Note: Descriptions are shown in the official language in which they were submitted.
F. HOFFMANN-LA ROCHE AG Basle/Switzerland RAN 6103/49
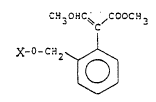
The present invention relates to aromatic
compounds, namely substituted methyl 2-phenyl-3-methoxy-
acrylates of the general formula
C!3_OHC~ ~C!~OC~3 3
X-o-c:~2~ . I
15~J
where X represents an aldimino or ketimino group, in
particular a group
20R1
C=N-
where R1 and R2 independently of one another denote
hydrogen, C1_12-alkyl, C1_4-haloalkyl, C1_4-alkoxy-
C1_4-alkyl, C1_4-alkylthio-C1_4-alkyl, aryl-C1_4-alkyl,
aryloxy-C1_4-alkyl, arylthio-C1_4-alkyl, heteroaryl-C1_4-
alkyl, C2_12-alkenyl, aryl-C2_4-alkenyl, heteroaryl-C2_4-
alkenyl, C2_12-alkynyl, C3_6-cycloalkyl, aryl, heteroaryl,
cyano or one of the groups (a) to (d)
CooR3 (a) CoNR4R5 (b)
coR6 (c) CR7=NoR8 (d)
or R1 and R2 together with the carbon atom to which they
are attached denote a 4-7-membered ring which optionally
. : , :
, .. , , , . ,. .:
.
~Q~ 5
-- 2
contains an oxygen or sulphur atom and which can contain
one or two fused aromatic rings, for example optionally
substituted benzene rings,
and R3, R4, R5, R6, R7 and R8 in each case denote
hydrogen,C1_4-alkyl, aryl or heteroaryl.
The compounds according to the invention have
fungicidal properties and are suitable as fungicidal active
substances, in particular Xor use in agriculture and
horticulture.
The invention furthermore relates to a process for
the manufacture of the compounds according to the
invention, to fungicidal compositions which contain such
compounds as active substances, and to the use of such
compounds and compositions for controlling fungi in
agriculture and horticulture.
In the above formula I, all the "alkyl", "alkenyl"
and "alkynyl" groups, as such or as a component of larger
groups, for example heteroarylalkyl, can be straight-chain
or branched, depending on the number of carbon atoms they
contain. In addition, the alkenyl and alkynyl groups can
in each case have more than one double or triple bond. A
halogen atom which may be present is fluorine, chlorine,
bromine or iodine, fluorine, chlorine and bromine being
preferred. A group, such as, for example, alkyl, as such
or as a component of a larger group which in each case has
more than one halogen substituent, can have identical or
different halogen atoms. Aryl is taken to mean, in
particular, phenyl, naphthyl, phenanthryl or fluorenyl,
heteroaryl is taken to mean a heterocyclic group having
aromatic character, such as pyrrolyl, pyridyl, furyl,
thienyl, isoxazolyl, thiazolyl, imidazolyl, pyrimidinyl or
triazolyl, or such a group with a benzene nucleus fused to
it, for example quinolinyl, benzofuryl, benzothienyl or
dibenzofuryl. This also applies to aryl or heteroaryl as
part of a larger group, for example aralkyl or
heteroarylalkyl. The aryl and heteroaryl groups can in
,
.
: , :
35~5
-- 3 --
each case have one or more substituents, this substituent
or these substituen~s being suitably selected from amongst
halogen, C1_4-alkyl, C1_4-haloalkyl, C1_4-alkoxy-C1_4-
alkyl, aryl-C1_4-alkyl, heteroaryl-C1_4-alkyl, aryloxy-
C1_4-alkyl, heteroaryloxy-C~_4-alkyl, C2_4-alkenyl,
aryl-C2_4-alkenyl, heteroaryl-C2_~-alkenyl, C2_~-alkynyl,
C3_6-cycloalkyl, aryl, heteroaryl, C1_4-alkoxy,
C1_4-haloalkoxy, aryl-C1_4-alkoxy, heteroaryl-C1_~-alkoxy,
C2_4-alkenyloxy, C2_4-alkynyloxy, aryloxy, heteroaryloxy,
C1_4-alkylthio, cyano, nitro, a group NR9R10, a group COR11
and a group COOR12 (where R9, R10, R11 and R12 in each case
denote C1_~-alkyl, aryl or heteroaryl).
If the compounds of the formula I have asymmetric
carbon atoms, the compounds occur in optically active form.
By virtue of the aliphatic and the imino double bond alone,
the compounds occur in any casa in the [E] or [Z] form. It
is also possible that atropisomerism occurs. Formula I is
intended to embrace all these possible isomeric forms as
well as their mixtures, for example racemic mixtures and
any [E/Z] mixtures.
A particular group of compounds of the formula I
consists of those compounds of the formula I in which X
denotes a group R1R2C=N-, where R1 and R2 independently of
one another denote hydrogen, C1_12-alkyl, C1_4-haloalkyl,
aryl-Cl_4-alkyl, heteroaryl-C1_4-alkyl, C2~12-alkenyl,
aryl-C2_4-alkenyl, heteroar~l-C2_4-alkenyl, C2_12-alkynyl,
C3_6-cycloalkyl, aryl, heteroaryl, cyano or one of the
abovementioned groups (a) to (d), or R1 and R2 together
with the carbon atom to which they are attached denote a
ring as defined in greater detail above.
In the group R1R2C=N-, R1 and R2 being independent
of one another, R1 preferably denotes optionally substi-
tuted phenyl, any substituents which may be present
preferably being up to three identical or different halogen
atoms (in particular fluorine,. chlorine and/or bromine),
C1_4-alkyl groups (in particular methyl), C1_4-haloalkyl
. . . , , :
: - : . : , . . :
: . . .
. . . " , ~: .,: "
~: :, : :,:: : : . .: ,
: ,. ..
-- 4 --
groups (in particular trifluoromethyl) and/or C1_4-halo-
alkoxy groups (in particular trifluoromethoxy), and R2
preferably denotes hydrogen, C1-12-alkyl (in particular
methyl or ethyl), C1-4-haloalkyl (in particular
trifluoromethyl) or C3_6-cycloalkyl (in particular cyclo-
propyl).
R1 likewise preferably denotes heteroaryl, in
particular furyl which is optionally substituted with up to
two methyl groups, thienyl which is optionally substituted
with chlorine or methyl, or benzofuryl, and R2 likewise
preferably denotes methyl.
In general, the ~E] forms of the compounds of the
formula I are preferred to the [X] forms.
Particularly preferred individual compounds of the
formula I are:
methyl 3-methoxy-2-[a-{[(a~methyl-benzyl)imino]-
oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[a-{[(a-methyl-3,5-di-tri-
fluoromethyl-benzyl)imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[a-{[(-methyl-2-fluoro-5-5 methyl-benzyl)imino]oxy}-o-tolyl]-acrylate,
methyl 1-[a-{~(1-[2-benzofuryl]-ethyl)imino]oxy}-
o-tolyl]-3-methoxy-acrylate,
methyl 3-methoxy-2-[a-{[(3-nitrobenzyl)imino]oxy}-
o-tolyl]-acrylate,
methyl 3-methoxy-2-[-{[(a-trifluoromethyl-
benzyl)imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[-{[(a-methyl-4-fluorobenzyl)-
imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[-{[(1-[2-thienyl]-~thyl)-5 imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[a-{[(a-methyl-4-chlorobenzyl)-
imino]oxy}-o-tolyl]-acrylate,
methyl 2-[a-{[(a-cyclopropyl-benzyl)imino]oxy}-o-
tolyl~-3-methoxy-acrylate,
. ~ . . . :
"
" ; ,, ; ; : .
;,
~:C~3'~5
methyl 2-[a-{[(1-[5-chloro-2-thienyl]-ethyl)-
imino]oxy}-o-tolyl]-3-methoxy-acrylate,
methyl 3-methoxy-2-[a-{[(a-methyl-3-trifluoro-
methyl-benzyl~imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[-{[(-methyl-3-bromobenzyl)-
imino]oxy}-o-tolyl]-acrylate~
methyl 2-[a-{[(1-[3,5-dimethyl-2-furyl]-ethyl)-
imino]oxy}-o-tolyl]-3-methoxy-acrylate,
methyl 2-[a--{[(1-[2,5-dimethyl-3-thienyl]-ethyl)-0 imino]oxy}-o-tolyl]-3-methoxy-acrylate,
methyl 3-methoxy-2-[a-{[(~-methyl-3-chlorobenzyl)-
imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[~-{[(~-methyl-3-methylbenzyl)-
imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[a-{[(-methyl-4-methylbenzyl)-
imino]oxy}-o-tolyl]-acrylate,
methyl 2-[-{~(3-fluorobenzyl)imino]oxy}-o-tolyl]-
3-methoxy-acrylate,
methyl 3-methoxy-2-[~-~[(-methyl-4-trifluoro-0 methyl-benzyl)imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[-{[(-methyl-3-fluoro-5-tri-
fluoromethyl-benzyl)imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[-{[(-methyl-3-fluorobenzyl)-
imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[~-{[(a-methyl-3-trifluoro-
methoxy-benzyl)imino]oxy}-o-tolyl]-acrylate and
methyl 2-[a-{[(-ethyl-3-trifluoromethyl-benzyl)-
imino]oxy}-o-tolyl]-3-methoxy-acrylate.
Other preferred individual compounds of the
formula I are:
methyl 2-[~-{[(1-[2-furyl]-ethyl)imino]oxy}-o-
tolyl]-3-methoxy-acrylate,
methyl 3-methoxy-2-1~-{~(1-[2-naphthyl]-ethyl)-
imino]oxy}-o-tolyl]-acrylate,
methyl 2-[a-{[(4-chlorobenzyl)imino]oxy}-o-tolyl]-
3-methoxy-acrylate,
methyl 2-la-{1(2,4-dichlorobenzyl~imino]oxy~-o-
: : . . : ::.: : - . : ;
. ; - . ~ :
- j :: : . , ; : :, , ~
- ~: , . , , . ;. .
. -, .~, - . - ~, . . . . .
2~
-- 6 --
tolyl]-3-methoxy-acrylate,
methyl 3-methoxy-2-[a-{[(a-methyl-4-nitrobenzyl)-
imino]oxy}-o-tolyl]-acrylate,
methyl 2-[a-{(benzylimino)oxy}-o-tolyl]-3-methoxy-
acrylate,methyl 3-methoxy-2-[a-{~(2-pyridylmethyl)imino]-
oxy}-o-tolyl]-acrylate,
methyl 2-[a-{[(a-ethyl-benzyl)imino]oxy}-o-tolyl]-
3-methoxy-acrylate,
methyl 2-[a-{[(a-ethyl-4-chlorobenzyl)imino]oxy}-
o-tolyl]-3-methoxy-acrylate,
methyl 3-methoxy-2-[a-{[(a-[n-propyl]-benzyl)-
imino]oxy}-o-tolyl]-acrylate,
methyl 2-[-{[(1-[benzoyl3-ethyl)imino]oxy}-o-5 tolyl]-3-methoxy-acrylate,
methyl 3-methoxy-2-[a-l[(a-methyl-~-phenyl-allyl)-
imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[a-{[(-methyl-4-phenoxy-
benzyl)imino]oxy}-o-tolyl]-acrylate,
methy.l 2-[a-{[(a-cyclopropyl-4-chlorobenzyl)-
imino]oxy}-o-tolyl]-3-methoxy-acrylate,
methyl 3-methoxy 2-[a-{[(1-~2-pyridyl]-ethyl)-
imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[~-{[~1-[3-pyridyl]-ethyl)-5 imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[a-{[(1-[5-methyl-2-furyl]-
ethyl)imino]oxy}-o-tolyl]-acrylate,
methyl 2-[a~t[(dicyclopropylmethyl)imino]oxy}-o-
tolyl]-3-methoxy-acrylate,
methyl 3-methoxy-2-[a-{[(2-naphthylmethyl)imino]-
oxy}-o-tolyl]-3-methoxy-acrylate,
methyl 3-methoxy-2-Cu-{[(a-methyl-3-nitrobenzyl)-
imino~oxy}-o-tolyl]-acrylate,
methyl 2-[a-~[(1-[2,4-dimethyl-5-thiazolyl]-5 ethyl)imino]oxy}-o-tolyl]-3-methoxy-acrylate,
methyl 3-methoxy-2-[a-t[(-methyl-4-methoxy-
benzyl)imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[a-{[(a-methyl-3,4-dichloro-
benzyl)imino]oxy}-o-tolyl]-acrylate,
; ,
.
2~ i3~
-- 7
methyl 3-methoxy-2-[a-{[(a-methyl-2-fluorobenzyl)-
imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[a-{[~a-methyl-2,4-dimethyl-
benzyl)imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[a-{[(a-methyl-4-ethylbenzyl)-
imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[a-{[(a-methyl-3-ethylbenzyl)-
imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[a-{[(a-methyl-3,4-dimethyl-0 benzyl)imino]oxy}-o-tolyl]~acrylate,
methyl 2-[a-{[(1-[5-bromo-2-thi~nyl]-ethyl)imino]-
oxy}-o-tolyl]-3-methoxy-acrylate,
methyl 3-methoxy-2-[a-{[(1-[2-naphthyl]-propyl~-
imino]oxy}-o-tolyl]-acrylate and
methyl 2-[a-{[(a-cyclopropyl-2-naphthylmethyl)-
imino]oxy}-o-tolyl]-3-methoxy-acrylate.
Other representatives of compounds of the formula
I are:
methyl 3-methoxy-2-la-{[(a-methyl-2,3-di~luoro-
benzyl)imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-~a-{[(a-methyl-2,4-difluoro-
benzyl)imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[a-{[(a-~methyl-2,5-difluoro-
benzyl)imino~oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[~-{[(a-methyl-3,~-difluoro-
benzyl)imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[a-{[(a-trifluoromethyl-0 carbonyl-2,3-dichlorobenzyl)imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[a-{[(a-trifluoromethyl-2,3-
dichlorobenzoylmethyl)imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[a-{[(1-methyl-2-phenoxy-
propyl)imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[a-{[(1-methyl-2-~2-chloro-
phenoxy]-propyl)imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-[a-{[(1-methyl-2-phenylthio-
propyl)imino]oxy}-o-tolyl]-acrylate,
methyl 3-methoxy-2-~a-{[(~-trifluoromethyl-
,
' '- .~ ' . O ' '
. .
: . : .
. . ...
-- 8 --
carbonyl-2-naphthylmethyl)imino~oxy}-o-tolyl~-acrylate,
methyl 2-[~-{[(a-cyclopropylcarbonyl-2-naphthyl-
methyl)imino]oxy}-o-tolyl]-3-methoxy-acrylate,
methyl 2-[~-{[la-isopropyl-2-naphthylmethyl)-
imino]oxy}-o-tolyl]-3-methoxy-acrylate,
methyl 2-[a-{[(-dimethylaminomethyl-2-naphthyl-
methyl)imino]oxy}-o-tolyl]-3-methoxy-acrylate and
methyl 3-methoxy-2-la-{t(2,4,4-trimethyl-1-cyclo-
hexen-6-yl)imino]oxy}-o-tolyl]-acrylate.
The process according to the invention for the
manufacture of the compounds according to the invention is
characterized in that an oxime X-OH t in particular an oxime
of the general formula
R1
~ C=N-OH II
where R1 and R2 have the abovementioned meanings
is reacted with a benzyl alcohol derivative of the general
formula
C.;3~:)'nC~ ~COOCH3
UC:' ~
~ ~ ~ I III
where U denotes a leaving group.
This reaction is a nucleophilic substitution which
can be carried out under the reaction conditions customary
for such substitutions. The leaving group U in the ben7yl
alcohol derivative of the formula III is preferably taken
to mean chlorine, bromine, iodine, mesyloxy or tosyloxy.
The reaction is suitably carried out in an inert organic
diluent, such as a cyclic ether, for example tetrahydro-
furan or dioxan, acetone, dimethyl formamide or dimethyl
sulphoxide, in the presence of a base, such as sodium
- , .. .
. ~ : , ,
hydride, sodium carbonate or potassium carbonate, of a
tertiary amine, for example a trialkyl amine, in particular
diazabicyclononane or diazabicycloundecane, or silver
oxide, at temperatures between -20C and 80C, preferably
in the temperature range from 0C to 20C.
Alternatively, the reaction can be carried out
under phase transfer catalysis at room temperature in an
or~anic solvent, such as, for example, methylene chloride,
in the presence of an aqueous basic solution, for example
sodium hydroxide solution, and of a phase transfer
catalyst, such as, for example, tetrabutylammonium
bisulphate.
The resulting compounds of the formula I can be
isolated and purified by methods known per se. Any
mixtures of isomers which are obtained, for example
mixtures of E/Z isomers, can be separated to give the pure
isomers likewise by methods known per se, for example by
chromatography or fractional crystallisation.
The oximes X-OH which are used as starting
materials in the process according to the invention, for
example those of the formula II, are either known or they
can be produced by methods known per se, for example by
reacting the corresponding carbonyl compound R1R2C-O with
hydroxylamine hydrochloride in the presence of a base, for
example sodium hydroxide, potassium hydroxide or pyridine.
For other methods, see Houben-Weyl, "Methoden der
Organischen Chemie" ["Methods of Organic Chemistry"],
volume X/4, pages 3-308 (1968) ("Herstellung und Umwandlung
von Oximen" ~"Preparation and Conversion of Oximes"]).
Likewise, the starting materials of the formula
III can be produced in a manner known per se, for example
as described in European Patent Publication No. 203,606
(BASF) and in the references quoted therein.
.
.: ~ ~: ' : ~ '
:
2~
-- 10 --
The compounds according to the invention have
fungicidal activity and can accordingly be used for
controlling fungi in agriculture, horticulture and wood
processing. They are particularly suitable for inhibiting
the growth of, or destroying, phytopathogenic fungi on
parts of plants, for example leaves, stems, roots, tubers,
fruits or flowers, and on seeds, and for inhibiting the
growth of, or destroying, pathogenic fungi which occur in
the soil. It is furthermore possible to control wood-
destroying and wood-discolouring fungi with the compounds
accordin~ to the invention. For example, the compounds
according to the invention are active in the control of
fungi of the classes of the Deuteromycetes, Ascomycetes,
Basidiomycetes and Phycomycetes.
The compounds according to the invention are
particularly suitable for controlling the following
pathogens:
Powdery mildews (for example Erysiphe graminis,
Erysiphe cichoracearum, Podosphaera leucotricha, Uncinula
necator, Sphaerotheca spp.)
Rusts (for example Puccinia tritici, Puccinia
recondita, Puccinia hordei, Puccinia coronata, Puccinia
striiformis, Puccinia arachidis, Hemileia vastatrix,
Uromyces fabae)
Scabs (for example Venturia inaequalis)
Cercospora spp. (for example Cercospora
arachidicola, Cercospora beticola)
Mycosphaerella spp. (for example Mycosphaerella
fijiensis)
Alternaria spp. (for example Alternaria brassicae,
Alternaria mali)
.. : - : , - .
,
Septoria spp. (for example Septoria nodorum)
Helminthosporium spp. (for example
Helminthosporium teres, Helminthosporium oryzae)
Plasmopara spp. (for example Plasmopara viticola)
Psèudoperonospora spp. (for exa~ple
Pseudoperonospora cubensis)
Phytophtora spp. (for e~ample Phytophtora
infestans)
Pseudocexcosporella spp. (for example
Pseudocercosporella herpotrichoides)
Pyricularia spp. (for example Pyricularia oryzae)
Furthermore, the compounds are active against, for
example, fungi of the genera Ti.lletia, Ustilago,
Rhizoctonia, Verticillium, Fusarium, Pythium,
Gaeumannomyces, Sclerotinia, Monilia, Botrytis,
Peronospora, Bremia, Gloeosporium, Cercosporidium,
Penicillium, Ceratocystis, Rhynchosporium, Pyrenophora,
Diaporthe, Ramularia and Leptosphaeria. Certain
representatives of the compounds according to the invention
have an additional action against wood-destroying fungi,
such as, for example, those of the genera Coniopho~a,
Gloeophyllum, Poria, Merulius, Trametes, Aureobasidium,
Sclerophoma and Trichoderma.
The compounds according to the invention are
distinguished by a prophylactic and curative action.
Under greenhouse conditions, the compounds
according to the invention are active against
phytopathogenic fungi at concentrations of as little as 0.5
mg to 500 mg of active substance per litre of spray liquor.
In the field, it is advantageous to apply dosages o 20 g
,
.. ~
,: . :
:".
2~ 3S3d~5i
- 12 -
to 1 kg of active substance of the ~ormula I per hectare
per treatment. To control seed-borne fungi or fungi which
occur in the soil by seed-dressing, it is advantageous to
use dosages of 0.01 g to 1.0 g of active substance of the
formula I per kg of seed.
The compounds according to the invention can be
formulated to give a range of compositions, for example
solutions, suspensions, emulsions, emulsifiable
1Q concentrates and preparations in the form of powders. The
fungicidal compositions according to the invention are
characterized in that they contain an effective amount of
at least one compound of the general formula I, as defined
above, in addition to formulation adjuvants. It is
expedient for the compositons to contain at least one of
the following formulation adjuvants:
Solid carriers; solvents or dispersion media;
tensides (wetting agents and emulsifiers); dispersing
agents (without tenside action); and stabilizers.
Solid carriers which are suitable are, mainly,
natural mineral substances, such as kaolin, clays,
kieselguhr, talc, bentonite, chalk, for example whiting,
magnesium carbonate, limestone, quartz, dolomite,
attapulgite, montmorillonite and diatomaceous earth;
synthetic mineral substances, such as highly-dispersed
silica, alumina and silicates; organic substances, such as
cellulose, starch, urea and synthetic resins; and
fertilizers, such as phosphates and nitrates, it being
possible for such carriers to be present, for example, as
granules or powders.
Suitable solvents or dispersion media are, mainly,
aromatic substances, such as toluene, xylenes, benzene and
alkylnaphthalines; chlorinated aromatic substances and
chlorinated aliphatic hydrocaxbons, such as chlorobenzenes,
chloroethylenes and methylene chloride; aliphatic
hydrocarbons, such as cyclohexane, and paraffins, for
- : -. - , . , :
. ~ - i, - , ~- :' ~
- 13 -
example mineral oil fractions; alcohols, such as butanol
and glycol, as well as their ethers and esters; ketones,
such as acetone, methyl ethyl ketone, methyl isobutyl
ketone and cyclohexanone; and strongly polar solvents or
dispersion media, such as dimethylformamide, N-
methylpyrrolidone and dimethyl sulphoxide, these solvents
or dispersion media preferably having flashpoints of at
least 30C and boiling points of at least 50C, and water.
Suitable amon~ the solvents or dispersion media are also
so-called liquefied gaseous extenders or carriers, which
are those products which are gaseous at room temperature
and under atmospheric pressure. Examples of such products
are, in particular, aerosol propellants, such as
halohydrocarbons, for example dichlorodifluoro-
methane. If water is used as the solvent, it is also
possible to use, for example, organic solvents as auxiliary
solvents.
The tensides (wetting agents and emulsifiers) can
be nonionic compounds, such as condensation products of
fatty acids, fatty alcohols or aliphatically substituted
phenols with ethylene oxide; fatty acid esters and fatty
acid ethers of sugars or polyhydric alcohols; the products
which are obtained from sugars or polyhydric alcohols by
condensation with ethylene oxide, block polymers of
ethylene oxide and propylene oxide; or alkyldimethylamine
oxides.
The tensides can also be anionic compounds, such
as soaps; fatty sulphate esters, for example dodecyl sodium
sulphate, octadecyl sodium sulphate and cetyl sodium
sulphate; alkylsulphonates, arylsulphonates and aliphatic-
aromatic sulphonates, such as alkylben~enesulphonates, for
example calcium dodecylbenzenesulphonate, and butyl-
naphthalenesulphonates; and more complex fatty sulphonates,for example the amide condensation product of oleic acid
and N-methyltaurine, and the sodium sulphonate of dioctyl
succinate.
-
~, :
,
Z~ 3 ~
- 14 -
Finally, the tensides can be cationic compounds,
such as alkyldimethylbenzylammonium chlorides, dialkyl-
dimethylammonium chlorides, alkyltrimethylammonium
chlorides and ethoxylated quaternary ammonium chlorides.
Suitable dispersing agents (without surfactant
action) are mainly lignin, sodium salts and ammonium salts
of lignin sulphonic acid, sodium salts of maleic
anhydride/diisobutylene copolymers, sodium salts and
ammonium salts of sulphonated polycondensation products of
naphthalene and formaldehyde, and sulphite waste liquors.
Examples of dispersing agents which are parti-
cularly suitable as thickeners and anti-settling agents are
methylcellulose, carboxymethylcellulose, hydroxyethyl-
cellulose, polyvinyl alcohol, alginates, caseinates and
blood albumin.
Examples of suitable stabilizers are acid-binding
agents, for example epichlorohydrin, phenyl glycidyl ether
and soya epoxides; antioxidants, for example gallic esters
and butyl hydroxytoluene; UV absorbers, for example
substituted benzophenones, diphenylacrylonitrile acid
esters and cinnamic esters; and deactivators, for example
salts of ethylenediamine tetraacetic acid, and polyglycols.
In addition to the active substances of the
ormula I, the fungicidal compositions according to the
invention can also contain other active substances, for
example further fungicidal agents, insecticidal and
acaricidal agents, bactericides, plant growth regulators
and fertilizers. Such combination compositions are
suitable for broadening the spectrum of activity or for
specifically influencing plant growth.
In general, the fungicidal compositions according
to the invention contain, depending on their type, between
0.0001 and 85 percent by weight of compound according to
the invention, or compounds according to the invention~ as
: . ~ . . .
,: :
~. .
.
active substance(s). They can be in a form which is
suitable for storage and transport. The concPntration of
active substance in such forms, for example emulsifiable
concentrates, is usually in the higher range of the above
concentration interval. These forms can then be diluted
with the same or different formulation adjuvants down to
concentrations of active substance which are suitable for
use in practice, and such concentrations are usually in the
lower range of the above concentration interval. In
general, emulsifiable concentrates contain 5 to 85 percent
by weight, preferably 25 to 75 percent by weight, of the
compound(s) according to the invention. Suitable use forms
are, inter alia, ready-to-use solutions, emulsions and
suspensions, which are suitable, for example, as spray
liquors. Concentrations of between 0.0001 and 20 percent
by weight, for example, can be present in such spray
liquors. When using the ultra-low-volume method, it is
possible to formulate spray liquors in which the concen-
tration of active substance is preferably from 0.5 to 20
percent by weight, while the spray liquors formulated for
the low-volume method and the high-volume method preferably
have a concentration of active substance of 0.02 to 1.0
percent by weight, or 0.002 to 0.1 percent by weight,
respectively.
The fungicidal compositions according to the
invention can be prepared by a process in which at least
one compound according to the invention is mixed with
formulation adjuvants.
The compositions can be prepared in a known
manner, for example by mixing the active substance with
solid carriers, by dissolving or suspending the active
substance in suitable solvents or dispersion media, if
appropriate with the use of tensides as wetting agents or
emulsifiers, or of dispersing agents, or by diluting
already prepared emulsifiable concentrates with solvents or
dispersion media, etc.
.: .
, ' ,. ' ::' ': -
:, ,
2~ 3~45
- 16 -
In the case of compositions in the form of
powders, the active substance can be mixed with a solid
carrier, for example by grinding the active substance
together with the carrier; or the solid carrier can be
impregnated with a solution or suspension of the active
substance, and the solvent or dispersion media can then be
removed by evaporating, heating or by filtering off with
suction, under reduced pressure. By adding tensides or
dispersing agents, such compositions in the form of powders
can be rendered readily wettable with water, so that they
can be converted into aqueous suspensions which are
suitable, for example, as sprays.
The compounds according to the invention can also
be mixed with a tenside and a solid carrier to form a
wettable powder which is dispersible in water, or they can
be mixed with a solid, pre-granulated carrier to foxm a
product in the form of granules.
If desired, a compound according to the invention
can be dissolved in a water-immiscible solvent, such as,
for example, an alicyclic ketone, and this solvent suitably
contains a dissolved emulsifier, so that the solution is
self-emulsifying on addltion to water. Alternatively, the
active substance can be mixed with an emulsifier, and the
mixture can then be diluted with water to the desired
concentration. In addition, the active substance can be
dissolved in a solvent, and the solution can then be mixed
with an emulsifier. Such a mixture can likewise be diluted
with water to the desired concentration. In this manner,
emulsifiable concentrates or ready-to-use emulsions are
obtained.
The compositions according to the invention can be
applied by the application methods customary in plant
protection or agriculture. The process according to the
invention for controlling fungi is characterized in that
the material to be protected, for example plantsl parts of
plants or seeds, is treated with an effective amount of a
.
. ~
'2(~ S
- 17 -
compound according to the invention or a composition
according to the invention.
The examples which follow illustrate the
invention.
I.Manufacture of the active substances of the formula I:
Example 1
1 0
2.0 g (7.0 mmol) of methyl 2-ta-bromo-o-tolyl)-3-
methoxyacrylate and 0.95 g (7.0 mmol~ of acetophenone oxime
are added at 0C to 0.19 g (7.7 mmol) of sodium hydride in
20 ml of dimethylformamide. After the reaction mixture has
been stirred for 10 minutes, saturated sodium bicarbonate
solution is added, and the mixture is extracted three times
using ethyl acetate. The organic phases are dried over
anhydrous sodium sulphate. After the solvent has been
distilled off, the oil which remains is purified by
chromatography on silica gel using diethyl ether/n-hexane
(1:1) as the eluent. In this manner, methyl [E] -3
-methoxy-2-[a-{[(-methylbenzyl)imino]oxy}-o-tolyl]-
acrylate is obtained as a yellow oil.
Example 2
3.67 g (13.2 mmol) of methyl 2-(~-bromo-o-tolyl)-
3-methoxy-acrylate and 3.0 g (13.2 mmol) of 4-phenoxyaceto-
phenone oxime in 13 ml of methylene chloride are stirred
vigorously for 10 minutes at room temperature with 13 ml of
2.2N sodium hydroxide solution and 5.7 g of tetrabutyl
ammonium bisulphate. Identical amounts of methylene
chloride, 2.2N sodium hydroxide solution and tetrabutyl-
ammonium bisulphate are then added, and stirring is
continued for 10 minutes. Again, identical amounts of 2.2N
sodium hydroxide solution and tetrabutylammonium bisulphate
are subse~uently added, and, after the mixture has been
stirred for a further 10 minutes, saturated sodium
bicarbonate solution.
~OC~3~5
- 18 -
The mixture is extracted three times with ethyl
acetate, and the combined organic phases are washed with
saturated sodium chloride solution and dried over anhydrous
sodium sulphate. After the solvent has been distilled off,
the oil which remains is purified by chromatography on
silica gel using n-hexane/diethyl ether (4:1) as the
eluent
In this manner, methyl [E~3-methoxy-2-ta-l[(a-
methyl-4-phenoxybenzyl)imino]oxy}-o-tolyl]-acrylate is
obtained as a yellow oil.
Examples 3-244
Starting from methyl 2-(a-bromo-o-tolyl)-3-
methoxyacrylate and the appropriate oxime of the formula
II, the compounds of the formula I listed in the table
below are obtained in analogy to the process described in
Example 1 ("Method 1") or Example 2 ("Method 2"):
C~jGHC~ ~c~cH3
Rl ¦ I'
2 C=~l--C~
''` , :
.
.
, . . . .
-. . ~ :,
-- 19 --
~le
. ~ . __
Ex~)le Rl ~2 Pl~ical M3tld
~ata 1/2
. _ _ ___ .
3 ca~l ~ ~p. 136-137~
4 2-furyl ~1 ~p. 77C
bE~yl ethoxy - oil 1
cas~l
63,5~(tri- ~yl np. 11?-113C 1
fluQra~t~yl)-
~1
72--fll~5-- n oi l
m3tl~1-Ei~l
82-b~zofurar~l .- oi 1
9 4~1O~1 ~yd~ ~p. 119~
2,4-dichlar~t. ~. 111-112C 1
E~!l
11 3-nit3:Q~l ., oil 1
12 2~inoliny1 .- ~. 114-115C 1
13 5~3thyl-3- mE~yl oi ~ 1
~lthlo-4-
i~oxazolyl
14 pih~#nylt~if luoro- oil
~1
4-fl~rq~yl me~yl oi ~ 1
16 4-nit~l .. Ilp. 145-146C
17 pheny 1 ~ o; l 1
18 2-~yric~yl ll o; 1 1
~9 3~ yl .. oil
2--chlor~yl n ~p~ 97--98 C
21 bEnzo~rl . " oil 1
22 ,B-pt~yle~yl ll ~. 151-~52C 1
23 2-~chi~yl ~yl ~ 0-61C 1
24 4--chlo~1 n n~ 119 C 1
25 E~Yl et~l oil
26 4-chlorc~1 .. oi ~
27 E~yl n-Ex~F~rl oil
Z~ 5
_ 20 --
. _ e ~ _, _
Exan~ple ~ R2 ~?~ysical ~gbthod
data 1/2
~ . . _ _
28 Ehe~l ~ oil 1
E~fl
29 qc~l ~yano ~ 1-9~C
30 bE~s!l ~yl O;l 1
31 ~-Eihe~leth~l .. Dæ. 11~-114C
32 ~,~,~iflunro-- ~ ~p~, 127--128aC
~tolyl
33 2-E~1 me~yl ~po 65-f;7"C
34 3--~i~l n oi 1 1
4-E~i~l ~ ~e- 101-1031
36 5~t~1-2~ ~l oi1
37 5-chla~:2- ~ ~ 1:
th~l ~p. 91-9~C
is~ 2 oil
38 2,4-din~t~1-5- ~. oi 1
~lyl
39 o--tolyl n oi 1 1
P~l n llç~ 89--90 C . 1
41 4-biEhelylyl n ~ 117--118C 1
42 cy~:l~yl c~:lc~c~yl oil 1
43 ~-(2-fu~l)- m~t}yl oi l 1
~yl
44 ~ (3,4,5--tri-- n oi 1
me~-~l)-
~th~yl
4-t4-chla3:o-3- .. oil 1
rli~l)-3-
n~ 1,3-h~
diyl
46 3,4-di~1~1 ~ ~p. 95-g6~
47 2-na~t~l .. ol 1
48 2-thiazolyl .. oi ~
49 ~,~t~-trifluo~ met~l ~p. 55-56C
m-tolyl
: , , ~ ... .
.: : ~ . .:: , .
-- 21 --
_ .- _ .
Exan~le I~ R2 P~sical ~d
data 1/2
3~q1 methyl ~p. 73-74~C
51 2~al:~1 n oil
52 3--llit~l n o~ l
53 6 c:hl~2- ~- ~p. 129-130C
di~zof~l c~l
54 ~Tl E~l oil
5~hlo~3- ~ yl ~. 11~-113C
D~y1-2~
~hi~l
56 3--chlod:t~l n ~i 1
57 3,4 di~lo~1 .. -~p~ 8~-83C 1
53 2,4 di~lor~1 .. ~ær 1: oil 1
i~r 2: o~
59 2-na ~t~yl - oil
60 ~-meth~y- ~ hydrDgen oil
2,4-dichlornbenzy1
61 ~henyl n-butyl oil
62 2,5-d~methy1-3- methyl mp. ~8-69C 2
~1
63 phenyl isopn~yl oil
64 phenyl cyclohexyl o;l 1
65 2-(p-ter~.~utyl- hydro~en oil
Ehe~
~1
66 2,4-dichlorbbenzy1 3-p~ridyl oi
67 2,4-dichloro- 30p~ridy1- oi
~yl ~1
68 3~methy1-2- mEthyl mp. 8~-86C
b~hier~yl
69 3,5-dim~thy1-2- .. ~p. 96-97C 1
furyl
70 1-naFh~hyl hydrogen oil
71 ~ (4imethlxy- H ~p. 83-87 C
p~l)-e~l
:: . , , :
5~'15
~ 22 --
Table (cca~ati~
~ _ __ _ __
EsE~ple ~ R2 P~sical ~tbod
data 1/2
_ _ ~
72 m-tolyl ~ o~l
73 2~ ~1 n l~p~ 83--~14 C
74 3~ ~l n 3~p~ 82--B3C
4--fll~l n ~ 1
. Il~p. 92-931::
i~aner 2~ o;l
76 ~ 2-- n Qil
. ~h~l
77 3~hl~1 n o; 1
:15 7~ 3-}~1 ~1 Oil
79 4-h~yl ll ~p. 142-143C
3~y1-2 ~ ., Oil
~1
81 213-difll~l ~ oil
82 p--~olyl n ~ 93~94 s~
83 2-t~i~l .. oil
84 2,5-diflu~y1 ll oil 1
85 2-fluor~6~hlcxm- ll n~p. 70-73t::
E~1
86 3-phe~-~1 ~t oil
87 4~1E~1 n oil 1
88 3--thi~yl n iK~ 1: 1
~p. 125-126C
. ~2: oi
89 ~~ ( 2~1i~Dli~l ) ~ n oil
~1
~_ ( 3_th~1 ) n O i l
e~l
91 Q,~2,c~-trifluaro- .. oi
m-tolyl
92 ~~ (~hyl--2 n oil
E~olyl)~l
93 4-~1 met~l mp~ 123-124C
"
ZO~i3~5i
23
~le 1~ ~_
data 1/2
S . . ~ _
94 2,5-di~l~ ~}~yl i~ lo
~yl ~p. 92-93C
~ ~: oi~
95 4~1~1 N I:)il 2
96 3~1Ehel~Tl 1~ oil 2
97 3 ~ 4--dii~l-- n ~ 88--89 C 2
~1
9B 2,4~met~1- - oi 1 2
EhE~yl
99 2-fl~yl ., 73~4t'C
100 m-tolyl .. ~p. 7~C 2
101 ~tolyl .. 77-78C 2
102 6~t~1--2-- n oi 1 2
r~
103 5-~2-~hi~l .. i~r 1:
np. 98C
~2: o;
104 2-~hi~l t~rt.h~l oil
105 l-na~l ~yl ~p. 11~.5-121C 1
106 2-na~ yl ~lc~yl oil
107 2-na~ yl ~1 oil
108 4-diflu~ ~1 ~. 102-103C
~1
109 4{:hl~1 ~1 oil
110 2-naE~t}~l n-E~l oi l 1
111 3,5~i(tr~f1uoro- et~l ~ 1: oil 1
mE~yl)-Eit~l is~ 2: oil
112 5,6,7~8-t~ ~yl llp. 97-98C 2
~r~2-rla~t}yl
113 c~ fluo2:~ ll ~p. 123-124C
p-tolyl
114 3-Eihenanth~l .. oi l ~
115 2-fluar~l ll oi 1 2
116 ~1 .l oil 2
~ .
`~ ;,, ~ .
.
Z C~ 5
~ 24 ~
~le (c~t~i~n)
` .......... _ ~ . _
Ex3~e ~ R2 P~si~l ~thod
data 1/2
S . __ ~. . . ,, _
117 ~chlc~l ~p~l oil
118 4~hlo~1 ~:l~yl oil
119 3~1 ~yl ~p. 86-87C
120 3~1 ~1 o;~
1 7l 4--fll~l n i8~æ 1 oil
i~2. oil .
122 4-bromophenyl ~ mp. 108C l
123 4-tert.h~t~yl- ~yl ~p. 105-106C 2
E~l
124 3-thif~l .. mp. ~7-88C
125 cy~:l~l .. oil 2
126 2-quinDli~r1- ,. oil 2
~1
127 3_f~5-tri- - ~p. m2-103C
fl~l-E~l
128 3-fl~yl ll Oil
129 3,5~ 1u~y1 n oil 1
130 3,5~1u~y1 e~yl oil
131 2--fl~l n isa~ 1: i 1 .
~ 2~
132 3,4-di~- ~yl oil 2
~1
133 p-tolyl ~yl Oil 2
134 2,5~1-~- ~:}~1 c)il
fu~!l
135 ~1 ~tl~lthi~ ~ 1: oi
~yl i~ 2: oi~
136 2-thiazoli~lmQ~lthiD ~p. 81-83~C
137 bel~zyl ~}~yl ~il
138 3-tri~luo~ ll oi 1
metho~_~yl
13g 3-flu~5~ e~yl ~ 1: oil 1
fluDmnet~yl- isc~er 2: oil
p~l .
.
2~ 5
-- 25 --
~le ~a~
, . ~ . _ ~
Ex3n ple ~ R2 P~;i~:al ~tl~d
data1/2
S . ~__ _
1~0 3,4,5~ ~1 O;~ 2
E~l
141 2-1li~1 ~ oil 1
142 ~ lolle~l n oil 2
143 ~lthi~l n L~Ic 1 o oil 2
i~CG 2 t~il
1ds4 4-dll0~1 tri fltX~ oi l
qyl
145 2-thi~l c~clc~l oi 1 1
146 3/4 dineb~9!~ m~yl oil
b~l
1~7 ~ trifluar~ tri~luo~ Oi 1
m-tolyl me~l
148 ~ ri1u~ e~yl oi 1 1
m-tolyl
14g 3,5-di~lo~ mEt:}~l o; 1 1
Ehe~yl
150 ~-(2-S~yl)- tri~lu~ oi l .
~1 ~1
151 2-na~ l .. oi1
152 E~yl ~Y~ i~ 1: oil
mEt:}~l ~ 2s oil
153 E~yl m~yl oil
154 ma~yl .. oil 2
155 ~ _triflu~ n-E~Yl oil 1
m-tolyl
156 ~,~f~-trifluaro- ~~ i~ 1: oil 1
m~olyl me~ yl ~ 2: oil
157 2~ y1 n~ oil 1
158 c~ _triflu~ cy~l~l ~il 1
m-tolyl
159 2-E~yri~l .. o~l 1
160 2-E~yric~yl m3th~ - oil
~1 ....
5~
-- 26 ~
~e . _ R~ P~ ical 1/2
S _ . _ . _, ~ ~
161 2~chlcLe~5-tri- ~yl i~3aær 1: oil
f~ i~ 2~
E~l
162 2~1tbiQ--5~ n is~ 1 oi:L
0 ~fl~ ~ 20 oil
~yl
163 4~1ar~3~ .. oil
f~ b~l
164 4~1 .. oil 1
16S 3~ yl ~t~yl
166 ~,,c~-trifluo3:0- iEc~l o;
n~tolyl
167 a~ ifluo~ me~ yl oil 1
o-tolyl ~
168 ~ uam- u~ z- oi l
m-tolyl triflu~
m-tolyl
169 4~y1 ~yl m.p. 79-80C 2
170 N~t~ 2- .. oil 1
E~lyl -
171 10, 11--di~5H-- n oil
d~be0zo[b. f ]aze-
pin-2-yl
172 3-chlor~4-_1~ n m.p. 85C
Eihenyl
173 2 t 3~ hl~1 n i~ 1 oil
isc~ 2: Oi
174 ~toly~l triflu~ o;l
~1
175 4-~lthio- met~l oil 2
. ~1
176 4-(2,4-dichl~ .. c~il 2
~)-~1
177 4-(4-n~t~) .. oil 2
40 ~yl
,, : .
;.,, . , : .
. .
- , `
'; ~
2~ 5
-- 27 --
qable (cc~i~)
_ I __ _. __
E~le Rl lR~ Pl~ al ~5ethDd
data 1/2
_ _ _ ~
178 4_(4~y_ ~yl Ojl 2
E~)-Ehe~yl
179 3, 4~t~y1ene- n oi 1 2
di~yl
180 7--bellzodi~l n oil ;~
181 2~5-dil hlo~3- ~t i~ il
~1 is~ 2: oil
182 2-thi~l e~l i~æ ~: oi~ 1
is~ 2: oil
183 2-chl~y m~l oil 1
~t3~yl
184 o-~olyl me~_ i~nEr 1:
ca~l mOp. 101-103C
isa~r2:
2Q m.p. 85-86 C
185 3-~rl ~yl Ojl
186 3-~luaro-5-tri-~lc~yl oil 1
fl~l~
187 2,3,4-tri~:hlo~ me~!l m.p. 96-97C 1
E~yl
188 4~hlo~3~y1- u m.p. 88C
E~l
189 p-tolyl n-E~fl oil
190 5~t}~y1-2- ~yl i6~r 1: oil 1
~ch~l i~ 2: oil
191 2-~chi~yl n~ is~ l: oil 1
. i~ 2: o;~
192 4-~y-p~l ~ycl~l oil 2
193 3-}~yl .. oil
194 3 chl~1 ., oil 1
195 3--fll~l n oi 1
196 2--thiazolyl n ~ 1: c)i 1
isa~ 2
m.~. 71-72C
., ~ ,; . .
- . . .
~: . .
.. : ., .. : .
: . .. ~ , :
.- .,, .. :
2~
-- 28 --
q~ble (ccnti~mati~)
.... _ ~ _ ._ ,. .. . ..
Ex~mple Rl R2 P~sical ~d
~ata 1/2
~ _ _
197 3-l~rl n-~s~rl o;l
lg8 3~yl .. oil
199 4~f1~ i n oil
f~
200 3~ ~1 n oil 1
201 4--EdY~ p~l n Qil 2
202 3--f 1 uQr~5~ n oil
fl~e}y~
203 2--~ol~l n oil 1
204 3~1 triflu~ oil
m~l
205 3~l1a~1 n oi 1
206 2-thiazolyl n m.p, 97-98C
207 4--Ehe~--~1 n oi 1
208 2-E~yric~yl " ojl 1
209 3-l~yl ~11 O;l 2
210 3~y1 n oil 2
211 2-E~ic~yl ,. i~mer 1: oil
isa~3r 2: oil
~12 4~ ~l ., oil 2
213 3-l~yl methD~- isa~ lo oil
~yl is~ 2: oil
214 4--fluQ~3~ n ~ iUllEI:' 1 oil
fluaw~yl- ~ 2: oil
E~yl
215 4-fluoro-3-tri- ~:lc~rl oi
~1~1-
~1 .
216 2-~1 met~l oil
217 4-fl~yl c~:lo~l ~il 1
218 4- (n-~Yl ) - me~l oil 2
E~
219 4~-3-ru~ ll m.p. 131-132C 1
~rl
- . ~ .. . . .
. - . . : - ~ :, , . , : ,
,
2~S3~
~ 29
Table (~i~ati~)
. _ ~.................. ~ . _ .
Exa;~le ~ ~Z P~sical 2kth~d
data 1/2
,, _ ~ __
220 2,3-dil~5- m~yl oil . 2
benzolb]fu:~l
221 2~1 .. o~l
222 2,4-di~y_ .. oil 2
~1
223 4-~1~3_~._ ~1 oil 1
fl~lyl- .
E~l
224 3-chlorc~l ~ ~ jRr~nPr 1: o; l 1
m~l isa~ 2: oil
225 3-~1 ~yl m.p. 103C
226 4-~yl .. m.p. 124C
227 2-na~tl~yl ~~ oil
~1
228 2-(4~_ ~1 oil .
E~~
229 1,4,8-tri~yl- .. O;
s~na-1,3,7-t3~i~1
230 1~1--2--( 3 ~ ~~ n oil 1
~yl~nedi~_
p~l)~l
231 (4-flu~l)- }~ O;l 2
carbamoyl
_ _ , ., ,_ . .. , .
- : ;, .. ... .. . ~ ~ . i . . ... . . .
~, . ... . .. .
~::
.
- .. .. . ~ . :
:,
-- 30 --
. _ . . _ __
~P~ ~ ~ ~hod
I /C da~a ï/2
. . _ _ ~ .
232 ~? oll 1
233 c~
23~ ~ ~ ~ ~
236 o;l ~ 1
~3 ~S~
i.
31
E:x~le ~}~ P}~ . l~th~d
(~R2/ d~ta 1/2
S ~T~3
240 ~ ~ oil
241 ~ o
10 ~ ~ ,
0~ o i 1 ¦ 1
.
j: ,: ,
,~
,~ .: : ~ .
2~
_ 32 _
II. Formulation exam~es
Example 245
An emulsifiable concentrate has the following
composition:
q/litre
Active substance (compound according to the invention) 100
Nonylphenol (10)ethoxylate (nonionic emulsifier) 50
Calcium dodecylbenzenesulphonate (anionic emulsifier) 25
N-methyl-2-pyrrolidone (solubilizer) 200
Mixture of alkylbenzenes (solvent) to 1 l
The active substance and the emulsifiers are
dissolved in the solvent and the solubilizer. A ready-
to-use spray liquor of any desired concentration can be
prepared by emulsifying this concentrate in water.
.-. . . ~ - . ;
~:: i ,,
.
20 0~ ~r
- 33 -
ExamPle 246
A wettable powder has the following composition:
Percent bY
weiqht
Active substance (compound according to the
invention) 25.0
Silica (hydratised; carrier) 20.0
10 Sodium lauryl sulphate (wetting agent) 2.0
Sodium lignosulphonate (dispersing agent) 4.0
Kaolin (carrier) 49.0
15 The components are mixed with each other, and the
mixture is ground finely in a suitable mill. Dispersing
the mixture in water results in a suspension which is
suitable as a ready-to-use spray liquor.
, , :., , : . . ~, , ~