Note: Descriptions are shown in the official language in which they were submitted.
2~ 3
HOECHST ARTIENGESELLSCHAFT HOE 88/F 336 Dr.WI/PP
Description
~-Amino-boronic acid derivative~
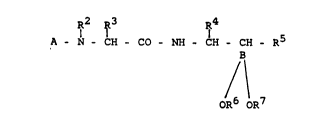
The invention relates to compounds of the formula I
R2 R3 R4
A - N - CH - Co - NH - CH - ~H - R5
/~
oR6 oR7
in which
A denotes a radical of the formula II, III or IV
R9 R8 O
R1 N - CH - C - (II)
~10 R8 o
R1 CH - CH - C - (II~)
R11 - (CH2)n - CH - C ~ (IV)
( CH2 ) m
in which
R1 al) denotes hydrogen, (Cl-Cl2)-alkyl, which i8 option-
ally mono- or diunsaturated and i8 optionally
sub~tituted by up to 3 identical or different
radical~ from the series comprising hydroxyl,
(Cl-C~)-alkoxy, carbamoyl, (Cl-C~)-alkanoyloxy,
carboxyl, (Cl-C7)-alkoxycarbonyl, F, Cl, Br, I,
amino, amidino, which can optionally be sub~ti-
tuted by one, two or three (Cl-C8)-alkyl radical~,
Z C3 ~ ) 3
-- 2 --
guanidino, which can optionally be sub6tituted by
one, two, three or four (Cl-CB)-alkyl radicals,
(Cl-C7)-alkylalllino, di-(Cl-C7)-alkylalllino, (C~-C5)-
alkoxycarbonylEmino, (C7-Cl5)-aralkoxycarbonyl-
amino and 9-fluorenylmethoxycarbonylamino;
mono-, bi- or tricyclic (C3-Cl8)-cycloalkyl,
(C3-Cl8)-cycloalkyl-(Cl-C6)-alkyl or (C6-Cl;)-aryl,
the cycloalkyl part optionally being sub~tituted
by (Cl-C6)-alkyl and 8ryl optionally being ~ub~ti-
tuted by one or two identical or different
radicals from the series comprising F, Cl, Br, I,
hydroxyl, (Cl-C7)-alkoxy, (Cl~C7)-alkyl, (Cl C7 ) -
alkoxycarbonyl, amino and trifluoromethyl;
(C6-Cl~)-aryl-(C1-C6)-alkyl, in which the aryl part
is optionally substituted by one or two identical
or different radicals from the series comprising
F, Cl, Br, I, hydroxyl, (Cl-C7)-alkoxy, (Cl-C7)-
alkyl, (Cl-C7)-alkoxycarbonyl, amino, (Cl-C,)-
alkylamino, di-(Cl-C7)-alkylamino, carboxyl,
carboxymethoxy, amino-(C1-C7)-alkyl, (Cl-C7)-
alkylamino-(Cl-C7)-alkyl, -di-(Cl-C7)-alkylamino-
(Cl-C7)-alkyl, (Cl-C7)-alkoxycarbonylmethoxy, car
bamoyl, sulfamoyl, (C1-C7)-alkoxysulfonyl and
sulfo- and guanidino-(Cl-CB)-alkyl; or represents
the radical of a 5- or 6-membered monocycl$c or
9- or 10-membered bicyclic partly or completely
hydrogenated heteroaromatic having at least 1
carbon atom, 1 - 4 nitrogen atom6 and/or 1 or 2
~ulfur atomfi and/or 1 or 2 oxygen atoms as ring
member~ and which is optionally mono-, di- or
trisub~tituted like the (C6-Cl~)-aryl defined
under al), or
a2) denotes a radical of the formula V
Rl' W (V)
in which R1 i~ defined as R1 under a1) and W
reprefient~ -CO-, -O-CO-, -S02-, -SO-, -NH-SOz-,
-NH-CO-, -CH~OH)- or -N(OH)-;
2~ 3~3
-- 3 --
R2 denote~ hydrogen or (Cl-C8)-alkyl, or, together with
~ R3 and the atoms carrying these, form~ a mono- or
bicyclic, saturated or partly unsaturated ring
system having 5-12 ring members;
R3 and R8 independently of one another are defined as
under a~) or,
together with R2 or with R9 and the atoms carrying
these, form ring systems having 5-12 ring members,
as defined above;
R4 denote~ (C3-Cl2)-alkyl, mono-, bi- or tricyclic
(C3-Cl8~-cycloalkyl, (C3-Cl8~-cycloalkylmethyl or
(C3-Cl8)-cycloalkylethyl, the cycloalkyl part option-
ally beiny substituted by tCl-C6)-alkyl; dithiolanyl;
( C6-C14 )-arylmethyl; dithiolanylmethyl; di~hiolanyl-
ethyl; dithianyl; dithianylmethyl or dithianylethyl;
R5 denote~ hydrogen; azido; azido-(C~-C~)-alkyl;
(C,-Cl2)-alkyl, which can optionally be ~ubstituted
by hydroxyl, azido or hydroxyl and azido; (C3-C~)-
cycloalkyl; ( C3-C~2) -cycloalkyl-( Cl-C6) -alkyl;
(C3-C12) -cycloalkylsulfonyl-(C~-C~)-alkyl; (C~-C6) -
alkylsulfonyl-(C,-C6)-alkyl; (C6-Cl4)-aryl or (C6-C~
aryl-(Cl-C~)-alkyl, it also being pos~ible for aryl
to represent the radical of a 5- or 6-membered
monocyclic or 9- or 10-membered bicyclic hetero-
aromatic having at least carbon atom, 1 - 4 nitrogen
atoms and/or 1 or 2 sulfur atoms and/or 1 or 2
oxygen atoms as ring members and which can also be
partly or completely hydrogenated, and it is like-
wise possible that aryl and heteroaryl can be
substitut2d as defined under al),
R6 and R7 independently of one another dznote hydrogen or
(Cl-C6) -alkyl; or,
together with the boron atom and the oxygen atoms,
form a ~ono-, bi- or tricyclic, saturated or partly
unsaturated, mono-, di-, tri- or tetra-(Cl-C6)-
ZC~3~)3
- 4 -
alkylated or -phenylated ring sy~tem having 5-18
ring members, which, in addition to the boron atom,
the oxygen atoms and the carbon atom(s), can al80
contain an -O- member, an -NRl3- member or a -CRl4Rl5-
member;
R8 represents hydrogen or (Cl-Ca)-alkyl, or, together
with Ra and the atom~ carrying the~e, form~ a mono-
or bicyclic, ~aturated or partly unsaturated ring
system having 5-12 ring members;
0 Rl iB hydrogen or (Cl-C8)-alkyl, or, together with Rl or
R8 and the atoms carrying these, form~ a mono- or
bicyclic, saturated or partly un~aturated ring
By~tem having 5-12 rins members, which, in addition
to the carbon atom, can also contain 1 sulfur atom,
which can optionally be oxidized to the ~ulfoxide or
sulfone;
Rl1 and R~ independently of one another denote hydrogen,
hydroxyl or (C6-Cl4)-aryl, aryl optionally being sub-
stituted by one or two identical or different
radical~ from the ~eries compri~ing F, Cl, Br, I,
hydroxyl, (Cl-C7)-alkoxy, ( C,-C7 )-alkyl, (Cl-C7)-
alkoxycarbonyl,amino,(Cl-C,)-alkylamino,di-(Cl-C7)-
alkylamino, carboxyl, carboxymethoxy, amino-(C1-C~)-
alkyl, (Cl-C7)-alkylamino-(Cl-C7)-alkyl, di-(Cl-C~)-
alkylamino-(C1-C~)-alkyl, (cl-c~)-alkoxycarbonyl-
methoxy,c~rbamoyl, sulfamoyl, (Cl-C7~-alkoxysulfonyl
and sulfo- and guanidino-(C1-C8)-alkyl; or represent
the radical of a 5- or 6-membered monocyclic or 9-
or 10-membered bicyclic, optionally partly or com-
pletely hydrogenated heteroaromatic having at least
1 carbon atom, 1 - 4 nitrogen atom~ and/or 1 or 2
~ulfur atom~ and/or 1 or 2 oxygen atom~ a~ r~ng
m~mbers and which is optionally mono- or di~ubsti-
tuted like (C6-C~)-aryl abo~e,
n and m independently of one another can be 0, 1, 2,
2~ 3
-- 5 --
3 or 4,
R13 denotes hydrogen or (C1-C1z)-alkyl, which i8 option-
ally mono- or diunsaturated and i8 optionally
substituted by up to 3 identical or different
radical6 from the ~eries comprisinq hydroxyl,
(Cl-C7)-alkoxy, zmino and mono- or di-tCl-C7~-alkyl-
amino, and
R14 and R15 independently of one another denote hydrogen,
(Cl-Ct)-alkyl, hydroxymethyl, 2-hydroxyethyl, (3-
hydroxysulfonyl, 2-hydroxypropyl)amino, (2-hydroxy-
sulfonylethyl)amino, (2-hydroxysulfonylpropyl)amino,
(carboxymethyl)amino or bis(2-hydroxyethyl)amino,
and physiologically tolerated salts thereof.
A radical of a 5- or 6-membered monocyclic or 9- or 10-
membered bicyclic heteroaromatic having at leas~ 1 carbon
atom, 1 - 4 nitrogen atoms and/or 1 or 2 sulfur atoms
and/or 1 or 2 oxygen atoms as ring member~ is understood
a~ meaning radicals of heteroaromatics such a~ are
defined, for example, in Ratritzky and Lagow~ki, Chemie
der Heterocyclen tChemistry of the ~eterocyclics]~
Berlin, Heidelberg 1968. The heteroaromatic radical can
be substituted by one, two or three, preferably one or
two, identical or different radicals from the series
compri~ing F, Cl, Br, I, hydroxyl, (Cl-C7)-alkoxy, (Cl-
C7)-alkyl, (Cl-C7)-alkoxycarbonyl, amino and trifluoro-
methyl. Examples of monocyclic heteroaromatic~ are
thiophene, furan, pyrrole, imidazole, pyrazole, pyridine,
pyrazine, pyrimidine, pyridazine, 1,2,4-triazole, thia-
zole, tetrazole, isothiazole, oxazole and isoxazole.
Example3 of bicyclic heteroaromatics are benzothiophene,
benzofuran, indole, i30indole, indazole, benzimidazole,
quinoline, isoguinoline, phthalazine, quinoxaline,
quinazoline and cinnoline. Corresponding statemants apply
to radicals derived from heteroaryl, such as, for
example, completely or partly hydrogenated heteroaryl,
inter alia al~o, for exa~ple, benzodioxolane, hetero-
~C~ )3
-- 6 --
aryloxy, heteroarylthio and heteroaryl-alkyl.
Alkyl can be straiqht-chain or branched. The correspond-
ing ~tatement applies to radicals derived therefr~m, ~uch
a~, for example, alkoxy, alkylthio, alkylamino, dialkyl-
amino, alkylsulfinyl, alkyl~ulfonyl, alkanoyl or aralkyl.
( C6-C14 ) -Aryl i5, for example, phenyl, naphthyl, bi-
phenylyl or fluorenyl; phenyl and naphthyl are preferred.
Corresponding statements apply to radicals derived
therefrom, such as, for example, aryloxy, aroyl, aralkyl
and aralXyloxy. Aralkyl is understood as meaning an
unsubstituted or ~ubstituted (C6-C,;)-aryl radical linked
to (Cl C6)-alkyl, such as, for example, benzyl, 1- and 2-
naphthylmethyl, halobenzyl and alkoxybenzyl, but aralkyl
would not be limited to the radicals mentioned.
Salts of compounds of the formula I are to be under~tood
as meaning, in particular, pharmaceutically u6able or
non-toxic salts.
Such salt~ are formed, for example, from compounds of the
formula I which contain acid groups, for example car-
boxyl, with alkali metal~ or alkaline earth metals, sucha~ Na, R, Mg and Ca, and with physiologically tolerated
organic amines, such as, for example, triethylamine and
tri-(2-hydroxy-ethyl)-amine.
Compounds of the formula I which contain ba~ic groups,
for oxample an amino group or a guanidino group, form
salts with inorganic acids, ~uch as, for ex~mple, hydro-
chloric acid, sulfuric acid or phosphoric acid, snd with
organic carboxylic or sulfonic acids, such as, for
example, acetic acid, citric acid, benzoic acid, maleic
acid, fumaric acid, tartaric acid and p-toluenesulfonic
acid.
Compounds of the formula I in which the radical~ are
defined as follows are preferred:
2C t~3~3
-- 7 --
A is as defined on page 1,
Rl preferably denotes hydrogen or represents ~Cl-C,0)-
alkyl; cyclopentyl; cyclohexyl; cyclopentyl-(C1-C10)-
alkyl; cyclohexyl-(C1-C10)-alXyl; optionally BUbsti
tuted phenyl-( Cl-C8 3-alkyl; 2-pyridyl-(Cl-C~)-alkyl;
3-pyridyl-(Cl-C~)-alkyl;4-pyridyl-( Cl-Ca ) -alk~l;X2N~
(C~-clO)-alkyl; H0-(C1-C10)-alkyl;( Cl-C4 )-alkoxy-
(C1-C~0)-alkyl; ( Cl-C4 )-alkoxycarbonyl-(cl-clo)-alkyl;
(Cl-Ca)-alkylsulfonyl; (C,-C~)-alkylsulfinyl; (C,-C8)-
hydroxyalkylsulfonyl;( Cl-C8 )-hydroxy-alkylsulfinyl;
hydroxy-(C~-C~0)-alkanoyl, such a~ 2-hydroxypropio-
nyl, 3-hydroxypropionyl, 3-hydroxybutyryl or 2-
hydroxy-3-methyl-butyryl; ( C1_CB) -alkanoyloxy-(
Clo)-alkyl; (Cl-C~,)-alkanoyl, such as n-decanoyl,
formyl, acetyl, propionyl, pivaloyl, isovaleryl or
isobutyryl; optionally protected amino-(C,-C")-
alkanoyl, such a~ (3-amino-3,3-dLmethyl)propionyl,
4-aminobutyryl, 5-aminopentanoyl, 6-aminohexanoyl,
4-N-tert.-butoxycarbonylaminobutyryl, S-N-t~rt.-
butoxycarbonylaminopentanoyl or 6-N-tert.-butoxy-
carbonylaminohexanoyl; di-( Cl-C7 ) -alkylamino-( C2-
Cl1)-alkanoyl, such as dimethylaminoaGetyl; piperi-
dino-4-carbonyl; morpholino-4-carbonyl; (C3-ca)-
cycloalkylcarbonyl, such as cyclopropylcarbonyl,
cyclobutylcarbonyl, cyclopentylcarbonyl or cyclo-
hexylcarbonyl;(C~-C~ aryl-( C2-Cll )-alkanoyl,sucha~
phenylacetyl, phenylpropanoyl or phenylbutanoyl; 2-
pyridyl-(C1-Ca)-alkanoyl;3-pyridyl-( C1_CB ) -a1kanOY1;
4-pyridyl-(C1-C~)-alkanoyl; benzoyl which is option-
ally Eub~tituted by halogen, (C1-C7)-alkyl, (C1-C7)-
alkoxy or (Cl-C7)-alkoxycarbonyl, such as 4-chloro-
benzoyl, 4-methylbenzoyl, 2-methoxycarbonylbenzoyl
or 4-methoxybenzoyl; pyrrolyl-2-carbonyl, pyridyl-
3-carbonyl;benzenesulfonyl;(C1-C10)-alkoxycarbonyl,
such as methoxycarbonyl, ethoxycarbonyl, isobutoxy-
carbonyl or tert.-butoxycarbonyl; substituted (Cl-
C10)-alkoxycarbonyl, such as 2-(trLme~hyl~ilylJ-
8 --
ethoxycarbonyl, 2,2,2-trichloroethoxycarbonyl or
~ 1,1-dimethyl-2,2,2-trichloroethoxycarbonyl;( C6-C14 ) -
aryl-(C~-C6)-alkoxycarbonyl, such a~ benzyloxycar-
bonyl, 1- or 2-naphthylmethoxycarbonyl or 9-
fluorenylmethoxycarbonyl; or R1, together with R10,
preferably forms a mono- or bicyclic ~aturated or
partly unsaturated ring system having 5-12 ring
members, which, in addition to carbon, can also
contain 1 sulfur atom, which can optionally be
oxidized to the sulfoxide or ~ulfone,
R2 i8 preferably hydrogen, methyl or ethyl, or,
together with R3 and the atoms carrying the~e,
preferably forms pyrrolidine or piperidine, each of
which can also be fused with cyclopentyl, cyclohexyl
or phenyl,
R3 and R8 independently of one another are preferably
hydrogen; (Cl-C~O)-alkyl, which i~ optionally mono-
or diunsaturated and which i8 optionally substituted
by up to 3 identical or different radicals from the
series comprising hydroxyl, (C1-C~)-alkoxy, (Cl-C7)
alkanoyloxy, carboxyl, (Cl-C7)-alkoxycarbonyl, Cl,
Br, amino, amidino, guanidino, carbamoyl, (Cl-C5)-
alkoxycarbonylamino, (C6-C15)-aralkosycarbonylamino
and 9-fluorenylmethoxycarbonyl2mino; (C3-C~)-cyclo-
alkyl, (C3-Cl2)-cycloalkyl-(Cl-C3)-alkyl or mono- or
bicyclic (C6-C~ aryl-(C1-C3)-alkyl, which iB option-
ally substituted by one or two identical or dif-
ferent radicals from the serie~ comprising F, Cl,
Br, I, hydroxyl, (Cl-C7)-alkoxy, (Cl-C,)-alkyl, (Cl-
C7)-alkylcarbonyl, amino and trifluoromethyl; or
preferably represent~ (Cl-C3)-alkyl, sub~titu~ed by
the radical of 8 5- or 6-membered monocyclic or 9-
or 10-membered bicyclic, optionally partly or com-
pletely hydrogenated heteroaro~atic having st least
1 carbon atom, 1 - 4 nitrogen ato~s nnd/or 1 or 2
sulfur atoms and/or 1 or 2 oxygen atoms A8 ring
members and which is optionally mono- or disubsti-
3~)3
_ g
tuted as described for the aryl part on pag2 2, or,
together with R2 or R~, form ring systems as
described above under R2;
R4 i~ preferably (C3-Cl2)-alkyl; mono-, bi- or tricyclic
S (C3-Cl8)-cycloalkyl or (C3-C18~-cycloalkylmethyl, the
cycloalkyl part optionally being ~ubstituted by
(C1-C4)-alkyl; (C6-C14)-arylmethyl; dithiolanyl;
dithiolanylmethyl; dithianyl or dithianylmethyl;
R5 preferably represent~ hydrogen, azido, azido-(Cl-C4)-
alkyl, (Cl-Clz)-alkyl, (C3-Cl2)-cycloalkyl, (C3-Cl2)-
cycloalkyl-(Cl-C6)-alkyl, (2-pyridyl)-(Cl-C4)-alkyl,
(3-pyridyl)-(Cl-C4)-alkyl,(4-pyridyl)-(Cl-C~)-alkyl,
imidazol-2-yl, imidazol-l-yl, imidazol-4-yl,
(imidazol-2-yl)-(Cl-C~)-alkyl, (imidazol-l-yl)-
(Cl-C4)-alkyl, (imidazol-4-yl)-(Cl-C4)-alkyl, [1-
(Cl-C6)-alkylimidazol-2-yl]-(Cl-C~)-alkyl, (Lmid-
azolin-2-yl)-(Cl-C4)-alkyl or [1-(Cl-C6)-alkylimida-
zolin-2-yl]-(C1-C4)-alkyl;
R6 and R7 independently of one another are preferably
hydrogen or, together with the boron atom and the
oxygen atoms, form a mono-, bi- or tricyclic,
saturated or partly unsaturated mono-, di-, tri- or
tetraalkylated or -phenylated ring ~y~tem having
5 - 18 ring members, which, in addition to the boron
atom, the oxygen atoms and the carbon atom(s), can
al~o contain an -O- member, an -NRl3- m~mber or a -
CR14Rl5- member;
R~ i~ preferably hydrogen, methyl or ethyl, or, to-
gether with R8 and the atoms carrying these, forms
pyrrolidine or piperidine, each of which can ~ddi-
tionally be fused with cyclopentyl, cyclohexyl or
phenyl;
Rl i8 preferably hydrogen or methyl, or, together with
R1 or R8 and the atoms carrying these, forms a mono-
Z~CO ~3~)3
-- 10 --
or bicyclic saturated or partly unsaturated ring
8y8tem having 5 - 12 ring member~, which, in addi-
tion to carbon, can also contain 1 ~ulfur atom,
which can optionally be oxidized to the ~ulfoxide or
sulfone;
R1l and R12 independently of one another are preferably
hydrogen, hydroxyl, phenyl, 2- or 3-thienyl, 2-, 3-
or 4-pyridyl, 1-, 2- or 4-imidazolyl, 1- or 2-
naphthyl or 2- or 3-benzo[b]thienyl,
n and m independently of one another can denote 0,
1, 2, 3 or 4,
Rl3 is preferably hydrogen or (Cl-Cl2)-alkyl; and
R14 and R15 independently of one another preferably denote
hydrogen, hydroxymethyl, 2-hydroxyethyl, (3-
hydroxysulfonyl, 2-hydroxypropyl~amino, (2-hydroxy-
sulfonylethyl)amino, (2-hydroxysulfonylpropyl) ~ino,
(carboxymethyl)amino or bis(2-hydroxyethyl)amino.
R1 particularly preferably denotes (Cl-C8)-alkylsul-
fonyl; ( Cl-C9 ) -alkyl8ulfinyl; ( Cl-C8 )-hydroxyalkylsul-
fonyl, in particular 2-hydroxyethylsulfonyl or 2-
hydroxypropylsulfonyl; hydroxy-(C1-C10)-alkanoyl,
such as 2-hydroxypropionyl, 3-~ydroxypropionyl, 3-
hydroxybutyryl or2-hydroxy-3-methylbutyryl; (Cl-Ca)-
alkanoyloxy-(Cl-C10)-alkyl; (C1-C11)alkanoyl, such a~
n-decanoyl, for~yl, acetyl, propionyl, pivaloyl,
isovaleryl or isobutyryl; amino-tCl-C11)-alkanoyl,
such a~ (3-amino, 3,3-dimethyl)propionyl, 4-amino-
butyryl, 5-aminopentanoyl, 6-aminohexanoyl; di-(C1-
C7 )-alkylamino-tC2-C11)-alkanoyl, such as dimethyl-
aminoacetyl; piperidino-4-carbonyl; morpholino-4-
carbonyl; (C3-C9)-cycloalkylcarbonyl, ~uch as cyclo-
propylcarbonyl, cyclobutylcarbonyl, cyclpentylcar-
bonyl or cyclohexylcarbonyl; ( C6-C10 ) -aryl-( C2-Cll ) -
alkanoyl, such as phenylacetyl; phenylpropanoyl or
2(:~0 ~3~3
-- 11 --
phenylbutanoyl; 2-pyridyl-( C1_CB )-alkanoyl; 3-pyr-
idyl-(C1-C~)-alkanoyl; 4-pyridyl-(Cl-C~)-alkanoyl;
benzoyl which i8 optionally ~ubstituted by halogen,
(Cl-C7)-alkyl, (Cl-C7)-alkoxy or (Cl-C7)-alkoxycar-
bonyl, such as 4-chlorobenzoyl, 4-methylbenzoyl, 2-
methoxycarbonylbenzoyl or 4-methoxybenzoyl; pyr-
rolyl-2-carbonyl; pyridyl-3-carbonyl; benzene6ul-
fonyl; (Cl-C1O)-alkoxycarbonyl, ~uch a~ methoxycar-
bonyl, ethoxycarbonyl, isobutoxycarbonyl or tert.-
butoxycarbonyl; substituted (C,-ClD)-alkoxycarbonyl,
such a6 2-(trimethylsilyl)-etho~ycarbonyl, 2,2,2-
trichloroethoxycarbonyl or 1,1-dLmethyl-2,2,2-
trichloroethoxycarbonyl; or ( C6-C14 ) -aryl-( ~1-c6 ) -
alkoxycarbonyl, such as benzyloxycarbonyl, 1- or 2-
naphthylmethoxycarbonyl or 9-fluorenylmethoxycar-
bonyl,
R2 particularly preferably denote~ hydrogen or methyl,
or, together with R3 and the -~-CH- group carrying
these radicals, form~ a tetrahydroi~oquinoline or
azabicyclooctane ~keleton,
R3 and R4 independently of one another are particularly
preferably hydrogen, methyl, ethyl, i~opropyl, n-
propyl, n-butyl, isobutyl, zec.-butyl, 3-guanidino-
propyl, carbamoylmethyl, 2-carbamoylethyl, carboxy-
methyl, 2-carboxyethyl, mercaptomethyl, 2-(methyl-
thio)ethyl, (1-mercapto,l-methyl)ethyl, hydroxy-
methyl, 1-hydroxyethyl, amino, aminomethyl, 2-
bminoethyl, 3-aminopropyl, 4-aminobutyl, N,~-
dimethylamino, cyclohexylmethyl, imidazol-4-yl-
methyl, benzyl, 2-methylbenzyl, 3-methylbenzyl,
indol-3-yl-methyl,4-hydroxybenzyl,4-methoxybenzyl,
3,4-dihydroxybenzyl, 3,4-dimethoxybenzyl, (benzodi-
oxolan-5-yl)methyl, 2-thienyl, 2-thienyl~ethyl, 2-
(2-thienyl)-ethyl, 3-thienyl, 3-thienylmethyl, 2-(3-
thienyl)ethyl, 4-chlorobenzyl, 2-tmethylsulfinyl)-
ethyl, 2-(methylsulfonyl)ethyl, 2-pyridylmethyl, 3-
pyridylmethyl, 4-pyridylmethyl, cyclohexyl, (1-
2 0 ~
- 12 -
methyl-imidazol-4-yl)methyl, (3-methyl-imidazol-4-
yl)methyl, phenyl, l-naphthylmethyl, 2-naphthyl-
methyl, 2-phenylethyl, 2-thiazolylmethyl, 4-thi-
azolylmethyl, 3-pyrazolylmethyl, 4-pyrimidinyl-
methyl, indol-2-yl-methyl, 2-benzo[b]thienylmethyl,
3-benzotb]thienylmethyl or 2-furylmethyl,
or with R2 or R~ form ring systems as defined above
under R2,
R~ is particularly preferably (C3-Cl2)-alkyl; mono- or
bicyclic (C3-Cl2)-cycloalkyl or (C3-C,2)-cycloalkyl-
methyl, the cycloalkyl part optionally being 6ub~ti-
tuted by (Cl-C~)-aryl; ( C6-clo )-arylmethyl; dithio-
lanyl; dithiolanylmethyl; dithianyl or dithianyl-
methyl,
R5 iB particularly preferably hydroqen, azido, azido-
methyl, 2-azidoethyl, (Cl~C6)-alkyl, (C3-C12)-cyclo-
alkyl, (C3-Cl2)-cycloalkyl-(Cl-C3)-alkyl, 3-(2-pyri-
dyl)-propyl, 3-(3-pyridyl)-propyl, 3-(4-pyridyl)-
propyl, imidazol-2-yl, imidazol-1-yl, imidazol-4-yl,
(imidazol-2-yl)-propyl, (imidazol-l-yl)-propyl,
(imidazol-4-yl)-propyl, [1-(Cl-C4)-alkylimidazol-2-
yl]-propyl or (imidazolin-2-yl)-propyl,
R6 and R7 are as defined on page 9,
R~ particularly preferably denotes hydrogen or methyl,
or, together with R~ and the -~-CH- group carrying
these radical~, forms a tetrahydroi~oquinoline or
azabicyclooctane ~keleton,
Rl particularly preferably denote6 hydrogen, or,
together with R1 and the atoms carrying these, forms
a mono- or b~cyclic saturnted or partly un~aturated
ring sy~tem having 5-12 ring m~mber~, which, in
addition to carbon, also contain~ 1 ~ulfur atom,
which i~ particularly preferably oxidized to the
sulfone, or, together with R8 and the atoms carrying
2 ~ )3
- 13 -
these, forms a thiochroman system, the ~ulfur atom
of which i8 particularly preferably oxidized to the
sulfone,
R~1 and R12 independently of one another are particularly
preferably hydrogen, hydroxyl, phenyl, 2-thienyl,
2-, 3- or 4-pyridyl, 1- or 2-imidazolyl, l-naphthyl
or 2- or 3-benzo[b]thienyl,
n and m independently of one another are particularly
preferably 0, 1 or 2,
Rl3 is particularly preferably hydrogen or [C~-C4)-alkyl,
and
R1~ and R15 are as defined on page 10.
The invention furthermore relates to a process for the
preparation of compounds of the formula I, which com-
prises coupling a fragment havin~ a terminal hydroxyl
group or a reactive derivative thereof with a correspond-
ing fragment having a free amino group, if appropriate
splitting off (a) protective group(s) temporarily intro-
duced to protect other functional groups, and if appro-
priate converting the compound thus obtained into its
physiologically tolerated ~alt.
Fragments of a compound of the formula I having a ter-
minal carboxyl group have the following formulae VI and
VII
A - OH (VI)
R2 R3
A - N - CH - C - OH (VII)
Fragments of a compound of the formula I having a ter-
minal amino group have the following formulae VIII to X
2C~'~*3~3
-- 14 --
R9 R8 o R2 R3 R4
P ~ ( YI I I )
HN - CH - C - N - CH - C - HN - C~ - CH - R5
oR6 oR7
R2 R3 R4 ( I2~ )
HN - CH - C - HN - CH - CH - R
B
oR6 oR7
.4
~2N - C}I - CH - R5
B (X)
6 7
OR OR
Methods which are suitable for the production of an emide
bond are described, for exzmple, in Houben-Weyl, Methoden
der oryanischen Chemie [Method~ of Organic Chemistry~,
Volume 15/2; and Bodanszky et al., Peptide Synthesis, 2nd
ed. (Wiley & Sons, New York 1976) or Gro~s and
Meienhofer, The Peptides: Analysis, Synthesis, Biology
(Academic Pre6s, New York 1979). The following method~
are preferably u~ed: active ester methods with N-hydroxy-
succinhmide, l-hydroxybenzotriazole or 3-hydroxy-4-oxo-
3,4-dihydro-1,2,3-benzotriazine as the alcohol co~ponent,
coupling with a carbodiimide, such as dicyclohexylcarbo-
di~mide, or with propanephosphonic anhydride and the
mixed anhydride method with pivaloyl chloride or ethyl or
isobutyl chloroformate.
Pragments of the formula VI where they
a) fall under formula II are ~nthesized by the gener-
ally known methods for the preparation of amino
ZC~ )3
- 15 -
acids;
b) fall under formula III are synthesized ~tarting from
the corresponding amino acids, the chirality center
thereof being retained. Diazotization at -20C to
50C in dilute mineral acids leads to ~bromo-
carboxylic acids or, via the lactic acids~ to ~-
trifluoromethanesulfonyloxy-carboxylic acids, which
can be reacted with 2 nucleophile carrying Rl and
Rl;
c) fall under formula IV are prepared ~tartinq from
malonic ester~, alkylation of which with arylalkyl
halides gives mono- or disub~tituted malonic ester~,
which, after hydrolysis, can be converted into the
desired derivatives by decarboxylation. In the ca e
where one of the radicals Rll or Rl2 denotes hydroxyl,
the corresponding amino acid is used as the starting
~ubstance and diazotization (as de~cribed above)
gives the lactic acid (number of CH2 groups carrying
Rll or Rl2 = 0), or the substituted malonic acid i8
used as the ~tarting substance, monohydrolysis and
~elective reduction (with, for example, diborane or
LiAlH4) giving the 2-Eubstituted 3-hydroxy-propionic
acid.
Fragments of the formula VII are synthesized by the
generally known methods for ~he preparation of amino
acids and peptides.
For the synthesis of the fragments of the formula X, N-
protected ~-amino acid~ are converted into the ~-amino-
aldehydes in Accordance with the method of B. Castro et
al. (Synthesis 1983, 676). A Wittig reaction with a
pho~phonium ~alt carrying the radical R5 then ~akes place,
followed by hydroboronation.
The preliminary and ~ubsequent operations required for
the preparation of compound3 of the formula I, such as
2C~ )3
- 16 -
introduction and splitting off of protec~ive groups, are
known from the literature and are described, for example,
in T.W. Greene "Protective Groups in Organic Synthesi~"
(John Wiley ~ Sons, New York, 1981). Salts of compounds
of the formula I with salt-forming qroups are prepared in
a manner which is known per ~e, for example by reacting
a compound of the formula I having a ba~ic group with a
stoichiometric amount of a suitable acid, or reactin~
compounds of ~he formula I having an acid group with a
stoichiometric amount of a suitable base. Stereoisomer
mixtures, in particular diastereomer mixture~, which are
obtained, if appropriate, in the synthe6i6 of compounds
of the formula I, can be resolved in a manner which i~
known per se by fractional crystallization or by chroma-
tography.
The compounds of the formula I accordin~ to the inventionexhibit enzyme-inhibiting properties, and in particular
they inhibit aspartyl proteases, such a~ renin,
Renin i8 secreted into the blood circulation by the
~uxtaglomerular cells of the kidneys as a result of
Yarious stimuli (volume depletion, sodium deficiency, ~-
receptor ~timulation). In the blood circulation it splits
off the decapeptide angiotensin I from the angioten-
sinogen secreted from the liver. This angioten~in I i8converted into angiotensin II by "angiotensin converting
enzymen (ACE~. An~iotensin II plays an essential role in
blood pre~sure regulation, since it increases the blood
pressure directly by vasoconstriction. In addition, it
stimulates the ~ecretion of aldosterone from the adrenal
gland and in this way, via inhibition of the excretion of
sodium, increases the extracellular fluid volume, which
in turn contributes towards increa~ing the blood pres-
sure. Inhibitors of the enzymatic activity of renin cause
reduced formation of angiotensin I, which results in
reduced formation of angiotensin II. The reduction ~n the
concentration of this active peptide hormone i3 the
20~3~:3
- 17 -
direct cause of the antihypertensive action of renin
inhibitors.
The activity of renin inhibitors can be investigated by
in vitro te6ts. In these, the reduction of the formation
S of angioten~in I i8 measured in various ~ystems ~human
plasma and purified human renin).
1. Test principle
For example, human plasma which contains both renin and
angiotensinogen i8 incubated at 37C with the compound to
be tested. During this incubation, angiotensin I i8
liberated from the angiotensinogen under the action of
renin, and can ~ubsequently be measured with a commer-
cially available radioimmunoas6ay method. This angio-
tensin liberation i8 inhibited by renin inhibitors.
2. Isolation of the pla~ma
The blood is obtained from volunteer sub~ects (about
0.5 1 per person; ~luko withdrawal apparatus from ASID
Bonz und Sohn, Unterschleissheim) and is collected in
partially evzcuated bottles, while coolin~ with ice. The
coagulation is prevented by addition of EDTA (final
concentration 10 mM). After centrifu~ation (Rotor HS 4
(Sorvall), 3500 r.p.m., 0-4C, 15 minutes; repeat if
neces~ary), the plasma i8 carefully pipetted off and
frozen in suitable portion at -30C. Only plasmas with a
sufficiently high renin activity are used for the test.
Pla~mas with a lower renin activity are activated
(prorenin -> renin) by a low temperature treatment (-4C,
3 day8).
3. Te~t procedure
Angiotensin I i6 determined with the Renin-MaiaO kit
(Seronon Diagnostics S.A., Coinsins, Switzerland). The
plasma i8 incubated in accordance with the instructions
2~
- 18 -
given with the kit:
Incubation batch: 1000 ~1 of pla~ma (thawed at 0-4C)
100 ~1 of phosphate buffer (pH 7.4)
addition of 10-~ ~ ramipri
late)
100 ~1 of PMSF solution
10 ~1 of 0.1 % strength Genapol ~FIC
12 ~1 of DMS0 or test preparation
The test preparation~ are in general di~601ved in a
concentration of 10-~ M in 100 ~ pure dLmethyl ~ulfoxide
(DNS0~ and the solutions are diluted appropriately with
DMS0; the incubation batch contains not more than 1 % of
DNS0.
The batche~ are mixed with ice and placed in a water bath
(37C) for 1 hour for incubation. A total of 6 ~amples
(in each case 100 ~1) are taken from an additional batch
without inhibitor and without further incubation in order
to determine the initial angiotensin I content of the
plasma used.
The concentrations of the test preparations are chosen ~o
that approxlmately the range from 10 - 90 % enzyme
inhibition is ccvered ~at least five concentrations). At
the end of the incubation time, three 100 ~1 ~amples from
each batch are frozen on dry ice in pre-cooled Eppendorf
vessels and kept at about -25C for the angiotensin I
determination (mean value of three individual ~ample~).
An~iotensin I radioim~unoas~ay tRIA)
The use instructions of the RIA kit (Renin Maie0 kit,
Serono Diagno~tics S.A., Coinsins, Swit~erland) are
followed exac~ly.
The calibration curve cover~ the range from O . 2 to 25 . O
ng of angioten~in I per ml. ~he base angiotensin I
~c~
-- 19 --
content of the plasma is subtracted from all the measure-
ment values. The plasma renin activity (PRA) is given in
ng of Ang I/ml x hour. The PRA values in the presence of
the test substances are based on a batch without in-
hibitor (= 100 %) and given as % residual activity. TheIC5~ value is read off from the plot of the % residual
activity against the concentration (M) of the test
preparation (logarithmic scale).
The compounds of the general formula I described in the
present invention exhibit inhibiting actions in the in
vitro test at concentrations of about 10-5 to 10-1 mol/l.
Renin inhibitors cause a reduction in blood pressure in
animals with salt depletion. Since human renin differs
from the renin of other species, primates, such as, for
example, rhesus monkeys, are used for the in vivo test of
renin inhibitors. Primate renin and human renin are
largely homologous in their sequence. An endogenous renin
secretion is stimulated by intravenous in~ection of
furosemide. The test compounds are then administered and
their action on the blood pressure and heart rate is
measured. The compounds of the present invention are
active here in a dose range of about ~ S mg/kg
intravenously, on intraduodenal administration by a
gastroscope in the dose range of about l - 50 mg/kg. The
compounds of the general formula I described in the
present invention can be used as antihypertensives and
for the treatment of cardiac insufficiency.
- 20 ~
2C0~
The invention furthermore relates to the use of compound6
of the formula I for the preparation of pharmaceuticals
for the therapy of high blood pressure and treatment of
congestive cardiac insufficiency
and to the pharmaceuticals mentioned.
Pharmaceutical preparations contain an effective amount
of the acti~e compounds of the formula I together wi~h an
inorganic or organic pharmaceutically usable excipient.
They can be administered intranasally, intravenou61y,
subcutaneously, perorally or intraduodenally. The dosage
of the active compound depends on the warm-blooded
species, the body weight, the age and the mode of admini-
6tration.
~he pharmaceutical preparations of the present invention
are prepared by dissolving, mixing, granulating or
tablet-coating proce~ses which are known per se.
For the oral use form, the active compounds are mixed
with the additives customary for these, ~uch as excipi-
ents, stabilizer6 or tnert diluent~, and the ~ixture is
brought by cufitomary methods into suitable presentation
forms, such a~ tablets, coated tablets, hard gelatine
push-fit capsules, aqueous, alcoholic or oily sus~en~ion~
or aqueous, alcoholic or oily solutions. Examples of
inert excipients which can be u~ed are gum arabic,
magnesia, magnesium carbonate, potas~ium phosphate,
~C~ )3
- 21 -
lactose, glucose, magnesium stearylfumarate or starch, inparticular maize starch. Formulation can be effected here
either in the form of dry granules or in the form of
moist granules. Examples of pos~ible oily excipients or
solvents are vegetable or anLmals oil~, such as ~unflower
oil and cod-liver oil.
For subcu~aneou~ or intravenous administration, the
active compounds or physiolo~ically tolerated salt~
thereof are brought into solutions, suspension~ or
emulsions, if desired with the substances customary for
these, such as solubilizing agents, emulsifiers or other
additives. Examples of po~sible solvent~ are: water,
physiological saline solutions or alcohols, for example
ethanol, propanediol or glycerol, and in addition also
sugar solutions, such as glucose solutions or mannitol
solutions, or a mixture of the various solvents men-
tioned.
Li~t of abbreviations used
Ac acetyl
20Boc tert.-butoxycarbonyl
bp~ boiling point under xx mm Hg
BuLi n-butyllithium
DCC dicyclohexylcarbodiimide
DCI desorption chemical ionization
25DIP dii~opropyl ether
DMF dimethylformamide
DNSO dimethyl ~ulfoxide
DNP 2,4-dinitrophenyl
EA ethyl acetate
EI electron impact
Etoc ethoxycarbonyl
FAB fast atom bombardment
H hexane
HOBt l-hydroxybenzotriazole
Iva i~ovaleryl
M molecular peak
2C~
- 22 -
M.p. melting point
MeOH methanol
NS mass spectrum
MTB methyl tert.-butyl ether
Nle norleucine
Nva norvaline
R . T . room temperature
THF tetrahydrofuran
Thi ~-2-thienylalanine
TLC thin layer chromatography
Z benzyloxycarbonyl.
The other abbreviations used for amino acids correspond
to the three-letter code customary in peptide chemistry,
such afi is described in Eur J. Biochem. 138, 9 - 37
(1984). Unles~ expres~ly indicated otherwise, the amino
acids are always in the L-confiquration.
The following examples ~erve to illustrate the present
invention, without this being limited thereto.
~sample 1
t3-tert.-Butylsulfonyl, 2-(2)-thienylmethyl]propionyl-
Nva-[l-cyclohexylmethyl, 2-(4,4,5,5-tetramethyl-1,3,2-
dio~aborolan-2-yl), 5-methyl]hexylamide
346 mg of [3-tert.-butyl6ulfonyl,2-(2)-thienylmethyl]-
propionyl-Nva-OH are dissolved in 40 ml of THF and first
98 ~1 of N~methylmorpholine and then 115 ~1 of isobutyl
chloroformate are in~ected in at -20C. After 10 minute~
at -20C, 123 ~1 of triethylamine are added. The mixed
anhydride thus obtained is in~ected into a solution of
400 mg of tl-cyclohexylmethyl, 2-(4,4,5,5-tetramathyl-
1,3,2-dioxaborolan-2-yl), 5-methyl]hexylammoniu~ tri-
fluoroacetate in 40 ml of THF. The mixture is ~tirred at
-20C for 1 hour and left to stand at ~.T. for 22 hours.
It i~ diluted with 100 ml of NTB and wa~hed twice with
30 ~1 of 5 ~ ~trenqth aqueous NaHSO~ solution each time
~0~ )3
-- 23 --
and twice with 30 ml of 5 % strength aqueous Na2CO3
601ution each time. The organic phase i8 dried over Na2SO4
and the solvent is removed in vacuo. Chromatography on
silica gel with NTB/DIP = 1:1 give~ 160 mg of the title
S compound as ~olorless cry~tals.
Rf (MTB/DIP 1:1) = 0.50 MS (FAB): 709 (M+l)
a~[3-t-Butyl~ulfonyl,2-(2-thienylmethyl)]propionyl-Nva-
OH
490 mg of [3-t-butylsulfonyl, 2-(2-thienylmethyl)lpropio-
nyl-Nva-ONe are dissolved in 4 ml of methanol, 0.4 ml of
H20 and 0.7 ml of 2N NaOH are added and the mixture is
stirred at R.T. for 5 hours. It is acidified to pH = 2
with NaHSO4 solution and extracted 3x with 50 ml of EA.
The extract is then dried over Na2SO4 and the solvent i~
removed in vacuo. 470 mg of pale yellow resin are ob-
tained.
R (EA) = 0.24 MS (FAB): 390 (M+1)
b)[3-t-Butylsulfonyl,2-t2-thienylmethyl)]propionyl-Nva-
OMe
1.5 g of t3-t-butyl~ulfonyl, 2-t2-thienylmethyl)]pro-
pionic acid and 0.95 g of Nva-OMe x HCl are di~solved in
40 ml of CH2Cl2, and first 3.8 ml of triethylamine and
then 3.5 ml of 8 50 ~ strength solution of propanephos-
phonic anhydride in CH2Cl2 are hdded at lO~C. The mixture
i6 stirred at R.T. for 20 hour~, the 601vent is removed
in vacuo, the residue is taken up in 100 ml of NTB and
the mixture is washed with 100 ml each of NaHSO~ 301ution
and NaHCO3 solution. It is dried over Na2SO~, the solvent
is removed in vacuo and the re~idue is chromatographed
with ~/H 1:1. 2 diastereomeric oils are obtained and are
further processed separately.
)3
- 24 -
Diastereomer 1 Rr ( EA/H 1: 1 ) = O . 3 0 0 . 9 5 g
Diastereomer 2 R~ (EA/H 1:1) = 0.20 O.9S g
MS (DCI, the same for both diastereom0rs): 404 (~+1)
c) ~3-t-Butylsulfonyl,2-(2 thienylmethyl)]propionic acid
4.4 g of met~yl [3-t-butylsulfonyl,2-(2-thienylmethyl)]-
propionate are suspended in 50 ml of 5N HCl and the
suspension i8 heated under reflux for 2 hours. After
cooling, it is extracted 3x with 50 ml of EA, the extract
is dried over Na2SO4 and the solvent i8 removed in vacuo.
4.0 g of the title compound are obtained a~ a pale yellow
oil.
R~ (MTB) = 0.15 - 0.25 MS (DCI): 291 (M~l)
d) Methyl t3-t-butylsulfonyl, 2-(2-thienylmethyl)]pro-
pionate
8.4 g of methyl [3-t-butylsulfonyl,2-(2-thienylmethyl)]-
propionate are dis~olved in 100 ml of CH2Cl2, and 10.6 g
of m-chloroperbenzoic acid are added in portiGns, while
cooling with ice. The mixture i~ stirred at R.T. for 1
hour and then washed first with 100 ml of 10 % strength
Na2SO3 and then with 100 ml of NaHCO3. It i8 dried over
Na2SO~ and the solvent is removed in vacuo. 7.2 g of the
title compound are obtained as a colorle~ oil.
R~ (MTB) = 0.56 MS (DCI)s 305 tM~l)
e) Nethyl t3-t-butylthio, 2-(2-thienylmethyl)]propionate
6.1 ml of t-butyl ~ercaptan are di~solved in 100 ml of
MeOH (anhydrou~) and 130 mg of ~aH are added under argon.
7.6 g of methyl 2-(2-thienylmethyl)acrylatR are then
added dropwise and the mixture i8 ~tirred at R.T. for 4
hours. The ~olvent i8 removed in vacuo, the residue is
2IG~
- 25 -
taken up in 100 ml of MTB and the mixture iB washed with
100 ml of 5 % ~trength NaHSO4 solution. It i8 dried over
Na~SO~ and the solvent is removed in vacuo. 10.4 g of the
title compound are obtained as a pale yellow liquid,
which is used further without purification and charac-
terization.
R~ (DIP/H 1:5) = 0.31
f) Methyl 2-(2-thienylmethyl)acrylate
11.8 g of monomethyl 2-thienylmethylmalonate, 5.8 ml of
diethylamine and 5.0 ml of 36 ~ strength aqueous form-
aldehyde fiolution are stirred at R.T. under argon for 1
hour. The water is then removed in vacuo and the residue
i~ chromatographed. 7.6 g of the title compound are
obtained as a colorless liquid.
~k (NTB/H 1:5) = 0.49
g) Monomethyl 2-thienylmethylmalonate
20.1 g of dimethyl 2-thienylmethylmalonate are di~solved
in 300 ml of MeOH and 5.0 g of ROH are added. The mi~ture
i8 ~tirred at R.T. for 7 hour~, the ~olvent i8 removed in
vacuo and the residue i~ taken up with 100 ml each of 5 %
strength Na2CO3 solution and EA. The aqueous phase i8 then
~cidified to pH = 2 and extracted 3x with 100 ml of EA
each time. The ~xtract i8 dried over Na2SO4 and the
solvent i8 r~moved in vacuo. 16.0 g of the title compound
are obtained a~ a pale yellow oil.
F4 (EA/MeOH 6:1) = 0.3 - 0.4
h) Dimethyl 2-thienylmethylmalonate
87.8 g of dimethylmalonate and 41.0 g of pota88ium t-
butylate are dissolved in 1.1 1 of THF (anhydrous), while
cooling with ice, and 44.1 g of 2-thienylmethyl chloride
21~3~3
- 26 -
in 500 ml of THF are added dropwi6e, under argon. The
mixture i8 stirred at R.T. for 3 hours, the ~Cl is
filtered off, the solvent is removed in vacuo and the
residue is chromatographed. 33.8 ~ of the title compound
S (I) are obtained as a colorless oil alongside 8.8 g of
dimethyl bis-(2-thienylmethyl)malonate (II)
Rf (I) (toluene/DIP 20:1) = G.35
R~ (II) (toluene/DIP 20:1) = 0.44
g) 2-Thienylmethyl chloride
252 g of thiophene are su6pended in 128 ml of concen-
trated aqueous HCl and HCl gas is passed in at 0C for 1
hour. 255 ml of 35 % strength aqueous formaldehyde
~olution are then added dropwi6e without interrupting the
stream of HCl, and the mixture i6 stirred at 0C for a
further 15 minutes. After removal of the organic phase,
the aqueous pha6e i8 extracted twice more with 600 ml of
CH2Cl2. The extract i8 then wa6hed twice with 600 ml of
saturated aqueous Na2CO3 solution and dried over Na2SO~,
the solvent i8 removed in vacuo and the residue is
distilled. 174 g of the title compound are obtained as a
colorless liquid.
bp~ = 81 - 84C
h) [1-Cyclohexylmethyl, 2-(4,4,5,5-~etramethyl-1,3,2-
dioxaborolan-2-yl), 5-methyl]hexylammonium trifluoro-
acetate
520 mg of Z-~1-cyclohexylmethyl, 2-(4,4,5,5-tetramethyl-
1,3,2-dioxaborolan-2-yl), 5-methyl]hexylamine and 95 ~1
of trifluoroacetic acid are di~solved in 10 ml of EtOH,
105 mg of Pd/C are added and hydrogenation i6 carried out
at R.T. under a pres~ure of 1 bar for 2.5 hourg. The
c~talyst i6 then filtered off and the solvent is r~moved
in vacuo. 400 mg of the title compound are obtained a~ a
colorle~ oil which i8 u~ed further without purification.
~C~ )3
-- 27 --
MS (DCI) s 338 (M+l)
i) Z-[l-Cyclohexylmethyl, 2-(4,4,5,5-tetramethyl-1,3,2-
dioxaborolan-2-yl), 5-methyl]hexylamine
6.5 g of Z-(l-cyclohexylmethyl, 5-methyl)-hex-2-en-amine
are dissolved in 190 ml of THF under argon. 5.7 ml of
BH3-S(CH3)2 are added dropwise at 0C and the mixture is
stirred at R.T. for 1 hour. Water is then 810wly added
dropwise until the evolution of hydrogen has ended. The
solvent i8 removed in vacuo and subsequently evaporated
twice on a rotary evaporator with 100 ml of toluene each
time. The residue is dissolved in 120 ml of CH2Cl2 and
11.2 g of pinacol are added. The mixture is boiled for 4
hours over a water separator, the CH2C12 i8 removed in
vacuo and the residue is chromatographed on silica gel
with EA/n-hexane = 1:8. 4.7 g of the title compound are
obtained as a diastereomer mixture, which can be separ-
ated by renewed chromatography.
h~ (EA/n-hexane = 1:8) = 0.25 NS (DCI) s 472 (N+l)
0.22
k~ Z-(l-Cyclohexylmethyl, 5-methyl)-hex-2-en-amine
13.1 g of (isopentyl, triphenyl)phosphonium bromide arç
suspended in 280 ml of THF, and 3.2 g of potassium tert.-
butylate are added. The mixture i~ stirred at R.T. for
2.5 hours and then cooled to 0C, snd 8.3 g of Z-(2(S)-
cyclohexylmethyl)-aminoacetaldehyde in 100 ml of THF are
added dropwise. The mixture i8 ~tirred at R.T. for 1
hour, 200 ml of NTB are added and the mixture i~ washed
twice with 50 ml of 5 ~ strength aqueous NaHS0~ ~olution
each time and twice with 50 ml of 5 % ~trength aqueous
Na~C03 solution each time. The organic phase is dried over
Na2S04 and the solvent i~ removed in vacuo~ Chromatography
on silica gel give~ 6.5 g of the title compound as a
colorles~ oil.
2C~3~
- 28 -
R~ ~EA/n-hexane = 1:~) = 0.28 MS (DCI) : 344 (M+1~
xamples 2 and 3 were ~ynthesized analogously to Fsa~ple
~ample 2
[2-(S)-Benzyl,3-tert.-butylsulfonyl]propionyl-Nva-[l-
cyclohexylmethyl,2-(4,4,5,5-tetramethyl-1j3,2-dioxa-
borolan-2-yl),5-methyl]hexylamide
F~ ~NTB/DIP 1:1) = 0.52 MS (FAB) : 703 (M+1)
~xample 3
~3-tert.-Butylsulfonyl,2-(1-n~phthylmethyl)]propionyl-
Val-[l-cyclohexylmethyl,2-(4,4,5,5-tetramethyl-1,3,2-
dioxaborolan-2-yl),2-cyclopentyl]methylamide
F~ (MTB/DIP 1:1) = 0.42 MS (FAB) : 751 (M+l)
~sample 4
Boc-Ph0-His-[l-Cyclohexylmethyl,2-(4,4,5,5-tetramethyl-
1,3,2-dioxaborolan-2-yl),5-methyl]hexylamide
300 mg of Boc-Phe-His(DNP)-[Cyclohexylmethyl,2-(4,4,5,5-
tetramethyl-1,3,2-dioxaborolan-2-yl),5-methyl]-hexylEmide
are di~solved in 5 ml of acetonitrile and 281 ~1 of
thiophenol are added. The mixture i8 ~tirred for 3 hours
at R.T., the solvent iB removed in vacuo and the re~idue
i8 chromatographed on silica gel with MeOH/EA 1:20. 130
mg of the title compound are obtained as a colorless
amorphou~ powder.
R~ (EA/MeOH 20:1) = 0.35 MS (FAB) : 722 ~+1)
2C~ 3~:~
- 29 -
a) Boc-Phe-His(DNP)-[1-Cyclohexylmethyl,2-(4,4,5,5-
tetramethyl-1,3,2-dioxaborolan-2-yl),5-methyl]hexylamide
The title compound was ~ynthesized analogously to Example
1.
Rf (MTB) = 0.27 MS (FAB) : 872 (M+l)
Example 5 wss synthesized analogou~ly to Example 4:
Example 5
[2-(S)-Benzyl,3-tert.-butylsulfonyl]propionyl-His-[l-
cyclohexylmethyl,2-(4,4,5,5-tetramethyl-1,3,2-dioxa-
borolan-2-yl),5-methyl]hexylamide
R~ (EA/M~OH 10:1) = 0.20 NS (FAB~ : 741 (M+l)
E~ample 6
[2-(S)-Benzyl,3-tert.-butylsulfonyl]propionyl-His-[1-
cyclohexylmethyl,2-dihydroxyboryl,5-methyl]hexylamide
217 mg of the title compound of Example 5 and 220 ~1 of
titanium isopropoxide are di~solved in 100 ml of n-
propanol and boiled for 8 hours under a Soxhlet extractor
which i8 filled with 0.3 nm molecular ~ieve. The reaction
~olution is poured onto 100 ml of saturated NaHCO3 801u-
tion, the TiO2 i8 filtered off, the solution is extracted
3 time~ with 50 ml of EA and dried over Na2SO~. The
solvent is removed in vacuo and chromatographed on 8ilica
gel with CH2Cl2/MeOH 10:1. 43 mg of the title compound are
obtained aR a white amorphous powder.
Rf (CH2Cl2/MeOH 10:1) = 0.28 MS tFAB) : 659 (M+l),