2~
Calculated (~): C, 48.42; H, 6.01; N, 14.73.
Found (%): C, 48.14; H, 5.82; N, 14.32.
Compound 121 (R7, R8 and R9 are as defined with
respect to Compound 113):
Melting point: 182-183~C (decomposed).
IR vmax (cm 1): 3350, 2550, 1690, 1630,
1540, 1350, 1290, 1100,
810, 770.
Elemental analysis for C25H34N6O5 ( 2 2
Calculated (%): C, 50.46; H, 6.59; N, 13.08.
Found (%): C, 51.04; H, 6.77; N, 12.90.
Compound 122 (R7, R8 and R9 are as defined with
respect to Compound 114):
Melting point: Amorphous.
Y 26 29N6O5Cl (COOH)2 ~H2O
Calculated (%): C, 52.54; H, 5.04; N, 13.13;
Cl, 5.54.
Found (%): C, 52.36; H, 5.05; N, 13.14;
Cl, 5.23.
2~01389
- 222 -
Compound 123 (R7, R8 and R9 are as defined with
respect to Compound 115):
Melting point: 151~C (decomposed).
IR vmax (cm 1): 3440, 1706, 1645, 1588,
1496, 1441, 1333, 1200,
768, 722, 500.
Elemental analysis for C25H29N7O5~ COOH)2-H2O:
Calculated (%): C, 50.91; H, 5.19; N, 14.84.
Found (%): C, 51.12; H, 5.10; N, 14.81.
Compound 124 (R7, R8 and R9 are as defined with
respect to Compound 116):
Melting point: 162-165~C (decomposed).
IR vKBr (Cm-l~ 3400, 1690, 1600, 1550,
max
1350, 1260, 1150, 780,
700.
e en y 25 29 7~5 ( )2 2
Calculated (%): C, 48.00; H, 4.83; N, 13.06.
Found (%): C, 48.38; H, 4.92; N, 12.91.
Compound 125 (R7, R8 and R9 are as defined with
respect to Compound 117):
Melting point: 148-150~C (decomposed).
IR vKBr (cm 1): 3350, 2600, 1690, 1640,
1530, 1330, 1240, 980,
760, 700.
Elemental analysis for C25H34N6O5-(COOH)2-3H2O:
Calculated (%): C, 50.46; H, 6.59; N, 13.08.
26g~1389
- 223 -
Found (%): C, 51.04; H, 6.77; N, 12.90.
Compound 126 (R7, R8 and R9 are as defined with
respect to Compound 118):
Melting point: 202-203~C (decomposed).
IR vKBr (cm-l) 3560, 2550, 1700, 1610,
lS30, 1320, 1280, 1110,
810, 710.
Y 26 30 6~5 (C ~ )2 2
Calculated (%): C, 54.72; H, 5.58; N, 13.67.
Found (%): C, 54.68; H, 5.21; N, 13.47.
Compound 127 (R7, R8 and R9 are as defined with
respect to Compound 119):
Melting point: about 140~C (amorphous).
IR vKBr (cm~l) 3420, 2600, 1710, 1640,
max
1540, 1320, 1220, 1110,
810, 710.
Y 21 28 6~5 (C ~ )2 2 2
Calculated (%): C, 48.24; H, 5.57; N, 14.06.
Found (%): C, 44.11; H, 5.90; N, 13.89.
Example 68:
Production of tablets containing as an effective
ingredient 1,3-dimethyl-6-{4-t3-(2-benzoyl-4-
nitroanilino)propyl]piperazin-l-yl}-2,4(1H,3H)-
pyrimidinedione oxalate (Compound 111) available
by the process of Example 66:
2~1389
- 224 -
1 g of the pyrimidinedione derivative oxalate
tCompound 111), 123 g of lactose and 20 g of corn
starch were finely mixed. Using a solution of S g of
hydroxypropylcellulose in 100 m~ of water, the
resultant mixture was granulated. The resultant
particles were dried at 50~C for 4 hours and then
mixed thoroughly with 1 g of magnesium stearate. The
thus-prepared mixture was then compressed into tablets,
each containing 150 mg, by a tablet machine.
Example 69:
Production of capsules containing as an
effective ingredient 1,3-dimethyl-6-{4-[3-(2-
acetyl-4-nitroanilino)propyl]piperazin-1-yl}-
2,4(1H,3H)-pyrimidinedione oxalate (Compound
120) available by the process of Example 67:
5 g of the pyrimidinedione derivative oxalate
(Compound 120), 120 g of lactose and 25 g of corn
starch were finely mixed. The resulting mixture was
filled into hard capsules, each containing 150 mg, by a
capsule filling machine.
Example 70:
Production of injection containing as an
effective ingredient 1,3-dimethyl-6-{4-t3-(2-
benzoyl-4-nitroanilino]propyl]piperazin-1-
yl}-2,4-(lH,3H~-pyrimidinedione oxalate
(Compound 111) available by the process of
2(~ 389
- 225 -
Example 66:
20 mg of the pyrimidinedione derivative oxalate
(Compound 111) and 0.85 g of sodium chloride were
weighed. They were dissolved in distilled water for
injection to give a total volume of 100 m~, thereby
preparing a formulation suitable for injection.
Pharmacological Test 5:
Similarly to Pharmacological Test 1, the ADP75
and ERP of each of the compounds shown in Table 10 and
obtained in the corresponding examples described above
were determined. The results are summarized in Table
10 .
Table 10
15 Result of Pharmacological Test
75 (%) ERP (%)
Compound Dose (~g/m~) Dose (mg/kg, i.v.)
No.
1.0 3.0 10.0 0.1 0.3 1.0 3.0
111 13.0 21.0 - 7.710.9 14.0 21.0
120 16.0 27.0 29.0
125 - 10.0 21.0
126 23.0 45.0 48.0 7.014.0 21.0
Toxicity Test 5:
Similarly to Toxicity Test 1, the toxicity of
each of the compounds shown in Table 11 and obtained in
20~389
- 226 -
the corresponding examples described above was tested
to determine the mortality rate of mice. The results
are summarized in Table 11.
Incidentally, the administration of each
compound was conducted orally (p.o.) at a dose of
300 mg/Kg.
Table ll
Compound No.Mortality rate (%)
122 o
126 0
Example 71:
Preparation of 1,3-dimethyl-6-{4-(2-benzoyl-4-
nitrophenyl)piperazin-l-yl}-2,4(lH,3H)-
pyrimidinedione oxalate (Compound 128):
C ~
02N~CO-~> + HN N~l-CH3 --
(COOH) 2/CH30H,
2~1!~i~89
CO / O
CH3 ~(COOH)2
~ (Compound 128)
0.5 g of 1,3-dimethyl-6-(piperazin-1-yl)-
S 2,4(1H,3H)-pyrimidinedione (Compound 157) and 0.6 g of
3-benzoyl-4-chloronitrobenzene were added to S ml of
dimethylsulfoxide. The resultant mixture was heated at
110~C for 20 hours. After allowing the reaction
mixture to cool down, the reaction mixture was poured
into a saturated aqueous solution of sodium bicarbonate
and the resulting mixture was extracted with
chloroform.
An organic layer tchloroform layer) obtained by
the extraction was separated, washed with water, dried
over anhydrous sodium sulfate, and then concentrated to
dryness. The residue was purified by chromatography on
a silica gel column (eluent: chloroform/methanol = 50/1
- 20/1, by volume), thereby obtaining 0.2 g of 1,3-
dimethyl-6-[4-(2-benzoyl-4-nitrophenyl)piperazin-1-yl]-
2,4(lH,3H)-pyrimidinedione.
Analytical results of the pyrimidinedione derivative
thus obtained:
NMR (d6-DMso)~ ~ ppm: 2.64(m,2H), 3.18(m,6H),
3.28(s,3H), 3,17(s,3H),
S.OO(s,lH),
7.3-8.0(m,5H),
~1389
- 228 -
-
8.2-8.6(m,3H).
Further, the pyrimidinedione derivative was
treated in an oxalic acid/methanol solution by a method
known per se in the art to obtain 0.18 g of 1,3-
dimethyl-6-[4-(2-benzoyl-4-nitrophenyl)piperazin-1-
yl]-2,4(lH,3H)-pyrimidinedione oxalate (Compound 128).
Analytical results of Compound 128 thus obtained:
Melting point: 178-179~C (decomposed).
IR vmBax (cm 1): 3430, 1700, 1660, 1600,
1520, 1320, 1260, 1130,
950, 860, 760, 700.
Elemental analysis for C23H23N5O5-~(COOH)2-~H2O:
Calculated (%): C, 57.25; H, 5.01; N, 13.91.
Found (%): C, 57.55; H, 4.80; N, 13.56.
Example 72:
Preparation of 1,3-dimethyl-6-{3-[4-nitro-2-(3-
pyridinecarbonyl)anilino]propylamino}-
2,4(lH,3H)-pyrimidinedione oxalate
(Compound 129):
0
~ Cl ~ + H2NCHzCH2CHzNH- ~ -CH3
, (COOH)2/CH30H,
2~ 389
- 229 -
2 ~ 2 2CH2NH ~ CH3
CH3 ~(COOH)2
N
(Compound 129)
0.67 g of 4-chloro-3-(3-pyridinecarbonyl)nitro-
benzene and 0.6 g of 1,3-dimethyl-6-(3-aminopropyl-
amino)-2,4(1H,3H)-pyrimidinedione were dissolved in
3 m~ of dimethylformamide, followed by the addition of
0.79 m~ of triethylamine. The resultant mixture was
heated under stirring at 80~C for 5 hours.
After completion of the heating, the reaction
mixture was allowed to cool down and the solvent was
then distilled off. The residue was purified by
chromatography on a silica gel column (eluent:
chloroform/methanol = 40/1, by volume) to obtain 0.39 g
lS of 1,3-dimethyl-6-{3-[4-nitro-2-(3-pyridinecarbonyl)-
anilino]propylamino}-2,4(1H,3H)-pyrimidinedione as an
oily substance.
Analytical results of the pyrimidinedione derivative
thus obtained:
NMR (CDC13), ~ ppm: 9.40(m,lH), 8.84(m,6H),
7.86-8.S7(m,3H),
7.50(dd,1H), 6.85(d,1H),
5.90(m,1H), 4.86(s,1H),
3.23(s,3H), 3.38(s,3H),
3.40(m,4H), 2.1(m,2H).
2~31389
- 230 -
Further, the pyrimidinedione derivative was
treated in an oxalic acid/methanol solution by a method
known per se in the art to obtain 0.36 g of 1,3-
dimethyl-6-{3-t4-nitro-2-(3-pyridinecarbonyl)anilino~ -
propylamino}-2,4(1H,3H)-pyrimidinedione oxalate
(Compound 129).
Analytical results of Compound 129 thus obtained:
Melting point: 136-139~C (decomposed).
IR vmBax (cm 1): 3350, 2550, 1690, 1640,
1550, 1330, 1260, 1110,
770, 700.
Elemental analysis for C21H22N6O5-(COOH)2:
Calculated (%): C, 52.27; H, 4.58; N, 15.90.
Found (%): C, 52.25; H, 4.92; N, 15.51.
~5 Example 73:
Preparation of 1,3-dimethyl-6-t3-(2-benzoyl-4-
nitroanilino)propylamino]-2,4(1H,3H)-
pyrimidinedione oxalate (Compound 130):
~ Cl ~ NH2CHzCHzCHzNH ~ ~-CH3 >
OzN~ CO- ~ ,N~o
CH3
(COOH)2/CH30H
>
389
- 231 -
2 ~ 2C 2CH2NH ~ N CH3
CH3 ~(COOH)2
(Compound 130)
By a similar procedure to Example 72 except for
the use of 3-benzoyl-4-chloronitrobenzene in place of
4-chloro-3-(3-pyridinecarbonyl)nitrobenzene, 1,3-
dimethyl-6-[3-(2-benzoyl-4-nitroanilino)propyl-
amino]-2,4(1H,3H)-pyrimidinedione oxalate (Compound
130) was obtained.
Analytical results of Compound 130 thus obtained:
Melting point: amorphous.
IR vmax (cm 1): 3300, 1690, 1620, 1540,
1330, 1110, 990, 850,
760, 700.
Elemental analysis for C22H23N505-~(COOH)2:
Calculated (%): C, 57.26; H, 5.01; N, 14.52.
Found (%): C, 57.16; H, 4.63; N, 14.76.
Example 74:
Preparation of 1,3-dimethyl-6-[2-(2-benzoyl-4-
nitroanilino)ethylamino]-2,4(1H,3H)-pyrimidine-
dione oxalate (Compound 131):
~ Cl + NH2CH2CH2NH ~ -CH
ozlYJ~-~'C0 ~ N 0
CH3
-
2C~0~389
- 232 -
02N~NHCH2CH2NH~ CH3
CO C/H3
(Compound 131)
By a similar procedure to Example 73 except tha~
1,3-dimethyl-6-(2-aminoethylamino)-2,4(lH,3H)-
pyrimidinedione was reacted in place of 1,3-dimethyl-6-
(3-aminopropylamino)-2,4(1H,3H)-pyrimidinedione with
3-benzoyl-4-chloronitrobenzene, 1,3-dimethyl-6-[2-(2-
benzoyl-4-nitroanilino)ethylamino]-2,4(lH,3H)-
pyrimidinedione (Compound 131) was obtained.
Analytical results of Compound 131 thus obtained:
Melting point: 207-209~C (decomposed).
IR vmBr (cm 1): 3050, 2950, 1760, 1680,
1560, 1340, 1110, 760,
700.
Y 21H21N5~5 (COOH)2 H20
Calculated (%): C, 51.98; H, 4.74; N, 13.18.
Found (%): C, 51.81; H, 4.87; N, 12.97.
Example 75:
Preparation of 1,3-dimethyl-6-{2-[4-nitro-2-
(3-pyridiencarbonyl)phenylamino]ethylamino}-
2,4(lH,3H)-pyrimidinedione oxalate
(Compound 132):
o
CO-~ + NHzCH2CH2NH ~N-CH3
2~01~89
- 233 -
021~NHCH2CH2NH~N--CH3
CO CH3
(Compound 132)
By a similar procedure to Example 72 except for
the use of 1,3-dimethyl-6-(2-aminoethylamino)-
2,4(lH,3H)-pyrimidinedione in place of 1,3-dimethyl-6-
(3-aminopropylamino)-2,4(1H,3H)-pyrimidinedione, 1,3-
dimethyl-6-{2-[4-nitro-2-(3-pyridinecarbonyl)phenyl-
amino]ethylamino}-2,4(lH,3H)-pyrimidinedione (Compound
132) was obtained.
Analytical results of Compound 132 thus obtained:
Melting point: 148~C.
IR VmBax (cm ): 3380, 3200, 1692, 1630,
1587, 1333, 1265, 1155,
1115 .
Elemental analysis for C20H20N6O5-H2O:
Calculated (%): C, 54.30; H, 5.01; N, 19.00.
Found (%): C, 54.04; H, 4.71; N, 18.84.
Example 76:
- Preparation of 1,3-dimethyl-6-{4-[2-(2-benzoyl-
4-nitrophenyl)ethyl]piperazin-1-yl)-2,4(lH,3H)-
pyrimidinedione oxalate (Compound 133):
HN NH ((CH 3 ) 3 COCO ) 2 0
02N ~ CHzCHz-Br > >
N0z ~ HzCHzN N-COC(CH 3) 3
- 234 - 20~3~9
~N-CH3
~COCl H~ C)~O (COOH) z/CH30H
CH3
~ 2 2~_, '1~ 3
CE13 ~ (COOEI)2
\ /
(Compound 133)
(1) Preparation of l-[2-(4-nitrophenyl)ethyl]-4-tert-
butyloxycarbonylpiperazine:
1.7 g of 4-nitrophenethyl bromide and 5 g of
piperazine were dissolved in 15 ml of chloroform. The
( resultant solution was heated under reflux for 3 hours.
After completion of the reaction, the reaction
mixture was allowed to cool down and washed three times
with water to remove excess piperazine from the
reaction mixture. Then, the chloroform layer was
separated.
Next, the solvent was removed under reduced
pressure from the chloroform layer. The residue was
dissolved in 15 me of dry tetrahydrofuran, followed by
the addition of 1.6 g of di-tert-butyl dicarbonate.
~. .
2~C~1389
- 235 -
The mixture thus obtained was stirred at room
temperature for 1 hour and the solvent was distilled
off from the reaction mixture.
A hexane/ethanol mixed solvent was added to the
residue. The thus-precipitated crystals were collected
by filtration and then recrystallized from a
hexane/ethanol mixed solvent, thereby obtaining 2.4 g
of l-[2-(4-nitrophenyl)ethyl]-4-tert-butyloxycarbonyl-
piperazine.
Analytical results of the piperazine derivative thus
obtained:
Melting point: amorphous.
NMR (CDC13), ~ ppm: 8.14(d,2H), 7.34(d,2H),
3.41(m,4H), 2.74(m,4H),
2.43(m,4H), 1.45(s,9H).
(2) Preparation of 1,3-dimethyl-6-{4-t2-(2-benzoyl-4-
nitrophenyl)ethyl]piperazin-l-yl}-2,4(1H,3H)-
pyrimidinedione oxalate (Compound 133):
0.8 g of 1-[2-(4-nitrophenyl)ethyl]-4-tert-
butyloxycarbonylpiperazine was dissolved in 20 m~ ofdry tetrahydrofuran, followed by the addition of 1 m~
of hexamethylphosphorus triamide. While chilling the
resultant mixture at -78~C, 2.9 m~ of a 1 M solution
of lithium diisopropylamide in dry tetrahydrofuran were
added.
Z¢~ 89
- 236 -
Upon an elapsed tie of 1 hour after completion
of the addition, the temperature of the reaction
mixture was maintained at -78~C and 0.83 m~ of
benzoyl chloride was then added dropwise.
After completion of the dropwise addition, the
temperature of the reaction mixture was gradually
raised to -30~C, at which the reaction mixture was
stirred for 3 hours. The reaction mixture was then
poured into ice water, followed by extraction with
chloroform.
The extract was washed with water, dried over
anhydrous sodium sulfate, and then concentrated under
reduced pressure to obtain a syrupy substance.
The syrupy substance was dissolved in 30 m~ of
ethyl ether, followed by the addition of 2 m~ of
hydrochloric acid/dioxane (4 N). The resultant mixture
was stirred at room temperature for 30 minutes.
The resultant crystals were collected by filtra-
tion and then washed with ether.
The crystals were next dissolved in 20 m~ of
isopropanol, to which 0.5 g of 1,3-dimethyl-6-chloro-
2,4(1H,3H)-pyrimidinedione and 3 m~ of triethylamine.
The thus-prepared mixture was stirred under reflux for
6 hours.
After completion of the stirring, the solvent
was distilled off from the reaction mixture. The
Z~1389
- 237 -
residue was dissolved in chloroform. The resultant
chloroform solution was washed with water, dried over
anhydrous sodium sulfate, and then concentrated under
reduced pressure.
The residue was purified by chromatography on a
silica gel column (eluent: chloroform/methanol = 50/1,
by volume) to obtain 0.33 g of 1,3-dimethyl-6-{4-[2-(2-
benzoyl-4-nitrophenyl)ethyl]piperazin-1-yl}-2,4(lH,3H)-
pyrimidinedione.
Analytical results of the pyrimidinedione derivative
thus obtained:
NMR (CDC13), ~ ppm: 7.3-8.2(m,8H),
5.06(s,1H), 3.28(s,3H),
3.39(s,3H),
2.4-3.0(m,12H).
Next, the pyrimidinedione derivative was treated
in an oxalic acid/methanol solution by a method known
E~ se in the art to obtain 0.34 g of 1,3-dimethyl-6-
{4-[2-(2-benzoyl-4-nitrophenyl)ethyl]piperazin-1-yl}-
2,4(lH,3H)-pyrimidinedione oxalate (Compound 133):
Analytical results of Compound 133 thus obtained:
Melting point: 183-185~C (decomposed).
IR vmax (cm 1): 3350, 2850, 2500, 1710,
1660, 1520, 1340, 1210,
840, 800, 750, 700.
Elemental analysis for C25H27N5O5 ( 2 2
2~01389
- 238 -
Calculated (%): C, 55.38; H, 5.34; N, 11.96.
Found (%): C, 55.16; H, 5.11; N, 12.01.
Example 77:
Preparation of 1,3-dimethyl-6-{4-t(2-benzoyl-4-
nitrophenyl)methyl]piperazin-1-yl}-2,4(lH,3H)-
pyrimidinedione oxalate (Compound 134):
HN NH ((CH 3 ) 3 CO CO ) 2 0
NO2~&:H2Br > >
NO 2~3CH2N~,N-COOC (CH3 ) 3
~N-CH3
(~COCl H~ Cl Nl o (COOH) 2/CH30H
CH3
> > > >
O ~ C ~ 4~
~ CH3 ~(COOH)2
tCompound 134)
(1) Preparation of l-t(4-nitrophenyl)methyl]-4-tert-
butyloxycarbonylpiperazine:
By a similar procedure to Example 76-(1) except
for the use of 1.6 g of 4-nitrobenzyl bromide in place
of 4-nitrophenethyl bromide, 1.1 g of 1-[(4-nitro-
phenyl)methyl~-4-tert-butyloxycarbonylpiperazine.
2~
Analytical results of the piperazine derivative thus
obtained:
NMR (CDC13), ~ ppm: 8.11(d,2H), 7.44(d,2H),
2.96(m,4H), 2.73(m,4H),
1.51(s,9H).
(2) Preparation of 1,3-dimethyl-6-{4-[(2-benzoyl-4
nitrophenyl)methyl]piperazin-l-yl}-2,4(1H,3H)-
pyrimidinedione oxalate (Compound 134):
In a manner similar to Example 76-(2) except for
the use of 0.77 g of 1-[(4-nitrophenyl)methyl]-4-tert-
butyloxycarbonylpiperazine obtained in the above
procedure (1) in place of 1-[2-(4-nitrophenyl)ethyl]-4-
tert-butyloxycarbonylpiperazine, 0.10 g of 1,3-
dimethyl-6-{4-[(2-benzoyl-4-nitrophenyl)methyl]-
piperazin-1-yl}-2,4(lH,3H)-pyrimidinedione oxalate
(Compound 134) was obtained.
Analytical results of Compound 134 thus obtained:
Melting point: amorphous.
IR vKBr (cm~l~ 3350, 1700, 1630, 1530,
max
1360, 1120, 860, 760,
700.
y s 24H25N505 1/2(COOH)2 3/2H20:
Calculated (~): C, 56.42; H, 4.92; N, 12.65.
Found (~): C, 56.12; H, 4.77; N, 12.39.
Example 78:
Preparation of 1,3-dimethyl-6-{2-[(2-benzoyl-4-
2~1389
- 240 -
nitrophenyl)methylamino]ethylamino}-2,4-(lH,3H)-
pyrimidinedione oxalate (Compound 135):
OzN ~ Cl CH2(S ~ )2 HgCl2 H~
S CO~
02N ~ CHO
CO,-~>
~
HzNCHzCHzNH-y N-CH3
N~o ( co OH~2/MeOH
CH3
02N ~ CH2NHc~2cH2NH ~ -CH3
C~3 ~(COOH)2
(Compound 135)
(l) Preparation of 2-benzoyl-4-nitrobenzaldehyde:
3.72 g of bisthiophenylmethane were dissolved in
40 mQ of tetrahydrofuran and the resultant solution
was chilled to -20~C. 12 m~ of n-butyl lithium
(1.6 M solution in hexane) were added dropwise,
followed by stirring at -20~C for l hour.
After completion of the stirring, a solution of
4 g of 3-benzoyl-4-chloronitrobenzene in 20 ml of
2(~ 389
- 241 -
tetrahydrofuran was added dropwise while maintaining
the temperature of the reaction mixture at -20~C.
Subsequent to completion of the dropwise addition, they
were reacted for 1 hour while controlling the
temperature of the reaction mixture within a range of
from -78~C to -20~C.
After completion of the reaction, the reaction
mixture was poured into water and then extracted with
400 m~ of ether. The extract layer (ether layer) was
washed twice with 200 m~ of water and dried over
anhydrous magnesium sulfate.
The solvent was next distilled off from the
extract under reduced pressure and the residue was
purified by chromatography on a silica gel column
(eluent: hexane/ethyl acetate = lO/l, by volume) to
obtain an oily substance.
The oily substance was dissolved in 40 m~ of
acetonitrile, to which a solution of 2.0 g of mercuric
chloride in 20 m~ of water was added dropwise.
Subsequent to completion of the dropwise
reaction, 0.1 g of p-toluenesulfonic acid was added
further and was reacted at 60~C for 4 hours.
After completion of the reaction, insoluble
matters were filtered off from the reaction mixture and
the filtrate was concentrated. The resultant concent-
2001389
- 242 -
rate was purified by chromatography on a silica gel
column (eluent: hexane/ethyl acetate = 10/1, by volume)
to obtain 1.1 g of crude 2-benzoyl-4-nitrobenzaldehyde
as an oily substance.
(2) Preparation of 1,3-dimethyl-6-{2-t(2-benzoyl-4-
nitrophenyl)methylamino]ethylamino}-2,4(1H,3H)-
pyrimidinedione oxalate (Compound 135):
A solution of 1.0 g of 1,3-dimethyl-6-(2-amino-
ethylamino)-2,4(lH,3H)-pyrimidinedione in 20 m~ of
methanol, a solution of 1.1 g of the crude product of
2-benzoyl-4-nitrobenzaldehyde in 10 m~ methanol and
0O3 mQ of a 4 N solution of hydrochloric acid in
dioxane were separately cooled to 0~C, mixed together
and then reacted at 0~C for 1 hour.
After completion of the reaction, 0.1 g of
sodium cyanoborohydride, followed by a reaction at
0 + 5~C for 3 hours.
After the reaction was completed, the reaction
mixture was acidified with 1 N hydrochloric acid and
then poured into ice water. The resultant mixture was
rendered basic with sodium bicarbonate and then
extracted twice with 50 mQ portions of chloroform.
Subsequent to completion of the extraction, the
chloroform layer was washed with water, dried over
anhydrous magnesium sulfate and then concentrated under
reduced pressure.
- 2~1il389
- 243 -
The thus-obtained concentrate was purified by
chromatography on a silica gel column (eluent:
chloroform/methanol = 10/1, by volume) to obtain 0.7 g
of 1,3-dimethyl-6-{2-t(2-benzoyl-4-nitrophenyl)methyl-
amino]ethylamino}-2,4(1H,3H)-pyrimidinedione as an oily
substance.
Analytical results of the pyrimidinedione derivative
thus obtained:
NMR (CDC13), ~ ppm: 7.2-8.1(m,8H),
5.68(b,1H), 4.89(s,1H),
3.61(s,2H), 3.34(s,3H),
3.39(s,3H), 3.06(m,4H).
Further, 0.5 g of the pyrimidinedione derivative was
treated in an oxalic acid/methanol solution by a method
known Per se in the art to obtain 0.11 g of 1,3-
dimethyl-6-{2-[(2-benzoyl-4-nitrophenyl)methylamino]-
ethylamino}-2,4(lH,3H)-pyrimidinedione oxalate
(Compound 135).
Analytical results of Compound 135 thus obtained:
Melting point: hygroscopic amorphous.
IR vmax (cm 1): 3400(br), 2700, 2550,
1700, 1620, 1530, 1340,
1120, 820, 720, 700.
Y 22H23N5~5 2(cooH)2 3H20
Calculated (%): C, 46.50; H, 4.95; N, 10.43.
Found (%): C, 46.82; H, 4.65; N, 10.25.
Z~)1389
- - 244 -
Example 79:
Preparation of 1,3-dimethyl-6-{4-t3-(4-benzoyl-
2-nitrophenyl)propyl~piperazin-1-yl}-2,4(lH,3H)-
pyrimidinedione hydrochloride (Compound 136):
O ' O
C ~ Cl I, ~ C ~ I H2C=CH-COOCH3
NOz NO2
~ -C ~ -CH=CH-COOCH3
NO2
OH
> ~ -CH ~ -CH=CH-COOCH3
'NO2
o
~ -C ~ CH2CHzCHzOH
NO2
HN N ~ -CH3
CH3 ~ SOzCl'J N~o HCl/CH30H
CH3
CH2CH2N~ ~N~N-CH3
CH3 HC1
(Compound 136)
~1) Preparation of 4-iodo-3-nitrobenzophenone:
89
- 245 -
A mixture of 10.44 g of 4-chloro-3-nitrobenzo-
phenone, 60 g of sodium iodide and 60 m~ of dimethyl-
formamide was heated under reflux for 15 hours.
The reaction mixture was cooled and then poured
into 800 m~ of water. Precipitated crystals were
collected by filtration.
The precipitated crystals were washed with
water, dried under reduced pressure and then purified
by chromatography on a silica gel column (eluent:
c~loroform/hexane = 2/1 - 10/1, by volume), thereby
obtaining 10.17 g of 4-iodo-3-nitrobenzophenone as
yellow crystals.
Analytical results of the benzophenone derivative thus
obtained:
i5 NMR (CDC13), ~ ppm: 8.35(d,1H),
8.26(d,1H),
7.40-8.10(m,6H).
(2) Preparation of methyl 4-benzoyl-2-nitrocinnamate:
10.17 g of 4-iodo-3-nitrobenzophenone obtained
in the above procedure (1), 4.10 g of methyl acrylate
and 4.70 g of triethylamine were dissolved in 130 m~
of acetonitrile and then degasified. The resultant
solution was added with 0.65 g of palladium (II)
acetate and heated under reflux for 6 hours under a
nitrogen gas stream, whereby a reaction was conducted.
After allowing the reaction mixture to cool down, the
2~1389
- 246 -
solvent was distilled off from the reaction mixture
under reduced pressure. Benzene was added to the
residue. The resultant solution was washed
successively with water, 1 N hydrochloric acid, a
saturated solution of sodium bicarbonate and a
saturated NaCl solution.
The thus-washed organic layer was dried over
anhydrous sodium sulfate and the solvent was then
distilled off under reduced pressure. The residue was
thereafter purified by chromatography on a silica gel
column (eluent: chloroform) to obtain 7.12 g of methyl
4-benzoyl-2-nitrocinnamate as pale yellow crystals.
Analytical results of the cinnamic acid derivative thus
obtained:
NMR (CDC13), ~ ppm: 8.43(d,1H),
7.48-8.38(m,8H).
6.51(d,1H), 3.84(s,3H).
(3) Preparation of 2-nitro-4-(a-hydroxybenzyl)-
cinnamic acid:
7.12 g of methyl 4-benzoyl-2-nitrocinnamate
obtained in the above procedure (2) were dissolved in
120 m2 of methanol, followed by the addition of 0.90 g
of sodium borohydride at room temperature. After
stirring the resultant mixture at the same temperature
for 10 hours, a small amount of water was added to
terminate the reaction.
2~ 89
- 247 -
The solvent was distilled off from the reaction
mixture under reduced pressure and the residue was
dissolved in benzene. The resultant solution was
washed successively with 200 m~ of water and 200 mQ
of a saturated NaCl solution.
The thus-washed organic layer was dried over
anhydrous sodium sulfate and the solvent was distilled
off under reduced pressure, thereby obtaining 7.12 g of
methyl 2-nitro-4-(a-hydroxybenzyl)cinnamate as an pale
yellow oily substance.
Further, 7.12 g of the pale yellow oily
substance were dissolved in 60 m~ of methanol,
followed by the addition of 40 m~ of a 1 N aqueous
solution of potassium hydroxide at room temperature.
The resultant solution was vigorously stirred for 3
hours at the same temperature. After completion of the
stirring, the reaction mixture was poured into water
and then neutralized with 1 N hydrochloric acid.
Precipitated crystals were collected by filtration and
then washed with water.
The crystals thus obtained were dried under
reduced pressure to obtain 6.95 g of 2-nitro-4-(a-
hydroxybenzyl)cinnamic acid in a purified form.
Analytical results of the product thus obtained in the
purified form:
Elemental analysis for C16H13NO5:
Z001389
- 248 -
Calculated (%): C, 64.21; H, 4.38; N, 4.68.
Found (%): C, 63.79; H, 4.95; N, 4.21.
(4) Preparation of 3-(4-benzoyl-2-nitrophenyl)-1-
propanol:
3.54 g of 2-nitro-4-(a-hydroxybenzyl)cinnamic
acid obtained in the above procedure (3), 13.38 g of
hydroxyamine-O-sulfonic acid and 9.69 g of hydroxyl-
amine sulfate were suspended in 180 m~ of water. The
resulting suspension was cooled and while maintaining
its internal temperature at 20-30~C, a 50% aqueous
solution of sodium hydroxide (about 22 m~ in total)
was added dropwise thereto.
After the mixture was stirred at the same
temperature for 3 hours to react them, the reaction
mixture was ice-cooled. The reaction mixture was then
added with 6 N sulfuric acid to acidify same, and
precipitated crystals were collected by filtration.
The thus-obtained crystals were washed with water and
then dried under reduced pressure to obtain 3.48 g of
3-[2-nitro-4-(a-hydroxybenzyl)phenyl]propionic acid as
crystals.
3.3 g of the crystals of the phenylpropionic
acid derivative thus obtained were dissolved in 70 mQ
of tetrahydrofuran. The resultant solution was added
with 1.4 me of boran-dimethylsulfide complex,
followed by stirring. Upon an elapsed time of 6 hours,
2~01389
- 49 -
5 mQ of water was added to the reaction mixture to
terminate the reaction. The solvent was distilled off
from the reaction mixture under reduced pressure and
the residue was dissolved in 100 m~ of chloroform.
The chloroform solution was washed successively
with water, a lN aqueous solution of sodium hydroxide
and a saturated saline solution. The thus-washed
organic layer was dried over anhydrous sodium sulfate
and the solvent was distilled off under reduced
pressure to obtain 2.90 g of crude 3-[2-nitro-4-
(~-hydroxybenzyl)phenyl]propanol.
2.90 g of the crude product were dissolved in
100 m~ of chloroform, followed by the addition of 20 g
of celite and 9.0 g of manganese dioxide. The
resultant mixture was vigorously stirred for 24 hours
at room temperature.
After completion of the stirring, the reaction
mixture was filtered and the filtrate was concentrated
to dryness to obtain 2.87 g of 3-(4-benzoyl-2-nitro-
phenyl)-l-propanol.
Analytical results of the phenylpropanol derivative
thus obtained:
NMR (CDC13), ~ ppm: 8.12(d,1H), 7.80(dd,1H),
7.00-7.72(m,6H).
3.64(t,2H), 3.00(t,2H),
1.92(m,2H), 1.84(brs,1H).
2~ 89
- - 250 -
(5) Preparation of 1,3-dimethyl-6-{4-~3-(4-benzoyl-
2-nitrophenyl)propyl]piperazin-1-yl}-2,4(lH,3H)-
pyrimidinedione hydrochloride (Compound 136):
1.0 g of 3-(4-benzoyl-2-nitrophenyl)-1-propanol
obtained in the above procedure(4) and 1.38 g of
pyridine were dissolved in 20 m~ of chloroform. The
resultant mixture was added under ice-cooling with
1.34 g of p-toluenesulfonyl chloride. The resultant
mixture was stirred for 10 minutes at the same
temperature and for additional 16 hours at room
temperature.
The thus-obtained reaction mixture was added
with 2 m~ of water and then stirred for 3 hours at
room temperature. After addition of 50 m~ of
chloroform, the reaction mixture was poured into 50 mQ
of water. The thus-formed mixture was allowed to
separate into layers. The organic layer was washed
successively with 1 N hydrochloric acid, a 1 N aqueous
solution of sodium hydroxide and a saturated saline
solution and was then dried over anhydrous sodium
sulfate. The solvent was distilled off under reduced
pressure to obtain 1.51 g of 3-(4-benzoyl-2-nitro-
phenyl)-l-propyl p-toluenesulfonate as an oily yellow
substance.
Z~)1389
- - 251 -
1.51 g of the oily yellow substance were
dissolved in 10 m~ of dimethyl sulfoxide. The
resultant solution was added with 1.10 g of 1,3-
dimethyl-6-(1-piperazinyl)-2,4(lH,3H)-pyrimidinedione
and then heated at 80~C for 6 hours to react them.
The resultant reaction mixture was poured into a
solution which had been obtained by adding 2.0 g of
potassium carbonate to 100 m~ of water. The thus-
prepared mixture was extracted twice with 50 m~
portions of chloroform.
The organic layers separated by the two
extraction operations were combined, washed with water,
and then dried over anhydrous sodium sulfate. The
solvent was distilled off under reduced pressure and
the residue was purified by chromatography on a silica
gel column (eluent: chloroform/methanol = 50/1 - 20/1,
by volume), thereby obtaining 0.92 g of 1,3-dimethyl-
6-{4-[3-(4-benzoyl-2-nitrophenyl)propyl]piperazin-
l-yl}-2,4(lH,3H)-pyrimidinedione as pale yellow
crystals.
Analytical results of the pyridine derivative thus
obtained:
NMR (CDC13), ~ppm: 8.23(d,1H),
7.16-8.10(m,7H),
5.21(s,1H), 3.36(s,3H),
3.29(s,3H),
-- - 252 - 2 ~ O ~ 3 8 9
2.26-3.26(m,12H),
1.96(m,2H).
Next, the pyrimidinedione derivative was treated
in a hydrochloric acid/methanol solution by a method
S known per se in the art to obtain 0.9 g of 1,3-
dimethyl-6-{4-[3-(4-benzoyl-2-nitrophenyl)propyl]-
piperazin-l-yl}-2,4(1H,3H)-pyrimidinedione hydro-
( chloride (Compound 136) as white crystals:
Analytical results of Compound 136 thus obtained:
Elemental analysis for C26H29N5O5-HCl:
Calculated (%): C, 59.14; H, 5.73; N, 13.26;
Cl, 6.71.
Found (~): C, 59.56; H, 6.09; N, 13.16;
Cl, 6.21.
Example 80:
Preparation of 1,3-dimethyl-6-{4-[2-(4-benzoyl-
- 2-nitrophenyl)ethyl]piperazin-1-yl}-2,4(1H,3H)-
pyrimidinedione oxalate (Compound 137):
O O
--'~ ~ HOCHzCH2CH20H ~ \/ ~
25'~CO-,/,~Cl ~ ~C~Cl
NO2 NO2
_ - 253 - 2 G ~ ~ ~ 8 9
~rCH 2 CO OCzH 5 \ / 1) HCl
> ~ C- ~ CH2COOC2H5 >
NOz ~)NdOH
0 0
C ~ CHzCOOH ~ ~ C ~ CHzCH20H
NO2 NO2
HN N- ~ N-CH3
10 CH3- ~ SOzCl '' ~o (COOH)2/CH30H
CH3 >
C ~ C 2CH2N~_~N~ N-CH3
CH3 ~(COOH)2
( Compound 137)
(1) Preparation of 2-(4-chloro-3-nitrophenyl)-2-phenyl-
1,3-dioxane:
10 g of 4-chloro-3-nitrobenzophenone, 15 ml of
1,3-propanediol and 0.5 g of camphorsulfonic acid were
dissolved in 50 ml of benzene and while eliminating
water by a molecular sieve ("Molecular Sieves 3A",
trade mark; 1/16 pellets; product of Junsei Chemical
Co., Ltd.), were reacted under reflux for 12 hours.
The resultant reaction mixture was allowed to
cool down, washed with a 1 N aqueous solution of sodium
, ~ .
Z~)1389
- 254 -
hydroxide, washed further with water, dried and then
concentrated, thereby obtaining an oily substance.
Next, the oily substance was purified by
chromatography on a silica gel column (eluent:
hexane/ethyl acetate = 4/1, by volume) to obtain 7.8 g
of 2-(4-chloro-3-nitrophenyl)-2-phenyl-1,3-dioxane.
Analytical results of the compound thus obtained:
Elemental analysis for C16H13NO4Cl:
Calculated (%): C, 60.29; H, 4.11; N, 4.39;
Cl, 11.12.
Found (%): C, 60.09; H, 4.56; N, 4.01;
Cl, 11.29.
(2) Preparation of ethyl 4-(2-phenyl-1,3-dioxan-2-yl)-
2-nitrophenylacetate:
lS 5.2 g of zinc powder were suspended in a mixed
solution of 10 m~ of benzene and 10 m~ of trimethyl
borate. In a nitrogen gas atmosphere, 9 m~ of ethyl
bromoacetate were added dropwise at room temperature to
the suspension to react them.
Upon an elapsed time of 1 hour, the reaction
mixture was added further with a solution of 7.8 g of
2-(4-chloro-3-nitrophenyl)-2-phenyl-1,3-dioxane in
40 m~ of benzene, 0.9 m~ of dimethylformamide and
0.22 g of tris(triphenylphosphine) palladium(II)
chloride. The resultant mixture was allowed to stand
overnight at room temperature to react them.
Z6~1389
- - 255 -
Insoluble matters were then filtered off and the
filtrate was concentrated. The thus-obtained filtrate
was purified by chromatography on a silica gel column
(eluent: hexane/ethyl acetate = 3/1, by volume),
thereby obtaining 4.4 g of ethyl 4-(2-phenyl-1,3-
dioxan-2-yl)-2-nitrophenylacetate in an oily form.
Analytical results of the ethyl phenylacetate
derivative thus obtained:
NMR (CDC13), ~ppm: 7.3-8.2(m,8H),
4.15(q,2H), 3.22(s,2H),
1.40(t,3H).
(3) Preparation of 4-benzoyl-2-nitrophenylacetic acid:
4.4 g of ethyl 4-(2-phenyl-1,3-dioxan-2-yl)-2-
nitrophenylacetate obtained in the above procedure (2)
were dissolved in 30 m~ of methanol, followed by the
addition of 15 m~ of 1 N hydrochloric acid. They were
reacted under reflux for 1 hour.
After completion of the reaction, the reaction
mixture was allowed to cool down and the solvent was
distilled off. The residue was extracted with ether.
The organic layer obtained by the extraction was
washed with water and then concentrated, whereby 3.5 g
of an oily crude substance.
Next, the oily substance was dissolved in 20 m~
of ethanol. The resulting solution was added with
5 m~ of a 10% aqueous solution of sodium hydroxide,
2~3~1389
- - 256 -
and they were reacted overnight at room temperature.
The solvent was distilled off from the reaction
mixture. The residue was added with 10 m~ of water,
followed by the addition of 3 N hydrochloric acid to
acidify the resultant solution. Precipitated crystals
were collected by filtration, thereby obtaining 2.8 g
of 4-benzoyl-2-nitrophenylacetic acid.
Analytical results of the nitrophenyl acetic acid
derivative thus obtained:
NMR (CDC13), ~ppm: 7.2-8.3(m,8H),
3.73(s,2H).
(4) Preparation of 2-(4-benzoyl-2-nitrophenyl)ethyl
alcohol:
A solution of 2.8 g of 4-benzoyl-2-nitrophenyl-
acetic acid, which had been obtained in the above
procedure (3), in SO m~ of tetrahydrofuran was ice-
cooled, to which 2.3 mQ of borane-methyl sulfide
complex were added dropwise. The resultant mixture was
left over.
Upon an elapsed time of 1 hour, water was added
to terminate the reaction. The solvent was distilled
off from the reaction mixture. The residue was added
with ether and the resulting ether solution was washed
with a dilute hydrogen peroxide solution.
After the washing, the ether solution was
concentrated. The resulting syrup was added along with
Z~}0~8~
- 257 -
17 g of activated manganese dioxide into S0 m~ of
chloroform. The mixture was vigorously stirred for 30
hours.
After completion of the stirring, manganese
dioxide was filtered off from the reaction mixture and
the filtrate was concentrated. The concentrate was
purified by chromatography on a silica gel column
(eluent: hexane/ethyl acetate = 3/1, by volume),
thereby obtaining 1.9 g of 2-(4-benzoyl-2-nitrophenyl)-
ethyl alcohol in an oily form.Analytical results of the compound thus obtained:
NMR (CDC13), ~ppm: 7.2-8.3(m,8H),
4.05(t,2H), 3.11(t,2H).
(5) Preparation of 1,3-dimethyl-6-{4-[2-(4-benzoyl-2-
nitrophenyl)ethyl]piperazin-1-yl}-2,4(1H,3H)-pyrimidine-
dione oxalate (Compound 137):
1.9 g of 2-(4-benzoyl-2-nitrophenyl)ethyl
alcohol obtained in the above procedure (4), 1.5 g of
p-toluenesulfonyl chloride and 1.8 m~ of pyridine were
dissolved in 30 mQ of chloroform and reacted at room
temperature for 24 hours. Water was then added to the
reaction mixture. After vigorously stirring the
reaction mixture for 3 hours, the chloroform layer was
separated, washed first with a 1 N aqueous solution of
hydrochloric acid and then a 1 N aqueous solution of
sodium hydroxide, washed with water, concentrated over
Z~ .89
- 258 -
sodium sulfate, and then concentrated to obtain a brown
oily substance.
The brown oily substance, 0.6 g of 1,3-dimethyl~
6-(1-piperazLnyl) -2,4(lH,3H)-pyrimidinedione and 3 mQ of
triethylamine were reacted for 6 hours under reflux in
20 m~ of isopropanol.
The reaction mixture was allowed to cool down,
concentrated, and then dissolved in chloroform~ The
resultant chloroform solution was washed with water,
concentrated and then purified by chromatography on a
silica gel column (eluent: chloroform/methanol ~ 40~1
by volume), thereby obtaining 0.7 g of 1,3-dime~yl~6
{4-[2-(4-benzoyl-2-nitrophenyl)ethyl]piperazin-l~yl~
2,4(lH,3H)-pyrimidinedione in an oily form.
Analytical results of the pyrimidinedione deriva~i~e
thus obtained:
NMR (CDC13), ~ppm: 8.01(d,1H),
7.3-7.8(m,7H),
5.12(s,1H), 4.55(m,2H),
3.22(s,3H), 3.30(s,2H),
2.6-3.3(m,10H).
Next, the oily pyrimidinedione derivative was
treated in an oxalic acid/methanol solution by a method
known ~ se in the art to obtain 0.6 g of 1,3-
dimethyl-6-{4-t2-(4-benzoyl-2-nitrophenyl)ethyl]-
2C~
- 259 -
piperazin-l-yl}-2,4(lH,3H)-pyrimidinedione oxalate
(Compound 137).
Analytical results of Compound 137 thus obtained:
IR vmBax (cm 1): 2550, 1740, 1700, 1650,
1530, 1340, 1200, 1030,
830, 760, 700.
Elemental analysis for C25H27N5O5-(COOH)2-3H2O:
Calculated (~): C, 52.17; H, 5.68; N, 11.27.
Found (%): C, 52.57; H, 6.01; N, 11.30.
~0 Example 81:
Production of tablets containing as an effective
ingredient 1,3-dimethyl-6-{4-[2-(2-benzoyl-4-
nitrophenyl)ethyl~piperazin-l-yl}-2,4-(lH,3H)-
pyrimidinedione oxalate (Compound 133) available
by the process of Example 76:
1 g of the pyrimidinedione derivative oxalate
(Compound 133), 123 g of lactose and 20 g of corn
starch were finely mixed. Using a solution of 5 g of
hydroxypropylcellulose in 100 m~ of water, the
resultant mixture was granulated. The resultant
particles were dried at 50~C for 4 hours and then
mixed thoroughly with 1 g of magnesium stearate. The
thus-prepared mixture was then compressed into tablets,
each containing 150 mg, by a tablet machine.
Example 82:
Production of capsules containing as an
2Q01389
- 260 -
effective ingredient 1,3-dimethyl-6-t2-(2-
benzoyl-4-nitroanilino)ethylamino]-
2,4(1H,3H)-pyrimidinedione (Compound 131)
available by the process of Example 74:
5 g of the pyrimidinedione derivative oxalate
(Compound 131), 120 g of lactose and 25 g of corn
starch were finely mixed. The resulting mixture was
filled into hard capsules, each containing 150 mg, by a
capsule filling machine.
Example 83:
Production of injection containing as an
effective ingredient 1,3-dimethyl-6-{3-[4-nitro-
2-(3-pyridinecarbonyl)anilino]propylamino}-
2,4-(lH,3H)-pyrimidinedione oxalate (Compound
129) available by the process of Example 72:
20 mg of the pyrimidinedione derivative oxalate
(Compound 129) and 0.85 g of sodium chloride were
weighed. They were dissolved in distilled water for
injection to give a total volume of 100 m~, thereby
preparing a formulation suitable for injection.
Pharmacological Test 6:
Similarly to Pharmacological Test 1, the ADP75
and ERP of each of the compounds shown in Table 12 and
obtained in the corresponding examples described above
were determined. The results are summarized in Table
12.
2~ 8g
- 261 -
Table 12
Result of Pharmacological Test
75 ( ) ERP (%)
5 Compound Dose (~g/ml)Dose (mg/kg, i.v.)
No.
1.0 3.0 10.0 0.1 0.3 1.0 3.0
133 - - - 6.3 6.318.8
136 11.0 14.0 - - 7.715.4 15.4
137 32.0 51.0 - 6.3 6.312.5 18.8
Toxicity Test 6:
Similarly to Toxicity Test 1, the toxicity of
each of the compounds shown in Table 13 and obtained in
the corresponding examples described above was tested
to determine the mortality rate of mice. The results
are summarized in Table 13.
Incidentally, the administration of each
compound was conducted orally (p.o.) at a dose of
300 mg/Kg.
Table 13
Compound No.Mortality rate (%)
128 0
131 0
133 0
134 0
26~ 89
- 262 -
Example 84:
Preparation of 1,3-dimethyl-6-{4-[3-(3-methyl-4-
nitrophenyloxy)propyl]piperazin-l-yl}-2,4~
(lH,3H)-pyrimidinedione hydroxide (Compound
138):
r-~ ~ 2 2 2
HN N N-CH3
C/H3
. (Compound 157)
~ ~
--/ N~o
CH3
(Compound 139)
~3
O2N ~ OH Hcl/cH3oH
> >
3 ,O
2 ~ 2C~2CH2~ ~ -CH3 HCl
C~3
(Compound 138)
(1) Preparation of 1,3-dimethyl-6-[4-(3-hydroxypropyl)-
piperazin-l-yl]-2,4(lH,3H)-pyrimidinedione (Compound
139):
14.1 g of 1,3-dimethyl-6-(1-piperazinyl)-
2,4(1H,3H)-pyrimidinedione (Compound 157), 11.7 g of 3-
2~
- 263 -
bromo-l-propanol and 13 g of triethylamine were reacted
under reflux for 20 hours in 250 ml of ethanol. After
completion of the reaction, the reaction mixture was
concentrated to dryness. The residue was dissolved in
300 m~ of chloroform. The chloroform solution was
washed twice with 100 m~ portions of water and the
thus-washed organic layer was dried over anhydrous
magnesium sulfate. The organic layer was heated under
reduced pressure to distill off the solvent, whereby
20.5 g of a crude product were obtained. Ether was
added to the crude product to have it crystallized.
The resultant crystals were collected, washed and then
dried, thereby obtaining 12.4 g of 1,3-dimethyl-6-[4-
(3-hydroxylpropyl)piperazin-1-yl]-2,4(lH,3H)-pyridine-
dione ICompound 139) (yield: 69.8%).
Analytical results of crystals of Compound 139 thus
obtained:
Melting point: 119-121~C.
NMR ( CDC13), ~ppm: 1.8(d,lH), 2.7(m,6H),
3.02(m,4H), 3.36(s,3H),
3.43(s,3H), 3.82(t,2H),
4.34(br.1H), 5.26(s,1H),
IR vmax (cm 1): 3380, 3180, 2830, 1695,
1650, 1605, 1440, 1213,
1068, 1000, 921, 750.
(2) Preparation of 1,3-dimethyl-6-{4-[3-(3-methyl-4-
2~1389
- 264 -
nitrophenyloxy)propyl]piperazin-1-yl}-2,4(lH,3H)-
pyrimidinedione hydrochloride (Compound 138):
1.13 g of Compound 139 obtained in the above
procedure, 1.21 g of triphenylphosphine and 0.70 g of
3-methyl-4-nitrophenol were suspended and mixed in
15 m~ of anhydrous tetrahydrofuran. Into the
resultant suspension, a solution of 0.8 g of diethyl
azodicarboxylate in 10 m~ of anhydrous tetrahydrofuran
was added at room temperature.
After stirring the resultant mixture for 10
minutes, it was concentrated to dryness. The residue
was purified by chromatography on a silica gel column
(eluent: methanol/ethyl acetate = 1/15 - 1/7, by
volume), thereby obtaining 0.92 g of 1,3-dimethyl-6-{4-
[3-(3-methyl-4-nitrophenyloxy)propyl]piperazin-1-yl}-
2,4(lH,3H)-pyrimidinedione.
Analytical results of crystals of the pyrimidinedione
derivative thus obtained:
Melting point: 178.5-181~C.
Elemental analysis for C20H27N5O5:
Calculated (%): C, 57.54; H, 6.52; N, 16.78.
Found (%): C, 57.30; H, 6.56; N, 16.64.
NMR (CDC13), ~ppm: 1.9-2.3(m,2H), 2.6-2.8(m,9H),
3.0-3.2(m,4H), 3.44(s,3H),
3.54(s.3H), 4.28(t,2H),
5.44(s,1H), 7.0-7.16(m,2H),
Z00~89
- 265 -
8.40(d,lH).
Next, 0.80 g of 1,3-dimethyl-6-{4-[3-(3-methyl-
4-nitrophenyloxy)propyl~-piperazin-1-yl}-2,4(lH,3H)-
pyrimidinedione was treated in an HCl/methanol solution
by a method known per se in the art to obtain 0.60 g
of 1,3-dimethyl-6- {4-[3-(3-methyl-4-nitrophenyloxy)-
propyl]piperazin- l-yl}-2,4(1H,3H)-pyrimidinedione
hydrochloride (Compound 138).
Analytical results of Compound 138 thus obtained:
Melting point: 224~C (decomposed1.
Elemental analysis for C2oH27N5O5-HCl-2H2O:
Calculated (%): C, 49.03; H, 6.58; N, 14.29;
Cl, 7.24.
Found (%): C, 48.82; H, 6.88; N, 14.18;
Cl, 7.31.
Example 85:
Preparation of 1,3-dimethyl-6-{4-[3-(4-chloro-2-
nitrophenoxy)propyl]piperazin-l-yl}-2,4(lH,3H)-
pyrimidinedione hydrochloride (Compound 140):
C1 ~ OH+HOC~2CH2CH2N N ~ -CH3
2 CH3
(Compound 139
HCl/CH3OH
2~389
- 266 -
Cl ~ OCH2CH2CH2N~_~N ~ N--CH3 HC1
NO2 c~3
(Compound 140)
0.80 g of 4-chloro-2-nitrophenol, 1.13 g of 1,3-
dimethyl-6-[4-(3-hydroxypropyl)piperazin-1-yl}-
2,4(1H,3H)-pyrimidinedione (Compound 139) and 1.21 g of
triphenylphosphine were suspended in 15 mQ of
anhydrous tetrahydrofuran, followed by the addition of
0.80 g of diethyl azodicarboxylate. The resultant
mixture was treated in a similar manner to Example
84-(2~, thereby obtaining 1.43 g of 1,3-dimethyl-6-{4-
[3-(4-chloro-2-nitrophenyloxy)propyl]piperazin-1-yl}-
2,4(lH,3H)-pyrimidinedione as crystals.
Analytical results of crystals of the pyrimidinedione
derivative thus obtained:
Melting point: 162-163~C.
Elemental analysis for ClgH24N5O5Cl-~H2O:
Calculated (%): C, 51.07; H, 5.64; N, 15.67;
Cl, 7.93.
Eound (%): C, 51.11; H, 5.68; N, 15.64;
Cl, 7.94.
NMR (CDC13), ~ppm: 1.8-2.2(m,2H), 2.4-2.8(m,6H),
2.8-3.04(m,4H), 3.2-3.4(m,2H),
z~
- 267 -
3.52(s.3H), 3.80(s,3H),
4.18(t,2H), 5.20(s,1H),
7.20(d,lH), 7.45(dd,lH).
7.81(d,1H).
5Next, 1.2 g of the pyrimidinedione derivative
were treated in a 15% HCl/methanol solution by a method
known E~ se in the art to obtain 1.13 g of 1,3-
dimethyl-6-{4-[3-(4-chloro-2-nitrophenyloxy)propyl]-
piperazin-l-yl}-2,4(lH,3H)-pyrimidinedione hydrochloride
(Compound 140).
Analytical results of Compound 140 thus obtained:
Melting point: 271~C (decomposed).
Elemental analysis for ClgH24N5O5Cl-HCl:
Calculated (%): C, 48.11; H, 5.31; N, 14.76;
Cl, 14.95.
Found (%): C, 47.80; H, 5.33; N, 14.62;
Cl, 14.86.
Example 86:
Preparation of 1,3-dimethyl-6-{4-[3-(2-chloro-4-
20nitrophenyloxy)propyl~piperazin-1-yl}-2,4-
(lH,3H)-pyrimidinedione hydrochloride
(Compound 141):
O2N ~ OH+HOCR2CH2C~2N N ~ O-CH3
(Compound 139)
HCl/CH3OH
~00~389
- 268 -
2 ~ Oc~2cH2cH2N~-~N ~ -CH
(Compound 141)
2.4 g of 2-chloro-4-nitrophenol, 3.38 g of 1,3-
dimethyl-6-[4-(3-hydroxypropyl)piperazin-1-yl]-
2,4(1H,3H)-pyrimidinedione (Compound 139) and 3.62 g of
triphenylphosphine were suspended in 70 me of
anhydrous tetrahydrofuran, followed by the addition of
2.4 g of diethyl azodicarboxylate. The resultant
mixture was treated in a similar manner to Example
84-(2), thereby obtaining 2.5 g of pale yellow
crystals. Those crystals were recrystallized from
ethanol so that 2.07 g of 1,3-dimethyl-6-{4-t3-(2-
chloro-4-nitrophenyloxy)propyl]piperazin-1-yl}-
2,4(lH,3H)-pyrimidinedione were obtained as crystals.
Analytical results of crystals of the pyrimidinedione
derivative thus obtained:
Melting point: 133-134~C.
Elemental analysis for ClgH24N5O5Cl:
Calculated (%): C, 52.12; H, 5.52; N, 15.99;
C1, 8.10.
Found (%): C, 51.99; H, 5.72; N, 15.70;
ZQ~89
- 269 -
Cl, 8.06.
NMR (CDC13), ~ppm: 1.8-2.3(m,2H), 2.3-3.1(m,10H),
3.33(s,3H), 3.41(s,3H), 4.24(t,2H),
5.26(s,1H), 7.0-8.4(m,3H).
Next, 0.5 g of the pyrimidinedione derivative
was treated in a 15% HCl/methanol solution by a method
known ~ se in the art to obtain 0.5 g of 1,3-
dimethyl-6-{4-[3-(2-chloro-4-nitrophenyloxy)propyl]-
piperazin-l-yl}-2,4(lH,3H)-pyrimidinedione hydrochloride
(Compound 141).
Analytical results of Compound 141 thus obtained:
Melting point: 115-120~C.
Elemental analysis for ClgH24N5O5Cl-HCl-H2O:
Calculated (%): C, 46.35; H, 5.53; N, 14.22;
Cl, 14.40.
Found (%): C, 46.25; H, 5.29; N, 14.46;
Cl, 14.69.
IR vmaBr (cm 1): 1700, 1345, 1285, 1200, 1130,
1055, 900, 830, 750.
Example 87:
Preparation of 1,3-dimethyl-6-{4-t3-(4-methane-
sulfonamido-2-nitrophenyloxy)propyl]piperazin-1-
yl}-2,4(1H,3H)-pyrimidinedione hydrochloride
(Compound 142~:
Z(~01389
- 270 -
H2N ~ OH ~CH3CONH ~ OH
N02 N02
(Compound 143)
s
BrCH2CH2CH2Br
) CH3CONH ~ 2 2 2
N02
1) 2N HCl
2) CH3S02Cl
CH3S02NH~OCH2CH2CH2C
N02
HN N ~ N-CH
~-~ N-~o 3
CH3 HCl/CH30H
(Compound 157)
CH3S02NH ~ O~H2CH2C~2N~_~N ~ N CH3
N02 CH3
(Compound 142)
(1) Preparation of 4-acetamido-2-nitrophenol (Compound
143):
Z~i1389
- 271 -
10.1 g of 4-amino-2-nitrophenol were dissolved
in 80 m~ of acetic acid, followed by the addition of
6.7 g of acetic anhydride over 5 minutes. After
stirring the resultant mixture for 1 hour, the solvent
S was distilled off under reduced pressure. Water was
added to the residue, whereby a solid of a dark brown
color precipitated. The solid was collected by
filtration. The solid was washed with water and then
recrystallized from water-containing ethanol, thereby
obtaining 10.2 g of 4-acetamido-2-nitrophenol (Compound
143) as crystals.
Analytical results of crystals of Compound 143 thus
obtained:
Melting point: 158-159~C.
(2) Preparation of 3-(4-acetamido-2-nitrophenyloxy)-
propyl bromide:
A mixture consisting of 10.2 g of 4-acetamido-2-
nitrophenol (Compound 143) obtained by the above
procedure, 13.9 g of potassium carbonate, 100 m~ of
methyl ethyl ketone and 40.2 g of 1,3-dibromopropane
was reacted under reflux for 6 hours. Insoluble
matters were filtered off from the reaction mixture and
the filtrate was concentrated under reduced pressure.
The residue was dissolved in chloroform. The
chloroform solution was washed with water and then
dried over anhydrous magnesium sulfate. The solvent
Z~0~389
- - 272 -
was distilled off under reduced pressure. The residue
was then purified by chromatography on a silica gel
column leluent: chloroform/methanol = 100/1, by
volume), followed by recrystallization from dichloro-
s methane-hexane to obtain 5.2 g of 3-(4-acetamido-2-
nitrophenyloxy)propyl bromide as crystals.
Analytical results of crystals of the propyl bromide
derivative thus obtained:
Melting point: 134~C.
Elemental analysis for CllH13N2O3Br:
Calculated (%): C, 41.66; H, 4.13; N, 8.83;
Br, 25.13.
Found (%): C, 41.84; H, 4.35; N, 8.92;
Br, 24.73.
(3) Preparation of 3-(4-methanesulfonamido-2-nitro-
phenyloxy)propyl chloride:
1.0 g of 3-(4-acetamido-2-nitrophenyloxy)propyl
bromide obtained by the above procedure was suspended
in 15 m~ of 2N hydrochloric acid. After heating the
resultant mixture under reflux for 30 minutes, 10 m~
of acetic acid were added, followed by heating under
reflux for additional 4 hours. The reaction mixture
was concentrated under reduced pressure and water was
added to the residue. Sodium carbonate was added to
the resultant aqueous mixture to adjust the pH to 7-8.
The aqueous mixture was then extracted with chloroform.
~i1;389
- 273 -
The extract was dried over anhydrous sodium sulfate.
The drying agent was filtered off. The filtrate was
added with 1 m~ of pyridine. A solution of 0.5 g of
mesyl chloride in 5 m~ of chloroform was added
dropwise under ice-cooling. After stirring the thus-
obtained mixture for 2 hours under ice-cooling, it was
stirred at room temperature for 2 hours and then
extracted twice with a 2 N solution of sodium
hydroxide. The resultant aqueous layer was washed with
chloroform. This aqueous layer was acidified with 6 N
hydrochloric acid and then extracted with ethyl
acetate. The extract was concentrated under reduced
pressure and the residue was recrystallized from
ethanol, thereby obtaining 0.7 g of 3-(4-methanesulfon-
amido-2-nitrophenyloxy)propyl chloride as crystals.
Analytical results of crystals of the propyl chloride
derivative thus obtained:
Elemental analysis for CloH13ClN2O5S:
Calculated (%): C, 38.90 H, 4.24; N, 9.07;
Cl, 11.49; S, 10.38.
Found (%): C, 38.90; H, 4.30; N, 8.94;
Cl, 10.72; S, 10.03.
(4) Preparation of 1,3-dimethyl-6-{4-t3-(4-methane-
sulfonamido-2-nitrophenyloxy)propyl]piperazin-1-yl}-
2,4(lH,3H)-pyrimidinedione hydrochloride
(Compound 142):
2~1389
- 274 -
0.31 g of 3-(4-methanesulfonamido-2-nitro-
phenyloxy)propyl chloride, 0.22 g of 1,3-dimethyl-6-(1-
piperazinyl)-2,4(1H,3H)-pyrimidinedione (Compound 157)
and O.S ml of triethylamine were dissolved under heat
S in 10 m~ of ethanol to give a uniform solution. The
solvent was distilled off under reduced pressure.
After heating the residue at 110~C for 2 hours, 10 m~
of n-butanol were added and the resulting mixture was
heated under stirring at 110~C for S hours. The
reaction mixture was concentrated under reduced
pressure and the residue was dissolved in a dilute
aqueous solution of sodium hydroxide. The thus-
prepared solution was washed with chloroform. The
water layer was then adjusted to pH 1-2 with dilute
lS hydrochloric acid, washed with ethyl acetate, and then
added with sodium hydrogencarbonate to raise the pH to
7-8. Precipitated insoluble matters were collected by
filtration. The thus-collected substance was dried
under reduced pressure and then dissolved in methanol.
15% HCl/methanol was added to convert it into the
hydrochloride by a method known per se in the art,
thereby obtaining 0.2 g of 1,3-dimethyl-6-{4-[3-(4-
methanesulfonamido-2-nitrophenyloxy)propyl]piperazin-1-
yl}-2,4(lH,3H)-pyrimidinedione hydrochloride (Compound
142) as crystals.
- Z~01389
-- - 275 -
Analytical results of crystals of Compound 142 thus
obtained:
Melting point: 239~C.
Elemental analysis for C20H28N607S~HCl-~H20:
Calculated (%): C, 44.32; H, 5.58; N, 15.51;
Cl, 6.54; S, 5.92.
Found (%): C, 44.30; H, 5.58; N, 15.45;
Cl, 6.96; S, 5.94.
Example 88:
Preparation of 1,3-dimethyl-6-{4-[3-(4-
acetamido-2-nitrophenyloxy)propyl]piperazin-1-
yl}-2,4(lH,3H)-pyrimidinedione hydrochloride
(Compound 144):
CH3CONH ~ OH+HOCH2CH2CH2N N ~ N-CH3
2 C~3
(Compound 143) (Compound 139)
HCl/CH30H
>
CH3CONH ~ ~C~2c~'2c~'2N ~ h-C~3
(Compound 144)
2.71 g of 4-acetamido-2-nitrophenol (Compound
143) o~tained in Example 87-(1), 3.38 g of 1,3-
- Z~01~89
- 276 -
dimethyl-6-[4-(3-hydroxypropyl)piperazin-1-yl3-
2,4(1H,3H)-pyrimidinedione (Compound 139) and 3.62 g of
triphenylphosphine were suspended in 70 mQ of
anhydrous tetrahydrofuran, followed by the addition of
2.4 g of diethyl azodicarboxylate. The thus-prepared
mixture was treated in a similar manner to Example
84-(2), thereby obtaining 2.7 g of pale yellow
crystals. 2.0 g of the crystals were treated in a
15% HCl/methanol solution by a method known ~er se in
the art to obtain 2.5 g of 1,3-dimethyl-6-{4-[3-(4-
acetamido-2-nitrophenyloxy)propyl]piperazin-1-yl}-
2,4(lH,3H)-pyrimidinedione hydrochloride (Compound
144).
Analytical results of Compound 144 thus obtained:
Melting point: 228.5-230~C.
Elemental analysis for C21H28N6O6-HCl:
Calculated (%): C, 50.76; H, 5.88; N, 16.91;
Cl, 7.13.
Found (%): C, 50.67; H, 6.27; N, 17.07;
Cl, 6.81.
Example 89:
Preparation of 1,3-dimethyl-6-{4-[3-(2-
hydroxy-5-nitrophenyloxy)propyl]piperazin-1-
yl}-2,4(1H,3H)-pyrimidinedione hydrochloride
(Compound 145):
89
- 277 -
O2N ~ OH+HOCH2CH2C~2N N ~ -CH3
(Compound 139J
02N ~ OCH2CH2CH2N~ N CH3
OH CH3
(Compound 145 - free form)
HC1/CH30H
H2~ ~ ~ -C~3.
OH CH3
(Compound 145)
0.63 g of 2-nitrocatechol, 1.0 g of 1,3-
dimethyl-6-[4-(3-hydroxypropyl)piperazin-1-yl]-
2,4(1H,3H)-pyrimidinedione (Compound 139) and 1.06 g of
triphenylphosphine were suspended in 15 ml of
anhydrous tetrahydrofuran, followed by the addition of
0.71 g of diethyl azodicarboxylate. The thus-prepared
mixture was treated in a similar manner to Example
84-(2), thereby obtaining 0.65 g of pale yellow
crystals. The crystals were recrystallized form
ethanol to obtain 0.55 g of 1,3-dimethyl-6-{4-[3-
(2-hydroxy-5-nitrophenyloxy)propyl]piperazin-1-yl}-
89
- 278 -
2,4(1H,3H)-pyrimidinedione (Compound 145 - free form)
as crystals.
Analytical results of crystals of the pyrimidinedione
derivative thus obtained:
Melting point: 171-173~C.
Elemental analysis for C19H25N5O5:
Calculated (%): C, 54.40; H, 6.00; N, 16.69.
Found (%): C, 54.40; H, 6.28; N, 16.48.
NMR (CDC13), ~ppm: 1.9-2.2(m,2H), 2.6-3.2(m,10H),
3.2(m,10H), 3.3(s,3H), 3.4(s,3H),
4.1(t,2H), 5.24(s,1H),
6.88-7.08(m,lH), 7.60-7.80(m,2H).
IR vKBr (cm~l) 1700, 1620, lS00, 1340, 1285,
max
1280, 1140, 990, 870.
Next, 0.5 g of the pyrimidinedione derivative
were treated in a 20% HCl/methanol solution by a method
known per se in the art to obtain 0.5 g of 1,3-
dimethyl-6-{4-[3-(2-hydroxy-5-nitrophenyloxy)propyl]-
piperazin-l-yl}-2,4(1H,3H)-pyrimidinedione
hydrochloride (Compound 145) as crystals.
Analytical results of crystals of Compound 145 thus
obtained:
Melting point: 246-249~C.
Elemental analysis for ClgH25N5O5-HCl-2H2O:
Calculated (%): C, 46.39; H, 6.15; N, 14.24;
Cl, 7.21.
~ - 279 - ~ a ~ 89 -
Found (%): C, 46.83; H, 5.97; N, 14.55;
Cl, 7.77.
Example 90:
Preparation of 1,3-dimethyl-6-{4-[3-(2-
allyloxy-5-nitrophenyloxy)propyl]piperazin-1-
yl}-2,4(lH,3H)-pyrimidinedione hydrochloride
(Compound 146):
(
~0CH2CR2CH2N~N~N-C~3
OH CH3
(Compound 145 - free form)
HCl/CH30H
> >
02N ~ OC~2C~2C~2~_JN ~ N CB3 HC
( OCH2CH=CH2 H3C
(Compound 146)
1 g of 1,3-dimethyl-6-{4-13-(2-hydroxy-5-nitro-
phenyloxy)propyl]piperazin-l-yl}-2,4(1H,3H)-pyrimidine-
dione (Compound 145 - free form), 0.31 g of allyl
bromide and 0.36 g of potassium carbonate were
suspended in 10 ml of dry acetone and heated under
reflux for 5 hours. The reaction mixture was poured
, ~ .....
Z~1389
- 280 -
into 60 m~ of water and then extracted three times
with 30 m~ portions of chloroform. The extract was
washed first with a 0.5 N aqueous solution of sodium
hydroxide and then with water. The chloroform solution
was dried over anhydrous sodium sulfate. The solvent
was distilled off under reduced pressure and the
residue was purified by chromatography on a silica gel
column (eluent: chloroform/methanol = 20/1, by volume).
The resulting oily substance was crystallized from
ethanol, thereby obtaining 0.78 g of 1,3-dimethyl-6-{4-
[3-(2-allyloxy-5-nitrophenyloxy)propyl]piperazin-1-yl}-
2,4(lH,3H)-pyrimidinedione as crystals.
Analytical results of crystals of the pyrimidinedione
derivative thus obtained:
Melting point: 120-121~C.
NMR (CDC13), ~ppm: 2.08(t,2H), 2.5-3.0(m,10H),
3.3(s,3H), 3.36(s,3H), 4.16(t,2H),
4.5-4.7(m,2H), 5.2(s,1H),
5.16-5.6(m,2H), 5.8-6.24(m,1H),
6.9(d,lH), 7.68-7.92(m,2H).
IR vmaBxr (cm 1): 1690, 1630, 1510, 1330,
1280, 1000.
Next, 0.5 g of the pyrimidinedione derivative
was treated in a 20~ HCl/methanol solution by a method
known per se in the art to obtain 0.5 g of 1,3-
dimethyl-6-{4-t3-(2-allyloxy-5-nitrophenyloxy)propyl]-
2~)1389
- - 281 -
piperazin-l-yl}-2,4(lH,3H)-pyrimidinedione hydro-
chloride (Compound 146) as crystals.
Analytical results of Compound 146 thus obtained:
Melting point: 161.5-180~C.
~xample 91:
Preparation of 1,3-dimethyl-6-{4-[3-(4-
methylthio-2-nitrophenyloxy)propyl]piperazin-1-
yl}-2,4(lH,3H)-pyrimidinedione hydrochloride
(Compound 147):
o
CH3S ~ OH+HOCH2C~2CH2N~--J~ ~ CH3
N~2 CH3
(Compound 139)
HCl/CH30H
CH3S ~ 0cH2cH2cH2N~~ C~3 HC
N~2 C/H3
(Compound 147)
1.5 g of 4-methylthio-2-nitrophenol, 2.0 g of
1,3-dimethyl-6-[4-(3-hydroxypropyl)piperazin-1-yl]-
2,4(1H,3H)-pyrimidinedione (Compound 139) and 2.2 g of
triphenylphosphine were suspended in 30 m~ of
2~1389
- 282 -
anhydrous tetrahydrofuran, followed by the addition of
1.35 m~ of diethyl azodicarboxylate. The thus-
prepared mixture was treated in a similar manner to
Example 84-(2), thereby obtaining 2.9 g of 1,3-
dimethyl-6-{4-[3-(4-methylthio-2-nitrophenyloxy)propyl]-
piperazin-l-yl}-2,4(lH,3H)-pyrimidinedione as crystals.
Analytical results of crystals of the pyrimidinedione
derivative thus obtained:
NMR (CDC13), ~ppm: 1.9-2.2(m,2H), 2.48(s,3H),
2.4-2.7(m,6H), 2.8-3.0(m,4H),
3.28(s,3H), 3.36(s,3H),
4.14(m,2H), 5.16(s,1H), 7.0(d,1H),
7.36(dd,1H), 7.62(d,1H).
Next, 0.5 g of the pyrimidinedione derivative
were treated in a 20% HCl/methanol solution by a method
known E~ se in the art to obtain 0.4 g of 1,3-
dimethyl-6-{4-t3-(4-methylthio-2-nitrophenyloxy)propyl]-
piperazin-l-yl}-2,4(1H,3H)-pyrimidinedione hydro-
chloride (Compound 147) as crystals.
Analytical results of crystals of Compound 147 thus
obtained:
Melting point: 224-226~C.
Elemental analysis for C20H27N5O5S-HCl:
Calculated (%): C, 49.43; H, 5.81; N, 14.41;
Cl, 7.30; S, 6.60.
Found (%): C, 49.20; H, 5.97; N, 14.28;
2()0~389
- 283 -
Cl, 7.23; S, 6.84.
Example 92:
Preparation of 1,3-dimethyl-6-{4-<3-t2~
hydroxybenzyl)-4-nitrophenyloxy]propyl>pipera-
zin-1-yl}-2,4(lH,3H)-pyrimidinedione oxalate
(Compound 148):
o2~ ~ o~+~oC~2c~2c~2~ C~3
(Compound 139)
2 ~ 2 2 ~2N~ J ~ ~ C~3
~ C~3
(Compound 149)
Oxalic acid/CH30H
02N ~ OCH2CH2CE2N~_~N ~ N CH3 ( 2
OH c~3
(Compound 148)
(1) Preparation of 1,3-dimethyl-6-{4-[3-(2-benzoyl-4-
nitrophenyloxy)propyllpiperazin-1-yl}-2,4(1H,3H)-
pyrimidinedione (Compound 149)
389
- 284 -
3.74 g of 2-benzoyl-4-nitrophenol, 3.5 g of 1,3-
dimethyl-6-[4-(3-hydroxypropyl)piperazin-1-yl]-
2,4(1H,3H)-pyrimidinedione (Compound 139) and 3.3 g of
triphenylphosphine were suspended in 50 m~ of
anhydrous tetrahydrofuran, followed by the addition of
2.40 m~ of diethyl azodicarboxylate. The thus-
prepared mixture was treated in a similar manner to
Example 84-(2), thereby obtaining 3.9 g of 1,3-
dimethyl-6-{4-t3-(2-benzoyl-4-nitrophenyloxy)propyl]-
piperazin-1-yl}-2,4(1H,3H)-pyrimidinedione (Compound
149) as crystals.
Analytical results of crystals of the pyrimidinedione
derivative (Compound 149) thus obtained:
NMR (CDC13), ~ppm: 1.9-2.2(m,2H), 2.4-2.7(m,6H),
2.8-3.0(m,4H), 3.26(s,3H),
3.36(s,3H), 4.14(m,2H), 5.14(s,1H),
7.0-7.8(m,8H), 8.36(d,2H).
(2) Preparation of 1,3-dimethyl-6-{4-<3-[2-(a-hydroxy-
benzyl)-4-nitrophenyloxy]propyl>piperazin-1-yl}-
2,4(lH,3H)-pyrimidinedione oxalate (Compound 148):
0.2 g of the pyrimidinedione derivative
(Compound 149) obtained in the above procedure and
15 mg of sodium borohydride were dissolved in ethanol.
The resultant solution was stirred at room temperature
for 6 hours. The reaction mixture was poured into
water and then extracted with chloroform. The
2~0~389
- 285 -
chloroform layer was washed with water and then dried
over anhydrous sodium sulfate. The solvent was
distilled off and the residue was purified by
chromatography on a silica gel column (eluent:
chloroform/methanol = 40/1, by volume), thereby
obtaining 0.15 g of 1,3-dimethyl-6-{4-<3-[2-(a-hydroxy-
benzyl)-4-nitrophenyloxy]propyl>piperazin-1-yl}-
2,4(lH,3H)-pyrimidinedione.
Analytical results of crystals of the pyrimidinedione
derivative thus obtained:
NMR (CDC13), ~ppm: 2.0(m,2H), 2.5-2.9(m,10H),
3.26(s,3H), 3.37(s,3H), 4.11(m,2H),
5.16(s,1H), 6.04(s,1H), 6.88(d,1H),
8.14(dd,lH), 8.52(d,lH).
Next, 0.15 g of the pyrimidinedione derivative
was treated in an oxalic acid/methanol solution by a
method known per se in the art to obtain 0.15 g of 1,3-
dimethyl-6-{4-<3-[2-(~-hydroxybenzyl)-4-nitrophenyloxy]-
propyl>piperazin-l-yl}-2,4(1H,3H)-pyrimidinedione oxalate
(Compound 148) as crystals.
Analytical results of crystals of Compound 148 thus
obtained:
Melting point: 111-115~C.
Elemental analysis for C26H31N5O6 2 2
Calculated (%): C, 52.91; H, 5.87; N, 11.02.
Found (%): C, 52.74; H, 5.65; N, 10.94;
IR vKBr (cm-l) 3460, 1700, 1640, 1610, 1510,
max
2~1389
- 286 -
1340, 760, 700.
Example 93:
Preparation of 1,3-dimethyl-6-{4-[3-(3-
trifluoromethyl-4-nitrophenyloxy)propyl]-
piperazin-l-yl}-2,4(lH,3H)-pyrimidinedione
oxalate (Compound 150):
~2N ~ 2 2 2N~_~N ~ ~ C~3
CF3 c/~3
(Compound 139)
Oxalic acid/CH3OH
>
O2N ~ OC~2CH2C~2U N ~ N-C~3-(COOH)2
3 C~3
(Compound 150)
l.9 g of 4-nitro-3-trifluoromethylphenol, 2.25 g
of 1,3-dimethyl-6-[4-(3-hydroxypropyl)piperazin-l-yl]-
2,4(lH,3H)-pyrimidinedione (Compound 139) and 2.41 g of
triphenylphosphine were suspended in 60 m~ of
anhydrous tetrahydrofuran, followed by the addition of
1.6 g of diethyl azodicarboxylate. The thus-prepared
mixture was treated in a similar manner to Example
2~ 389
- 287 -
84-(2), thereby obtaining 3.50 g of pale yellow
crystals. The crystals were recrystallized from
ethanol to obtain 3.31 g of 1,3-dimethyl-6-{4-[3-(3-
trifluoromethyl-4-nitrophenyloxy)propyl]piperazin-1-yl}-
2,4(lH,3H)-pyrimidinedione as crystals.
Analytical results of crystals of the pyrimidinedione
derivative thus obtained:
NMR (CDC13), ~ppm: 1.9-2.2(m,2H), 2.5-2.75(m,6H),
2.9-3.1(m,4H), 3.3(s,3H),
3.4(s,3H), 4.15(t,2H), 5.3(s,1H),
7.1-7.4(m,2H), 8.15(d,1H).
Next, 3.2 g of the pyrimidinedione derivative
were treated in an oxalic acid/methanol solution by a
method ~nown Per se in the art to obtain 3.22 g of 1,3-
dimethyl-6-{4-[3-(3-trifluoromethyl-4-nitrophenyloxy)-
propyl]piperazin-l-yl}-2,4(1H,3H)-pyrimidinedione oxalate
(Compound 150) as crystals.
Analytical results of crystals of Compound 150 thus
obtained:
Melting point: 187-189~C.
Elemental analysis for C20H24F3N5O5-(COOH)2:
Calculated (%): Ct 47.06; H, 4.67; N, 12.47;
F, 10.15.
Found (~): C, 47.51; H, 5.24; N, 12.67;
F, 10.49.
- 288 - ~ O ~ ~ 3 8 ~
Example 94:
Preparation of 1,3-dimethyl-6-{4-13-(2-
methoxycarbonyl-4-nitrophenyloxy)propyl~-
piperazin-l-yl}-2,4(lH,3H)-pyrimidinedione
oxalate (Compound 151):
02N~OH ~ 02N~OH
COOH COOCH3
~ ~~
2 2 2 ~ ~0 3
c~3
(Compound 139)
; >
02N ~ OC~2C~2CH2N~_~N ~ N--C~3
COOCH3 3
(Compound 151 - free form)
Oxalic acid/CH30H
2 ~ OC-2CH2C22N~_~N ~ -C~3~(COOH)2
COOCH3
(Compound 151)
(1) Preparation of methyl 5-nitrosalicylate:
,~ .
. " . . ~
Z~389
- 289 -
80 m~ of methanol and 10 g of concentrated
sulfuric acid were added to 5 g of 5-nitrosalicylic
acid. The resultant mixture was heated under stirring
for 8 hours whlle distilling methanol off. The solvent
was then distilled off under reduced pressure and the
residue was dissolved in chloroform. The resultant
chloroform solution was washed with water and then
concentrated to dryness. The resultant residue was
purified by chromatography on a silica gel column
(eluent: chloroform), followed by recrystallization
from chloroform-ether to obtain 5.1 g of methyl
5-nitrosalicylate as crystals.
Analytical results of crystals-of methyl 5-nitro-
salicylate thus obtained:
Melting point: 114-116~C.
(2) Preparation of 1,3-dimethyl-6-{4-[3-(2-methoxy-
carbonyl-4-nitrophenyloxy)propyl]piperazin-1-yl}-
2,4(1H,3H)-pyrimidinedione oxalate (Compound 151):
2.96 g of methyl 5-nitrosalicylate obtained by
the above procedure, 4.23 g of 1,3-dimethyl-6-t4-
(3-hydroxypropyl)piperazin-1-yl]-2,4(1H,3H)-pyrimidine-
dione (Compound 139) and 3.93 g of triphenylphosphine
were suspended in 50 m~ of anhydrous tetrahydrofuran,
followed by the addition of 2.6 g of diethyl azo-
dicarboxylate. The thus-prepared mixture was treated
in a similar manner to Example 84-(2), thereby
-
~9~ 89
obtaining 6.5 g of pale yellow crystals. The crystals
were recrystallized from methanol to obtain 5.74 g of
1,3-dimethyl-6-{4-~3-(2-methoxycarbonyl-4-nitro-
phenyloxy)propyl]piperazin-l-yl}-2,4(lH,3H)-pyrimidine-d
ione (Compound 151 - free form) as crystals.
Analytical results of crystals of the pyrimidinedione
derivative (Compound 151 - free form) thus obtained:
Melting point: 145-146~C.
NMR (CDC13), ~ppm: 1.8-2.2(m,2H), 2.4-3.0(m,10H),
3.27(s,3H), 3.36(s,3H), 3.87(s,3H),
4.17(t,2H), 5.24(s,1H),
7.0-8.7(m,2H).
Next, 5.5 g of the pyrimidinedione derivative
were treated in an oxalic acid/methanol solution by a
method known per se in the art to obtain 5.6 g of 1,3-
dimethyl-6-{4-[3-(2-methoxycarbonyl-4-nitrophenyloxy)-
propyl]piperazin-l-yl}-2,4(lH,3H)-pyrimidinedione
oxalate (Compound 151) as crystals.
Analytical results of crystals of Compound 151 thus
obtained:
Melting point: 186-187~C (decomposed).
Y 21 27 5 7 ( )2 3
Calculated (%): C, 47.92; H, 5.86; N, 11.64.
Found (%): C, 47.74; H, 5.66; N, 11.43.
IR vmax ~cm 1): 1655, 1520, 1345, 1200, 1130,
1080, 820, 765, 750.
c~l3~9
- - 291 -
Example 95:
Preparation of 1,3-dimethyl-6-{4-[3-(2-
carboxy-4-nitrophenyloxy)propyl]piperazin-1-yl}-
2,4(lH,3H)-pyrimidinedione oxalate
(Compound 152):
2 ~ 0C~2C~2C~2N~_~N ~ _CH3
COOCH3
(Compound 151 - free form)
Oxalic acid/CH30H
2 ~ 2C 2CH2~_~N ~ CH3
(Compound 152)
1.38 g of 1,3-dimethyl-6-{4-[3-(2-methoxy-
carbonyl-4-nitrophenyloxy)propyl]piperazin-1-yl}-
2,4(lH,3H)-pyrimidinedione (Compound 151 - free form)
were dissolved in 200 ml of methanol, followed by the
addition of 100 m~ of a 1.5 N aqueous solution of
sodium hydroxide. The resultant mixture was stirred at
60~C for 30 minutes. The reaction mixture was allowed
to cool down, neutralized with dilute hydrochloric
acid, and then concentrated to a total volume of 50 mQ
under reduced pressure. The concentrate was ice-cooled
6)1389
- 292 -
and precipitated crystals were collected by filtration,
thereby obtaining 1.16 g of 1,3-dimethyl-6-{4-[3-(2-
carboxy-4-nitrophenyloxy)propyl]piperazin-1-yl}-
2,4(lH,3H)-pyrimidinedione.
Analytical results of crystals of the pyrimidinedione
derivative thus obtained:
Melting point: 155-158~C.
NMR (DMSO-d6), ~ppm: 1.8-2.3(m,2H), 2.4-3.2(m,10H),
3.13(s,3H), 3.27(s,3H), 4.2(t,2H),
5.2(s,1H), 7.3-8.5(m,3H).
Next, 1.0 g of the pyrimidinedione derivative
was treated in an oxalic acid/methanol solution by a
method known per _ in the art to obtain 0.9 g of 1,3-
dimethyl-6-{4-[3-(2-carboxy-4-nitrophenyloxy)propyl]-
piperazin-1-yl}-2,4(lH,3H)-pyrimidinedione oxalate
(Compound 152) as crystals.
Analytical results of crystals o~ Compound 152 thus
obtained:
Melting point: 191-193~C (decomposed).
Elemental analysis for C20H25N5O7-(COOH)2-H2O:
Calculated (~): C, 47.57; H, 5.26; N, 12.61.
Found (~): C, 47.85; H, 5.32; N, 12.49.
IR vmax (cm 1): 1700, 1650, 1500, 1345, 1290,
1135, 1075, 760, 750.
Example 96:
Preparation of 1,3-dimethyl-6-{4-[3-(2-amino-4-
-
2~01389
-
- - 293 -
nitrophenyloxy)propyl]piperazin-l-yl}-
2,4(lH,3H)-pyrimidinedione oxalate
(Compound 153):
o
2 ~ 2 2 2 ~_~ ~ 3
NH2 c~3
(Compound 139)
Oxalic acid/CH30H
. o
2 ~ OC~2CH2C~2N~ N--CB3~(COOH)2
NH2 c/~3
(Compound 153)
1.54 g of 2-amino-4-nitrophenol, 2.82 g of
1,3-dimethyl-6-[4-(3-hydroxypropyl)piperazin-1-yl]-
2,4(1H,3H)-pyrimidinedione (Compound 139) and 2.88 g of
triphenylphosphine were suspended in 20 m~ of
anhydrous tetrahydrofuran, followed by the addition of
1.92 g of diethyl azodicarboxylate. The thus-prepared
mixture was stirred for 1 hour at room temperature and
a precipitate was collected by filtration. The
precipitate was washed with chloroform-ether to obtain
1.44 g of crytals. The crystals were recrystallized
from ethanol-chloroform, thereby obtaining 0.6 g of
1,3-dimethyl-6-{4-[3-(2-amino-4-nitrophenyloxy)propyll-
- 29i~QO1389
piperazin-l-yl}-2, 4tlH,3H)-pyrimidinedione as crystals.
Analytical results of crystals of the pyrimidinedione
derivative thus obtained:
Melting point: 188~C.
NMR (DMSO-d6), ~ppm: 1.9-2.0(m,2H), 2.4-3.0(m,10H),
3.1(s,3H), 3.24(s,3H), 4.08(m,2H),
5.15(s,1H), 5.35(br.2H),
6.8-7.5(m,3H).
Next, 0.2 g of the pyrimidinedione derivative
was treated in an oxalic acid/methanol solution by a
method known per se in the art to obtain 0.21 g of 1,3-
dimethyl-6-{4-[3-(2-amino-4-nitrophenyloxy)propyl]
piperazin-l-yl}-2,4(lH,3H)-pyrimidinedione oxalate
(Compound 153) as crystals.
Analytical results of crystals of Compound 153 thus
obtained:
Melting point: 145~C.
Elemental analysis for ClgH26N6O5-(COOH)2:
Calculated (~): C, 49.60; H, 5.55; N, 16.53.
Found (%): C, 49.53; H, 5.96; N, 16.49.
IR vmax (cm 1): 3430, 3340, 2940, 1690, 1650,
1510, 1435, 1340, 1290, 1235,
1075.
Example 97:
Preparation of 1,3-dimethyl-6-{4-[3-(4-
methoxycarbonyl-2-nitrophenyloxy)propyl]-
-- 2~Q~3as
piperazin-l-yl}-2,4(lH,3H)-pyrimidinedione
oxalate (Compound 154):
Ho~CoocH3+HocH2cH2cH2N~N~ - cH3
02N CH3
(Compound 139)
Oxalic acid/CH30H
H300C ~ OC~2CH2C~2N~_~N ~ -C~3 ~COOH)2
(Compound 154)
0.84 g of methyl 4-hydroxy-3-nitrobenzoate,
1.5 n . 8 g of 1,3-dimethyl-6-[4-(3-hydroxypropyl)piperazin-
l~yl]-2,4(1H,3H)-pyrimidinedione (Compound 139) and
0.88 g of triphenylphosphine were suspended in 14 m~
of anhydrous tetrahydrofuran, followed by the addition
of 0.54 g of diethyl azodicarboxylate. The thus-
prepared mixture was treated in a similar manner to
Example 84-(2), thereby obtaining 1.1 g of 1,3-
dimethyl-6-{4-[3-(4-methoxycarbonyl-2-nitrophenyloxy)-
propyl]piperazin-l-yl}-2,4(1H,3H)-pyrimidinedione as an
oily substance.
Analytical results of the pyrimidinedione derivative
thus obtained:
ZO~)138~
- 296 -
NMR (CDC13), ~ppm: 2.1(m,2H), 2.3-3.0(m,10H),
3.3(s,3H), 3.4(s,3H), 3.95(s,3H),
4.3(t,2H), 5.25(s,1H), 7.25(d,1H),
8.27(dd,lH), 8.61(d,lH).
Next, 1.0 g of the pyrimidinedione derivative
was treated in an oxalic acid/methanol solution by a
method known per _ in the art to obtain 1.0 g of 1,3-
dimethyl-6-{4-[3-(4-methoxycarbonyl-2-nitrophenyloxy)-
propyl]piperazin-l-yl}-2,4(1H,3H)-pyrimidinedione
oxalate ~Compound 154) as crystals.
Analytical results of crystals of Compound 154 thus
obtained:
Melting point: 190-193~C (decomposed).
Elemental analysis for C21H27N5O7-(COOH)2-3H2O:
Calculated (%): C, 45.62; H, 5.83; N, 11.57.
Found (%): C, 45.92; H, 5.21; N, 11.84.
IR vmax (cm 1): 2900, 2500, 1740, 1720, 1700,
1640, 1620, 1530, 1340, 800.
Example 98:
Preparation of 1,3-dimethyl-6-{4-[3-(2-
cyano-4-nitrophenyloxy)propyl]piperazin-1-yl}-
2,4(lH,3H)-pyrimidinedione oxalate
(Compound 155):
2~1389
- - 297 -
02N ~ Cl+HOCX2C~2Ca2N~_~N ~ C 3
CN C~3
(Compound 139)
Oxalic acid/CH30H
02N ~ OCH2CX2C~2N~_~N ~ N C~3 (C )2
CN C/H3
(Compound 155)
0.82 g of 2-chloro-5-nitrobenzonitrile, 1.0 g of
1,3-dimethyl-6-[4-(3-hydroxypropyl)piperazin-1-yl]-
2,4(1H,3H)-pyrimidinedione (Compound 139) and O.Z2 g of
sodium hydride were reacted at 0~C for 1 hour in 5 m~
of dimethylformamide. Water was then added.
Precipitated crystals were collected by filtration and
washed with water. The thus-obtained crystals were
dried in vacuum, thereby obtaining 0.9 g of 1,3-
dimethyl-6-{4-[3-(2-cyano-4-nitrophenyloxy)propyl]-
piperazin-l-yl}-2,4(lH,3H)-pyrimidinedione.
Analytical results of crystals of the pyrimidinedione
derivative thus obtained:
NMR (CDC13), ~ppm: 2.1(m,2H), 2.6-3.0(m,10H),
3.3(s,3H), 3.35(s,3H), 4.6(t,2H),
5.1(s,1H), 7.5(d,1H), 8.6(m,2H).
2~)1389
- 298 -
Next, 0.9 g of the pyrimidinedione derivative
was treated in an oxalic acid/methanol solution by a
method known ~ se in the art to obtain 0.87 g of 1,3-
dimethyl-6-{4-[3-(2-cyano-4-nitrophenyloxy)propyl]-
piperazin-1-yl}-2,4(lH,3H)-pyrimidinedione oxalate
(Compound 155) as crystals.
Analytical results of crystals of Compound 155 thus
obtained:
Melting point: 191-193~C (decomposed).
Elemental analysis for C20H24N6~5-(C~~H)2-3H2~
Calculated (%): C, 46.15; H, 5.63; N, 14.68.
Found (%): C, 45.89; H, 5.38; N, 14.29.
IR Vmax (cm ): 2200, 1680, 1630, 1600, 1580,
1520, 1340, 840.
~5 Example 99:
Preparation of 1,3-dimethyl-6-{4-[3-(2-cyano-
4-nitroanilino)propyl]piperazin-1-yl}-
2,4(lH,3H)-pyrimidinedione oxalate
(Compound 156):
O O
BrCH2CH2CH2Br ~J~
NII ~ ~ N-CH2CH2cH2sr
O O
- 2~ )1389
- 299 -
~ ~4
N
CH3
(Compound 157) Hydrazine H2O
> >
H2NCH2CH2CH2N~_~N N_~N CH3
CH3
(Compound 158)
Oxalic acid/CH OH
CN 3
O2N ~ NHCH2C~2C~2~_~N ~ ~N-C~3 (COOH)2
CH3
(Compound 156)
(1) Preparation of 1,3-dimethyl-6-t4-(3-aminopropyl)-
piperazin-l-yl]-2,4(1H,3H)-pyrimidinedione (Compound
158):
A solution in which 18.52 g of potassium
phthalimide and 200 g of 1,3-dibromopropane were
suspended in 100 mQ of dimethylformamide was heated
under stirring at 120~C for 6 hours, so that potassium
;~;)0~L389
- 300 -
phthalimide and 1,3-dibromopropane were reacted. Next,
insoluble matters were filtered off from the reaction
mixture and the filtrate was concentrated to dryness
under reduced pressure. The residue was washed with
hexane and then recrystallized from ethanol-water.
Crystals-thus obtained were collected by filtration,
washed and then dried, thereby obtaining 13.8 g of
N-(3-bromopropyl)phthalimide.
Next, 13.0 g of N-(3-bromopropyl)phthalimide,
10.3 g of 1,3-dimethyl-6-(1-piperazinyl)-2,4(1H,3H)-
pyrimidinedione (Compound 157) and 20 g of triethyl-
amine were suspended in 200 m~ of dioxane. The
suspension was thereafter heated under reflux for 6
hours.
Further, insoluble matters were filtered off
from the reaction mixture and the filtrate was
concentrated to dryness under reduced pressure. The
residue (dry concentrate) was recrystallized from ethyl
acetate/n-hexane. Resultant crystals were collected by
filtration, washed and then dried, thereby obtaining
1.25 g of 1,3-dimethyl-6-[4-(3-phthaloylaminopropyl)-
piperazin-l-yl]-2,4(1H,3H)-pyrimidinedione.
12.5 g of the crystals and 6.0 g of hydrazine
hydrate were next suspended in 200 m~ of ethanol. The
suspension was heated for 4 hours under reflux. After
allowing the suspension to cool down, resultant
Z~0~389
~ - 301 -
insoluble matters were filtered off. The filtrate was
concentrated to dryness under reduced pressure. Then,
the residue (dry concentrate) was dissolved in water,
to which dilute hydrochloric acid was added to adjust
the pH to about 3. Insoluble matters formed upon the
pH adjustment were filtered off. The filtrate was
added with a large amount of potassium carbonate and
then extracted with chloroform. After completion of
the extraction, the resultant organic layer was dried
over anhydrous sodium sulfate and then heated under
reduced pressure to distill the solvent off, whereby
6.80 g of 1,3-dimethyl-6-[4-(3-aminopropyl)piperazin-
l-yl]-2,4(lH,3H)-pyrimidinedione (Compound 158) were
obtained as a colorless syrupy substance. The syrupy
substance was crystallized when allowed to stand
overnight.
Analytical results of crystals of Compound 158 thus
obtained:
Melting point: 85-88~C.
NMR (CDC13), ~ppm: 1.58(br.2H), 1.66(m,2H),
2.48(t,2H), 2.59(m,4H), 2.78(t,2H),
2.97(m,4H), 3.32(s,3H), 3.38(s,3H),
5.24(s,1H).
(2) Preparation of 1,3-dimethyl-6-{4-[3-(2-cyano-
4-nitroanilino)propyl]piperazin-1-yl}-2,4(1H,3H)-
pyrimidinedione oxalate (Compound 156):
389
- 302 -
0.3 g of Compound 158 obtained beforehand, 0.3 g
of 2-chloro-5-nitrobenzonitrile and 0.31 m~ of
triethylamine were stirred at 80~C for 1 hour in 5 m~
of dimethylformamide. The reaction mixture was concell~dLed
under reduced pressure and the residue was purified by
chromatography on a silica gel column (eluent:
chloroform/methanol = 40/1, by volume), thereby
obtaining 0.4 g of 1,3-dimethyl-6-{4-t3-(2-cyano-
4-nitroanilino)propyl]piperazin-1-yl}-2,4(1H,3H)-
pyrimidinedione.
Analytical results of the pyrimidinedione derivative
thus obtained:
NMR (CDC13), ~ppm: 2.0(m,2H), 2.6-3.3(m,12H),
3.35(s,3H), 3.45(s,3H), 5.33(s,1H),
7.28(m,1H), 8.4-8.6(m,2H).
Next, 0.4 g of the pyrimidinedione derivative
was treated in an oxalic acid/methanol solution by a
method known per _ in the art to obtain 0.41 g of 1,3-
dimethyl-6-{4-[3-(2-cyano-4-nitroanilino)propyl]-
piperazin-1-yl}-2,4(1H,3H)-pyrimidinedione oxalate
(Compound 156) as crystals.
Analytical results of crystals of Compound 156 thus
obtained:
Melting point: 156-158~C (decomposed).
Elemental analysis for C20H25N7O4 2 2
Calculated (%): C, 49.41; H, 5.43; N, 17.94.
Z~
- 303 -
Found (~): C, 49.34; H, 5.46; N, 18.31.
IR vmax (cm 1): 3300, 2250, 2200, 1530, 1340,
1160, 800, 750, 700.
Example 100:
Preparation of 1,3-dimethyl-6-{4-t3-(2-chloro-
4-nitroanilino)propyl]piperazin-1-yl}-
2,4(lH,3H)-pyrimidinedione oxalate
(Compound 159):
10 0 N~Cl+H2NCH2 CH2CE~2N~N~N--CH3
(Compound 158)
Oxalic acid/CH30H
>
O2N ~ NHCH2CH2CH2N~_~N ~ C~3 (C )2
(Compound 159)
A mixture consisting of 0.4 g of Compound 158
obtained in Example 99-(1), 0.4 g of 3,4-dichloro-
nitrobenzene, 0.43 ml of triethylamine and 6 ml of
dimethylformamide was treated in a manner similar to
Example 99-(2), thereby obtaining 0.55 g of 1,3-
Z~1389
- 304 -
dimethyl-6-{4-[3-(2-chloro-4-nitroanilino)propyl]-
piperazin-l-yl}-2,4(1H,3H)-pyrimidinedione.
Analytical results of crystals of the pyrimidinedione
derivative thus obtained:
NMR (CDC13), ~ppm: 2.0(m,2H), 2.5-3.3(m,10H),
3.28(s,3H), 3.39(s,3H), 5.23(s,1H),
6.77(m,1H), 8.11(m,1H), 8.20(m,1H).
Next, 0.5 g of the pyrimidinedione derivative
was treated in an oxalic acid/methanol solution by a
method known per se in the art to obtain 0.53 g of 1,3-
dimethyl-6-{4-[3-(2-chloro-4-nitroanilino)propyl]-
piperazin-l-yl}-2,4(lH,3H)-pyrimidinedione oxalate
(Compound 159) as crystals.
Analytical results of crystals of Compound 159 thus
obtained:
Melting point: 153-155~C (decomposed).
Y 19 2sC 6 4 (C )2 ~ 2
Calculated (%): C, 44.10; H, 5.64; N, 14.69;
Cl, 6.20.
Found (%): C, 44.14; H, 5.20; N, 14.63;
Cl, 6.13.
IR vmBax (cm 1): 2250, 1690, 1640, 1630, 1590,
1530, 1330, 800, 740.
Example 101:
Preparation of 1,3-dimethyl-6-{4-[3-(2-methoxy-
5-nitrophenyloxy)propyl]piperazin-1-yl}-
200138~
- 305 -
2,4(lH,3H)-pyrimidinedione hydrochloride
(Compound 160)
OH~HOC~2CH2CH2N~_J~ ~ -CH3
(Compound 139)
HCl/CH30H
02N ~ ~
OC~2C~2C~2N~ N Ca3 H
OCH3 CH 3
~ (Compound 160)
0.9 g of 2-methoxy-5-nitrophenol, 1.0 g of 1,3-
dimethyl-6-[4-(3-hydroxypropyl)piperazin-1-yl]-
2,4(lH,3H)-pyrimidinedione (Compound 139) and 1.1 g of
triphenylphosphine were suspended in 20 m~ of
anhydrous tetrahydrofuran, followed by the addition of
0.67 m~ of diethyl azodicarboxylate. The thus-
prepared mixture was treated in a simila-r manner to
Example 84-(2), thereby obtaining 1.2 g of 1,3-
dimethyl-6-{4-[3-(2-methoxy-5-nitrophenyloxy)propyl]-
piperazin-l-yl}-2,4(1H,3H)-pyrimidinedione as an oily
substance.
Analytical results of the pyrimidinedione derivative
thus obtained:
2~
NMR (CDC13), ~ppm: 2.15(m,2H), 2.5-3.2(m,10H),
3.3(s,3H), 3.4(s,3H), 3.97(s,3H),
4.23(t,2H), 5.26(s,1H), 6.96(d,1H),
7.86(m,2H).
Next, 1.1 g of the pyrimidinedione derivative
was treated in a 10% HCl/methanol solution by a method
known per se in the art to obtain 1.17 g of 1,3-
dimethyl-6-{4-[3-(2-methoxy-5-nitrophenyloxy)propyl]-
piperazin-l-yl}-2,4(lH,3H)-pyrimidinedione hydro-
chloride (Compound 160) as crystals.
Analytical results of crystals of Compound 160 thus
obtained:
Melting point: 158-162~C (decomposed).
Elemental analysis for C20H27N5O6-HCl-H2O:
Calculated (%): C, 49.23; H, 6.20; N, 14.35;
Cl, 7.27.
Found (%): C, 49.04; H, 6.28; N, 14.26;
Cl, 7.54.
IR vKBr (cm~l) 3400, 2950, 1660, 1620, 1540,
max
1350, 1260, 1010, 810, 770.
Example 102:
Preparation of 1,3-dimethyl-6-{4-[3-(2-allyloxy-
4-nitrophenyloxy)propyl]piperazin-1-yl}-
2,4(lH,3H)-pyrimidinedione hydrochloride
(Compound 161):
~ 307 ~ ~ O ~ ~ ~ 89
02N ~ OH + H~CH2CH=CH2 2 ~
OH OCH2CH=cH2
HOCH2CH2CH2N N ~ N-CH3
(Compound 139) 3
02N ~ OCH2CH2CH2N~_~N ~ C~3
OCH2CH=CH2 c~3
(Compound 161 - free form)
HCl/CH30H
( 2 ~ 2C~2c~2~ N ~ N-C~3 HC1
OCH2CH=CH2 CH~
(Compound 161)
(1) Preparation of 2-allyloxy-4-nitrophenol:
1.7 g of 4-nitrocatechol, 0.74 ml of allyl
alcohol and 2.4 g of triphenylphosphine were suspended
in 40 ml of anhydrous tetrahydrofuran, followed by the
addition of 1.5 m~ of diethyl azodicarboxylate. The
resultant mixture was stirred at room temperature for
B
2Q~1389
- 308 -
11 hours, followed by the addition of 5 g of silica
gel. The thus-prepared mixture was concentrated under
reduced pressure. The residue was purified by
chromatography on a silica gel column (eluent:
hexane/ethyl acetate = 3/1, by volume), thereby
obtaining 1.5 g of 2-allyloxy-4-nitrophenol as an oily
substance.
Analytical results of the phenol derivative thus
obtained:
NMR (CDC13), ~ppm: 4.76(m,2H), 5.22(m,lH),
5.35(m,1H), 5.96(m,1H),
6.99(d,1H), 7.91(m,2H).
(2) Preparation of 1,3-dimethyl-6-{4-[3-(2-allyloxy-4-
nitrophenyloxy)propyl]piperazin-l-yl}-2,4(lH,3H)-
pyrimidinedione hydrochloride (Compound 161):
1.5 g of 2-allyloxy-4-nitrophenol, 1.0 g of 1,3-
dimethyl-6-[4-(3-hydroxypropyl)piperazin-1-yl]-
2,4(lH,3H)-pyrimidinedione (Compound 139) and 1.1 g of
triphenylphosphine were suspended in 20 m~ of
anhydrous tetrahydrofuran, followed by the addition of
0.67 mQ of diethyl azodicarboxylate. The thus-
prepared mixture was treated in a similar manner to
Example 84-(2), thereby obtaining 1.1 g of 1,3-
dimethyl-6-{4-[3-(2-allyloxy-4-nitrophenyloxy)propyl]-
piperazin-1-yl}-2,4(1H,3H)-pyrimidinedione (Compound
161 - free form) as an oily substance.
0~389
- 309 -
Analytical results of the pyrimidinedione derivative
(Compound 161 - free form) thus obtained:
NMR (CDC13), ~ppm: 2.1(m,2H), 2.5-3.3(m,10H),
3.35(s,3H), 3.43(s,3H), 4.76(m,2H),
5.3(s,lH), 5.6(m,2H), 6.15(m,lH),
7.0(m,1H), 7.8(m,2H).
Next, 0.5 g of the pyrimidinedione derivative
was treated in a 10% HCl/methanol solution by a method
known E~ se in the art to obtain 0.4 g of 1,3-
dimethyl-6-{4-[3-(2-allyloxy-4-nitrophenyloxy)propyl]-
piperazin-l-yl}-2,4(1H,3H)-pyrimidinedione hydro-
chloride (Compound 161) as crystals.
Analytical results of crystals of Compound 161 thus
obtained:
Melting point: 134-136~C (decomposed).
Elemental analysis for C22H29N5O6-HCl-2~H2O:
Calculated (%): C, 48.84; H, 6.52; N, 12.95;
Cl, 6.55.
Found (%): C, 48.97; H, 6.27; N, 12.74;
Cl, 6.75.
IR vmax (cm 1): -~3350, 2900, 1700, 1640, 1510,
1340, 1280, 1090, 810, 760.
Example 103:
Preparation of 1,3-dimethyl-6-{4-t3-(2-hydroxy-
4-nitrophenyloxy)propyl]piperazin-1-yl}-
2,4(lH,3H)-pyrimidinedione hydrochloride
2~01389
- 310 -
(Compound 162):
2 ~ OCH2CH2CH2N N ~ N-CH
OCH2CH=CH2 C/H3
(Compound 161 - free form)
HCl/CH30H
~
02N ~ oc~2c~2c 2N N ~ -C~3 HC
(Compound 162)
0.6 g of 1,3-dimethyl-6-~4-[3-(2-allyloxy-4-
nitrophenyloxy)propyl]piperazin-l-yl}-2,4(lH,3H)-
pyrimidinedione (Compound 161 - free form) was
dissolved in 10 ml of methanol, followed by the
addition of 0.1 g of 10~ Pd/activated carbon, 0.1 g of
p-toluenesulfonic acid monohydrate and 2 ml of water.
The resultant mixture was heated for 15 hours under
stirring and reflux. After allowing the reaction
mixture to cool down, insoluble matters were filtered
off and the filtrate was concentrated. The residue was
purified by chromatography on a silica gel column
2 ~3Q~ ~ 8 9
(eluent: chloroform/methanol = 50/1, by volume),
thereby thereby obtaining 0.4 g of 1,3-dimethyl-6-{4-
[3-(2-hydroxy-4-nitrophenyloxy)propyl]piperazin-1-yl}-
2,4(lH,3H)-pyrimidinedione.
Analytical results of the pyrimidinedione derivative
thus obtained:
NMR (CDC13), ~ppm: 2.15(m,2H), 2.7-3.3(m,10H),
3.34(s,3H), 3.43(s,3H), 4.13(t,2H),
5.34(s,1H), 7.0(m,1H), 8.03(m,2H).
Next, 0.4 g of the pyrimidinedione derivative
was treated in a 10% HCl/methanol solution by a method
known ~ se in the art to obtain 0.33 g of 1,3-
dimethyl-6-{4-[3-(2-hydroxy-4-nitrophenyloxy)propyl]-
piperazin-l-yl}-2,4(1H,3H)-pyrimidinedione hydro-
chloride (Compound 162) as amorphous powder.
Analytical results of amorphous powder of Compound 162
thus obtained:
Elemental analysis for ClgH25N5O6-HCl-l~H2O:
Calculated (%): C, 47.26; H, 6.05; N, 14.05;
Cl, 7.34.
Found (%): C, 47.53; H, 6.21; N, 13.75;
Cl, 7.59.
IR Vmax (cm ) 3350, 1680, 1630, 1530, 1340,
1220, 1000, 750, 710.
Example 104:
Preparation of 1,3-dimethyl-6-{4-[3-(2-benzyl-
2n ~ ~ 3 8 9 ~i
- 312 -
amino-4-nitrophenyloxy)propyl]piperazin-1-yl}-
2,4(lH,3H)-pyrimidinedione oxalate
(Compound 163):
02N ~ OH + ~ CHO ~ 02N ~ OH
NH2 HCH2 ~
o
(, IOCH2C~2CF12 ~ N~N--cH3
c~3
(Compound 139) Oxalic acid/CH30H
02N ~ OC~2C zCH2N~ CH3~(COOH)2
(Compound 163)
(1) Preparation of 2-benzylamino-4-nitrophenol:
4.4 g of 2-amino-4-nitrophenol, 5.2 g of
benzaldehyde and 0.4 g of p-toluenesulfonic acid
monohydrate were dissolved in 300 ml of benzene. The
resultant mixture was heated under reflux for 5 hours
while removing water. The solvent was distilled off
under reduced pressure and hexane is added to the
residue. Precipitated crystals were collected by
filtration to obtain 6.6 g of crystals. The crystals
were dissolved in S5 ml of dimethylformamide, followed
~....
. ~ ,
- Z~1~)138~
- 313 -
by the addition of 2.2 g of sodium borohydride under
ice-cooling. The resultant mixture was stirred at the
same temperature for 2 hours. Ether was added to the
reaction mixture. The resultant ether solution was
washed with water and then dried over anhydrous sodium
sulfate. The solvent was distilled off to obtain 4-.2 g
of 2-benzylamino-4-nitrophenol. This product was
provided for the subsequent reaction without
purification.
Analytical results of 2-benzylamino-4-nitrophenol thus
obtained:
NMR (CDC13), ~ppm: 4.5(s,2H), 6.8-7.0(d,1H),
7.3-7.6(m,2H), 7.4(s,1H).
(2) Preparation of 1,3-dimethyl-6-{4-[3-(2-benzylamino-
4-nitrophenyloxy)propyl]piperazin-1-yl}-2,4(1H,3H)-
pyrimidinedione oxalate (Compound 163):
0.7 g of 2-benzylamino-4-nitrophenol, 0.8 g of
1,3-dimethyl-6-[4-(3-hydroxypropyl)piperazin-1-yl]-
2,4(lH,3H)-pyrimidinedione (Compound 139) and 0.9 g of
triphenylphosphine were suspended in 20 m~ of
anhydrous tetrahydrofuran, followed by the addition of
0.65 g of diethyl azodicarboxylate. The thus-prepared
mixture was treated in a similar manner to Example
84-(2), thereby obtaining 0.93 g of 1,3-dimethyl-6-{4-
[3-(2-benzylamino-4-nitrophenyloxy~propyl]piperazin-1-
yl}-2,4(lH,3H)-pyrimidinedione as crystals.
2Q~ 8~
- - 314 -
Analytical results of the pyrimidinedione derivative
thus obtained:
NMR (CDC13), ~ppm: 2.05(m,2H), 2.6(m,6H),
2.95(m,4H), 3.3(s,3H),
3.35(s,3H), 4.15(t,2H),
4.35(d,2H), 4.8(m,1H),
5.25(s,1H), 6.75(d,1H),
7.2-7.45(m,1H),
7.35(s,5H), 7.6(m,1H).
Next, 0.8 g of the pyrimidinedione derivative
was treated in an oxalic acid/methanol solution by a
method known per se in the art to obtain 0.81 g of 1,3-
dimethyl-6-{4-[3-(2-benzylamino-4-nitrophenyloxy)-
propyl~piperazin-l-yl}-2,4(1H,3H)-pyrimidinedione
oxalate (Compound 163) as crystals.
Analytical results of crystals of Compound 163 thus
obtained:
Y 26 32N6 5 (COO )2 ~ 2~
Calculated (%): C, 55.35; H, 5.81; N, 13.83.
Found (%): C, 55.73; H, 5.78; N, 13.87.
Example 105:
Preparation of 1,3-dimethyl-6-{4-[3-(2-methoxy-
4-nitrophenyloxy)propyl]piperazin-1-yl}-
2,4(lH,3H)-pyrimidinedione hydrochloride
(Compound 164):
~01389
. - 315-
~ 2 N ~ OH
OCH3 OCH3
\-- N~~o
c~3
(Compound 139) HCl/CH30H
~2N ~ 2C''2C~2N~_J~ ~ C 3 HCl
OCH3 CH3
(Compound 164)
(1) Preparation of 2-methoxy-4-nitrophenol:
50 g of 2-amino-5-nitroanisole, 50 g of sodium
hydroxide were dissolved in 450 ml of water. The
resultant mixture was heated under reflux for 3 hours
and then ice-cooled. Precipitated crystals were
collected by filtration, followed by dissolution in
water. The thus-prepared solution was neutralized with
6 N hydrochloric acid and crystals thus precipitated
were collected by filtration. The crystals were
dissolved in chloroform. The resultant solution was
washed with water and dried over anhydrous sodium
- 316 2 ~ ~ 1 3 8 9
sulfate. By concentrating the chloroform layer under
reduced pressure, 4.5 g of 2-methoxy-4-nitrophenol were
obtained as crystals.
Analytical results of 2-methoxy-4-nitrophenol thus
5 obtained: -
Melting point: 102-103~C.
(2) Preparation of 1,3-dimethyl-6-{4-[3-(2-methoxy-4-
nitrophenyloxy)propyl]piperazin-l-yl}-2,4(1H,3H)-
pyrimidinedione hydrochloride (Compound 164):
0.68 g of 2-methoxy-4-nitrophenol, 1.0 g of
1,3-dimethyl-6-[4-(3-hydroxypropyl)piperazin-1-yl]-
2,4(1H,3H)-pyrimidinedione (Compound 139) and 1.1 g of
triphenylphosphine were suspended in 20 mQ of
anhydrous tetrahydrofuran, followed by the addition of
0.71 g of diethyl azodicarboxylate. The thus-prepared
mixture was treated in a similar manner to Example
84-(2), followed by recrystallization from methanol,
whereby 1.35 g of 1,3-dimethyl-6-{4-[3-(2-methoxy-4-
nitrophenyloxy)propyl]piperazin-l-yl}-2,4(1H,3H)-
pyrimidinedione as crystals.
Analytical results of the pyrimidinedione derivative
thus obtained:
NMR (CDC13), ~ppm: 1.9-3.3(m,12H),
3.43(s,3H), 3.5(s,3H),
4.06(s,3H), 4.26(t,2H),
5.35(s,1H), 7.05(d,1H),
389
- 317 -
7.8-8.15(m,2H).
Next, 1.3 g of the pyrimidinedione derivative
was treated in a lO~HCl/methanol solution by a method
known per se in the art to obtain 1.17 g of 1,3-
dimethyl-6-{4-t3-(2-methoxy-4-nitrophenyloxy)propyl]-
piperazin-l-yl}-2,4(lH,3H)-pyrimidinedione
hydrochloride (Compound 164) as crystals.
Analytical results of crystals of Compound 164 thus
obtained:
Melting point: 135-138~C.
Elemental analysis for C20H27N5O6-HCl-l~H2O
Calculated (%): C, 48.34; H, 6.29; N, 14.09;
Cl, 7.13.
Found (%): C, 48.20; H, 6.61; N, 14.27;
Cl, 7.38.
Example 106:
Preparation of 1,3-dimethyl-6-{4-[3-(2,6-
dichloro-4-nitrophenyloxy)propyl]piperazin-1-
yl}-2,4(1H,3H)-pyrimidinedione hydrochloride
(Compound 165):
02N~OH + BrCH2CH2CH2Br
Cl
02N~OCH2CH2CH2Br
~1389
- 318 -
~0
CH3
HCl/CH30H
(Compound 157)
~C , / 11~
(Compound 165)
(1) Preparation of l-bromo-3-(2,6-dichloro-4-nitro-
phenyloxy)propane:
A mixture of 4.16 g of 2,6-dichloro-4-
nitrophenol, 40.4 g of 1,3-dibromopropane, 2.76 g of
potassium carbonate and 2.46 ~-of potassium t-butoxide
was heated under reflux for 4 hours in 50 ml of methyl
ethyl ketone. The reaction mixture was allowed to cool
down and insoluble matters were filtered off. The
filtrate was concentrated under reduced pressure. The
residue was dissolved in chloroform. The thus-prepared
chloroform solution was washed with water, and the
chloroform was then distilled off. The residue was
purified by chromatography on a silica gel column
(eluent: chloroform/hexane = 4/1, by volume) to obtain
6.24 g of 1-bromo-3-(2,6-dichloro-4-nitrophenyloxy)-
propane. This compound was employed in the nextreaction without any further purification.
1389
- 319 -
(2) Preparation of 1,3-dimethyl-6-{4-[3-(2,6-dichloro-
4-nitrophenyloxy)propyl]piperazin-1-yl}-2,4(lH,3H)-
pyrimidinedione hydrochloride (Compound 165):
4.94 g of 1-bromo-3-(2,6-dichloro-4-nitro-
phenyloxy)propane obtained by the above procedure,
3.36 g of 1,3-dimethyl-6-(1-piperazinyl)-2,4(lH,3H)-
pyrimidinedione (Compound 157) and 4 ml of triethyl-
amine were dissolved in 100 m~ of dioxane. The
thus-prepared mixture was heated for 2 hours under
stirring and reflux. The reaction mixture was allowed
to cool down and then filtered to remove insoluble
matters. ~he filtrate was concentrated to dryness
under reduced pressure and the residue was dissolved in
chloroform. The resultant chloroform solution was
washed with water and the solvent was distilled off.
The residue was purified by chromatography on a silica
gel column (eluent: chloro~orm/methanol = 100/0-2, by
volume), followed by recrystallization from ethanol,
whereby 3.57 g of 1,3-dimethyl-6-{4-[3-(2,6-dichloro-
4-nitrophenyloxy)propyl]-piperazin-1-yl}-2,4(1H,3H)-
pyrimidinedione as crystals.
Analytical results of the pyrimidinedione derivative
thus obtained:
NMR (CDC13), ~ppm: 1.95-2.25(m,2H), 2.5-2.8(m,6H),
2.9-3.1(m,4H), 3.3(s,3H), 3.4(s,3H),
4.2(t,2H), 5.35(s,1H), 8.25(s,2H).
389
- 320 -
Next, 0.5 g of the pyrimidinedione derivative
was treated in a 10%HCl/methanol solution by a method
known ~ se in the art to obtain 0.45 g of 1,3-
dimethyl-6-{4-t3-(2,6-dichloro-4-nitrophenyloxy)propyl]-
piperazin-1-yl}-2,4(lH,3H)-pyrimidinedione hydro-
chloride (Compound 165) as crystals.
Analytical results of crystals of Compound 165 thus
obtained:
Melting point: 200-202~C.
~0 Elemental analysis for ClgH23cl2N5O5-Hcl-4H2o:
Calculated (%): C, 44.46; H, 4.81; N, 13.64;
Cl, 20.72.
Found (%): C, 44.47; H, 4.87; N, 13.55;
Cl, 20.73.
~5 Example 107:
Production of tablets containing as an effective
ingredient 1,3-dimethyl-6-{4-[3-t4-chloro-2-
nitrophenyloxy)propyl]piperazin-l-yl}-
2,4(lH,3H)-pyrimidinedione hydrochloride
(Compound 140) available by the process of
Example 85:
1 g of the pyrimidinedione derivative
hydrochloride (Compound 140), 123 g of lactose and 20 g
of corn starch were finely mixed. Using a solution of
5 g of hydroxypropylcellulose in 100 m~ of water, the
resultant mixture was granulated. The resultant
-
89
- 321 -
particles were dried at 50~C for 4 hours and then
mixed thoroughly with 1 g of magnesium stearate. The
thus-prepared mixture was then compressed into tablets,
each containing 150 mg, by a tablet machine.
Example 108:
Production of capsules containing as an
effective ingredient 1,3-dimethyl-6-{4-[3-
(2-chloro-4-nitrophenyloxy)propyl]piperazin-1-
yl}-2,4(1H,3H)-pyrimidinedione hydro-
chloride ~Compound 141) available by the process
of Example 86:
5 g of the pyrimidinedione derivative hydro-
chloride ~Compound 141), 120 g of lactose and 25 g of
corn starch were finely mixed. The resulting mixture
was filled into hard capsules, each containing 150 mg,
by a capsule filling machine.
Bxample 109:
Production of injection containing as an
effective ingredient 1,3-dimethyl-6-{4-[3-~4-
methanesulfonamido-2-nitrophenyloxy)propyl]-
piperazin-l-yl}-2,4~lH,3H)-pyrimidinedione
hydrochloride ~Compound 142) available by the
process of Example 87:
20 mg of the pyrimidinedione derivative hydro-
25 chloride (Compound 142) and 0.85 g of sodium chloride
were weighed. They were dissolved in distilled water
Z~ 389
- 322 -
for injection to give a total volume of 100 m~,
thereby preparing a formulation suitable for injection.
Pharmacological Test 7:
Similarly to Pharmacological Test 1, the ADP75
and ERP of each of the compounds shown in Table 14 and
obtained in the corresponding examples described above
were determined. The results are summarized in Table
14.
Table 14
Result of Pharmacological Test
Effects to duration Effects to refractory
time of myocardinal period of ventricular
action potential muscle
Compound75 t ) ERP (%)
~Dose (~g/m~) Dose (mg/kg, i.v.)
1.0 3.0 10.0 0.1 0.3 1.0 3.0
140 6 17 29 0 3.55 3.55
141 27 41 - 0 2.1 2.1 15
145 7 16 23 0 6.7 6.7 20
147 14 18 - 0 0 0
150 - 16 - 0 0 6.3 6.3
153 20 25 - 6.1 9.1 18.3
154 9 12 20 0 0 5.9 11.8
161 26 39 - 0 6.7 6.7 6.7
162 19 41 60 0 0 0 0
164 - 8 12 0 7.7 15.4 15.4
2(~1389
- 323 -
Toxicity Test 7:
Similarly to Toxicity Test 1, the toxicity of
each of the compounds shown in Table 15 and obtained in
the corresponding examples described above was tested
to determine the mortality rate of mice. The results
are summarized in Table 15.-
Incidentally, the administration of each
compound was conducted orally (p.o.) at a dose of
300 mg/Kg.
Table 15
Results of Toxicity Test on Novel
Pyrimidine Derivative Compounds
Compound No. Mortality rate (%) .
141 0
145 0
147 0
148 0
161 0
~0 Example 110:
Preparation of 1,3-dimethyl-6-{2-[N-methoxy-
carbonylmethyl-3-(4-nitroanilino)propylamino]-
ethylamino}-2,4(lH,3H)-pyrimidinedione
hydrochloride (Compound 166):
-
2001389
- 324 -
o o
~C:~3 HocH2cH2NH2 CH3 ~ 2 ~ N~C~3
cl N~o ~ ~ CH3~S03CH2cH2NH N~o
c~3 CH3
> C ¢N~o
CH3 ~ ~ -CH
(Compound a) N ~ 3
N02-0-F T NH2-CH2CH2CH2NH2 ~I N~2-~- NHCH2CH2CH2NH2
N~2 ~ 2 2 2NHcH2cH2NH ~ CH Cl-CH2COOCH3 HCl/MeOH
(Compound b) CH3 ~
N~2 ~ CH2coocH3 ~ 3 HCl
(Compound 166) CH3 ~
(1) Preparation of 1,3-dimethyl-6-[2-(p-toluene-
sulfonyloxy)ethylamino]-2,4(1H,3H)-pyrimidinedione:
35.0 g of 2-aminoethanol were heated to 90~C
and then removed from an oil bath. 50.0 g of 6-chloro-
1,3-dimethyl-2,4-(lH,3H)-pyrimidinedione were then
added to react them to each other. The addition was
conducted at such a rate that the reaction temperature
was maintained within the range of 90-110~C. After
completion of the addition, the reaction mixture was
stirred for 10 minutes, followed by the addition of 300
m~ of dioxane/methanol (= 10/1, by volume). The
resultant mixture was allowed to stand overnight.
2~:)1389
- 325 -
Crystals thus obtained were washed with a small amount
of dioxane and then dried to obtain 49.0 g of 1,3-
dimethyl-6-(2-hydroxyethylamino)-2,4-(lH,3H)-
pyrimidinedione as white crystals.
Next, a suspension of 49.0 g of the white
crystals in 200 ml of pyridine was chilled to -5~C,
to which 40.0 g of p-toluenesulfonyl chloride were
added at a rate slow enough to maintain the reaction
temperature below 5~C. To eliminate cloudiness from
the reaction mixture, 51.0 g of p-toluenesulfonyl
chloride were used additionally.
The reaction mixture was poured into 1.5 1 of
ice water in which 70 g of K2CO3 were contained.
The thus-obtained mixture was allowed to stand
overnight. Resulting crystals were collected by
filtration, washed with water, and then dried under
reduced pressure, thereby obtaining 50.5 g of 1,3-
dimethyl-6-[2-(p-toluenesulfonyloxy)ethylamino]-
2,4(lH,3H)-pyrimidinedione as pale yellow crystals.
Analytical results of crystals of the pyrimidinedione
derivative thus obtained:
Melting point: 146.0-149.0~C.
IR vmax (cm ): 3270, 1682, 1615, 1550,
1480, 1435, 1360, 1190,
1178, 1010, 903, 780.
(2) Preparation of 6-(1-aziridinyl)-1,3-dimethyl-
ZC~01389
_ 326 -
2,4(lH,3H)-pyrimidinedione (Compound a):
To a solution of 47.2 g of 1,3-dimethyl-6-[2-
(p-toluenesulfonyloxy)ethylamino]-2,4(lH,3H)-
pyrimidinedione, which had been obtained in the above
procedure, in 150 mQ of anhydrous dimethylsulfoxide,
6.24 g of 60% oil-base sodium hydride were gradually
added at room temperature. The resultant mixture was
vigorously stirred at room temperature for 5 hours and
then cooled. A small amount of water was added to
terminate the reaction. The thus-obtained mixture was
poured into 1 ~ of water which contained 70 g of
potassium carbonate. The resultant mixture was
extracted 3 times with 200 m~ portions of chloroform.
The extracts were combined into an organic layer. The
lS organic layer was dried over anhydrous sodium sulfate
and then concentrated. The concentrate thus prepared
was added with 300 m~ of ether and the resulting
solution was allowed to stand overnight.
Pale yellow crystals which had precipitated by
while the solution was allowed to stand overnight were
collected by filtration, washed with ether and then
dried under reduced pressure, whereby 15.2 g of 6-(1-
aziridinyl)-1,3-dimethyl-2,4(lH,3H)-pyrimidinedione
(Compound a) were obtained.
Analytical results of crystals of Compound a thus
obtained:
- 327 - a o Q 1 3 8 9
Melting point: 126.0-126.5~C.
I~ vmBax (cm 1): 1705, 1650, 1612, 1470, 1440,
1305, 1160, 783, 490.
lH-NMR (CDC13), ~ ppm: 2.34(s,4H), 3.35(s,3H),
3.56(s,3H), 5.25(s,1H).
(3) Preparation of 1,3-dimethyl-6-{2-[3-(4-nitro-
anilino)propylamino]ethylamino}-2,4(lH,3H)-pyrimidine-
dione (Compound b):
2.8 g of 4-nitrofluorobenzene were heated to
80~C in 29.6 g of propylenediamine and then stirred
for 1 hour at the same temperature. The reaction
mixture was poured into water and precipitated crystals
were collected by filtration, thereby obtaining 3.6 g
of N-(4-nitrophenyl)propylenediamine as crystals.
After dissolving 1.2 g of N-(4-nitrophenyl)-
propylenediamine and 1.1 g of 6-(1-aziridinyl)-1,3-
dimethyl-2,4(lH,3H)-pyrimidinedione (Compound a),
which were obtained in the above procedure (2), in
5 ml of chloroform, the resultant mixture was
concentrated under reduced pressure. The residue was
added with 10 mg of "Amberlist 15" (trade mark; product
of Rohm & Hass Co.) and stirred at 80~C for 1 hour.
The thus-obtained mixture was dissolved in 20 ml of
chloroform and "Amberlist 15" was filtered off. The
filtrate was washed with water and dried over anhydrous
sodium salfate. The solvent was then distilled off
-
2001389
- 328 -
under reduced pressure to obtain 1,3-dimethyl-6-
{2-[3-(4-nitroanilino)propylamino~ethylamino}-
2,4(1H,3H)-pyrimidinedione (Compound b) in a crude
form. Although the reaction product obtained by the
above procedure contained impurities, it was provided
for the next reaction without purification.
(4) Synthesis of 1,3-dimethyl-6-{2-~N-methoxycarbonyl-
methyl-3-(4-nitroanilino)propylamino]ethylamino}-
2,4(lH,3H)-pyrimidinedione hydrochloride (Compound
166):
1.5 g of 1,3-dimethyl-6-{2-[3-(4-nitroanilino)-
propylamino]ethylamino}-2,4(lH,3H)-pyrimidinedione
(Compound b) obtained in the above procedure (3) were
dissolved in 12 m~ of DMSO, followed by the addition
of 1.2 g of methyl chloroacetate and 1.5 m~ of
triethylamine. The resultant mixture was stirred at
50~C for 2 hours, dissolved in chloroform, washed with
water and then dried over anhydrous sodium sulfate.
The solvent was thereafter distilled off under reduced
pressure. The residue was purified by chromatography
on a silica gel column (eluent: chloroform/methanol =
30/1, by volume), thereby obtaining 1.8 g of 1,3-
dimethyl-6-{2-[N-methoxycarbonylmethyl-3-(4-nitro-
anilino)propylamino]ethylamino}-2,4(1H,3H)-
pyrimidinedione.
- 2~0138~
- 329 -
Analytical results of crystals of the pyrimidinedione
derivative thus obtained:
NMR (DMSO-d6), ~ ppm: 1.70(m,2H), 2.6-2.9(m,4H),
2.95-3.4(m,4H), 3.25(s,3H),
3.3(s,3H), 3.45(s,2H),
3.65(s,3H), 4.6(s,1H),
6.45(m,1H), 6.6(d,2H),
7.2(m,1H), 7.95(d,2H).
Further, 1.5 g of the pyrimidinedione derivative
were treated in an HCl/methanol solution by a method
known ~ se in the art to obtain 1.4 g of
1,3-dimethyl-6-{2-[N-methoxycarbonylmethyl-3-(4-
nitroanilino)propylamino]ethylamino}-2,4(1H,3H)-
pyrimidinedione hydrochloride (Compound 166) as
amorphous powder.
Elemental analysis for C20H28N6O6-HCl-H2O:
Calculated (%): C, 47.76; H, 6.21; N, 16.71;
Cl, 7.05.
Found (%): C, 47.06; H, 5.91; N, 16.05;
Cl, 7.58.
Example 111:
Preparation of 1,3-dimethyl-6-{2-[N-(2-acetoxy-
ethyl)-3-(4-nitrophenyl)propylamino]ethylamino}-
2,4(lH,3H)-pyrimidinedione oxalate
(Compound 167~:
;~0~3~9
- 330 -
SOC 2 2 2 2
N02 ~ CH2CH2C~~H > ' N02 ~ cH2CH2coNHcH2CH20H
> N02 ~ CH2CH2CH2NHCH2CH20H
O O
~C%3 NH2CH2~H2~H CH35~2Cl ~N~CH3 K2C03
cl I ~o > CH3502-ocH2cH2NH N~o >
CH3 (Compound c) c~3
No2~CH2CH2cH2NHcH2cH20 CH2CH20H ,~11~
No2~cH2cH2cH2N-cH2cH2 N~o
(Compound dJ C~3
o
CH2CH20CCH3
(CH3CO)20 (COOH)2/MeOH ¦ ~ N,CH3
> ~ N02~CH2CH2CH2NCH2cH2NH N~o ~ ( COOH)2
(Compound 167) CH3
(1) Preparation of N-(2-hydroxyethyl)-3-(4-nitro-
phenyl)propionamide:
60 g of 3-(4-nitrophenyl)propionic acid were
suspended in 360 m~ of chloroform, followed by the
addition of 2.25 g of dimethylformamide. The thus-
prepared reaction mixture was heated at 50-60~C, to
which 33.5 m~ of thionyl chloride were gradually added
dropwise. After the dropwise addition, the resultant
mixture was heated under reflux for 1 hour, the solvent
was distilled off under reduced pressure, and the
resultant oily substance was dissolved in 150 m~ of
chloroform. The chloroform solution was added dropwise
Z~)0~38~3
- 331 -
under ice cooling into a solution which had been formed
by dissolving 28.2 g of ethanolamine and 42.5 g of
potassium carbonate in 450 m~ of water. After
completion of the dropwise addition, the resultant
mixture was stirred for 1 hour. Precipitated crystals
were collected by filtration and then recrystallized
from 1 ~ of ethyl acetate, thereby obtaining 56.1 g of
N-(2-hydroxyethyl)-3-(4-nitrophenyl)propionamide as
- crystals.
Melting point: 122-125~C.
(2) Preparation of N-(2-hydroxyethyl)-3-(4-nitro-
phenyl)propylamine:
50 g of N-(2-hydroxyethyl)-3-(4-nitrophenyl)-
propionamide obtained in the above procedure and 31.8 g
of sodium borohydride were suspended in 500 m~ of
tetrahydrofuran, followed by the dropwise addition of
50.5 g of acetic acid under ice-cooling. After
completion of the dropwise addition, the resultant
mixture was heated under reflux for 2 hours. It was
again ice-cooled, followed by the dropwise addition of
500 m~ of water. 4 N hydrochloric acid was added to
adjust the pH to 5-6 and tetrahydrofuran was distilled
off under reduced pressure. The resultant aqueous
solution was added with 425 m~ of 4 N hydrochloric
acid and the mixture thus prepared was heated at
60-70~C for 1 hour under stirring. The reaction
~ 9
- - 332 -
mixture was allowed to cool down to room temperature
and washed with chloroform. The resultant aqueous
solution was adjusted to pH 11 with a 16% aqueous
solution of sodium hydroxide and then extracted twice
with 500 mQ portions of chloroform. The chloroform
extracts were combined together and then concentrated
under reduced pressure. The residue was crystallized
from 900 mQ of toluene, thereby obtaining 38.6 g of
N-(2-hydroxyethyl)-3-(4-nitrophenyl)propylamine as
crystals.
Melting point: 82.5-84.5~C.
(3) Preparation of 1,3-dimethyl-6-(2-methanesulfonyl-
oxyethylamino)-2,4(1H,3H)-pyrimidinedione (Compound c):
52.4 g of 6-chloro-1,3-dimethyl-2,4(lH,3H)-
pyrimidinedione were dissolved in 280 mQ of pyridine,
followed by the addition of 45.5 g of triethylamine and
21.3 g of aminoethanol. The thus-prepared mixture was
heated under reflux at 90~C for 4 hours. The reaction
mixture was ice-cooled and while maintaining the
internal temperature at 0-4~C, 55.8 g of methane-
sulfonyl chloride were added dropwise. The resultant
mixture was stirred for 3 hours at the same tempera-
ture. 1.2 Q of methanol were added, followed by
stirring for additional 2 hours. Crytals precipitated
in the reaction mixture were collected by filtration
Z001389
- 333 -
and then recrystallized from 3.5 ~ of methanol,
thereby obtaining 70.0 g of 1,3-dimethyl-6-(2-methane-
sulfonyloxyethylamino)-2,4(lH,3H)-pyrimidinedione
(Compound c) as crystals.
Melting point: 169-170~C.
(4) Preparation of 1,3-dimethyl-6-{2-tN-(2-hydroxy-
ethyl)-3-(4-nitrophenyl)propylamino]ethylamino}-
2,4(lH,3H)-pyrimidinedione (Compound d):
20.2 g of 1,3-dimethyl-6-(2-methanesulfonyloxy-
ethylamino)-2,4(1H,3H)-pyrimidinedione (Compound c),
which had been synthesized in the above procedure (3),
and 15.1 g of potassium carbonate were suspended in
300 m~ of acetonitrile. The resultant mixture was
heated under reflux for 4 hours. Insoluble matters
were filtered off, and the filtrate was concentrated
under reduced pressure to the total volume of about
60 m~, followed by the addition of 18 g of N-(2-
hydroxyethyl)-3-(4-nitrophenyl)propylamine obtained in
the above procedure (2), 36 m~ of DMF and 0.69 g of
p-toluenesulfonic acid monohydrate. Under reduced
pressure, acetonitrile was distilled off. The residue
was heated under stirring at 80~C for 2 hours. The
reaction mixture was cooled to room temperature, to
which 900 m~ of 0.1 N hydrochloric acid were added to
dissolve insoluble matters. Thereafter, a 0.5 M
aqueous solution of potassium carbonate was added to
~1389
- 334 -
render the mixture alkaline. The thus-prepared mixture
was stirred at room temperature for 3 hours.
Precipitated crystals were collected by filtration,
dried and then recrystallized from ethanol, thereby
obtaining 26.6 g of 1,3-dimethyl-6-{2-[N-(2-hydroxy-
ethyl)-3-(4-nitrophenyl)propylamino]ethylamino}-
2,4(1H,3H)-pyrimidinedione (Compound d) as crystals.
Melting point: 125-126~C.
(5) Preparation of 1,3-dimethyl-6-{2-[N-(2-acetoxy-
ethyl)-3-(4-nitrophenyl)propylamino]ethylamino}-
2,4(lH,3H)-pyrimidinedione oxalate (Compound 167):
1.6 g of 1,3-dimethyl-6-{2-[N-(2-hydroxyethyl)-
3-(4-nitrophenyl)propylamino]ethylamino}-2,4(1H,3H)-
pyrimidinedione (Compound d) and 0.8 g of acetic
anhydride were dissolved in 5 m~ of pyridine. The
resultant mixture was stirred at room temperature for
24 hours and then at 60~C for 1 hour. The reaction
mixture was poured into water and extracted with
chloroform. The extract was washed with water and then
concentrated. The residue was purified by chromato-
graphy on a silica gel column (eluent: chloroform/
methanol = 25/1-10/1, by volume), thereby obtaining
1.65 g of 1,3-dimethyl-6-{2-[N-(2-acetoxyethyl~-3-
(4-nitrophenyl)propylamino]ethylamino}-2,4(1H,3H)-
pyrimidinedione as crystals.
2001389
- - 335 -
Analytical results of crystals of the pyrimidinedione
derivative thus obtained:
Melting point: 90.5-92.0~C.
NMR (CDC13), ~ ppm: 1.85 (m,2H), 2.15(s,3H),
2.5-3.3(m,10H), 3.26(s,3H),
3.37(s,3H), 4.3(t,2H),
4.78(s,1H), 7.2-8.2(m,4H).
- Further, 1.5 g of the pyrimidinedione derivative
were treated in an oxalic acid/methanol solution by a
method known E~ se in the art to obtain 1.6 g of
1,3-dimethyl-6-{2-tN-(2-acetoxyethyl)-3-(4-nitrophenyl)-
propylamino]ethylamino}-2,4(1H,3H)-pyrimidinedione
oxalate (Compound 167) as crystals.
Analytical results of crytals of Compound 167 thus
obtained:
Y 21 29N5~6 (COOH)2 H2O
Calculated (%): C, 49.73; H, 5.99; N, 12.61.
Found (%): C, 50.22; H, 5.75; N, 12.98.
Example 112:
Preparation of 1,3-dimethyl-6-{2-[N-(2-benzoyl-
oxyethyl)-3-(4-nitrophenyl)propylamino]ethyl-
amino}-2,4(1H,3H)-pyrimidinedione oxalate
(Compound 168):
2~1389
-- - 336 -
CH2c~20~ ~( ~ CO)2o HCl/MeOH
No2~cH2cH2cH2N-cH2cH2 N~o >
(Compound d) CH3
C~12CH20C~
o
N~2 ~ CH2CH2CH2NCH2cH2NH ~N ~ .(COOH)2
(Compound 168) c~3
In a similar manner to Example 111-(5) except
for the use of 1.6 g of benzoic anhydride in place of
acetic anhydride, 1.85 g of 1,3-dimethyl-6-{2-[N-(2-
benzoyloxyethyl)-3-(4-nitrophenyl)propylamino]ethyl-
amino}-2,4(1H,3H)-pyrimidinedione were obtained.
Analytical results of the pyrimidinedione derivative
thus obtained:
NMR (CDC13), ~ ppm: 1.93 (m,2H), 2.5-3.4(m,10H),
3.29(s,3H), 3.38(s,3H),
4.45(t,2H), 4.77(s,1H),
7.0-8.2(m,9H).
Further, 1.5 g of the pyrimidinedione derivative
were treated in an oxalic acid/methanol solution by a
method known per se in the art to obtain 1.4 g of
1,3-dimethyl-6-{2-[N-(2-benzoyloxyethyl)-3-(4-nitro
phenyl)propylamino]ethylamino}-2,4(1H,3H)-pyrimidine-
dione oxalate (Compound 168) as crystals.
Analytical results of crytals of Compound 168 thus
obtained:
2Q01389
- 337 -
ement na ysis for C26 31N5~6 (C ~ )2 2
Calculated (%): C, 54.45; H, 5.71; N, 11.34.
Found (%): C, 54.90; H, 5.55; N, 11.55.
Example 113:
Preparation of 1,3-dimethyl-6-{2-<N-[2-(4-
fluorobenzoyloxy)ethyl]-3-(4-nitrophenyl)propyl-
amino>ethylamino}-2,4(lH,3H)-pyrimidinedione
oxalate (Compound 169):
I NH ~ N_CH
N02~CH2CH2cH2NcH2cH2 N~o
CH3
CH2cH2oc ~ F
(COOH)2/MeOH ~ N_CH3
N02~CH2CH2CH2N-cH2cH2 I~N~O ~ ( COOH)2
(Compound 169) CH3
1.0 g of 1,3-dimethyl-6-{2-[N-(2-hydroxyethyl)-
3-(4-nitrophenyl)propylamino]ethylamino}-2,4(lH,3H)-
pyrimidinedione (Compound d), which had been obtained
in Example 111-(4), and 0.8 g of p-fluorobenzoyl
chloride were dissolved in 5 ml of pyridine. The
resultant mixture was stirred at room temperature for
12 hours, poured into water, and then extracted with
Z~1389
- - 338 -
chloroform. The extract was washed with water and then
concentrated. The residue was purified by chromato-
graphy on a silica gel column (eluent: chloroform/
methanol = 40/1, by volume), thereby obtaining 1.0 g of
S 1,3-dimethyl-6-{2-<N-[2-(4-fluorobenzoyloxy)ethyl]-3-
(4-nitrophenyl)propylamino>ethylamino}-2,4(lH,3H)-
pyrimidinedione.
Analytical results of crystals of the pyrimidinedione
derivative thus obtained:
NMR (CDC13), ~ ppm: 1.9-3.1(m,12H), 3.29(s,1H),
3.38(s,3H), 4.59(t,2H),
4.78(s,1H), 7.1-8.0(m,8H).
Further, 0.95 g of the pyrimidinedione deriva-
tive was treated in an oxalic acid/methanol solution by
a method known per se in the art to obtain 1.07 g
of 1,3-dimethyl-6-{2-<N-[2-(4-fluorobenzoyloxy)ethyl]-
3-(4-nitrophenyl)propylamino>ethylamino}-2,4(1H,3H)-
pyrimidinedione oxalate (Compound 169) as crystals.
Analytical results of crytals of Compound 169 thus
obtained:
Melting point: 96-99~C (decomposed).
Y 26 30 5~6 (COO )2 2H2o
Calculated (%): C, 51.45; H, 5.55; N, 10.71.
Found (%): C, 51.82; H, 5.40; N, 10.82.
IR vmax (cm 1): 3350, 2950, 1740, 1700, 1620, 1530,
1330, 1000, 850, 760.
200~389
- 339 -
Example 114:
Preparation of 1,3-dimethyl-6-{2-<N-[2-(4-
methoxybenzoyloxy)ethyl]-3-(4-nitrophenyl)-
propylamino>ethylamino}-2,4(lH,3H)-pyrimidine-
dione oxalate (Compound 170):
CH2CH2OH l~U~ CH3O~COCl
No2~cH2cH2cH2NcH2cH2 N~o
CH3
CH2CH20C ~ oCH3
( COOH ) 2 /MeOH ¢I~N~CH 3
N02~CH2CH2CH2NcH2cH2NH N~o ~ ~ COOH ) 2
( C~ ~und 170 ) C~3
In a similar manner to Example 113 except for
the use of 0.63 g of p-methoxybenzoyl chloride instead
of p-fluorobenzoyl chloride, 1.1 g of 1,3-dimethyl-6-
{2-<N-[2-(4-methoxybenzoyloxy)ethyl]-3-(4-nitrophenyl)-
propylamino>ethylamino}-2,4(lH,3H)-pyrimidinedione were
obtained.
Analytical results of the pyrimidinedione derivative
thus obtained:
NMR (CDC13), ~ ppm: 2.0(m,2H), 2.4-3.0(m,10H),
3.31(s,3H), 3.42(s,3H),
3.50(s,3H), 4.4(t,2H),
4.77(s,1H), 5.31(br,1H),
2~01389
- 340 -
7.18(m,4H~, 8.0(m,4H).
Further, 1.0 g of the pyrimidinedione derivative
were treated in an oxalic acid/methanol solution by a
method known E~ se in the art to obtain 1.05 g of
1,3-dimethyl-6-{2-<N-[2-(4-methoxybenzoyloxy)ethyl]-3-
(4-nitrophenyl)propylamino>ethylamino}-2,4(lH,3H)-
pyrimidinedione oxalate ~Compound 170) as crystals.
Analytical results of crytals of Compound 170 thus
obtained:
Elemental analysis for C27H33N5O7-(COOH)2-~H2O:
Calculated (%): C, 54.54; H, 5.68; N, 10.97.
Found (%): C, 54.21; H, 5.99; N, 10.92.
IR vmBx (cm 1): 3300, 2950, 1690, 1600, 1520, 1350,
1250, 1020, 850, 760.
Example 115:
Preparation of 1,3-dimethyl-6-{2-[N-(2-methoxy-
ethyl)-3-(4-nitrophenyl)propylamino]ethylamino}-
2,4(lH,3H)-pyrimidinedione oxalate
(Compound 171):
O
¢)~N--CH3
3 2 2 2 CH3 ( Compound a )
No2~cH2cH2cH2oso2~ 3 >
( COOH ) 2/MeCH fH2CH2~CH~
~, NO 2 ~ CH 2 CH 2 CH 2 NCH 2 C 2 N~o ~ ( COOH ) 2
( Compound 171 ) CH3
200~389
- 341 -
A mixture consisting of 1 g of 3-(4-nitro-
phenyl)propyl p-toluenesulfonate, 6.5 ml of 2-methoxy-
ethylamine and 5 m~ of dioxane was heated under reflux
for 3 hours, followed by the addition of 100 m~ of
chloroform. The resultant mixture was washed with
water and then dried over anhydrous sodium sulfate.
The thus-prepared chloroform solution was added with
10 mg of p-toluenesulfonic acid and 0.54 g of 6-(1-
- aziridinyl)-1,3-dimethyl-2,4(1H,3H)-pyrimidinedione
(Compound a) obtained in Example 110-~2), followed by
the distillation of the solvent under reduced pressure.
The mixture was then heated at 80~C for 2 hours and
then cooled down to the room temperature. The thus-
obtained mixture was directly subjected to chromato-
graphic purification on a silica gel column (eluent:chloroform/methanol = 40/1, by volume), thereby
obtaining 0.82 g of 1,3-dimethyl-6-{2-[N-(2-methoxy-
ethyl)-3-(4-nitrophenyl)propylamino]ethylamino}-
2,4(lH,3H)-pyrimidinedione as an oily substance.
Analytical results of the pyrimidinedione thus
obtained:
NMR (CDC13), ~ ppm: l.9(m,2H), 2.4-3.6(m,12H),
3.23(s,3H), 3.30(s,3H),
3.33(s,3H), 4.71(s,1H),
5.74(br,1H), 7.19(d,2H),
8.03(d,2H).
2001389
- 342 -
Further, 0.80 g of the pyrimidinedione deriva-
tive was treated in an oxalic acid/methanol solution by
a method known E~ se in the art to obtain 0.85 g of
1,3-dimethyl-6-{2-[N-(2-methoxyethyl)-3-(4-nitrophenyl)-
propylamino~ethylamino}-2,4(1H,3H)-pyrimidinedione
oxalate (Compound 171) as crystals.
Analytical results of crytals of Compound 171 thus
obtained:
Elemental analysis for C20H29N5O5-(COOH)2-H2O:
Calculated (~): C, 50.09; H, 6.31; N, 13.28.
Found (%): C, 50.14; H, 6.39; N, 13.30.
IR vmax (cm 1): 3300, 3000, 1680, 1630, 1510, 1430,
1350, 1200, 770, 700.
Example 116:
Preparation of 1,3-dimethyl-6-{2-[N-benzyl-3-(4-
nitrophenyl)propylamino]ethylamino}-2,4(lH,3H)-
pyrimidinedione oxalate (Compound 172):
2 0 ~ 2 2 ¢U'N~CH3
No2~CHzCH2CH20502~CH3 > >
O
( COOH ) 2 /MeOH ICH2 ~N~CH 3
> N02~CH2CH2cH2N-cH2cH2 ~N~o ~(COOH)2
( Compound 172 ) CH3
- 343 -
In a similar manner to Example 115 except for
the use of 0.36 m~ of benzylamine instead of 2-
methoxyethylamine and the addition of 1 ml of tri-
ethylamine, 1.6 g of 1,3-dimethyl-6-{2-[N-benzyl-3-
(4-nitrophenyl)propylamino]ethylamino}-2,4(1H,3H)-
pyrimidinedione were obtained.
Analytical results of crystals of the pyrimidinedione
derivative thus obtained:
NMR (CDC13), ~ ppm: 2.0(m,2H), 2.4-3.3(m,8H),
3.21(s,3H), 3.25(s,3H),
3.58(s,2H), 4.67(s,1H),
5.22(br,1H), 7.22(d,2H),
7.30(s,5H), 8.03(d,2H).
Further, 1.50 g of the pyrimidinedione deriva-
tive were treated in an oxalic acid/methanol solutionby a method known E~ se in the art to obtain 1.55 g of
1,3-dimethyl-6-{2-[N-benzyl-3-(4-nitrophenyl)propylamino]-
ethylamino}-2,4(lH,3H)-pyrimidinedione oxalate (Compound
172) as crystals.
Analytical results of crytals of Compound 172 thus
obtained:
Y 24 29 5 4 (C ~ )2 ~ 2~
Calculated t%): C, 56.72; H, 5.86; N, 12.72.
Found (%): C, 56.41; H, 5.82; N, 12.24.
IR vmax (cm 1): 3300, 2950, 1680, 1630, 1540, 1340,
1200, 770, 700.
- 344 - ~ 3 8 9
Example 117:
Preparation of 1,3-dimethyl-6-{2-[N-(t-butoxy-
carbonyl)-3-(4-nitrophenyl)propylamino~ethyl-
amino}-2,4(1H,3H)-pyrimidinedione
(Compound 173):
~ NH4OH/H O N~ElH
N02~3CH2CH2CC1 2 ~ N02~CH2CH2CH2NH2
C ~N
l O O
C~3 ~(CH3)3Coc0120 1 ~Y3 ~N~ 3
~,~ NC2~CH2CH2C~2N-cH2cH2NH I~N~O
(C~ 173)CS~3
(1) Preparation of 3-(4-nitrophenyl)propylamine:
A solution of 21 g of 3-~4-nitrophenyl)propionyl
chloride in 30 ml of chloroform was added dropwise
into a mixture of 75 ml of concentrated aqueous
ammonia and 75 ml of ice water. The resultant mixture
was then stirred for 2 hours under ice-cooling. The
resultant precipitate was collected by filtration,
dried in air and recrystallized from ethyl acetate,
thereby obtaining 13.7 g of 3-(4-nitrophenyl)propan-
amide.
10.0 g of the amide compound and 9.S g of sodium
borohydride were suspended in 250 ml of dioxane,
followed by the dropwise addition of 15 ml of acetic
acid. The resultant mixture was stirred for 10 hours
under reflux. The reaction mixture was added with
B
~r, .
2001389
- 345 -
10 mQ of methanol and then concentrated to dryness.
The residue was dissolved in chloroform. The solution
thus obtained was washed with water and then
concentrated to dryness, thereby obtaining 6.0 g of
3-(4-nitrophenyl)propylamine as an oily substance.
This compound was provided for the next reaction
without any further purification.
NMR (CDC13), ~ ppm: l.9(m,2H), 2.4-3.0(m,4H),
- 6.70(br,2H), 8.31(d,2H),
8.10(d,2H).
(2) Preparation of 1,3-dimethyl-6-{2-[N-(t-butoxy-
carbonyl)-3-(4-nitrophenyl)propylamino]ethylamino}-
2,4(lH,3H)-pyrimidinedione (Compound 173):
6.0 g of 3-(4-nitrophenyl)propylamine obtained
in the above procedure (1) and 3.98 g of 6-(1-
aziridinyl)-1,3-dimethyl-2,4(1H,3H)-pyrimidinedione
(Compound a) obtained in Example 110-(2) were
dissolved in 50 ml of chloroform, and the resultant
mixture was concentrated to dryness under reduced
pressure. The residue was added with 50 mg of
p-toluenesulfonic acid monohydrate and the mixture thus
prepared was stirred at 90~C for 2 hours. The mixture
was dissolved in 60 ml of tetrahydrofuran, followed by
the addition of 3.0 g of di-tert-butyldicarbonate. The
thus-obtained mixture was stirred at room temperature
~or 1 hour and then concentrated. The residue was
26:~1389
- 346 -
purified by chromatography on a silica gel column
(eluent: chloroform/methanol = 40/1, by volume),
thereby obtaining 5.0 g of 1,3-dimethyl-6-{2-[N-
(t-butoxycarbonyl)-3-(4-nitrophenyl)propylamino]ethyl-
amino}-2,4(lH,3H)-pyrimidinedione (Compound 173) as
crystals.
A~alytical results of Compound 173 thus obtained:
Melting point: 111-112~C.
NMR (CDC13), ~ ppm: 1.51(s,9H), l.9(m,2H),
2.5-3.0(m,6H), 3.3-3.4(m,2H),
3.28(s,3H), 3.36(s,3H),
4.68(s,1H), 6.54(br,1H),
7.39(d,2H), 8.20(d,2H).
Elemental analysis for C22H31N5O6:
Calculated (%): C, 57.25; H, 6.77; N, 15.17.
Found (%): C, 57.29; H, 6.76; N, 15.13.
Example 118:
Preparation of 1,3-dimethyl-6-{2-[N-(4-methoxy-
benzyl)-3-(4-nitrophenyl)propylamino]ethyl-
amino}-2,4(lH,3H)-pyrimidinedione oxalate
(Compound 174):
COOC~ CH3 ) 3 ~
N02~ 2CH2CH2N-CH2CH2NH-~,~ 3 HCl/O/--\O
CH 3
( Compound 173 )
- 2~1~1389- - 347 -
Z~CH2cH2cH2NH-cH2cH2NH~
(Compound e)~HCl ~H3
OCH3
O
1 ~ CH2Br (COOH)2/MeOH NO2 ~ CH2CH2CH2N-CH2cH2NH ~ ~ ~(COOH)
(Compound 174) CH3
(1) Preparation of 1,3-dimethyl-6-{2-[3-(4-nitro-
phenyl)propylamino]ethylamino}-2,4(1H,3H)-pyrimidine-
dione hydrochloride (Compound e):
30 m~ of 0.-5 N HCl/dioxane were added to 4.9 g
of 1,3-dimethyl-6-{2-[N-(t-butoxycarbonyl~-3-(4-nitro-
phenyl)propylamino]ethylamino}-2,4(1H,3H)-pyrimidine-
dione (Compound 173). The resultant mixture was
stirred at room temperature for 24 hours. Precipitated
crystals were collected by filtration and then washed
with a chilled solvent consisting of a 1:2 mixture (by
volume) of ethanol and ether, thereby obtaining 3.9 g
of 1,3-dimethyl-6-{2-[3-(4-nitrophenyl)propyl-
amino]ethylamino}-2,4(1H,3H)-pyrimidinedione
hydrochloride (Compound e).
Analytical results of crystals of Compound e thus
obtained:
Melting point: 230-231~C (decomposed).
Elemental analysis for C17H23N5O4-2HCl-H2O:
Calculated (%): C, 45.14; H, 6.02; N, 15.48;
26~389
- 348 -
Cl, 15.68.
Found (%): C, 45.31; H, 5.87; N, 15.54;
Cl, 15.67.
(2) Preparation of 1,3-dimethyl-6-{2-[N-(4-methoxy-
benzyl)-3-(4-nitrophenyl)propylamino]ethylamino}-
2,4(lH,3H)-pyrimidinedione oxalate (Compound 174):
1.4 g of 1,3-dimethyl-6-{2-[3-~4-nitrophenyl)-
propylamino]ethylamino}-2,4(1H,3H)-pyrimidinedione
hydrochloride (Compound e) were dissolved in 5 m~ of
water, followed by the addition of potassium carbonate
to render the solution alkaline. The solution was then
extracted with chloroform. The thus-obtained chloro-
form solution was concentrated to dryness, followed by
the addition of 0.6 g of p-methoxybenzyl bromide, 3 m~
of triethylamine and 20 m~ of isopropanol. The
resultant mixture was heated for 8 hours under reflux.
The solvent was distilled off under reduced pressure
and the residue was directly subjected to chromato-
graphic purification on a silica gel (eluent: chloro-
form/methanol = 40/1, by volume), thereby obtaining0.55 g of 1,3-dimethyl-6-{2-[N-(4-methoxybenzyl)-3-
(4-nitrophenyl)propylamino]ethylamino}-2,4(1H,3H)-
pyrimidinedione.
Analytical results of the pyrimidinedione derivative
thus obtained:
NMR (CDC13), ~ ppm: 2.0(m,2H), 2.5-3.1(m,8H),
2Q01389
- 349 -
3.24(s,3H), 3.30(s,3H),
3.64(s,2H), 4.59(s,1H),
5.16(m,1H), 7.16(m,4H),
7.94(m,4H).
Further, 0.52 g of the pyrimidinedione deriva-
tive was treated in an oxalic acid/methanol solution
by a method known ~ se in the art to obtain 0.54 g of
1,3-dimethyl-6-{2-[N-(4-methoxybenzyl)-3-(4-nitro-
phenyl)propylamino]ethylamino}-2,4(1H,3H)-pyrimidine-
dione oxalate (Compound 174) as crystals.
Analytical results of crytals of Compound 174 thus
obtained:
Melting point: 104-106~C (decomposed).
IR vmax (cm 1): 3300, 2950, 1710, 1700, 1630, 1520,
1340, 1250, 1070, 770, 700.
Y 25 31 5 5 ( )2 2
Calculated (%): C, 54.18; H, 6.06; N, 11.70.
Found (%): C, 54.74; H, 5.97; N, 11.37.
Example 119:
Preparation of 1,3-dimethyl-6-{2-tN-ethoxy-
carbonylmethyl-3-(4-nitrophenyl)propylamino]-
ethylamino}-2,4(lH,3H)-pyrimidinedione oxalate
(Compound 175):
2Q01389
- 350 -
N02 ~ CH2CH2CH2NHCH2cH2NH ~N
(Compound e) HCl C 3
Br-CH COOC H (COOH) /MeOH CH2cooc2H5 ~
2 2 5 2 > NO2 ~ C~2cH2cH2NcH2cH2NH ~N~ ~ (COOHJ
(Compound 175) 1~3~ 2
In a similar manner to Example 118-(2) except
for the use of 0.4 m~ of ethyl bromoacetate in place
of p-methoxybenzyl bromide, 0.77 g of 1,3-dimethyl-6-
~2-[N-(2-ethoxycarbonylmethyl-3-(4-nitrophenyl)propyl-
amino]ethylamino}-2,4(1H,3H)-pyrimidinedione was
obtained.
Analytical results of crystals of the pyrimidinedione
derivative thus obtained:
NMR (CDC13), ~ ppm: 1.30(t,3H), l.9(m,2H),
2.4-3.0(m,6H), 3.0-3.4(m,4H),
3.3(s,3H), 3.5(s,3H),
4.22(q,2H), 4.74(s,1H),
7.31(d,2H), 8.12(d,2H).
Further, 0.7 g of the pyrimidinedione derivative
was treated in an oxalic acid/methanol solution by a
method known ~ se in the art to obtain 0.72 g of
1,3-dimethyl-6-{2-[N-ethoxycarbonylmethyl-3-(4-nitro-
phenyl)propylamino]ethylamino~-2,4(lH,3H)-pyrimidine-
26~389
- 351 -
dione oxalate (Compound 175) as crystals.
Analytical results of crytals of Compound 175 thus
obtained:
Melting point: 148-149~C (decomposed).
IR Vmax (cm ): 2930, 2450, 1730, 1700, 1600, 1540,
1340, 1250, 1200, 850, 750.
Element ana ys s r 21 29N5O6 ( )2 ~ 2
Calculated (%): C, 50.55; H, 5.90; N, 12.81.
- Found (%): C, 50.89; H, 5.81; N, 12.62.
Example 120:
Preparation of 1,3-dimethyl-6-{N-(2-phenyl-
ethyl)-2-[3-(4-nitrophenyl)propylamino]-
ethylamino}-2,4(lH,3H)-pyrimidinedione
hydrochloride (Compound 176):
o
Cl ~¢DN~o CH 2CH2-~
NH2CH2 2 CH3 HocH2cH2N~
~>-cH2cH2-Br ~ 3 O
(COc1)2-DMso 02N~CH2CH2CH2NH2 HCl/MeOH
? ~ >
o
02N~CH2CH2CH2NHcH2cH2N -~N~ ~ HCl
(Compound 176) CH3
(1) Preparation of 1,3-dimethyl-6-[N-(2-hydroxyethyl)-
ZQ01389
- 352 -
2-phenylethylamino]-2,4(1H,3H)-pyrimidinedione:
A mixture consisting of 3 mO of phenethyl
bromide, 13 m~ of ethanolamine and 15 m~ of
isopropanol was heated for 2 hours under reflux. After
allowing the reaction mixture to cool down, 50 mQ of
chloroform were added. The thus-prepared mixture was
washed with water and then concentrated to obtain an
oily substance. To the oily substance, 3.5 g of
6-chloro-1,3-dimethyl-2,4(lH,3H)-pyrimidinedione and
3.3 m~ of triethylamine were added. The resultant
mixture was dissolved in 15 m~ of dimethylformamide
and stirred at 110~C for 4 hours. The solution was
cooled down to room temperature, followed by the
addition of 150 mQ of chloroform. The resultant
chloroform solution was washed with water and then
concentrated to obtain an oily substance. By
crystallizing the oily substance from ether, 4.1 g of
1,3-dimethyl-6-[N-(2-hydroxyethyl)-2-phenyl-
ethylamino]-2,4(lH,3H)-pyrimidinedione were obtained as
crystals.
~nalytical results of crystals of the pyrimidinedione
derivative thus obtained:
Melting point: 77-78~C.
NMR (CDC13), ~ ppm: 2.4-3.2(m,6H), 3.18(s,3H),
3.22(s,3H), 3.50(t,2H),
5.28(s,1H), 7.18(br,5H).
1389
- 353 -
Elemental analysis for C16H21N3O3:
Calculated (%): C, 63.35; H, 6.98; N, 13.85.
Found (%): C, 63.01; H, 6.78; N, 13.91.
(2) Preparation of 1,3-dimethyl-6-{N-(2-phenylethyl)-
2-[3-(4-nitrophenyl)propylamino]ethylamino}-2,4(1H,3H)-
pyrimidinedione hydrochloride (Compound 176):
0.26 m~ of oxalyl chloride was dissolved in
5 m~ of dichloromethane. The resultant mixture was
- chilled to -78~C, followed by the dropwise addition of
a solution of 0.21 m~ of dimethylsulfoxide in 5 m~ of
dichloromethane. The thus-prepared mixture was stirred
for 15 minutes at the same temperature, followed by the
dropwise addition of a solution of 0.6 g of 1,3-
dimethyl-6-[N-(2-hydroxyethyl)-2-phenylethylamino]-
2,4(1H,3H)-pyrimidinedione in 10 m~ of dichloro-
methane. The mixture was stirred for further 15
minutes. After adding 0.84 ml of triethylamine, the
mixture was gradually cooled to room temperature and
then stirred for 5 minutes. 30 m~ of chloroform were
added. The mixture thus obtained was washed with water
and then dried over anhydrous sodium sulfate. The
solvent was distilled off under reduced pressure to
obtain 0.51 g of an oily substance.
The oily substance was dissolved in 10 m~ of
methanol, followed by the addition of 0.72 g of 3-(4-
2001389
- 354 -
nitrophenyl)propylamine obtained in Example 117-(1) and
0.5 mQ of 4 N-HCl/dioxane. The resulting mixture was
stirred at room temperature for 30 minutes and 1 g of
sodium cyanoborohydride was added in several small
portions. After stirring the mixture at room
temperature for 12 hours, 1 N hydrochloric acid was
added to acidify the reaction mixture. Methanol was
distilled off and potassium carbonate was added to
neutralize the residue. The thus-prepared solution was
extracted with chloroform and the chloroform layer was
concentrated to dryness. The residue was purified by
chromatography on a silica gel column (eluent:
chloroform/methanol = 40/1, by volume), thereby
obtaining 0.2 g of 1,3-dimethyl-6-{N-(2-phenylethyl)-
2-[3-(4-nitrophenyl)propylamino]ethylamino}-2,4(1H,3H)-
pyrimidinedione.
Analytical results of crystals of the pyrimidinedione
derivative thus obtained:
NMR (CDC13), ~ ppm: l.9(m,2H), 2.6-3.6(m,12H),
3.18(s,3H), 3.26(s,3H),
5.28(s,1H), 7.18(br,5H),
7.30(d,2H), 8.09(d,2H).
Further, 0.19 g of the pyrimidinedione deriva-
tive was treated in a 1 N-HCl/methanol solution by a
method known ~ se in the art to obtain 0.12 g of
1,3-dimethyl-6-{N-(2-phenylethyl)-2-[3-(4-nitrophenyl)-
26~C)1389
- 355 -
propylamino]ethylamino}-2,4(lH,3H)-pyrimidinedione
hydrochloride (Compound 176) as crystals.
Analytical results of crystals of Compound 176 thus
obtained:
Elemental analysis for C25H31N5O4-HC1-3H2O:
Calculated (%): C, 54.00; H, 6.89; N, 12.59;
Cl, 6.38.
Found (%): C, 53.81; H, 6.74; N, 12.11;
- Cl, 5.91.
10IR vKBr (cm~l) 3420, 2610, 1700, 1650, 1620, 1550,
max
1320, 1010, 820, 780, 750.
Example 121:
Preparation of 1,3-dimethyl-6-{2-tN-allyl-3-
(4-nitrophenyl)propylamino]ethylamino}-
15 2,4(lH,3H)-pyrimidinedione oxalate
(Compound 177):
~ N_CH3 ~\" Br (COOH)2/MeOH
N02~CH2CH2cH2NHcH2cH2NHJ~N~o
(Compound e) HCl CH3
N02~ CH2-CH-CH2~
~Compound 177) CH3
In a similar manner to Example 118-(2) except
Eor the use of 0.38 m2 of allyl bromide in place of
p-methoxybenzyl bromide, 0.9 g of 1,3-dimethyl-6-{2-
2~1389
- 356 -
[N-allyl-3-(4-nitrophenyl)propylamino]ethylamino}-
2,4(lH,3H)-pyrimidinedione was obtained.
Analytical results of crystals of the pyrimidinedione
derivative thus obtained:
Melting point: 109-111~C.
NMR (CDC13), ~ ppm: 2.0(m,2H), 2.5-3.1(m,10H),
3.26(s,3H), 3.34(s,3H),
4.75(s,1H), 5.16(m,2H),
5.63(m,1H), 7.25(d,2H),
8.04(d,2H).
Further, 0.80 g of the pyrimidinedione deriva-
tive was treated in an oxalic acid/methanol solution by
a method known E~ se in the art to obtain 0.75 g of
1,3-dimethyl-6-{2-[N-allyl-3-(4-nitrophenyl)propylamino]-
ethylamino}-2,4(lH,3H)-pyrimidinedione oxalate (Compound
177) as crystals.
Analytical results of crytals of Compound 177 thus
obtained:
Melting point: 85-90~C.
Elemental analysis for C20H27N5O4-(COOH)2-l~H2O:
Calculated (%): C, 50.96; H, 6.22; N, 13.51.
Found (%): C, 51.30; H, 6.26; N, 13.24.
IR vmax (cm 1): 3250, 2940, 1690, 1620, 1540, 1340,
1160, 990, 850, 770, 700.
Example 122:
Preparation of 1,3-dimethyl-6-{2-[N-propargyl-3-
~ 357 ~ 2~Q~38g
(4-nitrophenyl)propylamino]ethylamino}-
2,4(1H,3H)-pyrimidinedione oxalate
(Compound 178):
~ N_cH3 ~r-CH2-C~CH (COOH)2/MeOH
N02~C~2CH2c~2NHcH2c~2NH N~o
(C. ,-~nd e) CH3 ~HCl
C~12-C-CH ~
N02 ~ CH2cH2cH2NcH2cH2NH ~ ~COOH~
(C~ , ~ 178~ CN3
In a similar manner to Example 118-(2) except
for the use of 0.37 ml of propargyl bromide in place
of p-methoxybenzyl bromide, 0.85 g of 1,3-dimethyl-
6-{2-tN-propargyl-3-(4-nitrophenyl)propylamino]ethyl-
amino}-2,4(1H,3H)-pyrimidinedione was obtained.
Analytical results of crystals of the pyrimidinedione
derivative thus obtained:
Melting point: 156-157~C.
NMR (CDC13), ~ ppm: l.9(m,2H), 2.23(m,1H),
2.4-3.1(m,10H), 3.25(s,3H),
3.34(s,3H), 4.73(s,1H),
5.22(br,1H), 7.27(d,2H),
8.04(d,2H).
Further, 0.80 g of the pyrimidinedione deriva-
tive was treated in an oxalic acid/methanol solution by
~=
2~ 389
- 358 -
a method known per se in the art to obtain 0.75 g of
1,3-dimethyl-6-{2-[N-propargyl-3-(4-nitrophenyl)propyl-
amino]ethylamino}-2,4(lH,3H)-pyrimidinedione oxalate
(Compound 178) as crystals.
Analytical results of crytals of Compound 178 thus
obtained:
Melting point: 170-172~C.
Element n y 20 25 5 4 ( 2 ~ 2
Calculated (%): C, 53.01; H, 5.66; N, 14.05.
Found (%): C, 53.31; H, 5.63; N, 14.18.
IR vmax (cm 1): 3250, 2600, 1640, 1620, 1530, 1340,
770, 700.
Example 123:
Production of tablets containing as an effective
ingredient 1,3-dimethyl-6-{2-[N-(2-acetoxy-
ethyl)-3-(4-nitrophenyl)propylamino]ethylamino}-
2,4-(lH,3H)-pyrimidinedione oxalate (Compound
167) available by the process of Example 111:
1 g of the pyrimidinedione derivative oxalate
(Compound 167), 123 g of lactose and 20 g of corn
starch were finely mixed. Using a solution of 5 g of
hydroxypropylcellulose in 100 m~ of water, the
resultant mixture was granulated. The resultant
particles were dried at 50~C for 4 hours and then
mixed thoroughly with 1 g of magnesium stearate. The
2~1389
- 359 -
thus-prepared mixture was then compressed into tablets,
each containing 150 mg, by a tablet machine.
Example 124:
Production of capsules containing as an
effective ingredient 1,3-dimethyl-6-
{2-[N-benzyl-3-(4-nitrophenyl)propyl-
amino]ethylamino}-2,4(1H,3H)-pyrimidinedione
oxalate (Compound 172) available by the process
of Example 116:
5 g of the pyrimidinedione derivative oxalate
(Compound 172), 120 g of lactose and 25 g of corn
starch were finely mixed. The resulting mixture was
filled into hard capsules, each containing 150 mg, by a
capsule filling machine.
Example 125:
Production of injection containing as an
effective ingredient 1,3-dimethyl-6-{2-[N-
ethoxycarbonylmethyl-3-(4-nitrophenyl)propyl-
amino]ethyl-2,4~1H,3H)-pyrimidinedione oxalate
(Compound 175) available by the process of
Example 119:
20 mg of the pyrimidinedione derivative oxalate
(Compound 175) and 0.85 g of sodium chloride were
weighed. They were dissolved in distilled water for
injection to give a total volume of 100 mQ, thereby
preparing a formulation suitable for injection.
Z~01389
- 360 -
Pharmacological Test 8:
Similarly to Pharmacological Test 1, the ADP75
and ERP of each of the compounds shown in Table 16 and
obtained in the corresponding examples described above
were determined. The results are summarized in Table
16.
Table 16
Results of Pharmacological Test
75 ( ) ERP (%)
CompoundDose (~g/m~)Dose (mg/kg, i.v.)
No.
1.0 3.0 10.0 0.1 0.3 1.0 3.0
166 8 18 - 6.7 6.7 6.7 6.7
167 16 21 26 8 8 15 15
168 48 55 68 6 12 18 18
171 19 23 38 - - - -
175 8 16
Toxicity Test 8:
Similarly to Toxicity Test 1, the toxicity of
each of the compounds shown in Table 17 and obtained in
the corresponding examples described above was tested
to determine the mortality rate of mice. The results
are summarized in Table 17.
Incidentally, the administration of each
compound was conducted orally (p.o.) at a dose of
300 mg/Kg.
2~)01389
- 361 -
Table 17
Compound No.Mortality rate (%)
165 ~
166 ~
167 ~
175 0
23~1389
Example 126
Preparation of 6-{2-[N-(2-hydroxyethyl)-3-(4-nitrophenyl)
propylaminoJethylamino}-1,3,5-trimethyl-2,4(1H,3H)-
pyrimidinedlone hydrochloride
CH3 CH3
Cl N ~ HOCHzCHzNH N ~
CH CH3 CH3 1l 'CH3
H CH3
CH3SO2-OCHzCHzN N ~
> ~
CH3 o CH3
NOz ~ CH2CH2CH2NHCH2CH20H HCI/CH30H
> >
CH2CH20H
NOz ~ CH2CH2CHzNCH2CH2NH ~ NCH3 HCl
CH3 0 (Compound 179)
(1) Preparation of 6-~2-hydroxyethylamino)-1,3,5-trimethyl-
2,4(1~,3H)-pyrimidinedione
6-chloro-1,3,5-trimethyl-2,4(1H,3H~-pyrimidinedione
(3.4g) was suspended in 30 ml of isopropanol, and the
suspension was added with 1.26 ml of ethanolamine and 3.8 ml
of triethylamine and then the resultant mixture was refluxed
fvr 3 hours. The reaction mixture was allowed to stand
overnight at room temperature, and thereafter crystals
formed were collected by filtration, washed with water and
then recryst~llized l}sing a water-ethanol solution (1:1,
Z001389
- 363 -
vJv~, thereby 2.9g of 5-(2-hydroxyethylamino)~ ,5-
trimethyl-2,4(1H,3H)-pyrimidinedione being obtained.
Results of analysis of the pyrimidinedione derivative thus
obtained:
Melting point: ]59-160~C
N'MR (CDCI3), ~ ppm: 4.71 (t,2H), 3.55 (m, 2H), 3.21 (s, 3H),
3.39 (s, 3H), 1 93 (s, 3H)
Elemental analysis: CgH15N303
Calculated (X): C, 50.69; H, 7.09: N, 19.71
10- Analyzed (x): C, 50.89; H, 7.11: H, 19.90
(2) Preparation of 6-(2-methanesulfonyloxyethylamino)-1,3,5-
trimethyl-2,4(1H,3H)-pyrimidinedione
6-(2-hydroxyethylamino)-1,3,5-trimethyl-2,4(1H,3H)-
pyrimidinedione (2.9g) was dissolved in 20 ml of pyridine
and added with 1.20 ml of methanesulfonyl chloride drop by
drop at 0~C. After the reaction at 0~C for 4 hours, the
reaction mixture was poured into lO ml of ice water and then
subjected to chloroform extraction. The extract was washed
with water, dried over sodium sulfate anhydrous,
concentrated ln vacuo. The resultant oily substance was
subjected to silica gel column chromatograph
(chloroform/methanol = 30:1, v/v) for purification. Thus,
3.0 g of 6-(2-methanesulfonyloxyethylamino~-1,3,5-trimethyl-
2,4~1H,3H)-pyrimidinedione was obtained.
Results of analysis of the pyrimidinedione derivative thus
obtained:
389
- 364 -
NMR (CDC13), ~ ppm: 5.02 (t,2H), 3.~ (m, 2H), 3.30 (s, 3H),
3.41 (s, 3H), 2.14 (s, 3H), 1.99(s, 3H)
Elemental analYSiS C10~17N3~5S
Calculated ~): C, 41.23; H, 5.88: N, 14.42;S, 11.01
Analy7.ed (x~: C, 40.97; H, 5.91: N, 14.65; S, 11.07
(3) Preparation of 6-{2-[N-(2-hydroxyethyl)-3-(4-
nitrophenyl)propylamino]ethylamino}-1,3,5-trimethyl-
2,4~1H,3H)-pyrimidinedione hydrochloride ~compound 179)
6-(2-methanesulfonyloxyethylamino)-1,3,5-trimethyl-
2,4(1H,3H)-pyrimidinedione (3.0 g)-was dissolved in 3~ ml of
acetonitrile, added with 2.1 g potassium carbonate and then
allowed to react under heating with reflux for 6 hours.
After the reaction, the reaction mixture was allowed to
stand overnight at room temperature. Insoluble materials
were removed by filtration and the filtrate was made to 100
ml by addition of acetonitrile. A portion of 10 ml was
taken, and 0.23 g of N-~2-hydroxyethyl)-3-t4-
nitrophenyl~propylamine and 50 mg of p-toluenesulfonic acid
monohydrate was dissolved in this portion. The solution was
concentrated in vacuo and the resultant oily substance was
allowed to react at 80~C for 6 hours. The reaction mixture
was then subjected to silica gel column chromatograph
(chloroform/methanol = 40:1, v/v~ for purification. Thus,
0.29 g of 6-{2-~N-(2-hydroxyethyl)-3-(4-
nitrophenyl)propylamino]ethylamino}-1,3,5-trimethyl-
2,4(1~,3~)-pyrimidinedione was obtained.
2001389
- 365 -
Results of the analysis of the pyrimidinedivne derivative
thus obtained:
NMR (CDCl~ ppm: 8.05 (d, 2H), 7.30 (d, 2H), 4.32 (m,
2H), 3.25 ~s, 3H), 3.31 (s, 3H), 2.6-3.1(m, lOH), 2.06 (S,
3H), 1.9 (m, 2H)
Next, this pyrimidlnedione derivative was treated with
HCl/methanol in an ordinary method and thereby 0.12 g o~ a
hygroscopic amorphous compound, 6-{2-[N-(2-hydroxyethyl)-3-
(4-nitrophenyl)propylamino]ethylamino}-1,3,5-trimethyl-
10- 2,4(1H,3H?-pyrimidinedione hydrochloride (compound 179), was
obtained.
Results of the analysis of the compound 179 thus obtained:
IR ~ KBr max (cm 1): 3300, 2640, 1690? 1590, 1550, 1340,
1220, 930, 750, 700
Elemental analysis: C20H2~N505 HCl H20
Calculated (x): C, 50.68; H, 6.81: N, 14.78; Cl, 7.48
Analyzed (x): C, 51.22; H, 6.94: N, 14.29; Cl, 7.73
Example 127
Preparation of 1,3-dimethyl-6-~2-~N-(2-hydroxyethyl~-3-(4-
nitrophenyl)propylamino~ethylamino~-5-nitro-2,4(1H,3H)-
pyrimidinedione hydrochloride (compound 180)
CH3 CH3
Cl ~ O CH3SO2-OCH2CH2N ~ O
NO2 CH3 NO 'CH
2~01389
- 366 -
CH3
,0 No2~CH2CH2CH2NHcH2CH20H
N0 \CH
HCI/CH3OH CH2CH20H 2
N02~CH2CH2CH2NCH2CH2NH~NCH3 ~HCl
(Compound 180)
(1) Preparation of 6-~2-methanesulfonyloxyethylamino)-1,3-
dimethyl-5-nitro-2,4(1H,3H)-pyrimidinedione
6-chloro-1,3-dimethyl-5-nitro-2,4(1H,3H)-
pyrimidinedione (4.0 g) was dissolved in a solution of 13 ml
of dichloromethane and added to a solution of 1.26 ml of
ethanolamine and 3.8 ml of triethylamine in dichloromethane
(13 ml) gradually drop by drop at 0~C. The reaction was
carried out at 0~C for 1 hour; the reaction mixture was
allowed to stand at room temperature for 5 hours.
The reaction mixture was again cooled down to 0~C and
added with 3.8 ml of triethylamine and with 2.28 ml of
methanesulfonyl chloride drop by drop. After the reaction
at 0~C for 5 hours, 30 ml of ice water was added, and the
mixture was stirred vigorously for 30 minutes. The
dichloromethane layer fraction was removed, and the water
layer fraction was subiected to extraction with chloroform.
The extract combined with the organic layer fraction was
washed with water. dried over sodium sulfate anhydrous and
X~1389
- 367 -
then concentrated in _a~uo. The resultant oily substance
was subjected to silica gel column chromatograph
(chloroform/methanol = 30:1 (v/v)~ for purification. Thus,
5.8 g of 6-(2-methanesulfonyloxyethylamino)-1,3-dimethyl-5-
nitro-2,4(1H,3H)-pyrimidlnedione was obtained.
Results of the analysis of the pyrimidinedione
derivative thus obtained:
NMR (CDC13), ~ ppm: 5.12 (t, 2~, 3.44 (s, 3H), 3.22 (m,
2H~, 3.16 (s, 3H)
(2) Preparation of 6-(aziridin-1-yl)-1,3-dimethyl-5-nitro-
2,4(1H,3H)-pyrimidinedione
6-(2-methanesulfonyloxyethylamino)-1,3-dimethyl-5-
nitro-2,4(1H,3H)-pyrimidinedione ~5.8 g) was dissolved in 70
ml of acetonitrile and added with 3.8 g of potassium
carbonate. The reaction was carried out under heating with
reflux for 3 hours. The reaction mixture was cooled down to
room temperature; insoluble materials were removed by
filtration. The filtrate was concentrated to a volume of 20
ml, added with 100 ml of ether and then stored refrigerated
for 2 days. Crystals formed were collected by filtration
and washed with hexane, thus 1.0 g of a powdery compound, 6-
(aziridin-l-yl)-1,3-dimethyl-5-nitro-2,4(1H,3H)-
pyrimidinedione, was obtained.
Results of the analysis of the pyrimidinedione derivative
thus obtained:
NMR C~C13, ~ ppm: 3.37 (s, 3H), 3.18 (s, 3H), 2.1-2 2
2001389
- 368 -
(m, ~lH)
~3) Preparation of 1,3-dimethYl-6-~2-~N-(2-hydroxyethyl)-
3-(4-nitrophenyl)propylamino]ethylamino}-5-nitro-2,4(lH,3H)-
pyrimidinedione hydrochloride (compound 180)
An oily substance obtained by dissolving 1.0 g of 6-
(aziridin -l-yl)-1,3-dimethyl-5-nitro-2,4~1H,3H)-
pyrimidinedione, 0.99 g of N-(2-hydroxyethyl)-3-(4-
nitrophenyl)propylamine and 50 mg of p-toluenesulfonic acid
in l.V ml of dimethylformamide was allowed to react at 90~C
for 3 hours. The reaction mixture was cooled down to room
temperature, added with 10 ml of water and then vigorously
stirred. Crystals formed were collected by filtration,
dissolved in chloroform and then subjected to silica gel
chromatograph (chloroform/methanol = 40:1 (v/v)) for
purification. Thus, 0.44 g of 1,3-dimethyl-6-{2-[N-(2-
hydroxyethyl)-3-(4-nitrophenyl)propylamino]ethylamino~-5-
nitro-2,4(1H,3H)-pyrimidinedione (a free form of compound
180) was obtained.
Results of the analysis of the pyrimidinedione derivative
thus obtained:
NMR (CDC13), ~ ppm: 8.01 (d, 2H), 7.31 (d, 2H), 3.66 (t,2H)
3.45 (5, 3H), 3.35 (s, 3H), 2.6-3.6 (m, lOH), 2.0(m, 2H)
Next, this pyrimidinedione derivative was treated with
HCI/methanol in an ordinary method; thus 0.11 g o~ a
hygroscopic amorphous compound, 1,3-dimethyl-6-{2-[N-(2-
hydroxyethyl)-3-(4-nitrophenYl)propylamino]ethylamino}-5-
2(~01389
- 369 -
nitro-2,4(1H,3H)-pyrimidinedione hydrochloride ~compound
180), was obtained.
Results of the analysis of the compound 180 thus obtained:
IR ~ KBr max (cm 1): 3300, 2550, 1700, 1650, 1530, 1350,
1110, 930, 750
Elemental analysis: C1gH26N607 HCl 3H20
Calculated (x): C, 42.19; H, 6.15: N, 15.54; Cl, 6.55
Analyzed ~X): C, 41.79; H, 6.41: N, 14.83; CI, 6.02
Example 128
Preparation of tablets containing, as an active ingredient,
1,~-dimethyl-6-{2-[N-(2-hydroxyethyl)-3-(4-
nitrophenyl)propylamino]ethylamino}-5-nitro-2,4(1H,3H)-
pyrimidinedione (free form of compollnd 180), which can be
obtained by the method of Example 127.
Said pyrimidinedione derivative (free form of compound
180) ~1 g), 123 g of lactose and 20 g of corn starch were
thoroughly mixed. The mixture was mixed in an aqueous
solution of 5 g of hydroxypropylcellulose (100 ml) was
granulated and dried at 50~C for 4 hours. The granules were
20 thoroughly blended with 1 g of magnesium stearate and then
compressed into tablets using a tablet machine with a
pressure of 150 mg/tablet.
Example 129
Production of capsules containing, as an active ingredient,
6-~2-[N-(2-hydroxyethyl~-3-(4-nitrophenyl)propylamino]
ethylamino}-1,3,5-trimethyl-2,4(1H,3H)-pyrimidinedione
ZQ~1389
- 370 -
hydrochloride (compound 179), which can be obtained by the
method of Example 12~
Said pyrimidinedione hydrochloride derivative (compound
179) (5 g), 120 g of lactose and 25 g of corn starch were
thoroughly mixed, and the resultant mixture was formulated
into hard capsules (150 mg per capsule~ using a capsule
filling machine.
Example 130
Production of an injection containing, as an active
ingredient, 1,~-dimethyl-6-~2-~N-(2-hydroxyethyl)-3-(4-
nitrophenyl)propylamino~ethylamino}-5-nitro-2,4(1H,3H)-
pyrimidinedione hydrochloride (compound 180) which can be
obtained by the method of Example 127.
Said pyrimidinedione derivative hydrochloride (compound
180) (20 mg) and 0.85 g of sodium chloride were dissolved in
an appropriate amount of distilled water for injection to
total the volume 100 ml and thus formulated into an
injection.
Pharmacological test example 9:
A pharmacological tests with the compound 180 obtained
by the method of Example 127 was carried out in the same
manner as described in Pharmacological test 1. As a result,
the ADP75 values as shown in Table 18 were obtained.
- 371 - ~ 3 8 9
Table 18
ADP75 (~)
Compound No.Concentration
(Jug/ml )
0.3 1.0 3.0 10.0
180 - 11 20 26
Examele 131
Preparation of 1,3-dimethyl-6-~4-<3-~2-nitro-4-(2-
pyridinecarbonyl)phenoxy]propyl>piperazin-l-yl~-2~4~1H,3H~-
pyrimidinedione oxalate (compound 181)
Cl
'o~ C
CN
15z z ~ ~N~ (COOH)2/cH3oH
(Compound 36) CH3 >
<~C~-OCHzCH2CHzN N ~NCH3
(COOH) 2
(Com~ound 181)
... ... . ..
2~11389
- 372 -
(1~ Preparation of 2-chloro-5-(2-pyridinecarbonyl)
nitrobenzene
2-bromopyridine ~3.3 ml) was dissolved in 30 ml of
ether and added with 22.1 ml of butyllithium (1.6M, in
hexane~ drop by drop at -30~C. To this mixture were added a
solution of 5.0 g of 4- chlorobenzonitrile in ether (10 ml)
drop by drop at the same temperatllre. The reaction mixture
was stirred for 1 hour and then poured into ice water.
Ether was removed In vacuo. The resultant water-layer
fraction was acidified by adding 6N HCl, stirred at 100~C
for 1 hour, cooled, alkalized by adding sodium hydroxide and
then extracted with ether. The resultant ether-layer
fraction was concentrated and the oil thus obtained was
dissolved in 10 ml of fuming sul~uric acid at 0~C. To this
solution, added 1.2 ml of fuming nitric acid drop by drop
and the resultant solution was stirred at the same
temperature for 1 hour. The reaction mixture was poured
into ice water and crystals formed were collected by
filtration. The crystals were further recrystallized using
ethanol, and 3.6 g of crystalline 2-chloro-5-(2-
pyridinecarbonyl)nitrobenzene was thus obtained.
(2) Preparation of 1,3-dimethyl-6-~4-<3-[2-nitro-4-(2-
pyridinecarbonYl)PhenoXy]propyl>piperazin-l-yl}-2~4(lH~3H)
pyrimidinedione oxalate (compound 181)
Sodium hydride (60 ~ dispersion in mineral oil) (0 17 g) was
washed with hexane to remove oil and added with 8 ml of DMF.
200:~389
- 373 -
The mixture was cooled down to V~C, added with 1.0 g of
1,3-dimethyl-6-[4-~3-hydroxypropyl>piperazin-1-yl]-
2,4~lH,3H)-pyrimidinedione (compound 36) and stirred for 30
minutes. To this mixture was added 1.1 g of 2-chloro-5-(2-
pyridinecarbonyl)nitrobenzene, and the resultant mixture wasstirred for 1 hour. The reaction mixture was poured into
ice water and crystals formed were collected by filtration,
washed with water, dried and 1.0 g of 1,3-dimethyl-6-{4-<3-
t2-nitro-4-(2-pyridinecarbonyl)phenoxy]propyl>piperazin-1-
yl~-2,4~1H,3H)-pyrimidinedione in an amorphous powder form
was thus obtained.
Results of the analysis of the pyrimidinedione derivative
thus obtained:
NMR ~CDCI3~, ~ ppm: 2.1 (m, 2H), 2.4-3.1 (m, lOH), 3.25 (s,
3H), 3.34 (s, 3H), 4.28(t, 2H), 5.14 (s, lH), 7.1-8.9 (m,
7H)
Next, this pyrimidinedione deri~ative (0.98 g) was
treated with an oxalic acid/methanol solution in the
ordinary method; thus 0.76 g of crystals of 1,3-dimethyl-6-
{4-<3-t2-nitro-4-(2-pyridinecarbonyl)phenoxy~propyl>
piperazin-l-yl}-2,4(1H,3H)-pyrimidinedione oxalate
(compound 181) was obtained.
Results of the analysis of the compound 181 thus obtained:
lR ~ KBr max (cm 1): 3450, 2500, 1700, 1650, 1600, 1520,
1310, 1280, 800, 740, 700
Elemental analYSis: C2~H28~6~6 (C~~~)2 2
- 374 - ~ ~ Q~ 389
-
Calculated (X): C, Sl.10; H, 5.40: N, 13.24;
Analyzed (X): C, 51.32; H, 5.40: N, 13.15
Example 132
Preparation of 1,3-dimethyl-6-~2-[N-(3-hydroxypropyl)-3-(4-
nitrophenyl)propylamino]ethylamino~ 2,4(1H,3H)-
pyrimidinedione oxalate (compound 182)
N02 ~ CH2CH2CH20S- ~ CH 3
~ ~o
CH3
N02 ~ ~ CH2CH2CH2NHCH2CH2CH20H >
(Compound 6)
(COOH)/~OH
S CH2CH2CHz0H o
N02 ~ CH2CH2CH2NCH2CHzNH- ~ CH3
CH 3 ' (C 00H) 2
(Compound 182)
( (1) Preparation of N-(3-hydroxypropyl)-3-(4-
nitrophenyl)propylamine
3-(4-nitrophenyl)propyl p-toluenesulfonate (1 g) and
4.1 ml of 3-hydroxypropylamine were dissolved in 10 ml of
dioxane and the solution was stirred at 80~C for 30 minutes.
To the solution was added 100 ml of water and 100 ml of
chloroform, and the mixture was thoroughly mixed for
separation. The chloroform-layer fraction was taken. washed
, .,
.~
389
_ - 375 -
with water and then dried over sodium snlfate anhydrous.
The solvent was removed in _acuo and 0.7 g of an oil of N-
~3-hydroxypropyl)-3-(4-nitrophenyl)propy1amine was vbtained.
Results of the analysis of the amine derivative thus
obtained:
NMR (CDC13), ~ ppm: 1.9-2.1 (m, 4H), 2.8-3.2 (m, 6H), 4.18
(t, 2~), 7.62 (d, 2H), 8.01 (d, 2H)
(2) Preparation of 1,3-dimethyl-6-{2-[N-(3-hydroxypropyl)-3-
(4-nitrophenyl)propylamino]ethylamino}-2,4(1H,3H~-
pyrimidinedione oxalate (compound 182)
N-(3-hydroxypropyl~-3-(4-nitrophenyl)propylamine ~0.7
g), 0.53 g of 6-(1-aziridinyl)-1,3-dimethyl-2,4~1H,3H)-
pyrimidinedi.one (compound 6) and 50 m~ of p-toluenesul.fonic
acid monohydrate were dissolved in 30 ml acetonitrile, and
then the solvent was removed ln vacuo. The resultant oil
was allowed to react at 80~C for 3 hours and subjected to
silica gel column chromatograph (chloroform/ methanol = 40:1
(vJv)) for purification: 0.68 g of 1,3-dimethyl-6-~2-[N-(3-
hydroxypropyl)-3-(4-nitrophenyl)propylamino]ethylamino}-
2,4(1.H,3H)-pyrimidinedione was thus obtained.
Results of the analysis of the pyrimidinedione derivative
. .
thus obtained:
NMR (CDCl~ ppm: 1.7 (m, 4H}, 2.4-3.1 (m, 8H), 3.21 (s,
3H~, 3.34 (s, 3~), 3.70 (br, 2H), 4.0 (m, 2H), 4.71 (s, IH),
5.81 (br, lH~, 7.25 (d, 2H), 8.06 (d, 2H)
Next, this pyrimidinedione derivative (0.65 g) was
376 - ~ 0 0 ~ 3 8 9 ~
treated with an oxalic acid/methanol solution in the
ordinary method; thus 0.62 g of crystalline 1,3-dimethyl-6-
{2-[N-(3-hydroxypropyl)-3-(4-
nitrophenyl~propylamino]ethylamino}-2,4(1H,3H)-
pyrimidinedione oxalate ~compound 182) was obtained.
Results of the analysis o~ the compound 182 thus obtained:
IR ~ KBr max (cm 1): 3300, 1690, 1620, 1540, ~410, 1330,
1050, 850, 770, 740, 690
Elemental analysis: C20H29N5o5 (C~~~)2 H20
Calculated (%): C, 50.09; H, 6.31: N, 13.28
Analyzed (%): C, 50.37; H, 6.25: N, 12.79
Example 133
Preparation of 1,3-dimethyl-6-~2-~N-(l-methylethyl~-3-(4-
nitrophenyl)propylamino]ethylamino~-2,4(1H,3H)-
pyrimidinedione oxalate (compound 183)
N0z ~ CH2CH2CHIoll ~ CH3
~N- ~ NCH3
,N~o
N0z ~ CHzCHzCH2NHCH2(CH3~2 CH3
(Compound 6)
(COOH)2/CH3oH
) CH(CH3~2 0
N02- ~ CH2CH2CHzNCH2CH2NH ~ NCH3
IN~o
CH3 ~(C00H~ 2
(C~mpound 183)
- Z~01389
- 377 -
(1) Preparation of N-(1-methylethyl)-3-(4-
nitr()phenyl)propylamine
3-(4-nitrophenyl~propyl p-toluenesulfonate (1 g) and 5
ml of isopropylamine were dissolved in 15 ml of dioxane, and
the solutlon was stirred under heating with reflux for 4
hours. To the solution were added 100 ml of water and 100
ml of chloroform, and the mixture was thoroughly mixed for
extraction. The chloroform-layer fraction was taken, washed
with water and dried over sodium sulfate anhydrous. The
solvent was removed in vacuo, and thus 0.61 g of an oil of
N~ methylethyl)-3-~4-nitrophenyl)propylamine was obtained.
Results of the analysis of the amine derivative thus
obtained:
NMR ~CDC13), ~ ppm: 1.22 (d, 6H), 2.0 (m, 2H), 2.9-3.3 (m,
i5 5H), 7.28(d. 2H), 8.0g (d, 2~)
(2~ Preparation of 1,3-dimethyl-6-{2-[N-(l-methylethyl)-3-
(4 nitrophenyl)propylamino~ethylamino}-2,4(1H,3H)-
Pyrimidinedione oxalate (compound 183)
N-~l-methylethyl~-3-(4-nitrophenyl)propylamine (0.6 g),
0.5 g of 6-(1-aziridinyl)-1,3-dimethyl-2,4(1H,3~)-
pyrimidinedione (compound 6) and 50 mg of p-toluenesulfonic
acid monohydrate was dissolved in 30 ml acetonitrile, and
then the solvent was removed in vacuo. The resultant oil
was alLowed to react at 80~C for 3 hours and subiected to
silica gel column chromatograph (chloroform/ methanol = 40:1
(v/v)~ for purification. Thus, 1 0 g of 1,3-dimethyl-6-12-
~)1389
- 378 -
[N-~1-methylethyl)-3-~4-nitrophenyl)propylamino]ethyl~mino~-
2,4(1H,3H)-pyrimidinedione was obtained.
Results of the analysis of the pyrimidinedione derivative
thus obtained:
NMR ~GDCl3), ~ ppm: I.45~d, 6~), 2.0 ~m, 2H), 3.29 (s, 3H),
3.40 (s, 3H), 2.5-3.1 (m, ~H), 3.8-4.1 ~m, 4H), 4.80 (s,
lH), 5.5 (m, lH), 7.31 (d, 2H), 8.00 (d, 2H)
This pyrlmidinedione derivative ~0.95 g) was treated
with an oxalic acid/methanol solution in the ordinary
method; thus 0.88 g of crystals of 1,3-dimethyl-6-~2-[N-(1-
methylethyl)-3-(4-nitrophenyl)propylamino]ethylamino}-
2,4(1H,3H)-pyrimidinedione oxalate (compound 183) was
obtained.
Results of the analysis of the compound 183 thus obtained:
IR ~ KBr max ~cm 1) 3250, 2600, 1700, 1640, 1590, 1510,
1~30, 1220, 760, 750, 700
Elemental analysis: C20ff29N50~ 1.5~COOH)2 0.5H20
Calculated (X): C, 50.45; H, 6.08: N, 12.79
Analy~ed (X): C, 50.25; H, 5.81: N, 12.52
Example 134
Preparation of 1,3-dimethyl-6-{2-rN-(2-hydroxy-1-
methylethyl~-3-(4-nitrophenyl)propylamino]ethylamino~-
2,4~1H,3H)-pyrimidinedione hydrochloride (compound 187)
_ - 379 ~ a 0 n ~ ~ 8 9
0 CH3
NOz ~ CHzCHzCOzH ~NO2 ~ CHzCHzCNHCHCHzOH >
C ~J
CH3 N O
r~ I CH3
NOz- ~ CH2CH2CHzNHCHCHzOH
(Compound 6)
HCl /CH30H
> CH(CH3)cH2oH O
NO2 ~ CHzCHzCHzNCHzCHzN~ ~ NC~3
~HCl
(Compound 187)
(1) Preparation of N-(2-hydroxy-1-methylethyl)-3-(4-
nitrophenyl)propionamide
3-(4-nitrophenyl)propionic acid (5 g) was suspended in
40 ml of thionylchloride, and the suspension was stirred
under heating with reflux for 2 hours. Excessive
thionylchloride was removed in vacuo and the oil thus
( obtained was dissolved in 15 ml of chloroform.
Separately, 3.8 g of 2-amino-1-propanol and 3.5 g of
potassium carbonate were dissolved in 35 ml water, and the
resultant solution was cooled down to 0~C and added drop by
drop with the chloroform solution which had been obtained
previously. The mixture was stirred maintaining at ~~C for
1 hour. Crystals formed were collected by filtration and
washed with water. Furthermore, the crystals were
, ~ .. .
2~)1389
- 380 -
recrystallized using an ethyl acetate/chloroform ~1:1 (v/v)~,
and thus 3.46 g of crystalline N-(~-hydroxy-
l-methylethyl)-3-(4-nitrophenyl)prupionamide was obtained.
Results of the analysis of the amine derivative thus
obtained:
Melting point: 174~C
(2) Preparation of N-(2-hydroxy-1-methylethyl)-3-(4-
nitrophenyl)propylamine
N-(2-hydroxy-1-methylethyl)-~-(4-nitrophenyl)
propionamide obtained in (1) above (3.4 g) and sodium
borohydride (2.1 g) was suspended in 32 ml of THF. To the
mixture was added drop by drop with a solution of 3.3 g
acetic acid in THF ~32 ml~. The mixture was stirred under
heating with reflux for 10 hours. The reaction mixture was
then cooled down to 5~C, added with 20 ml of methanol,
stirred for 30 minutes and then concentrated by drying. To
the resultant residue was added 50 ml of lN-HCl and 50 ml of
chloroform, and the mixture was vigorously stirred. The
mixture was allowed to stand for extraction then
fractionated. The water-layer fraction was alkalinized with
sodium hydroxide and extracted with chloroform. The
chloroform-layer fraction was washed with water, dried over
sodium sulfate anhydrous and then concentrated by drying;
thus, 2.96 g of an oily compound, N-(2-hydroxy-1-
methylethyl)-3-(4-nitrophenyl)propylamine, was obtained.
This was used for the following reaction without further
2C~1389
- 381 -
purification.
~3) Preparation of 1,3-dimethyl-6-{2-[N-(3-hydroxy-1-
methylethyl)-3-(4-nitrophenyl)propylamino]ethylamino}-
?~4(lH~3H)-pyrimidinedione hydr-ochloride (compound 187)
N-(2-hydroxy-1-methylethyll-3-~4-nitrophenyl)
propylamine (2.2 g) obtained in ~2~ above, 1.6 g of 6-(1-
aziridinyl)-1,3-dimethyl-2,4(1H,3HS-pyrimidinedione
(compound 6) and 0.1 g of p-toluenesulfonic acid mono-
hydrate were dissolved in 100 ml of acetonitrile, and then
the solvent was removed n vacuo. The resultant oil was
allowed to react at 80~C for 3 hours and subjected to silica
gel column chromatograph tchloroform/ methanol = 40:1 ~v/v))
for purification; thus, 3.0 g of 1,~-dimethyl-6-{2-~N-(2-
hydroxy-l-methylethyl~-3-(4-nitrophenyl)propylamino~
ethylamino}-2,4(1H,3H)-pyrimidinedione was obtained.
Results of the analysis o~ the pyrimidinedione derivative
thus obtained:
NMR (CDC13), ~ ppm: 1.04 (d, 3H), 1.9 (m, 2H), 2.5-3.0 (m,
9H), 3.28 (s, 3H), 3.40 (s, 3H), 4.13 (m, 2H), 4.69 (s, lH),
6.47 (m, lH), 7.29 (d, 2H), 8.0fi (d, 2H)
This pyrimidinedione derivative (2.95 g) was treated
with a HCI/methanol solution in the ordinary method; thus
3.02 g of crystalline 1,3-dimethyl-fi-~2-rN-~2-hydroxy-1-
methylethyl)-3-(4-nitrophenyl)propylamlno]ethylamino}-
2,~(lH,3H)-pyrimidinedione hydrochloride (compound 187) was
obtained.
200~389
-- 382 --
Resul ts of the analysis of the compound 187 thus obtained:
Melting point: 154 - 155~C
IR ~ KBr max (cm 1) 3250, 2~00, 1690, 1600, 1550, 1340,
1240, 1060, 840, 760, 700
Example 135
Preparation of 1,3-dimethyl-6-~4-(3-methyl-4-
nitrobenzylpiperazin-1-yl~-2,4~1H,3H)-pyrimidinedione
hydrochloride ~compound 188)
HN N~CH3
- ~ N~o
N02~CH20H > NO~CHzCl CH3
CH3 CH3(Compound 2)
HCl /CH30H
> N02- ~ CH2N ~ - ~NCH3
CH3
~ HCl
(Compound 188
~1) Preparation of 3-methyl-4-nitrobenzyl chloride
3-methyl-4-nitrobenzylalcoho~ (2 g) and 0.2 ml of DMF
were dissolved in 20 ml of toluene. The solution was added
with 1 ml of thionyl chloride and stirred under heating with
reflux for 3 hours. The reaction mi~ture was concentrated
and thus 2.2 g of an oil, 3-methyl-4-nitrobenzyl chloride,
281)1389
- 383-
was obtained. This compound was used for the following
reaction without further purification.
(2~ Preparation of 1,3-dimethyl-6-t4-(3-methyl-4-
nitrobenZYl)piperazin-l-yl~-2,4~1H,3H~-pyrimidinedione
S chloride (compound 188)
3-methyl-4-nitrobenzyl chloride (2.2 g) obtained in ~1
above, 2.2 g of 1,3-dimethyl-6-(piperazin-1-yl3-2,4(1H,3H)-
pyrimidinedione (compound 2) and 4.1 ml of triethylamine
were dissolved in 40 ml of isopropanol, and the solution was
stirred under heating with reflux for 2.5 hours. After the
reaction, the solvent was removed in vacuo, and the residue
was dissolved in 50 ml of chloroform. The chloroform
solution was washed with water and dried over sodium sulfate
anhydrous and then the solvent was removed in vacuo; thereby
2.76 g ~f crystalline 1,3-dimethyl-6- r 4-(3-methyl-4-
nitrobenzyl)Piperazin-l-yl]-2~4(lH~3H)-pyrimidinedione was
obtained.
Results of the analysis of the pyrimidinedione derivative
thus obtained:
Melting point: 162 -164~C
NMR (CDC13~, ~ ppm: 2.5-3.3 (m, 8H). 2.61 (s, 3H), 3.26 ~s,
3H~, 3.36 (s, 3H), 3.61 ~br, 2H), 5.15 (s, lH~, 7.35 (m,
2H), 7.87 (d, LH)
This pyrimidinedione derivative (2.4 g) was treated
with a hydrochloric acid/methanol solution by the ordinary
method; thus 2.34 g of crystalline 1,3-dimethyl-6-[4-(3-
- 384 - 2 ~ 0 1 3 8 9
methyl-4-nitrobenzyl)piperazin-l-yl]-2~4(lH~3H)-
pyrimidinedione hydrochloride (compound 188) was obtained.
Results of the analysis of the comPound 188 thus obtained:
lR ~ KBr max (cm 1~ 3360, 25~0, 1690, 1640, 1520, 1440,
1340, 1200, 980, 840, 760, 700
Elemental analYsis: C18H23N504 HCI H20
Calculated (X): C, 50.52; H, 6.12: N, 16.36; Cl, 8.28
Analyzed (X): C, 50.87; H, 6.64: N, 16.44; Cl, 7.68
Example 136
Preparation of 1,3-dimethyl-6-{2-~N-(2-hydro~yethyl~-3-(4-
benzoyl-2-nitrophenoxy)propylamino]ethylamino}-2,4(1H,3H)-
pyrimidinedione oxalate ~compound 189)
~N ~CH3 ~ HOCH2CH2NHCH2CH2NH ~ CH3
(Compound 6) ~ >
~ C ~ OH ~ ~ -C ~ OCH2CH2CHzBr
( O 02 0 NOz
(COOH)2/CH30H
> CH2CH20H o
C, ~ OCH2CHzCH2NCHzCH2NH- ~ CH3
CH3 ~(COOH) 2
(Compound 189)
(1) Preparation o~ 1,3-dimethyl-6-{2-(2-hydroxyethylamino)
B
.. . .
2~01389
- - 385 -
ethylamino~-2,~(1H,3H)-pyrimidinedione
6-(1-aziridinyl)-1,3-dimethyl-2-4(1H,3H~-
pyrimidinedione (compound 6) ~0.81 g)~ 1 ml of ethanolamine
and 50 mg of p-toluenesulfonic acid monohydrate were
dissolved in 200 ml of acetonitrile and then the solvent was
removed in vacuo. The resultant oil was allowed to react at
90~C for 3 hours. The reaction mixture was cooled down to
room temperature and then dissolved by adding ethanol.
Ether was added to this soiution for crystallization. The
crystals formed were collected by filtration and further
recrystallized using an ethanol/ether mixed solvent; thereby
1.02 g of crystalline 1,3-dimethyl-6-~2-(2-
hydroxyethylamino)ethylamino~-2,4(1~,3H)-pyrimidinedione was
obtained.
Results of the analysis of the crystals of pyrimidinedione
derivative
Melting point: 146-148~C
~2) Preparation of 4-(3-bromopropyloxy)-3-nitrobenzophenone
4-hydroxy-3-nitrobenzophenone (2.5 g) and 7.1 g of
potassium carbonate were suspended in 15 ml
methylethylketone. The suspension was stirred under heating
with reflux for 30 minutes, added with 1,3-dibromopropane
and then heated with reflux for 6 hours. The reaction
mixture was cooled down to room temperature, and insoluble
substances were removed by filtration. The filtrate was
then concentrated, and the resultant precipitate was
- 2C~01389
- 386 -
subjected to silica ~el column chromatograph (hexane/ethyl
acetate =3:1 (v/v)~ for purification; thereby 2.08 g of an
oil, 4-(3-bromopropyloxy)-3-nitrobenzophenone, was obtained
NMR ~CDC13~, ~ ppm: 2.40(m, 2H), 3.66 ~t, 2H), 4.39 (t, 2H),
7.13-8.34 (m, 8H)
(3) Preparation of 1,3-dimethyl-6-~2-[N-(2-hydroxyethyl)-3-
(4-benzoyl-2-nitrophenoxy~propylamino]ethylamino~-
2,4(1H,3H)-pyrimidinedione oxalate (compound 189)
A mixture of 0.67 g of 1,3-dimethyl-6-{2-(2-
hydroxyethylamino)ethylamino}-2,4(1H,3H)-pyrimidinedione
obtained in (1) above, 1.0 g of 4-(3-bromopropyloxy)-3-
nitrobenzophenone obtained in (2) above, 1.5 ml of
triethylamine and 3 ml of D~F were stirred under heating at
100~C for 1 hour. The mixture was cooled down to room
temperature, added with 50 ml chloroform, washed with water
and then dried over anhydrous sodium sulfate. The solvent
was removed and the resultant residue was crystallized using
an ethanol/ether mixed solvent; 0.59 g of crystalline 1,3-
dimethyl-6-~2-~N-(2-hydroxyethyl)-3-(4-benzoyl-2-
nitrophenoxy)propylamino]ethylamino}-2,4(1H,3H~-
pyrimidinedione was thus obtained.
Results of the analysis of the pyrimidinedione derivative
thus obtained:
Melting point: 180 -181~C
NMR ~CDC13/DMSO-d6=1/1, v/v~, ~ ppm: 2.0 (m, 2H), 2.fi-3.1
(m, 8H~, 3.21 (s, 3H), 3.34 (s, 3H?, 3.67 (t, 2H~, 4.39 (t,
38 72~1389
2H), 4~58 (s, lH), 6.25 (br, lH~, 7.31 (d, lH), 7.5-8.1 (m,
6H), 8.26 (d, lH)
This pyrimidinedione derivative (0.55 g) was treated
with an oxalic acid/methanol solution in the ordinary
method; 0.62 g of crystalline 1,3-dimethyl-6-{2-[N-(2-
hydroxyethyl)-3-(4-benzoyl-2-nitrophenoxy)
propylamino~ethylamino}-2,4(1H,3H)-pyrimidinedione oxalate
(compound 189) was thus obtained.
Results of the analysis of the compound 189 thus obtained:
Melting point: 144 - 147~C (Decomposed~
IR ~> KBr max ~cm 1): 3300, 2590, 1720, 1680, 1630, 1530,
1340, 1280, 1070, 7B0, 7tO
Elemental analysis: C26H31N507 (COOH)~ 2
Calculated (X): C, 53.84; H, 5.49: N, 11.21;
Analyzed ~x): C, 53.~8; H, 5.?6: N, 11.27
Example 1~7
Preparation of 1,3-dimethyl-6-{2-[N-(2-hydroxyethyl)-3-~3-
methoxy-4-nitrophenyl~propylamino]ethylamino}-2,4(1H,3H)-
pyrimidinedione oxalate (compound 1~0
N0z ~ CH=CHC02H , N02 ~ CH2CHzCOzH
CH30 CH30
> N02 ~ CH2CH2CNHCH2CH20H
CH30 0
¦ N- ~ CH3
> N02 ~ CHzCH2CH2NHCHzCHzOII CH3
CH30 / (Compound 6)
- 388 - 20 n ~ 3 8 9 !
(COOH)2/CH30H
CH2CHzOH o
--~ NOz ~ -CHzCHzCHzNCHzCH2NH- ~ NCH3
N o (COOH) 2
CH30 CH3
(Compound 190)
(1) Preparation of 3-(3-metho~y-4-nitrophenyl)propionic acid
3-methoxy-4-nitrocinna~ic acid (6.2 g) and 12.3 g of
hydroxyamine sulfate were dissolved in 250 ml of water. To
the solution was added 7.1 g of sodium hydroxide, and
further alternately added a solution of 18 g of sodium
hydroxide in water (27 ml) and 19.2 g of hydroxyamine-o-
sulfonic acid little by little, maintaining the p~ at 9.
The mixture was stirred at 5~C for 6 hours and then
insoluble substances were removed by filtration. The
resultant filtrate was cooled on ice and was added with 6N
sulfuric acid to adjust the pH to 2. Crystals formed were
collected by filtration, washed with water and dried; 1.~7 g
of crystalline 3-(3-methoxy-4-nitrophenyl)propionic acid was
thus obtained.
Results of the analysis of the crystalline propionic acid
derivative thus obtained:
Melting point: 137-139~C
(2) Preparation of N-(2-hydrnxyethyl)-3-~3-methoxy-4-
nitrophenyl)propanamide
3-(3-methoxy-4-nitrophenyl~propionic acid obtained in
'
, ,
r- .i
2~01389
- 389 -
(1) above (1 2 g) was suspended in 20 ml of thionyl
chloride. The suspension was stirred for 2 hours under
heating with reflux and then the solvent was removed. The
resultant residue was dissolved in 3 ml of chloroform.
Separately, 0.51 g of ethanolamine and 0.78 g of
potassium carbonate were dissolved in 8 ml of water, and the
resultant solution was cooled down to 0~C and added drop by
drop with the chloroform solutinn which had been obtained
previously. The mixture ~as vigorously for 1 hour at the
temperature maintained at 0~C. Crystals formed were
collected by filtration, washed with water and dried.
Furthermore, the crystals were recrystallized using an ethyl
acetate solution and thus l.14 g of crystalline N-(2-
hydroxyethyl)-3-(3-methoxy-4-nitrophenyl~propanamide was
obtained.
Results of the analysis of the crystalline amide thus
obtained:
Melting point: 155~C (Decomposed)
(3) Preparation of N-t2-hydroxyethyl)-3-(3-methoxy-4-
nitrophenyl)propylamine
Sodium borohydride (0.78 g) was suspended in 12 ml ofTHF. To this suspension were added ~.1 g of N-(2-
hydroxyethyl)-3-(3-methoxy-4-nitrophenyl)propanamide
obtained in (2~ above, and further added little by little
1.2 ml of acetic acid. The mixture was stirred until
bubbling stopped and then stirred under heating with reflux
2(~01389
- 390 -
for 10 hours. The mixture was cooled down to room
temperature and added with lO ml of methanol little by
little. The solvent was removed in vacuo. The residue was
added with 20 ml of chloroform and then extracted with a lN-
HCI solution. The extracted acidic water phase wasalkalinized by adding sodium hydroxide on ice and extracted
with chloroform. The chloroform-layer fraction was washed
with a saturated sodium chloride solution and dried over
anhydrous sodium sulfate, and chloroform was removed;
thereby V.72 g of an oily compound, N-(2-hydroxyethyl)-3-(3-
methoxy-4-nitrophenyl~propylamine, was obtained. This
compound was used for the following reaction without further
purification.
(4) Preparation of 1,3-dimethyl-6-{2-~N-(2-hydroxyethyl)-3-
(3-methoxy-4-nitrophenyl)propylamino~ethylamino}-2,4(1H,3H~-
pyrimidinedione oxalate (compound 190)
6-(1-aziridinyl~-1,3-dimethyl-2,4(1H,3H)-
pyrimidinedione (compound 6~ (0.45 g), 0.7 g of N-(2-
hydroxyethyl)-3-(3-methoxy-4-nitrophenyl)propylamine and 50
mg of p-toluenesulfonic acid monohydrate were dissolved in
25 ml of acetonitrile and then the solvent was removed in
vacuo. The resultant oil was allowed to react at 80~C for 3
hours and then subjected to silica gel column chromatograph
(chloroform~methanol = 40/1, v/v~ for purification; thereby
0.38 g of an oil, 1,3-dimethyl-6-{2-[N-(2-hydroxyethyl)-3-
(3-methoxy-4-nitrophenyl)propylamino]ethylamino}-2,4~1H,3H)-
200138~
- 391 -
pyrimidinedjone, was obtained.
Results of the analysis of the pyrimidinedione derivative
thus obtained:
NMR (CDCl3) ~~ ppm: 1.9 (m, 2H), 2.4-3.8 (m, 12H), 3.20 (s,
3H), 3.33 (s, 3H), 3.83 (s, 3H), 4.63 (s, lH), 6.11 (m, lH),
6.75 (m, 2H), 7.26 (m, lH~
This pyrimidinedione derivative (0.33 g) was treated
with an oxalic acid/methanol solution in the ordinary
method; 0.22 g of an amorphous powder of 1,3-dimethyl-6-{2-
~N-(2-hydroxyethyl)-3-(3-methoxy-4-
nitrophenyl)propylamino]ethylamino}-2,4(1H,~H)-
pyrimidinedione oxalate (compound 1gO~ was thus obtained.
Results of the analysis of the compound 190 thus obtained:
IR ~ KBr max (cm 1): 3300, 2550, 1690, 1640, 1540, 1350,
1280, 760, 700
Elemental analysis: C20H29N5O6 2(COOH)2 2H20
Calculated (x): C, 44.24; H, 5.72: N, 10.75
Analyzed (X): C, 43.93; H, 5.85; N, 11.10
- 392 - 20 ~ ~ 3 89 ~
Example 138
Production of 1,3-dimethyl-6-l2-[N- (2-dieth
amino) ethyl-3-(4-nitrophenyl)propylamino]ethylaminoJ-
2,4(1H,3H)-pyrimidinedione oxalate (Compound 191)
o
N~2- ~ -CH2CH2CH2NHCH2CH2NH- ~ -CH3
CH ~
(Compound e) 3
( ClCH2CH2N(C2H5)2 HCl (COOH)2/CH30H
CH2CH2N(C2H5)2 0
N~2- ~_CH2CH2 CH2N_CH2CH2NH_~N-CH3
(CompOund 191) CH3 ~ (C~2 )2
First, 1.4g of 1,3-dimethyl-6-l2-[3-(4-nitro-
phenyl) propylamino]ethylamino~-2,4(1H,3H)-pyrimidine-
dione hydrochloride (Compound e) was dissolved with 5 ml of
water, followed by the addition of K2C03 to render the
solution alkaline.
The resulting solution was then extracted with
CHCl3. The combined layers were concentrated in vacuo,
followed by the addition of 1.0 g of 2-d;ethylamino ethyl-
chloride, 3 ml of triethylamine and 20 ml of isopropanol.
The reaction mixture was refluxed for 10 hours.
Solvent was removed in vacuo, and then the resulting residue
was directly purified by a silica gel chromatography
(eluent: CHCl3/CH30H=20/1, by volume) thereby obtaining 0.6 g
-,~
2g~ 89
- 393 -
of 1,3-dimethyl-6-t2-[N-diethylamino)ethyl-3-(4-nitro-
phenyl)propylamino~-2,4(1 H,3H)-pyrimidinedione.
Further, 0.6 g of the pyrimidinedione derivative
was treated with an oxalic acid/methanol solution by a
method known per se in the art to obtain 0.52 g of 1,3-
dimethyl-6-t2-[N-(2-diethylamino)ethyl-3-(4-nitrophenyl)-
propylamino] ethylamino~-2,4(1 H,3H)-pyrimidinedione (Compound
191) as pale yellow crystals.
Analytical results of crystals of compound 191
thus obtained:
Melting point: 197 - 199~C
IR vKBr(cm 1 ); 3000, 2950, 1720, 1700, 1600, 1340
max
852, 700