Note: Descriptions are shown in the official language in which they were submitted.
0~ 7
A method of combus-tion for ~educing the formation of
nitrogen oxides during combustion and an apparatus
for applying the method
The invention relates to a method of combustion
for reducing the formation of nitrogen oxides during
combustion, wherein air required for the combustion
of a fuel is introduced in at least two steps, the
air being introduced understoi hiometrically in the
first step, preferably with an air coefficient
ranging from 0.80 to 0.95, and a gas or gas mixture
substantially free from elementary oxygen is mixed
with the air to be introduced into the first step.
The invention is also concerned with an ap-
paratus for applying the method comprising means for
introducing air into a furnace, means for introducing
fuel into the furnace, and means for mixing a gas or
gas mixture containing less oxygen than air with the
air to be introduced into the first understoichio-
metric combustion step before the air is introduced
into tha furnace.
All combustion processes produce nitrogen
oxides when the nitrogen of both air and fuel com-
bines with oxygen to form oxides of different kinds.
In the reducing flame, N0x is derived mainly from the
nitrogen of the fuel through rapid formation, that
is, so called prompt N0x is obtained. At high tem-
peratures, mostly nitrogen oxide (N0) is obtained.
When ~the temperature drops, N0 is easily co~verted
into the other nitrogen oxides in the presence of
oxygen, mainly into nitrogen dioxide (N02). The for-
mation of nitrogen oxides occurs at a rapid reaction
rate as soon as the required chemical equilibrium
conditions are met, i.e., mainly at high temperature
and in the presence of oxygen. If the equilibrium
.~ . .
' ~'"
`~': .:;,'
Z~ 9~7
.. -- .
..
conditions are altered after the formation of nitro-
gen oxi~es so as to cause decomposition of the nitro-
gen oxides, the reaction cate o the decomposing pro-
cess is very slow, the clecomposing requiring mainly
time, catalysts or additional chemicals. From the
environmental point of view, nitrogen oxides are
highly disadvantageous. They are formed abundantly in
industrial processes as well as in power plants and
other boiler works, and one of the most important ob-
jectives of environmental protection is to reduce NOx
emissions to the atmosphere.
In an attempt to reduce NOx emissions, nitrogen
oxides are converted into another form in various
ways. Such techniques include various reduction
methods based on the use of catalysts, and the use of
absorbing agents for simultaneous absorption of
sulphur and nitrogen oxides in various ~ays. These
methods involve various problems difficult to solve,
such as the high price and difficult availability of
precious metals used as catalysts and the poor
absorption properties of ~he absorbing agents. More-
over, it is often difficult to dimension the ap-
paratus when applying absorption methods due to
variation in boiler capacities and other such
factors.
Technically, it is more advantageous to try to
prevent the formation of nitrogen oxides during the
combustion step instead of removing them. For this
purpose, a variety of low NOx burners have been de-
veloped, and attemp-ts have been made to carry out -the
combustion in a pressurized space, in addition to
which supply of air into the boiler has been carried
~ out in stages before the superheaters. Contrary to
i expectations, these methods, however, have not pro-
~ vided any particularly good results, because in prac-
i
~00~9~7
tice the operation of -the methods has been prevented
or substantially deteriorated by such factors as
variation in the formation conditions of nitrogen ox-
ides, reaction kinetics, operational conditions of
boilers and variation occurring therein. Furthermore,
removal of nitrogen oxides has been attempted by
means of circulation bed furnaces operating at very
low temperatures (about 800C), that is, at condi-
tions disadvantageous for the formation of NOx. This,
however, has deteriorated the efficiency of the fur-
naces as well as their ability to burn different
kinds of fuel as it has been necessary to drop the
temperature as low as near the minimum temperature
required for continuously maintaining the combustion
process. The methods described above are widely known
and therefore will not be described more closely
(Finnish Ministry of Trade and Industry/Energy De-
partment D:140, Helsinki 1987).
DE Offenlegungsschrift 30 40 830 discloses a
method ln which completely combusted cooled flue gas
obtained from a gas flue after the boiler is mixed
with the air to be introduced into the under-
stoichiometric first combustion zone in order to re-
duce the amount of nitrogen oxides. Even though this
.,: ~ ,..
method helps to prevent the formation of nitrogen
oxides to some extent, it does not enable sufficient
control of the amount of nitrogen oxides. In addi-
tion, the recycling of the flue gas increases the
mass flow of gas flowing through the boiler, thus re-
~uiring a somewhat larger combustion space and larger
conduits everywhere in the boiler.
The NO content is usually low within the re-
ducing area, depending on the reducing effect of
hydrogen (H2) and carbon monoxide (C02). These sub-
stances decompose the possibly formed NO roughly ac-
1. '""'.'-.'' '
z~
cording to the following reactions:
NO ~ CO -> 1/2 N2 + C2
NO + H2 -~ 1/2 N2 ~ H2O
In combustion carried ou-t understoichiometric-
ally in a manner known per se, the NO concentra-tion
can, in principle, be kept on a low level. Problems
occur only when the conditions become reducing or
when very high temperatures occur, that is, over
1500C. The problems result even ~rom a minor excess
of air, which under furnace conditions causes rapid
formation of NO, or from very high temperatures (over
1500C) at which H2 and CO cannot any more prevent
the formation of NO due to their reduced reducing
po-tential. In prior art apparatuses such situations
occur particularly in the primary flame but also in
connection with the introduction of secondary and
tertiary air. One of the most important reasons for
the formation of NO in the primary flame of prior art
apparatuses is that the heterogeneous flame contains,
e.g., oil drops or carbon particles and, as a conse-
quence, there occurs high concentration gradients of
oxygen and burning gases as well as high temperature
gradients. Thereby it is always possible that minor
temperature peaks occur locally at phase boundaries,
for instance, if the amount of oxygen at such a point
is stoichiometric or slightly overstoichiometric. In
a typical combustion apparatus, the temperature may
rise instantaneously and locally up to about 2000C.
As a result, the local NO concentration rises rapidly
up to about 3500 ppm (prompt NO~). The NO so formed
will not decompose to any greater degree under boiler
conditions. Accordingly, it is obvious that even
minor locally and instantaneously occurring tempera-
ture peaks increase rapidly the average NO value of
exhaust gas, which should remain on a concen-tration
~0~345~
level of about 100 ppm.
The object of the present invention is to pro-
vide a method by means of which the formation of ~x
during a reducing combustion step, usually in so
called primary combustion, particularly in the flame,
can be minimized and in which conditions prerequisite
for the formation of NOx are prevented without any
complicated apparatuses. Removal of NOx after combus-
tion is not required. The method is characterized in
that a gas or gas mixture containing reducing agents,
such as H2 and CO, is mixed with the air to be intro-
duced into the first step, that the oxygen content of
the gas mixture introduced into the first step is
preferably 12-19%, and that the oxygen content and
reducing potential of the air mixture to be intro-
duced are adjus~ed so that the nitrogen oxide con-
centration of flue gas from combustion carried out at
the adiabatic combustion temperature of the fuel
used, corresponding to the supplied oxygen content
and reducing potential, is no more than a prede-
termined concentration value.
The basic idea of the invention is that air is
introduced into the combustion process in such a man-
; ~:
ner that the NOx formation in the reducing part ofthe furnace, particularly in the difficultly con-
trollable flame, remains on a sufficiently low level
at all temperatures and oxygen/fuel ratios possibly
occurring during this combustion step. This is
achieved by carrying out the combustion under re-
ducing conditions by using a gas or a gas mixture
having an oxygen content lower than that of ordinary
air and containing reducing agents. By means of the
method according to the invention, the concentration
of nitrogen oxides can be controlled so that the
equilibrium concentration of the nitrogen oxides in
,,"~.
Z0~9637
: :
the flue gas, in practice, also the maximum con-
centration, remains all the time at a very low value.
A further object of the invention is to provide
an apparatus for applying the method. The apparatus
is characterized in -that the mixing means comprise at
least one gas flue for passing part of the flue gas
from the first combustion step into the air to be
introduced into the first step to be mixed with it.
The basic idea of the apparatus of the inven-
tion is that the reducing gas or gas mixture, that
is, non-oxygen-containing or low-oxygen-content gas
containing reducing agents, and air are mixed
thoroughly with each other and introduced at least
into the boiler zone in which fuel and air are nor-
mally poorly mixed with each other so that local tem-
perature peaks are likely to occur. Typically, this
zone is the reducing combustion zone of the boiler,
mainly the flame.
The invention will be described in greater de-
tail in the attached drawings, wherein
Figure 1 illustrates the interdependence of the ;
temperature and the air coefficient (ratio of oxygen
to the amount of theoretical oxygen required for the
combustion irrespective of the other components pre-
sent in the gas mixture, such as inert components and
reducing agents) in a prior art application with re-
spect to a predetermined NO concentration level when
the combustion is carried out normally with air, and
the interdependence of the adiabatic temperature and
the air coefficient in a typical oil combustion pro-
cess carried out normally with air and with a mixture
of air and gas consisting of completely combusted
flue gas from a boiler, the mixture having an oxygen
content of 17~ (see DE Patent Application 3 040 830);
r igure 2 illustrates by way of example the
, ':
;~;(3~14907
~`
interdependence of the maximal NO amount obtained in
the combustion of pure methane (CH4) and the air co-
efficient when the gas maintaining the combustion is
air, a mixture of completely combusted flue gas and
air, as disclosed in Figure 1, or a mixture of cooled
gas recycled from the reducing combustion and air;
and
Figure 3 is a schematic view of an apparatus
for applying the method of the invention.
In Figure 1, the curve A-B shows by way of
example the adiabatic combustion temperature of a
widely used oil type as a function of the air coeffi~
cient when the combustion is carried out normally
with air. The curve C-D illustrates by way of example
the adiabatic combustion temperature of the same oil
type as a function of the air coefficient when the
combustion is carried out with air diluted with com~
pletely combusted flue gas, the oxygen content of the
mixture being 17%. The curve E-F shows by way of
example the pairs of temperature and air coefficient
values corresponding to the N0 concentration 100 ppm
when the combustion is carried out normally with air.
Above the curve the N0 concentration is more than 100
ppm. Very high temperatures (over 1500C) in par~
ticular are important for the invention. As the local
temperatures in the hottest portions of the flame may
rise very close to the adiabatic temperature, it is
to be seen from the figure that the N0 concentration
of 100 ppm (point G) can be achieved with an air co-
efficient as low as 0.82 when the combustion is
carried out normally with air. When using air diluted
with completely combusted flue gas, the concentration
of 100 ppm is achieved with an air coefficient of
0.93 (point H). At worst, the maximum N0 concentra-
tion within the reducing area is about 2700 ppm when
.
. . ~.
- ;~0~49~37 :-
the combustion is carried out normally with air and
only ~00 ppm when the co~bustion is carried out with
diluted air as disclosed in the example. The first-
mentioned value is represented by point I and the
last-mentioned by point J in Figure 1.
It has now been found unexpectedly that the NO
formati~n can be prevented within the reducing area
of the burner, particularly in the flame, when oxy-
gen required by the combustion is introduced under-
stoichiometrically and at an uniform oxygen content
of less than 21% by mixing a gas or gas mixture con-
taining considerable amounts of reducing agents with
the combustion air. In this way the combustion tem-
perature, particularly that of the flame, can be de-
creased, simultaneously increasing reducing potential
so that abundant formation of NOx is no more
possible, not even locally or instantaneously. This
preferably takes place so that the local temperature
peak of the flame does not exceed about 1500C and
the oxygen concentration is reduced by means of cool-
ed flue gas recycled from the reducing combustion
step, typically containing hydrogen (H2) and carbon
monoxide (CO). The formation of NOx is efficiently
prevented both by the temperature drop and the in-
crease in the reducing potential.
In Figure 2, the curve K-L illustrates the
maximum NO concentration obtained in the combustion
of pure methane (CH4) when the combustion is carried
out normally with air and the burning takesi place
adiabatically. The curve M-N illustrates the maximum
NO concentration when completely combusted flue gas
has been mixed with the combustion air. The curve 0-
N, in turn, illustrates an example where the oxygen
content of the combustion air has been decreased by
adding to it properly cooled flue gas from the re-
,. . .
~0~9~
g
ducing step, operated wit:h the same air coefficient,in an amount of 24% on the volume flow of the primary
air, whereby reducing agents H2 and C0 will also be
recycled to a considerable degree. It clearly appears
from the figure that the recycling of reducing gases
decreases greatly the N0 concentration while the tem~
perature is decreased and the reducing potential in-
creased. For instance, the maximum decrease in N0
concentxation with the air coefficient 0.80 is as
great as 97% with the air coefficient 0.80 as com-
pared with combustion with air, that is, the con-
centration drops from 0.048 mol N0/kg CH4 (point P in
Figure 2) to 0.0012 mol N0/kg CH4 (point Q in Figure
2) and about 73~ on the value obtained when complete-
ly combusted flue gas is mixed with the combustion
air (corresponding to the ratio between Q and R).
When the method of the invention is applied, the oxy-
gen concentration as well as the amount and reduction
capability of the reducing agents, that is, their
reducing potential, can be adjusted in a desired man-
ner according to the used fuel and other combustion
conditions.
It has been unexpectedly found that the maximum
efficacy of the method of the present invention, that
is, the greatest decreasa in N0 formation as compared
with the prior art, is to be obtained with air co-
efficients ranging from 0.80 to 0.95. It is likewise
unexpected that the maximum decrease in N0 concentra-
tion occurs within the air coefficient range conven-
tionally applied in typical prior art primary combus-
tion in power plant boilers. Accordingly, the method
of the present invention decisively decreases N0x
formation in the flame of the burner, that is, the
formation of prompt N0x, which has been most diffi-
cult if not impossible to prevent in prior art appar-
: ", ~
:-`'''' .:;
~,' ,',
,.'.'~,"
~0~ 7
, ~ .
, . . .~,
~.
atuses. When comparing the curves M-N and O-N of Fig-
ure 2, it appears that when a gas or gas mixture con-
taining reducing agents :is mixed with air according
to the invention, the air coefficient 0.95 still
gives a NOx concentration (point S) which is about
92~ lower than that obtained by combustion with ordi-
nary air (point T) and 40~ lower than the value ob-
tained by adding completely combusted flue gas
(corresponding to the ratio between points U and S).
Furthermore, the use of completely combusted flue gas
increases the amount of gas used, which requires a
greater boiler and greater gas flues, whereas in the
method of the invention the increased amount of gas
and the greater space requirement concern only that
part of the boiler in which the reducing combustion
takes place. As further appears from Figure 2, the
curves M-N and O-N join when the air coeficient is
1, which is due to the fact that flue gas from fuel
burned with stoichiometric ratio cannot any more con-
tain reducing agents to any greater degree. This,
however, is unimportant ~or the end result in combus-
tion processes carried out with lower air coeffi-
cients. Essential in the invention is that local
overheating is prevented in an understoichiometric
combustion so that nitrogen oxides will not be form-
ed.
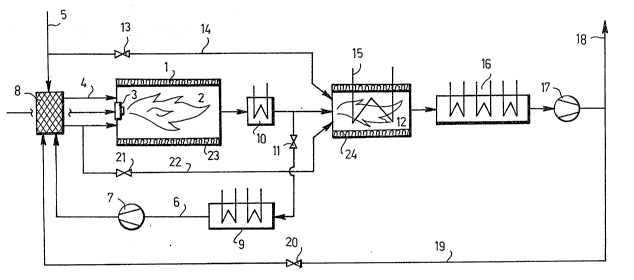
Figure 3 shows schematically an apparatus for
applying the method of the invention. The apparatus
comprises a burner such as a boiler 1 with a furnace
2. Fuel is introduced into the furnace 2 by means of
one or more feeding devices 3. Oxygen-containing gas
mixture required for the combustion is introduced
into the same part of the furnace 2 through a conduit
4 belonging to air supply means. Air is supplied into
the conduit 4 through a conduit 5, while reducing
Z(~4~7 :
!
1 1
gas, that is, at least substantially non-oxygen-con-
taining gas mixture containing substantial amounts of
reducing agents, mainly H2 and C0, is supplied
through a conduit 6 belonging to mixing means via a
blower 7 and a gas mi~er 8. The gas to be mixed is
preferably flue gas deri~ed from the furnace 2, in
which raducing combustion takes place. The flue gas,
which contains reducing agents, is cooled by means of
coolers 9 and 10 and its amount is controlled by
means of a valve 11. The reducing flue gas is mixed
in the mixer 8 with the air to be introduced into the
furnace. A major portion of the flue gas produced
during the reducing combustion step i5 passed into
subsequent combustion steps, shown schematically with
a single combustion step 12. During the subsequent
combustion steps, additional air is introduced into
the boiler by means of a valve 13 through a conduit
14, whereby the fuel will be combusted as completely
as possible. At this stage the 1ue gas can be cooled
b~ means of a heat exchanger 15 and thereafter by
means o coolers 16, whereafter it is passed through
a blower 17 into a gas flue 18. Depending on the re-
quired amount of reducing agents, final cooled flue
gas can be mixed with the air to be introduced into
the furnace 2 during the first step in a manner known
per se through a conduit 19 in addition to the flue
gas from the reducing step introduced through the
conduit 6, the amount of the final flue gas being
adjusted by a valve 20. In this way, both the oxygen
content of the mixture of air and gas to be intro-
duced into the furnace and the concentration of the
reducing agents in it can be adjusted according to
the combustion conditions and fuel used. If there
exists a danger that the flame or a portion of it
becomes too hot at the beginning of the reducing com-
,' '
:, .' ~'.~.
':~ ` ` ','. '. .- j .' . ~ .,; , . '
:
12
bustion step 12 with resultant excessive formation of
nitrogen oxide, the flame temperature during this
step can also be decreased by feeding reducing gas
through a valve 21 and a conduit 22 at the beginning
of the reducing combustion step 12. Heat losses from
the burner can be decreased by insulating the combus-
-tion chambers by insulations 23 and 24, shown schema-
tically in the figure~
I~ is to be understood that some of the devices
described above can be combined into a single entity
to obtain a structurally more advantageous solution.
For instance, the parts 2, 10, 12, 15 and 16 can be
easily combined.
It is essential in the apparatus of the inven-
tion that the air and reducing gas are mixed properly
before their introduction into the reducing part of
the furnace and that the temperature of the flame or
a flame portion is decreased only to such an extent
as is required for preventing the formation of NO
without any risk of the combustion process being
interrupted. In the present method the mix ratio of
air and fusl is ~etermined, e.g., by the thermal
value of the fuel used, the minimum temperature re-
quired for maintaining combustion, the chemical
analysis of the gas, the desired NOx level, the di-
mensions of the heat surfaces of the boiler, the de-
gree of cooling (temperature~ of the recycled gas,
and the positions of the gas introduction steps. As a
consequence, this ratio may vary widely; typically,
the amount of gas is 10 to 70% on the amount of air
supplied.
It is obvious that when the same combustion
efficiency is to be obtained, the mass volume of the
gas used in the apparatus of the invention is greater
as compared with prior art apparatuses, though mainly
i ,, . . ~ ., , ~ , ., " ,. . .- , . . .
;;~ 9L9~7 ::
13
only in the reducing part of the boiler. However, the
dimensions of the boiler will not change to any
greater degree because the recycling preferably takes
place only during the understoichiometric combustion
step(s) and because increase in the flow volume of
gas is for a major part compensated for by change in
the gas density caused by the temperature drop. It is
also obvious that, theoretically, the recycling of
gas does not reduce the efficiency of the boiler;
varying heat losses, however, may resul-t in slightly
reduced efficiency. In view of the advantages ob-
tained, this drawback is not of any greater im-
portance.
The invention has the advantage that the ap-
paratus can be constructed by means of well-known
inexpensive constructions and no separate expensive
means for removing NOx are needed because the forma-
tion of NOx has been prevented sufficiently. Further,
the method of the invention is easy to realize and
very easy to control when its principles are applied
to apparatuses and control systems known per se. It
is likewise possible to control the high local forma-
tion o~ NOx in the hottest, spotlike portions of the
flame of the burner because the formation of NOx is
so restricted that its concentration cannot exceed a
set limit value. The NO concentration of the flue gas
emission to the surrounding is, of course, dependent
on the operational properties and structure of the
oxidizing part of the boiler.
.., ".