Note: Descriptions are shown in the official language in which they were submitted.
Z~S3~
~OECHST-ROUSSEL PH~RMACEUTICALS INC. Dr- LA HOE B8/S 031
1,2,3,4-Tetrahydro-l,9-acridinediamines, a process for
their preparation and their use as meclicaments
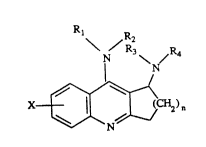
This invcntion relates to compounds having the forrnula
- N \ N~' 4
x~ ) n
(I)
where
nis 1,20r3,
X is hydrogen, lowcraL~yl, loweraL~coxy, halogen, hydroxy, nitro or
~ifluvromethyl;
Rl and R2 arc each independently hydrogcn, loweralkyl or aryllowelallcyl, but
both may not be aryllowerallcyl simul~ancously;
R3 and R4 are each indcpcndcntly hydrogen, loweralkyl, aryllowcsallyl,
fo~myl or loweraL1~y]carbonyl, or alternadvcly the group -NR3R4 takcn ~s a
whole constitutes
~)(3Z~S3~.
-- 2
--N~ --N~ --N N--CH3 --N N~
--N O --N S --N/ _
/, / ~ V \=/;
stereo isomers thereof and pharrnaceutically acceptable acid addition salts
thereof, which are useful for the treatrnent cf various memory dysfunctions
characterized by decreased cholinergic function, such as Alzheimer's disease.
3~iL
Throughout the specification and the appended elaims, a given ehemical
formula or narne shall encompass all stereo, geomet~ic~ and optical isomers thereof
where such isomers exist, as well as phaJrnaceu~ically aceeptable acid addition salts
thereof and solvates thereof such as for instance hydrates.
The following definitions shall apply throught the specification and the
appended clairns.
Unless otherwise stated or indicated, the te~m loweraLkyl denotes a straight or
branched alkyl group having from 1 to 6 carbon atoms. Examples of said loweraL~cyl
include methyl, ethyl, n-propyl, iso butyl, pentyl and hexyl.
Unless otherwise stated or indicated, the term eyeloalkyl denotes an alieyelic
hydroearbon group eontaining 3 to 7 earbon atoms. Exsn ples of said eyeloaLkyl
include eyclopropyl, eyelohexyl and eycloheptyl.
Unless otherwise sta~ed or indicated, the term halogen shall mean fluorine,
ehlorine, bromine or iodine.
Unless otherwise statcd or indicsted, the term aryl shall mean an unsubstituted
phenyl gr~up OI a phenyl group substituted widl 1, 2 or 3 substituents eaeh of whieh
being independently loweraLlcyl, loweraL~oxy, halogen, hydroxy or trifluoromethyl.
The eompounds of this invention are prepared by u~lizing one or more of the
steps deseribed below.
ll rou~hout the description of the synthetic steps, t~e definitions of n, X, R
through R4 are as given above unless otherwise stated or indieated
4 Z~z~
STEP A:
A compound of Forrnula Il is reacted with a diketone of Formula m tO afford
a compound of Formula IV. Typically~ said reaction is conducted in a suitable
solvent such as an etheral solvent including tetrahydrofuran, diethyl ether and dioxane
at a ~emperature of about 30-1 00C.
X~ ~ H2)n X~ J~
NH2
H
(Il) (I~ (IV)
Sll~P B:
A compound of Formula V is prepared by cyclizing compound IV in the
presence of a metallic halide such as cuprous chloride, cuprous bromide or cuprous
iodide or the like used as a catalyst. Typically said cyclization reaction is conducted
in a suitable solvent such as an ethercal solvent including tetrahydrofuran, diethyl
cther and dioxane and in the presence of a ca~alys~ and a basic inorganic salt such as
potassium carbonate, pvtassiurn bicarbonate, sodium carl~onate, sodium bicarbona~e
or the like, at a temperature of about 30-100C.
~(lZ531
NH2
( IV ) p X~H~n
(V)
STEP C: -
A compound of Fo~nula VI (where X is not N02) is prepared by reacting
compound V with a suitable metal hydride such as LiAlH4 in a suitable solven~ such
as an ethereal solvent including ~etrahydrofuran, diethyl ether, dioxane and mixtures
thereof at a temperature of from about -20 to about 20C., and thercafter hydrolyzing
the product.
NH2 OH
( V ~ + ~AIH4 ~ ~
( or N~BH4 ) X~ ~l~CH2)n
( VI)
STEP D:
A compound of Formula V~ is prcpared by reacting compound Vl widl an
~In2s~l
/R3
amine of the Fonnula H--N (where R3 and R4 are not formyl or
R4
loweraLlcylcarbonyl) in a suitable medium, for instance, an aromatic hydrocarbon such
as toluene and preferably in the presence of a suitable catalyst such as
p-toluenesulfonic acid. Typically the reaction is conducted a~ a temperature of 50 to
200~C.
R3 R4
( Vl ) + H-N _~ X~2)n
R3, R4 ~ ~ormyl or loweral~lc~nyl
(vn
STEP E:
A compound of Formula VIII (where Rl is not hydrogen and X is not OH~ is
prepared by reacting compound V with a compound of the fonnula RIW, W being Cl,
Br, 1 or OS03CH3 (mesyloxy). Typically, said reactiorl is oonductcd in a biphasic
systcm comprising a suitable organic solvent such as dichloromethane, chloroform,
benzene, toluene or thc likc, a strongly alkaline aqueous phase such as 509~o aqueous
7 ~s~,n23~3l
NaOH or the like, the starting compounds and a phase transfer catalyst such as
tetrabutylammonium hydrogensulfate at a temperature of about 0-50~C.
Rl /H
\N o
( V ) + R~W X~ ~FH2)~,
( R~ ~ H ) N
( VIII )
Where X is OH, the excluded compound can be prepared by subjecting a compound
of Formula V where X is a loweraL~oxy group (e.g. methoxy group) to a eleavage
reaction eonducted, for instance, with the aid of pyridine hydroehloride at a
temperature of around 180C.
STEP F:
In a manner sirnilar to the one deseribed abovc as STEP C, a compound of
Formula IX (where Rl and R2 may not be bodl aryllowcralkyl) is obtained by further
rcaeting eompound Vlll ~,vith a eompound of the formula R2W, W being the same asdcfincd above.
- 8 - ;~ )2~S3~l
\ /
( VI~ 2W 1~ X ¦ S )n
( Rl and R2 may not be both nyDow~mLI~yl ) N
(IX)
STEP G:
Compound IX obtained in STEP E or F is reduced with LiAlH4 or NaBH4 in
substantially the sarne manner as in STEP C above to afford a compound of formula
X.
\ /
(IX) ~ LiAlH4 ID X~C~2)n
( or NaBH4 ) N
( X)
STEP H:
Compound X is rcacted with aD amine of the Fo~mula
- 9 ~ n~
/R3
H--N ~where R3 and R4 are not forrnyl or lowcraLl~ylcarbonyl) in
R4
subs~an~ially the same manner as in STEP D above to afford a compound of Forrnula
x.
~ ,~ 2
D N
~~ ~ n
R4
R3, R4 ~ formyl or bw L~ylccrbonyl
(XI)
STEP I:
Compound Vl is allowed to rcact with a mixn~e of diphenylphosphorylazide,
triphenylphosphine and diethyl azodicarboxylate to afFord an azide compound of
Forrnula X.
-- 10 -
Z0~1253~1
(C6H50)2poN3 N3
(V~
(C6H5)3P X~ l I ¦ (CH2)n
C2H50cO - N = N - C2C2H5 ~ ~/
(XIT )
Typically, this reac~ion is conducted in a suitable solvent such as tctrahydrofuran at a
temperature of 0 to 80C.
STEP J:
Compound XII is catalytically hydrogenated with a suitable catalys~ such as
palladium on carbon in a routinc manner known to the an to afford a diamine
compound of Formula XI.
~32 NH2
(J~ 11 h H2 x~H2),,
(XIII)
STEPK:
Compound XIII is allowed to react with an acyl
chloride of the formula ~5COCl
11- zon~
where R5 is hydrogen or loweralkyl in a routine manner known to the art to afford a
compound of Forrnula X I V .
\ N/ C\R
X I I I + RCOCl X~--I ~ J: ~H2)n
(XIV )
STEP L:
Compound XIV is reduced with LiAlH4 in a routine manner h~own to the art to
afford a compound of Formula X V .
H CH2Rs
NH2 \ N/
(XIV)+ LL~IH.~ X~H~)A
~XV )
STEP M:
As a special case, a comp~und of Farmula ~ V where Rs is methyl can be
prepared in thc ~ollowing manner.
- 1 2 - Z00z~'~3~
First, compound Vl is allowed to react with acetonitrile in a suitable acidic
medium, for instance, CF3COOH containing a lower concentration of sulfuric acid to
afford a compound of Formula XVI. Typically, this rc,action is conductcd at a
temperature of 0 to 100~C.
CH3
N~ll
(Vl) + CH3CI`I ~ X ~ ~ CH2)n
(XVI )
Secondly, compound XVI obtained above is allowed to react with liAlH~ in a
suitable medium such as diethyl edler or tetrahydrofuran to afford a compound ofFormula ( X V a ) .
H ~C2H5
NH2 \ N
( X V I ) ~ LiAlH4 X~c/H2)n
~XVa)
The compounds of Formula (I) of the present inven~ion can be used for lhe
treatment of various memory dysfunctions characterized by d~creased cholinergic
functions, such as Alzheimer's disease.
This utility can be ascertained by deterrnining the ability of these compounds
to inhibit the activity of the enzyme acetylcholinesterase and thereby increase the
acetylcholine levels in the brain.
CHOLINESTERASE INHIBITlON ASSAY
The ability to inhibit acetylchlincsterase was deterrnined by the photometric
method of Ellman et al., Biochem. Pharrnacol. 7, 88 (1961). Results of some of the
compounds of this invention are presented in Table 1 below along with those of some
reference compounds.
- 14 Z0
TABLE 1 Cholinesterase Inhibilion
~ompound IC5o (molar conc.)
1 -(4-morpholinyl)- 1,2,3,4-
tetrahydro-9-acridinamine >1.0 x 10-3
l -(1 -piperidinyl)- 1,2,3,4-
tetrahydro-9-acridinamine 8.4 x 10-6
1-(1 -pyrrolidinyl)- 1,2,3,4-
tetrahydro-9-acridinamine 1.8 x 10-5
1 -(4-phenyl- 1 -piperazinyl)-
1,2,3,4 tetrahydr~9 acridinamine,>1.0x 10-3
1-(4-methyl- l-piperazinyl)-
1,2,3,4-tetrahydro-9-acridinamine>1.0 x 10-3
Nl-butyl-l ,2,3,4-tetrahydro-
l,9-acridinediamine 4.9 x 10-5
N-benzyl-1,2,3,4-tctrahydro-
l,~-acridinediamine 2.0 x -10-
N-propyl-1,2,3,4-tetrahydro-
I,~-acridinediam~ne 1.3 x 10-
Nl -(2-phenylethyl)- 1 ,2,3,4-tetrahydr~
l,9-acridinediamine 1.9 x 10-5
Nl-ethyl-l ,2,3,4-tetrahydr~1,9-
acridinediaminc, furnarate, hcmi-hydrate 4.8 x 10-6
Nl -(3,4-dimethoxyben2yl)- 1,2,3 ,4-
tetrahydro-l ,9-acridinediamine 3.9 x 10-5
1 -azido- 1 ,2,3,4-tetrahydro-9-
acndinamine, malcate 1.2 x 10-5
'~04~ 3
Reference Cornpounds)
9-Amino-1,2,3,4-tetrahydroacridine 3.1 x 10-7
Physostigrnine 6.0 x 10 9
This utility can also be ascertained by determining the ability of these compounds
~o restore cholinergically deficient memory in the Dark Avoidance Assay. ln this assay
rnice are tested for their ability to remember an unpleasant stimulus for a period of 24
hours. A mouse is placed in a chamber that contains a dark cornpar~nent; a strong
incadescent light drives it to the dark compartment, wherc an electric shock is
administered through metal plates on the floor. The animal is rcmoved from the testing
apparatus and tested again, 24 hours later, for the ability tO remembcr thc electric
shock.
If scopolarninc, an anticholinergic that is known tO cause memory impailment7 isadministered beforc an animal's initial exposure to the test chamber, thc animalrc-enters thc dark compartment shortly after being placed in the test chambcr 24 hours
later. This effect of scopolamine is blocked by an activc tcst compound, rcsulting in a
grcater intcrval before rc~ntry into the daric compartment.
The rcsults for an active compound arc cxpressed as the percen~ of a group of
animals in which the cffect of scopolarnine is blocked, as manifcsted by an increascd
interval bctwcen bcing placcd in the test chamber and re-entcring the dark
compartment. Results oi Dark Avoidance Assay for reprcsentative compounds of this
invention and a rcferencc compound arc prcsentcd in Table 2.
- 1 6 zO~
-
TABLE 2 Dark Avoidance Assay
% of an~mals with
Dose scopolamine induced
Compound mg/kg body weight memory deficit
reversal
Physosdgrnine (Reference) 0.31 20%
l-(l-piperidinyl)-l ,2,3,4-
tetrahydro-9-acridinamine 0.16 13%
Nl-propyl- 1 ,2,3,4-tetrahydro-
l,9-acridinediamine Q.31 20%
Effective quandties of the compounds of the invendon may be administered to a
padent by any of the various methods, for example, orally as in capsule or tablets,
parenterally in the form of sterile solutions or suspensions, and in some cases
intravenously in the form of sterile soludons. Thc rce base final products, while
effecdve themsclves, may bc formulatcd and administered in the form of their
pharmaceudcally acceptablc acid addition salts for purposes of stability, convenience
of crystallizadon, increased solubility and the like.
Acids useful for preparing the pharmaceutically acceptable acid addidon salts ofthe invendon include inorganic acids such as hyd~chloqic, hydrobrornic, sulfwic,nitric, phosphoric and perchlor~c acids, as well as organic acids such as fartaric, citric,
acetic, succinic, maleic, fumaric and oxalic acids.
The acdve compounds of the present invendon may be o;ally administered, for
Z~ 3~l
example, with an inen diluent or with an edible carrier, or they may be enclosed in
gelatin capsules, or they may be cornpressed into tablets. For the purpose of oral
therapeutic administration, the active compounds of the invention may be ineo~rated
with excipients and used in the forrn of tablets, troches, capsules, elixirs, suspensions,
syrups, wafers, chewing gum and the like. These preparations should eontain at least
0.5% of active compounds, but may be varied depending upon the particular form and
may conveniently be between 4% to about 70% of the weight of the unit. The amount
of active compound in such compositions is such that a suitable dosage will be
obtained. Preferred compositions and preparations according to the present invention
are prepared so that an oral dosage unit folTn eontains between 1.0 - 300 milligrams of
active compound.
The tablets, pills, capsules, troches and the like may also eontain the following
ingredients: a binder sueh as micro-crystalline eellulose, gum tragacanth or gelatin; an
excipient such as starch or laetose, a disintegrating agent sueh as alginie aeid,
Prirnogel, eornstareh and the like; a lubneant sueh as magrlesium stearate or Sterotex; a
glidant sueh as eolloidal silicon dioxide; and a sweeting agent such as sucrose or
saeeharin may be added or a flavoring agent sueh as peppertnint, methyl salieylate, or
orange flavoring. When the dosage unit form is a eapsule, it may eontain, in addition to
materials of the above type, a liquid earrier such as a fatty oil. Other dosage unit forms
may eontain other various materials whieh modify the physieal form of the dosage unit,
for example, as eoatings. Thus, tablets or pills may bc coated with sugar, shellac, or
other enteric eoating agents. A s~up may eontain, in addition to the aetive eompounds,
sucrose as a sweetening agent and ccrtain prcservativcs, dyes, colo~ing and flavors.
Materials used in preparing these valious eompositions should be pharmaeeutieally
- 18 2~J~i;
pure and non-toxic in the amounts used.
For the purpose of parenteral therapeutic administration, thc active compounds of
the invention may be incolporated into a solution or suspension. These preparations
should contain at least 0.1% of active compound, but may be varied betwcen 0.5 and
about 30% of the weight thereof. The arnount of ac~ive compound in such
compositions is such that a suitable dosage ~.vill be obtained. Preferred compositions
and preparations according to the present inventions are prepared so that a parenteral
dosage unit contains between 0.5 to 100 milligrams of active compound.
Examples of the compounds of this invention include
pyrrolidinyl)- 1 ,2,3,4-tetrahydro-9-acridinamine;
1-(1 -piperidinyl)- I ,2,3,4-tetrahydro-9-acridinan~ine;
1-(4-morpholinyl)-1,2,3,4-tetrahydro 9-acrdinamine; and
I -(4-phenyl- 1 -piperidinyl)- 1 ,2,3,4-tetrahydro-9-acrdinamine;
Nl-propyl- 1 ,2,3,4-tetrahydro- 1 ,9-acridinediarnine;
Nl-butyl- 1 ,2,3,4-tetrahydro-1 ,9-acridinediamine;
Nl-benzyl- 1 ,2,3,4-tetrahydro- 1 ,9-acridinediamine;
Nl-(3,4-dimethoxybenzyl)- 1 ,2,3,4-tetrahydro-1 ,9-acridinediamine;
Nl-(2-phenylethyl)- 1 ,2,3,4-tetrahydr~ 1 ,9-acridinediamine;
1-(4-methyl- 1-piperazinyl)- 1 ,2,3,4-tetrahydro-9-acridinamine;
1-(4-phenyl- 1 -pipe~azinyl)- 1 ,2,3,4-tetrahydro 9-acridinamine,;
N1-hexanoxyl- 1 ,2,3,4-tetrahydr~ 1 ,9-acridinediamine;
Nl-acetyl-l,2,3,4-tetrahydro 1,9-acridinediamine;
1 -azido- 1 ,2,3,4-tetrahydT~9-acridinamine;
1,2,3 ,4-tetrahydr~ 1 ,9-acridinediamine;
Nl -ethyl-1 ,2,3,4-tetrahydro- 1 ,9-acridinediamine;
ZOQ~
The following exarnples are presente~ in order to illustrate this invention.
EXAMlPLE 1
PvrrolidinYI)- 1,2,3,4-tetrahvdro-l9-acridinamine
9-Amino-1,2,3,4-tetrahydroacridin-1~1 ~5.36 g) was refluxed ovemight in
250 ml of toluene that contained pyrrolidine (7.10 g) and p-toluenesulfonic acidmonohydrate (9.5 g). At the cnd of this eime the reaction mixture was washed with an
aqueous K2CO3 solution and then the organic phase was concentrated. The residue
obtained in this manner was purifled by flash chromatography (5% Et3N, toluene) to
give, after recrystallization fr~m benzenetpentane, 2.91 g, m.p. 201-203.
ANALYSIS:
Calculated for Cl7H21N3: 76.37%C 7.92%H 15.72%N
Found: 76.13%C 8.01%H 15.33%N
EXAMPLE 2
1-(1-Pipcridinyl)-1.2,3.4~ ol~vdro 9-acridinamine
9-Amino-1,2,3,4-tetrahydroacridin-1-ol (10.32 g) was refluxed in 500 ml of
toluene containing 8.5 g of piperidine and 19.0 g of p-toluenesulfonic acid
monohydrate. The reaction mixture was refluxcd overnight and thereaftcr
conccntratcd and tnturated with aqueous NH3. The crude product obtained in this
manner was filtered off, trituratcd with Et20, and then recrystallized ~om benzene to
give 7.02 g of analy~ically pure product, m.p. 21~-217 (d).
- 20 -
~'~0~ 3
ANALYSIS:
Calculated for Cl8H23N3: 76.82%C 3.24%H 14.94%N
Found: 76~99%C 8.20%H 14.81%N
EXAMPLE 3
1 -(4-Mo~pholinyl)- 1 ,2,3t4-tetrahvdro-9-acridinarr~ine
9-Amino-1,2,3,4-tetrahydroacridin-1-ol (15.0 g3 was refluxed in 1000 ml of
toluene that contained 12.18 g of morpholine and 9.285 g of benzaldehyde that had
been freshly washed in K2C03. The reaction mixture was refluxed oven~ight and
allowed to cool. It was then filtered off and the crude product was purificd by flash
chromatography (20% PrOH/EtOAc) to give 2.20 g of analytically purc p~uct after
recrystallization ~om benzene/pentane, m.p. 215-217.
ANALYSIS:
Calculated for Cl7H21N3O: 72.05%C 7.47%H 14.83%N
Found: 71.92%C 7.44%H 14.70%N
EXAMPLE 4
1-(4-Phenvl-l-~iPeridinvl)-1 2~3~4-tetrah~dr
9-acridinamine
9-Amino-1,2,3,4tctrahydroacridin-1~1 (5.36 g) was refluxed for 48 hours in
300 ml of tolucne that contained 4-phcnylpiperidine (8.06 g) and p-tolucnesulfonic
acid monohydratc (9.5 g). At the end of this time the rcaction mixture was
- 21 -
Z(~0%531
concentrated and the residue purified by flash chromatography (5% Et3N/toluene) to
give, after concentration of the product-containing fractions and recrystalli~tion from
EtOAc/pentane, 3.22 g of analytically pure product, m.p. 189-190.
ANALYSIS:
Calculated for C24H2~N3: 80.63%C 7.61%H 11.75%N
Found: 80.79%C 7.72%H 11.72%N
EXAMPLE 5
Nl-Pro~yl-l.2.3,4-tetrahvdro-1,9-acridinediamine
A mixture of 9-amino-1,2,3,4-tetrahydroacridin-1-ol (8.66 g),
p-toluensulfonic acid (8.5 g) and propylaminc (19.9 ml) in 250 rnl of toluene was
refluxed with removal of water for twenty hours. An additional 4 ml of the aminewas then added and reflux was continued for thirty hours.
The rnixture was treated with a dilute NaOH solution and extrac~ed with ethyl
acetate (3x). The organics wcrc thcn washcd with water and dried (saturated NaCIsolution, MgSO4).
Thc desircd am~ne was purificd via flash ch~matography t7.5%
Et3N/C6H5CH3) to give 7.8 g of a ye110w solid, m.p. 171-177C. A 3.8S g portion
was recrystallized from methanol/watcr to give 2.79 g of ye~low crystals, ~p.
175-177C.
ANALYSIS:
~0~1;253~
Calculated for C16H21N3: 75.26%C 8.29%H 16.45%N
Found: 75.40%C 8.39%H 16.52%N
EXAMPLE 6
Nl-Butvl- I ,2,3.4-tetrahvdro- 1 ,9-acridinediamine
A mixture of 9-amino-1,2,3,4-tetrahydroacridin-1-ol (8.26 g), n-butylamine
(15.2 ml) and p-toluenesulfonic acid (8.8 g) in 250 ml of toluene was refluxed with
removal of water for sixteen hours.
The mixture was thcn added to a dilute NaOH solution and extracted with
cthyl acetate (3X). The combined organics were washl with water and dried
(MgS04).
The arnine was purified via flash chromatography (5% Et3N/C6H5CH3) to give
8.0 g of an off-whitc solid, m.p. 162-166~C. A 3.9 g portion was rcclystallized from
methanol/water to give 3.41 g of a white powder, m.p. 164-166~C.
ANALYSIS:
Calculated for C~7H23N3: 75.80%C 8.61%H 15.60%N
Found: 75.86%C 8.61%H 15.64%N
EXAMPLE 7
Nl-Benzvl- 1 .2.3.4-tetrahydro- 1 9-acridinediarr~ne
A mixtore of 9-amino-1,2,3,4tetrahydroacridin-1-ol (7.19 g), ben~ylamine
(14.7 rnl) and p-~oluensuifonic ~d ('7.6 g) in 250 rnl of xylencs was refluxcd with
ZOOZ~3
removal of water for eighteen hours.
The reaction rnixture was quenched into a dilute K2CO3 solu~on and extracted
with ethyl acetate (2X). The organics wele washed wilh water and dricd (saturated
NaCI, MgSO4).
The amine was purified via flash chromatography (5% Et3N/C6HsCH3) to give
6.1 g of an off-white solid m.p. 161-167C. A 4.0 g portion was recrystallized from
ethyl acetate/hexane to give 3.24 g of a white powder, m.p.: 16~168C.
ANALYSIS:
Calculated for C20H21N3: 79.17%C 6.98%H 13.85%N
Found: 79.15%C 7.10%H 13.75%N
EXAMPLE 8
Nl-(3~4-Dimethoxvbenzvl~-1,2~3,4-
tetrahvdro- 1 .9-acridinediarnine
A rnix~ure of 9-amino-1,2,3,4-tetrahydroacridin-1-ol (8.43 g),
3,4-dimethoxybenzylarnine (11.9 ml) and p-toluenesulfonic acid (9.0g) in 250 ml of
toluene was refluxed with removal of water overnight.
The rnixture was then added so iccd NaOH solution and extracted with ethyl
acetate (3x). Thc organics were washed with water and dried (MgS04).
The compound was purified via flash chromatography (2.5% Et3N/EtOAc) to
give 6.65 g of an off-white solid. After attempted purification via she fumaric acid
addition salt, the free base was twice recrystallizcd from dichloromethane/hexane to
give 2.65 g of a yellowish solid, m.p. 16~162C.
ZC
ANALYSIS:
Calculated for C22H25N302: 72.70%C 6.93%H 11.56%N
Found: 72.66%C 6.88%H 11.54%N
EXAMPLE 9
Nl -(2-Phenylethvl)- 1.2,3~4-tetrahvdro- 1 ~9-acridinediallune
A mixture of 9-amino-1,2,3,4-tetrahydroacridin-1-ol (8.82 g), phenethylamine
(15.6 rnl) and p-toluensulfonic acid (8.7 g) in 250 rnl of toluene was refluxed with
removal of water for twenty hours. The mixture was thcn treated with a dilule NaOH
solution, and extrac~ed with ethyl acctate (3x). The combined organics were washed
with water and dried (saturated NaCI solution, MgSO4).
The arnine was purified via flash chromatography (7.5% Et3N/C6HsCH3) to
give 10.6 g (81%) of a yellowish solid, m.p. 124-128C. A 4.02 g portion was
recrystallized from ethyl acetate/hexane to give 3.05 g (61 %) of a white powder, m.p.
120-122C.
ANALYSIS:
Calculated for C2,H23N3: 79.46%C 7.30%H 13.24%N
Found: 79.63%C 7.26%H 13.37%N
EXAMPLE 10
1-(4-Methyl~ icerazinyl)-1,2~3,4-
tetrahydro 9-acndinamine
- 2 5 - '~ Z~3~
9-Amino-1,2,3,4-tetrahydroacridin-1-ol (5.36 g~ was refluxed for 48 h in 300
;nl of toluene that contained l-methylpiperazine (5.06 g) and p-toluenesulfonic acid
monohydrate (9.5 g). At the end of this time the reaction rnixture was distributed
between EtOAc and aqueous K2CO3 and then the combined ~rganic phase was
concentrated and the residue purified by flash chromatography (59to Et3N/EtOAc) to
give, after concentration of the product-containing fractions and recrystallization from
EtOAc, 2.30 g of analytically pure product, m.p. 200 202.
ANALYSIS:
Calculated for Cl8H24N4: 72.94%C 8.16%H 18.90%N
Found: 72.92%C 8.27%H 18.80%N
EXAMPLE 1 1
1 -(4-Phenyl- I -piPerazinvl)- 1.2,3.4-
tetrahvdro-9-acridinamine. maleate
9-Aminio-1,2,3,4-tetrahydroacridin-1-ol (5.36 g) was ref,luxed for 24 h in 300
ml of tcluene that contained 1-phenylpiperazine (8.10 g) and p-toluensulfonic acid
monohydrate (9.5 g). At the end of this time the reaction mLsture was distributed
between EtOAc and aqueous K2CO3 and dlen the combined organic phase was
concentrated and the residue pulified by flash chromatography (5% Et3N/toluene) to
give, after concentration of ~e product-containing fracdons and rçcrystallizadon ~om
CH2CI2-pentane, a product that contained a high rnass weight impurity by mass
spcctrometry. llle maleale was fomled in isopro~anol, filtered off and recrystallized
from MeOHlE~20 to give 3.03 g of analyticaMy pure pr~duct, m.p. 205-207.
ANALYSIS:
- 26 - ',~0~Z`~3~
Calculatedforc23H26N4c4H4o4: 68.34%C 6.37%H 11.81%N
Found: 68.25%C 6.36%H 11.71%N
EXAMPLE 12
Nl-Hexanovl- 1,2.3,4-tetrahydro- 1 ~9-acridinediamine
To a chilled solution of 1,2,3,4-~etrahydro- 1,9-acridinediamine (4.96 g) and
triethylamine ~3.6 rnl) in 100 ml of tefrahydrofuran was added hexanoyl chloride (3.6
rnl). This was stirred for five minutes, then quenched into a dilutc NaOH soluion.
The aqueous phase was extracted with ethyl acetate (3x) and dried (MgSO4). The
organics were conccntrated to a scmi-solid which was triturated with cthyl cther to
give 3.32 g of a pale yellow powdcr, m.p. 205-211C (dec.). This was recrystallized
from methanoVwater to give 2.54 g of a palc yellow powder, m.p. 214-217 (dec.).
ANALYSIS:
Calculated for ClgH2sN30: 73.28%C 8.09%H 13.49%N
Found: 73.02%C 7.94%H 13.33%N
EXAMPLE 13
N~ AcetYl-1.2.3A-tetrahvdro 1~9-acridinedian~ine~
hemi-fumerate. hvdrate
To a chilled rnixture of 1,2,3,4tetrahydro-1,9-acridinedian~ine (3.3 g) and
triethylan~ine (2.4 ml) in 100 ml of tctrahydrofi~ran wss added a solution of acetyl
chloride (1.2 ml) in S ml of THF. This was stirred for 1.5 hours, then qucnched into
- 27 -
dilute NaOH solution and extracted with ethyl acetate (3x). The organics were
washed with water and dried (saturated NaCI, MgS04). This was conccntrated to a
solid which was triturated with ethyl ether to give an off-white powder. This solid
was combined with another lot (3.06 g total) and the hemi-fumerate was fom~ed inisopropanol to give 3.14 g of a white powder. This was twice recrystallizcd frommethanol/ethyl ether to give 2.25 g of a white powder, m.p. 190-193C (dec.).
ANALYSIS:
Calculated for Cl5Hl7N300~C4H404-H20:
61.61%C 6.39%H 12.68%N
Found: 61.17%C 6.13%H 12.53%N
EXA~LE 14
1-Azido-1,2,3,4-tetrahvdro 9-acridinarnine, maleate
A mixture of 9-amino-1,2,3,4-tetrahydroacridin-1-ol (17.2g),
triphenylphosphine (23.2 g), diethyl azodicarboxylate (13.9 ml) and
diphenylphosphorylazide (19.0 ml) in 500 ml of tctrahydrofuran was heated at 65C
for 65 hours. The solution was then concentrated off and the desired acid was
purified via flash chrometography (EtOAC--2%Et3N/EtOAC) to give 9.48 g of a
yellow solid, m.p. 124-126C (dec.).
A 3.13 g portion of the free base was dissolvcd in isopropanol and I
equivalent of maleic acid was added. The resulting solid was collected and twicerccrystallized from mcthanoUethyl ethcr to give 1.75 g of a yellow powder, ~p.
163-165C (dcc.).
B
ANALYSIS:
Calculated for Cl3HI3~154C4H404:
57.46%C 4.82%H lg.71%N
Found: 57.84%C' 4.84%H 19.27%N
EXAMPLE 15
1,2,3,4-Tetrahydro-1.9-acridinediamine, dirnaleate
To a suspension of 10% palladium on charcoal (314 mg) in 200 ethanol
charged in a Parr pressure vessel was added a solution of l-azid~ 1 ,2,3,4-tetrahydr~
9-acndinamine (4.7 g) in 125 rnl of ethanol. This was pressurized to 50 psi withhydrogen and shaken for 2.5 hours.
The catalyst was filtered and the desired amino compound was purified via
flash chromatography (EtOAC/Et3NtMeOH; 90:5:5) to give 2.72 g of an off-white
powder, m.p.l67-171C. This was dissolved in isopropanol and ~eated with 2
equivalents of rnaleic acid in isopropanol. The dirnaleate was collected and
recrystallized from methanoUethyl cther tO give 4.27 g of a white powder, ~p.
187-190C (dec.).
ANALYSIS:
Calculated for C,3H,sN3-2.0C4H404:
56.63%C 5.20%H 9.43%N
Found: 56.40%C 5.07%H 9.32%N
- 2 9 -
EXAMPLE 16
Nl-Ethvl-1.2,3,4-tetrahvdrol~ 9-acridinediamine,
fumarate, hemi-hydrate
2-Methyl-3a,4,5,6-tetrahydro-(3H)-pyrirnidino[4,5,6-k,l]acr idine (4.5 g) was
suspended in 100 ml of tetrahydrofuran and treated with 39 ml of a 1 molar lithium
aluminum hydride solution in tetrahydrofuran. This was refluxed overnight.
The reaction was quenched with a saturated NH4CI solution, the resulting
precipitate filtered away and the filtrate was dried (MgSO4).
The desired compound was pu~ified via flash chromatography (2.5%
Et3N/EtOAc) to give 2.9 g of a yellow powder. This was dissolved in isopropanol
and treated with solid fumaric acid to g~ve 3.75 g of a white solid. This was twice
rccrystallized from methanoVethyl ether to givç 2.68 g of a white powder m.p.
164-167~ (dec.).
ANALYSIS:
Calculated for C~sHI9N3-C4H404 0.5H20:
62.28%C 6.33%H 11.47%N
Found: 62.35%C 6.27%H 11.40%N