Note: Descriptions are shown in the official language in which they were submitted.
60412-2012
~ 1 --
SMALL DIAMETER GUIDEWIRES
This invention relates to medicaL guidewires,
including medical injection wires.
Medical devices consisting of elongated spring
coils are employed widely as guidewires, e.g., ~or
negotiating narrow, ~ortuous passageways of the body to a
site to be treated, and then serving as guides for
cathetsrs or oth~r larger diameter devic~s advanced over
the guidewires. In order to obtain maximum performance
and patient sa~ety, it i5 important t~a~ the guidewire be
as small in diameter as possible, particularly in the tip
region, but not so small as to create a danger of the tip
breaking loose in the body; that the distal tip region be
highly flexible to permit negotiation of difficult turns
within the body; that the guidewire also be stiff enough
axially to be advancad by pressure from the proximal end
outside the body; and that the guidewixe have good
steerability or torque response, i.e., the tip-to-handle
turn ratio should be as clo~e to 1:1 as possible, ~ithout
whipping. Most prior art guidewire~ offer a compro~isa
of these desired featuras, e.g., trading tip flexibility
for good torque response.
Another use of sprinq coils is ln ca~eter-like
medical injection wires or t~e like which require
characteristics similar to those described above. An
exampls o~ such a device is described in Tate U.S.
3,841,308 as havin~ a spring coil covered with a
polyfluoroethylene ~lexible coating or sheath ~or0 delivery o~ ~luid to ports adjacent the distal end.
sulmnarv Qf ~he l~yentiQn
According to one asp~ct o~ the invention, a
~nedlcal guidewirs having a di~tal tip portion ~or
a~vancement through a bo~y by application oS ~orc~ to a
proximal end portion comprise~ an elongated multi-filar
-- 2
coil structural element, and a sheath disposed thereabout
and along a substantial portion of the length of the
structural elemant, the sheath formed of material that is
non-corrosive within the body, and the sheath beiny
adapted to flex in unison with the structural element
without kinking, the sheath and structural element in
combination having a torque response along the joined
length approaching l:l, thereby allowing control of the
distal tip of the guidawire within a body by application
of rotational force to the proximal end portion outside
the body.
According to one preferred embodiment of this
aspect of the invention, a core element formed of
elongated metal is disposed within the structural
element. According to another preferred embodiment of
this aspect of the invention, the distal end of the
structural element and the sheath define an orifice for
delivery of a fluid through the element to a body cavity.
Preferred embodiments of each of the above may
include one or more of the following features. The
sheath is disposed along the majority of the element, and
has a wall thickness o~ less than 0.0025 inch (0.064 mm).
The material is able to withstand a pressure of at least
300 psi (21.1 kg/cm~). The material is able to withstand
a pressure of at least about 700 psi (50 kg/cm~) and/or
the material has a tensile strength of at least lS,000
psi (1,050 kg/cm2), is not brittle, has a low elongation
factor and is dimensionally stable; whereby the material
can allow radiopaque fluid to be inserted into a body
cavity through the guidewire in sufficient amount to
provide good X-rav contrast at the site of insertion.
The material is resistant to heat at temperatures
suitable for soldering, to allow soldering of material to
the structural element. The material has a uniform wall
thickness so that the guidewire provides as little trauma
)3~
to the body cavity a~ possible as lt passes through the
body cavity. The material has no pin holes so that fluid
._.
does not leak through the material. The sheath has a
wall thickness of between 0.00075 inch (0.019 mm) and
0.0015 inch (0.038 mm). The wall thickness varies less
than 0.0002 inch (0.005 mm) along the length of the
sheath. The sheath has an inner diameter of at least
0.0075 inch (0.19 mm). The material comprises polyimide.
The core element has a distal tapered region, and the
core element is fixedly attached within the structural
element proximally from the tapered region. The
structural element has an inner diameter greater than the
outer diameter of the core element by about 0.0005 inch
tO.013 mm). The guidewire further comprises an electron
dense material, e.g. platinum, in its distal region. The
guidewire further comprises a sleeve positioned about the
distal tapered region of the core element, the sleeve
providing a transition between the core element and the
tip of the guidewire. The sleeve comprises polyimide
material. The structural element is a cross-~ound multi-
filar element. The cross-wound multi-filar element
comprises an inner coil formed of wire having a flat
cross-section. The core element is soldered to the
structural element. The sheath is fixed to the
structural element, e.g. by glue. The sheath extends
along the distal end of the structural element and is
fixed at the distal end of the element. The structural
element has an inner diameter of greater than 0.022 inch
(O.56 mm) and the guidewire has an outer diameter of less
than 0.040 inch (1.02 mm). The structural element
comprises a wound multi filar element. The distal end of
the sheath is spaced from the distal end of the
structural elemcnt.
According to another aspect of the invention, a
method for forming a guidewire comprises the steps of
~0~3~
60412-201
- 4 -
providing an elongated structural element, di6posing a
sheath thereabout and along a substantial portion of the
element, and gluing the sheath to the structural element.
GuidewirPs of this invention can be made to
extremely small dia~eter (les~ than 0.018 inch) and
provide high torgue response (e.g., at least approaching
1:1) o~ proximal to distal ends with high visibility o~
the tip region. The polyimide sheath acts in conjunction
with a woun~ multi-filar coil ~o provide thi~ torqu~.
The distal tip of the guidewire is without a sheath to
provide a softer tip region. A core provided within a
guidewixe i5 fixed to the inner coil of the guidewire but
is separated along the majority of its length from that
coil by about 0.0005 inch. Thus, the core eiement only
contacts the inner coil constantly when the guidewire is
caused to bend, for example, around a curve in a body
cavity. At these curves the contact with the core
element provides better torque to the guidewire.
A polyimide sheath is particularly suited for use
in this invention becaus~ it provides th~ features
describad above, and can be formed into an extre~Rly thin
walled ma~erial with small inner diameter. Injection
wires of this invention provide means by which an
extremely small diam~ter tube can be inserted within a
body cavity and still allow a signi~icant amount of fluid
to be placed within the cavity at a desired site~ since
the injection wir~ has a relatively large lumen and can
withstand high ~luid prassure.
Thes~ and other features and advantages of the
invention will be seen fro~ the following dascript.ion of
presently pr~erred embodiments thereof, and from the
claims.
We fir~t brla~ly describe the drawing~.
Z~)34L47
Figs. 1 and 2 are sectional views of multi-filar
cross-wound spring coil high torque guidewires of the
. _
invention; and
Figs. 3 and 4 are sectional views, partly in
isometric view, of wound multi-filar guidewires formed as
injection wires.
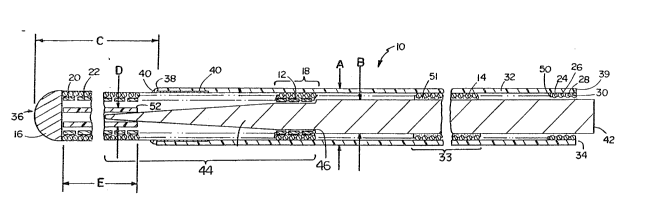
Referring to Fig. 1, torqueable coronary guidewire
10 has a length of about 145 cm, an outer diameter A,
about 0.018 inch (0.46 mm), and is formed of an inner
coil 12 and an outer coil 14 joined ~istally at a ball
tip element 16, and joined proximally, e.g., by
soldering, at a region 18. Inner coil 12 is bifilar,
formed of two flat platinum wires, 20, 22, e.g., about
0.002 inch (0.05 mm) by 0.006 inch (0.15 mm), closely
wound at a pitch ratio of about 2:1. Inner coil 12
extends only abou 6-~ inches (15 to 20 cm) from ball tip
element 16. Outer coil 14 is quadrifilar, formed of four
stainless steel circular cross-sectional wires 24, 26,
28, 30 of between 0.002 inch (0.05 mm) and 0.003 inch
(0.075 mm) diameter, which are closely wound a~out inner
coil 12, but in a direction opposite to the winding
direction of inner coil 12, with a pitch ratio of about
4:1. Outer coil 14 extends the length of guidewire 10.
A sheath 32 formed of polyimide of thickness
0.00075 inch (0.019 mm) is provided tightly fitted around
outer coil 14. Sheath 32 extends from proximal end 34 to
a distance C, about 2 to 3 cm, from distal end 36.
Distal end 38 of sheath 32 is fixed by glue 40 (e.g.,
cyanoacrylate) to outer coil 14, and along a length of
about 3 to 4 cm in the nearby distal region 33. Proximal
end 39 o~ sheath 32 i~ bonded by cyanoacrylate 50 to ~he
proximal end of outer coil 14.
Disposed within inner coil 12 is a core 42 formed
of a stainless steel rod of outer base diameter B, about
~)3~4~
-- 6 --
0.010 inch (0.25 mm), with about a 0.0005 inch (0.013 mm)
clearance from inner coil 12. Core 42 and inner coil 12
_
interact by close fit interference. Core 42 has a distal
tapered portion 4~ of length about ~ to 8 inches (15 to
20 cm) corresponding generally to the length of inner
coil 12, beginning at step 46. Core 42 is fixed to outer
coil 14 proximally from tapered portion 44 by solder or
adhesive 51.
Also provided is a polyimide sleeve 52 of length
E, e.g. about 2 cm, outer diameter D, 0.0095 inch (0.24
mm), and wall thickness 0.001 inch (0.025 mm), slid onto
the distal end of tapered portion 44 to provide a smooth
transition from tapered portion 44 to ball tip element
16, and thereby lncrease tor~ue transmission to ball tip
element 16. Sleeve 52 is not fixed to ball tip element
16.
Referring to Fig. 2, there is shown another
guidewire 60, of diameter H, about 0.018 inch (0.46 mm),
having a construction similar to guidewire 10, shown in
Fig. 1 and described above. Guidewire 60 is formed with
an innex coil 62 formed of a flat wire, and outer coil
64, formed o~ a circular wire, both extending the lenyth
of guidewire 60 and being encased within a polyimide
sheath 66 along their length, except for a distance G of
3 to 5 cm at the distal tip. Sheath 66 is bonded by
cyanoacrylate (50') to outer coil 64 at its distal encl,
and inner coil 62 is soldered (51') at its proximal end
to core 68, as described above. Inner core 68, of outer
diameter I, about 0.006 inch (0.15 mm), has a tapered tip
69 having a tapered region 70 of length L, about 3 cm,
and a flat tip portion 72 of length F, about 2 cm.
Proximal end 74 o~ core 68 has a single ~ilar coil 73
attached to it (e.g., by adhesive) to provide a handle
74. As above, core 68 has a clearance ~rom inner coil 62
of about 0.0005 inch (0.013 mm). Inner coil 62 and outer
)3~47
60412-2012
-- 7 --
coil 64 are formed of stainless steel except for a distal
region M, of length about 5 cm, f~r~ed of platinum and
glued or soldered (not shown) to ~he stainles~ steel
coils .
R~f erring to Figs . 3 and 4, ther~ arla sh~wn
embodiment of a injection wir~3 form~d fro~ a ~nulti-filar
coil having a polyimide shea~. As shown in Fig. 3,
injection wire 80 has a length of about 100 to 150 cr~,
and iE ar~ixed at its p~oximal end to ~luid d6~1ivery
10 device 82, for example, a syringe. Injection wire 80 is
formed of a bi~ilar or quadrifilar coil 84 of wire
diameter 0.005 inch (O.13 mm) ~ormed with a lumen of
diamQter Q, 0.027 inch (0.69 mm), and enveloped by a
polyimid~ sheath of nominal outer diameter P, abou~ 0.038
inch (0.97 mm). Polyimide sheath 86 and coil 84 are
fixed together at the extremities by glue 88 such that
polyimide sheath ~6 extends a distance N, about 0.5 t~
2.0 mm, beyond a tip 89 of coil S4.
Refexring to Fig. 4, injection wire 90 is ~ormed
as described above for injection wire 80, except coils 92
aro ~lx~d together by ~old~r 93 to each other and
subsequently bonded by cyanoacrylate to polyimide sleeve
94 in d~stal region 96 such that th~ tip of coil 92 and
the tip og pQlyimide sheath 94 ~re adjacent and
coaxtensive.
Thes~ injection wires are able to with~tand hiqh
pressure fluid and allow delivery of substantial amounts
of ~luid to any desired region within a body cavity.
Thes~ wires may be used in conjunction with a movable and
removablo core, or a standard 0.025 inch ~0.64 mm)
guidowir0.